Abstract
The US EPA’s ToxCast program is designed to assess chemical perturbations of molecular and cellular endpoints using a variety of high-throughput screening (HTS) assays. However, existing HTS assays have limited or no xenobiotic metabolism which could lead to false positive (chemical is detoxified in vivo) as well as false negative results (chemical is bioactivated in vivo) and thus potential mischaracterization of chemical hazard. To address this challenge, the ten most prevalent human liver cytochrome P450 (CYP) enzymes were introduced into a human cell line (HEK293T) with low endogenous metabolic capacity. The CYP enzymes were introduced via transfection of modified mRNAs as either singlets or as a mixture in relative proportions as expressed in human liver. Initial experiments using luminogenic substrates demonstrate that CYP enzyme activities are significantly increased when co-transfected with an mRNA encoding a CYP accessory protein, P450 oxidoreductase (POR). Transfected HEK293T cells demonstrate the ability to produce predicted metabolites following treatment with well-studied CYP substrates for at least 18 hours post-treatment. As a demonstration of how this method can be used to retrofit existing HTS assays, a proof-of-concept screen for cytotoxicity in HEK293T cells was conducted using 56 test compounds. The results demonstrate that the xenobiotic metabolism conferred by transfection of CYP-encoding mRNAs shifts the dose-response relationship for some of the tested chemicals such as aflatoxin B1 (bioactivation) and fenazaquin (detoxification). Overall, transfection of CYP-encoding mRNAs is an effective and portable solution for retrofitting existing cell-based HTS assays with metabolic competence.
Keywords: Metabolism, mRNA, cell-based assay, high-throughput screening, cytochrome P450, biotransformation
Introduction
Biotransformation is a fundamental principle of toxicology and a critical consideration in the evaluation of any chemically-induced perturbation of a molecular and/or cellular endpoint. However, the in vitro assay systems widely used high-throughput screening (HTS) campaigns to identify potential toxicants often lack xenobiotic metabolism capabilities (Westerink and Schoonen, 2007; Gotz et al., 2012; Soltanpour et al., 2012; Thomas et al., 2012; Garcia-Canton et al., 2013). The insufficiency or lack of metabolic competence in these in vitro systems can lead to false positive (if the chemical is detoxified in vivo) as well as false negative results (if the chemical is bioactivated in vivo) and a potential mischaracterization of chemical hazard. The widespread acknowledgement of this issue has prompted calls from the Organisation for Economic Co-operation and Development (OECD) and others for improved in vitro methods that incorporate xenobiotic metabolism (Kirkland et al., 2007; OECD, 2008; Jacobs et al., 2013).
In vitro metabolic systems can be broadly categorized into two groups based on where metabolism of the parent compound occurs – either outside of the cell (extracellular) or inside the cell (intracellular). Extracellular metabolism systems typically utilize hepatic subcellular fractions such as microsomes or the post mitochondrial supernatant (S9) to model the effects of circulating metabolites on distal target tissues. For example, direct S9 addition to recombinant U2OS cells in culture has been used to demonstrate the formation of estrogen receptor (ER)-active metabolites (Mollergues et al., 2017). The disadvantages of this system include cytotoxicity of the microsome/S9 subcellular fraction as well as the requirement for metabolites to cross the cell membrane in order to interact with the target of interest. In contrast, intracellular metabolism systems, such as primary cells, model the effects of direct-acting metabolites. Although newer approaches are overcoming some of the limitations of using primary cells and 3-D culture systems, these models can be difficult to deploy in high-throughput toxicity testing, have limited availability, are slow growing, and often suffer from high inter-individual variability (Donato et al., 2008; Chen, 2010).
Apart from the use of primary cells, one long-standing solution is the introduction of xenobiotic metabolizing enzyme (XME)-encoding complementary DNA (cDNA) genes into immortalized cell lines with low or no endogenous metabolic capacity (Kitamura et al., 1992; Doehmer, 1993; Mace et al., 1997; Bull et al., 2001; Vignati et al., 2005; Xuan et al., 2016). Two disadvantages of cDNA-driven expression are that relatively few genes can be multiplexed for co-expression and the expression of each gene cannot be tightly controlled to mimic tissue-specific metabolism. To avoid the challenges associated with XME cDNA expression, the use of messenger RNAs (mRNA) has been proposed, although historically, the direct introduction of mRNAs into cells lead to activation of the interferon response and cytotoxicity (Diebold et al., 2004; Warren et al., 2010). Advances in the development of chemically-modified mRNAs have significantly reduce the immunogenicity and cytotoxicity typically associated with mRNA transfections, as recently demonstrated for stem cell reprogramming (Warren et al., 2010; Preskey et al., 2016). The direct introduction of mRNA transcripts into the cell provides rapid protein expression without the need for transcription and the relative expression of each mRNA can be tightly controlled by varying the relative or absolute amount of transfected mRNA. Transfection of mRNA also avoids cell-type dependent differences in transcription. As such, mRNA transfection is an attractive solution for retrofitting HTS assays with metabolic competence due to the ease and portability by which XME can be introduced into existing cell-based assays.
The ToxCast and Tox21 portfolio of high-throughput screening assays contain over 300 cell-based assay endpoints. In both ToxCast and Tox21, thousands of industrial and environmental chemicals have been tested, thus highlighting the significant investment in characterizing the biological activities associated with these chemicals. Given the existing investment in ToxCast and Tox21, and the broad range of cellular assays employed, a flexible approach to retrofit the existing cell-based high-throughput screening assays with metabolic competence was investigated. In this report, we utilize chemically-modified mRNAs to transiently express ten human cytochrome P450 (CYP) enzymes along with an accessory protein, P450 oxidoreductase (POR) in human embryonic kidney cells (HEK293T). In doing so, we describe the optimization and validation process for imbuing metabolic competence to a cell line with little to no intrinsic metabolic capability in a manner that is portable to other cell types and amenable to high-throughput screening.
Materials & Methods
Plasmid Constructs.
Human cDNAs for the cytochrome P450 (CYP) and P450 oxidoreductase (POR) enzymes were purchased from Open Biosystems (Dharmacon, Lafayette, CO). Clone Identification (ID) and accession numbers are listed in Supplementary Table S1. Each cDNA, with the exception of CYP2A6, was PCR amplified using Q5 High-Fidelity 2X Master Mix (New England Biolabs, Ipswich, MA) to incorporate 5’ (BbsI, BsmBI, or BsaI) and 3’ (NotI) restriction enzyme sites (forward and reverse primers are presented in Supplementary Table S1). The resulting PCR fragments were digested, purified, and ligated into the NcoI/NotI sites of the plasmid pmRNA (TriLink Biotechnologies, San Diego, CA). CYP2A6 cDNA was PCR amplified using the Q5 High-Fidelity 2X Master Mix and a 5’-phosphorylated forward primer. The PCR product was purified and cloned into pmRNA between a 5’ blunted NcoI site (Quick Blunting Kit, New England Biolabs) and a complementary 3’ NotI site. Plasmids were purified by cesium chloride-ethidium bromide gradient centrifugation (Sambrook and Russell, 2001) and sequence verified by fluorescent DNA capillary sequencing.
In vitro Transcription.
The pmRNA vector (TriLink Biotechnologies) served as a template for in vitro transcription and contains a T7 RNA polymerase promoter, a proprietary 5’ untranslated region (UTR) with a strong Kozak sequence and an alpha globin 3’ UTR. Eleven purified cDNA plasmid constructs as described above were sent to TriLink Biotechnologies for the synthesis of chemically-modified mRNA. β-galactosidase (βgal) mRNA, used as a negative transfection control, was transcribed using a vendor-supplied plasmid. The final mRNA transcripts, including βgal, were fully substituted with 5-methylcytidine (5meC) and pseudouridine (ψ), contained a poly(A) tail, and were capped with an anti-reverse cap analog (ARCA). All mRNA transcripts were processed via standard procedures including enzymatic treatment with DNase and phosphatase to remove the DNA template and the terminal 5’ triphosphate, respectively. Upon arrival, mRNAs were aliquoted into single use portions prior to the first use and stored at −80°C
Cell Culture and Transfection Workflow.
The human HEK293T cell line (GenHunter; Nashville, TN) was maintained in Dulbecco’s Modified Eagle’s Medium (DMEM; ThermoFisher, Grand Island, NY) containing 10% fetal bovine serum (FBS, HyClone, Logan, UT), 1X penicillin-streptomycin (HyClone), and 5 mM HEPES in a humidified 37°C atmosphere containing 5% CO2. To increase HEK293T cell adhesion, cell culture flasks for routine maintenance and 384-well microplates for transfection experiments were coated with collagen I (from rat tail; prepared at 50 μg/mL in 0.05 N acetic acid, Corning, Corning, NY) and rinsed with sterile water prior to use. To initiate mRNA transfection experiments, cells were seeded at a density of 25,000 cells per well in 384-well, solid white assay plates (Greiner Bio-One, Monroe, NC) in 40 μL DMEM supplemented with 2% FBS, and allowed to attach for 6 hours. Preparation of the mRNA:lipid complexes was per the manufacturer’s recommendations (Lipofectamine MessengerMAX, ThermoFisher), with specific exceptions and optimizations discussed in the text. Briefly, MessengerMAX transfection reagent was diluted 1:20 into Opti-MEM Reduced Serum Medium (ThermoFisher) and allowed to incubate for 10 minutes at room temperature. Separately, mRNA was also diluted into Opti-MEM Reduced Serum Medium and then mixed with the diluted MessengerMax transfection reagent in equal volumes and allowed to incubate for an additional 5 minutes at room temperature. The mRNA:lipid complexes were dispensed by single channel pipet for Figures 1–4, and with a Certus Flex automated liquid dispensing system (Fritz Gyger AG, Switzerland) for Figures 5–7. Following mRNA transfection, the 384-well plates were returned to a 37°C/5% CO2 humidified atmosphere to allow for protein expression. For the experiments shown in Figures 4–7, the ten most prevalent human liver CYP-encoding mRNAs were transfected as singlets or in a 10-CYP mixture. This CYP combination designated as “liver mix” is pooled in a ratio based on mRNA expression patterns in a series of human liver samples (Zanger and Schwab, 2013). The constituent CYP mRNA levels present in the liver mix are presented in Table 1. At 6 hours post-transfection, cells were treated with various luminogenic substrates or test compounds as described below.
Figure 1. CYP mRNA transfection and optimization of POR co-expression.
(A) HEK293T cells were transfected with P450 oxidoreductase (middle) or CYP3A4 mRNA (right) and CYP3A4 activity (y-axis; note log-scale) was measured by the addition of luciferin-IPA for 12 hours at 6 hours post-transfection and compared to un-transfected control cells (left). Data expressed as mean CYP3A4 activity ± sd (dashed lines) of three replicate experiments. * indicates p-value > 0.001 for %Mean CYP3A4 activity compared to untransfected control using Welch two sample t-test. (B) HEK293T cells were transfected with 12.5 ng CYP3A4 mRNA per well and co-transfected with POR mRNA from 12.5 pg −12.5 ng per well (expressed as % of CYP3A4 mRNA on x-axis) and βgalactosidase mRNA to maintain total mRNA transfected per well. CYP3A4 activity (y-axis) was measured by the addition of luciferin-IPA for 12 hours at 6 hours post-transfection and expressed as mean % CYP3A4 (no POR) activity ± sd (dashed lines) of three replicate experiments. * indicates p-value > 0.001 for %POR compared to CYP3A4 only (No POR) using Welch two sample t-test.
Figure 4. Metabolism of luminogenic CYP substrates.
HEK293T cells were transfected with 25 ng of β-galactosidase (A), 24 ng of CYP1A2 (B), CYP2A6 (C), CYP2B6 (D), CYP2C8 (E), CYP2C9 (F), CYP2C19 (G), CYP2D6 (H), CYP2E1 (I), CYP2J2 (J), CYP3A4 (K), or a 10-CYP liver mix (L; Table 1 for ratio) + 1 ng P450 oxidoreductase mRNA at a lipid:mRNA ratio of 2.5 and delivered at 5 μL per well. Luminogenic CYP substrates (x-axis) were added as 10 μL of 5.5x stocks at 6 hours post-transfection, excepting luciferin-1A2 and −2B6 were added as 55 μL of 1x stocks made in Krebs-Henseleit buffer supplemented with 3 μM salicylamide after a 40 μL rinse with Krebs-Henseleit buffer supplemented with 3 μM salicylamide. Substrates were incubated for 12 hours prior to luminescent detection. CYP activity (y-axis) was expressed as mean RLU ± sd of three replicate experiments. * indicates p-value > 0.001 for enzyme-substrate pair compared to βgal control using Welch two sample t-test.
Figure 5. Metabolism of luminogenic CYP substrates over time course.
HEK293T cells were transfected with 25 ng of βgal or 24 ng of CYP3A4, CYP2J2 or a 10-CYP liver mix (A; Table 1 for ratio), CYP2D6 (B), CYP2C8 (C), CYP2C9 (D), CYP1A2, CYP2A6, or CYP2E1 (E), CYP2B6 or CYP2C19 (F) + 1 ng P450 oxidoreductase mRNA at a lipid:mRNA ratio of 2.5 and delivered at 5 μL per well. Luciferin-IPA (A), -ME EGE (B), -ME (C), or -H (D) were added as 10 μL of 5.5x stocks at 6 hours post-transfection, whereas luciferin-1A2 (E) and −2B6 (F) were added as 55 μL of 1x stocks made in Krebs-Henseleit buffer supplemented with 3 μM salicylamide after a 40 μL rinse with Krebs-Henseleit buffer supplemented with 3 μM salicylamide. Substrates were incubated for 1–18 hours (x-axis) prior to luminescent detection. CYP activity (y-axis) was expressed as mean RLU ± sd of three replicate experiments.
Figure 7. Cytotoxicity screen to identify chemicals bioactivated and detoxified by CYP metabolism.
HEK293T cells were transfected with 25 ng of βgalactosidase or 24 ng of CYP1A2, CYP2A6, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, CYP2E1, CYP2J2, CYP3A4, or a 10-CYP liver mix (Table 1 for ratio) + 1 ng P450 oxidoreductase mRNA at a lipid:mRNA ratio of 2.5 and delivered at 5 μL per well. Fifty-six test compounds (Table S5) were delivered at a volume of 225 nL with an acoustic dispenser (0.5% DMSO final) using randomized plate designs at final concentrations ranging from 1–100 μM (x-axis) at 6 hours post-transfection and incubated for 36 hours. Cytotoxicity (y-axis) was measured in three replicate experiments and normalized data fit with a 3-parameter Hill model (bottom constrained to zero, dashed black line). Red dashed line showed the mean ± 2*sd for the DMSO vehicle control wells. Shown are example curves for aflatoxin B1 (A), amodiaquin (B), amitripytyline (C), 8-quinolinol (D), azobenzene (E), and fenazaquin (F). All ten CYP singlets and liver mix were run for each chemical; however, for clarity only βgal, liver mix and those CYP singlets showing bioactivation or detoxification were plotted. See Supplemental Figure 1 for all curves and bio-groups.
Table 1:
mRNA Composition of Transfection Groups
| Gene | % as Singlet | % as Liver Mix(Zanger and Schwab, 2013) |
|---|---|---|
| Cytochrome P450 1A2 | 96 | 11.6 |
| Cytochrome P450 2A6 | 96 | 9.9 |
| Cytochrome P450 2B6 | 96 | 3.8 |
| Cytochrome P450 2C8 | 96 | 5.7 |
| Cytochrome P450 2C9 | 96 | 20.6 |
| Cytochrome P450 2C19 | 96 | 2.7 |
| Cytochrome P450 2D6 | 96 | 3.1 |
| Cytochrome P450 2E1 | 96 | 11.7 |
| Cytochrome P450 2J2 | 96 | 0.7 |
| Cytochrome P450 3A4 | 96 | 26.3 |
4% of singlets and mix reserved for POR co-transfection
Luminogenic P450 Activity Assays.
CYP activity was assessed using P450-Glo assays (Promega, Madison, WI). Luminogenic substrates were prepared as 5.5x concentrated solutions in DMEM/2% FBS, and added directly to the cells (10 μL/well) at 6 hours post-transfection. The final concentrations of the substrates were as follows: luciferin-CEE (100 μM); luciferin-H (100 μM); luciferin-H EGE (10 μM); luciferin-IPA (3 μM); luciferin-ME (100 μM) or luciferin-ME EGE (30 μM). For assays using luciferin-1A2 or −2B6 substrates, the culture medium was removed and the cells rinsed once with 40 μL Krebs-Henseleit buffer containing 3 mM salicylamide. Luciferin-1A2 and −2B6 substrates were prepared as 1X solutions in Krebs-Henseleit buffer (with 3 mM salicylamide) and added to directly to the cells (55 μL/well). Final concentrations of luciferin-1A2 and luciferin-2B6 substrates were 6 μM and 3 μM, respectively. All assays, excepting time course experiments, were allowed to proceed for 12 hours at 37°C/5% CO2 followed by the addition (40 μL/well) of Luciferin Detection Reagent (LDR), LDR with esterase (luciferin-H EGE, -ME EGE, and -IPA) or LDR containing D-cysteine (luciferin-1A2, 2B6). Assay plates were allowed to equilibrate to room temperature (5 minutes) and read on a microplate reader (CLARIOstar, BMG Labtech, Cary, NC) using an endpoint luminescent protocol (top read) with an integration time of 0.2 seconds, a 384 aperture spoon to minimize signal interference from neighboring wells and unrestricted gain to maximize signal detection (gain = 3600). Raw data was expressed as mean relative light units (RLU) ± SD (Figures 1A, 2, 4 and 5) or normalized to control wells and expressed as % control: CYP3A4 only (no POR) in Figure 1B and CYP3A4 only (no CYP2C9) in Figure 3. Data were analyzed using R (Version 3.2.2) and RStudio (Version 0.99.467) and plotted using the ggplot2 package. Statistical significance was determined using a Welch two sample t-test. All source files and code are made available at ftp://newftp.epa.gov/COMPTOX/NCCT_Publication_Data/Simmons_Steve/Metabolism_mRNA_transfection/.
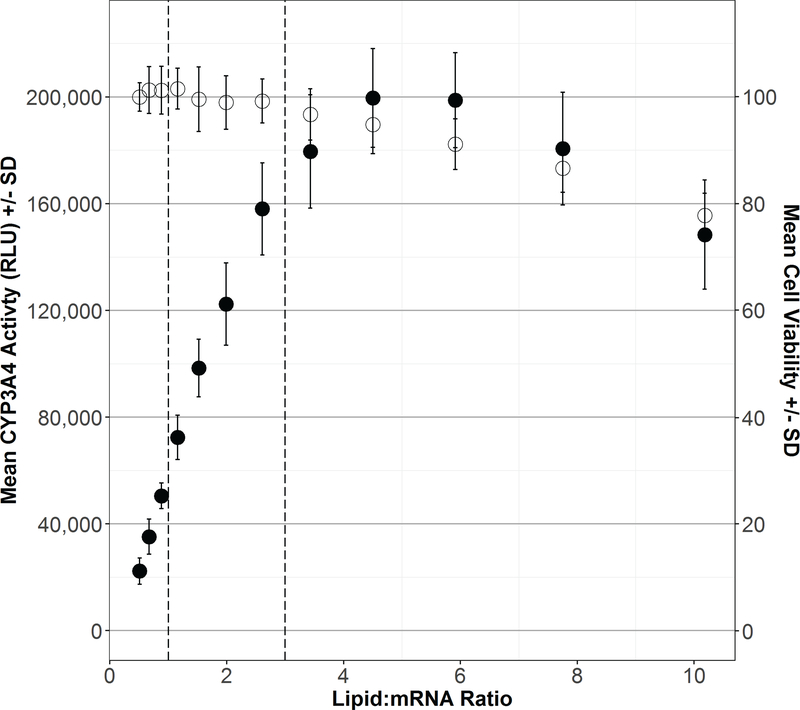
Figure 2. mRNA transfection lipid:RNA ration delivery volume optimization.
(A) HEK293T cells were transfected with 24 ng of CYP3A4 and 1 ng P450 oxidoreductase mRNA with RNA Lipofectamine lipid:mRNA ratios ranging from 0.5 to 10 (x-axis) and delivered at 2.5 μL per well. CYP3A4 activity (left y-axis, solid black circles) was measured by the addition of luciferin-IPA for 12 hours at 6 hours post-transfection as was cell viability at 18 hours post-transfection in parallel wells (right y-axis, open circles). Dashed lines represent the manufacturer’s suggested lipid:mRNA ratios. CYP3A4 activity (RLU) and cell viability (% viability) are expressed as mean ± sd of three replicate experiments. (B) HEK293T cells were transfected with 24 ng of CYP3A4 and 1 ng P450 oxidoreductase mRNA at a lipid:mRNA ratio of 2.5 and delivered in volumes ranging from 1.25 to 10 μL per well (x-axis). CYP3A4 activity (left y-axis, solid black circles) was measured by the addition of luciferin-IPA for 12 hours at 6 hours post-transfection as was cell viability at 18 hours post-transfection in parallel wells (right y-axis, open circles). Dashed line represent the manufacturer’s suggested delivery volume. CYP3A4 activity (RLU) and cell viability (% viability) are expressed as mean ± sd of three replicate experiments.
Figure 3. Effect of competing CYP expression on CYP3A4 activity.
(A) HEK293T cells were transfected with 6.6 ng of CYP3A4, 1 ng P450 oxidoreductase mRNA, and CYP2C9 mRNA ranging from 66 pg to 16.6 ng per well (x-axis, expressed as CYP2C9(ng):CYP3A4(ng) ratio ) at a lipid:mRNA ratio of 2.5 and delivered at 5 μL per well. βgalactosidase mRNA was used to maintain total mRNA transfected per well. CYP3A4 activity (y-axis) was measured by the addition of luciferin-IPA for 12 hours at 6 hours post-transfection and is expressed as mean % CYP3A4 activity (no CYP2C9) ± sd (dashed lines) of three replicate experiments. * indicates p-value > 0.001 for CYP2C9:CYP3A4 ratio compared to CYP3A4 only (no CYP2C9) using Welch two sample t-test.
Cell Viability Assay.
CellTiter-Glo (Promega, Madison, WI) was used to assess cell viability by luminescent quantitation of ATP. For optimization experiments in Figure 2, HEK293T cells were seeded in 384-well solid white assay plates and transfected as described above. Half of the wells for each treatment group were treated with luciferin-IPA to assay for CYP3A4 activity, with the other half left untreated for determination of cell viability. Following a 12-hour incubation at 37°C, CellTiter-Glo (20 μL/well) was added to the untreated wells. Assay plates were equilibrated to room temperature for 10 minutes before being read on a CLARIOstar microplate reader using an endpoint luminescent protocol (top read) with an integration time of 0.2 seconds, a 384 aperture spoon to minimize signal interference from neighboring wells and a restricted gain to avoid signal saturation (gain = 3200). Raw data were normalized using the following equation: norm = 100 * Raw.Data / bval, where Raw.Data is the raw data value and bval is the mean raw data value of the lowest tested lipid:RNA ratio (Figure 2A) or volume of transfection mix (Figure 2B) for each replicate experiment. Data were analyzed using R and RStudio and plotted using the ggplot2 package. All source files and code are made available at ftp://newftp.epa.gov/COMPTOX/NCCT_Publication_Data/Simmons_Steve/Metabolism_mRNA_transfection/.
Substrate Depletion and Metabolite Formation by Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS)
Materials –
Substrates standards, metabolite standards, and internal standards were obtained from the following sources: diclofenac, 4’-hydroxydiclofenac, [13C6]4’-hydroxydiclofenac, dextromethorphan, dextrorphan, [D3]dextromethorphan, [D3]dextrorphan, testosterone, 6β- hydroxytestosterone, [2,3,4-13C3 ]testosterone, and [D3]6β-hydroxytestosterone, (Cerilliant Corp., Round Rock, TX); diclofenac-(acetophenyl ring-[13C6]) sodium salt hemi(nonahydrate), chlorzoxazone, and 6-hydroxychlorzoxazone (Sigma-Aldrich, St. Louis, MO); [D2]6-hydroxychlorzoxazone (Medical Isotopes, Inc., Pelham, NH); and [4,6,7-D3]chlorzoxazone (C/D/N Isotopes Inc., Pointe-Claire, Quebec, Canada). Purities of standards were defined by the manufacturer, other reagents and solvents were reagent or HPLC grade or higher. Stock solutions were prepared in solvent (acetonitrile or methanol) and stored at −20°C. Mixed intermediate standards and matrix-matched standards were prepared fresh daily from stocks.
Sample Collection & Sample Preparation –
Diclofenac sodium salt (15307–79-6), dextromethorphan hydrobromide (6700–34-1), chlorzoxazone (95–25-0), and testosterone (58–22-0) stocks were prepared in 100% DMSO and stored at −80°C until use. Each compound was added to an Echo qualified source plate and acoustically-dispensed to pre-transfected HEK293T cells using an Echo 555 (Labcyte, San Jose, CA) at a final DMSO concentration of 0.5% (v/v). Final concentrations of each substrate were as follows: diclofenac sodium salt 25 μM, dextromethorphan hydrobromide 15 μM, chlorzoxazone 250 μM, and testosterone 160 μM. Samples were collected at the time points indicated in the text and stored a −80°C until processed for analytical analysis as described below. Matrix-matched calibration standards and quality control samples were prepared by spiking blank sample matrix formulated with DMEM cell culture medium supplemented with 2% fetal bovine serum, 5 mM HEPES buffer, 2.78 μL/mL Lipofectamine MessengerMax (ThermoFisher, Waltham, MA), 1.11 μg/mL chemically-modified βgal mRNA, and 0.5% DMSO. Standards, controls, and samples were prepared by adding internal standards in 1% formic acid and acetonitrile at 3x the sample volume, vortexing to mix, and centrifuged at 10,000 rpm. The supernatant was filtered through Phree™ Phospholipid Removal, 30 mg / well, 96-Well Plate (Phenomenex, Torrance, CA) using a positive pressure manifold at 2 – 5 psi and transferred to LC vials for analysis. Samples were prepared in batches with reagent blank, blank matrix, method blank, eight matrix-matched calibration standards, and matrix-matched quality control samples at low, medium, and high concentrations.
Instrumentation –
All analytical methods were conducted by LC-MS/MS on an 1100 Series LC (Agilent Technologies, Santa Clara, CA) with API3000 triple quadrupole mass spectrometer or API4000 triple quadrupole mass spectrometer (SCIEX, Framingham, MA). LC separation was achieved using a Kinetex C18 LC column (100 × 2.1 mm, 2.6 μm, 100 å, P/N 00D-4725-AN; Phenomenex, Torrance, CA) with gradient elution at a flow rate of 350 μL/min using 0.1 % formic acid in 5% acetonitrile and water (A) and 0.1 % formic acid in 5% water and acetonitrile (B). Analytical parameters for target analysis are listed in Supplementary Table S2.
Data Analysis –
Integration, calibration, and quantitation were performed using Analyst 1.3.2 or Analyst 1.6.2 (SCIEX, Framingham, MA). Calibration standard data was fit to quadratic curves using 1/x2 weighting. Batch results were accepted based on the following criteria: calibration curves used a minimum of 7 standards with a correlation coefficient of > 0.99, standards and quality control (QC) accuracy tolerance ≤ 20% (30% at lower limit of quantification [LLOQ]), QC precision expressed as % relative standard deviation (%RSD) ≤ 15% (20% LLOQ), > 67% of all standards and QC standards satisfy accuracy criteria, reagent blank and blank matrix response free of target analyte, and method blank < limit of detection (LOD). Supplementary Table S3 lists the analytical method performance characteristics for target analytes. Parent and metabolite concentration data were plotted using R and RStudio with the ggplot2 package. All samples for which no analyte was detected was set to zero for statistical purposes. All source files and code are made available at ftp://newftp.epa.gov/COMPTOX/NCCT_Publication_Data/Simmons_Steve/Metabolism_mRNA_transfection/.
Cytotoxicity Chemical Test Set.
Stock solutions of 56 chemicals (Supplementary Table S4) were prepared in 100% dimethyl sulfoxide (DMSO) at 20mM, and stored at −80°C. For preparation of compound plates (Echo Qualified; Labcyte, San Jose, CA) chemical stocks were thawed at room temperature and diluted using 100% DMSO to prepare a titrated series at 200X of the final assay concentrations which ranged from 1–100 μM. Compound plates containing control and test compounds were sealed, stored in a desiccator and protected from light when not in use. All experiments were conducted within one week of compound plate preparation.
Cytotoxicity Screen.
Following the transfection workflow as described above, HEK293T cells were dosed with test compounds using an Echo 555 acoustic liquid handler running Cherry Pick software (Labcyte, San Jose, CA). Test chemicals were administered (225 nL; 0.5% DMSO final concentration (v/v)) using a randomized plate design. Three biological replicates of technical singles were run for every chemical concentration tested in every bio-group (βgal, ten CYP singlets, and liver mix). The same randomized plate design was used for the delivery of all concentrations of all compounds within any one biological replicate, but a different randomized design was used for each of the three bioreplicates. Cells were returned to a humidified 37°C/5% CO2 incubator for 36 hours. CellTiter-Glo reagent (35 μL/well) was added using a BioTek MicroFlo, and cells incubated at room temperature for 10 minutes. Plates were read on a CLARIOstar microplate using an endpoint luminescent protocol (top read) with an integration time of 0.2 seconds, a 384 aperture spoon to minimize signal interference from neighboring wells and a restricted gain to avoid signal saturation (gain = 3200). Raw data were normalized using the following equation: resp = 100 * (1 – ((Raw.Data – controlmin) / (vmed – controlmin), where Raw.Data is the raw data value, controlmin is the minimum raw data value of the four well per plate of cytotoxicity positive control (Bisphenol AF) at the highest tested concentration (100 μM) for each assay plate and vmed is the median raw data value of the 60 DMSO vehicle control wells on each plate. Data were analyzed using R and RStudio. Curves were fit to a 3-parameter Hill model (bottom constrained to zero) using tcpl_lite R source code and plotted using the ggplot2 package. All source files and code are made available at ftp://newftp.epa.gov/COMPTOX/NCCT_Publication_Data/Simmons_Steve/Metabolism_mRNA_transfection/.
Results
CYP-POR Ratio Optimization.
An initial transfection of 25 ng/well CYP3A4 mRNA significantly increased CYP3A4 activity in HEK293T cells (Figure 1A) compared to un-transfected control cells. However, co-factors essential to CYP activity may be limited or absent in cell lines used in high-throughput screening applications. Cytochrome P450 oxidoreductase (POR) is a membrane-bound enzyme required for the electron transfer from NADPH to cytochrome P450 enzymes in the endoplasmic reticulum (Riddick et al., 2013). POR is expressed in human liver (Zanger and Schwab, 2013), but it is unclear whether the levels of endogenous POR expression in HEK293T cells are sufficient for maximal P450 activity from transiently transfected CYP-encoding mRNAs. As highlighted in Figure 1A, co-transfection of POR alone resulted in a moderate increase in endogenous CYP3A4 activity in HEK293T cells, but was significantly less than that observed with CYP3A4 mRNA transfection. HEK293T cells were then co-transfected with a constant amount of CYP3A4 mRNA (12.5 ng/well) and increasing amounts of POR mRNA (12.5 pg-12.5 ng/well). βgalactosidase (βgal) mRNA was added to maintain the total amount of transfected mRNA at 25 ng/well. As Figure 1B illustrates, a co-transfection consisting of 50% CYP3A4, 4% POR, and 46% βgal mRNAs increased the CYP3A4 activity by 33.5% compared to CYP3A4 mRNA transfection alone. Increasing POR mRNA beyond 4% significantly decreased CYP3A4 activity; thus, all subsequent experiments were performed using 4% POR mRNA.
Transfection Optimization.
With the optimal amount of POR mRNA co-expression established, parameters in the transfection protocol were further optimized to generate maximal CYP3A4 activity. First, the ratio of μL lipid:ng mRNA was varied from 0.5 to 10 in an attempt to balance maximal CYP3A4 activity with post-transfection cell viability (Figure 2A). Increasing the lipid:mRNA ratio outside of the manufacturer’s suggested range (dotted lines) did produce a marked increase in CYP3A4 activity, but was offset by a loss of cell viability at ratios > 4. The optimal lipid:mRNA ratio was determined to be 2.5:1. Using the 2.5 ratio of lipid:mRNA, a titrated range of total transfection volumes from 1.25 to 10 μL per well were tested for enhancement of CYP3A4 activity and concurrent effects on cell viability (Figure 2B). Increasing the transfection volume from the recommended 2.5 μL/well (dotted line) to 5.0 μL/well resulted in a 40% increase in CYP3A4 activity with minimal impact on cell viability. A lipid:mRNA ratio of 2.5 and transfection volume of 5.0 μL per well were utilized for the remainder of the study.
Cell Resource Competition in CYP Combinations.
Cytochrome P450 enzymes require multiple factors for optimal activity, including, but not limited to proper subcellular location, sufficient cofactors (e.g., POR), and ample capacity for heme protein synthesis. To determine any effects ectopic CYP enzyme expression may have on rate-limiting resources in HEK293T cells, a titration of CYP2C9 mRNA was co-transfected with a constant amount of CYP3A4 mRNA, and resultant CYP3A4 activity was measured using a luminogenic substrate. For this experiment, the transfected CYP3A4 mRNA was held constant at 27% of the total transfected mRNA (Table 1), and this level of activity was normalized to 100%. βgal mRNA was co-transfected to maintain a constant amount of total mRNA transfected in each treatment. As shown in Figure 3, CYP3A4 activity begins to decrease at a CYP2C9:CYP3A4 ratio of 0.5, and becomes significant at a ratio of 2.6, where CYP2C9 mRNA comprises 69% of the total mRNA transfected. At the highest CYP2C9:CYP3A4 ratio (2.6), CYP3A4 activity is decreased by 21%, indicating that co-transfection of maximized multiple-CYP payloads does have a small, but significant (p <0.001; Supplemental Table S6) impact on the activity of any single CYP enzyme due to rate-limiting resources in HEK293T cells.
Cytochrome P450-Luminogenic Substrate Matrix
To determine an optimal luminogenic substrate and identify potential confounding cross-reactivity, eight luminogenic substrates were evaluated against 10 human liver CYP-encoding mRNAs transfected as singlets or in a 10-CYP mixture to mimic the ratio of human liver tissue (Figure 4). This CYP combination designated as “liver mix” is pooled in a ratio based on mRNA expression patterns in a series of human liver samples (Zanger and Schwab, 2013). The constituent CYP mRNA levels present in the liver mix are presented in Table 1, and the assay conditions are listed in Supplemental Table S7. Transfection with βgal alone demonstrated the presence of little or no CYP activity in the native HEK293T cell line with the exception of a small amount of CYP1A2 and CYP2B6. CYP1A2 activity was only slightly increased above the βgal background using the luciferin-1A2 substrate; however, CYP2A6 gave a robust signal using the same substrate (compare Figures 4B and 4C). The most robust signals for CYP2B6, CYP2C9, CYP2D6, and CYP3A4 were obtained with the manufacturer’s recommended substrates. Interestingly, CYP2E1 produced a strong signal with the luciferin-1A2 substrate while CYP2J2 reacted with luciferin-IPA albeit to a small extent. The response of CYP2C19 indicated multiple cross-reactions primarily with four substrates only one of which (luciferin-H EGE) was expected. The results from the transfected liver mix indicate that the highest activity was produced using luciferin-IPA substrate, followed by luciferin-1A2, 2B6, H, and ME in descending order. Activity with luciferin substrates CEE, H EGE, and ME EGE were minimally detectable.
Temporal Resolution of CYP Enzymatic Activity from mRNA Transfections
With luminogenic substrates identified for each CYP singlet as well the liver mix, time course experiments were conducted with the best-performing substrate to determine the duration of CYP activities in transfected HEK293T cells. Previous experiments had shown that d-luciferin, the native substrate of firefly luciferase and presumed metabolite of the pro-luciferin luminogenic substrates, was stable when co-incubated with cultured HEK293T cells for at least 12 hours (data not shown). The stability of the luciferin metabolite was further supported by the robust signal observed for many of the substrates incubated for 12 hours as shown in Figure 4. Cells transfected with each of ten CYP singlets or the liver mix were treated with the one substrate identified as optimal for that enzyme (Figure 4) and then sampled for luminescent activity at 1, 2, 4, 10 and 18 hours (Figure 5). Luciferin-IPA was used to measure CYP3A4, CYP2J2 and the liver mix (Figure 5A). CYP3A4 (blue) activity was robust through 10 hours but plateaued as evidenced by the small increase in luminescent signal between 10 and 18 hours. Liver mix (green) which is comprised of 27% CYP3A4 mRNA also had strong activity through 18 hours, but without the diminution of activity observed for CYP3A4 between 10 and 18 hours. CYP2J2 (red) also had significant luciferin-IPA metabolism through 18 hours compared to βgal control (p < 0.001) but not to the extent of CYP3A4 or the liver mix. Luciferin-ME EGE was used to measure CYP2D6 activity (Figure 5B, red). Although CYP2D6 activity rapidly increased the luminescent signal through 2 hours, the signal decreased from the peak signal observed at 2 hours through 18 hours. However, at 18 hours CYP2D6 activity was still significantly different from βgal controls (p < 0.001). This decrease in signal suggests that luciferin-ME EGE is not directly metabolized to d-luciferin and that the luciferin-ME EGE intermediate is not as stable as d-luciferin in prolonged cell culture incubations. Luciferin-ME was used to measure CYP2C8 activity (Figure 5C, red), which showed a slow increase over 4 hours but then a more rapid increase in signal from 4 to 18 hours. Luciferin-H was used to measure CYP2C9 activity (Figure 5D, red) which had a nearly linear increase from 1 to 18 hours. Luciferin-1A2 was used to measure CYP1A2, CYP2A6, and CYP2E1 activity (Figure 5E). CYP2A6 (blue) had robust activity through 10 hours that plateaued by 18 hours. CYP2E1 (green) also had a continuous increase in activity over 10 hours that slowed between 10 to 18 hours. CYP1A2 (green) had a marginal increase in activity over 18 hours, although it was significantly different from βgal controls (p < 0.001) at 18 hours. Luciferin-2B6 was used to measure CYP2B6 and CYP2C19 activity (Figure 5F). CYP2B6 (red) had robust activity through 10 hours that decreased by 18 hours while CYP2C19 (blue) displayed a modest increase in activity over the first 4 hours that escalated slightly from 4 to 18 hours.
Metabolism of CYP Substrates to Predicted Metabolites
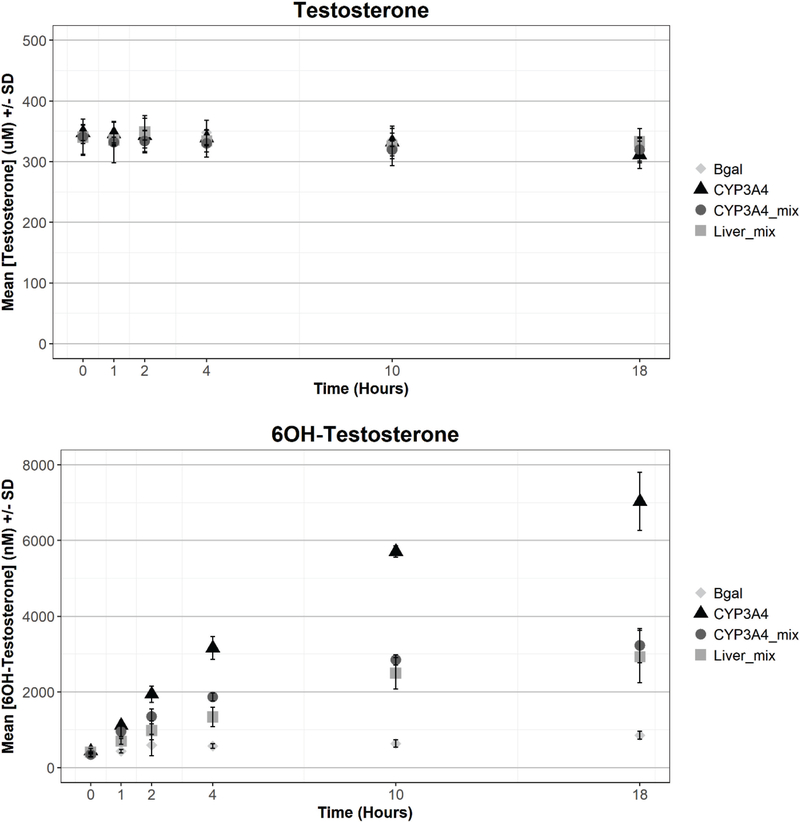
In order to further characterize the activity of the CYP-encoding mRNA, transfected HEK293T cells were treated with four well-characterized substrates (diclofenac, dextromethorphan, chlorzoxazone or testosterone) which are converted via CYP-specific reactions to known metabolites. For each substrate, four groups of transfected mRNA were tested: a single CYP (96% of total transfected mRNA; designated as CYP-singlet), a single CYP at its corresponding percentage in liver mix (designated as CYP-mix, the remainder comprised of βgal mRNA), the complete liver mix, and the βgal control. Figure 6 shows the time course of transfected CYP-encoding mRNA activity for the four substrate/metabolite pairs. Overall, the data demonstrates that each transfected CYP-encoding mRNA was able to effectively metabolize its substrate to the predicted metabolite with formation of the metabolite continuing for the duration of the time course, for each CYP-substrate combination tested. Due to the reported Km values for testosterone and chlorzoxazone (Walsky and Obach, 2004), any appreciable depletion of these parent compounds was obscured by the high test concentrations used (Figure 6A and 6C). While parent depletion was negligible, metabolites of testosterone and chlorzoxazone were readily detected. The CYP3A4 singlet metabolized testosterone to 6β-hydroxytestosterone at a rate of 26.1 pmol/min/million cells through 1 hour to a final concentration of 7.03 μM at 18 hours (Figure 6B). Both liver mix and a CYP3A4- mix comprised of CYP3A4/βgal/POR (27/69/4%) to mimic liver concentrations of CYP3A4 but without the competing influences of co-expressed CYPs, also metabolized testosterone to 6β-hydroxytestosterone, albeit at less than half the rate of the CYP3A4 singlet. CYP2E1 (singlet) converted chlorzoxazone to 6-hydroxychlorzoxazone at a rate of 26.1 pmol/min/million cells through 18 hours to a final concentration of 16.4 μM (Figure 6D). Liver mix and a CYP2E1- mix also generated the 6-hydroxychlorzoxazone metabolite, but at 36% and 28% of the CYP2E1 singlet, respectively. Diclofenac was extensively converted to its CYP2C9 metabolite, 4’-hydroxydiclofenac, for all transfection groups tested except the βgal control. The CYP2C9 singlet metabolized more than 95% of the diclofenac parent by 18 hours (Figure 6E), whereas the liver mix and CYP2C9- mix had metabolized 33% and 26% by this same time. The disappearance of diclofenac parent was accounted for by a commensurate increase in 4’-hydroxydicofenac metabolite (Figure 6F) at a rate of 41.2 pmol/min/million cells with the CYP2C9 singlet. Similarly, dextromethorphan was depleted 51% by the CYP2D6 singlet steadily over 18 hours (Figure 6G). There was no observable depletion of dextromethorphan by the liver mix or CYP2D6- mix. The dextrorphan metabolite was formed at 7.34 pmol/min/million cells over 18 hours by the CYP2D6 singlet, but only 0.95 and 0.45 pmol/min/million cells for the liver mix and CYP2D6- mix, respectively (Figure 6H).
Figure 6. Metabolism of benchmark compounds to form predicted metabolites.
HEK293T cells were transfected with 25 ng of βgalactosidase or 24 ng of a 10-CYP liver mix (Table 1 for ratio) or 24 ng of CYP3A4 (A and B), CYP2E1 (C and D), CYP2C9 (E and F), CYP2D6 (F and G) + 1 ng P450 oxidoreductase mRNA at a lipid:mRNA ratio of 2.5 and delivered at 5 μL per well. Test compounds testosterone (A and B; 160 μM final concentration), chlorzoxazone (C and D; 250 μM final concentration), diclofenac (E and F; 25 μM final concentration) and dextromethorphan (G and H; 15 μM final concentration) were delivered at a volume of 225 nL with an acoustic dispenser (0.5% DMSO final) at 6 hours post-transfection and incubated for 0–18 hours (x-axis). Samples were prepared by protein precipitation and phospholipid removal and analyzed by LC-MS/MS. Parent compound depletion (μM) and targeted metabolite formation (nM) are plotted on the y-axis as mean ± sd of three replicate experiments.
Cytotoxicity Screen to Identify CYP-Bioactivated and -Detoxified Chemicals
In order to identify chemicals either bioactivated or detoxified by the ectopic expression of human liver CYPs and to demonstrate the HTS retrofitting capability of mRNA-transfection, HEK293T cells were transfected with 10 human liver CYP-encoding mRNAs as singlets and also in the 10-CYP liver mix and then treated with 56 test compounds. Cells were exposed to the test compounds for 36 hours and then assayed for cytotoxicity. The cytotoxicity assay performed as expected using DMSO and bisphenol AF as vehicle and positive controls, respectively. The mean robust coefficient of variation (rCV) was 6.4% ± standard deviation (sd) of 1.5% across all plates. The mean robust Z’ factor (rZ’) was 0.80 ± sd of 0.05 across all plates. The 3-parameter Hill model fits of all 56 test compounds across all twelve bio-groups (including βgal as a negative transfection control) are shown in Supplemental Figure 1. Figure 7 highlights the more pronounced responses observed from the cytotoxicity screen. For instance, aflatoxin B1, a potent rodent carcinogen, was bioactivated from a non-toxic parent (Figure 7A, black) to a potent cytotoxic metabolite by CYP3A4 (green; AC50 = 5.16 μM) and to a lesser extent by CYP2J2 (blue; AC50 = 32.5 μM). The liver mix (red; AC50 = 12.7 μM), which is comprised of roughly 27% CYP3A4 and 1% CYP2J2, bioactivated aflatoxin B1 but to a lesser degree than the CYP3A4 singlet. Amodiaquin, an anti-malarial drug, was bioactivated to a more toxic species by CYP2C8 (Figure 7B, blue) compared to the βgal control (black), although the effect manifested primarily in efficacy (not potency) at the highest tested concentrations (97.9% versus 62.6% for CYP2C8 and βgal, respectively). Conversely, amodiaquin was detoxified by CYP2J2 (green) at all but the highest tested concentrations producing a pronounced right shift in the Hill model fit. Interestingly, the liver mix (red) which is comprised of less than 6% CYP2C8 mRNA and 1% CYP2J2 mRNA had no net effect on amodiaquin-induced cytotoxicity and was indistinguishable from βgal control. Amitriptyline, a drug used to treat a number of mental illnesses, was bioactivated by CYP2C19 (Figure 7C, blue) compared to βgal control (black) as indicated by the efficacy shift at all but the highest tested concentrations and the left shift in the Hill model fit (AC50 values of 26.0 μM and 70.1 μM for CYPC19 and βgal, respectively). Liver mix (red) which is comprised of less than 3% CYP2C19 mRNA, did not shift either potency or efficacy of amitriptyline-induced cytotoxicity compared to βgal control. 8-quinolinol, a heterocycle quinoline used as a pesticide and antiseptic, was bioactivated by CYP2A6 (Figure 7D, blue) as indicated by an increase in mean cytotoxicity from 29.1% for βgal (black) at 100 μM to 76.2%. The liver mix (red) which is comprised of 10% CYP2A6 mRNA produced only a modest increase (39.2%) in 8-quinolinol-induced cytotoxicity at the highest tested concentration over βgal control (29.1%). Azobenzene, a commercial chemical used in polymer synthesis, was bioactivated from a non-toxic parent (Figure 7E, black) to a toxic metabolite by CYP2E1 (blue) and to a lesser extent by the liver mix (red) which is comprised of 12% CYP2E1 mRNA. Fenazaquin, a pesticide and known mitochondrial toxicant, was one of only a few test chemicals that appeared to be detoxified by CYP enzyme activity in HEK293T cells. CYP2B6 (Figure 7F, blue) and the liver mix (red) both produced subtle right shifts in the Hill model fits of fenazaquin-induced cytotoxicity compared to βgal control (AC50 values of 48.4 μM and 43.3 μM for CYP2B6 and liver mix respectively compared to 35.0 μM for βgal).
Discussion
Many of the 700 cell-based and cell-free HTS assays currently in use in the EPA’s ToxCast program do not possess endogenous xenobiotic metabolic activity. Thus, the current screening assays only evaluate the biological activity of the parent chemical and not those of potential metabolites generated in metabolically-competent models or in vivo. This lack of metabolic capability can lead to the mischaracterization of chemical hazards, overestimating the toxicity of readily-metabolized parent chemicals and underestimating the hazards posed by toxic metabolites, and is a common criticism of using such in vitro approaches to predict in vivo toxicities. The work presented herein describes a novel approach to retrofit existing cell-based assays with metabolic competence using transfection of chemically-modified mRNAs. The results show that mRNA transfection is a fast and effective way to imbue cells that have little or no intrinsic metabolic capacity with robust XME activity. While this study focuses on CYP-encoding mRNAs, this method could be expanded to include other Phase I, II and III gene families.
Pre-optimized transfection of human CYP3A4 mRNA produced a marked increase in CYP3A4 activity over un-transfected cells and cells transfected with POR alone. Optimization of the CYP mRNA transfection protocol in HEK293T cells was performed using CYP3A4 as the sentinel for two reasons. First, CYP3A4 is the predominant CYP enzyme expressed in human liver and is known to be important in the metabolism of many clinical drugs and environmental chemicals (Hodgson and Rose, 2007; Zanger and Schwab, 2013). Second, while it was possible to optimize for several of the major liver CYPs in parallel, either there would be broad agreement among those CYPs, in which optimizing for any single CYP would suffice, or there would be marked and perhaps mutually-exclusive differences between those CYPs, in which case optimization would be geared toward the CYP enzyme(s) deemed most important (i.e. CYP3A4). Optimization of the mRNA transfection protocol first examined whether P450 oxidoreductase was rate-limiting in HEK293T cells. When POR mRNA was co-transfected in a titrated manner using a constant level of CYP3A4 mRNA, there was an increase in CYP3A4 activity compared to cells transfected with only CYP3A4 mRNA (no POR) from 0.1%−4% relative to CYP3A4 mRNA levels. As POR mRNA levels were increased to > 4%, CYP3A4 activity decreased. When POR mRNA was co-transfected at the same concentration relative to CYP3A4 mRNA (100%), CYP3A4 activity was decreased by 60%, indicating that POR co-expression is required in HEK293T cells to maximize CYP3A4 activity, but the benefit from POR co-expression quickly diminishes at higher POR mRNA levels. This inhibition of CYP activity by POR co-expression has been previously reported (Lengler et al., 2006) and was found to be cell-type dependent. With proper POR co-expression ratios established, routine transfection parameters such a lipid:mRNA ratio and total delivery volume were optimized.
One of the aims of this project was to establish whether multiple XME-encoding mRNAs could be effectively co-transfected together to mimic the expression patterns observed in situ for tissues such as human liver. While co-transfection of multiple, pooled CYP mRNAs is technically feasible, the impact of ectopic co-expression of multiple CYPs on the activity of any single CYP within the pool was not known. It is possible that in a cell line such as HEK293T, resources such as cytochrome b5, heme protein synthesis, and localization space on the endoplasmic reticulum, etc. quickly become rate-limiting. As these resources are exhausted with high levels of transfected CYP-encoding mRNAs, the overall activity of any single CYP enzyme would decrease. CYP2C9 was selected for competition with CYP3A4 as it does not cross-react with the luminogenic substrate used to measure CYP3A4 activity (luciferin-IPA) and it is the second most abundant CYP expressed in human liver(Zanger and Schwab, 2013). A titration of CYP2C9 mRNA co-transfected with a constant amount of CYP3A4 mRNA revealed that as the total mRNA payload was comprised of a greater amount of a competing CYP-encoding mRNA, CYP3A4 activity decreased. With a maximal CYP2C9:CYP3A4 ratio (69%:27%), CYP3A4 activity was decreased by 21%, indicating that co-transfection of maximized multiple-CYP mRNA payloads does have a small, but significant impact on the activity of any single CYP enzyme due to rate-limiting resources in HEK293T cells.
A panel of eight luminogenic CYP substrates were tested against the top ten human liver CYP enzymes as singlets as well as a 10-CYP mixture to mimic the ratio of human liver tissue. While there were pronounced differences in the overall luminescent signals generated by the eight CYP substrates, all but two were optimal for at least one CYP enzyme or the liver mix. As expected, luciferin-IPA was the best substrate for measuring CYP3A4 activity among those tested, but was also the preferred substrate of CYP2J2 and the liver mix, unsurprisingly since the plurality of the liver mix is comprised of CYP3A4 mRNA (27%). Luciferin-ME EGE is marketed as a CYP2D6 substrate and was the best substrate among those tested for CYP2D6. Likewise, luciferin-H, -ME, −1A2 and −2B6 are billed as sensitive for CYP2C9, CYP2C8, CYP1A2 and CYP2B6, respectively and all four of these probes were the preferred substrate for their designated enzymes. The robust signals stemming from CYP2A6 and CYP2E1 using the luciferin-1A2 substrate and from CYP2C19 using the luciferin-2B6 substrate were unexpected. Luciferin-H EGE is marketed as the prime substrate for measuring CYP2C19 in cell-free applications (S9, microsomes, recombinant enzyme) and is not recommended for cell-based assays, so it not entirely unforeseen that luciferin-H EGE did not perform well in cells expressing CYP2C19. With suitable luminogenic substrates identified for each of the ten human liver CYP enzymes and the liver mix, a time course study was conducted to measure enzyme activity durations. CYP3A4, CYP2A6 and CYP2B6 showed a strong signal increase through 10 hours but diminished increases in signal from 10 to 18 hours. It is difficult to know whether the plateau in signal from 10 to 18 hours represents decreased enzyme activity after 10 hours or if substrate depletion reduces enzyme activity after 10 hours. CYP3A4, CYP2A6 and CYP2B6 were the only enzymes to produce mean activities in excess of 3×104 RLU and luciferin-IPA, −1A2, and −2B6 substrates are used at the lowest concentrations (Table S7; 3–6 μM). It also worth noting that other CYP enzymes with lower activities using the same three substrates (CYP2J2 with luciferin-IPA, CYP1A2 and CYP2E1 with luciferin-1A2, and CYP2C19 with luciferin-2B6) had linear signal increases through 18 hours. Since the substrate concentrations are used at the Km for their designated enzyme (excepting CYP2A6 with luciferin-1A2), depletion of the substrate below the Km over the first several hours would rapidly decrease enzyme activity below ½ Vmax even if the enzyme is active throughout the 18-hour time course. This question could be addressed by either using increased concentrations of the luminogenic substrates or using analytical methods with known parent-metabolite pairs (e.g. coumarin for CYP2A6 and bupropion for CYP2B6), but is beyond the scope of this study.
While the use of luminogenic substrates provides a fast and effective way to optimize and characterize CYP activities in mRNA-transfected cells, these substrates are not yet widely accepted as evidence of CYP activity, particularly because the metabolism of these substrates is not well-understood compared to well-studied parent-metabolite pairs commonly used to characterize metabolically-competent systems such as primary human hepatocytes. Five benchmark parent compounds were used to analyze CYP1A2, CYP2C9, CYP2D6, CYP2E1 and CYP3A4 metabolism using standard liquid chromatography-tandem mass spectrometry methods. There was little evidence of testosterone or chlorzoxazone depletion after 18 hours for CYP3A4 and CYP2E1, respectively; however, the parent compound concentrations used in this study (160 and 250 μM) exceed those commonly used in toxicity HTS screening campaigns (~100 μM). CYP3A4 did catalyze a marked increase in 6β-hydroxytestosterone formation through 18 hours. Likewise, CYP2E1 activity remained strong through 18 hours, evidenced by the formation of 6-hydroxychlorzoxazone. While the formation of these predicted metabolites is encouraging, the 7 μM 6β-hydroxytestosterone and 14 μM 6-hydroxychlorzoxazone levels at 18 hours represent metabolism of only 4.4% and 5.6% of parent compound. Conversely, the metabolism of diclofenac by CYP2C9 was nearly complete by 18 hours with more than 95% of the parent metabolized to the predicted metabolite 4’-hydroxydiclofenac. The metabolism of dextromethorphan by CYP2D6 was also extensive by 18 hours with 50% of the parent metabolized and most of that to the predicted metabolite dextrorphan. While the metabolism of both diclofenac and dextromethorphan were thorough after 18 hours, it is important to highlight that these compounds were used at 25 μM and 15 μM, respectively, well below the typical upper dose range of a toxicity HTS study. At upper HTS test concentrations, the metabolism of diclofenac and dextromethorphan would only constitute approximately 25% and 7% of the parent after 18 hours. A useful metric to further gauge the performance of this platform is to compare the formation the predicted metabolites against other in vitro systems which are metabolically competent. Overall, metabolite formation using transfected CYP-encoding mRNA at levels found in human liver (i.e. liver mix) compares favorably, if not exceeds, rates in systems such as HepaRG cells and sandwich-culture PHHs, but is lower than rates observed in suspension PHHs. For CYP2C9, transfected liver mix formed 4’-hydroxydiclofenac at a rate of 5.3 pmol/million cells/minute (1 hour incubation) compared to 3.9 and 5.4 pmol/million cells/minute for HepaRG and sandwich culture PHH.(Jackson et al., 2016) Similarly, at 1 hour, liver mix transfections resulted in the formation of dextrorphan and 6β-hydroxytestosterone at 0.96 and 16.3 pmol/million cells/minute, respectively. For comparison, the rates in HepaRG cultures were 0.40 pmol/million cells/minute for dextrorphan and 248 pmol/million cells/minute for 6β-hydroxytestosterone. Sandwich PHH cultures formed dextrorphan at 1.18 pmol/million cells/minute and 6β-hydroxytestosterone at 55.2 pmol/million cells/minute. A meta-analysis of PHH suspension cultures reports median rates of formation for 4’-hydroxydicofenac, dextrorphan, and 6β-hydroxytestosterone as 88.2, 21.1, and 407 pmol/million cells/minute (Jackson et al., 2016). 6-hydroxychlorzoxazone formation in liver mix transfections was 4.09 pmol/million cells/minute at 1 hour, but increased to 8.10 pmol/million cells/minute at the 10 hour time point and dropped only slightly through the 18 hour time point. This difference likely results from low metabolite recovery during sample preparation rather than a true increase in metabolite formation over time. Comparison of the rates of formation of 6-hydroxchlorzoxazone to other metabolically competent in vitro systems is hindered by the sensitivity of CYP2E1 to DMSO (Chauret et al., 1998). Thus, the rates reported in this study are likely to be lower than those reported elsewhere. However, the distinctions with respect to how these experiments are typically conducted likely impact any meaningful comparative consideration. The benchmark compound experiments conducted in this study were performed under typical HTS conditions, namely parent compounds were dissolved in DMSO and delivered at a final DMSO concentration of 0.5%. While it is known that DMSO inhibits CYP enzyme activity (Chauret et al., 1998), it was important to gauge how extensive the metabolism from mRNA-transfected cells withstood HTS screening conditions. Also, due to logistical constraints of simultaneously handling a large number of samples in parallel, no LC-MS/MS samples were collected prior to 1 hour except the initial (t0) samples. Typical benchmark compound experiments use a variety of solvents to solubilize parent compounds, but never DMSO. Also, incubations for PHH characterization are generally much shorter than one hour (15–30 minutes) when enzymatic activity is at its highest level. Nevertheless, the extent of metabolism for any potential CYP enzyme-substrate pairing will depend on a number of factors such as the concentration of substrate tested, the relationship of that tested concentration to the Km for that enzyme-substrate pairing, and the level of enzyme present which impacts the Vmax; therefore, it is difficult to make generalizations about how extensive the metabolism of test compounds in a HTS campaign might be. However, if the metabolism of the benchmark compounds in this study serve as any guide, achieving complete metabolism for a significant percentage of parent compounds in an HTS-compatible format seems unlikely.
Having optimized the mRNA transfection method and having demonstrated ample metabolism of both luminogenic substrates and benchmark compounds through 18 hours of chemical exposure, HTS deployment was demonstrated using a cytotoxicity assay to measure the effect of CYP metabolism on 56 test chemicals. The cytotoxicity assay performed well, each plate exceeding the quality control acceptance criteria for rCV and rZ’. Overall, CYP metabolism did not impact the cytotoxicity for most of the tested compounds. However, cytotoxicity was discernably impacted by CYP metabolism for several test compounds. At least two chemicals, notably aflatoxin B1 and azobenzene were bioactivated from non-toxic parents to toxic metabolites by CYP3A4/CYP2J2 and CYP2E1, respectively. CYP3A4-mediated bioactivation of aflatoxin B1 is well documented and the AC50 value of 5.16 μM is consistent with that reported by others (Jackson et al., 2016). Bioactivation of aflatoxin B1 by CYP2J2 has not been previously reported. Azobenzene has been shown to induce CYP1A gene expression and is mutagenic (Ioannides and Lewis, 2004), but there are no published reports of cytotoxicity via bioactivation of azobenzene by CYP2E1. The toxicity of other tested parent compounds like amodiaquin, amitriptyline, and 8-quinolinol were intensified by CYP2C8, CYP2C19 and CYP2A6 metabolism. Amodiaquin has been identified as a substrate of CYP2C8 and the resulting metabolite, N-desethylamodiaquin, is a suspected hepatotoxicant (Jewell et al., 1995). Detoxification of amodiaquin by CYP2J2 has not previously reported. CYP2C19-mediated metabolism of amitriptyline is well-studied and its metabolites have been linked to cardiotoxicity (Dean, 2012). CYP2A6 is known to form a reactive quinoline-1-oxide from quinolone (Reigh et al., 1996), but this is the only known report of 8-quinolinol (8-hydroxyquinoline) bioactivation by CYP2A6. One notably inactive test chemical was cyclophosphamide (Supplemental Figure 1), known to be bioactivated to a cytotoxic mustard by CYP2B6, and to a lesser extent by CYP3A4, but was not active in this study. The reasons CYP2B6 failed to bioactivate cyclophosphamide are unclear, but since both CYP2B6 and CYP3A4 were shown to be active throughout this study, it seems likely that either the neat cyclophosphamide compound has expired or perhaps the highest tested concentrations were too low to support robust metabolism (i.e. below the Km).
Several compounds, including amodiaquin (CYP2J2) and fenazaquin (CYP2B6), were detoxified by CYP metabolism; however, the magnitude of the detoxifying effects of CYP metabolism were minimal compared to the toxifying effects of bioactivation. In light of the parent depletion observations from the benchmark compound study, the paucity of detoxifications from the cytotoxicity screen should not be entirely unanticipated. If a parent compound induces pronounced cytotoxicity, and only a small portion (4–20% estimated from the benchmark compound study) of that parent is metabolized, then the rightward AC50 shift in a cytotoxicity assay would likely be negligible (assuming the metabolite itself is also not cytotoxic). Conversely, if a non- or minimally-cytotoxic parent is bioactivated to form a potent toxic metabolite (i.e. aflatoxin B1 or azobenzene), the leftward AC50 shift in a cytotoxicity assay would be more pronounced. This is precisely the observation of the cytotoxicity screen reported herein. It should be noted that relatively short-term assays in metabolically competent cells (e.g., primary hepatocytes) would suffer from the same challenges. Longer-term maintenance of metabolic activity and extended exposure times may be required to observe more detoxifying effects in vitro.
Whether apical in vitro assay endpoints, such as cytotoxicity, are the most sensitive tool to detect toxic metabolite formation, much less detoxification of toxic parent compounds, is an open question. While such apical endpoints integrate many possible mechanisms into a definitive and easily discernable event, cells are dynamic systems with many adaptive responses to abrogate the effects of toxic metabolite formation, rendering bioactivation difficult to detect in all but the most extreme cases. Some of this can be overcome through pre-treatments designed to hamper phase II metabolism, such as buthionine sulfoximine (BSO) to neutralize glutathione conjugation and salicylamide to abrogate glucuronide conjugation. Other possible solutions include genomic manipulation of cell models, for instance the use of CRISPR/Cas9 to target regulatory control genes like NFE2L2 (Nrf2) to disable oxidative stress response triggered by bioactivation. As xenobiotic metabolism is coupled to HTS toxicity assays, the metabolism observed will inescapably be strained through the lens of the bioassay with which metabolism is coupled. In order to gain a more comprehensive understanding of bioactive metabolites (as well as detoxification), more mechanistically-tailored HTS assays need to be developed for endpoints not well-addressed by the current in vitro assay portfolio and for which metabolism is known to play a major role (e.g., mutagenicity in human cells). That said, the mRNA transfection HTS retrofit method can be immediately deployed to other endpoints, such as estrogen receptor transactivation assays, that are predictive of in vivo toxicities.
Of the ten predominant human liver CYP enzymes, CYP1A2 was notably non-functional in this study. In the luminogenic substrate experiments using the luciferin-1A2 substrate, CYP1A2 had a minimal increase in activity over 18 hours. Although it was significantly different from βgal controls (p < 0.001) at 18 hours, the signal generated by CYP1A2 metabolism of the luciferin-1A2 substrate paled in comparison to the signal generated by CYP2A6 and CYP2E1 using the same substrate. CYP1A2 failed to catalyze the formation of any detectable acetaminophen from phenacetin in the benchmark compound testing (data not shown). With the exception of folpet bioactivation (Supplemental Figure 1), there was no evidence of CYP1A2 bioactivity in the cytotoxicity screen. The inactivity of CYP1A2 was unanticipated since the cDNA clone was full-length and sequence verified prior to purchase. After cloning, the pmRNA-CYP1A2 plasmid was re-sequenced to confirm no artifacts were introduced by PCR amplification or cloning. CYP1A2 was cloned in the exact manner into the same pmRNA vector as eight of the other nine CYP-encoding genes. Evidence of bioactivity of all nine of the other CYP enzymes are derived from the luminogenic substrate, benchmark compound and/or cytotoxicity screen experiments. Alignment with the canonical “wild-type” CYP1A2 sequence revealed that the BC067427 clone used in this study harbors a single nucleotide change (248G>A) that encodes a change in amino acid 81 from glycine to aspartic acid (G81D; Supplemental Figure 2). This nucleotide change was verified in the BC067427 reference sequence and was re-confirmed after subcloning CYP1A2 into the pmRNA plasmid. Although many CYP1A2 polymorphisms have been reported (https://www.pharmvar.org/gene/CYP1A2), this specific nucleotide change has not been reported, and therefore nothing is known about the functional impact of the resulting G81D amino acid change. CYP1A2 is important for the metabolism of many known drugs and environmental chemicals, so it is imperative that a wild-type CYP1A2 cDNA be procured, re-cloned and transcribed to mRNA to re-test with a functional enzyme. The aberrant CYP1A2 clone highlights both a caveat and an opportunity for the use of transfected XME-encoding mRNAs to retrofit cells with metabolic activity. The results present herein or in other gene delivery XME studies, are dependent on the cDNA sequences used and it is known that for most CYP genes, many genetic variants exist. The mRNA transfection method described herein could however facilitate the wide-scale study of how CYP gene variants differentially metabolize environmental chemicals, helping to expand our understanding of interindividual differences in toxicant susceptibility.
The expression of multiple XME-encoding mRNAs could be effectively achieved by co-transfecting an mRNA pool to mimic the expression patterns observed in situ for tissues such as human liver. This study relied upon a single review to demonstrate how XME-encoding mRNAs might be pooled to approximate liver tissue. However, there is evidence that trying to architype the “typical” human liver may very difficult due to inter-individual XME gene expression pattern and that perhaps maybe as many as five broad liver “types” exist (Slatter et al., 2006). Modeling multiple liver “types” is easily achieved using the mRNA transfection approach. Many other studies have looked at XME expression patterns in extrahepatic tissues which could also be modeled using this approach. The use of a liver mix when compared to CYP singlets in this study was instructive. Some of the predominant CYP enzymes (CYP3A4, CYP2C9, CYP2E1) were capable of bioactivating test compounds in the cytotoxicity screen even when reduced to their liver mix percentage and despite the activity penalty stemming from co-expression of multiple CYPs in HEK293T cells. The liver mix always induced a muted bioactivity shift compared to the CYP singlet suspected of driving the response, meaning with the 56 chemicals tested, no synergistic or non-additive effects were observed from multiple CYP co-expression.
In conclusion, we have successfully demonstrated mRNA transfection as a rapid and efficient method for introducing xenobiotic metabolism into HEK293T cells. Currently, the ToxCast program uses HEK293 cells for several important assay endpoints including many of the nuclear receptors (such as estrogen and androgen receptor) and certain stress response pathway assays (e.g. DNA damage response). While the mRNA transfection protocol will have to be re-optimized for each additional cell line used in ToxCast (i.e. HepG2), this study provides a roadmap for imbuing metabolic competence to a cell line with little to no intrinsic metabolic capability in a manner that is portable to other cell types and amenable to high-throughput screening.
Supplementary Material
Acknowledgements
The authors are grateful to Dr. Brian Chorley of the National Health and Environmental Effects Research Laboratory and Dr. John Cowden of the National Center for Computational Toxicology of the US EPA for critical review of the manuscript and helpful comments. This work was funded in part by a Cooperative Research and Development Agreement (CRADA) between Unilever U.K. Central Resources Limited and the United States Protection Agency. The full text of the CRADA can be found at: https://www.epa.gov/sites/production/files/2015-9/documents/unilever_crada_linda_edwards.pdf
Footnotes
Supplementary Data
Supplemental Figure 1 and Supplemental Figure 2/Supplemental Tables 1–6 are provided as separate files. Supplemental Figure 1 presents concentration-response curves for 56 chemicals tested for cytotoxicity in HEK293T cells transfected with ten human liver CYP-encoding mRNAs as singlets, a 10-CYP mixture to mimic human liver and βgal control mRNA. Supplemental Figure 2 shows a protein alignment of wild-type CYP1A2 and the CYP1A2 enzyme resulting from the BC067427 clone used in this study. Supplemental Table 1 lists the Dharmacon clone ID and Genbank Accession ID and forward and reverse PCR primers used to subclone all eleven cDNAs used in this study. Supplemental Table 2 lists the analytical standards and LC-MS/MS conditions used to quantify each parent/metabolite pair examined. Supplemental Table 3 lists the limits of detection and recoveries for parent/metabolite pair examined. Supplemental Table 4 lists the 56 chemicals tested in the cytotoxicity screen. Supplemental Table 5 lists the POR optimization data from Figure 1B with p-values. Supplemental Table 6 lists the CYP competition data from Figure 3 with p-values. Supplemental Table 7 lists the luminogenic CYP substrate assay conditions used in Figure 4. Supplemental Table 8 lists the p-values for each enzyme-substrate pair examined in Figure 5 compared to βgal controls.
Disclaimer:
The views expressed in this paper are those of the authors and do not necessarily represent the views or policies of the U.S. Environmental Protection Agency.
References
- Bull S, Langezaal I, Clothier R, Coecke S, 2001. A Genetically engineered cell-based system for detecting metabolism-mediated toxicity. Altern. Lab. Anim 29, 703–716. [DOI] [PubMed] [Google Scholar]
- Chauret N, Gauthier A, Nicoll-Griffith DA, 1998. Effect of common organic solvents on in vitro cytochrome P450-mediated metabolic activities in human liver microsomes. Drug Metab. Dispos 26, 1–4. [PubMed] [Google Scholar]
- Chen T (Ed.), 2010. A Practical Guide to Assay Development and High-Throughput Screening in Drug Discovery, first ed. CRC Press, Boca Raton. [Google Scholar]
- Dean L, 2012. Amitriptyline Therapy and CYP2D6 and CYP2C19 Genotype. In Pratt V, McLeod H, Dean L, Malheiro A, Rubinstein W, (Eds.), Medical Genetics Summaries [Internet]. Available from: https://www.ncbi.nlm.nih.gov/pubmed/28520380 [Google Scholar]
- Diebold SS, Kaisho T, Hemmi H, Akira S, Reis e Sousa C, 2004. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science 303, 1529–1531. [DOI] [PubMed] [Google Scholar]
- Doehmer J, 1993. V79 Chinese hamster cells genetically engineered for cytochrome P450 and their use in mutagenicity and metabolism studies. Toxicology 82, 105–118. [DOI] [PubMed] [Google Scholar]
- Donato MT, Lahoz A, Castell JV, Gomez-Lechon MJ, 2008. Cell lines: A tool for in vitro drug metabolism studies. Curr. Drug Metab 9, 1–11. [DOI] [PubMed] [Google Scholar]
- Garcia-Canton C, Minet E, Anadon A, Meredith C, 2013. Metabolic characterization of cell systems used in in vitro toxicology testing: lung cell system BEAS-2B as a working example. Toxicol. In Vitro 27, 1719–1727. [DOI] [PubMed] [Google Scholar]
- Gomez-Foix AM, Coats WS, Baque S, Alam T, Gerard RD, Newgard CB, 1992. Adenovirus-mediated transfer of the muscle glycogen phosphorylase gene into hepatocytes confers altered regulation of glycogen metabolism. J. Biol. Chem 267, 25129–25134. [PubMed] [Google Scholar]
- Gotz C, Pfeiffer R, Tigges J, Blatz V, Jackh C, Freytag EM, Fabian E, Landsiedel R, Merk HF, Krutmann J, Edwards RJ, Pease C, Goebel C, Hewitt N, Fritsche E, 2012. Xenobiotic metabolism capacities of human skin in comparison with a 3D epidermis model and keratinocyte-based cell culture as in vitro alternatives for chemical testing: activating enzymes (Phase I). Exp. Dermatol 21, 358–363. [DOI] [PubMed] [Google Scholar]
- Hodgson E, Rose RL, 2007. Human metabolic interactions of environmental chemicals. J. Biochem. Mol. Toxicol 21, 182–186. [DOI] [PubMed] [Google Scholar]
- Ioannides C, Lewis DF, 2004. Cytochromes P450 in the bioactivation of chemicals. Curr. Top. Med. Chem 4, 1767–1788. [DOI] [PubMed] [Google Scholar]
- Jackson JP, Li L, Chamberlain ED, Wang H, Ferguson SS, 2016. Contextualizing Hepatocyte Functionality of Cryopreserved HepaRG Cell Cultures. Drug Metab. Dispos 44, 1463–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs MN, Laws SC, Willett K, Schmieder P, Odum J, Bovee TF, 2013. In vitro metabolism and bioavailability tests for endocrine active substances: what is needed next for regulatory purposes? ALTEX 30, 331–351. [DOI] [PubMed] [Google Scholar]
- Jewell H, Maggs JL, Harrison AC, O’Neill PM, Ruscoe JE, Park BK, 1995. Role of hepatic metabolism in the bioactivation and detoxication of amodiaquine. Xenobiotica 25, 199–217. [DOI] [PubMed] [Google Scholar]
- Kirkland D, Pfuhler S, Tweats D, Aardema M, Corvi R, Darroudi F, Elhajouji A, Glatt H, Hastwell P, Hayashi M, Kasper P, Kirchner S, Lynch A, Marzin D, Maurici D, Meunier JR, Muller L, Nohynek G, Parry J, Parry E, Thybaud V, Tice R, van Benthem J, Vanparys P, White P, 2007. How to reduce false positive results when undertaking in vitro genotoxicity testing and thus avoid unnecessary follow-up animal tests: Report of an ECVAM Workshop. Mutat. Res 628, 31–55. [DOI] [PubMed] [Google Scholar]
- Kitamura R, Sato K, Sawada M, Itoh S, Kitada M, Komori M, Kamataki T, 1992. Stable expression of cytochrome P450IIIA7 cDNA in human breast cancer cell line MCF-7 and its application to cytotoxicity testing. Arch. Biochem. Biophys 292, 136–140. [DOI] [PubMed] [Google Scholar]
- Lahoz A, Vila MR, Fabre M, Miquel JM, Rivas M, Maines J, Castell JV, Gomez-Lechon MJ, 2013. An in vitro tool to assess cytochrome P450 drug biotransformation-dependent cytotoxicity in engineered HepG2 cells generated by using adenoviral vectors. Toxicol. In Vitro 27, 1410–1415. [DOI] [PubMed] [Google Scholar]
- Lengler J, Omann M, Duvier D, Holzmuller H, Gregor W, Salmons B, Gunzburg WH, Renner M, 2006. Cytochrome P450 reductase dependent inhibition of cytochrome P450 2B1 activity: Implications for gene directed enzyme prodrug therapy. Biochem. Pharmacol 72, 893–901. [DOI] [PubMed] [Google Scholar]
- Mace K, Aguilar F, Wang JS, Vautravers P, Gomez-Lechon M, Gonzalez FJ, Groopman J, Harris CC, Pfeifer AM, 1997. Aflatoxin B1-induced DNA adduct formation and p53 mutations in CYP450-expressing human liver cell lines. Carcinogenesis 18, 1291–1297. [DOI] [PubMed] [Google Scholar]
- Matuszewski BK, Constanzer ML, Chavez-Eng CM, 2003. Strategies for the assessment of matrix effect in quantitative bioanalytical methods based on HPLC-MS/MS. Anal. Chem 75, 3019–3030. [DOI] [PubMed] [Google Scholar]
- Mollergues J, van Vugt-Lussenburg B, Kirchnawy C, Bandi RA, van der Lee RB, Marin-Kuan M, Schilter B, Fussell KC, 2017. Incorporation of a metabolizing system in biodetection assays for endocrine active substances. ALTEX 34, 389–398. [DOI] [PubMed] [Google Scholar]
- OECD: Organisation for Economic Co-operation and Development, 2008. Detailed review paper on the use of metabolising systems for in vitro testing of endocrine disruptors, pp. 1–79.
- Preskey D, Allison TF, Jones M, Mamchaoui K, Unger C, 2016. Synthetically modified mRNA for efficient and fast human iPS cell generation and direct transdifferentiation to myoblasts. Biochem. Biophys. Res. Commun 473, 743–751. [DOI] [PubMed] [Google Scholar]
- Qin JY, Zhang L, Clift KL, Hulur I, Xiang AP, Ren BZ, Lahn BT, 2010. Systematic comparison of constitutive promoters and the doxycycline-inducible promoter. PLoS One 5, e10611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reigh G, McMahon H, Ishizaki M, Ohara T, Shimane K, Esumi Y, Green C, Tyson C, Ninomiya S, 1996. Cytochrome P450 species involved in the metabolism of quinoline. Carcinogenesis 17, 1989–1996. [DOI] [PubMed] [Google Scholar]
- Riddick DS, Ding X, Wolf CR, Porter TD, Pandey AV, Zhang QY, Gu J, Finn RD, Ronseaux S, McLaughlin LA, Henderson CJ, Zou L, Fluck CE, 2013. NADPH-cytochrome P450 oxidoreductase: roles in physiology, pharmacology, and toxicology. Drug Metab. Dispos 41, 12–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Russell D (Eds.), 2001. Molecular Cloning: A Laboratory Manual, third ed. Cold Spring Harbor Laboratory Press, New York. [Google Scholar]
- Slatter JG, Templeton IE, Castle JC, Kulkarni A, Rushmore TH, Richards K, He Y, Dai X, Cheng OJ, Caguyong M, Ulrich RG, 2006. Compendium of gene expression profiles comprising a baseline model of the human liver drug metabolism transcriptome. Xenobiotica 36, 938–962. [DOI] [PubMed] [Google Scholar]
- Smith RL, Traul DL, Schaack J, Clayton GH, Staley KJ, Wilcox CL, 2000. Characterization of promoter function and cell-type-specific expression from viral vectors in the nervous system. J. Virol 74, 11254–11261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltanpour Y, Hilgendorf C, Ahlstrom MM, Foster AJ, Kenna JG, Petersen A, Ungell AL, 2012. Characterization of THLE-cytochrome P450 (P450) cell lines: gene expression background and relationship to P450-enzyme activity. Drug Metab. Dispos 40, 2054–2058. [DOI] [PubMed] [Google Scholar]
- Thomas RS, Black MB, Li L, Healy E, Chu TM, Bao W, Andersen ME, Wolfinger RD, 2012. A comprehensive statistical analysis of predicting in vivo hazard using high-throughput in vitro screening. Toxicol. Sci 128, 398–417. [DOI] [PubMed] [Google Scholar]
- Tolosa L, Donato MT, Perez-Cataldo G, Castell JV, Gomez-Lechon MJ, 2012. Upgrading cytochrome P450 activity in HepG2 cells co-transfected with adenoviral vectors for drug hepatotoxicity assessment. Toxicol. In Vitro 26, 1272–1277. [DOI] [PubMed] [Google Scholar]
- Tolosa L, Gomez-Lechon MJ, Perez-Cataldo G, Castell JV, Donato MT, 2013. HepG2 cells simultaneously expressing five P450 enzymes for the screening of hepatotoxicity: identification of bioactivable drugs and the potential mechanism of toxicity involved. Arch. Toxicol 87, 1115–1127. [DOI] [PubMed] [Google Scholar]
- Tolosa L, Jimenez N, Perez G, Castell JV, Gomez-Lechon MJ, Donato MT, 2017. Customised in vitro model to detect human metabolism-dependent idiosyncratic drug-induced liver injury. Arch. Toxicol, [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignati L, Turlizzi E, Monaci S, Grossi P, Kanter R, Monshouwer M, 2005. An in vitro approach to detect metabolite toxicity due to CYP3A4-dependent bioactivation of xenobiotics. Toxicology 216, 154–167. [DOI] [PubMed] [Google Scholar]
- Walsky RL, Obach RS, 2004. Validated assays for human cytochrome P450 activities. Drug Metab. Dispos 32, 647–660. [DOI] [PubMed] [Google Scholar]
- Warren L, Manos PD, Ahfeldt T, Loh YH, Li H, Lau F, Ebina W, Mandal PK, Smith ZD, Meissner A, Daley GQ, Brack AS, Collins JJ, Cowan C, Schlaeger TM, Rossi DJ, 2010. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell 7, 618–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerink WMA, Schoonen W, 2007. Cytochrome P450 enzyme levels in HepG2 cells and cryopreserved primary human hepatocytes and their induction in HepG2 cells. Toxicol. In Vitro 21, 1581–1591. [DOI] [PubMed] [Google Scholar]
- Xuan JK, Chen S, Ning BT, Tolleson WH, Guo L, 2016. Development of HepG2-derived cells expressing cytochrome P450s for assessing metabolism-associated drug-induced liver toxicity. Chem.-Biol. Interact 255, 63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanger UM, Schwab M, 2013. Cytochrome P450 enzymes in drug metabolism: regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol. Ther 138, 103–141. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.