Abstract
Patients with relapsed or refractory (r/r) acute myeloid leukemia (AML) have a poor prognosis and treatment remains challenging. For the majority of r/r patients, allogeneic hematopoietic stem cell transplantation (HSCT) is the only curative treatment approach. Salvage therapy is given in order to reduce the leukemia load prior to transplantation. Patients achieving complete remission prior to allogeneic HSCT have a more favorable outcome. Intensive salvage regimens commonly consist of an anthracycline and high-dose cytarabine backbone. Donor lymphocyte infusions have shown efficacy in patients relapsing after allogeneic HSCT. For patients who cannot be intensively treated (eg, elderly AML patients), outcome is generally very poor and combinations with novel agents are currently under investigation. Mutational analysis should be repeated at the time of relapse to identify aberrations that can be targeted with new agents. For r/r AML patients with mutated fms-related tyrosine kinase 3 (FLT3), gilteritinib has shown superior results to intensive salvage regimens. The US Food and Drug Administration (FDA) and European Medicines Agency (EMA) approved gilteritinib for FLT3 mutated r/r AML patients. Ivosidenib and enasidenib, inhibitors for mutated isocitrate dehydrogenase (IDH) 1 and 2, respectively, have received approval for IDH1/IDH2 mutated r/r AML by the FDA (not EMA). APR-246 restores the function of mutated TP53 and early study results are promising. Other agents targeting CD47, menin, neural-precursor-cell-expressed developmentally down-regulated 8, as well as bispecific antibodies or chimeric antigen receptor T cells are under investigation. Further trials are needed to understand how to best combine novel agents with each other or with chemotherapy.
Introduction
Over recent years, treatment options for patients with newly diagnosed acute myeloid leukemia (AML) have evolved beyond 7 + 3. For AML patients who harbor a mutation in fms-related tyrosine kinase 3 (FLT3) and are eligible for intensive treatment, midostaurin in combination with 7 + 3 has become the standard of care.1 Gemtuzumab-ozogamicin received approval in combination with intensive chemotherapy for patients with CD33 positive AML. It shows a benefit in patients with good and intermediate AML risk.2 For patients with newly diagnosed therapy-related AML (t-AML), secondary (s-AML), or AML with myelodysplasia-related changes, treatment with CPX-351—a liposomal formulation of daunorubicin and cytarabine in a fixed combination—is now available.3 In elderly patients, the addition of venetoclax to hypomethylating agents (HMAs)/low-dose Cytarabine (LDAC) has resulted in impressive survival benefits.4,5 Despite these advancements, a significant number of AML patients die from the disease, many due to relapse and some by being refractory to frontline-treatment. The European LeukemiaNet defines primary refractory disease as failure to achieve complete remission (CR) after 2 courses of intensive induction therapy.6 An operational definition of primary refractory AML has been suggested by Ferguson et al7 when analyzing outcome in 8907 AML patients. Here, patients who had an insufficient response to the first induction—defined as a less than 50% proportional reduction in blasts and the presence of more than 15% blasts—or a failure to achieve CR after 2 courses of induction, showed a very poor outcome and were defined as primary refractory patients.7 In contrast, relapse is diagnosed in AML patients who have achieved CR but show an increase of blasts in the bone marrow to ≥5%, reappearance of blasts in the blood or development of extramedullary disease. Of note, molecular relapse has been well studied in certain molecular subgroups including patients with core binding factor leukemia and patients with mutated nucleophosmin 1 (NPM1).8,9 Furthermore, progressive disease describes an increase in bone marrow blast percentage and/or increase of absolute blast counts in the blood.6 It is frequently referred to elderly patients not receiving intensive therapy who fail to achieve a CR and instead develop an increase in the leukemia load in the course of the disease. Thus, the situation of patients with refractory/relapsed (r/r) AML is complex and diverse. The prognosis of r/r AML patients depends on many factors including age, prior therapy including allogeneic hematopoietic stem cell transplantation (HSCT), timing of relapse, and the mutational as well as cytogenetic profile of the disease.10,11 In general, allogeneic HSCT achieves survival in 20%–35% of r/r AML patients at 4 years.12,13 Thus, relapse after allogeneic HSCT is a common problem and occurs in 25%–55% of AML patients.14 These numbers underscore that clinical trials for r/r AML patients are highly needed due to the grim prognosis of many r/r AML patients. Therefore, enrolling r/r AML patients into clinical trials has a high priority. There is no single standard treatment for r/r AML patients. Instead, many factors need to be considered when choosing therapy for these patients as outlined in Figure 1, and therapeutic strategies have diverse cellular targets (Figure 2).
Figure 1.
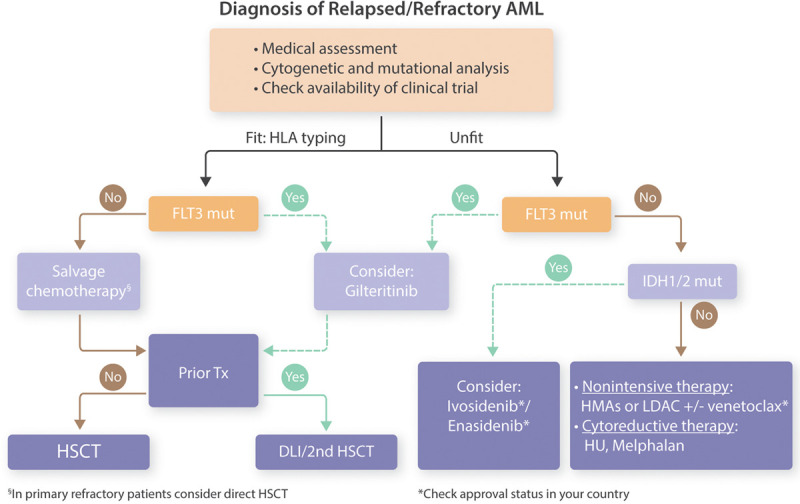
Possible treatment algorithm in relapsed/refractory AML patients. AML = acute myeloid leukemia; DLI = donor lymphocyte infusions; FLT3 = fms-related tyrosine kinase 3; HLA = human leukocyte antigen; HMA = hypomethylating agent; HSCT = hematopoietic stem cell transplantation; HU = hydroxyurea; IDH = isocitrate dehydrogenase; LDAC = low-dose cytarabine; mut = mutated; Tx = transplantation.
Figure 2.
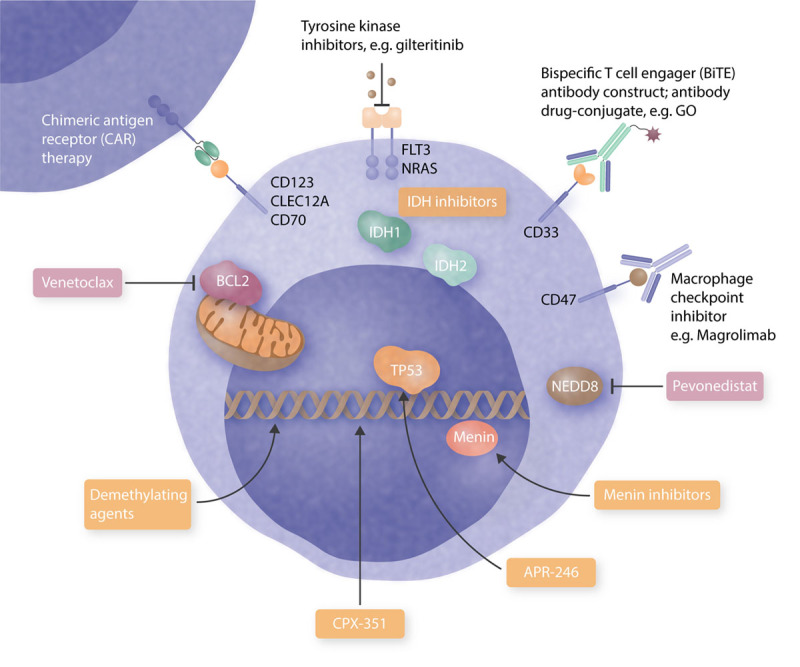
Evolving and established targets in relapsed/refractory AML. BCL2 = B-cell leukemia/lymphoma-2; BiTE = bispecific T-cell engager; CAR = chimeric antigen receptor; CLEC12A = C-type lectin domain family 12 member A; FLT3 = fms-related tyrosine kinase 3; GO = gemtuzumab ozogamicin; IDH = isocitrate dehydrogenase; NEDD8 = neural-precursor-cell-expressed developmentally down-regulated 8.
Treatment choice in fit/transplant eligible patients
Allogeneic HSCT is the treatment of choice for AML patients relapsing after chemotherapy. Transplant eligibility depends on patients’ age as well as comorbidities (eg, as calculated by the Hematopoietic Cell Transplantation-specific Comorbidity Index15). Thus, in case not already done at the time of diagnosis, human leukocyte antigen (HLA) typing should be performed in all transplant-eligible patients at time of relapse. Prior to allogeneic HSCT, salvage therapy is necessary, especially for patients with high blast percentage in the bone marrow. A subgroup of patients (eg, those unlikely to benefit from salvage therapy) who have primary refractory disease might benefit from direct allogeneic HSCT.7 Several intensive treatment protocols are established as salvage therapy. Anthracyclines and high-dose cytarabine are usually the backbone of these salvage regimens. Examples of commonly used regimens include Fludarabine, Cytarabine, Idarubicin, and granulocyte-colony stimulating factor (FLAG-IDA) and Mitoxantrone, Etoposide, and Cytarabine (MEC). Expected CR rates with these regimens are around 29%–66%.16 None of these regimens have shown superiority over the others, again highlighting how little progress has been made over the years.17 CR rates of r/r AML patients with salvage therapy are considerably lower as compared to rates achieved in front-line treatment.6 Furthermore, remissions are usually not long sustained, making allogeneic HSCT a critical element for cure. The combination of these salvage regimens with novel drugs is currently under investigation. In 2 smaller studies, venetoclax, an oral highly selective small-molecule B-cell leukemia/lymphoma-2 inhibitor, was combined with the intensive salvage regimen FLAG-IDA. In our observational study, 13 r/r AML patients received FLA-V-IDA (FLAG-IDA plus venetoclax given on days 1–7).18 The outcomes of these 13 FLA-V-IDA patients were retrospectively compared to 81 r/r AML patients treated with conventional FLAG-IDA. Importantly, the addition of 7-day venetoclax did not result in excess hematological toxicity. The overall response rate (ORR) was 69% in patients treated with FLA-V-IDA versus 47% in patients treated with FLAG-IDA.18 In a similar phase Ib/II study, FLAG-IDA was combined with venetoclax. Initially, patients received a 21-day course of venetoclax.19 However, after observing sepsis or bacteremia in 5 of 6 patients, the schedule of venetoclax was reduced to a 14-day course. Twenty-six out of 35 (74%) r/r AML patients achieved an overall response (CR/CR with incomplete hematologic recovery [CRi]/CR with partial hematologic recovery) and 54% achieved CR/CRi.19 Both studies, despite being small and early, are encouraging for the combination therapy. CPX-351, the liposomal formulation of daunorubicin and cytarabine, has also been studied in r/r AML patients. In a phase II trial, 125 r/r AML patients were randomized 1:2 to receive conventional salvage therapy or CPX-351.20 One-year survival was similar in both treatment arms. Interestingly, in the subset of patients with poor-risk cytogenetics, CPX-351 demonstrated higher response rates (39.3% versus 27.6%) and improved overall survival (OS) (hazard ratio [HR], 0.55; P = 0.02). However, to date, CPX-351 has no approval in the r/r setting. Instead, CPX-351 has received approval as front-line treatment for patients with s-AML, t-AML, as well as AML with MRC. These examples demonstrate how novel agents can be added to established salvage therapies. Nevertheless, further studies are highly needed in order to understand how novel agents can be combined with each other or with standard therapy in r/r AML to improve outcome of r/r AML.
Measurable residual disease (MRD) assessment prior to allogeneic HSCT has demonstrated that a lower leukemia burden has been associated with a more favorable outcome after allogeneic HSCT,21,22 emphasizing the significance of effective salvage therapy. In the setting of r/r AML, allogeneic HSCT is mostly performed from matched related as well as unrelated donors in the United States and in Europe. However, haploidentical donors have become good alternatives due to advancement in prophylaxis of graft-versus-host-disease, for example, application of post-transplantation cyclophosphamide.23 Early data suggests that MRD-positive patients show a lower relapse rate if transplanted from a haploidentical versus a matched related/unrelated donor.24 Prospective trials addressing this question are currently underway and will hopefully give us future guidance. In general, allogeneic HSCT achieves survival in 20%–35% of these patients at 4 years.12,13 Thus, relapse after allogeneic HSCT is a common problem and occurs in 25%–55% of AML patients.14
Post-allograft relapse
Prognosis is very poor for those patients with post-allograft relapsed AML, that is, relapsing after allogeneic HSCT. Unfortunately, this is a common clinical scenario given the relatively high relapse rate described above. The 2-year OS of relapsed patients following allogeneic HSCT ranges between 14% and 25% with even fewer of them achieving a long-term cure.14 There is no established standard therapy for patients with post-allograft relapse, and continued research is urgently needed in order to improve their dismal outcome. Importantly, a second HSCT is associated with markedly higher transplant-related mortality as the first transplantation.25 Donor lymphocyte infusions (DLIs) may represent an alternative strategy to second allogeneic HSCT as DLIs can also achieve a graft-versus-leukemia (GvL) effect. A retrospective registry study in relapsed AML patients compared the effect of DLIs versus second HSCT.25 In the study, 137 patients were treated with a second HSCT while 281 patients received a DLI. Both interventions achieved better outcome if applied after achieving CR and dismal outcome if applied in patients relapsing less than 6 months after initial transplant. OS was comparable for patients treated with DLIs and second HSCT, with a 2-year OS of 25% versus 26%, and 5-year OS of 15% versus 19%, respectively.
Nonintensive therapy
Nonintensive therapy is the treatment of choice for elderly AML patients or patients with significant comorbidities. Since the approval of decitabine and azacitidine in AML, HMAs have become the standard of care for elderly AML patients. In a phase III trial with 488 elderly AML patients, azacitidine improved median OS from 6.5 to 10.4 months with conventional treatments (eg, LDAC).26 Outside clinical trials, treatment with HMAs was associated with a median OS of 7–8 months.27
HMAs
In addition, HMAs are also being employed as second- or third-line treatment for r/r AML patients after intensive treatments. In this patient group, response rates are lower compared to front-line treatment with HMAs. A study based on an international multicenter retrospective database evaluated the effectiveness of HMAs in 655 r/r AML patients.28 Patients who relapsed after HSCT or who were refractory to induction therapy were included. In this trial, best response to HMAs was CR in 11% and CRi in 5.3%. The median OS was 6.5 months for all patients, while the OS for patients achieving CR/CRi was 21 months. Patients with a low proliferative disease showed a superior OS. This data underscores that HMAs monotherapy does not result in long-term remissions neither as front-line treatment in elderly AML patients nor in younger r/r AML patients.
Venetoclax
For these patients, outcome is generally very grim. In this context, the approval of venetoclax for elderly AML patients by the US Food and Drug Administration (FDA) in 2018 (with European Medicines Agency [EMA] approval still pending) has positively changed the treatment landscape for elderly AML patients. Approval was based on the encouraging results of venetoclax in combination with LDAC5 and HMAs.4 Fifty-four percent of AML patients treated with venetoclax and LDAC achieved either a CR or CRi with a median OS of 10.1 months.5 For patients who received this combination front-line (ie, without prior HMA exposure), CR/CRi rates were 64% with a median OS of 13.5 months.5 In a randomized trial comparing HMAs in combination with venetoclax or placebo, median OS was 14.5 months in the venetoclax arm compared to 9.6 months in the placebo arm. Furthermore, the CR/CRi rate was increased from 28.3 in the placebo arm to 66.4 in the venetoclax arm. Response and durable remission have been associated with mutations in NPM1 and isocitrate dehydrogenase (IDH) 2 while mutations in FLT3, RAS, and biallelic TP53 mutations are increased in patients refractory to venetoclax.29 Many elderly patients (especially in the United States, and likely soon more globally) will have received venetoclax combinations front-line. As these combinations are relatively novel, we still lack comprehensive and long-term data of patients relapsing after initial venetoclax combinations. However, a retrospective analysis suggests that once patients become r/r to HMA/venetoclax, the prognosis is dismal.30 Importantly, in this setting, salvage therapy seems to be ineffective as patients who receive salvage therapy after HMA and venetoclax failure showed a median OS of only 2.9 months30 as compared to 9.5 months when being r/r to HMA alone as previously reported.31 This suggests a very aggressive disease biology arising after HMA/venetoclax failure. This is supported by the observation that patients with favorable predictive markers (eg, mutations in IDH1/2 or NPM1) at the initiation of treatment developed high-risk cytogenetic and molecular features (eg, mutations in TP53, N/KRAS, and/or KIT) when being r/r to HMA/venetoclax.30 In elderly patients who fail HMA/venetoclax, the first choice of salvage treatments are clinical trials and targeted approaches, which are described below. Of note, r/r AML patients who are venetoclax-naive may benefit from venetoclax treatment as second- or third-line treatment, but not as single agent as it has no significant activity as monotherapy.32 In a single-center retrospective study, 33 patients with r/r AML (20 patients who failed HMA, 13 patients who relapsed after allogeneic HSCT) were treated with HMA and venetoclax.33 ORRs were 64%, with 30% of patients achieving a CR, 21% a CRi, and 12% a morphological leukemia-free state (MLFS). Importantly, the best response was obtained after 2 cycles of therapy, which is more rapid as compared to HMA alone.33 Response rates were similar between patients with prior allogeneic HSCT or HMA therapy. Further retrospective studies involving between 21 and 90 r/r AML patients have also studied venetoclax in combination with HMA/LDAC.34–37 Here, CR rates were between 38% and 46% with a median OS of 5.6 to 7.8 months.34–37 In a prospective trial, venetoclax was added to a 10-day schedule of decitabine.38 Fifty-five of 168 AML patients (33%) in the trial had r/r AML. The ORR was 62% in this cohort of patients (34/55 patients). A frequent side effect was febrile neutropenia, occurring in 29% of patients. The median OS was 7.8 months in r/r AML patients as compared to 18.1 months in newly diagnosed AML patients.38 These early data suggest that venetoclax combinations may also play a role in the r/r setting, but are unlikely to lead to long-term remissions or cure.
Miscellaneous
A non-targeted approach that is approved in the United States is gemtuzumab-ozogamicin monotherapy for induction followed by cytarabine consolidation, with a CR/CRi rate of 33% and a median relapse-free survival (RFS) of 10 months.39 Cladribine in combination with low-dose cytarabine alternating with cladribine/decitabine has resulted in a CR/CRi rate of 68% and a median OS of 13.8 months in the front-line setting, making it an attractive combination for evaluation in patients who fail HMA/venetoclax.40 Another therapeutic target is Pevonedistat, a first-in-class neural-precursor-cell-expressed developmentally down-regulated 8-activating enzyme inhibitor. It has shown modest single-agent activity in relapsed AML patients41 and is currently studied in combination with azacitidine as well as azacitidine and venetoclax.
Targeted therapy
Clonal diversity and evolution is an important driver of r/r AML. While some mutations are stable and remain present at diagnosis and relapse, other mutations can be lost or gained so that the molecular profile at diagnosis and relapse can differ. Due to prognostic and therapeutic implications, it is recommended to repeat mutational profiling at the time of relapse.
FLT3 inhibitors
FLT3-internal tandem duplications (ITDs) and FLT3-tyrosine kinase domain (TKD) mutations occur in 20%–30% and <10% of AML patients, respectively.42 Of note, these mutations can be unstable during clonal evolution and therefore disappear or emerge at relapse. The prognosis of patients with relapsed FLT-ITD positive AML is poor.43 Standard treatment with intensive chemotherapy rarely leads to long-term survival.43 The introduction of first- and second-generation tyrosine kinase inhibitors (TKIs) with activity against FLT3 has changed treatment options for FLT3 mutated patients. However, we are just beginning to understand the mechanisms of resistance to TKIs.44 While primary resistance occurs in patients who are TKI-naive, secondary resistance describes resistance in patients already treated with TKI and who become resistant due to clonal evolution.44 Midostaurin—a first-generation TKI—in combination with intensive therapy has become standard of care for newly diagnosed fit FLT3 mutated AML patients.1 Nevertheless, midostaurin has no activity as a single agent and currently plays no role in r/r AML. Second-generation TKIs, which are more specifically targeting FLT3, have shown promising results in r/r AML patients. In a large phase III trial (ADMIRAL trial), 371 relapsed AML patients with mutations in FLT3 (FLT3-ITD and FLT3-TKD) were randomized 2:1 to receive gilteritinib versus salvage therapy (FLAG-IDA, MEC, LDAC, or azacitidine according to local investigators choice). CR or CRi was achieved in 34% versus 15.3% in patients treated in the gilteritinib versus standard arm. Patients treated with gilteritinib demonstrated a significantly longer median OS with 9.3 months as compared to 5.6 months in the standard arm.45 Severe events were less commonly seen in the gilteritinib arm. Of note, patients were allowed to have received midostaurin during front-line treatment. However, this was only the case in a limited number of patients (5.7%). This small fraction of patients does not allow a valid subgroup analysis for possible implications of previous midostaurin treatment. However, a retrospective analysis of 13 US medical centers investigated how gilteritinib impacts survival in r/r FLT3 mutated AML patients previously treated with TKIs.46 For patients who remained r/r after 7 + 3 + midostaurin (n = 46), gilteritinib produced composite CR rates of 58% and OS of 7.8 months. The encouraging results of gilteritinib in the ADMIRAL trial has led to the approval of the drug by the FDA and EMA for FLT3 mutated r/r AML patients. While monotherapy with gilteritinib is unlikely to lead to cure for relapsed patients, it can be used as a bridge to allogeneic HSCT. Thus, gilteritinib treatment should be followed by allogeneic HSCT in all transplant-eligible patients (preferably at time of CR). For elderly r/r patients who are not transplant-eligible, gilteritinib can be continued until patients lose response. The benefit of combining gilteritinib with other agents is currently being investigated in clinical trials. For instance: in a multicenter, open-label, phase 1b clinical trial (NCT03625505) evaluating the safety and efficacy of venetoclax in combination with gilteritinib for patients with r/r AML.47 For patients with mutated FLT3, the ORR was 90% with 50% achieving a CR/CRi and 40% showing a MLFS. Importantly, a substantial number of patients have received prior TKI treatment and the response rates were still high. These early data suggest a high efficacy of this combination for r/r FLT3 mutated AML patients. Quizartinib, another second-generation TKI, was also studied in r/r FLT3 mutated AML patients. The QuANTUM-R trial was a randomized phase 3 trial enrolling 367 FLT3-ITD positive (not FLT3-TKD) r/r AML patients.48 Of note, in this study as compared to the ADMiRAL trial, only patients with a duration of first composite CR ≤6 months were included. While 245 patients were randomly allocated to quizartinib, 122 patients received chemotherapy (LDAC, MEC, or FLAG-IDA according to investigators choice). Median OS in the quizartinib arm was 6.2 versus 4.7 months in the chemotherapy arm (HR, 0.76, P = 0.02). While quizartinib received approval in Japan for r/r FLT3-ITD positive AML patients, it is currently not approved by the FDA nor EMA. Sorafenib, a multikinase inhibitor that among other kinases also targets FLT3, has not only been studied in front-line AML treatment but also in relapse after allogeneic HSCT. Here, an intriguing finding is that sorafenib has been shown to promote GvL activity in mice and humans through interleukin-15 production in FLT3-ITD-mutant leukemia cells.49 This might also be reflected by the encouraging results of sorafenib in combination with azacitidine in patients with relapsed FLT3-mutated AML after allogeneic HSCT.50 Furthermore, in the SORMAIN trial FLT3-ITD positive patients were randomized to receive sorafenib or placebo maintenance following allogeneic HSCT.51 In the sorafenib arm, the 24-month RFS probability was increased from 53.3% in the placebo arm to 85% (HR, 0.256; P = 0.002). In summary, TKIs show encouraging results in r/r FLT3 mutated AML and gilteritinib is approved in this setting.
IDH1/2 inhibitors
Targeted therapy has also been developed for AML patients harboring mutations in IDH1 or IDH2 occurring in 6%–10% of AML patients, respectively.42,52,53 Enasidenib, an oral inhibitor of mutated IDH2, has been studied as monotherapy in r/r IDH2 mutated AML patients.54 In this phase I/II trial, ORR among these r/r AML patients was 40.3% (median duration of response 5.8 mo). Median OS was 9.3 months for all r/r IDH2 mutated AML patients, and 19.7 months for the 19.3% of AML patients that attained CR. Specific side effects of enasidenib include differentiation syndrome in 7% of patients and hyperbilirubinemia in 12% of patients. Monotherapy of enasidenib has received approval for r/r IDH2 mutated AML patients by the FDA (but not EMA). Similarly, ivosidenib has been developed as an oral inhibitor of mutant IDH1. In a phase I trial, 179 r/r IDH1 mutated AML patients were treated.55 ORR was 39.1% and 21.8% of patients achieved a CR. The safety profile was similar to that already seen with enasidenib. The median OS was 8.8 months in the primary efficacy population. Ivosidenib was approved by the FDA (not EMA) for r/r IDH1 mutated AML patients in 2018, and since 2019 also as front-line treatment for elderly IDH1 mutated AML patients.
Additional targets
TP53 is another gene mutation where targeted therapy is evolving. It is the single most mutated gene in cancers and is found to be mutated in 10%–20% of AML patients.42 TP53 mutations are associated with poor outcomes and are increasingly found in patients with r/r AML.56 APR-246 targets mutated TP53 and restores its function as a tumor suppressor gene.57 Initial trials have studied APR-246 in combination with azacitidine in myelodysplastic syndrome (MDS) and AML. In a phase I/II trial, Sallman et al58 have studied this combination in 40 MDS patients and 11 AML (with <30% of blasts) patients with mutated TP53 as front-line therapy. In AML, the ORR was 64% with a 36% CR rate, which is only slightly lower as compared to MDS patients (here ORR 73%, CR 50%). Median OS was 10.8 months for the whole cohort. A very similar study was conducted by the French Groupe Francophone des Myélodysplasies in high-risk MDS and AML patients harboring a TP53 mutation. In this trial, the CR rate was found to be 56% at 6 cycles. Currently, APR-246 is neither approved by the FDA nor EMA. However, it has received orphan drug and fast track designations from the FDA for MDS, and orphan drug designation from the EMA for AML and MDS. These preliminary data raise hope for TP53 mutated r/r AML patients in the future. The MLL gene, located at 11q23 band, can be disrupted by different chromosomal rearrangements in AML (MLL-r). Menin is an exciting target for AML patients with MLL-translocations.59 SNDX-5613 and KO-539, potent, highly selective oral menin inhibitors, inhibit menin-MLL binding interactions. They are currently studied in r/r AML patients with MLL-rearrangements as well as NPM1 mutations (NCT04065399, NCT04067336). For those patients with MLL-r or mutant NPM1 and an additional FLT3 mutation, the combination of a menin inhibitor with an FLT3 inhibitor appears to be promising.60
Future directions
Novel strategies are arising for AML treatment. Some of them are still in preclinical development while others have already shown promising results in phase I trials. In order to find the best treatment approach for each individual patient, ex vivo drug sensitivity screening studies such as patient-derived xenograft models are under investigation and might be helpful for selected patients.61 However, more globally, many of the novel strategies are targeting or promoting the immune system in a sophisticated way. For instance, magrolimab is a novel immunotherapy that targets CD47 (anti-CD47 antibody). CD47 is known to be a macrophage immune checkpoint that functions as a “don’t eat me” signal on cancers. By blocking CD47 magrolimab can induce tumor phagocytosis and promotes elimination of leukemia stem cells by macrophages. In a phase IB trial, 52 treatment-naive AML patients (including a high number of patients with poor risk cytogenetics and/or mutated TP53) received magrolimab with azacitidine.62 Sixty-five percent of patients achieved an objective response, with 44% of patients achieving a CR. Time to response was approximately 2 months and therefore more rapid than with azacitidine alone. These encouraging results prompt further studies including in the r/r AML population. In 2020, magrolimab was granted orphan drug designation by the FDA for MDS and AML, and by the EMA for AML. Similar to acute lymphoblastic leukemia, where the CD19 directed bispecific antibody blinatumomab has become successfully embedded into treatment strategies for relapsed disease, CD33- and CD123-directed bispecific antibodies have been developed for the treatment of AML.63 In a phase I trial, AMG 330, an anti-CD33 bispecific T cell engager antibody construct was studied in r/r AML patients.64 Disappointingly, 35 of 40 patients discontinued therapy after a median of 1 cycle, in 77% of these cases due to disease progression. However, Flotetuzumab, a humanized dual-affinity re-targeting antibody-based molecule that both recognizes CD3 and CD123, has shown encouraging activity in an early trial including 38 AML patients with primary induction failure or early relapse. Here, 42.1% of patients achieved a CR and over half of those received allogeneic HSCT.65 Chimeric antigen receptor T cell (CAR-T) cell constructs for AML are also in development. The challenges for the success of CAR-T cells and bispecific antibody constructs in AML is the lack of a myeloid equivalent to a ubiquitously expressed antigen like CD19. Further research is currently on the way in order to identify the ideal myeloid target. But, it is conceivable that in AML, more than a single target exists for all patients. Other immune modulating approaches include the application of checkpoint inhibitors. Here, nivolumab as well as other checkpoint inhibitors have been added to azacitidine in r/r AML patients.66,67 While some patients demonstrated encouraging responses, the overall results in AML have been much less impressive as compared to solid tumors.66 Vaccines, for example, wilms tumor 1 peptide vaccines, are also under investigation.68 However, the study population of many vaccine trials are AML patients in CR (for relapse prevention) rather than r/r AML patients.69 In summary, r/r disease is still a very common problem and the most challenging scenario in AML therapy requiring novel treatment strategies.
Disclosures
The authors have no conflicts of interest to disclose.
References
- 1.Stone RM, Mandrekar SJ, Sanford BL, et al. Midostaurin plus chemotherapy for acute myeloid leukemia with a FLT3 mutation. N Engl J Med. 2017; 377:454–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hills RK, Castaigne S, Appelbaum FR, et al. Addition of gemtuzumab ozogamicin to induction chemotherapy in adult patients with acute myeloid leukaemia: a meta-analysis of individual patient data from randomised controlled trials. Lancet Oncol. 2014; 15:986–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lancet JE, Uy GL, Cortes JE, et al. CPX-351 (cytarabine and daunorubicin) liposome for injection versus conventional cytarabine plus daunorubicin in older patients with newly diagnosed secondary acute myeloid leukemia. J Clin Oncol. 2018; 36:2684–2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DiNardo CD, Jonas BA, Pullarkat V, et al. Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N Engl J Med. 2020; 383:617–629. [DOI] [PubMed] [Google Scholar]
- 5.Wei AH, Strickland SA, Jr, Hou JZ, et al. Venetoclax combined with low-dose cytarabine for previously untreated patients with acute myeloid leukemia: results from a phase Ib/II study. J Clin Oncol. 2019; 37:1277–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Döhner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017; 129:424–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferguson P, Hills RK, Grech A, et al. An operational definition of primary refractory acute myeloid leukemia allowing early identification of patients who may benefit from allogeneic stem cell transplantation. Haematologica. 2016; 101:1351–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krönke J, Schlenk RF, Jensen KO, et al. Monitoring of minimal residual disease in NPM1-mutated acute myeloid leukemia: a study from the German-Austrian acute myeloid leukemia study group. J Clin Oncol. 2011; 29:2709–2716. [DOI] [PubMed] [Google Scholar]
- 9.Schuurhuis GJ, Heuser M, Freeman S, et al. Minimal/measurable residual disease in AML: a consensus document from the European LeukemiaNet MRD Working Party. Blood. 2018; 131:1275–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Breems DA, Van Putten WL, Huijgens PC, et al. Prognostic index for adult patients with acute myeloid leukemia in first relapse. J Clin Oncol. 2005; 23:1969–1978. [DOI] [PubMed] [Google Scholar]
- 11.Chevallier P, Labopin M, Turlure P, et al. A new Leukemia Prognostic Scoring System for refractory/relapsed adult acute myelogeneous leukaemia patients: a GOELAMS study. Leukemia. 2011; 25:939–944. [DOI] [PubMed] [Google Scholar]
- 12.Schlenk RF, Döhner K, Mack S, et al. Prospective evaluation of allogeneic hematopoietic stem-cell transplantation from matched related and matched unrelated donors in younger adults with high-risk acute myeloid leukemia: German-Austrian trial AMLHD98A. J Clin Oncol. 2010; 28:4642–4648. [DOI] [PubMed] [Google Scholar]
- 13.Duval M, Klein JP, He W, et al. Hematopoietic stem-cell transplantation for acute leukemia in relapse or primary induction failure. J Clin Oncol. 2010; 28:3730–3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loke J, Malladi R, Moss P, et al. The role of allogeneic stem cell transplantation in the management of acute myeloid leukaemia: a triumph of hope and experience. Br J Haematol. 2020; 188:129–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005; 106:2912–2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thol F, Schlenk RF, Heuser M, et al. How I treat refractory and early relapsed acute myeloid leukemia. Blood. 2015; 126:319–327. [DOI] [PubMed] [Google Scholar]
- 17.Roboz GJ, Rosenblat T, Arellano M, et al. International randomized phase III study of elacytarabine versus investigator choice in patients with relapsed/refractory acute myeloid leukemia. J Clin Oncol. 2014; 32:1919–1926. [DOI] [PubMed] [Google Scholar]
- 18.Shahswar R, Beutel G, Klement P, et al. FLA-IDA salvage chemotherapy combined with a seven-day course of venetoclax (FLAVIDA) in patients with relapsed/refractory acute leukaemia. Br J Haematol. 2020; 188:e11–e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lachowiez C, Konopleva M, Kadia TM, et al. Interim analysis of the phase 1b/2 study of the BCL-2 inhibitor venetoclax in combination with standard intensive AML induction/consolidation therapy with FLAG-IDA in patients with newly diagnosed or relapsed/refractory AML. Presented at: 62nd ASH Annual Meeting and Exposition; December 5–8, 2020: Virtual: Abstract 332. [Google Scholar]
- 20.Cortes JE, Goldberg SL, Feldman EJ, et al. Phase II, multicenter, randomized trial of CPX-351 (cytarabine:daunorubicin) liposome injection versus intensive salvage therapy in adults with first relapse AML. Cancer. 2015; 121:234–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walter RB, Buckley SA, Pagel JM, et al. Significance of minimal residual disease before myeloablative allogeneic hematopoietic cell transplantation for AML in first and second complete remission. Blood. 2013; 122:1813–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thol F, Gabdoulline R, Liebich A, et al. Measurable residual disease monitoring by NGS before allogeneic hematopoietic cell transplantation in AML. Blood. 2018; 132:1703–1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCurdy SR, Luznik L. How we perform haploidentical stem cell transplantation with posttransplant cyclophosphamide. Hematology Am Soc Hematol Educ Program. 2019; 2019:513–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang YJ, Wang Y, Liu YR, et al. Haploidentical allograft is superior to matched sibling donor allograft in eradicating pre-transplantation minimal residual disease of AML patients as determined by multiparameter flow cytometry: a retrospective and prospective analysis. J Hematol Oncol. 2017; 10:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kharfan-Dabaja MA, Labopin M, Polge E, et al. Association of second allogeneic hematopoietic cell transplant vs donor lymphocyte infusion with overall survival in patients with acute myeloid leukemia relapse. JAMA Oncol. 2018; 4:1245–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dombret H, Seymour JF, Butrym A, et al. International phase 3 study of azacitidine vs conventional care regimens in older patients with newly diagnosed AML with >30% blasts. Blood. 2015; 126:291–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zeidan AM, Wang R, Wang X, et al. Clinical outcomes of older patients with AML receiving hypomethylating agents: a large population-based study in the United States. Blood Adv. 2020; 4:2192–2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stahl M, DeVeaux M, Montesinos P, et al. Hypomethylating agents in relapsed and refractory AML: outcomes and their predictors in a large international patient cohort. Blood Adv. 2018; 2:923–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.DiNardo CD, Tiong IS, Quaglieri A, et al. Molecular patterns of response and treatment failure after frontline venetoclax combinations in older patients with AML. Blood. 2020; 135:791–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maiti A, Rausch CR, Cortes JE, et al. Outcomes of relapsed or refractory acute myeloid leukemia after frontline hypomethylating agent and venetoclax regimens. Haematologica. 2021; 106:894–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nanah R, McCullough K, Hogan W, et al. Outcome of elderly patients after failure to hypomethylating agents given as frontline therapy for acute myeloid leukemia: single institution experience. Am J Hematol. 2017; 92:866–871. [DOI] [PubMed] [Google Scholar]
- 32.Souers AJ, Leverson JD, Boghaert ER, et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat Med. 2013; 19:202–208. [DOI] [PubMed] [Google Scholar]
- 33.Aldoss I, Yang D, Aribi A, et al. Efficacy of the combination of venetoclax and hypomethylating agents in relapsed/refractory acute myeloid leukemia. Haematologica. 2018; 103:e404–e407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aldoss I, Yang D, Pillai R, et al. Association of leukemia genetics with response to venetoclax and hypomethylating agents in relapsed/refractory acute myeloid leukemia. Am J Hematol. 2019; 94:E253–E255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Byrne M, Danielson N, Sengsayadeth S, et al. The use of venetoclax-based salvage therapy for post-hematopoietic cell transplantation relapse of acute myeloid leukemia. Am J Hematol. 2020; 95:1006–1014. [DOI] [PubMed] [Google Scholar]
- 36.Ram R, Amit O, Zuckerman T, et al. Venetoclax in patients with acute myeloid leukemia refractory to hypomethylating agents-a multicenter historical prospective study. Ann Hematol. 2019; 98:1927–1932. [DOI] [PubMed] [Google Scholar]
- 37.Wang YW, Tsai CH, Lin CC, et al. Cytogenetics and mutations could predict outcome in relapsed and refractory acute myeloid leukemia patients receiving BCL-2 inhibitor venetoclax. Ann Hematol. 2020; 99:501–511. [DOI] [PubMed] [Google Scholar]
- 38.DiNardo CD, Maiti A, Rausch CR, et al. 10-day decitabine with venetoclax for newly diagnosed intensive chemotherapy ineligible, and relapsed or refractory acute myeloid leukaemia: a single-centre, phase 2 trial. Lancet Haematol. 2020; 7:e724–e736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taksin AL, Legrand O, Raffoux E, et al. High efficacy and safety profile of fractionated doses of Mylotarg as induction therapy in patients with relapsed acute myeloblastic leukemia: a prospective study of the alfa group. Leukemia. 2007; 21:66–71. [DOI] [PubMed] [Google Scholar]
- 40.Kadia TM, Cortes J, Ravandi F, et al. Cladribine and low-dose cytarabine alternating with decitabine as front-line therapy for elderly patients with acute myeloid leukaemia: a phase 2 single-arm trial. Lancet Haematol. 2018; 5:e411–e421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Swords RT, Watts J, Erba HP, et al. Expanded safety analysis of pevonedistat, a first-in-class NEDD8-activating enzyme inhibitor, in patients with acute myeloid leukemia and myelodysplastic syndromes. Blood Cancer J. 2017; 7:e520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Papaemmanuil E, Gerstung M, Bullinger L, et al. Genomic classification and prognosis in acute myeloid leukemia. N Engl J Med. 2016; 374:2209–2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wagner K, Damm F, Thol F, et al. FLT3-internal tandem duplication and age are the major prognostic factors in patients with relapsed acute myeloid leukemia with normal karyotype. Haematologica. 2011; 96:681–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scholl S, Fleischmann M, Schnetzke U, et al. Molecular mechanisms of resistance to FLT3 inhibitors in acute myeloid leukemia: ongoing challenges and future treatments. Cells. 2020; 9:E2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Perl AE, Martinelli G, Cortes JE, et al. Gilteritinib or chemotherapy for relapsed or refractory FLT3-mutated AML. N Engl J Med. 2019; 381:1728–1740. [DOI] [PubMed] [Google Scholar]
- 46.Numan Y, Rahman ZA, Grenet J, et al. Gilteritinib remains clinically active in relapsed/refractory FLT3 mutated AML previously treated with FLT3 inhibitors. Presented at: 62nd ASH Annual Meeting and Exposition; December 5–8, 2020: Virtual: Oral Presentation 262. [Google Scholar]
- 47.Daver N, Altman JK, Maly J, et al. Efficacy and safety of venetoclax in combination with gilteritinib for relapsed/refractory FLT3-mutated acute myeloid leukemia in the expansion cohort of a phase 1b study. Presented at: 62nd ASH Annual Meeting and Exposition; December 5–8, 2020: Virtual: Oral Presentation 333. [Google Scholar]
- 48.Cortes JE, Khaled S, Martinelli G, et al. Quizartinib versus salvage chemotherapy in relapsed or refractory FLT3-ITD acute myeloid leukaemia (QuANTUM-R): a multicentre, randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2019; 20:984–997. [DOI] [PubMed] [Google Scholar]
- 49.Mathew NR, Baumgartner F, Braun L, et al. Sorafenib promotes graft-versus-leukemia activity in mice and humans through IL-15 production in FLT3-ITD-mutant leukemia cells. Nat Med. 2018; 24:282–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rautenberg C, Nachtkamp K, Dienst A, et al. Sorafenib and azacitidine as salvage therapy for relapse of FLT3-ITD mutated AML after allo-SCT. Eur J Haematol. 2017; 98:348–354. [DOI] [PubMed] [Google Scholar]
- 51.Burchert A, Bug G, Fritz LV, et al. Sorafenib maintenance after allogeneic hematopoietic stem cell transplantation for acute myeloid leukemia with FLT3-internal tandem duplication mutation (SORMAIN). J Clin Oncol. 2020; 38:2993–3002. [DOI] [PubMed] [Google Scholar]
- 52.Wagner K, Damm F, Göhring G, et al. Impact of IDH1 R132 mutations and an IDH1 single nucleotide polymorphism in cytogenetically normal acute myeloid leukemia: SNP rs11554137 is an adverse prognostic factor. J Clin Oncol. 2010; 28:2356–2364. [DOI] [PubMed] [Google Scholar]
- 53.Thol F, Damm F, Wagner K, et al. Prognostic impact of IDH2 mutations in cytogenetically normal acute myeloid leukemia. Blood. 2010; 116:614–616. [DOI] [PubMed] [Google Scholar]
- 54.Stein EM, DiNardo CD, Pollyea DA, et al. Enasidenib in mutant IDH2 relapsed or refractory acute myeloid leukemia. Blood. 2017; 130:722–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.DiNardo CD, Stein EM, de Botton S, et al. Durable remissions with ivosidenib in IDH1-mutated relapsed or refractory AML. N Engl J Med. 2018; 378:2386–2398. [DOI] [PubMed] [Google Scholar]
- 56.Kadia TM, Jain P, Ravandi F, et al. TP53 mutations in newly diagnosed acute myeloid leukemia: clinicomolecular characteristics, response to therapy, and outcomes. Cancer. 2016; 122:3484–3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang Q, Bykov VJN, Wiman KG, et al. APR-246 reactivates mutant p53 by targeting cysteines 124 and 277. Cell Death Dis. 2018; 9:439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sallman DA, DeZern AE, Garcia-Manero G, et al. Eprenetapopt (APR-246) and azacitidine in TP53-mutant myelodysplastic syndromes. J Clin Oncol. 2021: January 15. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Krivtsov AV, Evans K, Gadrey JY, et al. A menin-MLL inhibitor induces specific chromatin changes and eradicates disease in models of MLL-rearranged leukemia. Cancer Cell. 2019; 36:660–673.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dzama MM, Steiner M, Rausch J, et al. Synergistic targeting of FLT3 mutations in AML via combined menin-MLL and FLT3 inhibition. Blood. 2020; 136:2442–2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mambet C, Chivu-Economescu M, Matei L, et al. Murine models based on acute myeloid leukemia-initiating stem cells xenografting. World J Stem Cells. 2018; 10:57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sallman DA, Asch AS, Al Malki MM, et al. The first-in-class anti-CD47 antibody magrolimab (5F9) in combination with azacitidine is effective in MDS and AML patients: ongoing phase 1b results. Blood. 2019; 134(suppl_1):569. [Google Scholar]
- 63.Krupka C, Kufer P, Kischel R, et al. CD33 target validation and sustained depletion of AML blasts in long-term cultures by the bispecific T-cell-engaging antibody AMG 330. Blood. 2014; 123:356–365. [DOI] [PubMed] [Google Scholar]
- 64.Ravandi F, Stein AS, Kantarjian HM, et al. A phase 1 first-in-human study of AMG 330, an anti-CD33 bispecific T-cell engager (BiTE®) antibody construct, in relapsed/refractory acute myeloid leukemia (R/R AML). Blood. 2018; 132(suppl_1):25. [Google Scholar]
- 65.Aldoss I, Uy GL, Vey N, et al. Flotetuzumab as salvage therapy for primary induction failure and early relapse acute myeloid leukemia. Presented at: 62nd ASH Annual Meeting and Exposition; December 5–8, 2020: Virtual: Oral Presentation 331. [Google Scholar]
- 66.Daver N, Boddu P, Garcia-Manero G, et al. Hypomethylating agents in combination with immune checkpoint inhibitors in acute myeloid leukemia and myelodysplastic syndromes. Leukemia. 2018; 32:1094–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Daver N, Garcia-Manero G, Basu S, et al. Efficacy, safety, and biomarkers of response to azacitidine and nivolumab in relapsed/refractory acute myeloid leukemia: a nonrandomized, open-label, phase II study. Cancer Discov. 2019; 9:370–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Maslak PG, Dao T, Bernal Y, et al. Phase 2 trial of a multivalent WT1 peptide vaccine (galinpepimut-S) in acute myeloid leukemia. Blood Adv. 2018; 2:224–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Agrawal V, Gbolahan OB, Stahl M, et al. Vaccine and cell-based therapeutic approaches in acute myeloid leukemia. Curr Cancer Drug Targets. 2020; 20:473–489. [DOI] [PubMed] [Google Scholar]


