Abstract
Allogeneic hematopoietic stem cell transplantation (allo-HCT) is a potentially curative therapy for patients suffering from hematological malignancies, and its therapeutic success is based on the graft-versus-leukemia (GvL) effect. Severe acute and chronic graft-versus-host disease (GvHD) are life-threatening complications after allo-HCT. To date, most of the approved treatment strategies for GvHD rely on broadly immunosuppressive regimens, which limit the beneficial GvL effect by reducing the cytotoxicity of anti-leukemia donor T-cells. Therefore, novel therapeutic strategies that rely on immunomodulatory rather than only immunosuppressive effects could help to improve patient outcomes. Treatments should suppress severe GvHD while preserving anti-leukemia immunity. New treatment strategies include the blockade of T-cell activation via inhibition of dipeptidyl peptidase 4 and cluster of differentiation 28-mediated co-stimulation, reduction of proinflammatory interleukin (IL)-2, IL-6 and tumor necrosis factor-α signaling, as well as kinase inhibition. Janus kinase (JAK)1/2 inhibition acts directly on T-cells, but also renders antigen presenting cells more tolerogenic and blocks dendritic cell-mediated T-cell activation and proliferation. Extracorporeal photopheresis, hypomethylating agent application, and low-dose IL-2 are powerful approaches to render the immune response more tolerogenic by regulatory T-cell induction. The transfer of immunomodulatory and immunosuppressive cell populations, including mesenchymal stromal cells and regulatory T-cells, showed promising results in GvHD treatment. Novel experimental procedures are based on metabolic reprogramming of donor T-cells by reducing glycolysis, which is crucial for cytotoxic T-cell proliferation and activity.
Introduction
Allogeneic hematopoietic stem cell transplantation (allo-HCT) is the only potentially curative treatment for many malignant and non-malignant hematologic diseases by stimulating a graft-versus-leukemia (GvL) immune response.1 However, a life-threatening complication is graft-versus-host disease (GvHD), a process of donor-T-cells recognizing the recipient’s tissue as foreign.2 The conditioning regimen before transplantation causes tissue damage and host antigen presenting cell (APC) activation in GvHD target organs. APCs express foreign major and minor histocompatibility molecules, thereby stimulating graft-derived T-cell activation and proliferation.3 Afterwards, donor-T-cells exhibit a cytotoxic immune response to eliminate foreign antigens. GvHD can be either acute or chronic, and major target organs involved in acute GvHD include skin, liver, and intestines.1–3 Given the high mortality of patients suffering from acute GvHD, novel approaches are urgently needed to reduce GVHD-related mortality. Until now, approved treatments for GvHD rely on broadly immunosuppressive mechanisms, which also suppress the beneficial anti-leukemia immune response.4 To maintain GvL effects, it is crucial to identify novel therapeutic strategies, which should rather be immunomodulatory than only immunosuppressive. A variety of novel concepts emerged during the last years and are reviewed in the following article and summarized in Figure 1.
Figure 1.
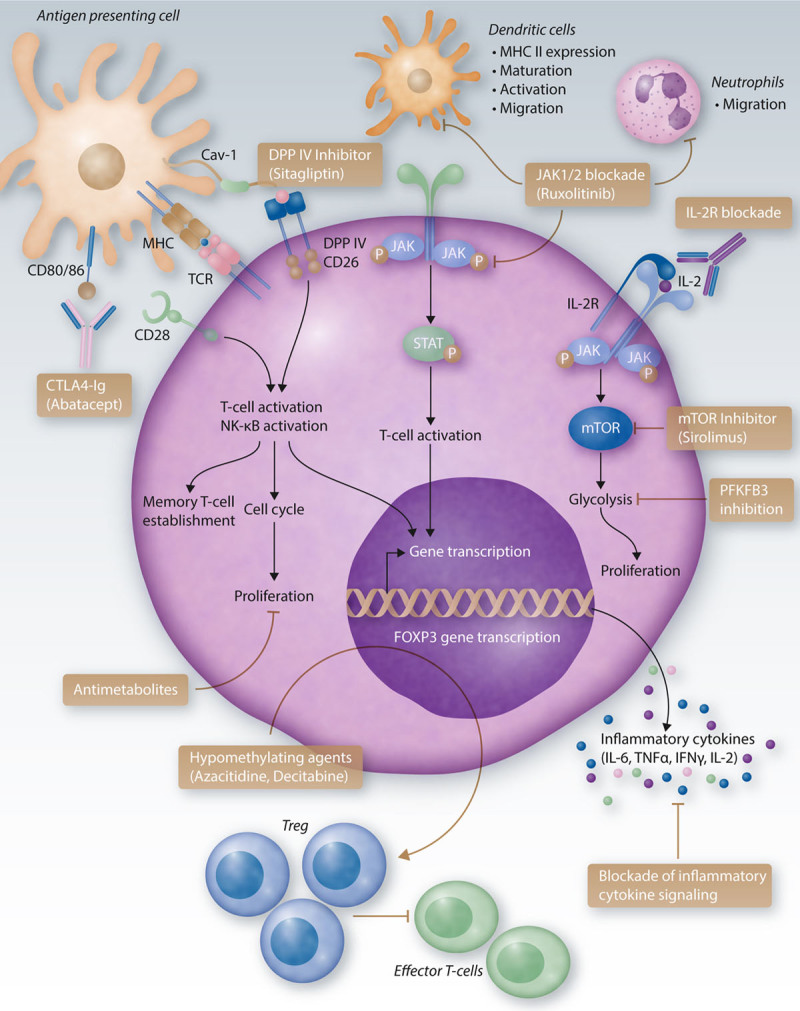
Immunomodulatory therapies in GvHD. Immunomodulatory therapies aim to reduce increased cytotoxic T-cell activities seen in acute and chronic GvHD. Novel approaches include the blockade of T-cell co-stimulation using a CTLA4-Ig fusion protein, which binds to CD80/86 on the APC surface. Thereby, binding to CD2, which is important for T-cell activation, is blocked. Further, DPPIV (DPP4) can be inhibited with sitagliptin to block T-cell activation. T-cell proliferation can be reduced using antimetabolites. JAK1/2 inhibition does not only reduce STAT phosphorylation, T-cell activation, and gene transcription, but also reduces neutrophil migration into GvHD target organs and mesenteric lymph nodes, as well as MHC II expression on dendritic cells and dendritic cell maturation, activation, and migration. Reduced T-cell activation also results in decreased secretion of pro-inflammatory cytokines. A blockade of the IL-2 receptor inhibits IL-2-mediated T-cell activation and subsequent mTOR signaling. The latter leads to increased glycolysis and enhanced T-cell activity and can be blocked by mTOR inhibitors, eg, sirolimus. Elevated glycolytic activity could also be reduced in an experimental setup by PFKFB3 inhibition. Inflammatory cytokine signaling can be targeted by blocking cytokines directly or using approaches to block their respective receptors. Hypomethylating agents such as azacitidine and decitabine inhibit DNA-methyltransferases, leading to DNA and promotor hypomethylation and increased gene expression. Increased expression of FOXP3 results in higher frequencies of Treg cells, which reduce activation of alloreactive T-cells. APC = antigen-presenting cell; Cav = Caveolin; CD = cluster of differentiation; CTLA4-Ig = cytotoxic T-cell-lymphocyte-4-immunoglobulin; DPP4 = dipeptidyl peptidase 4; FOXP3 = forkhead box P3; GvHD = graft-versus-host disease; IFN = interferon; IL = interleukin; JAK = Janus kinase; MHC = major histocompatibility complex; mTOR = mechanistic target of rapamycin; NK = natural killer; PFKFB3 = 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3; STAT = signal transducer and activator of transcription; TCR = T cell receptor; TNF = tumor necrosis factor.
Blockade of T-cell stimulation with abatacept
Abatacept, known as cytotoxic T-cell-lymphocyte-4-immunoglobulin (CTLA4-Ig), is a fusion protein between the extracellular domain of human CTLA4 and a modified Fc-region of human IgG, and blocks co-stimulation of T-cells.5 High-affinity binding of abatacept to cluster of differentiation (CD) 28 interrupts binding of this receptor-like protein to its ligands CD80/CD86 on APC surface, thereby preventing CD28-mediated T-cell activation.6 Administration of a CTLA4-Ig fusion protein prevented acute and chronic GvHD in a mouse model by reducing donor T-cell activation. Proinflammatory cytokine secretion and memory T-cell establishment was blocked.7 However, when CTLA4-Ig was not applied early at GvHD onset, but rather at a later time point, the blockade did not affect acute GvHD but reduced chronic GvHD.7 In a model of collagen-induced arthritis, CTLA4-Ig suppressed the disease in mice through increased regulatory T-cell (Treg) numbers. CTLA4-Ig rendered dendritic cells (DCs) from mice more tolerogenic, thereby stimulating the Treg population.8 In a first-in-disease trial of abatacept for acute GvHD prophylaxis (NCT01012492), patients received cyclosporine/methotrexate as prophylaxis (StdRx cohort) or additionally abatacept (ABA cohort). Compared to StdRx, the ABA cohort showed a significant inhibition of early CD4+ T-cell proliferation and activation, mainly affecting effector memory T-cells. The treatment had minor effects on CD8+ T-cells, implicating that combination therapies should be considered in future studies. GvHD analysis revealed only a low rate of acute GvHD in the ABA group compared to the StdRx cohort, whereas all patients showed immune reconstitution. The investigators only reported 2 cases of grade II–IV acute GvHD until day +100. The reconstitution of natural killer (NK) cells occurred relatively fast upon abatacept treatment, while T-cell proliferation was reduced. Abatacept did not affect long-lasting immune reconstitution. In summary, blocking the interaction of CD28 and CD80/CD86 is a promising option to reduce acute GvHD after allo-HCT.9 The ABA2 trial (NCT01743131) was initiated to test the hypothesis that abatacept could reduce the risk for severe acute GvHD in patients receiving unrelated donor hematopoietic cell transplantation. The phase II trial evaluated safety, efficacy, and immunological effects of abatacept when combined with a calcineurin inhibitor and methotrexate-based GvHD prophylaxis to test whether abatacept can reduce acute GvHD severity. Acute GvHD was significantly decreased in the abatacept treatment group compared to the placebo group. The day +180 severe-GvHD-free-survival was significantly higher in abatacept treated patients as compared to standard prophylaxis only, but chronic GvHD was not improved. Abatacept treatment neither increased the relapse risk, nor impaired engraftment and immune reconstitution.5 A major challenge of novel GvHD prophylaxis and treatment strategies is to simultaneously decrease severe inflammation while preserving an effective GvL immunity. Ohata et al10 reported in 2002 that the blockade of CD28-mediated T-cell activation maintains the GvL effect and should therefore be favored over CD40 blockade. Although the latter did also induce tolerance in a GvHD model, it suppressed a proper anti-leukemia immune reaction.10 Clinical trials reported in this review are summarized in Table 1.
Table 1.
Selected Clinical Trials of Immunomodulatory Therapies in GvHD.
| Trial Number | Treatment | Trial Description | Status, Outcome Measures, Comments |
|---|---|---|---|
| NCT 01012492a | CTLA4-Ig (Abatacept) | Abatacept-based immunosuppression to prevent aGvHD during URD-HCT | Completed |
| Safety and tolerability of additional Abatacept (to cyclosporine/methotrexate) in aGvHD prophylaxis after URD-HCT with BM or PB grafts | |||
| Phase 2 | |||
| GvHD severity and incidence by day +100; hematologic/immune reconstitution; protective immunity | |||
| NCT 01743131a | CTLA4-Ig (Abatacept) | Abatacept as GvHD prophylaxis | Active |
| Randomized | |||
| Phase 2 | Evaluation of Abatacept with calcineurin inhibitors and methotrexate to protect aGvHD without causing more infections | ||
| GvHD incidence and severity at day +100 and +180; incidence of infections, engraftment, relapse, OS; GvHD-free survival | |||
| NCT 02683525a | DPP-4 inhibition (Sitagliptin) | Sitagliptin to prevent aGvHD after allo-HCT | Completed |
| Efficacy of DPP-4 inhibition (Sitagliptin) to reduce grade II–IV aGvHD by day +100 with sirolimus/tacrolimus GvHD prophylaxis | |||
| Phase 2 | |||
| Neutrophil and platelet counts; infections, NRM; cGvHD incidence; relapse rate | |||
| NCT 00862719a | DPP-4 inhibition (Sitagliptin) | Sitagliptin to speed up engraftment after UCB-transplant | Completed |
| Non-randomized | |||
| Phase 2 | Recovery of blood counts after UCB-transplantation | ||
| Patients with engraftment at day +30; time to neutrophil and platelet engraftment; treatment-related adverse events (grade 3); non-hematological toxicities | |||
| NCT 04448587a | DPP-4 inhibition (Sitagliptin) | Sitagliptin for treatment of grade III/IV and refractory aGvHD | Recruiting |
| Safety and efficacy of sitagliptin in severe and refractory aGvHD | |||
| Phase 2 | Response (CR, VGPR, PR) by day +28 and +56 | ||
| Treatment-related adverse events (safety/tolerability); GvHD-free survival at 6 months; biomarker blood profiling | |||
| NCT 02953678a | JAK1/2 inhibition (Ruxolitinib) | Ruxolitinib with corticosteroids in SR-aGvHD (REACH-1) | Completed |
| Ruxolitinib with prednisolone/methylprednisolone in grade II-IV SR-aGvHD | |||
| Phase 2 | ORR (CR, VGPR, PR) at day +28, +56 and +100 | ||
| Three- and 6-mo DOR; relapse rate; NRM; relapse-related mortality; FFS; OS, adverse events | |||
| NCT 02913261a | JAK1/2 inhibition (Ruxolitinib) | Safety/efficacy of ruxolitinib vs BAT in SR-aGvHD after allo-HCT (REACH2) | Active |
| Randomized | |||
| ORR (CR, PR) at day +28 | |||
| Phase 3 | Durable ORR (patients with CR/PR at day +28 maintaining until day +56; OR at day +14; DOR; OS; cumulative steroid dose; event-free survival; FFS; NRM; MR; incidence of cGvHD; PK parameters: plasma concentration peak, AUC, total body clearance; exposure-efficacy relationship of Ruxolitinib in SR-aGvHD; BOR until day +28 | ||
| NCT 01747499a | HMA (Azacitidine) | Azacitidine in patients undergoing MUD-HCT | Terminated |
| Non-randomized | |||
| Phase 1/2 | Determination of MTD of 5-AzaC and effect on grade II-IV GvHD in MUD-transplantation | ||
| Grade II-IV aGvHD until day +180 with 5-AzaC treatment; OS; TRM; cGvHD incidence | |||
| NCT 01390311a | HMA (Azacitidine) | Azacitidine in relapsed AML and MDS after allo-HCT | Completed |
| Randomized | |||
| Phase 1 | Aza after chemotherapy and DLI in relapse AML/MDS previously received allo-HCT | ||
| MTD | |||
| Grade II-IV aGvHD (day +100 post-DLI); ORR (1 y); OS (day +100 post-DLI); effects of Aza dose increase on resting and activated Tregs | |||
| ISRCTN 36825171b | HMA (Azacitidine) | Azacitidine in reduced-intensity conditioned allo-HCT | Completed |
| Aza treatment in AML and MDS patients undergoing reduced-intensity allo-HCT | |||
| Phase 2 | RR at 12 mo post-HCT; OS at 3 y post-HCT | ||
| NCT 00529035a | Ultra-low dose IL-2 | Ultra-low dose IL-2 in refractory cGvHD | Completed |
| MTD and toxicity profile of IL-2 in cGvHD with inadequate response to steroids; ORR (CR and PR); immune cell phenotyping (CD4, CD8, Treg, Tcon, NK, B cells); Treg/Tcon ratio | |||
| Phase 1 | |||
| NCT 00539695a | Low Dose IL-2 | IL-2 for GvHD | Completed |
| Phase 2 | Safety and efficacy of low-dose IL-2 in aGvHD; rate of dose-limiting toxicities; rate of severe aGvHD (grade III/IV, 12 wk on treatment); reconstitution of Tregs; suppressive activities of Tregs, cytokine secretion, immune phenotypes of PBMCS, NK analysis | ||
| NCT 01927120a | IL-2 tacrolimus, sirolimus | In vivo Treg expansion and GvHD prophylaxis; | Completed |
| Determination if GvHD prophylaxis with IL-2/tacrolimus/sirolimus enhances Treg reconstitution and differentiation after allo-HCT; safety and effect on aGvHD/cGvHD; evaluation of T-cell specific signaling | |||
| Phase 2 | |||
| Treg frequencies day +30 and +90; 1-y OS; relapse rate; aGvHD (grade II–IV) day +100; cGvHD (day +365); non-relapse mortality; adverse events; phosphorylation of STAT3, STAT5 and S6 | |||
| NCT 02318082a | IL-2 | Individual dose-escalated IL-2 in refractory cGvHD | Completed |
| MTD 8-wk dose-escalated IL-2 (adult and pediatric patients); DLT; cGvHD ORR; OS (1 y); malignancy relapse rate (1 y) | |||
| Phase 1 | |||
| ACTRN 12614000266662c | Anti-IL6R (Tocilizumab) | Tocilizumab to prevent aGvHD after allo-HCT | Completed |
| Tocilizumab at 8 mg/kg on day -1 of conditioning | |||
| Phase 3 | Control to placebo (saline) | ||
| Incidence of grade II–IV GvHD at day +100 post allo-HCT; IL-6/IL-6R levels in serum; immune reconstitution; infection rate; PFS, TRM; OS | |||
| NCT 00726375a | TNFα blockade (Etanercept) | Etanercept (Enbrel) as sole treatment for grade I aGvHD | Completed |
| Treatment of early skin GvHD (grade I) with Etanercept instead of high-dose steroid | |||
| Phase 3 | Disease progression within 28 d of Etanercept treatment; CR at 4 wk | ||
| NCT 00602693a | Treg (cellular therapy) | Treg infusion post UCB-transplant in advanced hematologic cancer | Completed |
| MTD of UCB-derived Tregs (dose escalation, DLTs); Treg numbers in PB; grade II–IV aGvHD (day +100); donor engraftment; chimerism; neutrophil/platelet recovery; cGvHD incidence; infectious complications; relapse | |||
| Phase 1 | |||
| NCT 01911039a | Treg (cellular therapy) | Treg infusion in SR-cGvHD | Safety and tolerability of Treg infusion in SR-cGvHD; adverse events; infusion-related toxicities; Treg counts in PB; FFS; ORR; improvement of quality of life |
| Phase 1 | |||
| NCT 01903473a | Treg (cellular therapy) | Donor-Treg infusion in cGvHD patients | Recruiting |
| Phase 2 | Non-randomized | ||
| Safetly evaluation of rapamycin with donor-Treg infusion and low-dose IL-2 in SR-cGvHD patients; immunological changes, Treg counts, Treg phenotype | |||
| Efficacy of Treg selection; response of cGvHD to Treg + IL-2 + rapamycin; infectious complications; OS (1 y); PFS (1 y); ORR (1 y) |
aRegistered at https://clinicaltrials.gov.
bRegistered at http://isrctn.com.
cRegistered at https://www.anzctr.org.au/.
5-AzaC = 5-Azacitidine; aGvHD = acute graft-versus-host disease; AML = acute myeloid leukemia; AUC = area under the curve; BAT = best available therapy; BM = bone marrow; BOR = best overall response; CD = cluster of differentiation; cGvHD = chronic graft-versus-host disease; CR = complete remission; CTLA4-Ig = cytotoxic T-cell-lymphocyte-4-immunoglobulin; DLI = donor lymphocyte infusion; DLT = dose-limiting toxicities; DOR = duration of response; DPP4 = dipeptidyl peptidase 4; FFS = failure-free survival; GvHD = graft-versus-host disease; HCT = hematopoietic stem cell transplantation; HMA = hypomethylating agents; IL = interleukin; JAK = Janus kinase; MDS = myelodysplastic syndrome; MR = malignancy relapse/progression; MTD = maximum tolerated dose; MUD = matched unrelated donor; NK = natural killer; NRM = non-relapse mortality; ORR = overall response rate; OS = overall survival; PB = peripheral blood; PBMCs = peripheral blood mononuclear cells; PFS = progression free survival; PK = pharmacokinetic; PR = partial response; RR = response rate; SR = steroid refractory; STAT = signal transducer and activator of transcription; Tcon = conventional T-cells; TNF = tumor necrosis factor; Tregs = regulatory T-cells; TRM = treatment-related mortality; UCB = umbilical cord blood; URD = unrelated donor; VGPR = very good partial response.
Dipeptidyl peptidase 4 inhibition
Dipeptidyl peptidase 4 (DPP4, also known as DPPIV or CD26), a homodimeric type-II transmembrane receptor with serine peptidase activity in its extracellular domain, is widely expressed on hematopoietic, endothelial, and epithelial cells, but also found as a soluble and enzymatically active form in the plasma.11 DPP4/CD26 has costimulatory functions and enhances T-cell activation, which is its most important effect in the context of GvHD. CD26 on T-cells binds to its ligand caveolin-1 on APCs, leading to enhanced costimulatory signals GvHD.11 A role for caveolin-1 in acute GVHD had been previously reported.12 CD26high CD8+ T-cells belong to the early effector memory T-cell subset. Moreover, CD26-mediated co-stimulation of CD8+ T-cells leads to increased cytotoxic responses trough release of granzyme-B, tumor necrosis factor (TNF)α, and interferon (IFN)γ.13 Confirmatory DPP4 inhibition could ameliorate inflammatory processes.14 In GvHD, CD26+ T-cells accumulate in target organs, making it an interesting approach for GvHD prophylaxis.15 A humanized GvHD model highlighted the potential of CD26 blockade to suppress the inflammatory response and GvHD severity without interfering with anti-leukemia immunity.15 In a clinical trial, DPP4 was inhibited with its selective inhibitor, sitagliptin, as GvHD prophylaxis, which is already approved in type-2 diabetes mellitus patients (NCT02683525).11 Sitagliptin had acceptable side effects if applied with cord blood transplantation to enhance engraftment. Patients with sitagliptin treatment showed reduced incidence of acute GvHD compared to historical controls (NCT00862719).16 Randomized trials are required to validate these findings. Based on previous findings, a phase II trial was conducted to evaluate the efficacy of sitagliptin in combination with other immunosuppressive treatments to prevent acute GvHD after allo-HCT.11 Patients underwent myeloablative conditioning before transplantation of peripheral-blood stem cells. Prophylaxis started on day −3 with tacrolimus/sirolimus, followed by sitagliptin from day −1 to 14. All enrolled patients had engraftment and at least 95% donor chimerism on day 30. In total, 2 of 36 patients developed acute GvHD by day 100, which affected the skin, liver, and gut with grade II or IV. Treatment-related non-hematologic toxicities and infections were manageable and reversible. Nine patients had a relapse and 15 of 34 patients developed chronic GvHD. The 1-year GvHD-free, relapse-free survival was 46%. Overall survival (OS) at 1 year was 94%. Sitagliptin in combination with immunosuppressive therapy is feasible, the treatment is associated with a low incidence of acute GvHD, and the results were in line with preclinical data of an anti-CD26 antibody to prevent GvHD.11,15 Although no control group was included, the investigators state that the incidence of acute GvHD was lower with sitagliptin than other treatments. DPP4 inhibition achieved the lowest levels of acute GvHD in comparison to other new agents, is a promising prophylaxis treatment for acute GvHD, and should be further evaluated in additional trials.5,11 One currently ongoing trial evaluates sitagliptin in grade III–IV and refractory acute GvHD patients (NCT04448587). DPP4 inhibition might have further benefits by blocking glucagon-like-peptide 2 degradation,17 which plays a pivotal role for regeneration of intestinal stem cells and paneth cells after GvHD-induced intestinal damage.18
Extracorporeal photopheresis
Extracorporeal photopheresis (ECP), an immunomodulatory and immunosuppressive regimen, was first described in 1987 as treatment of cutaneous T-cell lymphoma.19,20 Today, the treatment is used in more diseases, for example in organ transplantation, chronic GvHD, and checkpoint inhibitor–induced adverse events.21–23 During ECP, peripheral blood mononuclear cells are collected by leukapheresis, treated with 8-methoxypsoralen (8MOP), exposed to ultraviolet A (UVA) light and transfused back to the patient.20 8MOP is photoactivated upon exposure to UVA light, resulting in DNA cross-linking and rapid cell death within 72 hours after the procedure.20 Although this direct cell death induction could account for the therapeutic effects in cutaneous T-cell lymphoma and the reduction of inflammatory cells in GvHD, ECP acts in a more sophisticated, yet not completely understood manner (Figure 2).20 The application of ECP was shown to be a promising treatment strategy for chronic GvHD patients20 and it was found to modulate the T-cell response towards a T helper type 2 (Th2) immune response with increased serum levels of interleukin (IL)-4 and IL-10, as well as changes in the NK cell and DC compartment.24 In a preclinical setting, the transplantation of autologous ECP-treated apoptotic cells prolonged the survival of mice in severe GvHD models trough immunomodulation, decreased NF-κB levels, suppressed DC maturation, and reduced CD80 expression on host cells.25 Besides suppressing APCs in vivo, ECP-treated cells also rendered DCs more immunosuppressive in vitro in mixed lymphocyte reactions (MLR). Co-cultures with allo-T-cells resulted in decreased T-cell proliferation and pro-inflammatory cytokine secretion. ECP treatment of donor splenocytes and transplantation of these cells modulated the recipient’s immune system by increasing forkhead box P3 positive (FoxP3+) Tregs and IL-10 secretion.25 Comparable, a preclinical GvHD model showed that ECP treatment reduced GvHD severity through reduced IFNγ secretion by donor cytotoxic T-cells and increased Treg frequencies in recipient animals.26 The importance of antigen-specific Tregs upon ECP treatment was further confirmed in mice and humans.27 Besides induction of Tregs, ECP also increased myeloid-derived suppressor cells in the peripheral blood (PB) of GvHD patients. These cells were confirmed to have high arginase-1 activity and could dampen T-cell proliferation, as well as IFNγ and IL-17 secretion, in co-culture experiments.28 Although confirming experiments are missing, one could hypothesize that arginase-1 expression is upregulated via Th2 cytokines induced by ECP treatment, thereby depleting arginine in the micromilieu of cytotoxic T-cells. The depletion of arginine results in limited T-cell proliferation and could even cause apoptosis of T-cells in vivo.29 Besides its role in acute GvHD, recent data suggest a therapeutic role for ECP in immune checkpoint inhibitor–induced colitis.22
Figure 2.
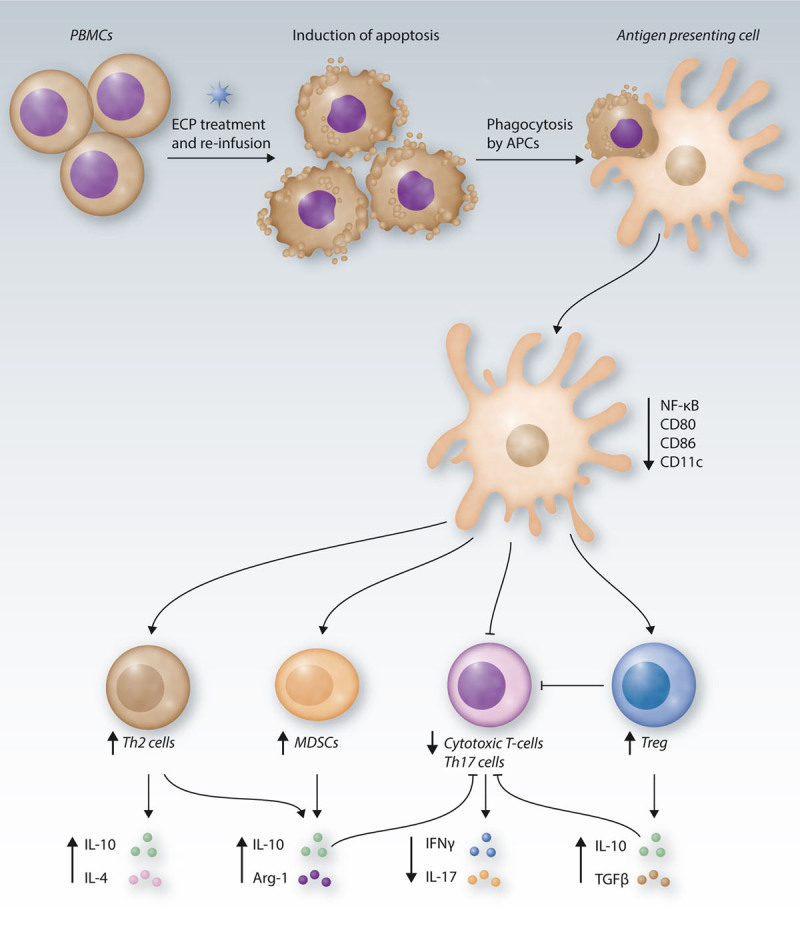
ECP-mediated immune modulation. During ECP, patient-derived PBMCs are treated with 8-MOP and UVA light, inducing rapid cell death. Upon re-infusion, the apoptotic cells are phagocytosed by APCs and macrophages, leading to reduced NF-κB signaling and reduced dendritic cell maturation. Following, the number of MDSCs increases, accompanied by an elevated secretion of the immunosuppressive regulators IL-10 and arginase-1. The T-cell response is modulated towards a Th2 phenotype with enhanced production of the cytokines IL-10 and IL-4. Moreover, the Treg compartment is increased. The effects exhibited by Th2 cells, Tregs, and MDSCs synergize to reduce the proliferation, activation and cytotoxic activity of Th17 and cytotoxic T-cells, seen by reduced pro-inflammatory cytokine secretion. In summary, ECP induces tolerogenic events at multiple levels of the immune response. APC = antigen-presenting cell; CD = cluster of differentiation; ECP = extracorporeal photopheresis; IFN = interferon; IL = interleukin; MDSCs = myeloid-derived suppressor cells; MOP = methoxypsoralen; NF-κB = nuclear factor kappa-light-chain-enhancer of activated B cells; PBMCs = peripheral blood mononuclear cells; TGF = transforming growth factor; Th2 = T helper type 2; Treg = regulatory T-cell; UVA = ultraviolet A.
Janus kinase 1/2 blockade
Signaling via Janus kinase (JAK) 1/2 and signal transducer and activator of transcription (STAT) pathways are crucial for immune cell activation and stimulation of pro-inflammatory cytokine production during acute GvHD.30 Ruxolitinib is a potent selective JAK1/2 inhibitor, which was first approved in myelofibrosis, aiming to reduce inflammatory cytokine signaling.31 JAK/STAT signaling is important for T-cell activation and changes of T-cell phenotypes, including STAT1 and STAT3 being activated early after acute GvHD onset.32 Additionally, ruxolitinib has also major effects on APCs by impairing DC development, maturation, activation and migration.33,34 JAK inhibition interferes with the cytokine activity that is mediated by the early tissue damage. This early tissue damage involves the release of damage-associated patterns35,36 and neutrophil granulocyte-induced intestinal barrier damage.2,37,38 JAK1/2 inhibition blocks inflammatory neutrophil migration, which causes major tissue damage in acute GvHD.2 In T cells, JAK inhibition interferes with common gamma chain downstream effects, which were shown to be required for acute GVHD pathogenesis.30 In a murine GvHD model, ruxolitinib significantly increased the survival and reduced GvHD severity due to lower T-cell proliferation, and decreased organ infiltration of donor T-cells. Moreover, pro-inflammatory cytokines were reduced in MLR and in the serum of mice upon JAK1/2 inhibition. Mechanistically, the treatment increased FoxP3+ Tregs in the spleen and the intestine and reduced IFNγ production in vivo and in vitro. The proliferation of T-cells was reduced upon co-culture with ruxolitinib-treated allogeneic DCs. Comparable results were seen as a direct effect on T-cells when cultured with activation beads. JAK inhibition reduced STAT3 phosphorylation in T-cells, thereby reducing T-cell activation and proliferation. In a first trial, ruxolitinib improved skin GvHD and intestinal symptoms in patients while decreasing serum levels of proinflammatory cytokines.39 In a first retrospective study, 95 patients were included receiving ruxolitinib as a salvage therapy for steroid-refractory GvHD. The overall response rate (ORR) was 81.5% and patients having severe intestinal or skin GvHD showed impressive response to the treatment. The reduction of the inflammatory disease was linked to decreased proinflammatory serum cytokines and reduced numbers of activated T-cells. Although cytopenia was observed in some patients, ruxolitinib was found safe to use and well tolerated. OS of ruxolitinib-treated steroid-refractory acute GvHD patients was higher than ever reported for any other therapy.40 The REACH1 phase II trial (NCT02953678) was initiated including patients who underwent their first HCT from any donor source with the development of steroid-refractory acute GvHD. The ORR was 73.2% with a median time to response of 7 days. The 6- and 12-month OS was 51.0% and 42.6%, respectively. OS was reduced in patients with grade III/IV acute GvHD and longer corticosteroid treatment before ruxolitinib. Biomarker analysis revealed an upregulation of proteins associated with hematopoiesis and a reduction of inflammatory pathway proteins in ruxolitinib-treated patients.41 The follow-up REACH2 trial (NCT02913261) was initiated to compare the efficacy of ruxolitinib with the best available care for patients with steroid-refractory acute GvHD. ORR at day 28 was significantly increased upon JAK1/2 inhibition compared to control and the percentage of patients with a complete response (CR) was higher (34% vs 19%). Ruxolitinib treatment significantly increased the median failure-free survival and OS compared to control treatment. However, as seen in previous trials, ruxolitinib was associated with thrombocytopenia and a slightly increased incidence of infections compared to control treatment.42 Interestingly, a novel attempt combined ruxolitinib together with ECP in steroid-refractory chronic GvHD patients, as both treatment regimens alone already showed activity against the disease. The best response was 74% and the 2-year survival rate was 75%. This novel strategy is safe and has activity in at least a part of steroid-refractory chronic GvHD patients; however, more detailed validation is needed in a prospective trial.21
Metabolic reprogramming of T-cells
It was well-known that alloreactive T-cells drive GvHD, whereas the changes of T-cell metabolism after allo-HCT were largely unknown. A metabolic reprogramming of T-cells after allo-HCT from fatty acid-oxidation towards aerobic glycolysis was reported.43 The requirement of high glycolytic activity is typical for effector T-cells, whereas Tregs rather rely on lipid oxidation.44 Donor T-cells were isolated from syngeneic or allogeneic recipients on day 14 after transplantation. The extracellular acidification rate—reflecting the rate of glycolysis—and the oxygen consumption rate (OCR) were higher in donor T-cells from transplanted mice compared to naive T-cells. While OCR was comparable between syngeneic and allogeneic recipients, glycolysis was significantly higher in T-cells from allogeneic recipients. Moreover, the glycolytic activity of allogeneic T-cells infiltrating GvHD target organs was higher compared to those isolated from the spleen. T-cells from transplanted mice showed increased anabolism and glycolysis intermediates and regulators of mRNA and metabolite levels. Findings were confirmed by higher expression of Raptor and Rictor and increased S6 phosphorylation, indicating enhanced mechanistic target of rapamycin (mTOR) signaling in T-cells isolated from allogeneic recipients.43 Confirming the role of mTOR, several chronic GvHD patients were identified with activating mTOR mutations, thereby driving clonal CD4+ T-cell expansion and chronic GvHD development.45 Inhibition of mTOR signaling by rapamycin reduced GvHD in mice, and reduced pro-inflammatory cytokines in the serum and alloreactive T-cell infiltration in target organs.43,46 Additionally, rapamycin treatment reduced glycolytic activity of donor T-cells. The role of this pathway was confirmed using mTOR-deficient T-cells as donors, whereas further detailed analysis with Raptor-deficient T-cells revealed that T-cell pathogenicity is only dependent on mTORC1 but not on mTORC2. The in vitro findings were evaluated in vivo by inhibition of glycolysis through blockade of 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3, a rate-limiting enzyme in glycolysis.43 Limited donor T-cell glycolysis significantly improved the survival of mice suffering from acute GvHD. In summary, the glycolytic activity in donor T-cells is crucial for GvHD induction and a specific blockade could be a novel therapeutic approach to reduce GvHD severity. However, it is not yet known if a reduction of glycolytic activity also impairs alloreactivity against leukemia cells.43 The importance of the glycolytic activity of T-cells to enhance GvL immunity was recently demonstrated.47 T-cells from patients relapsing after allo-HCT showed reduced glycolysis that was mechanistically linked to increased lactic acid secreted by leukemia cells leading to a reduction of the intracellular pH in T-cells. Metabolic reprogramming of donor T-cells enhanced GvL activity and prolonged the survival of leukemia-bearing mice.47 Based on the findings that highly glycolytic donor T-cells accumulate in GvHD target organs, a novel method was established applying hyperpolarized 13C-pyruvate magnetic resonance imaging to detect high rates of glycolysis in vivo. The lactate/pyruvate ratio significantly increased in the liver of allo-HCT mice compared to syngeneic transplanted mice. Although few patients were included in the pilot study, increased transcription of glycolytic enzymes was found in CD4+ donor T-cells prior to acute GvHD onset. The method is a novel, noninvasive technique to determine acute GvHD onset in allo-HCT patients, which could be used to follow GvHD development over time and to identify a suitable time point for glycolysis inhibition to reduce overactive immunity.48
Hypomethylating agent treatment
The hypomethylating agents (HMAs) azacitidine and decitabine act as inhibitors of DNA-methyltransferases, thereby causing global DNA hypomethylation and alterations in gene expression control.49 Azacitidine was established in hematologic diseases with aberrant DNA methylation, such as myelodysplastic syndrome (MDS) or acute myeloid leukemia (AML).49 Besides direct cytostatic activities, induction of apoptosis leads to expansion of Tregs by increasing FOXP3 expression through FOXP3-promoter demethylation.50 Regarding the importance of Tregs in GvHD, azacitidine is a promising strategy to ameliorate GvHD after allo-HCT. Indeed, HMA treatment prevented GvHD in major mismatch models in mice through increasing the numbers of Tregs, inhibition of T-cell proliferation, and rendering T-cells less pro-inflammatory.50 This was attributed to expression of genes involved in cell-cycle progression and T-cell activation rather than induction of apoptosis. Additionally, longer exposure times affected FOXP3 gene expression, resulting in higher Treg numbers.50 However, strong effects on T-cell activation led to major concerns about the preservation of anti-leukemia immunity after transplantation. Although HMAs prolong survival after allo-HCT through Treg induction, the GvL effect was unaffected.51 Cooper et al,52 who further highlighted the importance of natural Treg cells (nTregs) in the donor graft to mitigate severe GvHD by azacitidine treatment, confirmed these findings. They proposed that nTregs are resistant against the anti-proliferative effect of azacitidine as compared to effector T-cells.52 Using a xenogeneic GvHD model, azacitidine prolonged the survival and reduced GvHD scores and lymphocyte infiltration into target organs in a dose-dependent manner.53 Gene expression and cytokine analyses revealed reduced gene expression and secretion of IFNγ, TNFα, and perforin upon azacitidine treatment, underlining its important immunomodulatory role. Consistent with other reports, azacitidine increased the Treg frequency and Tregs kept their immunosuppressive phenotype when transplanted into secondary recipients. The preservation of GvL activity was confirmed.53 A phase II trial investigating HMA effects in patients after allo-HCT was terminated (NCT01747499). However, another trial evaluated effects of azacitidine after donor lymphocyte infusion (DLI) for relapsed AML after allo-HCT (NCT01390311) and found azacitidine safe to use, without causing cytopenia or significant toxicities. Surprisingly, Tregs were not increased in PB upon azacitidine treatment, which might be due to the conditioning regimen, CD4+ frequencies in DLI, or a migration of these cells into GvHD target organs. The investigators did not identify grade III/IV GvHD or acute GvHD-related death, whereas all patients relapsed after disease. Conclusions are difficult due to the small patient number enrolled in the trial. Further studies are needed to evaluate the optimal biological dose for azacitidine after allo-HCT.54 A trial evaluating azacitidine effects after reduced-intensity allo-HCT found lower GvHD incidence in the azacitidine group besides increased Treg numbers (#ISRCTN36825171).55 Based on the knowledge that azacitidine can upregulate the expression of tumor antigens,56 specific CD8+ T-cell responses were investigated and increased cytotoxic response against several tumor antigens were found, supporting previous findings about the persistent GvL effect after allo-HCT during azacitidine treatment.55 Although the results are highly promising, additional trials with larger patient cohorts need to confirm the findings.
IL-2 treatment
Sykes et al57 already proposed in 1990 that exogenous IL-2 administration could prevent GvHD while keeping the anti-leukemia immunity effective. Depletion of IL-2 by calcineurin inhibition reduced Treg activity in mice developing acute GVHD,58 while mTOR inhibition did not interfere with Treg function.59 Low-dose IL-2 was effective to increase the Treg frequencies in chronic GvHD patients60 and it is known that IL-2 is important for development, expansion, and activity of Tregs.61 Low-dose IL-2 was clinically tolerated and immunologically active in patients undergoing T-cell depleted allo-HCT. Within the cohort, only a low acute GvHD incidence was reported and relapse rates were lower upon IL-2 application. Compared to control, IL-2–treated patients had longer disease-free survival and a lower relapse risk, and IL-2 increased NK cell frequencies over time.62 Another study highlighted the importance of IL-2 to increase FOXP3 expression in CD4+CD25+ Tregs through enhanced STAT binding. The results were confirmed in vivo in patients who underwent allo-HCT. IL-2 treatment increased the frequencies of CD4+CD25+ T-cells, upregulated FOXP3 expression, and expanded NK cells, confirming the aforementioned study.63 However, it was largely unknown if IL-2 application also increases Tregs in chronic GvHD patients without an expansion of conventional T-cells. A phase I trial evaluated the efficacy of ultra-low dose IL-2 in patients with refractory chronic GvHD (NCT00529035). It was shown that Tregs increased upon treatment without affecting conventional CD4+ T-cells and without impairing the immune response in general. Functional assays proved that the expanded cells inhibit the proliferation of autologous T-cells.61 Mechanistic analyses revealed a selective increase of STAT5 phosphorylation in Tregs and dephosphorylation in conventional T-cells (Tcon) upon ultra-low dose IL-2 application, together with increasing proliferation and apoptosis-resistance in Tregs, thereby rendering the CD4 T-cell compartment more tolerogenic.60 Similar results of low-dose IL-2 were also seen in other clinical trials and the cytotoxic reactivity against a variety of viral and leukemic agents was unchanged (NCT00539695).64 Although IL-2 significantly elevated Tregs if added to sirolimus and tacrolimus treatment at day +30 after allo-HCT, Treg numbers decreased by day +90 and only slightly reduced acute GvHD or chronic GvHD (NCT01927120). The investigators claimed that soluble IL-2 receptors accumulate in the circulation and neutralize IL-2, thereby leading to a progressive loss of Tregs. Further studies are necessary to evaluate the optimal dosing of IL-2 and to overcome IL-2 neutralization.65 A dose-escalating study was conducted in children and adults, aiming to escalate the dose of IL-2 at the time of anticipated falls in plasma levels (NCT02318082). Although dose-escalation was well-tolerated by children with partial response (PR) in 82% of patients, only 2/7 evaluable adult patients showed PR and a dose-escalation above the previously defined maximum tolerated dose could not improve the clinical outcome.66
Blockade of proinflammatory cytokines
GvHD is associated with an increase of proinflammatory cytokines, including TNFα, IFNγ and IL-6, secreted by effector cells.67 These inflammatory regulators enhance GvHD severity through direct tissue damage or further immune cell activation.68,69 Of high importance is IL-6 as it is known to regulate the immune response away from a regulatory towards a more inflammatory one.70 Although an increase of IL-6 and its receptor (IL-6R) during GvHD was reported, the absence of IL-6 in neither the donor nor the recipient compartment was sufficient to ameliorate murine GvHD. However, a blockade of the IL-6R could significantly prolong the survival of mice after allo-HCT and reduce GvHD severity. Better GvHD outcome was accompanied by increased Tregs and a loss of Th1 and Th17 cells in GvHD target organs.70 Another preclinical study confirmed that IL-6R blockade successfully reduced GvHD while preserving anti-tumor immunity.71 The humanized anti-IL-6R monoclonal antibody tocilizumab was found to be a promising treatment strategy for patients with steroid-refractory GvHD. Its addition to standard GvHD prophylaxis reduced the incidence of acute GvHD in 2 trials.72 A phase III trial (ACTRN12614000266662) investigated the beneficial effect of tocilizumab added to cyclosporine/methotrexate GvHD prophylaxis in MDS and AML patients after allo-HCT.72 Grade II–IV acute GvHD at day +100 was decreased in the tocilizumab versus placebo group; however, treatment-related mortality was slightly increased upon tocilizumab treatment, and neutrophil and platelet engraftment was delayed.72 In summary, tocilizumab partly reduced GvHD severity, but could not improve long-term survival.
Another strategy to reduce proinflammatory signaling is to block the formation of the IL-2 receptor (IL-2R) by targeting CD25, the IL-2R alpha chain expressed on Tregs and recently antigen-activated T-cells.73 Complexation of receptor subunits and ligands leads to JAK1/3 activation, followed by phosphoinositide-3-kinase/AKT and rat sarcoma/mitogen-activated protein kinase signaling or STAT phosphorylation, ultimately leading to T-cell activation and gene transcription.74 Anti-CD25 in combination with other GvHD prophylaxis did not affect engraftment after allo-HCT, and patients did not develop grade II–IV acute GvHD. Although the treatment did not affect hematopoietic stem and progenitor cells, it decreased the number of cytotoxic T-lymphocyte progenitors.75 Treatment with the IL-2R antagonist daclizumab resulted in 9/11 PR in children with steroid-refractory acute GvHD. In chronic GvHD, daclizumab only reduced skin GvHD, and 7 of 9 patients showed a response-experienced recurrence of GvHD.76 Daclizumab was combined with the TNF-receptor fusion-protein etanercept in steroid-refractory acute GvHD patients. Recent data have shown a role of TNF for GVHD of the central nervous system.77 Two thirds had at least PR; however, treatment-related mortality remained high due to infections and GvHD organ failure.78 Although IL-2 and TNFα signaling blockade had activity in steroid-refractory GvHD, the survival of patients remained poor.79 Importantly, studies on IL-2R blockade were performed before the importance of Tregs in GvHD was understood, and it is crucial to understand whether IL-2 signaling blockade interferes with Treg activity and expansion. The risk for acute GvHD did not differ between daclizumab and placebo, but blood analysis and long-term follow-up showed decreased Treg frequencies and a higher risk for chronic GvHD development under daclizumab treatment. Anti-CD25 therapy could be beneficial in delaying Treg reconstitution after allo-HCT and favoring CD4 memory T-cells, thereby supporting anti-leukemia immunity.80 However, this is still speculative and needs validation in clinical trials.
TNFα is a third proinflammatory cytokine with importance in GvHD.67 Although the anti-TNFα antibody infliximab was reported to have activity in acute GvHD, results are controversial.73,81 Retrospective analyses claimed that, especially in patients with severe intestinal steroid-refractory acute GvHD, infliximab has activity and potential infections would be worth weighing the benefits.82,83 However, a phase III trial of Infliximab/steroid combination did not result in a difference in GvHD-related mortality, non-relapse mortality, or OS between infliximab/steroid and steroid alone–treated patients.84 An explanation is the importance of TNFα to increase Treg frequencies through binding to TNFR2 on the Treg surface, which is crucial for modulating Treg numbers to control GvHD severity and anti-leukemia immunity.73,85 Blockade of TNFα with etanercept in steroid-refractory GvHD patients resulted in a response in 52% of the patients, whereas the best responses were seen in patients with gut GvHD.86 A preferential GvHD control in skin and gut was also seen in another trial, whereas hepatic GvHD was not affected.87 Promising results from a phase III trial (NCT00726375) showed that patients with early acute GvHD are at a lower risk to progress to grade II–IV acute GvHD if corticosteroids are combined with etanercept. However, additional studies are needed to confirm the findings.88
Cellular therapies to prevent GvHD
Given the importance of Tregs in GvHD, adoptive transfer is a promising strategy to reduce GvHD severity and Tcon effector functions. The addition of CD4+CD25+FOXP3+ Treg to the graft reduced GvHD development without interfering with anti-leukemia immunity.89 GvHD severity was reduced and immune-reconstitution and tissue regeneration supported when ex vivo expanded Tregs were given after disease onset in a mouse model.90 Tregs expanded from umbilical cord blood grafts significantly reduced acute GvHD and chronic GvHD onset and severity without affecting immune-reconstitution and relapse after allo-HCT (NCT00602693).91 Several trials in chronic GvHD proved that Treg transfer reduces GvHD severity without impairing GvL activity if compared to historical controls (NCT01911039, NCT01903473).92 However, Treg isolation is difficult since percentages in PB are low and ex vivo expansion is critical regarding the risk of Tcon expansion.93 Transfer of IL10-producing FOXP3− Tregs (Tr1) was associated with immunosuppression and tolerance induction in vitro and in vivo.93,94 In 5 of 12 patients, Tr1-infusion enhanced immune reconstitution without increasing the risk for GvHD.95 Although results sound promising, other trials could not confirm the findings96 and more research is needed to identify the perfect cellular source, cell purity, transplantation time-point, and the cell number needed to exhibit a biological response in individual patients.93 Besides Tregs, mesenchymal stromal cells (MSCs) were tested in GvHD and clinical studies showed impressive results.97 MSCs down-regulate immune reactions by interacting with T-cells and other immune cells (Figure 3).73 MSC treatment resulted in GvHD reduction in 6 of 8 patients with grade III–IV GvHD and better survival98 or CR in 30 of 55 steroid-refractory GvHD patients resulting in 55% OS at 2 years.99 Preclinical models proved that MSCs inhibit the proliferation and activation of CD8+ T-cells through increased indoleamine-2,3-dioxygenase 1, prostaglandin E2, and transforming growth factor-β and by Treg stimulation.94 MSCs further stimulated Th2-polarization, increased IL-4 and IL-10, reduced IFNγ and IL-2 secretion, and inhibited T-bet and RAR-related orphan receptor-γt expression.100 Comparable with Treg transfusion, study results were contradictory and more trials are needed to evaluate the best MSC preparation procedure and transfer timepoint.3
Figure 3.
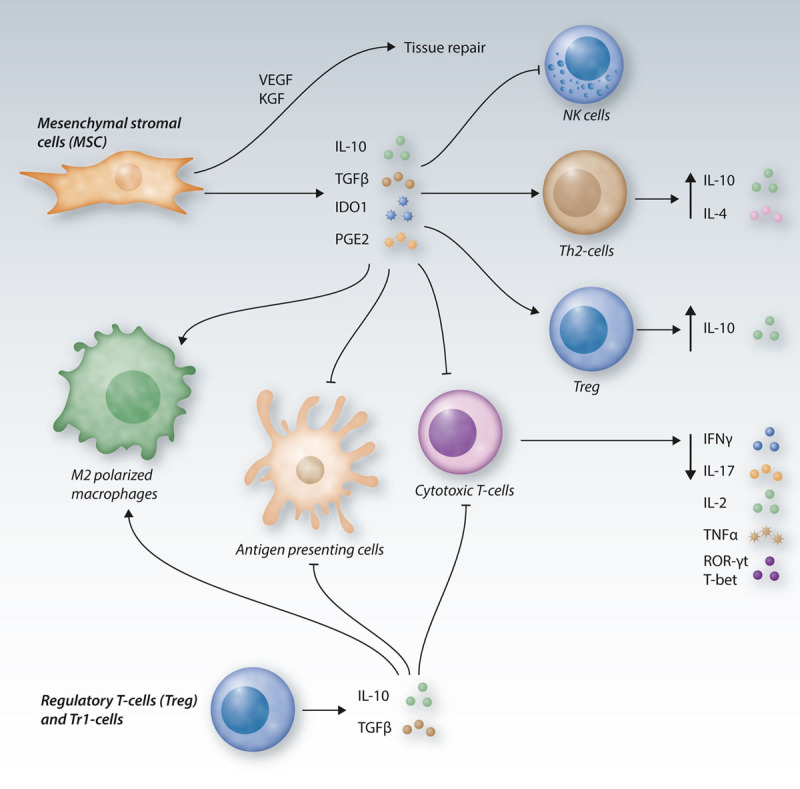
Immunomodulatory cellular therapies in GvHD.The adoptive transfer of MSCs, Tregs and Tr1-cells can be a promising strategy to reduce GvHD severity. Tregs and Tr1-cells secrete high levels of IL-10 and TGFβ, thereby inhibiting APC and cytotoxic T-cell functions, as well as polarizing macrophages towards an immunosuppressive M2 phenotype. MSC secrete high amounts of IL-10, TGFβ and PGE2 and exhibit high IDO1 activity, thereby reducing the function, activity and proliferation of cytotoxic T-cells, leading to reduced secretion of the inflammatory cytokines IFNγ, IL-17, IL-2 and TNFα and to decreased ROR-γt and T-bet expression. Moreover, immunomodulation by MSC leads to a blockade of APCs and NK cells, it promotes M2 polarization of macrophages and an increase of Treg and Th2-cells with increased production of the immunosuppressive cytokines IL-10 and IL-4. MSC can further contribute to tissue repair through secretion of the growth factors VEGF and KGF (FGF7). APC = antigen-presenting cell; FGF7 = fibroblast growth factor 7; GvHD = graft-versus-host-disease; IDO1 = indoleamine-2,3-dioxygenase 1; IFN = interferon; IL = interleukin; KGF = keratinocyte growth factor; MSCs = mesenchymal stromal cells; NK = natural killer; PGE2 = prostaglandin E2; ROR = RAR-related orphan receptor; TGF = transforming growth factor; Th2 = T helper type 2; TNF = tumor necrosis factor; Treg = regulatory T-cells; VEGF = vascular endothelial growth factor.
Conclusions
Acute GvHD is a life-threatening complication after allo-HCT that involves several layers of innate and adaptive immune responses. Although many mechanisms underlying GvHD have been clarified and novel therapies have shown activity, GvHD-related mortality remains high. Novel therapeutic concepts should not only suppress the inflammatory immune response, but rather modulate the immune reaction to preserve GvL effects. Here, we presented and discussed recently developed concepts to modulate the immune response after allo-HCT to prevent life-threatening GvHD. Although many therapeutic approaches are promising in early clinical trials, randomized phase-III trials are needed to identify the best strategies to prevent or treat GvHD.
Acknowledgments
We apologize to all authors whose work could not be cited due to space and citation restrictions.
Sources of funding
This article was supported by the DFG under Germany’s Excellence Strategy – EXC 2189 Project ID: 390939984, TRR167 to RZ, SFB1160 TP B09 to RZ, SFB850 TP C06 to RZ, the European Union: Proposal n 681012 GvHDCure (ERC consolidator grant to RZ), the Deutsche Krebshilfe (grant number 70113473), the Jose-Carreras Leukemia foundation (grant number DJCLS 01R/2019) (RZ).
Disclosures
The authors have no conflicts of interest to disclose.
References
- 1.Ferrara JL, Levine JE, Reddy P, et al. Graft-versus-host disease. Lancet. 2009; 373:1550–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hülsdünker J, Ottmüller KJ, Neeff HP, et al. Neutrophils provide cellular communication between ileum and mesenteric lymph nodes at graft-versus-host disease onset. Blood. 2018; 131:1858–1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zeiser R, Blazar BR. Acute graft-versus-host disease - biologic process, prevention, and therapy. N Engl J Med. 2017; 377:2167–2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haring E, Uhl FM, Andrieux G, et al. Bile acids regulate intestinal antigen presentation and reduce graft-versus-host disease without impairing the graft-versus-leukemia effect. Haematologica. 2020 July 16. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Watkins B, Qayed M, McCracken C, et al. Phase II trial of costimulation blockade with abatacept for prevention of acute GVHD. J Clin Oncol. 2021 January 15. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khoury SJ, Akalin E, Chandraker A, et al. CD28-B7 costimulatory blockade by CTLA4Ig prevents actively induced experimental autoimmune encephalomyelitis and inhibits Th1 but spares Th2 cytokines in the central nervous system. J Immunol. 1995; 155:4521–4524. [PubMed] [Google Scholar]
- 7.Via CS, Rus V, Nguyen P, et al. Differential effect of CTLA4Ig on murine graft-versus-host disease (GVHD) development: CTLA4Ig prevents both acute and chronic GVHD development but reverses only chronic GVHD. J Immunol. 1996; 157:4258–4267. [PubMed] [Google Scholar]
- 8.Ko HJ, Cho ML, Lee SY, et al. CTLA4-Ig modifies dendritic cells from mice with collagen-induced arthritis to increase the CD4+CD25+Foxp3+ regulatory T cell population. J Autoimmun. 2010; 34:111–120. [DOI] [PubMed] [Google Scholar]
- 9.Koura DT, Horan JT, Langston AA, et al. In vivo T cell costimulation blockade with abatacept for acute graft-versus-host disease prevention: a first-in-disease trial. Biol Blood Marrow Transplant. 2013; 19:1638–1649. [DOI] [PubMed] [Google Scholar]
- 10.Ohata J, Sakurai J, Saito K, et al. Differential graft-versus-leukaemia effect by CD28 and CD40 co-stimulatory blockade after graft-versus-host disease prophylaxis. Clin Exp Immunol. 2002; 129:61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Farag SS, Abu Zaid M, Schwartz JE, et al. Dipeptidyl peptidase 4 inhibition for prophylaxis of acute graft-versus-host disease. N Engl J Med. 2021; 384:11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schönle A, Hartl FA, Mentzel J, et al. Caveolin-1 regulates TCR signal strength and regulatory T-cell differentiation into alloreactive T cells. Blood. 2016; 127:1930–1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hatano R, Ohnuma K, Yamamoto J, et al. CD26-mediated co-stimulation in human CD8(+) T cells provokes effector function via pro-inflammatory cytokine production. Immunology. 2013; 138:165–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li YC, Sung PH, Yang YH, et al. Dipeptidyl peptidase 4 promotes peritoneal fibrosis and its inhibitions prevent failure of peritoneal dialysis. Commun Biol. 2021; 4:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hatano R, Ohnuma K, Yamamoto J, et al. Prevention of acute graft-versus-host disease by humanized anti-CD26 monoclonal antibody. Br J Haematol. 2013; 162:263–277. [DOI] [PubMed] [Google Scholar]
- 16.Farag SS, Srivastava S, Messina-Graham S, et al. In vivo DPP-4 inhibition to enhance engraftment of single-unit cord blood transplants in adults with hematological malignancies. Stem Cells Dev. 2013; 22:1007–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ning MM, Yang WJ, Guan WB, et al. Dipeptidyl peptidase 4 inhibitor sitagliptin protected against dextran sulfate sodium-induced experimental colitis by potentiating the action of GLP-2. Acta Pharmacol Sin. 2020; 41:1446–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Norona J, Apostolova P, Schmidt D, et al. Glucagon-like peptide 2 for intestinal stem cell and Paneth cell repair during graft-versus-host disease in mice and humans. Blood. 2020; 136:1442–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Edelson R, Berger C, Gasparro F, et al. Treatment of cutaneous T-cell lymphoma by extracorporeal photochemotherapy. Preliminary results. N Engl J Med. 1987; 316:297–303. [DOI] [PubMed] [Google Scholar]
- 20.Cho A, Jantschitsch C, Knobler R. Extracorporeal photopheresis-an overview. Front Med (Lausanne). 2018; 5:236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maas-Bauer K, Kiote-Schmidt C, Bertz H, et al. Ruxolitinib-ECP combination treatment for refractory severe chronic graft-versus-host disease. Bone Marrow Transplant. 2021; 56:909–916. [DOI] [PubMed] [Google Scholar]
- 22.Apostolova P, Unger S, von Bubnoff D, et al. Extracorporeal photopheresis for colitis induced by checkpoint-inhibitor therapy. N Engl J Med. 2020; 382:294–296. [DOI] [PubMed] [Google Scholar]
- 23.Isenring B, Robinson C, Buergi U, et al. Lung transplant recipients on long-term extracorporeal photopheresis. Clin Transplant. 2017; 31. [DOI] [PubMed] [Google Scholar]
- 24.Alcindor T, Gorgun G, Miller KB, et al. Immunomodulatory effects of extracorporeal photochemotherapy in patients with extensive chronic graft-versus-host disease. Blood. 2001; 98:1622–1625. [DOI] [PubMed] [Google Scholar]
- 25.Florek M, Sega EI, Leveson-Gower DB, et al. Autologous apoptotic cells preceding transplantation enhance survival in lethal murine graft-versus-host models. Blood. 2014; 124:1832–1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gatza E, Rogers CE, Clouthier SG, et al. Extracorporeal photopheresis reverses experimental graft-versus-host disease through regulatory T cells. Blood. 2008; 112:1515–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maeda A, Schwarz A, Kernebeck K, et al. Intravenous infusion of syngeneic apoptotic cells by photopheresis induces antigen-specific regulatory T cells. J Immunol. 2005; 174:5968–5976. [DOI] [PubMed] [Google Scholar]
- 28.Rieber N, Wecker I, Neri D, et al. Extracorporeal photopheresis increases neutrophilic myeloid-derived suppressor cells in patients with GvHD. Bone Marrow Transplant. 2014; 49:545–552. [DOI] [PubMed] [Google Scholar]
- 29.Bronte V, Serafini P, Mazzoni A, et al. L-arginine metabolism in myeloid cells controls T-lymphocyte functions. Trends Immunol. 2003; 24:302–306. [DOI] [PubMed] [Google Scholar]
- 30.Hechinger AK, Smith BA, Flynn R, et al. Therapeutic activity of multiple common γ-chain cytokine inhibition in acute and chronic GVHD. Blood. 2015; 125:570–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Verstovsek S, Kantarjian H, Mesa RA, et al. Safety and efficacy of INCB018424, a JAK1 and JAK2 inhibitor, in myelofibrosis. N Engl J Med. 2010; 363:1117–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schindler C, Levy DE, Decker T. JAK-STAT signaling: from interferons to cytokines. J Biol Chem. 2007; 282:20059–20063. [DOI] [PubMed] [Google Scholar]
- 33.Stickel N, Hanke K, Marschner D, et al. MicroRNA-146a reduces MHC-II expression via targeting JAK/STAT signaling in dendritic cells after stem cell transplantation. Leukemia. 2017; 31:2732–2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heine A, Held SA, Daecke SN, et al. The JAK-inhibitor ruxolitinib impairs dendritic cell function in vitro and in vivo. Blood. 2013; 122:1192–1202. [DOI] [PubMed] [Google Scholar]
- 35.Wilhelm K, Ganesan J, Müller T, et al. Graft-versus-host disease is enhanced by extracellular ATP activating P2X7R. Nat Med. 2010; 16:1434–1438. [DOI] [PubMed] [Google Scholar]
- 36.Jankovic D, Ganesan J, Bscheider M, et al. The Nlrp3 inflammasome regulates acute graft-versus-host disease. J Exp Med. 2013; 210:1899–1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schwab L, Goroncy L, Palaniyandi S, et al. Neutrophil granulocytes recruited upon translocation of intestinal bacteria enhance graft-versus-host disease via tissue damage. Nat Med. 2014; 20:648–654. [DOI] [PubMed] [Google Scholar]
- 38.Hülsdünker J, Thomas OS, Haring E, et al. Immunization against poly-N-acetylglucosamine reduces neutrophil activation and GVHD while sparing microbial diversity. Proc Natl Acad Sci U S A. 2019; 116:20700–20706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spoerl S, Mathew NR, Bscheider M, et al. Activity of therapeutic JAK ½ blockade in graft-versus-host disease. Blood. 2014; 123:3832–3842. [DOI] [PubMed] [Google Scholar]
- 40.Zeiser R, Burchert A, Lengerke C, et al. Ruxolitinib in corticosteroid-refractory graft-versus-host disease after allogeneic stem cell transplantation: a multicenter survey. Leukemia. 2015; 29:2062–2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jagasia M, Perales MA, Schroeder MA, et al. Ruxolitinib for the treatment of steroid-refractory acute GVHD (REACH1): a multicenter, open-label phase 2 trial. Blood. 2020; 135:1739–1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zeiser R, von Bubnoff N, Butler J, et al. ; REACH2 Trial Group. Ruxolitinib for glucocorticoid-refractory acute graft-versus-host disease. N Engl J Med. 2020; 382:1800–1810. [DOI] [PubMed] [Google Scholar]
- 43.Nguyen HD, Chatterjee S, Haarberg KM, et al. Metabolic reprogramming of alloantigen-activated T cells after hematopoietic cell transplantation. J Clin Invest. 2016; 126:1337–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chang CH, Curtis JD, Maggi LB, Jr, et al. Posttranscriptional control of T cell effector function by aerobic glycolysis. Cell. 2013; 153:1239–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim D, Park G, Huuhtanen J, et al. Somatic mTOR mutation in clonally expanded T lymphocytes associated with chronic graft versus host disease. Nat Commun. 2020; 11:2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Blazar BR, Taylor PA, Panoskaltsis-Mortari A, et al. Rapamycin inhibits the generation of graft-versus-host disease- and graft-versus-leukemia-causing T cells by interfering with the production of Th1 or Th1 cytotoxic cytokines. J Immunol. 1998; 160:5355–5365. [PubMed] [Google Scholar]
- 47.Uhl FM, Chen S, O’Sullivan D, et al. Metabolic reprogramming of donor T cells enhances graft-versus-leukemia effects in mice and humans. Sci Transl Med. 2020; 12:eabb8969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Assmann JC, Farthing DE, Saito K, et al. Glycolytic metabolism of pathogenic T cells enables early detection of GVHD by 13C-MRI. Blood. 2021; 137:126–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gardin C, Dombret H. Hypomethylating agents as a therapy for AML. Curr Hematol Malig Rep. 2017; 12:1–10. [DOI] [PubMed] [Google Scholar]
- 50.Sánchez-Abarca LI, Gutierrez-Cosio S, Santamaría C, et al. Immunomodulatory effect of 5-azacytidine (5-azaC): potential role in the transplantation setting. Blood. 2010; 115:107–121. [DOI] [PubMed] [Google Scholar]
- 51.Choi J, Ritchey J, Prior JL, et al. In vivo administration of hypomethylating agents mitigate graft-versus-host disease without sacrificing graft-versus-leukemia. Blood. 2010; 116:129–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cooper ML, Choi J, Karpova D, et al. Azacitidine mitigates graft-versus-host disease via differential effects on the proliferation of T effectors and natural regulatory T cells in vivo. J Immunol. 2017; 198:3746–3754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ehx G, Fransolet G, de Leval L, et al. Azacytidine prevents experimental xenogeneic graft-versus-host disease without abrogating graft-versus-leukemia effects. Oncoimmunology. 2017; 6:e1314425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ghobadi A, Choi J, Fiala MA, et al. Phase I study of azacitidine following donor lymphocyte infusion for relapsed acute myeloid leukemia post allogeneic stem cell transplantation. Leuk Res. 2016; 49:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Goodyear OC, Dennis M, Jilani NY, et al. Azacitidine augments expansion of regulatory T cells after allogeneic stem cell transplantation in patients with acute myeloid leukemia (AML). Blood. 2012; 119:3361–3369. [DOI] [PubMed] [Google Scholar]
- 56.Weber J, Salgaller M, Samid D, et al. Expression of the MAGE-1 tumor antigen is up-regulated by the demethylating agent 5-aza-2’-deoxycytidine. Cancer Res. 1994; 54:1766–1771. [PubMed] [Google Scholar]
- 57.Sykes M, Romick ML, Sachs DH. Interleukin 2 prevents graft-versus-host disease while preserving the graft-versus-leukemia effect of allogeneic T cells. Proc Natl Acad Sci U S A. 1990; 87:5633–5637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zeiser R, Nguyen VH, Beilhack A, et al. Inhibition of CD4+CD25+ regulatory T-cell function by calcineurin-dependent interleukin-2 production. Blood. 2006; 108:390–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zeiser R, Leveson-Gower DB, Zambricki EA, et al. Differential impact of mammalian target of rapamycin inhibition on CD4+CD25+Foxp3+ regulatory T cells compared with conventional CD4+ T cells. Blood. 2008; 111:453–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Matsuoka K, Koreth J, Kim HT, et al. Low-dose interleukin-2 therapy restores regulatory T cell homeostasis in patients with chronic graft-versus-host disease. Sci Transl Med. 2013; 5:179ra43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Koreth J, Matsuoka K, Kim HT, et al. Interleukin-2 and regulatory T cells in graft-versus-host disease. N Engl J Med. 2011; 365:2055–2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Soiffer RJ, Murray C, Gonin R, et al. Effect of low-dose interleukin-2 on disease relapse after T-cell-depleted allogeneic bone marrow transplantation. Blood. 1994; 84:964–971. [PubMed] [Google Scholar]
- 63.Zorn E, Nelson EA, Mohseni M, et al. IL-2 regulates FOXP3 expression in human CD4+CD25+ regulatory T cells through a STAT-dependent mechanism and induces the expansion of these cells in vivo. Blood. 2006; 108:1571–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kennedy-Nasser AA, Ku S, Castillo-Caro P, et al. Ultra low-dose IL-2 for GVHD prophylaxis after allogeneic hematopoietic stem cell transplantation mediates expansion of regulatory T cells without diminishing antiviral and antileukemic activity. Clin Cancer Res. 2014; 20:2215–2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Betts BC, Pidala J, Kim J, et al. IL-2 promotes early Treg reconstitution after allogeneic hematopoietic cell transplantation. Haematologica. 2017; 102:948–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Whangbo JS, Kim HT, Mirkovic N, et al. Dose-escalated interleukin-2 therapy for refractory chronic graft-versus-host disease in adults and children. Blood Adv. 2019; 3:2550–2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hill GR, Crawford JM, Cooke KR, et al. Total body irradiation and acute graft-versus-host disease: the role of gastrointestinal damage and inflammatory cytokines. Blood. 1997; 90:3204–3213. [PubMed] [Google Scholar]
- 68.Mowat AM. Antibodies to IFN-gamma prevent immunologically mediated intestinal damage in murine graft-versus-host reaction. Immunology. 1989; 68:18–23. [PMC free article] [PubMed] [Google Scholar]
- 69.Nestel FP, Price KS, Seemayer TA, et al. Macrophage priming and lipopolysaccharide-triggered release of tumor necrosis factor alpha during graft-versus-host disease. J Exp Med. 1992; 175:405–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen X, Das R, Komorowski R, et al. Blockade of interleukin-6 signaling augments regulatory T-cell reconstitution and attenuates the severity of graft-versus-host disease. Blood. 2009; 114:891–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tawara I, Koyama M, Liu C, et al. Interleukin-6 modulates graft-versus-host responses after experimental allogeneic bone marrow transplantation. Clin Cancer Res. 2011; 17:77–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kennedy GA, Tey SK, Buizen L, et al. A phase 3 double-blind study of the addition of tocilizumab vs placebo to cyclosporin/methotrexate GVHD prophylaxis. Blood. 2021; 137:1970–1979. [DOI] [PubMed] [Google Scholar]
- 73.Zhang L, Yu J, Wei W. Advance in targeted immunotherapy for graft-versus-host disease. Front Immunol. 2018; 9:1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Malek TR, Castro I. Interleukin-2 receptor signaling: at the interface between tolerance and immunity. Immunity. 2010; 33:153–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chen HR, Ji SQ, Wang HX, et al. Humanized anti-CD25 monoclonal antibody for prophylaxis of graft-vs-host disease (GVHD) in haploidentical bone marrow transplantation without ex vivo T-cell depletion. Exp Hematol. 2003; 31:1019–1025. [DOI] [PubMed] [Google Scholar]
- 76.Teachey DT, Bickert B, Bunin N. Daclizumab for children with corticosteroid refractory graft-versus-host disease. Bone Marrow Transplant. 2006; 37:95–99. [DOI] [PubMed] [Google Scholar]
- 77.Mathew NR, Vinnakota JM, Apostolova P, et al. Graft-versus-host disease of the CNS is mediated by TNF upregulation in microglia. J Clin Invest. 2020; 130:1315–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wolff D, Roessler V, Steiner B, et al. Treatment of steroid-resistant acute graft-versus-host disease with daclizumab and etanercept. Bone Marrow Transplant. 2005; 35:1003–1010. [DOI] [PubMed] [Google Scholar]
- 79.Rager A, Frey N, Goldstein SC, et al. Inflammatory cytokine inhibition with combination daclizumab and infliximab for steroid-refractory acute GVHD. Bone Marrow Transplant. 2011; 46:430–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Locke FL, Pidala J, Storer B, et al. CD25 blockade delays regulatory T cell reconstitution and does not prevent graft-versus-host disease after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2017; 23:405–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kobbe G, Schneider P, Rohr U, et al. Treatment of severe steroid refractory acute graft-versus-host disease with infliximab, a chimeric human/mouse antiTNFalpha antibody. Bone Marrow Transplant. 2001; 28:47–49. [DOI] [PubMed] [Google Scholar]
- 82.Patriarca F, Sperotto A, Damiani D, et al. Infliximab treatment for steroid-refractory acute graft-versus-host disease. Haematologica. 2004; 89:1352–1359. [PubMed] [Google Scholar]
- 83.Couriel D, Saliba R, Hicks K, et al. Tumor necrosis factor-alpha blockade for the treatment of acute GVHD. Blood. 2004; 104:649–654. [DOI] [PubMed] [Google Scholar]
- 84.Couriel DR, Saliba R, de Lima M, et al. A phase III study of infliximab and corticosteroids for the initial treatment of acute graft-versus-host disease. Biol Blood Marrow Transplant. 2009; 15:1555–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Leclerc M, Naserian S, Pilon C, et al. Control of GVHD by regulatory T cells depends on TNF produced by T cells and TNFR2 expressed by regulatory T cells. Blood. 2016; 128:1651–1659. [DOI] [PubMed] [Google Scholar]
- 86.Busca A, Locatelli F, Marmont F, et al. Recombinant human soluble tumor necrosis factor receptor fusion protein as treatment for steroid refractory graft-versus-host disease following allogeneic hematopoietic stem cell transplantation. Am J Hematol. 2007; 82:45–52. [DOI] [PubMed] [Google Scholar]
- 87.Park JH, Lee HJ, Kim SR, et al. Etanercept for steroid-refractory acute graft versus host disease following allogeneic hematopoietic stem cell transplantation. Korean J Intern Med. 2014; 29:630–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gatza E, Braun T, Levine JE, et al. Etanercept plus topical corticosteroids as initial therapy for grade one acute graft-versus-host disease after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2014; 20:1426–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Edinger M, Hoffmann P, Ermann J, et al. CD4+CD25+ regulatory T cells preserve graft-versus-tumor activity while inhibiting graft-versus-host disease after bone marrow transplantation. Nat Med. 2003; 9:1144–1150. [DOI] [PubMed] [Google Scholar]
- 90.Riegel C, Boeld TJ, Doser K, et al. Efficient treatment of murine acute GvHD by in vitro expanded donor regulatory T cells. Leukemia. 2020; 34:895–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Brunstein CG, Miller JS, McKenna DH, et al. Umbilical cord blood-derived T regulatory cells to prevent GVHD: kinetics, toxicity profile, and clinical effect. Blood. 2016; 127:1044–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Martelli MF, Di Ianni M, Ruggeri L, et al. HLA-haploidentical transplantation with regulatory and conventional T-cell adoptive immunotherapy prevents acute leukemia relapse. Blood. 2014; 124:638–644. [DOI] [PubMed] [Google Scholar]
- 93.Schneidawind D, Pierini A, Negrin RS. Regulatory T cells and natural killer T cells for modulation of GVHD following allogeneic hematopoietic cell transplantation. Blood. 2013; 122:3116–3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Blazar BR, MacDonald KPA, Hill GR. Immune regulatory cell infusion for graft-versus-host disease prevention and therapy. Blood. 2018; 131:2651–2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bacchetta R, Lucarelli B, Sartirana C, et al. Immunological outcome in haploidentical-HSC transplanted patients treated with IL-10-anergized donor T cells. Front Immunol. 2014; 5:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Theil A, Tuve S, Oelschlägel U, et al. Adoptive transfer of allogeneic regulatory T cells into patients with chronic graft-versus-host disease. Cytotherapy. 2015; 17:473–486. [DOI] [PubMed] [Google Scholar]
- 97.Le Blanc K, Rasmusson I, Sundberg B, et al. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004; 363:1439–1441. [DOI] [PubMed] [Google Scholar]
- 98.Ringdén O, Uzunel M, Rasmusson I, et al. Mesenchymal stem cells for treatment of therapy-resistant graft-versus-host disease. Transplantation. 2006; 81:1390–1397. [DOI] [PubMed] [Google Scholar]
- 99.Solenberg PJ, Baltz RH. Transposition of Tn5096 and other IS493 derivatives in streptomyces griseofuscus. J Bacteriol. 1991; 173:1096–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yang D, Wang LP, Zhou H, et al. Inducible costimulator gene-transduced bone marrow-derived mesenchymal stem cells attenuate the severity of acute graft-versus-host disease in mouse models. Cell Transplant. 2015; 24:1717–1731. [DOI] [PubMed] [Google Scholar]


