Abstract
Background
The use of insulin‐sensitising agents, such as metformin, in women with polycystic ovary syndrome (PCOS) who are undergoing ovulation induction or in vitro fertilisation (IVF) cycles has been widely studied. Metformin reduces hyperinsulinaemia and suppresses the excessive ovarian production of androgens. It is suggested that as a consequence metformin could improve assisted reproductive techniques (ART) outcomes, such as ovarian hyperstimulation syndrome (OHSS), pregnancy, and live birth rates.
Objectives
To determine the effectiveness and safety of metformin as a co‐treatment during IVF or intracytoplasmic sperm injection (ICSI) in achieving pregnancy or live birth in women with PCOS.
Search methods
We searched the Cochrane Gynaecology and Fertility Group Specialised Register, CENTRAL via the Cochrane Register of Studies Online (CRSO), MEDLINE, Embase, PsycINFO, LILACS, the trial registries for ongoing trials, and reference lists of articles (from inception to 13 February 2020).
Selection criteria
Types of studies: randomised controlled trials (RCTs) comparing metformin treatment with placebo or no treatment in women with PCOS who underwent IVF or ICSI treatment.
Types of participants: women of reproductive age with anovulation due to PCOS with or without co‐existing infertility factors.
Types of interventions: metformin administered before and during IVF or ICSI treatment.
Primary outcome measures: live birth rate, incidence of ovarian hyperstimulation syndrome.
Data collection and analysis
Two review authors independently selected the studies, extracted the data according to the protocol, and assessed study quality. We assessed the overall quality of the evidence using the GRADE approach.
Main results
This updated review includes 13 RCTs involving a total of 1132 women with PCOS undergoing IVF/ICSI treatments. We stratified the analysis by type of ovarian stimulation protocol used (long gonadotrophin‐releasing hormone agonist (GnRH‐agonist) or short gonadotrophin‐releasing hormone antagonist (GnRH‐antagonist)) to determine whether the type of stimulation used influenced the outcomes. We did not perform meta‐analysis on the overall (both ovarian stimulation protocols combined) data for the outcomes of live birth and clinical pregnancy rates per woman because of substantial heterogeneity.
In the long protocol GnRH‐agonist subgroup, the pooled evidence showed that we are uncertain of the effect of metformin on live birth rate per woman when compared with placebo/no treatment (risk ratio (RR) 1.30, 95% confidence interval (CI) 0.94 to 1.79; 6 RCTs; 651 women; I2 = 47%; low‐quality evidence). This suggests that if the chance for live birth following placebo/no treatment is 28%, the chance following metformin would be between 27% and 51%. Only one study used short protocol GnRH‐antagonist and reported live birth rate. Metformin may reduce live birth rate compared with placebo/no treatment (RR 0.48, 95% CI 0.29 to 0.79; 1 RCT; 153 women; low‐quality evidence). This suggests that if the chance for live birth following placebo/no treatment is 43%, the chance following metformin would be between 13% and 34% (short GnRH‐antagonist protocol). We found that metformin may reduce the incidence of OHSS (RR 0.46, 95% CI 0.29 to 0.72; 11 RCTs; 1091 women; I2 = 38%; low‐quality evidence). This suggests that for a woman with a 20% risk of OHSS without metformin, the corresponding risk using metformin would be between 6% and 14%. Using long protocol GnRH‐agonist stimulation, metformin may increase clinical pregnancy rate per woman compared with placebo/no treatment (RR 1.32, 95% CI 1.08 to 1.63; 10 RCTs; 915 women; I2 = 13%; low‐quality evidence). Using short protocol GnRH‐antagonist, we are uncertain of the effect of metformin on clinical pregnancy rate per woman compared with placebo/no treatment (RR 1.38, 95% CI 0.21 to 9.14; 2 RCTs; 177 women; I2 = 87%; very low‐quality evidence).
We are uncertain of the effect of metformin on miscarriage rate per woman when compared with placebo/no treatment (RR 0.86, 95% CI 0.56 to 1.32; 8 RCTs; 821 women; I2 = 0%; low‐quality evidence). Metformin may result in an increase in side effects compared with placebo/no treatment (RR 3.35, 95% CI 2.34 to 4.79; 8 RCTs; 748 women; I2 = 0%; low‐quality evidence).
The overall quality of evidence ranged from very low to low. The main limitations were inconsistency, risk of bias, and imprecision.
Authors' conclusions
This updated review on metformin versus placebo/no treatment before or during IVF/ICSI treatment in women with PCOS found no conclusive evidence that metformin improves live birth rates. In a long GnRH‐agonist protocol, we are uncertain whether metformin improves live birth rates, but metformin may increase the clinical pregnancy rate. In a short GnRH‐antagonist protocol, metformin may reduce live birth rates, although we are uncertain about the effect of metformin on clinical pregnancy rate. Metformin may reduce the incidence of OHSS but may result in a higher incidence of side effects. We are uncertain of the effect of metformin on miscarriage rate per woman.
Keywords: Female; Humans; Pregnancy; Abortion, Spontaneous; Abortion, Spontaneous/epidemiology; Abortion, Spontaneous/prevention & control; Bias; Confidence Intervals; Fertilization in Vitro; Hyperandrogenism; Hyperandrogenism/drug therapy; Hyperinsulinism; Hyperinsulinism/drug therapy; Hypoglycemic Agents; Hypoglycemic Agents/adverse effects; Hypoglycemic Agents/therapeutic use; Live Birth; Live Birth/epidemiology; Metformin; Metformin/adverse effects; Metformin/therapeutic use; Ovarian Hyperstimulation Syndrome; Ovarian Hyperstimulation Syndrome/epidemiology; Ovarian Hyperstimulation Syndrome/prevention & control; Ovulation Induction; Ovulation Induction/methods; Placebos; Placebos/therapeutic use; Polycystic Ovary Syndrome; Polycystic Ovary Syndrome/complications; Pregnancy Rate; Randomized Controlled Trials as Topic; Sperm Injections, Intracytoplasmic
Plain language summary
Metformin in women with polycystic ovary syndrome (PCOS) for improving fertility
Review question
The aim of this review was to determine if metformin improves live birth and clinical pregnancy rates and whether it reduces the incidence of ovarian hyperstimulation syndrome (OHSS) in women with PCOS undergoing in vitro fertilisation (IVF) or intracytoplasmic sperm injection (ICSI).
Background
In women with PCOS there is a chronic failure or absence of ovulation (anovulation) and excessive production of male hormones (hyperandrogenism). The main symptoms of the disorder are irregular periods, infertility, hirsutism (excessive facial and body hair growth), and acne. PCOS is the most common endocrine disorder in women, affecting 5% to 10% of women of reproductive age. IVF could be an effective treatment option for infertility in women with PCOS who do not respond to ovulation induction treatments. In the first part of IVF treatment, ovarian stimulation using gonadotrophins is necessary to develop more mature oocytes in order to produce more good‐quality embryos to be transferred into the uterus. This overstimulation increases the risk of developing a serious complication known as ovarian hyperstimulation syndrome (OHSS). Strategies used during IVF treatments to decrease the risk of OHSS include: low‐dose gonadotrophin ovarian stimulation, metformin co‐treatment, use of gonadotrophin‐releasing hormone (GnRH)‐antagonist protocol instead of GnRH‐agonist, and use of GnRH‐agonist trigger to final oocyte maturation rather than the usual human chorionic gonadotrophin (hCG)‐trigger.
Study characteristics
We included 13 randomised controlled trials (a type of study in which participants are assigned to one of two or more treatment groups using a random method) involving a total of 1132 women assigned to receive either metformin (570) or placebo (dummy treatment)/no treatment (563). The evidence is current to 13 February 2020.
Key results
We divided the analysis by type of ovarian stimulation protocol used during the IVF treatment (long GnRH‐agonist or short GnRH‐antagonist) to determine whether the type of stimulation used influenced the outcomes. We are uncertain of the effect of metformin using long protocol GnRH‐agonist on live birth rates compared with placebo or no treatment, but metformin may increase clinical pregnancy rate with this type of ovarian stimulation protocol. Metformin may reduce the incidence of OHSS. We estimated that for a woman with a 28% chance of achieving a live birth (long protocol GnRH‐agonist) following placebo or no treatment, the chance following metformin would be between 27% and 51%. For a woman with a 28% chance of achieving a clinical pregnancy in long protocol GnRH‐agonist without metformin, the chance using metformin would be between 30% and 45%.
For the short protocol GnRH‐antagonist, metformin may reduce live birth rate, and we are uncertain of its effect on clinical pregnancy and OHSS rates compared with placebo/no treatment.
Overall, metformin may reduce the incidence of OHSS when compared with placebo/no treatment. For a woman with a 20% risk of OHSS without metformin, the corresponding risk using metformin would be between 6% and 14%. Side effects (mostly gastrointestinal) may be more common with metformin. We are uncertain of the effect of metformin on miscarriage rates when compared with placebo/no treatment.
Quality of the evidence
The overall quality of evidence for the primary outcomes live birth rate and incidence of OHSS was low. We assessed the evidence as low for the secondary outcomes clinical pregnancy rate (long protocol GnRH‐agonist), miscarriage rate, and side effects, and very low for clinical pregnancy rate (short protocol GnRH‐antagonist). The main limitations were risk of bias and imprecise results.
Conclusion
This updated review on metformin versus placebo/no treatment before or during IVF/ICSI treatment in women with PCOS found no clear evidence that metformin improves live birth rates: the effect of metformin is uncertain using long protocol GnRH‐agonist, but live birth rates may be reduced using short protocol GnRH‐antagonist. Metformin may increase clinical pregnancy rates using long protocol GnRH‐agonist, but we are uncertain of the effect using short protocol GnRH‐antagonist. Metformin may reduce the incidence of OHSS, but may result in a higher incidence of side effects. We are uncertain of the effect of metformin on miscarriage rate.
Summary of findings
Summary of findings 1. Metformin compared to placebo or no treatment in women with polycystic ovary syndrome.
| Metformin compared to placebo or no treatment in women with polycystic ovary syndrome | ||||||
| Patient or population: women with polycystic ovary syndrome Setting: Human reproduction center Intervention: metformin Comparison: placebo or no treatment | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with placebo or no treatment | Risk with metformin | |||||
| Live birth rate per woman ‐ long protocol GnRH‐agonist | Study population | RR 1.30 (0.94 to 1.79) | 651 (6 RCTs) | ⊕⊕⊝⊝ LOW 1 2 | The evidence for the effect of metformin on live birth rate per woman ‐ long protocol GnRH‐agonist is uncertain. | |
| 283 per 1000 | 368 per 1000 (266 to 507) | |||||
| Live birth rate per woman ‐ short protocol GnRH‐antagonist | Study population | RR 0.48 (0.29 to 0.79) | 153 (1 RCT) | ⊕⊕⊝⊝ LOW 3 | Metformin may reduce live birth rate per woman ‐ short protocol GnRH‐antagonist. | |
| 434 per 1000 | 208 per 1000 (126 to 343) | |||||
| Incidence of OHSS per woman | Study population | RR 0.46 (0.29 to 0.72) | 1091 (11 RCTs) | ⊕⊕⊝⊝ LOW 1 4 | Metformin may reduce incidence of OHSS per woman. | |
| 196 per 1000 | 90 per 1000 (57 to 141) | |||||
| Clinical pregnancy rate per woman ‐ long protocol GnRH‐agonist | Study population | RR 1.32 (1.08 to 1.63) | 915 (10 RCTs) | ⊕⊕⊝⊝ LOW 1 2 | Metformin may increase clinical pregnancy rate per woman ‐ long protocol GnRH‐agonist. | |
| 275 per 1000 | 363 per 1000 (297 to 449) | |||||
| Clinical pregnancy rate per woman ‐ short protocol GnRH‐antagonist | Study population | RR 1.38 (0.21 to 9.14) | 177 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW 5 6 7 | The evidence for the effect of metformin on clinical pregnancy rate per woman ‐ short protocol GnRH‐antagonist is uncertain. | |
| 443 per 1000 | 612 per 1000 (93 to 1000) | |||||
| Miscarriage rate per woman | Study population | RR 0.86 (0.56 to 1.32) | 821 (8 RCTs) | ⊕⊕⊝⊝ LOW 1 8 | The evidence for the effect of metformin on miscarriage rate per woman is uncertain. | |
| 106 per 1000 | 91 per 1000 (59 to 141) | |||||
| Side effects per woman | Study population | RR 3.35 (2.34 to 4.79) | 748 (8 RCTs) | ⊕⊕⊝⊝ LOW 1 4 | Metformin may result in an increase in side effects per woman. | |
| 88 per 1000 | 296 per 1000 (207 to 424) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; GnRH: gonadotrophin‐releasing hormone; OHSS: ovarian hyperstimulation syndrome; RCT: randomised controlled trial; RR: risk ratio | ||||||
|
GRADE Working Group grades of evidence
High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
1Evidence downgraded by one level for serious risk of bias: the majority of the RCTs have unclear or high risk of bias. 2Evidence downgraded by one level for serious imprecision: low number of events (total number of events < 300) and 95% CI includes both appreciable effect and little or no effect. 3Evidence downgraded by two levels for very serious imprecision: low number of events (total number of events < 300) and data based on one small RCT. 4Evidence downgraded by one level for serious imprecision: low number of events (total number of events < 300). 5Evidence downgraded by one level for serious risk of bias: all studies considered as at unclear risk of bias for at least one domain. 6Evidence downgraded by two levels for serious inconsistency (I2 = 87%) as unexplained heterogeneity (i.e. heterogeneity not explained by subgrouping of data according to stimulation protocol). 7Evidence downgraded by two levels for very serious imprecision: low number of events (total number of events < 300) and data based on two RCTs, and 95% CI includes both appreciable benefit and harm. 8Evidence downgraded by one level for serious imprecision: low number of events (total number of events < 300), and 95% CI includes both appreciable benefit and harm.
Background
Description of the condition
Polycystic ovary syndrome (PCOS) is a disorder characterised by chronic anovulation (failure or absence of ovulation) and hyperandrogenism (excessive production of male hormones in women) and is associated with irregular menstrual cycles, infertility, hirsutism, and acne (Speroff 1995). This condition is the most common endocrine disorder in women, affecting approximately 5% to 10% of all women of reproductive age (Frank 1995; Knochenhauer 1998).
PCOS is a heterogenous condition, from a clinical as well as from a biochemical perspective. According to the recommendations proposed by an international consensus group (ESHRE/ASRM 2003), the diagnosis of PCOS is made when at least two of the following criteria are met:
oligo‐ or anovulation (infrequent or no ovulation);
clinical or biochemical signs of hyperandrogenism, or both;
polycystic ovaries on ultrasound.
Other causes of hyperandrogenism that mimic PCOS (such as congenital adrenal hyperplasia, Cushing's syndrome, or androgen‐secreting tumours) were excluded.
Although the primary aetiology of PCOS is unknown (Balen 2004), insulin resistance with compensatory hyperinsulinaemia is a prominent feature of the syndrome and seems to play an important physiopathological role in hyperandrogenism, in both lean and obese women with PCOS (Dunaif 1989; Tsilchorozidou 2004). Hyperinsulinaemia increases ovarian androgen biosynthesis, both in vivo and in vitro (Adashi 1985; Barbieri 1986), and decreases the hepatic production of sex hormone‐binding globulin (SHBG) (Nestler 1991), thus leading to increased bioavailability of free androgens.
Description of the intervention
Several treatments have been used to induce ovulation and pregnancy in infertile anovulatory women with PCOS. The use of clomiphene citrate as first‐line treatment leads to modest pregnancy rates (Barbieri 2000; Kocak 2002; Thessaloniki ESHRE/ASRM‐Sponsored PCOS 2008). Based on the association between insulin resistance and anovulation in women with PCOS, insulin‐sensitising agents, such as metformin, have recently been added to the treatment protocols of these women (Costello 2007; Jungheim 2010; Nestler 2002).
How the intervention might work
Metformin is an orally active, water‐soluble biguanide used to treat type 2 diabetes mellitus. The drug has an antihyperglycaemic effect and does not cause hypoglycaemia. It enhances insulin sensitivity both in the liver, by inhibiting hepatic glucose production, and in peripheral tissues, such as muscle cells, by increasing glucose uptake and utilisation (Barbieri 1986; Dunn 1995; Nardo 2001). There is a good physiological rationale for believing that suppression of insulin levels, through the use of insulin‐sensitising agents such as metformin, may be useful in women with PCOS who are undergoing in vitro fertilisation (IVF) with or without intracytoplasmic sperm injection (ICSI). Suppression of insulin levels might ameliorate the adverse effects of ovarian stimulation and improve treatment outcomes such as ovulation and pregnancy rates (Dunaif 1989; Tang 2006). In addition, metformin may also act directly on ovarian thecal cells, decreasing androgen production (Attia 2001; Palomba 2010).
Why it is important to do this review
Due to a large cohort of antral follicles sensible to gonadotrophins in PCOS women, the risk of developing ovarian hyperstimulation syndrome (OHSS) is high in this population who are undergoing ovarian stimulation with follicle‐stimulating hormone (FSH). Ovarian hyperstimulation syndrome is a life‐threatening iatrogenic condition, and therefore one of the most important and serious complications of assisted reproductive technology (ART). Higher total FSH doses lead to a larger number of follicles and oocytes, high serum oestradiol (E2) levels, increased risk of OHSS, elevated cancellation rates, and lower conception rates (Aboulghar 2003; Yarali 2004). It is therefore important to assess the effects of metformin on the clinical, biochemical, and laboratory profiles of PCOS women undergoing ART cycles. Several adequately designed trials have addressed this question (Kjotrod 2004; Kjotrod 2011; Palomba 2011; Tang 2006).
Objectives
To determine the effectiveness and safety of metformin as a co‐treatment during IVF or ICSI in achieving pregnancy or live birth in women with PCOS.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) comparing metformin treatment with placebo or no treatment in women with PCOS undergoing IVF or ICSI treatment.
We did not include non‐randomised and quasi‐randomised trials due to their high risk of bias. We considered only the first part of cross‐over trials in the meta‐analysis.
Types of participants
Women of reproductive age with anovulation attributed to PCOS, with or without another cause of couple infertility, who were treated with metformin before and during an IVF or ICSI cycle were eligible.
The aetiology of infertility leading to treatment by IVF or ICSI was defined by the individual study authors. The diagnosis of PCOS was based on the European Society of Human Reproduction and Embryology/American Society for Reproductive Medicine (ESHRE/ASRM) criteria (ESHRE/ASRM 2003). Due to the wide variation of diagnostic criteria used for PCOS, studies that used different diagnostic criteria were included in the review if the broad definition used in the study matched the ESHRE/ASRM criteria. According to the recommendations proposed by that group, the diagnosis of PCOS is made when at least two of the following criteria are met:
oligo‐ or anovulation (infrequent or no ovulation);
clinical or biochemical (or both) signs of hyperandrogenism;
polycystic ovaries on ultrasound.
Other causes of hyperandrogenism that mimic PCOS (such as congenital adrenal hyperplasia, Cushing's syndrome, or androgen‐secreting tumours) should have been excluded.
Types of interventions
Metformin versus placebo or no treatment before and during IVF or ICSI treatment.
Types of outcome measures
Primary outcomes
Live birth rate (per woman), defined as a baby born after 20 weeks of gestation.
Incidence of OHSS (per woman), defined according to the definition of reporting authors.
Secondary outcomes
Clinical pregnancy rate (per woman), defined as the identification of an intrauterine gestational sac on ultrasound scan.
Miscarriage rate (per woman and per pregnancy), defined as the involuntary loss of a pregnancy before 20 weeks gestation.
Incidence of participant‐reported side effects (especially gastrointestinal symptoms, e.g. nausea, vomiting, and diarrhoea).
Number of oocytes retrieved.
Total dose of FSH (in international units (IU)).
Number of days of gonadotrophin treatment.
Cycle cancellation rate (per woman).
Serum oestradiol level (pg/mL) on the day of human chorionic gonadotrophin (hCG) trigger injection.
Serum androgen level (total testosterone, sex hormone‐binding globulin (SHBG) or free‐androgen index).
Fasting insulin and glucose levels.
Fertilisation rate, defined as normal fertilisation with two pronuclei‐stage embryos. The fertilisation rate was defined as the number of normally fertilised oocytes divided by the number of oocytes retrieved per cycle.
Search methods for identification of studies
We sought all relevant RCTs of metformin co‐treatment (prior to and during ovarian stimulation) in women with PCOS undergoing IVF or ICSI treatment, without language restriction. Our original search was conducted in 2008, with updated searches carried out in November 2012, September 2013, October 2014, and March 2019. The searches were performed in consultation with the Cochrane Gynaecology and Fertility Group Information Specialist.
Electronic searches
In order to identify relevant studies, we developed detailed search strategies for each specific database.
We searched the following databases:
Cochrane Gynaecology and Fertility Group Specialised Register; ProCite platform, searched 13 February 2020 (Appendix 1);
the Cochrane Central Register of Controlled Trials (CENTRAL) via the Cochrane Register of Studies Online (CRSO); Web platform, searched 13 February 2020 (Appendix 2);
MEDLINE; Ovid platform, searched from 1946 to 13 February 2020 (Appendix 3);
Embase; Ovid platform, searched from 1980 to 13 February 2020 (Appendix 4);
PsycINFO; Ovid platform, searched from 1806 to 13 February 2020 (Appendix 5);
LILACS (Latin American and Caribbean Health Science Information database); Web platform, searched 13 February 2020 (Appendix 6).
We also searched the trial registries (until 13 February 2020) for ongoing and registered trials:
ISRCTN registry (www.controlled-trials.com);
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (clinicaltrials.gov);
World Health Organization International Clinical Trials Registry Platform (www.who.int/trialsearch/Default.aspx).
Searching other resources
We checked the citation lists of relevant publications, review articles, and included studies. We handsearched references of identified selected articles for additional relevant citations. We also contacted experts in the field for additional relevant citations.
Data collection and analysis
We analysed data using Review Manager 5 (Review Manager 2014).
Selection of studies
For the 2020 update, two review authors (LOT and MFC) independently selected trials for inclusion in the review in accordance with the aforementioned criteria. Any disagreements were settled by a third review author (CRM).
Data extraction and management
Two review authors (LOT and LETA) independently extracted data using forms designed according to Cochrane guidelines. We sought additional information from the authors of trials that appeared to meet the eligibility criteria but for which the methodological details were unclear. We also sought further trial data when data in the reports were presented in a form that was unsuitable for meta‐analysis.
Differences of opinion were registered and resolved by consensus. We planned to perform a series of analyses on the results; however, these analyses were not always possible due to an insufficient number of trials reporting on a given outcome.
We extracted the information presented in Appendix 7 from the included studies, which is presented in the Characteristics of included studies table.
Assessment of risk of bias in included studies
Two review authors (LOT and CRM) independently assessed the risk of bias of the included studies using the tools described in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019). We assessed selection bias (random sequence generation and allocation concealment); performance bias (blinding of participants and personnel); detection bias (blinding of outcome assessment); attrition bias (completeness of outcome data); reporting bias (selective reporting); and other bias, and summarised our judgements in the 'Risk of bias' table in Characteristics of included studies. Any disagreements were resolved by discussion. We incorporated the 'Risk of bias' assessment into the interpretation of review findings by means of sensitivity analyses.
Measures of treatment effect
For dichotomous data, we expressed the results for each study as risk ratios (RR) with 95% confidence intervals (CIs). For continuous data, we measured the mean post‐treatment intervention values and standard deviations for each group and calculated mean differences (MDs) with 95% CIs. If similar outcomes were reported using different scales, we calculated the standardised mean differences (SMDs) with 95% CIs.
Unit of analysis issues
We analysed the primary outcomes (live birth rate and OHSS), clinical pregnancy rate, and cycle cancellation rate outcomes per woman. We expressed miscarriage rate per woman as well as per pregnancy. Some of the included studies reported our primary outcomes using other units of analysis (e.g. per cycle, per embryo transfer). These data were not included in the review because they were not randomised comparisons, but applied only to selected subsets of participants, such as those who underwent repeated cycles or those who underwent embryo transfer.
We reported and pooled the review outcomes 'number of gonadotrophin units used' and 'number of days of gonadotrophin treatment', because all women underwent one treatment cycle. For studies that performed more than one cycle per woman, only the data from the first cycle were included in the meta‐analyses.
Dealing with missing data
To the greatest degree possible, we analysed data on an intention‐to‐treat (ITT) basis and made attempts to obtain missing data from the original trials. When this information was not available, we performed the analysis using the original number of women randomised.
Assessment of heterogeneity
We considered whether the clinical and methodological characteristics of the included studies were sufficiently similar for meta‐analysis to provide a clinically meaningful summary. We assessed statistical heterogeneity using the I2 statistic, interpreting I2 > 50% as being indicative of substantial heterogeneity amongst studies (Higgins 2019).
Assessment of reporting biases
In view of the difficulty of detecting and correcting for publication bias and other reporting biases, we aimed to minimise their potential impact by undertaking a comprehensive search for eligible studies and by paying attention to data duplication. We planned to use a funnel plot to explore the possibility of publication bias if sufficient studies (10 or more) were found for either of the primary outcomes.
Data synthesis
We combined data for meta‐analysis using Review Manager 5 to perform all the statistical analyses (Review Manager 2014).
We employed RR with 95% CI as the measure of effect for each dichotomous outcome using the Mantel‐Haenszel method, and reported continuous outcome differences between groups as MD with 95% CI. We used a random‐effects model in the analysis.
Subgroup analysis and investigation of heterogeneity
We performed a stratified meta‐analysis according to the type of stimulation protocol (long gonadotrophin‐releasing hormone (GnRH)‐agonist or short GnRH‐antagonist) for all outcomes that presented different types of stimulation protocol used. This stratification was added in the 2014 update of the review, to examine any possible difference in effect related to the type of stimulation.
We planned that if there was a clinically important difference in drug regimen (outside of normal clinical practice) amongst studies, we would examine the possible effects by performing subgroup analyses.
Sensitivity analysis
We conducted sensitivity analyses for the primary outcomes to determine whether the conclusions were robust to arbitrary decisions made regarding eligibility and analysis. We considered whether the review conclusions would have differed if:
eligibility was restricted to studies without high risk of bias (high risk of bias in sequence generation, allocation concealment, or blinding method, or any substantial methodological or clinical characteristic);
a fixed‐effect model had been adopted;
the summary effect measure had been odds ratio rather than risk ratio.
Summary of findings and assessment of the certainty of the evidence
We generated a 'Summary of findings' table using GRADEpro GDT software and Cochrane methods (GRADEpro GDT; Higgins 2019). This table evaluates the overall quality of the body of evidence for the main review outcomes (live birth rate, OHSS, clinical pregnancy rate, miscarriage, side effects) using GRADE criteria (study limitations, i.e. risk of bias, consistency of effect, imprecision, indirectness, and publication bias). Judgements regarding evidence quality (high, moderate, low, or very low) were justified, documented, and incorporated into the reporting of results for each outcome.
Results
Description of studies
See Characteristics of included studies and Characteristics of excluded studies.
Results of the search
The 2020 search retrieved 156 citations (up to 13 February 2020). We identified 13 additional records through other sources. After title and abstract screening, we selected 12 citations for full‐text reading, of which we excluded seven studies. One study is awaiting classification (Sun 2016). Thirteen studies matched the selection criteria and were included in the review (nine from the previous version plus four new studies) (see Figure 1 for details of the study selection process). There were five duplicate publications: Stadtmauer 1999 and Stadtmauer 2000, the latter being a continuation of the former; Visnova 2002 and Visnova 2003, one in English and the other in Czech; Kjotrod 2003a, Kjotrod 2004, and Kjotrod 2008a, all generated from the same trial; and Cheraghi 2013, Cheraghi 2014, and Cheraghi 2018, all generated from the same trial. In the 2014 version of the review, Tang 2010 was assessed as a study awaiting classification; the author answered and clarified that this study was part of another included study, Tang 2006.
1.

Study flow diagram.
We included four new studies in this 2020 review update (An 2014; Cheraghi 2018; Jacob 2016; Kim 2016). Nine studies were included in 2014. Therefore 13 studies in total met the inclusion criteria and were included in the review (An 2014; Cheraghi 2018; Doldi 2006; Fedorcsak 2003; Jacob 2016; Kim 2016; Kjotrod 2004; Kjotrod 2011; Onalan 2005; Palomba 2011; Qublan 2009; Tang 2006; Visnova 2003). We emailed the authors of the newly included studies to obtain more details on study characteristics and methodological quality that were unclear in the published article. Four author groups answered our queries (Cheraghi 2018; Fedorcsak 2003; Onalan 2005; Tang 2006). See Characteristics of included studies and Characteristics of excluded studies. All trials reported that only one cycle per participant was permitted, with the exception of Fedorcsak 2003 (a cross‐over trial).
Included studies
Study design and setting
We included 13 parallel‐design RCTs and one cross‐over trial in the review. A total of 1132 participants were randomised.
Ten studies were prospective, randomised, double‐blind, placebo‐controlled trials (metformin versus placebo) (An 2014; Cheraghi 2018; Jacob 2016; Kim 2016; Kjotrod 2004; Kjotrod 2011; Onalan 2005; Palomba 2011; Qublan 2009; Tang 2006).
One study was a prospective, open‐label, randomised, placebo‐controlled, cross‐over trial (Fedorcsak 2003). Only data from the pre‐cross‐over phase of this study were considered for meta‐analysis.
Two studies were prospective RCTs (metformin versus no treatment) (Doldi 2006; Visnova 2003).
Participants
All participants were women undergoing IVF or ICSI treatments. A total of 1132 women were randomised: 562 to placebo or no treatment, and 570 to metformin.
Baseline characteristics of the studied groups
Twelve studies met the Rotterdam criteria, ESHRE/ASRM 2003, for PCOS (An 2014; Cheraghi 2018; Doldi 2006; Fedorcsak 2003; Jacob 2016; Kim 2016; Kjotrod 2004; Kjotrod 2011; Onalan 2005; Palomba 2011; Qublan 2009; Tang 2006). One study did not meet the Rotterdam criteria because other causes for hyperandrogenism that mimic PCOS (such as congenital adrenal hyperplasia, Cushing's syndrome, or androgen‐secretin tumours) were not reported as excluded (Visnova 2003).
Seven studies did not report the causes of infertility (Cheraghi 2018; Doldi 2006; Fedorcsak 2003; Kim 2016; Palomba 2011; Tang 2006; Visnova 2003).
Nine studies provided full baseline characteristics of the women in both groups (age, body mass index (BMI), duration of infertility, previously used treatment) (An 2014; Jacob 2016; Kim 2016; Kjotrod 2004; Kjotrod 2011; Onalan 2005; Palomba 2011; Qublan 2009; Tang 2006). In two studies the baseline characteristics of women were incomplete (only age and BMI provided) (Fedorcsak 2003; Visnova 2003). Two studies did not report any baseline characteristics of participating women (Cheraghi 2018; Doldi 2006). Three studies did not report exclusion criteria (Cheraghi 2018; Doldi 2006; Kim 2016).
Interventions
Two studies started metformin on the day of ovarian stimulation with FSH (Jacob 2016; Visnova 2003). The remaining 11 studies used metformin before and during ovarian stimulation for IVF or ICSI treatment. Metformin commencement varied from 16 weeks before (earliest) to the first day (latest) of GnRH‐agonist administration in the studies reporting metformin use before FSH treatment and continued at least until the day of the hCG trigger.
Metformin was used 500 mg twice daily in three studies (Kim 2016; Kjotrod 2004; Visnova 2003); 850 mg twice daily in two studies (Jacob 2016; Tang 2006); and 500 mg three times daily in five studies (An 2014; Cheraghi 2018; Doldi 2006; Fedorcsak 2003; Palomba 2011). Onalan 2005 used metformin 850 mg twice daily (BMI < 28 kg/m2) or three times daily (BMI >= 28 kg/m2); Qublan 2009 used metformin 850 mg three times daily; and Kjotrod 2011 gradually increased the dose of metformin from 500 mg to 2 g per day during the first week of treatment.
Eight of the 13 included studies used long protocol GnRH‐agonist with recombinant FSH (rec‐FSH), whilst three studies used short protocol GnRH‐antagonist with rec‐FSH (Doldi 2006; Jacob 2016; Kim 2016). Only Visnova 2003 used either rec‐FSH or highly purified FSH (hp‐FSH), and only Qublan 2009 used HMG (hp‐ human menopausal gonadotrophin) in long protocol GnRH‐agonist ovarian stimulation protocol.
The method of oocyte fertilisation varied amongst the trials and included IVF alone (Doldi 2006), ICSI alone (Cheraghi 2018; Onalan 2005), or a combination of IVF and ICSI, depending on the cause of infertility (An 2014; Fedorcsak 2003; Jacob 2016; Kjotrod 2004; Kjotrod 2011; Qublan 2009; Palomba 2011; Tang 2006). Two trials did not report whether IVF or ICSI was performed (Kim 2016; Visnova 2003).
A maximum of two embryos were transferred on day two after oocyte retrieval by Tang 2006 and on day three by Fedorcsak 2003 and Kjotrod 2004. Jacob 2016 transferred up to two embryos, but on day three or day five (blastocyst stage). A maximum of three embryos were transferred on day two by Doldi 2006 and on day three by Onalan 2005. An 2014 and Kjotrod 2011 transferred up to two embryos on day two or three. Qublan 2009 transferred two to four embryos on day three. Cheraghi 2018 transferred a maximum of four embryos on day two or day three. Palomba 2011 transferred a maximum of two embryos on day two, three, or five (blastocyst stage). Kim 2016 and Visnova 2003 did not report the number of embryos transferred. Three studies reported performing embryo transfer under ultrasound guidance (Doldi 2006; Jacob 2016; Tang 2006).
The type of luteal phase support also varied amongst the trials and included vaginal progesterone capsules (Progestan 200 mg three times daily) (Kjotrod 2004), vaginal progesterone gel (Crinone 90 mg ‐ 8% daily) (Doldi 2006), vaginal progesterone pessaries (Cyclogest 400 mg daily) (Qublan 2009; Tang 2006), intramuscular progesterone (25 mg, 50 mg, or 100 mg daily) (Cheraghi 2018; Fedorcsak 2003; Palomba 2011), and progesterone, with type and dose selected by the physician (Kjotrod 2011). Three studies did not report what type of medication was used for luteal phase support (Kim 2016; Onalan 2005; Visnova 2003).
Onalan 2005 performed selective assisted hatching with laser when: the woman was over 35 years of age; the zona pellucida was considered thick; an abnormally shaped zona was present; or excessive embryo fragmentation or slowly developing embryos were noted. We considered this procedure to be substantially different from that used in the other trials.
Outcomes
Primary outcomes
7/13 studies reported live birth rate (per woman) (An 2014; Jacob 2016; Kjotrod 2004; Kjotrod 2011; Onalan 2005; Palomba 2011; Tang 2006).
11/13 studies reported OHSS (An 2014; Cheraghi 2018; Doldi 2006; Jacob 2016; Kjotrod 2004; Kjotrod 2011; Onalan 2005; Palomba 2011; Qublan 2009; Tang 2006; Visnova 2003).
The publication by Onalan 2005 did not provide the live birth rate; we obtained this information after contacting the author by email.
Secondary outcomes
12/13 studies reported clinical pregnancy rate (An 2014; Cheraghi 2018; Fedorcsak 2003; Jacob 2016; Kim 2016; Kjotrod 2004; Kjotrod 2011; Onalan 2005; Palomba 2011; Qublan 2009; Tang 2006; Visnova 2003).
8/13 studies reported miscarriage rate (An 2014; Fedorcsak 2003; Jacob 2016; Kjotrod 2004; Kjotrod 2011; Onalan 2005; Palomba 2011; Tang 2006). Miscarriage was defined as the involuntary loss of a pregnancy before 20 weeks gestation.
8/13 studies reported participant‐reported side effects (An 2014; Cheraghi 2018; Jacob 2016; Kim 2016; Kjotrod 2004; Kjotrod 2011; Onalan 2005; Tang 2006).
10/13 studies reported the number of oocytes retrieved, total dose of FSH per woman (An 2014; Doldi 2006; Fedorcsak 2003; Jacob 2016; Kjotrod 2004; Kjotrod 2011; Onalan 2005; Qublan 2009; Tang 2006; Visnova 2003).
9/13 studies reported the number of days of gonadotrophin treatment per woman (Doldi 2006; Fedorcsak 2003; Jacob 2016; Kjotrod 2004; Kjotrod 2011; Onalan 2005; Qublan 2009; Tang 2006; Visnova 2003).
Only Palomba 2011 did not report secondary outcomes, and we were unsuccessful in contacting the author.
8/13 studies reported cancellation rates (An 2014; Doldi 2006; Jacob 2016; Kjotrod 2004; Kjotrod 2011; Palomba 2011; Tang 2006; Visnova 2003).
6/13 studies reported serum oestradiol level on the day of hCG (An 2014; Doldi 2006; Kjotrod 2004; Onalan 2005; Qublan 2009; Visnova 2003).
3/13 studies reported fertilisation rate (An 2014; Jacob 2016; Tang 2006).
Of the four newly included studies (An 2014; Cheraghi 2018; Jacob 2016; Kim 2016), only An 2014 reported all the main clinical outcomes (live birth rate, incidence of OHSS, clinical pregnancy rate, miscarriage rate, and side effects). We contacted the authors by email to obtain more information about the outcomes not reported; however, only Cheraghi 2018 answered with clarification.
Excluded studies
We excluded 17 studies after full‐text review. The reasons for exclusion were as follows:
He 2019 (retrospective data analysis);
Abdalmageed 2019 (not an RCT);
Akbari 2010 (abstract of a congress oral presentation with data not available; we contacted the authors but received no response);
Demirol 2006 (not an RCT);
Egbase 2001 (data irregularities);
Geusa 2002 (data irregularities);
Ghasemi 2012 (women had a history of unexplained recurrent pregnancy loss and PCOS);
Immediata 2014 (different interventions);
Kahraman 2001 (control group treated with oral contraceptives rather than placebo or no treatment);
Palomba 2011a (women were poor responders);
Pourmatroud 2015 (different interventions);
Schachter 2007 (women specifically undergoing ICSI were not randomised separately);
Stadtmauer 1999 (women acted as their own control);
Stadtmauer 2001 (retrospective data analysis);
Stadtmauer 2002 (not an RCT);
Swanton 2011 (women with ovaries of polycystic morphology (PCO) on ultrasound were included, not PCOS women);
Tasdemir 2004 (women undergoing ovulation induction cycles, not IVF or ICSI cycles).
Risk of bias in included studies
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation
Eight trials reported acceptable methods of sequence generation and were judged as being at low risk of bias for this domain (An 2014; Fedorcsak 2003; Jacob 2016; Kjotrod 2004; Kjotrod 2011; Onalan 2005; Palomba 2011; Tang 2006). Six studies used computer randomisation (An 2014; Jacob 2016; Kjotrod 2004; Kjotrod 2011; Onalan 2005; Palomba 2011), and two a random numbers table (Fedorcsak 2003; Tang 2006). The other five studies did not report what methods were used for sequence generation and were classified as being at unclear risk of bias for this domain (Cheraghi 2018; Doldi 2006; Kim 2016; Qublan 2009; Visnova 2003).
Allocation concealment
Seven studies were at low risk of bias for allocation concealment because they used either sequentially numbered, sealed, opaque envelopes (Fedorcsak 2003), or codes kept by a third party such as the pharmacy department, Jacob 2016; Kjotrod 2004; Kjotrod 2011; Palomba 2011, or a trial office (An 2014; Tang 2006). Five studies did not report allocation concealment method used and were judged as having an unclear risk of bias (Cheraghi 2018; Doldi 2006; Onalan 2005; Qublan 2009; Visnova 2003). One study reported using sealed envelopes, but it was unclear whether or not they were opaque, even after contacting the authors via email, thus it was also classified as having an unclear risk of bias (Kim 2016).
Blinding
We did not consider that blinding was likely to influence findings for the main clinical review outcomes (live birth rate, clinical pregnancy rate, and incidence of OHSS). However, blinding status could potentially affect findings for side effects.
For performance bias:
Eight studies reported double‐blinding and were classified as being at low risk of bias for this domain (An 2014; Jacob 2016; Kim 2016; Kjotrod 2004; Kjotrod 2011; Onalan 2005; Palomba 2011; Tang 2006). Three studies were open‐label comparisons (Doldi 2006; Fedorcsak 2003; Visnova 2003), and one was single‐blind (Qublan 2009), and were therefore classified as being at high risk of bias. One study was described as double‐blind (Cheraghi 2018); however, the blinding method used was not reported, therefore we judged this study to be at unclear risk of performance bias.
For detection bias:
Only one study reported blinding of outcome assessment (An 2014), and was classified as being at low risk of bias for this domain. Eight studies did not report blinding of assessors and were therefore assessed as being at unclear risk of detection bias (Cheraghi 2018; Jacob 2016; Kim 2016; Kjotrod 2004; Kjotrod 2011; Onalan 2005; Palomba 2011; Tang 2006). Three studies were open‐label comparisons (Doldi 2006; Fedorcsak 2003; Visnova 2003), and one was single‐blind (Qublan 2009); we classified these studies as being at high risk of detection bias.
Incomplete outcome data
We judged 10 studies to be at low risk of bias because they analysed their data on an ITT basis (trial participants were analysed in the groups to which they had been randomised; all participants were included as there were no withdrawals) (An 2014; Cheraghi 2018; Fedorcsak 2003; Jacob 2016; Kjotrod 2004; Kjotrod 2011; Palomba 2011; Qublan 2009; Tang 2006; Visnova 2003).
One study did not report the reasons for withdrawals and was judged to be at unclear risk of bias for this domain (Doldi 2006).
One study did not perform ITT analysis and was therefore classified being at unclear risk of attrition bias (Kim 2016).
One study conducted available‐case analyses (trial participants were analysed in the groups to which they had been randomised, and only participants who completed the trials were included) and was judged to be at high risk of bias for this domain (Onalan 2005).
Selective reporting
Seven studies reported live birth and OHSS rates (the primary outcomes of this review) and were therefore classified as being at low risk of bias (An 2014; Jacob 2016; Kjotrod 2004; Kjotrod 2011; Onalan 2005; Palomba 2011; Tang 2006).
We judged five studies to be at unclear risk for reporting bias because they failed to report at least one of the following outcomes: live birth or OHSS rates (the primary outcomes of this review) (Doldi 2006; Fedorcsak 2003; Kim 2016; Qublan 2009; Visnova 2003).
Cheraghi 2018 did not report either of the primary outcomes of this review and was classified as being at high risk of bias.
Other potential sources of bias
Four studies did not report the causes of infertility and were thus deemed as at unclear risk of other bias (Doldi 2006; Fedorcsak 2003; Kim 2016; Visnova 2003).
We rated Palomba 2011 as being at high risk of bias for this domain due to a data discrepancy. We attempted to contact the authors for clarification, without success.
We assessed the remaining eight studies as being at low risk of other bias (An 2014; Cheraghi 2018; Jacob 2016; Kjotrod 2004; Kjotrod 2011; Onalan 2005; Qublan 2009; Tang 2006).
Effects of interventions
See: Table 1
1. Comparison of metformin versus placebo or no treatment
Primary outcomes
1.1 Live birth rate (per woman)
Seven studies reported live birth (An 2014; Jacob 2016; Kjotrod 2004; Kjotrod 2011; Onalan 2005; Palomba 2011; Tang 2006). We detected substantial heterogeneity (I2 = 77%; P < 0.001), which may be explained by the difference in effect of the interventions between the study protocol subgroups (test for subgroup difference: P = 0.001, I2 = 90.5%). We therefore analysed the results per study protocol subgroup, that is studies that used a long protocol GnRH‐agonist and those using a short protocol GnRH‐antagonist.
Six studies used a long protocol GnRH‐agonist (An 2014; Kjotrod 2004; Kjotrod 2011; Onalan 2005; Palomba 2011; Tang 2006). Pooled evidence from these six studies showed that we are uncertain of the effect of metformin on live birth rate per woman when compared with placebo/no treatment (risk ratio (RR) 1.30, 95% confidence interval (CI) 0.94 to 1.79; 6 RCTs; 651 women; I2 = 47%; low‐quality evidence; Analysis 1.1; Figure 4). We estimated that for a woman with a 28% chance of achieving a live birth using placebo or no treatment, the chance using metformin would be between 27% and 51%.
4.

Forest plot of comparison: 1 Metformin versus placebo or no treatment, outcome: 1.1 Live birth rate per woman.
We conducted a post hoc sensitivity analysis due to a data discrepancy (suspected risk of bias) in one of the studies (Palomba 2011). According to the study publication, in both the metformin group and placebo/no treatment group the clinical pregnancy rate (26/60 and 24/60) was lower than the live birth rate (29/60 and 27/60). Our attempts to contact the first author have to date received no response. Sensitivity analysis excluding this study yielded an RR of 1.38 (95% CI 0.91 to 2.10; I2 = 52%) for live birth, which did not substantially change our findings (Figure 5).
5.

Sensitivity analysis by excluding Palomba 2011 due to a data discrepancy (suspected risk of bias).
Only one study used a short protocol GnRH‐antagonist and reported live birth rate (Jacob 2016). Metformin may reduce live birth rate compared with placebo/no treatment (RR 0.48, 95% CI 0.29 to 0.79; 1 RCT; 153 women; low‐quality evidence; Analysis 1.1). This suggests that if the chance for live birth following placebo/no treatment is 43%, the chance following metformin would be between 13% and 34%.
1.1. Analysis.

Comparison 1: Metformin versus placebo or no treatment, Outcome 1: Live birth rate per woman
We also performed sensitivity analysis using a fixed‐effect model and observed a difference in results for this outcome when a long protocol GnRH agonist was used (RR 1.30, 95% CI 1.04 to 1.62; 6 RCTs; 651 women; I2 = 47%) which could be due to the heterogeneity observed. It is known that when heterogeneity is present, CIs are wider when a random‐effects method is used rather than a fixed‐effect method, and the estimated effect is more conservative (McKenzie 2020).
Sensitivity analysis using odds ratio rather than RR did not lead to a difference in results.
1.2 Incidence of OHSS
When we combined data from 11 studies (An 2014; Cheraghi 2018; Doldi 2006; Jacob 2016; Kjotrod 2004; Kjotrod 2011; Onalan 2005; Palomba 2011; Qublan 2009; Tang 2006; Visnova 2003), we found that metformin may reduce the incidence of OHSS when compared with placebo/no treatment (RR 0.46, 95% CI 0.29 to 0.72; 11 RCTs; 1091 women; I2 = 38%; low‐quality evidence; Analysis 1.2; Figure 6). This suggests that for a woman with a 20% risk of OHSS without metformin, the corresponding risk using metformin would be between 6% and 14%. Sensitivity analysis including only studies with a low risk of bias resulted in an RR of 0.48 (95% CI 0.25 to 0.91; 6 RCTs; 696 women; I2 = 51%; Figure 7) (An 2014; Jacob 2016; Kjotrod 2004; Kjotrod 2011; Palomba 2011; Tang 2006), which did not influence the findings.
1.2. Analysis.

Comparison 1: Metformin versus placebo or no treatment, Outcome 2: Incidence of OHSS per woman
6.

Forest plot of comparison: 1 Metformin versus placebo or no treatment, outcome: 1.2 Incidence of OHSS per woman.
7.
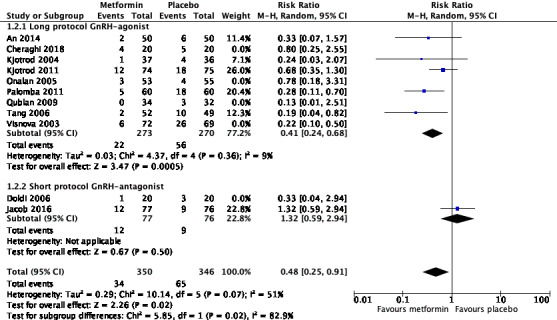
Sensitivity analysis including only studies with low risk of bias (An 2014; Jacob 2016; Kjotrod 2004; Kjotrod 2011; Palomba 2011; Tang 2006).
The funnel plot showed asymmetry, which may have reflected publication bias or smaller studies of lower methodological quality, hence producing a large intervention effect estimate (i.e. Doldi 2006; Qublan 2009), or chance (Figure 8). Sensitivity analysis adopting a fixed‐effect model and summary effect measure risk ratio did not lead to a change in the results.
8.

Funnel plot of comparison: 1 Metformin versus placebo or no treatment, outcome: 1.2 Incidence of OHSS per woman.
Secondary outcomes
1.3 Clinical pregnancy rate (per woman)
We included data from 12 studies for this outcome (An 2014; Cheraghi 2018; Fedorcsak 2003; Jacob 2016; Kim 2016; Kjotrod 2004; Kjotrod 2011; Onalan 2005; Palomba 2011; Qublan 2009; Tang 2006; Visnova 2003). The data were pooled only within the study protocol subgroups due to substantial heterogeneity observed (I2 = 57%; P = 0.008), although the detected heterogeneity was not explained by the difference in effect of the interventions between the study protocol subgroups (test for subgroup difference: P = 0.97, I2 = 0%). This outcome was analysed according to the two subgroups, that is studies using a long protocol GnRH‐agonist and those using a short protocol GnRH‐antagonist.
Using a long protocol GnRH‐agonist (An 2014; Cheraghi 2018; Fedorcsak 2003; Kjotrod 2004; Kjotrod 2011; Onalan 2005; Palomba 2011; Qublan 2009; Tang 2006; Visnova 2003), metformin may increase the clinical pregnancy rate per woman compared with placebo/no treatment (RR 1.32, 95% CI 1.08 to 1.63; 10 studies; 915 women; I2 = 13%; low‐quality evidence; Analysis 1.3; Figure 9). This suggests that for a woman with a 28% chance of achieving a clinical pregnancy using a long protocol GnRH‐agonist with placebo/no treatment, the chance with metformin would be between 30% and 45%.
1.3. Analysis.

Comparison 1: Metformin versus placebo or no treatment, Outcome 3: Clinical pregnancy rate per woman
9.

Forest plot of comparison: 1 Metformin versus placebo or no treatment, outcome: 1.3 Clinical pregnancy rate per woman.
As noted above, we conducted a post hoc sensitivity analysis due to a data discrepancy in one of the studies (Palomba 2011). Sensitivity analysis excluding this study yielded an RR of 1.39 (95% CI 1.10 to 1.76; I2 = 15%) for pregnancy, which did not substantially change our findings.
Using a short protocol GnRH‐antagonist (Jacob 2016; Kim 2016), we are uncertain of the effect of metformin on clinical pregnancy rate per woman compared with placebo/no treatment (RR 1.38, 95% CI 0.21 to 9.14; 2 studies; 177 women; I2 = 87%; very low‐quality evidence; Analysis 1.3). Most of the heterogeneity appeared to be due to Jacob 2016, as this was the only study in which clinical pregnancy rates were higher in the placebo/no treatment group compared with the metformin group.
The funnel plot showed asymmetry, which in this case may be due to publication bias or smaller studies of lower methodological quality, hence producing a larger intervention effect estimate (i.e. Cheraghi 2018; Kim 2016; Qublan 2009), or chance (Figure 10).
10.
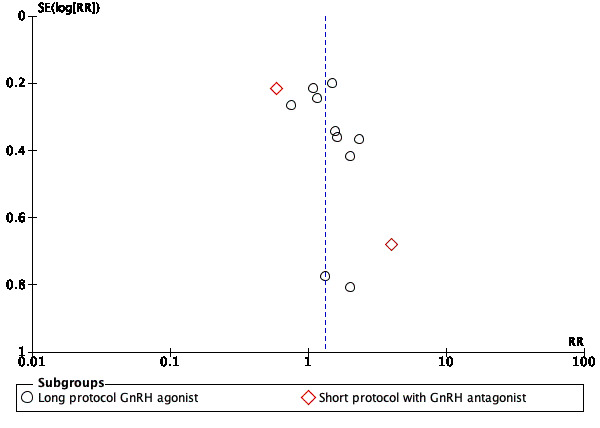
Funnel plot of comparison: 1 Metformin versus placebo or no treatment, outcome: 1.3 Clinical pregnancy rate per woman.
1.4 Miscarriage rate (per woman)
We included eight studies in this analysis (An 2014; Fedorcsak 2003; Jacob 2016; Kjotrod 2004; Kjotrod 2011; Onalan 2005; Palomba 2011; Tang 2006). We are uncertain of the effect of metformin on miscarriage rate per woman when compared with placebo/no treatment (RR 0.86, 95% CI 0.56 to 1.32; 8 RCTs; 821 women; I2 = 0%; low‐quality evidence; Analysis 1.4). This suggests that if the risk of miscarriage with placebo/no treatment is 11%, the risk with metformin would be between 6% and 14%.
1.4. Analysis.

Comparison 1: Metformin versus placebo or no treatment, Outcome 4: Miscarriage rate per woman
1.5 Miscarriage rate (per pregnancy)
We included eight studies in this analysis (An 2014; Fedorcsak 2003; Jacob 2016; Kjotrod 2004; Kjotrod 2011; Onalan 2005; Palomba 2011; Tang 2006). We are uncertain of the effect of metformin on miscarriage rate per pregnant woman when compared with placebo/no treatment (RR 0.81, 95% CI 0.51 to 1.31; 8 RCTs; 312 women; I2 = 23%; low‐quality evidence; Analysis 1.5).
1.5. Analysis.

Comparison 1: Metformin versus placebo or no treatment, Outcome 5: Miscarriage rate per pregnant woman
1.6 Incidence of participant‐reported side effects
Eight studies reported this outcome (An 2014; Cheraghi 2018; Jacob 2016; Kim 2016; Kjotrod 2004; Kjotrod 2011; Onalan 2005; Tang 2006).
Metformin (116/375, 30.9%) may result in an increase in side effects compared with placebo/no treatment (33/373, 8.8%) (RR 3.35, 95% CI 2.34 to 4.79; 8 RCTs; 748 women; I2 = 0%; low‐quality evidence; Analysis 1.6). This suggests that if the risk of side effects with placebo/no treatment is 9%, the risk with metformin would be between 21% and 42%.
1.6. Analysis.

Comparison 1: Metformin versus placebo or no treatment, Outcome 6: Side effects per woman
Kjotrod 2004 and Kjotrod 2011 reported that the most frequent side effects associated with metformin were gastrointestinal and included nausea, vomiting, diarrhoea, abdominal discomfort or pain.
1.7 Number of oocytes retrieved per woman
We included 11 studies in this analysis (An 2014; Cheraghi 2018; Doldi 2006; Fedorcsak 2003; Jacob 2016; Kjotrod 2004; Kjotrod 2011; Onalan 2005; Qublan 2009; Tang 2006; Visnova 2003). The mean number of oocytes retrieved per woman did not differ between groups (mean difference (MD) 0.03, 95% CI −1.42 to 1.48; 11 RCTs; 890 women; I2 = 56%; Analysis 1.7).
1.7. Analysis.

Comparison 1: Metformin versus placebo or no treatment, Outcome 7: Number of oocytes retrieved per woman
The 11 studies were subdivided into two subgroups: those using a long protocol GnRH‐agonist and those using short protocol GnRH‐antagonist. Doldi 2006 and Jacob 2016 used a short protocol GnRH‐antagonist. There was no difference between the results of the two subgroups, and only one individual trial, Qublan 2009, demonstrated a significant difference in the number of oocytes collected between the two treatment groups, with fewer oocytes collected in the metformin group.
1.8 Total dose of FSH (IU) per woman
We included 10 studies in this analysis (An 2014; Doldi 2006; Fedorcsak 2003; Jacob 2016; Kjotrod 2004; Kjotrod 2011; Onalan 2005; Qublan 2009; Tang 2006; Visnova 2003). Due to extreme heterogeneity (I2 = 96%), we did not pool data (Analysis 1.8). Most of the heterogeneity appeared to be due to Qublan 2009; exclusion of this study reduced heterogeneity to I2 = 43%. However, we did not identify any clear methodological difference between this study and the others. Eight of 10 studies found no evidence of a difference between groups (An 2014; Doldi 2006; Fedorcsak 2003; Jacob 2016; Kjotrod 2004; Kjotrod 2011; Onalan 2005; Tang 2006).
1.8. Analysis.

Comparison 1: Metformin versus placebo or no treatment, Outcome 8: Mean total dose of FSH (IU) per woman
1.9 Mean number of days of gonadotrophin treatment
Nine studies reported this outcome (Doldi 2006; Fedorcsak 2003; Jacob 2016; Kjotrod 2004; Kjotrod 2011; Onalan 2005; Qublan 2009; Tang 2006; Visnova 2003). The mean number of days of gonadotrophin treatment did not differ significantly between groups (MD −0.17 days, 95% CI −0.71 to 0.37; 9 studies; 796 women; I2 = 49%; Analysis 1.9). There was statistical heterogeneity in this comparison (I2 = 49%), which disappeared (I2 = 0%) when we excluded Qublan 2009, but we could not identify any clear methodological difference between this study and the others.
1.9. Analysis.

Comparison 1: Metformin versus placebo or no treatment, Outcome 9: Mean days of gonadotrophin per woman
We also analysed this outcome by subdividing studies into two subgroups: those using a long protocol GnRH‐agonist and those using short protocol GnRH‐antagonist. Seven studies used long protocol GnRH‐agonist (Fedorcsak 2003; Kjotrod 2004; Kjotrod 2011; Onalan 2005; Qublan 2009; Tang 2006; Visnova 2003), and there were no statistically significant differences between the groups (MD −0.22 days, 95% CI −0.89 to 0.45; 7 RCTs; 603 women; I2 = 62%). Two studies used short protocol GnRH‐antagonist and found no evidence of a difference between groups (MD 0.00 days, 95% CI −1.04 to 1.04; 2 RCTs; 193 women; I2 = 0%) (Doldi 2006; Jacob 2016).
1.10 Cycle cancellation rate
We included eight studies in this analysis (An 2014; Doldi 2006; Jacob 2016; Kjotrod 2004; Kjotrod 2011; Palomba 2011; Tang 2006; Visnova 2003). Due to wide CI crossing the line of no effect, we could not determine if there is a difference between the metformin group (58/442, 13.1%) and the placebo/no treatment group (69/435, 15.8%) in cancellation rates (RR 0.74, 95% CI 0.43 to 1.29; 8 RCTs; 877 women; I2 = 46%; Analysis 1.10).
1.10. Analysis.

Comparison 1: Metformin versus placebo or no treatment, Outcome 10: Cycle cancellation rate (after ovulation induction)
We also analysed this outcome according to subgroups, that is long protocol GnRH‐agonist, An 2014; Kjotrod 2004; Kjotrod 2011; Palomba 2011; Tang 2006; Visnova 2003, and short protocol GnRH‐antagonist, Doldi 2006; Jacob 2016, finding no substantial heterogeneity between them (I2 = 0%; P = 0.56).
1.11 Serum oestradiol level (on the day of hCG): mean level per woman
Six studies reported this outcome (An 2014; Doldi 2006; Kjotrod 2004; Onalan 2005; Qublan 2009; Visnova 2003). Due to very high heterogeneity (I2 = 89%) that could not be explained, we did not pool the studies. Three of the six studies reported lower serum oestradiol levels in the metformin group, whilst the other three studies found no evidence of a difference between groups (Analysis 1.11).
1.11. Analysis.

Comparison 1: Metformin versus placebo or no treatment, Outcome 11: Serum oestradiol level (nmol/L) per woman
Tang 2006 reported serum oestradiol levels using multiple linear regression analysis. After adjustment for the total FSH dose and the number of follicles, metformin treatment reduced oestradiol concentration on the day of hCG administration (coefficient = −35.6, P = 0.048).
1.12 Serum androgen levels (testosterone, SHBG, free‐androgen index)
Onalan 2005 and Tang 2006 reported serum androgen levels on the day of hCG. We were unable to pool these data because they were reported as median and range by Onalan 2005 and as geometric measures by Tang 2006.
Onalan 2005 found no difference in total testosterone between the metformin group (median 3.1, range 2.5 to 3.9) and the placebo/no treatment group (median 3.1, range 2.4 to 3.9, P = 0.646). Tang 2006 reported that whilst testosterone levels did not change in the metformin group (baseline geometric mean: 2.03 nmol/L, geometric mean on the day of hCG administration: 1.97 nmol/L; P = 0.892), the placebo/no treatment group had an increase in testosterone levels (baseline geometric mean: 2.06 nmol/L, geometric mean on the day of hCG administration: 2.52 nmol/L; P = 0.040). In the metformin group, on the day of hCG administration there was a decrease in testosterone concentration (geometric mean: 1.96 versus 2.52 nmol/L; P = 0.029) and in the free‐androgen index (geometric mean: 2.43 versus 3.34; P = 0.004). See Analysis 1.12.
1.12. Analysis.
Comparison 1: Metformin versus placebo or no treatment, Outcome 12: Mean or median serum androgen levels per woman
| Mean or median serum androgen levels per woman | |||
| Study | Results | Metformin | Placebo |
| Onalan 2005 | No significant differences in total testosterone measures from women treated with placebo (P = 0.646). | Median 3.1; range 2.5 to 3.9 | Median 3.1; range 2.4 to 3 |
| Tang 2006 | Testosterone levels did not change significantly in the group taking metformin (P = 0.892); however, participants in the placebo group had a significant increase in testosterone levels (P = 0.040). In the metformin group, on the day of hCG administration, there was a significant decrease in testosterone concentration (P = 0.029) and in the free‐androgen index (P = 0.004). | Baseline geometric mean: 2.03 nmol/l, geometric mean on the day of hCG administration: 1.97 nmol/l. Testosterone concentration (geometric mean: 1.96 nmol/l). Free‐androgen index (geometric mean: 2.43) | Baseline geometric mean: 2.06 nmol/l, geometric mean on the day of hCG administration: 2.52 nmol/l. Testosterone concentration (geometric mean: 2.52 nmol/l). Free‐androgen index (geometric mean: 3.34) |
1.13 Fasting insulin and glucose levels
Onalan 2005 and Tang 2006 reported fasting insulin and glucose levels on the day of hCG. Data could not be pooled because they were reported as glucose/insulin ratio (median and range) by Onalan 2005 and as Quantitative Insulin Sensitivity Check Index (QUICKI) by Tang 2006.
Onalan 2005 found no difference in the glucose/insulin ratio between the metformin group (median 6; range 2.4 to 8.8) and the placebo/no treatment group (median 6; range 3 to 10, P = 0.81). Tang 2006 found no difference in the insulin sensitivity test results (QUICKI) between baseline and the day of oocyte retrieval in either the metformin group (baseline 0.377 and 0.417 at the day of oocyte retrieval (P = 0.2)) or the placebo/no treatment group (baseline 0.386 and 0.400 at the day of oocyte retrieval (P = 0.572)). See Analysis 1.13.
1.13. Analysis.
Comparison 1: Metformin versus placebo or no treatment, Outcome 13: Mean or median fasting insulin and glucose levels per woman
| Mean or median fasting insulin and glucose levels per woman | |||
| Study | Results | Metformin | Placebo |
| Onalan 2005 | There were no significant changes in the glucose/insulin ratio between groups (P = 0.81). | Median 6; range 2.4 to 8.8 | Median 6; range 3 to 10 |
| Tang 2006 | There were no significant changes in the insulin sensitivity test (QUICKI) between baseline and the day of oocyte retrieval in the metformin group (P = 0.200) and the placebo group (P = 0.572). | Baseline: 0.377 At the day of oocyte retrieval: 0.417 |
Baseline: 0.386 At the day of oocyte retrieval: 0.400 |
1.14 Fertilisation rate
Four studies reported fertilisation rate with two pronuclei‐stage embryos, but in different ways (An 2014; Cheraghi 2018; Jacob 2016; Tang 2006). We therefore pooled only An 2014 and Jacob 2016. The mean fertilisation rate did not differ between groups (MD −4.78, 95% CI −12.21 to 2.65; 2 RCTs; 225 women; I2 = 19%; Analysis 1.14).
1.14. Analysis.

Comparison 1: Metformin versus placebo or no treatment, Outcome 14: Fertilisation rate
An 2014 (metformin: 57.5 ± 25.9; placebo: 56.8 ± 26.3) and Jacob 2016 (metformin: 53.3 ± 25.4; placebo: 60.5 ± 22.2) reported fertilisation rate as percentage of fertilisation.
Cheraghi 2018 reported fertilisation rate as the number of oocytes fertilised (metformin: 9.2 ± 4.5; placebo: 5.6 ± 2.2).
Tang 2006 reported fertilisation rate per oocyte retrieved. Metformin did not improve the overall fertilisation rate (52.9% versus 54.9%, P = 0.641) (data not shown).
Discussion
Summary of main results
Reproductive outcomes
Due to substantial heterogeneity, we did not perform meta‐analysis on the overall (both ovarian stimulation protocols combined) data for the effect of metformin on the outcomes of live birth and clinical pregnancy rates per woman in women with PCOS undergoing IVF/ICSI treatment.
With the use of the long protocol GnRH‐agonist ovarian stimulation, we are uncertain of the effect of metformin on live birth rate per woman when compared with placebo/no treatment (Analysis 1.1), but metformin may increase the clinical pregnancy rate per woman (Analysis 1.3).
Using the short protocol GnRH‐antagonist ovarian stimulation, metformin may reduce live birth rate compared with placebo/no treatment (Analysis 1.1), and we are uncertain of the effect of metformin on clinical pregnancy rate per woman (Analysis 1.3). The authors of the one RCT assessing the effect of metformin on live birth rate stated that the finding of a reduced live birth rate with metformin should be viewed with caution, as this outcome was not the primary outcome for the study (i.e. the RCT was not powered for this outcome) and may represent a purely chance outcome.
Overall, metformin may reduce the incidence of OHSS when compared with placebo/no treatment; this finding was demonstrated in the long protocol GnRH‐agonist ovarian stimulation protocol but not the short GnRH‐antagonist ovarian stimulation protocol, for which we are uncertain of the effect of metformin on OHSS (Analysis 1.2). We are uncertain of the effect of metformin on miscarriage rates per woman and per pregnancy when compared with placebo/no treatment.
Metformin reduced the risk of OHSS by approximately 55%, but increased the risk of side effects three‐fold. Although the reason metformin reduces the risk of OHSS is not clear, it has been hypothesised that since it decreases hyperinsulinaemia, it could also reduce the production of vascular endothelial growth factor (VEGF), one of the most important factors involved in the pathophysiology of the syndrome. In addition, metformin is associated with a statistically significant effect on oestradiol levels, an important risk factor for OHSS.
Metformin co‐treatment appeared to decrease serum oestradiol levels on the day of hCG (Analysis 1.11), but there was no evidence that it had an effect on other ovarian stimulation parameters (number of oocytes retrieved, total dose of gonadotrophin, number of days of gonadotrophin stimulation, and cycle cancellation rate) or embryological outcomes (fertilisation rate) compared with placebo/no treatment (Analysis 1.7; Analysis 1.8; Analysis 1.9; Analysis 1.14).
Side effects
Metformin may result in an increase in side effects (mostly gastrointestinal) compared with placebo/no treatment (Analysis 1.6).
Biochemical outcomes
This review included studies that specifically reported reproductive outcomes in IVF/ICSI treatments where women had taken metformin in an attempt to improve reproductive profiles and conceive. We excluded studies that compared metformin with placebo or no treatment to improve metabolic or biochemical outcomes only, without attempting to conceive. We therefore cannot provide a robust analysis of the impact of metformin compared with placebo or no treatment on biochemical outcomes (serum oestradiol, androgen, and insulin levels) (Analysis 1.11; Analysis 1.12; Analysis 1.13).
Overall completeness and applicability of evidence
The increased number of studies included in this updated review has improved the statistical power and external validity of our findings. Five of the 13 trials performed a priori sample size calculations to assess their primary outcome measures. A total of 1132 participants under 40 years of age were included. Eleven of the 13 included studies met the Rotterdam consensus criteria, ESHRE/ASRM 2003, for the diagnosis of PCOS and excluded other causes of hyperandrogenism. The only study that did not meet the Rotterdam consensus criteria may have included women with other causes of hyperandrogenism that mimic PCOS (such as congenital adrenal hyperplasia, Cushing's syndrome, and androgen‐secreting tumours) (Visnova 2003). None of the 13 trials reported previous ovarian surgery in their baseline characteristics, and five trials did not report the cause of infertility. Twelve of the 13 trials included in this review provided data on clinical pregnancy. However, only seven trials reported live birth, and no trials reported the rate of healthy take‐home baby, which is considered by consumers to be the most important long‐term outcome of interest. The primary endpoints of the three trials (Doldi 2006; Tang 2006; Visnova 2003) were either not clearly reported or were related to ovarian response parameters.
We observed significant heterogeneity in many of the analyses. This could have been due to many factors, such as: dose and duration of metformin, different protocols of ovarian stimulation, and number of embryos transferred. However, the heterogeneity remained unchanged, even after sensitivity analysis.
This review confirms the findings of the previous review that there is a large reduction in incidence of OHSS and a potential benefit of using metformin in long protocol GnRH‐agonist in IVF/ICSI treatments when compared with placebo or no treatment. These positive findings were not observed when metformin was used in short protocol GnRH‐antagonist. The type of ovarian stimulation protocol used in different studies, that is long protocol GnRH‐agonist or short protocol GnRH‐antagonist, is an important factor that must be discussed. Jacob 2016 was included in this updated version, and this study contributed some interesting findings and raised questions that must be considered in the interpretation of data and conclusions regarding the use of metformin (especially for a short period of time) with GnRH‐antagonist. One point of discussion is the different effects of metformin co‐treatment in long protocol GnRH‐agonist and in short protocol GnRH‐antagonist. Regardless of metformin effect, it is well known that OHSS incidence in women receiving GnRH‐antagonist is significantly lower compared with the GnRH‐agonist, although there are no differences in live birth rates and clinical pregnancy rates for the two protocols (Al‐Inany 2011; Griesinger 2006). Jacob 2016 is the largest randomised, placebo‐controlled trial to study the adjuvant effect of metformin on the risk of OHSS (the study was powered to assess this outcome) using GnRH‐antagonist in IVF cycles. Surprisingly, unlike the tendency of reduction in OHSS found by Doldi 2006 (another study that used short protocol GnRH‐antagonist), metformin had no impact on the incidence of this complication in Jacob 2016. It is uncertain why this difference occurred. One possible reason is the duration of metformin pretreatment: whilst Doldi 2006 used 1.5 g/day for two months, Jacob 2016 chose a different approach, using a short regimen of 1.7 g/day for only 16 days. On the other hand, Visnova 2003 used the shortest course of metformin, but in a long protocol GnRH‐agonist, and despite that showed a significant reduction in OHSS rate. There is a good pharmacological rationale for believing that different doses and durations of metformin co‐treatment as well as different types of ovarian stimulation protocol (different doses of gonadotrophin and different types of pituitary GnRH receptor inhibitors) will impact the outcomes differently.
Based on our findings, routine co‐administration of metformin to PCOS women undergoing IVF/ICSI treatments may offer benefits only in cycles in which long protocol GnRH‐agonist was used in terms of increasing clinical pregnancy rate and, especially, reducing the incidence of OHSS. Few data are available regarding the effect of metformin in IVF/ICSI cycles using short protocol GnRH‐antagonist, and the results are contradictory.
Quality of the evidence
See Table 1.
We classified the quality of evidence as low for the main outcomes live birth rate (long and short protocols) and incidence of OHSS; low for the secondary outcomes clinical pregnancy rate (long protocol GnRH‐agonist), miscarriage rate, and side effects; and very low for the secondary outcome clinical pregnancy rate (short protocol GnRH‐antagonist).
Overall, we graded only one study, An 2014, out of the 13 included studies as having low risk of bias for sequence generation, allocation concealment, and blinding (performance and detection bias). See Figure 2 and Figure 3 for the 'Risk of bias' graph and summary. The main limitations of the comparisons in this review are risk of bias and imprecision. We conducted a post hoc sensitivity analysis for our primary outcomes after noting a data discrepancy in one of the studies (Palomba 2011), and another sensitivity analysis including only studies with low risk of bias. However, both sensitivity analyses on the studies did not substantially change our findings.
Heterogeneity was low or moderate for the primary clinical outcomes. However, due to substantial heterogeneity between both subgroups (long and short protocols), the data for live birth and clinical pregnancy rates were pooled only according to the type of ovarian stimulation protocol. Besides that, the data for two of the laboratory outcomes (FSH dose and serum oestradiol level) were not pooled due to very high levels of unexplained heterogeneity.
We observed extreme heterogeneity (I2 = 72%; P = 0.002) when Jacob 2016 was included in the live birth rate analysis. One plausible reason (amongst others) for this heterogeneity is the difference between the short and long GnRH protocol adopted. Most of the heterogeneity both in live birth rate and in clinical pregnancy rate seemed to be due to Jacob 2016 which was the only study in which live birth rate and clinical pregnancy rate were higher in the placebo/no treatment group than in the metformin group. This study was powered to assess the ability of metformin to reduce OHSS as an adjunct in an IVF cycle. Consequently, Jacob 2016 was not powered to assess live birth or clinical pregnancy rates, which is another possible explanation for these inconsistencies.
Potential biases in the review process
A limitation of this review is the lack of full data from some studies, despite our attempts to obtain missing information from study authors. Whenever possible, we performed analyses based on intention‐to‐treat, to minimise bias.
We conducted a sensitive search and are confident that we have included in the review all existing randomised trials assessing the use of metformin in PCOS women undergoing ART cycles and which reported clinically relevant outcomes (live birth rate, clinical pregnancy rate, and incidence of OHSS).
Agreements and disagreements with other studies or reviews
We are unaware of any systematic reviews evaluating the effect of metformin in women with PCOS undergoing IVF/ICSI treatment since the previous version of this review was published in 2014. The first evidence‐based international guidelines on PCOS were recently published (Teede 2018). These guidelines assessed the role of adjuvant metformin for women with PCOS undergoing IVF/ICSI treatment and made a conditional recommendation based on low‐quality evidence that stated: "Adjunct metformin therapy could be used before and/or during follicle stimulating hormone ovarian stimulation in women with PCOS undergoing a IVF ± ICSI therapy with a GnRH agonist protocol, to improve the clinical pregnancy rate and reduce the risk of OHSS". Our findings that the use of metformin in the long GnRH‐agonist ovarian stimulation protocol may increase the clinical pregnancy rate and may reduce the rate of OHSS would support this conditional recommendation. In addition, the guidelines made a clinical practice point that in IVF ± ICSI cycles, women with PCOS could be counselled on the potential benefits of adjunct metformin in a GnRH‐antagonist protocol to reduce risk of OHSS. Clinical practice points are made where evidence was not sought (i.e. are not evidence based) and are established where important clinical issues arose from discussion of evidence‐based or clinical consensus recommendations. In this review we are uncertain of the effect of metformin on the risk of OHSS for women with PCOS undergoing IVF/ICSI treatment. As such, this would still be consistent with the guideline clinical practice point of discussion of the potential, although uncertain, benefits of adjunct metformin in PCOS women undergoing IVF ± ICSI treatment with the GnRH‐antagonist protocol.
Authors' conclusions
Implications for practice.
This updated review on metformin versus placebo/no treatment before and during in vitro fertilisation/intracytoplasmic sperm injection (IVF/ICSI) treatment in women with polycystic ovary syndrome (PCOS) found no conclusive evidence that metformin improves live birth rates. In a long gonadotrophin‐releasing hormone (GnRH)‐agonist protocol, we are uncertain whether metformin improves live birth rates, but metformin may increase clinical pregnancy rate. In a short GnRH‐antagonist protocol, metformin may reduce live birth rates, although we are uncertain of the effect of metformin on clinical pregnancy rate. Metformin may reduce the incidence of ovarian hyperstimulation syndrome (OHSS), but may result in a higher incidence of side effects. We are uncertain of the effect of metformin on miscarriage rate per woman.
Implications for research.
Further large, well‐designed, and well‐executed randomised controlled trials with adequate power are needed to definitively answer the question of whether the use of metformin in women with PCOS undergoing assisted reproductive techniques (ART) improves live birth rate. All studies should report live birth rate (per woman), OHSS (per woman), and other adverse events. Only a few studies using short protocol GnRH‐antagonist comparing metformin with placebo or no treatment exist, therefore further studies using this type of protocol are required to determine the effect this comparison has on reproductive outcomes.
What's new
| Date | Event | Description |
|---|---|---|
| 8 May 2020 | New citation required but conclusions have not changed | The addition of four new studies, An 2014; Cheraghi 2018; Jacob 2016; Kim 2016, did not lead to a change in the conclusions of the review. |
| 8 May 2020 | New search has been performed | We updated the literature search. We included four studies that compared metformin with placebo. We changed 'clinical pregnancy' from a primary outcome to a secondary outcome. |
History
Protocol first published: Issue 3, 2006 Review first published: Issue 2, 2009
| Date | Event | Description |
|---|---|---|
| 16 April 2015 | Amended | Correction of text error in the Abstract and Plain language summary, and addition of search result numbers in Appendix 1. |
| 15 October 2014 | New search has been performed | One new co‐author was added for the update of the review (Cristiane R Macedo). Three new trials were added. |
| 15 October 2014 | New citation required and conclusions have changed | With the addition of three new studies (Kjotrod 2011; Palomba 2011; Qublan 2009), totalling 816 participants, the conclusions of the review have changed. |
| 1 November 2012 | New search has been performed | The search was updated on 5 November 2012. Three new trials were included (Kjotrod 2011; Palomba 2011; Qublan 2009), totalling 801 participants. The conclusions of the review have changed: the clinical pregnancy rate was significantly higher in the metformin group. Two new co‐authors were added (Luiz Eduardo T Albuquerque and Cristiane R Macedo). |
| 31 August 2008 | Amended | The 'Risk of bias' table was completed. |
| 13 April 2008 | Amended | The review was converted to the new format. |
| 28 February 2008 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
The review authors wish to express their gratitude to the Brazilian Cochrane Centre and the Gynaecology and Fertility Group, especially the managing editor, Elena Kostova, for her help and support throughout the review process, and to Marian Showell, for conducting the database searches and providing assistance with retrieval of studies.
We would also like to thank the peer reviewers: Annika Strandell, Jack Wilkinson, Rebecca Harmston, Thomas Tang, and the co‐ordinating editor Madelon van Wely.
We acknowledge the contribution of Professor Vilmon de Freitas who contributed to the protocol and the initial version of the review. Professor Vilmon de Freita passed away on June 22, 2010.
Appendices
Appendix 1. Cochrane Gynaecology and Fertility Group Specialised Register search strategy
Procite platform
Searched 13 February 2020
Keywords CONTAINS "polycystic ovary syndrome" or "PCOS" or Title CONTAINS or "polycystic ovary syndrome" or "PCOS"
AND
Keywords CONTAINS "IVF" or "in‐vitro fertilisation " or "in vitro fertilization" or "ICSI" or "intracytoplasmic morphologically selected sperm injection" or "intracytoplasmic sperm injection" or "Embryo Transfer" or "ovulation" or "ovarian stimulation" or Title CONTAINS "IVF" or "in‐vitro fertilisation " or "in vitro fertilization" or "ICSI" or "intracytoplasmic morphologically selected sperm injection" or "intracytoplasmic sperm injection" or "Embryo Transfer" or "ovulation" or "ovarian stimulation"
AND
Keywords CONTAINS "metformin" or "glucophage" or Title CONTAINS "metformin" or "glucophage"
(213 records )
Appendix 2. CENTRAL via Cochrane Register of Studies Online (CRSO) search strategy
Web platform
Searched 13 February 2020
#1 MESH DESCRIPTOR Polycystic Ovary Syndrome EXPLODE ALL TREES 1447
#2 (Polycystic Ovar*):TI,AB,KY 3426
#3 (PCOS or PCOD):TI,AB,KY 2815
#4 (stein‐leventhal or leventhal):TI,AB,KY 60
#5 #1 OR #2 OR #3 OR #4 3778
#6 MESH DESCRIPTOR Metformin EXPLODE ALL TREES 3917
#7 metformin:TI,AB,KY 9887
#8 (dimethylbiguanidium or dimethylguanylguanidine or glucophage or glucovance):TI,AB,KY 196
#9 #6 OR #7 OR #8 9894
#10 #5 AND #9 1143
#11 MESH DESCRIPTOR Fertilization in Vitro EXPLODE ALL TREES 2021
#12 (in vitro fertili*):TI,AB,KY 3391
#13 (intra?cytoplasmic sperm*):TI,AB,KY 1932
#14 (ivf or icsi):TI,AB,KY 6445
#15 MESH DESCRIPTOR Embryo Transfer EXPLODE ALL TREES 1072
#16 #11 OR #12 OR #13 OR #14 OR #15 7891
#17 #10 AND #16 104
Appendix 3. MEDLINE search strategy
OVID platform
Searched from 1946 to 13 February 2020
1 exp Polycystic Ovary Syndrome/ (14088) 2 Polycystic Ovar$.tw. (16192) 3 (PCOS or PCOD).tw. (11120) 4 (stein‐leventhal or leventhal).tw. (724) 5 (ovar$ adj (scelerocystic or polycystic or degeneration)).tw. (93) 6 or/1‐5 (19290) 7 exp metformin/ (12919) 8 metformin.tw. (19312) 9 (dimethylbiguanidium or dimethylguanylguanidine or glucophage or glucovance).tw. (129) 10 or/7‐9 (21138) 11 exp fertilization in vitro/ (35157) 12 (ivf or icsi).tw. (26952) 13 (in vitro fertil$ or intra?cytoplasmic sperm$).tw. (27835) 14 exp embryo transfer/ or exp sperm injections, intracytoplasmic/ (20586) 15 or/11‐14 (54026) 16 6 and 10 and 15 (119) 17 randomized controlled trial.pt. (500622) 18 controlled clinical trial.pt. (93579) 19 randomized.ab. (470075) 20 placebo.tw. (210862) 21 clinical trials as topic.sh. (190144) 22 randomly.ab. (327266) 23 trial.ti. (213342) 24 (crossover or cross‐over or cross over).tw. (83467) 25 or/17‐24 (1299415) 26 exp animals/ not humans.sh. (4673209) 27 25 not 26 (1194045) 28 16 and 27 (46)
Appendix 4. Embase search strategy
OVID platform
Searched from 1980 to 13 February 2020
1 exp ovary polycystic disease/ (25946) 2 polycystic ovar$.tw. (22411) 3 (PCOD or PCOS).tw. (16914) 4 (stein‐leventhal or leventhal).tw. (305) 5 (ovar$ adj (scelerocystic or polycystic or degeneration)).tw. (94) 6 or/1‐5 (30089) 7 exp METFORMIN/ (60705) 8 metformin.tw. (33522) 9 (dimethylbiguanidium or dimethylguanylguanidine or glucophage or glucovance).tw. (1718) 10 or/7‐9 (62889) 11 exp fertilization in vitro/ (67828) 12 (ivf or icsi).tw. (46523) 13 in vitro fertil$.tw. (30651) 14 ((intracytoplasmic or intra‐cytoplasmic) adj sperm$).tw. (10062) 15 exp intracytoplasmic sperm injection/ (20373) 16 exp embryo transfer/ (30409) 17 or/11‐16 (92397) 18 6 and 10 and 17 (805) 19 Clinical Trial/ (952109) 20 Randomized Controlled Trial/ (585105) 21 exp randomization/ (85854) 22 Single Blind Procedure/ (37795) 23 Double Blind Procedure/ (166174) 24 Crossover Procedure/ (61930) 25 Placebo/ (331619) 26 Randomi?ed controlled trial$.tw. (220568) 27 Rct.tw. (35629) 28 random allocation.tw. (1966) 29 randomly allocated.tw. (34141) 30 allocated randomly.tw. (2501) 31 (allocated adj2 random).tw. (808) 32 Single blind$.tw. (23985) 33 Double blind$.tw. (198653) 34 ((treble or triple) adj blind$).tw. (1089) 35 placebo$.tw. (296356) 36 prospective study/ (578090) 37 or/19‐36 (2132117) 38 case study/ (66726) 39 case report.tw. (391258) 40 abstract report/ or letter/ (1076971) 41 or/38‐40 (1524866) 42 37 not 41 (2079925) 43 18 and 42 (395)
Appendix 5. PsycINFO search strategy
OVID platform
Searched from 1806 to 13 February 2020
1 exp Endocrine Sexual Disorders/ (1752) 2 Polycystic Ovar$.tw. (409) 3 (PCOS or PCOD).tw. (280) 4 (stein‐leventhal or leventhal).tw. (299) 5 (ovar$ adj (scelerocystic or polycystic or degeneration)).tw. (0) 6 or/1‐5 (2317) 7 metformin.tw. (455) 8 (dimethylbiguanidium or dimethylguanylguanidine or glucophage or glucovance).tw. (2) 9 or/7‐8 (455) 10 exp Reproductive Technology/ (1799) 11 (ivf or icsi).tw. (583) 12 (in vitro fertil$ or ((intracytoplasmic or intra‐cytoplasmic) adj sperm$)).tw. (759) 13 or/10‐12 (2146) 14 6 and 9 and 13 (0)
Appendix 6. LILACS search strategy
Web platform
Searched 13 February 2020
((MH:C04.182.612.765$) OR (MH:C13.351.500.056.630.580.765$) OR (MH:C19.391.630.580.765$) OR (TW:"Polycystic Ovary Syndrome") OR (TW:"Síndrome del Ovario Poliquístico") OR (TW:"Síndrome do Ovário Policístico") OR (TW:"Stein‐Leventhal Syndrome") OR (TW:"Síndrome de Stein‐Leventhal") OR (TW:stein‐leventhal) OR (TW:leventhal) OR (TW:PCOS) OR (TW:PCOD) OR (TW:Ovar$ AND (Poliquístico OR Sclerocystic OR Polycystic OR Degeneration OR Policístico OR Degeneração))) AND ((MH:D02.078.370.141.450$) OR (TW:Metformin) OR (TW:METFORMINA) OR (TW:Dimethylguanylguanidine) OR (TW:"Dimetil Guanil Guanidina") OR (TW:Dimetilguanilguanidina) OR (TW:Glucophage) OR (TW:Glucovance)) AND ((MH:E02.875.800.500$) OR (MH:E05.820.800.500$) OR (TW:Embryo Transfer) OR (TW:Transferencia de Embrión) OR (TW:Transferência Embrionária) OR (TW:Blastocyst Transfer) OR (TW:Tubal Embryo Transfer) OR (TW:Transferencia de Blastocitos) OR (TW:Transferencia Tubaria del Embrión) OR (TW:Transferência de Blastócitos) OR (TW:Transferência Tubária de Embrião) OR (TW:Transferência de Embrião) OR (MH:E02.875.800.750$) OR (MH:E05.820.800.750$) OR (TW:Fertilization in Vitro) OR (TW:Fertilización In Vitro) OR (TW:Fertilização In Vitro) OR (TW:Test‐Tube Fertilization) OR (TW:Fecundación In Vitro) OR (TW:Fecundación en Probeta) OR (TW:Fertilización en Probeta) OR (TW:Fecundação In Vitro) OR (TW:Fecundação em Tubo de Ensaio) OR (TW:Fertilização em Tubo de Ensaio) OR (MH:E02.875.800.750.700$) OR (MH:E05.820.800.750.700$) OR (TW:Inyecciones de Esperma Intracitoplasmáticas) OR (TW:Injeções de Esperma Intracitoplásmicas) OR (TW:Intracytoplasmic Sperm Injections) OR (TW:ICSI) OR (TW:Inyecciones Intracitoplasmáticas de Esperma) OR (TW:IICE) OR (TW:Injeções Intracitoplásmicas de Esperma) OR (TW:assisted reproductive technique) OR (TW:in vitro fertilization) OR (TW:in vitro fertilisation) OR (TW:FIV) OR (TW:IVF)) (4)
Appendix 7. Data extraction
-
Trial characteristics
Randomisation
Allocation concealment
Trial design: multicentre or single centre, single‐phase or cross‐over design
Number of participants randomised, excluded, and analysed
Duration, timing, and location of the trial
Source of funding
-
Baseline characteristics of the studied groups
Definition of PCOS and duration of pre‐existing infertility
Age of participants
Investigative work‐up
Other causes of infertility
Previously administered infertility treatment(s)
BMI
-
Interventions
Type of intervention and control
Dose regimen and duration
-
Outcomes
Outcomes reported
Definition of outcomes
Measurement of outcomes
Timing of outcome measurement
Data and analyses
Comparison 1. Metformin versus placebo or no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Live birth rate per woman | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1.1 Long protocol GnRH‐agonist | 6 | 651 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [0.94, 1.79] |
| 1.1.2 Short protocol GnRH‐antagonist | 1 | 153 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.29, 0.79] |
| 1.2 Incidence of OHSS per woman | 11 | 1091 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.29, 0.72] |
| 1.2.1 Long protocol GnRH‐agonist | 9 | 898 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.26, 0.60] |
| 1.2.2 Short protocol GnRH‐antagonist | 2 | 193 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.32, 2.98] |
| 1.3 Clinical pregnancy rate per woman | 12 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.3.1 Long protocol GnRH‐agonist | 10 | 915 | Risk Ratio (M‐H, Random, 95% CI) | 1.32 [1.08, 1.63] |
| 1.3.2 Short protocol GnRH‐antagonist | 2 | 177 | Risk Ratio (M‐H, Random, 95% CI) | 1.38 [0.21, 9.14] |
| 1.4 Miscarriage rate per woman | 8 | 821 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.56, 1.32] |
| 1.4.1 Long protocol GnRH‐agonist | 7 | 668 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.51, 1.26] |
| 1.4.2 Short protocol GnRH‐antagonist | 1 | 153 | Risk Ratio (M‐H, Random, 95% CI) | 1.48 [0.43, 5.04] |
| 1.5 Miscarriage rate per pregnant woman | 8 | 312 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.51, 1.31] |
| 1.5.1 Long protocol GnRH‐agonist | 7 | 253 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.43, 1.00] |
| 1.5.2 Short protocol GnRH‐antagonist | 1 | 59 | Risk Ratio (M‐H, Random, 95% CI) | 2.52 [0.80, 7.97] |
| 1.6 Side effects per woman | 8 | 748 | Risk Ratio (M‐H, Random, 95% CI) | 3.35 [2.34, 4.79] |
| 1.6.1 Long protocol GnRH‐agonist | 6 | 571 | Risk Ratio (M‐H, Random, 95% CI) | 3.07 [1.79, 5.24] |
| 1.6.2 Short protocol GnRH‐antagonist | 2 | 177 | Risk Ratio (M‐H, Random, 95% CI) | 3.68 [1.92, 7.04] |
| 1.7 Number of oocytes retrieved per woman | 11 | 890 | Mean Difference (IV, Random, 95% CI) | 0.03 [‐1.42, 1.48] |
| 1.7.1 Long protocol GnRH‐agonist | 9 | 697 | Mean Difference (IV, Random, 95% CI) | 0.24 [‐1.44, 1.93] |
| 1.7.2 Short protocol GnRH‐antagonist | 2 | 193 | Mean Difference (IV, Random, 95% CI) | ‐1.00 [‐3.76, 1.76] |
| 1.8 Mean total dose of FSH (IU) per woman | 10 | 868 | Std. Mean Difference (IV, Random, 95% CI) | 0.24 [‐0.23, 0.70] |
| 1.8.1 Long protocol GnRH‐agonist | 8 | 675 | Std. Mean Difference (IV, Random, 95% CI) | 0.32 [‐0.28, 0.92] |
| 1.8.2 Short protocol GnRH‐antagonist | 2 | 193 | Std. Mean Difference (IV, Random, 95% CI) | 0.00 [‐0.28, 0.28] |
| 1.9 Mean days of gonadotrophin per woman | 9 | 796 | Mean Difference (IV, Random, 95% CI) | ‐0.17 [‐0.71, 0.37] |
| 1.9.1 Long protocol GnRH‐agonist | 7 | 603 | Mean Difference (IV, Random, 95% CI) | ‐0.22 [‐0.89, 0.45] |
| 1.9.2 Short protocol GnRH‐antagonist | 2 | 193 | Mean Difference (IV, Random, 95% CI) | 0.00 [‐1.04, 1.04] |
| 1.10 Cycle cancellation rate (after ovulation induction) | 8 | 877 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.43, 1.29] |
| 1.10.1 Long protocol GnRH‐agonist | 6 | 684 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.33, 1.19] |
| 1.10.2 Short protocol GnRH‐antagonist | 2 | 193 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.25, 4.45] |
| 1.11 Serum oestradiol level (nmol/L) per woman | 6 | 484 | Mean Difference (IV, Random, 95% CI) | ‐2.43 [‐4.59, ‐0.26] |
| 1.11.1 Long protocol GnRH‐agonist | 5 | 444 | Mean Difference (IV, Random, 95% CI) | ‐2.21 [‐4.75, 0.33] |
| 1.11.2 Short protocol GnRH‐antagonist | 1 | 40 | Mean Difference (IV, Random, 95% CI) | ‐3.56 [‐5.30, ‐1.82] |
| 1.12 Mean or median serum androgen levels per woman | 2 | Other data | No numeric data | |
| 1.13 Mean or median fasting insulin and glucose levels per woman | 2 | Other data | No numeric data | |
| 1.14 Fertilisation rate | 2 | 225 | Mean Difference (IV, Random, 95% CI) | ‐4.78 [‐12.21, 2.65] |
| 1.14.1 Long protocol GnRH‐agonist | 1 | 72 | Mean Difference (IV, Random, 95% CI) | 0.70 [‐11.38, 12.78] |
| 1.14.2 Short protocol GnRH‐antagonist | 1 | 153 | Mean Difference (IV, Random, 95% CI) | ‐7.40 [‐14.96, 0.16] |
| 1.15 Sensitivity analysis live birth by excluding Palomba 2011 due to a data discrepancy (suspected risk of bias) | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.15.1 Long protocol GnRH‐agonist | 5 | 531 | Risk Ratio (M‐H, Random, 95% CI) | 1.38 [0.91, 2.10] |
| 1.15.2 Short protocol GnRH‐antagonist | 1 | 153 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.29, 0.79] |
1.15. Analysis.

Comparison 1: Metformin versus placebo or no treatment, Outcome 15: Sensitivity analysis live birth by excluding Palomba 2011 due to a data discrepancy (suspected risk of bias)
Characteristics of studies
Characteristics of included studies [ordered by study ID]
An 2014.
| Study characteristics | ||
| Methods |
|
|
| Participants | 150 PCOS participants were randomised (50 in the placebo group, 50 in the metformin group, and 50 in the berberine group) Diagnosis of PCOS followed the Rotterdam criteria (ESHRE/ASRM) A total of 128 of 150 randomised women (85%) completed the first 12 weeks of pretreatment using metformin, berberine, or placebo before starting controlled ovarian stimulation for IVF/ICSI. These 14 women were excluded due to voluntary dropout or incomplete data (5 in the PLC group, 6 in the MET, group and 3 in the BBR group), lost to follow‐up (2 in the PLC group, 1 in the MET group, and 2 in the BBR group), or gastrointestinal side effects (2 in the PLC group, 2 in the MET group, and 1 in the BBR group). In total, 109 women had an embryo to be transferred and completed IVF/ICSI cycle (34 in the PLC group, 38 in the MET group, and 37 in the BBR group) and were analysed. ITT analysis was not performed but could be calculated based on extracted data. Infertility factors were reported. Primary outcomes (live birth rate, clinical pregnancy rate, and OHSS rate) were reported.
a) congenital adrenal hyperplasia b) Cushing's syndrome c) androgen‐producing tumours d) hyperprolactinaemia e) liver or kidney disease f) diabetes mellitus g) alcoholism h) drug abuse Oestradiol concentration on hCG day was reported in nmol/L and needed to be converted into pg/mL. |
|
| Interventions |
Duration of metformin treatment: metformin 500 mg 3 times daily started on the same day of oral contraceptive commencement and continued to the day of oocyte retrieval. All women were pretreated with berberine, metformin, or placebo for 12 weeks before starting controlled ovarian stimulation for IVF/ICSI. Protocol for controlled ovarian hyperstimulation: long protocol GnRH‐agonist was used in all participants. For ovarian downregulation, daily injections of triptorelin (0.1 mg Beaufour, Ipsen, France) were administered subcutaneously from day 20 of the preceding menstrual cycle followed by at least 14 days of downregulation, when rec‐FSH (Gonal‐f, Merck Serono SA, Switzerland) was used for ovarian stimulation. The starting dose of rec‐FSH was 150 IU daily and was further adjusted according to serum oestradiol levels and measurements of follicular development obtained by vaginal ultrasound. “Coasting” was permitted if considered necessary to prevent OHSS. When more than 3 follicles had reached mean diameter over 17 mm, hCG was administered (hCG 5000 or 10000 IU, Li Zhu Corp, Zhu Hai, China), and 34 to 36 hours after that transvaginal follicle aspiration was performed under ultrasound guidance and general anaesthesia. Assisted reproductive technology: IVF or ICSI. A maximum of 2 embryos at cleavage stage (day 2 or day 3) were transferred. Catheter used for transfer: not reported. Luteal phase support: luteal phase was supported with progesterone intramuscularly for 2 weeks (progesterone injection, 60 mg 4 times a day, Xian Ju Corp, Zhe Jiang, China). |
|
| Outcomes |
a) live birth rate (per woman) b) clinical pregnancy rate (per woman) c) incidence of OHSS (per woman)
a) miscarriage rate (per woman) b) incidence of participant‐reported side effects (per woman) c) number of oocytes retrieved d) total dose of FSH (IU) given during stimulation e) number of days of gonadotrophin treatment f) cycle cancellation rate (per woman) g) serum oestradiol level on the day of hCG trigger (pg/mL) h) fertilisation rate (2 pronuclei‐stage embryos) |
|
| Notes | Country of the study: China Registration number of the trial: HMU‐2009037 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer randomisation system. |
| Allocation concealment (selection bias) | Low risk | Random allocation sequence was concealed at the clinical trial office in the pharmacy department. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded assessor. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Number and reasons for withdrawals were reported. |
| Selective reporting (reporting bias) | Low risk | All main outcomes reported. |
| Other bias | Low risk | None suspected. |
Cheraghi 2018.
| Study characteristics | ||
| Methods |
|
|
| Participants | 80 PCOS women were randomised (20 in the MET group, 20 in the placebo group, 20 in the NAC group, and 20 in the MET + NAC group). Diagnosis of PCOS followed the Rotterdam criteria (ESHRE/ASRM). No participant withdrew for personal reasons. Dropouts were as follows.
In total, 60 women had an embryo to be transferred (15 in each group) and were analysed. ITT analysis was not performed. Infertility factors were not reported. Primary outcomes (live birth rate, clinical pregnancy rate, and OHSS rate) were not reported.
a) congenital adrenal hyperplasia b) Cushing's syndrome c) androgen‐producing tumours d) hyperprolactinaemia e) thyroid dysfunction Number of oocytes retrieved and number of good‐quality embryos were reported, but these data could not be included in the meta‐analysis. |
|
| Interventions |
Duration of metformin treatment: metformin 500 mg 3 times daily started on the same day of OC commencement and continued to the day of oocyte retrieval. All women were pretreated for 3 weeks with OC starting simultaneously with placebo, metformin, N‐acetylcysteine, or MET + NAC. Protocol for controlled ovarian hyperstimulation: 5 days after OC discontinuation daily FSH‐rec was started. GnRH‐agonist protocol was used in all women. For ovarian downregulation, daily injections of buserelin (1 mg Suprefact, Aventis, Germany) were administered from day 19 of the preceding menstrual cycle until day 2 of the next cycle, when the dose of buserelin was reduced to 0.5 mg. FSH‐rec (Gonal‐f 150 IU, Merck Serono SA, Switzerland) was used for ovarian stimulation and started on day 2 until hCG day. When at least 3 follicles had reached mean diameter of 16 to 18 mm, hCG was administered, and 36 hours after that transvaginal follicle aspiration was performed under ultrasound guidance and general anaesthesia. Assisted reproductive technology: ICSI. Routinely, a maximum of 4 embryos were transferred for each woman. It was not reported if the transfer was performed under abdominal ultrasound guidance. Catheter used for transfer: Labotec (Gotitingen, Germany) Luteal phase support: intramuscular daily injections of progesterone 100 mg (Gestone, London, UK). |
|
| Outcomes |
a) clinical pregnancy rate (per woman) b) incidence of OHSS (per woman)
a) incidence of participant‐reported side effects (per woman) b) number of oocytes retrieved c) fertilisation rate (2 pronuclei‐stage embryos) |
|
| Notes | Country of the study: Iran Registration number: IRCT201204159476N1 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blinded, but blinding method not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Number and reasons for withdrawals were reported. |
| Selective reporting (reporting bias) | High risk | No main outcomes were reported. |
| Other bias | Low risk | None suspected. |
Doldi 2006.
| Study characteristics | ||
| Methods |
|
|
| Participants | 40 PCOS participants were randomised (20 in the metformin group and 20 in the placebo group). Diagnosis of PCOS followed the Rotterdam criteria (ESHRE/ASRM).
a) congenital adrenal hyperplasia b) Cushing's syndrome c) androgen‐producing tumours d) hyperprolactinaemia e) thyroid dysfunction f) participant age older than 40 years g) FSH > 12 mIU/mL The causes of infertility were not reported. Participants did not take any ovulation drugs or hormones for at least 3 months prior to the trial. |
|
| Interventions | Group A was pretreated for 2 months with metformin 1.5 g/day until embryo transfer day. Protocol for controlled ovarian hyperstimulation: short protocol GnRH‐antagonist (cetrorelix, Cetrotide) with step‐up rec‐FSH (Gonal F ‐ starting dose 150 IU). GnRH‐antagonist, cetrorelix 0.25 mg/day, was started when the leading follicle reached 14 mm in diameter on ultrasound scan and stopped on the day of hCG. Recombinant hCG (Ovitrelle 250 μg) was given when 2 or 3 follicles reached 16 mm in diameter on ultrasound scan. Oocyte retrieval was performed within 36 h of hCG injection. No more than 3 oocytes were fertilised (in accordance with Italian law). Assisted reproductive technology: IVF Embryo transfer: maximum of 3 embryos were transferred per participant on day 2 after oocyte retrieval under abdominal US guidance. Catheter used for transfer: not reported Luteal phase support: progesterone 90 mg (Crinone 8) was given on the day of oocyte retrieval and was continued until menstruation or a positive pregnancy test |
|
| Outcomes |
a) incidence of OHSS
a) number of ampoules of rec‐FSH b) oestradiol levels c) cancelled cycles d) number of mature oocytes |
|
| Notes | Country of the study: Italy Clinical trial registration number not provided. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Reasons for withdrawals were not reported. |
| Selective reporting (reporting bias) | Unclear risk | Live birth and clinical pregnancy rates were not assessed. |
| Other bias | Unclear risk | The causes of infertility were not reported. |
Fedorcsak 2003.
| Study characteristics | ||
| Methods |
|
|
| Participants | 17 PCOS participants were randomised (9 in the metformin group and 8 in the placebo group). Diagnosis of PCOS followed the Rotterdam criteria (ESHRE/ASRM). All women had insulin‐resistance, based on an insulin resistance index.
a) congenital adrenal hyperplasia b) Cushing's syndrome c) androgen‐producing tumours d) hyperprolactinaemia Age: 23 to 35 years (median 31) The causes of infertility were not reported. Only the first arm was compared: 8 participants in the no‐treatment group versus 9 participants in the metformin group. |
|
| Interventions | Metformin 500 mg 3 times a day was started 3 weeks before downregulation with GnRH‐agonist began and was continued until the day of hCG injection.
Protocol for controlled ovarian hyperstimulation: long luteal phase pituitary downregulation using the GnRH analogue buserelin 600 μg (Suprefact) with step‐up rec‐FSH (Gonal F ‐ starting dose 150 IU). hCG (Profasi 10,000 IU) was administered when at least 2 follicles were larger than 18 mm. Oocyte retrieval was performed within 34 to 38 h of hCG injection.
Assisted reproductive technology: IVF or ICSI
Embryo transfer: maximum of 2 embryos were transferred per participant on day 3 after oocyte retrieval Catheter used for transfer: not reported Luteal phase support: intramuscular progesterone (25 mg/day) until day 14 after follicle puncture |
|
| Outcomes |
a) clinical pregnancy rate per woman b) incidence of OHSS
a) total dose of FSH (IU) given during stimulation b) number of collected oocytes c) number of days of gonadotrophin d) fertilisation rate e) number of embryos transferred f) miscarriage rate g) incidence of adverse side effects |
|
| Notes | Country of the study: Norway Clinical trial registration number not provided. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate ‐ random numbers table. |
| Allocation concealment (selection bias) | Low risk | Adequate ‐ sealed, opaque envelopes serially numbered. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label cross‐over trial. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label cross‐over trial. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no withdrawals in the phase analysed (pre‐cross‐over phase). |
| Selective reporting (reporting bias) | Unclear risk | Live birth rate was not evaluated. |
| Other bias | Unclear risk | The causes of infertility were not reported. |
Jacob 2016.
| Study characteristics | ||
| Methods |
|
|
| Participants | 153 PCOS participants were randomised (77 in the metformin group and 76 in the placebo group). Diagnosis of PCOS followed the Rotterdam criteria (ESHRE/ASRM). No participant withdrew for personal reasons. 2 women in the metformin group and 2 in the placebo group became pregnant spontaneously and did not start ovarian stimulation for IVF/ICSI cycles. 149 women started ovulation induction (75 in the metformin group and 74 in the placebo group). In total, 122 women had an embryo transfer (58 in the MET group and 64 in the PLC group): 1 woman in the MET group did not have embryo transfer, 9 had poor ovarian response pre‐oocyte retrieval (6 in the MET group and 3 in the PLC group), 3 had over response pre‐oocyte retrieval (2 in the MET group and 1 in the PLC group), 9 had OHSS and freeze all embryos (4 in the MET group and 5 in the PLC group), and 5 women had total failed of fertilisation/cleavage (4 in the MET group and 1 in the PLC group). ITT analysis was performed for clinical pregnancy rate and implantation rate. Infertility factors were reported. Both groups were matched for age, cause, and duration of infertility, BMI, and gravity.
a) concomitant use of medication that could interfere with the absorption, metabolism, and excretion of metformin including antivirals, cimetidine, and other oral antidiabetic medication b) any significant systemic disease c) diabetes (type 1 or 2) |
|
| Interventions |
Duration of metformin treatment: metformin 850 mg twice a day started either 7 days prior to the woman’s anticipated menstruation in those with a regular menstrual cycle (mid‐luteal) or day 1 of the period for women with an irregular cycle or after a progesterone (Provera, Pharmacia, USA) induced withdrawal bleed, with the intention of maximising study drug use. The medication was continued until the day before egg collection. Protocol for controlled ovarian hyperstimulation: daily rec‐FSH was started from day 2 of the menstrual cycle, at a dose adjusted for patient age, ovarian reserve, and BMI (starting range 100 to 150 IU; Puregon). A GnRH‐antagonist (250 μg; Orgalutron) was added on day 6 of the cycle. The dose of rec‐FSH was adjusted depending upon ovarian response, with a reduction if there was risk of over response. Oocyte retrieval was performed within 35 to 37 h after hCG injection (Pregnyl 5000 to 10,000 IU). Assisted reproductive technology: IVF or ICSI. Embryo transfer: maximum of 2 embryos were transferred on day 3 or day 5 after oocyte retrieval under abdominal ultrasound guidance. Catheter used for transfer: Cook embryo replacement catheter. Luteal phase support: vaginal progesterone (Cyclogest, 400 to 800 mg/day or intramuscular Gestone or Prontogest, 50 to 100 mg/day), which varied depending upon patient/clinician preference. |
|
| Outcomes |
a) clinical pregnancy rate per woman b) incidence of OHSS per woman (moderate to severe)
a) incidence of participant‐reported side effects (per woman) ‐ gastrointestinal side effect rate b) number of oocytes retrieved c) total dose of FSH (IU) given during stimulation d) number of days of gonadotrophin treatment e) cycle cancellation rate (per woman) f) serum androgen level g) fertilisation rate h) live birth rate (per embryo transfer) i) serum E2 levels on the day of hCG |
|
| Notes | Country of the study: United Kingdom Prospectively registered in the ISRCTN registry (ISRCTN21199799) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random permuted blocks method with a 50:50 allocation ratio. |
| Allocation concealment (selection bias) | Low risk | Codes were kept by a third party in the pharmacy department. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Number and reasons for withdrawals were reported. |
| Selective reporting (reporting bias) | Unclear risk | Live birth rate (per woman) was not reported, |
| Other bias | Low risk | None suspected. |
Kim 2016.
| Study characteristics | ||
| Methods |
|
|
| Participants | 24 PCOS participants were randomised (12 in the metformin group and 12 in the placebo group). Diagnosis of PCOS followed the Rotterdam criteria (ESHRE/ASRM). In the metformin group, 2 women suffered nausea and diarrhoea and were withdrawn. In the control group, 1 woman was withdrawn for personal reasons. ITT analysis was not performed for clinical pregnancy rate and implantation rate. Infertility factors were not reported. There were no significant differences in participant characteristics between the metformin and placebo groups.
|
|
| Interventions |
Duration of metformin treatment: metformin 500 mg twice a day started on the same day as OC commencement and continued to the day of oocyte retrieval. All participants were pretreated for 3 weeks with OC before controlled ovarian hyperstimulation. Protocol for controlled ovarian hyperstimulation: 5 days after OC discontinuation daily FSH‐rec was started. GnRH‐antagonist protocol was used in all participants. The study reported neither the kind of hCG used nor the timing of oocyte retrieval. Assisted reproductive technology: IVF or ICSI. The study did not report the number and stage of embryos transferred or whether abdominal ultrasound guidance was used. Catheter used for transfer: not reported Luteal phase support: not reported |
|
| Outcomes |
a) clinical pregnancy rate (per woman)
a) incidence of participant‐reported side effects (per woman) b) number of oocytes retrieved c) total dose of FSH (in IU) |
|
| Notes | Country of the study: Seoul, Korea Prospectively registered at ClinicalTrials.gov (NCT03086005) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelopes (not reported if they were opaque). |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | ITT analysis was not performed. Number and reasons for withdrawals were reported. |
| Selective reporting (reporting bias) | Unclear risk | Live birth rate (per woman), incidence of OHSS (per woman), and miscarriage rate (per woman) were not reported. Live birth rate (per embryo transfer) was reported. |
| Other bias | Unclear risk | Infertility factors were not reported. |
Kjotrod 2004.
| Study characteristics | ||
| Methods |
|
|
| Participants | 73 PCOS participants were randomised (37 in the metformin group and 36 in the placebo group).
Diagnosis of PCOS followed the Rotterdam criteria (ESHRE/ASRM).
4 participants withdrew for personal reasons.
4 women in the metformin group and 2 in the placebo group became pregnant spontaneously.
63 women started ovulation induction (31 in the metformin group and 32 in the placebo group).
2 women were excluded before oocyte retrieval (1 poor responder and 1 OHSS).
4 women dropped out before embryo transfer (2 due to OHSS and 2 due to lack of good‐quality embryos).
57 women received embryos. ITT analysis was performed for the primary outcomes. Infertility factors were reported. Both groups were matched for age, cause and duration of infertility, BMI, and gravity.
a) diabetes mellitus b) renal insufficiency c) liver disease d) treatment with oral glucocorticoids e) congenital adrenal hyperplasia g) androgen‐producing tumours h) hyperprolactinaemia f) thyroid dysfunction |
|
| Interventions | Metformin 500 mg twice a day (gradually increasing the dose during the first 2 weeks) for at least 16 weeks until the day of hCG injection
Protocol for controlled ovarian hyperstimulation: long luteal phase pituitary downregulation using the GnRH analogue nafarelin 800 μg daily (Synarela) with rec‐FSH (Puregon 100 IU daily in normal‐weight women or 150 IU in obese women). Oocyte retrieval was performed within 34 to 36 h after hCG injection (Pregnyl 5000 IU).
Assisted reproductive technology: IVF or ICSI
Embryo transfer: maximum of 2 embryos were transferred per participant on day 3 after oocyte retrieval Catheter used for transfer: not reported Luteal phase support: vaginal progesterone (Progestan) for 2 weeks (200 mg 3 times a day) |
|
| Outcomes |
a) live birth rate per woman b) clinical pregnancy rate per woman c) incidence of OHSS
a) total dose of FSH given during stimulation b) number of collected oocytes c) fertilisation rate d) number of good‐quality embryos e) pregnancy rate per woman f) number of days of gonadotrophins g) serum E2 levels on the day of hCG |
|
| Notes | Country of the study: Norway Clinical trial registration number not provided. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate ‐ computer randomisation system. |
| Allocation concealment (selection bias) | Low risk | Adequate ‐ codes kept by a third party in the pharmacy department. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Number and reasons for withdrawals were reported. |
| Selective reporting (reporting bias) | Low risk | All main outcomes were reported. |
| Other bias | Low risk | None suspected. |
Kjotrod 2011.
| Study characteristics | ||
| Methods |
|
|
| Participants | 150 PCOS participants were randomised (74 in the metformin group and 76 in the placebo group); however, 1 woman in the placebo group withdrew her consent just after randomisation. The ITT population in the study consisted of 149 women (74 in the metformin group, 75 in the placebo group). Diagnosis of PCOS followed the Rotterdam criteria (ESHRE/ASRM). A total of 53 women withdrew during the study period. The most common reason for withdrawal was spontaneous pregnancy (n = 23). 56 women in each group (metformin and placebo) started ovulation induction (ART population: 112 women). Infertility factors were reported. Both groups were matched for age, cause and duration of infertility, weight, and BMI.
a) contraindication for starting dose of 112.5 IU recombinant human follicle‐stimulation hormone b) baseline FSH serum level > 10 IU/L c) liver or kidney diseases d) treatment with oral glucocorticoids e) congenital adrenal hyperplasia f) androgen‐producing tumours or Cushing's syndrome g) hyperprolactinaemia h) thyroid dysfunction i) alcoholism or drug abuse j) diabetes mellitus |
|
| Interventions | The dose of metformin was gradually increased from 500 to 2000 mg per day during the first 2 weeks of treatment for at least 12 weeks prior to controlled ovarian hyperstimulation.
Protocol for controlled ovarian hyperstimulation: long luteal phase pituitary downregulation using the GnRH analogue nafarelin 800 μg daily (Synarela) with rec‐FSH (Gonal‐f starting dose of 112.5 IU daily and adjusted according to ovarian response). To induce final follicular maturation, a single dose of hCG (Pregnyl 5000 or 10,000 IU; or Ovitrelle 250 μg) was given when at least 1 follicle reached a diameter > 17 mm.
Assisted reproductive technology: IVF, ICSI or both procedures
Embryo transfer: maximum of 2 embryos were transferred per participant on day 2 or 3 after oocyte retrieval Catheter used for transfer: not reported Luteal phase support: progesterone, but the type and dose were selected by the physician |
|
| Outcomes |
a) live birth rate per woman b) clinical pregnancy rate per woman (ITT population) c) incidence of OHSS
a) pregnancy rate b) spontaneous pregnancy rate c) number of collected oocytes d) number of good‐quality embryos g) total dose of FSH given during stimulation h) number of days of gonadotrophins i) miscarriage rate j) incidence of side effects |
|
| Notes | Country of the study: Norway Prospectively registered at ClinicalTrials.gov (NCT00159575) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate ‐ computer randomisation system. |
| Allocation concealment (selection bias) | Low risk | Adequate ‐ codes kept by a third party in the pharmacy department. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Number and reasons for withdrawals were reported. |
| Selective reporting (reporting bias) | Low risk | All main outcomes were reported. |
| Other bias | Low risk | None suspected. |
Onalan 2005.
| Study characteristics | ||
| Methods | Generation of the allocation sequence: computer randomisation system Allocation concealment method: not reported Blinding method: yes (participants and personnel) Number and reasons for withdrawals: reported ITT analysis: no Prospective, randomised, double‐blind, placebo‐controlled trial Metformin versus placebo |
|
| Participants | 110 PCOS participants without concomitant causes of infertility Diagnosis of PCOS followed the Rotterdam criteria (ESHRE/ASRM). 2 women withdrew for personal reasons. 108 women were randomised (53 in the metformin group and 55 in the placebo group). Women < 40 years Both groups were matched for age, duration of infertility, BMI, and insulin resistance. All other causes of hyperandrogenism were ruled out before diagnosis of PCOS.
|
|
| Interventions | Metformin 850 mg twice or 3 times daily (according to BMI) for 8 weeks before their first ICSI cycle, through the luteal phase and until a positive pregnancy test
Protocol for controlled ovarian hyperstimulation: long luteal phase pituitary downregulation using the GnRH analogue triptorelin 0.1 mg (Decapeptyl) with rec‐FSH (Gonal F starting dose of 150 IU or 300 IU). Oocyte retrieval was performed within 36 hours after hCG injection (Pregnyl 10,000 IU).
Assisted reproductive technology: ICSI
Embryo transfer: maximum of 3 embryos were transferred per participant on day 3 after oocyte retrieval
A selective assisted hatching procedure with laser was used if the woman was > 35 years, the zona pellucida was considered to be thick, abnormally shaped zona, and excessive fragmentation or slowly developing embryos were noted. Catheter used for transfer: not reported Luteal phase support: not reported |
|
| Outcomes |
a) clinical pregnancy rate per woman b) incidence of OHSS
a) number of days of gonadotrophins b) number of ampoules of gonadotrophins c) number of follicles (> 16 mm) d) number of mature oocytes e) fertilisation rate f) number of embryos transferred g) pregnancy rate per woman h) miscarriage rate i) serum E2 levels j) glucose/insulin rate |
|
| Notes | Country of the study: Turkey Clinical trial registration number not provided. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate ‐ computer randomisation system. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | ITT analysis was not performed. |
| Selective reporting (reporting bias) | Low risk | Live birth rate was not reported; however, we were able to obtain this information after contacting the author by email. |
| Other bias | Low risk | None suspected. |
Palomba 2011.
| Study characteristics | ||
| Methods |
|
|
| Participants | Number of eligible cycles: 120 infertile women with PCOS who had a history of 1 previous cancelled cycle due to a high risk of OHSS or history of moderate or severe case of OHSS during their previous IVF cycle
Diagnosis of PCOS followed the Rotterdam criteria (ESHRE/ASRM).
120 PCOS participants were screened and underwent 120 consecutive IVF/ICSI cycles.
60 women were randomised to each group (metformin and placebo).
No women dropped out.
Age: younger than 35 years
Causes of infertility not reported.
Both groups were matched for age, median duration of infertility, BMI, and hirsutism according to modified Ferriman‐Gallwey score.
Inclusion criteria: only women who had received in the previous cycle mid‐luteal long GnRH‐agonist and a gonadotrophin step‐down stimulation protocol with a starting dose of 225 IU daily.
a) age > 35 years b) FSH level of > 10 c) BMI > 30 kg/m2 d) neoplastic, metabolic, hepatic, or cardiovascular disorders or other concurrent medical illness e) hypothyroidism f) hyperprolactinaemia h) Cushing's syndrome i) non‐classic congenital adrenal hyperplasia j) alcohol abuse k) current or previous: a wash‐out period of at least 6 months without use of any antidiabetic, obesity, or hormonal drugs except those used during the previous IVF cycle l) male factor infertility |
|
| Interventions | Metformin 500 mg 3 times a day and placebo tablets were started on the same day that downregulation with GnRH‐agonist began and were continued until a positive pregnancy test was obtained or menstrual bleeding appeared.
Protocol for controlled ovarian hyperstimulation: long luteal phase pituitary downregulation using the GnRH analogue Enantone 1 mg (0.5 mg twice daily, SC) and reduced to 0.5 mg (0.25 mg twice daily) after pituitary suppression with step‐down rec‐FSH (Gonal F ‐ starting dose 150 IU). hCG (Profasi 10,000 IU) was administered when at least 3 follicles were larger than 18 mm. Oocyte retrieval was performed within 36 h after hCG injection.
Assisted reproductive technology: IVF or ICSI
Embryo transfer: maximum of 2 embryos were transferred per participant on day 2, 3, or 5 after oocyte retrieval without ultrasonographic guidance Catheter used for transfer: not reported Luteal phase support: intramuscular progesterone (Prontogest 50 mg/day) |
|
| Outcomes |
a) live birth rate per woman b) clinical pregnancy rate per woman c) incidence of OHSS
a) number of collected oocytes b) number of good‐quality embryos c) total dose of FSH given during stimulation d) number of days of gonadotrophins |
|
| Notes | Country of the study: Italy ClinicalTrials.gov identifier: NCT01233206 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate ‐ computer randomisation system. |
| Allocation concealment (selection bias) | Low risk | Random allocation sequence was concealed in the central pharmacy. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no withdrawals. |
| Selective reporting (reporting bias) | Low risk | All main outcomes were reported. |
| Other bias | High risk | There is a discrepancy in the data in the published study: in both the metformin group and the placebo group the clinical pregnancy rate is lower than the live birth rate (pregnancy 26/60, 24/60; live birth 29/60, 27/60). We have attempted to contact the first author (emailed 19 August 2014) but have not received a response. |
Qublan 2009.
| Study characteristics | ||
| Methods |
|
|
| Participants | 66 clomiphene‐resistant PCOS participants were randomised (34 in the metformin group and 32 in the placebo group).
Diagnosis of PCOS followed the Rotterdam criteria (ESHRE/ASRM).
34 women in the metformin group and 32 in the placebo group started controlled ovarian hyperstimulation (66 participants ‐ 4 women in the metformin group and 2 in the placebo group became pregnant spontaneously) There were no withdrawals. Infertility factors were reported. Both groups were matched for age, cause and duration of infertility, BMI, and hormonal/ biochemical profiles. All women were required to have a normal uterine cavity. |
|
| Interventions | Metformin 850 mg 3 times a day for a month before their first ICSI cycle, through the luteal phase and until a positive pregnancy test. If the test was positive, metformin was continued until 12 weeks of gestation. Protocol for controlled ovarian hyperstimulation: long luteal phase pituitary downregulation using the GnRH analogue triptorelin (Decapeptyl) with hMG (Menogon) (starting dose of 150 IU daily and adjusted according to ovarian response). Oocyte retrieval was performed within 36 h after hCG injection (10,000 IU). Assisted reproductive technology: IVF or ICSI Embryo transfer: 2 to 4 embryos were transferred per participant on day 3 after oocyte retrieval Luteal phase support: progesterone pessaries (Cyclogest) | |
| Outcomes |
a) clinical pregnancy rate per woman (ITT population) b) incidence of OHSS
a) number of days of gonadotrophins b) number of ampoules of gonadotrophins c) number of follicles (> 14 mm) d) number of mature oocytes e) fertilisation rate f) number of embryos transferred g) pregnancy rate per woman h) miscarriage rate i) serum E2 levels on day of hCG j) glucose/insulin rate |
|
| Notes | Country of the study: Jordan Prospectively registered at ClinicalTrials.gov (NCT01233206) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Single‐blinded (participants only). |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis was performed. |
| Selective reporting (reporting bias) | Unclear risk | Live birth rate was not reported. |
| Other bias | Low risk | None suspected. |
Tang 2006.
| Study characteristics | ||
| Methods |
|
|
| Participants | Diagnosis of PCOS followed the Rotterdam criteria (ESHRE/ASRM).
101 PCOS participants were randomised (52 in the metformin group and 49 in the placebo group). 5 cycles in the metformin group and 2 in the placebo group were abandoned due to poor response. 47 cycles in each arm completed through to oocyte retrieval. Age: 20 to 39 years Causes of infertility not reported. Both groups were matched for mean age, median duration of infertility, BMI, nulliparity, participants who had previous IVF cycle, ICSI cycles. Inclusion criteria: a) serum testosterone concentration < 5.0 nmol/L b) normal prolactin concentration, thyroid, renal, and haematological indices.
a) concurrent hormone therapy within the previous 6 weeks b) any chronic disease that could interfere with the absorption, distribution, metabolism, or excretion of metformin c) renal or liver disease d) systemic disease or diabetes (types 1 and 2) |
|
| Interventions | Metformin 850 mg twice a day from the first day of downregulation GnRH‐agonist to the day of oocyte retrieval Protocol for controlled ovarian hyperstimulation: long luteal phase pituitary downregulation using the GnRH analogue nafarelin 600 μg daily (Synarel) with step‐up rec‐FSH (Puregon ‐ starting dose of 100 IU). hCG (Profasi 10,000 IU) was administered when there were more than 3 follicles over 17 mm in diameter. Oocyte retrieval was performed within 36 to 38 h after hCG injection. All follicles with a diameter over 14 mm were aspirated and flushed twice with normal saline when oocytes were not found in the first aspirate. Assisted reproductive technology: IVF or ICSI (4 hours after oocyte retrieval) Embryo transfer: maximum of 2 embryos were transferred per participant on day 2 after follicle puncture under abdominal US guidance Catheter used for transfer: Wallace Luteal phase support: daily Cyclogest pessary (400 mg) was used until the day of pregnancy test |
|
| Outcomes |
a) fertilisation rate
a) number of days of gonadotrophins b) total dose of FSH given during stimulation c) number of follicles (> 14 mm) d) number of oocytes e) number of embryos transferred f) implantation rate g) pregnancy rate per woman h) clinical pregnancy rate per woman i) pregnancy rate per transfer j) clinical pregnancy rate per transfer k) live birth rate l) incidence of OHSS that required hospitalisation m) side effects n) fasting insulin o) fasting glucose p) SHBG q) free androgen index r) testosterone |
|
| Notes | Country of the study: United Kingdom Clinical trial registration number not provided. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate ‐ random numbers table. |
| Allocation concealment (selection bias) | Low risk | Adequate ‐ codes kept by a third party in the trial office. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reasons for withdrawals were reported. ITT analysis was performed. |
| Selective reporting (reporting bias) | Low risk | All main outcomes were reported. |
| Other bias | Low risk | None suspected. |
Visnova 2003.
| Study characteristics | ||
| Methods |
|
|
| Participants | 172 participants were selected, but only 141 were randomised because they met the inclusion criteria for PCOS according to the Rotterdam criteria (ESHRE/ASRM).
72 women in the metformin group (I), 69 in the no‐treatment group (II), and 31 in the no‐PCOS participants group (III) started controlled ovarian hyperstimulation.
1 woman in the metformin group (I) and 3 in the no‐treatment group (II) were excluded due to poor response to ovulation induction.
Infertility factors were not reported.
Both groups were matched for age, BMI, and hormonal parameters.
|
|
| Interventions | Metformin 500 mg twice a day from the first day of ovulation induction to the day of oocyte retrieval Protocol for ovulation induction: long protocol GnRH‐agonist with rec‐FSH or hp‐FSH (150 to 225 IU/day) The following were not reported: a) which hCG was used; b) when the oocyte retrieval was performed; c) how many embryos were transferred; d) luteal phase support medication used. | |
| Outcomes |
a) incidence of OHSS
a) pregnancy rate b) number of oocytes retrieved c) total dose of FSH (IU) d) number of days of gonadotrophin treatment |
|
| Notes | Country of the study: the Czech Republic Clinical trial registration number not provided. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reasons for withdrawals were reported. However, 4 women were excluded due to poor response to ovulation induction after randomisation and were not included in the ITT analysis in the study. |
| Selective reporting (reporting bias) | Unclear risk | Live birth rate was not assessed. |
| Other bias | Unclear risk | Infertility factors were not reported. |
ART: assisted reproductive technology BMI: body mass index CI: confidence interval E2: oestradiol ESHRE/ASRM: European Society of Human Reproduction and Embryology/American Society for Reproductive Medicine FSH: follicle‐stimulating hormone GnRH: gonadotrophin‐releasing hormone hCG: human chorionic gonadotrophin hMG: human menopausal gonadotrophin hp‐FSH: highly purified follicle stimulating hormone ICSI: intracytoplasmic sperm injection ITT: intention‐to‐treat IU: international units IVF: in vitro fertilisation mIU: milli‐international units OC: oral contraceptive OCP: oral contraceptive pill OHSS: ovarian hyperstimulation syndrome OR: odds ratio PCOS: polycystic ovary syndrome RCT: randomised controlled trial rec‐FSH: recombinant human follicle stimulating hormone SC: subcutaneous SD: standard deviation SE: standard error SHBG: sex hormone‐binding globulin US: ultrasonography
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abdalmageed 2019 | Not a randomised controlled trial. |
| Akbari 2010 | The study was published only as the abstract of a congress oral presentation and the authors did not answer our email to send study data. |
| Demirol 2006 | Not a randomised controlled trial. |
| Egbase 2001 | Data were not properly reported. The study did not state how many participants withdrew from each group (1 and 2). Moreover, the authors do not present means and standard deviations or standard errors. |
| Geusa 2002 | The study was excluded because of data irregularities (summarised reported data were not consistent with table data). We were not successful in contacting the authors for clarification. |
| Ghasemi 2012 | Women who had a history of unexplained recurrent pregnancy loss and PCOS, not just PCOS, were enrolled. |
| He 2019 | Retrospective data analysis. |
| Immediata 2014 | Metformin versus myo‐inositol. Different interventions were used. |
| Kahraman 2001 | Control group was treated with oral contraceptives instead of placebo or no treatment. |
| Palomba 2011a | Women as potential poor ovarian responders, not just PCOS women, were enrolled. |
| Pourmatroud 2015 | Metformin versus simvastatin. Different interventions were used. |
| Schachter 2007 | Women specifically undergoing ICSI were not randomised separately. |
| Stadtmauer 1999 | Not a randomised controlled trial. Prospective controlled analysis. Participants acted as their own control, when metformin was used in the subsequent cycle. |
| Stadtmauer 2001 | Not a randomised controlled trial. Retrospective data analysis. |
| Stadtmauer 2002 | Not a randomised controlled trial. |
| Swanton 2011 | No PCOS women. Only women with ovaries of polycystic morphology were included. |
| Tasdemir 2004 | Women undergoing ovulation induction cycles, not IVF or ICSI cycles. |
ICSI: intracytoplasmic sperm injection IVF: in vitro fertilisation PCOS: polycystic ovary syndrome
Characteristics of studies awaiting classification [ordered by study ID]
Sun 2016.
| Methods | Prospective randomised trial. |
| Participants | 120 PCOS participants scheduled for first IVF/ICSI were randomly divided into 2 groups: 60 in the metformin group and 60 in the placebo group. Information on diagnosis of PCOS was not provided. |
| Interventions | Metformin versus placebo. |
| Outcomes | Incidence of OHSS, clinical pregnancy rate. |
| Notes | Information was obtained from an abstract of a poster presentation in the ASRM Congress 2015. This abstract was published in the Supplement of Fertility and Sterility September 2016. We contacted the authors for more information about participant characteristics and methodology details. |
ASRM: American Society for Reproductive Medicine ICSI: intracytoplasmic sperm injection IVF: in vitro fertilisation OHSS: ovarian hyperstimulation syndrome PCOS: polycystic ovary syndrome
Differences between protocol and review
The following outcomes were in the original protocol and have since been removed: clinical pregnancy rate (per transfer), pregnancy rate (per transfer and per woman), number of follicles, and embryo quality (Methods; Types of outcome measures). Absolute risk was calculated for the primary outcomes.
After the publication of the protocol we decided to stratify the main analysis by type of stimulation protocol used (long GnRH‐agonist or short GnRH‐antagonist) in order to determine whether the type of stimulation used influenced the outcomes.
In the 2020 updated version we made the following changes:
clinical pregnancy rate per woman was changed to a secondary outcome;
estimate effects were changed from odds ratio to risk ratios;
miscarriage rate was reported as per woman and per pregnancy.
Contributions of authors
For the current update of this review, the contributions are as follows.
LT: contributed to the protocol and the initial version of the review; revised and updated the review.
MFC: contributed to the protocol and the initial version of the review; revised and updated the review.
LA: initiated and conceptualised the protocol and the initial version of the review; revised and updated the review.
RBA: initiated and conceptualised the protocol and the initial version of the review; revised and updated the review.
CRM: revised and updated the review.
Sources of support
Internal sources
Federal University of São Paulo (UNIFESP/EPM), Brazil
External sources
Nuffield Department of Obstetrics and Gynaecology, UK
School of Women's and Children's Health, Division of Obstetrics and Gynecology, Royal Hospital for Women, Australia
Declarations of interest
Leopoldo de Oliveira Tso: none known.
Dr Michael Costello has declared shares in Virtus Health and past sponsorship from Merck Serono for scientific conference presentations. These relationships are declared in the interests of transparency and do not constitute a conflict of interest in this review.
Luiz Eduardo T Albuquerque: none known.
Regis B Andriolo: none known.
Cristiane R Macedo: none known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
An 2014 {published data only}
- An Y, Sun Z, Zhang Y, Liu B, Guan Y, Lu M. The use of berberine for women with polycystic ovary syndrome undergoing IVF treatment. Clinical Endocrinology 2014;80(3):425-31. [PMID: ] [DOI] [PubMed] [Google Scholar]
Cheraghi 2018 {published data only}
- Cheraghi E, Mehranjani MS, Shariatzadeh MA, Esfahani MH, Ebrahimi Z. N-Acetylcysteine improves oocyte and embryo quality in polycystic ovary syndrome patients undergoing intracytoplasmic sperm injection: an alternative to metformin. Reproduction, Fertility, and Development 2016;28(6):723-31. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Cheraghi E, Soleimani Mehranjani M, Shariatzadeh MA, Nasr Esfahani MH, Ebrahimi Z. Co-Administration of metformin and N-Acetyl cysteine fails to improve clinical manifestations in PCOS individual undergoing ICSI. International Journal of Fertility & Sterility 2014;8(2):119-28. [PMID: ] [PMC free article] [PubMed] [Google Scholar]
- *.Cheraghi E, Soleimani Mehranjani M, Shariatzadeh SMA, Nasr Esfahani MH, Alani B. N-Acetylcysteine compared to metformin, improves the expression profile of growth differentiation factor-9 and receptor tyrosine kinase c-Kit in the oocytes of patients with polycystic ovarian syndrome. International Journal of Fertility & Sterility 2018;11(4):270-8. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Doldi 2006 {published and unpublished data}
- *.Doldi N, Persico P, Di Sebastiano F, Marsiglio E, Ferrari A. Gonadotropin-releasing hormone antagonist and metformin for treatment of polycystic ovary syndrome patients undergoing in vitro fertilization-embryo transfer. Gynecological Endocrinology 2006;22(5):235-8. [DOI] [PubMed] [Google Scholar]
Fedorcsak 2003 {published data only}
- *.Fedorcsak P, Dale PO, Storeng R, Abyholm T, Tanbo T. The effect of metformin on ovarian stimulation and in vitro fertilization in insulin-resistant women with polycystic ovary syndrome: an open-label randomized cross-over trial. Gynecological Endocrinology 2003;17:207-14. [DOI] [PubMed] [Google Scholar]
Jacob 2016 {published data only}
- Jacob SL, Brewer C, Tang T, Picton HM, Barth JH, Balen AH. A short course of metformin does not reduce OHSS in a GnRH antagonist cycle for women with PCOS undergoing IVF: a randomised placebo-controlled trial. Human reproduction (Oxford, England) 2016;31(12):2756-64. [ISRCTN21199799] [PMID: ] [DOI] [PubMed] [Google Scholar]
Kim 2016 {published data only}
- Kim C, Jeung Y, Moon J, Kang B. Effect of metformin treatment on intrafollicular cytokines, ovarian response to gonadotropin and in vitro fertilization outcome in patients with polycystic ovary syndrome. Fertility and Sterility 2016;106(3 Suppl):e259-60. [CTG: ] [CN-01200008]
Kjotrod 2004 {published data only}
- Kjøtrød SB, Romundstad P, Düring V, Sunde A, Carlsen SM. Prospective, randomized trial of metformin and vitamins for the reduction of plasma homocysteine in insulin-resistant polycystic ovary syndrome. Fertility and Sterility 2008;89(3):635-41. [Google Scholar]
- Kjotrod S, Düring V, Sunde A, Carlsen SM. Metformin treatment before IVF/ICSI in polycystic ovary syndrome women: a prospective, randomized, double-blind study. Human Reproduction 2003;18 Suppl 1:42-3. [DOI] [PubMed] [Google Scholar]
- *.Kjotrod SB, Düring V, Carlsen SM. Metformin treatment before IVF/ICSI in women with polycystic ovary syndrome; a prospective, randomized, double blind study. Human Reproduction 2004;19(6):1315-22. [DOI] [PubMed] [Google Scholar]
Kjotrod 2011 {published data only}
- Kjotrod S, Carlsen SM, Rasmussen PE, Holst-Larsen T, Mellembakken J, Thurin-Kjellberg A, et al. Metformin treatment before and during IVF or ICSI in PCOS women with BMI < 28 kg/m2. Human Reproduction 2010;25(Suppl 1):i286-7. [Google Scholar]
- *.Kjotrod SB, Carlsen SM, Rasmussen PE, Holst-Larsen T, Mellembakken J, Thurin-Kjellberg A, et al. Use of metformin before and during assisted reproductive technology in non-obese young infertile women with polycystic ovary syndrome: a prospective, randomized, double-blind, multi-centre study. Human Reproduction 2011;26(8):2045-53. [CTG: ] [PMID: ] [DOI] [PubMed] [Google Scholar]
Onalan 2005 {published data only}
- *.Onalan G, Pabuccu R, Goktolga U, Ceyhan T, Bagis T, Cincik M. Metformin treatment in patients with polycystic ovary syndrome undergoing in vitro fertilization: a prospective randomized trial. Fertility and Sterility 2005;84(3):798-801. [DOI] [PubMed] [Google Scholar]
Palomba 2011 {published data only}
- *.Palomba S, Falbo A, Carrillo L, Villani MT, Orio F, Russo T, et al. Metformin reduces risk of ovarian hyperstimulation syndrome in patients with polycystic ovary syndrome during gonadotropin-stimulated in vitro fertilization cycles: a randomized, controlled trial. Fertility and Sterility 2011;96:1384-90. [CTG: ] [DOI] [PubMed] [Google Scholar]
Qublan 2009 {published data only}
- Qublan HS, Al-Khaderei S, Abu-Salem AN, Al-Zpoon A, Al-Khateeb M, Al-Ibrahim N, et al. Metformin in the treatment of clomiphene citrate-resistant women with polycystic ovary syndrome undergoing in vitro fertilisation treatment: a randomised controlled trial. Journal of Obstetrics and Gynecology 2009;29(7):651-5. [DOI] [PubMed] [Google Scholar]
Tang 2006 {published data only}
- Tang T, Barth JH, Balen AH. Effect of metformin on follicular anti-Mullerian hormone concentrations in women with PCOS undergoing IVF treatment. Human Reproduction 2010;25(Suppl 1):i68. [Google Scholar]
- *.Tang T, Glanville J, Orsi N, Barth JH, Balen AH. The use of metformin for women with PCOS undergoing IVF treatment. Human Reproduction 2006;21(6):1416-25. [DOI] [PubMed] [Google Scholar]
Visnova 2003 {published data only}
- *.Visnova H, Ventruba P, Crha I, Zakova J. Importance of sensitization of insulin receptors in the prevention of ovarian hyperstimulation syndrome [Význam senzitizace inzulinových receptoru pro prevenci ovariálního hyperstimulacního syndromu]. Ceská Gynekologie 2003;68(3):155-62. [PubMed] [Google Scholar]
- Visnova H, Ventruba P, Crha I, Zakova J. The impact of insulin receptor sensitization on prevention of ovarian hyperstimulation syndrome. Human Reproduction 2002;17 Suppl:180. [Google Scholar]
References to studies excluded from this review
Abdalmageed 2019 {published data only}
- Abdalmageed OS, Farghaly TA, Abdelaleem AA, Abdelmagied AE, Ali MK, Abbas AM. Impact of Metformin on IVF Outcomes in Overweight and Obese Women With Polycystic Ovary Syndrome: a Randomized Double-Blind Controlled Trial. Reproductive Sciences (Thousand Oaks, Calif.) 2019;26(10):1336-42.. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Abdalmageed OS, Farghaly TA, Ismail AM, Hurd WW. Impact of metformin on in vitro fertilization outcomes in overweight and obese polycystic ovary syndrome women: a prospective cohort Study. Fertility and Sterility 2016;106(3 Suppl):e262. [Google Scholar]
Akbari 2010 {published data only}
- Akbari AF. Evaluating the effect of the metformin treatment on ICSI in infertile PCOS women. Iranian Journal of Reproductive Medicine 2010;8:8. [Google Scholar]
Demirol 2006 {published data only}
- Demirol A, Sari T, Girgin B, Kent E, Gurgan T. The effect of metformin treatment on the ICSI cycle outcome in PCOS patients. Human Reproduction 2006;21(Suppl 1):i88. [Google Scholar]
Egbase 2001 {published data only}
- *.Egbase PE, Al Sharhan M, Buzaber M, Grudzinskas JG. Prospective randomised study of metformin in IVF and embryo transfer treatment cycles in obese patients with polycystic ovarian syndrome. Human Reproduction 2001;16 Suppl 1:202. [Google Scholar]
Geusa 2002 {published data only}
- *.Geusa S, Stanziano A, Causio F, Pansini N, Sarcina E. The efficacy of insulin-sensitizing agent (metformin) in PCOS and insulin resistance patients undergoing IVF treatment. Human Reproduction 2002;17 Suppl:115. [Google Scholar]
Ghasemi 2012 {published data only}
- Ghasemi N, Jahaninejad T, Sheida E, Aflatoonian A. Effectiveness of Metformin in treatment of infertility and recurrent pregnancy loss in polycystic ovarian syndrome. Iranian journal of reproductive medicine 2012;10:8. [PMC free article] [PubMed] [Google Scholar]
He 2019 {published data only}
- He Y, Lu Y, Zhu Q, Wang Y, Lindheim SR, Qi J, et al. Influence of metabolic syndrome on female fertility and in vitro fertilization outcomes in PCOS women. American journal of obstetrics and gynecology 2019;221(2):138. [DOI] [PubMed] [Google Scholar]
Immediata 2014 {published data only}
- Immediata V, Romualdi D, De Cicco S, Tagliaferri V, Di Florio C, Cirella E, et al. Metformin versus myoinositol: which one is better in obese PCOS patients - a crossover study on clinical, endocrine and metabolic effects. Human Reproduction (Oxford, England) 2014;29:i334. [Google Scholar]
Kahraman 2001 {published data only}
- *.Kahraman S, Vanlioglu F, Yakin K, Cengiz S, Karlikaya G. A comparative trial of metformin and oral contraceptive pretreatment in patients with polycystic ovary syndrome undergoing ICSI for severe male factor infertility. Fertility and Sterility 2001;76 Suppl 1(3):67. [Google Scholar]
Palomba 2011a {published data only}
- Palomba S, Falbo A, Di Cello A, Cappiello F, Tolino A, Zullo F. Does metformin affect the ovarian response to gonadotropins for in vitro fertilization treatment in patients with polycystic ovary syndrome and reduced ovarian reserve? A randomized controlled trial. Fertility and sterility 2011;96(5):1128-33. [PMID: ] [DOI] [PubMed] [Google Scholar]
Pourmatroud 2015 {published data only}
- Pourmatroud E, MohamadJafari R. Comparison of metformin and simvastatin administration in women with polycystic ovary syndrome before intracytoplasmic sperm injection cycle; a prospective, randomized, clinical trial. Human Reproduction (Oxford, England) 2014;29:i334-5. [DOI: 10.1093/humrep/29.Supplement_1.1] [CN-01473786] [DOI] [PMC free article] [PubMed]
- Pourmatroud E, Mohammadjafari R, Roozitalab M. Comparison of Metformin and Simvastatin Administration in Women With Polycystic Ovary Syndrome Before Intra-Cytoplasmic Sperm Injection Cycle: a Prospective, Randomized, Clinical Trial Study. Iranian Red Crescent medical journal 2015;17(12):e20082. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourmatroud E, Mohammad Jafari R. Two protocols for pretreatment in women with polycystic ovary syndrome before intracytoplasmic sperm injection cycle; a prospective, randomized, clinical trial. Iranian journal of reproductive medicine 2014;12(6 Suppl 1):4-5. [CN-01107870]
Schachter 2007 {published data only}
- *.Schachter M, Raziel A, Strassburger D, Rotem C, Ron-El R, Friedler S. Prospective, randomized trial of metformin and vitamins for the reduction of plasma homocysteine in insulin-resistant polycystic ovary syndrome. Fertility and Sterility 2007;88(1):227-30. [DOI] [PubMed] [Google Scholar]
Stadtmauer 1999 {published data only}
- Stadtmauer L, Riehl R, Toma S, Talbert L. Use of metformin in patients with PCOS undergoing IVF-ET improves outcomes. International Journal of Gynecology and Obstetrics 2000;70:B41. [Google Scholar]
- *.Stadtmauer LA, Riehl RM, Toma SK, Huang S, Barker S, Talbert LM. Metformin treatment of patients with polycystic ovarian syndrome undergoing IVF increases the number of mature oocytes, the fertilization rate and the number of embryos with changes in the levels of insulin-like growth factor. Fertility and Sterility 1999;72 Suppl 1(3):12. [Google Scholar]
Stadtmauer 2001 {published data only}
- *.Stadtmauer LA, Toma SK, Riehl RM, Talbert LM. Metformin treatment of patients with polycystic ovary syndrome undergoing in vitro fertilization improves outcomes and is associated with modulation of the insulin-like growth factors. Fertility and Sterility 2001;75(3):505-9. [DOI] [PubMed] [Google Scholar]
Stadtmauer 2002 {published data only}
- *.Stadtmauer LA, Wong BC, Oehninger S. Impact of metformin therapy on ovarian stimulation and outcome in 'coasted' patients with polycystic ovary syndrome undergoing in-vitro fertilization. Reproductive Biomedicine Online 2002;5(2):112-6. [DOI] [PubMed] [Google Scholar]
Swanton 2011 {published data only}
- Swanton A, Lighten A, Granne I, McVeigh E, Lavery S, Trew G, et al. Do women with ovaries of polycystic morphology without any other features of PCOS benefit from short-term metformin co-treatment during IVF? A double-blind, placebo-controlled, randomized trial. Human Reproduction (Oxford, England) 2011;26(8):2178-84. [DOI: 10.1093/humrep/der120] [CN-01261056] [DOI] [PubMed]
Tasdemir 2004 {published data only}
- Tasdemir S, Ficicioglu C, Yalti S, Gurbuz B, Basaran T, Yildirim G. The effect of metformin treatment to ovarian response in cases with PCOS. Archives of Gynecology and Obstetrics 2004;269(2):121-4. [PMID: ] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Sun 2016 {published data only}
- Sun D, Yao G, Wu L, Wang J, Zhao Z, Zhai J. The effect of metformin on pregnancy outcome, endometrial receptivity & MIRNAs in endometrium of patients with PCOS undergoing IVF/ICSI. Fertility and sterility 2016;106(3):e260. [CN-01200009] [Google Scholar]
Additional references
Aboulghar 2003
- Aboulghar MA, Mansour RT. Ovarian hyperstimulation syndrome: classifications and critical analysis of preventive measures. Human Reproduction Update 2003;9:275-89. [DOI] [PubMed] [Google Scholar]
Adashi 1985
- Adashi EY, Resnick CE, D'Ercole AJ, Svoboda ME, Van Wyk JJ. Insulin-like growth factors as intraovarian regulators of granulosa cell growth and function. Endocrine Review 1985;6:400-20. [DOI] [PubMed] [Google Scholar]
Al‐Inany 2011
- Al-Inany HG, Youssef MA, Aboulghar M, Broekmans F, Sterrenburg M, Smit J, et al. GnRH antagonists are safer than agonists: an update of a Cochrane review. Human Reproduction Update 2011;17:435. [DOI] [PubMed] [Google Scholar]
Attia 2001
- Attia GR, Rainey WE, Carr BR. Metformin directly inhibits androgen production in human thecal cells. Fertility and Sterility 2001;76:517-24. [DOI] [PubMed] [Google Scholar]
Balen 2004
- Balen A. The pathophysiology of polycystic ovary syndrome: trying to understand PCOS and its endocrinology. Best Practice & Research. Clinical Obstetrics & Gynaecology 2004;18:685-6. [DOI] [PubMed] [Google Scholar]
Barbieri 1986
- Barbieri RL, Makris A, Randall RW, Daniels G, Kistner RW, Ryan KJ. Insulin stimulates androgen accumulation in incubations of ovarian stroma obtained from women with hyperandrogenism. Journal of Clinical Endocrinology and Metabolism 1986;62:904-10. [DOI] [PubMed] [Google Scholar]
Barbieri 2000
- Barbieri RL. Induction of ovulation in infertile women with hyperandrogenism and insulin resistance. American Journal of Obstetrics and Gynecology 2000;183:1412-8. [DOI] [PubMed] [Google Scholar]
Costello 2007
- Costello M, Shrestha B, Eden J, Sjoblom P, Johnson N. Insulin-sensitising drugs versus the combined oral contraceptive pill for hirsutism, acne and risk of diabetes, cardiovascular disease, and endometrial cancer in polycystic ovary syndrome. Cochrane Database of Systematic Reviews 2007, Issue 1. Art. No: CD005552. [DOI: 10.1002/14651858.CD005552.pub2] [DOI] [PubMed] [Google Scholar]
Dunaif 1989
- Dunaif A, Segal KR, Futterweit W, Dobrjansky A. Profound peripheral insulin resistance, independent of obesity, in polycystic ovary syndrome. Diabetes 1989;38:1165-74. [DOI] [PubMed] [Google Scholar]
Dunn 1995
- Dunn CJ, Peters DH. Metformin. A review of its pharmacological properties and therapeutic use in non-insulin-dependent diabetes mellitus. Drugs 1995;49:721-49. [DOI] [PubMed] [Google Scholar]
ESHRE/ASRM 2003
- Rotterdam ESHRE/ASRM - Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertility and Sterility 2004;81:19-25. [DOI] [PubMed] [Google Scholar]
Frank 1995
- Frank S. Polycystic ovary syndrome. New England Journal of Medicine 1995;333:853-61. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro GDT. Version accessed 7 February 2019. Hamilton (ON): McMaster University (developed by Evidence Prime). Available at gradepro.org.
Griesinger 2006
- Griesinger G, Diedrich K, Tarlatzis BC, Kolibianakis EM. GnRH-antagonists in ovarian stimulation for IVF in patients with poor response to gonadotrophins, polycystic ovary syndrome, and risk of ovarian hyperstimulation: a meta-analysis. Reproductive Biomedicine Online 2006;13:628-38. [DOI] [PubMed] [Google Scholar]
Higgins 2019
- Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook. [DOI] [PMC free article] [PubMed]
Jungheim 2010
- Jungheim ES, Odibo AO. Fertility treatment in women with polycystic ovary syndrome: a decision analysis of different oral ovulation induction agents. Fertility and Sterility 2010;94(7):2659-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Knochenhauer 1998
- Knochenhauer ES, Key TJ, Kahsar-Miller M, Waggoner W, Boots LR, Azziz R. Prevalence of the polycystic ovary syndrome in unselected black and white women of the southeastern United States: a prospective study. Journal of Clinical Endocrinology and Metabolism 1998;83:3078-82. [DOI] [PubMed] [Google Scholar]
Kocak 2002
- Kocak M, Caliskan E, Simsir C, Haberal A. Metformin therapy improves ovulatory rates, cervical scores and pregnancy rates in clomiphene citrate-resistant women with polycystic ovary syndrome. Fertility and Sterility 2002;77:101-6. [DOI] [PubMed] [Google Scholar]
McKenzie 2020
- McKenzie JE, Brennan SE, Ryan RE, Thomson HJ, Johnston RV. Summarizing study characteristics and preparing for synthesis. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors(s). version 6.1 edition. Cochrane, 2020. [Google Scholar]
Nardo 2001
- Nardo LG, Rai R. Metformin therapy in the management of polycystic ovary syndrome: endocrine, metabolic and reproductive effects. Gynecology and Endocrinology 2001;15:373-80. [DOI] [PubMed] [Google Scholar]
Nestler 1991
- Nestler JE, Powers LP, Matt DW, Steingold KA, Plymate SR, Rittmaster RS, et al. A direct effect of hyperinsulinemia on serum sex hormone-binding globulin levels in obese women with the polycystic ovary syndrome. Journal of Clinical Endocrinology and Metabolism 1991;72:83-9. [DOI] [PubMed] [Google Scholar]
Nestler 2002
- Nestler JE, Stovall D, Akhter N, Iuorno MJ, Jakubowicz DJ. Strategies for the use of insulin-sensitizing drugs to treat infertility in women with polycystic ovary syndrome. Fertility and Sterility 2002;77:209-15. [DOI] [PubMed] [Google Scholar]
Palomba 2010
- Palomba S, Falbo A, Russo T, Orio F, Tolino A, Zullo F. Systemic and local effects of metformin administration in patients with polycystic ovary syndrome (PCOS): relationship to the ovulatory response. Human Reproduction 2010;25(4):1005-13. [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Speroff 1995
- Speroff L, Glass RH, Kase NG. Anovulation and polycystic ovary [Anovulação e o ovário policístico]. In: Clinical Gynecology, Endocrinology and Infertility. 5th edition. São Paulo: Manole, 1995:477-502. [Google Scholar]
Teede 2018
- Teede HJ, Misso ML, Costello MF, Dokras A, Laven J, Moran L, Piltonen T, Norman RJ and International PCOS Network. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Human reproduction 2018;33(9):1602-18. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Thessaloniki ESHRE/ASRM‐Sponsored PCOS 2008
- Thessaloniki ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Consensus on infertility treatment related to polycystic ovary syndrome. Fertility and Sterility 2008;89(3):505-22. [DOI] [PubMed] [Google Scholar]
Tsilchorozidou 2004
- Tsilchorozidou T, Overton C, Conway GS. The pathophysiology of polycystic ovary syndrome. Clinical Endocrinology 2004;60(1):1-17. [DOI] [PubMed] [Google Scholar]
Yarali 2004
- Yarali H, Zeyneloglu HB. Gonadotrophin treatment in patients with polycystic ovary syndrome. Reproductive Biomedicine Online 2004;8:528-37. [DOI] [PubMed] [Google Scholar]


