Abstract
Renal disease is a common health condition that leads to loss of physical function, frailty, and premature loss of independence in addition to other severe comorbidities and increased mortality. Increased levels of physical activity and initiation of exercise training is recommended in the currently guidelines for all patients with renal disease, but participation and adherence rates are low. The barriers to exercise and physical activity in patients with renal disease are not well defined and currently based on patient provider perception and opinion. There have been no published reviews that have synthesized published findings on patient reported barriers to exercise. This integrative literature review therefore aimed to identify the current understanding of patient reported barriers to regular exercise. This integrative review found that patient perceived barriers to exercise are not consistent with the barriers that have been identified by renal disease specialists and healthcare providers, which were disinterest, lack of motivation, and being incapable of exercise. The patient reported barriers identified through this review were complex and diverse, and the most frequently reported patient perceived barrier to exercise was low energy levels and fatigue. It is clear that additional research to identify patient perceived barriers to exercise is needed and that patient directed interventions to address these barriers should be developed. This integrative review provides information to the interdisciplinary nephrology team that can be used to tailor their assessment of barriers to exercise and provide exercise education for patients with renal disease.
Keywords: Renal disease, chronic kidney disease, exercise, barriers
Introduction
Renal disease is a common chronic condition, with 15.23% of the total United States population having diagnosed chronic kidney disease (CKD) stages I through IV [1] and 648,538 people have end stage renal disease (ESRD) [2]. Chronic kidney disease has an estimated worldwide prevalence of 11-13% [3]. Despite medical treatment advances, renal disease remains a debilitating disease with a myriad of consequences, including depression, fatigue, and decreased quality of life [4]. Patients with renal disease are also at risk for developing co-morbid conditions, cardiovascular disease, reduced physical function, and frailty leading to premature loss of independence [5].
Although renal disease symptoms and associated co-morbidities have the potential to be improved with lifestyle interventions, the majority of patients with renal disease are not exercising and daily physical activity levels are below population norms [5-10]. The National Kidney Foundation (NKF) and the Kidney Disease Improving Global Outcomes (KDIGO) have developed physical activity recommendations for patients with renal disease, which are similar to those for other chronic disease populations. Current recommendations include aerobic exercise 30 minutes on most days of the week [11,12]. Observational and epidemiological investigations have demonstrated that patients with CKD participate in physical activity approximately nine days per month [13] and 43.9% of patients with ESRD reported not exercising [14].
Attempts have been made to make exercise more convenient for patients with renal disease, particularly for patients with ESRD on hemodialysis (HD). Intervention studies have instituted intradialytic exercise programs using cycle ergometers and various resistance exercises [15-17]. Despite these attempts at tailored interventions, patients are not adhering to exercise regimens. When Konstantinidou and colleagues compared intradialytic exercise, home-based exercise, and rehabilitation center-based exercise, the group that participated in the intradialytic exercise program had a dropout rate of 16.7%, as compared to 23.8% dropout rate for patients in the rehabilitation center-based group and 16.7% in the home-based exercise group [18].
Despite the recommendations and attempts to integrate exercise into plans of care, it is clear that patients with renal disease are not exercising. In order for clinicians to create tailored exercise interventions that promote exercise adherence, further examination of barriers to exercise is needed. We examined previously published meta-analyses, systematic reviews, and integrative reviews on this topic area. Although several reviews on the benefits of exercise for patients with kidney disease have been published, the search did not yield any meta-analyses, or systematic or integrative reviews dedicated to examining patient reported barriers to regular exercise. Therefore, the aim of this integrative review is to ascertain patient reported barriers to regular exercise for adult patients with CKD and ESRD.
Methods
Seven electronic databases were searched to locate studies on patient reported barriers to exercise: Medline via PubMed, Medline via Ovid, CINAHL via EBSCO, PsychInfo via EBSCO, Embase, ProQuest Dissertations and Theses, and Scopus. The searches included all available literature from the start of the databases through September 2016 to obtain the broadest collection of studies possible. The search terms were determined based on a preliminary review of the literature (see Table 1). The search terms used in each database were chosen based on database preference language (i.e. MeSH, Emtree, CINAHL headings, etc.).
Table 1.
Search Terms
| Exercise OR physical activity OR motor activity |
| AND |
| End stage renal disease OR kidney disease OR chronic renal failure OR hemodialysis OR ESRD OR dialysis |
| OR |
| Chronic kidney disease OR chronic renal insufficiency OR CKD |
| AND |
| Barriers OR contraindications OR hurdles OR compliance OR patient compliance OR adherence OR concordance OR guideline adherence OR self perception OR self concept OR treatment refusal OR motivation OR Health knowledge, attitudes, practice |
We developed inclusion criteria to capture the most relevant research on adult patients with kidney disease and the barriers they report that prevent them from exercising. The inclusion criteria were articles that a) included patients 18 years and older b) included patients with CKD Stage 3-5 or ESRD requiring hemodialysis or peritoneal dialysis c) addressed patient reported barriers to regular exercise d) and were available in English. Studies were excluded if they a) discussed epidemiological associations of exercise limitations and exercise frequency b) listed reasons for not participating in or withdrawing from an exercise intervention study c) focused solely on healthcare provider identified barriers d) or included post-kidney transplant recipients, since this patient population has different characteristics that should be considered separately.
After conducting the search strategy and applying filters (age over 18, English), each title and abstract was reviewed for eligibility. If the abstract was unavailable or if it was unclear if it met criteria, the entire article was obtained and reviewed. After the initial search was concluded, the reference lists of the eligible articles were hand searched for additional studies. The articles were independently reviewed by both authors before inclusion. The final search strategy is outlined in the flow diagram displayed in Figure 1 [19].
Figure 1.
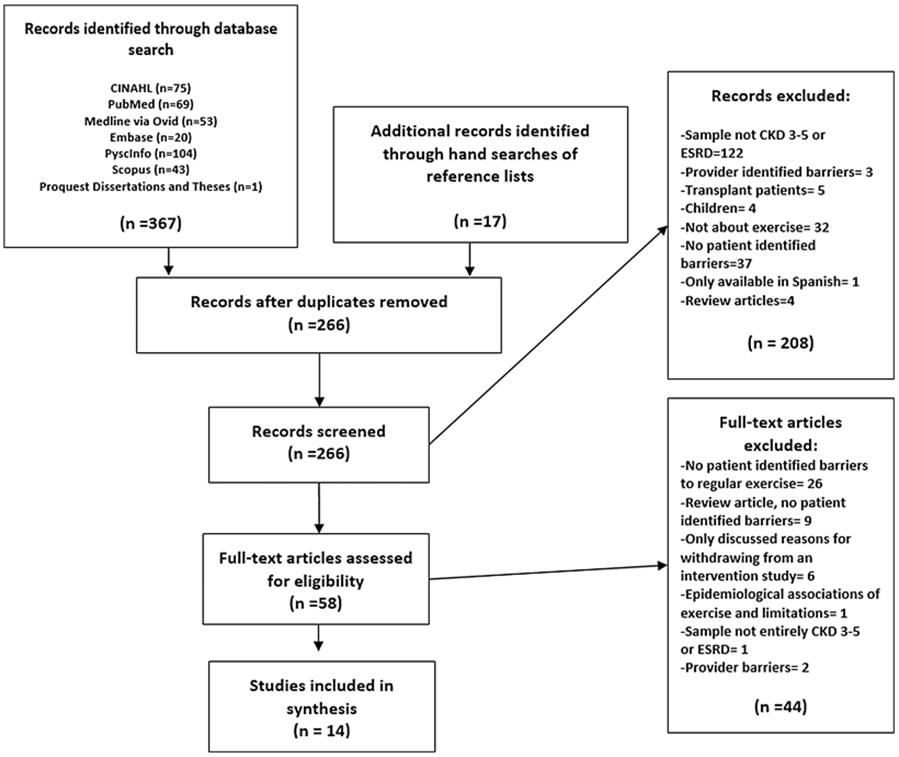
Prisma Flowchart
Results
Through the search and analysis process, 14 papers were identified that covered a 14-year period, from 2001 to 2015, that discussed patient identified barriers to regularly exercising. The final date for the search strategy was September 30, 2016. The individual details of each study are included in Table 2.
Table 2.
Matrix
| Authors, country |
Year | CKD or ESRD |
Purpose | Design related to barriers identification |
Patient Sample | Tool or data collection method utilized |
Most Frequently Reported Barriers |
|---|---|---|---|---|---|---|---|
| Boller United States | 2001 | ESRD- HD | To examine the lived experience of patients on HD and their exercise practices | Mixed method | N= 24; mean age 54.6; 58% male; 21% black, 25% Caucasian, 17% Latino, 38% Asian | Exercise Benefits/Barriers scale (EBBS) and interviews | "Exercise tires me", "I am fatigued by exercise", concerns about vascular access |
| Allen. & Gappmaier United States | 2001 | ESRD-HD | To assess characteristics and attitudes of HD patients that exercise | Mixed method | N= 135; mean age 56+/−17, years; 58% male; 82% Caucasian non-Hispanic | Author created exercise questionnaire; open ended question about barriers | Diabetic neuropathy, visual impairment, weakness, other orthopedic problems |
| Goodman & Ballou United States | 2004 | ESRD- HD | To identify motivators and barriers to exercise for patients on HD | Quantitative Descriptive | N=50; mean age 67.8 +/−10; 60% male; 88% Caucasian | Author developed "The Barriers and Motivators Questionnaire"; Godin Leisure-Time Exercise Questionnaire (GLTEQ) | Lack of motivation (60%), bad weather (50%), fear of falling (50%), being too fatigued(50%), co-morbid health problems (50%) |
| Kolewaski et al. Canada | 2005 | ESRD-HD | To explore the perceptions of patients with ESRD on HD after an 8-week intradialytic exercise program | Qualitative | N=7; mean age 45 +/− 19; sex not specified; race not specified | Interviews | "I just don't have the time to…go to a gym or exercise…" |
| Kontos et al. Canada | 2007 | ESRD-HD | To explore motivators and barriers to exercise participation in patients on HD from the perspective of patients, their family members, and nurses | Qualitative | N= 17 (patients); mean age 74.8+/−6; sex not specified; race not specified | Focus groups | Fatigue, comorbidities, pain, depression, transportation, lack of time |
| Zheng et al. China | 2010 | ESRD-HD | To develop and test the properties of the Dialysis patient-perceived Exercise Benefits and Barriers Scale (DPEBBS) | Mixed method | N=269; mean age 59.67+/− 14.28; 55.39% male; all Chinese | Focus group; Creation of the Dialysis Patient-perceived Exercise Benefits and Barriers Scale (DPEBBS) |
Open ended questions-Environmental factors, lack of time, concern of complications, comorbidities On the DPEBBS- frequent tiredness, worry about falling, "lack of understanding about exercise" |
| Byrne & Russell Ireland | 2011 | ESRD- HD and PD | To determine self-reported activity levels and barriers to increasing physical activity for patients on dialysis | Quantitative Descriptive | N= 78; age range 40-80; sex not specified; race not specified | Author created questionnaire | Being too tired (40%), being incapable of physical activity (15%), lack of time (8%), feeling too out of shape or overweight (8%) |
| Delgado & Johansen United States | 2012 | ESRD- HD | To determine what are the barriers patients on HD have to physical activity | Quantitative Descriptive | N= 100; mean age 60 +/− 15; 73% male; 27% white, 30% African American 21% Hispanic | Author created questionnaire; Physical Activity Scale for the Elderly (PASE); 7-day physical activity recall | Fatigue on HD days (67%), shortness of breath (48%), ‘I don’t want to’ (42%), fatigue on non-HD days (40%) |
| Darawad & Khalil Jordan | 2012 | ESRD-HD | To determine patients' on HD perceived barriers and benefits to exercise and their correlation to demographic characteristics and labs | Quantitative Descriptive | N= 190, mean age 48.2 +/− 14.9; 54.3% male; Jordanian | DPEBBS | "frequent tiredness impedes my exercise participation"(83.6%), "frequent lower extremity fatigue impedes my exercise participation" (79.3%), "body pain impedes my exercise participation"(68.8%), " I worry about a fall during exercise" (70.1%) |
| Bossola et al. Italy | 2014 | ESRD-HD | To assess barriers for patients on HD that do not exercise or exercise minimally | Quantitative Descriptive | N= 105; mean age of inactive subjects 72.8 +/− 10.9, active subjects 64.7+/−15; inactive subjects 52% male, active subjects 51% male; race not specified | Author created questionnaire; Charlson Comorbidity Index (CCI) | Fatigue on dialysis days (67%), reduced walking ability (48%), muscle pain (42%), pain during walking (36%), too many medical problems (36%) |
| Fiaccadori et al. Italy | 2014 | ESRD-HD | To evaluate social, psychological, and clinical barriers to physical activity in patients on HD | Quantitative Descriptive | N=104 (patients); mean age 69; 65% male; race not specified | Katz Independence in daily living questionnaire, Human Activity Profile (HAP); questionnaire created by another author; healthcare provider questionnaire | Fatigue on dialysis days (56.7%), feeling like they have too many medical problems 54.8%), sadness (50%) |
| Clarke et al. United Kingdom | 2015 | CKD Stages 3-5 | To understand beliefs, barriers, and motivators about exercise in patients with CKD | Qualitative | N= 30; mean age 68.6(focus group), 64.1(interviews); focus group 54% male, interview group 65% male; 83% Caucasian, 3% black, 13% Asian | Focus groups and interviews | Comorbid conditions and symptom burden, especially fatigue; fear of injury and aggravating their medical conditions, lack of healthcare provider guidance, weather and lack of facilities |
| Rosa et al. Brazil | 2015 | ESRD- HD | To determine factors associated with leisure time activities | Quantitative Descriptive | N= 98, mean age 51.6+/− 15.7; 58% male; 60% White/other, 40% black | Author created questionnaire; other author created questionnaire; Actigraphy | Feeling too tired (63.3%), CKD (51%), HD treatment (48%), lack of time (39.8%), lack of company (37.8%), lack of money (36/7%), fear of injury (35.7%), disliking exercise (25.5%) |
| Sieverdes et al. United States | 2015 | ESRD- HD | To explore barriers and perceptions of exercise for patients on HD and to determine patient interest in mobile health | Qualitative | N= 22; mean age 46(+/−10.7); 55% male; 82% African American | Interview | Time on dialysis, motivation, fatigue, problems with joints, muscle weakness, pain in the lower extremities during physical activity, stress, depression, employment status, not enough time |
Overview of the studies
Of the 14 studies, five studies took place in the United States [20-24], with the majority of these US studies taking place between 2001 and 2004. Two studies took place in Canada [25,26], two in Italy [27,28], one in the United Kingdom [29], one in Ireland [30], one in Jordan [31], one in Brazil [32], and one in China [33]. Only one study evaluated patients with CKD stages three through five [29]. Only one study evaluated ESRD patients on peritoneal dialysis (PD) and hemodialysis [30]. The remaining twelve studies were solely on patients with ESRD on hemodialysis.
Purpose
For nine of the studies, one of the primary purposes was to determine patient reported barriers to exercise [21,23,24,26-31]. The remaining five studies reported patient identified barriers to exercise, but it was not a primary purpose of the study [20,22,25,32,33].
Study design
Descriptive quantitative studies were utilized by eight of the 14 studies [20,21,23,27,28,30-32]. Survey was the quantitative descriptive research tool utilized in all eight studies. Four studies applied a qualitative method to evaluate patient reported barriers to exercise [24-26,29]. The qualitative studies utilized individual interviews and focus groups. Two studies used a mixed quantitative and qualitative descriptive method [22,33], consisting of interviews or a focus group and surveys. There were no randomized controlled trials or experimental studies.
Quality of Studies
The studies’ quality was assessed utilizing a technique ideal for studies of various designs in an integrative review [34]. The level of quality of the studies ranged from 6-9 (mean= 7.5, SD=1.09). Convenience sampling was utilized by all included studies. Every study had a description of the methodology utilized. Five studies reported narrative statistics [24-26,29,30] and nine studies reported the most common barrier via descriptive statistics [20-23,27,28,31-33]. The full details of the quality assessment are in Table 3.
Table 3.
Quality scoring
| First Author | Study typea | Samplingb | Methodc | Statistical analysis- related to the review questiond |
Score |
|---|---|---|---|---|---|
| Zheng, 2010 | 5 | 1 | 1 | 2 | 9 |
| Boller, 2001 | 5 | 1 | 1 | 2 | 9 |
| Allen, 2001 | 4 | 1 | 1 | 2 | 8 |
| Bossola, 2014 | 4 | 1 | 1 | 2 | 8 |
| Darawad, 2012 | 4 | 1 | 1 | 2 | 8 |
| Delgado, 2012 | 4 | 1 | 1 | 2 | 8 |
| Fiaccadori, 2014 | 4 | 1 | 1 | 2 | 8 |
| Goodman, 2004 | 4 | 1 | 1 | 2 | 8 |
| Rosa, 2015 | 4 | 1 | 1 | 2 | 8 |
| Byrne, 2011 | 4 | 1 | 1 | 1 | 7 |
| Clarke, 2015 | 3 | 1 | 1 | 1 | 6 |
| Kolewaski, 2005 | 3 | 1 | 1 | 1 | 6 |
| Kontos, 2007 | 3 | 1 | 1 | 1 | 6 |
| Sieverdes, 2015 | 3 | 1 | 1 | 1 | 6 |
| Range | 3-5 | 1-1 | 1-1 | 1-2 | 6-9 |
| Mean | 3.86 | 1 | 1 | 1.64 | 7.5 |
Study type: 3=qualitative design; 4=quantitative descriptive design; 5=mixed qualitative and quantitative descriptive; 6=quantitative experimental and quasi-experimental
Sampling: 0=not explained; 1=convenience; 2=purposive or case matching/cohort; 3=random or 100%
Method: 1=methods and tools explained; 0=not explained
Analysis:1=narrative statistics; 2=descriptive statistics; 3= inferential statistics
Sample characteristics
The sample sizes and sample characteristics of the studies varied, as displayed in Table 4. The sample sizes ranged from seven subjects in a qualitative study that utilized interviews [25] to 269 subjects in a descriptive, mixed method study [33]. The average ages of the samples were between 45-80 years old. In studies that reported sample race, the majority of the patients were Caucasian, except for two studies [21,24]. Three studies did not report full demographic information, including gender and race [25,26,30].
Table 4.
Sample Characteristics
| Authors, country |
Year | Sample Size |
Mean Age | Gender | Race |
|---|---|---|---|---|---|
| Boiler United States | 2001 | 24 | 54.6 | 58% male | 21% black, 25% Caucasian, 17% Latino, 38% Asian |
| Allen. & Gappmaier United States | 2001 | 135 | 56+/−17 years | 58% male | 82% Caucasian non-Hispanic |
| Goodman & Ballou United States | 2004 | 50 | 67.8 +/−10 | 60% male | 88% Caucasian |
| Kolewaski et al. Canada | 2005 | 7 | 45 +/−19 | Gender not specified | Race not specified |
| Kontos et al. Canada | 2007 | 17 | 74.8+/−6 | Gender not specified | Race not specified |
| Zheng et al. China | 2010 | 269 | 59.67+/− 14.28 | 55.39% male | All Chinese |
| Byrne & Russell Ireland | 2011 | 78 | Age range 40-80 | Gender not specified | Race not specified |
| Delgado & Johansen United States | 2012 | 100 | 60 +/− 15 | 73% male | 27% white, 30% African American 21% Hispanic |
| Darawad & Khalil Jordan | 2012 | 190 | 48.2 +/− 14.9 | 54.3% male | All Jordanian |
| Bossola et al. Italy | 2014 | 105 | inactive subjects 72.8 +/− 10.9, active subjects 64.7+/−15 | Inactive subjects 52% male, active subjects 51% male | Race not specified |
| Fiaccadori et al. Italy | 2014 | 104 | 69 | 65% male | Race not specified |
| Clarke et al. United Kingdom | 2015 | 30 | 68.6(focus group), 64.1(interviews) | Focus group 54% male, interview group 65% male | 83% Caucasian, 3% black, 13% Asian |
| Rosa et al. Brazil | 2015 | 98 | 51.6+/− 15.7 | 58% male | 60% White/other, 40% black |
| Sieverdes et al. United States | 2015 | 22 | 46 +/−10.7 | 55% male | 82% African American |
Tools utilized
For the descriptive studies, the most prevalent tool utilized was author created questionnaires that assessed patient reported barriers to exercise [20,21,23,27,28,30,32]. Only two of the seven author created questionnaires conducted validity testing prior to survey administration [23,27]. When details were provided, the questions on the author created questionnaires were varied. For example, Allen & Gappmaier’s questionnaire focused on physical limitations as barriers, whereas Goodman and Ballou’s survey assessed physical, emotional, environmental, and financial barriers [20,23]. If an author created tool was not utilized, the Exercise Benefits and Barriers Survey (EBBS) and the Dialysis Patient Exercise Benefits and Barriers Survey (DPEBBS) were the commonly used tools [22,31,33]. In the qualitative and mixed method studies, interviews were the qualitative tool utilized in four studies.
Barriers identified
Patient reported barriers to exercise were identified in all studies. The most common barrier identified was fatigue or lack of energy, which was reported as a barrier in 12 of the 14 studies (see Table 5). The second most commonly reported barrier was co-morbid health conditions, which was noted in eight of the 14 studies [20,23,24,26-29,33]. The third most common barrier was lack of time or access, which was noted in seven of the 14 studies [24-26,29,30,32,33]. Lack of motivation was only, reported in three of the studies [21,23,24].
Table 5.
Fatigue listed as a patient reported barrier to exercise
| Authors | Country | Was fatigue or lack of energy identified as a barrier? |
|---|---|---|
| Boller | United States | Yes |
| Allen. & Gappmaier | United States | No |
| Goodman & Ballou | United States | Yes |
| Kolewaski et al. | Canada | No |
| Kontos et al. | Canada | Yes |
| Zheng et al. | China | Yes |
| Byrne & Russell | Ireland | Yes |
| Delgado & Johansen | United States | Yes |
| Darawad & Khalil | Jordan | Yes |
| Bossola et al. | Italy | Yes |
| Fiaccadori et al. | Italy | Yes |
| Clarke et al. | United Kingdom | Yes |
| Rosa et al. | Brazil | Yes |
| Sieverdes et al. | United States | Yes |
Barriers and physical activity level
Six studies evaluated patient perceived barriers to exercise and self-reported physical activity {21, 23, 27, 28, 30, 32]. One of these studies evaluated these two factors and found that 58% of their sample exercised two days per week or less and the sample’s most frequently reported barrier was ‘being too tired’, which is consistent with our primary findings of fatigue and lack of energy being primary contributors to exercise barriers. The authors did not make influential inferences from the data [30]. Of the remaining five studies that attempted to make associations between barriers and level of physical activity, three studies found that barriers related to medical conditions and symptoms (‘having too many medical problems’ (p<.05), leg and feet ulcers (p<.05), shortness of breath (p=.012), chest pain (p=.029)) were significantly associated with inactivity level [ 21, 27, 28], but these were not the most frequently reported barriers in the samples.
Discussion
We identified fatigue and low energy levels as the most frequently reported barriers to regular exercise in the literature (see table 5). This is not consistent with the barriers that have previously been identified by healthcare providers of patients with renal disease, which were disinterest, lack of motivation, and being incapable of exercise [35-37]. This integrative review suggests that provider perceived barriers to exercise are not the primary barriers to exercise. We were unable to find any exercise intervention research that addressed fatigue as a barrier to exercise. As shown in a Cochrane review of exercise in renal disease, much of the current research on exercise interventions in patients with ESRD focuses on making exercise more convenient by having it occur during, before, or after hemodialysis [38]. These interventions appear to address barriers such as lack of access, transportation, lack of time, and fear of exercising alone, which were not the most commonly identified barriers to exercise in this integrative review. Although these interventions may overcome barriers for some patients, they are not addressing the barrier most commonly reported by patients in the literature.
Fatigue is a well-known, established consequence of renal disease [39]. The pathophysiologic cause of fatigue in patients with renal disease is thought to be multi-factorial, including anemia, uremia, malnutrition, and multiple associated medical conditions [40]. Despite practice changing advances such as the routine use of erythropoietin stimulating agents (ESAs) and improved dialysis clearance with high flux dialyzers, fatigue remains a debilitating symptom that affects the daily lives of patients with renal disease [39]. Through this review, it is evident that fatigue and low energy levels create barriers for participating in self-care activities like exercise. These findings will enable providers to target patient-perceived barriers to exercise and investigate interventions to combat excessive fatigue and low energy level.
Commonly Reported Barriers
The barriers patients with renal disease report that prevent them from regularly exercising are complex and diverse. Twenty-four unique barriers were elucidated through this integrative review (see table 6). There were two other barriers that were most commonly identified in the literature besides low energy and fatigue. Lack of time or access as a barrier was reported in seven of the 14 studies [24-26,29,30,32,33]. This finding, mostly from studies of patient with ESRD, is not surprising given the amount of time patients with ESRD spend receiving dialysis. As previously described, this barrier is being addressed in exercise intervention research. The second most commonly reported barrier to exercise for patients with renal disease was their perception of having too many medical problems. Although it is valid that patients with renal disease have many co-morbidities [41], having multiple medical problems is not an outright contraindication to exercise. This highlights a need for more patient and provider education. It is unclear if the patients in the studies were told they could not exercise because they had too many medical problems or that they assumed they could not exercise due to their medical conditions. This barrier needs exploration with qualitative research to better understand its meaning for patients with renal disease.
Table 6.
Patient reported barriers
| Reported Barrier | Number of Times Found in the Literature |
|---|---|
| Fatigue | 12 |
| Co-morbid Health Conditions | 8 |
| Lack of Time or Access | 7 |
| Fear of Falling | 6 |
| Pain | 5 |
| Depression | 3 |
| Lack of Motivation | 3 |
| Being Incapable of Exercise | 2 |
| Environmental Limitations (weather, air quality, etc.) | 2 |
| "Renal disease" (CKD or HD) | 2 |
| "Being out of shape" | 1 |
| Concern or Complications | 1 |
| Dislike of Exercise | 1 |
| Employment | 1 |
| Exercise Is Tiring | 1 |
| Healthcare Provider Guidance | 1 |
| Lack of Company | 1 |
| Lack of Interest | 1 |
| Lack of Money | 1 |
| Lack of Understanding | 1 |
| Shortness of Breath | 1 |
| Stress | 1 |
| Vascular Access | 1 |
| Weakness | 1 |
Geographic Variations in Barriers
Another interesting finding of this review was the geographic variations found in barriers to exercise. Three of the five United States studies found lack of motivation as a barrier to exercise [21,23,24], which was not a commonly reported barrier in studies that took place outside of the United States. Similarly, one barrier was unique to studies outside of the United States. Two studies that occurred outside of the United States cited access to exercise facilities as a commonly reported barrier to exercise [26,29], which was not one of the most frequently reported barriers in any United States studies. These differences may be related to regional differences, as opposed to cultural or racial differences, since Americans are generally more inactive than residents of other developed countries [42].
Barriers and physical activity level
Six studies attempted to evaluate associations of activity level and number of barriers identified, in addition to frequency of barriers. The barrier that was associated most frequently with inactivity levels in three of the six studies that attempted to analyze these factors was related to medical conditions and symptoms, including chest pain and shortness of breath. Since this was a correlational analysis, causticity cannot be determined. It is therefore unclear if these barriers were uniquely identified by the most inactive patients, or if barriers related to medical conditions and symptoms lead to the greatest inactivity level. It should be noted that self-reported physical activity is notoriously inaccurate with frequently higher physical activity reports, which could significantly influence the results. More research is needed to determine if a patient’s usual physical activity levels, using objective measure of daily physical activity, significantly influence the specific barriers to exercise that they identify.
Barriers and the opinions of the dialysis team
One of the exclusion criteria of our review was papers that solely focused on provider perceived barriers to exercise for patients with renal disease. Three of our included studies that focused on patient barriers to exercise also evaluated provider opinions about exercise [22, 26, 28]. The studies sought out provider perspectives about exercise benefits [28], exercise counseling patterns [28], in-center exercise programs [22] and barriers to exercise [26, 28]. None of the studies drew conclusions about the opinions of the dialysis team influencing the barriers identified by the patients. One study revealed that the nurses and patients reported two similar barriers (lack of time and lack of equipment), but it was not clear if the dialysis teams’ opinions influenced the patients perceived barriers [26]. This is an opportunity for more research to be done if dialysis team opinions directly influenced the barriers identified by patients.
Barriers in patients with access to exercise professionals
One of our exclusion criteria was studies that focused on barriers and reasons for not participating in exercise intervention studies. Two studies in our review assessed barriers to exercise after patients had completed an eight-week intervention exercise program [22, 25]. In both studies, the barriers assessment was done after the completion of the exercise intervention study. These studies identified lack of time [25], fatigue [22], and concerns about vascular access [22] as the most prevalent barriers. Two of these barriers were some of the most frequently reported barriers in our review. Based on this limited evaluation of barriers in patients who had access to exercise professionals and an exercise program, it does not appear that perceived barriers to exercise were significantly different than those who did not have access to these resources. Further investigation is needed to determine if barriers differ for patients who have access to an exercise program and exercise professionals as part of their nephrology care.
Limitations Across Studies
Although rich in information, the studies in this integrative review have some limitations. Some studies did not fully report their demographic data, including sample race and gender [25-28,30,31]. In studies that reported race, there was often a lack of diversity, and the most represented race was Caucasian, ranging from 60% to 88% of the sample [20,23,29,32]. Samples that lack diversity are not representative of the United States dialysis population. The prevalence of ESRD in African Americans is 3.7 times greater as compared to Caucasians [2], which was only represented in one study’s demographics [24]. There is a clear need to investigate barriers to exercise in African Americans, which may differ from those reported in Caucasians. Exercise habits of the general population differ based on age, sex, education, income, and race [43]. It is likely, but not yet clear, that these variations occur in patients with renal disease as well. Delgado and Johansen’s study with a diverse sample of 100 patients refutes this conjecture [21]. Their United States study found that the specific barriers and number of barriers identified by their diverse sample were no different based on race, gender, or income level, although the statistical significance of this finding was not provided [21]. It should be noted that this was a secondary analyses of a small sample size of 100, which is likely too low to enable identification of statistically significant differences in these areas. Further research is needed to assess if barriers are unique to different demographic characteristics in various countries for patients with renal disease.
A limitation of all the studies is their lack of generalizability to the renal disease population at large, since they all utilized convenience sampling methods. Some convenience samples were larger, covering multiple clinics [20,21,24,26,27,29,31-33], whereas others were single clinics [22,23,25,28,30]. Another limitation in sampling was one study’s utilization of only patients on the transplant list [24]. Pre-transplant patients tend to be more active [23], since they must meet strict medical and psychosocial criteria before being placed on the transplant list. Therefore, the results of this study may not be applicable to all patients with renal disease, but it does highlight the need for more research on the exercise habits of pre-transplant patients and what they have done to overcome the common barriers to exercise [24].
Despite the availability of standardized tools to assess barriers to exercise, such as the Exercise Benefits/Barriers Scale [43]and the Dialysis Exercise Benefits/Barriers Scale [33], many authors created their own barriers questionnaires [20,21,23,27,28,30,32]. Only two of the author created questionnaires conducted validity testing prior to administration [23,27]. Five questionnaires were not validated before they were administered [20,21,28,30,32]. Since various barriers surveys were utilized and some were not validated, it is difficult to compare results across studies and determine the strongest barrier.
Assessing patient identified barriers to exercise is complicated, since exercise and health behaviors appear to be influenced by personality traits and external factors [45]. Surveys are beneficial in their ability to obtain information about large numbers of patients, but they do not provide self-report of the behavioral components of exercising or not exercising [43]. This issue was highlighted in Zheng and colleagues’ study which found that patients were more responsive in the open-ended questions rather than the multiple-choice questions in their questionnaire [33]. The authors concluded that this was due to exercise barriers being diverse and complex, which is not as easily captured in a survey. Correlational, survey studies with the addition of open-ended questions or mixed method studies may be the preferred method to obtain depth and breadth on barriers to exercise, but more research is needed to determine the ideal research method.
Future Research
This integrative review highlights the need for more research on patient reported barriers to exercise. The lack of investigations that include patients with CKD highlight an important area of focus for future research. Further research is needed to determine if the barriers for patients with CKD are unique, and whether exercise interventions can be tailored to the needs of patients with CKD. Goodman & Ballou found that 83% of their sample of patients with ESRD reported being more active prior to being on HD [23], which suggests the number of barriers increase and exercise frequency decreases with the initiation of renal replacement therapy. Exercise habits prior to a patient having ESRD were also found to be predictive of exercise habits after a patient has ESRD [20,32]. If a patient exercised three times per week prior to HD initiation, they were twice as likely to continue exercise after HD initiation [20]. This important finding suggests that having patients start exercise regimens prior to initiating dialysis would improve the likelihood of exercise continuation when they have ESRD. This is an area where more investigational and intervention research is greatly needed.
The lack of studies of patients on PD highlights another important area of future research. Our search only yielded one study that included patients on PD, but it was not solely focused on patients on PD [31]. More research is needed on patients on PD, since these patients are generally more active than their counterparts on HD [31,46-48]. It needs to be determined why patients on PD are more active and if they have fewer barriers or more promoters to exercise. If it is determined that patients on PD have fewer barriers to exercise, research is needed to evaluate what this population has done to successfully overcome the barriers to exercise that other patients with renal disease experience.
Implications for Practice
This integrative review highlights important areas where providers can tailor patient education on lifestyle therapies such as exercise. The barriers identified by healthcare providers as why patients don’t exercise do not seem to be the barriers most frequently reported by patients [35,36]. Barriers should not be assumed, but should be investigated with each individual patient. Although a patient may not have barriers at one time, there is no guarantee that barriers won’t develop or change over time. Providers should assess barriers to exercise along the entire continuum and progression of a patient’s renal disease [37].
Providers in the health care team such as nephrologists, nephrology nurses, and allied health professionals have an important role in the assessment and care planning of patients with renal disease who identify fatigue as a barrier to exercise. If fatigue is elucidated as a barrier, further assessment is needed. The characteristics of the fatigue need to be fully assessed and evaluation is needed into reversible causes of fatigue, such as medication side effects, shift work, and sleep patterns [49]. If all reversible causes are ruled out, the nephrology interdisciplinary team should evaluate the patient’s symptoms to determine if there are relieving therapies or interventions that may benefit the patient. In addition to assessment, patient education is a critical component of the nephrology healthcare provider’s role in encouraging exercise and clarifying barriers to exercise [50]. Providers should include self-care teaching, including exercise teaching, in all patient encounters.
Limitations of the Review
Despite efforts for thoroughness and rigor in this integrative review, there are limitations. The criteria omitted articles that were not available in English, which may have excluded pertinent, international studies. The search for grey literature was not exhaustive, since only dissertations and theses were searched. Another limitation was the utilization of the term barrier. Barrier is not the only word used to determine reasons for exercise non-participation. In our search, the term barrier was expanded to include complimentary and synonymous terms preferred by each database (see table 1). Although this process was done, an article may not have been found by the search methods if an author utilized a more abstract term for barriers. The final limitation relates to the quality scoring method. Given the integrative nature of this review, all articles were kept despite their quality and level of evidence. This may have allowed for the inclusion of lower rigor or not representative studies. Despite this fact, the studies with the highest rigor found fatigue and low energy to be one of the most commonly reported barriers [21-23,27,28,31-33]. This integrative review was strong in its search of seven databases, the inclusion of grey literature, and the addition of hand searching references for additional relevant studies.
Conclusion
This is the first integrative review that explored patient perceived barriers to exercise in patients with renal disease and elucidated that barriers to exercise are intricate and varied. We identified for the first time that fatigue and low energy are the most commonly reported barriers to exercise in the literature, which are barriers that are not currently being addressed in exercise intervention research. To develop interventions and provide education to promote exercise in patients with renal disease, knowing the most frequent barriers to exercise is essential for health care providers.
Acknowledgements:
Portions of this manuscript are to be presented at the Sigma Theta Tau International's 28th International Nursing Research Congress, July 2017.
Footnotes
There are no potential conflicts of interest to disclose.
References
- 1.Centers for Disease Control and Prevention (CDC) (2016) Chronic Kidney Disease (CKD) Surveillance Project. https://nccd.cdc.gov/ckd/. Accessed 30 September 2016
- 2.United States Renal Data System (USRDS) (2015) Chapter 1: ESRD Incidence, Prevalence, Patient Characteristics and Modalities. https://www.usrds.org/2015/view/v2_01.aspx. Accessed 30 September 2016
- 3.Hill NR, Fatoba ST, Oke JL, Hirst JA, O'Callaghan CA et al. (2016) Global Prevalence of Chronic Kidney Disease - A Systematic Review and Meta-Analysis. PLoS One 11(7): e0158765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murtagh FEM, Addington-Hall J, Higginson IJ (2007) The Prevalence of Symptoms in End-Stage Renal Disease: A Systematic Review. Adv Chronic Kidney Dis 14(1):82–99. [DOI] [PubMed] [Google Scholar]
- 5.Johansen KL (2007) Exercise in the end-stage renal disease population. J Am Soc Nephrol 18(6):1845–1854. [DOI] [PubMed] [Google Scholar]
- 6.O'Sullivan D, McCarthy G (2007) An exploration of the relationship between fatigue and physical functioning in patients with end stage renal disease receiving haemodialysis. J Clin Nurs 16(11c):276–284. [DOI] [PubMed] [Google Scholar]
- 7.Miller BW, Cress CL, Johnson ME, Nichols DH, Schnitzler MA (2002) Exercise during hemodialysis decreases the use of antihypertensive medications. Am J Kidney Dis 39(4):828–833. [DOI] [PubMed] [Google Scholar]
- 8.Momeni A, Nematolahi A, Nasr M (2014) Effect of intradialytic exercise on echocardiographic findings in hemodialysis patients. Iran J Kidney Dis 8(3):207–211. [PubMed] [Google Scholar]
- 9.Painter P, Carlson L, Carey S, Paul SM, Myll J (2000) Physical functioning and health-related quality-of-life changes with exercise training in hemodialysis patients. Am J Kidney Dis 35(3):482–492. [DOI] [PubMed] [Google Scholar]
- 10.Smart N, McFarlane J, Cornelissen V (2010) The Effect of Exercise Therapy on Physical Function, Biochemistry and Dialysis Adequacy in Haemodialysis Patients: A Systematic Review and Meta-Analysis. Open J Nephrol 03(01):25. [Google Scholar]
- 11.National Kidney Foundation (NKF) (2005) KDOQI Clinical Practice Guidelines for Cardiovascular Disease in Dialysis Patients. Am J Kid Dis 45(Supplement): 3S1–S154. [PubMed] [Google Scholar]
- 12.Kidney Disease Improving Global Outcomes (KDIGO) (2012) KDIGO Clinical Practice Guideline for the Management of Blood Pressure in Chronic Kidney Disease. Kidney Int 2(5):337–405. [Google Scholar]
- 13.Finkelstein J, Joshi A, Hise MK (2006) Association of Physical Activity and Renal Function in Subjects With and Without Metabolic Syndrome: A Review of the Third National Health and Nutrition Examination Survey (NHANES III) Am J Kidney Dis 48(3):372–382. [DOI] [PubMed] [Google Scholar]
- 14.Tentori F, Elder SJ, Thumma J, Pisoni RL, Bommer J, Fissell RB et al. (2010) Physical exercise among participants in the Dialysis Outcomes and Practice Patterns Study (DOPPS): correlates and associated outcomes. Nephrol Dial Transplant 25(9):3050–3062. [DOI] [PubMed] [Google Scholar]
- 15.Deligiannis A, Kouidi E, Tourkantonis A (1999( Effects of physical training on heart rate variability in patients on hemodialysis. Am J Cardiol 84(2):197–202. [DOI] [PubMed] [Google Scholar]
- 16.Kouidi EJ, Grekas DM, Deligiannis AP (2009) Effects of exercise training on noninvasive cardiac measures in patients undergoing long-term hemodialysis: a randomized controlled trial. Am J Kidney Dis 54(3):511–521. [DOI] [PubMed] [Google Scholar]
- 17.Parsons TL, Toffelmire EB, King-VanVlack CE (2004) The effect of an exercise program during hemodialysis on dialysis efficacy, blood pressure and quality of life in end-stage renal disease (ESRD) patients. Clin Nephrol 61(4):261–274. [DOI] [PubMed] [Google Scholar]
- 18.Konstantinidou E, Koukouvou G, Kouidi E, Deligiannis A, Tourkantonis A (2002) Exercise training in patients with end-stage renal disease on hemodialysis: comparison of three rehabilitation programs. J Rehab Med 34(1):40–45. [DOI] [PubMed] [Google Scholar]
- 19.Moher D, Tetlzlaff J, Altman DG, The Prisma Group (2009) Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med 6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Allen K, Gappmaier E (2001) Exercise habits and attitudes of patients undergoing hemodialysis. Cardiopulm Phys Ther J 12(1):11. [Google Scholar]
- 21.Delgado C, Johansen KL (2012) Barriers to exercise participation among dialysis patients. Nephrol Dial Transplant 27(3):1152–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boller JE (20010 The ecology of exercise: an interpretive phenomenological account of exercise in the lifeworld of persons on maintenance hemodialysis. Dissertation, University of California, San Francisco [Google Scholar]
- 23.Goodman ED, Ballou MB (2004) Perceived barriers and motivators to exercise in hemodialysis patients. Nephrol Nurs J 31(1):23–29. [PubMed] [Google Scholar]
- 24.Sieverdes JC, Raynor PA, Armstrong T, Jenkins CH, Sox LR, Treiber FA (2015) Attitudes and perceptions of patients on the kidney transplant waiting list toward mobile health-delivered physical activity programs. Prog Transplant 25(1):26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kolewaski CD, Mullally MC, Parsons TL, Paterson ML, Toffelmire EB, King-Van Vlack CE (2005) Quality of life and exercise rehabilitation in end stage renal disease. CANNT Journal 15(4):22–29. [PubMed] [Google Scholar]
- 26.Kontos PC, Miller KL, Brooks D, Jassal SV, Spanjevic L, Devins GM et al. (2007). Factors influencing exercise participation by older adults requiring chronic hemodialysis: a qualitative study. Int Urol Nephrol 39(4):1303–1311. [DOI] [PubMed] [Google Scholar]
- 27.Bossola M, Pellu V, Di Stasio E, Tazza L, Giungi S, Nebiolo PE (2014) Self-reported physical activity in patients on chronic hemodialysis: correlates and barriers. Blood Purif 38(1):24–29. [DOI] [PubMed] [Google Scholar]
- 28.Fiaccadori E, Sabatino A, Schito F, Angella F, Malagoli M, Tucci M et al. (2014) Barriers to physical activity in chronic hemodialysis patients: a single-center pilot study in an Italian dialysis facility. Kidney Blood Press Res 39(2-3):169–175. [DOI] [PubMed] [Google Scholar]
- 29.Clarke AL, Young HML, Hull KL, Hudson N, Burton JO, Smith AC (2015) Motivations and barriers to exercise in chronic kidney disease: A qualitative study. Nephrol Dial Transplant 30(11):1885–1892. [DOI] [PubMed] [Google Scholar]
- 30.Byrne K, Russell M (2011) Physical activity levels of patients with chronic kidney disease requiring dialysis. Physiotherapy Ireland 32(2):29–33. [Google Scholar]
- 31.Darawad MW, Khalil AA (2013) Jordanian dialysis patients' perceived exercise benefits and barriers: a correlation study. Rehabil Nurs 38(6):315–322. [DOI] [PubMed] [Google Scholar]
- 32.Rosa CSC, Bueno DR, Souza GD, Gobbo LA, Freitas IF, Sakkas GK et al. (2015) Factors associated with leisure-time physical activity among patients undergoing hemodialysis. BMC Neph 16(192). doi: 10.1186/s12882-015-0183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zheng J, You LM, Lou TQ, Chen NC, Lai DY, Liang YY et al. (2010) Development and psychometric evaluation of the Dialysis patient-perceived Exercise Benefits and Barriers Scale. Int J Nurs Stud 47(2):166–180. [DOI] [PubMed] [Google Scholar]
- 34.Olsen J, Baisch MJ (2014) An integrative review of information systems and terminologies used in local health departments. J Am Med Inform Assoc 21(e1):e20–e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johansen KL, Sakkas GK, Doyle J, Shubert T, Dudley RA (2003) Exercise counseling practices among nephrologists caring for patients on dialysis. Am J Kidney Dis 41(1):171–178. [DOI] [PubMed] [Google Scholar]
- 36.Young HM, Hudson N, Clarke AL, Dungey M, Feehally J, Burton JO et al. (2015) Patient and Staff Perceptions of Intradialytic Exercise before and after Implementation: A Qualitative Study. PLoS One 10(6):e0128995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aucella F, Battaglia Y, Bellizzi V, Bolignano D, Capitanini A, Cupisti A. Physical exercise programs in CKD: lights, shades and perspectives [corrected]. J Nephrol. 2015;28(2):143–150. [DOI] [PubMed] [Google Scholar]
- 38.Heiwe S, Jacobson SH (2011) Exercise training for adults with chronic kidney disease. Cochrane DB Syst Rev 10. doi:_ 10.1002/14651858.CD003236.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Polaschek N (2003) Living on dialysis: concerns of clients in a renal setting. J Adv Nurs 41(1):44–52. [DOI] [PubMed] [Google Scholar]
- 40.McCann K, Boore JRP (2000) Fatigue in persons with renal failure who require maintenance haemodialysis. J Adv Nurs 32(5):1132–1142. [DOI] [PubMed] [Google Scholar]
- 41.National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (2016) Manage Patients with CKD: Treat Complications and Comorbidities. https://www.ncbi.nlm.nih.gov/pubmed/. Accessed 11 November 2016.
- 42.Hallal PC, Andersen LB, Bull FC, Guthold R, Haskell W, Ekelund U et al. (2012) Global physical activity levels: surveillance progress, pitfalls, and prospects. Lancet 380(9838):247. [DOI] [PubMed] [Google Scholar]
- 43.Sallis JF, Hovell MF (1990) Determinants of exercise behavior. Exerc Sport Sci Rev 18(1):307–330. [PubMed] [Google Scholar]
- 44.Sechrist KR, Walker SN, Pender NJ (1987) Development and psychometric evaluation of the exercise benefits/barriers scale. Res Nurs Health 10(6):357–365. [DOI] [PubMed] [Google Scholar]
- 45.Ingledew DK, Markland D, Sheppard KE (2004) Personality and self-determination of exercise behaviour. Pers Indiv Differ 36(8):1921–1932. [Google Scholar]
- 46.Wight JP, Edwards L, Brazier J, Walters S, Payne JN, Brown CB (1998) The SF36 as an outcome measure of services for end stage renal failure. Qual Health Care 7(4):209–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hayhurst WSG, Ahmed A (2015) Assessment of physical activity in patients with chronic kidney disease and renal replacement therapy. SpringerPlus 4(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cobo G, Gallar P, Gama-Axelsson T, Di Gioia C, Qureshi AR, Camacho R et al. (2015) Clinical determinants of reduced physical activity in hemodialysis and peritoneal dialysis patients. J Nephrol 28(4):503–510. [DOI] [PubMed] [Google Scholar]
- 49.Newton JL, Jones DEJ (2010) Making sense of fatigue. Occup Med 60(5):326–329. [DOI] [PubMed] [Google Scholar]
- 50.Davies N (2011) Healthier lifestyles: behaviour change. Nursing Times (107)23: 20–23. [PubMed] [Google Scholar]


