Abstract
Anaplasma platys is a tick-transmitted rickettsial pathogen, which is known to be the etiologic agent for cyclic thrombocytopenia in its primary canine host. Infections with this pathogen are also reported in cats, cattle and people. Similarly, Ehrlichia canis is another tick-borne rickettsial pathogen responsible for canine monocytic ehrlichiosis and is also reported to cause infections in people. We describe infections in dogs with these two pathogens on the Caribbean island of Grenada, West Indies by detection using molecular methods. We utilized a 16S rRNA gene-based PCR assay to detect both Ehrlichia and Anaplasma species by screening 155 canine blood samples from asymptomatic dogs. We found 18.7 % of the dogs to be positive for A. platys and 16.8 % for E. canis. Samples that tested positive for A. platys were further assessed by sequence analysis targeting 16S rRNA, 23S rRNA, citrate synthase (gltA) and heat shock protein (groEL) genes. Phylogenetic analysis revealed high correlation of A. platys 16S rRNA and gltA gene sequences with the geographic origins, while 23S rRNA and groEL gene sequences clustered independent of the geographic origins. This study represents an important step in defining the widespread distribution of active rickettsial infections in Caribbean dogs with no apparent clinical signs, thus posing a high risk for canine health and to a lesser extent to humans, as most dogs in the Caribbean are free-roaming.
Keywords: Anaplasma platys, Ehrlichia canis, 16S rRNA, 23S rRNA, Citrate synthase (gltA) and heat shock protein (groEL), Tick-borne diseases, PCR 1
1. Introduction
Anaplasma platys is an obligate intracellular, tick-borne rickettsial pathogen of the family Anaplasmataceae (Dumler et al., 2001), which infects platelets of its primary canine host (Sainz et al., 2015; Harvey et al., 1978). It is the causative agent of infectious canine cyclic thrombocytopenia, which was first described in 1978 by Harvey and colleagues in a dog infection from Florida, USA (Harvey et al., 1978). The biological vector for the pathogen is likely the brown dog tick, Rhipicephalus sanguineus sensu lato (Yuasa et al., 2017; Cicuttin et al., 2015; Ramos et al., 2014; Ybañez et al., 2012), although there is scant evidence for this (Gaunt et al., 2010; Simpson et al., 1991). One recent vector competence study, however, seems to provide strong evidence implicating R. sanguineus sensu stricto as a biological vector of A. platys (Snellgrove et al., 2020). This tick is also the vector for Ehrlichia canis, another Anaplasmataceae pathogen closely related to A. platys. Co-infections with A. platys and E. canis are commonly observed in dogs (Lanza-Perea et al., 2014; Yabsley et al., 2008).
In dogs, clinical signs with A. platys and E. canis infections may vary from asymptomatic to fever, weight loss, depression, weakness/lethargy, anorexia, lymphadenomegaly, splenomegaly, thrombocytopenia and weight loss (Mylonakis and Theodorou, 2017; Sainz et al., 2015; Harrus and Waner, 2011; Komnenou et al., 2007; Harrus et al., 1997a; Harvey et al., 1978). Anaplasma platys infections may result in petechiae, while E. canis may cause nose bleeds in some dog breeds. Ecchymoses and cyclical changes in platelet counts, possibly resulting from infection-associated drop in thrombocytes and host-induced immune countering, occur in dogs with A. platys infections. While it is likely that the canine immune system naturally controls the infection, the infection-associated immune suppression and fluctuating platelet counts may take a serious toll on the animal’s health.
Recent molecular evidence from several studies suggests that A. platys has a global presence in R. sanguineus s.l. ticks and dogs (Latrofa et al., 2014) including in the USA (Diniz et al., 2010; Kordick et al., 1999), Argentina (Cicuttin et al., 2015; Eiras et al., 2013), Brazil (Soares et al., 2017), Grenada (Wilkerson et al., 2017; Lanza-Perea et al., 2014; Yabsley et al., 2008), St. Kitts (Loftis et al., 2013), Haiti (Starkey et al., 2016), Italy (Ramos et al., 2014), Portugal (Cardoso et al., 2010), Malaysia (Low et al., 2018), and Taiwan (Yuasa et al., 2017). Anaplasma platys is also reported to cause infections in domestic cattle in Algeria (Dahmani et al., 2015a), goats in China (Wei et al., 2020), and in cats (Qurollo et al., 2014; Lima et al., 2010) as well as in people in some South American and Caribbean countries (Arraga-Alvarado et al., 2014; Maggi et al., 2013), thus posing threat to wider host species, including humans. Similarly, E. canis infections are frequently reported in various host species in parts of South America (Arroyave et al., 2020; Dumler, 2013; Eiras et al., 2013; Vieira et al., 2011; Vinasco et al., 2007), and Caribbean countries (Gondard et al., 2017; Kelly et al., 2017; Wilkerson et al., 2017; Loftis et al., 2013; Yabsley et al., 2008), as well as in Central America (Springer et al., 2019; Zhang et al., 2015; Romero et al., 2011). Dogs with persistent infections of either pathogen are highly susceptible to secondary infections, in addition to serving as sources of infection for the pathogen acquisition by naïve ticks and their subsequent transmission to naïve dogs as well as to people (Sainz et al., 2015). The brown dog tick, R. sanguineus s.l. has been implicated as the vector for these two pathogens as well as many other zoonotic rickettsial pathogens such as Rickettsia conorii and Rickettsia rickettsii (Dantas-Torres, 2010; Yabsley et al., 2008; Skotarczak, 2003; Lewis et al., 1977; Groves et al., 1975). This ectoparasite is also known to feed on people, although less frequently (Mentz et al., 2016; Dantas-Torres, 2010).
A vast amount of information can be gathered from performing systematic phylogenetic analyses of related species or strains of a species to make inferences on phylogeographic and evolutionary relationships (Martin, 2002). There is little or no information in literature that comprehensively elucidates the phylogenetic relationships between A. platys strains using several genes. Furthermore, the same holds true for phylogenetic sequence analysis comparing A. platys gene sequences with sequences from the Caribbean and other regions of the world. One study was recently carried out in dogs from the French Guyana region of the Caribbean and focused on detecting A. platys from dogs using conventional and quantitative PCR (Dahmani et al., 2015b). This, like other studies, did not perform any phylogenetic analyses, although a few 23S rRNA and 16S rRNA gene sequences are deposited in the NCBI database as unpublished work.
The current study was focused on assessing the infection status in dogs with no apparent clinical signs for A. platys and E. canis from the Caribbean island of Grenada and then mapping sequence similarities of A. platys isolates with other geographic locations around the world. Phylogenetic analyses were performed for A. platys sequences targeting 16S rRNA, 23S rRNA, gltA (citrate synthase), and groEL (Heat-shock protein) genes. Genetic diversity was compared between the newly reported sequences from this study, and other sequences of A. platys reported previously.
2. Materials and methods
2.1. Samples and genomic DNA extraction
A total of 155 blood samples drawn from the cephalic veins of apparently healthy dogs into EDTA tubes were collected from four parishes (St. Andrew, St. David, St. George, St. Mark) in Grenada during the years of 2017 and 2018. The samples were collected at the Junior Surgery laboratory and the St. George’s University Small Animal Clinic. Genomic DNA was extracted from 100 μL volume of blood using the DNeasy Blood and Tissue kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions and eluted in a 100 μL volume of elution buffer, then stored at −20 °C.
2.2. PCR amplification of A. platys 16S rRNA, 23S rRNA, gltA and groEL genes
Firstly, dog genomic DNA samples were screened for the presence of any Ehrlichia and Anaplasma species using a generic primer pair (EHR16S forward and reverse) (Inokuma et al., 2000; Parola et al., 2000) (Table 1). A negative control PCR with no template DNA added was included each time a PCR assay was set up. Similarly, a known positive control reaction was set up, which contained A. platys genomic DNA as the template. PCRs were performed using a GeneAmp 9700 thermocycler (Applied Biosystems, Foster City, California, USA). Briefly, the first PCR was carried out in a 25 μl reaction mixture containing Platinum Taq DNA polymerase (Life Technologies, Carlsbad, CA, USA), 1X PCR Buffer (Life Technologies, Carlsbad, CA, USA), 100 μM dNTPs, 0.25 μM of each primer and 2 μl of DNA template (approximately 100 ng). PCR cycling conditions were as follows; initial denaturation at 94 °C for 3 min followed by 40 cycles of denaturation at 94 °C for 30 s, annealing at 55 °C for 30 s, and extension at 72 °C for 60 s, and then one cycle of extension at 72 °C for 2 min. The products were then stored at 4 °C.
Table 1.
PCR primers used for amplifying various gene segments of Ehrlichia and Anaplasma species in this study.
| Name | Sequence | Annealing Temperature | Size (bp) | Reference(s) |
|---|---|---|---|---|
| EHR16SF | GGTACCYACAGAAGAAGTCC | 55 °C | 345 | Inokuma et al., 2000; Parola et al., 2000 |
| EHR16SR | TAGCACTCATCGTTTACAGC | |||
| Aplatys.16S-F | TTTGTCGTAGCTTGCTAT | 50 °C | 349 | Matei et al., 2016 |
| Aplatys.16S-R | CTTCTGTGGGTACCGTC | |||
| Aplatys-23sF2 | TCGATGGGAATCAGGTTAATATTCCTG | 55 °C | 800 | This study |
| Aplatys-23sR2 | TTGTACTATAAAAGCTGATTTCCG | |||
| Aplatys-groEF | AAGGCGAAAGAAGCAGTCTTA | 54 °C | 724 | Inokuma et al., 2002 |
| Aplatys-groER | CATAGTCTGAAGTGGAGGAC | |||
| Aplatys-gltAF | GACCTACGATCCGGGATTCA | 61 °C | 580 | Silva et al., 2016 |
| Aplatys-gltAR | CCGCACGGTCGCTGTT | |||
| E. canis F | CAATTATTTATAGCCTCTGGCTATAGGA | 58 °C | 475 | Rufino et al., 2013 |
| E. canis R | ATAGGGAAGATAATGACGGTACCTATA |
The second PCR to detect A. platys or E. canis was carried out similarly as previously stated, with the only exception being gene/target specific primers. PCR thermal cycling conditions were done according to published protocols (Table 1). As A. platys 23S rRNA gene sequences were not available in the GenBank database at the time of our investigation, we compared 23S rRNA gene sequences representing Anaplasma marginale and Anaplasma phagocytophilum by sequence alignment to design primers from regions conserved in both species. Primers from the Anaplasma genus-specific regions were then used to amplify A. platys 23S rRNA gene segments. Sequences for oligonucleotide primers used to perform the above outlined PCRs and their predicted annealing temperatures as well as PCR product sizes are presented in Table 1. Products from the first and second PCR were resolved on a 1.5 % agarose gel to identify predicted amplicons. All PCR amplicons were purified using the QIAquick Gel Extraction kit (Qiagen, Hilden, Germany) and quantified using a Nanodrop ND-1000 spectrophotometer (NanoDrop Technologies, Inc., Wilmington, DE, USA). Thereafter, both strands of the amplicons were sequenced by the Sanger sequencing method at MCLAB sequencing laboratory (San Francisco, CA, USA). Samples that tested positive for Anaplasma species by PCR were subsequently analysed for 16S rRNA, citrate synthase (gltA) and heat shock protein (groEL) gene sequences.
2.3. Sequence analysis and phylogenetic tree construction
Nucleotide sequences for the above-mentioned gene targets were edited, assembled and trimmed using Geneious Prime v2020.0.4 (Biomatters Ltd., Auckland, New Zealand,) and subsequently compared with existing sequences in the GenBank database using the BLAST algorithm (Altschul et al., 1990) on NCBI (https://blast.ncbi.nlm.nih.gov/Blast.cgi). Sequences with the highest percentage identities with A. platys sequences on GenBank were aligned using the MUSCLE algorithm in MEGA X v10.1.7 (Kumar et al., 2018). Pairwise distances were computed in MEGA X for the A. platys 16S, 23S, gltA and groEL nucleotide sequences and also compared with A. marginale, A. phagocytophilum, Ehrlichia ruminantium, E. canis and Ehrlichia chaffeensis. Additionally, variance estimation for pairwise analysis for all four gene sequences was carried out by the bootstrap method with 1000 replicates and uniform evolutionary rates among sites. The 16S rDNA, 23S rDNA, gltA and groEL nucleotide sequences were further model tested for Maximum Likelihood (ML), Neighbour Joining (NJ) and Unweighted Pair Group Method with Arithmetic mean (UPGMA) phylogeny methods in MEGA X. The Kimura 2-parameter model (K-2) (Kimura, 1980) with uniform evolutionary rates among sites and the K-2 model with discrete Gamma distribution among sites (K-2 + G) were found to be the best models for ML phylogeny for the 16S rDNA and 23S rDNA sequences, respectively. For both 16S and 23S sequences, NJ and UPGMA were performed using the K-2 model with uniform evolutionary rates as a basis. For gltA and groEL sequences, the T-92 parameter (Tamura 3-parameter) model (Tamura, 1992) with an evolutionary variable rate (T-92 + I) was determined to be the most appropriate for these data sets for ML, while the T-92 model with uniform evolutionary rates was best for NJ and UPGMA analysis. All phylogenetic analysis was implemented using the bootstrap method with 1000 replicates per tree. In toto, for each of the phylogenetic tree building methods (ML, NJ and UPGMA) the dataset with each of the four genes were tested in MEGA X to ascertain the model that was most suitable in generating the most reliable phylogenetic tree.
Homologous sequences from E. ruminantium (strain Welgevonden) were used as an outgroup for A. platys 16S rDNA, 23S rDNA, gltA and groEL (Accession numbers: NR 07413.2, NR 077000.1, NC005295.2 and CR767821.1 respectively) for ML and NJ analysis with rooted trees generated. We also used A. platys 16S rDNA, 23S rDNA, gltA and groEL sequences from the recently published S3 strain of A. platys (Accession number CP04639) from St. Kitts, West Indies (Llanes and Rajeev, 2020), which represents the first A. platys genome to be sequenced. Additionally, homologous A. marginale (Accession no.: CP001079.1 and CP001079), A. phagocytophilum (Accession no.: CP000235.1 and APHH0100001.1), E. canis (Accession no.: CP000107.1, NR 076375 and CP025749), and E. chaffeensis (Accession no.: CP000236.1 and NR 076400) whole genome sequences were included in the phylogenetic analysis to increase tree robustness.
3. Results
3.1. Assessment of canine blood samples randomly collected from Grenada for Ehrlichia and Anaplasma species pathogen infections
One hundred and fifty-five canine blood samples randomly collected from different locations in St. Georges, Grenada, West Indies were assessed by genera-specific PCR targeting Ehrlichia and Anaplasma species 16S rRNA gene segment. Sixty-six samples (42.6 %) tested positive for the predicted amplicons of 345 bp, which were then subjected to DNA sequence analysis. Twenty-nine of the 66 positive samples tested positive for A. platys, while 26 of the remaining samples were identified as E. canis sequences. Due to poor sequence quality, we were unable to obtain the identities of the remaining 11 samples. The overall infection prevalence rate of 18.7 % (29/155) for A. platys and 16.8 % (26/155) for E. canis (Table 2). The A. platys-positive samples were further evaluated by subjecting them to sequence analysis targeting 23S rRNA, gltA and groEL genes. Anaplasma platys 16S rDNA sequences showed >99 % sequence identity with previously reported sequences representing diverse geographic regions (Table 3). Samples from the St. George parish of Grenada had the highest overall A. playts infection prevalence; 30.8 % (12/39), which is nearly double the overall infection prevalence observed for the samples representing other parishes of the island (Table 2). Although St. David parish samples had a higher prevalence (50 %), the number of samples from this location were low (Table 2). Samples from St. Andrews, St. Mark and of unknown origin in Grenada had comparable infection prevalences ranging from 11 to 17%.
Table 2.
Location and number data for canine blood samples used in this study.
| Parish | Total samples | Ehrlichia/ Anaplasma spp. (%) | E. canis (%) | A. platys (%) |
|---|---|---|---|---|
| St. Andrew | 54 | 15 (27.8) | 9 (16.6) | 6 (11.1) |
| St. David | 4 | 3 (75.0) | 1 (25.0) | 2 (50.0) |
| St. George | 39 | 20 (51.3) | 8 (20.5) | 12 (30.8) |
| St. Mark | 12 | 6 (50.0) | 3 (25.0) | 2 (16.7) |
| Unknown | 46 | 22 (48.0) | 5 (10.9) | 7 (15.2) |
| Total | 155 | 66 (42.6) | 26 (16.8) | 29 (18.7) |
NB: Ehrlichia and Anaplasma species., E. canis and A. platys % values were calculated as a % of total number of samples.
Table 3.
Selected 16S rRNA gene sequences from GenBank matching A. platys isolates from this study.
| Accession numbers | Identity (%) | Country |
|---|---|---|
| MK506833 | 99.48 | Cuba |
| MF153975 | 99.48 | Peru |
| MK814421 | 99.22 | South Africa |
| LC269821 | 99.22 | Zambia |
| KU500912 | 99.22 | Malaysia |
| JX118826 | 99.22 | Brazil |
| AF286699 | 99.22 | Thailand |
| KX818218 | 98.92 | India |
| AF303467 | 99.48 | France |
| AF536828 | 99.48 | Japan |
| M82801 | 99.48 | USA |
| KY010669 | 99.48 | Trinidad |
3.2. Phylogenetic analysis
A total of 28, 12, 21 and 18 sequences were used in the generation of the final phylogenetic trees for 16S, 23S, gltA and groEL sequences, respectively. A robust ML phylogenetic trees (and NJ and UPGMA trees also) for A. platys and other Anaplasma species sequences were generated as supported by the high bootstrap values of 99–100 % on the main internal tree nodes for the 16S rRNA, 23S rRNA, gltA and groEL gene sequences. The data correspond well with pairwise analysis data, which show low genetic distance or high similarity between A. platys sequences. Pairwise comparison of A. platys sequences with other closely related Ehrlichia and Anaplasma species from GenBank are presented as supplementary data set (Supplementary Tables 1–4 and Supplementary Data 1–4). The pairwise comparisons showed average nucleotide substitutions of: 0.000–0.059 (16S), 0.051–0.111 (23S), 0.000–0.977 (gltA) and 0.000–0.391 (groEL). As a general observation, Ehrlichia and Anaplasma species sequences clustered into distinct branches according to species and geographic origin for 16S rDNA and gltA phylogenetic trees (Figs. 1 and 3, respectively), whereas the 23S rDNA and groEL gene sequences (Figs. 2 and 4, respectively) grouped together into distinct clusters by species, regardless of the region of the world they originate from. All representative A. platys sequences generated in this study were submitted to GenBank under the accession numbers MW450800–MW450814.
Fig. 1.
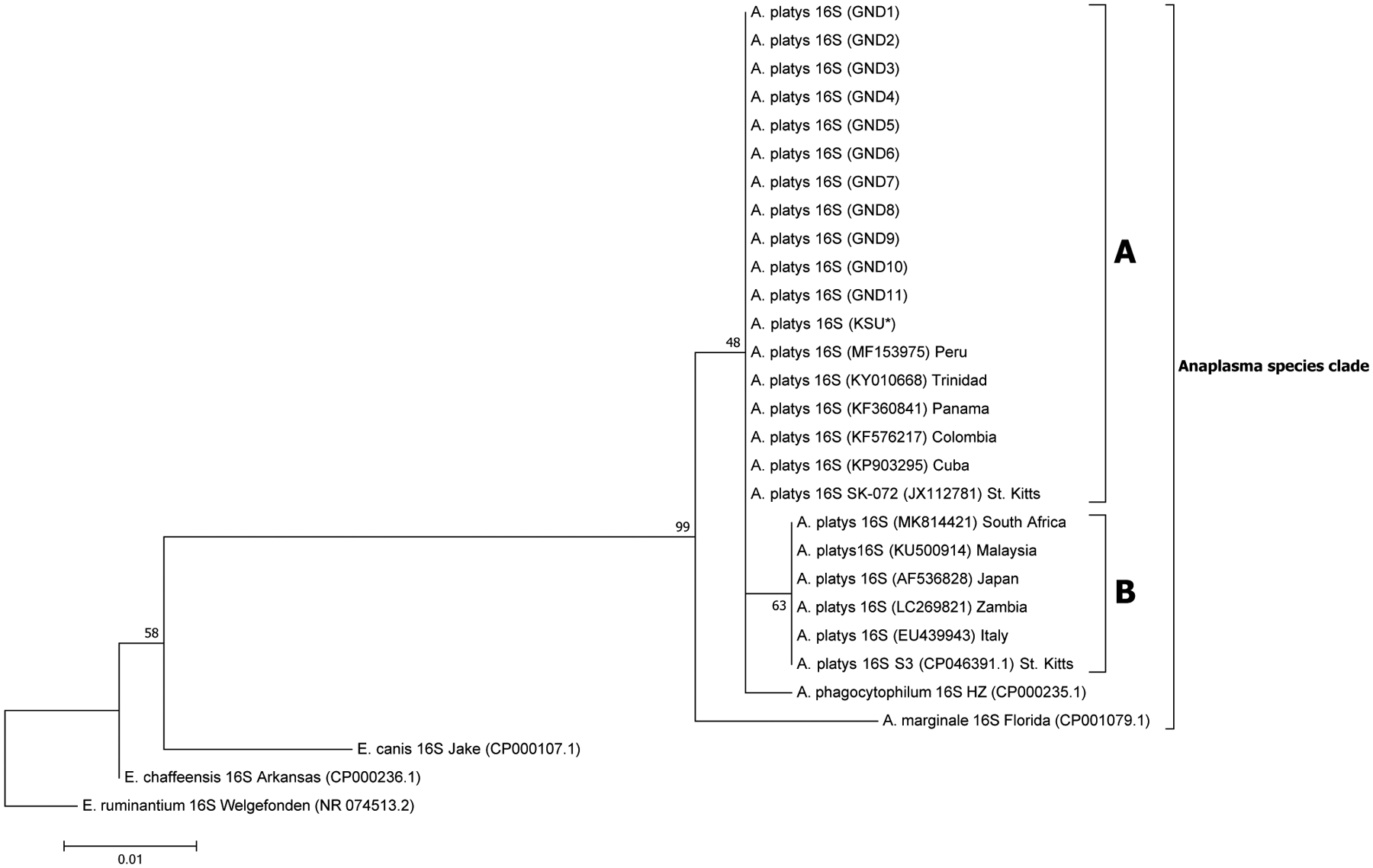
Maximum likelihood (ML) phylogenetic analysis of A. platys 16S rRNA gene sequences. Phylogenetic relationships between A. platys and homologous 16S sequences from A. marginale, A. phagocytophilum, E. canis, E. chaffeensis and E. ruminantium were established. Bootstrap values are shown by numbers at each internal node and these represent the percentage of 1000 replicates for which the same branching pattern was obtained. There were a total of 1456 positions in the dataset. Both NJ and UPGMA analyses for 16S sequences generated trees with similar topology with those derived from ML analysis (Supplementary Fig. 1a and b). A total of 29 sequences were used in the final analysis and the scale-bar represents a 1% nucleotide sequence divergence. Pairwise identity results of these data are given in Supplementary Table 1 and Data 1. (KSU*) = sample obtained from a dog tested positive in Florida, USA. GND = Grenada samples.
Fig. 3.
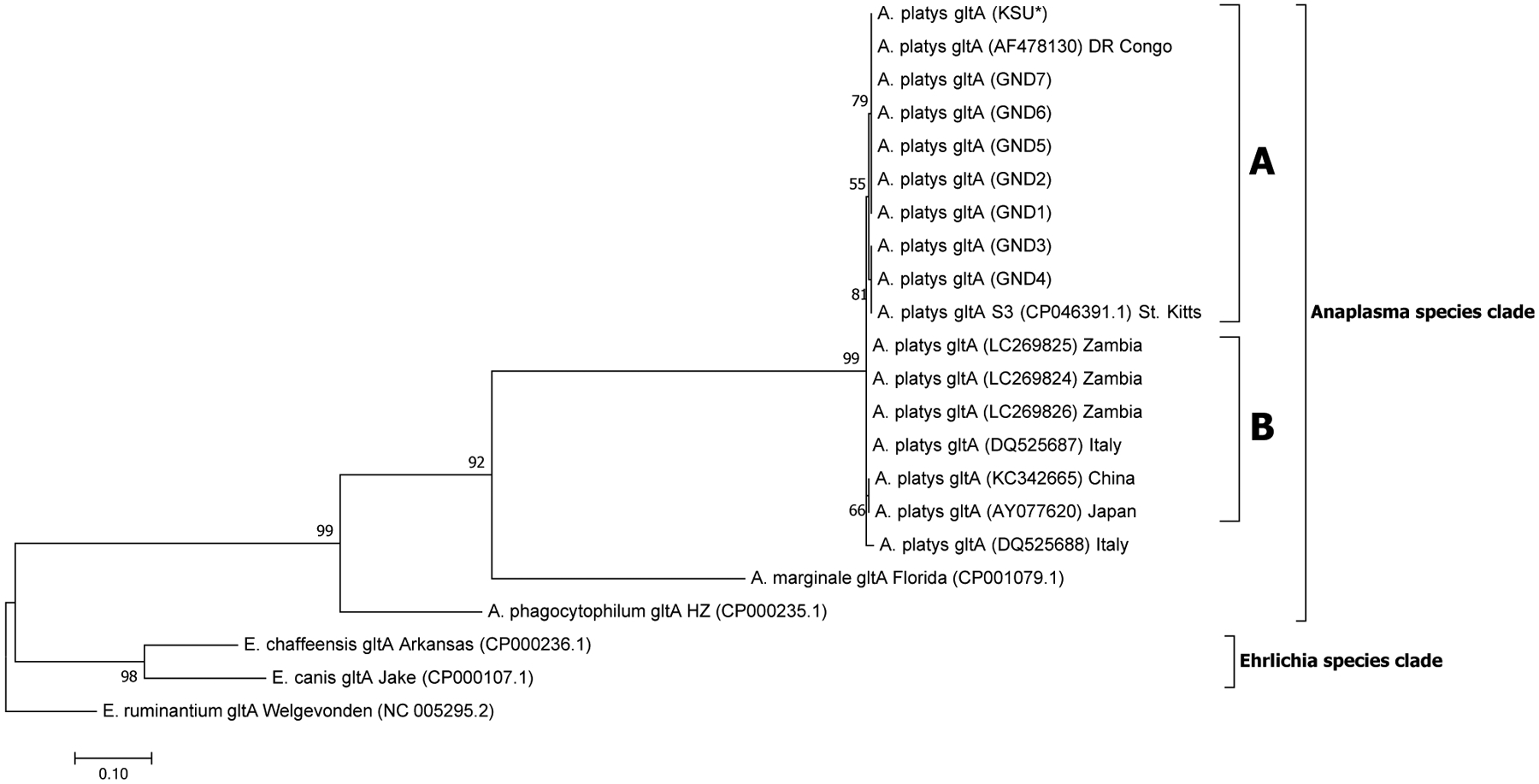
Phylogenetic analysis of A. platys gltA gene sequences. Phylogenetic relationships between A. platys and homologous gltA sequences from A. marginale, A. phagocytophilum, E. canis, E. chaffeensis and E. ruminantium were established. Bootstrap values are shown by numbers at each internal node and represent the percentage of 1000 replicates for which the same branching pattern was obtained. There were a total of 1287 positions in the dataset. The analysis was performed as in Fig. 2. For additional details, please refer to Supplementary Fig. 3a and b. A total of 21 nucleotide sequences were used in the final analysis and the scale-bar represents a 10 % nucleotide sequence divergence. Pairwise identity results are included in Supplementary Table 3 and Data 3.
Fig. 2.
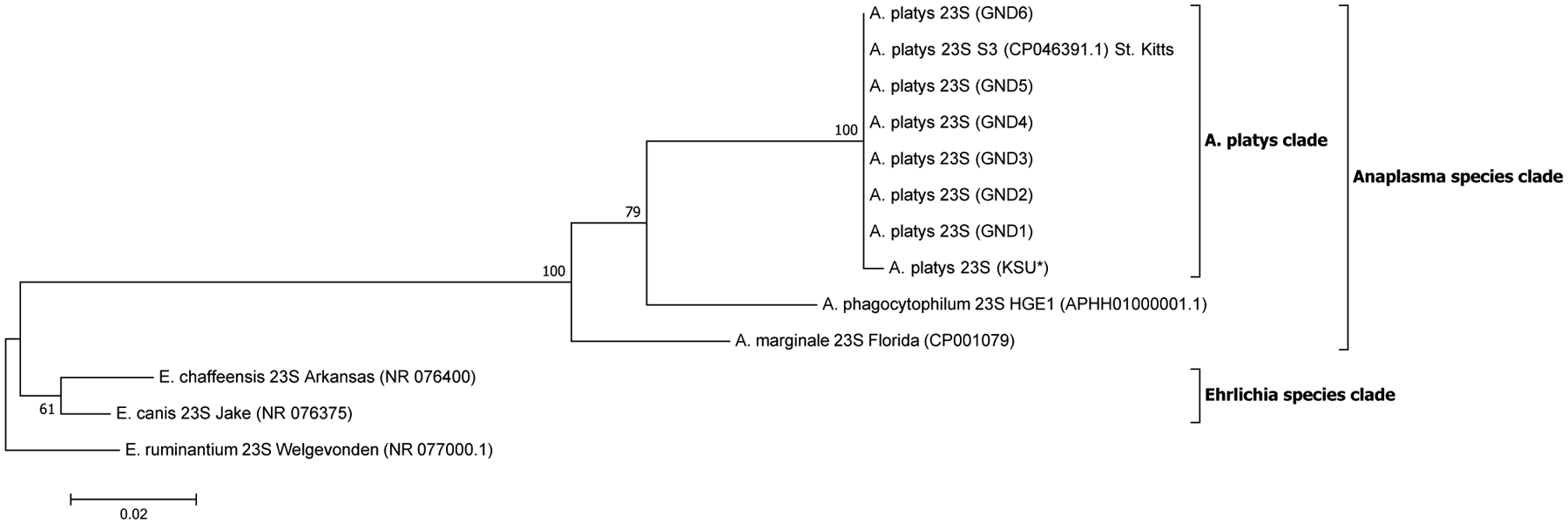
Maximum likelihood (ML) phylogenetic analysis of A. platys 23S rRNA gene sequences. Phylogenetic relationships between A. platys and homologous 23S sequences from A. marginale, A. phagocytophilum, E. canis, E. chaffeensis and E. ruminantium were established. Bootstrap values are shown by numbers at each internal node and represent the percentage of 1000 replicates for which the same branching pattern was obtained. There were a total of 2794 positions in the dataset. Both NJ and UPGMA analyses for 23S sequences generated trees with similar topology with those derived from ML analysis (Supplementary Fig. 2a and b). A total of 13 nucleotide sequences were used in the final analysis and the scale-bar represents a 2% nucleotide sequence divergence. Pairwise identity results of these data are given in Supplementary Table 2 and Data 2. (KSU*) = sample obtained from a dog tested positive in Florida, USA. GND = Grenada samples.
Fig. 4.
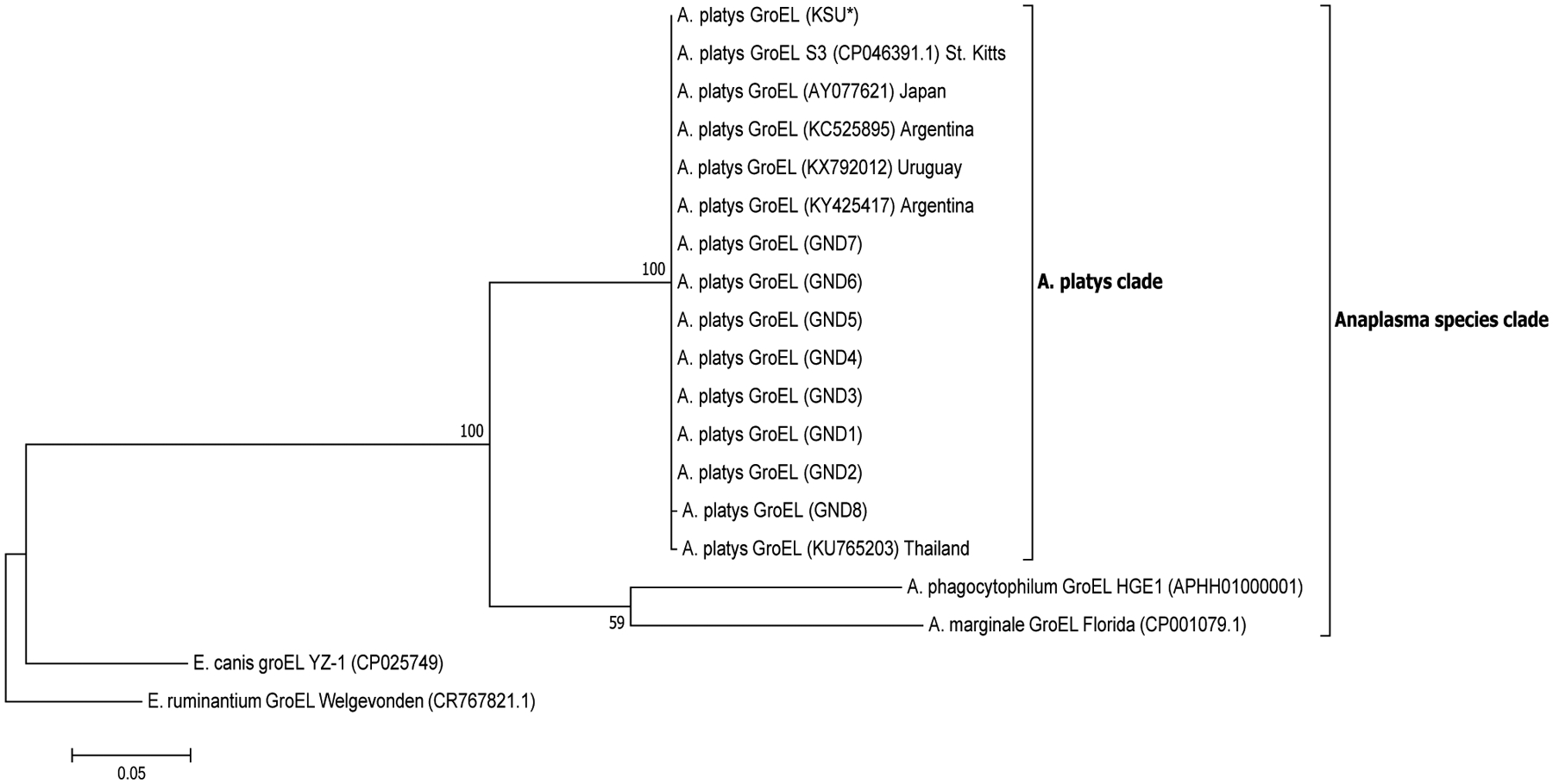
Phylogenetic analysis of A. platys groEL gene sequences. Phylogenetic relationships between A. platys and homologous groEL sequences from A. marginale, A. phagocytophilum, E. canis, E. chaffeensis and E. ruminantium were established. Bootstrap values are shown by numbers at each internal node and represent the percentage of 1000 replicates for which the same branching pattern was obtained. There were a total of 1653 positions in the dataset. The analysis was performed as in the previous two figures. Additional details are provided in Supplementary Fig. 4a and b. A total of 19 nucleotide sequences were used in the final analysis and the scale-bar represents a 5% nucleotide sequence divergence. Pairwise identity results are included in Supplementary Table 4 and Data 4.
4. Discussion
The current study demonstrates the high prevalence of circulating A. platys and E. canis organisms in dogs and validates prior studies regarding the pathogens’ presence identified mostly by serology from the Caribbean island of Grenada (Wilkerson et al., 2017; Lanza-Perea et al., 2014; Yabsley et al., 2008). Further, the study demonstrates that some dogs on the island have active infections while exhibiting no apparent clinical signs. Similar studies reporting PCR positives in asymptomatic dogs have also been conducted in the region, in St. Kitts (Lara et al., 2020; Kelly et al., 2013; Loftis et al., 2013) and Nicaragua (Springer et al., 2018; Wei et al., 2015). These studies further highlight the occurrence and extent of this phenomenon as well as the continued need to monitor such asymptomatic dogs in Grenada and in other parts of the world where the infections may be prevalent. The study also builds on the previous data through targeted sequence analysis of four different A. platys genes, which further confirms the pathogen’s existence by phylogenetic analysis. The data also suggest the importance of rickettsial infections in mostly free-roaming dogs, with A. platys and E. canis serving as potential sources for zoonotic infections for people. No observable and obvious clinical manifestations were apparent in dogs, and these tested positive for the circulating A. platys and/or E. canis organisms, which is similar to a previous study in Grenada (Lanza-Perea et al., 2014). The current study tested for the presence of organisms detectable by molecular analysis and differs from the previous study, which reported seropositives in apparently healthy dogs. Thus, the present study is the first in Grenada documenting detailed molecular evidence for actively circulating A. platys and E. canis organisms in dogs with no observable clinical signs.
Infected animals pose a potential public health problem as they could be asymptomatic, thus posing a challenge for diagnosis, since they may be erroneously missed and not be part of routine investigations (Harrus et al., 1997b; Harvey et al., 1978). Previous studies demonstrate that A. platys infects a range of host species. Besides dogs, the pathogen is reported to infect cats, cattle and people (Dahmani et al., 2015a; Arraga-Alvarado et al., 2014; Qurollo et al., 2014; Maggi et al., 2013; Lima et al., 2010). Human infections with E. canis have also been recorded in Venezuela and the USA (Perez et al., 2006, 1996; Taylor et al., 1988; Fishbein et al., 1987; Maeda et al., 1987)
Dogs with high amounts of actively circulating rickettsial pathogens may serve as important reservoir hosts, thus enabling their consistent spread among dogs, other vertebrates and ticks. The risk of humans acquiring A. platys and E. canis is, therefore, enhanced because dogs are the closest companion animals, independent of their status as free-roaming dogs owned by people in places such as in the Caribbean. Dogs may thus represent an environmental and public health sentinel species to track potential human infections (Reif, 2011; Cleaveland et al., 2006).
The current study location was in tropical climatic conditions where infections with rickettsial pathogens are known to occur in many climatically similar geographic regions of the world in India, Argentina, Brazil and the French Guyana (Manoj et al., 2020; Soares et al., 2017; Cicuttin et al., 2015; Dahmani et al., 2015b). Anaplasma platys and E. canis infections are expected in the Caribbean as recent studies also demonstrated their existence in Grenadian dogs (Wilkerson et al., 2017; Lanza-Perea et al., 2014; Yabsley et al., 2008). Our study revealed an infection prevalence of 18.7 % for A. platys and this was comparable to data from another earlier study from Grenada (Yabsley et al., 2008) and French Guyana (Dahmani et al., 2015b). The aforementioned studies reported infection prevalences of 19.2 % (14/73) for A. platys by PCR targeting a segment of the 16S rRNA gene (Yabsley et al., 2008) and similarly, 15.4 % (10/65) for the 23S rRNA gene segment, also by PCR analysis (Dahmani et al., 2015b). In our current study, we performed a detailed molecular analysis involving PCRs targeting four different gene segments followed by DNA sequence analysis and sequence comparisons to diverse geographic isolates of A. platys, using 155 dog blood samples. Prior PCR-based evidence is also documented for A. platys infection in dogs from various geographic regions of the world, including Brazil (approximately 16 %) (Soares et al., 2017), Mexico (approximately 10 %) (Almazán et al., 2016), USA (4.5–8.3 %) (Diniz et al., 2010), Portugal (three out of four clinically sick animals tested positive) (Cardoso et al., 2010), Malaysia (about 3%) (Low et al., 2018), India (45/230; about 20 %) (Manoj et al., 2020). Infection prevalence in Brazil and India appear to be similar to the current data. Co-infection of dogs with both E. canis and A. platys is not uncommon. In the current study, our data showed a co-infection rate of 4.5 % for A. platys and E. canis. Three PCR-based canine studies in this region of the world also reveal E. canis and A. platys co-infection rates of 19 % in St. Kitts (Lara et al., 2020), 4.7 % in Nicaragua (Springer et al., 2018) and 5% in Costa Rica (Wei et al., 2015). While the St. Kitts study shows a much higher co-infection rate, the Nicaragua and Costa Rica studies show very similar co-infection rates with our study. In a previous study in Grenada using ELISA and IFAT, we reported co-seropositivity in dogs for A. platys and E. canis to be 13 % and 9%, respectively (Wilkerson et al., 2017).
In the recent aforementioned study in St. Kitts (Lara et al., 2020), an antibody-based bead assay detected a co-seropositivity rate of 22 % in dogs. These two antibody based test results are expected as immunological assays are more sensitive and also known to yield more positives due to higher cross-reactions with related rickettsia compared to PCR-based assays (Biggs et al., 2016). Furthermore, the accuracy of these serological assays can vary widely between testing laboratories primarily due to the lack of standardized antigenic targets, cross-reactivity, and subjective establishment of positivity thresholds (Biggs et al., 2016). The difference in results in this and other aforementioned studies may also be explained by variations in experimental factors such as sample numbers, types of assays used to assess infection status and seasonal factors supporting different tick abundances.
The brown dog tick is regarded as the most widespread tick species in the world (Dantas-Torres, 2010) and is often associated with dogs (Latrofa et al., 2014), including Grenada (Yabsley et al., 2008). This tick is the likely vector for A. platys transmission and is also the known vector for E. canis and other important tick-borne pathogens impacting canine and human health (Inokuma et al., 2000; Kordick et al., 1999). In this study, we observed the presence of this tick on nearly all the dogs sampled. Warm and humid tropical climatic conditions that exist in Grenada are favourable for survival and spread of the brown dog tick. This is supported by the previous study reporting the prevalence of R. sanguineus s.l. from nearby Caribbean islands; St. Kitts (Loftis et al., 2013) and Haiti (Starkey et al., 2016). Likewise, the tick’s prevalence is reported from a southern part of India where comparable climatic conditions exist (Manoj et al., 2020). This tick prefers to feed on one host, the domesticated dog, and it is ubiquitous in and around homes. Nonetheless, it is adaptable to parasitizing people in the event that it is unable to find a canine host or when humans are encountered accidentally (Dantas-Torres, 2010; Dantas-Torres et al., 2006). Indeed, several studies documented parasitism of this tick on humans, mostly from the western hemisphere; in Brazil (Dantas-Torres et al., 2006), Uruguay (Venzal et al., 2003), Argentina (Guglielmone et al., 1991), USA (Carpenter et al., 1990) and Italy (Manfredi et al., 1999). Studies also reported fatal outbreaks of Rocky Mountain spotted fever resulting from R. rickettsii-infected R. sanguineus s.l. in the Americas, i.e. in Arizona in the USA (Eremeeva, 2012; Openshaw et al., 2010; Demma et al., 2006, 2005; Nicholson et al., 2006), northern Mexico (Álvarez-Hernández et al., 2017; Eremeeva et al., 2011; Tinoco-Gracia et al., 2009) and Brazil (Cunha et al., 2009; Moraes-Filho et al., 2009). Importantly, recent evidence suggests that this tick vector is likely more significant as a human ectoparasite and also in transmitting pathogens than previously regarded (Paddock et al., 2016; Eremeeva, 2012). The presence of this tick is, therefore, a significant threat to both canine and human health, and thus requires monitoring in and around human dwellings as well as on their companion animals, particularly on dogs (Dantas-Torres et al., 2013).
The phylogenetic data presented in this study for the four gene targets; 16S and 23S rRNA, gltA and groEL genes of A. platys, are in agreement with the taxonomic separation of members of the family Anaplasmataceae into the Ehrlichia and Anaplasma genera, as well as other genera (Neorickettsia and Wolbachia) (Dumler et al., 2001). Our phylogenetic analysis demonstrates that A. platys sequences cluster tightly into an A. platys subclade, followed by A. marginale along with A. phagocytophilum clustering into their own subclades, in accordance with previously identified Anaplasma species similarities (Llanes and Rajeev, 2020; Dumler et al., 2001; Inokuma et al., 2001). Additionally, the A. platys clade containing nucleotide sequences of both 16S rRNA and gltA genes showed similar topology for these phylogenetic trees as they branched out into two distinct sub-groups/clades, A and B (Figs. 1 and 3), in which sequences grouped together according to geographic origin of the samples. The SK-072 and S3 strains of A. platys from St. Kitts, which sit in different clades seem to be indicative of sufficient genetic divergence for these to be placed together with strains from different geographical locations of the world, according to the 16S rRNA gene phylogenetic tree. Despite the accuracy of these sequences, however, this result would have been more reliable if 23S rRNA, gltA and groEL gene sequences from the SK-072 were also available for an assessment of where they sat relative to sequences from other geographic locations, in the respective phylogenetic trees. Subclade A, which we designated the ‘Americas group’, contained the Grenada sequences reported in this study, as well as other Carribean origin sequences reported earlier from Trinidad and St. Kitts (16S rRNA gene sequence from the St. Kitts SK-072 strain A. platys with accession number, JX112781). Sequences in this clade also included sequences from these regions of the world as well those documented from Panama, Peru, Colombia, Cuba and USA (Central, South and North America). Subclade B contained sequences from Africa, Europe and Asia (with the exception of one 16S sequence from the St. Kitts strain of A. platys; accession number CP046391) and therefore, it was designated the ‘Africa/Eurasia group’, with some of the geographically representative sequences being from South Africa, Italy and Japan, respectively. This clustering of A. platys 16S rRNA and gltA gene sequences into subclades A and B suggests a high phylogeographic sequence similarity for these gene sequences, which may point to common origins/ancestry of the two major representative strains documented from this region of the world. This separation into subclades A and B in the 16S rRNA and gltA phylogenetic trees may also imply that two major genetic strains of A. platys with minor variations are circulating in the world, although more data from other regions of the world are needed to reach a more informative conclusion. Genome sequencing or mutli-locus sequence typing of several global strains may also be conducted to shed light on the variation in these and other world strains of A. platys. Furthermore, high sequence similarities observed in strains from different geographical regions in the aforementioned Americas and Africa/Eurasia group clades, may also indicate recent movements of the A. platys pathogen via infected vertebrate hosts and/or tick vectors.
The A. platys 23S rRNA and groEL gene sequences each grouped into one main A. platys subclade regardless of geographic origin, which was not expected. This may be explained by the fact that the sequence variations in the gene loci we used for the phylogenetic tree construction may have had inadequate discriminatory power to separate the A. platys strains. In particular, for the 23S rRNA gene, only few sequences were available in the GenBank database. A potential solution, which may be explored in future studies, is to generate longer PCR amplicons to get more informative sequence data, which may prove to have higher discriminatory power to separate the strains based on 23S rRNA and groEL genes.
5. Conclusion
This work corroborates findings from previous studies as it provides more detailed molecular evidence of A. platys and E. canis infections in apparently healthy dogs from the St. George’s region of Grenada, and also sheds light on the phylogenetic relationships between the Caribbean and other global strains of A. platys, while highlighting the importance and continued utility of molecular methods in performing such studies. Additionally, this study reiterates the need to raise awareness about the presence of A. platys and E. canis, which are among important rickettsial zoonotic pathogens in this area. We anticipate that the information gleaned from this study will build on current data and provide further information on the presence and genetic diversity of A. platys in dogs in the Caribbean, including in Grenada and the regions of the world where these pathogens are more prevalent.
Supplementary Material
Acknowledgements
The authors gratefully acknowledge the assistance of clinicians and technicians at St. George’s University Small Animal Veterinary Clinic and the Junior Surgery laboratory for their assistance with sample collection.
Funding
This study was funded by the Small Research Grant Initiative (GSP\SRGI\16002) and the Postdoctoral Scholars Program (PSP), St. George’s University and the Center of Excellence for Vector-Borne diseases (CEVBD), Kansas State University. This work was also partially supported by the PHS grant AI070908 from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, USA (https://www.niaid.nih.gov/) to R.R.G.
Footnotes
Ethics statement
This study was performed in accordance with guidelines and approval given by the Institutional Animal Care and Use Committee (IACUC-17,006-R) at St. George’s University, School of Veterinary Medicine.
Declaration of Competing Interest
The authors report no declarations of interest.
Appendix A. Supplementary data
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.ttbdis.2021.101727.
References
- Almazán C, Gonźalez-Álvarez VH, Fernández de Mera IG, Cabezas-Cruz A, Rodríguez-Martínez R, de la Fuente J, 2016. Molecular identification and characterization of Anaplasma platys and Ehrlichia canis in dogs in Mexico. Ticks Tick. Borne. Dis 7, 276–283. 10.1016/j.ttbdis.2015.11.002. [DOI] [PubMed] [Google Scholar]
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ, 1990. Basic local alignment search tool. J. Mol. Biol 215, 403–410. 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Álvarez-Hernández G, Roldán JFG, Milan NSH, Lash RR, Behravesh CB, Paddock CD, 2017. Rocky Mountain spotted fever in Mexico: past, present, and future. Lancet Infect. Dis 17, e189–e196. 10.1016/S1473-3099(17)30173-1. [DOI] [PubMed] [Google Scholar]
- Arraga-Alvarado CM, Qurollo BA, Parra OC, Berrueta MA, Hegarty BC, Breitschwerdt EB, 2014. Case report: Molecular evidence of Anaplasma platys infection in two women from Venezuela. Am. J. Trop. Med. Hyg 91, 1161–1165. 10.4269/ajtmh.14-0372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arroyave E, Rodas-González JD, Zhang X, Labruna MB, González MS, Fernández-Silva JA, McBride JW, 2020. Ehrlichia canis TRP36 diversity in naturally infected-dogs from an urban area of Colombia. Ticks Tick. Borne. Dis 11, 101367 10.1016/j.ttbdis.2019.101367. [DOI] [PubMed] [Google Scholar]
- Biggs HM, Behravesh CB, Bradley KK, Dahlgren FS, Drexler NA, Dumler JS, Folk SM, Kato CY, Lash RR, Levin ML, Massung RF, Nadelman RB, Nicholson WL, Paddock CD, Pritt BS, Traeger MS, 2016. Diagnosis and management of tickborne rickettsial diseases: Rocky Mountain spotted fever and other spotted fever group rickettsioses, ehrlichioses, and anaplasmosis — United States. MMWR Recomm. Rep 65, 1–44. 10.15585/mmwr.rr6502a1. [DOI] [PubMed] [Google Scholar]
- Cardoso L, Tuna J, Vieira L, Yisaschar-Mekuzas Y, Baneth G, 2010. Molecular detection of Anaplasma platys and Ehrlichia canis in dogs from the north of Portugal. Vet. J 183, 232–233. 10.1016/j.tvjl.2008.10.009. [DOI] [PubMed] [Google Scholar]
- Carpenter TL, McMeans MC, McHugh CP, 1990. Additional instances of human parasitism by the brown dog tick (Acari: Ixodidae). J. Med. Entomol 27, 1065–1066. 10.1093/jmedent/27.6.1065. [DOI] [PubMed] [Google Scholar]
- Cicuttin GL, Tarragona EL, De Salvo MN, Mangold AJ, Nava S, 2015. Infection with Ehrlichia canis and Anaplasma platys (Rickettsiales: Anaplasmataceae) in two lineages of Rhipicephalus sanguineus sensu lato (Acari: Ixodidae) from Argentina. Ticks Tick. Borne. Dis 6, 724–729. 10.1016/j.ttbdis.2015.06.006. [DOI] [PubMed] [Google Scholar]
- Cleaveland S, Meslin FX, Breiman R, 2006. Dogs can play useful role as sentinel hosts for disease. Nature 440. 10.1038/440605b, 605–605. [DOI] [PubMed] [Google Scholar]
- Cunha NC, Fonseca AH, Rezende J, Rozental T, Favacho ARM, Barreira JD, Massard CL, Lemos ERS, 2009. Primeira identificação de infecção natural por Rickettsia rickettsii no carrapato Rhipicephalus sanguineus no Rio de Janeiro. Pesqui. Vet. Bras 29, 105–108. 10.1590/S0100-736X2009000200003. [DOI] [Google Scholar]
- Dahmani M, Davoust B, Benterki MS, Fenollar F, Raoult D, Mediannikov O, 2015a. Development of a new PCR-based assay to detect Anaplasmataceae and the first report of Anaplasma phagocytophilum and Anaplasma platys in cattle from Algeria. Comp. Immunol. Microbiol. Infect. Dis 39, 39–45. 10.1016/j.cimid.2015.02.002. [DOI] [PubMed] [Google Scholar]
- Dahmani M, Maríe J-L, Mediannikov O, Raoult D, Davoust B, 2015b. First identification of Anaplasma platys in the blood of dogs from French Guiana. Vector-Borne Zoonotic Dis. 15, 170–172. 10.1089/vbz.2014.1720. [DOI] [PubMed] [Google Scholar]
- Dantas-Torres F, 2010. Biology and ecology of the brown dog tick, Rhipicephalus sanguineus. Parasit. Vectors 3, 26. 10.1186/1756-3305-3-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantas-Torres F, Figueredo LA, Brandão-Filho SP, 2006. Rhipicephalus sanguineus (Acari: Ixodidae), the brown dog tick, parasitizing humans in Brazil. Rev. Soc. Bras. Med. Trop 39, 64–67. 10.1590/S0037-86822006000100012. [DOI] [PubMed] [Google Scholar]
- Dantas-Torres F, Latrofa MS, Annoscia G, Giannelli A, Parisi A, Otranto D, 2013. Morphological and genetic diversity of Rhipicephalus sanguineus sensu lato from the New and Old Worlds. Parasit. Vectors 6, 213. 10.1186/1756-3305-6-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demma LJ, Traeger MS, Nicholson WL, Paddock CD, Blau DM, Eremeeva ME, Dasch GA, Levin ML, Singleton J, Zaki SR, Cheek JE, Swerdlow DL, McQuiston JH, 2005. Rocky Mountain spotted fever from an unexpected tick vector in Arizona. N. Engl. J. Med 353, 587–594. 10.1056/NEJMoa050043. [DOI] [PubMed] [Google Scholar]
- Demma LJ, Traeger M, Blau D, Gordon R, Johnson B, Dickson J, Ethelbah R, Piontkowski S, Levy C, Nicholson WL, Duncan C, Heath K, Cheek J, Swerdlow DL, McQuiston JH, 2006. Serologic evidence for exposure to Rickettsia rickettsii in eastern Arizona and recent emergence of Rocky Mountain spotted fever in this region. Vector-Borne Zoonotic Dis. 6, 423–429. 10.1089/vbz.2006.6.423. [DOI] [PubMed] [Google Scholar]
- Diniz PPVP, Beall MJ, Omark K, Chandrashekar R, Daniluk DA, Cyr KE, Koterski JF, Robbins RG, Lalo PG, Hegarty BC, Breitschwerdt EB, 2010. High prevalence of tick-borne pathogens in dogs from an Indian reservation in northeastern Arizona. Vector-Borne Zoonotic Dis. 10, 117–123. 10.1089/vbz.2008.0184 [DOI] [PubMed] [Google Scholar]
- Dumler JS, 2013. Ehrlichiosis y anaplasmosis humanas en América (Human Ehrlichiosis and Anaplasmosis in America). Acta Med. Costarric 1, 34–40. [Google Scholar]
- Dumler JS, Barbet AF, Bekker CP, Dasch GA, Palmer GH, Ray SC, Rikihisa Y, Rurangirwa FR, 2001. Reorganization of genera in the families Rickettsiaceae and Anaplasmataceae in the order Rickettsiales: unification of some species of Ehrlichia with Anaplasma, Cowdria with Ehrlichia and Ehrlichia with Neorickettsia, descriptions of six new species combinations and designation of Ehrlichia equi and ‘HE agent’ as subjective synonyms for Ehrlichia phagocytophila. Int. J. Syst. Evol. Microbiol 51, 2145–2165. 10.1099/00207713-51-6-2145. [DOI] [PubMed] [Google Scholar]
- Eiras DF, Craviotto MB, Vezzani D, Eyal O, Baneth G, 2013. First description of natural Ehrlichia canis and Anaplasma platys infections in dogs from Argentina. Comp. Immunol. Microbiol. Infect. Dis 36, 169–173. 10.1016/j.cimid.2012.11.008. [DOI] [PubMed] [Google Scholar]
- Eremeeva ME, 2012. Molecular epidemiology of rickettsial diseases in North America. Ticks Tick. Borne. Dis 3, 332–337. 10.1016/j.ttbdis.2012.10.022. [DOI] [PubMed] [Google Scholar]
- Eremeeva ME, Zambrano ML, Anaya L, Beati L, Karpathy SE, Santos-Silva MM, Salceda B, Macbeth D, Olguin H, Dasch GA, Aranda CA, 2011. Rickettsia rickettsii in Rhipicephalus ticks, Mexicali, Mexico. J. Med. Entomol 48, 418–421. 10.1603/ME10181. [DOI] [PubMed] [Google Scholar]
- Fishbein DB, Sawyer LA, Holland CJ, Hayes EB, Okoroanyanwu W, Williams D, Sikes RK, Ristic M, McDade JE, 1987. Unexplained febrile illnesses after exposure to ticks: Infection with an Ehrlichia? JAMA 257, 3100–3104. 10.1001/jama.1987.03390220098028. [DOI] [PubMed] [Google Scholar]
- Gaunt S, Beall M, Stillman B, Lorentzen L, Diniz P, Chandrashekar R, Breitschwerdt E, 2010. Experimental infection and co-infection of dogs with Anaplasma platys and Ehrlichia canis: hematologic, serologic and molecular findings. Parasit. Vectors 3, 33. 10.1186/1756-3305-3-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gondard M, Cabezas-Cruz A, Charles RA, Vayssier-Taussat M, Albina E, Moutailler S, 2017. Ticks and tick-borne pathogens of the Caribbean: Current understanding and future directions for more comprehensive surveillance. Front. Cell. Infect. Microbiol 7, 1–16. 10.3389/fcimb.2017.00490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves MG, Dennis GL, Amyx HL, Huxsoll DL, 1975. Transmission of Ehrlichia canis to dogs by ticks (Rhipicephalus sanguineus). Am. J. Vet. Res 36, 937–940. [PubMed] [Google Scholar]
- Guglielmone AA, Mangold AJ, Viñabal AE, 1991. Ticks (Ixodidae) parasitizing humans in four provinces of north-western Argentina. Ann. Trop. Med. Parasitol 85, 539–542. [DOI] [PubMed] [Google Scholar]
- Harrus S, Waner T, 2011. Diagnosis of canine monocytotropic ehrlichiosis (Ehrlichia canis): An overview. Vet. J 187, 292–296. 10.1016/j.tvjl.2010.02.001. [DOI] [PubMed] [Google Scholar]
- Harrus S, Aroch I, Lavy E, Bark H, 1997a. Clinical manifestations of infectious canine cyclic thrombocytopenia. Vet. Rec 141, 247–250. 10.1136/vr.141.10.247. [DOI] [PubMed] [Google Scholar]
- Harrus Shimon, Bark H, Waner T, 1997b. Canine monocytic ehrlichiosis: An update. Compend. Contin. Educ. Pract. Vet 19, 431–444. [Google Scholar]
- Harvey JW, Simpson CF, Gaskin JM, 1978. Cyclic thrombocytopenia induced by a Rickettsia-like agent in dogs. J. Infect. Dis 137, 182–188. 10.1093/infdis/137.2.182. [DOI] [PubMed] [Google Scholar]
- Inokuma H, Raoult D, Brouqui P, 2000. Detection of Ehrlichia platys DNA in brown dog ticks (Rhipicephalus sanguineus) in Okinawa Island. Japan. J. Clin. Microbiol 38, 4219–4221. 10.1128/jcm.38.11.4219-4221.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inokuma H, Terada Y, Kamio T, Raoult D, Brouqui P, 2001. Analysis of the 16S rRNA gene sequence of Anaplasma centrale and its phylogenetic relatedness to other ehrlichiae. Clin. Diagn. Lab. Immunol 8, 241–244. 10.1128/CDLI.8.2.241-244.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inokuma H, Fujii K, Okuda M, Onishi T, Beaufils J, Raoult D, Brouqui P, 2002. Determination of the nucleotide sequences of heat shock operon groESL and the citrate synthase gene (gltA) of Anaplasma (Ehrlichia) platys for phylogenetic and diagnostic studies. Clin. Vaccine Immunol 9, 1132–1136. 10.1128/CDLI.9.5.1132-1136.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly PJ, Xu C, Lucas H, Loftis A, Abete J, Zeoli F, Stevens A, Jaegersen K, Ackerson K, Gessner A, Kaltenboeck B, Wang C, 2013. Ehrlichiosis, babesiosis, anaplasmosis and hepatozoonosis in dogs from St. Kitts, West Indies. PLoS One 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly PJ, Köster L, Li J, Zhang J, Huang K, Branford GC, Marchi S, Vandenplas M, Wang C, 2017. Survey of vector-borne agents in feral cats and first report of Babesia gibsoni in cats on St Kitts, West Indies. BMC Vet. Res 13, 331. 10.1186/s12917-017-1230-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura M, 1980. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol 16, 111–120. 10.1007/BF01731581. [DOI] [PubMed] [Google Scholar]
- Komnenou AA, Mylonakis ME, Kouti V, Tendoma L, Leontides L, Skountzou E, Dessiris A, Koutinas AF, Ofri R, 2007. Ocular manifestations of natural canine monocytic ehrlichiosis (Ehrlichia canis): a retrospective study of 90 cases. Vet. Ophthalmol 10, 137–142. 10.1111/j.1463-5224.2007.00508.x. [DOI] [PubMed] [Google Scholar]
- Kordick SK, Breitschwerdt EB, Hegarty BC, Southwick KL, Colitz CM, Hancock SI, Bradley JM, Rumbough R, Mcpherson JT, MacCormack JN, 1999. Coinfection with multiple tick-borne pathogens in a Walker hound kennel in North Carolina. J. Clin. Microbiol 37, 2631–2638. 10.1128/JCM.37.8.2631-2638.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Stecher G, Li M, Knyaz C, Tamura K, 2018. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol 35, 1547–1549. 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanza-Perea M, Zieger U, Qurollo BA, Hegarty BC, Pultorak EL, Kumthekar S, Bruhl-Day R, Breitschwerdt EB, 2014. Intraoperative bleeding in dogs from Grenada seroreactive to Anaplasma platys and Ehrlichia canis. J. Vet. Intern. Med 28, 1702–1707. 10.1111/jvim.12442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lara B, Conan A, Thrall MA, Ketzis JK, Branford GC, Rajeev S, 2020. Serologic and molecular diagnosis of Anaplasma platys and Ehrlichia canis infection in dogs in an endemic region. Pathogens 9, 1–9. 10.3390/pathogens9060488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latrofa MS, Dantas-Torres F, Giannelli A, Otranto D, 2014. Molecular detection of tick-borne pathogens in Rhipicephalus sanguineus group ticks. Ticks Tick. Borne. Dis 5, 943–946. 10.1016/j.ttbdis.2014.07.014. [DOI] [PubMed] [Google Scholar]
- Lewis GE, Ristic M, Smith RD, Lincoln T, Stephenson EH, 1977. The brown dog tick Rhipicephalus sanguineus and the dog as experimental hosts of Ehrlichia canis. Am. J. Vet. Res 38, 1953–1955. [PubMed] [Google Scholar]
- Lima ML, Soares P, Ramos CA, Araújo F, Ramos RA, Souza II, Faustino MA, Alves LC, 2010. Molecular detection of Anaplasma platys in a naturally-infected cat in Brazil. Braz. J. Microbiol 41, 381–385. 10.1590/S1517-83822010000200019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llanes A, Rajeev S, 2020. First whole genome sequence of Anaplasma platys, an obligate intracellular rickettsial pathogen of dogs. Pathogens 9, 277. 10.3390/pathogens9040277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loftis AD, Kelly PJ, Freeman MD, Fitzharris S, Beeler-Marfisi J, Wang C, 2013. Tick-borne pathogens and disease in dogs on St. Kitts, West Indies. Vet. Parasitol 196, 44–49. 10.1016/j.vetpar.2013.01.024. [DOI] [PubMed] [Google Scholar]
- Low VL, Prakash BK, Lim YA-L, Tan TK, Vinnie-Siow WY, Sofian-Azirun M, AbuBakar S, 2018. Detection of Anaplasmataceae agents and co-infection with other tick-borne protozoa in dogs and Rhipicephalus sanguineus sensu lato ticks. Exp. Appl. Acarol 75, 429–435. 10.1007/s10493-018-0280-9. [DOI] [PubMed] [Google Scholar]
- Maggi RG, Mascarelli PE, Havenga LN, Naidoo V, Breitschwerdt EB, 2013. Co-infection with Anaplasma platys, Bartonella henselae and Candidatus Mycoplasma haematoparvum in a veterinarian. Parasit. Vectors 6, 103. 10.1186/1756-3305-6-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manfredi MT, Dini V, Piacenza S, Genchi C, 1999. Tick species parasitizing people in an area endemic for tick-borne diseases in north-western Italy. Parassitologia 41, 555–560. [PubMed] [Google Scholar]
- Manoj RRS, Iatta R, Latrofa MS, Capozzi L, Raman M, Colella V, Otranto D, 2020. Canine vector-borne pathogens from dogs and ticks from Tamil Nadu. India. Acta Trop 203, 105308 10.1016/j.actatropica.2019.105308. [DOI] [PubMed] [Google Scholar]
- Martin AP, 2002. Phylogenetic approaches for describing and comparing the diversity of microbial communities. Appl. Environ. Microbiol 68, 3673–3682. 10.1128/AEM.68.8.3673-3682.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matei IA, D’Amico G, Yao PK, Ionică AM, Kanyari PWN, Daskalaki AA, Dumitrache MO, Sándor AD, Gherman CM, Qablan M, Modrý D, Mihalca AD, 2016. Molecular detection of Anaplasma platys infection in free-roaming dogs and ticks from Kenya and Ivory Coast. Parasit. Vectors 9, 157. 10.1186/s13071-016-1443-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mentz MB, Trombka M, da Silva GL, Silva CE, 2016. Rhipicephalus sanguineus (Acari: Ixodidae) biting a human being in Porto Alegre City, Rio Grande do Sul, Brazil. Rev. Inst. Med. Trop. Sao Paulo 58, 2–4. 10.1590/S1678-9946201658035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moraes-Filho J, Pinter A, Pacheco RC, Gutmann TB, Barbosa SO, Gonźales MARM, Muraro MA, Cecílio SRM, Labruna MB, 2009. New epidemiological data on Brazilian Spotted Fever in an endemic area of the state of São Paulo. Brazil. Vector-Borne Zoonotic Dis. 9, 73–78. 10.1089/vbz.2007.0227. [DOI] [PubMed] [Google Scholar]
- Mylonakis ME, Theodorou KN, 2017. Canine monocytic ehrlichiosis: an update on diagnosis and treatment. Acta Vet. Brno 67, 299–317. 10.1515/acve-2017-0025. [DOI] [Google Scholar]
- Nicholson WL, Paddock CD, Demma L, Traeger M, Johnson B, Dickson J, McQuiston J, Swerdlow D, 2006. Rocky Mountain spotted fever in Arizona: Documentation of heavy environmental infestations of Rhipicephalus sanguineus at an endemic site. Ann. N. Y. Acad. Sci 1078, 338–341. 10.1196/annals.1374.065. [DOI] [PubMed] [Google Scholar]
- Openshaw JJ, Swerdlow DL, Krebs JW, Holman RC, Mandel E, Harvey A, Haberling D, Massung RF, McQuiston JH, 2010. Rocky Mountain spotted fever in the United States, 2000–2007: Interpreting contemporary increases in incidence. Am. J. Trop. Med. Hyg 83, 174–182. 10.4269/ajtmh.2010.09-0752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paddock CD, Lane RS, Staples JE, Labruna MB, 2016. Changing paradigms for tick-borne diseases in the Americas. In: Mack A (Ed.), Global Health Impacts of Vector-Borne Diseases: Workshop Summary. National Academies Press, Washington, D.C, pp. 221–258. 10.17226/21792. [DOI] [Google Scholar]
- Parola P, Roux V, Camicas J-L, Baradji I, Brouqui P, Raoult D, 2000. Detection of ehrlichiae in African ticks by polymerase chain reaction. Trans. R. Soc. Trop. Med. Hyg 94, 707–708. 10.1016/S0035-9203(00)90243-8. [DOI] [PubMed] [Google Scholar]
- Perez M, Rikihisa Y, Wen B, 1996. Ehrlichia canis-like agent isolated from a man in Venezuela: antigenic and genetic characterization. J. Clin. Microbiol 34, 2133–2139. 10.1128/jcm.34.9.2133-2139.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez M, Bodor M, Zhang C, Xiong Q, Rikihisa Y, 2006. Human infection with Ehrlichia canis accompanied by clinical signs in Venezuela. Ann. N. Y. Acad. Sci 1078, 110–117. 10.1196/annals.1374.016. [DOI] [PubMed] [Google Scholar]
- Qurollo BA, Balakrishnan N, Cannon CZ, Maggi RG, Breitschwerdt EB, 2014. Co-infection with Anaplasma platys, Bartonella henselae, Bartonella koehlerae and ‘Candidatus Mycoplasma haemominutum’ in a cat diagnosed with splenic plasmacytosis and multiple myeloma. J. Feline Med. Surg 16, 713–720. 10.1177/1098612X13519632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos RAN, Latrofa MS, Giannelli A, Lacasella V, Campbell BE, Dantas-Torres F, Otranto D, 2014. Detection of Anaplasma platys in dogs and Rhipicephalus sanguineus group ticks by a quantitative real-time PCR. Vet. Parasitol 205, 285–288. 10.1016/j.vetpar.2014.06.023. [DOI] [PubMed] [Google Scholar]
- Reif JS, 2011. Animal sentinels for environmental and public health. Public Health Rep. 126, 50–57. 10.1177/00333549111260S108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero LE, Meneses AI, Salazar L, Jiménez M, Romero JJ, Aguiar DM, Labruna MB, Dolz G, 2011. First isolation and molecular characterization of Ehrlichia canis in Costa Rica, Central America. Res. Vet. Sci 91, 95–97. 10.1016/j.rvsc.2010.07.021. [DOI] [PubMed] [Google Scholar]
- Rufino CP, Moraes PHG, Reis T, Campos R, Aguiar DCF, McCulloch JA, Meneses AMC, Gonçalves EC, 2013. Detection of Ehrlichia canis and Anaplasma platys DNA using multiplex PCR. Vector-Borne Zoonotic Dis. 13, 846–850. 10.1089/vbz.2013.1303Sainz. [DOI] [PubMed] [Google Scholar]
- Sainz Á, Roura X, Miró G, Estrada-Peña A, Kohn B, Harrus S, Solano-Gallego L, 2015. Guideline for veterinary practitioners on canine ehrlichiosis and anaplasmosis in Europe. Parasit. Vectors 8, 75. 10.1186/s13071-015-0649-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva C.Bda, Santos HA, Navarrete MG, Ribeiro CCDU, Gonzalez BC, Zaldivar MF, Pires MS, Peckle M, Costa R.Lda, Vitari GLV, Massard CL, 2016. Molecular detection and characterization of Anaplasma platys in dogs and ticks in Cuba. Ticks Tick. Borne. Dis 7, 938–944. 10.1016/j.ttbdis.2016.04.012. [DOI] [PubMed] [Google Scholar]
- Simpson RM, Gaunt SD, Hair JA, Kocan KM, Henk WG, Casey HW, 1991. Evaluation of Rhipicephalus sanguineus as a potential biologic vector of Ehrlichia platys. Am. J. Vet. Res 52, 1537–1541. [PubMed] [Google Scholar]
- Skotarczak B, 2003. Canine ehrlichiosis. Ann. Agric. Environ. Med 10, 137–141. [PubMed] [Google Scholar]
- Snellgrove AN, Krapiunaya I, Ford SL, Stanley HM, Wickson AG, Hartzer KL, Levin ML, 2020. Vector competence of Rhipicephalus sanguineus sensu stricto for Anaplasma platys. Ticks Tick. Borne. Dis 11, 101517 10.1016/j.ttbdis.2020.101517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares R, Ramos CA, Pedroso T, Babo-Tera V, Cleveland H, de Araujo F, 2017. Molecular survey of Anaplasma platys and Ehrlichia canis in dogs from Campo Grande, Mato Grosso do Sul, Brazil. An. Acad. Bras. Cienc 89, 301–306. 10.1590/0001-3765201720150556Starkey [DOI] [PubMed] [Google Scholar]
- Springer A, Montenegro VM, Schicht S, Pantchev N, Strube C, 2018. Seroprevalence and current infections of canine vector-borne diseases in Nicaragua. Parasit. Vectors 11, 1–9. 10.1186/s13071-018-3173-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer A, Montenegro VM, Schicht S, Globokar Vrohvec M, Pantchev N, Balzer J, Strube C, 2019. Seroprevalence and current infections of canine vector-borne diseases in Costa Rica. Front. Vet. Sci 6, 1–10. 10.3389/fvets.2019.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkey LA, Newton K, Brunker J, Crowdis K, Edourad EJP, Meneus P, Little SE, 2016. Prevalence of vector-borne pathogens in dogs from Haiti. Vet. Parasitol 224, 7–12. 10.1016/j.vetpar.2016.04.017. [DOI] [PubMed] [Google Scholar]
- Tamura K, 1992. Estimation of the number of nucleotide substitutions when there are strong transition-transversion and G+C-content biases. Mol. Biol. Evol 9, 678–687. 10.1093/oxfordjournals.molbev.a040752. [DOI] [PubMed] [Google Scholar]
- Taylor JP, Betz TG, Fishbein DB, Roberts MA, Dawson J, Ristic M, 1988. Serological evidence of possible human infection with Ehrlichia in Texas. J. Infect. Dis 158, 217–220. 10.1093/infdis/158.1.217. [DOI] [PubMed] [Google Scholar]
- Tinoco-Gracia L, Quiroz-Romero H, Quintero-Martinez MT, Renteria-Evangelista TB, Gonzalez-Medina Y, Barreras-Serrano A, Hori-Oshima S, Moro MH, Vinasco J, 2009. Prevalence of Rhipicephalus sanguineus ticks on dogs in a region on the Mexico-USA border. Vet. Rec 164, 59–61. 10.1136/vr.164.2.59. [DOI] [PubMed] [Google Scholar]
- Venzal JM, Guglielmone AA, Estrada Peña A, Cabrera PA, Castro O, 2003. Ticks (Ixodida: Ixodidae) parasitising humans in Uruguay. Ann. Trop. Med. Parasitol 97, 769–772. 10.1179/000349803225002327. [DOI] [PubMed] [Google Scholar]
- Vieira R.Fda C., Biondo AW, Guimarães AMS, Dos Santos AP, Dos Santos RP, Dutra LH, Diniz PPV, de P, de Morais HA, Messick JB, Labruna MB, Vidotto O, 2011. Ehrlichiosis in Brazil. Rev. Bras. Parasitol. Vet 20, 1–12. 10.1590/s1984-29612011000100002. [DOI] [PubMed] [Google Scholar]
- Vinasco J, Li O, Alvarado A, Diaz D, Hoyos L, Tabachi L, Sirigireddy K, Ferguson C, Moro MH, 2007. Molecular evidence of a new strain of Ehrlichia canis from South America. J. Clin. Microbiol 45, 2716–2719. 10.1128/JCM.01102-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei L, Kelly P, Ackerson K, El-Mahallawy HS, Kaltenboeck B, Wang C, 2015. Molecular detection of Dirofilaria immitis, Hepatozoon canis, Babesia spp., Anaplasma platys and Ehrlichia canis in dogs on Costa Rica. Acta Parasitol. 60, 21–25. 10.1515/ap-2015-0003. [DOI] [PubMed] [Google Scholar]
- Wei W, Li J, Wang Y-W, Jiang B-G, Liu H-B, Wei R, Jiang R-R, Cui X-M, Li L-F, Yuan T-T, Wang Q, Zhao L, Xia L-Y, Jiang J-F, Qiu Y-F, Jia N, Cao W-C, Hu Y-L, 2020. Anaplasma platys-like infection in goats, Beijing. China. Vector-Borne Zoonotic Dis. 20, 755–762. 10.1089/vbz.2019.2597. [DOI] [PubMed] [Google Scholar]
- Wilkerson MJ, Black KE, Lanza-Perea M, Sharma B, Gibson K, Stone DM, George A, Nair ADS, Ganta RR, 2017. Initial development and preliminary evaluation of a multiplex bead assay to detect antibodies to Ehrlichia canis, Anaplasma platys, and Ehrlichia chaffeensis outer membrane peptides in naturally infected dogs from Grenada, West Indies. J. Vet. Diagn. Invest 29, 109–114. 10.1177/1040638716671979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabsley MJ, McKibben J, Macpherson CN, Cattan PF, Cherry NA, Hegarty BC, Breitschwerdt EB, O’Connor T, Chandrashekar R, Paterson T, Perea ML, Ball G, Friesen S, Goedde J, Henderson B, Sylvester W, 2008. Prevalence of Ehrlichia canis, Anaplasma platys, Babesia canis vogeli, Hepatozoon canis, Bartonella vinsonii berkhoffii, and Rickettsia spp. in dogs from Grenada. Vet. Parasitol 151, 279–285. 10.1016/j.vetpar.2007.11.008. [DOI] [PubMed] [Google Scholar]
- Ybañez AP, Perez ZO, Gabotero SR, Yandug RT, Kotaro M, Inokuma H, 2012. First molecular detection of Ehrlichia canis and Anaplasma platys in ticks from dogs in Cebu. Philippines. Ticks Tick. Borne. Dis 3, 288–293. 10.1016/j.ttbdis.2012.10.032. [DOI] [PubMed] [Google Scholar]
- Yuasa Y, Tsai Y-L, Chang C-C, Hsu T-H, Chou C-C, 2017. The prevalence of Anaplasma platys and a potential novel Anaplasma species exceed that of Ehrlichia canis in asymptomatic dogs and Rhipicephalus sanguineus in Taiwan. J. Vet. Med. Sci 79, 1494–1502. 10.1292/jvms.17-0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Kelly P, Guo W, Xu C, Wei L, Jongejan F, Loftis A, Wang C, 2015. Development of a generic Ehrlichia FRET-qPCR and investigation of ehrlichioses in domestic ruminants on five Caribbean islands. Parasit. Vectors 8, 506. 10.1186/s13071-015-1118-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


