Abstract
Purpose:
Randomized, multicenter, open-label, phase 2/3 trial investigating lenalidomide versus investigator’s choice (IC) in relapsed/refractory diffuse large B-cell lymphoma (DLBCL).
Experimental Design:
Patients with DLBCL who received ≥2 prior therapies were stratified by DLBCL subtype [germinal center B-cell (GCB) vs. non-GCB; determined by immunohistochemistry (IHC)] and then randomized 1:1 to lenalidomide (25 mg/day, 21 days of 28-day cycle) or IC (gemcitabine, rituximab, etoposide, or oxaliplatin). Crossover to lenalidomide was permitted for IC-treated patients with radiologically confirmed progressive disease. The primary endpoint was overall response rate (ORR). Progression-free survival (PFS), overall survival, and subtype analysis [GCB vs. activated B-cell (ABC)] using gene expression profiling (GEP) were exploratory endpoints.
Results:
Stage 1: 102 DLBCL patients (by IHC: non-GCB, n = 54; GCB, n = 48) received ≥1 dose of lenalidomide or IC. Hematologic treatment-emergent adverse events with lenalidomide versus IC included neutropenia (42.6%; 36.4%), anemia (33.3%; 47.3%), thrombocytopenia (24.1%; 43.6%), and leukopenia (5.6%; 12.7%), respectively. Overall, lenalidomide-treated patients had an ORR of 27.5% versus 11.8% in IC (ORRs were similar regardless of IHC-defined DLBCL subtype). Median PFS was increased in patients receiving lenalidomide (13.6 weeks) versus IC (7.9 weeks; P = 0.041), with greater improvements in non-GCB patients (15.1 vs. 7.1 weeks, respectively; P = 0.021) compared with GCB (10.1 vs. 9.0 weeks, respectively; P = 0.550).
Conclusions:
The clinical benefit of lenalidomide monotherapy in DLBCL patients was more evident in the non-GCB subtype. Exploratory analyses suggest that this preferential benefit was more pronounced in the GEP-defined ABC population, demonstrating a need for additional studies of lenalidomide in DLBCL using GEP subtyping.
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common subtype of non-Hodgkin lymphoma (NHL) and is aggressive in nature (1). Overall survival (OS) rates range from 30% to 50% over 5 years (1), and approximately 60% of patients will remain disease-free following standard immunochemotherapy (2, 3). Although front-line R-CHOP (rituximab-cyclophosphamide, doxorubicin, vincristine, prednisone) can improve clinical outcomes in DLBCL, 20% to 25% of patients relapse after initial response to therapy (4, 5). Currently, no agents are approved for relapsed/refractory DLBCL by the FDA. The European Medicines Agency has granted conditional approval for the cytotoxic azaanthracenedione pixantrone for multiply relapsed/refractory NHL (6).
DLBCL is a heterogeneous malignancy comprising multiple subtypes based on cell-of-origin that influence clinical presentation, prognosis, and treatment response (7, 8). Germinal center B-cell (GCB) and non-GCB subtypes can be distinguished using immunohistochemistry (IHC; ref. 9), whereas the more precise, gold-standard method of gene expression profiling (GEP) is capable of distinguishing three categories—GCB, activated B-cell (ABC), and unclassified (8, 10). Patients with ABC subtypes have an inferior outcome versus GCB patients when treated with immunochemotherapy. In addition, a subset of DLBCL patients (~20% to 30%; characterized by an aggressive clinical course and poor response to conventional chemotherapy) express high levels of MYC and BCL-2 proteins by IHC, and are termed double-expressors (3, 11).
Lenalidomide (Revlimid) is an IMiD immunomodulatory agent with activity in multiple NHL subpopulations (12), including heavily pretreated, relapsed/refractory DLBCL (13–15). In a phase 2 trial investigating lenalidomide monotherapy, patients with DLBCL (N = 108) achieved a 28% overall response rate (ORR) and 2.7 months’ median progression-free survival (PFS; ref. 14). In a retrospective analysis (N= 40), an ORR of 27.5% was observed in lenalidomide-treated patients with DLBCL; patients with the non-GCB subtype (by IHC) achieved higher ORR (52.9%) than the GCB subtype (8.7%; P = 0.006; ref. 13).
The antilymphoma activity of lenalidomide is mediated through multiple mechanisms including inhibiting proliferation of ABC-subtype DLBCL cells (16), increased T-cell activation and cytokine production (17), and enhancement of antibody-dependent cellular cytotoxicity (ADCC; ref. 18). Initial observations of lenalidomide’s mechanism of action showed the importance of decreased expression of interferon regulatory factor 4 (IRF4) and Spi-B transcription factor (SPIB), as well as inhibition of B-cell receptor–dependent NFκB activation in ABC-subtype DLBCL cell lines (19). Subsequent preclinical studies revealed that the cell-autonomous antilymphoma activity of lenalidomide is derived from ubiquitination and subsequent proteasomal degradation of the transcription factors Aiolos and Ikaros by the CRL4CRBN E3 ligase complex. Aiolos functions as a direct transcriptional repressor of interferon-stimulated genes (ISG), and Aiolos degradation by lenalidomide treatment results in upregulated ISG levels, independent of interferon beta production (20). Expression of CRBN/Aiolos and lenalidomide sensitivity in DLBCL is currently unknown.
Based on prior clinical observations of enhanced benefit in non-GCB patients, and to define activity of lenalidomide relative to double-expressor status or CRBN and Aiolos levels, the current study evaluated the efficacy and safety of lenalidomide versus investigator’s choice (IC) in relapsed/refractory DLBCL patients.
Patients and Methods
Patient eligibility
Eligible patients were adults (≥18 years old) with histologically confirmed DLBCL who had relapsed or were refractory to 1 chemotherapy regimen containing rituximab and an anthracycline/anthracycline equivalent as well as ≥1 additional combination chemotherapy regimen, which had to include ≥1 treatment of ifosfamide, gemcitabine, etoposide, or a platinum agent, and, if not previously administered, rituximab; or conditioning regimen containing an alkylating agent followed by autologous or allogeneic stem cell transplant (SCT). Patients could be exempted from the additional treatment requirement if they were documented as being ineligible for both the second combination chemotherapy and SCT at the time of inclusion in the study. Other requirements were DLBCL subtype results by IHC from central pathology; measurable disease (≥2 cm longest diameter); Eastern Cooperative Oncology Group performance status 0–2; life expectancy >3 months; and a formalin-fixed paraffin-embedded (FFPE) tumor block or if possible, fresh frozen tumor sample. Patients with a diagnosis of NHL other than DLBCL or previous lenalidomide treatment were excluded.
Study design
DLC-001 was a phase 2/3, randomized, multicenter, open-label, two-stage trial to determine the efficacy and safety of single-agent lenalidomide versus IC in relapsed/refractory DLBCL patients. The objective of stage 1 was to select appropriate DLBCL subtypes for testing in stage 2. Stage 1 results are presented herein. The stage 1 primary endpoint ORR was determined by an Independent Response Assessment Committee (IRAC) and is defined by the sum of complete response (CR), CR unconfirmed (CRu), and partial response (PR) rates as recommended by the International Workshop Response Criteria (IWRC 1999; ref. 21). No secondary endpoints were defined for stage 1. Exploratory endpoints for stages 1 and 2 included analyses of CR rate, duration of overall response, duration of CR/objective response, PFS, OS, and DLBCL subtype using GEP. The intent-to-treat (ITT) population was defined as all randomized patients. The safety population was defined as all randomized patients who received ≥1 dose of study treatment. The primary efficacy analysis was performed on the modified ITT (mITT) population, defined as all randomized patients who had confirmed DLBCL and GCB or non-GCB subtype diagnosis and received ≥1 dose of study drug. This study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. Prior to study commencement, the protocol, the proposed informed consent form, and other information for patients was reviewed and approved by a properly constituted Institutional Review Board/Independent Ethics Committee at each participating institution. All patients provided written informed consent.
Before stage 1 randomization, IHC was conducted by central pathology to confirm DLBCL diagnosis and subtype. Patients were stratified based on DLBCL subtype (GCB or non-GCB) and randomized 1:1 to receive lenalidomide or IC (Supplementary Fig. S1). Lenalidomide dose was based on creatinine clearance (CrCl)—patients received either 25 mg (CrCl ≥60 mL/min) or 10 mg (CrCl ≥30 mL/min but <60 mL/min) once daily for 21 days (day 1 to day 21) in each 28-day cycle until progressive disease (PD), unacceptable toxicity, or voluntary withdrawal. Patients randomized to IC (single-agent gemcitabine, rituximab, etoposide, or oxaliplatin) were treated following a suggested standard regimen (Supplementary Table S1) until treatment completion, PD, unacceptable toxicity, or voluntary withdrawal. At the time of radiologically documented relapse or PD, patients receiving IC had the option to receive crossover lenalidomide.
The independent reviewers interpreted imaging studies and relevant clinical data for study subjects using an adaptation of the response criteria for NHLs from the IWRC 1999 for the primary assessment (21). The IWRC 1999 criteria for assessment were selected based on investigator consensus and the availability of standardized imagining modalities at the various study sites.
Adverse events (AE) and serious AE (SAE) were graded using the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) Version 4.03. Tumor flare reaction was graded using NCI CTCAE Version 3.0.
Stage 2 of the study was not opened for enrollment because the stage 1 efficacy results as assessed by the IRAC did not meet the protocol-specified threshold.
Dose modification/interruption criteria
Dose modification/interruption of lenalidomide was required in the event of specific toxicities such as grade 2 allergic reaction; grade ≥2 tumor lysis syndrome; or grade ≥3 neutropenia (grade 3 sustained ≥7 days or associated with fever or any grade 4), thrombocytopenia, or venous thrombosis/embolism. Lenalidomide discontinuation was required in the event of desquamating grade ≥3 or non-desquamating grade 4 rash, or grade ≥3 allergic reaction. Dose interruption or modification of IC treatment was permitted under the clinical practice of the investigator’s institution.
Immunohistochemistry
Subtyping on FFPE or fresh-frozen lymph node/tumor tissues was performed per patient at study entry using the Hans algorithm (9). Central pathology laboratories included the Centre for Lymphoid Cancers, British Columbia Cancer Agency (Vancouver, Canada), and CHU Toulouse Purpan, Laboratoire d’Anatomie Pathologique. Four-micron-thick FFPE tumor sections were stained with antibodies to c-myc (clone Y69; Abcam), BCL-2 (clone 124; DAKO), CRBN (rabbit monoclonal antibody; Celgene CRBN65), and Aiolos (rabbit monoclonal antibody; Celgene Clone 9B-9–7), using the Bond-Max automated slide strainer (Leica Microsystems) and the Bond Polymer Refine Detection Kit. Antigen retrieval was performed with Epitope Retrieval 2 (pH 9.0) for 20 minutes at 100°C on the instrument. The slides were blocked for endogenous peroxidase activity with Peroxide Block for 5 minutes at room temperature. Sections were then incubated with primary antibodies for 15 minutes at room temperature. Horseradish peroxidase–labeled Polymer was applied at the instrument’s default conditions, and diaminobenzidine tetrahydrochloride was used as the enzyme substrate to visualize specific antibody localization. Slides were counterstained with hematoxylin. Markers used to distinguish GCB from non-GCB subtypes were CD10, BCL6, and MUM1. For distinguishing GCB from non–GCB-based levels of CD10, BCL6, and MUM-1, a priori scoring criteria were established before trial enrollment and the first 50 cases were used for a cross-laboratory IHC validation analysis. H-scores for CRBN and Aiolos were generated with H-score =Σ(1 + i)pi, where i is the intensity score and pi is the percentage of the cells with the corresponding intensity.
Molecular characterization
Gene expression profiling subtyping on fresh-frozen lymph/node/tumor tissues was batch performed at study conclusion. RNA samples were extracted using the AllPrep DNA/RNA Kit (Qiagen). Total RNA was amplified and labeled using Sensation-Plus FFPE Reagent Kit and then hybridized on Affymetrix U133 Plus 2.0 GeneChips (Affymetrix) following vendor instructions. Samples were classified as ABC, GCB, or unclassified DLBCL in a blinded fashion using a Bayesian model based on a linear predictor score formed from the expression of genes that distinguish these two subtypes as previously described (7, 22).
DNA sequencing of mutations in lymphoma-associated genes
Genomic DNA from patient samples was extracted with the AllPrep DNA/RNA Kit (Qiagen) according to the manufacturer’s instructions. PCR was performed with a GeneAmp XL PCR kit (Applied Biosystems) as previously described (23, 24). The sequences for primers applied to amplify MYD88, CD79A, CD79B, CARD11, and TNFAIP3 are summarized in Supplementary Table S2. The PCR products were visualized by electrophoresis on a 1% agarose gel and ethidium bromide staining. The templates were purified using the QuickStep2 96-well PCR purification Kit (Edge BioSystems) and subsequently sequenced (BigDye sequencing system, Applied Biosystems). Mutations were confirmed on independent PCR products and sequenced from both strands.
Genetic mutations in the B-cell receptor (BCR) pathway have been shown to result in constitutive activity of NFκB, leading to deregulated proliferation and survival signals (23, 25). In addition, mutations in this pathway are predicted to result in intrinsic resistance to targeted agents such as ibrutinib. Indeed, mutations in CARD11 or TNFAIP3 have been shown to inhibit clinical response to ibrutinib in R/R DLBCL (26). We therefore performed targeted sequencing of genes in the BCR pathway (MYD88, CD79A, CD79B, CARD11, and TNFAIP3) to understand if mutations abrogated lenalidomide activity.
Statistical analysis
All statistical analyses were conducted using SAS Version 9.1.3 or higher. Efficacy evaluations were conducted using the mITT and ITT populations for the primary and supportive analyses, respectively. Statistical comparisons were made between lenalidomide and IC groups according to initial randomized treatment. The Kaplan–Meier method was used to estimate survival distribution functions for each treatment group; median of the survival distribution along with associated two-sided 95% confidence intervals (CI) was estimated. A Cox proportional hazards model was used to estimate the HR along with 95% CIs. In stage 1, a sample size of 25 patients per subtype per treatment group provided 90% power to detect a 35% difference in ORR between lenalidomide and IC at 2-sided α = 0.15 level assuming a 15% ORR in the IC group and 1 interim analysis planned at 60% information level. For the primary endpoint, P values were presented for comparison of the best response rates between treatment groups. The O’Brien–Fleming approach was used to control the overall type-I error of 15%.
Results
Patient demographics
The ITT population consisted of 111 patients randomized to lenalidomide (n = 54) or IC (n = 57). Of these, DLBCL subtyping was not feasible for 9 patients because of technical difficulties, resulting in a mITT population of 102 patients with DLBCL diagnosis and subtype confirmation of non-GCB (n = 54) or GCB (n = 48) by IHC and who received ≥1 dose of lenalidomide (n = 51) versus IC (n = 51). The IHC analysis for DLBCL subtyping was conducted by three independent laboratory facilities; the agreement rate among the laboratories was 87.5% to 97.9%. Overall, baseline characteristics in the mITT population were similar between treatment groups (Table 1). The majority of patients received≥2 previous systemic chemotherapies (90.2% in the lenalidomide group vs. 92.2% in IC; P = 0.7270), and nearly half had received ≥3 prior systemic chemotherapies (49.0% vs. 62.7%, respectively; P = 0.1627).
Table 1.
Demographic characteristics in the overall population and DLBCL subtypes as determined by IHC
| Overall |
GCB |
Non-GCB |
||||
|---|---|---|---|---|---|---|
| Len n = 51 | IC n = 51 | Len n = 23 | IC n = 25 | Len n = 28 | IC n = 26 | |
| Median age, years (min, max) | 69.0 | 65.0 | 70.0 | 64.0 | 67.5 | 66.5 |
| (28.0, 84.0) | (20.0, 84.0) | (37.0, 84.0) | (28.0, 84.0) | (28.0, 78.0) | (20.0, 80.0) | |
| Age, n (%) | ||||||
| <65 years | 16 (31.4) | 25 (49.0) | 8 (34.8) | 13 (52.0) | 8 (28.6) | 12 (46.2) |
| ≥65 years | 35 (68.6) | 26 (51.0) | 15 (65.2) | 12 (48.0) | 20 (71.4) | 14 (53.8) |
| Sex, n (%) | ||||||
| Male | 30 (58.8) | 31 (60.8) | 13 (56.5) | 16 (64.0) | 17 (60.7) | 15 (57.7) |
| Female | 21 (41.2) | 20 (39.2) | 10 (43.5) | 9 (36.0) | 11 (39.3) | 11 (42.3) |
| ECOG PS, n (%)a | ||||||
| 0 | 18 (35.3) | 15 (29.4) | 6 (26.1) | 9 (36.0) | 12 (42.9) | 6 (23.1) |
| 1 | 24 (47.1) | 28 (54.9) | 12 (52.2) | 12 (48.0) | 12 (42.9) | 16 (61.5) |
| 2 | 7 (13.7) | 8 (15.7) | 4 (17.4) | 4 (16.0) | 3 (10.7) | 4 (15.4) |
| Systemic anticancer therapy, n (%) | ||||||
| 1b | 5 (9.8) | 4 (7.8) | 2 (8.7) | 0 (0.0) | 3 (10.7) | 4 (15.4) |
| 2 | 21 (41.2) | 15 (29.4) | 7 (30.4) | 10 (40.0) | 14 (50.0) | 5 (19.2) |
| ≥3 | 25 (49.0) | 32 (62.7) | 14 (60.9) | 15 (60.0) | 11 (39.3) | 17 (65.4) |
| ASCT | 13 (25) | 17 (33.3) | 6 (26.1) | 8 (32.0) | 7 (25.0) | 9 (34.6) |
| Prior anticancer therapies, n (%)c,d | ||||||
| Patients with ≥1 therapy | 51 (100) | 51 (100) | 23 (100) | 25 (100) | 28 (100) | 26 (100) |
| R-CHOP | 38 (74.5) | 35 (68.6) | 15 (65.2) | 12 (48.0) | 23 (82.1) | 23 (88.5) |
| R-ICE | 9 (17.6) | 15 (29.4) | 3 (13.0) | 6 (24.0) | 6 (21.4) | 9 (34.6) |
| R-DHAP | 8 (15.7) | 9 (17.6) | 4 (17.4) | 6 (24.0) | 4 (14.3) | 3 (11.5) |
| R-GemOx | 5 (9.8) | 4 (7.8) | 3 (13.0) | 3 (12.0) | 2 (7.1) | 1 (3.8) |
| Other | 5 (9.8) | 4 (7.8) | 4 (17.4) | 3 (12.0) | 1 (3.6) | 1 (3.8) |
One patient (GCB subtype) missing from lenalidomide arm and 1 patient (non-GCB subtype) in the lenalidomide arm entered with ECOG PS2 at screening but had 4 at C1D1.
These patients were exempt from the requirement for second combination chemotherapy or stem cell transplant on the basis of advanced age alone (n = 1) or in combination with poor performance status (n = 1), major organ dysfunction or significant medical condition that placed the patient at unacceptable risk at time of study enrollment (n = 4), or patient decision to decline second-line combination chemotherapy (n = 3).
A patient with the same regimen multiple times was counted only once. All regimens are listed in descending order of frequency based on the overall ITT population.
Prior systemic anticancer therapy for DLBCL received by ≥10% of patients in the overall treatment phase (mITT population).
Abbreviations: ASCT, autologous stem cell transplant; ECOG, Eastern Cooperative Oncology Group; PS, performance status; R-CHOP, rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone; R-DHAP, rituximab, dexamethasone, high-dose cytarabine, cisplatin; R-GemOx, rituximab, gemcitabine, oxaliplatin; R-ICE, rituximab, ifosfamide, carboplatin, etoposide.
Efficacy
IRAC review demonstrated that in the core treatment phase, 14 patients (27.5%) had a clinical response to lenalidomide versus 6 (11.8%) treated with IC (P = 0.079; Table 2). Following lenalidomide treatment, ORR was higher in both DLBCL subtypes versus IC. Based on subtyping by IHC (Table 2), non-GCB patients treated with lenalidomide had an ORR of 28.6% [n= 8; CR = 14.3% (n 4)] versus 11.5% [n = 3; CR = 3.8% (n = 1)] for IC; a similar pattern was observed in GCB patients, with ORRs of 26.1% [n = 6; CR = 4.3% (n = 1)] and 12.0% (n = 3; no CRs), respectively.
Table 2.
Response rate based on IRAC assessment (IWRC 1999)a (mITT Population)
| Immunohistochemistry |
Gene expression profiling |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Overall |
GCB |
Non-GCB |
GCB |
ABC |
||||||
| Len n = 51 | IC n = 51 | Len n = 23 | IC n = 25 | Len n = 28 | IC n = 26 | Len n = 14 | IC n = 16 | Len n = 11 | IC n = 16 | |
| ORR, n (%) | 14 (27.5) | 6 (11.8) | 6 (26.1) | 3 (12.0) | 8 (28.6) | 3 (11.5) | 3 (21.4) | 2 (12.5) | 5 (45.5) | 3 (18.8) |
| [95% CI]b | [15.9–41.7] | [4.4–23.9] | [10.2–48.4] | [2.5–31.2] | [13.2–48.7] | [2.4–30.2] | [4.7–50.8] | [1.6–38.3] | [16.7–76.6] | [4.0–45.6] |
| P valuec | 0.079 | 0.279 | 0.179 | 0.642 | 0.206 | |||||
| CRd, n (%) | 5 (9.8) | 1 (2.0) | 1 (4.3) | 0 (0.0) | 4 (14.3) | 1 (3.8) | 1 (7.1) | 0 (0.0) | 3 (27.3) | 1 (6.3) |
| PR, n (%) | 9 (17.6) | 5 (9.8) | 5 (21.7) | 3 (12.0) | 4 (14.3) | 2 (7.7) | 2 (14.3) | 2 (12.5) | 2 (18.2) | 2 (12.5) |
| SD, n (%) | 13 (25.5) | 11 (21.6) | 5 (21.7) | 7 (28.0) | 8 (28.6) | 4 (15.4) | 4 (28.6) | 3 (18.8) | 2 (18.2) | 3 (18.8) |
| PD/death, n (%) | 24 (47.1) | 33 (64.7) | 12 (52.2) | 14 (56.0) | 12 (42.9) | 19 (73.1) | 7 (50.0) | 11 (68.8) | 4 (36.4) | 10 (62.5) |
| Unknown, n (%) | 0 (0.0) | 1 (2.0) | 0 (0.0) | 1 (4.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| PFS, weeks | 13.6 | 7.9 | 10.1 | 9.0 | 15.1 | 7.1 | 13.2 | 7.1 | 82.0 | 6.2 |
| HR (95% CI) | 0.64 (0.41-0.99) | 0.82 (0.43–1.57) | 0.50 (0.27-0.92) | 0.77 (0.35–1.68) | 0.44 (0.15–1.23) | |||||
| P value | 0.041 | 0.550 | 0.021 | 0.506 | 0.105 | |||||
| OS, weeks | 31.0 | 24.6 | 30.0 | 24.9 | 32.3 | 20.4 | 30.0 | 20.1 | 108.4 | 18.6 |
| HR (95% CI) | 0.91 (0.59–1.41) | 1.23 (0.65–2.34) | 0.70 (0.38–1.30) | 1.12 (0.52–2.42) | 0.47 (0.17–1.33) | |||||
| P value | 0.673 | 0.526 | 0.253 | 0.767 | 0.144 | |||||
mITT population; defined as all randomized patients who had confirmed DLBCL and GCB or non-GCB subtype diagnosis and received ≥1 dose of study drug.
Exact CI based on binomial distribution.
P value derived from the Fisher exact test.
No CR unconfirmed observed.
Abbreviation: Len, lenalidomide
Median duration of response based on IRAC review was longer in the lenalidomide-treated patients (73.9 weeks; 95% CI, 16.4 weeks–not yet reached) than in IC-treated patients (29.2 weeks; 95% CI, 7.0–43.9 weeks; P = 0.138). Median PFS was 13.6 weeks in patients treated with lenalidomide and 7.9 weeks in those treated with IC (HR, 0.64; P = 0.041; Fig. 1; Table 2). An increase in median PFS was observed in non-GCB patients treated with lenalidomide (15.1 weeks) versus IC (7.1 weeks; HR, 0.50; P = 0.021), compared with median PFS of 10.1 and 9.0 weeks, respectively, in GCB patients (HR, 0.82; P = 0.550; Fig. 1; Table 2).
Figure 1.

PFS in DLBCL subtype populations after treatment with lenalidomide or IC. Kaplan–Meier estimates of PFS are shown for (A) overall population, (B) GCB DLBCL, and (C) non-GCB DLBCL analyzed by IHC, as well as (D) GCB DLBCL and (E) ABC DLBCL analyzed by GEP.
Patients treated with lenalidomide versus IC achieved similar OS, irrespective of IHC-defined DLBCL subtype (Table 2); median OS was 31.0 and 24.6 weeks in lenalidomide and IC arms, respectively (HR, 0.91; P = 0.673). In non-GCB patients, median OS with lenalidomide was 32.3 weeks versus 20.4 with IC (HR, 0.70; P = 0.253), compared with 30.0 versus 24.9 weeks (HR, 1.23; P = 0.526) in GCB patients (Fig. 2; Table 2).
Figure 2.
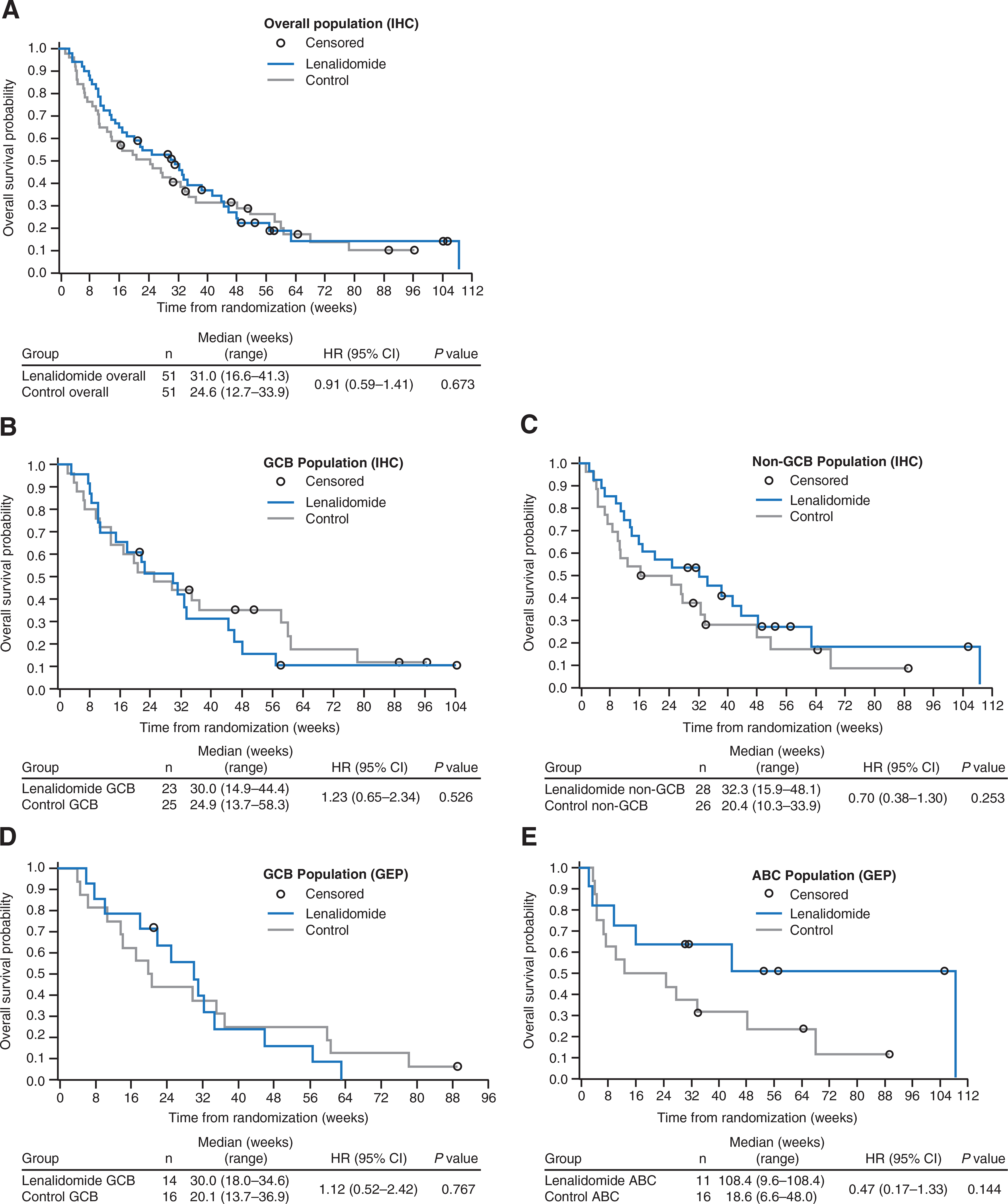
OS in DLBCL subtype populations after treatment with lenalidomide or IC. Kaplan–Meier estimates of OS are shown for (A) overall population, (B) GCB DLBCL, and (C) non-GCB DLBCL analyzed by IHC, as well as (D) GCB DLBCL and (E) ABC DLBCL analyzed by GEP.
Safety evaluation
The safety population consisted of 109 patients. Median treatment duration was 7.4 weeks in lenalidomide-treated patients [n = 54; 7.1 weeks non-GCB (n = 28); 9.1 weeks GCB (n = 24)] versus 5.1 weeks in the IC group [n = 55; 4.1 weeks non-GCB (n = 28); 5.1 weeks GCB (n = 25)]. Overall, similar proportions of patients treated with lenalidomide [31 (57.4%)] versus IC [30 (54.5%)] required ≥1 dose interruption for AEs. All patients, irrespective of study treatment or DLBCL subtype, had ≥1 treatment-emergent adverse event (TEAE). Grade ≥3 TEAEs (Supplementary Table S3) were reported in 43 patients in both lenalidomide (79.6%) and IC (78.2%) groups. Incidence of SAEs was similar across groups: 30 patients (55.6%) treated with lenalidomide and 30 (54.5%) with IC. Among common TEAEs reported in ≥10% of patients (Table 3), nausea, anemia, thrombocytopenia, leukopenia, back pain, hypokalemia, and hyperglycemia were observed more frequently (difference of ≥5%) in patients treated with IC, whereas fatigue, constipation, diarrhea, dry mouth, neutropenia, cough, bronchitis, rash, and tumor flare reaction were more frequent in the lenalidomide group.
Table 3.
Treatment-emergent AEs reported in ≥20% of patients in any arm in the overall population (safety population)
| Overall |
GCB |
Non-GCB |
||||
|---|---|---|---|---|---|---|
| TEAEa | Len n = 54 n (%) | IC n = 55 n (%) | Len n = 24 n (%) | IC n = 25 n (%) | Len n = 28 n (%) | IC n = 28 n (%) |
| Patients with ≥1 AE | 54 (100) | 55 (100) | 24 (100) | 25 (100) | 28 (100) | 28 (100) |
| General disorders and administration-site conditions | 40 (74.1) | 34 (61.8) | 20 (83.3) | 14 (56.0) | 18 (64.3) | 18 (64.3) |
| Fatigue | 18 (33.3) | 15 (27.3) | 10 (41.7) | 6 (24.0) | 7 (25.0) | 8 (28.6) |
| Pyrexia | 16 (29.6) | 16 (29.1) | 7 (29.2) | 6 (24.0) | 9 (32.1) | 9 (32.1) |
| Gastrointestinal disorders | 39 (72.2) | 37 (67.3) | 20 (83.3) | 14 (56.0) | 18 (64.3) | 21 (75.0) |
| Constipation | 16 (29.6) | 12 (21.8) | 9 (37.5) | 4 (16.0) | 7 (25.0) | 8 (28.6) |
| Diarrhea | 15 (27.8) | 12 (21.8) | 8 (33.3) | 5 (20.0) | 7 (25.0) | 6 (21.4) |
| Nausea | 10 (18.5) | 20 (36.4) | 5 (20.8) | 7 (28.0) | 4 (14.3) | 11 (39.3) |
| Blood and lymphatic system disorders | 34 (63.0) | 41 (74.5) | 14 (58.3) | 18 (72.0) | 19 (67.9) | 21 (75.0) |
| Neutropenia | 23 (42.6) | 20 (36.4) | 11 (45.8) | 10 (40.0) | 11 (39.3) | 10 (35.7) |
| Anemia | 18 (33.3) | 26 (47.3) | 6 (25.0) | 13 (52.0) | 12 (42.9) | 11 (39.3) |
| Thrombocytopenia | 13 (24.1) | 24 (43.6) | 4 (16.7) | 15 (60.0) | 9 (32.1) | 8 (28.6) |
| Respiratory, thoracic, and mediastinal disorders | 29 (53.7) | 22 (40.0) | 12 (50.0) | 10 (40.0) | 16 (57.1) | 10 (35.7) |
| Cough | 13 (24.1) | 6 (10.9) | 5 (20.8) | 5 (20.0) | 8 (28.6) | 0 (0.0) |
| Musculoskeletal and connective tissue disorders | 26 (48.1) | 20 (36.4) | 12 (50.0) | 10 (40.0) | 14 (50.0) | 10 (35.7) |
| Infections and infestations | 25 (46.3) | 32 (58.2) | 11 (45.8) | 12 (48.0) | 14 (50.0) | 18 (64.3) |
Safety population, defined as all randomized patients who received ≥1 dose of study treatment.
Efficacy in the GEP population
In an exploratory analysis, DLBCL subtype was retrospectively analyzed using GEP in a subset of patients (n = 92). Of 57 patients with clinical outcome data and GEP classification, 25 (n = 11 ABC; n = 14 GCB) were treated with lenalidomide and 32 (n = 16 ABC; n = 16 GCB) with IC. Sixty-nine patients whose biopsies were interrogated for subtype by IHC and GEP revealed a concordance between the 2 methods of 82.6%, with 57 of the 69 being correctly identified. The discordance was the result of 5 non-GCB IHC samples being GEP subtyped as GCB and 7 GCB IHC samples being GEP subtyped as unclassified (n = 5) or ABC (n = 2).
Lenalidomide treatment resulted in an ORR of 45.5% (n = 5) in ABC patients versus 18.8% (n = 3) with IC (P = 0.206; Table 2). In GCB patients, ORR was 21.4% (n = 3) for lenalidomide and 12.5% (n = 2) for IC (P = 0.642; Table 2). Median PFS in ABC patients was 82.0 weeks with lenalidomide (n = 11) versus 6.2 weeks with IC (n = 16; HR, 0.44; P = 0.105), compared with 13.2 weeks with lenalidomide and 7.1 weeks with IC (HR, 0.77; P = 0.506), in the GCB DLBCL patients (Fig. 2; Table 2). In patients with ABC DLBCL treated with lenalidomide, median OS was 108.4 weeks versus 18.6 weeks in IC (HR, 0.47; P = 0.144). Median OS in GCB patients treated with lenalidomide versus IC was 30.0 and 20.1 weeks, respectively (HR, 1.12; P = 0.767).
Efficacy in the crossover population
A total of 29 patients (56.9%) from the IC group crossed over to lenalidomide after radiologically confirmed PD: 16 (55.2%) from the non-GCB population and 13 (44.8%) from the GCB population. ORR in the crossover patients was modest (n = 1; 3.4%). None of the 29 crossover patients achieved CR, 1 achieved PR, 5 had stable disease, and the majority (n = 22) had PD. The only responder was a patient with non-GCB (ABC by GEP) DLBCL who maintained a PR for 11.7 weeks. The median treatment duration for crossover patients was 6.0 weeks, and, based on investigator’s assessment, median PFS was 8.3 weeks. In crossover patients with non-GCB and GCB DLBCL subtypes, median PFS durations were 6.1 and 8.6 weeks, respectively. In lenalidomide-crossover patients (n = 29), median OS was 32.7 weeks versus 10.1 weeks in the remaining IC-treated patients (n = 22; P = 0.201).
Correlation of cereblon and Aiolos protein expression in tumor biopsies with response to therapy
In 38 evaluable biopsy samples (19 in each arm), staining intensities for CRBN (total, nuclear, and cytoplasmic) or Aiolos (nuclear) did not correlate with response to lenalidomide or IC therapy (Fig. 3A and B). In addition, there was little correlation between Aiolos and CRBN protein expression in tumor biopsies (Fig. 3C).
Figure 3.
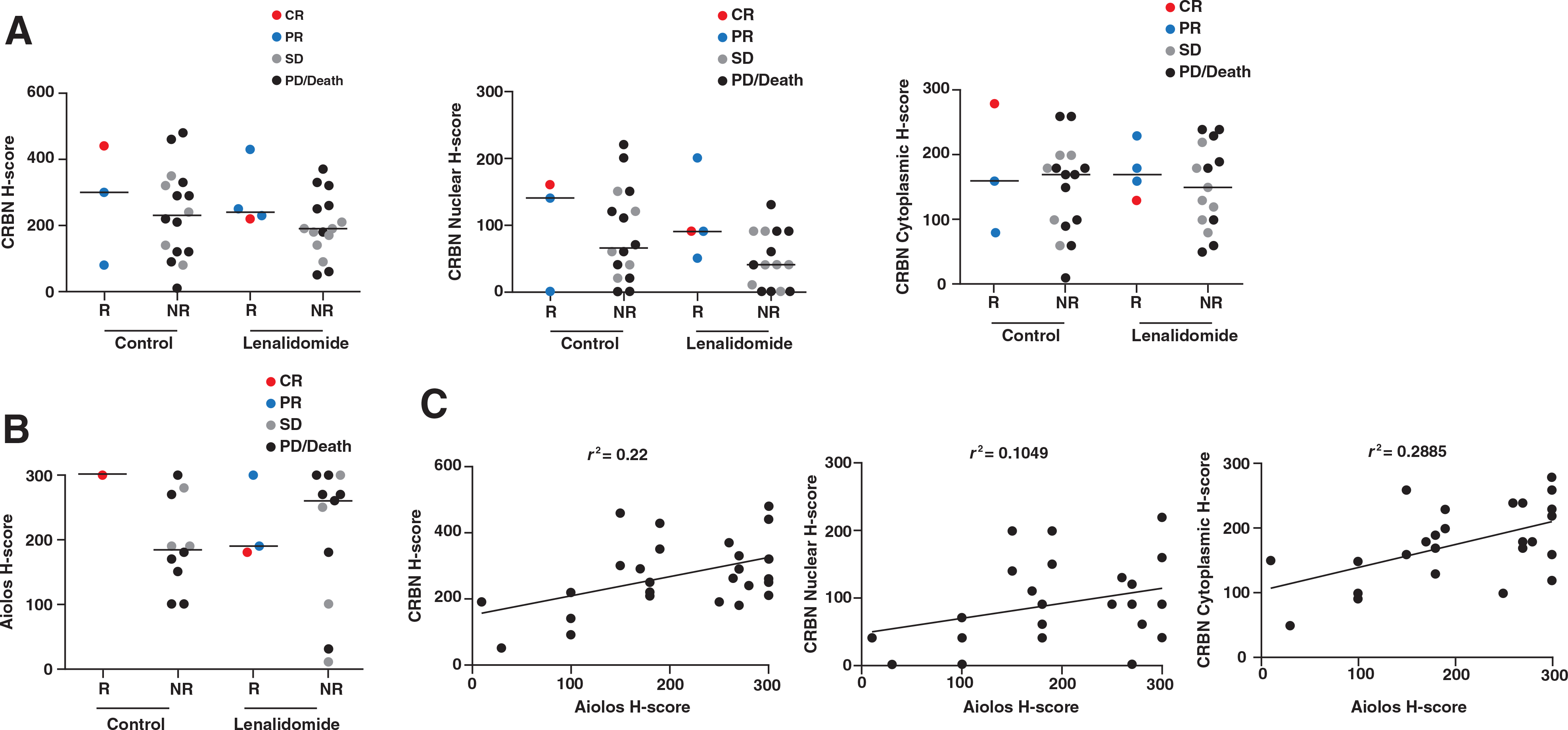
Expression of Cereblon and Aiolos protein levels in lymph node biopsies and correlation with clinical response. A and B, Correlation between immunohistochemical H-scores for Cereblon (nuclear, cytoplasmic, or total) and Aiolos protein expression in FFPE lymph node biopsies versus best response (CR = red, PR = blue, SD = gray, PD/Death = black). C, H-score for each marker is graphically represented in a scatter plot, x-axis Aiolos H-score versus y-axis CRBN (nuclear, cytoplasmic, or total) H-score. r2 values were generated using linear regression analysis. Abbreviations: R, responder; NR, nonresponder.
Efficacy in patients with concurrent expression of MYC and BCL-2, and in patients with mutations in the B-cell receptor/NFκB pathway
Thirty-three patients (39.3%) were double-expressor for MYC and BCL-2 by IHC. In the lenalidomide-treated arm, ORR was similar for double-expressors (33.3%; n = 6, 2 PR) and non–double-expressors (33.3%; n = 12, 1 CR, 3 PR). Median PFS was 17.9 weeks (95% CI, 7.3–27.0) for double-expressors and 16.3 weeks (95% CI, 6.4–25.1) for non–double-expressors.
In patients with an identified mutation in the BCR pathway, ORR with lenalidomide was 28.6% (n = 7; 1 CR, 1 PR) versus 18.75% for IC-treated patients (n = 16; 1 CR, 1 PR). In patients with no mutations identified, ORR was 29.6% with lenalidomide (n = 27; 3 CR, 5 PR) versus 8.6% with IC (n = 23; 2 PR). Median PFS based on mutational status demonstrated no statistical significance between lenalidomide and IC-treated patients (data not shown).
Discussion
In DLC-001, lenalidomide treatment resulted in higher ORR and longer PFS compared with IC in patients with heavily pretreated DLBCL. When analyzed using IHC, the non-GCB population benefited from use of lenalidomide monotherapy and achieved an ORR of 28.6% versus 11.5% in IC. However, exploratory analysis using GEP shows a more pronounced benefit after lenalidomide treatment compared with IC (ORR 45.5% and 18.8%, respectively) that is associated with longer PFS and OS in ABC patients and supports further investigation of GEP-guided treatment in DLBCL patients. Additional exploratory investigations examining lenalidomide response in MYC/BCL-2 positive patients revealed similar ORR compared with non–double-expressor patients (33.3% vs. 33.3%), respectively. Although lenalidomide activity appeared to be independent of the mutational status of genes involved in the BCR/NFκB pathways, such as MYD88, CD79A, CD79B, CARD11, and TNFAIP3, the small sample size necessitates additional studies in larger patient populations as the context of these mutations relative to cell-of-origin is an important contributor to the resulting biology.
The ORR in patients who crossed over to lenalidomide was modest, which might be expected from heavily pretreated patients who progressed after additional therapy; nonetheless, increased OS was observed in lenalidomide-crossover patients (32.7 weeks) versus non-crossover patients (10.1 weeks). Although the use of IC as the control arm in this study could be considered a limitation (especially in the context of the IC regimens including agents that were used in previous lines of therapy), and patient numbers were small, these results are promising and warrant further investigation. Results of this study are consistent with previous investigations, including a phase 2 study that investigated lenalidomide monotherapy in patients with DLBCL and reported an ORR of 28% with a similar AE profile (14). Another phase 2 trial recently showed that lenalidomide is also effective in relapsed/refractory DLBCL when combined with the anti-CD20 antibody obinutuzumab, with 35.2% ORR, 16.9% CR/Cru rate, and 10.6 months median OS (95% CI, 6.5–NR; ref. 27).
Non-GCB as defined by IHC remains a heterogeneous NHL population as classic IHC methods cannot distinguish ABC from other unclassified non-GCB subtypes. GEP is more accurate than IHC for predicting patient response to R-CHOP therapy (28). In this study, obvious differences in lenalidomide treatment response were observed in non-GCB versus ABC, supporting use of GEP methods over IHC in DLBCL subtype analysis. Use of GEP can be restricted due to limited accessibility to equipment and cost. However, newer technologies are being developed that utilize the more readily available FFPE tissue samples and produce robust, consistent results with speed and high accuracy (29). The 20-gene Lymph2Cx expression assay utilizes FFPE tissue and has demonstrated >95% concordance with previously published methods for DLBCL subtype determination, along with applicability to large patient cohorts (29).
Compared with GCB populations, patients with non-GCB/ABC DLBCL have decreased response to standard chemotherapy regimens and poor prognosis (8–10, 30). Clinical trials have demonstrated preferential activity by subtypes with agents such as lenalidomide, bortezomib, and ibrutinib, supporting the need to develop personalized therapies effective in high-risk populations. In one retrospective analysis of relapsed/refractory DLBCL patients treated with lenalidomide monotherapy (N = 40), non-GCB patients had more favorable ORR and OS, and significantly longer PFS versus GCB (6.2 vs. 1.7 months, respectively; P = 0.004; ref. 13). In a second, recently published retrospective analysis in 123 patients with relapsed/refractory DLBCL at median follow-up of 4.5 years, lenalidomide treatment was associated with significantly higher response rates in the non-GCB population compared with GCB (CR: 32% vs. 0; PR: 33% vs. 3%, respectively; P < 0.001 for both). Median PFS was also longer with lenalidomide in the non-GCB population (37 vs. 30 months for GCB; P< 0.001; ref. 31). The proteasome inhibitor bortezomib in combination with DA-EPOCH (dose-adjusted etoposide, vincristine, doxorubicin, cyclophosphamide, and prednisone) showed significantly higher ORR (83% vs. 13%; P < 0.001) and median OS (10.8 vs.3.4 months; P = 0.003) in relapsed/refractory ABC versus GCB DLBCL subpopulations (N = 49) (32). In a phase 2 trial (N = 70), ibrutinib, a Bruton’s tyrosine kinase inhibitor, elicited higher ORRs among patients with relapsed/refractory ABC DLBCL versus GCB (40% and 5.3%, respectively), and PFS of 2.5 and 1.3 months, respectively (33).
Preclinical studies have demonstrated modulation of CRL4CRBN E3 ligase activity by lenalidomide, resulting in ubiquitination and subsequent proteasomal degradation of Aiolos and Ikaros, leading to decreased proliferation of ABC-DLCBL cell lines and activation of immune cells such as T and natural killer cells (16–18, 20). However, in this study, investigation of Aiolos and CRBN levels in tumor biopsies revealed a range of expression for each protein and, more importantly, a lack of correlation between expression and response to lenalidomide treatment.
Lenalidomide is further being investigated as front-line therapy in a phase 3 trial, ROBUST (NCT02285062), which will evaluate the efficacy and safety of lenalidomide plus R-CHOP (R2-CHOP) versus placebo plus R-CHOP in treatment-naïve ABC DLBCL as determined by GEP subtype analysis. DLC-001 results suggest that DLBCL subtyping by GEP may facilitate patient selection, and the ROBUST trial could provide further evidence for this approach. In addition, the phase 3 REMARC (NCT01122472) trial evaluated maintenance therapy with lenalidomide versus placebo in responding elderly patients with DLBCL treated with R-CHOP, and recently reported improved PFS with 2 years of lenalidomide maintenance therapy versus placebo (34); further analyses from this study (including subsets based on cell-of-origin subtyping) are awaited.
Supplementary Material
Translational Relevance.
Diffuse large B-cell lymphoma (DLBCL) is a heterogeneous malignancy comprising multiple subtypes based on cell-of-origin that influence clinical presentation, prognosis, and treatment response. Immunohistochemistry can be used to distinguish germinal center B-cell (GCB) and non-GCB subtypes, whereas gene expression profiling (GEP) can distinguish three categories—GCB, activated B-cell (ABC), and unclassified. The ABC subtype in particular is poorly responsive to standard immunochemotherapy, highlighting the need for additional treatment options. In this study, we report promising clinical activity with lenalidomide monotherapy in patients with DLBCL, especially in the GEP-defined ABC population. These data underscore a need for additional studies of lenalidomide in DLBCL using GEP subtyping and provide additional rationale for studies such as the ongoing phase 3 trial, ROBUST (NCT02285062) comparing lenalidomide plus immunochemotherapy versus immunochemotherapy alone in patients with treatment-naïve ABC-subtype DLBCL selected by GEP.
Acknowledgments
Disclosure of Potential Conflicts of Interest
M.S. Czuczman is an employee of Celgene. M. Trnĕný reports receiving speakers bureau honoraria from and is a consultant/advisory board member for Celgene. A. Davies is a consultant/advisory board member for Celgene, CTI, Gilead, Janssen, Karyopharma, Mundipharma, Roche and Takeda, and reports receiving commercial research grants from Acerta Pharma, Bayer, Celgene, Gilead, Karyopharma, Roche and Takeda. S. Rule is a consultant/advisory board member for Celgene. N. Wagner-Johnston reports receiving speakers bureau honoraria from Gilead, is a consultant/advisory board member for Gilead, Juno and Pharmacyclics, and reports receiving commercial research grants from Celgene. R.D. Gascoyne is a consultant/advisory board member for Celgene. G.W. Slack is a consultant/advisory board member for Seattle Genetics Inc. T.E. Witzig is a consultant/advisory board member for Celgene. G.W. Wright is listed as a co-inventor on lymphoma-related patents that are owned by the US Federal Government and licensed to NanoString. J. Russo has ownership interest (including patents) in Celgene. P. Hagner has ownership interest (including patents) in Celgene Corporation. No potential conflicts of interest were disclosed by the other authors.
Acknowledgments
The authors received medical editorial assistance from Stephanie K. Doerner, PhD, of ProEd Communications, Inc.
Grant Support
This work was supported by Celgene Corporation, Inc.
Footnotes
Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Trial registration ID: EudraCT NUMBER: 2009–013483-38 https://www.clinical-trialsregister.eu/ctr-search/search?query=2009–013483-38
References
- 1.Cultrera JL, Dalia SM. Diffuse large B-cell lymphoma: Current strategies and future directions. Cancer Control 2012;19:204–13. [DOI] [PubMed] [Google Scholar]
- 2.Maurer MJ, Ghesquieres H, Jais JP, Witzig TE, Haioun C, Thompson CA, et al. Event-free survival at 24 months is a robust end point for disease-related outcome in diffuse large B-cell lymphoma treated with immunochemotherapy. J Clin Oncol 2014;32:1066–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson NA, Slack GW, Savage KJ, Connors JM, Ben-Neriah S, Rogic S, et al. Concurrent expression of MYC and BCL2 in diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Clin Oncol 2012;30:3452–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hitz F, Connors JM, Gascoyne RD, Hoskins P, Moccia AA, Savage KJ, et al. Outcome of patients with chemotherapy refractory and early progressive diffuse large B cell lymphoma after R-CHOP treatment. Blood. 2010;116: abstract 1751. [DOI] [PubMed] [Google Scholar]
- 5.Sehn LH. Paramount prognostic factors that guide therapeutic strategies in diffuse large B-cell lymphoma. Hematology Am Soc Hematol Educ Program 2012;2012:402–9. [DOI] [PubMed] [Google Scholar]
- 6.Pean E, Flores B, Hudson I, Sjoberg J, Dunder K, Salmonson T, et al. The European Medicines Agency review of pixantrone for the treatment of adult patients with multiply relapsed or refractory aggressive non-Hodgkin’s B-cell lymphomas: Summary of the scientific assessment of the committee for medicinal products for human use. Oncologist 2013;18:625–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lenz G, Wright G, Dave SS, Xiao W, Powell J, Zhao H, et al. Stromal gene signatures in large-B-cell lymphomas. N Engl J Med 2008;359:2313–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosenwald A, Wright G, Chan WC, Connors JM, Campo E, Fisher RI, et al. The use of molecular profiling to predict survival after chemotherapy for diffuse large-B-cell lymphoma. N Engl J Med 2002;346:1937–47. [DOI] [PubMed] [Google Scholar]
- 9.Hans CP, Weisenburger DD, Greiner TC, Gascoyne RD, Delabie J, Ott G, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood 2004;103:275–82. [DOI] [PubMed] [Google Scholar]
- 10.Alizadeh AA, Eisen MB, Davis RE, Ma C, Lossos IS, Rosenwald A, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature 2000;403:503–11. [DOI] [PubMed] [Google Scholar]
- 11.Green TM, Young KH, Visco C, Xu-Monette ZY, Orazi A, Go RS, et al. Immunohistochemical double-hit score is a strong predictor of outcome in patients with diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Clin Oncol 2012;30:3460–7. [DOI] [PubMed] [Google Scholar]
- 12.Witzig TE, Nowakowski GS, Habermann TM, Goy A, Hernandez-Ilizaliturri FJ, Chiappella A, et al. A comprehensive review of lenalidomide therapy for B-cell non-Hodgkin lymphoma. Ann Oncol 2015;26:1667–77. [DOI] [PubMed] [Google Scholar]
- 13.Hernandez-Ilizaliturri FJ, Deeb G, Zinzani PL, Pileri SA, Malik F, Macon WR, et al. Higher response to lenalidomide in relapsed/refractory diffuse large B-cell lymphoma in nongerminal center B-cell-like than in germinal center B-cell-like phenotype. Cancer 2011;117: 5058–66. [DOI] [PubMed] [Google Scholar]
- 14.Witzig TE, Vose JM, Zinzani PL, Reeder CB,Buckstein R, Polikoff JA, et al. An international phase II trial of single-agent lenalidomide for relapsed or refractory aggressive B-cell non-Hodgkin’s lymphoma. Ann Oncol 2011; 22:1622–7. [DOI] [PubMed] [Google Scholar]
- 15.Wiernik PH, Lossos IS, Tuscano JM, Justice G, Vose JM, Cole CE, et al. Lenalidomide monotherapy in relapsed or refractory aggressive non-Hodgkin’s lymphoma. J Clin Oncol 2008;26:4952–7. [DOI] [PubMed] [Google Scholar]
- 16.Zhang LH, Kosek J, Wang M, Heise C, Schafer PH, Chopra R. Lenalidomide efficacy in activated B-cell-like subtype diffuse large B-cell lymphoma is dependent upon IRF4 and cereblon expression. Br J Haematol 2013; 160:487–502. [DOI] [PubMed] [Google Scholar]
- 17.Gandhi AK, Kang J, Havens CG, Conklin T, Ning Y, Wu L, et al. Immunomodulatory agents lenalidomide and pomalidomide co-stimulate T cells by inducing degradation of T cell repressors Ikaros and Aiolos via modulation of the E3 ubiquitin ligase complex CRL4(CRBN.). Br J Haematol 2014;164:811–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu L, Adams M, Carter T, Chen R, Muller G, Stirling D, et al. Lenalidomide enhances natural killer cell and monocyte-mediated antibody-dependent cellular cytotoxicity of rituximab-treated CD20+ tumor cells. Clin Cancer Res 2008;14:4650–7. [DOI] [PubMed] [Google Scholar]
- 19.Yang Y, Shaffer AL 3rd, Emre NC, Ceribelli M, Zhang M, Wright G, et al. Exploiting synthetic lethality for the therapy of ABC diffuse large B cell lymphoma. Cancer Cell 2012;21:723–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hagner PR, Man HW, Fontanillo C, Wang M, Couto S, Breider M, et al. CC-122, a pleiotropic pathway modifier, mimics an interferon response and has antitumor activity in DLBCL. Blood 2015;126:779–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheson BD, Horning SJ, Coiffier B, Shipp MA, Fisher RI, Connors JM, et al. Report of an international workshop to standardize response criteria for non-Hodgkin’s lymphomas. NCI Sponsored International Working Group. J Clin Oncol 1999;17:1244. [DOI] [PubMed] [Google Scholar]
- 22.Wright G, Tan B, Rosenwald A, Hurt EH, Wiestner A, Staudt LM. A gene expression-based method to diagnose clinically distinct subgroups of diffuse large B cell lymphoma. Proc Natl Acad Sci U S A 2003;100:9991–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lenz G, Davis RE, Ngo VN, Lam L, George TC, Wright GW, et al. Oncogenic CARD11 mutations in human diffuse large B cell lymphoma. Science 2008;319:1676–9. [DOI] [PubMed] [Google Scholar]
- 24.Ngo VN, Young RM, Schmitz R, Jhavar S, Xiao W, Lim KH, et al. Oncogenically active MYD88 mutations in human lymphoma. Nature 2011;470:115–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vaque JP, Martinez N, Batlle-Lopez A, Perez C, Montes-Moreno S, Sanchez-Beato M, et al. B-cell lymphoma mutations: improving diagnostics and enabling targeted therapies. Haematologica 2014;99:222–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilson WH, Young RM, Schmitz R, Yang Y, Pittaluga S, Wright G, et al. Targeting B cell receptor signaling with ibrutinib in diffuse large B cell lymphoma. Nat Med 2015;21:922–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morschhauser M, Cartron G, Salles GA, Bijou F, Fruchart C, Bouabdallah K, et al. A phase II LYSA study of obinutuzumab combined with lenalidomide for relapsed or refractory aggressive B-cell lymphoma. Blood 2016;128:abstract 4202.27827828 [Google Scholar]
- 28.Read JA, Koff JL, Nastoupil LJ, Williams JN, Cohen JB, Flowers CR. Evaluating cell-of-origin subtype methods for predicting diffuse large B-cell lymphoma survival: A meta-analysis of gene expression profiling and immunohistochemistry algorithms. Clin Lymphoma Myeloma Leuk 2014; 14:460–7e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scott DW, Wright GW, Williams PM, Lih CJ, Walsh W, Jaffe ES, et al. Determining cell-of-origin subtypes of diffuse large B-cell lymphoma using gene expression in formalin-fixed paraffin-embedded tissue. Blood 2014;123:1214–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thieblemont C, Briere J, Mounier N, Voelker HU, Cuccuini W, Hirchaud E, et al. The germinal center/activated B-cell subclassification has a prognostic impact for response to salvage therapy in relapsed/refractory diffuse large B-cell lymphoma: A bio-CORAL study. J Clin Oncol 2011;29:4079–87. [DOI] [PubMed] [Google Scholar]
- 31.Mondello P, Steiner N, Willenbacher W, Ferrero S, Ghione P, Marabese A, et al. Lenalidomide in relapsed or refractory diffuse large B-cell lymphoma: Is it a valid treatment option? Oncologist 2016;21:1107–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dunleavy K, Pittaluga S, Czuczman MS, Dave SS, Wright G, Grant N, et al. Differential efficacy of bortezomib plus chemotherapy within molecular subtypes of diffuse large B-cell lymphoma. Blood 2009; 113:6069–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilson WH, Gerecitano JF, Goy A, de Vos S, Kenkre VP, Barr PM, et al. The Bruton’s tyrosine kinase (BTK) inhibitor, ibrutinib (PCI-32765), has preferential activity in the ABC subtype of relapsed/refractory de novo diffuse large B-cell lymphoma (DLBCL): Interim results of a multicenter, open-label, phase 2 study. Blood 2012;120:abstract 686. [Google Scholar]
- 34.Thieblemont C, Tilly H, Gomez da Silva M, Casasnovas R-H, Fruchart C, Morschhauser F, et al. First analysis of an international double-blind randomized phase III study of lenalidomide maintenance in elderly patients with DLBCL treated with R-CHOP in first line, the REMARC study from LYSA. Blood 2016;128:abstract 471.27827828 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


