Abstract
Objective
STAT 3 deficiency (autosomal dominant hyper immunoglobulin E syndrome (AD-HIES)) is a primary immunodeficiency disorder with multi-organ involvement caused by dominant negative signal transducer and activator of transcription gene 3 (STAT3) mutations. We sought to describe the gastrointestinal (GI) manifestations of this disease.
Methods
Seventy subjects aged five to 60 years with a molecular diagnosis of AD-HIES were evaluated at the National Institutes of Health (NIH). Data collection involved a GI symptom questionnaire and retrospective chart review.
Results
In our cohort of 70 subjects, we found that 60% had GI symptoms (42/70). The most common manifestations were gastroesophageal reflux disease (GERD) observed in 41%, dysphagia in 31%, and abdominal pain in 24%. The most serious complications were food impaction in 13% and colonic perforation in 6%. Diffuse esophageal wall thickening in 74%, solid stool in the right colon in 50% (12/24), and hiatal hernia in 26% were the most prevalent radiologic findings. Esophagogastroduodenoscopy (EGD) demonstrated esophageal tortuosity in 35% (8/23), esophageal ulceration in 17% (4/23), esophageal strictures requiring dilation in 9% (2/23), and gastric ulceration in 17% (4/23). Esophageal eosinophilic infiltration was an unexpected histologic finding seen in 65% (11/17).
Conclusion
The majority of AD-HIES subjects develop GI manifestations as part of their disease. Most notable are the symptoms and radiologic findings of GI dysmotility, as well as significant eosinophilic infiltration, concerning for a secondary eosinophilic esophagitis. These findings suggest that the STAT3 pathway may be implicated in a new mechanism for the pathogenesis of several GI disorders.
Keywords: Autosomal dominant hyper immunoglobulin E syndrome, eosinophilic esophagitis, STAT3, gastrointestinal dysmotility
Introduction
STAT3 deficiency, also known as autosomal dominant hyper-IgE syndrome (AD-HIES), is a primary immunodeficiency characterized by eczema, recurrent infections, and multiple connective tissue, skeletal, and vascular abnormalities [1–3]. STAT3 is expressed widely and mediates varied pathways from wound healing, host defense, and vascular remodeling, thus explaining the multi-system clinical phenotype. Although there have been a few case reports of perforation and infection of the gastrointestinal tract [5–7], the prevalence and spectrum of gastrointestinal (GI) manifestations in STAT3 deficiency have never been systematically described.
In our cohort of patients with STAT3 deficiency, we have noted GI presentations that are consistent with some of the defined immune abnormalities of STAT3 deficiency as well as the connective tissue findings. These include both Candida and endemic fungal infections of the GI tract, likely related to the impaired development of TH17 lymphocyte that impairs epithelial host immunity [8–10], as well as symptoms of connective tissue disorders such as Marfan’s and Ehlers-Danlos syndromes [4], with diverticuli and perforation.
Therefore, we systemically examined the GI manifestations of STAT3 deficiency through an adapted questionnaire as well as review of medical history, laboratories, endoscopies, and pathology.
Methods
Subjects
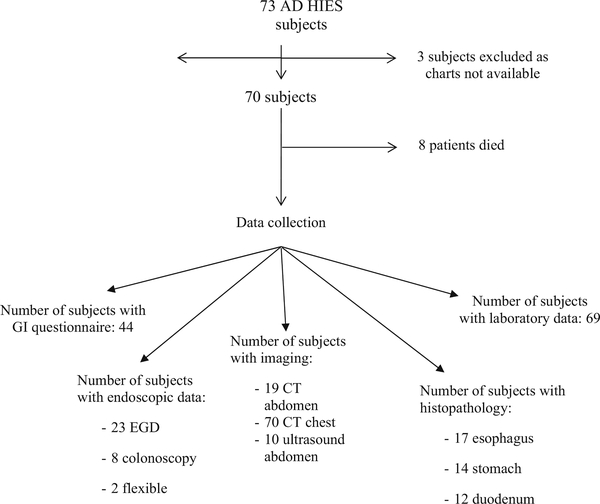
The charts of 70 subjects with loss of function STAT3 mutations enrolled in a National Institutes of Health (NIH) Institutional Review Board approved protocol (00-I-0159, NCT00006150) were reviewed (Fig. 1). Forty-four of these 70 subjects completed an adaptation of a published dysphagia questionnaire with additional upper and lower GI symptom questions [11] (Supplemental Appendix 1). The retrospective chart review focused on GI and allergic history (foods, medications, and environmental) and relevant laboratory data.
Fig. 1.
Study overview
Radiology
Twenty-five subjects had dedicated abdominal imaging for clinical evaluation (some subjects underwent more than one imaging modality): 19 underwent computed tomography (CT) scans and 10 had abdominal ultrasounds. All 70 subjects had CT imaging of the chest done for routine pulmonary evaluation and these were assessed specifically for esophageal abnormalities. Radiologic imaging was analyzed by a single radiologist (AMV), who was blinded to subjects’ individual clinical, endoscopic, and pathologic findings.
Endoscopy
Twenty-three patients underwent clinically indicated EGD, eight underwent colonoscopy, and two underwent flexible sigmoidoscopy. EGD indications included dysphagia, odynophagia, history of food impaction, reflux, failure to thrive, nausea, vomiting, early satiety, melena, or abdominal pain. Indications for lower endoscopy included iron deficiency anemia, hematochezia, abdominal pain, and follow up of perianal fistula. Two subjects had incomplete colonoscopies due to poor bowel preparation. All endoscopic procedures were performed under appropriate sedation. All studies were performed and analyzed by the same gastroenterologist (TH).
Pathology
Seventeen patients had histopathology from biopsies taken during endoscopy available for review, including esophageal tissue (17 patients), gastric tissue (14 patients), duodenal tissue (12 patients), and colonic tissue (6 patients). All specimens were reviewed by two GI pathologists (DEK, MQ) and included documentation of tissue eosinophil counts averaged over 10 high power fields (×40).
Statistical Analysis
Data analyses included descriptive analyses such as percentages, mean and standard deviation, and range (minimum and maximum). Fisher’s exact test was used in analyses of association between two categorical variables. Data analysis was performed using SAS, Version 9.1.3 (SAS Institute Inc., Cary, NC) and JMP 8.0 (SAS Institute Inc., Cary, NC).
Results
Demographics
The patient cohort was 59% female (41/70), with ages ranging from 5 to 60 years (Table 1). Fifty-nine percent of patients (41/70) had medication allergies, and 34% (19/70) were found to have one or more food allergies, which is consistent with a prevalence of 38% we reported in a previous paper [20].
Table 1.
Study population demographics
| Number of subjects (living) | 70 (59) |
|---|---|
| Age—median (range) | 29 (5–60) |
| Sex | |
| Female | 41 |
| Male | 29 |
| Race | |
| Black | 9 |
| Asian | 3 |
| Caucasian | 51 |
| Othera | 7 |
| Mutation domain | |
| DNA domain | 37 |
| SH2 domain | 32 |
| Transactivation domain | 1 |
| Symptoms, N (%) | |
| GERD | 29 (40) |
| Odynophagia | 4 (6) |
| Dysphagia | 22 (31) |
| Abdominal pain | 17 (24) |
| Nausea | 13 (19) |
| Vomiting | 15 (21) |
| Constipation | 10 (14) |
| Early satiety | 7 (10) |
| Chest pain | 6 (9) |
| Diarrhea | 5 (7) |
Race mixed or unknown
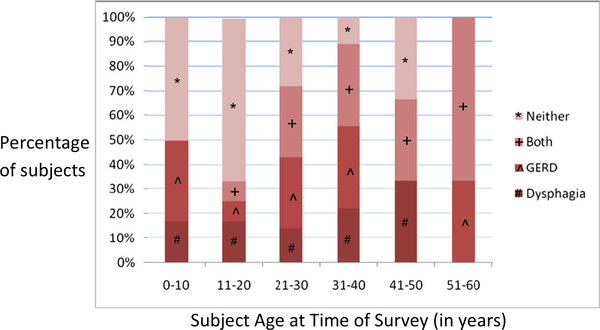
Sixty percent of patients (42/70) complained of one or more GI symptoms, with the most prevalent being gastroesophageal reflux disease (GERD), and dysphagia in 22/70 (31 (Table 1). Both of these symptoms were found to have progressively increasing incidence with age (Fig. 2).
Fig. 2.
STAT3-deficient subjects experience esophageal GI disease early in life
One of the most predominant clinical findings identified from the patient histories was food impaction in 13% (9/70) of the cohort. Other significant findings included intestinal perforation in 6% of the subjects (4/70), rectal prolapse in 3% (2/70), and colonic diverticulosis in 3% (2/70). Among the four patients with bowel perforation, two cases were spontaneous and the other two were attributed to diverticular disease.
Gastrointestinal infections were also observed in this cohort, with the most common being esophageal candidiasis in 6% of patients (4/70). Other infections included esophageal cryptococcus (1/70, 1%), esophageal EBV (1/70, 1%), and Histoplasma capsulatum infection of the terminal ileum and colon (1/70, 1%).
Laboratory Data Review
Elevated serum IgE was found in all but one subject and this subject had an elevated serum IgE in the past. Peripheral eosinophilia was observed in 76% of subjects (53/70). Additionally, 25% of the cohort (18/70) had liver chemistry abnormalities, predominantly characterized by transaminitis (Supplemental Table 1).
Radiologic Findings
The most common radiologic finding was esophageal wall thickening, observed on chest CTs of 74% of patients (52/70). The wall thickening was characterized as mild in 60% (31/52), moderate in 35% (18/52), and severe in 6% (3/52) of patients.
Gastric and intestinal (small and large) bowel wall thickening was also observed, seen in 11% (2/19) and 16% (3/19) of patients, respectively, on abdominal CT. There was also evidence of increased echogenicity of the liver parenchyma, suggestive of steatosis, on abdominal ultrasonography and CT, in 24% of patients (6/25). Other findings included gastric distension in 11% (2/19), diverticulosis in 5% (1/19), and moderate to extensive amounts of stool in the right colon in 63% (12/19) of patients.
Upper Gastrointestinal Tract Endoscopic Findings
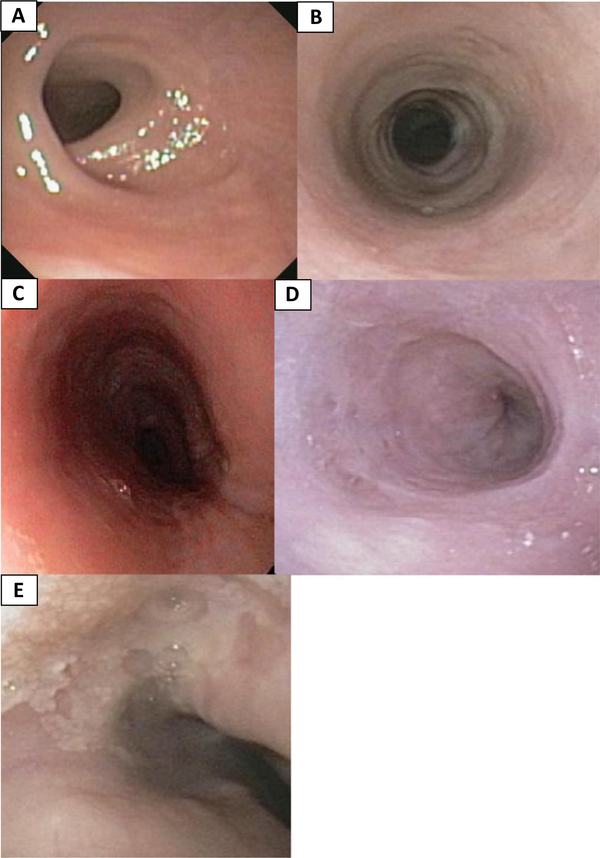
Noteworthy, esophageal endoscopic findings included tortuosity in 39% of subjects (9/23) and esophageal ulcers in 26% (6/23). Significant upper esophageal strictures were seen in 9% of subjects (2/23). Other findings suggestive of eosinophilic infiltration included intramural diverticula (2/23, 9%), white plaques (2/23, 9%), esophageal rings (2/23, 9%), and linear furrows (1/23, 4%) [(Fig. 3).
Fig. 3.
Endoscopic views of the esophagus: a upper esophageal strictures, b rings, c linear furrows, d diverticuli, and e white exudate
In the stomach and duodenum, erythema was the most prevalent finding, present in 30% (7/23) and 17% (4/23) of patients, respectively (Supplemental Table 2). Gastric and duodenal ulceration was also common and observed in 17% (4/23) and 13% (3/23) of the cohort, respectively. Retained food and/or bilious fluid was found in the stomach or duodenum of 26% of the patients (6/23).
Lower Gastrointestinal Tract Endoscopic Findings
One subject had a perianal ulcer, internal hemorrhoids, and a sigmoid nodule, whereas another, with a family history of Crohn’s, had rectal erythema with ulcers. Other colonic findings included a perianal cyst and a subcentimeter sigmoid polyp. Two subjects had incomplete colonoscopies due to poor bowel preparation (Supplemental Table 3).
Histopathology Findings
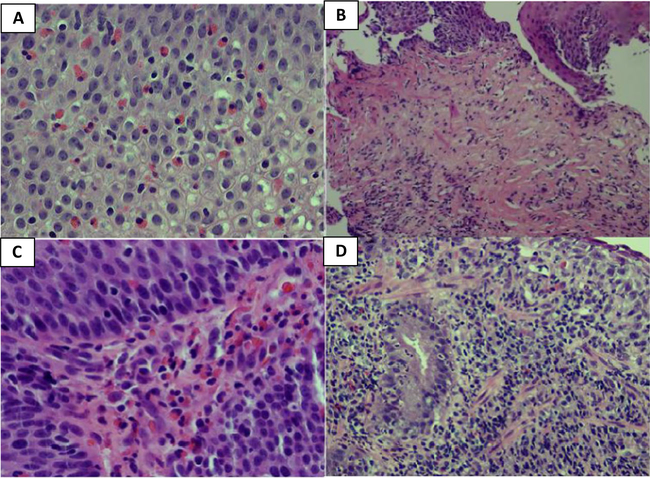
The most impressive esophageal histologic finding was the presence of eosinophils, with greater than 15 eosinophils/hpf found in 65% (11/17) of the patients, as well as inflammation, found in 59% (10/17) of patients (Fig. 4). Other notable findings included varying degrees of papillary lengthening, basal cell hyperplasia, and lamina propria fibrosis (Supplemental Table 4).
Fig. 4.
Esophageal biopsies (H&E, ×40) showing a intraepithelial eosinophilic infiltration (> 20/hpf), b prominent subepithelial fibrosis and scattered eosinophilic infiltration (×10), c lamina propria with eosinophilic infiltration (×40), and d mixed inflammation in the subepithelial tissue (×20)
Gastric, duodenal, and colonic mucosa were also found to have varying degrees of eosinophilic infiltration (Fig. 4). Greater than 20 eos/hpf were observed in 50% (7/14) of the gastric biopsies, 42% (5/12) duodenal biopsies, and 50% (3/6) colon biopsies (Supplemental Table 5).
Seven patients underwent clinically indicated liver biopsies. Although no identifiable pattern of abnormality was found, one of the patients, with evidence of increased liver echogenicity on imaging, was confirmed to have steatohepatitis on biopsy (Supplemental Table 6).
Analysis of Association Among Symptoms
An elevated peripheral eosinophil count was associated with dysphagia (p = 0.01), but not with high serum IgE (p = 0.5), eosinophilic infiltration of the esophagus (p = 1.0), or other GI symptoms (p = 0.2). Additionally, no correlation was found between elevated serum IgE and dysphagia, other GI symptoms or eosinophilic infiltration of the esophagus (p = 1.0, p = 0.5, p = 0.3, respectively). Correlation was found between a history of food impaction and eosinophilic infiltration of the esophagus (p = 0.004).
Discussion
STAT3 deficiency presents primarily with dermatologic, infectious, and connective tissue complications, but its GI involvement has remained largely undefined. The existing literature is only limited to a small number of case reports describing GI infections and intestinal perforations [6, 7, 12–17].
We found a high overall rate of eosinophilic infiltration throughout the GI tract. In the esophagus, > 15 eos/hpf were observed in 65% (11/17) of subjects, which is consistent with eosinophilic esophagitis (EoE) [18, 19]. This number was likely an underestimation as not all with symptoms had endoscopy and biopsies performed. Although GERD may explain some of the eosinophilic infiltration, the majority of endoscopies were performed while on acid suppression, and the high numbers of eosinophils seen was more consistent with EoE [21]. Chronic EoE also likely contributed to the esophageal rings, linear furrows, diverticula, and upper esophageal strictures seen. A correlation between esophageal eosinophilia and food impaction is also not surprising, as studies have shown that up to 10% of patients presenting with food impactions are ultimately diagnosed with EoE [24, 33]. The lack of association between peripheral and esophageal eosinophilia suggests a propensity for eosinophils to infiltrate the esophagus independent of peripheral levels [1]. This may be partially from fibrosis masking/replacing esophageal eosinophils, as one subject with three upper esophageal strictures had only rare eosinophils on pathology.
Multiple subjects also had evidence of underlying GI dysmotility and colonic perforation. The pathophysiology for these manifestations may be related to abnormal connective tissue remodeling and impaired mucosal healing in patients with STAT3 deficiency. Similar presentations have been observed in patients with known connective tissues disorders, including Marfan’s and Ehlers-Danlos Syndromes, which share clinical features with STAT3 deficiency [8, 22, 23]. STAT3 signaling plays a role in the regulation of matrix metalloproteinases (MMPs), and subjects with STAT3 deficiency have been shown to have abnormal levels of MMPs [25–27]. In addition, impaired mucosal wound healing has been seen in murine models with deletion of STAT3 [28]. One subject who developed a colon perforation showed multiple ulcers at the site of perforation. Additionally, impaired mucosal healing may also explain the severity of the pathology on EGD compared to a relative paucity of clinical findings.
Mild transaminitis was also common, and radiologic findings suggestive of hepatic steatosis were found in six patients, with one confirmed to have steatohepatitis on biopsy. This suggests a possible correlation between STAT3 deficiency and fatty liver. However, it is also possible that the fatty liver could be related to obesity and metabolic syndrome, which has a higher prevalence in AD-HIES patients, though further studies are needed to look at this correlation more closely.
Other than one subject with a self-limited proctitis and a family history of Crohn’s disease, we found no other inflammatory bowel disease (IBD) in STAT3 deficiency despite severe colitis developing in STAT3 hematopoietic deficient mouse and presence of IBD in many other primary immunodeficiencies [30]. Furthermore, the recent description of severe colitis in subjects with IL-10 ligand and receptor deficiencies indicates a critical role for IL-10 in the control of gut inflammation, a pathway impaired in AD-HIES [29–31]. The absence of IBD may be explained by the absence of Th17 cells in STAT3 deficiency, as increased Th17 cells have been observed in IBD [10].
Optimal therapy for these GI disorders remains to be determined. One subject was treated with swallowed fluticasone, resulting in a decrease of his esophageal eosinophil count, as well as resolution of dysphagia. However, the use of oral or topical corticosteroid therapy is limited in this population by the high risk of infection. The use of long-term acid suppression for GERD, both with proton pump inhibitors (PPIs) and H2 blockers, also needs to be weighed against the risk of osteoporosis [32]. The efficacy of some antifungal prophylactic therapy with azole medications, which are commonly prescribed to these subjects, may also be compromised when co-administered with PPIs.
To our knowledge, this is the first major review of GI symptoms, endoscopic abnormalities, and histopathological findings in STAT3 deficiency. It seems likely that these manifestations have been previously underappreciated [15]. GI symptoms are common in STAT3 deficiency, particularly GERD (42%), dysphagia (32%), and abdominal pain (23%), and were found to be associated with a number of GI conditions, including gut dysmotility, bowel perforation, and eosinophilic esophagitis. This association indicates that the gastrointestinal tract is impacted by STAT3 signaling, suggesting that this organ system is important in further understanding of this disease entity.
Supplementary Material
Funding
The study was funded by the intramural programs of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), National Institute of Allergy and Infectious Disease (NIAID), Clinical Center (CC), and National Cancer Institute (NCI) of the NIH.
Footnotes
Compliance with Ethical Standards
Conflict of Interest The authors declare that they have no conflict of interest.
Electronic supplementary material The online version of this article (doi:10.1007/s10875-017-0429-z) contains supplementary material, which is available to authorized users.
References
- 1.Grimbacher B, et al. Hyper-IgE syndrome with recurrent infections—an autosomal dominant multisystem disorder. N Engl J Med. 1999;340(9):692–702. [DOI] [PubMed] [Google Scholar]
- 2.Freeman AF, Holland SM. Clinical manifestations of hyper IgE syndromes. Dis Markers. 2010;29(3–4):123–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buckley RH, Wray BB, Belmaker EZ. Extreme hyperimmunoglobulinemia E and undue susceptibility to infection. Pediatrics. 1972;49(1):59–70. [PubMed] [Google Scholar]
- 4.Solomon JA, Abrams L, Lichtenstein GR. GI manifestations of Ehlers-Danlos syndrome. Am J Gastroenterol. 1996;91(11):2282–8. [PubMed] [Google Scholar]
- 5.Butterworth SA, Webber EM. Meconium thorax: a case of Bochdalek hernia and cecal perforation in a neonate with Job’s syndrome. J Pediatr Surg. 2002;37(4):673–4. [DOI] [PubMed] [Google Scholar]
- 6.Chen CM, et al. Colon perforation in a patient with hyperimmunoglobulin E (Job’s) syndrome. J Pediatr Surg. 1995;30(10):1479–80. [DOI] [PubMed] [Google Scholar]
- 7.Jacobs DH, et al. Esophageal cryptococcosis in a patient with the hyperimmunoglobulin E-recurrent infection (Job’s) syndrome. Gastroenterology. 1984;87(1):201–3. [PubMed] [Google Scholar]
- 8.Levy DE, Loomis CA. STAT3 signaling and the hyper-IgE syndrome. N Engl J Med. 2007;357(16):1655–8. [DOI] [PubMed] [Google Scholar]
- 9.Milner JD, Sandler NG, Douek DC. Th17 cells, Job’s syndrome and HIV: opportunities for bacterial and fungal infections. Curr Opin HIV AIDS. 2010;5(2):179–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Milner JD, et al. Impaired T(H)17 cell differentiation in subjects with autosomal dominant hyper-IgE syndrome. Nature. 2008;452(7188):773–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilkins T, et al. The prevalence of dysphagia in primary care patients: a HamesNet Research Network study. J Am Board Fam Med. 2007;20(2):144–50. [DOI] [PubMed] [Google Scholar]
- 12.Steiner SJ, et al. Ileocecal histoplasmosis simulating Crohn disease in a patient with hyperimmunoglobulin E syndrome. Pediatr Infect Dis J. 2009;28(8):744–6. [DOI] [PubMed] [Google Scholar]
- 13.Gotzberger M, et al. A rare cause of gastrointestinal bleeding in a patient with hyper-IgE syndrome. Gut. 2004;53(10):1430–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Watanabe T, et al. Pancreatitis and cholangitis due to cytomegalovirus in a patient with hyperimmunoglobulin E syndrome. Pancreas. 2010;39(6):940–2. [DOI] [PubMed] [Google Scholar]
- 15.Chandesris MO, et al. Autosomal dominant STAT3 deficiency and hyper-IgE syndrome: molecular, cellular, and clinical features from a French national survey. Medicine (Baltimore). 2012;91(4):e1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hwang EH, et al. Colon perforation in hyperimmunoglobulin E syndrome. J Pediatr Surg. 1998;33(9):1420–2. [DOI] [PubMed] [Google Scholar]
- 17.Stover DG, et al. Diverticulitis in a young man with hyper-IgE syndrome. South Med J. 2010;103(12):1261–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liacouras CA. Eosinophilic esophagitis. Gastroenterol Clin N Am. 2008;37(4):989–98. xi [DOI] [PubMed] [Google Scholar]
- 19.Dellon ES, et al. ACG clinical guideline: evidenced based approach to the diagnosis and management of esophageal eosinophilia and eosinophilic esophagitis (EoE). Am J Gastroenterol. 2013;108(5): 679–92. quiz 693 [DOI] [PubMed] [Google Scholar]
- 20.Siegel AM, Stone KD, Cruse G, Lawrence MG, Olivera A, Jung MY, et al. Diminished allergic disease in patients with STAT3 mutations reveals a role for STAT3 signaling in mast cell degranulation. J Allergy Clin Immunol. 2013. December;132(6):1388–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Camilleri M, et al. Prevalence and socioeconomic impact of upper gastrointestinal disorders in the United States: results of the US Upper Gastrointestinal Study. Clin Gastroenterol Hepatol. 2005;3(6):543–52. [DOI] [PubMed] [Google Scholar]
- 22.Minegishi Y, et al. Human tyrosine kinase 2 deficiency reveals its requisite roles in multiple cytokine signals involved in innate and acquired immunity. Immunity. 2006;25(5):745–55. [DOI] [PubMed] [Google Scholar]
- 23.Renner ED, et al. STAT3 mutation in the original patient with Job’s syndrome. N Engl J Med. 2007;357(16):1667–8. [DOI] [PubMed] [Google Scholar]
- 24.Sperry SL, Crockett SD, Miller CB, Shaheen NJ, Dellon ES. Esophageal foreign-body impactions: epidemiology, time trends, and the impact of the increasing prevalence of eosinophilic esophagitis. Gastrointest Endosc. 2011;74(5):985–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lagente V, Boichot E. Role of matrix metalloproteinases in the inflammatory process of respiratory diseases. J Mol Cell Cardiol. 2010;48(3):440–4. [DOI] [PubMed] [Google Scholar]
- 26.Lopez-Otin C, Palavalli LH, Samuels Y. Protective roles of matrix metalloproteinases: from mouse models to human cancer. Cell Cycle. 2009;8(22):3657–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sekhsaria V, et al. Plasma metalloproteinase levels are dysregulated in signal transducer and activator of transcription 3 mutated hyper-IgE syndrome. J Allergy Clin Immunol. 2011;128(5):1124–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pickert G, et al. STAT3 links IL-22 signaling in intestinal epithelial cells to mucosal wound healing. J Exp Med. 2009;206(7):1465–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Agarwal S, Mayer L. Gastrointestinal manifestations in primary immune disorders. Inflamm Bowel Dis. 2010;16(4):703–11. [DOI] [PubMed] [Google Scholar]
- 30.Glocker EO, et al. Inflammatory bowel disease and mutations affecting the interleukin-10 receptor. N Engl J Med. 2009;361(21): 2033–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Welte T, et al. STAT3 deletion during hematopoiesis causes Crohn’s disease-like pathogenesis and lethality: a critical role of STAT3 in innate immunity. Proc Natl Acad Sci U S A. 2003;100(4):1879–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Corley DA, et al. Proton pump inhibitors and histamine-2 receptor antagonists are associated with hip fractures among at-risk patients. Gastroenterology. 2010;139(1):93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Byrne KR, Panagiotakis PH, Hilden K, Thomas KL, Peterson KA, Fang JC. Retrospective analysis of esophageal food impaction: differences in etiology by age and gender. Dig Dis Sci. 2007;52(3): 717–21. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.