Abstract
Purpose
This research aims to investigate the predictive capacity of PET/CT quantitative parameters combined with haematological parameters in advanced lung cancer patients treated with immune checkpoint inhibitor (ICI) plus chemotherapy.
Methods
A total of 120 patients who underwent 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) were enrolled before therapy. The following parameters were calculated: the maximum, mean, and peak standardized uptake value (SUVmax, SUVmean, and SUVpeak, respectively); total tumour volume (MTV) and total lesion glycolysis (TLG); and whole-body metabolic values (MTVwb, TLGwb, SUVmeanwb, and SUVmaxwb). Lactate dehydrogenase (LDH) levels, absolute neutrophil count, absolute platelet count, albumin levels and derived neutrophil to lymphocyte ratio (dNLR) were also computed. The associations between the variables and therapy outcome (evaluated by iRECIST) were analyzed.
Results
Based on iRECIST, 32 of 120 patients showed iPD, 43 iSD, 36 iPR and 9 iCR. Multivariate analysis found that SUVmax, MTVwb, LDH and absolute platelet count were associated with treatment response (P =0.015, P =0.005, P <0.001 and P =0.015, respectively). Kaplan-Meier survival analyses showed that SUVmax ≥11.42 and LDH ≥245 U/L were associated with shorter OS (P = 0.001 and P = 0.004, respectively). Multivariate Cox regression revealed that SUVmax and LDH alone were not correlated with survival prognosis (p>0.05), but the combination of SUVmax and LDH was independently associated with OS (P=0.015, P=0.001, respectively). The median survival time (MST) for the low (LDH<245 and SUVmax<11.42), intermediate(LDH<245 or SUVmax<11.42), and high(SUVmax≥11.42 and LDH≥245) groups was 24.10 months (95% CI: 19.43 to 28.77), 17.41 months (95% CI: 15.83 to 18.99), and 13.76 months (95% CI: 12.51 to 15.02), respectively.
Conclusion
This study identified that SUVmax plus LDH correlated with the survival outcome in patients with advanced lung cancer receiving PD-1/PD-L1 blockade plus chemotherapy.
Keywords: prognosis, hematology, immunotherapy, lung cancer, SUVmax
Introduction
Recently, GLOBOCAN reported that lung cancer has the highest rate of incidence and mortality for men and women in the world (1). It has a poor prognosis, with a 5-year survival rate of 15% (2, 3). Unfortunately, a large population of primary lung cancer patients are diagnosed at stage IV (4). The 5‐year survival rate of metastatic lung cancer is no more than 5% because of the lack of appropriate treatment options. Therefore, systemic therapy has become the primary treatment option.
Today, systemic therapy for advanced lung cancer mainly includes immunotherapy and chemotherapy, as well as their combination. Many preclinical studies have shown the immunomodulatory effects of cytotoxic chemotherapy. Whether for non-small-cell lung cancer (NSCLC) or small cell lung cancer (SCLC), chemotherapy combined with PD-1 receptor or ligand inhibitor plays an important role in first-line therapy, and this approach has been undertaken to improve treatment responses and prolong survival (5–11).
Immune checkpoint inhibitors (ICIs) plus chemotherapy are recommended as the optimal first‐line therapy for patients with advanced NSCLC (9). A meta-analysis (12) found that overall survival (OS) and progression-free survival (PFS) advantages of ICI therapies were observed in patients with NSCLC with low or high programmed cell death 1 ligand 1 (PD‐L1) expression levels but not in intermediate PD‐L1 TPS patients. Update data for the KEYNOTE-189 study found that regardless of PD-L1 positivity, both median OS and PFS improved in the pembrolizumab combination chemotherapy group in patients with metastatic NSCLC (13). Interestingly, the HRs (hazard ratios) for PFS were similar among PD-L1-expressing and PD-L1-negative patients (13). Therefore, PD‐L1 alone is not recommended as a molecular biomarker to identify eligible patients for immunotherapy plus chemotherapy in routine clinical practice.
According to research, the inflammation process is associated with the mechanism of oncogene signaling pathway activation and immunoresistance in the cancer population (14). Of note, a pro-inflammatory status is connected with poor outcomes in cancer patients (15–17). The hematological parameters circulating white blood cells, absolute neutrophil count, absolute platelet count, lactate dehydrogenase (LDH) level and derived neutrophil-to-lymphocyte ratio (dNLR; absolute neutrophil count/[white blood cell concentration − absolute neutrophil count]) have been proposed as potential inflammatory biomarkers in cancer patients and are also correlated with poor outcomes in several solid tumors (17–21).
As an advanced imaging examination, [18F]F-FDG PET/CT (18F-fluorodeoxyglucose positron emission tomography/computed tomography) is widely used for response monitoring and prognostication for locally advanced NSCLC (22–24). The study found that SUVpeak, MTV, and TLG has predictive significance in the response to immunotherapy in patients with melanoma (25).Another study showed that baseline MTVwb and SUVmean correlate with survival in advanced non-small cell lung cancer patients treated with pembrolizumab (26). At the same time, the entire tumor burden evaluated by 18F-FDG PET/CT was proved to be the Predictors to immunotherapy in patients with metastatic lung cancer (27). Soussan et al. found that SUVmax, SUVpeak, SUVmean, TLG were prognostic factors for EFS(event-free survival) in lung cancer after chemotherapy, but MTV is not (28).A study that included 60 patients with lung cancer who received chemotherapy alone, and finally found that the whole-body PET/CT parameters (MTV,TLG) significantly associated with overall survival. However, SUVmaxwb and SUVmeanwb were not statistically significant association with OS (29). The uptake of FDG by malignant tissues as well as in inflammatory disorders is quantified by various parameters of [18F]F-FDG PET/CT, such as the standardized uptake value (SUV) (30–32).
Published retrospective studies reported that a pro-inflammatory status was associated with poor outcomes of immunotherapy in melanoma patients (33, 34). Mezquita et al. found that combining a dNLR greater than 3 with LDH greater than the upper limit of normal (ULN) could identify advanced NSCLC patients who would have poor outcomes from immunotherapy (21). We hypothesized that combining baseline parameters of hematology and [18F] F-FDG PET/CT would be correlated with a poor outcome of ICI therapy combined with chemotherapy in patients with advanced lung cancer.
Materials and methods
Population
This retrospective study enrolled 120 patients with advanced lung cancer at our institute from January 2017 to January 2020 who underwent pretreatment 18F-FDG PET/CT before receiving combination treatment of ICI plus chemotherapy. The inclusion criteria were as follows: (1) histologically or cytologically proven lung cancer; (2) TNM stage IV in the American Joint Committee on Cancer (AJCC) 8th (35) staging system; (3) Eastern Cooperative Oncology Group Performance Status of 0 to 1; (4) more than 4 cycles (3 weeks to a cycle) of ICI plus chemotherapy; and (5) more than 18 years old. Exclusion criteria:
This study was approved by the Institutional Review Board of the Shandong Cancer Hospital and Institute (Jinan, China). All patients provided informed consent before treatment.
PET-CT Imaging
In the Department of Nuclear Medicine and PET-CT Centre, all patients had to have serum glucose levels less than 11 mol/L and at least 6 h of fasting before intravenous administration of 370 MBq (10 mCi) of FDG. After resting in a lounge chair for a minimum of 60 min, all patients underwent 5 min whole-body emission scanning from the skull base to the upper femur. PET images were obtained with a dedicated PET/CT scanner (GEMINI TF Big Bore; Philips Healthcare). Under 4.25 mm/slice axial sampling thickness and 0.8 s rotation speed per rotation, spiral CT was performed.
All subjects were asked to maintain tidal breathing during PET scanning. The images were reconstructed by ordered-subset expectation maximization (OSEM) after attenuation correction. Then, the corresponding PET and CT images, as well as fused PET/CT images, were observed on a dedicated workstation (Xeleris; GE Healthcare) in the transverse, coronal, and sagittal planes. [18F]F-FDG PET/CT scans for all patients were performed before they received the combined treatment.
Image Analysis
Two experienced nuclear medicine physicians outlined the regions of interest (ROIs) separately according to 3-dimensional CT scans and PET/CT fusion images by using MIM software (MIM, 6.2.8, Cleveland, OH, USA). The automated contouring program was set to a fixed standardized uptake value (SUV) threshold of 2.5 (34–36). Under the fixed threshold, the maximum, mean, and peak standardized uptake values (SUVmax, SUVmean, and SUVpeak, respectively), as well as total tumour volume (MTV) and total lesion glycolysis (TLG), were acquired. The whole-body burden values of SUVmax, SUVmean, SUVpeak, MTV, and TLG were named SUVmaxwb, SUVmeanwb, MTVwb, and TLGwb, which were defined as their respective summations.
Hematological Parameters
We also collected hematological parameters within 3 days before the start of combination treatment by searching the patient’s electronic medical records: lactate dehydrogenase (LDH) levels (the normal reference range was 109–245 U/L), absolute neutrophil count, absolute platelet count, albumin levels and dNLR [absolute neutrophil count/(white blood cell concentration − absolute neutrophil count)].
Response Evaluation
Every subject’s best treatment response was evaluated by iRECIST (Immune Response Evaluation Criteria in Solid Tumors) (37) according to their every-6-weekly clinical and radiological follow-up. Patients were grouped as experiencing progression of disease (iPD), stable disease (iSD) or partial response or complete response (iPR/iCR). Clinical benefit (CB) was grouped as iPR or iCR, and no clinical benefit (no-CB) was grouped as iPD or iSD.
Statistical Analyses
Overall survival (OS) was computed as the start of combination therapy until death for any reason or the date of the last follow-up. To summarize the results of this study, descriptive statistics are reported as mean ± standard deviation. Statistical analysis tried to solve several objectives. The independent sample Student’s t-test was used for continuous variables. Kaplan-Meier analyses and the log-rank test were used to quantify the associations with survival. Cox proportional hazards regression aimed to distinguish variables independently correlated with survival. Statistically significant variables in the univariate analysis (P <0.10) were included in the final multivariate model. Logistic regression analysis with the upward elimination method was performed to further verify the relationships found. Cut-off points were obtained by receiver operating characteristic (ROC) curve analysis. SPSS Statistics version 26.0 (IBM Corporation, Armonk, NY, USA) was used for statistical analyses. P value < 0.05 was considered statistically significant.
Results
Characteristics of Patients
Table 1 shows the patient demographics and baseline characteristics.
Table 1.
Baseline characteristics of all 120 patients.
| Characteristic | Total (n=120) | CB (Clinical Benefit) (n=45) | no-CB (no-Clinical Benefit) (n=75) |
|---|---|---|---|
| Age (years) | |||
| Median age, year (range) | 60 (37-81) | 60 (43-76) | 60 (37-81) |
| Gender n. (%) | |||
| Male | 83 (69.2) | 35 (77.8) | 48 (64.0) |
| Female | 37 (30.8) | 10 (22.2) | 27 (36.0) |
| Performance status (ECOG) n. (%) | |||
| 0,1 | 120 (100) | 45 (100) | 75 (100) |
| ≥2 | 0 (0) | 0 (0) | 0 (0) |
| Histology n. (%) | |||
| Small cell lung cancer | 33 (27.5) | 11 (24.4) | 22 (29.3) |
| Non-Small cell lung cancer | |||
| Squamous cell carcinoma | 33 (27.5) | 13 (28.9) | 20 (26.7) |
| Non-Squamous cell carcinoma | 54 (45.0) | 21 (46.7) | 33 (44.0) |
| Previously treated n. (%) | 34 (28.3) | 14 (31.1) | 20 (26.7) |
| Previous therapy n. (%) | |||
| Thoracic radiotherapy | 26 (21.7) | 11 (24.4) | 15 (20.0) |
| Target therapy | 15 (12.5) | 7 (15.6) | 8 (10.7) |
| Chemotherapy | 32 (26.7) | 12 (26.7) | 20 (26.7) |
| Smoking status n. (%) | |||
| Former or current | 60 (50.0) | 29 (64.4) | 31 (41.3) |
| Never | 60 (50.0) | 16 (35.6) | 44 (58.7) |
| PD-L1 expression n. (%) | |||
| ≥1% | 34 (28.3) | 14 (31.1) | 20 (26.7) |
| <1% | 39 (32.5) | 12 (26.7) | 27 (36.0) |
| NA | 47 (39.2) | 19 (42.2) | 28 (37.3) |
ECOG, Eastern Cooperative Oncology Group; PD-L1, programmed death ligand 1, was acquired by immunohistochemistry; NA, not available.
This retrospective study comprised 120 patients. The patients were predominantly male (69.2%, 83/120), with a median age of 60 years (range 37–81 years) at the time of diagnosis. Thirty-five (77.8%) of 45 patients were male in the CB group, and 48 (64.0%) of 75 patients were male in the no-CB group. Out of 120 patients, 33 had small cell lung cancer (SCLC), 33 had squamous cell lung carcinoma, and 54 had non-squamous-cell lung carcinoma. They had predominantly NSCLC, at 21 (46.7%) and 33 (44%) in the CB group and no-CB group, respectively. The ECOG performance status for all patients was 0 or 1. Before starting ICI plus chemotherapy, 26 patients received thoracic radiotherapy, 15 received targeted therapy, and 32 patients received chemotherapy. Some 28.3% (34/120) of the sample showed a PD‐L1 tumour proportion score (TPS) ≥1% by immunohistochemical analysis, and 32.5% (39/120) showed a PD‐L1 TPS <1%. The smoking status was similar in the two groups: 29 (64.4%) patients in the CB group were former or current smokers, versus 31 (41.3%) in the no-CB group. Staging was carried out on the basis of the 8th edition of the American Joint Committee on Cancer tumour, node and metastasis staging system.
Radiological Outcome and Prognosis
Radiological follow-up was available in all patients. Based on iRECIST, 32 of 120 patients showed iPD, 43 iSD, 36 iPR and 9 iCR. The proportion of patients in the CB group was 37.5% (45/120), and that in the no-CB group was 62.5% (75/120). The difference in outcomes that were continuous variables between the CB group and no-CB group, as calculated by the independent-sample Student’s t-test, is shown in Table 2 .
Table 2.
Independent sample student t-test of groups difference (CB vs no-CB).
| Parameters | no-CB (n=75) (mean ± SD) | CB (n=45) (mean ± SD) | P value |
|---|---|---|---|
| SUVmax | 12.83 ± 5.31 | 10.82 ± 5.27 | 0.046* |
| SUVpeak | 9.43 ± 5.15 | 7.75 ± 4.12 | 0.052 |
| SUVmean | 5.24 ± 2.16 | 5.25 ± 1.95 | 0.982 |
| MTV | 92.56 ± 131.68 | 46.22 ± 61.22 | 0.010* |
| TLG | 713.35 ± 822.01 | 342.10 ± 734.80 | 0.055 |
| SUVmaxwb | 22.13 ± 13.72 | 18.80 ± 21.58 | 0.302 |
| SUVmeanwb | 6.55 ± 2.93 | 6.82 ± 2.32 | 0.600 |
| MTVwb | 97.53 ± 120.14 | 53.38 ± 59.93 | 0.009* |
| TLGwb | 495.88 ± 668.78 | 271.28 ± 576.55 | 0.066 |
| LDH,U/L | 291.96 ± 106.70 | 204.84 ± 59.85 | <0.001* |
| Absolute neutrophil count,×109/L | 5.03 ± 3.04 | 4.40 ± 1.24 | 0.113 |
| Absolute platelet count,×109/L | 239.56 ± 70.24 | 201.44 ± 78.10 | 0.007* |
| Albumin levels, g/L | 43.71 ± 3.97 | 43.85 ± 3.78 | 0.851 |
| dNLR | 2.43 ± 1.30 | 2.67 ± 1.46 | 0.360 |
no-CB, no-Clinical Benefit, was defined as complete or partial response; CB, Clinical Benefit, was defined as stable disease or progressive disease response; SUVmax, maximum standardized uptake value; SUVpeak, peak standardized uptake value; SUVmean, mean standardized uptake value; MTV, metabolic tumor volume; TLG, total lesion glycolysis; SUVmaxwb, whole-body maximum standardized uptake value; SUVmeanwb, whole-body mean standardized uptake value; MTVwb, whole-body metabolic tumor volume; TLGwb, whole-body total lesion glycolysis; LDH, lactate dehydrogenase; dNLR was defined as absolute neutrophil count/[white blood cell concentration − absolute neutrophil count];* p<0.05.
Patients in the CB group had a significantly lower SUVmax, MTV, MTVwb, LDH and absolute platelet count than patients in the no-CB group (10.82 ± 5.27 vs 12.83 ± 5.31, p=0.046; 46.22 ± 61.22 vs 92.56 ± 131.68, p=0.010; 53.38 ± 59.93 vs. 97.53 ± 120.14, p=0.009; 204.84 ± 59.85 vs. 291.96 ± 106.70, p<0.001; 201.44 ± 78.10 vs. 239.56 ± 70.24, p=0.007, respectively) ( Table 2 ). In multivariate analysis, SUVmax, MTVwb, LDH and absolute platelet count were associated with progressive disease, with an OR of 4.44 (95% CI, 1.33-14.80; P =0.015), 12.63 (95% CI, 2.17-73.56; P =0.005), 22.20 (95% CI, 6.31-78.05; P <0.001), and 4.85(95% CI, 1.60-14.76; P =0.015), respectively ( Table 3 ).
Table 3.
Logistic regression of clinical benefit in 120 patients.
| Variables | OR (95% CI) | P value |
|---|---|---|
| Gender(vs) | ||
| Male | ||
| Female | 0.67 (0.12-3.68) | 0.643 |
| Age | ||
| <60 | ||
| ≥60 | 1.15 (0.38-3.48) | 0.805 |
| Histology | ||
| Squamous NSCLC | ||
| Non-squamous NSCLC | ||
| Small cell lung cancer | 1.26 (0.60-2.62) | 0.545 |
| Smoking status | ||
| Never | ||
| Former or current | 0.15 (0.03-0.77) | 0.024* |
| PD-L1 expression | ||
| <1% | ||
| ≥1% | ||
| NA | 0.15 (0.03-0.77) | 0.701 |
| SUVmax | ||
| <11.42 | ||
| ≥11.42 | 4.44 (1.33-14.80) | 0.015* |
| MTV | ||
| <40.36 | ||
| ≥40.36 | 0.99 (0.22-4.41) | 0.990 |
| MTVwb | ||
| <61.45 | ||
| ≥61.45 | 12.63 (2.17-73.56) | 0.005* |
| LDH,U/L | ||
| <245 | ||
| ≥245 | 22.20 (6.31-78.05) | <0.001* |
| Absolute platelet count,×109/L | ||
| <177 | ||
| ≥177 | 4.85 (1.60-14.76) | 0.005* |
OR, odds ratio; NSCLC, non‒small-cell lung cancer; PD-L1, programmed death ligand 1, was acquired by immunohistochemistry; NA, not available; SUVmax, maximum standardized uptake value; MTV, metabolic tumor volume; MTVwb, whole-body metabolic tumor volume; LDH lactate dehydrogenase; * p<0.05.
Survival Analysis
At the time of analysis, 76 patients (63.3%) had died. The median follow-up time was 15.40 months (range, 1.37–29.43 months). The estimated median survival time (MST) for the entire cohort was 16.67 months (95% CI: 15.41–17.93 months), with estimated 6-month, 12-month and 18-month OS rates of 96.6%, 80.0% and 41.9%, respectively.
Univariate analysis of OS among patients with advanced lung cancer revealed that smoking status, SUVmax and LDH were significant predictors, whereas PD-L1 expression, sex, age, histology, SUVmaxwb, SUVpeak, SUVmean, SUVmeanwb, MTV, MTVwb, TLG, TLGwb, absolute platelet count, absolute neutrophil count, albumin levels and dNLR were not significant prognostic factors ( Table 4 ). Based on the ROC curve, the optimal cut-off value of SUVmax was 11.42.
Table 4.
Univariate analysis of overall survival in all patients.
| Variables | OS | |
|---|---|---|
| HR (95% CI) | P value | |
| Gender (Male vs Female) | 0.730 (0.387-1.379) | 0.332 |
| Age (<60 vs ≥60) | 0.624 (0.355-1.096) | 0.101 |
| Histology (Squamous NSCLC vs Non-squamous NSCLC vs Small cell lung cancer) | 1.213 (0.576-2.554) | 0.612 |
| Smoking status (Never vs Former or current) | 1.385 (0.387-1.379) | 0.025* |
| PD-L1 expression (<1% vs ≥1% vs NA) | 1.206 (0.620-2.343) | 0.581 |
| SUVmax | 1.059 (1.005-1.117) | 0.033* |
| SUVmaxwb | 1.010 (0.998-1.022) | 0.119 |
| SUVpeak | 1.030 (0.975-1.089) | 0.293 |
| SUVmean | 1.077 (0.920-1.261) | 0.357 |
| SUVmeanwb | 0.998 (0.881-1.131) | 0.981 |
| MTV | 1.000 (0.997-1.002) | 0.864 |
| MTVwb | 0.999 (0.881-1.131) | 0.486 |
| TLG | 1.000 (0.998-1.003) | 0.987 |
| TLGwb | 1.000 (0.999-1.000) | 0.906 |
| LDH,U/L | 1.003 (1.000-1.005) | 0.049* |
| Absolute platelet count,×109/L | 0.999 (0.996-1.003) | 0.660 |
| Absolute neutrophil count,×109/L | 0.968 (0.850-1.103) | 0.628 |
| Albumin levels, g/L | 0.968 (0.900-1.040) | 0.374 |
| dNLR | 0.974 (0.791-1.199) | 0.803 |
HR, hazard ratio; NSCLC non‒small-cell lung cancer; PD-L1, programmed death ligand 1, was acquired by immunohistochemistry; NA, not available; SUVmax maximum standardized uptake value; SUVpeak peak standardized uptake value; SUVmean mean standardized uptake value; MTV metabolic tumor volume; TLG total lesion glycolysis; SUVmaxwb whole-body maximum standardized uptake value; SUVmeanwb whole-body mean standardized uptake value; MTVwb, whole-body metabolic tumor volume; TLGwb, whole-body total lesion glycolysis; LDH lactate dehydrogenase; dNLR was defined as absolute neutrophil count/[white blood cell concentration − absolute neutrophil count]; OS, overall survival;* p<0.05.
According to the above, SUVmax and LDH were combined for analysis and were used to classify the patients into 3 groups: the high group (SUVmax≥11.42 and LDH≥245), 2 risk factors; intermediate group (LDH<245 or SUVmax<11.42), 1 risk factor; and low group (LDH<245 and SUVmax<11.42), 0 risk factors ( Figure 1 ).
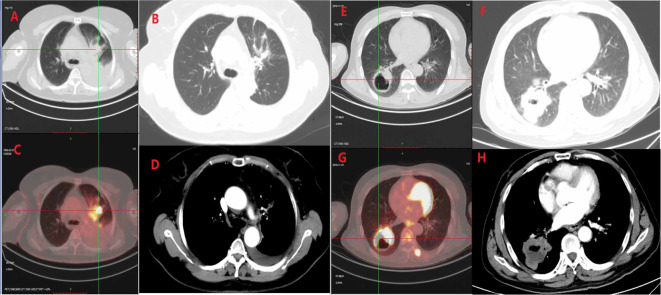
Figure 1.
(A) A 63-year-old female patient with stage IVa left upper lobe adenocarcinoma. PET-CT before immunization and chemotherapy showed a SUVmax of 11.0 and an LDH of 196 (A, C). The imaging efficacy evaluation after 6 cycles of treatment showed PR (B, D). (B) A 58-year-old male patient with squamous cell carcinoma of the right lower lobe, PET-CT showed SUVmax of 22.8, LDH of 196 (E, G), and disease progression (F, H) after 4 cycles of treatment.
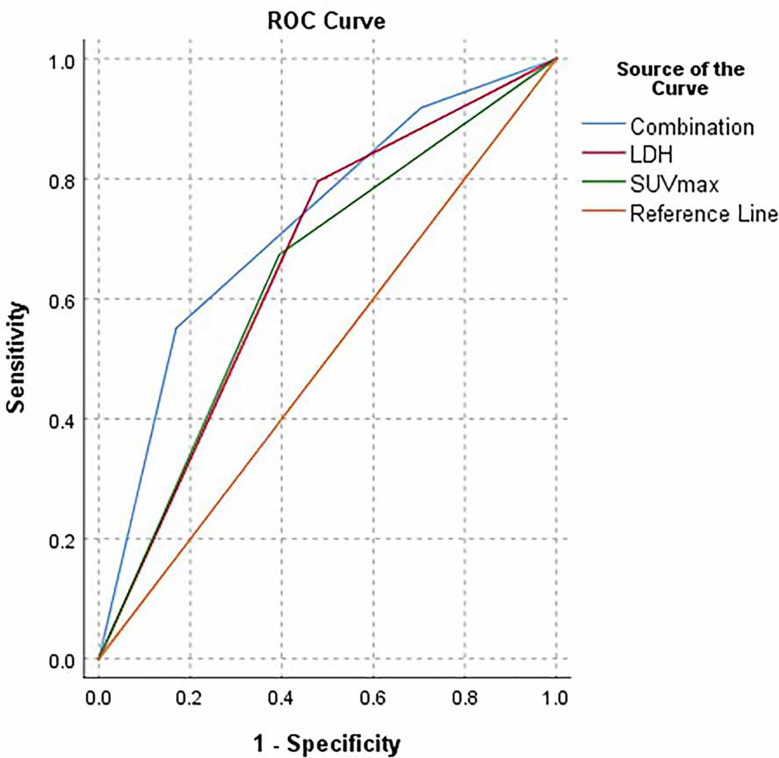
We further analyzed these selected parameters and found that the AUC of the combination of SUVmax and LDH was greater than that of SUVmax or LDH alone, with values of 0.723, 0.640 and 0. 659, respectively ( Figure 2 ).
Figure 2.
ROC curve of LDH (<245 VS ≥245), SUVmax(<11.42 vs ≥11.42), Combination group (Low group, LDH<245 and SUVmax<11.42; intermediate group, LDH<245 or SUVmax<11.42; high group, SUVmax≥11.42 and LDH≥245), respectively.
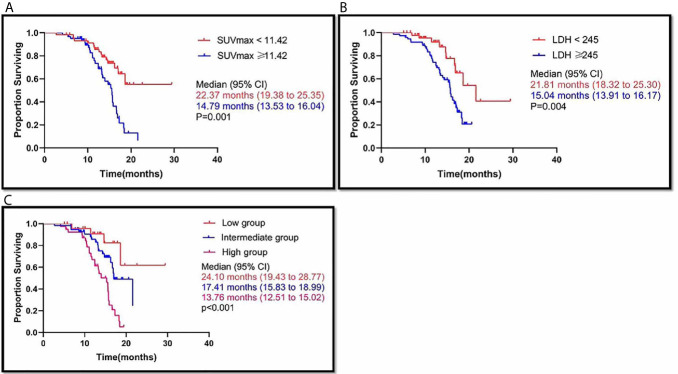
The difference in MST between SUVmax ≥11.42 patients and SUVmax <11.42 patients was statistically significant (P=0.001), and the median (95% CI) survival time was 14.79 (13.53 to 16.04) months in the SUVmax ≥11.42 group and 22.37 (19.38 to 25.35) months in the SUVmax <11.42 group ( Figure 3A ). LDH ≥245 and LDH <245 were also associated with different MSTs (P=0.004). The median (95% CI) survival time was 15.04 (13.91 to 16.17) months in the LDH ≥245 group and 21.81 (18.32 to 25.30) months in the LDH <245 group ( Figure 3B ).
Figure 3.
SUVmax≥11.42, LDH≥245 and high group (SUVmax≥11.42 and LDH≥245), was associated with poor outcome. Kaplan-Meier analysis for Overall survival (OS) in the SUVmax ≥11.42 vs. SUVmax <11.42 (A); LDH ≥245 vs. LDH <245 (B); high group (SUVmax≥11.42 and LDH≥245) vs. intermediate group (LDH<245 or SUVmax<11.42) vs. low group (LDH<245 and SUVmax<11.42) (C), respectively.
Out of all the patients, there were 25 patients in the low group, who had a median survival time of 24.10 months (95% CI: 19.43 to 28.77). There were 56 patients in the intermediate group, and their MST was 17.41 months (95% CI: 15.83 to 18.99). The 39 patients in the high group had an MST of 13.76 months (95% CI: 12.51 to 15.02). The difference in MST between these groups in the study was statistically significant (p<0.001) ( Figure 3C ).
In multivariate analysis, an independent prognostic factor associated with OS was the combination of primary tumour SUVmax and LDH (the high group had HR 2.397, 95% CI 0.808-7.112, P=0.015; the intermediate group had HR 6.399; 95% CI 2.201-18.602; P =0.001) ( Table 5 ).
Table 5.
Multivariate analysis of overall survival in all patients.
| Characteristics | OS | |
|---|---|---|
| HR (95%CI) | P value | |
| Combination groups | ||
| Low group | ||
| Intermediate group | 2.397 (0.808-7.112) | 0.015* |
| High group | 6.399 (2.201-18.602) | 0.001* |
OS, overall survival; HR, hazard ratio; * p<0.05.
Subgroup Analysis
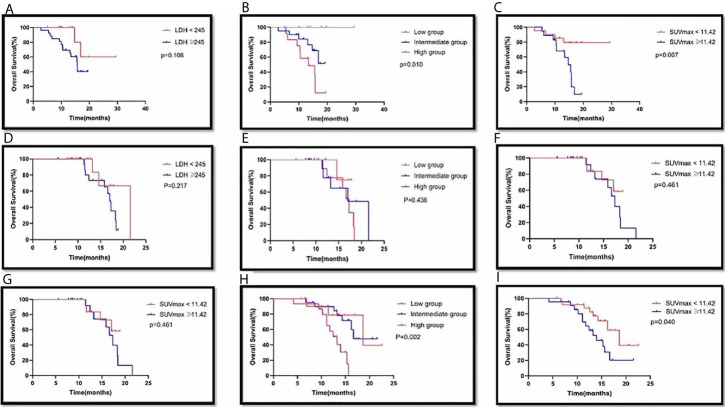
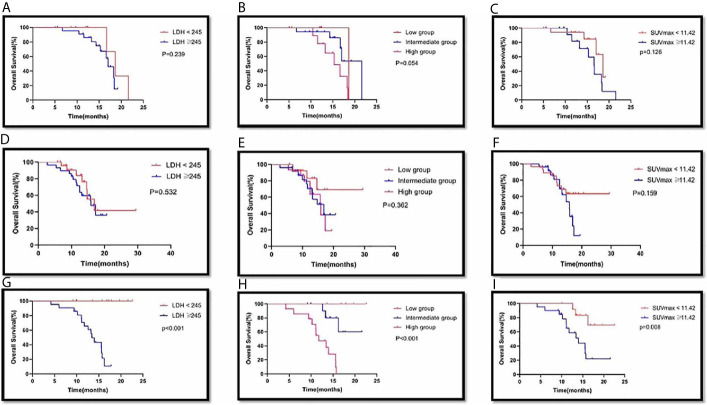
Among the 39 patients with PD-L1 expression <1%, there were 13 patients in the LDH <245 group, with an MST of 23.98 months (95% CI: 18.09-29.86), and 26 patients in the LDH ≥245 group, with an MST of 14.50 months (95% CI: 12.44-16.55). There was no significant difference in MST between the two groups (p=0.108) ( Figure 4A ). There were 7 patients in the low group, and none died; of the 20 patients in the intermediate group, 6 died; of the 12 patients in the high group, 9 died. There was a significant difference in MST among the different groups (p=0.010). There were 21 patients in the SUVmax <11.42 group, with an MST of 25.05 months (95% CI: 21.18-28.92); there were 18 patients in the SUVmax ≥11.42 group, with an MST of 13.62 months (95% CI: 11.65-15.59). There was a significant difference in MST between the two groups (p=0.007) ( Figure 4C ).
Figure 4.
Survival curves of LDH(<245 VS ≥245), combination group(Low group, LDH<245 and SUVmax<11.42; intermediate group, LDH<245 or SUVmax<11.42; high group, SUVmax≥11.42 and LDH≥245) and SUVmax(<11.42 vs ≥11.42) of primary tumor in PD-L1 expression <1% (A,B,C), PD-L1 expression ≥1% (D,E,F) and PD-L1 expression not available (G,H,I) with advanced lung cancer, respectively.
In Cox multivariate analysis, the only independent prognostic factor associated with OS was SUVmax (HR 4.359, 95% CI: 1.373-13.837, p=0.012)
Among the 34 patients with PD-L1 expression ≥1%, there were 16 patients in the LDH <245 group, with an MST of 19.02 months (95% CI: 15.47-22.58). The 18 patients in the LDH ≥245 group had an MST of 16.06 months (95% CI: 14.64-17.48). There was no significant difference in MST between the two groups (p=0.217). There were 7 patients in the low group, with an MST of 16.66 months (95% CI: 14.74-18.57); 15 patients in the intermediate group, with an MST of 17.51 months (95% CI: 14.20-20.83); and 12 patients in the high group, with an MST of 16.21 months (95% CI: 14.47-17.95). There was no significant difference in MST among the different groups (p=0.436). There were 13 patients in the SUVmax <11.42 group, with an MST of 16.95 months (95% CI: 15.31-18.59), and 21 patients in the SUVmax ≥11.42 group, with an MST of 16.66 months (95% CI: 14.74-18.57). There was no significant difference in MST between the two groups (p=0.461). ( Figure 4F )
Among the 47 patients whose PD-L1 expression was not available, there were 18 patients in the LDH <245 group, with an MST of 18.20 months (95% CI: 15.25-21.16), and 29 patients in LDH ≥245 group, with an MST of 14.61 months (95% CI: 12.83-16.38). There was no significant difference of MST between the two groups (p=0.064). There were 11 patients in the low group, with an MST of 18.21 months (95% CI: 14.51-21.92); 21 patients in the intermediate group, with an MST of 17.51 months (95% CI: 17.45); and 15 patients in the high group, with an MST of 12.43 months (95% CI: 10.81-14.05). There was a significant difference in MST among the different groups (p=0.002). There were 25 patients in the SUVmax <11.42 group, with an MST of 17.75 months (95% CI: 15.25-20.25), and 22 patients in the SUVmax ≥11.42 group, with an MST of 14.31 months (95% CI: 12.12-16.51). There was a significant difference in MST between the two groups (p=0.040). In Cox multivariate analysis, independent prognostic factors associated with OS included combination group. Taking the low group as a reference, the HR of the high group was 5.356 (95% CI: 1.336-21.466, p=0.018), and the HR of the intermediate group was 1.144 (95% CI: 0.292-4.481, p=0.005) ( Figure 4I ).
In 33 patients with squamous non–small-cell lung cancer, there were 10 patients in the LDH <245 groups, with an MST of 18.96 months (95% CI: 16.17-21.75). There were 23 patients in the LDH ≥245 group, and their MST was 16.19 months (95% CI: 14.74-17.64). The difference in MST between these two groups was not statistically significant (p=0.239) ( Figure 5A ). There were 5 patients in the low group, with an MST of 18.63 months (95% CI: 18.63-18.63). There were 18 patients in the intermediate group, and their MST was 18.61 months (95% CI: 16.01-21.20). There were 10 patients in the high group, and their MST was 15.28 months (95% CI: 13.18-17.38). The difference in MST between these different groups was not statistically significant (p=0.054). There were 18 patients in the SUVmax <11.42 group, with an MST of 17.35 months (95% CI: 15.73-18.98), and 15 patients in the SUVmax ≥11.42 group, with an MST of 16.10 months (95% CI: 13.96-18.25). There was no significant difference in MST between the two groups (p=0.126). ( Figure 5C )
Figure 5.
Survival curves of LDH(<245 VS ≥245), combination group(Low group, LDH<245 and SUVmax<11.42; intermediate group, LDH<245 or SUVmax<11.42; high group, SUVmax≥11.42 and LDH≥245) and SUVmax(<11.42 vs ≥11.42) of primary tumor in Squamous NSCLC (A,B,C), Non-Squamous NSCLC (D,E,F) and SCLC (G,H,I) with advanced lung cancer, respectively.
In 54 patients with non-squamous non–small-cell lung cancer, there were 25 patients in the LDH <245 group, with an MST of 20.16 months (95% CI: 15.29-25.03). There were 29 patients in the LDH ≥245 group, and their MST was 15.27 months (95% CI: 13.23-17.30). The difference in MST between these two groups was not statistically significant (p=0.532). There were 14 patients in the low group, with an MST of 23.99 months (95% CI: 18.72-29.25). There were 25 patients in the intermediate group, and their MST was 15.32 months (95% CI: 12.97-17.67). There were 15 patients in the high group, and their MST was 14.75 months (95% CI: 12.60-16.90). The difference in MST between these different groups was not statistically significant (0.362). There were 28 patients in the SUVmax <11.42 group, with an MST of 22.38 months (95% CI: 18.63-26.13), and 26 patients in the SUVmax ≥11.42 group, with an MST of 14.52 months (95% CI: 12.90-16.14). There was no significant difference in MST between the two groups (p=0.159). ( Figure 5F )
In 33 patients with small cell lung cancer, there were 12 patients in the LDH <245 group, and no patients died. Twenty-one patients were included in the LDH ≥245 group. The difference in MST between these two groups was statistically significant (p<0.001). There were 6 patients in the low group, 13 patients in the intermediate group, and 14 patients in the high group. The difference in MST between these different groups was statistically significant (p<0.001). There were 13 patients in the SUVmax <11.42 group, with an MST of 20.10 months (95% CI: 17.70-22.51), and 20 patients in the SUVmax ≥11.42 group, with an MST of 14.07 months (95% CI: 11.67-16.48). There was a significant difference in MST between the two groups (p=0.008). In the Cox multivariate analysis, neither SUVmax nor LDH nor SUVmax plus LDH was an independent prognostic factor for OS. (all p>0.05).
Discussion
The tumour metabolic activity assayed by the advanced noninvasive examination method 18F-FDG PET/CT plays an important role in the diagnosis, staging, and evaluation of the treatment response and prognosis of lung cancer. Although some studies have shown the value of 18F-FDG PET/CT in immunotherapy for advanced lung cancer (27, 38), few data are available about its potential utility in the response evaluation of immunotherapy plus chemotherapy. It is worth mentioning that our study explored the prognostic evaluation and curative effect of SUVmax and LDH in patients treated with ICI plus chemotherapy. SUVmax ≥11.42 and LDH ≥245 were associated with significantly shorter OS (HR, 2.397, 95% CI, 0.808-7.112, P=0.015 and HR, 6.399; 95% CI, 2.201-18.602; P =0.001, respectively). No OS differences were observed in our cohort according to histology (SCLC/squamous NSCLC/nonsquamous NSCLC).
Our findings show that among all enrolled 18F-FDG PET imaging parameters and hematological parameters at baseline, SUVmax and LDH predicted the response to PD-L1/PD-1 blockade plus chemotherapy. This study suggested that a higher SUVmax or LDH at baseline led to a worse response to therapy, which implies that they may be promising parameters for the identification of patients who have a higher chance of not responding to ICI plus chemotherapy. Our findings likely reflect the potential correlation between inflammatory biology and the steady-state biological activity of tumors in response to combination therapy of ICI plus chemotherapy. 18F-FDG PET/CT is usually used to monitoring the response to immunotherapy or chemotherapy (39–42). A preliminary analysis found that the SUVmax at baseline was significantly different between responders to immunotherapy among patients with NSCLC (43). However, a prospective study of 32 patients (44) suggested that pretreatment SUVmax as shown by 18F-FDG PET/CT was not able to predict the response to chemotherapy in advanced NSCLC patients. These findings indicate that the presence of areas of high metabolic activity in tumors, which may be associated with histological differentiation (45), predicts PD-1/PD-L1 inhibitor activity at baseline and consequently the response to PD-1 blockade, but not to chemotherapy.
In our study, univariate analysis showed that not only SUVmax but also LDH was an independent prognostic factor for OS, and patients with higher SUVmax (≥11.42) or LDH (≥245) had a significantly shorter median survival time (P=0.001, P=0.004, respectively) than patients with lower SUVmax (<11.42) or LDH (<245), respectively. Previous studies also confirmed the prognostic significance of the SUVmax on 18F-FDG PET/CT in patients with lung cancer (45–48). However, some studies have not found the same (49–51).
After adjusting for age, sex, smoking status, PD-L1 expression, and histology, both SUVmax and LDH were not independent prognostic factors. The role of SUVmax in lung cancer remains controversial. A previous study revealed that the SUVmax of the primary tumour before treatment had no prognostic value in patients with locally advanced NSCLC (52). In the present study, subgroup analysis found that among people with PD-L1 expression less than 1, SUVmax was an independent prognostic factor for OS.
SUVmax has following limitations. First, it gives a single-pixel value representing the most intense 18F-FDG uptake by the tumour and may not be a strong surrogate marker of tumour biology (53). It may not reveal the heterogeneous nature of the tumour, and it is affected by statistical noise and pixel size (54). Furthermore, SUVmax is affected by a variety of factors, such as the physique of the subject, the level of blood glucose, and the time after the injection when imaging is done. Therefore, further large-sample and multicentre studies are needed to confirm our conclusions.
Previous studies revealed that inflammatory status was associated with worse prognosis in advanced disease patients treated with chemotherapy (55–57). Several studies have demonstrated that elevated LDH was significantly associated with shorter survival (21, 58–60). Our study found that in patients with advanced lung cancer, the median (95% CI) survival times of patients with LDH ≥245 U/L and LDH <245 U/L were 15.04 months (95% CI: 13.91 to 16.17) and 21.81 months (95% CI: 18.32 to 25.30), respectively. These times were not significantly different. We suppose that combining SUVmax with LDH can provide a more accurate prediction of prognosis than SUVmax or LDH alone.
Our findings show that the combination of SUVmax and LDH was an independent prognostic factor for survival, with patients in the high group (SUVmax≥11.42 and LDH≥245) being more likely to have progressive disease (P =0.001) and having a shorter survival time (median, 13.76 months) than those in the intermediate or low group (P <0.05). The risk of death was 6.339 times and 2.397 times higher in the high and intermediate groups, respectively, than in the low group (LDH<245 and SUVmax<11.42). In our subgroup analysis, the combination of SUVmax and LDH was also an independent prognostic factor of survival for patients whose PD-L1 expression was not available. Based on the above results, we concluded that this combination was a predictor of poor outcome from ICI plus chemotherapy. SUVmax plus LDH may be more relevant to prognosis than SUVmax or LDH alone because it not only reflects tumour metabolic activity but also reflects the inflammatory status. SUVmax, which is a functional metabolism biomarker derived from 18F-FDG PET, can be used to estimate the survival value in a noninvasive manner. Additionally, SUVmax reflects metabolic activity in malignant cancerous cells, which has been significantly correlated with PD-1/PD-L1 status and CD8+ tumour-infiltrating lymphocytes (TILs) (61). One study showed that high pretreatment LDH levels were significantly associated with lower overall survival (62). Although the associations had statistical significance, there is no doubt that the relationship between SUVmax or LDH alone and the survival prognosis is relatively weak, reflecting that additional, uncertain factors exist, impossibly explained by the combination of these two markers.
Our study has several limitations. First, the sample size was relatively small. Second, this study was a retrospective analysis and hence cannot exclude potential biases. Third, 18F-FDG is not a cancer-specific imaging molecule, especially for outcome evaluation during immunotherapy. The question is whether ICIs are related to inflammatory responses, which stimulate neutrophils and macrophages and activate T cells around the tumour site. The high metabolism aroused by immune cells makes this radiotracer inadequately specific. Further research with a larger number of subjects is needed to verify our conclusions. Fourth, because of different histologies and various therapeutic methods, patients are highly heterogeneous, which limits the applicability of our results. Last but not least, both hematologic parameters and metabolic parameters are often influenced by other, uncontrollable factors; therefore, some underlying confusion cannot be avoided. Nonetheless, these weaknesses do not decrease the contributions of our research or minimize the significance of the factors associated with survival.
Conclusion
This study identified both a new noninvasive imaging-based biomarker and a hematological parameter that were correlated with poor outcomes after PD-1/PD-L1 blockade plus chemotherapy in patients with advanced-stage NSCLC. The negative correlation between combined therapy and the FDG-avid tumour metabolism parameters expressed by SUVmax could reveal intratumoural necrotic and/or apoptotic changes, and LDH could show a relationship with tumour inflammation. Further investigation is needed to confirm these possible associations and to elucidate the role of SUVmax plus LDH as a predictor of clinical response to targeted anti-PD-1/PD-L1 therapy combined with chemotherapy in a larger number of patients.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics Statement
The studies involving human participants were reviewed and approved by Shandong Cancer Hospital affiliated to Shandong University Ethical Committee. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
XM and JY contributed conception and design of the study. LK organized the database. LK performed the statistical analysis. LK, XM, and JY wrote the first draft of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study is supported by the Innovation Project of Shandong Academy of Medical Sciences (2019-04), and the Academic Promotion Program of Shandong First Medical University (2019ZL002), National Natural Science Foundation of China (81972864), Academic Promotion Program of Shandong First Medical University (2019RC002), Science and Technology Support Plan for Youth Innovation Teams of Universities in Shandong Province (2019KJL001) and Science and Technology Plan of Jinan (201907113).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin (2018) 68(6):394–424. 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2. Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2016. CA Cancer J Clin (2016) 66:7–30. 10.3322/caac.21332 [DOI] [PubMed] [Google Scholar]
- 3. Didkowska J, Wojciechowska U, Manczuk M, Lobaszewski J. Lung Cancer Epidemiology: Contemporary and Future Challenges Worldwide. Ann Transl Med (2016) 4:150. 10.21037/atm.2016.03.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brahmer JR, Govindan R, Anders RA, Antonia SJ, Sagorsky S, Davies MJ, et al. The Society for Immunotherapy of Cancer Consensus Statement on Immunotherapy for the Treatment of non-Small-Cell Lung Cancer (NSCLC). J Immunotherapy Cancer (2018) 6(1):75–4. 10.1186/s40425-018-0382-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pfirschke C, Engblom C, Rickelt S, Cortez-Retamozo V, Garris C, Pucci F, et al. Immunogenic Chemotherapy Sensitizes Tumors to Checkpoint Blockade Therapy. Immunity (2016) 44(2):343–54. 10.1016/j.immuni.2015.11.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lesterhuis WJ, Punt CJ, Hato SV, Eleveld-Trancikova D, Jansen BJ, Nierkens S, et al. Platinum Based Drugs Disrupt STAT6-mediated Suppression of Immune Responses Against Cancer in Humans and Mice. J Clin Invest (2011) 121(8):3100–8. 10.1172/JCI43656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ramakrishnan R, Assudani D, Nagaraj S, Hunter T, Cho HI, Antonia S, et al. Chemotherapy Enhances Tumor Cell Susceptibility to CTL-mediated Killing During Cancer Immunotherapy in Mice. J Clin Invest (2010) 120(4):1111–24. 10.1172/JCI40269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, et al. Pembrolizumab Plus Chemotherapy in Metastatic non-Small-Cell Lung Cancer. N Engl J Med (2018) 378(22):2078–92. 10.1056/NEJMoa1801005 [DOI] [PubMed] [Google Scholar]
- 9. Paz-Ares L, Luft A, Vicente D, Tafreshi A, Gümüş M, Mazières J, et al. Pembrolizumab Plus Chemotherapy for Squamous non-Small-Cell Lung Cancer. N Engl J Med (2018) 379(21):2040–51. 10.1056/NEJMoa1810865 [DOI] [PubMed] [Google Scholar]
- 10. Horn L, Mansfield AS, Szczęsna A, Havel L, Krzakowski M, Hochmair MJ, et al. First-Line Atezolizumab Plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. N Engl J Med (2018) 379:2220–9. 10.1056/NEJMoa1809064 [DOI] [PubMed] [Google Scholar]
- 11. Goldman JW, Dvorkin M, Chen Y, Reinmuth N, Hotta K, Trukhin D, et al. Durvalumab, With or Without Tremelimumab, Plus Platinum-Etoposide Versus Platinum-Etoposide Alone in First-Line Treatment of Extensive-Stage Small-Cell Lung Cancer (CASPIAN): Updated Results From a Randomised, Controlled, Open-Label, Phase 3 Trial. Lancet (2021) 22(1):51–65. 10.1016/S1470-2045(20)30539-8 [DOI] [PubMed] [Google Scholar]
- 12. Wang C, Qiao W, Jiang Y, Zhu M, Shao J, Wang T, et al. The Landscape of Immune Checkpoint Inhibitor Plus Chemotherapy Versus Immunotherapy for Advanced non-Small-Cell Lung Cancer: A Systematic Review and Meta-Analysis. J Cell Physiol (2020) 235:4913–27. 10.1002/jcp.29371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gadgeel S, Rodríguez-Abreu D, Speranza G, Esteban E, Felip E, Dómine M, et al. Updated Analysis From KEYNOTE-189: Pembrolizumabor Placebo Plus Pemetrexed and Platinum for Previously Untreated Metastatic Nonsquamous non-Small-Cell Lung Cancer. J Clin Oncol (2020) 38:1505–17. 10.1200/JCO.19.03136 [DOI] [PubMed] [Google Scholar]
- 14. Hanahan D, Weinberg RA. Hallmarks of Cancer: The Next Generation. Cell (2011) 144(5):646–74. 10.1016/j.cell.2011.02.013 [DOI] [PubMed] [Google Scholar]
- 15. Zhu L, Li X, Shen Y, Cao Y, Fang X, Chen J, et al. A New Prognostic Score Based on the Systemic Inflammatory Response in Patients With Inoperable non-Small-Cell Lung Cancer. Onco Targets Ther (2016) 9:4879–86. 10.2147/OTT.S107279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Laird BJA, Fallon M, Hjermstad MJ, Tuck S, Kaasa S, Klepstad P, et al. Quality of Life in Patients With Advanced Cancer: Differential Association With Performance Status and Systemic Inflammatory Response. J Clin Oncol (2016) 34(23):2769–75. 10.1200/JCO.2015.65.7742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. McMillan DC. The Systemic Inflammation-Based Glasgow Prognostic Score: A Decade of Experience in Patients With Cancer. Cancer Treat Rev (2013) 39(5):534–40. 10.1016/j.ctrv.2012.08.003 [DOI] [PubMed] [Google Scholar]
- 18. Petrelli F, Cabiddu M, Coinu A, Borgonovo K, Ghilardi M, Lonati V, et al. Prognostic Role of Lctate Dehydrogenase in Solid Tumors: A Systematic Review and Meta-Analysis of 76 Studies. Acta Oncol (2015) 54(7):961–70. 10.3109/0284186X.2015.1043026 [DOI] [PubMed] [Google Scholar]
- 19. Paramanathan A, Saxena A, Morris DL. A Systematic Review and Meta-Analysis on the Impact of Pre-Operative Neutrophil Lymphocyte Ratio on Long Term Outcomes After Curative Intent Resection of Solid Tumours. Surg Oncol (2014) 23(1):31–9. 10.1016/j.suronc.2013.12.001 [DOI] [PubMed] [Google Scholar]
- 20. Templeton AJ, Ace O, McNamara MG, Al-Mubarak M, Vera-Badillo FE, Hermanns T, et al. Prognostic Role of Platelet to Lymphocyte Ratio in Solid Tumors: A Systematic Review and Meta-Analysis. Cancer Epidemiol Biomarkers Prev (2014) 23(7):1204–12. 10.1158/1055-9965.EPI-14-0146 [DOI] [PubMed] [Google Scholar]
- 21. Mezquita L, Auclin E, Ferrara R, Charrier M, Remon J, Planchard D, et al. Association of the Lung Immune Prognostic Index With Immune Checkpoint Inhibitor Outcomes in Patients With Advanced non-Small Cell Lung Cancer. [Multicenter Study]. JAMA Oncol (2018) 4(3):351–7. 10.1001/jamaoncol.2017.4771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Salavati A, Duan F, Snyder BS, Wei B, Houshmand S, Khiewvan B, et al. Optimal FDG PET/CT Volumetric Parameters for Risk Stratification in Patients With Locally Advanced non-Small Cell Lung Cancer: Results From the ACRIN 6668/RTOG 0235 Trial. Eur J Nucl Med Mol Imaging (2017) 44:1969–83. 10.1007/s00259-017-3753-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Simone CB2, Houshmand S, Kalbasi A, Salavati A, Alavi A. PET-Based Thoracic Radiation Oncology. PET Clin (2016) 11:319–32. 10.1016/j.cpet.2016.03.001 [DOI] [PubMed] [Google Scholar]
- 24. Geiger GA, Kim MB, Xanthopoulos EP, Pryma DA, Grover S, Plastaras JP, et al. Stage Migration in Planning PET/CT Scans in Patients Due to Receive Radiotherapy for non–Small-Cell Lung Cancer. Clin Lung Cancer (2014) 15:79–85. 10.1016/j.cllc.2013.08.004 [DOI] [PubMed] [Google Scholar]
- 25. Ayati N, Sadeghi R, Kiamanesh Z, Lee ST, Zakavi SR, Scott AM, et al. The Value of F-FDG PET/CT for Predicting or Monitoring Immunotherapy Response in Patients With Metastatic Melanoma: A Systematic Review and Meta-Analysis. .Eur J Nucl Med Mol Imaging (2021) 48:428–48. 10.1007/s00259-020-04967-9 [DOI] [PubMed] [Google Scholar]
- 26. Seban RD, Assie JB, Giroux-Leprieur E, Massiani MA, Soussan M, Bonardel G, et al. Fdg-PET Biomarkers Associated With Long-Term Benefit From First-Line Immunotherapy in Patients With Advanced non-Small Cell Lung Cancer. Ann Nucl Med (2020) 34:968–74. 10.1007/s12149-020-01539-7 [DOI] [PubMed] [Google Scholar]
- 27. Evangelista L, Cuppari L, Menis J, Bonanno L, Reccia P, Frega S, et al. 18f-Fdg PET/CT in non-Small-Cell Lung Cancer Patients: A Potential Predictive Biomarker of Response to Immunotherapy. Nucl Med Commun (2019) 40:802–7. 10.1097/MNM.0000000000001025 [DOI] [PubMed] [Google Scholar]
- 28. Soussan M, Chouahnia K, Maisonobe JA, Boubaya M, Eder V, Morère JF, et al. Prognostic Implications of Volume-Based Measurements on FDG PET/CT in Stage III non-Small-Cell Lung Cancer After Induction Chemotherapy. Eur J Nucl Med Mol Imaging (2013) 40:668–76. 10.1007/s00259-012-2321-7 [DOI] [PubMed] [Google Scholar]
- 29. Sharma A, Mohan A, Bhalla AS, Sharma MC, Vishnubhatla S, Das CJ, et al. Role of Various Metabolic Parameters Derived From Baseline 18f-Fdg PET/CT as Prognostic Markers in Non-Small Cell Lung Cancer Patients Undergoing Platinum-Based Chemotherapy. Clin Nucl Med (2018) 43:e8–e17. 10.1097/RLU.0000000000001886 [DOI] [PubMed] [Google Scholar]
- 30. Basu S, Zhuang H, Torigian DA, Rosenbaum J, Chen W, Alavi A. Functional Imaging of Inflammatory Diseases Using Nuclear Medicine Techniques. Semin Nucl Med (2009) 39:124–45. 10.1053/j.semnuclmed.2008.10.006 [DOI] [PubMed] [Google Scholar]
- 31. Cheng G, Alavi A, Del Bello CV, Akers SR. Differential Washout of FDG Activity in Two Different Inflammatory Lesions: Implications for Delayed Imaging. Clin Nucl Med (2013) 38:576–9. 10.1097/RLU.0b013e318292efc8 [DOI] [PubMed] [Google Scholar]
- 32. Nahmias C, Wahl LM. Reproducibility of Standardized Uptake Value Measurements Determined by 18F-FDG PET in Malignant Tumors. J Nucl Med (2008) 49:1804–8. 10.2967/jnumed.108.054239 [DOI] [PubMed] [Google Scholar]
- 33. Ferrucci PF, Ascierto PA, Pigozzo J, Del Vecchio M, Maio M, Antonini Cappellini GC, et al. Baseline Neutrophils and Derived Neutrophil-to-Lymphocyte Ratio: Prognostic Relevance in Metastatic Melanoma Patients Receiving Ipilimumab. Ann Oncol (2016) 27(4):732–8. 10.1093/annonc/mdw016 [DOI] [PubMed] [Google Scholar]
- 34. Weide B, Martens A, Hassel JC, Berking C, Postow MA, Bisschop K, et al. Baseline Biomarkers for Outcome of Melanoma Patients Treated With Pembrolizumab. Clin Cancer Res (2016) 22(22):5487–96. 10.1158/1078-0432.CCR-16-0127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Detterbeck FC, Boffa DJ, Kim AW, Tanoue LT. The Eighth Edition Lung Cancer Stage Classification. Chest (2017) 151(1):193–203. 10.1016/j.chest.2016.10.010 [DOI] [PubMed] [Google Scholar]
- 36. Luan X, Huang Y, Gao S, Sun X, Wang S, Ma L, et al. (18) F-alfatide PET/CT may Predict Short-Term Outcome of Concurrent Chemoradiotherapy in Patients With Advanced non-Small Cell Lung Cancer. Eur J Nucl Med Mol Imaging (2016) 43:2336–42. 10.1007/s00259-016-3505-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jahangiri P, Pournazari K, Torigian DA, Werner TJ, Swisher-McClure S, Simone CB, et al. A Prospective Study of the Feasibility of FDG-PET/CT Imaging to Quantify Radiation-Induced Lung Inflammation in Locally Advanced non-Small Cell Lung Cancer Patients Receiving Proton or Photon Radiotherapy. Eur J Nucl Med Mol Imaging (2019) 46(1):206–16. 10.1007/s00259-018-4154-5 [DOI] [PubMed] [Google Scholar]
- 38. Jreige M, Letovanec I, Chaba K, Renaud S, Rusakiewicz S, Cristina V, et al. (18)F-Fdg PET Metabolic-to-Morphological Volume Ratio Predicts PD-L1 Tumour Expression and Response to PD-1 Blockade in non-Small-Cell Lung Cancer. Eur J Nucl Med Mol Imaging (2019) 46(9):1859–68. 10.1007/s00259-019-04348-x [DOI] [PubMed] [Google Scholar]
- 39. Covington MF, Curiel CN, Lattimore L, Avery RJ, Kuo PH. Fdg-PET/CT for Monitoring Response of Melanoma to the Novel Oncolytic Viral Therapy Talimogene Laherparepvec. Clin Nucl Med (2017) 42:114–5. 10.1097/RLU.0000000000001456 [DOI] [PubMed] [Google Scholar]
- 40. Wachsmann JW, Ganti R, Peng F. Immune-Mediated Disease in Ipilimumab Immunotherapy of Melanoma With FDG Pet-Ct. Acad Radiol (2017) 24:111–5. 10.1016/j.acra.2016.08.005 [DOI] [PubMed] [Google Scholar]
- 41. Wong AM, McArthur GA, Hofman MS, Hicks RJ. The Advantages and Challenges of Using Fdg PET/CT for Response Assessment in Melanoma in the Era of Targeted Agents and Immunotherapy. Eur J Nucl Med Mol Imaging (2017) 44:67–77. 10.1007/s00259-017-3691-7 [DOI] [PubMed] [Google Scholar]
- 42. Li X, Wang D, Yu L. Prognostic and Predictive Values of Metabolic Parameters of (18)F-FDG PET/CT in Patients With Non-Small Cell Lung Cancer Treated With Chemotherapy. Mol Imaging (2019) 18:1536012119846025. 10.1177/1536012119846025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Grizzi F, Castello A, Lopci E. Is it Time to Change Our Vision of Tumor Metabolism Prior to Immunotherapy? Eur J Nucl Med Mol Imaging (2018) .45:1072–5. 10.1007/s00259-018-3988-1 [DOI] [PubMed] [Google Scholar]
- 44. Sharma A, Mohan A, Bhalla AS, Vishnubhatla S, Pandey AK, Bal CS, et al. Role of Various Semiquantitative Parameters of 18F-FDG PET/CT Studies for Interim Treatment Response Evaluation in non-Small-Cell Lung Cancer. Nucl Med Commun (2017) 38(10):858–67. 10.1097/MNM.0000000000000723 [DOI] [PubMed] [Google Scholar]
- 45. Zhang M, Wang D, Sun Q, Pu H, Wang Y, Zhao S, et al. Prognostic Significance of PD-L1 Expression and 18 F-FDG PET/CT in Surgical Pulmonary Squamous Cell Carcinoma. Oncotarget (2017) 8:51630–40. 10.18632/oncotarget.18257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Liu J, Dong M, Sun X, Li W, Xing L, Yu J, et al. Prognostic Value of 18F-FDG PET/CT in Surgical non-Small Cell Lung Cancer: A Meta-Analysis. PloS One (2016) 11(1):e0146195. 10.1371/journal.pone.0146195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bille A, Okiror L, Skanjeti A, Errico L, Arena V, Penna D, et al. The Prognostic Significance of Maximum Standardized Uptake Value of Primary Tumor in Surgically Treated non-Small-Cell Lung Cancer Patients: Analysis of 413 Cases. Clin Lung Cancer (2013) 14:149–56. 10.1016/j.cllc.2012.04.007 [DOI] [PubMed] [Google Scholar]
- 48. Lee DS, Kim SJ, Jang HS, Yoo Ie R, Park KR, Na SJ, et al. Clinical Correlation Between Tumor Maximal Standardized Uptake Value in Metabolic Imaging and Metastatic Tumor Characteristics in Advanced non-Small Cell Lung Cancer. Medicine (2015) 94:e1304. 10.1097/MD.0000000000001304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hyun SH, Choi JY, Kim K, Kim J, Shim YM, Um SW, et al. Volume Based Parameters of 18F FDG-PET/CT Improve Outcome Prediction in Early-Stage Nonsmall Cell Lung Cancer After Surgical Resection. Ann Surg (2013) 257(2):364–70. 10.1097/SLA.0b013e318262a6ec [DOI] [PubMed] [Google Scholar]
- 50. Hyun SH, Ahn HK, Kim H, Ahn MJ, Park K, Ahn YC, et al. Volume Based Assessment by 18F-FDG PET/CT Predicts Survival in Patients With Stage III Nonsmall-Cell Lung Cancer. Eur J Nucl Med Mol Imaging (2014) 41(1):50–8. 10.1007/s00259-013-2530-8 [DOI] [PubMed] [Google Scholar]
- 51. Liao S, Penney BC, Wroblewski K, Zhang H, Simon CA, Kampalath R, et al. Prognostic Value of Metabolic Tumor Burden On18f-FDG PET in Nonsurgical Patients With non-Small Cell Lung Cancer. Eur J Nucl Med Mol Imaging (2012) 39(1):27–38. 10.1007/s00259-011-1934-6 [DOI] [PubMed] [Google Scholar]
- 52. Yılmaz U, Batum Ö, Koparal H, Özbilek E, Kıraklı E. Prognostic Value of Primary Tumor SUV on Pre-Treatment F-FDG PET/CT Imaging in Patients With Stage Iii non-Small Cell Lung Cancer. Rev Esp Med Nucl Imagen Mol (2018) S2253-654X(17)30216-0. 10.1016/j.remn.2017.11.006 [DOI] [PubMed] [Google Scholar]
- 53. Dibble EH, Alvarez AC, Truong MT, Mercier G, Cook EF, Subramaniam RM. 18f-FDG Metabolic Tumor Volume and Total Glycolytic Activity of Oral Cavity and Oropharyngeal Squamous Cell Cancer: Adding Value to Clinical Staging. J Nucl Med (2012) 53:709–15. 10.2967/jnumed.111.099531 [DOI] [PubMed] [Google Scholar]
- 54. Soret M, Bacharach SL, Buvat I. Partial-Volume Effect in PET Tumor Imaging. J Nucl Med (2007) 48(6):932–45. 10.2967/jnumed.106.035774 [DOI] [PubMed] [Google Scholar]
- 55. Song YJ, Wang LX, Hong YQ, Lu ZH, Tong Q, Fang XZ, et al. Lymphocyte to Monocyte Ratio is Associated With Response to First-Line Platinum-Based Chemotherapy and Prognosis of Early-Stage non-Small Cell Lung Cancer Patients. Tumour Biol (2016) 37(4):5285–93. 10.1007/s13277-015-4397-8 [DOI] [PubMed] [Google Scholar]
- 56. Chen YM, Lai CH, Chang HC, Chao TY, Tseng CC, Fang WF, et al. Baseline and Trend of Lymphocyte-to-Monocyte Ratio as Prognostic Factors in Epidermal Growth Factor Receptor Mutant non-Small Cell Lung Cancer Patients Treated With First-Line Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors. PloS One (2015) 10(8):e0136252. 10.1371/journal.pone.0136252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Han Y, Wang J, Hong L, Sun L, Zhuang H, Sun B, et al. Platelet Lymphocyte Ratio is an Independent Prognostic Factor in Patients With ALK-positive non-Small-Cell Lung Cancer. Future Oncol (2017) 13(1):51–61. 10.2217/fon-2016-0317 [DOI] [PubMed] [Google Scholar]
- 58. Fiala O, Pesek M, Finek J, Topolcan O, Racek J, Svaton M, et al. Change in Serum Lactate Dehydrogenase is Associated With Outcome of Patients With Advanced-Stage NSCLC Treated With Erlotinib. Anticancer Res (2016) 36(5):2459–65. 10.1007/s13277-015-3660-3 [DOI] [PubMed] [Google Scholar]
- 59. Inomata M, Hayashi R, Tanaka H, Shimokawa K, Tokui K, Taka C, et al. Elevated Levels of Plasma Lactate Dehydrogenase is an Unfavorable Prognostic Factor in Patients With Epidermal Growth Factor Receptor Mutation-Positive non-Small Cell Lung Cancer, Receiving Treatment With Gefitinib or Erlotinib. Mol Clin Oncol (2016) 4(5):774–8. 10.3892/mco.2016.779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Koo PJ, Klingensmith WC, Lewis KD, Bagrosky BM, Gonzalez R. Anti-CTLA4 Antibody Therapy Related Complications on FDG Pet/Ct. Clin Nucl Med (2014) 39:e93–6. 10.1097/RLU.0b013e318292a775 [DOI] [PubMed] [Google Scholar]
- 61. Lopci E, Toschi L, Grizzi F, Rahal D, Olivari L, Castino GF, et al. Correlation of Metabolic Information on FDG-PET With Tissue Expression of Immune Markers in Patients With non-Small Cell Lung Cancer (NSCLC) Who are Candidates for Upfront Surgery. Eur J Nucl Med Mol Imaging (2016) 43:1954–61. 10.1007/s00259-016-3425-2 [DOI] [PubMed] [Google Scholar]
- 62. de Jong C, Deneer VHM, Kelder JC, Ruven H, Egberts TCG, Herder GJM. Association Between Serum Biomarkers CEA and LDH and Response in Advanced non-Small Cell Lung Cancer Patients Treated With Platinum-Based Chemotherapy. Thorac Cancer (2020) 11:1790–800. 10.1111/1759-7714.13449 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.