Abstract
Clinical features of Kawasaki disease (KD) display overlap with common pediatric viral illnesses, leading some to hypothesize that a viral infection is the inciting event for KD. To investigate viral infection history in KD patients, we performed comprehensive serological profiling using a high-throughput phage immunoprecipitation sequencing assay covering the complete reference protein sequences of known viruses with human tropism. KD and matched febrile control sera did not demonstrate differences in antiviral antibody profiles. We conclude that in the acute and subacute phases of disease, KD patients do not exhibit serologic evidence of exposure to known viruses that differs from controls.
Keywords: Kawasaki disease, virus, antibody
High-throughput antibody profiling of acute sera from patients with Kawasaki disease demonstrates no evidence of a shared antibody response to known human viruses.
Despite important advances in the effective treatment of KD, the etiology of the disease has remained elusive. Infectious, environmental, genetic, and immunologic factors have been reported as potential contributing factors to the development of KD [1]. Epidemiologic findings, including seasonal variation in KD with peak incidence in the winter, occurrence of KD clusters [2], and associations with antecedent upper respiratory illness [3], support the hypothesis that a novel virus is involved in the pathogenesis of KD. Additionally, similarities between the clinical phenotype of KD and common pediatric viral exanthems [4], and histopathologic findings of antigen-driven immunoglobulin A (IgA) immune response and intracytoplasmic inclusion bodies [5, 6], lend additional support to an inciting viral infection as the cause of KD. Considerable investigative effort has focused on the potential relationship between infections by known viral agents and the development of KD, with no conclusive causative association to date. The majority of these studies have required a priori specification of a small subset of viruses of interest, and comprehensive profiling of viral exposure in KD has not previously been performed.
Recently, a high-throughput phage immunoprecipitation assay called VirScan was developed to detect antibodies directed against viral peptides in patient serum [7]. VirScan utilizes a programmable DNA microarray to create a phage display library expressing 56-residue peptide sequences that span the proteomes of 206 species and > 1000 strains of viruses with human tropism. Patient sera are added to the phage display library, and enrichment of antibody-mediated library precipitates is detected by high-throughput sequencing. This methodology creates a serologic profile that reflects a patient’s viral exposure history and is capable of detecting temporal changes in the presence of antiviral antibodies [8, 9].
In this study, we performed serological profiling in KD and control subjects with VirScan to investigate patterns of viral exposure and any potential association with the development of KD.
METHODS
Patient Selection Criteria and Sample Collection
Patients fulfilling established clinical criteria for KD, including fever for > 5 days and at least 4 of 5 physical examination findings, were prospectively identified at 2 pediatric tertiary care centers (Boston Children’s Hospital, Boston, Massachusetts; and Rady Children’s Hospital, San Diego, California) and had serum collected for research purposes in accordance with institutional research guidelines following signed parental consent and subject assent as appropriate [4]. Only serum samples collected prior to treatment with intravenous immunoglobulin were included for analysis. All KD patients underwent evaluation with echocardiography. The internal dimension of the left anterior descending and right coronary arteries were measured and expressed as standard deviations from the mean (z score) normalized for body surface area. KD patients were selectively enriched for 2 populations: patients with coronary involvement (z score > +2.5) to increase specificity of diagnosis and/or patients with a delayed diagnosis in the subacute phase who would be more likely to have a rise in antibody titer. Eight of 37 KD patients underwent viral testing prior to diagnosis (2 by viral culture, 3 by respiratory viral panel [respiratory syncytial virus {RSV}, adenovirus, influenza viruses A and B, parainfluenza virus 1–3], 3 by monospot test), and all tests were negative. Control subjects were children who underwent emergency department evaluation for febrile illnesses characterized by at least 3 days of fever and at least 1 of the 5 clinical criteria for KD and were matched 1:1 with KD patients based on age, sex, year of sample collection, and when possible, season of presentation. Matching by age was prioritized over sex when necessary. Ages were considered matched if within 6 months for patients < 1 year of age and within 1 year for patients > 1 year of age. Diagnoses of the febrile controls were as follows: 23 viral syndrome, 3 adenovirus, 2 herpes simplex virus (HSV), and 1 each with enterovirus, erythema multiforme, rotavirus, parainfluenza 3, RSV, Henoch–Schonlein purpura, scarlet fever, and bacterial lymphadenitis.
PhIP-seq (VirScan) Assay
Sera from patients and control subjects were analyzed by VirScan (PhIP-seq) as previously described [7, 10]. All samples were analyzed in duplicate. Detection of library member enrichment and calculation of a “viral score” were performed as previously described [7, 10]. In brief, to calculate the viral score, positive peptide hits that overlapped by at least 7 amino acids were assumed to be due to cross-reactivity and were ignored, yielding a viral score that indicates the number of unique viral peptides identified per virus for each patient sample. Samples were considered positive for viral infection if they had a minimum viral score of 3 and had an antibody directed against a known immunodominant target peptide (data available for 58 viruses).
Statistical Analysis
Categorical variables are displayed as frequencies or percentages, and a Fisher exact test was used for statistical comparison. Continuous variables are displayed as median with interquartile range (IQR) or as mean with standard deviation where appropriate, and a Wilcoxon rank-sum test was used for statistical comparison. Bonferroni adjustment of P values was applied to account for multiple testing. A P value of < .05 was considered statistically significant.
RESULTS
The median age for patients with KD was 4.2 years and 62% of the patients were male (Table 1). The median day of illness at the time of KD sample collection was 10 days (range, 5–32 days; IQR, 7–20 days). Twenty-six of 37 (70%) KD patients demonstrated coronary artery changes on echocardiography during the course of their illness (Table 1 and Supplementary Figure 1), defined as having coronary artery z scores for the left anterior descending or right coronary artery > +2.5. Matched control subjects had a median age of 3.6 years and a median day of illness at sample collection of 5 days (range, 1–16 days; IQR, 3–6 days) (Table 1).
Table 1.
Demographic and Clinical Characteristics of Patients With Kawasaki Disease and Matched Controls
| Variable | Patients With Kawasaki Disease | Controls | P Valueb | ||
|---|---|---|---|---|---|
| All | With Coronary Involvementa | Without Coronary Involvementa | |||
| No. of participants | 37 | 26 | 11 | 37 | |
| Age, y, median (IQR) | 4.2 (1.8–5.8) | 4.0 (1.6–5.2) | 5.7 (2.0–6.4) | 3.6 (1.8–6.2) | .94 |
| Male sex, No. (%) | 23 (62) | 18 (69) | 5 (45) | 23 (62) | 1 |
| Day of illness at sample collection | < .01 | ||||
| Median (IQR) | 10 (7–20) | 7 (6–10) | 21 (18–24) | 5 (3–6) | |
| Range | 5–32 | 5–27 | 14–32 | 1–16 | |
| Coronary artery z score, mean (SD) | +3.0 (2.5) | +3.9 (2.4) | +0.8 (0.6) |
Abbreviations: IQR, interquartile range; SD, standard deviation.
aCoronary involvement was defined as a z score of the left anterior descending or right coronary artery > +2.5.
b P value for comparison between all Kawasaki disease samples and controls.
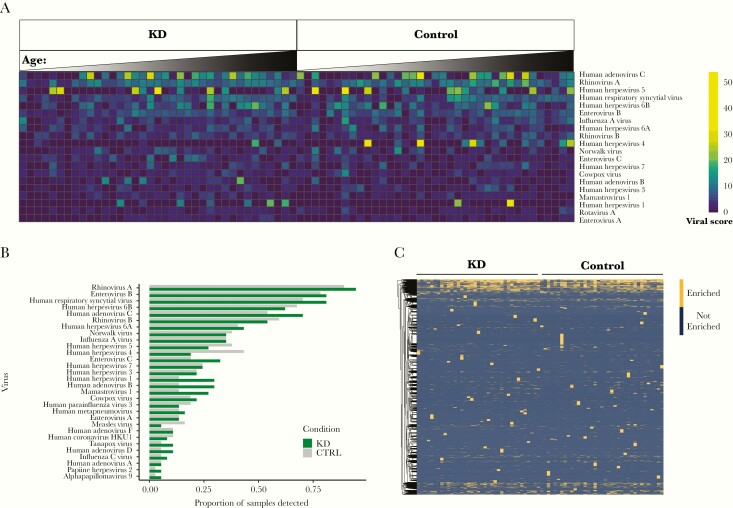
KD and matched control sera enriched the largest number of peptides from common viral pathogens, including RSV, rhinovirus A/B, enterovirus A/B/C, influenza A/C, adenovirus B/C/D, and multiple human herpesviruses (Supplementary Figure 2). To account for cross-reactive antibodies within an individual sample, a viral score was calculated for each virus (see Methods). Consistent with the absolute number of viral peptides detected, viral scores were highest for common viral pathogens and did not differ between cases and controls (Figure 1A and Supplementary Table 1). The viral score and the presence of an antibody against an immunodominant viral epitope were used to determine positive infection status, and the frequency of infection by detected viruses was similar between KD and matched control samples (Figure 1B and Supplementary Figure 3). Finally, a total of 10 201 of the 108 603 individual viral peptides in the VirScan library were enriched by KD and/or control sera, and there were no significant differences in enrichment of individual peptides based on KD status (Bonferroni-corrected P value > .05) (Figure 1C and Supplementary Table 2).
Figure 1.
Detection of antiviral antibodies by VirScan. A, Heatmap of viral scores for detected viruses with the highest median viral scores. Cases and controls are in order (left to right) of increasing age. B, Frequency of samples testing “positive” for viral pathogens, defined by a viral score > 3 and antibody against an immunodominant epitope. Viruses present in at least 5% of Kawasaki disease (KD) samples are shown, and there were no significant differences between cohorts (Fisher exact test, all P values > .05). C, Heatmap depicting enrichment for antibodies against approximately 10 201 individual viral peptides detected in KD samples and controls. No individual peptide was enriched in KD samples vs controls (Fisher exact test, all samples with a Bonferroni-corrected P > .05).
DISCUSSION
We investigated the history of viral exposure in KD patients in an unbiased, high-throughput manner to gain insight into the role of viral infections as inciting events in KD. Our data indicate that in the acute and subacute phases of illness, patients with KD do not exhibit serological evidence of a unique pattern of infection by common viral pathogens or infection by a shared viral pathogen that differs from control subjects.
Given the overlap in clinical features between common pediatric viral infections and KD, as well as the defined seasonality of KD, numerous avenues of investigation have focused on the possible link between infectious agents and KD. In addition, investigators have postulated that KD patients are more likely to have had an antecedent common viral infection compared to controls and that this in some way primes the immune system to subsequently respond to the KD agent [3]. The data from this study represent an effort to comprehensively study viral infections in KD patients through use of a high-throughput assay to screen patient sera for the presence of antibodies directed against viral peptides. The unique strengths of this research tool include the ability to screen for antibodies directed against all known peptides from viruses with human tissue tropism, from common to rare pathogens, and in theory, the ability to detect the presence of any antibody directed against an unknown human viral pathogen that shares cross-reactive epitopes with known viral peptides. Antiviral antibodies in KD subjects did not differ from controls. There was no evidence to suggest a novel infectious agent with cross-reactivity to known human viral peptides, or the generation of antiviral antibodies with cross-reactivity between the known human viral proteome and the human proteome as a pathogenetic mechanism in KD.
Another, more direct approach to detection of viral pathogens as the cause of KD would be direct high-throughput sequencing of patient and control body fluids. This was attempted in 7 acute KD patients with high-throughput sequencing performed on serum or plasma samples. Rhinovirus and bocavirus species were identified, but there was no uniform viral sequence shared by all the subjects [11].
A recent report by Rowley et al identified an epitope targeted by antibodies identified in plasmablasts and sera from KD patients [12]. While this specific epitope is not present in the VirScan library, a sequence of critical amino acids identified by protein substitution in the Rowley et al study (E/P-X-L-X-Q-E/D/T/S-F/I/V) is present in 51 VirScan library peptides representing 20 different viral species (Supplementary Table 3). The most frequently enriched peptides with this motif in our study were from the hemagglutinin-neuraminidase protein of parainfluenza 3, and were enriched in a similar number of cases and controls (Supplementary Table 3).
KD samples analyzed in this study were collected prior to treatment with intravenous immunoglobulin, as presence of exogenous pooled immunoglobulin G (IgG) would preclude meaningful analysis by PhIP-seq. One limitation of this timing of sample collection is that sera collected in the acute and subacute phases of illness may precede a detectable antibody titer rise, resulting in false-negative results. In an effort to allow for a titer rise, we preferentially selected samples with delayed diagnosis, with a median day of illness of 10 days. The immunoprecipitation step of the PhIP-seq assay uses protein A and protein G–coated beads, which preferentially bind IgG antibodies, but also detect the IgA antibody repertoire. Rowley and colleagues previously used molecular methods to create IgA antibodies that specifically recognized intracytoplasmic inclusions in numerous tissues from autopsies on patients who died of acute KD [13]. The use of protein A–coated beads with affinity for IgA in our study should allow for detection of any KD-associated IgA antibody signature directed against known viral species.
Potential limitations to the use of VirScan to detect viral infections in our study include a scope limited to viruses with known tropism for humans and utilization of synthetic peptide antigens that may not recapitulate immunogenic epitopes of intact viral proteins. These caveats notwithstanding, VirScan has been used as a powerful tool to investigate viral exposure history [7, 10], including the ability to perform with high sensitivity and specificity in the detection of hepatitis C virus (HCV) and human immunodeficiency virus in comparison to commercial assays and the ability to differentiate between HCV genotypes and HSV serotypes. Another important limitation to the VirScan method relevant to this study is the lack of temporal resolution of viral infection history. Detection of IgG documents a past viral exposure, but cannot discriminate between acute vs remote infection.
In conclusion, sera from patients with KD collected in the acute and early subacute phases of illness showed no signature of an antibody response to a known viral agent or individual viral peptides that differed from controls, although the timing of sample collection in some cases may have preceded an antibody titer rise. Further investigation of viral infections in patients with KD should utilize other techniques that do not require a priori specification of viruses of interest, such as high-throughput sequencing from clinical samples during the acute phase of the illness.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Financial support. This work was supported in part by the National Heart, Lung, and Blood Institute of the National Institutes of Health (grant numbers T32 HL 7572-33 to D. Q. and HL 140898 to J. C. B., A. H. T., and C. S.).
Potential conflicts of interest. S. J. E. has filed for a patent on VirScan. All other authors report no potential conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Newburger JW, Takahashi M, Burns JC. Kawasaki disease. J Am Coll Cardiol 2016; 67:1738–49. [DOI] [PubMed] [Google Scholar]
- 2. Rypdal M, Rypdal V, Burney JA, et al. Clustering and climate associations of Kawasaki disease in San Diego County suggest environmental triggers. Sci Rep 2018; 8:16140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Manlhiot C, Mueller B, O’Shea S, et al. Environmental epidemiology of Kawasaki disease: linking disease etiology, pathogenesis and global distribution. PLoS One 2018; 13:e0191087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McCrindle BW, Rowley AH, Newburger JW, et al. American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee of the Council on Cardiovascular Disease in the Young; Council on Cardiovascular and Stroke Nursing; Council on Cardiovascular Surgery and Anesthesia; and Council on Epidemiology and Prevention . Diagnosis, treatment, and long-term management of Kawasaki disease: a scientific statement for health professionals from the American Heart Association. Circulation 2017; 135:e927–99. [DOI] [PubMed] [Google Scholar]
- 5. Rowley AH, Shulman ST, Mask CA, et al. IgA plasma cell infiltration of proximal respiratory tract, pancreas, kidney, and coronary artery in acute Kawasaki disease. J Infect Dis 2000; 182:1183–91. [DOI] [PubMed] [Google Scholar]
- 6. Rowley AH, Baker SC, Shulman ST, et al. Ultrastructural, immunofluorescence, and RNA evidence support the hypothesis of a “new” virus associated with Kawasaki disease. J Infect Dis 2011; 203:1021–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Xu GJ, Kula T, Xu Q, et al. Viral immunology. Comprehensive serological profiling of human populations using a synthetic human virome. Science 2015; 348:aaa0698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bender Ignacio RA, Dasgupta S, Stevens-Ayers T, et al. Comprehensive viromewide antibody responses by systematic epitope scanning after hematopoietic cell transplantation. Blood 2019; 134:503–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Isnard P, Kula T, Avettand Fenoel V, et al. Temporal virus serological profiling of kidney graft recipients using VirScan. Proc Natl Acad Sci U S A 2019; 116:10899–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mina MJ, Kula T, Leng Y, et al. Measles virus infection diminishes preexisting antibodies that offer protection from other pathogens. Science 2019; 366:599–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. L’Huillier AG, Brito F, Wagner N, et al. Identification of viral signatures using high-throughput sequencing on blood of patients with Kawasaki disease. Front Pediatr 2019; 7:524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rowley AH, Baker SC, Arrollo D, et al. A protein epitope targeted by the antibody response to Kawasaki disease [manuscript published online ahead of print 13 February 2020]. J Infect Dis 2020. doi:10.1093/infdis/jiaa066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rowley AH, Baker SC, Orenstein JM, Shulman ST. Searching for the cause of Kawasaki disease—cytoplasmic inclusion bodies provide new insight. Nat Rev Microbiol 2008; 6:394–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.