Abstract
Antiretroviral therapy effectively controls human immunodeficiency virus (HIV) replication but it is unable to fully eradicate the HIV reservoir and treatment must be life-long. Progress toward a strategy for HIV remission will require overcoming key hurdles to fill gaps in our understanding of HIV persistence, but the identification of individuals who have attained sterilizing or functional HIV cure show that such a goal is achievable. In this review, we first outline challenges in targeting the HIV reservoir, including difficulties identifying HIV-infected cells, ongoing work elucidating the complex intracellular environment that contribute to HIV latency, and barriers to reactivating and clearing the HIV reservoir. We then review reported cases of HIV sterilizing cure and explore natural models of HIV remission and the promise that such HIV spontaneous and posttreatment controllers may hold in our search for a broadly-applicable strategy for the millions of patients living with HIV.
Keywords: HIV reservoir, latency, post-treatment controller, provirus, spontaneous controller
The introduction of combination antiretroviral therapy (ART) has turned human immunodeficiency virus (HIV) infection into a chronic, manageable disease. People with HIV (PWH) can enjoy a near-normal life expectancy if treated at an earlier stage with life-long ART and durable viral suppression [1]. However, ART is not curative and once interrupted, viral rebound occurs in nearly all patients [2, 3]. This is largely due to the establishment of a latent HIV reservoir in multiple anatomic compartments [4]. Unfortunately, this reservoir is not effectively eliminated by either ART or the immune response. For example, despite long-term suppressive ART, HIV decay can be divided by different phases, and the rate of decay has been found to level off in the setting of prolonged ART [5, 6].
Attempts to alter the decay of the HIV reservoir by ART intensification have largely been disappointing [7, 8], and together, these results raise a series of fundamental questions that remain unanswered about HIV persistence, including what fuels HIV persistence despite durable HIV viral suppression and how the HIV reservoir evades eradication by the host immune response. Understanding these questions will be paramount to our efforts to accelerate the development of novel strategies for HIV remission. In this review, we will first discuss the challenges of HIV persistence despite ART, with a focus on recent literature about virological and immunological mechanisms, and how they interact to promote HIV persistence. Then we will review the promise of HIV remission based on a number of reported cases.
PART I: THE CHALLENGE OF IDENTIFYING AND ERADICATING HIV-INFECTED CELLS
Rarity and Inaccessibility of HIV-Infected Cells
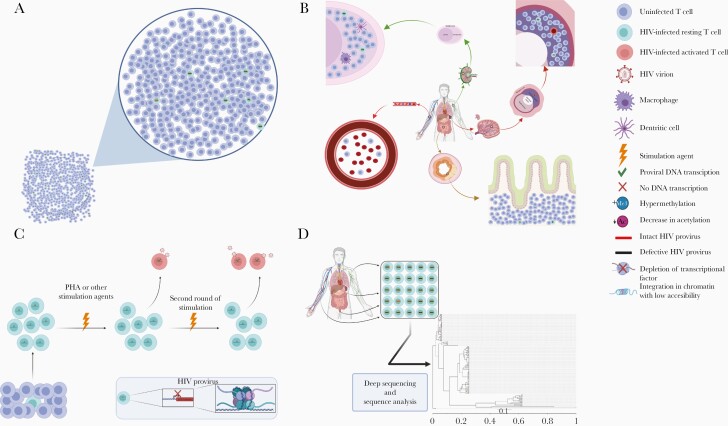
The identification and study of HIV-infected cells is challenging. First, CD4+ T cells harboring integrated intact proviruses are rare. Early studies have shown that only 0.03–3 infectious unit per million of HIV latent reservoir can be isolated from chronically infected participants receiving suppressive ART, measured by quantitative viral outgrowth assay (QVOA), which has historically been regarded as the reference standard for identifying the frequency of the inducible HIV reservoir [4, 9]. Using polymerase chain reaction–based methods, however, the pool of CD4+ T cells harboring HIV DNA is far greater, ranging from an estimate of 2 to >1000 cells per 1 million CD4+ T cells [10]. This discrepancy was partially reconciled by the discovery that the vast majority of HIV proviruses are defective [11]. In PWH who initiated treatment either during the early or chronic phases of infection, >90% of HIV proviral genomes are defective, including deletions, inversions, hypermutations, and other defects (Figure 1A) [11].
Figure 1.
Challenge of identifying and reactivating human immunodeficiency virus (HIV)–infected cells. A, CD4+ T cells harboring HIV proviral DNA are rare among the total CD4+ T-cell population; most of the proviral DNA is defective, and only <10% is intact. B, HIV-infected cells are mostly located in difficult-to-study anatomic sites, including lymph nodes, intestinal lymphoid tissue, and spleen. C, Multiple potential mechanisms contribute to HIV latency, including epigenetic modifications, depletion of transcriptional factors, and integration in dense regions of the chromosome. Even the most potent CD4+ T-cell–activating agents (eg, phytohemagglutinin [PHA]) are only able to activate a small proportion of CD4+ T cells harboring intact HIV proviruses, and the underlying mechanisms remain elusive. D, HIV achieves sequence diversity very early during infection; sequences obtained from different cell and anatomic compartments demonstrate substantial diversity as shown in this example phylogenetic analysis of intact HIV proviral sequences from 1 participant. Figures were generated with BioRender.com.
A second hurdle to identifying HIV-infected cells is that the HIV proviral genome is often transcriptionally quiescent. Using a single-cell analysis technique, Wiegand and colleagues [12] found that only an average of 7% of HIV proviruses expressed HIV RNA, with a similar level between defective and intact provirus. In lymph nodes (LNs) and intestinal lymphoid tissue (LT), which is estimated to harbor >90% of the HIV-infected CD4+ T cells in tissue (Figure 1B), only an estimated 0.2% of HIV DNA+ CD4+ cells expressed HIV RNA [13]. This, combined with the limited sensitivity of QVOA, has led to the estimate that the amount of proviruses that are genomically intact and capable of making replication-competent virus is likely to be ≥60-fold higher than estimates based on QVOA [14].
A third hurdle to the study of the HIV reservoir is that almost all of the HIV reservoir is located in difficult-to-sample anatomic regions, hindering our ability to fully quantify and characterize the anatomic compartments that harbor HIV-infected cells. While most HIV reservoir studies use peripheral blood-derived samples, there have been conflicting data on whether there is compartmentalization or evolution of HIV within certain tissues that is not reflected within the peripheral blood [15–17]. In a nonhuman primate model, Estes [13] and colleagues used in situ hybridization to show that >99% of cells expressing simian immunodeficiency virus or simian-human immunodeficiency virus viral RNA (vRNA) reside in LNs and LTs, including within the intestines, spleen, and lungs. Despite suppressive ART, the frequency of vRNA+ cells decreased only ≥2-fold in intestine or spleen compared with a 2-log10 reduction in LNs; correspondingly, intracellular ART levels in these LTs are lower than peripheral blood mononuclear cell ART levels. Similarly, in LNs and intestinal LTs samples from chronically HIV-infected participants, >2–6 years of suppressive ART was associated with an almost 3-log10 reduction in viral DNA (vDNA)+ cells in LNs but no significant decrease in vDNA+ cells in the intestinal LTs [13].
These findings from nonhuman primates and PWH indicate that ART alone cannot fully eradicate vRNA+ and vDNA+ cells in LTs. The importance of tissue reservoirs has also been demonstrated in treatment interruption studies, which have demonstrated the presence of multifocal origins of viral reactivation within tissue compartments after ART discontinuation [18] and shown that rebounding virus in plasma may originate from multiple tissue compartments [19].
Determining the Mechanisms Underlying HIV Latency and Reactivation
Understanding the pathways surrounding HIV latency and reactivation could accelerate progress for HIV latency reversal. There is evidence that HIV latency is established through a complex set of pathways [20], including epigenetic modifications that reduce histone acetylation [21], enhance histone/promoter methylation [22], and deplete transcriptional factors [23]. There is also evidence that the host chromosomal location of HIV integration may confer a deeper state of proviral latency [24] and that blocks in HIV transcriptional elongation and completion may also play a role in viral latency [25].
Assays like the QVOA use compounds including phytohemagglutinin to reverse proviral latency by inducing CD4+ T-cell activation and subsequently HIV provirus transcription [26]. However, even with maximal cellular activation in vitro, only a subset of proviruses can be induced during each round of immune stimulation (Figure 1C) [27]. This is despite the fact that intact noninduced HIV proviruses have the potential to be induced in vivo, because they harbor no lethal mutations, and most of them are integrated in transcriptionally active area with little cytosines followed by guanine residues (CpG) methylation [14]. With further modeling, these findings suggest that the “true” latent reservoir might be approximately 60-fold larger than what phytohemagglutinin stimulation QVOA can estimate [14]. Additional studies are needed to explore these findings and to determine the optimal latency pathways to target in the design of novel therapeutics.
Reactivating latent HIV provirus is challenging, and HIV-expressing cells may not be adequately cleared by the host immune response. There has been significant interest in ways to reactivate latent provirus [28, 29], potentially coupled with strategies to induce immune-mediated killing of reactivated cells in a strategy known as “kick and kill” [30, 31]. However, several early phase clinical trials with only administration of latency reversal agents (LRAs) and suppressive ART in the hope that preexisting HIV-specific immunity performs the killing have only reported moderate effects of “kick” and no clear evidence of reservoir elimination [32–37].
How can we explain the discrepancy between the in vivo and ex vivo studies? First, LRA administration alone is not sufficient to eliminate HIV reservoir. Huang and colleagues [38] used an ex vivo resting CD4+ T-cell model to show that cells harboring replication-competent HIV may be more resistant to CD8-mediated killing after exposure to LRAs. It is possible that HIV-specific cytotoxic T lymphocytes (CTLs) are diverted by CD4+ T cells harboring defective provirus; these CD4+ T cells with defective proviruses represent the majority of HIV-infected CD4+ T-cell populations [11], can still express HIV RNA, and can be recognized by HIV-specific CTLs [39].
In addition, different CD4+ T-cell subpopulations respond to LRAs differently. Noticeably, CD4+ T memory stem cells, a rare cell type that harbors a disproportionately high level of inducible HIV proviruses [40], is very resistant to LRA reactivation [41], which could contribute to the persistence of replication-competent reservoir despite “kick and kill.” Furthermore, several classes of currently studied LRA can potentially inhibit natural killer cells [42] and HIV-specific CTL function [43], thus jeopardizing the effector cells’ ability to eliminate the reactivated HIV reservoir. Thus, improved strategies are needed for both HIV latency reversal and the elimination of these reactivated cells. New classes of LRAs (eg, programmed cell death 1 protein blockade [44] and noncanonical NF-κB activation [45]) and reservoir clearance strategies (eg, convertible chimeric antigen receptor T cells [46]) are on the horizon, but further in vitro and clinical data are needed to evaluate their efficacy and safety profiles.
HIV Sequence Diversity and Immune Escape as Barriers to Achieving HIV Remission
The rapidity of HIV diversification and adaptation to the host immune response represent another challenge to viral eradication (Figure 1D) [47–49]. Strategies to stimulate the host immune response is a cornerstone of current strategies toward HIV remission. However, Deng and colleagues [50] have shown that unless ART is started early, the vast majority of HIV proviruses already harbor CTL escape mutations that is likely to adversely affect the host immune clearance of HIV-infected cells even after successful viral reactivation. In addition, HIV proviral sequence diversity could represent significant barriers to other HIV gene or protein-targeted interventions.
A 2019 study using long-acting slow-effective release antiviral therapy and clustered regularly interspaced short palindromic repeats (CRISPR)–CRISPR associated protein 9 (Cas9) targeting multiple segments of HIV genome resulted in depletion of the HIV reservoir and HIV remission in a subset of animals after ART interruption [51]. However, there was no assessment of proviral diversity in this study, which was likely limited as the humanized mice were treated with ART during acute infection. Although the target specificity of CRISPR-mediated DNA modification limits off-target effects, this may also hinder its ability to excise a diverse proviral reservoir, especially in individuals receiving long-term treatment and with relatively diverse reservoirs [52].
As shown in prior studies, intrapatient sequence diversity creates a barrier to efficient CRISPR-Cas9 induced cleavage [53]. Furthermore, previous in vitro studies demonstrated that HIV was capable of escaping CRISPR-Cas9 induced excision [54, 55]. Similarly, HIV sequence diversity also hampers antibody-based therapy. VRC01, a broadly neutralizing antibody, was shown to delay viral rebound after analytical treatment interruption; however, preexisting resistance mutations against VRC01 were detected and contributed to early viral rebound [56]. To overcome this barrier, triple, or even quadruple broadly neutralizing antibody combinations are likely needed [57].
PART II: THE PROMISE OF HIV REMISSION
Defining the Characteristics of an HIV Cure
There are 2 fundamental approaches to an HIV cure: sterilizing and functional cures. Sterilizing cure is defined as the state in which no replication-competent virus can be detected after treatment interruption. The cases of sterilizing cures represent some of the greatest success stories in the field but are challenging to replicate and to develop into a broadly applicable therapeutic option. Functional cure, also known as sustained HIV remission, refers to the ability of the patient to maintain viral control despite potentially low levels of detectable virus in the blood and tissues. In the development of broadly applicable therapeutics, the induction of sustained HIV remission represents a more realistic goal, as has already been described for HIV spontaneous (or elite) controllers and posttreatment controllers.
Success and Failures of Sterilizing Cure Approaches
The field has already identified at least 2 cases of apparent sterilizing cures. These include the “Berlin patient” [58] and the “London patient” [59, 60]. Both individuals underwent hematopoietic stem cell transplantation (HSCT) with donor cells harboring homozygous CCR5Δ32/Δ32 deletions. Apparent sterilizing cures were achieved despite important differences between the 2 patients, including the type of cancer and varying intensity of the conditioning regimens. Furthermore, at the 2019 Conference on Retroviruses and Opportunistic Infections, Björn-Erik et al [61] reported on a third possible case of sterilizing cure. The “Dusseldorf patient” is a 49-year-old man who received underwent HSCT, receiving cells from a CCR5Δ32/Δ32 donor in February 2013 because of acute myeloid leukemia. Repeated laboratory testing has not detected any remaining HIV reservoir, and ART was stopped in November 2018 with no evidence of HIV rebound to date [61].
These cases have elicited a great deal of hope for the HIV community and provided momentum to this scientific field. However, such an approach cannot be broadly applied to the general population of PWH, given the morbidity and mortality risk associated with stem cell transplants [62–65]. Furthermore, the identification of suitable CCR5Δ32/Δ32 donors can be challenging but appears to be a vital component to achieving a sterilizing cure. This is demonstrated by the report of the 2 “Boston patients,” both of whom underwent HSCT with donor cells harboring wild-type, functional CCR5. HSCT was associated with dramatic reductions in the size of the HIV reservoir, but eventual viral rebound occurred 12 and 32 weeks after discontinuation of ART [66]. There have also been reports of incomplete viral eradication and viral escape in the setting of HSCT with homozygous CCR5Δ32 donor cells [66–70].
Spontaneous Controllers as Possible Goal of HIV Remission?
Spontaneous controllers (SCs) are relatively rare patients with HIV who can maintain low or even undetectable levels of HIV RNA without needing to start ART. There is a wide range of definitions for this group of participants [71–73], but they are often classified as either elite controllers, if they can maintain viral loads below the limit of detection for commercial viral load assays, or viremic controllers, if they have low levels of detectable viremia. Host immunity appears to play a decisive and prime role in controlling HIV viral replication. They have been found to harbor a robust and polyfunctional HIV-specific T-cell response both in the peripheral blood [74, 75] and within tissue [76]. The importance of CTL-mediated viral suppression is supported by the enrichment of certain protective HLA alleles, such as HLA-B*27 and B*57 [77, 78]. It has been found that CTL targeting of highly networked epitopes can identify SCs, even for those without the usual protective HLA alleles [79].
While the studies of SCs have revealed much about effective natural immunity against HIV replication and disease progression, whether they represent the ideal end point for HIV curative strategies remains unclear. First, viral replication and viral evolution can be detected in SCs [80, 81] and loss of viral control and HIV disease progression occurs in a subset of them [72]. SCs have also been found to have increased levels of immune activation and chronic inflammation [73, 82], which may play a role in the reports that SCs may have a higher risk of cardiovascular disease or hospitalization in comparison to ART-treated HIV patients [83–85]. The initiation of ART in SCs is associated with further suppression of viral replication and reduction in immune activation and systemic inflammation [71, 86]. Although the discovery of treatment strategies that could induce spontaneous HIV control would undoubtedly represent a transformative advance, questions remain about whether this represents only an intermediate step toward a strategy that is not associated with higher risk of disease progression or adverse outcomes.
Posttreatment Controllers as Promising Model of HIV Remission
For most PWH, HIV plasma viral load rebounds within a few weeks after treatment interruption [3, 87]. However, a rare group of patients, termed posttreatment controllers (PTCs), are capable of suppressing the virus to low levels for ≥6 months after stopping ART [88, 89]. In 1999, Lisziewicz et al [90] described an HIV-infected individual who was able to control the infection after multiple treatment interruptions. Since then, several observational studies and clinical trials have been done on these individuals to define and determine their characteristics [8, 88, 91–101]. One of the first in-depth studies of PTCs was the VISCONTI study of 14 early-treated PTCs, with median of 89 months of HIV suppression after treatment interruption [89]. The largest study to date is the Control of HIV after Antiretroviral Medication Pause (CHAMP) study of 67 PTCs, aggregated from 14 clinical studies from AIDS Clinical Trials Group and other North American cohorts [88].
A number of factors seem to differentiate PTCs from SCs (Table 1). Unlike SCs, PTCs do not appear to be enriched for protective HLA alleles [89] and, intriguingly, they may be able to control viremia without increased immune activation [115]. Not only may early ART initiation increase the chances of HIV remission in adults [88], there have also been several reports of children who started ART during infancy and have subsequently exhibited delayed HIV rebound and posttreatment control [116, 117]. However, even among those treated during the earliest phases of HIV infection, posttreatment control can still be challenging to achieve, as was demonstrated in a Thai study of 8 participants who started ART at Fiebig stage I. Despite extremely low HIV reservoirs, none were able to achieve HIV remission, and all experienced viral rebound within 7 weeks after ART interruption [118]. Overall, PTCs seem to represent a promising model for HIV remission, but additional studies are needed to define the mechanisms of HIV control in these patients.
Table 1.
Comparison of Spontaneous and Posttreatment Controllers
| Finding | Description of Evidence | |
|---|---|---|
| SCs | PTCs | |
| Protective HLA alleles [78, 89] | Strong | Negative |
| CD4+ and CD8+ T-cell–mediated immunity [76, 79, 89, 102, 103] | Strong | Unclear or absent |
| Innate immune cells, eg, natural killer cells, involvement [104–106] | Modest | Modest |
| Immune activation [89, 107–109] | Modest | Unclear or absent |
| Antibody-mediated immunity [110–112] | Modest | Unclear or absent |
| High levels of defective provirus [75, 113] | Modest | Modest |
| Association of HIV proviral integration site [114] | Strong | Unclear or absent |
| Enhanced with early HIV treatment [88] | NA | Strong |
Abbreviation: HIV, human immunodeficiency virus; NA not applicable; PTCs, posttreatment controllers; SCs, spontaneous controllers.
CONCLUSIONS
To achieve an HIV cure or ART-free remission, it is crucial to understand the temporospatial distribution of HIV reservoir and the virologic and immunologic mechanisms that sustain HIV persistence. As we summarized in this review, there are still knowledge gaps in our understanding of the nature of HIV reservoir and mechanisms behind HIV persistence. Future research will need to focus on addressing HIV reservoir in tissue compartments and in the characterization of HIV-infected cells. Elucidating the mechanisms underlying HIV latency and reactivation will be crucial in the development new therapies for either the silencing or reduction of the viral reservoir. While the Berlin and London patients have provided proof that an HIV cure is possible, the identification of HIV PTCs offers a more feasible path toward sustained HIV remission. Understanding the mechanisms behind their ability to achieve posttreatment control underscores not only the challenges that remain, but also the promise that a successful strategy for HIV remission would have for the millions of PWH worldwide.
Notes
Financial support. This work was supported by Harvard University Center for AIDS Research (National Institute of Allergy and Infectious Diseases grant 5P30AI060354 to J.Z.L.).
Supplement sponsorship. This supplement is sponsored by the Harvard University Center for AIDS Research (CFAR), an NIH funded program (P30 AI060354), and the Ragon Institute of MGH, MIT and Harvard. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Potential conflicts of interest. J.Z.L. has consulted for AbbVie and JanBiotech. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Danforth K, Granich R, Wiedeman D, Baxi S, Padian N. Global mortality and morbidity of HIV/AIDS. In: Holmes KK, Bertozzi S, Bloom BR, Jha P. Major infectious diseases. 3rd ed. Washington, DC: International Bank for Reconstruction and Development/World Bank, 2017. [PubMed] [Google Scholar]
- 2. Sung JM, Margolis DM. HIV Persistence on antiretroviral therapy and barriers to a cure. Adv Exp Med Biol 2018; 1075:165–85. [DOI] [PubMed] [Google Scholar]
- 3. Li JZ, Etemad B, Ahmed H, et al. The size of the expressed HIV reservoir predicts timing of viral rebound after treatment interruption. AIDS 2016; 30:343–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Siliciano JD, Kajdas J, Finzi D, et al. Long-term follow-up studies confirm the stability of the latent reservoir for HIV-1 in resting CD4+ T cells. Nat Med 2003; 9:727–8. [DOI] [PubMed] [Google Scholar]
- 5. Besson GJ, Lalama CM, Bosch RJ, et al. HIV-1 DNA decay dynamics in blood during more than a decade of suppressive antiretroviral therapy. Clin Infect Dis 2014; 59:1312–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gandhi RT, McMahon DK, Bosch RJ, et al. ; ACTG A5321 Team . Levels of HIV-1 persistence on antiretroviral therapy are not associated with markers of inflammation or activation. PLoS Pathog 2017; 13:e1006285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chege D, Kovacs C, la Porte C, et al. Effect of raltegravir intensification on HIV proviral DNA in the blood and gut mucosa of men on long-term therapy: a randomized controlled trial. AIDS 2012; 26:167–74. [DOI] [PubMed] [Google Scholar]
- 8. Chaillon A, Gianella S, Lada SM, et al. Size, composition, and evolution of HIV DNA populations during early antiretroviral therapy and intensification with maraviroc. J Virol 2018; 92:e01589-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Finzi D, Blankson J, Siliciano JD, et al. Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nat Med 1999; 5:512–7. [DOI] [PubMed] [Google Scholar]
- 10. Eriksson S, Graf EH, Dahl V, et al. Comparative analysis of measures of viral reservoirs in HIV-1 eradication studies. PLoS Pathog 2013; 9:e1003174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bruner KM, Murray AJ, Pollack RA, et al. Defective proviruses rapidly accumulate during acute HIV-1 infection. Nat Med 2016; 22:1043–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wiegand A, Spindler J, Hong FF, et al. Single-cell analysis of HIV-1 transcriptional activity reveals expression of proviruses in expanded clones during ART. Proc Natl Acad Sci U S A 2017; 114:E3659–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Estes JD, Kityo C, Ssali F, et al. Defining total-body AIDS-virus burden with implications for curative strategies. Nat Med 2017; 23:1271–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ho YC, Shan L, Hosmane NN, et al. Replication-competent noninduced proviruses in the latent reservoir increase barrier to HIV-1 cure. Cell 2013; 155:540–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chaillon A, Gianella S, Dellicour S, et al. HIV persists throughout deep tissues with repopulation from multiple anatomical sources. J Clin Invest 2020; 130:1699–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fletcher CV, Staskus K, Wietgrefe SW, et al. Persistent HIV-1 replication is associated with lower antiretroviral drug concentrations in lymphatic tissues. Proc Natl Acad Sci U S A 2014; 111:2307–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bozzi G, Simonetti FR, Watters SA, et al. No evidence of ongoing HIV replication or compartmentalization in tissues during combination antiretroviral therapy: implications for HIV eradication. Sci Adv 2019; 5:eaav2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rothenberger MK, Keele BF, Wietgrefe SW, et al. Large number of rebounding/founder HIV variants emerge from multifocal infection in lymphatic tissues after treatment interruption. Proc Natl Acad Sci U S A 2015; 112:E1126–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. De Scheerder MA, Vrancken B, Dellicour S, et al. HIV rebound is predominantly fueled by genetically identical viral expansions from diverse reservoirs. Cell Host Microbe 2019; 26:347–58.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mbonye U, Karn J. Control of HIV latency by epigenetic and non-epigenetic mechanisms. Curr HIV Res 2011; 9:554–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Williams SA, Chen LF, Kwon H, Ruiz-Jarabo CM, Verdin E, Greene WC. NF-κB p50 promotes HIV latency through HDAC recruitment and repression of transcriptional initiation. EMBO J 2006; 25:139–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Blazkova J, Trejbalova K, Gondois-Rey F, et al. CpG methylation controls reactivation of HIV from latency. PLoS Pathog 2009; 5:e1000554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nguyen VT, Kiss T, Michels AA, Bensaude O. 7SK small nuclear RNA binds to and inhibits the activity of CDK9/cyclin T complexes. Nature 2001; 414:322–5. [DOI] [PubMed] [Google Scholar]
- 24. Einkauf KB, Lee GQ, Gao C, et al. Intact HIV-1 proviruses accumulate at distinct chromosomal positions during prolonged antiretroviral therapy. J Clin Invest 2019; 129:988–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yukl SA, Kaiser P, Kim P, et al. HIV latency in isolated patient CD4+ T cells may be due to blocks in HIV transcriptional elongation, completion, and splicing. Sci Transl Med 2018; 10:eaap9927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Siliciano JD, Siliciano RF. Enhanced culture assay for detection and quantitation of latently infected, resting CD4+ T-cells carrying replication-competent virus in HIV-1-infected individuals. Methods Mol Biol 2005; 304:3–15. [DOI] [PubMed] [Google Scholar]
- 27. Hosmane NN, Kwon KJ, Bruner KM, et al. Proliferation of latently infected CD4+ T cells carrying replication-competent HIV-1: potential role in latent reservoir dynamics. J Exp Med 2017; 214:959–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cillo AR, Sobolewski MD, Bosch RJ, et al. Quantification of HIV-1 latency reversal in resting CD4+ T cells from patients on suppressive antiretroviral therapy. Proc Natl Acad Sci U S A 2014; 111:7078–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Laird GM, Bullen CK, Rosenbloom DI, et al. Ex vivo analysis identifies effective HIV-1 latency-reversing drug combinations. J Clin Invest 2015; 125:1901–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shan L, Deng K, Shroff NS, et al. Stimulation of HIV-1-specific cytolytic T lymphocytes facilitates elimination of latent viral reservoir after virus reactivation. Immunity 2012; 36:491–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lehrman G, Hogue IB, Palmer S, et al. Depletion of latent HIV-1 infection in vivo: a proof-of-concept study. Lancet 2005; 366:549–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Siliciano JD, Lai J, Callender M, et al. Stability of the latent reservoir for HIV-1 in patients receiving valproic acid. J Infect Dis 2007; 195:833–6. [DOI] [PubMed] [Google Scholar]
- 33. Sagot-Lerolle N, Lamine A, Chaix ML, et al. ; ANRS EP39 study . Prolonged valproic acid treatment does not reduce the size of latent HIV reservoir. AIDS 2008; 22:1125–9. [DOI] [PubMed] [Google Scholar]
- 34. Archin NM, Liberty AL, Kashuba AD, et al. Administration of vorinostat disrupts HIV-1 latency in patients on antiretroviral therapy. Nature 2012; 487:482–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ke R, Lewin SR, Elliott JH, Perelson AS. Modeling the effects of vorinostat in vivo reveals both transient and delayed HIV transcriptional activation and minimal killing of latently infected cells. PLoS Pathog 2015; 11:e1005237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Elliott JH, McMahon JH, Chang CC, et al. Short-term administration of disulfiram for reversal of latent HIV infection: a phase 2 dose-escalation study. Lancet HIV 2015; 2:e520–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rasmussen TA, Tolstrup M, Brinkmann CR, et al. Panobinostat, a histone deacetylase inhibitor, for latent-virus reactivation in HIV-infected patients on suppressive antiretroviral therapy: a phase 1/2, single group, clinical trial. Lancet HIV 2014; 1:e13–21. [DOI] [PubMed] [Google Scholar]
- 38. Huang SH, Ren Y, Thomas AS, et al. Latent HIV reservoirs exhibit inherent resistance to elimination by CD8+ T cells. J Clin Invest 2018; 128:876–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pollack RA, Jones RB, Pertea M, et al. Defective HIV-1 proviruses are expressed and can be recognized by cytotoxic T lymphocytes, which shape the proviral landscape. Cell Host Microbe 2017; 21:494–506.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Buzon MJ, Sun H, Li C, et al. HIV-1 persistence in CD4+ T cells with stem cell-like properties. Nat Med 2014; 20:139–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Grau-Expósito J, Luque-Ballesteros L, Navarro J, et al. Latency reversal agents affect differently the latent reservoir present in distinct CD4+ T subpopulations. PLoS Pathog 2019; 15:e1007991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pace M, Williams J, Kurioka A, et al. ; CHERUB Investigators . Histone deacetylase inhibitors enhance CD4 T cell susceptibility to NK cell killing but reduce NK cell function. PLoS Pathog 2016; 12:e1005782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Walker-Sperling VE, Pohlmeyer CW, Tarwater PM, Blankson JN. The effect of latency reversal agents on primary CD8+ T cells: implications for shock and kill strategies for human immunodeficiency virus eradication. EBioMedicine 2016; 8:217–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fromentin R, DaFonseca S, Costiniuk CT, et al. PD-1 blockade potentiates HIV latency reversal ex vivo in CD4+ T cells from ART-suppressed individuals. Nat Commun 2019; 10:814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nixon CC, Mavigner M, Sampey GC, et al. Systemic HIV and SIV latency reversal via non-canonical NF-κB signalling in vivo. Nature 2020; 578:160–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Herzig E, Kim KC, Packard TA, et al. Attacking latent HIV with convertible CAR-T cells, a highly adaptable killing platform. Cell 2019; 179:880–94.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Iversen AK, Stewart-Jones G, Learn GH, et al. Conflicting selective forces affect T cell receptor contacts in an immunodominant human immunodeficiency virus epitope. Nat Immunol 2006; 7:179–89. [DOI] [PubMed] [Google Scholar]
- 48. Leslie AJ, Pfafferott KJ, Chetty P, et al. HIV evolution: CTL escape mutation and reversion after transmission. Nat Med 2004; 10:282–9. [DOI] [PubMed] [Google Scholar]
- 49. Bonsignori M, Liao HX, Gao F, et al. Antibody-virus co-evolution in HIV infection: paths for HIV vaccine development. Immunol Rev 2017; 275:145–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Deng K, Pertea M, Rongvaux A, et al. Broad CTL response is required to clear latent HIV-1 due to dominance of escape mutations. Nature 2015; 517:381–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Dash PK, Kaminski R, Bella R, et al. Sequential LASER ART and CRISPR treatments eliminate HIV-1 in a subset of infected humanized mice. Nat Commun 2019; 10:2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wertheim JO, Oster AM, Murrell B, et al. Maintenance and reappearance of extremely divergent intra-host HIV-1 variants. Virus Evol 2018; 4:vey030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Roychoudhury P, De Silva Feelixge H, Reeves D, et al. Viral diversity is an obligate consideration in CRISPR/Cas9 designs for targeting the HIV reservoir. BMC Biol 2018; 16:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wang Z, Wang W, Cui YC, et al. HIV-1 employs multiple mechanisms to resist Cas9/single guide RNA targeting the viral primer binding site. J Virol 2018; 92:e01135-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wang Z, Pan Q, Gendron P, et al. CRISPR/Cas9-derived mutations both inhibit HIV-1 replication and accelerate viral escape. Cell Rep 2016; 15:481–9. [DOI] [PubMed] [Google Scholar]
- 56. Bar KJ, Sneller MC, Harrison LJ, et al. Effect of HIV antibody VRC01 on viral rebound after treatment interruption. N Engl J Med 2016; 375:2037–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wagh K, Bhattacharya T, Williamson C, et al. Optimal combinations of broadly neutralizing antibodies for prevention and treatment of HIV-1 clade C infection. PLoS Pathog 2016; 12:e1005520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hütter G, Nowak D, Mossner M, et al. Long-term control of HIV by CCR5 Delta32/Delta32 stem-cell transplantation. N Engl J Med 2009; 360:692–8. [DOI] [PubMed] [Google Scholar]
- 59. Gupta RK, Abdul-Jawad S, McCoy LE, et al. HIV-1 remission following CCR5Δ32/Δ32 haematopoietic stem-cell transplantation. Nature 2019; 568:244–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Gupta RK, Peppa D, Hill AL, et al. Evidence for HIV-1 cure after CCR5Δ32/Δ32 allogeneic haemopoietic stem-cell transplantation 30 months post analytical treatment interruption: a case report. Lancet HIV 2020; 7:e340–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Björn-Erik O, Jensen EK, Lübke N, , et al. Analytic treatment interruption after allogeneic CCR5-D32 HSCT for AML in 2013. Presented at: 2019 Conference on Retroviruses and Opportunistic Infections (CROI); 4–7 March 2019; Seattle, Washington. [Google Scholar]
- 62. Dlamini PS, Wantland D, Makoae LN, et al. HIV stigma and missed medications in HIV-positive people in five African countries. AIDS Patient Care STDS 2009; 23:377–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Lv W, Qu H, Wu M, et al. Autoimmune hemolytic anemia after allogeneic hematopoietic stem cell transplantation in adults: a southern China multicenter experience. Cancer Med 2019; 8:6549–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Sakamoto H, Itonaga H, Sawayama Y, et al. Treatment with mogamulizumab or lenalidomide for relapsed adult T-cell leukemia/lymphoma after allogeneic hematopoietic stem cell transplantation: the Nagasaki transplant group experience. Hematol Oncol 2020; 38:162–70. [DOI] [PubMed] [Google Scholar]
- 65. Sanz J, Arango M, Carpio N, et al. Autoimmune cytopenias after umbilical cord blood transplantation in adults with hematological malignancies: a single-center experience. Bone Marrow Transplant 2014; 49:1084–8. [DOI] [PubMed] [Google Scholar]
- 66. Henrich TJ, Hanhauser E, Marty FM, et al. Antiretroviral-free HIV-1 remission and viral rebound after allogeneic stem cell transplantation: report of 2 cases. Ann Intern Med 2014; 161:319–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Verheyen J, Thielen A, Lübke N, et al. Rapid rebound of a preexisting CXCR4-tropic human immunodeficiency virus variant after allogeneic transplantation with CCR5 Δ32 homozygous stem cells. Clin Infect Dis 2019; 68:684–7. [DOI] [PubMed] [Google Scholar]
- 68. Kordelas L, Verheyen J, Beelen DW, et al. ; Essen HIV AlloSCT Group . Shift of HIV tropism in stem-cell transplantation with CCR5 Delta32 mutation. N Engl J Med 2014; 371:880–2. [DOI] [PubMed] [Google Scholar]
- 69. Petz LD, Redei I, Bryson Y, et al. Hematopoietic cell transplantation with cord blood for cure of HIV infections. Biol Blood Marrow Transplant 2013; 19:393–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Rothenberger M, Wagner JE, Haase A, et al. Transplantation of CCR532 homozygous umbilical cord blood in a child with acute lymphoblastic leukemia and perinatally acquired HIV infection. Open Forum Infect Dis 2018; 5:ofy090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Li JZ, Segal FP, Bosch RJ, et al. ART reduces T cell activation and immune exhaustion markers in HIV controllers. Clin Infect Dis 2020; 70:1636–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Leon A, Perez I, Ruiz-Mateos E, et al. ; EC and Immune Pathogenesis Working group of the Spanish AIDS Research Network . Rate and predictors of progression in elite and viremic HIV-1 controllers. AIDS 2016; 30:1209–20. [DOI] [PubMed] [Google Scholar]
- 73. Hunt PW, Brenchley J, Sinclair E, et al. Relationship between T cell activation and CD4+ T cell count in HIV-seropositive individuals with undetectable plasma HIV RNA levels in the absence of therapy. J Infect Dis 2008; 197:126–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Migueles SA, Osborne CM, Royce C, et al. Lytic granule loading of CD8+ T cells is required for HIV-infected cell elimination associated with immune control. Immunity 2008; 29:1009–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Casado C, Galvez C, Pernas M, et al. Permanent control of HIV-1 pathogenesis in exceptional elite controllers: a model of spontaneous cure. Sci Rep 2020; 10:1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Monel B, McKeon A, Lamothe-Molina P, et al. HIV controllers exhibit effective CD8+ T cell recognition of HIV-1-infected non-activated CD4(+) T cells. Cell Rep 2019; 27:142–53.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Avettand-Fenoel V, Bayan T, Gardiennet E, et al. ; CODEX ANRS Cohort Study Group . Dynamics in HIV-DNA levels over time in HIV controllers. J Int AIDS Soc 2019; 22:e25221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Pereyra F, Jia X, McLaren PJ, et al. The major genetic determinants of HIV-1 control affect HLA class I peptide presentation. Science 2010; 330:1551–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Gaiha GD, Rossin EJ, Urbach J, et al. Structural topology defines protective CD8+ T cell epitopes in the HIV proteome. Science 2019; 364:480–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Mens H, Kearney M, Wiegand A, et al. HIV-1 continues to replicate and evolve in patients with natural control of HIV infection. J Virol 2010; 84:12971–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. O’Connell KA, Brennan TP, Bailey JR, Ray SC, Siliciano RF, Blankson JN. Control of HIV-1 in elite suppressors despite ongoing replication and evolution in plasma virus. J Virol 2010; 84:7018–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Li JZ, Arnold KB, Lo J, et al. Differential levels of soluble inflammatory markers by human immunodeficiency virus controller status and demographics. Open Forum Infect Dis 2015; 2:ofu117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Crowell TA, Gebo KA, Blankson JN, et al. ; HIV Research Network . Hospitalization rates and reasons among HIV elite controllers and persons with medically controlled HIV infection. J Infect Dis 2015; 211:1692–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Noël N, Gominet M, Meyer L, Boufassa F, Lambotte O. Cardiovascular events in the French ANRS HIV controller cohort. J Acquir Immune Defic Syndr 2019; 82: e32–4. [DOI] [PubMed] [Google Scholar]
- 85. Pereyra F, Lo J, Triant VA, et al. Increased coronary atherosclerosis and immune activation in HIV-1 elite controllers. AIDS 2012; 26:2409–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Hatano H, Yukl SA, Ferre AL, et al. Prospective antiretroviral treatment of asymptomatic, HIV-1 infected controllers. PLoS Pathog 2013; 9:e1003691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Bongiovanni M, Casana M, Tincati C, d’Arminio Monforte A. Treatment interruptions in HIV-infected subjects. J Antimicrob Chemother 2006; 58:502–5. [DOI] [PubMed] [Google Scholar]
- 88. Namazi G, Fajnzylber JM, Aga E, et al. The control of HIV after antiretroviral medication pause (CHAMP) study: posttreatment controllers identified from 14 clinical studies. J Infect Dis 2018; 218:1954–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Sáez-Cirión A, Bacchus C, Hocqueloux L, et al. ; ANRS VISCONTI Study Group . Post-treatment HIV-1 controllers with a long-term virological remission after the interruption of early initiated antiretroviral therapy ANRS VISCONTI Study. PLoS Pathog 2013; 9:e1003211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Lisziewicz J, Rosenberg E, Lieberman J, et al. Control of HIV despite the discontinuation of antiretroviral therapy. N Engl J Med 1999; 340:1683–4. [DOI] [PubMed] [Google Scholar]
- 91. Assoumou L, Weiss L, Piketty C, et al. ; ANRS 116 SALTO study group . A low HIV-DNA level in peripheral blood mononuclear cells at antiretroviral treatment interruption predicts a higher probability of maintaining viral control. AIDS 2015; 29:2003–7. [DOI] [PubMed] [Google Scholar]
- 92. Calin R, Hamimi C, Lambert-Niclot S, et al. ; ULTRASTOP Study Group . Treatment interruption in chronically HIV-infected patients with an ultralow HIV reservoir. AIDS 2016; 30:761–9. [DOI] [PubMed] [Google Scholar]
- 93. Fidler S, Olson AD, Bucher HC, et al. Virological blips and predictors of post treatment viral control after stopping ART started in primary HIV infection. J Acquir Immune Defic Syndr 2017; 74:126–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Gianella S, Anderson CM, Richman DD, Smith DM, Little SJ. No evidence of posttreatment control after early initiation of antiretroviral therapy. AIDS 2015; 29:2093–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Goujard C, Girault I, Rouzioux C, et al. ; ANRS CO6 PRIMO Study Group . HIV-1 control after transient antiretroviral treatment initiated in primary infection: role of patient characteristics and effect of therapy. Antivir Ther 2012; 17:1001–9. [DOI] [PubMed] [Google Scholar]
- 96. Kilby JM, Bucy RP, Mildvan D, et al. ; Adult AIDS Clinical Trials Group A5024 Protocol Team . A randomized, partially blinded phase 2 trial of antiretroviral therapy, HIV-specific immunizations, and interleukin-2 cycles to promote efficient control of viral replication (ACTG A5024). J Infect Dis 2006; 194:1672–6. [DOI] [PubMed] [Google Scholar]
- 97. Lodi S, Meyer L, Kelleher AD, et al. Immunovirologic control 24 months after interruption of antiretroviral therapy initiated close to HIV seroconversion. Arch Intern Med 2012; 172:1252–5. [DOI] [PubMed] [Google Scholar]
- 98. Maenza J, Tapia K, Holte S, et al. How often does treatment of primary HIV lead to post-treatment control? Antivir Ther 2015; 20:855–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Perkins MJ, Bradley WP, Lalani T, et al. Brief report: prevalence of posttreatment controller phenotype is rare in HIV-infected persons after stopping antiretroviral therapy. J Acquir Immune Defic Syndr 2017; 75:364–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Stöhr W, Fidler S, McClure M, et al. Duration of HIV-1 viral suppression on cessation of antiretroviral therapy in primary infection correlates with time on therapy. PLoS One 2013; 8:e78287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Van Gulck E, Bracke L, Heyndrickx L, et al. Immune and viral correlates of “secondary viral control” after treatment interruption in chronically HIV-1 infected patients. PLoS One 2012; 7:e37792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Samri A, Bacchus-Souffan C, Hocqueloux L, et al. ; ANRS VISCONTI study group . Polyfunctional HIV-specific T cells in post-treatment controllers. AIDS 2016; 30:2299–302. [DOI] [PubMed] [Google Scholar]
- 103. Descours B, Avettand-Fenoel V, Blanc C, et al. ; ALT ANRS CO15 Study Group . Immune responses driven by protective human leukocyte antigen alleles from long-term nonprogressors are associated with low HIV reservoir in central memory CD4 T cells. Clin Infect Dis 2012; 54:1495–503. [DOI] [PubMed] [Google Scholar]
- 104. Daniel Scott-Algara CD, Arnold V, Cummings J-S, et al. Post-treatment controllers have particular NK cells with high anti-HIV capacity: VISCONTI study. Conference on Retroviruses and Opportunistic Infections; 23–26 February 2015; Seattle, Washington. [Google Scholar]
- 105. Jiang Y, Zhou F, Tian Y, et al. Higher NK cell IFN-γ production is associated with delayed HIV disease progression in LTNPs. J Clin Immunol 2013; 33:1376–85. [DOI] [PubMed] [Google Scholar]
- 106. Tomescu C, Duh FM, Hoh R, et al. Impact of protective killer inhibitory receptor/human leukocyte antigen genotypes on natural killer cell and T-cell function in HIV-1-infected controllers. AIDS 2012; 26:1869–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Krishnan S, Wilson EM, Sheikh V, et al. Evidence for innate immune system activation in HIV type 1-infected elite controllers. J Infect Dis 2014; 209:931–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Li JZ, Segal FP, Bosch RJ, et al. Antiretroviral therapy reduces T-cell activation and immune exhaustion markers in human immunodeficiency virus controllers. Clin Infect Dis 2020; 70:1636–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Strongin Z, Micci L, Fromentin R, et al. Virologic and immunologic features of simian immunodeficiency virus control post-ART interruption in rhesus macaques. J Virol 2020; 94:e00338-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Mendoza P, Gruell H, Nogueira L, et al. Combination therapy with anti-HIV-1 antibodies maintains viral suppression. Nature 2018; 561:479–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Lucar O, Su B, Potard V, et al. Neutralizing antibodies against a specific human immunodeficiency virus gp41 epitope are associated with long-term non-progressor status. EBioMedicine 2017; 22:122–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Carotenuto P, Looij D, Keldermans L, de Wolf F, Goudsmit J. Neutralizing antibodies are positively associated with CD4+ T-cell counts and T-cell function in long-term AIDS-free infection. AIDS 1998; 12:1591–600. [DOI] [PubMed] [Google Scholar]
- 113. Sharaf R, Lee GQ, Sun X, et al. HIV-1 proviral landscapes distinguish posttreatment controllers from noncontrollers. J Clin Invest 2018; 128:4074–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Jiang C, Lian X, Gao C, et al. Distinct viral reservoirs in individuals with spontaneous control of HIV-1. Nature 2020; 585:261–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Etemad B, Sun X, Lederman MM, et al. Viral and immune characteristics of HIV post-treatment controllers in ACTG studies. Presented at: Conference on Retroviruses and Opportunistic Infections; 22–25 February 2016;Boston, MA; abstract 347. [Google Scholar]
- 116. Persaud D, Gay H, Ziemniak C, et al. Absence of detectable HIV-1 viremia after treatment cessation in an infant. N Engl J Med 2013; 369:1828–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Frange P, Faye A, Avettand-Fenoël V, et al. ; ANRS EPF-CO10 Pediatric Cohort and the ANRS EP47 VISCONTI study group . HIV-1 virological remission lasting more than 12 years after interruption of early antiretroviral therapy in a perinatally infected teenager enrolled in the French ANRS EPF-CO10 paediatric cohort: a case report. Lancet HIV 2016; 3:e49–54. [DOI] [PubMed] [Google Scholar]
- 118. Colby DJ, Trautmann L, Pinyakorn S, et al. ; RV411 study group . Rapid HIV RNA rebound after antiretroviral treatment interruption in persons durably suppressed in Fiebig I acute HIV infection. Nat Med 2018; 24:923–6. [DOI] [PMC free article] [PubMed] [Google Scholar]