Abstract
Gastric cancer (GC) remains a leading cause of cancer morbidity and mortality worldwide. Outcomes from GC remain poor, especially in Western nations where cancer diagnosis is usually at advanced stages where curative resection is not possible. By contrast, nations of East Asia have adopted methods of population-level screening with improvements in stage of diagnosis and survival. In this review, we discuss the epidemiology of GC in Western populations, highlight at-risk populations who may benefit from screening, overview screening modalities, and discuss promising approaches to early GC detection.
Keywords: Helicobacter pylori, intestinal metaplasia, endoscopic screening, early detection, cancer stage, East Asia
Introduction
Every year 1.2 million persons are diagnosed with and 860,000 persons die from gastric cancer (GC) worldwide,1 making GC the fifth-leading cause of cancer incidence and third leading cause of cancer mortality, respectively.2 Outcomes from GC in most of the world remain poor, including in the United States (US). In the US, GC afflicts 27,000 each year3 and carries a dismal prognosis (5-year survival of 27%).4 These statistics reflect the fact that the majority of GCs in the US are diagnosed at advanced stages,4 where curative resection is unlikely. Strategies to improve the early diagnosis of GC are therefore crucial to improving survival.
GCs are classified as cardia or non-cardia based on the anatomic location of origin within the stomach. Cardia GCs, which share risk factors and natural history with esophageal adenocarcinomas, constitute approximately one-quarter of GCs worldwide.5 Non-cardia GCs constitute three-quarters of GCs worldwide, have witnessed improvements in outcomes following adoption of screening programs in nations of East Asia,6, 7 and will be the focus of this review.
Helicobacter pylori and Correa’s Cascade
Development of non-cardia GC has been linked in multiple epidemiologic studies with infection with the gram-negative, micro-aerophilic organism Helicobacter pylori (Hp).8, 9 Worldwide Hp prevalence rates range from <40% in industrialized nations of Western Europe and North America to >70% in areas of South America, Africa, Eastern Europe, and East Asia.10 Hp infection is associated with a three-fold increase in lifetime odds of development of non-cardia GC; moreover, Hp is believed responsible for 75-95% of all GC cases worldwide.11, 12 Colonization with Hp induces a state of chronic inflammatory insult which leads to a cascade of mucosa perturbations, termed Correa’s Cascade (Figure 1).13 In Correa’s cascade, chronic gastritis is followed progressive atrophy of the oxyntic or antral gastric mucosa, and then eventual replacement by intestinal mucosa consisting of Paneth, goblet, and absorptive cells. Intestinal metaplasia (IM) of the stomach is an important precursor lesion in the pathway to GC,14-17 and regional prevalence of IM correlates closely with incidence of GC worldwide.18 Even with decreasing prevalence of Hp, with the secular aging of the global population GC cases and deaths are expected to climb well into the 21st century.19, 20
Figure 1:
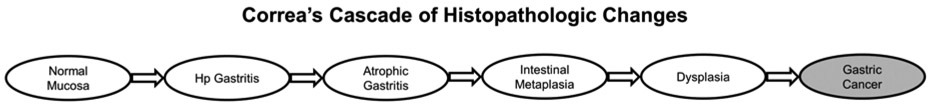
Proposed carcinogenic cascade induced by Helicobacter pylori (Hp) and other environmental insult. Patients with atrophic gastritis, intestinal metaplasia, and dysplasia remain at increased risk for gastric cancer even following Hp eradication.
Gastric Cancer Screening and Outcomes in East Asia
The incidence of GC is significantly higher in nations of East Asia compared to the US. While the incidence of GC is roughly 6 per 100,000 in the US, it is approximately 28 per 100,000 in Japan and 34 per 100,000 in South Korea. Yet while incidence of GC is much higher in these countries, survival from GC is also higher compared to the US or Western Europe (Figure 2). Five-year observed survival from GC exceeds 60% in both South Korea and Japan, compared to below 30% for the US and Western Europe.21, 22 These differences in survival are due in large part to differences in stage of diagnosis. While nearly 60% of GCs are diagnosed at a surgically or endoscopically curable stage in South Korea and Japan, fewer than a quarter of GCs are diagnosed at such stages in the West.4, 23-25
Figure 2:
Left panel depicts five-year observed survival following gastric cancer diagnosis in East Asia (South Korea, Japan) and Western nations (United States and Europe). Right panel depicts the proportion of all gastric cancers diagnosed at localized stage based on United States National Cancer Institute summary staging.
In Japan, a national screening program for GC was first introduced in 1983. This consisted of radiography-based screening of all adults ≥40 years old, with endoscopic examination performed on individuals with abnormal radiographic results.26 Based on the results of several rigorous observational studies, the national screening program was amended in 2016 to allow for either endoscopic or radiographic screening for adults ≥50 years old on a biennial basis.26 While endoscopic screening is rapidly being adopted throughout Japan, radiographic screening is still the predominant screening modality in most prefectures.27
South Korea initiated a biennial screening program consisting of either endoscopic or radiographic screening for adults ≥40 years old in 2002.28 In practice, endoscopic screening has been the predominant modality practiced in South Korea due to patient preference. Since the initiation of the national screening program, the proportion of gastric cancers diagnosed as early gastric cancer(defined as tumor with invasion limited to mucosa or submucosa) has increased from 39% in 2001 to 73% in 2016.29 Moreover, observed five-year survival has increased from 46% to 75%.29
Efficacy and Safety of Screening Modalities
Radiographic Screening
Radiographic screening involves the ingestion of a contrast agent (often barium), and subsequent fluoroscopic imaging of the gastric lumen. Contrast radiography allows for the detection of luminal pathology including ulcers, polyps, and masses. However, compared to modern endoscopy, radiography has both limited sensitivity and specificity.30 Cancer registry data suggests that the sensitivity of radiographic screening ranges from 60-80%, and specificity from 80-90%.6 It should also be noted that for early GC (where a luminal prominence or depression may be minimal), the sensitivity of radiography has been reported to be significantly lower (14-36%).31,32
The efficacy of radiographic screening has been assessed in several observational studies, including both cohort and case-control studies,26 though notably no randomized control trial has been conducted comparing radiographic screening with standard of care. From cohort studies from Japan33, 34 comparing radiographic screening with no screening over long-term follow-up (ranging from 11-13 years), receiving radiographic screening was associated with both reductions in GC-specific mortality (with relative risk ranging from 0.52-0.54) as well as all-cause mortality (relative risk 0.71-0.83). However, during the period of these studies radiography was also a standard test for assessment of gastrointestinal symptoms, introducing the possibility of confounding by indication. Moreover, receipt of subsequent screening (such as by endoscopy) in the follow-up period was not ascertained possibly causing overestimation of the effect size. The safety profile of radiographic screening is generally favorable, with mild risk of constipation or ileus and rare cases of aspiration pneumonia.6 In a report of over 3 million radiographic screening procedures performed, only a single death was attributed to an adverse event related to screening.26 Radiation exposure from photofluorographic screening is in the range of 0.6 mSv (by comparison a standard chest x-ray exposure is approximately 0.1 mSv).
As a relatively safe and inexpensive modality, radiography may continue to serve a role for GC screening in resource-limited settings. However, as the primary motivation for screening is to improve the detection of early-stage cancers, radiography has limited utility compared to modern, high-resolution gastrointestinal endoscopy. Moreover, when an abnormality is detected through radiography a confirmatory upper endoscopy is required for visualization and tissue acquisition.
Endoscopic Screening
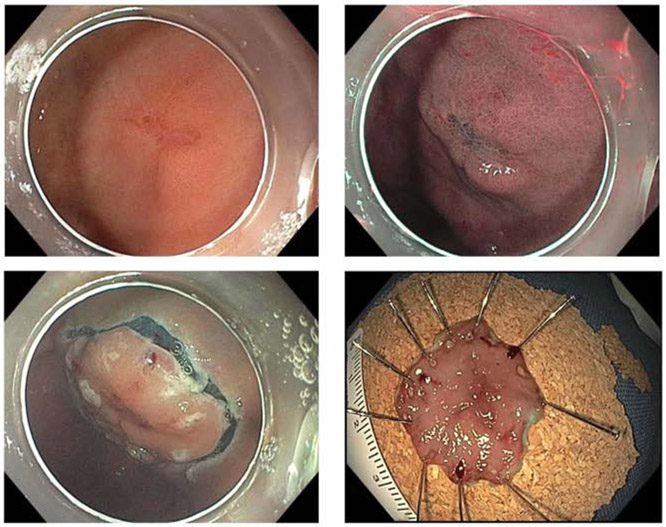
Since 2001 in South Korea and 2016 in Japan,29 endoscopic screening has been offered as an alternative modality to radiographic screening. Endoscopic screening offers several advantages to radiographic screening, including the ability to directly visualize the gastric mucosa and tissue sampling of abnormal-appearing tissue or visible lesions. Compared to radiographic screening, endoscopic screening demonstrates both better sensitivity and specificity.26, 35 This increased sensitivity is especially important for early GCs that demonstrate only subtle mucosal changes, and which may not have an elevated or depressed component visible on contrast radiography. Techniques to enhance mucosal contrast have been developed to improve detection of subtle lesions, such as narrow-band imaging and chromoendoscopy (Video 1). In narrow-band imaging, conventional white light is filtered into defined wavelengths in order to maximize absorption by hemoglobin, as well as limit penetration of light beyond the mucosal surface. Given this shorter wavelength, the resulting ‘blue’ light penetrates less deeply than conventional white light and may improve contrast of the mucosal surface. Chromoendoscopy also serves to amplify contrast of mucosal lesions through the use of dye-based staining of the gastric mucosa with biologically-compatible agents such as acetic acid or methylene blue.36, 37 Application of dilute acetic acid can modify the optical properties of the epithelium by slightly altering the pH or by reversibly altering the structure of cellular proteins to reflect white light. Methylene blue is actively absorbed by small intestinal epithelium but not normal gastric epithelium, enhancing contrast between metaplastic and normal gastric epithelium. Chromoendoscopy may improve the delineation of surface irregularities, which in turn may improve the diagnosis and staging of early GCs.38 Early GC detection may allow for opportunities for endoscopic resection through endoscopic submucosal dissection (ESD, Figure 3). For GCs confined to the mucosa or proximal submucosa (with invasion depth of <500 microns) and without lymph node involvement, ESD offers similar cure rate and fewer rates of adverse events compared to surgical gastrectomy based on retrospective series from East Asia.39, 40
Figure 3:
Top left panel depicts a subtle, flat, erythematous lesion which was biopsied to be gastric adenocarcinoma. Use of narrow-band imaging (top right) enhances visualization and delineation of the lesion. This lesion was staged as an early gastric cancer (tumor invasive to no deeper than mucosa or submucosa), and removed by endoscopic submucosal dissection (bottom left). En bloc resection specimen (bottom right) confirmed tumor confined to mucosal layer, without lymphovascular invasion, and with negative lateral and deep margins consistent with curative resection.
The efficacy of endoscopic screening in decreasing cancer-specific mortality have been evaluated in observational studies form East Asia. A systematic review and meta-analysis of the protective effect of endoscopic screening on cancer-specific mortality identified 10 studies (six cohort studies and four case-control studies) from South Korea, Japan, and China.41 Receipt of endoscopic screening was associated with an approximate 40% reduction in risk for GC-specific mortality in the pooled estimate, with a robust protective effect found compared both against no screening and radiographic screening controls.41 When reviewing the existing evidence in support of endoscopic screening, the Japanese Guideline Development Group initially found inadequate observational data to justify population-level endoscopic screening in 2008.6, 42 However, based on the results of numerous high-quality observational studies published after 2008, the Japanese guidelines were amended to favor endoscopic screening in 2018 with a evidence score of 2+ (moderate-quality case-control and cohort studies with a low risk for bias, confounding or chance and a moderate probability that the relation is causal).26 Notably the primary endpoint of these studies has been GC-specific mortality (as opposed to overall mortality). Currently no randomized control trial data exists for the benefits of endoscopic screening.
In the US and Europe most upper endoscopies are performed under sedation (either moderate or deep). The risk of cardiopulmonary events related to sedation have been estimated to be between 1 in 170 to 1 in 10,000, with the higher range of estimates incorporating minor events (such as changes in oxygen saturation or heart rate).43 The Japanese Association of Gastroenterological Cancer Screening has found an overall rate of complications of 87 per 100,000 for endoscopic screening and 43 per 100,000 for radiographic screening.26
Serologic Screening
Hp-induced inflammation begins in the antrum and proceeds upward to the corpus with chronic infection. Human pepsinogens are classified into two biologically distinct types, pepsinogen I and pepsinogen II. As inflammation proceeds toward the corpus with chronic Hp infection, levels of pepsinogen I (produced by chief cells in the corpus) decrease whereas levels of pepsinogen II remain more constant.44 As such, a decreased level of pepsinogen I and decreased pepsinogen I/II ratio may indicate advanced atrophic gastritis.44 Serum pepsinogens in combination with Hp IgG antibody have been evaluated as non-invasive screening tools in East Asian cohorts.45-47 However, use of these markers demonstrate significant limitations including a high degree of heterogeneity in reported testing characteristics between populations, differing cutoff points, and variability based on proton pump inhibitor use.48, 49 These methods are not currently used for population-level screening in either South Korea or Japan. Their use may also be limited in Western populations which differ in prevalence of Hp infection, proton pump inhibitor therapy use, and rates of autoimmune atrophic gastritis.49, 50
GASTRIC CANCER SCREENING IN THE UNITED STATES
At Risk Populations
In the US, GC survival is poor (5-year observed survival of 27%) and the majority of cancers are diagnosed at regional or distant stages.4 While overall incidence of GC is modest among the general population (~6 per 100,000), certain high-risk racial (Asians, Alaskan Indians, American Indians, Blacks/African Americans) and ethnic (Hispanics) groups may face significantly higher risk (Figure 4). Very high-risk subgroups such as Japanese and Korean Americans face an incidence six- to eight-fold higher than non-Hispanic Whites.51 Beyond race and ethnicity, Americans at increased risk for GC include those with a family history or with cancer-predisposing syndromes, recent immigrants form high-incidence regions of the world, those with a history of Hp infection, and those with precancerous changes of the stomach.29 It behooves both clinicians and policy makers to be cognizant of high-risk groups, and to offer appropriate counseling for the role of preventative strategies such as GC screening.
Figure 4:

Crude incidence of gastric cancer in United States (per 100,000; Y-axis) plotted by age group (X-axis). Asians, Blacks, Hispanics, and American Indian/Alaskan Natives face a several-fold increased risk compared to non-Hispanic Whites.
Data from the Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) Research Data (1973-2015), National Cancer Institute, DCCPS, Surveillance Research Program released in April 2018 based on the November 2017 submission.
IM is a critical precursor lesion to GC, and prevalence of IM in populations correlates with GC incidence. In the US, the prevalence of intestinal metaplasia has been estimated to be between 5-10% of the general population.52, 53 High-risk subgroups including certain racial and ethnic minorities may have IM prevalence several-fold higher.54, 55 Within the US, there appears to be a close association between prevalence of IM with incidence of GC within racial subgroups (Table 1). These data suggest that all racial and ethnic groups are at risk for GC once IM has developed. While gastritis and atrophy may reverse and normalize following Hp eradication, IM often persists.56, 57 Moreover, long-term clinical follow-up suggest that patients with IM remain at increased risk for GC even after eradication of Hp.58, 59
Table 1:
Estimated Prevalence of Intestinal Metaplasia and Incidence of Gastric Cancer in Racial/Ethnic Groups in the United States
| Racial/Ethnic Group | Prevalence of IM | Incidence of GC | References |
|---|---|---|---|
| Non-Hispanic Whites | 7-9% | 6-8 per 100,000 | 4, 21, 52, 54 |
| Non-Hispanic Black | 21% | 11 per 100,000 | 4, 21, 55 |
| Hispanics | 12-30% | 11 per 100,000 | 4, 21, 54, 55 |
| Chinese | 26% | 15 per 100,000 | 51, 54 |
| Koreans | 40% | 45 per 100,000 | 51, 54 |
IM,intestinal metaplasia; GC,gastric cancer.
It is estimated that the annual rate of progression onto GC from IM to be approximately 0.25%.60 However, this aggregation of risk does not capture the variability of presentation in IM histologic severity or topographic distribution. In order to estimate histologic severity, scoring systems such as the operative link for gastritis assessment (OLGA)61 for atrophic gastritis, and the operative link for gastric intestinal metaplasia (OLGIM) for IM have been developed (Figure 5).62 OLGA and OLGIM rely on both an assessment of the topographic extent of disease as well as the percentage of glandular involvement from each biopsy location utilizing a visual-analogue scale.63 The resultant stage score, ranging from 0 (no IM or no atrophy) to 4 (severe, extensive IM or atrophy) have been validated in several observational studies as risk-stratification tools for progression onto subsequent GC.64-67 Use of the OLGA and OLGIM systems is dependent on consistent sampling of multiple locations of the stomach (including antrum, incisura, and corpus/body) in a systematic manner termed the ‘Sydney Protocol’.63 Another promising method of histologic risk stratification is through distinguishing complete IM from incomplete IM. Complete IM is characterized by well-defined goblet cells and a well-developed brush border, whereas in incomplete IM mucin droplets of varying sizes and shapes can be found and there is an absence of a brush border.68 In specialized centers where mucin staining is available, complete IM will be found to display predominantly small intestinal phenotypic markers such as MUC2 and sucrase, whereas incomplete IM only selectively or incompletely expresses small intestinal markers but may express gastric phenotypic markers MUC5AC and large intestinal phenotypic markers such as Das-1.69
Figure 5:

Scoring of gastric precancerous lesions using the Operative Link systems. In these scoring systems, biopsies from the gastric antrum and body are individually scored for degree of atrophic gastritis and intestinal metaplasia utilizing a visual-analogue scale (none, mild, moderate, marked). A summary stage for both atrophy and intestinal metaplasia is then assigned.
Current Recommendations
Recommendations for GC screening or precancerous lesion surveillance by US-based professional societies are depicted in Table 2. Currently, the American Society of Gastrointestinal Endoscopy (ASGE) has recommended endoscopic screening for GC in first-generation immigrants from high-risk regions (i.e. Japan, China, Russia, and South America) over aged 40, in particular if there is a family history of GC in a first-degree relative.70 Regarding surveillance of patients with precancerous lesions, the ASGE recommends surveillance of patients with atrophic gastritis or IM when there is increased risk of GC due to ethnic/racial background, positive family history, or extensive anatomic distribution of disease.14, 70 By contrast, the American Gastroenterological Association (AGA) recommends against the routine use of endoscopic surveillance in patients with IM, but clarifies that this is a conditional recommendation based on very low quality of evidence.71 The AGA guidelines further state that “Patients with IM at higher risk for GC who put a high value on potential but uncertain reduction in GC mortality, and who put a low value on potential risks of surveillance endoscopies, may reasonably elect for surveillance.” The AGA guidelines identify patients with IM at higher risk for gastric cancer as those with incomplete IM, those with extensive IM, and those with a family history of gastric cancer. The AGA guidelines also identify patients at overall increased risk for GC including racial/ethnic minorities and immigrants from high-incidence regions. Notably neither the ASGE nor the AGA have made recommendation on the optimal interval for surveillance of IM if this strategy is pursued.
Table 2:
Guidelines Issued by United States Professional Societies
| Society | Year | Recommendation |
|---|---|---|
| Gastric Cancer Screening | ||
| American Society of Gastrointestinal Endoscopy70 | 2015 | Endoscopic screening for gastric cancer in first-generation immigrants from high-risk regions (e.g. Japan, China, Russia, and South America) may be considered for those aged 40 years, particularly if there is a family history of gastric cancer in a first-degree relative |
| Surveillance of Intestinal Metaplasia (IM) | ||
| American Society of Gastrointestinal Endoscopy14, 70 | 2015 | Endoscopic surveillance in patients with gastric atrophic gastritis or IM coupled with an increased risk of gastric cancer because of racial/ethnic background, extensive anatomic distribution, or family history |
| American Gastroenterological Association71 | 2019 | Recommends against routine use of endoscopic surveillance in patients with IM. Conditional recommendation, very low quality of evidence Patients with IM at higher risk for gastric cancer who put a high value on potential but uncertain reduction in gastric cancer mortality, and who put a low value on potential risks of surveillance endoscopies, may reasonably elect for surveillance. Patients with IM specifically at higher risk of gastric cancer include those with:
Patients at overall increased risk for gastric cancer include:
|
Existing recommendations from United States-based professional societies regarding screening of gastric cancer or surveillance of precancerous lesions such as intestinal metaplasia (IM).
FUTURE SCREENING MODALITIES
Promising biomarkers currently under development or validation may revolutionize the field of early GC detection and prevention. MicroRNAs (miRNAs) are small, non-coding molecules involved in biological processes including cell-cycle progression and apoptosis. Given their stability, presence in blood, and role in numerous pathways, miRNAs have been evaluated as potential biomarkers for GC. Five miRNAs have shown particular promise as screening tests for GC: miR-2172-74 (inhibitor of tumor suppressor genes), miR-106a75, 76 (cell proliferation signal), miR-106b77, 78 (inhibitor of apoptosis), miR-22373,79 (cell proliferation and invasion), and miR-421 80-82(apoptosis resistance). Notably these miRNAs have mostly been evaluated in cohorts from East Asia where the prevalence of Hp and incidence of GC is much higher. Additional prospective validation studies are required prior to translation of miRNAs to clinical practice.
Risk stratification of IM may also be improved through use of molecular markers. In a prospective cohort study of high-risk Singaporean Chinese patients, IM biopsy samples with shortened telomeres and chromosomal aberrations were found to be associated with subsequent progression to either dysplasia or frank carcinoma. By contrast, IM with normal-like epigenetic patterns were associated with stability or regression.65 While requiring validation, such a molecular signature for IM progression may serve as a valuable tool to allow for focused and highly-personalized strategies of surveillance while also avoiding unnecessary endoscopies in low-risk subjects.
SUMMARY
GC remains a devastating disease for the 27,000 Americans diagnosed each year. Compared to East Asia, survival from GC in the US and Europe are lower, reflecting a later stages of diagnosis. In high-incidence nations of East Asia, national screening programs have been adopted. An emerging body of observational data suggests that endoscopic screening may prevent GC-specific mortality in targeted populations. There exist high-risk populations within the US who may benefit from targeted screening, including racial/ethnic groups (American Indians, Alaska Indians, Asians, Blacks, Hispanics), first-generation immigrants from high-incidence regions, and those with a family history of GC. Individuals diagnosed with IM, particularly extensive IM or histologically-severe IM, may benefit from endoscopic surveillance. Emerging molecular technologies may help to identify high-risk individuals who should be screened, as well as stratify IM for risk of cancer progression.
Supplementary Material
Video 1: In this upper endoscopy, a subtle erythematous lesion is seen at the incisura which biopsies demonstrated to be gastric adenocarcinoma. White light endoscopic examination of the gastric antrum demonstrate subtle mucosa nodularity suggestive of intestinal metaplasia, which on narrow-band imaging (0:13) can be more clearly defined. Following application of dilute acetic acid (0:30) the areas of intestinal metaplasia become clearly defined. Finally application of methylene blue (0:45) which is selectively absorbed by intestinal epithelium, and not normal gastric epithelium, clearly delineates the areas of intestinal metaplasia.
Key Points:
Patients are diagnosed with gastric cancer at more advanced stages and have overall lower survival in the United States compared to East Asia
Observational data from Japan and South Korea, nations with national gastric cancer screening programs, show that endoscopic screening may improve gastric cancer mortality
In the United States high-risk racial/ethnic groups (Alaskan Natives, American Indians, Asians, Blacks, Hispanics), first-generation immigrants form high-incidence regions, and individuals with a family history may benefit from screening
Individuals with intestinal metaplasia, particularly extensive or histologically-severe disease, may benefit from endoscopic surveillance
A video of use of chromoendoscopy to enhance detection of gastric intestinal metaplasia accompanies this article.
Clinical Care Points.
Racial/ethnic minorities and first-generation immigrants are at increased risk for gastric cancer and may benefit from endoscopic cancer screening
Patients diagnosed with incomplete, extensive, or severe intestinal metaplasia should be offered endoscopic surveillance
Use of narrow-band imaging and chromoendoscopy during endoscopy can improve the detection of gastric cancer
Footnotes
Disclosures: The Authors have no disclosures to report with regards to this work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCE
- 1.Collaborators GBDSC. The global, regional, and national burden of stomach cancer in 195 countries, 1990-2017: a systematic analysis for the Global Burden of Disease study 2017. Lancet Gastroenterol Hepatol 2020;5:42–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization International Agency for Research on Cancer (IARC). GLOBOCAN 2012: estimated cancer incidence, mortality and prevalence worldwide in 2018 [homepage on the internet]; 2018. [cited 2019 Jan 23]. Available from: http://gco.iarc.fr/. [Google Scholar]
- 3.Cancer Facts and Figures 2019. American Cancer Society. Atlanta, GA. (https://www.cancer.org/cancer/stomach-cancer/about/key-statistics.html). [Google Scholar]
- 4.SEER*Explorer: An interactive website for SEER cancer statistics [Internet]. Surveillance Research Program, National Cancer Institute. [Cited 2019 Dec 30]. Available from https://seer.cancer.gov/explorer/. [Google Scholar]
- 5.Colquhoun A, Arnold M, Ferlay J, et al. Global patterns of cardia and non-cardia gastric cancer incidence in 2012. Gut 2015;64:1881–8. [DOI] [PubMed] [Google Scholar]
- 6.Hamashima C, Shibuya D, Yamazaki H, et al. The Japanese guidelines for gastric cancer screening. Jpn J Clin Oncol 2008;38:259–67. [DOI] [PubMed] [Google Scholar]
- 7.Jun JK, Choi KS, Lee HY, et al. Effectiveness of the Korean National Cancer Screening Program in Reducing Gastric Cancer Mortality. Gastroenterology 2017;152:1319–1328 e7. [DOI] [PubMed] [Google Scholar]
- 8.Nomura A, Stemmermann GN, Chyou PH, et al. Helicobacter pylori infection and gastric carcinoma among Japanese Americans in Hawaii. N Engl J Med 1991;325:1132–6. [DOI] [PubMed] [Google Scholar]
- 9.Parsonnet J, Friedman GD, Vandersteen DP, et al. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med 1991;325:1127–31. [DOI] [PubMed] [Google Scholar]
- 10.Hooi JKY, Lai WY, Ng WK, et al. Global Prevalence of Helicobacter pylori Infection: Systematic Review and Meta-Analysis. Gastroenterology 2017;153:420–429. [DOI] [PubMed] [Google Scholar]
- 11.Eslick GD, Lim LL, Byles JE, et al. Association of Helicobacter pylori infection with gastric carcinoma: a meta-analysis. Am J Gastroenterol 1999;94:2373–9. [DOI] [PubMed] [Google Scholar]
- 12.Peleteiro B, Bastos A, Ferro A, et al. Prevalence of Helicobacter pylori infection worldwide: a systematic review of studies with national coverage. Dig Dis Sci 2014;59:1698–709. [DOI] [PubMed] [Google Scholar]
- 13.Correa P Human gastric carcinogenesis: a multistep and multifactorial process--First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res 1992;52:6735–40. [PubMed] [Google Scholar]
- 14.Committee ASoP, Evans JA, Chandrasekhara V, et al. The role of endoscopy in the management of premalignant and malignant conditions of the stomach. Gastrointest Endosc 2015;82:1–8. [DOI] [PubMed] [Google Scholar]
- 15.Kim SG, Jung HK, Lee HL, et al. Guidelines for the diagnosis and treatment of Helicobacter pylori infection in Korea, 2013 revised edition. J Gastroenterol Hepatol 2014;29:1371–86. [DOI] [PubMed] [Google Scholar]
- 16.Leung WK, Sung JJ. Review article: intestinal metaplasia and gastric carcinogenesis. Aliment Pharmacol Ther 2002;16:1209–16. [DOI] [PubMed] [Google Scholar]
- 17.Uemura N, Okamoto S, Yamamoto S, et al. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med 2001;345:784–9. [DOI] [PubMed] [Google Scholar]
- 18.Sipponen P, Kimura K. Intestinal metaplasia, atrophic gastritis and stomach cancer: trends over time. Eur J Gastroenterol Hepatol 1994;6 Suppl 1:S79–83. [PubMed] [Google Scholar]
- 19.Carter AJ, Delarosa B, Hur H. An analysis of discrepancies between United Kingdom cancer research funding and societal burden and a comparison to previous and United States values. Health Res Policy Syst 2015;13:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maruthappu M, Head MG, Zhou CD, et al. Investments in cancer research awarded to UK institutions and the global burden of cancer 2000-2013: a systematic analysis. BMJ Open 2017;7:e013936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lui FH, Tuan B, Swenson SL, et al. Ethnic disparities in gastric cancer incidence and survival in the USA: an updated analysis of 1992-2009 SEER data. Dig Dis Sci 2014;59:3027–34. [DOI] [PubMed] [Google Scholar]
- 22.Anderson LA, Tavilla A, Brenner H, et al. Survival for oesophageal, stomach and small intestine cancers in Europe 1999-2007: Results from EUROCARE-5. Eur J Cancer 2015;51:2144–2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Minicozzi P, Walsh PM, Sanchez MJ, et al. Is low survival for cancer in Eastern Europe due principally to late stage at diagnosis? Eur J Cancer 2018;93:127–137. [DOI] [PubMed] [Google Scholar]
- 24.Hamashima C, Choi IJ, Jung HY. The 2020 Stanford Gastric Cancer Summit. Recording available at: https://med.stanford.edu/care/gastric-cancer-summit-at-stanford/Gastric-Cancer-Summit-Videos.html, Stanford, CA, 2020. [Google Scholar]
- 25.Jung KW, Won YJ, Kong HJ, et al. Survival of korean adult cancer patients by stage at diagnosis, 2006-2010: national cancer registry study. Cancer Res Treat 2013;45:162–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hamashima C Update version of the Japanese Guidelines for Gastric Cancer Screening. Japanese Journal of Clinical Oncology 2018;48:673–683. [DOI] [PubMed] [Google Scholar]
- 27.Hamashima C, Goto R. Potential capacity of endoscopic screening for gastric cancer in Japan. Cancer Sci 2017;108:101–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim Y, Jun JK, Choi KS, et al. Overview of the National Cancer screening programme and the cancer screening status in Korea. Asian Pac J Cancer Prev 2011;12:725–30. [PubMed] [Google Scholar]
- 29.Huang RJ, Koh H, Hwang JH, et al. A Summary of the 2020 Gastric Cancer Summit at Stanford University. Gastroenterology 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dooley CP, Larson AW, Stace NH, et al. Double-contrast barium meal and upper gastrointestinal endoscopy. A comparative study. Ann Intern Med 1984;101:538–45. [DOI] [PubMed] [Google Scholar]
- 31.Longo WE, Zucker KA, Zdon MJ, et al. Detection of early gastric cancer in an aggressive endoscopy unit. Am Surg 1989;55:100–4. [PubMed] [Google Scholar]
- 32.Choi KS, Jun JK, Park EC, et al. Performance of different gastric cancer screening methods in Korea: a population-based study. PLoS One 2012;7:e50041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee KJ, Inoue M, Otani T, et al. Gastric cancer screening and subsequent risk of gastric cancer: a large-scale population-based cohort study, with a 13-year follow-up in Japan. Int J Cancer 2006;118:2315–21. [DOI] [PubMed] [Google Scholar]
- 34.Miyamoto A, Kuriyama S, Nishino Y, et al. Lower risk of death from gastric cancer among participants of gastric cancer screening in Japan: a population-based cohort study. Prev Med 2007;44:12–9. [DOI] [PubMed] [Google Scholar]
- 35.Hamashima C, Okamoto M, Shabana M, et al. Sensitivity of endoscopic screening for gastric cancer by the incidence method. Int J Cancer 2013;133:653–9. [DOI] [PubMed] [Google Scholar]
- 36.Taghavi SA, Membari ME, Eshraghian A, et al. Comparison of chromoendoscopy and conventional endoscopy in the detection of premalignant gastric lesions. Can J Gastroenterol 2009;23:105–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Song KH, Hwang JA, Kim SM, et al. Acetic acid chromoendoscopy for determining the extent of gastric intestinal metaplasia. Gastrointest Endosc 2017;85:349–356. [DOI] [PubMed] [Google Scholar]
- 38.Dinis-Ribeiro M Chromoendoscopy for early diagnosis of gastric cancer. Eur J Gastroenterol Hepatol 2006;18:831–8. [DOI] [PubMed] [Google Scholar]
- 39.Chiu PW, Teoh AY, To KF, et al. Endoscopic submucosal dissection (ESD) compared with gastrectomy for treatment of early gastric neoplasia: a retrospective cohort study. Surg Endosc 2012;26:3584–91. [DOI] [PubMed] [Google Scholar]
- 40.Cho JH, Cha SW, Kim HG, et al. Long-term outcomes of endoscopic submucosal dissection for early gastric cancer: a comparison study to surgery using propensity score-matched analysis. Surg Endosc 2016;30:3762–73. [DOI] [PubMed] [Google Scholar]
- 41.Zhang X, Li M, Chen S, et al. Endoscopic Screening in Asian Countries Is Associated With Reduced Gastric Cancer Mortality: A Meta-analysis and Systematic Review. Gastroenterology 2018;155:347–354 e9. [DOI] [PubMed] [Google Scholar]
- 42.Hamashima C, Saito S, Nakayama T, et al. The Standardized Development Method of the Japanese Guidelinesfor Cancer Screening. Japanese Journal of Clinical Oncology 2008;38:288–295. [DOI] [PubMed] [Google Scholar]
- 43.Committee ASoP, Ben-Menachem T, Decker GA, et al. Adverse events of upper GI endoscopy. Gastrointest Endosc 2012;76:707–18. [DOI] [PubMed] [Google Scholar]
- 44.Miki K Gastric cancer screening by combined assay for serum anti-Helicobacter pylori IgG antibody and serum pepsinogen levels - "ABC method". Proc Jpn Acad Ser B Phys Biol Sci 2011;87:405–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Abnet CC, Zheng W, Ye W, et al. Plasma pepsinogens, antibodies against Helicobacter pylori, and risk of gastric cancer in the Shanghai Women's Health Study Cohort. Br J Cancer 2011;104:1511–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tu H, Sun L, Dong X, et al. A Serological Biopsy Using Five Stomach-Specific Circulating Biomarkers for Gastric Cancer Risk Assessment: A Multi-Phase Study. Am J Gastroenterol 2017;112:704–715. [DOI] [PubMed] [Google Scholar]
- 47.Wang X, Lu B, Meng L, et al. The correlation between histological gastritis staging- 'OLGA/OLGIM' and serum pepsinogen test in assessment of gastric atrophy/intestinal metaplasia in China. Scand J Gastroenterol 2017;52:822–827. [DOI] [PubMed] [Google Scholar]
- 48.Huang YK, Yu JC, Kang WM, et al. Significance of Serum Pepsinogens as a Biomarker for Gastric Cancer and Atrophic Gastritis Screening: A Systematic Review and Meta-Analysis. PLoS One 2015;10:e0142080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zagari RM, Rabitti S, Greenwood DC, et al. Systematic review with meta-analysis: diagnostic performance of the combination of pepsinogen, gastrin-17 and anti-Helicobacter pylori antibodies serum assays for the diagnosis of atrophic gastritis. Aliment Pharmacol Ther 2017;46:657–667. [DOI] [PubMed] [Google Scholar]
- 50.Castro C, Dinis-Ribeiro M, Rodrigues ANG, et al. Western long-term accuracy of serum pepsinogen-based gastric cancer screening. Eur J Gastroenterol Hepatol 2018;30:274–277. [DOI] [PubMed] [Google Scholar]
- 51.Huang RJ, Sharp N, Talamoa RO, et al. One Size Does Not Fit All: Marked Heterogeneity in Incidence of and Survival from Gastric Cancer among Asian American Subgroups. Cancer Epidemiol Biomarkers Prev 2020. [DOI] [PubMed] [Google Scholar]
- 52.Sonnenberg A, Lash RH, Genta RM. A national study of Helicobactor pylori infection in gastric biopsy specimens. Gastroenterology 2010;139:1894–1901 e2; quiz e12. [DOI] [PubMed] [Google Scholar]
- 53.Sonnenberg A, Genta RM. Changes in the Gastric Mucosa With Aging. Clin Gastroenterol Hepatol 2015;13:2276–81. [DOI] [PubMed] [Google Scholar]
- 54.Choi CE, Sonnenberg A, Turner K, et al. High Prevalence of Gastric Preneoplastic Lesions in East Asians and Hispanics in the USA. Dig Dis Sci 2015;60:2070–6. [DOI] [PubMed] [Google Scholar]
- 55.Huang RJ, Ende AR, Singla A, et al. Prevalence, risk factors, and surveillance patterns for gastric intestinal metaplasia among patients undergoing upper endoscopy with biopsy. Gastrointest Endosc 2020;91:70–77 e1. [DOI] [PubMed] [Google Scholar]
- 56.Hwang YJ, Kim N, Lee HS, et al. Reversibility of atrophic gastritis and intestinal metaplasia after Helicobacter pylori eradication - a prospective study for up to 10 years. Aliment Pharmacol Ther 2018;47:380–390. [DOI] [PubMed] [Google Scholar]
- 57.Chiang TH, Maeda M, Yamada H, et al. Risk stratification for gastric cancer after Helicobacter pylori eradication: A population-based study on Matsu Islands. J Gastroenterol Hepatol 2020. [DOI] [PubMed] [Google Scholar]
- 58.Ito M, Takata S, Tatsugami M, et al. Clinical prevention of gastric cancer by Helicobacter pylori eradication therapy: a systematic review. J Gastroenterol 2009;44:365–71. [DOI] [PubMed] [Google Scholar]
- 59.Take S, Mizuno M, Ishiki K, et al. Risk of gastric cancer in the second decade of follow-up after Helicobacter pylori eradication. Journal of Gastroenterology 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Song H, Ekheden IG, Zheng Z, et al. Incidence of gastric cancer among patients with gastric precancerous lesions: observational cohort study in a low risk Western population. BMJ 2015;351:h3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rugge M, Meggio A, Pennelli G, et al. Gastritis staging in clinical practice: the OLGA staging system. Gut 2007;56:631–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Capelle LG, de Vries AC, Haringsma J, et al. The staging of gastritis with the OLGA system by using intestinal metaplasia as an accurate alternative for atrophic gastritis. Gastrointest Endosc 2010;71:1150–8. [DOI] [PubMed] [Google Scholar]
- 63.Dixon MF, Genta RM, Yardley JH, et al. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol 1996;20:1161–81. [DOI] [PubMed] [Google Scholar]
- 64.den Hollander WJ, Holster IL, den Hoed CM, et al. Surveillance of premalignant gastric lesions: a multicentre prospective cohort study from low incidence regions. Gut 2018. [DOI] [PubMed] [Google Scholar]
- 65.Huang KK, Ramnarayanan K, Zhu F, et al. Genomic and Epigenomic Profiling of High-Risk Intestinal Metaplasia Reveals Molecular Determinants of Progression to Gastric Cancer. Cancer Cell 2018;33:137–150 e5. [DOI] [PubMed] [Google Scholar]
- 66.Rugge M, Genta RM, Fassan M, et al. OLGA Gastritis Staging for the Prediction of Gastric Cancer Risk: A Long-term Follow-up Study of 7436 Patients. Am J Gastroenterol 2018. [DOI] [PubMed] [Google Scholar]
- 67.Yue H, Shan L, Bin L. The significance of OLGA and OLGIM staging systems in the risk assessment of gastric cancer: a systematic review and meta-analysis. Gastric Cancer 2018;21:579–587. [DOI] [PubMed] [Google Scholar]
- 68.Correa P, Piazuelo MB, Wilson KT. Pathology of gastric intestinal metaplasia: clinical implications. Am J Gastroenterol 2010;105:493–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Huang RJ, Choi AY, Truong CD, et al. Diagnosis and Management of Gastric Intestinal Metaplasia: Current Status and Future Directions. Gut Liver 2019;13:596–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.ASGE Standards of Practice Committee, Wang A, Shaukat A, et al. Race and ethnicity considerations in GI endoscopy. Gastrointest Endosc 2015;82:593–9. [DOI] [PubMed] [Google Scholar]
- 71.Gupta S, Li D, El Serag HB, et al. AGA Clinical Practice Guidelines on Management of Gastric Intestinal Metaplasia. Gastroenterology 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zheng Y, Cui L, Sun W, et al. MicroRNA-21 is a new marker of circulating tumor cells in gastric cancer patients. Cancer Biomark 2011;10:71–7. [DOI] [PubMed] [Google Scholar]
- 73.Li BS, Zhao YL, Guo G, et al. Plasma microRNAs, miR-223, miR-21 and miR-218, as novel potential biomarkers for gastric cancer detection. PLoS One 2012;7:e41629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang B, Zhang Q. The expression and clinical significance of circulating microRNA-21 in serum of five solid tumors. J Cancer Res Clin Oncol 2012;138:1659–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhou H, Guo JM, Lou YR, et al. Detection of circulating tumor cells in peripheral blood from patients with gastric cancer using microRNA as a marker. J Mol Med (Berl) 2010;88:709–17. [DOI] [PubMed] [Google Scholar]
- 76.Yuan R, Wang G, Xu Z, et al. Up-regulated Circulating miR-106a by DNA Methylation Promised a Potential Diagnostic and Prognostic Marker for Gastric Cancer. Anticancer Agents Med Chem 2016;16:1093–100. [DOI] [PubMed] [Google Scholar]
- 77.Tsujiura M, Ichikawa D, Komatsu S, et al. Circulating microRNAs in plasma of patients with gastric cancers. Br J Cancer 2010;102:1174–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cai H, Yuan Y, Hao YF, et al. Plasma microRNAs serve as novel potential biomarkers for early detection of gastric cancer. Med Oncol 2013;30:452. [DOI] [PubMed] [Google Scholar]
- 79.Wang H, Wang L, Wu Z, et al. Three dysregulated microRNAs in serum as novel biomarkers for gastric cancer screening. Med Oncol 2014;31:298. [DOI] [PubMed] [Google Scholar]
- 80.Zhou H, Xiao B, Zhou F, et al. MiR-421 is a functional marker of circulating tumor cells in gastric cancer patients. Biomarkers 2012;17:104–10. [DOI] [PubMed] [Google Scholar]
- 81.Wu J, Li G, Yao Y, et al. MicroRNA-421 is a new potential diagnosis biomarker with higher sensitivity and specificity than carcinoembryonic antigen and cancer antigen 125 in gastric cancer. Biomarkers 2015;20:58–63. [DOI] [PubMed] [Google Scholar]
- 82.Zhao G, Xu L, Hui L, et al. Level of circulated microRNA-421 in gastric carcinoma and related mechanisms. Int J Clin Exp Pathol 2015;8:14252–6. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video 1: In this upper endoscopy, a subtle erythematous lesion is seen at the incisura which biopsies demonstrated to be gastric adenocarcinoma. White light endoscopic examination of the gastric antrum demonstrate subtle mucosa nodularity suggestive of intestinal metaplasia, which on narrow-band imaging (0:13) can be more clearly defined. Following application of dilute acetic acid (0:30) the areas of intestinal metaplasia become clearly defined. Finally application of methylene blue (0:45) which is selectively absorbed by intestinal epithelium, and not normal gastric epithelium, clearly delineates the areas of intestinal metaplasia.