Abstract
Background
Small-cell lung cancer (SCLC) is an aggressive disease with high metastatic potential and poor prognosis. Due to its low prevalence, epidemiological and clinical information of SCLC patients retrieved from lung cancer registries is scarce.
Patients and methods
This was an observational multicenter study that enrolled patients with lung cancer and thoracic tumors, recruited from August 2016 to January 2020 at 50 Spanish hospitals. Demographic and clinical data, treatment patterns and survival of SCLC patients included in the Thoracic Tumor Registry (TTR) were analyzed.
Results
With a total of 956 cases, the age of 64.7 ± 9.1 years, 78.6% were men, 60.6% smokers, and ECOG PS 0, 1 or ≥ 2 in 23.1%, 53.0% and 23.8% of cases, respectively. Twenty percent of patients had brain metastases at the diagnosis. First-line chemotherapy (CT), mainly carboplatin or cisplatin plus etoposide was administered to >90% of patients. In total, 36.0% and 13.8% of patients received a second and third line of CT, respectively. Median overall survival was 9.5 months (95% CI 8.8–10.2 months), with an estimated rate of 70.3% (95% CI 67.2–73.4%), 38.9% (95% CI 35.4–42.4%), and 14.8% (95% CI 11.8–17.8%) at 6, 12 and 24 months respectively. Median progression-free survival was 6.3 months. Higher mortality and progression rates were significantly associated with male sex, older age, smoking habit, and ECOG PS 1–2. Long-term survival (> 2 years) was confirmed in 6.6% of patients, showing a positive correlation with better ECOG PS, poor smoking and absence of certain metastases at diagnosis.
Conclusion
This study provides an updated overview of the clinical situation and treatment landscape of ES-SCLC in Spain. Our results might assist oncologists to improve current clinical practice towards a better prognosis for these patients.
Introduction
Lung cancer remains a major public health issue worldwide, with more than 2 million new cases and 1.8 million deaths estimated in 2018 [1]. Although smoking is known to be the primary risk factor for lung cancer, other factors such as asbestos, radiation, radon gas and environmental pollution have been also identified [2,3]. While non-small cell lung cancer (NSCLC) represents the majority of diagnosed lung cancer cases (80%), small-cell lung cancer (SCLC) occurs in approximately 15% of patients [4]. Nonetheless, SCLC is the most aggressive subtype of lung cancer, with a 5-year survival rate of below 7% [5].
SCLC is characterized by a rapid doubling time, high growth fraction, early development of widespread metastases, and endocrine paraneoplastic syndromes [6]. Patients usually present shortly after developing symptoms, and metastatic disease appears in approximately 65% of cases [7]. Moreover, nearly 70% of patients are diagnosed with advanced, i.e. extensive stage (ES), disease [8]. Comorbidities are very common and constitute an increasing burden among SCLC patients, often impacting prognosis negatively [9]. Its prevalence seems to increase with age and the presence of multimorbidity has been associated with poorer outcomes [9]. Older age, lower body mass index, poor performance status, ES disease, and best supportive care have emerged as predictors of mortality among SCLC patients [10].
Current therapeutic options for SCLC are limited and there is an unmet need for novel effective treatments to improve survival. Given the aggressive nature of the disease, first-line (1L) platinum-based chemotherapy (CT) has been the mainstay for both limited stage (LS) and ES-SCLC; cisplatin and carboplatin are the most recommended platinum agents in combination with etoposide [6] Recently, the addition of immunotherapy to platinum-etoposide has led to a significant increase in overall survival, thereby constituting the new standard of care in ES-SCLC [6]. While thoracic radiation therapy (RT) along with CT is also recommended to improve local control and survival in LS-SCLC patients [11], consolidative thoracic RT may be considered in selected cases of ES-SCLC who achieved complete response after CT [12,13]. Although 1L CT frequently results in high initial response rates, prognosis remains poor and most ES-SCLC patients experience early disease progression or recurrence [14].
The representation of SCLC patients in lung cancer registries is scarce and ranges between 13.5% and 19% [10,15]. Hence, the main evidence on epidemiological and clinical data of SCLC is often retrieved from hospital- or population-based studies [16–20]. The Thoracic Tumor Registry (TTR) is an observational, multicenter study of lung cancer and other thoracic tumors that was created by the Spanish Lung Cancer Group (GECP) in 2016 with the aim of collecting and unifying population-based data at a national level [21]. In a recent retrospective analysis, epidemiological and clinical data of 6,600 patients with NSCLC were reported [22]. Herein we present a descriptive analysis of the ES-SCLC population included in the TTR up to January 2020.
Methods
Study design and sponsor
Observational, prospective, registry-based study that enrolled patients with lung cancer and other thoracic tumors from August 2016 to date. The study was conducted in accordance with the Declaration of Helsinki. Protocol approval was obtained from the institutional review board of Hospital Universitario Puerta de Hierro-Majadahonda (No. PI 148/15). The registry was approved by the Spanish Agency for Medicines and Medical Devices (AEMPS) in 2016, and is registered in the ClinicalTrials.gov database (NCT02941458) [21].
The TTR-2 study was sponsored by the GECP, an independent, multidisciplinary oncology group that coordinates more than 400 experts and 160 hospitals across the Spanish territory. The registry creation was proposed by the steering committee with the aim of promoting lung cancer research and incorporating treatment advances into clinical practice.
Eligibility
Patients with histologically confirmed lung cancer or other types of thoracic tumors (NSCLC, SCLC, mesothelioma, thymic carcinoma, carcinoid cancer) were eligible for inclusion, without sex or age restrictions. Patients receiving active treatment or palliative care were included. Exclusion criteria were diagnosis of other types of tumors and healthy volunteers. All patients provided signed informed consent before their data were included in the TTR.
Information retrieval
Data were collected by research teams from patient medical records using an electronic data capture system (EDC). Sociodemographic, epidemiological, clinical, molecular and treatment outcome variables were recorded in an electronic case report form (eCRF). The information was classified into the following categories: (I) patient personal history, which included performance status (PS), tobacco use, and comorbidities; (II) diagnosis, including histological subtype, TNM classification of the tumor and location of metastases; (III) molecular profiling of the tumor; (IV) treatment patterns (surgery, CT, RT); (V) response and survival, including response rates, overall survival and progression-free survival (PFS); and (VI) prognostic factors.
Statistical analysis
A descriptive statistical analysis was performed. Quantitative variables are presented as mean, standard deviation (SD), median, interquartile range (IQR), minimum, and maximum. Qualitative variables are described as frequencies in the entire population and percentages. Pearson’s chi-square tests were used to compare patient characteristics according to the drug used in first-line CT (carboplatin vs. cisplatin), using Fisher’s exact tests when possible. The OS curve and PFS curves were estimated using the Kaplan-Meier method, evaluating the effect of different characteristics on diagnosis by adjusting univariate Cox regression models. The SPSS IBM Statistics 22 program was used for analysis, setting a significance level of 5% in all bilateral contrast tests. Comparison of 2-year OS rates was performed using the Fixpoint test (package ComparisonSurv of R, method cloglog), as previously described [23]. The Bonferroni correction was applied for multiple comparisons.
Results
Patient characteristics
At data cut-off (23rd January 2020), 1,658 (12.9%) SCLC patients were registered in the TTR database, which included a total of 12,867 patients. Of these, 1,037 (62.6%) patients had extensive-stage SCLC, 606 (36.6%) had limited-stage SCLC and 15 (0.9%) had unknown-stage SCLC. Based on data quality and availability, 956 patients with ES-SCLC were finally selected to perform this analysis and their characteristics are shown in Table 1.
Table 1. Patient characteristics at baseline (n = 956).
| Characteristic | n | % |
|---|---|---|
| Sex | ||
| Male | 751 | 78.6 |
| Female | 205 | 21.4 |
| Age at diagnosis | ||
| Mean (SD), years | 64.7 (9.1) | |
| Median [min-max], years | 65 [37–88] | |
| Distribution | ||
| <55 years | 117 | 12.2 |
| 55–64 years | 355 | 37.1 |
| 65–74 years | 335 | 35 |
| ≥75 years | 149 | 15.6 |
| Race | ||
| Caucasian | 929 | 97.2 |
| Other | 27 | 2.8 |
| Patient cancer history* | 110 | 11.5 |
| Head and neck | 21 | 2.2 |
| Bladder/urinary tract | 21 | 2.2 |
| Prostate | 14 | 1.5 |
| Non-melanoma skin | 10 | 1.0 |
| Smoking habit | ||
| Never smoker | 14 | 1.5 |
| Former smoker (> 1-year) | 357 | 37.3 |
| Smoker | 579 | 60.6 |
| Unknown | 6 | 0.6 |
| ECOG at diagnosis | ||
| 0 | 221 | 23.1 |
| 1 | 507 | 53.0 |
| ≥2 | 228 | 23.8 |
| Symptoms at diagnosis | ||
| Asymptomatic | 54 | 5.6 |
| Symptomatic | 882 | 92.3 |
| Unknown | 23 | 2.4 |
| Metastasis at diagnosis | 924 | 96.7 |
| Liver | 422 | 44.1 |
| Bone | 333 | 34.8 |
| Thoracic lymphadenopathy | 299 | 31.3 |
| Lung | 237 | 24.8 |
| Extrathoracic lymphadenopathy | 206 | 21.5 |
| Adrenal | 203 | 21.2 |
| CNS | 189 | 19.8 |
| Comorbidities* | 826 | 86.4 |
| Hypertension | 460 | 48.1 |
| Dyslipidemia | 330 | 34.5 |
| COPD | 248 | 25.9 |
| Diabetes mellitus | 248 | 25.9 |
| Heart disease | 180 | 18.8 |
CNS, central nervous system; COPD, chronic obstructive pulmonary disease; ECOG, Eastern Cooperative Oncology Group.
*The most frequent previous tumor locations and current comorbidities are shown.
Most patients (78.6%) were men, 60.6% smokers, with a mean age of 64.7 ± 9.1 years and ECOG PS 0, 1 or ≥ 2 in 23.1%, 53.0% and 23.8% of cases, respectively. According to the TNM classification (S1 Table) and the AJCC equivalence (8th edition) [24], most patients were diagnosed with stage IVA/B disease. Up to 88.5% did not have any previous cancer history, but the majority of patients presented symptoms at diagnosis, such as cough (39.4%), pain (36.7%), dyspnea (35.6%), and weight loss (28.6%). Mean time between developing symptoms and diagnosis was 1.09 ± 1.98 months. Metastases were observed in almost the entire population (96.7%), 19.8% of which corresponded to brain metastases. Comorbidities appeared in 86.4% of patients, the most common being hypertension, dyslipidemia, chronic obstructive pulmonary disease, and diabetes mellitus. Although molecular profiling of tumors was performed in a very low number of patients, positive results were observed for some biomarkers such as Ki67, TTF1, synaptophysin, enolase, and CD-56 (S2 Table).
Treatments and response
Mean time between diagnosis and first treatment was 0.68 ± 1.63 months. CT alone or in combination with RT was the treatment of choice in 415 (43.4%) and 449 (47.0%) patients, respectively; RT was mainly provided with palliative (30.9%) and cranial prophylactic (9.4%) intent (Table 2). Patients who were treated with CT and RT, received it sequentially and not concurrently treatment. All of them were cases selected by their oncologist after showing a good response to CT treatment.
Table 2. Treatment characteristics.
| Treatment | n | % |
|---|---|---|
| Single treatments | ||
| Radiotherapy (RT) | 18 | 1.9 |
| Chemotherapy (CT) | 415 | 43.4 |
| Surgery | 1 | 0.1 |
| Combined treatments | ||
| RT + CT | 449 | 47 |
| Surgery + RT | 2 | 0.2 |
| Surgery + CT | 5 | 0.5 |
| Surgery + RT + CT | 10 | 0.1 |
| Surgical intent | ||
| Diagnostic | 7 | 0.7 |
| Curative | 6 | 0.6 |
| Palliative | 5 | 0.5 |
| Radiotherapy intent | ||
| Radical | 67 | 7 |
| Adjuvant | 13 | 1.4 |
| Palliative | 295 | 30.9 |
| Prophylactic | 90 | 9.4 |
| Unknown | 14 | 1.5 |
| Chemotherapy | ||
| First line | 879 | 91.9 |
| Monotherapy | 19 | 2.2 |
| Combination of 2 drugs | 835 | 95 |
| Combination of 3 drugs | 25 | 2.8 |
| Second line | 344 | 36 |
| Third line | 132 | 13.8 |
In contrast, surgery was performed in less than 1% of patients. First-line (1L) CT was used in almost the entire population (91.9%), mostly using a combination of 2 drugs: carboplatin + etoposide (Car + E) (61.8%) or cisplatin + etoposide (Cis + E) (31.7%). In total, 36.0% and 13.8% of patients received a second (2L) and third line (3L) of CT, respectively. Consolidative RT was given to 262/879 (29.8%) patients treated with 1L CT; holocranial (58.8%) and thoracic (29.8%) were the most common irradiated areas.
Of the 879 patients who received 1L CT, 416 (52.7%) received 4 cycles or fewer, while 373 (47.3%) received 5 or more cycles. The mean number of cycles of CT was slightly higher in 1L (4.3) compared to 2L (3.7) and 3L (3.4) (Table 3).
Table 3. Chemotherapy treatment and response.
| First line (n = 879) | Second line (n = 344) | Third line (n = 132) | |
|---|---|---|---|
| Number of cycles | |||
| Mean (sd) | 4.3 (1.9) | 3.7 (3.3) | 3.4 (2.7) |
| Median [min-max] | 4 (1–12) | 3 (1–31) | 3 [1–16] |
| Duration of treatment (months) | |||
| Mean (sd) | 2.86 (1.78) | 2.39 (2.67) | 1.96 (2.11) |
| Median [min-max] | 3.0 [0–16.1] | 1.8 [0–18.9] | 1.4 [0–15.2] |
| Best response by RECIST 1.1 criteria, n (%) | |||
| CR | 24 (2.7%) | 9 (2.6%) | 1 (0.8%) |
| PR | 449 (51.5%) | 64 (18.7%) | 17 (12.9%) |
| SD | 72 (8.2%) | 55 (16.0%) | 22 (16.7%) |
| PD | 108 (12.3%) | 128 (37.3%) | 49 (37.1%) |
| NE | 87 (9.9%) | 48 (14.0%) | 24 (18.2%) |
| ND | 50 (5.7%) | 18 (5.2%) | 4 (3.0%) |
CR, complete response; ND, not determined; NE, not estimated; PD, progressive disease; PR, partial response; sd, standard deviation; SD, stable disease.
Likewise, duration of treatment was reduced in subsequent treatment lines from 2.86 months in 1L to 2.39 in 2L and 1.96 in 3L. The best response rates, mainly partial response (PR), were observed after 1L CT (51.5%), while rates of stable disease (SD) showed a 2-fold increase with 2L and 3L compared to 1L. Conversely, progressive disease (PD) was substantially higher in subsequent lines, being observed in more than one-third of patients.
Overall survival and progression-free survival
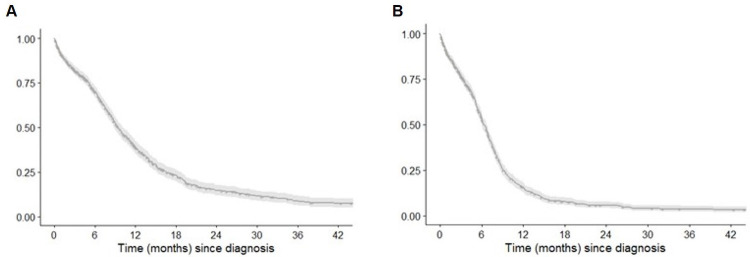
Median OS in the entire population was 9.5 months (95% CI 8.8–10.2 months), with an estimated OS rate of 70.3% (95% CI 67.2–73.4%) at 6 months, 38.9% (95% CI 35.4–42.4%) at 12 months, 26.2% (95% CI 22.8–29.6%) at 18 months, and 14.8% (95% CI 11.8–17.8%) at 24 months after diagnosis (Fig 1A). Greater survival was observed based on the number of CT lines received (p < 0.001); however, a selection bias should be noted as patients who received 3 or more lines survived longer. Higher mortality rates were significantly associated with male sex, older age, smoking habit, and ECOG PS 1–2 (S3 Table).
Fig 1. Overall survival (A) and progression-free survival (B) in the study population.
Overall, 649 (67.9%) patients progressed during follow-up, 105 of whom remained alive at the end of the study. Progressions were mostly distant and/or local, often affecting lung, thoracic lymph nodes, and liver. Median PFS was 6.3 months (95% CI 6.0–6.7 months), with an estimated PFS rate of 53.8% (95% CI 50.4–57.2%) at 6 months, 15.6% (95% CI 13.0–18.3%) at 12 months, 7.9% (95% CI 5.9–9.9%) at 18 months, and 5.8% (95% CI 4.0–7.6%) at 24 months (Fig 1B). As described for OS, sex, age, smoking and ECOG PS at diagnosis were factors significantly associated with poorer PFS outcomes (S4 Table).
Patients treated with Car/Cis + E in 1L
In total, 543 patients were treated with Car + E and 279 with Cis + E in 1L. S5 Table shows the distribution of patients according to their characteristics at diagnosis. A significantly higher proportion of women, younger age, better ECOG PS, and lower percentage of liver metastasis were observed among patients who received cisplatin. A similar distribution of brain metastases was observed between subgroups.
The number of cycles and duration of treatment did not differ among patients treated with Car + E or Cis + E (S6 Table). However, the latter showed a higher rate of response (complete or partial) and SD, with a lower proportion of patients who progressed. Overall, 703/822 (85.5%) patients finished 1L treatment, of whom 314 (44.7%) started a subsequent 2L: 193 (41.3%) with Car + E and 121 (51.3%) with Cis + E. Mean time from end of 1L to start of 2L CT ranged from 4.42 to 4.95 months.
Survival in patients treated with Car/Cis + E
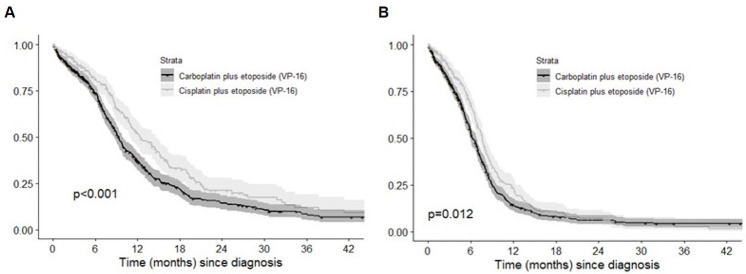
Of the 543 patients with Car + E, 366 (67.4%) died during follow-up, with a median OS of 9.3 months (95% CI 8.7–9.9 months) since diagnosis. A significantly lower rate of mortality was observed among patients treated with Cis + E (162/279, 58.1%), with a higher median OS at 12.5 months (95% CI 10.5–14.6) (p < 0.001) (Fig 2A). Likewise, median PFS was significantly higher among patients who received Cis + E (7.6 months [95% CI 7.1–8.2 months]) than those treated with Car + E (6.2 months [95% CI 5.7–6.7 months]) (p = 0.012) (Fig 2B). Differences among subgroups were also significant when OS was estimated from the start of 1L treatment: 11.5 (9.8–13.2) months in Cis + E and 8.8 (8.3–9.4) months in Car + E patients (p < 0.001). In total, 109/703 (15.5%) patients remained alive without a 2L treatment. Median survival of patients treated with 1L Car + E or Cis + E was 3.1 months (95% CI 2.7–3.5 months) and 3.9 months (95% CI 3.4–4.3 months), respectively.
Fig 2. Overall survival (A) and progression-free survival (B) in patients treated with first-line carboplatin/cisplatin + etoposide VP16.
Long-term survival
In total, 63/956 (6.6%) patients (44 men and 19 women) were alive after a 2-year follow-up. Most (95.2%) were smokers or former smokers and presented ECOG PS 0 (31.7%) or 1 (58.7%) and metastasis at diagnosis, liver (30.2%), bone (23.8%) and lung (22.2%) being the most frequent locations (S7 Table). A higher proportion of patients received Car + E than Cis + E (63.5% and 31.7%, respectively) as 1L treatment. Multivariate analysis of 2-year OS rates considering patient characteristics at diagnosis is shown in Table 4.
Table 4. Multivariate analysis of 2-year OS rates according to patient characteristics.
| 2-year OS rate (%) | 95% CI | p value | |
|---|---|---|---|
| Sex | 0.077 | ||
| Male | 13.4 | 10.5–17.1 | |
| Female | 20.3 | 14.1–29.0 | |
| Age | 0.117 | ||
| <65 years | 17.2 | 12.9–21.9 | |
| ≥65 years | 12.4 | 8.8–16.6 | |
| ECOG | 0.001 | ||
| 0 | 23 | 15.9–30.9 | |
| 1 | 14.9 | 11.1–19.2 | |
| ≥2 | 6.7 | 3.3–11.8 | |
| Smoking habit | 20.7 | 15.6–26.3 | 0.004 |
| Never/former smoker | 11.5 | 8.3–15.2 | |
| Smoker | |||
| Metastasis | |||
| Liver | 9.2 | 5.9–13.3 | 0.001 |
| Bone | 9.7 | 6.1–14.4 | 0.012 |
| Thoracic adenopathy | 9.6 | 5.5–15.1 | 0.031 |
| Lung | 14.3 | 8.8–21.0 | 0.85 |
| Extrathoracic adenopathy | 13.3 | 7.9–20.0 | 0.594 |
| Adrenal | 10.6 | 6.1–16.5 | 0.117 |
| CNS | 11.9 | 6.4–19.3 | 0.367 |
| Pleural effusion | 11.8 | 6.2–19.3 | 0.36 |
| Treatment | 0.078 | ||
| Carboplatin + Etoposide VP16 | 14.3 | 10.8–18.4 | |
| Cisplatin + Etoposide VP16 | 21 | 14.8–28.0 |
CI, confidence interval; CNS, central nervous system; ECOG, Eastern Cooperative Oncology Group; OS, overall survival.
A significantly greater long-term survival rate was associated with better ECOG PS, poor smoking habit, and the absence of liver, bone, and thoracic lymph node metastasis. Conversely, factors such as sex, age, other metastatic locations, or treatment failed to show statistical differences among this population.
Discussion
Following the recent publication of the TTR NSCLC population report [22], we analyzed the epidemiological and clinical data of 956 patients diagnosed with ES-SCLC who were recruited at 50 Spanish hospitals up to January 2020. To our knowledge, this is the first nationwide study providing an accurate description of the current situation, treatment patterns, and survival outcomes of SCLC patients. As reported in other registries [10,15], we identified more than 60% of patients with ES-SCLC (stage IV) among the Spanish SCLC population registered in the TTR. Most of these patients presented symptoms, metastases, and comorbidities at diagnosis, and had no previous history of cancer. In accordance with clinical guidelines for SCLC management [6,25], 1L CT alone or in combination with RT was administered to almost the entire population. With high progression and mortality rates, poor survival outcomes and associated negative prognostic factors were in line with reported evidence on this type of patients [6,10,14,18–20].
Cis + E has remained the standard of care for ES-SCLC patients for decades, while Car + E is alternatively advised in case of contraindications for Cis (level of evidence I, degree of recommendation A) [6]. Although the optimal duration of CT in these patients is not well defined, a maximum of 4–6 cycles is usually recommended [6]. Among our study population, a higher number of patients were treated with Car + E than Cis + E, and approximately half received 4 or fewer cycles of 1L CT. A systematic review of four randomized controlled trials (RCTs) compared Cis with Car + E in 1L treatment of SCLC, reporting no statistical differences in median OS (9.6 vs 9.4 months), median PFS (5.5 vs. 5.3 months), and overall response (67% vs. 66%) [26]. However, the safety profile differed between the treatments, with a higher hematologic toxicity being associated with Car and higher non-hematologic toxicity related to Cis [26]. Recently, these findings were corroborated in a meta-analysis of 12 RCTs, suggesting that treatment choice should be based on the toxicity profile of Cis/Car alongside patient’s comorbidities [27]. Interestingly, our study revealed that patients treated with Cis + E had higher response rates, fewer progressions, and significantly greater median OS and PFS compared to those who received Car + E. Nevertheless, it should be noted that these differences might be related more with the more favorable profile of ES-SCLC patients treated with Cis + E than with the treatment itself. Several factors have been identified as negative predictors of mortality in SCLC patients. Older age, male sex, poor ECOG PS, larger tumor size, multiple metastatic sites and increased creatinine levels are associated with poor prognosis of ES-SCLC disease [10,18,20]. Among the TTR SCLC population, patients who received Cis + E included a higher proportion of women, of a younger age, and with better ECOG PS and fewer liver metastasis than those treated with Car + E.
RT for thoracic lesions and metastatic sites, except for brain metastases, has been associated with improved OS and cancer-specific survival in patients with metastatic ES-SCLC [28]. As such, the Spanish Society of Medical Oncology (SEOM) recommends that consolidative thoracic RT should be considered in selected patients with ES-SLCL who have completed CT and achieved complete or near complete response, especially in patients with good extrathoracic response (I, B) [6]. In this study, RT was provided with palliative intent in nearly one-third of the population and consolidative RT, mostly holocranial, was given to nearly 30% of those treated with 1L CT. With a reported 20% incidence among this study population, it is estimated that approximately half of SCLC patients will develop brain metastases within 2 years after diagnosis [29]. Given that prophylactic cranial irradiation (PCI) has been shown to decrease the development of brain metastases by 25% [30], this strategy is advisable in ES-SCLC patients who respond to primary CT [6,29]. The roll of PCI in ES-SCLC was evaluated in a Japanese clinical trial with 224 patients (randomization 1:1). Median OS was 11.6 months (95% CI 9.5–13.3) in the first group versus 13.7 months in the control arm (10.2–16.4), HR 1, 27, 95% CI 0.96–1.68; p = 0.094. In our study, a small percentage of patients received PCI. Accordingly, the SEOM guidelines established that PCI should be evaluated in patients with good PS who achieve a response (I, B) [6]. Although more than 50% of SCLC patients showed CR/PR in our study, prophylactic RT was given to less than 10% of the population.
In line with previous studies, particularly those which included ES-SCLC patients [6,15,18], low survival and high progression rates were found among the TTR SCLC population. Median OS and PFS barely reached 9.5 months and 6.3 months, respectively, after 1L CT; male sex, older age, smoking habit and ECOG PS 1–2 were significantly associated with worse prognosis. Noticeably, evidence on survival trends shows an improvement in the prognosis of SCLC patients in the last decades. A previous analysis demonstrated significantly improved overall and stage-specific median survival times and 5-year survival rates of 1,032 SCLC patients treated at the Moffitt Cancer Center from 1986 to 2008 [19]. While the 5-year OS rate significantly increased from 8.3% to 11.0%, the median OS increased from 11.3 months to 15.2 months [19]. More recently, a study among the Japanese population reported that 5-year relative survival of 10,911 LS- and ES-SCLC patients significantly increased from 1999–2006 compared to 1993–1998 [16]. To date, our results indicate an estimated 2-year OS rate of 14.8% and PFS rate of 5.8% among the TTR SCLC Spanish population monitored since 2016. Furthermore, long-term survival (> 2 years), which was associated with better ECOG PS, poor smoking habit and absence of certain metastases at diagnosis, has been confirmed in 6.6% of patients. Close monitoring of these patients over a longer follow-up will help discern survival trends and potential improvements due to the implementation of novel therapies in current clinical practice.
Based on the encouraging results from phase III trials, the combination of CT and immunotherapy has been recently established as the first-line treatment of adult patients with ES-SCLC (I, A) [6]. In the IMpower133 trial, atezolizumab in combination with Car + E showed a significant benefit in median OS (12.3 vs. 10.3 months; HR 0.70) and OS rate at 18 months (34% vs. 21%) compared to Car + E [31]. This regimen, therefore, was first approved by the Food and Drug Administration (FDA), and later by the European Medicines Agency (EMA) in 2019. The updated data from this study were presented at ESMO 2020. With a follow-up of 22.9 months, OS at 24 months was 22% vs 16.8% in favor of the experimental arm (atezolizumab plus CT) [32]. On the other hand, the combination of durvalumab with Cis/Car + E is also a recommended treatment option for ES-SCLC (I, A) [6], as it demonstrated a significant improvement in median OS (13.0 vs. 10.3 months; HR 0.73) compared to CT in the CASPIAN trial [33]. In light of these findings, real-world evidence studies are warranted to confirm and further explore clinical benefits of checkpoint inhibition in this setting.
The main limitations of this study stem from its observational, retrospective design. Despite the potential bias in the recruited population among the participant centers, the sample size was large enough to provide an objective nationwide epidemiological overview of ES-SCLC status. Moreover, all patients had equal opportunities for diagnosis and treatment, as established by the universal coverage of the Spanish National Health System, and they were enrolled in the study over a short time period, thereby enabling a more reliable comparison of therapies and survival.
Conclusions
This study provides an accurate overview of the current clinical situation and treatment landscape of ES-SCLC in Spain. With a high proportion of patients diagnosed with metastatic disease and a very poor prognosis, epidemiological data and survival outcomes of this population are in line with those reported by previous studies across different countries. Since these results support current evidence on the aggressiveness of ES-SCLC and the need for more effective therapies, the development of further studies is warranted. Continuous monitoring of TTR data will help evaluate the impact of current and novel treatments used in clinical practice, with the aim of eventually improving the prognosis and survival of SCLC patients.
Supporting information
(DOCX)
ALK, anaplastic lymphoma kinase; BRAF, B-RAF proto-oncogene, serine/threonine kinase oncogene; EGFR, epidermal growth factor receptor; FISH, fluorescence in situ hybridization; FGFR1, fibroblast growth factor receptor type 1; HER2, human epidermal growth factor receptor type 2; IHC, immunohistochemistry; KRAS, Kirsten rat sarcoma viral oncogene homolog; MET, tyrosine-protein kinase MET/hepatocyte growth factor receptor; PD-L1, programmed death-ligand 1; RET, proto-oncogene tyrosine-protein kinase receptor; RNA, ribonucleic acid; ROS1, ROS proto-oncogene 1 receptor tyrosine kinase; TTF1, thyroid transcription factor 1.
(DOCX)
CNS, central nervous system; ECOG, Eastern Cooperative Oncology Group; HR, hazard ratio; CI, confidence interval; SD, standard deviation.
(DOCX)
CNS, central nervous system; ECOG, Eastern Cooperative Oncology Group; HR, hazard ratio; CI, confidence interval; SD, standard deviation.
(DOCX)
CNS, central nervous system; ECOG, Eastern Cooperative Oncology Group.
(DOCX)
CR, complete response; PR, partial response; sd, standard deviation; SD, stable disease; PD, progressive disease; NE, not estimated; ND, not determined.
(DOCX)
CNS, central nervous system; ECOG, Eastern Cooperative Oncology Group.
(DOCX)
Acknowledgments
The authors received medical writing support in the preparation of this manuscript from Celia Miguel Blanco (Medical Statistics Consulting, S.L., Valencia, Spain).
Data Availability
All relevant data are within the manuscript and its Supporting information files.
Funding Statement
The TTR registry was supported by Fundación GECP, AstraZeneca, Novartis, Roche, the European Union’s Horizon 2020 research and innovation program (CLARIFY 875160). The funders were not involved in writing the article or the decision to submit the article for publication.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. 10.3322/caac.21492 . [DOI] [PubMed] [Google Scholar]
- 2.Wang Y, Zou S, Zhao Z, Liu P, Ke C, Xu S. New insights into small-cell lung cancer development and therapy. Cell Biol Int. 2020;44(8):1564–76. 10.1002/cbin.11359 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fitzmaurice C, Allen C, Barber RM, Barregard L, Bhutta ZA, Brenner H, et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-years for 32 Cancer Groups, 1990 to 2015: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2017;3(4):524–48. 10.1001/jamaoncol.2016.5688 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Herbst RS, Heymach JV, Lippman SM. Lung Cancer. N Engl J Med. 2008;359(13):1367–80. 10.1056/NEJMra0802714 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karachaliou N, Pilotto S, Lazzari C, Bria E, de Marinis F, Rosell R. Cellular and molecular biology of small cell lung cancer: an overview. Transl Lung Cancer Res. 2016;5(1):2–15. 10.3978/j.issn.2218-6751.2016.01.02 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dómine M, Moran T, Isla D, Martí JL, Sullivan I, Provencio M, et al. SEOM clinical guidelines for the treatment of small-cell lung cancer (SCLC) (2019). Clin Transl Oncol. 2020;22(2):245–55. 10.1007/s12094-020-02295-w [DOI] [PubMed] [Google Scholar]
- 7.Bernhardt EB, Jalal SI. Small Cell Lung Cancer. Cancer Treat Res. 2016;170:301–22. 10.1007/978-3-319-40389-2_14 . [DOI] [PubMed] [Google Scholar]
- 8.Byers LA, Rudin CM. Small cell lung cancer: Where do we go from here? Cancer. 2015;121(5):664–72. 10.1002/cncr.29098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aarts MJ, Aerts JG, van den Borne BE, Biesma B, Lemmens VE, Kloover JS. Comorbidity in Patients With Small-Cell Lung Cancer: Trends and Prognostic Impact. Clin Lung Cancer. 2015;16(4):282–91. 10.1016/j.cllc.2014.12.003 . [DOI] [PubMed] [Google Scholar]
- 10.Choi CM, Kim HC, Jung CY, Cho DG, Jeon JH, Lee JE, et al. Report of the Korean Association of Lung Cancer Registry (KALC-R), 2014. Cancer Res Treat. 2019;51(4):1400–10. 10.4143/crt.2018.704 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun A, Durocher-Allen LD, Ellis PM, Ung YC, Goffin JR, Ramchandar K, et al. Initial management of small-cell lung cancer (limited- and extensive-stage) and the role of thoracic radiotherapy and first-line chemotherapy: a systematic review. Curr Oncol. 2019;26(3):e372–e84. 10.3747/co.26.4481 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Palma DA, Warner A, Louie AV, Senan S, Slotman B, Rodrigues GB. Thoracic Radiotherapy for Extensive Stage Small-Cell Lung Cancer: A Meta-Analysis. Clin Lung Cancer. 2016;17(4):239–44. 10.1016/j.cllc.2015.09.007 . [DOI] [PubMed] [Google Scholar]
- 13.Slotman BJ, van Tinteren H, Praag JO, Knegjens JL, El Sharouni SY, Hatton M, et al. Use of thoracic radiotherapy for extensive stage small-cell lung cancer: a phase 3 randomised controlled trial. Lancet. 2015;385(9962):36–42. 10.1016/S0140-6736(14)61085-0 [DOI] [PubMed] [Google Scholar]
- 14.Reck M, Luft A, Szczesna A, Havel L, Kim SW, Akerley W, et al. Phase III Randomized Trial of Ipilimumab Plus Etoposide and Platinum Versus Placebo Plus Etoposide and Platinum in Extensive-Stage Small-Cell Lung Cancer. J Clin Oncol. 2016;34(31):3740–8. 10.1200/JCO.2016.67.6601 . [DOI] [PubMed] [Google Scholar]
- 15.Sekine I, Shintani Y, Shukuya T, Takayama K, Inoue A, Okamoto I, et al. A Japanese lung cancer registry study on demographics and treatment modalities in medically treated patients. Cancer Sci. 2020;111(5):1685–91. 10.1111/cas.14368 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oze I, Ito H, Nishino Y, Hattori M, Nakayama T, Miyashiro I, et al. Trends in Small-Cell Lung Cancer Survival in 1993–2006 Based on Population-Based Cancer Registry Data in Japan. J Epidemiol. 2019;29(9):347–53. 10.2188/jea.JE20180112 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kourlaba G, Gkiozos I, Kokkotou E, Stefanou G, Papaspiliou A, Syrigos K. Lung cancer patients’ journey from first symptom to treatment: Results from a Greek registry. Cancer Epidemiol. 2019;60:193–200. 10.1016/j.canep.2019.04.014 . [DOI] [PubMed] [Google Scholar]
- 18.Fukui T, Itabashi M, Ishihara M, Hiyoshi Y, Kasajima M, Igawa S, et al. Prognostic factors affecting the risk of thoracic progression in extensive-stage small cell lung cancer. BMC Cancer. 2016;16:197. 10.1186/s12885-016-2222-4 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schabath MB, Nguyen A, Wilson P, Sommerer KR, Thompson ZJ, Chiappori AA. Temporal trends from 1986 to 2008 in overall survival of small cell lung cancer patients. Lung Cancer. 2014;86(1):14–21. 10.1016/j.lungcan.2014.07.014 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Foster NR, Mandrekar SJ, Schild SE, Nelson GD, Rowland KM Jr., Deming RL, et al. Prognostic factors differ by tumor stage for small cell lung cancer: a pooled analysis of North Central Cancer Treatment Group trials. Cancer. 2009;115(12):2721–31. 10.1002/cncr.24314 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spanish Lung Cancer Group (GECP). Thoracic Tumours Registry (RTT) (NCT02941458) at ClinicalTrials.gov; 2016 [cited August 2020]. https://clinicaltrials.gov/ct2/show/NCT02941458.
- 22.Provencio M, Carcereny E, Rodríguez-Abreu D, López-Castro R, Guirado M, Camps C, et al. Lung cancer in Spain: information from the Thoracic Tumors Registry (TTR study). Transl Lung Cancer Res. 2019;8(4):461–75. 10.21037/tlcr.2019.08.05 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klein JP, Logan B, Harhoff M, Andersen PK. Analyzing survival curves at a fixed point in time. Stat Med. 2007;26(24):4505–19. 10.1002/sim.2864 . [DOI] [PubMed] [Google Scholar]
- 24.Amin MB, Edge S, Greene F, Byrd DR, Brookland RK, Washington MK, et al. American Joint Committee on Cancer (AJCC) cancer staging manual. 8th ed. New York: Springer; 2017. 1024 p. [Google Scholar]
- 25.Kalemkerian GP, Loo BW, Akerley W, Attia A, Bassetti M, Boumber Y, et al. NCCN Guidelines Insights: Small Cell Lung Cancer, Version 2.2018. J Natl Compr Canc Netw. 2018;16(10):1171–82. 10.6004/jnccn.2018.0079 . [DOI] [PubMed] [Google Scholar]
- 26.Rossi A, Di Maio M, Chiodini P, Rudd RM, Okamoto H, Skarlos DV, et al. Carboplatin- or cisplatin-based chemotherapy in first-line treatment of small-cell lung cancer: the COCIS meta-analysis of individual patient data. J Clin Oncol. 2012;30(14):1692–8. 10.1200/JCO.2011.40.4905 . [DOI] [PubMed] [Google Scholar]
- 27.Griesinger F, Korol EE, Kayaniyil S, Varol N, Ebner T, Goring SM. Efficacy and safety of first-line carboplatin-versus cisplatin-based chemotherapy for non-small cell lung cancer: A meta-analysis. Lung Cancer. 2019;135:196–204. 10.1016/j.lungcan.2019.07.010 . [DOI] [PubMed] [Google Scholar]
- 28.Zhang R, Li P, Li Q, Qiao Y, Xu T, Ruan P, et al. Radiotherapy improves the survival of patients with extensive-disease small-cell lung cancer: a propensity score matched analysis of Surveillance, Epidemiology, and End Results database. Cancer Manag Res. 2018;10:6525–35. 10.2147/CMAR.S174801 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Manapov F, Käsmann L, Roengvoraphoj O, Dantes M, Schmidt-Hegemann NS, Belka C, et al. Prophylactic cranial irradiation in small-cell lung cancer: update on patient selection, efficacy and outcomes. Lung Cancer (Auckl). 2018;9:49–55. 10.2147/LCTT.S137577 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Slotman B, Faivre-Finn C, Kramer G, Rankin E, Snee M, Hatton M, et al. Prophylactic Cranial Irradiation in Extensive Small-Cell Lung Cancer. N Engl J Med. 2007;357(7):664–72. 10.1056/NEJMoa071780 . [DOI] [PubMed] [Google Scholar]
- 31.Horn L, Mansfield AS, Szczęsna A, Havel L, Krzakowski M, Hochmair MJ, et al. First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. N Engl J Med. 2018;379(23):2220–9. 10.1056/NEJMoa1809064 . [DOI] [PubMed] [Google Scholar]
- 32.Liu SV, Horn L, Mok T, Mansfield A, De Boer R, Losonczy G, et al. 1781MO IMpower133: Characterisation of long-term survivors treated first-line with chemotherapy ± atezolizumab in extensive-stage small cell lung cancer. Ann Oncol. 2020;31:S1032–S3. 10.1016/j.annonc.2020.08.1543 [DOI] [Google Scholar]
- 33.Paz-Ares L, Dvorkin M, Chen Y, Reinmuth N, Hotta K, Trukhin D, et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet. 2019;394(10212):1929–39. 10.1016/S0140-6736(19)32222-6 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
ALK, anaplastic lymphoma kinase; BRAF, B-RAF proto-oncogene, serine/threonine kinase oncogene; EGFR, epidermal growth factor receptor; FISH, fluorescence in situ hybridization; FGFR1, fibroblast growth factor receptor type 1; HER2, human epidermal growth factor receptor type 2; IHC, immunohistochemistry; KRAS, Kirsten rat sarcoma viral oncogene homolog; MET, tyrosine-protein kinase MET/hepatocyte growth factor receptor; PD-L1, programmed death-ligand 1; RET, proto-oncogene tyrosine-protein kinase receptor; RNA, ribonucleic acid; ROS1, ROS proto-oncogene 1 receptor tyrosine kinase; TTF1, thyroid transcription factor 1.
(DOCX)
CNS, central nervous system; ECOG, Eastern Cooperative Oncology Group; HR, hazard ratio; CI, confidence interval; SD, standard deviation.
(DOCX)
CNS, central nervous system; ECOG, Eastern Cooperative Oncology Group; HR, hazard ratio; CI, confidence interval; SD, standard deviation.
(DOCX)
CNS, central nervous system; ECOG, Eastern Cooperative Oncology Group.
(DOCX)
CR, complete response; PR, partial response; sd, standard deviation; SD, stable disease; PD, progressive disease; NE, not estimated; ND, not determined.
(DOCX)
CNS, central nervous system; ECOG, Eastern Cooperative Oncology Group.
(DOCX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting information files.