Abstract
Infective endocarditis (IE) is associated with relatively high morbidity and mortality and several risk factors have been identified in the past. Several predisposing factors for IE have been recognized in the literature, depending on the type of bacteria. Coronavirus disease 2019 (COVID-19) infection causes coagulopathy-associated complications and damage to many organ systems due to the inflammatory response induced by this viral infection. COVID-19 emerged only about a year ago and there are many unknown post-COVID-19 complications at this time. Here, we present the case of Streptococcus mitis IE in a patient with no prior predisposing factors other than diagnosis with COVID-19 a month ago.
Keywords: infective endocarditis, hypercoagulopathy, cytokine storms, sars-cov-2 infection, covid-19, covid-19 endocarditis, covid-19 coagulopathy side effects
Introduction
Infection of the endocardial surface of the native valve, prosthetic heart valve, or an implanted cardiac device is known as infective endocarditis (IE). IE is a disease with relatively high morbidity and mortality [1-6]. Risk factors for IE usually involve two elements, the first is a transient bacteremia that could originate from dental infections, surgical procedures in the oral cavity, indwelling intravenous catheters, intravenous drug abuse, or infections of the skin, lungs, intestine, and urinary tract; the second is an abnormal valve either secondary to a congenital heart disease, degenerative disease, or prior surgical intervention. Commonly reported organisms causing IE include Staphylococci, Streptococci, Enterococci, and HACEK (Haemophilus, Actinobacillus, Cardiobacterium, Eikenella, and Kingella). Staphylococcus aureus has surpassed viridans streptococci as the most common cause of IE in recent years [3-6]. Mitral and aortic valves are the most commonly infected valves [3-6]. We present a unique case of IE caused by Streptococcus mitis on a structurally normal native mitral valve, where the only risk factor the patient had was a recent coronavirus disease 2019 (COVID-19) infection.
Case presentation
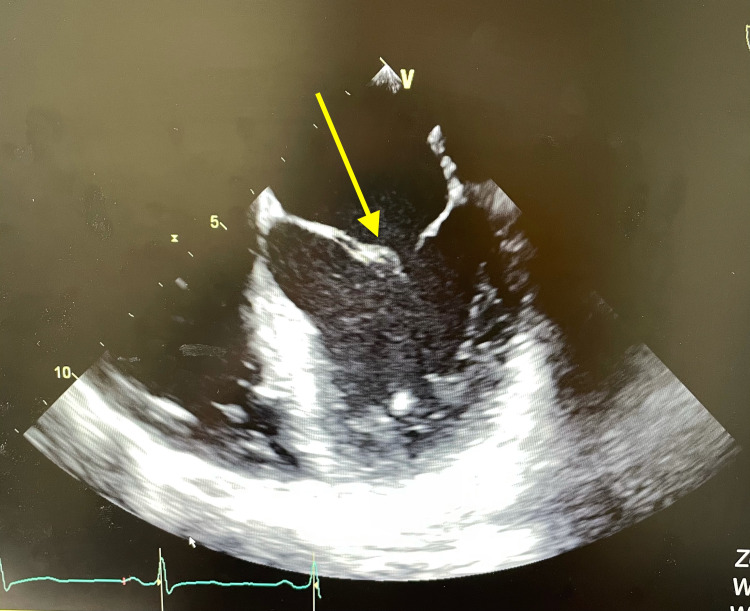
A 38-year-old male with no known past medical history (PMH) presented with fevers/chills, night sweats, and periumbilical, non-radiating, and dull pain for about a week associated with anorexia and fatigue. He reported 20-pound weight loss in the prior month and had tested positive for COVID-19 a month earlier. According to the patient, he was hospitalized for four to five days with hypoxic episodes, requiring supplemental oxygen and received dexamethasone as well as anticoagulation. He did not have any risk factors for IE such as intravenous drug use, congenital heart disease, prosthetic heart valves, prior IE, or any recent dental procedure. On physical examination, he did not have any findings of heart murmurs and immunological/vascular skin findings. A complete septic workup was performed and demonstrated elevated erythrocyte sedimentation rate at 105 mm/hour (normal: 0-30 mm/hour), C-reactive protein at 15.6 mg/dL (normal: 0.0-0.8 mg/dL), white blood cells 11.4 × 103/uL (normal 4.4-11 × 103/uL), hemoglobin 12.2 × 103/uL (normal: 13.5-17.5 × 103/uL), and platelets 317 × 103/uL (normal: 150-450 × 103/uL). Urinalysis was normal and one set of blood cultures grew half bottles of Streptococcus mitis/oralis,with the next set of blood cultures a day apart showing no growth. A computed tomography scan of the abdomen was also done as the patient was complaining of pain and showed a 11 mm hypodensity in the spleen, suggesting an infarction. Transthoracic echocardiogram was performed, which could not exclude mitral valve endocarditis, but transesophageal echocardiogram (TEE) showed vegetation on the anterior mitral valve leaflet (Figure 1). He tested positive for COVID-19 IgG and negative for IgM. Antigen and polymerase chain reaction tests were also negative. He was discharged on ceftriaxone 2 g intravenously for six weeks via a PICC line and was followed up in the outpatient setting. His weekly labs were unremarkable and his repeat TEE did not show any vegetations.
Figure 1. TEE showing a vegetation on the anterior mitral valve leaflet.
TEE: transesophageal echocardiogram
Discussion
Cardiovascular disease is the most common comorbidity associated with COVID-19 infection, as per the published literature to date. It is important to understand that severe COVID-19 disease can lead to serious long-term complications. Our patient had no significant PMH and no physical examination findings to suspect IE, but suspicion was high given the positive Duke’s score of one major criteria (TEE showing vegetation) and three minor criteria (pyrexia, vascular phenomena of splenic infarct, and one blood culture positive for Streptococcus mitis) [4-6]. In this case, as the patient did not have any risk factors for IE and had a history of hospitalization for severe COVID-19 disease, it can be suggested that the damage to the mitral valve structure by the cytokine storm with the systemic inflammation [1,2,7] and the hypercoagulable state induced by prior COVID-19 infection contributed as risk factors. The process of vegetation is initiated through a transient bacteremia, which causes organisms to adhere to the previously damaged endothelium [1,7]. The upregulated coagulation state by recent COVID-19 infection further helps microorganisms to encase in a platelet/fibrin matrix on the heart valve structure [1,7]. To the best of our knowledge, there have been no prior cases reported in the literature that proposes history of COVID-19 infection as a risk factor for future IE incidents.
Conclusions
COVID-19 has been known to cause hyperinflammatory response along with hypercoagulable state leading to various complications. This case highlights the importance of considering prior COVID-19 infection as a possible risk factor for IE, especially in the absence of other predisposing factors, due to the organ damage that can result from inflammatory response and hypercoagulable state secondary to COVID-19 infection.
This case significantly benefits the fields of cardiology, infectious diseases, as well as other fields of internal medicine in the face of unprecedented challenges brought by a global pandemic. We hope that this novel case influences the approach to diagnosing IE in patients who have a history of COVID-19 infection. More studies that explore advantages of receiving treatment for COVID-19 infection in regards to post-disease complications would be beneficial.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
The authors have declared that no competing interests exist.
Human Ethics
Consent was obtained or waived by all participants in this study
References
- 1.COVID-19 concomitant with infective endocarditis: a case report and review of management. Amir M, Djaharuddin I, Sudharsono A, Ramadany S. Int J Infect Dis. 2020;98:109–112. doi: 10.1016/j.ijid.2020.06.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.COVID-19 and cardiovascular disease. Clerkin KJ, Fried JA, Raikhelkar J, et al. Circulation. 2020;141:1648–1655. doi: 10.1161/CIRCULATIONAHA.120.046941. [DOI] [PubMed] [Google Scholar]
- 3.2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM) Habib G, Lancellotti P, Antunes MJ, et al. Eur Heart J. 2015;36:3075–3128. doi: 10.1093/eurheartj/ehv319. [DOI] [PubMed] [Google Scholar]
- 4.Pathology and pathogenesis of infective endocarditis in native heart valves. Thiene G, Basso C. Cardiovasc Pathol. 2006;15:256–263. doi: 10.1016/j.carpath.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 5.Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: the International Collaboration on Endocarditis-Prospective Cohort Study. Murdoch DR, Corey GR, Hoen B, et al. Arch Intern Med. 2009;169:463–473. doi: 10.1001/archinternmed.2008.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trends in infective endocarditis incidence, microbiology, and valve replacement in the United States from 2000 to 2011. Pant S, Patel NJ, Deshmukh A, et al. J Am Coll Cardiol. 2015;65:2070–2076. doi: 10.1016/j.jacc.2015.03.518. [DOI] [PubMed] [Google Scholar]
- 7.Cardiac complications in patients hospitalised with COVID-19. Linschoten M, Peters S, van Smeden M, et al. Eur Heart J Acute Cardiovasc Care. 2020;9:817–823. doi: 10.1177/2048872620974605. [DOI] [PMC free article] [PubMed] [Google Scholar]