Abstract
Although a growing number of studies suggest interactions between Schistosoma parasites and viral infections, the effects of schistosome infections on the host response to viruses have not been evaluated comprehensively. In this systematic review, we investigated how schistosomes impact incidence, virulence, and prevention of viral infections in humans and animals. We also evaluated immune effects of schistosomes in those coinfected with viruses. We screened 4,730 studies and included 103. Schistosomes may increase susceptibility to some viruses, including HIV and Kaposi’s sarcoma-associated herpesvirus, and virulence of hepatitis B and C viruses. In contrast, schistosome infection may be protective in chronic HIV, Human T-cell Lymphotropic Virus-Type 1, and respiratory viruses, though further research is needed. Schistosome infections were consistently reported to impair immune responses to hepatitis B and possibly measles vaccines. Understanding the interplay between schistosomes and viruses has ramifications for anti-viral vaccination strategies and global control of viral infections.
Author summary
Many studies have described the effects of parasitic Schistosoma worm infections on the way that humans and animals respond to a variety of viral infections. Our goal was to evaluate, in a systematic manner, how having a schistosome parasitic infection affects a host’s susceptibility to viral infections, the clinical disease course of viral infections, and prevention of viral infections by vaccines. We also assessed the effects of schistosome infection on the host immune response to viruses. We screened 4,730 studies for potential relevance and included 103 of them in this review. Overall, our analysis showed that schistosome infection impairs the host response to many viruses. This includes increasing host susceptibility to HIV and possibly Kaposi’s sarcoma-associated herpesvirus, worsening the severity of clinical disease in hepatitis B and C infections, and decreasing immune responses to vaccines for hepatitis B and possibly measles. The studies that we analyzed also suggested that schistosome infection may protect the host against poor clinical outcomes from some viral infections including Human T-cell Lymphotropic Virus-Type 1, respiratory viruses, and chronic HIV. We discuss how these findings might be interpreted, and the additional research needed, in order to improve anti-viral vaccination strategies and control of viral infections globally.
Introduction
Viral infections caused over 3 million deaths worldwide in 2017 [1,2], and will likely contribute over 1 million more in 2020 due to COVID-19 [3]. Global viral outbreaks including COVID-19, HIV, and influenza illustrate the critical need to understand how and why the same virus may cause mild disease in some hosts, yet trigger severe disease and death in others.
Helminth infections affect over 2 billion people worldwide and disproportionately infect the world’s poor [4]. A growing body of literature suggests that helminth infections, particularly schistosomes, affect both host susceptibility to viruses and the severity of viral illness via their impact on the host immune system. While previous reviews have investigated effects of schistosome co-infection on HIV [5–8] or hepatitis viruses [9–11], a more comprehensive analysis of the impact of schistosome infection on viral acquisition, virulence, and prevention has not been undertaken.
Schistosomes infect at least 229 million people globally and are treatable with praziquantel [12]. Parasitic worms lay eggs that damage tissue of the gastrointestinal (S. mansoni, S. japonicum) and genitourinary (S. haematobium) tracts, causing ~1.5 million disability-adjusted life years (DALYs) lost annually [13]. If schistosome infection additionally exacerbates viral susceptibility or virulence, the burden of DALYs lost may be higher. Conversely, if schistosomes benefit the host in some instances, this might hold potential for new therapeutic and prevention strategies against viral infections.
In this review, we systematically examine the effects of schistosome infection on viral infections. We hypothesized that this broader investigation would provide insights to guide future research, with potential to impact the public health effects of viruses. We focused on how schistosome co-infection impacts specific viral infections by examining: host anti-viral immune responses, prevalence and incidence of the viral infection, virulence, and anti-viral vaccination.
Methods
This systematic review utilized the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines [14] and was registered prospectively in PROSPERO (CRD42019129533).
Search strategy
Comprehensive literature searches were developed and performed by a medical librarian [KM]. The initial search was performed March 28, 2019 via OVID MEDLINE ALL (1946-March 27, 2019). The Cochrane Library (Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials (CENTRAL), Cochrane Methodology Register, Technology Assessments (HTA)), LILACS (Latin American and Caribbean Health Sciences Literature), and OVID EMBASE (1974-March 28, 2019) were searched on March 29, 2019; Scopus (Elsevier) on April 1, 2019. Follow-up searches were performed on December 9, 2019. Search terms included all subject headings and/or keywords associated with our research question, clustered as:
Parasite or proxy for parasitic infection (e.g. Schistosoma, Schistosomiasis);
Virus or vaccine-related headings (e.g. Virus, Virology, Vaccine, Vaccination);
Immune response or physiologic state indicative of host immune response (e.g. Immunomodulation, Lymphocyte Activation, Biomarkers, Disease Susceptibility, Cellular Immunity, Coinfection, HIV, T-Lymphocytes).
Boolean operators ‘OR’ and ‘AND’ were used as appropriate. Grey literature and bibliographies of included articles were also searched; see full search strategy in the Supplementary Appendix (S1 Text). Publication date or article-type restrictions were not imposed.
Study selection
After excluding duplicates, two investigators [BB, KK] independently screened titles and abstracts using Covidence, a systematic review tool. Discrepancies were resolved independently by a third investigator [JD]. We included studies describing the effects of schistosomes on viral infections, anti-viral immunity, or anti-viral vaccinations and defined virulence as severity of clinical disease in co-infected subjects [15]. This definition of virulence aligned with the definitions used in included manuscripts. Pre-defined exclusion criteria included: populations with and without viruses rather than with and without schistosome infection, focus on non-viruses, not available in English, and study design of case study or case review. We pre-specified that studies must test for active schistosome infection by microscopy or antigen and that we would exclude studies that relied on schistosome antibody testing due to antibodies remaining positive after clearance of infection (see S2 Text for full decision rules for data selection and extraction).
Full-text review followed the initial title and abstract screening phase, with data extracted into Microsoft Excel. Following Cochrane Collaboration guidance, included articles were evaluated for credibility, transferability, dependability, and confirmability [16] using Critical Appraisal Skills Programme checklists [17]. Discrepant analyses of study quality were resolved by discussion and author consensus. Due to heterogeneity of study designs, animal and human studies, and different viral infections, a meta-analysis was not possible.
Results
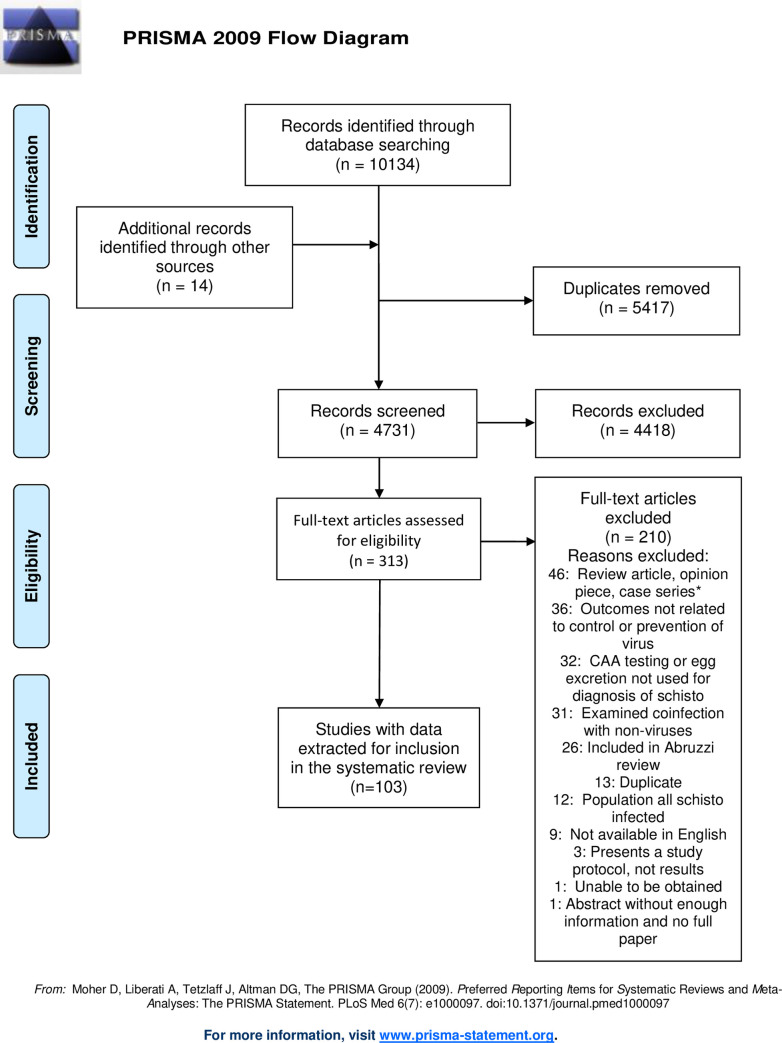
Titles and abstracts of 4,731 studies were screened, and 313 full-text papers were assessed for inclusion. 14 additional records were identified from the grey literature, expert recommendations, and screening bibliographies. Excluded studies are listed in S1 Table. A total of 103 studies were included (Fig 1 and S2 Table).
Fig 1. PRISMA flow diagram showing records identified by searching, screened, assessed for eligibility, and included in the systematic review.
We identified studies examining effects of schistosome coinfection on 7 categories of viruses: hepatotropic viruses such as Hepatitis B and C (HBV, HCV), Human Immunodeficiency Virus (HIV), Human papillomavirus (HPV), Human T-cell leukemia virus type 1 (HTLV-1), Kaposi’s sarcoma-associated herpesvirus (KSHV), respiratory viruses (influenza, murine herpesviruses), and rubeola virus vaccination.
The schistosome-infected host exhibits a dynamic immune response to schistosome infection that is initially dominated by Th1 with some contributions from Th2, and then shifts over time towards Th2 and T regulatory (T reg) dominance during chronic infection [18,19]. The concomitant decrease in Th1 cytokines during this chronic phase underlies the hypothesis that the schistosome-infected host may develop impaired Th1 anti-viral and cytotoxic immune function. Most studies in this review examined immune effects of schistosome-virus coinfection during chronic schistosome infection. We examine each category of virus separately, first reviewing reported immune effects of schistosome-virus coinfection and then examining effects on viral prevalence/incidence, virulence, and vaccination for each virus.
Hepatitis B and C viruses
Effects of schistosome infection on host immune response to HCV
In total, 15 studies described effects of schistosome infection on immune response to HCV and none focused on HBV (Table 1).
Table 1. Effects of Schistosome Coinfection on the Host Immune Response to Hepatitis C Virus.
| Study | Study Type | Sample Size | Sex (% Female) | Age in Years (Weighted Mean) | Country | Species | Immune and Viral Alterations in Schistosome Coinfected | Overall Effects of Schistosomes on HCV Infection |
|---|---|---|---|---|---|---|---|---|
| Kamal 2001 [20] | Human | 32 | 34% F | 28.0 | Egypt | S. mansoni |
↑ IL-4 ↑ IL-10 ↓ CD4+ |
Some studies report impaired anti-HCV responses of CD4+ helper T cells Decreased Th1 immune response (IFN-ɣ) and increased Th2 immune response (IL-4, IL-10) Increased HCV viral load and replication Impaired response and increased relapse after interferon therapy |
| Kamal 2001 [21] | Human | 85 | 35% F | 38.0 | Egypt | S. mansoni | ↓ CD4+ | |
| Kamel 2014 [22] | Human | 82 | 43% F | 51.2 | Egypt | S. mansoni | No difference in CD4+ or CD8+ | |
| Loffredo-Verde 2015 [23] | Human | 44 | 41% F | 39.5 | Egypt | S. mansoni |
No difference in CD4+
or CD8+ ↑ granzyme B+ T regulatory cells ↑ HCV viral load |
|
| Elrefaei 2003 [24] | Human | 24 | Not reported | 41.0 | Egypt | S. mansoni | ↓ Memory T-cells | |
| Kamal 2004 [25] | Human | 68 | 40% F | 34.3 | Egypt | S. mansoni |
↓ CD4+
Th1 intrahepatic T cells ↑ HCV viral load |
|
| El-Kady 2005 [26] | Human | 70 | 30% F | 44.3 | Egypt | S. mansoni |
↑ IL-4 ↑ IL-10 ↓ IFN-ɣ |
|
| Emam 2006 [27] | Human | 50 | 44% F | 44.7 | Egypt | S. mansoni |
↑ IL-4 ↑ IL-10 ↓ IFN-ɣ |
|
| Ahmed 2016 [28] | Human | 107 | 47% F | Range, 30–81 years | Egypt | S. mansoni |
↑ HCV viral load ↑ IL-28B No difference in IFN-ɣ |
|
| Elrefaei 2004 [29] | Human | 38 | Not reported | 42.5 | Egypt | S. mansoni |
No difference in IL-10 or IFN-ɣ with HCV peptide stim; ↑ IL-10 and ↓ IFN-ɣ with PHA stim* |
|
| Bahgat 2010 [30] | In vitro | Not reported | Not reported | Not reported | Egypt | S. mansoni | ↑ HCV viral replication | |
| El-Awady 2006 [31] | In vitro | 26 | Not reported | Not reported | Egypt | S. mansoni | ↑ HCV viral replication | |
| Edwards 2005 [32] | Animal | Not reported | Not reported | Not reported | Not reported | S. mansoni | ↑ HCV viral replication | |
| El-Shazly 1994 [33] | Human | 60 | 17% | 41.0 | Egypt | S. mansoni |
After interferon therapy: ↓ % ALT** normalization ↓ Response rate ↑ Relapse rate |
|
| Kamal 2000 [34] | Human | 62 | 35% | 43.1 | Egypt | S. mansoni |
After interferon therapy: ↓ Response rate ↑ Relapse rate |
*Phytohemagglutinin (PHA) stimulation, a positive control
**Alanine aminotransferase
Six studies investigated circulating T cells in HCV-schistosome coinfected individuals compared to those with HCV alone [20–24]. In one of these, S. mansoni-infected patients who acutely acquired HCV developed a Th2 and T reg-dominant response and 17/17 failed to clear HCV, while those without S. mansoni infection developed a Th1-dominant response and 5/15 cleared HCV infection [20]. Both this study and another documented lower HCV-specific CD4+ proliferative responses to HCV antigens in those with HCV-schistosome coinfection [20,21]. Two others reported no differences in CD4+ or CD8+ T cells, [22] or in CD4+ or CD8+ granzyme B+ effector T cells, although granzyme B+ effector T regs were increased in coinfection [23]. The fifth study reported fewer late differentiated HCV-specific memory T cells in coinfected individuals compared to those with HCV alone [24]. In the sixth study, S. mansoni-coinfected persons exhibited less frequent and smaller intrahepatic HCV-specific CD4+ Th1 responses than those with HCV mono-infection, and biopsy-confirmed liver fibrosis was inversely correlated with the Th1 immune response [25].
Five studies evaluated host cytokine responses to HCV in schistosome-HCV coinfection versus HCV-monoinfection [23,26–29]. Two of these reported higher serum IL-4 and IL-10 and lower interferon-gamma (IFN-γ) levels in coinfected individuals [26,27], although two others did not [23,28]. Another reported higher IL-10 and lower IFN-γ in response to generic mitogen stimulation although not to HCV-specific stimulation [29]. Together, these studies generally support impaired Th1 anti-viral immunity in patients with schistosome-HCV coinfection.
Two studies investigated the role of HCV-schistosome coinfection in viral propagation in humans [30,31] and one investigated an analogous virus in mice [32]. In humans, HCV-infected blood samples cultured with S. mansoni [30] or S. haematobium [31] egg antigens had increased intracellular HCV replication and earlier detectable HCV RNA than HCV-infected blood samples cultured without schistosome egg antigens. Concordantly, the study in mice reported increased intrahepatic viral replication and reduced type 1 IFN production [32]. These findings are in agreement with data from some [23–25,28], but not all [27,29], studies that reported higher HCV RNA viral loads in patients with schistosome-HCV coinfection.
Two additional studies reported that S. mansoni-HCV coinfected patients had poorer response to interferon therapy than HCV-monoinfected patients, marked by more frequent relapses and poorer hepatic histologic improvement six months after therapy [33,34]. Studies assessing effects of schistosome infection with newer, direct-acting antivirals for HCV have not been performed. Together, this body of data consistently suggests impaired antiviral immune responses and poorer viral control in HCV-S. mansoni coinfection than HCV alone.
Schistosomes and prevalence of HBV and HCV
Studies on associations between schistosomes and HBV or HCV prevalence reported up to 2015 were previously summarized by Abruzzi and colleagues [9] and therefore are not discussed in detail here. Briefly, schistosome infection is not associated with increased prevalence of HBV or HCV, aside from one study in Egypt where parenteral anti-schistosome treatment likely caused iatrogenic hepatitis infections. No additional studies of HBV or HCV prevalence or incidence that met inclusion criteria have been published since the 2015 review.
Schistosomes and virulence of HBV and HCV
Abruzzi and colleagues have meticulously summarized effects of schistosome-hepatitis virus coinfection on liver pathology [9], including several of the HCV immunology studies discussed above that also addressed virulence [20,21,25,27]. They concluded that studies in patients with schistosome-HCV coinfection indicate that schistosome-hepatitis coinfections have synergistic effects on liver disease. This was demonstrated by increased transaminases, cirrhosis, and/or portal hypertension in coinfected patients versus those with schistosomes or HCV alone. An additional HCV study published after the review also supports this finding [35].
Four studies in the Abruzzi review similarly reported that HBV-schistosome coinfected people had prolonged HbSAg antigenemia and greater liver fibrosis and splenomegaly compared to those with HBV alone. In a mouse model of HBV not included in the review, S. mansoni infection similarly promoted viral persistence, although liver pathology was decreased in coinfected compared to HBV-monoinfected mice during the T regulatory-dominant chronic phase of S. mansoni infection [36]. Interestingly, coinfected mice also cleared acute HBV infection more quickly during both the Th1-dominant acute phase of schistosome infection and the chronic T regulatory dominant phase [36]. These studies demonstrate the important role that timing of infections may have on viral virulence and the need for human data to clarify these findings.
Two small studies examined effects of praziquantel treatment on HBV disease course. In ten S. mansoni-HBV co-infected individuals, HBsAg remained positive in the four individuals with persistent S. mansoni six months post-treatment, versus in only one of six who were parasitologically cured [37]. The other study reported that praziquantel treatment of HBV-S. mansoni coinfected individuals led to clearance of HbSAg in 20% and normalization of liver transaminases in ~15% after 6 months, while those with HBV alone who were followed for 6 months had no clearance of HbSAg and ~10% developed abnormal transaminases [38].
Schistosomes and HBV vaccination
Eight studies investigated hepatitis B vaccine immunogenicity with and without schistosome coinfection (Table 2). Mice with chronic S. japonicum had significantly attenuated anti-hepatitis B surface (HBs) antibody response to hepatitis B vaccine, and anti-HBs antibody titers increased after praziquantel treatment [39]. Two studies reported lower post-vaccine anti-HBs titers in S. mansoni-infected children [40] and adults [41]. Titers in S. mansoni-infected adults were lower two weeks after the second hepatitis B vaccine dose, with a trend towards remaining lower after three doses [41]. Another study involving 504 children found no impact of S. mansoni infection on anti-HBs antibody titers when children were given praziquantel treatment and vaccinated concurrently [42]. These data suggest that hepatitis B vaccine response may be compromised by schistosome infection and improved after treatment. Notably, four studies reported that maternal schistosome infection does not impair neonatal response to hepatitis B vaccine [43–46].
Table 2. Effects of Schistosome Infection on Response to Hepatitis B Vaccination.
| Study | Subject Type | Sample Size | Sex (% Female) | Age in Years (Weighted Mean) | Country | Species† | Vaccine Response in Schistosome Infected | Overall Effects of Schistosomes on HBV Vaccine |
|---|---|---|---|---|---|---|---|---|
| Chen 2012 [39] | Mouse | 140 | 0% F | N/A | N/A | Sj |
↓ post-vaccine anti-HBs titers** Titers comparable to uninfected after praziquantel |
Lower post-vaccine anti-hepatitis B surface antigen antibody titers. Vaccine responsiveness may improve after praziquantel treatment. |
| Ghaffar 1990 [40] | Human | 80 | 0% F | Range, 8–12 years | Egypt | Sm | Mean anti-HBs titers 68 mIU/mL in Sm versus 335 in controls** | |
| Riner 2016 [41] | Human | 146 | 53% F | 21 | Kenya | Sm | Mean anti-HBs titers 158 mIU/mL in Sm versus 561 in controls* | |
| Bassily 1992 [42] | Human | 508 | 40% F | Range, 6–12 years | Egypt | Sm |
No difference in titers in Sm infected and uninfected Fewer children with hepatosplenomegaly had adequate titers** |
|
| Bassily 1997 [43] | Human | 385 | 49% F | Infants followed to age 9 months | Egypt | Sm | No difference in seroconversion or mean anti-HBs titers in infants of mothers with/without Sm | Maternal schistosome infection does not seem to impact infant response to hepatitis B vaccine. |
| Nash 2017 [44] | Human | 1379 | 50% F | Infants followed to age 1 year | Uganda | Sm | No effect of maternal praziquantel treatment on infant anti-HBs titers. | |
| Malhotra 2015 [45] | Human | 450 | Not reported | Infants followed to age 36 months | Kenya | Sh | No effect of maternal Sh infection on infant anti-HBs titers. | |
| Malhotra 2018 [46] | Human | 450 | Not reported | Infants followed to age 30 months | Kenya | Sh | Prenatal Sh exposure did not affect infant anti-HBs titers or trajectories of titers. |
†Sm = S. mansoni; Sh = S. haematobium; Sj = S. japonicum
*p<0.10
**p<0.05
Collectively, these data demonstrate that schistosome infection is associated with impaired anti-viral immune responses and subsequent increased viral replication and liver damage. This has been particularly well studied in adults with S. mansoni and HCV; very little is known in children who have acquired HBV perinatally. Schistosome infection does appear to depress the humoral HBV vaccine response in children. Importantly, praziquantel may aid viral control, decrease liver pathology, and improve HBV vaccine response and could be an effective means to decrease global burden of these viral infections in schistosomiasis endemic regions.
HIV
Effects of schistosome infection on host immune response to HIV
Three studies investigated the mucosal anti-HIV immune defense in HIV-uninfected women (Table 3). One study documented associations of S. haematobium infection with higher frequencies of CD14+ and CD4+ cells expressing the HIV coreceptor CCR5 in the genital tract and systemic circulation, which decreased after praziquantel treatment [47]. An in-vitro study found that HIV viral entry into cervical and systemic CD4+ cells was higher in S. mansoni-infected women and decreased after praziquantel treatment [48]. Treatment of S. mansoni infection also partially normalized dysregulated anti-viral interferon signaling [48]. This corresponds with observed lower levels of anti-viral cytokine interleukin-15 in cervicovaginal lavages of S. haematobium-infected women [49]. By contrast, immune cell populations were not altered in the foreskins of men with S. mansoni infection [50]. Taken together, these consistently report that schistosome-infected women have genital mucosal alterations in immune cell populations, anti-viral signaling, and HIV viral entry. Together with physical breaches of the mucosal barrier by schistosome eggs, these may contribute to increased susceptibility to mucosal HIV acquisition in women.
Table 3. Effects of Schistosome Infection on Host Immune Response to HIV in the Genital Mucosa and Systemic Circulation.
| Study | Sample Size | Sex (% Female) | Age in Years (Weighted Mean) | Country | Species† | Immune Alterations in Schistosome Coinfected | Overall Effects of Schistosome on HIV Infection |
|---|---|---|---|---|---|---|---|
| Genital Mucosal Immune Effects | |||||||
| Kleppa 2014 [47]* | 44 | 100% F | 18 | South Africa | Sh | ↑ cervical HIV target cells & coreceptors (CD14+, CD4+CCR5+) | Genital mucosal immune alterations are potentially consistent with increased HIV susceptibility in women. |
| Yegorov 2019 [48]* | 36 | 100% F | 24.5 | Uganda | Sm |
↑ HIV viral entry into cervical CD4+
cells ↑ IFN-α after schistosome treatment |
|
| Dupnik 2019 [49] | 97 | 100% F | 28.9 | Tanzania | Sh, Sm | ↓ Anti-viral cytokine (IL-15) in Sh but not Sm | |
| Prodger 2015 [50]* | 34 | 0% F | 24.6 | Uganda | Sm | No differences in frequencies or activation of HIV target cells in foreskin | |
| Systemic Circulation Immune Effects | |||||||
| Kleppa 2014 [47]* | 44 | 100% F | 18 | South Africa | Sh | ↑ blood HIV target cells & coreceptors (CD14+, CD4+CCR5+) | Peripheral blood immune alterations are potentially consistent with increased HIV susceptibility in both men and women. |
| Yegorov 2019 [48]* | 36 | 100% F | 24.5 | Uganda | Sm | ↑ HIV viral entry into blood CD4+ cells | |
| Prodger 2015 [50]* | 34 | 0% F | 24.6 | Uganda | Sm | ↑ frequencies of Th1, Th17, and Th22 CD4+ T cell subsets in blood | |
| Secor 2003 [51] | 42 | 0% F | Age > 18 years | Kenya | Sm | ↑ density of CXCR4 on CD4+ T cells and CD14+ monocytes | |
| McElroy 2005 [52] | 35 | Not reported | 36.6 | Uganda | Sm |
↓ HIV Gag-specific CD8+
cytolytic T-cell responses ↑ Gag-specific IL-10-positive CD8+ T-cell responses |
|
| Erikstrup 2008 [53] | 378 | 80% F | 33 | Zim-babwe | Sh, Sm |
↑ IL-8 in Sm but not Sh ↓ TNF-α receptor and IL-8 after schistosome treatment |
|
| Obuku 2016 [54] | 50 | At least 28% F | 29 | Uganda | Sm |
↑ TNF-α after non-viral stimulation ↑ CD8+IFN-ɣ+ and CD4+IFN-ɣ+TNF-α+ T cells after Gag stimulation |
|
| Mouser 2019 [63] | N/A–in vitro study | N/A | N/A | N/A | Sm | ↓ trans-HIV infection after Sm antigen stimulation in dendritic cells that matured in Th2 conditions | |
| Animal Studies | |||||||
| Dzhivhuho 2018 [55] | Not reported | Mice, 100% F | N/A | N/A | Sm | ↓ IFN-ɣ and IL-2 in response to challenge with candidate HIV vaccines | Animal models support human studies, demonstrating potentially impaired immunity to HIV. |
| Actor 1993 [56] | Not reported | Mice, not reported | N/A | N/A | Sm |
↓ IFN-ɣ and IL-2 in response to challenge with vaccinia virus expressing gp160 ↓ CD8+ anti-viral cytotoxicity ↓ viral clearance |
|
| Marshall 2001 [57] | Not reported | Mice, 100% F | N/A | N/A | Sm | ↓ CD8+ anti-viral cytotoxicity related to a splenic immune cell population | |
| Actor 1994 [58] | Not reported | Mice, not reported | N/A | N/A | Sm | ↓ anti-viral cytotoxicity and vaccinia viral clearance began 6 weeks after Sm infection, associated with egg granuloma formation | |
| Lacroix 1998 [59] | Not reported | Mice, 100% F | N/A | N/A | Sm | ↑ IL-4 in mice with Sm-murine HIV coinfection | |
| Chenine 2005 [60] | 8 | Maca-ques, not reported | N/A | N/A | Sm |
↑ IL-4 and IL-10 in Sm ↑ sHIV viral loads in Sm |
|
| Ayash-Rashkovsky 2007 [61] | 15 | Maca-ques, not reported | N/A | N/A | Sm |
↓ CD4+CD29+
memory T cells in Sm ↑ IL-4 in Sm ↑ reactivation of previously undetectable sHIV in Sm |
|
| Buch 2001 [62] | 8 | Maca-ques, not reported | N/A | N/A | Sm | ↑ sHIV replication in tissue macrophages in Sm accom-panied by ↑ IL-4 and ↓ IFN-ɣ | |
* indicates study examined both genital mucosa and systemic circulation
†Sm = S. mansoni; Sh = S. haematobium; Sj = S. japonicum
Five additional human studies examined host systemic immunity in HIV-schistosome coinfected patients, and all reported increased Th2 or Treg responses in those with coinfection versus those with HIV alone [50–54]. HIV-schistosome coinfected men had higher densities of HIV co-receptors CXCR4 and CCR5 on the surface of CD4+ T cells, which were upregulated by Th2/Treg cytokines IL-4 and IL-10 [51]. Higher systemic frequencies of HIV-susceptible Th1, Th17, and Th22 CD4+ T cell subsets were also observed in HIV-uninfected men with S. mansoni [50]. Stimulation of PBMCs with HIV-1 Gag peptides yielded decreased Gag-specific CD8+ cytolytic T-cell responses and increased Gag-specific IL-10-positive CD8+ T-cell responses in HIV-S. mansoni coinfected compared to HIV-monoinfected individuals, suggesting dysregulated cellular immunity to HIV [52]. These five studies thus demonstrate schistosome-associated alterations in host systemic immunity that could confer increased susceptibility to HIV.
Notably, some upregulations in pro-inflammatory markers IL-8, TNF receptor sTNF-rII, and TNF-α have been documented in schistosome-HIV coinfection, possibly consistent with heightened Th1 response following recent HIV acquisition [53,54]. However, overall, these human studies report that schistosome coinfection downregulates Th1 and cytotoxic immune responses and upregulates Th2 and Treg responses in HIV-infected individuals, and that treating the schistosome infection modulates these differences.
Eight animal studies reported decreased Th1 and increased Th2/T reg responses to HIV during schistosome infection [55–62]. In the first, splenocytes from S. mansoni-infected mice stimulated with HIV vaccine candidates produced less IFN-γ and IL-2 than S. mansoni-uninfected, vaccinated mice, and partial restoration was observed after anti-schistosome treatment [55]. In two other studies, mice with vaccinia-S. mansoni coinfection produced minimal IFN-γ and IL-2 in response to viral challenge with recombinant vaccinia virus expressing the HIV glycoprotein gp160, and exhibited delayed viral clearance, compared to mice with vaccinia alone [56,57]. Longitudinal mouse studies documented delayed viral clearance only after the onset of worm egg-laying, correlating with decreasing IFN-γ and IL-2, impaired viral-specific cytotoxic CD8+ T-cell responses [58], and increased IL-4 [59]. The Th2 predominance after egg-laying further links the impaired anti-viral response during schistosome infection to dwindling Th1 immune responses at the time of worm maturation and oviposition [63]. Elevated Th2/T reg cytokines and decreased IFN-ɣ have also been reported in macaques with sHIV-schistosome coinfection and have been linked to increased sHIV replication in macrophages [60–62]. Together, these animal studies support the human findings of Th2 predominance and potentially impaired anti-HIV immunity during chronic schistosome infection.
Finally, an in vitro study documented increased resistance to trans-infection in HIV-susceptible human dendritic cells that matured while exposed to schistosome egg antigens in a Th2-predominant environment compared to cells that matured in a Th1 environment [64]. This suggests that during schistosome-HIV coinfection, HIV may spread less readily from cell-to-cell via trans-infection than during HIV mono-infection, indicating a potential means by which schistosome coinfection, perhaps unexpectedly, could slow viral replication and disease progression.
Schistosomes and prevalence and incidence of HIV
Cross-sectional studies investigating HIV prevalence in schistosome-infected and -uninfected individuals have been inconclusive (Table 4). Four studies reported higher prevalence of HIV in schistosome-infected versus uninfected women [65–68], of which three focused on S. haematobium infection. Five others, conducted in S. mansoni-hyperendemic regions or in men only, found no difference by schistosome status [69–73], although at least one was under-powered.
Table 4. Prevalence and Incidence of HIV Among Schistosome-Infected and Uninfected Individuals.
| Study | Subject Type | Sample Size | Sex (% Female) | Age in Years (Weighted Mean) | Country | Species† | Reported Prevalence/Incidence | Overall Effects of Schisto-some Infection |
|---|---|---|---|---|---|---|---|---|
| HIV Prevalence–Cross-Sectional Studies | ||||||||
| Kjetland 2006 [65] | Human | 527 | 100% F | 34 | Zim-babwe | Sh | HIV prevalence: 41% among Sh 26% among uninfected* | Increased prevalence of HIV has been reported among women with Sh or mixed Sh/Sm infections. |
| Downs 2011 [66] | Human | 457 | 100% F | 30 | Tanzania | Sh Sm | HIV prevalence: 17% among Sh 6% among uninfected* | |
| Downs 2012 [67] | Human | 345 | 100% F | 30 | Tanzania | Sh and Sm | HIV prevalence: 9% among Sh or Sm 3% among uninfected* | |
| Mvumbi 2018 [68] | Human | 446 | 100% F | Not reported | Demo-cratic Republic of Congo | Sh | HIV prevalence: 28% among Sh 11% among uninfected* | |
| Downs 2017 [69] | Human | 674 | 0% F | 34 | Tanzania | Sh and Sm | HIV prevalence: 6.5% among Sh 6.4% among uninfected 5% among Sm 6% among uninfected |
No difference has been reported in HIV prevalence in men for Sh or Sm. No difference has been reported in HIV prevalence in many Sm studies. |
| Wood-burn 2009 [70] | Human | 2507 | 100% F | Median: 23 | Uganda | Sm | Odds of HIV in Sm infected: 1.06 (95% CI 0.78–1.51) | |
| Sanya 2015 [71] | Human | 1412 | 45% F | 30.3 | Uganda | Sm | HIV prevalence: 21% among Sm 18% among uninfected | |
| Mazigo 2014 [72] | Human | 1785 | 52.9% F | 35.6 | Tanzania | Sm | HIV prevalence: 6% among Sm 8% among uninfected | |
| De Lima e Costa 1988 [73] | Human | 180 | Not reported | 20.5 | Brazil | Sm | HIV prevalence: 3.6% among Sm 0 among uninfected | |
| HIV Incidence–Longitudinal Studies | ||||||||
| Ssetaala 2015 [74] | Human | 200 | Not reported | Range: (13–49) | Uganda | Sm | Sm prevalence was 49% among HIV-seroconverters and 52% among controls |
Several studies have shown no increased risk of HIV acquisition with Sm. Some studies suggest increased risk of HIV acquisition in women, more strongly with Sh. |
| Bochner 2020 [75] | Human | 2250 | 75% F | ~31# | Kenya & Uganda | Sh and Sm |
Sh/Sm prevalence was 27% among HIV-seroconverters and 25% among controls Trend toward increased risk for Sh in women (36% versus 25%). |
|
| Kroidl 2016 [76] | Human | 1055 | 51% F | 15.4 | Tanzania | Sh | HIV incidence per 100 person-years: 1.3 among Sh 1.2 among uninfected | |
| Downs 2017 [77] | Human | 338 | 61% F | Not reported | Tanzania | Sh and Sm |
Women: Sh/Sm prevalence was 43% among HIV-seroconverters and 30% among controls* Men: 29% among HIV-seroconverters and 38% among controls |
|
| Galla-gher 2005 [78] | Human | 936 | 100% F | Not reported | Kenya | Sh | Sh prevalence was 17% among mothers who transmitted HIV to children and 9% among mothers who did not [85% versus 40% for any helminth]* | |
| Chenine 2008 [79] | Animal | 17 | 100% F | N/A | N/A | Sm | 17-fold lower sHIV viral dose needed to achieve rectal transmucosal infection | Animal models support increased mucosal sHIV sus-ceptibility. |
| Siddappa 2011 [80] | Animal | 16 | 100% F | N/A | N/A | Sm | No difference in sHIV dose needed to achieve intravenous infection | |
† Sh = S. haematobium; Sm = S. mansoni; Sj = S. japonicum
* Difference was statistically significant.
# Approximate calculation; data was incomplete to determine precise weighted mean.
Five studies investigated effects of schistosome infection on HIV incidence. In a nested case-control study in Uganda, investigators found no increased incidence of HIV in S. mansoni-infected people, although the odds of incident HIV was eight-fold higher in those who had not received anti-schistosome treatment in the past two years [74]. Increased incidence of HIV was similarly not observed in serodiscordant couples and sex workers in Uganda and Kenya who had either S. haematobium or S. mansoni infection [75]. In a cohort in Tanzania, persons who had S. haematobium eggs in urine at any point during the study did not have increased HIV incidence [76]. In contrast, a nested case-control study in Tanzania reported increased odds of HIV acquisition (OR = 2.8 [95% confidence interval, 1.2–6.6]) in schistosome-infected women but not men [77]. One additional study reported no increased mother-to-child HIV transmission from schistosome-infected mothers, although infection with any helminth (including schistosomes) strongly increased odds of HIV transmission suggesting the study may have been underpowered to examine individual helminths [78]. Additional challenges with this body of evidence include lack of control for confounders including sexually-transmitted infections in high-risk cohorts [75], unclear timing of anti-schistosome treatment in relation to HIV acquisition [76], and use of assays unable to differentiate between schistosome species [77].
Studies in macaques more clearly support increased HIV susceptibility during schistosome infection. Investigators reported that S. mansoni-infected macaques, when rectally inoculated with increasing doses of simian HIV (sHIV), became infected at viral doses 17-fold lower than schistosome-uninfected macaques [79]. The same investigators found no difference in sHIV dose needed to achieve infection when schistosome-infected and uninfected macaques were infected intravenously, suggesting that the sHIV susceptibility is mediated by mucosal effects of S. mansoni [80]. Because macaques received rectal sHIV inoculation, S. mansoni in these studies may mimic the effects of S. haematobium infection on vaginal HIV exposure in humans. Although parasite eggs can be found in the genital tract in both S. haematobium and S. mansoni infections, they are more highly concentrated and associated with greater genital tract pathology in S. haematobium [81–84].
Overall, this body of evidence is inconclusive but suggests that schistosome infection may increase the risk for HIV acquisition in women, with a stronger signal toward increased risk with S. haematobium. The evidence for S. mansoni is mixed and an association with HIV, if present, may be lower in magnitude. This is corroborated by a third, larger nested case-control study, not eligible for inclusion in the review due to its use of antibody testing, which reported a moderately increased hazard of HIV acquisition in women with anti-S. haematobium antibodies and a smaller increased hazard for women with anti-S. mansoni antibodies that did not reach significance (hazard ratios = 1.44 [1.05–1.96] and 1.30 [0.91–1.87], respectively) [85].
Interestingly, that case-control study also indicated that the presence of anti-schistosome antibodies was associated with increased transmission of HIV from HIV-infected men and women to their sexual partners, as well as with increased hazard of death in HIV-infected women [85]. Men with S. haematobium infection have also been reported to have an increased concentration of leukocytes and eosinophils in semen, cell types that are permissive to HIV replication and may facilitate onward HIV transmission from men with HIV-S. haematobium coinfection to their sexual partners [86]. Further supporting this possibility, a small study of 6 HIV-S. haematobium coinfected men who were not on ART and were treated with praziquantel reported a decrease in HIV-1 RNA viral load in semen from baseline to 10 weeks post-treatment, though this did not reach significance [87].
Schistosomes and HIV virulence
One study reported that S. mansoni-HIV coinfection was associated with elevated liver transaminases after controlling for other known causes of transaminitis (HBV, HCV, alcohol, medications) in patients on ART [88]. This suggests that S. mansoni infection could potentiate direct hepatotoxicity of HIV on hepatocytes [89].
Thirteen studies examined effects of HIV-schistosome coinfection on CD4+ T cell counts (Table 5). Seven found no relationship [54,72,90–94], one reported higher CD4+ counts [95], and one reported lower increase in CD4+ counts following ART initiation in coinfected patients [96]. Four other studies found no difference in CD4+ counts before and 12 weeks after praziquantel treatment [95,97,98] or in those given praziquantel quarterly versus annually [99]. Another study reported that individuals successfully treated for schistosome infection had lower CD4+ counts after six months than those still infected [100]. Taken together, these studies indicate that schistosome infection does not consistently affect CD4+ count, and that treatment of schistosome infection seems not to increase, and could possibly decrease, CD4+ counts.
Table 5. Effects of Schistosome-HIV Coinfection on CD4+ T Cell Counts, HIV-1 RNA Viral Load, and HIV Disease Progression.
| Schistosome HIV Co-Infection Effects on CD4 Count | ||||||
| No relationship | Higher CD4 counts | Lower increase in CD4+ counts following ART initiation | No difference before and after praziquantel | No difference whether praziquantel is quarterly or annual | Lower CD4+ counts 6 months after schistosomiasis treatment | |
| Obuku 2016 [54] | Elliott 2003 [95] | Efraim 2013 [96] | Elliott 2003 [95] | Abaasa 2018 [99] | Brown 2005 [100] | |
| Mazigo 2014 [70] | ||||||
| Brown 2004 [90] | ||||||
| Kleppa 2015 [91] | Mulu 2013 [97] | |||||
| Colombe 2018 [92] | ||||||
| Colombe 2018 [93] | Mazigo 2016 [98] | |||||
| Idindili 2011 [94] | ||||||
| Schistosome Infection and HIV-1 Viral Load | ||||||
| Higher viral load | Lower viral load | No difference in viral load | Higher viral load, declining over time | No difference before and after praziquantel | Transient increase in viral load following praziquantel | Higher viral load >6 months after praziquantel |
| Downs 2017 [77] HIV recently acquired |
Bochner 2019 [101] HIV recently acquired |
Masikini 2019 [102] Patients failing first-line ART |
Chenine 2008 [79] Animal Study |
Brown 2004 [90] | Elliott 2003 [95] | Lawn 2000 [104] Pre-ART study |
| Brown 2004 [90] Chronic HIV |
Colombe 2018 [92] Chronic HIV |
Kallestrup 2005 [103] Pre-ART study; Also, viral loads increased pre-treatment those whose praziquantel was delayed |
Brown 2005 [100] | Abaasa 2018 [99] Patients not yet eligible for ART |
||
| Mazigo 2016 [98] Chronic HIV |
Elliott 2003 [95] Chronic HIV |
Mazigo 2016 [98] | ||||
| Slower HIV Disease Progression in those with Chronic HIV-Schistosome Coinfection | ||||||
| Colombe 2018 [93] Patients not yet on ART Outcome: CD4+ count < 350 cells/μL or death Hazard ratio = 0.31 [0.12–0.84] |
Abaasa 2018 [99] Patients not yet eligible for ART Outcome: progression to AIDS Log-rank chi-square (low-intensity versus high-intensity praziquantel) = 2.08, p = 0.15 |
Stete 2018 [105] Patients starting ART Outcome: death or loss-to-follow-up Hazard ratio = 0.58 [0.32–1.05] |
||||
Seven studies examined schistosome infection and HIV-1 viral load. In adults who had recently acquired HIV, median viral load was higher in those with HIV-schistosome coinfection than with HIV alone in one study [77] but lower in a combined analysis of four cohort studies in higher-risk populations [101]. Cervical HIV-1 viral loads were also unexpectedly lower in S. haematobium-coinfected patients [101]. In four studies among patients with advanced HIV infection, schistosome-HIV coinfected individuals had lower viral loads in one [92]. higher viral loads in one [98], and no significant differences in two [90,95]. Another study reported no difference in viral loads by schistosome status among patients failing first-line ART [102]. These discrepant results suggest a complex relationship between schistosome infection and HIV-1 viral load that may be influenced by duration of HIV infection. Macaques with S. mansoni infection also had higher HIV-1 viral loads shortly after mucosal HIV acquisition, which declined over time [79], though macaques naturally clear S. mansoni infection after several months and this precludes definite conclusions [60].
Examination of the effects of praziquantel treatment on viral load provides additional insight. In six studies of viral load in HIV-schistosome coinfected individuals within six months of praziquantel treatment, two reported no difference before and after treatment [90,98]. One small randomized trial reported higher viral loads in HIV-schistosome coinfected persons randomized to receive delayed treatment after three months than in persons who received praziquantel at baseline [103]. Two other studies reported increases in viral loads 4–5 weeks after treatment, which were not sustained when re-measured 4–5 months after treatment [95,100]. The transient increase in viral load was especially prominent in patients with high-intensity schistosome infection at baseline [100]. Together, these consistently report increased HIV-1 viral load shortly after praziquantel treatment, which decreased within three months. Notably, these studies neither categorized people by treatment success or failure, nor controlled for other possible causes of viral load elevations.
In contrast, two studies examined effects of praziquantel treatment over periods longer than six months and assessed for schistosome eradication. One pre-ART study reported increased viral load after effective treatment, particularly six or more months post-treatment [104]. In the other study, schistosome-infected persons not yet eligible for ART who received quarterly praziquantel treatment also tended towards higher viral loads, as compared to controls who received praziquantel once and among whom 73% remained infected one year post-treatment (mean viral load ratio 1.88 [0.78, 4.53] p = 0.16) [99]. Therefore, these two studies suggest, rather unexpectedly, that long-term successful treatment of schistosome coinfection could increase HIV-1 viral load.
Consistent with these findings, three longitudinal studies have suggested slower HIV disease progression in those with chronic HIV-schistosome coinfection, versus those with HIV infection alone—results that contradicted investigators’ original hypotheses. In the first study, people with schistosome coinfection not yet on ART had a lower hazard ratio of developing CD4+ counts <350 cells/μL or death (HR = 0.31 [0.12–0.84]) [93]. The study of quarterly praziquantel treatment described above also reported a trend toward increased risk of progression to AIDS in the intensively-treated, ART-naïve group (log-rank chi-square 2.08, p = 0.15) [99]. A third study reported a hazard ratio of 0.58 [0.32–1.05] for death or loss to follow-up in those with HIV-schistosome coinfection starting ART, almost reaching significance [105].
Differences in results reported in these studies highlight the complexity of the relationship between HIV-1 viral load and schistosome infection, which may vary by schistosome species, other coinfections, and parasite and/or viral dynamics. One possible hypothesis to harmonize these studies is that schistosome and HIV infections could interact differently over time, with HIV-1 viral load set-points elevated shortly after HIV acquisition in some groups of people with schistosome coinfection, and later becoming lower in coinfected people as HIV becomes chronic. Taken together, these data raise the possibility that, in people with chronic HIV-schistosome co-infection, viral loads may be lower and disease progression slower than in chronic HIV alone.
Schistosomes and HIV vaccination
Three murine studies reported lower magnitude of HIV-specific CD8+ T-cell responses to HIV vaccines in schistosome-infected than uninfected mice (Table 6) [55,106,107]. In one of these, mice given praziquantel two weeks before vaccination still had lower titers of gp140-specific IgG antibodies and only partial restoration of CD4+ and CD8+ T-cell responses 12 days after vaccination [55]. Further, the presence of schistosome eggs in tissue, even in the absence of active worm infection, attenuated anti-gp140 antibody responses [55]. Another study reported that S. mansoni infection attenuated HIV-specific CD8+ IFN-ɣ secretion [106]. Praziquantel treatment four weeks before vaccination caused partial restoration of this CD8+ response, and treatment eight weeks before vaccination completely restored this response [107]. These studies collectively demonstrate that schistosome infection could decrease CD8+ T-cell responses to an HIV vaccine, and that restoration of CD8+ responses occurs at least eight weeks after treatment, the approximate time over which the burden of eggs trapped in tissue decreases [108].
Table 6. Effects of Schistosoma mansoni Infection on Response to HIV Vaccine in Mice.
| Study | Study Design | Time Elapsed Between Vaccine and Assessment | Vaccine Response in Schistosome Infected | Overall Effects of S. mansoni on HIV Vaccine |
|---|---|---|---|---|
| Dzhivhuho 2018 [55] | Mice with and without Sm were given candidate HIV vaccines Some mice were treated with PZQ* pre-vaccine |
12 days post-vaccine (vaccinated 2 weeks after PZQ) | ↓ HIV-specific splenocyte IFN-ɣ and IL-2 secretion Partial restoration of IFN-ɣ and IL-2 after PZQ ↓ gp-140 IgG antibody production in Sm |
Impaired HIV-specific cellular immune response to HIV vaccine Cellular immune response was restored completely when PZQ treatment was provided at least 8 weeks prior to vaccination |
| Da’dara 2006 [106] | Mice with and without Sm were given candidate HIV vaccines | 2 weeks | ↓ HIV-specific IFN-ɣ secretion by CD8+ T cells in Sm | |
| Da’dara 2010 [107] | Mice with Sm, mice with prior Sm who were treated, and uninfected mice were given candidate HIV vaccines | 2 weeks post-vaccine (vaccinated 4 or 8 weeks after PZQ) | ↑ HIV-specific IFN-ɣ secretion by CD8+ T cells after PZQ (completely restored when vaccinated 8 weeks after PZQ) |
*PZQ = praziquantel
Herpesviruses in mice and humans
Herpesviruses, which cause lifelong infection marked by periods of latency and reactivation, may offer important insights into interactions between schistosomes and viruses. A study in mice with chronic latent murine ɣ-herpesvirus that were infected with S. mansoni developed systemic herpesvirus reactivation with concomitant increases in IL-4 and decreased IFN-γ compared to herpesvirus mono-infected mice (Table 7) [109].
Table 7. Effects of Schistosome Infection on the Host Immune Response to Herpesviruses, and on Prevalence of Kaposi’s Sarcoma-Associated Herpesvirus.
| Study | Subject Type and Virus | Sample Size | Sex (% Female) and Age | Country | Species† | Differences in Schistosome Infected Compared to Uninfected | Overall Effects of Schistosome Infection |
|---|---|---|---|---|---|---|---|
| Reese 2014 [109] | Mice; murine ɣ-herpes-virus 68 | Not reported | Not reported | N/A | Sm or Hp |
↑ systemic herpes-virus reactivation Reactivation was dependent on IL-4 and STAT-6 |
Increased Th2 cyto-kines were associated with systemic viral reactivation. Increased KSHV sero-prevalence in Sm, with evidence stronger for men. |
| Fu 2012 [110] | Human, KSHV | 105 | 47% F, Age range not reported | China | Sj |
KSHV prevalence: 8.4% among Sj 6.6% among uninfected (men: 8.4% versus 2.2%)* |
|
| Wakeham 2011 [111] | Human, KSHV | 1915 | 100% F, median age 23 | Uganda | Sm |
KSHV prevalence: 60% among Sm 61% among uninfected |
|
| Nalwoga 2019 [112] | Human, KSHV | 1137 | 44% F, median age 25 | Uganda | Sm |
KSHV prevalence: 89% among Sm 77% among uninfected* Odds increased after controlling for sex* |
†Sm = S. mansoni; Sh = S. haematobium; Sj = S. japonicum; Hp = Heligmosomoides polygyrus
*Statistically significant
In China, men with S. japonicum infection had higher KSHV seroprevalence compared to men without S. japonicum, although no difference was observed in women [110]. Similarly, no difference in KSHV seroprevalence was observed in pregnant women in Uganda based on S. mansoni status [111]. In contrast, in Ugandan fishing communities, seroprevalence of KSHV was 89% in those with S. mansoni infection versus 77% in those without, and odds of KSHV remained significant after adjusting for sex, age, and other parasitic infections [112]. In addition, elevated anti-K8.1 antibodies in S. mansoni infection suggested increased KSHV reactivation [112]. These studies, supported by mouse models discussed previously [109], suggest that KSHV is an additional virus for which schistosome infection may impair host anti-viral defense and subsequent control, with stronger evidence for this effect in men than in women.
Respiratory viruses
Effects of schistosome infection on host immune response to respiratory viruses
Five studies have investigated immune effects of S. mansoni infection on respiratory viruses in mice (Table 8). In the first study, mice with acute respiratory herpesvirus-S. mansoni coinfection had increased IL-4 and expanded CD8+ T-cell viral-specific effector responses in the lung, leading to enhanced control of the respiratory virus [113]. This suggests that schistosome-induced Th2 predominance, demonstrated here by increased IL-4, may be beneficial for viral control in the lung.
Table 8. Effects of Schistosome Infection on the Host Immune Response to Respiratory Viruses in Mice.
| Study | Virus | Sex (% Female) | Species† | Differences in Schistosome Infected Compared to Uninfected | Overall Effects of Schistosome Infection |
|---|---|---|---|---|---|
| Rolot 2018 [113] | Murine respiratory ɣ-herpes-virus 4 | 100% F | Sm |
In lung: ↑ IL-4 ↑ CD8+ T-cell viral-specific effector responses ↓ virus titers |
Increased Th2 responses and decreased Th1 responses were associated with better viral control in the lung. |
| Danz 2016 [114] | Influenza A | 100% F | Sm |
↓ mortality and morbidity ↓ influenza-specific IFN-ɣ secretion by mediastinal CD8+ T cells ↓ IFN-ɣ transcripts in lungs ↓ alveolar inflammation |
|
| Tundup 2017 [115] | Influenza A | Not reported | Sm |
↓ weight loss and lung injury ↑ dendritic cells and alternatively activated macrophages in lungs ↓ expression of lung IL-6, TNF-α |
|
| Kadl 2018 [116] | Influenza A | Not reported | Sm |
↓ mortality ↑ influenza-specific IgM & IgG ↑ influenza-specific CD8+ T cell responses ↑ epithelial regeneration |
|
| Scheer 2014 [117] | Influenza A + Pneumonia virus of mice | Not reported | Sm |
↓ mortality and weight loss ↑ mucus production and goblet cell hyperplasia in lungs ↓ lung viral burden |
†Sm = S. mansoni; Sh = S. haematobium; Sj = S. japonicum; Hp = Heligmosomoides polygyrus
*Statistically significant
A similar beneficial anti-viral effect in the lung has been observed in mice coinfected with S. mansoni plus influenza A. Mice with S. mansoni infection that were challenged with influenza A virus had decreased mortality, decreased weight loss, decreased alveolar inflammation, lower expression of pro-inflammatory cytokines IL-6 and TNF-α, and greater lung epithelial regeneration than mice without S. mansoni [114–116]. Comparable beneficial effects have been observed in mice with S. mansoni and pneumonia virus [117] and in studies of other helminths [118]. Proposed mechanisms for protection include goblet cell hyperplasia, excessive mucus secretion, and alterations in interferon levels. These intriguing data may be attributable to the unique environment of the lung compared to the rest of the body. Together with the studies of schistosome infection during chronic HIV, these mouse respiratory virus data exemplify another scenario in which schistosome-viral coinfection may be surprisingly beneficial compared to viral infection alone.
Human papillomavirus
Schistosomes and prevalence of HPV
Two small studies suggested possible interactions between S. haematobium and HPV. In 37 women followed for 5 years, S. haematobium was associated with development of high-grade squamous intraepithelial neoplasia but not with high-risk HPV persistence (Table 9) [119]. Genital HPV DNA was also associated with reported or confirmed S. haematobium in Tanzania, but only 6 women had confirmed schistosome infection [120]. Therefore, despite both pathogens affecting the genital mucosa, interactions between S. haematobium and HPV remain poorly understood.
Table 9. Schistosomes and Interactions with HPV, HTLV-1, and Measles.
| Study | Subject Type | Sample Size | Sex (% Female) | Age in Years (Weighted Mean) | Country | Species† | Differences in Schistosome Infected Compared to Uninfected | Overall Effects of Schisto-some Infection |
|---|---|---|---|---|---|---|---|---|
| HPV Prevalence | ||||||||
| Kjetland 2010 [119] | Human | 37 | 100% F | Range, 20–55 | Zimbab-we | Sh |
7 women developed HGSIL and 6 had Sh* No effect on HPV persistence (29% versus 19%) |
Possible increases in HPV prevalence and sequelae; larger studies needed |
| Petry 2003 [120] | Human | 218 | 100% F | Median,28.4 | Tanzaniaand Germany | Sh | Borderline ↑ HPV prevalence (83% in confirmed Sh vs 39% in uninfected) | |
| HPV Vaccine | ||||||||
| Gent 2019 [121] | Baboon | 10 | Not reported | Sub-adult | NA | Sm |
↓ HPV-specific IgG after vaccination in Sh IgG restored in baboons given PZQ before vaccine |
Discrepant data; large human study suggests no effect |
| Brown 2014 [122] | Human | 298 | 100% F | Range, 10–25 | Tanzania | Sh, Sm | No difference in HPV IgG antibody titers | |
| HTLV-1 | ||||||||
| Lima 2013 [125] | Human | 26 | 42% F | 48 | Brazil | Sm | ↓ IFN-ɣ and ↑ IL-10 after schistosome antigen stimulation | Decreased Th1 cytokines and HTLV-1 viral load in vitro, which may contribute to observed lower rates of tropical spastic paralysis |
| Lima 2017 [126] | Human | 38 | 76% F | 47.2 | Brazil | Sm | ↓ CXCL9 after schistosome antigen stimulation | |
| Santos 2004 [127] | Human | 120 | Not reported | Not reported | Brazil | Sm | ↓ IFN-ɣ and ↑ IL-10 in unstimulated cultures | |
| Porto 2005 [124] | Human | 70 | Approx-imately 15% F | Approx-imately 46 | Brazil | Sm |
↓ IFN-ɣ and ↑ IL-10 ↓ frequency of CD4+ and CD8* IFN-ɣ secreting cells ↓ HTLV-1 proviral DNA load in PBMCs |
|
| Response to Rubeola (Measles) Vaccine | ||||||||
| Tweyon-gyere 2019 [128] | Human | 239 | 53% F | 3.9 years | Uganda | Sm |
↓ anti-measles IgG in Sm at one week; lower IgG trend at 6 months ↓ % with protective antibody levels in Sm ↓ anti-measles IgG after PZQ |
Active schisto-some infection during measles vaccine may impair anti-measles antibody response. Maternal Sm infection did not consistently impact children’s measles vaccine response. Adults with Sm did not have decreased measles IgG. |
| Nono 2018 [129] | Human | 85 | Not reported for sub-study | Range, 5–18 | Came-roon | Sh, Sm | ↓ anti-measles IgG in Sm but not in Sh (children had been previously vaccinated) | |
| Jiz 2013 [130] | Human | 104 | Not reported | Range, 7–16 | Phili-ppines | Not listed | No difference in post-vaccine anti-MMR cytokine responses in schistosome infected | |
| Ondigo 2018 [131] | Human | 99 | Not reported | 2 years | Kenya | Sm | ↓ anti-measles IgG in 2-year-olds whose mothers had Sm during pregnancy | |
| Kizito 2013 [132] | Human | 711 | Not reported | 1 year | Uganda | Sm | No difference in post-vaccine anti-measles IgG in 1-year-olds whose mothers had Sm during pregnancy | |
| Storey 2017 [133] | Human | 100 | 82% F | 41 | Kenya | Sm | No difference in anti-measles IgG in HIV-infected adults with Sm | |
*HGSIL = High grade squamous intraepithelial lesion
†Sm = S. mansoni; Sh = S. haematobium
Schistosomes and HPV vaccination
Two studies have investigated the effect of schistosome infection on HPV vaccination. A small number of S. mansoni-infected baboons had lower HPV IgG antibody titers than baboons without schistosome infection, though praziquantel treatment prior to vaccination eliminated these differences [121]. However, a large study reported no difference in anti-HPV antibody titers seven and 12 months after HPV vaccination in 298 girls and women aged 10–25 with versus without S. mansoni infection [122]. Importantly, diagnosis of schistosomes by egg excretion, which is poorly sensitive in women [123], could have led to misclassification of infected women. Additional studies to understand schistosome-HPV interactions should be prioritized.
Human T-cell leukemia virus type 1 (HTLV-1)
Effects of schistosome infection on host immune response to HTLV-1
Three studies examined effects of S. mansoni coinfection on Th1 proinflammatory responses in HTLV-1, which are associated with development of tropical spastic paralysis [124]. In two studies, the addition of S. mansoni antigens to PBMC cultures from HTLV-1-infected adults caused increased IL-10 and decreased IFN-γ and CXCL9, important for Th1 cell recruitment [125,126]. Another reported decreased IFN-γ and increased IL-10 in unstimulated PBMC cultures of adults with HTLV-1-helminth co-infection versus with HTLV-1 alone [127], as well as decreased HTLV-1 proviral load in PBMCs of those with coinfection [124]. These data fit with one study’s clinical documentation of schistosome or strongyloides infections seven-fold more frequently in HTLV-1 disease carriers than in HTLV-1 infected persons who developed tropical spastic paralysis [124], suggesting that decreased Th1 and increased T regulatory could prevent the development of inflammatory paralysis. In this instance, HTLV-1 could be another viral infection that is positively impacted by schistosome coinfection and confirmatory studies are needed.
Rubeola virus (measles)
Schistosomes and measles vaccination
Five studies examined the effect of S. mansoni on measles vaccine response. In the first, preschool children with S. mansoni infection who received catch-up measles vaccination achieved lower anti-measles IgG titers. Children treated with praziquantel treatment before or at the time of catch-up measles immunization developed higher IgG titers than children treated after immunization, and children who remained schistosome-infected 24 weeks post-vaccination had lower IgG titers than those cured [128]. Lower anti-measles IgG titers were similarly documented in S. mansoni-infected schoolchildren [129], though a second study showed robust Th1 and pro-inflammatory post-vaccination cytokine responses to measles antigens [130]. Another study reported that two-year-old children born to mothers with S. mansoni had lower anti-measles IgG titers than those born to uninfected mothers [131], although no titer differences were observed in one-year-olds [132] and neither study determined schistosome infection status of the children. In HIV-infected antiretroviral therapy (ART)-naïve adults, anti-measles IgG titers were similar regardless of schistosome infection status [133]. Overall, these results suggest that active schistosome infection at the time of measles vaccination may impair anti-measles antibody response. Schistosome infection of the mother and schistosome infection acquired in adulthood seem to have limited effects compared to the relatively stronger negative impact of schistosome infection during childhood at the time of measles vaccination.
In summary, the 103 studies included in this review illustrate the breadth of data and the complex impact of schistosomes on host anti-viral immunity. Ramifications on viral acquisition, virulence, and prevention are diverse and vary not only with host factors but also with time-sensitive factors including the chronicity of the schistosome infection and elapsed time since anti-schistosome treatment. In addition, interactions of schistosomes with viruses such as HCV and HIV have been relatively well-studied, while only a few studies each have examined effects on other highly prevalent viruses including HPV, KSHV, and influenza.
Discussion
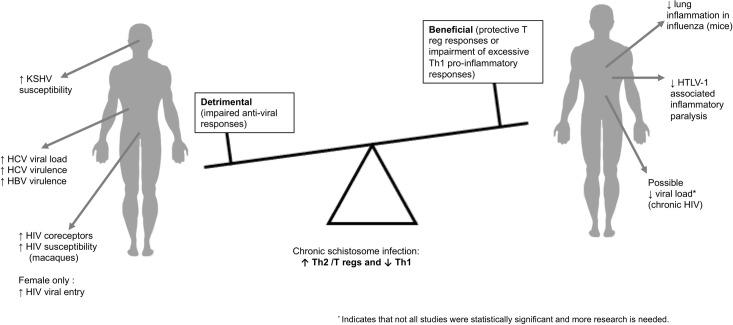
Studies included in this review consistently demonstrate that schistosome infection impairs host immune response to some anti-viral vaccines, skews anti-viral immunity, and impacts, possibly both positively and negatively, virulence of viral infections depending on the virus and the site of pathology (Fig 2). With regards to viral prevention, active schistosome infection seems to have mostly harmful effects by decreasing cellular and humoral responses to anti-viral vaccinations and increasing risk for acquisition of certain viruses, including HIV and KSHV. Once a virus has been acquired, schistosome coinfection induces Th2-dominant immune responses that may exacerbate severity of some viral infection outcomes, particularly hepatic damage by hepatidities and enteric viruses. However, in other viral infections including HIV, HTLV-1, and respiratory viruses, schistosome infection may mitigate disease severity. Taken together, these findings urge comprehensive consideration of effects of co-infections in endemic settings where viral and parasitic infections frequently converge, and point to the need for further research (Table 10). This is especially important given the high prevalence of schistosomiasis and helminth infections globally [134,135].
Fig 2. Chronic schistosome infection was associated with impaired anti-viral responses in some viral infections.
In others, chronic schistosome infection appeared to offer protection against viral infection attributable to T regulatory responses or to control of excessive Th1 inflammatory responses.
Table 10. Broad Priority Research Areas for Schistosome-Virus Coinfections.
| Topic | Priority Research Needs |
|---|---|
| Vaccines | Vaccine efficacy in schistosome-endemic settings before schistosome infection is acquired, or in LMIC settings in which schistosomes are endemic versus LMIC settings in which schistosomes are not endemic Effect of schistosome infection on other anti-viral vaccines, including rotavirus and poliovirus Optimal timing for praziquantel treatment prior to vaccination |
| Schistosome-Virus Immunology | Precise timing of the dynamics of the human immune response to schistosome infection, and to schistosome-virus coinfection Roles of mucosal and systemic immune cell populations in mediating response to virus in schistosome-infected populations |
| Schistosome-Virus Treatment | Antiviral effects of schistosome-induced host immunoregulatory molecules or cells Antiviral effects of immunomodulatory parasite effector molecules |
One of the most important potential public health impacts of our findings is that schistosome infection decreases the cellular and humoral immune responses to several anti-viral immunizations including hepatitis B and measles. Poorer responses to poliovirus, rotavirus, and Ebola vaccines have been widely reported in low-income helminth-endemic countries [136–138]. For example, oral rotavirus vaccine efficacy was 49% in Malawi versus 77% in higher-income countries [139], and the effects of schistosome infection are not known. Additional data are urgently needed to determine whether treatment of schistosome infection prior to vaccine administration could improve efficacy, as suggested by multiple studies in this review, and to optimize treatment timing in order to maximize resolution of immune alterations and elimination of schistosome eggs while limiting risk of schistosome re-infection. Coupling staged anti-schistosome treatment and subsequent vaccination has potential to confer the dual benefit of lowering schistosome-associated pathology and improving viral prevention. Schistosome treatment may improve responses to bacterial vaccines such as tetanus and BCG as well [41,140,141].
Studies have also consistently reported that schistosome-hepatitis coinfection increases severity of liver disease, and limited evidence suggests that anti-schistosome treatment could prevent or alleviate liver fibrosis in schistosome-hepatitis co-infected persons. Therefore, screening and treating for schistosome infection immediately in individuals diagnosed with hepatitis viruses could avert severe liver disease.
While many studies demonstrate potential benefits of anti-schistosome treatment, others invite further research in the context of some viral infections. Our synthesis of articles suggests that treatment of schistosome infection could increase HIV viral load in chronic HIV, and that coinfection with schistosomes could reduce mortality in HIV-infected individuals. Both findings are under-studied and lack statistical significance; further research is imperative to fully understand their implications. Their existence, however, does suggest that mechanistic studies on the potential beneficial effect of schistosome coinfection in chronic HIV are needed to pave the way for novel immunoregulatory interventions.
Across an array of viruses and mammals, articles in this review consistently report Th1-dominant immune responses to viral mono-infection, versus Th2 and T regulatory-dominant responses with schistosome-virus coinfection. These conserved immune responses to virus-parasite infections may suggest strategies that could harness the beneficial effects of parasites in some viral infections to improve clinical outcomes. The strong T regulatory component of chronic schistosome infection may be particularly important, and studies in this review have associated a T regulatory environment with decreased hepatic damage from HBV [36] and an HTLV-1 carrier rather than pro-inflammatory disease state [124]. T regs also have decreased HIV-1 R5 virus susceptibility [142], which could limit cell-cell spread and slow HIV disease progression. Other immune cell populations upregulated in chronic schistosome infection, such as alternatively activated macrophages [109], and immunomodulatory parasite effector molecules that incite Th2 and T regulatory responses (the subject of a recent comprehensive review) [143], may have promising anti-viral therapeutic efficacy. Efforts to identify and test immune cell products and schistosome immunomodulatory secretions should be included in ongoing global research to prevent and treat viral infections. In addition, recent developments in the area of controlled human schistosome challenge model [19] are expected to facilitate more precise mapping of the host response throughout acute and chronic schistosome infection. Although these controlled schistosome infections use only male worms and therefore may induce fewer Th2 and T reg responses because schistosome eggs are not produced, they nonetheless provide a potent resource to understand immune responses to coinfections.
Effects of timing, duration, and dose of both schistosome infection and viral infections could also influence the manifestation of schistosome-viral coinfection. Individuals may be less likely to become infected with schistosome infection before acquiring viral infections that are common in childhood, such as rotavirus. Conversely, they may be more likely to become infected with schistosome infection during childhood before acquiring sexually transmitted infections, such as HIV and HPV. Amount of time with untreated schistosome infection may also impact the host response to coinfection. Thus, incorporating age prevalence curves of viral infections in schistosome-endemic regions may be useful when considering public health interventions to address coinfection.
This review has limitations. Only studies that documented active schistosome infection by microscopy or antigen testing, but not by antibodies, were included due to antibodies remaining positive after clearance of infection. This may have caused us to underestimate effects of schistosome infection, because individuals who had cleared schistosome infections that previously affected their viral control would have been considered schistosome-uninfected. It also could preclude capturing long-term immunological effects of past infections. Effects of environmental, sociodemographic, and economic factors associated with schistosome infection also could not be evaluated [144]. Further, only studies examining the impact of schistosome infection on viral infections were included. Those assessing the impact of viruses on schistosome infection were beyond the scope of this review, as were those not published in English. Finally, publication bias could have led to the non-publication of studies that showed no impact of schistosomes on viral infections and thereby would not have been included.
In summary, this review highlights the major and complex effects of schistosome infection on host response to viruses, encompassing viral acquisition, immune response, virulence, and prevention. Considering the impact of parasitic infections on viral control could have important public health implications for anti-viral vaccination strategies and control of viral infections globally.
Supporting information
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
JAD received support from NIH K23 AI110238 (https://www.nih.gov/) and the Doris Duke Charitable Foundation 2017067 (https://www.ddcf.org/). The funders had no role in the study design, analysis, decision to publish, or preparation of the manuscript.
References
- 1.Roth GA, Abate D, Abate KH, Abay SM, Abbafati C, Abbasi N, et al. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392: 1736–1788. 10.1016/S0140-6736(18)32203-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Troeger CE, Blacker BF, Khalil IA, Zimsen SRM, Albertson SB, Abate D, et al. Mortality, morbidity, and hospitalisations due to influenza lower respiratory tract infections, 2017: an analysis for the Global Burden of Disease Study 2017. Lancet Respir Med. 2019;7: 69–89. 10.1016/S2213-2600(18)30496-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Center for Systems Science and Engineering. COVID-19 Dashboard. In: Johns Hopkins University and Medicine Coronavirus Resource Center [Internet]. 2020 [cited 9 May 2020]. Available: https://coronavirus.jhu.edu/map.html
- 4.Hotez PJ, Molyneux DH, Fenwick A, Kumaresan J, Sachs SE, Sachs JD, et al. Control of neglected tropical diseases. N Engl J Med. 2007;357: 1018–1027. 10.1056/NEJMra064142 [DOI] [PubMed] [Google Scholar]
- 5.O’Brien DP, Ford N, Djirmay AG, Calmy A, Vitoria M, Jensen TO, et al. Female Genital Schistosomiasis and HIV: Research Urgently Needed to Improve Understanding of the Health Impacts of This Important Coinfection. J Acquir Immune Defic Syndr. 2019;80: 489–493. 10.1097/QAI.0000000000001957 [DOI] [PubMed] [Google Scholar]
- 6.Bustinduy A, King C, Scott J, Appleton S, Sousa-Figueiredo JC, Betson M, et al. HIV and schistosomiasis co-infection in African children. Lancet Infect Dis. 2014;14: 640–649. 10.1016/S1473-3099(14)70001-5 [DOI] [PubMed] [Google Scholar]
- 7.von Braun A, Trawinski H, Wendt S, Lübbert C. Schistosoma and Other Relevant Helminth Infections in HIV-Positive Individuals—an Overview. Trop Med Infect Dis. 2019;4: 65. 10.3390/tropicalmed4020065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Furch BD, Koethe JR, Kayamba V, Heimburger DC, Kelly P. Interactions of Schistosoma and HIV in Sub-Saharan Africa: A Systematic Review. Am J Trop Med Hyg. 2020;102: 711–8. 10.4269/ajtmh.19-0494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abruzzi A, Fried B, Alikhan SB. Coinfection of Schistosoma Species with Hepatitis B or Hepatitis C Viruses. Adv Parasitol. 2016;91: 111–231. 10.1016/bs.apar.2015.12.003 [DOI] [PubMed] [Google Scholar]
- 10.Gasim GI, Bella A, Adam I. Schistosomiasis, hepatitis B and hepatitis C co-infection. Virol J. 2015;12: 19. 10.1186/s12985-015-0251-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van-Lume DS de M, Albuquerque M de FPM de, Souza AI de, Domingues ALC, Lopes EP de A, Morais CNL de, et al. Association between Schistosomiasis mansoni and hepatitis C: systematic review. Rev Saude Publica. 2013;47: 414–24. 10.1590/S0034-910.2013047004247 [DOI] [PubMed] [Google Scholar]
- 12.World Health Organization. Schistosomiasis. World Health Organization; 2020 [cited 8 May 2020]. Available: http://www.who.int/news-room/fact-sheets/detail/schistosomiasis
- 13.Wang H, Naghavi M, Allen C, Barber RM, Carter A, Casey DC, et al. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388: 1459–1544. 10.1016/S0140-6736(16)31012-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339: b2700. 10.1136/bmj.b2700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stedman’s Medical Dictionary for the Health Professions and Nursing. Philadelphia: Lippincott Williams and Wilkins; 2016. [Google Scholar]
- 16.Hannes K. Critical appraisal of qualitative research | University College London. Version 1. In: Noyes J, Booth A, Hannes K, Harden A, Harris J, Lewin S LC, editor. Supplementary Guidance for Inclusion of Qualitative Research in Cochrane Systematic Reviews of Interventions. Version 1. Cochrane Collaboration Qualitative Methods Group; 2011. [Google Scholar]
- 17.CASP Checklists—CASP—Critical Appraisal Skills Programme. [cited 4 Oct 2019]. Available: https://casp-uk.net/casp-tools-checklists/
- 18.Gatlin MR, Black CL, Mwinzi PN, Secor WE, Karanja DM, Colley DG. Association of the gene polymorphisms IFN-γ +874, IL-13-1055 and IL-4–590 with patterns of reinfection with schistosoma mansoni. PLoS Negl Trop Dis. 2009;3. 10.1371/journal.pntd.0000375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Langenberg MCC, Hoogerwerf MA, Koopman JPR, Janse JJ, Kos-van Oosterhoud J, Feijt C, et al. A controlled human Schistosoma mansoni infection model to advance novel drugs, vaccines and diagnostics. Nat Med. 2020;26: 326–332. 10.1038/s41591-020-0759-x [DOI] [PubMed] [Google Scholar]
- 20.Kamal SM, Rasenack JW, Bianchi L, Al Tawil A, El Sayed Khalifa K, Peter T, et al. Acute hepatitis C without and with schistosomiasis: correlation with hepatitis C-specific CD4(+) T-cell and cytokine response. Gastroenterology. 2001;121: 646–56. 10.1053/gast.2001.27024 [DOI] [PubMed] [Google Scholar]
- 21.Kamal SM, Bianchi L, Al Tawil A, Koziel M, El K, Khalifa S, et al. Specific Cellular Immune Response and Cytokine Patterns in Patients Coinfected with Hepatitis C Virus and Schistosoma mansoni. J Infect Dis. 2001;184: 972–82. 10.1086/323352 [DOI] [PubMed] [Google Scholar]
- 22.Kamel MM, Romeyia SA, Ali MM, Aziz HA, Abdel-Moneim AS. P selectin and T cell profiles provide verification to understand the pathogenesis of liver cirrhosis in HCV and Schistosoma mansoni infections. Microb Pathog. 2014;73: 19–24. 10.1016/j.micpath.2014.05.006 [DOI] [PubMed] [Google Scholar]
- 23.Loffredo-Verde E, Abdel-Aziz I, Albrecht J, El-Guindy N, Yacob M, Solieman A, et al. Schistosome infection aggravates HCV-related liver disease and induces changes in the regulatory T-cell phenotype. Parasit Immunol. 2015;37: 97–104. 10.1111/pim.12171 [DOI] [PubMed] [Google Scholar]
- 24.Elrefaei M, El-Sheikh N, Kamal K, Cao H. HCV-specific CD27- CD28- memory T cells are depleted in hepatitis C virus and Schistosoma mansoni co-infection. Immunology. 2003;110: 513–518. 10.1111/j.1365-2567.2003.01769.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kamal SM, Graham CS, He Q, Bianchi L, Tawil AA, Rasenack JW, et al. Kinetics of intrahepatic hepatitis C virus (HCV)-specific CD4+ T cell responses in HCV and Schistosoma mansoni coinfection: relation to progression of liver fibrosis. J Infect Dis. 2004;189: 1140–50. 10.1086/382278 [DOI] [PubMed] [Google Scholar]
- 26.El-Kady IM, Lotfy M, Badra G, El-Masry S, Waked I. Interleukin (IL)-4, IL-10, IL-18 and IFN-gamma cytokines pattern in patients with combined hepatitis C virus and Schistosoma mansoni infections. Scand J Immunol. 2005;61: 87–91. 10.1111/j.0300-9475.2005.01529.x [DOI] [PubMed] [Google Scholar]
- 27.Emam EA, Emam M, Shehata AE, Emara M. Impact of Schistosoma mansoni co-infection on serum profile of interferon-gamma, interleukin-4 and interleukin-10 in patients with chronic hepatitis C virus infection. Egypt J Immunol. 2006;13: 33–40. [PubMed] [Google Scholar]
- 28.Ahmed S. Retrospective and Prospective Study of the Interrelation between Interleukin-28B and Endogenous Interferon Gamma in Human Infected with Hepatitis-C Virus and/or Schistosoma mansoni. University of Cairo. 2016. Available at: http://erepository.cu.edu.eg/index.php/cutheses/article/view/7152 [Google Scholar]
- 29.Elrefaei M, El-sheikh N, Kamal K, Cao H. Analysis of T cell responses against hepatitis C virus genotype 4 in Egypt. J Hepatol. 2004;40: 313–318. 10.1016/j.jhep.2003.10.007 [DOI] [PubMed] [Google Scholar]
- 30.Bahgat MM, El-Far MA-E, Mesalam AA, Ismaeil AA-E, Ibrahim AA, Gewaid HE, et al. Schistosoma mansoni soluble egg antigens enhance HCV replication in mammalian cells. J Infect Dev Ctries. 2010;4: 226–34. 10.3855/jidc.522 [DOI] [PubMed] [Google Scholar]
- 31.El-Awady MK, Youssef SS, Omran MH, Tabll AA, El Garf WT, Salem AM. Soluble egg antigen of Schistosoma Haematobium induces HCV replication in PBMC from patients with chronic HCV infection. BMC Infect Dis. 2006;6: 91. 10.1186/1471-2334-6-91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Edwards MJ, Buchatska O, Ashton M, Montoya M, Bickle QD, Borrow P. Reciprocal Immunomodulation in a Schistosome and Hepatotropic Virus Coinfection Model. J Immunol. 2005;175: 6275–6285. 10.4049/jimmunol.175.10.6275 [DOI] [PubMed] [Google Scholar]
- 33.El-Shazly Y, Abdel-Salam AF, Abdel-Ghaffar A, Mohran Z, Saleh SM. Schistosomiasis as an important determining factor for the response of egyptian patients with chronic hepatitis c to therapy with recombinant human α-2 interferon. Trans R Soc Trop Med Hyg. 1994;88: 229–231. 10.1016/0035-9203(94)90310-7 [DOI] [PubMed] [Google Scholar]
- 34.Kamal SM, Madwar MA, Peters T, Fawzy R, Rasenack J. Interferon therapy in patients with chronic hepatitis C and schistosomiasis. J Hepatol. 2000;32: 172–4. 10.1016/s0168-8278(00)80207-x [DOI] [PubMed] [Google Scholar]
- 35.Attallah AM, El-Far M, Omran MM, Farid K, Attallah AA, Abd-Elaziz D, et al. Levels of Schistosoma mansoni Circulating Antigen in Chronic Hepatitis C Patients with Different Stages of Liver Fibrosis. J Immunoass Immunochem. 2016;37: 316–330. 10.1080/15321819.2015.1135163 [DOI] [PubMed] [Google Scholar]
- 36.Loffredo-Verde E, Bhattacharjee S, Malo A, Festag J, Kosinska AD, Ringelhan M, et al. Dynamic, Helminth-Induced Immune Modulation Influences the Outcome of Acute and Chronic Hepatitis B Virus Infection. J Infect Dis. 2020;221: 1448–1461. 10.1093/infdis/jiz594 [DOI] [PubMed] [Google Scholar]
- 37.Kotkat A, el-Masry S, Abdel-al Mahmoud N. Chronic hepatitis B antigenaemia in bilharzial patients treated with Praziquantel. J Egypt Public Health Assoc. 1990;65: 543–54. [PubMed] [Google Scholar]
- 38.Farghaly AG, Barakat RM. The effect of praziquantel administration on the course of hepatitis B among cases with concomitant schistosome infection. J Egypt Public Health Assoc. 1993;68: 81–100. [PubMed] [Google Scholar]
- 39.Chen L, Liu W, Lei J, Guan F, Li M, Song W, et al. Chronic Schistosoma japonicum infection reduces immune response to vaccine against hepatitis B in mice. PLoS One. 2012;7: e51512. 10.1371/journal.pone.0051512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ghaffar YA, Saleh MS, Dorgham LS, Wahab MFA, Kamel M, El Deeb AS. Hepatitis B Vaccination in Children Infected with Schistosoma Mansoni: Correlation with Ultrasonographic Data. Am J Trop Med Hyg. 1990;43: 516–519. 10.4269/ajtmh.1990.43.516 [DOI] [PubMed] [Google Scholar]
- 41.Riner DK, Ndombi EM, Carter JM, Omondi A, Kittur N, Kavere E, et al. Schistosoma mansoni Infection Can Jeopardize the Duration of Protective Levels of Antibody Responses to Immunizations against Hepatitis B and Tetanus Toxoid. PLoS Negl Trop Dis. 2016;10: e0005180. 10.1371/journal.pntd.0005180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bassily S, Strickland GT, Abdel-Wahab MF, Esmat GE, Narooz S, el-Masry NA, et al. Efficacy of hepatitis B vaccination in primary school children from a village endemic for Schistosoma mansoni. J Infect Dis. 1992;166: 265–8. 10.1093/infdis/166.2.265 [DOI] [PubMed] [Google Scholar]
- 43.Bassily S, Kotkat A, Hyams KC, Youssef FG, El-Masry NA, Arthur R, et al. Immunogenicity of recombinant hepatitis B vaccine among infants of mothers with active schistosomiasis. Am J Trop Med Hyg. 1997;57: 197–9. 10.4269/ajtmh.1997.57.197 [DOI] [PubMed] [Google Scholar]
- 44.Nash S, Mentzer AJ, Lule SA, Kizito D, Smits G, van der Klis FRM, et al. The impact of prenatal exposure to parasitic infections and to anthelminthic treatment on antibody responses to routine immunisations given in infancy: Secondary analysis of a randomised controlled trial. PLoS Negl Trop Dis. 2017;11: e0005213. 10.1371/journal.pntd.0005213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Malhotra I, McKibben M, Mungai P, McKibben E, Wang X, Sutherland LJ, et al. Effect of Antenatal Parasitic Infections on Anti-vaccine IgG Levels in Children: A Prospective Birth Cohort Study in Kenya. PLoS Negl Trop Dis. 2015;9: e0003466. 10.1371/journal.pntd.0003466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Malhotra I, LaBeaud AD, Morris N, McKibben M, Mungai P, Muchiri E, et al. Cord Blood Antiparasite Interleukin 10 as a Risk Marker for Compromised Vaccine Immunogenicity in Early Childhood. J Infect Dis. 2018;217: 1426–1434. 10.1093/infdis/jiy047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kleppa E, Ramsuran V, Zulu S, Karlsen GH, Bere A, Passmore J-AS, et al. Effect of female genital schistosomiasis and anti-schistosomal treatment on monocytes, CD4+ T-cells and CCR5 expression in the female genital tract. PLoS One. 2014;9: e98593. 10.1371/journal.pone.0098593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yegorov S, Joag V, Galiwango RM, Good S V., Mpendo J, Tannich E, et al. Schistosoma mansoni treatment reduces HIV entry into cervical CD4+ T cells and induces IFN-I pathways. Nat Commun. 2019;10: 2296. 10.1038/s41467-019-09900-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dupnik KM, Lee MH, Mishra P, Reust MJ, Colombe S, Haider SR, et al. Altered Cervical Mucosal Gene Expression and Lower Interleukin 15 Levels in Women With Schistosoma haematobium Infection but Not in Women With Schistosoma mansoni Infection. J Infect Dis. 2019;219: 1777–1785. 10.1093/infdis/jiy742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Prodger JL, Ssemaganda A, Ssetaala A, Kitandwe PK, Muyanja E, Mpendo J, et al. Schistosoma mansoni Infection in Ugandan Men Is Associated with Increased Abundance and Function of HIV Target Cells in Blood, but Not the Foreskin: A Cross-sectional Study. PLoS Negl Trop Dis. 2015;9: e0004067. 10.1371/journal.pntd.0004067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Secor WE, Shah A, Mwinzi PMN, Ndenga BA, Watta CO, Karanja DMS. Increased density of human immunodeficiency virus type 1 coreceptors CCR5 and CXCR4 on the surfaces of CD4(+) T cells and monocytes of patients with Schistosoma mansoni infection. Infect Immun. 2003;71: 6668–71. 10.1128/iai.71.11.6668-6671.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McElroy MD, Elrefaei M, Jones N, Ssali F, Mugyenyi P, Barugahare B, et al. Coinfection with Schistosoma mansoni is associated with decreased HIV-specific cytolysis and increased IL-10 production. J Immunol. 2005;174: 5119–23. 10.4049/jimmunol.174.8.5119 [DOI] [PubMed] [Google Scholar]
- 53.Erikstrup C, Kallestrup P, Zinyama-Gutsire RBL, Gomo E, van Dam GJ, Deelder AM, et al. Schistosomiasis and infection with human immunodeficiency virus 1 in rural Zimbabwe: systemic inflammation during co-infection and after treatment for schistosomiasis. Am J Trop Med Hyg. 2008;79: 331–7. [PubMed] [Google Scholar]
- 54.Obuku AE, Asiki G, Abaasa A, Ssonko I, Harari A, van Dam GJ, et al. Effect of Schistosoma mansoni Infection on Innate and HIV-1-Specific T-Cell Immune Responses in HIV-1-Infected Ugandan Fisher Folk. AIDS Res Hum Retroviruses. 2016;32: 668–75. 10.1089/AID.2015.0274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dzhivhuho GA, Rehrl SA, Ndlovu H, Horsnell WGC, Brombacher F, Williamson A-L, et al. Chronic schistosomiasis suppresses HIV-specific responses to DNA-MVA and MVA-gp140 Env vaccine regimens despite antihelminthic treatment and increases helminth-associated pathology in a mouse model. PLoS Pathog. 2018;14: e1007182. 10.1371/journal.ppat.1007182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Actor JK, Shirai M, Kullberg MC, Buller RM, Sher A, Berzofsky JA. Helminth infection results in decreased virus-specific CD8+ cytotoxic T-cell and Th1 cytokine responses as well as delayed virus clearance. Proc Natl Acad Sci USA. 1993;90: 948–52. 10.1073/pnas.90.3.948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Marshall MA, Jankovic D, Maher VE, Sher A, Berzofsky JA. Mice infected with Schistosoma mansoni develop a novel non-T-lymphocyte suppressor population which inhibits virus-specific CTL induction via a soluble factor. Microbes Infect. 2001;3: 1051–61. 10.1016/s1286-4579(01)01499-x [DOI] [PubMed] [Google Scholar]
- 58.Actor JK, Marshall MA, Eltoum IA, Buller RM, Berzofsky JA, Sher A. Increased susceptibility of mice infected with Schistosoma mansoni to recombinant vaccinia virus: association of viral persistence with egg granuloma formation. Eur J Immunol. 1994;24: 3050–6. 10.1002/eji.1830241220 [DOI] [PubMed] [Google Scholar]
- 59.Lacroix C, Akarid K, Chau F, Sinet M, Verola O, Carbon C, et al. The Th1 to Th2 shift induced by Schistosoma mansoni does not exacerbate murine aids (MAIDS). Parasite Immunol. 1998;20: 497–501. 10.1046/j.1365-3024.1998.00186.x [DOI] [PubMed] [Google Scholar]
- 60.Chenine A-L, Buckley KA, Li P-L, Rasmussen RA, Ong H, Jiang S, et al. Schistosoma mansoni infection promotes SHIV clade C replication in rhesus macaques. AIDS. 2005;19: 1793–7. 10.1097/01.aids.0000189857.51935.0b [DOI] [PubMed] [Google Scholar]
- 61.Ayash-Rashkovsky M, Chenine A-L, Steele LN, Lee SJ, Song R, Ong H, et al. Coinfection with Schistosoma mansoni reactivates viremia in rhesus macaques with chronic simian-human immunodeficiency virus clade C infection. Infect Immun. 2007;75: 1751–6. 10.1128/IAI.01703-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Buch S, Pinson D, King CL, Raghavan R, Hou Y, Li Z, et al. Inhibitory and enhancing effects of IFN-γ and IL-4 on SHIVKU replication in rhesus macaque macrophages: Correlation between TH2 cytokines and productive infection in tissue macrophages during late-stage infection. Cytokine. 2001;13: 295–304. 10.1006/cyto.2000.0829 [DOI] [PubMed] [Google Scholar]
- 63.Pearce EJ, Caspar P, Grzych JM, Lewis FA, Sher A. Downregulation of Th1 cytokine production accompanies induction of Th2 responses by a parasitic helminth, Schistosoma mansoni. J Exp Med. 1991;173: 159–66. 10.1084/jem.173.1.159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mouser EE, Pollakis G, Smits HH, Thomas J, Yazdanbakhsh M, de Jong EC, et al. Schistosoma mansoni soluble egg antigen (SEA) and recombinant Omega-1 modulate induced CD4+ T-lymphocyte responses and HIV-1 infection in vitro. Douek DC, editor. PLoS Pathog. 2019;15: e1007924. 10.1371/journal.ppat.1007924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kjetland EF, Ndhlovu PD, Gomo E, Mduluza T, Midzi N, Gwanzura L, et al. Association between genital schistosomiasis and HIV in rural Zimbabwean women. AIDS. 2006;20: 593–600. 10.1097/01.aids.0000210614.45212.0a [DOI] [PubMed] [Google Scholar]
- 66.Downs JA, Mguta C, Kaatano GM, Mitchell KB, Bang H, Simplice H, et al. Urogenital schistosomiasis in women of reproductive age in Tanzania’s Lake Victoria region. Am J Trop Med Hyg. 2011;84: 364–9. 10.4269/ajtmh.2011.10-0585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Downs JA, van Dam GJ, Changalucha JM, Corstjens PLAM, Peck RN, de Dood CJ, et al. Association of schistosomiasis and HIV infection in Tanzania. Am J Trop Med Hyg. 2012;87: 868–73. 10.4269/ajtmh.2012.12-0395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mvumbi GM, Gadoth A, Hoff NA, Musene K, Mukadi P, Martin-Blais RA, et al. Coinfections of Schistosoma haematobium amongst HIV-seropositive and -seronegative women: Kisantu Health Zone, Democratic Republic of Congo (Abstract 1971). American Society of Tropical Medicine and Hygiene Annual Meeting. 2018. p. 620. [Google Scholar]
- 69.Downs JA, de Dood CJ, Dee HE, McGeehan M, Khan H, Marenga A, et al. Schistosomiasis and Human Immunodeficiency Virus in Men in Tanzania. Am J Trop Med Hyg. 2017;96: 856–62. 10.4269/ajtmh.16-0897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Woodburn PW, Muhangi L, Hillier S, Ndibazza J, Namujju PB, Kizza M, et al. Risk factors for helminth, malaria, and HIV infection in pregnancy in Entebbe, Uganda. PLoS Negl Trop Dis. 2009;3. 10.1371/journal.pntd.0000473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sanya RE, Muhangi L, Nampijja M, Nannozi V, Nakawungu PK, Abayo E, et al. Schistosoma mansoni and HIV infection in a Ugandan population with high HIV and helminth prevalence. Trop Med Int Heal. 2015;20: 1201–8. 10.1111/tmi.12545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mazigo HD, Dunne DW, Wilson S, Kinung’hi SM, Pinot de Moira A, Jones FM, et al. Co-infection with Schistosoma mansoni and Human Immunodeficiency Virus-1 (HIV-1) among residents of fishing villages of north-western Tanzania. Parasit Vectors. 2014;7: 587. 10.1186/s13071-014-0587-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.De Lima-Costa MFF, Proietti FA, Paulino UHM, Antunes CMF, Guimaraes MDC, Rocha RS, et al. Absence of cross-reactivity between schistosoma mansoni infection and human immunodeficiency virus (HIV). Trans R Soc Trop Med Hyg. 1988;82: 262. 10.1016/0035-9203(88)90441-5 [DOI] [PubMed] [Google Scholar]
- 74.Ssetaala A, Nakiyingi-Miiro J, Asiki G, Kyakuwa N, Mpendo J, Van Dam GJ, et al. Schistosoma mansoni and HIV acquisition in fishing communities of Lake Victoria, Uganda: a nested case-control study. Trop Med Int Heal. 2015;20: 1190–1195. 10.1111/tmi.12531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bochner AF, Baeten JM, Secor WE, Dam GJ, Szpiro AA, Njenga SM, et al. Associations between schistosomiasis and HIV-1 acquisition risk in four prospective cohorts: a nested case-control analysis. J Int AIDS Soc. 2020;23. 10.1002/jia2.25534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kroidl I, Saathof E, Maganga L, Makunde W, Hoerauf A, Geldmacher C, et al. Effect of Wuchereria bancrofti infection on HIV incidence in southwest Tanzania (EMINI): a prospective cohort study. Lancet. 2016;388: 1912–20. 10.1016/S0140-6736(16)31252-1 [DOI] [PubMed] [Google Scholar]
- 77.Downs JA, Dupnik KM, van Dam GJ, Urassa M, Lutonja P, Kornelis D, et al. Effects of schistosomiasis on susceptibility to HIV-1 infection and HIV-1 viral load at HIV-1 seroconversion: A nested case-control study. PLoS Negl Trop Dis. 2017;11: e0005968. 10.1371/journal.pntd.0005968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gallagher M, Malhotra I, Mungai PL, Wamachi AN, Kioko JM, Ouma JH, et al. The effects of maternal helminth and malaria infections on mother-to-child HIV transmission. AIDS. 2005;19: 1849–1855. 10.1097/01.aids.0000189846.90946.5d [DOI] [PubMed] [Google Scholar]
- 79.Chenine A-L, Shai-Kobiler E, Steele LN, Ong H, Augostini P, Song R, et al. Acute Schistosoma mansoni infection increases susceptibility to systemic SHIV clade C infection in rhesus macaques after mucosal virus exposure. PLoS Negl Trop Dis. 2008;2: e265. 10.1371/journal.pntd.0000265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Siddappa NB, Hemashettar G, Shanmuganathan V, Semenya AA, Sweeney ED, Paul KS, et al. Schistosoma mansoni Enhances Host Susceptibility to Mucosal but Not Intravenous Challenge by R5 Clade C SHIV. PLoS Negl Trop Dis. 2011;5: e1270. 10.1371/journal.pntd.0001270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cheever AW, Kamel IA, Elwi AM, Mosimann JE, Danner R. Schistosoma mansoni and S. haematobium infections in Egypt. II. Quantitative parasitological findings at necropsy. Am J Trop Med Hyg. 1977;26: 702–16. 10.4269/ajtmh.1977.26.702 [DOI] [PubMed] [Google Scholar]
- 82.Gelfand M, Ross WF. II. The distribution of schistosome ova in the genito-urinary tract in subjects of bilharziasis. Trans R Soc Trop Med Hyg. 1953;47: 218–20. 10.1016/0035-9203(53)90006-6 [DOI] [PubMed] [Google Scholar]
- 83.Gelfand M, Ross MD, Blair DM, Weber MC. Distribution and extent of schistosomiasis in female pelvic organs, with special reference to the genital tract, as determined at autopsy. Am J Trop Med Hyg. 1971;20: 846–9. 10.4269/ajtmh.1971.20.846 [DOI] [PubMed] [Google Scholar]
- 84.Jourdan PM, Roald B, Poggensee G, Gundersen SG, Kjetland EF. Increased Vascularity in Cervicovaginal Mucosa with Schistosoma haematobium Infection. PLoS Negl Trop Dis. 2011;5: e1170. 10.1371/journal.pntd.0001170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wall KM, Kilembe W, Vwalika B, Dinh C, Livingston P, Lee Y-M, et al. Schistosomiasis is associated with incident HIV transmission and death in Zambia. PLoS Negl Trop Dis. 2018;12: e0006902. 10.1371/journal.pntd.0006902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Leutscher PDC, Pedersen M, Raharisolo C, Jensen JS, Hoffmann S, Lisse I, et al. Increased prevalence of leukocytes and elevated cytokine levels in semen from Schistosoma haematobium-infected individuals. J Infect Dis. 2005;191: 1639–47. 10.1086/429334 [DOI] [PubMed] [Google Scholar]
- 87.Midzi N, Mduluza T, Mudenge B, Foldager L, Leutscher PDC. Decrease in Seminal HIV-1 RNA Load After Praziquantel Treatment of Urogenital Schistosomiasis Coinfection in HIV-Positive Men—An Observational Study. Open Forum Infect Dis. 2017;4: ofx199. 10.1093/ofid/ofx199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Marti AI, Colombe S, Masikini PJ, Kalluvya SE, Smart LR, Wajanga BM, et al. Increased hepatotoxicity among HIV-infected adults co-infected with Schistosoma mansoni in Tanzania: A cross-sectional study. PLoS Negl Trop Dis. 2017;11: e0005867. 10.1371/journal.pntd.0005867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kim HN, Nance R, Van Rompaey S, Delaney JC, Crane HM, Cachay ER, et al. Poorly controlled HIV infection: An independent risk factor for liver fibrosis. J Acquir Immune Defic Syndr. 2016;72: 437–443. 10.1097/QAI.0000000000000992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Brown M, Kizza M, Watera C, Quigley MA, Rowland S, Hughes P, et al. Helminth Infection Is Not Associated with Faster Progression of HIV Disease in Coinfected Adults in Uganda. J Infect Dis. 2004;190: 1869–1879. 10.1086/425042 [DOI] [PubMed] [Google Scholar]
- 91.Kleppa E, Klinge KF, Galaphaththi-Arachchige HN, Holmen SD, Lillebø K, Onsrud M, et al. Schistosoma haematobium infection and CD4+ T-cell levels: a cross-sectional study of young South African women. PLoS One. 2015;10: e0119326. 10.1371/journal.pone.0119326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Colombe S, Corstjens PLAM, de Dood CJ, Miyaye D, Magawa RG, Mngara J, et al. HIV-1 Viral Loads Are Not Elevated in Individuals Co-infected With Schistosoma spp. After Adjustment for Duration of HIV-1 Infection. Front Immunol. 2018;9. 10.3389/fimmu.2018.02005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Colombe S, Machemba R, Mtenga B, Lutonja P, Kalluvya SE, de Dood CJ, et al. Impact of schistosome infection on long-term HIV/AIDS outcomes. PLoS Negl Trop Dis. 2018;12: e0006613. 10.1371/journal.pntd.0006613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Idindili B, Jullu B, Hattendorft J, Mugusi F, Antelman G, Tanner M. HIV and parasitic co-infections among patients seeking care at health facilities in Tanzania. Tanzan J Health Res. 2011;13. 10.4314/thrb.v13i4.68870 [DOI] [PubMed] [Google Scholar]
- 95.Elliott AM, Mawa PA, Joseph S, Namujju PB, Kizza M, Nakiyingi JS, et al. Associations between helminth infection and CD4+ T cell count, viral load and cytokine responses in HIV-1-infected Ugandan adults. Trans R Soc Trop Med Hyg. 2003;97: 103–8. 10.1016/s0035-9203(03)90040-x [DOI] [PubMed] [Google Scholar]
- 96.Efraim L, Peck RN, Kalluvya SE, Kabangila R, Mazigo HD, Mpondo B, et al. Schistosomiasis and impaired response to antiretroviral therapy among HIV-infected patients in Tanzania. J Acquir Immun Defic Syn. 2013;62: e153–6. 10.1097/QAI.0b013e318282a1a4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mulu A, Maier M, Liebert UG. Deworming of intestinal helminths reduces HIV-1 subtype C viremia in chronically co-infected individuals. Int J Infect Dis. 2013;17: e897–901. 10.1016/j.ijid.2013.03.022 [DOI] [PubMed] [Google Scholar]
- 98.Mazigo HD, Dunne DW, Morona D, Kinung’hi SM, Nuwaha F. Comparison of HIV-1 viral loads, CD4-Th2-lymphocytes and effects of praziquantel treatment among adults infected OR uninfected with Schistosoma mansoni in fishing villages of North-Western Tanzania. Tanzan J Health Res. 2016;18. [Google Scholar]
- 99.Abaasa A, Asiki G, Obuku Ekii A, Wanyenze J, Pala P, J van Dam G, et al. Effect of high-intensity versus low-intensity praziquantel treatment on HIV disease progression in HIV and Schistosoma mansoni co-infected patients: a randomised controlled trial. Wellcome Open Res. 2018;3: 81. 10.12688/wellcomeopenres.14683.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Brown M, Mawa PA, Joseph S, Bukusuba J, Watera C, Whitworth JAG, et al. Treatment of Schistosoma mansoni infection increases helminth-specific type 2 cytokine responses and HIV-1 loads in coinfected Ugandan adults. J Infect Dis. 2005;191: 1648–57. 10.1086/429668 [DOI] [PubMed] [Google Scholar]
- 101.Bochner AF, Secor WE, Baeten JM, Van Dam GJ, Szpiro AA, Njenga SM, et al. Schistosomiasis was not associated with higher HIV-1 plasma or genital set point viral loads among HIV seroconverters from four cohort studies. PLoS Negl Trop Dis. 2019;13. 10.1371/journal.pntd.0007886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Masikini P, Colombe S, Marti A, Desderius B, de Dood CJ, Corstjens PLAM, et al. Schistosomiasis and HIV-1 viral load in HIV-infected outpatients with immunological failure in Tanzania: a case-control study. BMC Infect Dis. 2019;19: 249. 10.1186/s12879-019-3876-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kallestrup P, Zinyama R, Gomo E, Butterworth AE, Mudenge B, van Dam GJ, et al. Schistosomiasis and HIV-1 Infection in Rural Zimbabwe: Effect of Treatment of Schistosomiasis on CD4 Cell Count and Plasma HIV-1 RNA Load. J Infect Dis. 2005;192: 1956–1961. 10.1086/497696 [DOI] [PubMed] [Google Scholar]
- 104.Lawn SD, Karanja DM, Mwinzia P, Andove J, Colley DG, Folks TM, et al. The effect of treatment of schistosomiasis on blood plasma HIV-1 RNA concentration in coinfected individuals. AIDS. 2000;14: 2437–43. 10.1097/00002030-200011100-00004 [DOI] [PubMed] [Google Scholar]
- 105.Stete K, Glass TR, van Dam GJ, Ntamatungiro A, Letang E, de Dood CJ, et al. Effect of schistosomiasis on the outcome of patients infected with HIV-1 starting antiretroviral therapy in rural Tanzania. PLoS Negl Trop Dis. 2018;12: e0006844. 10.1371/journal.pntd.0006844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Da’Dara AA, Lautsch N, Dudek T, Novitsky V, Lee T-H, Essex M, et al. Helminth infection suppresses T-cell immune response to HIV-DNA-based vaccine in mice. Vaccine. 2006;24: 5211–9. 10.1016/j.vaccine.2006.03.078 [DOI] [PubMed] [Google Scholar]
- 107.Da’dara AA, Harn DA. Elimination of helminth infection restores HIV-1C vaccine-specific T cell responses independent of helminth-induced IL-10. Vaccine. 2010;28: 1310–7. 10.1016/j.vaccine.2009.11.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Stete K, Krauth SJ, Coulibaly JT, Knopp S, Hattendorf J, Müller I, et al. Dynamics of Schistosoma haematobium egg output and associated infection parameters following treatment with praziquantel in school-aged children. Parasites and Vectors. 2012;5. 10.1186/1756-3305-5-298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Reese TA, Wakeman BS, Choi HS, Hufford MM, Huang SC, Zhang X, et al. Coinfection. Helminth infection reactivates latent γ-herpesvirus via cytokine competition at a viral promoter. Science (80-). 2014;345: 573–7. 10.1126/science.1254517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fu B, Yang R, Xia F, Li B, Ouyang X, Gao S-J, et al. Gender differences in Kaposi’s sarcoma-associated herpesvirus infection in a population with schistosomiasis in rural China. Jpn J Infect Dis. 2012;65: 350–3. 10.7883/yoken.65.350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wakeham K, Webb EL, Sebina I, Muhangi L, Miley W, Johnson WT, et al. Parasite infection is associated with Kaposi’s sarcoma associated herpesvirus (KSHV) in Ugandan women. Infect Agent Cancer. 2011;6: 15. 10.1186/1750-9378-6-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nalwoga A, Webb EL, Chihota B, Miley W, Walusimbi B, Nassuuna J, et al. Kaposi’s sarcoma-associated herpesvirus seropositivity is associated with parasite infections in Ugandan fishing communities on Lake Victoria islands. PLoS Negl Trop Dis. 2019;13. 10.1371/journal.pntd.0007776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rolot M, Dougall AM, Chetty A, Javaux J, Chen T, Xiao X, et al. Helminth-induced IL-4 expands bystander memory CD8+ T cells for early control of viral infection. Nat Commun. 2018;9: 4516. 10.1038/s41467-018-06978-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Danz H. Analysis of immune interactions, histopathology, and viral load during Schistosoma mansoni and Influenza A coinfection. University of Georgia. 2016. Available at: https://athenaeum.libs.uga.edu/handle/10724/36066 [Google Scholar]
- 115.Tundup S, Jayaguru P, Desmett A, Raje V, Harris T, Katl A. Modulation of lung inflammation ameliorates influenza-induced mortality, morbidity, and lung injury. American Thoracic Society International Conference. 2017. p. 125:A2923. [Google Scholar]
- 116.Kadl A, Tundup S, Jayaguru P. Helminth Egg Antigens Protect Mice from Influenza-Induced Mortality by Potentiate IFNAR1 Dependent Anti-Viral Immunity. American Thoracic Society International Conference. 2018. p. 197: A3840. [Google Scholar]
- 117.Scheer S, Krempl C, Kallfass C, Frey S, Jakob T, Mouahid G, et al. S. mansoni bolsters anti-viral immunity in the murine respiratory tract. PLoS One. 2014;9: e112469. 10.1371/journal.pone.0112469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.McFarlane AJ, McSorley HJ, Davidson DJ, Fitch PM, Errington C, Mackenzie KJ, et al. Enteric helminth-induced type I interferon signaling protects against pulmonary virus infection through interaction with the microbiota. J Allergy Clin Immunol. 2017;140: 1068–1078.e6. 10.1016/j.jaci.2017.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kjetland EF, Ndhlovu PD, Mduluza T, Deschoolmeester V, Midzi N, Gomo E, et al. The effects of genital Schistosoma haematobium on human papillomavirus and the development of cervical neoplasia after five years in a Zimbabwean population. Eur J Gynaecol Oncol. 2010;31: 169–173. [PubMed] [Google Scholar]
- 120.Petry KU, Scholz U, Hollwitz B, Von Wasielewski R, Meijer CJLM. Human papillomavirus, coinfection with Schistosoma hematobium, and cervical neoplasia in rural Tanzania. Int J Gynecol Cancer. 2003;13: 505–9. 10.1046/j.1525-1438.2003.13301.x [DOI] [PubMed] [Google Scholar]
- 121.Gent V, Waihenya R, Kamau L, Nyakundi R, Ambala P, Kariuki T, et al. An investigation into the role of chronic Schistosoma mansoni infection on Human Papillomavirus (HPV) vaccine induced protective responses. PLoS Negl Trop Dis. 2019;13: e0007704. 10.1371/journal.pntd.0007704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Brown J, Baisley K, Kavishe B, Changalucha J, Andreasen A, Mayaud P, et al. Impact of malaria and helminth infections on immunogenicity of the human papillomavirus-16/18 AS04-adjuvanted vaccine in Tanzania. Vaccine. 2014;32: 611–617. 10.1016/j.vaccine.2013.11.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Colombe S, Lee MH, Masikini PJ, Van Lieshout L, De Dood CJ, Hoekstra PT, et al. Decreased sensitivity of Schistosoma sp. egg microscopy in women and HIV-infected individuals. Am J Trop Med Hyg. 2018;98: 1159–64. 10.4269/ajtmh.17-0790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Porto AF, Santos SB, Muniz AL, Basílio V, Rodrigues W Jr., Neva FA, et al. Helminthic Infection Down-Regulates Type 1 Immune Responses in Human T Cell Lymphotropic Virus Type 1 (HTLV-1) Carriers and Is More Prevalent in HTLV-1 Carriers than in Patients with HTLV-1–Associated Myelopathy/Tropical Spastic Paraparesis. J Infect Dis. 2005;191: 612–618. 10.1086/427560 [DOI] [PubMed] [Google Scholar]
- 125.Lima LM, Santos SB, Oliveira RR, Cardoso LS, Oliveira SC, Góes AM, et al. Schistosoma antigens Downmodulate the in vitro Inflammatory Response in Individuals Infected with Human T Cell Lymphotropic Virus Type 1. Neuroimmunomodulation. 2013;20: 233–238. 10.1159/000348700 [DOI] [PubMed] [Google Scholar]
- 126.Lima LM, Cardoso LS, Santos SB, Oliveira RR, Oliveira SC, Góes AM, et al. Schistosoma antigens downregulate CXCL9 production by PBMC of HTLV-1-infected individuals. Acta Trop. 2017;167: 157–162. 10.1016/j.actatropica.2016.12.030 [DOI] [PubMed] [Google Scholar]
- 127.Santos SB, Porto AF, Muniz AL, De Jesus AR, Carvalho EM. Clinical and immunological consequences of human T cell leukemia virus type-I and Schistosoma mansoni co-infection. Memorias do Instituto Oswaldo Cruz. Fundacao Oswaldo Cruz; 2004. pp. 121–126. 10.1590/s0074-02762004000900022 [DOI] [PubMed] [Google Scholar]
- 128.Tweyongyere R, Nassanga BR, Muhwezi A, Odongo M, Lule SA, Nsubuga RN, et al. Effect of Schistosoma mansoni infection and its treatment on antibody responses to measles catch-up immunisation in pre-school children: A randomised trial. PLoS Negl Trop Dis. 2019;13: e0007157. 10.1371/journal.pntd.0007157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Nono JK, Kamdem SD, Netongo PM, Dabee S, Schomaker M, Oumarou A, et al. Schistosomiasis Burden and Its Association With Lower Measles Vaccine Responses in School Children From Rural Cameroon. Front Immunol. 2018;9: 2295. 10.3389/fimmu.2018.02295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Jiz M, Acosta L, Kurtis JD. Immune response to measles mumps and rubella (MMR) vaccination among schistosomiasis and geohelminth infected schoolchildren in Leyte, Philippines (Abstract 425). American Society of Tropical Medicine and Hygiene Annual Meeting. 2013. [Google Scholar]
- 131.Ondigo BN, Muok EMO, Oguso JK, Njenga SM, Kanyi HM, Ndombi EM, et al. Impact of mothers’ schistosomiasis status during gestation on children’s IgG antibody responses to routine vaccines 2 years later and anti-schistosome and anti-malarial responses by neonates in western Kenya. Front Immunol. 2018;9: 1402. 10.3389/fimmu.2018.01402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kizito D, Tweyongyere R, Namatovu A, Webb EL, Muhangi L, Lule SA, et al. Factors affecting the infant antibody response to measles immunisation in Entebbe-Uganda. BMC Public Health. 2013;13. 10.1186/1471-2458-13-619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Storey HL, Singa B, Naulikha J, Horton H, Richardson BA, John-Stewart G, et al. Soil transmitted helminth infections are not associated with compromised antibody responses to previously administered measles and tetanus vaccines among HIV-1 infected, ART naïve Kenyan adults. Parasite Epidemiol Control. 2017;2: 13–20. 10.1016/j.parepi.2016.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.World Health Organization. Schistosomiasis: Progress report 2001–2011, strategic plan 2012–2020. Geneva; 2013.
- 135.World Health Organization. Soil-transmitted helminth infections. 2020 [cited 29 Mar 2021]. Available: https://www.who.int/news-room/fact-sheets/detail/soil-transmitted-helminth-infections
- 136.Qadri F, Bhuiyan TR, Sack DA, Svennerholm A-M. Immune responses and protection in children in developing countries induced by oral vaccines. Vaccine. 2013;31: 452–460. 10.1016/j.vaccine.2012.11.012 [DOI] [PubMed] [Google Scholar]
- 137.Patel M, Shane AL, Parashar UD, Jiang B, Gentsch JR, Glass RI. Oral Rotavirus Vaccines: How Well Will They Work Where They Are Needed Most? J Infect Dis. 2009;200: S39–S48. 10.1086/605035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Venkatraman N, Ndiaye BP, Bowyer G, Wade D, Sridhar S, Wright D, et al. Safety and immunogenicity of a heterologous prime-boost ebola virus vaccine regimen in healthy adults in the United Kingdom and Senegal. J Infect Dis. 2019;219: 1187–1197. 10.1093/infdis/jiy639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Madhi S, Cunliffe N, Steele D, Witte D, Kirsten M, Louw C, et al. Effect of human rotavirus vaccine on severe diarrhea in African infants. N Engl J Med. 2010;362: 289–98. 10.1056/NEJMoa0904797 [DOI] [PubMed] [Google Scholar]
- 140.Sabin EA, Araujo MI, Carvalho EM, Pearce EJ. Impairment of tetanus toxoid-specific Th1-like immune responses in humans infected with Schistosoma mansoni. J Infect Dis. 1996;173: 269–72. 10.1093/infdis/173.1.269 [DOI] [PubMed] [Google Scholar]
- 141.Kabagenyi J, Natukunda A, Nassuuna J, Sanya RE, Nampijja M, Webb EL, et al. Urban-rural differences in immune responses to mycobacterial and tetanus vaccine antigens in a tropical setting: A role for helminths? Parasitol Int. 2020;78: 102132. 10.1016/j.parint.2020.102132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Moreno-Fernandez ME, Zapata W, Blackard JT, Franchini G, Chougnet CA. Human Regulatory T Cells Are Targets for Human Immunodeficiency Virus (HIV) Infection, and Their Susceptibility Differs Depending on the HIV Type 1 Strain. J Virol. 2009;83: 12925–12933. 10.1128/JVI.01352-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Maizels RM, Smits HH, McSorley HJ. Modulation of Host Immunity by Helminths: The Expanding Repertoire of Parasite Effector Molecules. Immunity. 2018;49: 801–818. 10.1016/j.immuni.2018.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Nkurunungi G, Lubyayi L, Versteeg SA, Sanya RE, Nassuuna J, Kabagenyi J, et al. Do helminth infections underpin urban-rural differences in risk factors for allergy-related outcomes? Clin Exp Allergy. 2019;49: 663–676. 10.1111/cea.13335 [DOI] [PMC free article] [PubMed] [Google Scholar]