Abstract
Insulin resistance early after acute myocardial infarction is associated with increased heart failure and mortality. OMEGA-REMODEL was a prospective double-blind 1:1 randomized control trial of patients with AMI. We reported that 6-month treatment with omega-3 fatty acid (O-3FA) 4 gm/day attenuated cardiac remodeling accompanied by reduction in inflammation. We hypothesized that insulin resistance modifies the therapeutic effect of O-3FA on post-MI cardiac remodeling. The OMEGA-REMODEL study group was dichotomized according to cohort- and gender-specific median cutoff value of leptin-to-adiponectin ratio (LAR) at baseline (LAR-Hi vs. LAR-Lo). Mixed model regression analyses was used to evaluate effect modification of O-3FA on reduction of left ventricular end-systolic volume index (LVESVI) by LAR status. Baseline LAR was evaluated on 325 patients (59 ± 11 years, 81% male). 168 patients were categorized in LAR-Lo, and 157 in LAR-Hi. O-3FA treatment resulted in significant LVESVI reduction in patients with LAR-Lo but not with LAR-Hi (p=0.0002 vs. 0.66, respectively). Mixed model regression analysis showed significant modification of LAR on O-3FA’s treatment effect in attenuating LVESVI (p=0.021). In conclusion, this post-hoc efficacy analysis suggests that LAR status significantly modified O-3FA’s treatment effect in attenuating cardiac remodeling. During the convalescent phase of acute infarct healing, patients with lower insulin resistance estimated by LAR appear to derive more therapeutic response from O-3FA towards improvement of LVESVI.
Keywords: Insulin resistance, leptin-to-adiponectin ratio, Omega-3 fatty acids, left ventricular remodeling
INTRODUCTION
Diabetes Mellitus represent one of the most significant risk factors for the development of heart failure (HF) following acute myocardial infarction (AMI), accounting for 66% of the mortality experienced within the first year.1 Several mechanisms predispose the diabetic heart to post-infarct adverse left ventricular (LV) remodeling, inclusive of myocardial energy demand/supply mismatch from increased vascular stiffness and endothelial dysfunction,2 upregulation of inflammatory mediators, such as tissue necrosis factor-α (TNF-α),3,4 and upregulation of adverse neurohormonal activation due to insulin resistance.5 The Omega-3 Acid Ethyl Esters on Left Ventricular Remodeling After Acute Myocardial Infarction (OMEGA-REMODEL) trial, demonstrated a significant attenuation of adverse LV remodeling using high-dose omega-3 fatty acids (O-3FA) for the first 6-months following AMI6. In this study, we evaluated the hypothesis that insulin resistance modifies the therapeutic response of O-3FA during the convalescent phase of acute MI by examining LV remodeling and inflammation.
METHODS
The main results of the Omega-3 Acid Ethyl Esters on Left Ventricular Remodeling After Acute Myocardial Infarction (OMEGA-REMODEL) trial have been published.6 OMEGA-REMODEL was a prospective, double-blind, placebo-controlled trial of 358 patients randomized to 4 grams/day of O-3FA for 6-months following an acute MI (2–4 weeks). Patient subjects were enrolled from 3 tertiary-care centers in Boston, Massachusetts. Inclusion criteria included all adult patients with symptoms of an acute coronary syndrome, serum troponin elevation consistent with acute myocardial injury, and angiographically significant obstructive coronary artery disease. Exclusion criteria included non-cardiac comorbidities with <1 year life expectancy, active pregnancy, and any absolute contraindications to contrast-enhanced cardiac MRI. The trial protocol included baseline cardiac MRI and plasma biomarker profile upon study entry with repeat after 6-months of study medication. Patients were followed for clinical outcomes and adverse events by physician investigators for a total period of up to 5 years. The study protocol was approved by the institutional review board of each enrolling site and all patients provided informed consent.
Blood samples were collected at both baseline and 6-month follow-up study visits immediately prior to cardiac MRI. The detailed method of sample collection is described elsewhere.6 All testing was performed by blinded laboratory personnel in a separate institution from clinical enrollment and follow-up. Given its known association with cardiovascular disease, systemic inflammation, and its relative freedom of influence from fasting to postprandial states, we estimated the degree of insulin resistance using leptin-to-adiponectin ratio (LAR), which has been well known to be gender-specific.11 Patients were grouped into high LAR (LAR-Hi) or low LAR (LAR-Lo) according to the corresponding gender-specific median cutoff value in the cohort. We also quantified other markers of insulin resistance including proinsulin, C-peptide, and homeostatic model assessment of insulin resistance (HOMA-IR). For these markers, high and low status were categorized using published and validated cut-off values of: proinsulin (20 pmol/L),12 c-peptide (3.1 ng/ml),13 and HOMA-IR as described in supplement table 1.14 Blood samples were assayed for red blood cell (RBC) fatty acid levels (OmegaQuant Analytics, LLC, Sioux Falls, SD). RBC fatty acid composition, which has been shown to correlate with myocardial O-3FA levels and unbiased by recent dietary intake,15,16 was evaluated using gas chromatography by flame ionization detection. The omega-3-index was calculated from the sum of DHA and EPA and expressed as a percentage of total RBC fatty acids.
All cardiac MRI studies were performed with a 3.0 Tesla scanner (Tim Trio or Verio, Siemens, Erlangen, Germany). The detailed cardiac MRI protocol is described elsewhere.6
The primary measure of our study was adverse LV remodeling as measured by change in LVESVI at 6-months of randomized study treatment from baseline (ΔLVESVI, ml/m2). Secondary measures included evaluation of changes in myocardial fibrosis measured as change in extracellular volume (by CMR) aftert 6-months of randomized study treatment from baseline (ΔECVRemote, %).
Continuous variables were summarized as means ± standard deviation. Categorical variables were presented as counts with percentages. Student’s t-test was used to calculate the improvement of LVESVI and ECVRemote by study treatment groups stratified by insulin resistance by IRLAR, proinsulin, C-peptide, and HOMA-IR. General linear mixed models (GLMM) were used to perform an intention-to-treat analysis that included patients missing follow-up visits for the primary and secondary endpoints. Restricted Maximum Likelihood (REML) estimation produces unbiased estimates under the assumption the missing responses are Missing At Random (MAR); i.e. the missing responses may be related to the observed responses, but are independent of the unobserved responses. This method alleviates the need for imputation. A compound symmetry correlation structure was used for the repeated measurements. A mixed model regression (PROC MIXED function, SAS version 9.4, Cary, NC) was performed to assess for the modification of study treatments’ longitudinal effect onto LVESVI (log transformed), by each of the 4 insulin resistance markers at baseline. Independent covariates in the mixed model included main effect terms of study treatment and insulin resistance, a time variable (0=baseline, 1=6-month), 2-way interaction terms, and a 3-way interaction term. Significant effect modification of study treatments’ effect onto LVESVI by insulin resistance was considered when the p-value of the 3-way interaction term was <0.05.
RESULTS
The whole cohort of 358 AMI patients enrolled in the OMEGA-REMODEL study were randomized into two groups; 180 patients in O-3FA treatment, and 178 patients in placebo. The baseline characteristics of patients were closely matched between O-3FA treatment and placebo groups (Supplemental Table 2).6 LAR was available in 325 patients and categorized as LAR-Lo (n=168) and LAR-Hi (n=157), respectively (Table 1).
Table 1.
Baseline characteristics of the patients comparing LAR-Lo vs. LAR-Hi.
| Variable | Entire Sample (N=325) | LAR-Lo (N=168) | LAR-Hi (N=157) | p-value |
|---|---|---|---|---|
| Age, year old ± SD | 58.9 ± 10.5 | 59.6 ± 10.7 | 58.0 ± 9.7 | 0.17 |
| Male | 263 (80.9 %) | 137 (81.6 %) | 126 (80.3 %) | 0.78 |
| Body mass index (kg/m2) | 29.0 ± 5.5 | 26.7 ± 4.3 | 31.5 ± 5.3 | <0.0001 |
| Hypertension | 212 (65.4 %) | 96 (57.1 %) | 116 (74.4 %) | 0.0015 |
| Diabetes Mellitus | 87 (26.9 %) | 37 (22.0 %) | 50 (32.1 %) | 0.045 |
| Hemoglobin A1c, % ± SD | 6.4 ± 1.4 | 6.1 ± 1.2 | 6.7 ± 1.4 | <0.0001 |
| Hyperlipidemia | 229 (70.7 %) | 109 (64.9 %) | 120 (76.9 %) | 0.020 |
| Smoker | 161 (49.7 %) | 82 (48.8 %) | 79 (50.6 %) | 0.82 |
| Prior Angina Pectoris | 76 (23.5 %) | 45 (26.8 %) | 31 (19.9 %) | 0.15 |
| Prior Myocardial Infarction | 195 (60.2 %) | 100 (59.5 %) | 95 (60.9 %) | 0.82 |
| Percutaneous coronary intervention | 234 (72.2 %) | 125 (74.4 %) | 109 (69.9 %) | 0.39 |
| Coronary artery bypass grafting | 35 (10.8 %) | 24 (14.3 %) | 11 (7.1 %) | 0.050 |
| Heart Failure | 9 (2.78 %) | 5 (3.0 %) | 4 (2.6 %) | 1.00 |
| Left ventricular ejection fraction (%) | 53.8 ± 9.8 | 52.8 ± 10.2 | 55.1 ± 8.5 | 0.035 |
| LVEDVI (ml/m2) | 83.5 ± 20.7 | 86.5 ± 21.4 | 79.3 ± 18.8 | 0.0018 |
| LVESVI (ml/m2) | 39.6 ± 17.2 | 41.9 ± 18.0 | 36.1 ± 14.4 | 0.0019 |
| Left ventricular mass index (grams/m2) | 59.3 ± 14.6 | 60.5 ± 15.0 | 58.5 ± 14.2 | 0.22 |
| ECV (%) | 33.8 ± 5.3 | 34.7 ± 5.7 | 33.0 ± 5.0 | 0.013 |
| Infarct size (grams) using FWHM Infarct percent (% left ventricular mass) |
17.3 ± 17.3 | 18.9 ± 19.3 | 15.1 ± 14.2 | 0.045 |
| using FWHM | 14.4 ± 13.2 | 15.5 ± 13.5 | 12.3 ± 11.2 | 0.020 |
| ACE/ARB | 240 (74.1 %) | 122 (72.6 %) | 118 (75.6 %) | 0.61 |
| Beta-Blocker | 297 (91.7 %) | 152 (51.2 %) | 145 (93.0 %) | 0.55 |
| Calcium Channel Blocker | 24 (7.4 %) | 10 (6.0 %) | 14 (9.0 %) | 0.40 |
| Statin | 313 (96.6 %) | 161 (95.8 %) | 152 (97.4 %) | 0.54 |
| Aspirin | 318 (98.2 %) | 163 (97.0 %) | 155 (99.4 %) | 0.22 |
In the study cohort, 87 (27%) had a history of diabetes mellitus. Of those with diabetes mellitus, 56 (64%), 12 (14%), and 19 (22%) were on either oral hypoglycemic or insulin, both, and neither medications, respectively. Using LAR level, 106 of 237 patients (44.7%) without a history of diabetes mellitus were categorized as LAR-Hi, which compared to 50 of 87 patients (57.5%) with diabetes mellitus (p=0.04). Neither a history of diabetes mellitus nor the type of diabetic treatment was an effect modifier to the treatment effect of O-3FA towards change in LVESVI or change of ECVRemote. In addition, baseline HbA1c did not correlate with %change in LVESVI (r2 = 0.009, p = 0.16).
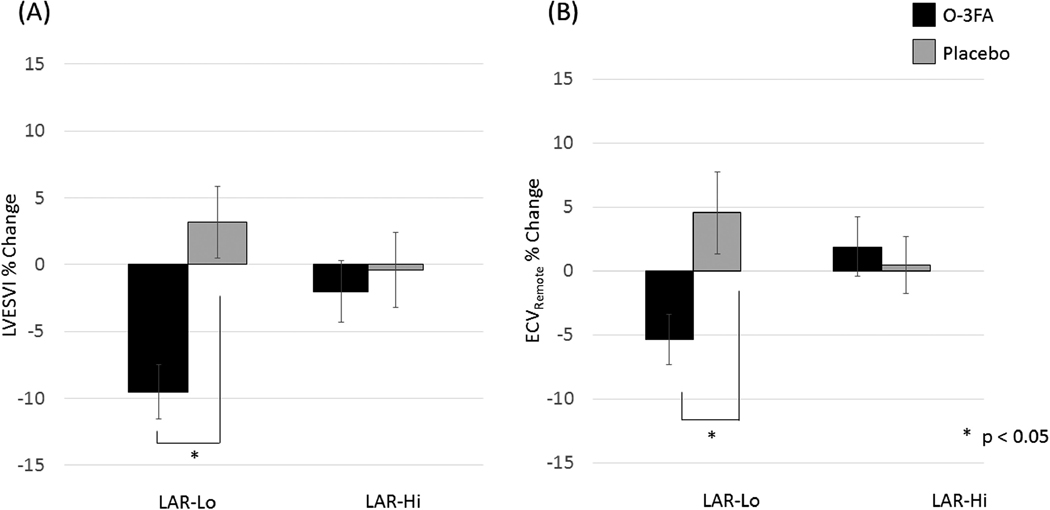
In patients with LAR-Lo, there was a significant improvement of LVESV from baseline to follow-up in the O-3FA group compared to placebo (p=0.0002). In contrast, in LAR-Hi patients, there was no therapeutic effect of O-3FA compared to placebo (p=0.66) (Figure 1A). Similarly, patients with LAR-Lo showed significant improvement of LVESV in the O-3FA group compared to placebo (−7.2 ± 14.1 % vs. −2.5 ± 11.1 %, p = 0.04). In contrast, the change of LVESV in O-3FA and placebo groups were similar (−1.1 ± 16.5 vs. −1.0 ± 13.9, p = 0.97). The odds of improving LVESVI by O-3FA was significant in LAR-Lo but not LAR-Hi group (p=0.025 vs. 1.0, respectively) (Figure 2A). Using mixed model regression, effect modification of O-3FA treatment to attenuate LVESVI, by LAR was significant (p=0.021). Similarly, there was improvement of ECVRemote from baseline to follow-up in the O-3FA group compared to placebo among LAR-Lo patients (p = 0.048) (Figure 1B). No benefit was seen in LAR-Hi patients (p = 0.36). The odds of improving ECVRemote by O-3FA was significant in LAR-Lo but not LAR-Hi group (p=0.028 vs. 0.64, respectively) (Figure 2B). Effect modification of LAR on O-3FA was also significant in reducing non-infarct myocardial fibrosis (p=0.0065).
Figure 1.
%change from baseline to follow-up in (A) LVESV and (B) ECV remote, comparing O-3FA treatment groug and placebo group. Significant change was observed in patients with LAR-Lo but not with LAR-Hi.
Figure 2.
The odds of improving (A) LVESVI and (B) ECVremote by O-3FA comparing patients high and low levels of LAR, proinsulin, C-peptide, and HOMA-IR. The odds of improving LVESVI and ECVremote was significant in LAR-Lo group but not significant in LAR-Hi group.
Using mixed model regression, effect modifications of O-3FA treatment to attenuate LVESVI were borderline significant, when severity of insulin resistance was assessed by either c-peptide (p=0.050) or HOMA-IR levels (p=0.054). The odds of improving LVESVI by O-3FA was significant in patients without insulin resistance by c-peptide but not so in patients with insulin resistance by c-peptide and HOMA-IR (Figure 2A).
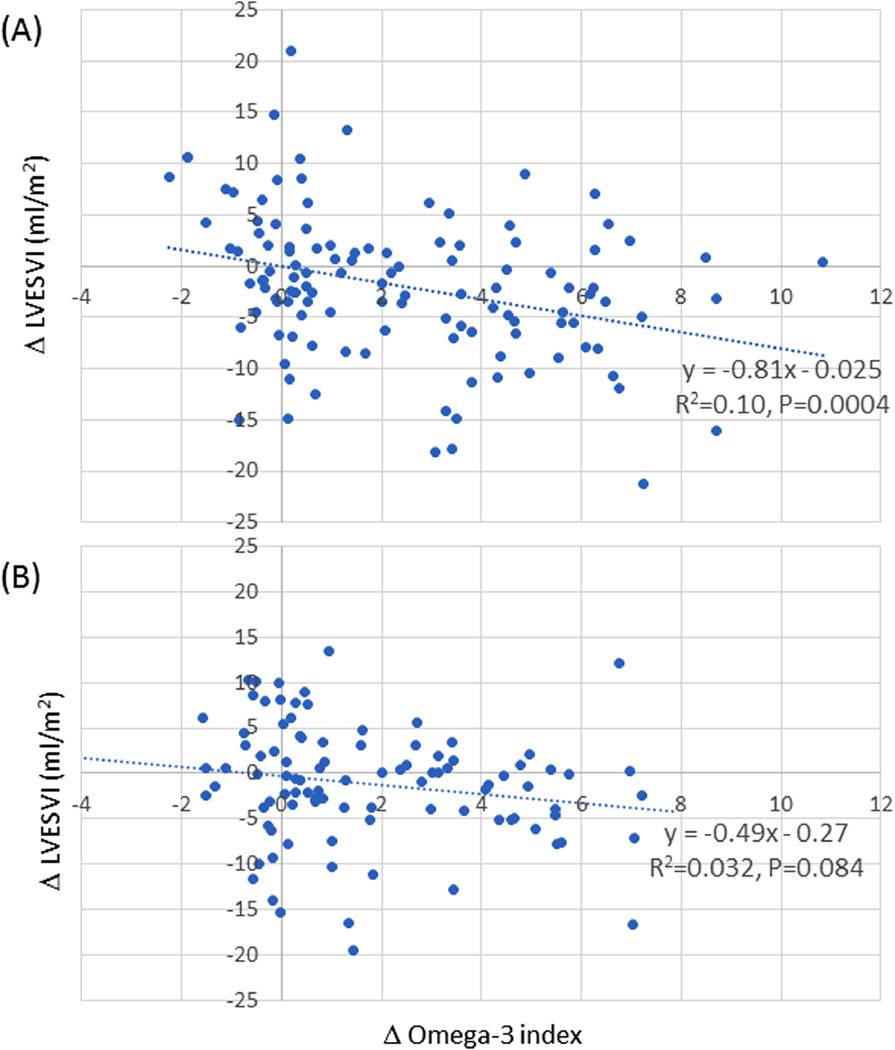
The difference of LVESVI between baseline and follow-up were both significantly correlated with the change of omega-3 index by the treatment in patients with LAR-Lo. However, no correlation was observed in patients with LAR-Hi (Figure 3).
Figure 3.
The difference of LVESVI between baseline and follow-up in patients with (A) LAR-Lo and (B) LAR-Hi.
Baseline high sensitivity c-reactive protein (hsCRP) was significantly higher among LAR-Hi patients compared to LAR-Lo (p=0.010). O-3FA treatment was associated with a reduction of hsCRP in LAR-Lo but not LAR-Hi patients (p=0.017 vs. 0.40, respectively). Among LAR-Lo patients, increase in O-3 index during study treatment demonstrated significant correlations with reduction of galectin-3, lipoprotein phospholipase A2 level, and lipoprotein mass (p=0.0042, p=0.003, and p=0.05, respectively). Among LAR-Hi patients, such correlations were not significant.
DISCUSSION
In patients suffering acute MI, we found that 6-months of high-dose O-3FA therapy was associated with attenuation of post AMI adverse remodeling in patients with low but not high LAR prior to initiation of therapy. Given the significance and impact of inflammation on adverse ventricular remodeling post-MI, we believe the observations from our study to have important therapeutic implications and support the notion that insulin resistance modifies the effectiveness of O-3FA toward promotion of post-MI cardiac remodeling.
After AMI, inflammation is induced as the initial phase of myocardial repairing process, and followed by resolution of inflammation, fibroblast proliferation, scar formation, and neovascularization. Overactive inflammation may cause adverse remodeling in patients with infarction, contributing to the pathogenesis of heart failure.22 Therefore, suppressing inflammation may be of vital importance in preventing cardiac remodeling. Our group demonstrated that high-dose O-3FA treatment was associated with significant reduction of both biomarkers of inflammation and myocardial fibrosis.6 Furthermore, we observed that the degree of LVESVI reduction correlated with the degree of O-3FA incorporation into the RBC membrane, suggesting RBC omega-3-index may serve as a useful marker of treatment efficacy.
In this study, we observed that patients with LAR-Hi had higher degree of systemic inflammation compared to LAR-Lo patients as measured by baseline hsCRP. Interestingly, treating patients with O-3FA at 4 grams/day resulted in attenuation of markers of inflammation and stretch (hsCRP, lipoprotein phospholipase A2, galectin-3), and reduction of remodeling in LAR-Lo, but not LAR-Hi patients. Although the exact mechanism is not well established, one possible explanation is that patients with relatively high magnitude of insulin resistance may have extensive underlying chronic, in addition to acute, inflammation, which may follow a different mechanistic pathway. For instance, previous studies which have examined the effect of intermediate dose O-3FA supplementation on systemic inflammatory markers in patients with impaired glucose metabolism or diabetes mellitus and stable atherosclerotic disease have failed to shown benefit.23,24
Independent of its effect on the inflammatory cascade, the presence of insulin resistance may impair myocardial function post AMI. In the ischemic myocardium, metabolism shifts from fatty acids to glucose as primary energy source. The effects of insulin, which regulate glucose transport into the cells, are therefore crucial.25 In this study, we observed that insulin resistance is a key factor prohibiting recovery of cardiac function following treatment with O-3FA.
Various biomarkers are available to assess insulin resistance such as LAR, proinsulin, c-peptide, and HOMA-IR. Leptin and adiponectin are adipokine mainly produced by adipose tissue that circulates in plasma in concentrations that are proportional to body fat. Therefore, LAR is relatively stable regardless of fasting state.26 Whereas, proinsulin,27 c-peptide,28 and HOMA-IR29 are directly associated with endogenous insulin, therefore it is important to assess them at fasting state. In our study, because the biomarkers were corrected at non-fasting state, LAR was considered as the most appropriate measure to assess insulin resistance.
Our study demonstrates that LAR is a parameter of insulin resistance which allows the selection of patients who responds most to O-3FA treatment after AMI in reducing of adverse remodeling. Leptin and adiponectin are adipokines secreted from adipose tissue. Leptin inhibits appetite, stimulates thermogenesis and enhances fatty acid oxidation. Adiponectin mediates tissue insulin sensitivity.26 In obesity, adipocytes secrete more leptin and less adiponectin, leading to the hypothesis that LAR is a useful biomarker of adipocyte hypertrophy, insulin resistance and cardiovascular risk.26,30 Interestingly, in our study cohort, 31.3% of patients without history of diabetes mellitus showed Glu > 100mg/dl. At the same time, 39.6% of patients without history of diabetes mellitus showed insulin resistance by LAR. Conversely, 45.8% of patients with history of diabetes mellitus showed Glu < 126 mg/ml. At the same time, 41.0% of patients with history of diabetes mellitus were categorized as low insulin resistance by LAR. This mismatch between history of diabetes mellitus and marker of insulin resistance may be important not only for understanding the effect modifying mechanism of insulin resistance on O-3FA treatment for infarct healing, but may also be relevant for general clinical management after AMI.
A few limitations to our study deserve mention. There are no firmly established cutoff values (for either gender) defining clinically significant insulin resistance. For this reason, we relied on gender-specific median cutoff values to explore the modifying effects of LAR onto post-MI cardiac remodeling. Furthermore, the absolute percent change of LVESVI and non-infarct myocardial fibrosis from O-3FA treatment, started at 2–4 weeks post-AMI, were modest incremental to guideline clinical care.6
In conclusion, our study demonstrated that patients with low levels of insulin resistance benefited from high-dose O-3FA treatment towards attenuation of left ventricular adverse remodeling after AMI; those with high levels of insulin resistance did not. Further investigations by prospective studies are warranted to evaluate the effect of insulin resistance to modify the benefit of O-3FA on cardiac remodeling after AMI.
Supplementary Material
Cut-off value of LAR and HOMA-IR
Baseline characteristics of the patients comparing O-3FA treatment vs. placebo groups.
ACKNOWLEDGEMENTS
The National Institutes of Health provided sole funding for this study, while GlaxoSmithKline (Research Triangle Park, NC) provided study medication. The National Heart, Lung, and Blood Institute of the National Institutes of Health (NIH) funded this study entirely (R01HL091157). Dr. Fujikura received salaray support from a T-32 training grant from NIH (T32HL094301-07). In addition, this work was funded in part by the Division of Intramural Research, NHLBI, NIH, United States Department of Health and Human Services (DHHS).
CLINICAL TRIAL REGISTRATION INFORMATION: National Heart, Lung, and Blood Institute (NHLBI), https://clinicaltrials.gov/ct2/show/NCT00729430, clinicaltrials.gov Identifier: NCT00729430.
GRANT SUPPORT
The National Institutes of Health provided sole funding for this study, while GlaxoSmithKline (Research Triangle Park, NC) provided study medication. The National Heart, Lung, and Blood Institute of the National Institutes of Health (NIH) funded this study entirely (R01HL091157). Dr. Fujikura received salaray support from a T-32 training grant from NIH (T32HL094301–07). In addition, this work was funded in part by the Division of Intramural Research, NHLBI, NIH, United States Department of Health and Human Services (DHHS).
Footnotes
DISCLOSURES
None of the authors have relevant and significant conflicts of interests including related consultancies and shareholdings.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Malmberg K, Ryden L, Hamsten A, Herlitz J, Waldenstrom A, Wedel H. Effects of insulin treatment on cause-specific one-year mortality and morbidity in diabetic patients with acute myocardial infarction. DIGAMI Study Group. Diabetes Insulin-Glucose in Acute Myocardial Infarction. Eur Heart J 1996;17:1337–1344. [DOI] [PubMed] [Google Scholar]
- 2.von Bibra H, St John Sutton M. Impact of diabetes on postinfarction heart failure and left ventricular remodeling. Curr Heart Fail Rep 2011;8:242–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuboki K, Jiang ZY, Takahara N, Ha SW, Igarashi M, Yamauchi T, Feener EP, Herbert TP, Rhodes CJ, King GL. Regulation of endothelial constitutive nitric oxide synthase gene expression in endothelial cells and in vivo : a specific vascular action of insulin. Circulation 2000;101:676–681. [DOI] [PubMed] [Google Scholar]
- 4.Jager J, Gremeaux T, Cormont M, Le Marchand-Brustel Y, Tanti JF. Interleukin-1beta-induced insulin resistance in adipocytes through down-regulation of insulin receptor substrate-1 expression. Endocrinology 2007;148:241–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Witteles RM, Fowler MB. Insulin-resistant cardiomyopathy clinical evidence, mechanisms, and treatment options. J Am Coll Cardiol 2008;51:93–102. [DOI] [PubMed] [Google Scholar]
- 6.Heydari B, Abdullah S, Pottala JV, Shah R, Abbasi S, Mandry D, Francis SA, Lumish H, Ghoshhajra BB, Hoffmann U, Appelbaum E, Feng JH, Blankstein R, Steigner M, McConnell JP, Harris W, Antman EM, Jerosch-Herold M, Kwong RY. Effect of Omega-3 Acid Ethyl Esters on Left Ventricular Remodeling After Acute Myocardial Infarction: The OMEGA-REMODEL Randomized Clinical Trial. Circulation 2016;134:378–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heydari B, Abdullah S, Shah R, Francis SA, Feng JH, McConnell J, Harris W, Antman EM, Jerosch-Herold M, Kwong RY. Omega-3 Fatty Acids Effect on Post-Myocardial Infarction ST2 Levels for Heart Failure and Myocardial Fibrosis. J Am Coll Cardiol 2018;72:953–955. [DOI] [PubMed] [Google Scholar]
- 8.Serhan CN, Petasis NA. Resolvins and protectins in inflammation resolution. Chem Rev 2011;111:5922–5943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buoite Stella A, Gortan Cappellari G, Barazzoni R, Zanetti M. Update on the Impact of Omega 3 Fatty Acids on Inflammation, Insulin Resistance and Sarcopenia: A Review. Int J Mol Sci 2018;19:E218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seropian IM, Toldo S, Van Tassell BW, Abbate A. Anti-inflammatory strategies for ventricular remodeling following ST-segment elevation acute myocardial infarction. J Am Coll Cardiol 2014;63:1593–1603. [DOI] [PubMed] [Google Scholar]
- 11.Finucane FM, Luan J, Wareham NJ, Sharp SJ, O’Rahilly S, Balkau B, Flyvbjerg A, Walker M, Hojlund K, Nolan JJ, Savage DB. Correlation of the leptin:adiponectin ratio with measures of insulin resistance in non-diabetic individuals. Diabetologia 2009;52:2345–2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murtha TD, Lupsa BC, Majumdar S, Jain D, Salem RR. A Systematic Review of Proinsulin-Secreting Pancreatic Neuroendocrine Tumors. J Gastrointest Surg 2017;21:1335–1341. [DOI] [PubMed] [Google Scholar]
- 13.Appendix: Normal Hormone Reference Ranges. In: Gardner DG, Shoback D, editors. Greenspan’s basic & clinical endocrinology. New York: the McGraw-Hill Education, 2018:848. [Google Scholar]
- 14.Gayoso-Diz P, Otero-Gonzalez A, Rodriguez-Alvarez MX, Gude F, Garcia F, De Francisco A, Quintela AG. Insulin resistance (HOMA-IR) cut-off values and the metabolic syndrome in a general adult population: effect of gender and age: EPIRCE cross-sectional study. BMC Endocr Disord 2013;13:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harris WS, Von Schacky C. The Omega-3 Index: a new risk factor for death from coronary heart disease? Prev Med 2004;39:212–220. [DOI] [PubMed] [Google Scholar]
- 16.Harris WS, Sands SA, Windsor SL, Ali HA, Stevens TL, Magalski A, Porter CB, Borkon AM. Omega-3 fatty acids in cardiac biopsies from heart transplantation patients: correlation with erythrocytes and response to supplementation. Circulation 2004;110:1645–1649. [DOI] [PubMed] [Google Scholar]
- 17.Kim RJ, Wu E, Rafael A, Chen EL, Parker MA, Simonetti O, Klocke FJ, Bonow RO, Judd RM. The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med 2000;343:1445–1453. [DOI] [PubMed] [Google Scholar]
- 18.Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, Pennell DJ, Rumberger JA, Ryan T, Verani MS, American Heart Association Writing Group on Myocardial S, Registration for Cardiac I. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation 2002;105:539–542. [DOI] [PubMed] [Google Scholar]
- 19.Deichmann R HA. Quantification of T1 Values by SNAPSHOT-FLASH NMR imaging. J Mag Res 1992;96:608–612. [Google Scholar]
- 20.Coelho-Filho OR, Mongeon FP, Mitchell R, Moreno H Jr., Nadruz W Jr., Kwong R, Jerosch-Herold M. Role of transcytolemmal water-exchange in magnetic resonance measurements of diffuse myocardial fibrosis in hypertensive heart disease. Circ Cardiovasc Imaging 2013;6:134–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jerosch-Herold M, Sheridan DC, Kushner JD, Nauman D, Burgess D, Dutton D, Alharethi R, Li D, Hershberger RE. Cardiac magnetic resonance imaging of myocardial contrast uptake and blood flow in patients affected with idiopathic or familial dilated cardiomyopathy. Am J Physiol Heart and Circ Physiol 2008;295:H1234–H1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prabhu SD, Frangogiannis NG. The Biological Basis for Cardiac Repair After Myocardial Infarction: From Inflammation to Fibrosis. Circ Res 2016;119:91–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sawada T, Tsubata H, Hashimoto N, Takabe M, Miyata T, Aoki K, Yamashita S, Oishi S, Osue T, Yokoi K, Tsukishiro Y, Onishi T, Shimane A, Taniguchi Y, Yasaka Y, Ohara T, Kawai H, Yokoyama M. Effects of 6-month eicosapentaenoic acid treatment on postprandial hyperglycemia, hyperlipidemia, insulin secretion ability, and concomitant endothelial dysfunction among newly-diagnosed impaired glucose metabolism patients with coronary artery disease. An open label, single blinded, prospective randomized controlled trial. Cardiovasc Diabetol 2016;15:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poreba M, Mostowik M, Siniarski A, Golebiowska-Wiatrak R, Malinowski KP, Haberka M, Konduracka E, Nessler J, Undas A, Gajos G. Treatment with high-dose n-3 PUFAs has no effect on platelet function, coagulation, metabolic status or inflammation in patients with atherosclerosis and type 2 diabetes. Cardiovasc Diabetol 2017;16:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jacoby RM, Nesto RW. Acute myocardial infarction in the diabetic patient: pathophysiology, clinical course and prognosis. J Am Coll Cardiol 1992;20:736–744. [DOI] [PubMed] [Google Scholar]
- 26.Bravo C, Cataldo LR, Galgani J, Parada J, Santos JL. Leptin/adiponectin ratios using either total or high-molecular-weight qdiponectin as biomarkers of systemic insulin sensitivity in normoglycemic women. J Diabetes Res 2017;2017:9031079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pfützner A, Kunt T, Honberg C, Mondok A, Pahler S, Konrad T, Lübben G, Forst T. Fasting intact proinsulin is a highly specific predictor of insulin resistance in type 2 diabetes. Diabetes Care 2004;27:682–687. [DOI] [PubMed] [Google Scholar]
- 28.McLaughlin T, Abbasi F, Cheal K, Chu J, Lamendola C RG. Use of Metabolic Markers To Identify Overweight Individuals Who Are Insulin Resistant. Ann Intern Med 2003;139:802–809. [DOI] [PubMed] [Google Scholar]
- 29.Matthews DR., Hosker JR., Rudenski AS, Naylor BA., Treacher DF, Turner RC Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–419. [DOI] [PubMed] [Google Scholar]
- 30.Yadav A, Kataria MA, Saini V, Yadav A. Role of leptin and adiponectin in insulin resistance. Clin Chim Acta 2013;417:80–84. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Cut-off value of LAR and HOMA-IR
Baseline characteristics of the patients comparing O-3FA treatment vs. placebo groups.