Abstract
APOBEC3 (A3) proteins are a family of host antiviral restriction factors that potently inhibit various retroviral infections, including human immunodeficiency virus (HIV)-1. To overcome this restriction, HIV-1 virion infectivity factor (Vif) recruits the cellular cofactor CBFβ to assist in targeting A3 proteins to a host E3 ligase complex for poly-ubiquitination and subsequent proteasomal degradation. Intervention of the Vif-A3 interactions could be a promising therapeutic strategy to facilitate A3-mediated suppression of HIV-1 in patients. In this structural snapshot, we review the structural features of the recently determined structure of human A3F in complex with HIV-1 Vif and its cofactor CBFβ, discuss insights into the molecular principles of Vif-A3 interplay during the arms race between the virus and host, and highlight the therapeutic implications.
Keywords: virus-host interaction, HIV-1 Vif, APOBEC3, E3 ligase complex, cryo-EM
Introduction
During the course of HIV infection, host cells employ a wide array of restriction factors to defend against the virus at different stages of the viral life cycle. APOBEC3 (A3) family of cytidine deaminases are among the most potent to restrict the virus during the early stage of HIV-1 infection. A3 enzymes from a virus-producing cell are incorporated into HIV virions and extensively hypermutate the viral genome during the next round of infection, by converting cytosine to uracil in the minus strand of the viral single stranded DNA (ssDNA) during reverse transcription [1–3]. A3 enzymes can also inhibit viral replication through deaminase-independent mechanisms by interfering with the functions of reverse transcriptase or integrase [4–12]. Humans express seven A3 proteins (A3A-A3H) with one or two structurally similar zinc-binding domains that are categorized into Z1, Z2, or Z3 phylogenies based on sequence[13]. Among them, A3C, A3D, A3F, A3G, and A3H have been found to inhibit HIV-1 replication, with the di-domain A3G and A3F enzymes being the most potent HIV-1 restrictors [14, 15]. The di-domain A3 enzymes normally have their C-terminal domain (CTD) catalytically active for deamination and their N-terminal domain (NTD) responsible for virion packaging, nucleic acid binding, enzyme processivity and oligomerization [16]. The catalytic domains of A3s all contain a well-conserved, zinc-coordinating H-X-E-X23–28-P-C-X2–4-C motif at the active site [13, 17–19], which preferentially selects different nucleotide motifs surrounding the sites of deamination [1, 20–24].
HIV-1 evades the restriction imposed by A3s through an accessory protein Vif, which hijacks the host ubiquitin-proteasome pathway to polyubiquitinate A3s for degradation [25–29]. To recruit the ubiquitin ligase, HIV-1 Vif mimics the substrate receptor component of the cellular Cullin-RING E3 ligase (CRL) machinery composed of the scaffold protein Cullin 5 (Cul5), the E2-activating RING finger protein Rbx2, and the adaptor proteins Elongin B (EloB) and Elongin C (EloC) that connect the substrate receptor to Cul5 (Fig. 1) [28]. This recruiting event also requires a non-canonical cellular cofactor core-binding factor beta (CBFβ) to stabilize Vif and its assembly with the E3 ligase [30–33]. Since the restriction by A3 is so potent, Vif expression is essential for HIV-1 infection in most cell types expressing A3 proteins. Disruption of the interaction between HIV-1 Vif and A3s would significantly impair HIV-1 infection. Therefore, the HIV-1 Vif-A3 interaction is an attractive target for potential therapeutic development.
Fig. 1. Models of Vif/CBFβ/A3F assemblies with or without CRL5.
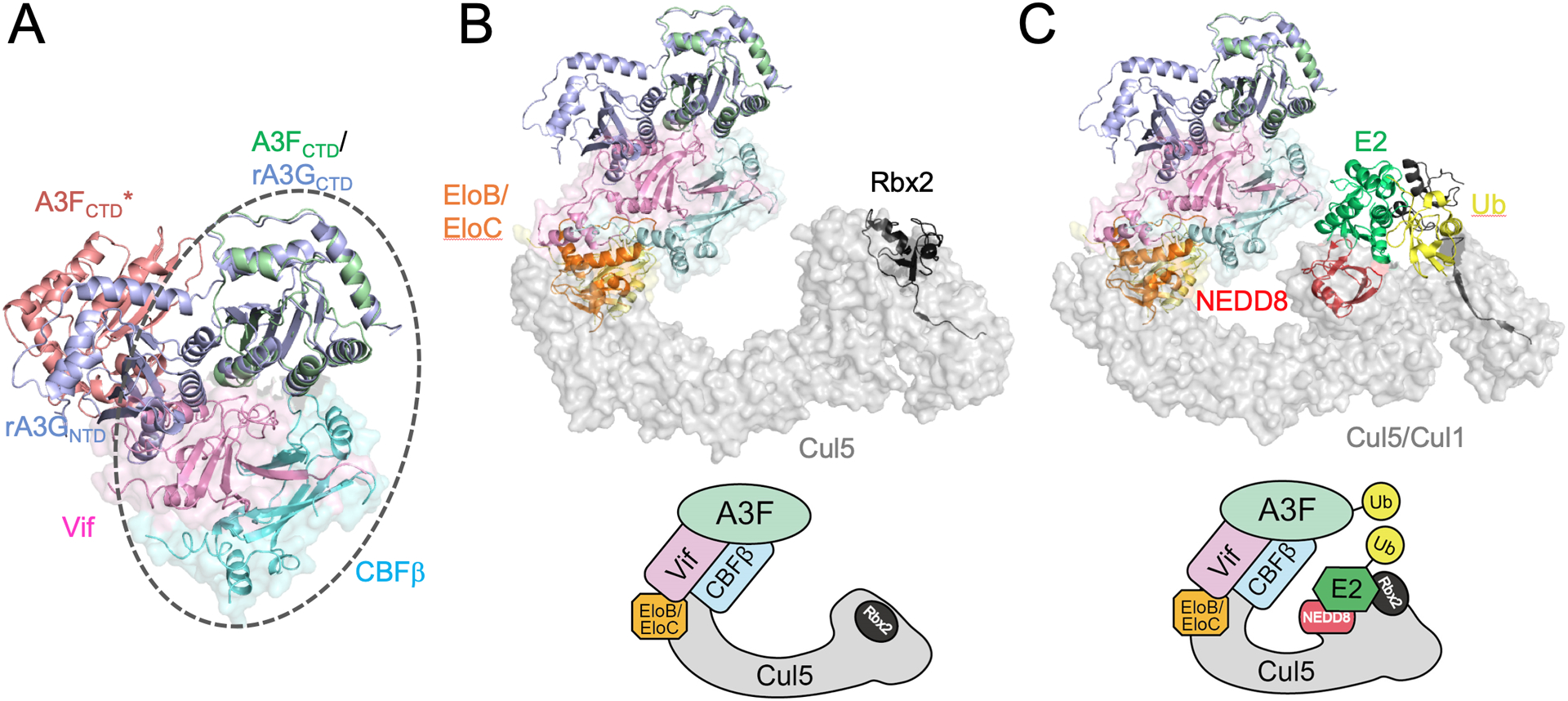
(A) Superposition of the crystal structure of rA3G (PDB 6P40, blue) onto the cryo-EM structure of Vif/CBFβ/A3FCTDternary complex (circled), where rA3G NTD is situated at a position similar to that of a neighboring A3FCTD (denoted by A3FCTD*, light red) observed in the cryo-EM structure, but with modest clashes with Vif. (B) Proposed model of Vif/CBFβ/A3F in complex with unneddylated CRL5 by overlaying the Vif/CBFβ/A3FCTD ternary complex with Vif/CBFβ/EloB/EloC/Cul5NTD(PDB 4N9F), Cul5CTD/Rbx2 (PDB 3DPL), and Cul1 FL/Rbx1 (PDB 1LDK). (C) Putative model of Vif/CBFβ/A3F in complex with the neddylated CRL5 with E2 and ubiquitin (Ub) by further overlaying the neddylated CRL1 structure (PDB 6TTU). The corresponding schematics of the models are shown in the lower panels of (B) and (C). The structural figures were generated using PyMol [103].
Vif interactions with host cofactors and E3 ubiquitin ligase machinery
Lentiviral Vifs recruit various host cofactors
Vif proteins from different lentiviruses have a conserved function of recruiting CRL complexes from the cognate hosts to initiate A3 degradation, but do so with different non-canonical cofactors [34]. For instance, primate lentiviral Vif proteins recruit CBFβ, which endogenously forms a heterodimer with the transcription factor RUNX1 to control expression of genes implicated in many cellular processes, including the immune system [35]. However, CBFβ is dispensable for non-primate Vifs. Bovine immunodeficiency virus (BIV) Vif assembles a host CRL complex without a cofactor, while maedi-visna virus (MVV) Vif requires a different cofactor Cyclophilin A (CypA), a prolyl isomerase that normally plays key roles in regulating immune responses in cells, as well as in multiple aspects of the HIV-1 life cycle [36]. Both CBFβ and CypA exist and are nearly identical in primates and sheep, so it is intriguing that HIV-1 and MVV Vif proteins have evolved to select host cofactors differentially. As the host CRL complexes are conserved among different species, it is possible that the divergent selection of host cofactors is primarily driven by the differences in Vif proteins themselves and the A3 proteins they target.
Structural features of primate lentiviral Vif-containing CRL5 complexes
To date, the crystal structures of two primate lentiviral (HIV-1 and simian immunodeficiency virus (SIVrcm)) Vif proteins in complex with various CRL5 assemblies have been determined, providing mechanistic insights into how primate lentiviral Vifs, facilitate by CBFβ, hijacks the host CRL5 complex [37, 38]. Both HIV-1 and SIVrcm Vif proteins adopt a similar di-domain architecture, with a smaller α domain containing two loosely-packed α helices and a larger α/β domain composed of an antiparallel β sheet tightly packed with three α helices. One helix of the α domain interacts with Cul5, and the other helix binds EloC through a conserved BC-box mimicking that of the natural substrate receptors SOCS-box proteins of CRL5 [39]. An extensive interaction between CBFβ and the antiparallel β sheet of the Vif α/β domain shields a large hydrophobic interface on Vif from solvent and stabilizes Vif and its assembly with CRL5. While both Vif and RUNX1 bind to CBFβ at the similar site, Vif buries a larger surface area, indicating a tighter binding affinity of Vif to CBFβ [37]. This sequestration of CBFβ by Vif also disrupts the interactions of CBFβ with RUNX transcription factors that regulate genes involved in various immune responses, including A3 proteins [32, 40, 41], which is potentially beneficial for the viruses. Although the overall structures are very similar, detailed conformational differences are still observed in the two primate lentiviral Vif-associated CRL5 complexes [37, 38]. These differences include variations of surface charge characters and the lengths of secondary structural elements at the interfaces, leading to modest changes in the packing between the interacting partners, all of which potentially results in the modulation of recognition and degradation of A3 proteins in distinct species.
Vif-mediated CRL-catalyzed ubiquitination
The CRL-dependent ubiquitination is specifically activated by the ubiquitin-like protein, NEDD8 [42], which forms an isopeptide bond with a specific lysine residue in the C-terminal WHB domain of Cullins [43]. The molecular mechanisms of the CRL-catalyzed ubiquitination and NEDD8 activation remains unclear until the recently determined cryo-EM structure capturing substrate ubiquitination by the neddylated CRL1 in complex with a ubiquitin-conjugating E2 enzyme [44]. Neddylation of Cul1 allows the complex to adapt a conformation that is amenable to the ubiquitination of the substrate. NEDD8 acts as a pivot to position the ubiquitin-carrying E2 and the E2-activating Rbx1 at the Cul1 C-terminus to be adjacent to the substrate receptor and the associated substrate at the Cul1 N-terminus for ubiquitination to occur. The NEDD8 activation of the CRL5-catalyzed ubiquitination is expected to work in a similar manner. Neddylation of Cul5 is required for HIV Vif-mediated degradation of A3G [45]. Additionally, a recent work showed that a member of the RING-Between-RING (RBR) E3 ligase family, ARIH2, is essential for the CRL5-based HIV infection in primary CD4+ T cells [46]. It is proposed that by interacting with the Vif-associated and neddylated CRL5, ARIH2 primes the initial ubiquitination of both A3F and A3G for further polyubiquitination by the E2 enzyme. ARIH2 is also found to be required for the ubiquitination of other CRL5 substrates, indicating that the priming regulation by ARIH2 may represent a universal mechanism of the CRL5-mediated ubiquitination.
The molecular mechanisms of APOBEC3 sequestration by Vif
A3 proteins are notoriously difficult to purify in vitro, because A3s usually associate tightly with nucleic acids and form higher ordered oligomers and soluble aggregates. Some soluble constructs of A3F and A3G have been engineered for biochemical and structural studies [47–53], but the yield of a wild type protein purified from a recombinant expression system is still limited. The topology of either single- or di-domain A3s are highly conserved, composed of five β strands surrounded by six α helices. The two most potent HIV-1 restrictors, A3G and A3F, are di-domain and interact with Vif through distinct domains: NTD for A3G and CTD for A3F [14, 54–56]. In addition, within the respective Vif-binding domains of A3G and A3F, separate interfaces have been mapped for Vif interaction through extensive mutagenesis studies [57, 58]. Similarly, Vif is also expected to recruit A3G and A3F through disparate, partially overlapping regions [58, 59], indicating that Vif may rely on multiple mechanisms to target A3s for degradation.
An atomic structure of the Vif/A3 complex with CRL5 has eluded the field for nearly two decades, primarily due to the poor solution behavior and flexible binding of Vif and A3s. Recently, we succeeded in obtaining a high resolution cryo-EM structure [60] focusing on the sub-complex of Vif/CBFβ with a solubility-enhanced A3F CTD (A3FCTD) [50]. A few specific construct design elements have improved the complex assembly to help achieve atomic resolution. First, we fused A3FCTD to CBFβ through an engineered linker to enhance the binding between Vif and A3F, which surprisingly stabilized a weak tetrameric form of the ternary complex. Moreover, we removed the α domain of Vif along with the associated C-terminal regions of CBFβ, which protrude away from the molecular core without being involved in the A3F interaction. This truncation further stabilized the tetramer formation of the ternary complex, resulting in a more rigid four-fold D2 symmetry which substantially improved the cryo-EM reconstruction.
CBFβ-A3FCTD interactions
A major finding from our Vif/CBFβ/A3FCTD complex structure is the direct participation of CBFβ in A3F recruitment. CBFβ is known to act as a molecular chaperone to enable initial Vif assembly with CRL5. CBFβ has also been suggested to bind Vif through a region partially overlapping with the A3F-binding interface, and therefore need to be dislodged from Vif to allow the A3F recruitment [31, 58]. The ternary complex structure reveals that CBFβ and Vif together form a wedge-like platform to accommodate A3FCTD with little conformational change. This observed novel CBFβ-A3FCTD interface has been further validated through biochemical and virology studies, including a charge-swapped CBFβ E54K/A3FCTDm R293D double mutation, which rescued the ternary complex formation in vitro, and restored the Vif-mediated A3F degradation and viral infectivity in vivo. Interestingly, the observed CBFβ interface exclusively binds A3F, but not A3G, indicating CBFβ either interacts with A3G through another interface, or does not participate in A3G recruitment at all. The roles of CBFβ in the Vif-mediated degradation of other A3 proteins warrant further investigation. The interactions between CBFβ and A3s are most likely modulated or stabilized by Vif, given the fact that the association of CBFβ and A3F was not detected in the absence of Vif [60].
Vif-A3FCTD interactions
The major Vif/CBFβ-A3FCTD binding interface within the ternary complex buries a relatively small surface area of 1004 Å2, explaining our observation of a relatively weak binding during complex reconstitution. This Vif-A3FCTD interface involves multiple electrostatic and hydrophobic regions of the Vif α/β domain located on the opposite side of its Cul5/EloC binding interface. In addition to the major interface, two potential minor Vif-A3FCTD interfaces are formed at the inter-ternary complex interfaces within the tetramer. One of the minor interfaces is located at the Vif α1 helix close to the major interface, while the other one is situated in the 55VxIPLx4–5L64 motif of Vif located on the opposite side. Extensive biochemical and virological studies have validated the importance of the observed major interface [14, 59, 61–66], with some data also support the interactions at the minor interfaces [59, 62, 67–70], although no evidence pointing to the presence of homo-tetramers in cells. Our in vivo A3F degradation data also supports that A3F is recruited by Vif to the CLR5 machinery primarily through interactions at the major interface. Whether the observed minor Vif-A3F interactions are involved in the Vif antagonism of A3F awaits further studies.
Degradation-dependent mechanism of Vif antagonization of APOBEC3s
The advances in the structural understanding of various subcomplexes help piece together an overall picture of the A3 degradation machinery. In addition to the Vif-containing complexes described above, recent crystal structures of a full-length rhesus A3G (rA3GFL, with various solubility-enhanced mutations) provided the first glimpse of di-domain A3 structures with various inter-domain interactions through a flexible linker [71]. When the rA3GFL structure is superimposed onto that of A3FCTD in the Vif complex, the rA3G NTD spatially collides with the Vif α1 helix (Fig. 1A). It has been reported that A3FNTD-A3GCTD chimeric proteins retain low-affinity binding to Vif, indicating potential Vif binding motifs in A3FNTD, as A3GCTD does not support the binding [55]. Therefore, the Vif α1 helix may be a potential site for interacting with A3FNTD, whose orientation may be adjusted through the inter-domain elasticity during the interaction. Aligning the Vif/CBFβ portion of the Vif/CBFβ/A3FCTD ternary complex structure with that of the Vif-containing CRL5 crystal structure [37] also shows no spatial conflict with the overlaid rA3GFL and Vif-interacting CRL5 components (Fig. 1B). By further superimposing the structure of neddylated CRL1 in complex with the ubiquitin-conjugating E2 [44] onto CRL5, we obtain a preliminary structural model for the Vif-mediated ubiquitination of A3F by neddylated CRL5 (Fig. 1C). In this model, there is considerable spatial distance between A3FCTD and the E2 enzyme, whose location is likely dictated by the large substrate recruited in the particular CRL1 complex structure [44]. However, conformational elasticity has been shown for the C-terminal WHB domain of Cullins and the RING domain of Rbx1/2 [44, 72]. Furthermore, ARIH2 may facilitate the juxtaposition of A3FCTDand E2 for priming the ubiquitination. Once the first ubiquitin is transferred onto A3FCTD, the geometry for subsequent polyubiquitination would be substantially less restricted due to the increased length, flexibility, and accessibility of the growing ubiquitin chain. Besides, the catalytic site of A3FCTD is located away from the major interface, exposing to solvent for substrate contact, indicating the binding of Vif/CBFβ may not directly affect the A3F deamination activity, therefore this proposed model could represent the primary degradation-dependent mechanism of Vif antagonization of A3F (Fig. 2).
Fig. 2. Schematics of the Vif-mediated CRL5-dependent polyubiquitination cycle of A3F.
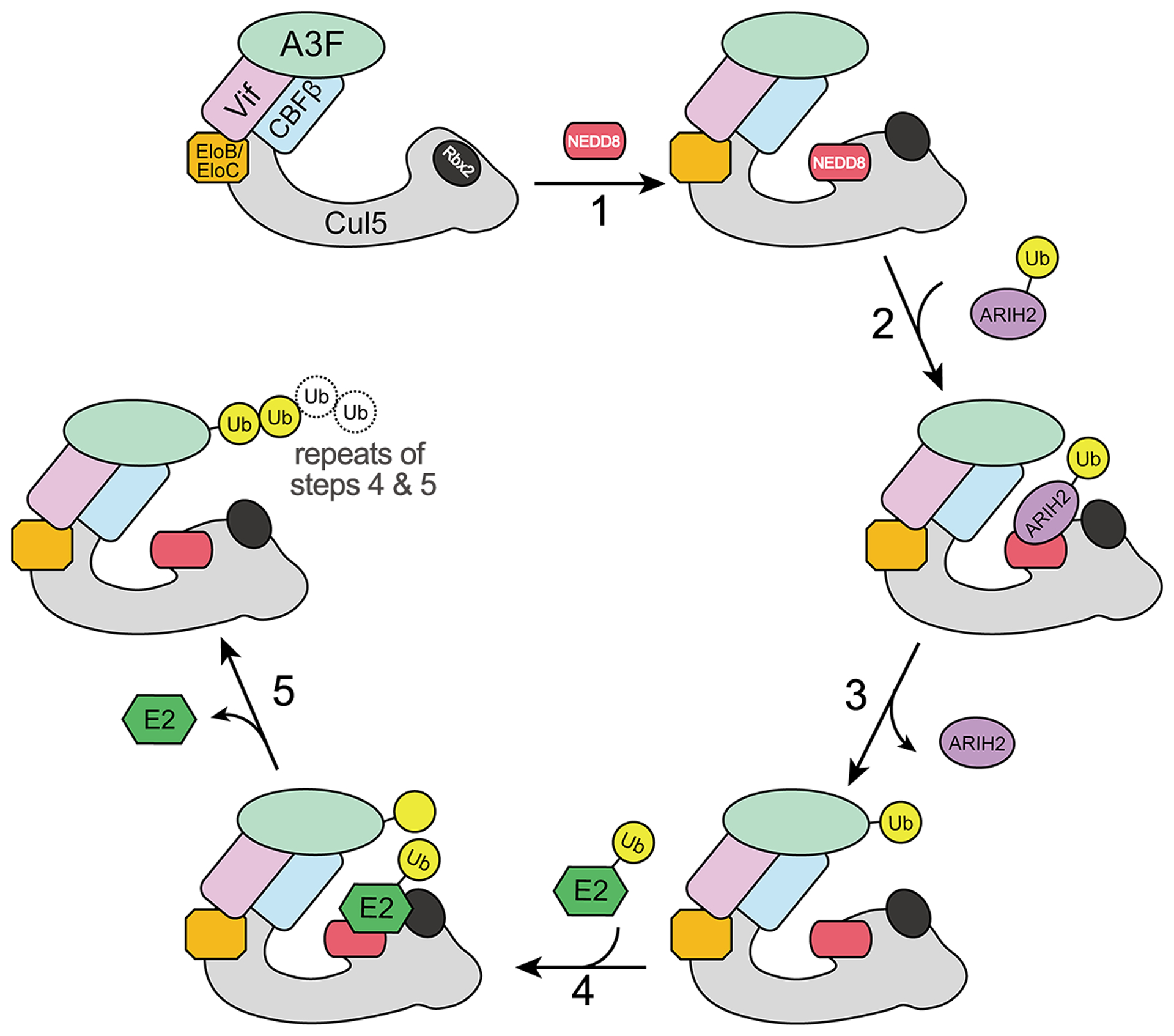
Upon A3F recruitment by Vif to CRL5 E3 ligase, neddylation of CRL5 by NEDD8 triggers conformational changes of Cul5 C-terminal domain and Rbx2. Ubiquitinated ARIH2 then interacts with the neddylated CRL5 and initializes the ubiquitination reaction by transferring the first ubiquitin to A3F. ARIH2 disassociates from CRL5 after priming the ubiquitination, followed by the association of a ubiquitin-carrying E2 enzyme to further catalyze the polyubiquitination reaction by transferring additional ubiquitin molecules to A3F in repeating cycles.
Degradation-independent mechanism of Vif antagonization of APOBEC3s
Vif can also counteract A3 restrictions through degradation-independent mechanisms. Vif does not appear to directly inhibit the A3F deamination activity by targeting the enzyme to the E3 ligase complex, as the catalytic site of A3F is still accessible in the observed major interaction mode. It was found that Vif can regulate the translation of A3G mRNA to lower A3G levels with an unknown mechanism [73–75]. Furthermore, blocking the Vif-mediated ubiquitination of A3C, A3F and A3G was shown to restore their cellular levels but without reinstating A3-mediated restriction of HIV-1 infection, as the restored A3s failed to package in viral like particles (VLPs) [76]. In addition, several enzymatic studies showed that Vif can either attenuate the processivity of A3G by disrupting its scanning on viral ssDNA [77], or inhibit the deaminase activity of A3G [78] potentially by competing for its binding to the ssDNA substrate. Our in vitro deamination study also indicated that, although not affected by Vif-CRL5 complex formation at low concentration, the A3FCTD enzymatic activity could be inhibited by the Vif complex at high concentration [60]. The inhibition is not revoked by the Vif R15E mutation disrupting the Vif/A3FCTD/CBFβ ternary complex, implying it is not mediated by A3F recruiting to the E3 ligase, consistent with our structural model that the catalytic site of A3FCTD is solvent-exposed in the ternary complex. Instead, the inhibition may come from the Vif interaction at one of the minor interfaces observed in our tetramer, which potentially confers weak binding but blocks the A3FCTDactive site at high concentrations. The exact mechanisms of the degradation-independent antagonization of A3s by Vif should be further investigated in vitro and in vivo.
Structural insights for Vif interactions with different APOBEC3s
The molecular details provided by our Vif/CBFβ/A3FCTD complex structure allow for a detailed scrutiny of the existing mutational analysis probing the Vif-A3F interface. Many of the Vif residues previously anticipated to interact with A3F, e.g. 11WQVD14, 74TGER77, 84GVSIEW89, and 96TQx5ADx2I107, are either buried in the molecular core or located at the Vif-CBFβ interface [59, 64, 66, 79, 80]. Mutations of these residues likely impair proper Vif folding or its stability maintained by CBFβ, rather than directly disrupting the Vif-A3F interaction. Furthermore, some A3F residues speculated to bind Vif [64, 65, 81] are actually interacting with CBFβ, all of which result in a much smaller Vif-A3F binding interface than predicted.
The A3-Vif interaction sites can be divided into three categories based on the prototypical A3s: A3C/D/F type, A3G type, and A3H type (Fig. 3). A3C, A3DCTD and A3FCTD have been predicted to share a similar binding interface to interact with Vif [14, 61, 62, 82], although local discrepancies still exist based on the mapping studies (Fig. 3). A3C residues C130/E133 are important for Vif interaction while the equivalent A3FCTD residues D313/E316 are not [83]. A residue cluster in A3FCTD (L291/A292/R293/E324) are found to be critical for Vif interaction, while the equivalent residues in A3C are dispensable for efficient binding [61, 62, 83]. Interestingly, our Vif/CBFβ/A3FCTD complex structure shows that this A3FCTD cluster contacts CBFβ rather than Vif, indicating the observed CBFβ interface may not be involved in A3C recruitment. Furthermore, a positively-charged patch on Vif (R17/E171/R173), which help position the Vif C-terminal loop for A3F interactions, is less critical for A3C degradation [62]. In contrast to the modest binding variations within the A3C/D/F type, A3GNTD employs an entirely distinct region at the opposite edge of the α-helical face of the molecule to interact with Vif, and correspondingly, Vif also uses a different interface to interact with A3GNTD [53, 84–89] (Fig. 3). Many of the Vif residues previously proposed for both A3F and A3G binding are found to play indirect structural roles, resulting in a more divergent A3F and A3G binding interfaces in Vif. In addition to these two types of interfaces, A3H haplotype II (hapII) has been mapped to interact with Vif through a third interface located between those of A3C/D/F-like and A3G [84, 90, 91] (Fig. 3). Accordingly, Vif is postulated to contact A3H hapII through a distinct interface located on the opposite side of that for A3C/D/F and A3G, partially overlapping with the A3G-interface [90, 92, 93]. These diverse interactions highlight that HIV-1 Vif is a versatile A3 antagonist, actively defending against various A3s through multifarious mechanisms.
Fig. 3. Structural analysis of different Vif/CBFβ-A3 interfaces.
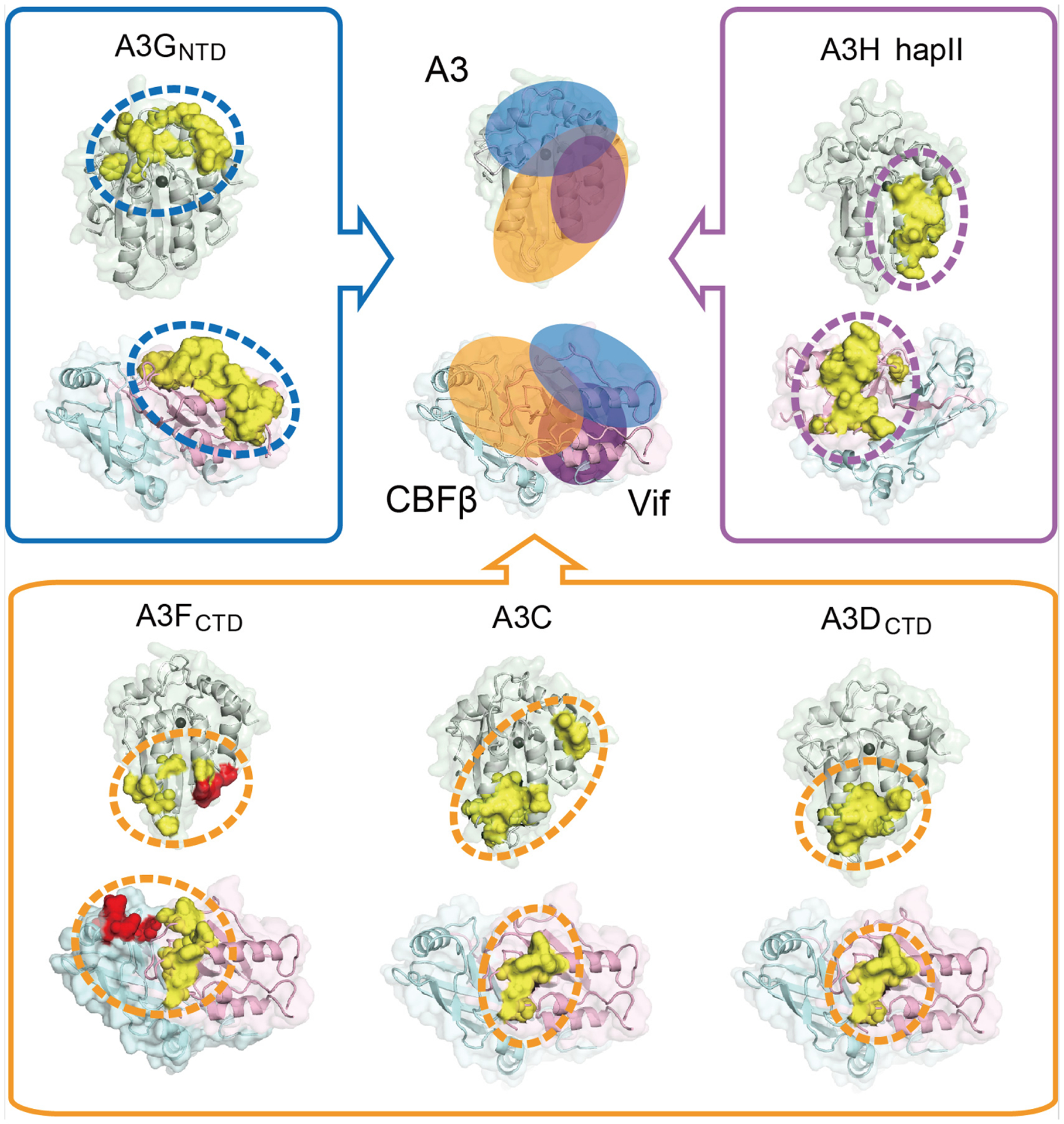
Comparison of the distinct Vif/CBFβ-A3 interfaces (marked with ovals of different colors) for A3FCTD (bottom left), A3C (bottom middle, PDB 3VOW), A3DCTD (bottom right, homology model built from A3FCTD (PDB 3WUS)), A3GNTD (top left, homology model built from rhesus A3GNTD (PDB 5K81)) and A3H hapII (top right, PDB 6BBO). Critical residues observed are highlighted in red at the CBFβ-A3F interface and in yellow at the Vif-A3 interfaces. The zinc ion at the ssDNA-binding or catalytic site is shown as a dark gray sphere. A summary of the diverse locations of the Vif/CBFβ-A3 interfaces is illustrated at top middle, with ovals of corresponding colors. The structural figures were generated using PyMol [103].
Therapeutic implications
Disrupting the Vif-mediated neutralization of A3 restrictions has the therapeutic potential to treat HIV-1 infections. Attempts at gene silencing [94, 95] and small molecule inhibitors directly targeting Vif [96] have been reported. However, as a viral protein which evolves rapidly, it may develop resistance to designed drugs, reducing the efficiency of HIV therapy. An alternative strategy is to suppress the host factors and/or their involvements in the Vif-mediated A3 degradation. It has been found that the pharmacological inhibition of NEDD8 or knockdown of its conjugating enzyme UBE2F or Rbx2 blocks HIV infectivity and restores A3G restriction [45]. A potent HIV-1 inhibitor ZBMA-1 is identified to potentially bind EloC, interrupting recruitment of Vif to the E3 ligase [97]. The small Vif-Cul5 interface could be another promising drug target, although Vif may still counteract A3 restrictions through degradation-independent mechanisms. Conversely, the extensive Vif-CBFβ interface is less feasible to be disrupted by a small drug molecule. There are also a few small molecule inhibitors reported to reduce viral infectivity by intervening Vif-A3G interaction [98–102], with unclear mechanism due to the lack of a Vif-A3 complex structure. However, a common problem for the strategies targeting host proteins is that it may also affect their normal cellular activities leading to cytotoxicity.
The molecular details elucidated by various Vif complexes may enable the design of inhibitors targeting unique host-viral interactions with minimal interference with cellular functions. For example, the novel CBFβ-A3F binding interface discovered in our Vif/CBFβ/A3F complex structure could open up a new avenue for anti-HIV therapeutic strategies. This interface is important for Vif-mediated degradation of A3F, with no conflict with the RUNX binding to CBFβ, and likely does not have an intrinsic cellular function. Another advantage of this interface is its small size, making it a druggable target to allow the design of small molecule compounds or peptides, which may effectively disrupt the CBFβ-A3F interactions without potential cytotoxic effect. In addition, the potential A3F/G/H overlapping binding regions on Vif could also be a promising drug target to inhibit Vif interactions with multiple A3 members, reducing the chance of drug-resistance. These highlight the importance of the structural details at the Vif/A3/E3 ligase interfaces in promoting the development of next generation of anti-HIV therapies.
Acknowledgements
KMK was supported by the predoctoral program in Biophysics NIH T32 GM008283. This work was supported by the National Institutes of Health grant AI116313 (Y.X.).
Abbreviations:
- APOBEC3
apolipoprotein-B mRNA-editing catalytic polypeptide-like 3
- HIV
human immunodeficiency virus
- MVV
maedi-visna virus
- SIV
simian immunodeficiency virus
- BIV
Bovine immunodeficiency virus
- Vif
viral infectivity factor
- CBFβ
Core binding factor beta
- RUNX
runt-related transcription factor
- ssDNA
single stranded DNA
- NTD
N-terminal domain
- CTD
C-terminal domain
- CRL
Cullin-RING E3 ligase
- Cul
cullin
- EloB
Elongin B
- EloC
Elongin C
- CypA
Cyclophilin A
- RBR
RING-Between-RING
- ARIH2
Ariadne RBR E3 Ubiquitin Protein Ligase 2
Footnotes
Conflicts of Interest: The authors declare no conflict of interest.
References
- 1.Mangeat B, Turelli P, Caron G, Friedli M, Perrin L & Trono D (2003) Broad antiretroviral defence by human APOBEC3G through lethal editing of nascent reverse transcripts, Nature. 424, 99–103. [DOI] [PubMed] [Google Scholar]
- 2.Zhang H, Yang B, Pomerantz RJ, Zhang C, Arunachalam SC & Gao L (2003) The cytidine deaminase CEM15 induces hypermutation in newly synthesized HIV-1 DNA, Nature. 424, 94–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lecossier D, Bouchonnet F, Clavel F & Hance AJ (2003) Hypermutation of HIV-1 DNA in the absence of the Vif protein, Science. 300, 1112. [DOI] [PubMed] [Google Scholar]
- 4.Holmes RK, Koning FA, Bishop KN & Malim MH (2007) APOBEC3F can inhibit the accumulation of HIV-1 reverse transcription products in the absence of hypermutation. Comparisons with APOBEC3G, J Biol Chem. 282, 2587–95. [DOI] [PubMed] [Google Scholar]
- 5.Li XY, Guo F, Zhang L, Kleiman L & Cen S (2007) APOBEC3G inhibits DNA strand transfer during HIV-1 reverse transcription, J Biol Chem. 282, 32065–74. [DOI] [PubMed] [Google Scholar]
- 6.Bishop KN, Verma M, Kim EY, Wolinsky SM & Malim MH (2008) APOBEC3G inhibits elongation of HIV-1 reverse transcripts, PLoS Pathog. 4, e1000231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iwatani Y, Chan DS, Wang F, Maynard KS, Sugiura W, Gronenborn AM, Rouzina I, Williams MC, Musier-Forsyth K & Levin JG (2007) Deaminase-independent inhibition of HIV-1 reverse transcription by APOBEC3G, Nucleic Acids Res. 35, 7096–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morse M, Huo R, Feng Y, Rouzina I, Chelico L & Williams MC (2017) Dimerization regulates both deaminase-dependent and deaminase-independent HIV-1 restriction by APOBEC3G, Nature communications. 8, 597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mbisa JL, Barr R, Thomas JA, Vandegraaff N, Dorweiler IJ, Svarovskaia ES, Brown WL, Mansky LM, Gorelick RJ, Harris RS, Engelman A & Pathak VK (2007) Human immunodeficiency virus type 1 cDNAs produced in the presence of APOBEC3G exhibit defects in plus-strand DNA transfer and integration, J Virol. 81, 7099–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang Y, Guo F, Cen S & Kleiman L (2007) Inhibition of initiation of reverse transcription in HIV-1 by human APOBEC3F, Virology. 365, 92–100. [DOI] [PubMed] [Google Scholar]
- 11.Guo F, Cen S, Niu M, Saadatmand J & Kleiman L (2006) Inhibition of tRNA(3)(Lys)-primed reverse transcription by human APOBEC3G during human immunodeficiency virus type 1 replication, J Virol. 80, 11710–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kobayashi T, Koizumi Y, Takeuchi JS, Misawa N, Kimura Y, Morita S, Aihara K, Koyanagi Y, Iwami S & Sato K (2014) Quantification of deaminase activity-dependent and -independent restriction of HIV-1 replication mediated by APOBEC3F and APOBEC3G through experimental-mathematical investigation, J Virol. 88, 5881–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Conticello SG, Thomas CJ, Petersen-Mahrt SK & Neuberger MS (2005) Evolution of the AID/APOBEC family of polynucleotide (deoxy)cytidine deaminases, Mol Biol Evol. 22, 367–77. [DOI] [PubMed] [Google Scholar]
- 14.Smith JL & Pathak VK (2010) Identification of specific determinants of human APOBEC3F, APOBEC3C, and APOBEC3DE and African green monkey APOBEC3F that interact with HIV-1 Vif, J Virol. 84, 12599–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hultquist JF, Lengyel JA, Refsland EW, LaRue RS, Lackey L, Brown WL & Harris RS (2011) Human and rhesus APOBEC3D, APOBEC3F, APOBEC3G, and APOBEC3H demonstrate a conserved capacity to restrict Vif-deficient HIV-1, J Virol. 85, 11220–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feng Y, Baig TT, Love RP & Chelico L (2014) Suppression of APOBEC3-mediated restriction of HIV-1 by Vif, Frontiers in microbiology. 5, 450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jarmuz A, Chester A, Bayliss J, Gisbourne J, Dunham I, Scott J & Navaratnam N (2002) An anthropoid-specific locus of orphan C to U RNA-editing enzymes on chromosome 22, Genomics. 79, 285–96. [DOI] [PubMed] [Google Scholar]
- 18.Wedekind JE, Dance GS, Sowden MP & Smith HC (2003) Messenger RNA editing in mammals: new members of the APOBEC family seeking roles in the family business, Trends Genet. 19, 207–16. [DOI] [PubMed] [Google Scholar]
- 19.LaRue RS, Andresdottir V, Blanchard Y, Conticello SG, Derse D, Emerman M, Greene WC, Jonsson SR, Landau NR, Lochelt M, Malik HS, Malim MH, Munk C, O’Brien SJ, Pathak VK, Strebel K, Wain-Hobson S, Yu XF, Yuhki N & Harris RS (2009) Guidelines for naming nonprimate APOBEC3 genes and proteins, J Virol. 83, 494–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harris RS, Bishop KN, Sheehy AM, Craig HM, Petersen-Mahrt SK, Watt IN, Neuberger MS & Malim MH (2003) DNA deamination mediates innate immunity to retroviral infection, Cell. 113, 803–9. [DOI] [PubMed] [Google Scholar]
- 21.Liddament MT, Brown WL, Schumacher AJ & Harris RS (2004) APOBEC3F properties and hypermutation preferences indicate activity against HIV-1 in vivo, Curr Biol. 14, 1385–91. [DOI] [PubMed] [Google Scholar]
- 22.Yu Q, Konig R, Pillai S, Chiles K, Kearney M, Palmer S, Richman D, Coffin JM & Landau NR (2004) Single-strand specificity of APOBEC3G accounts for minus-strand deamination of the HIV genome, Nat Struct Mol Biol. 11, 435–42. [DOI] [PubMed] [Google Scholar]
- 23.Dang Y, Wang X, Esselman WJ & Zheng YH (2006) Identification of APOBEC3DE as another antiretroviral factor from the human APOBEC family, J Virol. 80, 10522–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harari A, Ooms M, Mulder LC & Simon V (2009) Polymorphisms and splice variants influence the antiretroviral activity of human APOBEC3H, J Virol. 83, 295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marin M, Rose KM, Kozak SL & Kabat D (2003) HIV-1 Vif protein binds the editing enzyme APOBEC3G and induces its degradation, Nat Med. 9, 1398–403. [DOI] [PubMed] [Google Scholar]
- 26.Sheehy AM, Gaddis NC & Malim MH (2003) The antiretroviral enzyme APOBEC3G is degraded by the proteasome in response to HIV-1 Vif, Nat Med. 9, 1404–7. [DOI] [PubMed] [Google Scholar]
- 27.Stopak K, de Noronha C, Yonemoto W & Greene WC (2003) HIV-1 Vif blocks the antiviral activity of APOBEC3G by impairing both its translation and intracellular stability, Mol Cell. 12, 591–601. [DOI] [PubMed] [Google Scholar]
- 28.Yu X, Yu Y, Liu B, Luo K, Kong W, Mao P & Yu XF (2003) Induction of APOBEC3G ubiquitination and degradation by an HIV-1 Vif-Cul5-SCF complex, Science. 302, 1056–60. [DOI] [PubMed] [Google Scholar]
- 29.Conticello SG, Harris RS & Neuberger MS (2003) The Vif protein of HIV triggers degradation of the human antiretroviral DNA deaminase APOBEC3G, Curr Biol. 13, 2009–13. [DOI] [PubMed] [Google Scholar]
- 30.Zhang W, Du J, Evans SL, Yu Y & Yu XF (2011) T-cell differentiation factor CBF-beta regulates HIV-1 Vif-mediated evasion of host restriction, Nature. 481, 376–9. [DOI] [PubMed] [Google Scholar]
- 31.Fribourgh JL, Nguyen HC, Wolfe LS, Dewitt DC, Zhang W, Yu XF, Rhoades E & Xiong Y (2014) Core binding factor beta plays a critical role by facilitating the assembly of the Vif-cullin 5 E3 ubiquitin ligase, J Virol. 88, 3309–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim DY, Kwon E, Hartley PD, Crosby DC, Mann S, Krogan NJ & Gross JD (2013) CBFbeta stabilizes HIV Vif to counteract APOBEC3 at the expense of RUNX1 target gene expression, Mol Cell. 49, 632–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jager S, Kim DY, Hultquist JF, Shindo K, LaRue RS, Kwon E, Li M, Anderson BD, Yen L, Stanley D, Mahon C, Kane J, Franks-Skiba K, Cimermancic P, Burlingame A, Sali A, Craik CS, Harris RS, Gross JD & Krogan NJ (2011) Vif hijacks CBF-beta to degrade APOBEC3G and promote HIV-1 infection, Nature. 481, 371–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kane JR, Stanley DJ, Hultquist JF, Johnson JR, Mietrach N, Binning JM, Jonsson SR, Barelier S, Newton BW, Johnson TL, Franks-Skiba KE, Li M, Brown WL, Gunnarsson HI, Adalbjornsdottir A, Fraser JS, Harris RS, Andresdottir V, Gross JD & Krogan NJ (2015) Lineage-Specific Viral Hijacking of Non-canonical E3 Ubiquitin Ligase Cofactors in the Evolution of Vif Anti-APOBEC3 Activity, Cell reports. 11, 1236–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taniuchi I, Osato M, Egawa T, Sunshine MJ, Bae SC, Komori T, Ito Y & Littman DR (2002) Differential requirements for Runx proteins in CD4 repression and epigenetic silencing during T lymphocyte development, Cell. 111, 621–33. [DOI] [PubMed] [Google Scholar]
- 36.Watashi K & Shimotohno K (2007) Cyclophilin and viruses: cyclophilin as a cofactor for viral infection and possible anti-viral target, Drug target insights. 2, 9–18. [PMC free article] [PubMed] [Google Scholar]
- 37.Guo Y, Dong L, Qiu X, Wang Y, Zhang B, Liu H, Yu Y, Zang Y, Yang M & Huang Z (2014) Structural basis for hijacking CBF-beta and CUL5 E3 ligase complex by HIV-1 Vif, Nature. 505, 229–33. [DOI] [PubMed] [Google Scholar]
- 38.Binning JM, Chesarino NM, Emerman M & Gross JD (2019) Structural Basis for a Species-Specific Determinant of an SIV Vif Protein toward Hominid APOBEC3G Antagonism, Cell Host Microbe. 26, 739–747 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stanley BJ, Ehrlich ES, Short L, Yu Y, Xiao Z, Yu XF & Xiong Y (2008) Structural insight into the human immunodeficiency virus Vif SOCS box and its role in human E3 ubiquitin ligase assembly, J Virol. 82, 8656–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim JH, Jang JW, Lee YS, Lee JW, Chi XZ, Li YH, Kim MK, Kim DM, Choi BS, Kim J, Kim HM, van Wijnen A, Park I & Bae SC (2014) RUNX family members are covalently modified and regulated by PIAS1-mediated sumoylation, Oncogenesis. 3, e101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Collins A, Littman DR & Taniuchi I (2009) RUNX proteins in transcription factor networks that regulate T-cell lineage choice, Nat Rev Immunol. 9, 106–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Read MA, Brownell JE, Gladysheva TB, Hottelet M, Parent LA, Coggins MB, Pierce JW, Podust VN, Luo RS, Chau V & Palombella VJ (2000) Nedd8 modification of cul-1 activates SCF(beta(TrCP))-dependent ubiquitination of IkappaBalpha, Mol Cell Biol. 20, 2326–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wada H, Yeh ET & Kamitani T (1999) Identification of NEDD8-conjugation site in human cullin-2, Biochem Biophys Res Commun. 257, 100–5. [DOI] [PubMed] [Google Scholar]
- 44.Baek K, Krist DT, Prabu JR, Hill S, Klugel M, Neumaier LM, von Gronau S, Kleiger G & Schulman BA (2020) NEDD8 nucleates a multivalent cullin-RING-UBE2D ubiquitin ligation assembly, Nature. 578, 461–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stanley DJ, Bartholomeeusen K, Crosby DC, Kim DY, Kwon E, Yen L, Cartozo NC, Li M, Jager S, Mason-Herr J, Hayashi F, Yokoyama S, Krogan NJ, Harris RS, Peterlin BM & Gross JD (2012) Inhibition of a NEDD8 Cascade Restores Restriction of HIV by APOBEC3G, PLoS Pathog. 8, e1003085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huttenhain R, Xu J, Burton LA, Gordon DE, Hultquist JF, Johnson JR, Satkamp L, Hiatt J, Rhee DY, Baek K, Crosby DC, Frankel AD, Marson A, Harper JW, Alpi AF, Schulman BA, Gross JD & Krogan NJ (2019) ARIH2 Is a Vif-Dependent Regulator of CUL5-Mediated APOBEC3G Degradation in HIV Infection, Cell Host Microbe. 26, 86–99 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cheng C, Zhang TL, Wang CX, Lan WX, Ding JP & Cao CY (2018) Crystal Structure of Cytidine Deaminase Human APOBEC3F Chimeric Catalytic Domain in Complex with DNA, Chinese J Chem. 36, 1241–1248. [Google Scholar]
- 48.Holden LG, Prochnow C, Chang YP, Bransteitter R, Chelico L, Sen U, Stevens RC, Goodman MF & Chen XS (2008) Crystal structure of the anti-viral APOBEC3G catalytic domain and functional implications, Nature. 456, 121–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maiti A, Myint W, Kanai T, Delviks-Frankenberry K, Sierra Rodriguez C, Pathak VK, Schiffer CA & Matsuo H (2018) Crystal structure of the catalytic domain of HIV-1 restriction factor APOBEC3G in complex with ssDNA, Nature communications. 9, 2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bohn MF, Shandilya SM, Albin JS, Kouno T, Anderson BD, McDougle RM, Carpenter MA, Rathore A, Evans L, Davis AN, Zhang J, Lu Y, Somasundaran M, Matsuo H, Harris RS & Schiffer CA (2013) Crystal structure of the DNA cytosine deaminase APOBEC3F: the catalytically active and HIV-1 Vif-binding domain, Structure. 21, 1042–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xiao X, Li SX, Yang H & Chen XS (2016) Crystal structures of APOBEC3G N-domain alone and its complex with DNA, Nature communications. 7, 12193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ziegler SJ, Liu C, Landau M, Buzovetsky O, Desimmie BA, Zhao Q, Sasaki T, Burdick RC, Pathak VK, Anderson KS & Xiong Y (2018) Insights into DNA substrate selection by APOBEC3G from structural, biochemical, and functional studies, PLoS ONE. 13, e0195048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kouno T, Luengas EM, Shigematsu M, Shandilya SM, Zhang J, Chen L, Hara M, Schiffer CA, Harris RS & Matsuo H (2015) Structure of the Vif-binding domain of the antiviral enzyme APOBEC3G, Nat Struct Mol Biol. 22, 485–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Navarro F, Bollman B, Chen H, Konig R, Yu Q, Chiles K & Landau NR (2005) Complementary function of the two catalytic domains of APOBEC3G, Virology. 333, 374–86. [DOI] [PubMed] [Google Scholar]
- 55.Russell RA, Smith J, Barr R, Bhattacharyya D & Pathak VK (2009) Distinct Domains within APOBEC3G and APOBEC3F Interact with Separate Regions of Human Immunodeficiency Virus Type 1 Vif, J Virol. 83, 1992–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hache G, Liddament MT & Harris RS (2005) The retroviral hypermutation specificity of APOBEC3F and APOBEC3G is governed by the C-terminal DNA cytosine deaminase domain, J Biol Chem. 280, 10920–4. [DOI] [PubMed] [Google Scholar]
- 57.Kitamura S, Ode H & Iwatani Y (2011) Structural Features of Antiviral APOBEC3 Proteins are Linked to Their Functional Activities, Frontiers in microbiology. 2, 258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Aydin H, Taylor MW & Lee JE (2014) Structure-guided analysis of the human APOBEC3-HIV restrictome, Structure. 22, 668–84. [DOI] [PubMed] [Google Scholar]
- 59.Russell RA & Pathak VK (2007) Identification of two distinct human immunodeficiency virus type 1 Vif determinants critical for interactions with human APOBEC3G and APOBEC3F, J Virol. 81, 8201–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hu Y, Desimmie BA, Nguyen HC, Ziegler SJ, Cheng TC, Chen J, Wang J, Wang H, Zhang K, Pathak VK & Xiong Y (2019) Structural basis of antagonism of human APOBEC3F by HIV-1 Vif, Nat Struct Mol Biol. 26, 1176–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kitamura S, Ode H, Nakashima M, Imahashi M, Naganawa Y, Kurosawa T, Yokomaku Y, Yamane T, Watanabe N, Suzuki A, Sugiura W & Iwatani Y (2012) The APOBEC3C crystal structure and the interface for HIV-1 Vif binding, Nat Struct Mol Biol. 19, 1005–10. [DOI] [PubMed] [Google Scholar]
- 62.Nakashima M, Ode H, Kawamura T, Kitamura S, Naganawa Y, Awazu H, Tsuzuki S, Matsuoka K, Nemoto M, Hachiya A, Sugiura W, Yokomaku Y, Watanabe N & Iwatani Y (2016) Structural Insights into HIV-1 Vif-APOBEC3F Interaction, J Virol. 90, 1034–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Albin JS, LaRue RS, Weaver JA, Brown WL, Shindo K, Harjes E, Matsuo H & Harris RS (2010) A single amino acid in human APOBEC3F alters susceptibility to HIV-1 Vif, J Biol Chem. 285, 40785–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Siu KK, Sultana A, Azimi FC & Lee JE (2013) Structural determinants of HIV-1 Vif susceptibility and DNA binding in APOBEC3F, Nature communications. 4, 2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kim DY (2015) The assembly of Vif ubiquitin E3 ligase for APOBEC3 degradation, Arch Pharm Res. 38, 435–45. [DOI] [PubMed] [Google Scholar]
- 66.Dang Y, Davis RW, York IA & Zheng YH (2010) Identification of 81LGxGxxIxW89 and 171EDRW174 domains from human immunodeficiency virus type 1 Vif that regulate APOBEC3G and APOBEC3F neutralizing activity, J Virol. 84, 5741–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yamashita T, Kamada K, Hatcho K, Adachi A & Nomaguchi M (2008) Identification of amino acid residues in HIV-1 Vif critical for binding and exclusion of APOBEC3G/F, Microbes Infect. 10, 1142–9. [DOI] [PubMed] [Google Scholar]
- 68.Dang Y, Wang X, Zhou T, York IA & Zheng YH (2009) Identification of a novel WxSLVK motif in the N terminus of human immunodeficiency virus and simian immunodeficiency virus Vif that is critical for APOBEC3G and APOBEC3F neutralization, J Virol. 83, 8544–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen G, He Z, Wang T, Xu R & Yu XF (2009) A patch of positively charged amino acids surrounding the human immunodeficiency virus type 1 Vif SLVx4Yx9Y motif influences its interaction with APOBEC3G, J Virol. 83, 8674–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.He Z, Zhang W, Chen G, Xu R & Yu XF (2008) Characterization of conserved motifs in HIV-1 Vif required for APOBEC3G and APOBEC3F interaction, J Mol Biol. 381, 1000–11. [DOI] [PubMed] [Google Scholar]
- 71.Yang H, Ito F, Wolfe AD, Li S, Mohammadzadeh N, Love RP, Yan M, Zirkle B, Gaba A, Chelico L & Chen XS (2020) Understanding the structural basis of HIV-1 restriction by the full length double-domain APOBEC3G, Nature communications. 11, 632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Duda DM, Borg LA, Scott DC, Hunt HW, Hammel M & Schulman BA (2008) Structural insights into NEDD8 activation of cullin-RING ligases: conformational control of conjugation, Cell. 134, 995–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kao S, Khan MA, Miyagi E, Plishka R, Buckler-White A & Strebel K (2003) The human immunodeficiency virus type 1 Vif protein reduces intracellular expression and inhibits packaging of APOBEC3G (CEM15), a cellular inhibitor of virus infectivity, J Virol. 77, 11398–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stopak K, de Noronha C, Yonemoto W & Greene WC (2003) HIV-1 Vif blocks the antiviral activity of APOBEC3G by impairing both its translation and intracellular stability, Molecular cell. 12, 591–601. [DOI] [PubMed] [Google Scholar]
- 75.Mercenne G, Bernacchi S, Richer D, Bec G, Henriet S, Paillart JC & Marquet R (2010) HIV-1 Vif binds to APOBEC3G mRNA and inhibits its translation, Nucleic Acids Res. 38, 633–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Binning JM, Smith AM, Hultquist JF, Craik CS, Caretta Cartozo N, Campbell MG, Burton L, La Greca F, McGregor MJ, Ta HM, Bartholomeeusen K, Peterlin BM, Krogan NJ, Sevillano N, Cheng Y & Gross JD (2018) Fab-based inhibitors reveal ubiquitin independent functions for HIV Vif neutralization of APOBEC3 restriction factors, PLoS Pathog. 14, e1006830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Feng Y, Love RP & Chelico L (2013) HIV-1 viral infectivity factor (Vif) alters processive single-stranded DNA scanning of the retroviral restriction factor APOBEC3G, J Biol Chem. 288, 6083–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Britan-Rosich E, Nowarski R & Kotler M (2011) Multifaceted counter-APOBEC3G mechanisms employed by HIV-1 Vif, J Mol Biol. 410, 1065–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tian C, Yu X, Zhang W, Wang T, Xu R & Yu XF (2006) Differential requirement for conserved tryptophans in human immunodeficiency virus type 1 Vif for the selective suppression of APOBEC3G and APOBEC3F, J Virol. 80, 3112–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dang Y, Wang X, York IA & Zheng YH (2010) Identification of a critical T(Q/D/E)x5ADx2(I/L) motif from primate lentivirus Vif proteins that regulate APOBEC3G and APOBEC3F neutralizing activity, J Virol. 84, 8561–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Richards C, Albin JS, Demir O, Shaban NM, Luengas EM, Land AM, Anderson BD, Holten JR, Anderson JS, Harki DA, Amaro RE & Harris RS (2015) The Binding Interface between Human APOBEC3F and HIV-1 Vif Elucidated by Genetic and Computational Approaches, Cell reports. 13, 1781–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang W, Chen G, Niewiadomska AM, Xu R & Yu XF (2008) Distinct determinants in HIV-1 Vif and human APOBEC3 proteins are required for the suppression of diverse host anti-viral proteins, PLoS ONE. 3, e3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang Z, Gu Q, Jaguva Vasudevan AA, Jeyaraj M, Schmidt S, Zielonka J, Perkovic M, Heckel JO, Cichutek K, Haussinger D, Smits SHJ & Munk C (2016) Vif Proteins from Diverse Human Immunodeficiency Virus/Simian Immunodeficiency Virus Lineages Have Distinct Binding Sites in A3C, J Virol. 90, 10193–10208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nakashima M, Tsuzuki S, Awazu H, Hamano A, Okada A, Ode H, Maejima M, Hachiya A, Yokomaku Y, Watanabe N, Akari H & Iwatani Y (2017) Mapping Region of Human Restriction Factor APOBEC3H Critical for Interaction with HIV-1 Vif, J Mol Biol. 429, 1262–1276. [DOI] [PubMed] [Google Scholar]
- 85.Huthoff H & Malim MH (2007) Identification of amino acid residues in APOBEC3G required for regulation by human immunodeficiency virus type 1 Vif and Virion encapsidation, J Virol. 81, 3807–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bogerd HP, Doehle BP, Wiegand HL & Cullen BR (2004) A single amino acid difference in the host APOBEC3G protein controls the primate species specificity of HIV type 1 virion infectivity factor, Proc Natl Acad Sci U S A. 101, 3770–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mangeat B, Turelli P, Liao S & Trono D (2004) A single amino acid determinant governs the species-specific sensitivity of APOBEC3G to Vif action, J Biol Chem. 279, 14481–3. [DOI] [PubMed] [Google Scholar]
- 88.Schrofelbauer B, Chen D & Landau NR (2004) A single amino acid of APOBEC3G controls its species-specific interaction with virion infectivity factor (Vif), Proc Natl Acad Sci U S A. 101, 3927–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Xu H, Svarovskaia ES, Barr R, Zhang Y, Khan MA, Strebel K & Pathak VK (2004) A single amino acid substitution in human APOBEC3G antiretroviral enzyme confers resistance to HIV-1 virion infectivity factor-induced depletion, Proc Natl Acad Sci U S A. 101, 5652–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ooms M, Letko M & Simon V (2017) The Structural Interface between HIV-1 Vif and Human APOBEC3H, J Virol. 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhen A, Wang T, Zhao K, Xiong Y & Yu XF (2010) A single amino acid difference in human APOBEC3H variants determines HIV-1 Vif sensitivity, J Virol. 84, 1902–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Binka M, Ooms M, Steward M & Simon V (2012) The activity spectrum of Vif from multiple HIV-1 subtypes against APOBEC3G, APOBEC3F, and APOBEC3H, J Virol. 86, 49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ooms M, Letko M, Binka M & Simon V (2013) The resistance of human APOBEC3H to HIV-1 NL4–3 molecular clone is determined by a single amino acid in Vif, PLoS ONE. 8, e57744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Barnor JS, Miyano-Kurosaki N, Yamaguchi K, Sakamoto A, Ishikawa K, Inagaki Y, Yamamoto N, Osei-Kwasi M, Ofori-Adjei D & Takaku H (2004) Intracellular expression of antisense RNA transcripts complementary to the human immunodeficiency virus type-1 vif gene inhibits viral replication in infected T-lymphoblastoid cells, Biochem Biophys Res Commun. 320, 544–50. [DOI] [PubMed] [Google Scholar]
- 95.Barnor JS, Miyano-Kurosaki N, Yamaguchi K, Abumi Y, Ishikawa K & Yamamoto N (2005) Lentiviral-mediated delivery of combined HIV-1 decoy TAR and Vif siRNA as a single RNA molecule that cleaves to inhibit HIV-1 in transduced cells, Nucleosides Nucleotides Nucleic Acids. 24, 431–4. [DOI] [PubMed] [Google Scholar]
- 96.Bennett RP, Stewart RA, Hogan PA, Ptak RG, Mankowski MK, Hartman TL, Buckheit RW Jr., Snyder BA, Salter JD, Morales GA & Smith HC (2016) An analog of camptothecin inactive against Topoisomerase I is broadly neutralizing of HIV-1 through inhibition of Vif-dependent APOBEC3G degradation, Antiviral Res. 136, 51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang S, Zhong L, Chen B, Pan T, Zhang X, Liang L, Li Q, Zhang Z, Chen H, Zhou J, Luo H, Zhang H & Bai C (2015) Identification of an HIV-1 replication inhibitor which rescues host restriction factor APOBEC3G in Vif-APOBEC3G complex, Antiviral Res. 122, 20–7. [DOI] [PubMed] [Google Scholar]
- 98.Ali A, Wang J, Nathans RS, Cao H, Sharova N, Stevenson M & Rana TM (2012) Synthesis and structure-activity relationship studies of HIV-1 virion infectivity factor (Vif) inhibitors that block viral replication, Chemmedchem. 7, 1217–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pery E, Sheehy A, Nebane NM, Brazier AJ, Misra V, Rajendran KS, Buhrlage SJ, Mankowski MK, Rasmussen L, White EL, Ptak RG & Gabuzda D (2015) Identification of a novel HIV-1 inhibitor targeting Vif-dependent degradation of human APOBEC3G protein, J Biol Chem. 290, 10504–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nathans R, Cao H, Sharova N, Ali A, Sharkey M, Stranska R, Stevenson M & Rana TM (2008) Small-molecule inhibition of HIV-1 Vif, Nat Biotechnol. 26, 1187–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhou M, Luo RH, Hou XY, Wang RR, Yan GY, Chen H, Zhang RH, Shi JY, Zheng YT, Li R & Wei YQ (2017) Synthesis, biological evaluation and molecular docking study of N-(2-methoxyphenyl)-6-((4-nitrophenyl)sulfonyl)benzamide derivatives as potent HIV-1 Vif antagonists, European journal of medicinal chemistry. 129, 310–324. [DOI] [PubMed] [Google Scholar]
- 102.Mohammed I, Kummetha IR, Singh G, Sharova N, Lichinchi G, Dang J, Stevenson M & Rana TM (2016) 1,2,3-Triazoles as Amide Bioisosteres: Discovery of a New Class of Potent HIV-1 Vif Antagonists, J Med Chem. 59, 7677–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.DeLano W (2002) The PyMOL Molecular Graphics System DeLano Scientific, Palo Alto, CA, USA. [Google Scholar]


