Abstract
Background:
Though gemcitabine plus platinum chemotherapy is the established first line regimen for advanced biliary tract cancer (ABC), there is no standard second line therapy. We evaluated current practice and outcomes for second line chemotherapy in patients with ABC across three US academic medical centers.
Methods:
Institutional registries were reviewed to identify patients who had received second line chemotherapy for ABC from 4/2010 to 3/2015, along with demographics, diagnosis and staging, treatment history, and clinical outcomes. Overall survival from initiation of second line chemotherapy (OS2) was estimated using Kaplan-Meier methods.
Results:
We identified 198 patients with cholangiocarcinoma (intrahepatic, 61.1% and extrahepatic, 14.1%) and gallbladder carcinoma (24.8%); 52% received at least 3 lines of systemic chemotherapy. The median OS2 11 months (95% CI 8.8-13.1). Median OS2 for intrahepatic cholangiocarcinoma was 13.4 months (95% CI: 10.7-17.8), longer than extrahepatic or gallbladder with mOS2 of 6.8 months (95% CI: 5-10.5) and 9.4 months (95% CI: 7.2-12.3), respectively (p=0.018). The median time to second line treatment failure was 2.2 (95% CI: 1.8-2.7) months, similar across tumor locations (p=0.60).
Conclusions:
In this large cohort of ABC patients treated across three academic medical centers after failure of first line chemotherapy, the time to treatment failure on standard therapies was short, though median OS2 was longer than has been reported previously, and over half of patients received additional lines of treatment. This multicenter collaboration represents the largest cohort studied to date of second line chemotherapy in ABC and provides a contemporary benchmark for future clinical trials.
Keywords: Biliary cancer, bile duct cancer, cholangiocarcinoma, gallbladder cancer, chemotherapy, second line
Summary/precis:
In this large cohort of ABC patients treated across three academic medical centers after failure of first line chemotherapy, the overall survival after second line chemotherapy was higher than has been reported previously, and over half of patients received additional lines of treatment. This multicenter collaboration represents the largest cohort studied to date of second line chemotherapy in ABC and provides a contemporary benchmark for future clinical trials.
Introduction
Biliary cancers are a complex family of epithelial neoplasms, including cholangiocarcinoma that arises from the intrahepatic or extrahepatic bile ducts, and gallbladder carcinoma. Cholangiocarcinoma represents the most common primary biliary tract malignancy and the second most common primary cancer of the liver after hepatocellular carcinoma, and though it remains a rare cancer overall, the incidence of intrahepatic cholangiocarcinoma has risen significantly in recent years, while the incidence of extrahepatic cholangiocarcinoma appears static.1, 2 Gallbladder cancers also are a rare tumor type in the United States but account for an annual worldwide incidence of approximately 180,000 cases and almost 140,000 deaths based upon estimates from 2012.3
Most patients with biliary cancers are diagnosed with or progress to advanced stages of disease. In patients with advanced biliary cancers (ABC) not amenable to curative therapies, the ABC-02 trial established the regimen of gemcitabine plus cisplatin as a global standard of care in 2010, reporting significantly improved median overall survival of 11.7 months on the combination regimen compared to 8.1 months for gemcitabine alone (hazard ratio, 0.64; P<0.001).4 The median progression-free survival was 8.0 months for the combination of gemcitabine plus cisplatin, compared to 5.0 months for gemcitabine (P<0.001) in the first line setting.
After failure of first line therapy, there remains no standard second line regimen for ABC. Fluoropyrimidine-based regimens are commonly employed on the grounds of modest response rates reported in multiple retrospective analyses and single-arm prospective studies, though results are mixed and interpretation is limited without randomization to a control arm.5-9
To date, the largest retrospective analysis of second line chemotherapy in ABC examined outcomes from 603 patients treated with first line gemcitabine plus platinum chemotherapy across 17 centers in France between 2002-2013.10 Of these, 196 (32%) received second line chemotherapy after failure of a first line regimen, with a median second line progression-free survival (PFS2) of 3.2 months and median OS2 of 6.7 months. There was no difference in OS2 between chemotherapy regimens employed. Poor performance status, elevated bilirubin, and CA 19-9 tumor marker >400 IU/mL were associated with worse outcomes. In another retrospective analysis, 174 patients with ABC treated with second line chemotherapy after progression on first line gemcitabine plus platinum chemotherapy were identified across 10 Italian institutions between 2004 and 2013. In this cohort, fluoropyrimidine-based monotherapy or combinations were employed as the second line regimen in over 80% of cases. The overall response rate was only 3.4%, with median PFS2 of 3.0 months and median OS2 of 6.6 months.11 After second line chemotherapy, 43% of patients received a third line. We designed this retrospective, multicenter analysis to define contemporary practice patterns and treatment outcomes in second line ABC after the establishment of gemcitabine plus cisplatin as the standard first line regimen in ABC-02, with planned subanalyses including tumor location and treating institution to address the inherent heterogeneity of this family of cancers. The goal of this study is to inform the hypotheses and design of future second line systemic therapy trials in ABC, an area of significant and growing unmet need in oncology.
Materials and Methods
Study Overview
This multicenter, investigator-initiated, retrospective analysis was conducted across three study sites: Memorial Sloan Kettering Cancer Center (MSKCC), University of California, San Francisco (UCSF), and Vanderbilt-Ingram Cancer Center (VICC). IRB approval or exemption was obtained at each study site. This project was supported by a Cholangiocarcinoma Foundation grant awarded through a competitive review process.
Patient Selection
Patients were identified by review of consecutive Cancer Center registry records at each of the three study sites. Patients were included if they had the following characteristics: age 18 years or older; diagnosis of intrahepatic or extrahepatic (including distal and hilar/perihilar) cholangiocarcinoma or gallbladder cancer); locally-advanced or metastatic stage (American Joint Committee on Cancer (AJCC) stage III or IV, and/or assessed as incurable by treating providers); oncology care (consultation and/or any treatment) received at one of the study sites; treatment with first line systemic chemotherapy (including intra-arterial therapy) for advanced disease and/or treatment with adjuvant systemic therapy with recurrence of tumor ≤ 6 months from last dose; and receipt of second line chemotherapy after first line discontinuation. Patients with diagnoses of primary tumor of ampulla, duodenum, or pancreas were excluded. Additionally, overall survival and history of first and second line treatments had to be available per review of each patient’s medical records, local cancer registry abstract, or state cancer registry database.
Clinical Data Abstraction
De-identified clinical and pathologic data of patients with ABC were extracted from institutional medical records, and from case abstracts created under standards set for North American institutional and state-wide cancer registries and shared with institutional cancer registries. Data were compiled under a study identification number at each site and stored in HIPAA-compliant, password-protected, encrypted databases.
Statistical Analysis
The primary endpoint of the analysis was OS from start of second line systemic chemotherapy (OS2) after failure of first line chemotherapy. Subjects alive or with unknown vital status were censored at date last known alive. Secondary endpoints included: proportion of patients who received third-line chemotherapy after failure of first line gemcitabine plus any platinum regimen; incidence of specific chemotherapy regimens; OS for first line chemotherapy (OS1), and time to treatment failure from second line therapy (TTF2). OS1 was calculated from start date of first line chemotherapy to death. TTF2 was calculated from the start of second line chemotherapy to the date of discontinuation or death, whichever occurred first. Patients with ongoing treatment or unknown date of discontinuation and alive (n=5) were censored at date of last follow-up or at the cutoff date of August 2015. Exploratory endpoints included associations between OS, TTF2, and treatment regimen according to tumor location and other clinical and pathologic features.
Kaplan-Meier methods were used to summarize the OS2, OS1, and TTF2 endpoints with 95% confidence intervals (CI) and compared between patient subgroups using the log-rank test. Clinical and pathologic features of study population are reported using descriptive statistics (mean, median, standard deviations, and 95% CI when appropriate).
All statistical analyses were performed using SAS Version 9.3 (SAS Institute, Inc., Cary, NC, USA) or R Version 3.5.1 (R Foundation for Statistical Computing, Vienna, Austria). All P-values were two-sided. P-values of <0.05 were considered to indicate statistical significance
Results
Patient Characteristics
In total, 198 patients who had initiated first line systemic chemotherapy for ABC between 4/2010 and 3/2015 and were subsequently treated with second line chemotherapy were identified across the three institutions (MSKCC:123, UCSF:45, VICC: 30). Patient clinical and pathologic characteristics are described in Table 1. The majority of patients (61.1%) had tumors originating in an intrahepatic location. The American Joint Committee on Cancer (AJCC) stage at diagnosis was III/IV in over 80% of cases. Tumor mutation profiling for cholangiocarcinoma was not established as a standard practice during the timeframe of this retrospective analysis, thus the tumor genotypes of the study population are not known.
Table 1.
Patient characteristics
| MSKCC (n=123) |
UCSF (n=45) | VICC (n=30) | Total (n=198) | |
|---|---|---|---|---|
| Age at diagnosis, median (range) | 63 (21-84) | 60 (36-91) | 59 (36-85) | 62 (21-91) |
| Male | 57 (46.3%) | 19 (42.2%) | 10 (33.3%) | 86 (43.4%) |
| Primary tumor location | ||||
| Extrahepatic bile duct | 14 (11.4%) | 7 (15.6%) | 7 (23.3%) | 28 (14.1%) |
| Gallbladder | 34 (27.6%) | 7 (15.6%) | 8 (26.7%) | 49 (24.8%) |
| Intrahepatic bile duct | 75 (61.0%) | 31 (68.8%) | 15 (50.0%) | 121 (61.1%) |
| Adenocarcinoma | ||||
| Yes | 113 (91.8%) | 43 (95.5%) | 29 (96.7%) | 185 (93.4%) |
| AJCC stage at diagnosis | ||||
| I/II | 17 (13.8%) | 8 (17.8%) | 10 (33.3%) | 35 (17.7%) |
| III/IV | 103 (83.7%) | 37 (82.2%) | 20 (55.7%) | 160 (80.8%) |
| Unknown | 3 (2.4%) | 0 | 0 | 3 (1.5%) |
| First line regimen | ||||
| GEMICS/GEMOX | 94 (76.4%) | 29 (60.4%) | 21 (70.0%) | 144 (72.7%) |
| Intrahepatic FUDR* | 6 (4.9%) | 0 | 0 | 6 (3.0%) |
| Adjuvant <6 mos. prior | 0 | 10 (22.2%) | 4 (13.3%) | 14 (7.1%) |
| Others | 23 (18.7%) | 6 (13.3%) | 5 (16.7%) | 34 (17.2%) |
Key: AJCC=American Joint Committee on Cancer; GEMCIS=combination of gemcitabine plus cisplatin; GEMOX=combination of gemcitabine plus oxaliplatin; FUDR=fluorodeoxyuridine
4 additional patients received FUDR plus systemic chemotherapy in first line and are included under first line regimens used
Chemotherapy Regimens
Most patients (73%) received a gemcitabine plus platinum combination chemotherapy regimen as first line treatment (Table 1). In the second line setting, 5-fluorouracil was employed in over 60% of patients, either as single agent or in combination regimens (e.g. 5-fluorouracil plus irinotecan (FOLFIRI) or oxaliplatin (FOLFOX)), while 18% received gemcitabine in the second line setting (Table 2). None of the patients were known to have received second line targeted therapies directed against specific mutations; 12 patients were treated with unspecified regimens on clinical trial protocols (10) or another unknown regimen (2). Approximately half of the patients across the three institutions (103 patients, 52%) received a third line chemotherapy following disease progression on second line therapy.
Table 2.
Second line chemotherapy received by patients with advanced biliary cancer
| Regimen | N (%) |
|---|---|
| 5-fluorouracil-based | 124 (62.6%) |
| Gemcitabine-based | 36 (18.1%) |
| Intrahepatic FUDR | 11 (5.5%) |
| Other | 27 (13.6%) |
Overall Survival
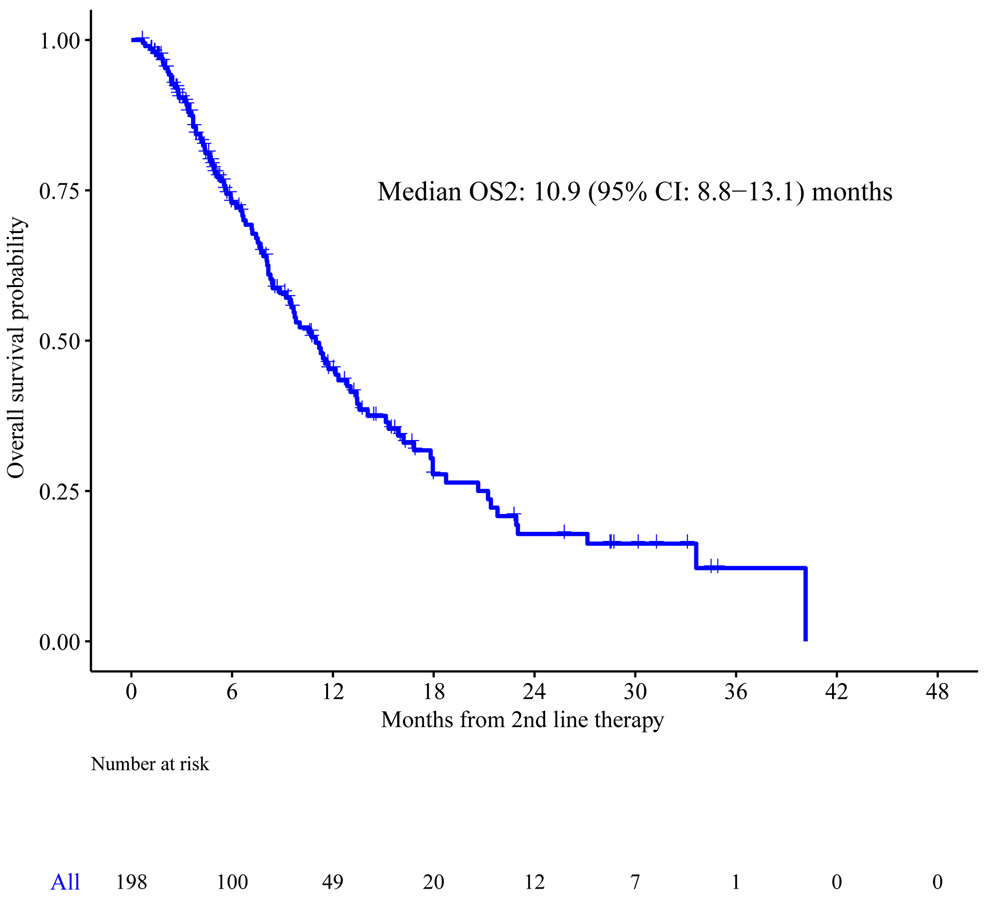
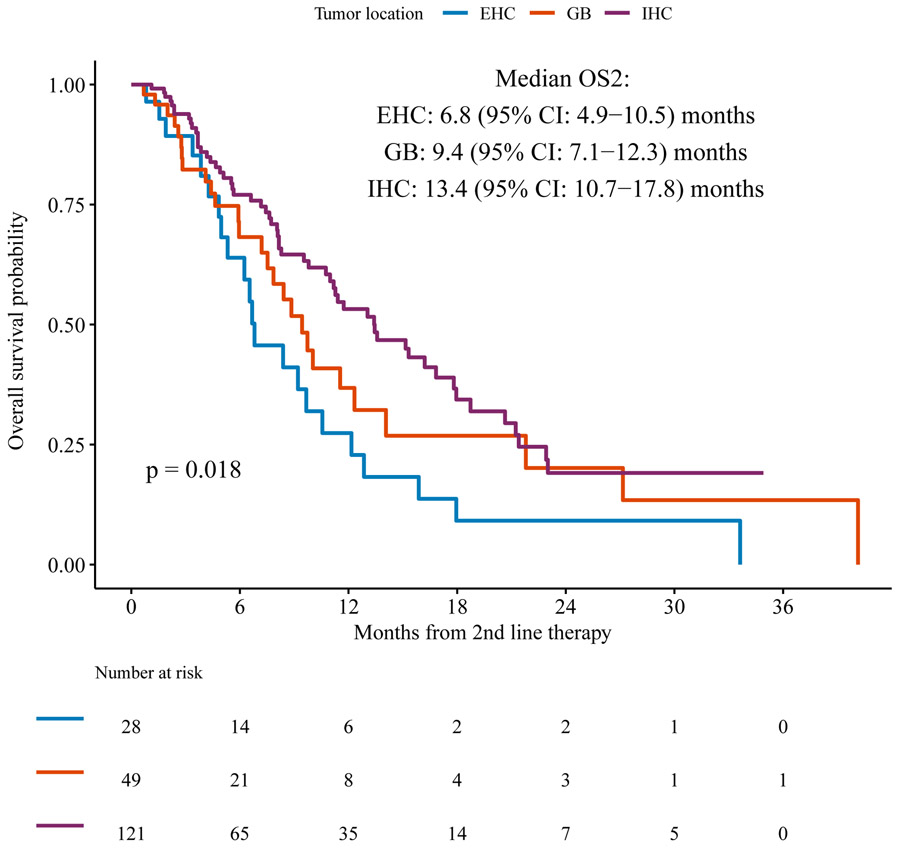
Median follow up from the start of second line therapy among survivors was 5 [range 1-36] months. At the time of study analysis, we observed a total of 106 deaths, and median OS2 was 11 months (95% CI: 8.8-13.1) (Figure 1a). For the subsets of patients with intrahepatic cholangiocarcinoma, gallbladder cancer, and extrahepatic cholangiocarcinoma, the median OS2 were 13.4 months (95% CI: 10.9-17.9), 9.4 months (95% CI: 7.2-12.3), and 6.8 months (95% CI: 7.5-21.8), respectively (p=0.018 for comparison between the three types of tumors) (Figure 1b). No significant difference in median OS2 was observed in the small number of patients (n=6) who received first line intrahepatic arterial chemotherapy with FUDR compared to those who received first line systemic chemotherapy.
Figure 1a.
Overall survival from the start of second line therapy (OS2)
Figure 1b.
OS2 according to primary tumor site
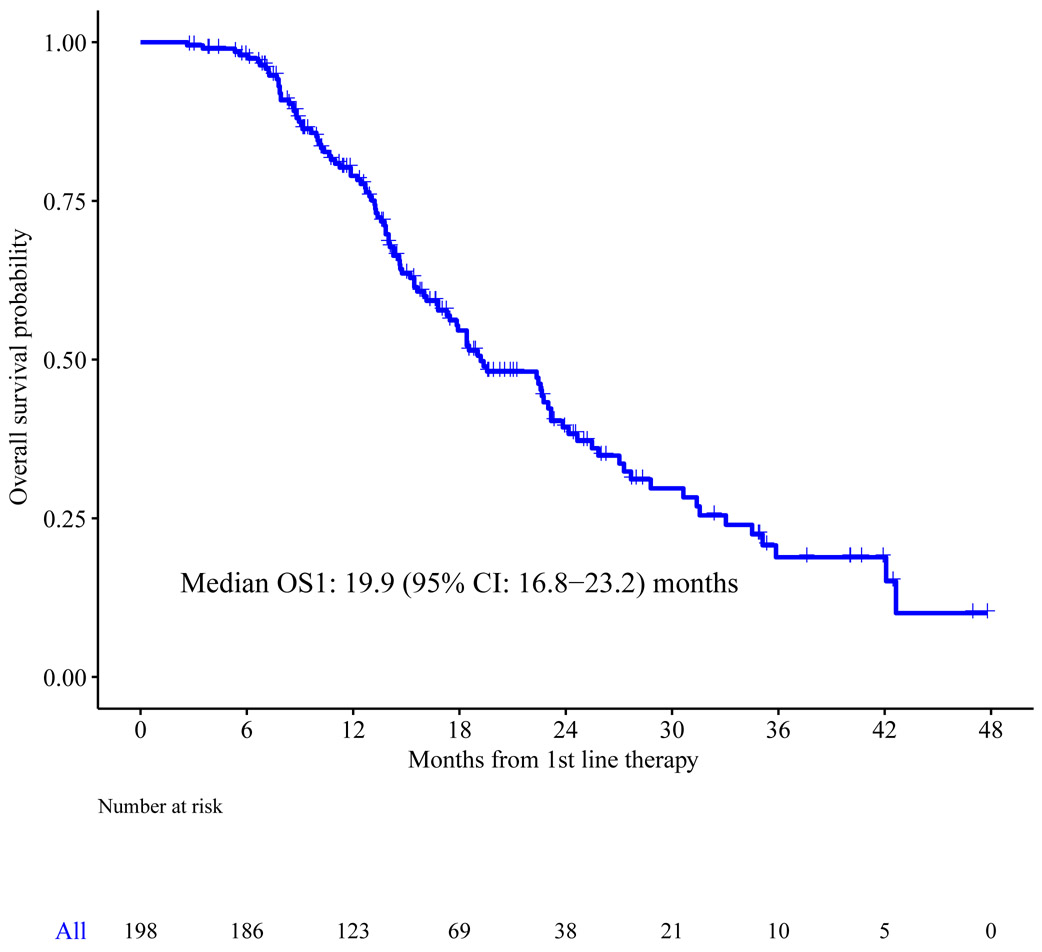
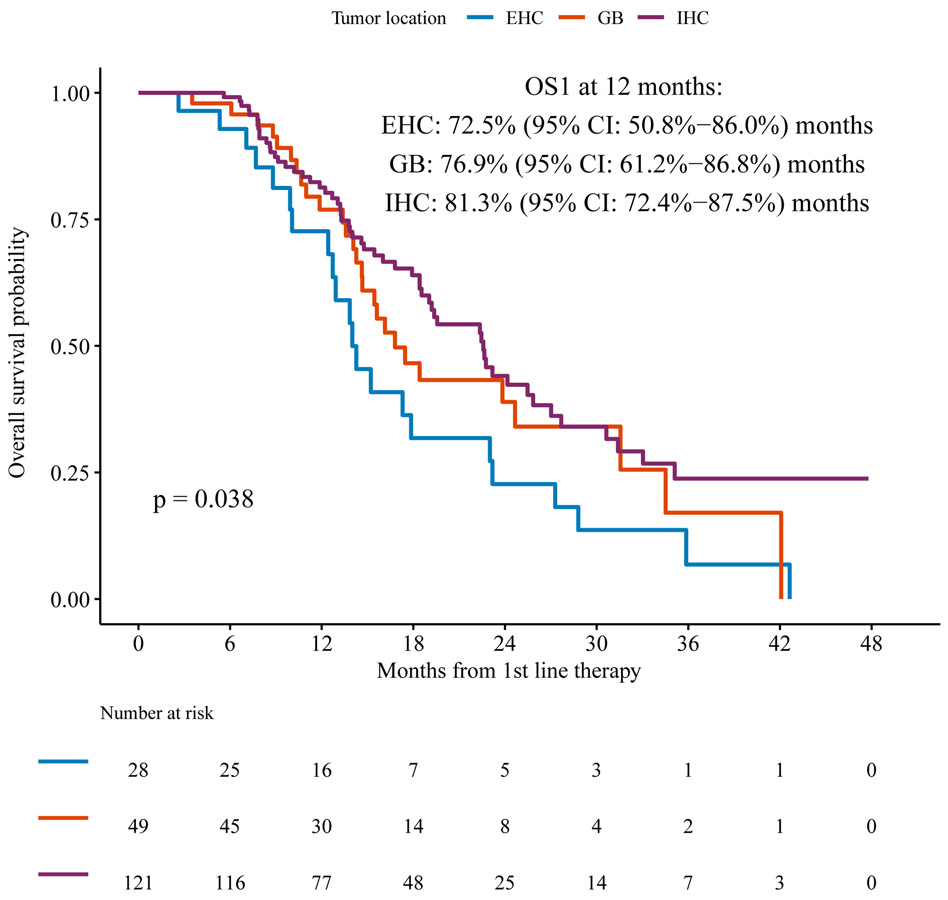
The median OS1 from the start of first line chemotherapy (n=198) was 19.2 months (95%CI: 16.8-23.2) (Figure 2a) in this population selected for receipt of subsequent second line chemotherapy, which is expected to be a positive prognostic factor. Intrahepatic cholangiocarcinoma was associated with longer OS1 at one year (81%) (95% CI: 72%-88%) compared to gallbladder and extrahepatic cholangiocarcinoma, 77% (95% CI: 61%-86%) and 73% (95% CI 51%-86%) respectively, p=0.038) using the log-rank test comparing the survival distributions between the three tumor sites (Figure 2b).
Figure 2a.
Overall survival following initiation of first line chemotherapy (OS1) for the overall cohort
Figure 2b.
OS1 according to primary tumor site
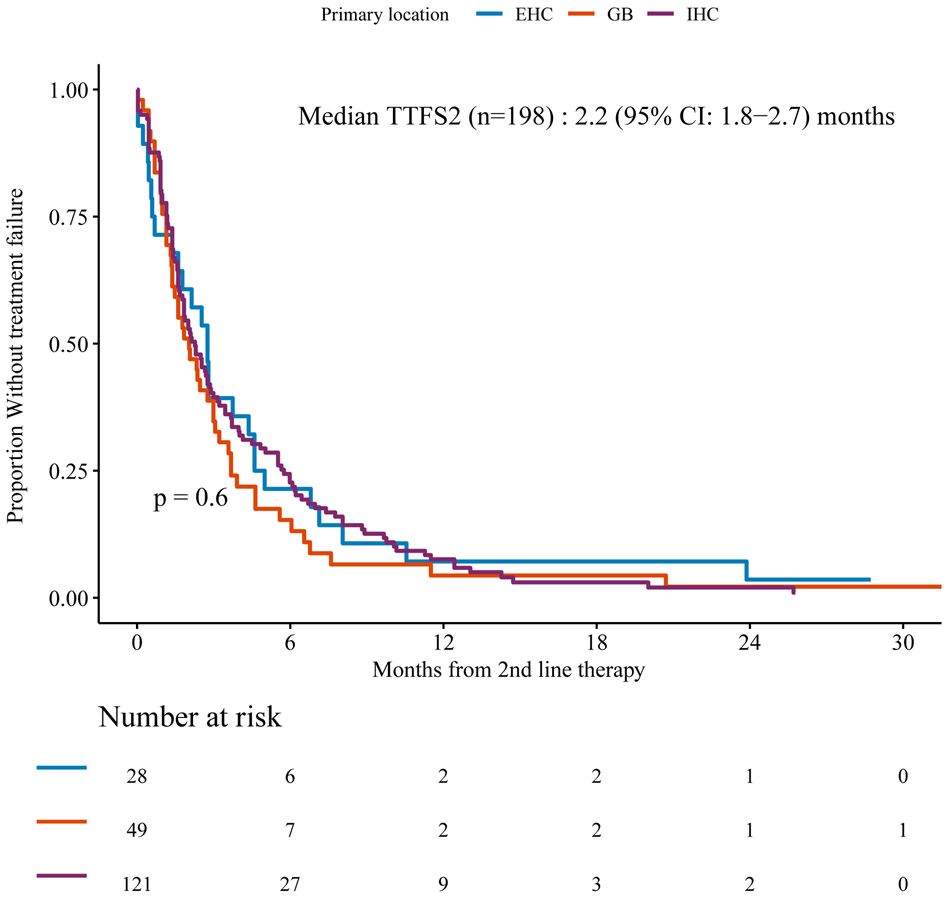
Time to Treatment Failure
Among patients who failed any first line chemotherapy, the median TTF2 on second line chemotherapy was 2.2 (95% CI: 1.8-2.7) months. At the time of analysis, we observed 193 had discontinued second line therapy or died with cumulative incidence of second line treatment failure at 6 months of 79.3% (95% CI: 73%-84%). There was no significant association between TTF2 and tumor location (Figure 3), age at diagnosis at least 60 or less than 60 years (p=0.65), or histology of adenocarcinoma versus other (p=0.274). TTF2 was longer in patients diagnosed with AJCC stage I/II tumors by comparison to stage III/IV tumors, with median 2.79 (95%CI: 1.87-6.71) months versus 2.07 (95%CI: 1.61-2.69) months, respectively (p=0.022).
Figure 3.
Time to treatment failure according to primary tumor site
Discussion
This multicenter, retrospective analysis of 198 patients with advanced biliary cancers represents the largest study to date of contemporary second line outcomes in the post-ABC-02 era. The relatively large size of this cohort, coupled with geographic and demographic diversity and subanalyses by tumor anatomic location and treating institution, support the robustness of the data.
The most important finding in this study is the extremely short duration of therapy received in the second line setting, with median TTF2 of only 2.2 (95% CI: 1.8 to 2.7) months suggesting limited efficacy of standard second line regimens and underscoring the urgent need for new, active therapies for this growing population. This TTF2 is somewhat shorter than the second line PFS reported in prior studies, which generally ranged from 2.7-3.2 months.9, 11, 12 The abbreviated TTF2 may reflect a tendency toward prompt treatment discontinuation at first evidence of progression in this population treated at tertiary centers with access to clinical trials and other later line treatments. TTF2 may also underestimate PFS by capturing treatment failures due to toxicity or complications of comorbid biliary obstruction, which are prevalent in this population. Though such events are often harbingers of underlying tumor progression, they are not captured consistently by the endpoint of PFS. For this retrospective analysis of registry-derived cases, TTF2 was selected as a “real world” secondary endpoint instead of PFS because dates of treatment were consistently available and because formal radiographic response assessment using RECIST 1.113 had not been performed for the majority of patients.
The primary endpoint of median OS2 was longer than expected in our study, measuring 11 months overall and as high as 13.4 months for patients with tumors arising from an intrahepatic location. This likely reflects a more favorable tumor biology among patients who were able to receive second line therapy following progression of disease on first line chemotherapy. Along with the substantial proportion (over 50%) receiving third line or later therapies, the prolonged median OS2, particularly for the intrahepatic cholangiocarcinoma subgroup, is an important index of the potential to conduct clinical trials of novel therapies after failure of first line regimens. The proportion of patients who received second line therapy after first line was unable to be estimated reliably under this retrospective design, owing to referral bias with a substantial proportion of patients referred to the three study sites after initiation of first line therapy.
Other important findings of this study include a numerically higher proportion of subjects with intrahepatic cholangiocarcinoma by comparison to extrahepatic or gall bladder locations, which may reflect the rising incidence of this subgroup,2 an increased likelihood for intrahepatic primary cholangiocarcinomas to maintain adequate liver function without obstruction, or a selection bias across the three centers.
There are inherent limitations to these findings, however, owing to the retrospective design without a control arm and selection of patients for receipt of second line therapy, likely to have more favorable biology, less comorbidity, and/or chemotherapy-responsive disease. The efficacy, or lack thereof, of second line chemotherapy cannot be inferred from a nonrandomized design, though the short TTF2 observed in this study, with similarly short PFS in other cohorts, suggests only modest activity in the overall population, without any differential benefit identified in anatomic subgroups. The inclusion of patients identified from the registries of three large academic centers may also introduce a selection bias towards patients with more favorable prognosis able to seek care at a referral center. The randomized, phase III ABC-06 trial recently reported a median overall survival of 6.2 months for ABC patients treated with FOLFOX as second line therapy at 20 centers across the U.K., achieving a small but significant improvement over active symptom control (hazard ratio 0.69, 95% CI: 0.50-0.97, p=0.031).14 Though the absolute survival outcomes were shorter than in our retrospective analysis and perhaps reflective of the selection bias inherent to tertiary referral center populations as discussed above, the progression free survival was similarly modest, measuring 4.0 months for FOLFOX (95% CI: 3.2-5.0) and reinforcing the need for more efficacious therapies for ABC after first line therapy.
Another important challenge is the inherent heterogeneity of biliary cancers, which may impact prognosis and clinical outcomes. This heterogeneity arises from clinical factors, including the variability in prior liver-directed therapies and degrees of hepatic dysfunction, anatomic location (which impacts risk for complications such as biliary obstruction), and the underlying tumor biology, with emerging evidence suggesting the existence of multiple molecular subtypes influenced by the etiology as well as anatomic location of biliary tract tumors.15-17 Some of these molecular subtypes, including tumors harboring FGFR2 fusions, IDH1 mutation, HER2 amplification, or mismatch repair deficiency, may be amenable to molecularly targeted therapies or immunotherapy, based upon promising evidence in case series and early phase clinical trials.18-26 Of note, however, this retrospective analysis preceded the activation of clinical trials of targeted inhibitors of FGFR2, mutant IDH1, or mismatch repair deficiency for cholangiocarcinoma at the study sites, thus the efficacy of targeted inhibitors in these contexts is not likely to have contributed to the more favorable outcomes for the intrahepatic subgroup of this study. Extrahepatic cholangiocarcinomas are associated with increased risk of biliary obstruction which can limit treatment options and lead to competing comorbidities such as cholangitis and sepsis, factors which are likely to have contributed to the poorer outcomes observed in the extrahepatic subgroup of this study and which underscore the importance of multidisciplinary supportive care in this population.
While randomization and stratification are the fundamental tools to study therapeutic efficacy and account for prognostic differences in a heterogeneous population such as ABC, the rarity of the population as a whole and of its individual subgroups require signal finding in single-arm studies, placed into the context of historical controls. Multiple single-arm studies of novel therapies including molecularly targeted agents are now ongoing in ABC, requiring context-dependent analysis of uncontrolled results and comparisons to historical controls such as this large retrospective cohort, to guide the decision to proceed to randomized phase III trials.
Conclusions
This study demonstrated that time to treatment failure on standard second line chemotherapy regimens for ABC was relatively short though a substantial proportion of patients treated across the three academic centers were fit to receive additional lines of therapy, reinforcing the urgent need for new, efficacious treatments. These outcomes overall and according to anatomic subgroups can inform the hypotheses and design for ongoing and future clinical trials in advanced biliary cancers.
Acknowledgment:
The authors thank the Cholangiocarcinoma Foundation for their financial support of this effort.
Funding statement:
This work was supported by a grant from the Cholangiocarcinoma Foundation.
Footnotes
Conflicts of interest: MAL reports consulting fees from Agios, Celgene, EMD Serono, and Roche. LWG reports consulting fees from Eli Lilly, Eisai, Bayer/Onyx, and Newlink Genetics, and research funding from Astellas Pharma, Pfizer, Onyx, Sun Pharma, Eli Lilly, Bristol-Myers Squibb, Agios, ArQule, H3 Biomedicine, Incyte, Leap Therapeutics, ASLAN Pharmaceuticals, BeiGene. EMO and GAA report consulting fees from 3DMedcare, Agios, Alignmed, Amgen, Antengene, Aptus, Aslan, Astellas, Astra Zeneca, Bayer, Beigene, Bioline, BMS, Boston Scientifc, Bridgebio, Carsgen, Casi, Celgene, Cipla, CytomX, Daiichi, Debio, Delcath, Eisai, Exelixis, Genoscience, Halozyme, Hengrui, Incyte, Inovio, Ipsen, Jazz, Jansen, Kyowa Kirin, LAM, Lilly, Loxo, Merck, Mina, Novella, Onxeo, PCI Biotech, Pfizer, Pieris, QED, Redhill, Sanofi, Servier, Silenseed, Sillajen, Sobi, Targovax, Tekmira, Twoxar, Vicus, Yakult, and Yiviva, and research funding from ActaBiologica, Agios, Array, Astra Zeneca, Bayer, Beigene, BMS, Casi, Celgene, Exelixis, Genentech, Halozyme, Incyte, Lilly, Mabvax, Novartis, OncoQuest, Polaris Puma, QED, Roche
JJH reports consulting fees from BMS, Eisai, Eli Lilly, and CytomX, and research funding from BMS. NK reports research funding from Amgen. RKK reports consulting fees from Agios, Astra Zeneca, Bristol-Myers Squibb, and Genentech/Roche, and research funding from Adaptimmune, Agios, Astra Zeneca, Bayer, Bristol-Myers Squibb, Eli Lilly, Exelixis, Medimmune, Merck, Novartis, QED, and Taiho. BK, EJ, RW, AGB, JFC, MC, ACG, JM, and APV report no conflicts.
References
- 1.Rizvi S, Gores GJ. Pathogenesis, Diagnosis, and Management of Cholangiocarcinoma. Gastroenterology. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saha SK, Zhu AX, Fuchs CS, Brooks GA. Forty-Year Trends in Cholangiocarcinoma Incidence in the U.S.: Intrahepatic Disease on the Rise. Oncologist. 2016;21: 594–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136: E359–386. [DOI] [PubMed] [Google Scholar]
- 4.Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362: 1273–1281. [DOI] [PubMed] [Google Scholar]
- 5.Hezel AF, Zhu AX. Systemic therapy for biliary tract cancers. Oncologist. 2008;13: 415–423. [DOI] [PubMed] [Google Scholar]
- 6.Kobayashi S, Ueno M, Sugimori K, et al. Phase II study of fixed dose-rate gemcitabine plus S-1 as a second-line treatment for advanced biliary tract cancer. Cancer Chemother Pharmacol. 2017. [DOI] [PubMed] [Google Scholar]
- 7.Unseld M, Scheithauer W, Weigl R, et al. Nab-paclitaxel as alternative treatment regimen in advanced cholangiocellular carcinoma. J Gastrointest Oncol. 2016;7: 588–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sebbagh S, Dreyer C, De Gramont A, et al. Effects of the sequential administration of GEMOX followed by FOLFIRI in cholangiocarcinoma. Gastrointestinal Cancers Symposium. San Francisco, CA: J Clin Oncol, 2014. [Google Scholar]
- 9.Rogers JE, Law L, Nguyen VD, et al. Second-line systemic treatment for advanced cholangiocarcinoma. J Gastrointest Oncol. 2014;5: 408–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chmielecki J, Hutchinson KE, Frampton GM, et al. Comprehensive genomic profiling of pancreatic acinar cell carcinomas identifies recurrent RAF fusions and frequent inactivation of DNA repair genes. Cancer Discov. 2014;4: 1398–1405. [DOI] [PubMed] [Google Scholar]
- 11.Fornaro L, Vivaldi C, Cereda S, et al. Second-line chemotherapy in advanced biliary cancer progressed to first-line platinum-gemcitabine combination: a multicenter survey and pooled analysis with published data. J Exp Clin Cancer Res. 2015;34: 156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brieau B, Dahan L, De Rycke Y, et al. Second-line chemotherapy for advanced biliary tract cancer after failure of the gemcitabine-platinum combination: A large multicenter study by the Association des Gastro-Enterologues Oncologues. Cancer. 2015;121: 3290–3297. [DOI] [PubMed] [Google Scholar]
- 13.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45: 228–247. [DOI] [PubMed] [Google Scholar]
- 14.Lamarca A, Palmer DH, Wasan HS, et al. ABC-06 ∣ A randomised phase III, multi-centre, open-label study of Active Symptom Control (ASC) alone or ASC with oxaliplatin / 5-FU chemotherapy (ASC+mFOLFOX) for patients (pts) with locally advanced / metastatic biliary tract cancers (ABC) previously-treated with cisplatin/gemcitabine (CisGem) chemotherapy. American Society of Clinical Oncology. Chicago, IL, 2019. [Google Scholar]
- 15.Jusakul A, Cutcutache I, Yong CH, et al. Whole-Genome and Epigenomic Landscapes of Etiologically Distinct Subtypes of Cholangiocarcinoma. Cancer Discov. 2017;7: 1116–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sia D, Hoshida Y, Villanueva A, et al. Integrative molecular analysis of intrahepatic cholangiocarcinoma reveals 2 classes that have different outcomes. Gastroenterology. 2013;144: 829–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu AX, Borger DR, Kim Y, et al. Genomic profiling of intrahepatic cholangiocarcinoma: refining prognosis and identifying therapeutic targets. Ann Surg Oncol. 2014;21: 3827–3834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Borger DR, Tanabe KK, Fan KC, et al. Frequent mutation of isocitrate dehydrogenase (IDH)1 and IDH2 in cholangiocarcinoma identified through broad-based tumor genotyping. Oncologist. 2012;17: 72–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Javle M, Lowery M, Shroff RT, et al. Phase II Study of BGJ398 in Patients With FGFR-Altered Advanced Cholangiocarcinoma. J Clin Oncol. 2017: JCO2017755009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Javle M, Rashid A, Churi C, et al. Molecular characterization of gallbladder cancer using somatic mutation profiling. Hum Pathol. 2014;45: 701–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Le DT, Uram JN, Wang H, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med. 2015;372: 2509–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roa I, de Toro G, Schalper K, de Aretxabala X, Churi C, Javle M. Overexpression of the HER2/neu Gene: A New Therapeutic Possibility for Patients With Advanced Gallbladder Cancer. Gastrointest Cancer Res. 2014;7: 42–48. [PMC free article] [PubMed] [Google Scholar]
- 23.Saha SK, Gordan JD, Kleinstiver BP, et al. Isocitrate Dehydrogenase Mutations Confer Dasatinib Hypersensitivity and SRC Dependence in Intrahepatic Cholangiocarcinoma. Cancer Discov. 2016;6: 727–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Subbiah IM, Subbiah V, Tsimberidou AM, et al. Targeted therapy of advanced gallbladder cancer and cholangiocarcinoma with aggressive biology: eliciting early response signals from phase 1 trials. Oncotarget. 2013;4: 156–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fan B, Mellinghoff IK, Wen PY, et al. Clinical pharmacokinetics and pharmacodynamics of ivosidenib, an oral, targeted inhibitor of mutant IDH1, in patients with advanced solid tumors. Invest New Drugs. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Javle M, Lowery M, Shroff RT, et al. Phase II Study of BGJ398 in Patients With FGFR-Altered Advanced Cholangiocarcinoma. J Clin Oncol. 2018;36: 276–282. [DOI] [PMC free article] [PubMed] [Google Scholar]