Abstract
Background.
The mechanisms governing extracellular matrix degradation and smooth muscle cell (SMC) loss in the ascending aorta of bicuspid aortic valve (BAV) patients are unknown. We recently reported that expression and induction of metallothionein, a reactive oxygen species scavenger, is reduced in BAV ascending aortic aneurysms relative to nonaneurysmal patients.
Methods.
Tissue and primary SMCs from patients with and without thoracic aortic aneurysms and metallothionein-null and wild-type mice were analyzed for cell viability, vascular endothelial growth factor (VEGF), and type I collagen gene expression during exposure to reactive oxygen species.
Results.
The BAV SMCs and metallothionein −/− mice failed to induce VEGF under conditions of oxidative stress in vitro. Exogenous VEGF restored resistance to oxidative stress in BAV SMCs to normal. Type I collagen gene induction was increased in BAV aorta.
Conclusions.
Lack of VEGF induction during exposure to reactive oxygen species suggest that the oxidative stress response is faulty upstream of metallothionein and VEGF in BAV SMCs. Improvement of cell viability with VEGF treatment suggests that the deficient pathway can be rescued by VEGF. Increased type I collagen in BAV suggests that lack of metallothionein/VEGF activation in response to reactive oxygen species may play a role in extracellular matrix homeostasis of the ascending aorta. These data continue to support our hypothesis that BAV SMCs lack sufficient resistance to reactive oxygen species to maintain extracellular matrix homeostasis, which imparts a predisposition to thoracic aortic aneurysms.
Bicuspid aortic valve (BAV) is the most common congenital cardiac malformation and occurs in 1% to 2% of the population [1]. In addition to development of valvular stenosis or insufficiency, BAV is distinctly associated with proximal ascending aortic pathologies including thoracic aortic aneurysm (TAA), dissection, and coarctation [2, 3].
The cause of BAV remains unknown, but the defect appears to arise during development of the aortic valvular cusps and aortic media from neural crest cells [1]. Linkage analyses distinguish that the BAV syndrome is heritable [3, 4]; however, the molecular basis for TAA formation in BAV patients remains unknown. A few studies have identified altered gene and protein expression profiles in aneurysmal tissue of BAV patients [5, 6]. The majority of our understanding of TAA pathogenesis is limited to histologic observations that occur irrespective of valvular pathology and are distinct from aneurysms of the descending aorta [2]. These changes include noninflammatory smooth muscle cell (SMC) loss, fragmentation of elastin, mucoid degeneration, and accumulation of basophilic ground substance within cell-depleted areas of the medial layer of the vessel wall [3]. Despite mounting circumstantial evidence of a molecular basis to extracellular matrix (ECM) disruption seen in BAV-associated TAA including recognizable patterns of ECM protein expression, there is a lack of understanding of the contribution of SMCs as contributors to ECM homeostasis and how their altered responsiveness in BAV patients may impact aneurysm formation.
Our group recently reported that several isoforms of the metallothionein (MT) family of genes are downregulated in aneurysmal ascending aortas, and primary SMCs from BAV patients demonstrated reduced cell viability during exposure to agents inducing reactive oxygen species (ROS) relative to donor aortas and aneurysmal aortas from patients with tricuspid aortic valve (TAV) [4]. Metallothioneins are a superfamily of intracellular cysteine-rich proteins of low (<7 kD) molecular weight, with high metal binding and redox capabilities. Metallothionein has been implicated in the pathogenesis of numerous diseases including oxidative stress-induced cardiac dysfunction [5]. Metallothionein was also shown to rescue hypoxia-inducible factor 1-α expression and to increase vascular endothelial growth factor (VEGF) expression in a mouse model of diabetic cardiomyopathy [6]. We hypothesize that reduced MT and inability to resist damage by ROS may contribute to compromised ECM homeostasis in the aortic wall of BAV patients. In this study, we explore the role of VEGF in the oxidative stress response of ascending aortic SMCs and Type I collagen expression in human TAA specimens and a MT−/− mice.
Material and Methods
For this study, we examined both human and mouse ascending aortic specimens and primary ascending aortic SMCs.
Patient Enrollment and Tissue Collection
To investigate the molecular basis for BAV-TAA, we actively maintain a tissue bank of ascending aorta from patients who underwent elective ascending aortic replacement, with informed patient consent and approval of the Institutional Review Board, and normal tissue from heart transplant donors and recipients (nonaneurysmal with TAV), acquired in conjunction with the Center for Organ Recovery and Education. For this study, all patient samples were obtained from males with no history of coarctation and minimal to no comorbidities. Samples were age matched (among all groups) and diameter matched (between TAV and BAV samples) within assays as closely as possible. One limitation to this study is that nonaneurysmal patients tended to be younger than TAA patients on average. Demographics and valvular pathophysiologies of all of the study samples used are presented in Table 1.
Table 1.
Demographics for Aortic Tissue Collected From Patients With TAV, BAV, and Nonaneurysmal Donors (Control) Included in Study
| All Samples | N | Age, Years (Mean ± SEM) | Aortic Diameter, mm (Mean ± SEM) | Hypertension | Aortic Insufficiency | Aortic Stenosis | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1+ | 2–3+ | 4+ | Mild | Moderate | Severe | |||||
| Control | 33 | 42.5 ± 2.41 | < 30 | 19.40% | 0 | 0 | 0 | 0 | 0 | 0 |
| TAV | 30 | 53.9 ± 2.00 | 51.0 ± 1.11 | 46.70% | 10.00% | 46.70% | 13.30% | 0.00% | 3.30% | 3.30% |
| BAV | 66 | 49.8 ± 1.11 | 51.2 ± 0.64 | 34.80% | 15.20% | 43.90% | 12.10% | 10.60% | 21.20% | 16.70% |
BAV = bicuspid aortic valve; TAV = tricuspid aortic valve.
Ascending aorta was also collected from MT−/− mice and wild-type litter mates under established Institutional Animal Care and Use Committee protocols. Wild-type and MT−/− mice (deletions in Mt-1 and Mt-2) that were derived from heterozygous mixed mating of 129 Ola and C57 BL/6 backgrounds have been extensively characterized for increased cell apoptosis under oxidative stress [7].
For both human and mouse specimens, a portion of each sample was placed in RNAlater solution (Ambion, Austin, TX) and stored at 4°C for 24 hours before long-term storage at −20°C. Additional portions of aorta were placed in DMEM/F12 containing 1% penicillin/streptomycin and Fungizone (Invitrogen, Carlsbad, CA) to create primary cultures of SMCs, as we have previously described [4]. The purity of the SMCs was confirmed by near-homogeneous positive staining with a smooth muscle α-actin monoclonal antibody (Sigma, St. Louis, MO [data not shown]) on fresh isolates. After cryopreservation, α-SMA expression was similar to fresh isolates and smooth muscle myosin heavy chain expression was near 100%, with no expression of the fibroblast marker, DDR2 (data not shown). Primary aortic SMCs were maintained in SMC growth medium (Cell Applications, San Diego, CA), and all experiments were performed using passage four or five.
RNA Extraction and Quantitative Real-Time PCR Analysis
For extraction of total RNA, tissue samples were pulverized under liquid nitrogen; then 25 to 30 mg of tissue was homogenized by passing through a QIAshredder column (Qiagen, Valencia, CA). The RNA was extracted by Trizol Reagent (Invitrogen) followed by purification using RNeasy Plus Mini kit (Qiagen) according to the manufacturers’ instructions. Total RNA was also isolated from primary SMC cultures using the RNeasy Plus Mini kit only, according to the manufacturer’s instructions. Quantitative real-time polymerase chain reaction (PCR) analysis was performed with Taqman One-step RT-PCR Master Mix (Applied Biosystems, Foster City, CA). The RNA samples (1 μL) were added to sequence-specific primers and Taqman probes (200 nM per 10-μL reaction). The primers and probe sequences are as follows: human VEGF165 (Genebank #NM 001033756; forward: CATGCA-GATTATGCGGATCAA; reverse: TTTGTTGTGCTG-TAGGAAGCTCAT; and probe: CCTCACCAAGGC-CAGCACATAGGAGA); human type I collagen (Genebank #NM 000088; forward: CACCAATCACCT-GCGTACAGA; reverse: GGCAGGGCTCGGGTTT; and probe: CGGCCTCAGGTACCATGACCGAGAC); mouse VEGF165 (Genebank #NM 009505; forward: AGCG-GAGAAAGCATTTGTTTG; reverse: CAACGC-GAGTCTGTGTTTTTG; and probe: CCAAGATCCGCA-GACGTGTAAATGTTCC); mouse type I collagen (Genebank #X54876; forward: CACGGCTGTGTGC-GATGA; reverse: TCGCCCTCCCGTCTTTG; and probe: TGCAATGAAGAACTGGACTGTCCCAACC). Target genes were normalized to 18S (Applied Biosystems). All target gene probes were labeled with FAM as the 5’ reporter dye and TAMRA as the 3’ quencher dye. The quantitative PCR assays were carried out in triplicate on an ABI Prism 7900HT sequence detection system in the Genomics and Proteomics Core Laboratories of the University of Pittsburgh. Data were analyzed using SDS 2.2.2 Software (Applied Biosystems).
Cell Viability
The SMCs were seeded at a density of 25,000 cells/cm2 in triplicate and allowed to attach overnight. The medium was then replenished in the presence or absence of 600 μM t-butyl hydroperoxide (tBHP) to induce generation of ROS. Cell viability was monitored after 3 hours of tBHP treatment by CellTiter 96 Aqueous One Solution Cell Proliferation Assay (Promega, Madison, WI) according to the manufacturer’s instructions. The RNA was harvested from parallel cultures after 6 hours of 600 μM tBHP and analyzed by quantitative real-time PCR, as described above. Pilot studies were conducted in triplicate and in-traassay variability was low (<5%), and subsequent exeriments were performed as a single well per patient.
Human SMCs were then seeded at a density of 25,000 cells/cm2 and allowed to attach overnight. Cells were then cultured in fresh medium in the presence or absence of 10 ng/mL recombinant VEGF (rVEGF [R&D Systems, Minneapolis, MN]) for 24 hours. The medium was then replenished in the presence and absence of 10 ng/mL rVEGF and 0 to 4.8 mM tBHP for 3 hours before quantification of cell viability.
Statistical Analysis
Experiments were repeated a minimum of three times, and data represent the mean of all observations. One-way analysis of variance, followed by Tukey honestly significant difference post-hoc test using SPSS software (SYSTAT software version 18.0; SPSS Inc, Chicago, IL), was performed to determine significance among treatment groups and appropriate age-matching among groups within experiments. Student’s t test (independent samples) was performed to determine differences between treatment groups, two tissue groups, or cell populations.
Results
To interrogate the cellular defense system of primary aortic SMCs exposed to ROS, we first quantified expression of the cell survival factor, VEGF. In the presence of tBHP, VEGF gene expression was increased in nonaneurysmal SMCs (p < 0.001) and TAV (p < 0.01) relative to unstressed (−tBHP) cells but not BAV (p = 0.132; Fig 1). There was no difference in basal VEGF gene expression in primary SMCs among nonaneurysmal (5.0 × 10−3 ± 2.14 × 10−3), TAV (6.93 × 10−3 ± 3.85 × 10−3), or BAV (5.42 × 10−3 ± 2.18 × 10−3; p = 0.879). There was no difference in VEGF gene expression among aneurysmal ascending aorta for patients with BAV (1.4 × 10−4 ± 3.55 × 10−5) or TAV (1.0 × 10−4 ± 4.33 × 10−5) relative to nonaneurysmal patients (1.1 × 10−4 ± 1.97 × 10−5; p = 0.2), nor between TAV and BAV patients (p = 0.9).
Fig 1.
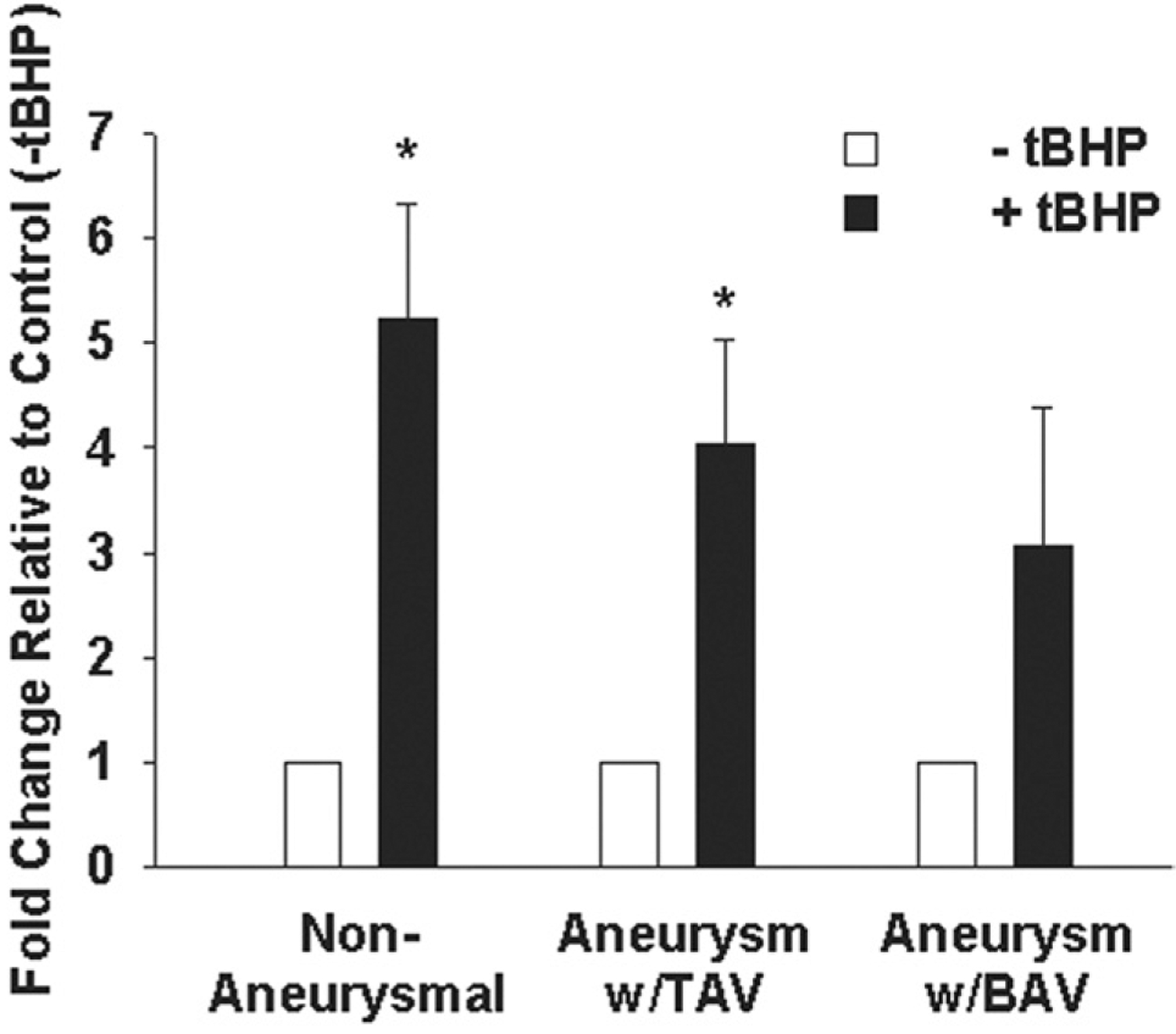
Expression of vascular endothelial growth factor (VEGF) gene expression in primary human ascending aortic smooth muscle cells (SMC). Cells from nonaneurysmal patients (n = 11), aneurysmal with tricuspid aortic valve (TAV) patients (n = 8), and aneurysmal with bicuspid aortic valve (BAV) patients (n = 9) were cultured in the presence or absence of 600 μM t-butyl hydroperoxide (tBHP [black bars]) for 6 hours. For all experiments, bars represent mean ± SEM. *Significant from control (−tBHP [white bars]), p less than 0.01.
We next asked the question: can cell viability be restored in BAV SMCs to levels comparable to nonaneurysmal SMCs? To attempt rescue of cell viability in BAV SMCs, we cultured human SMCs in the presence of rVEGF before exposure to ROS. Cell viability was increased in all three patient populations to a similar degree (p = 0.156). Treatment with rVEGF restored cell viability of BAV SMCs to a level equal to nonaneurysmal SMCs in the presence of ROS (p = 0.07; Fig 2).
Fig 2.
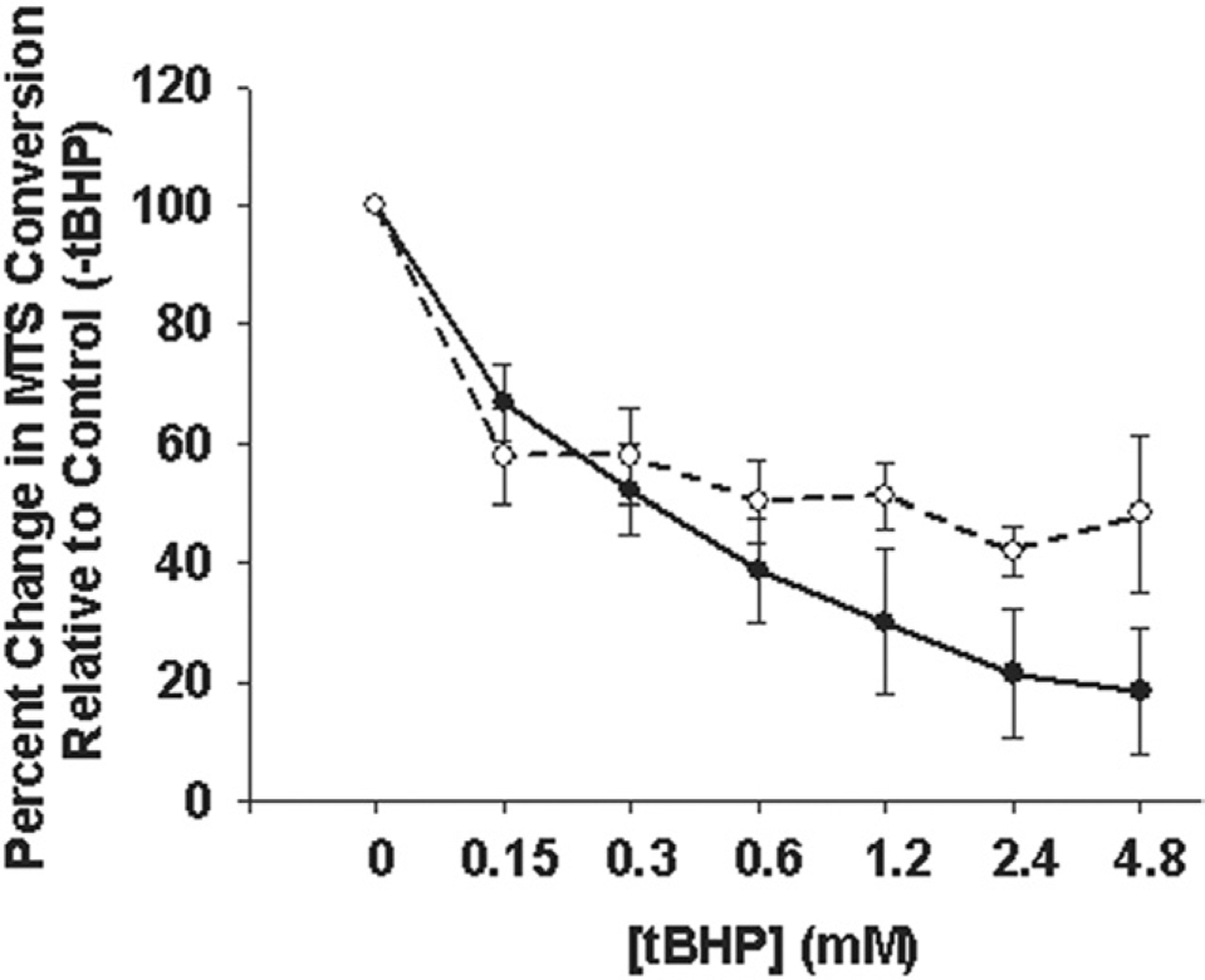
Cell viability in the presence of oxidative stress. Primary human ascending aortic smooth muscle cells (SMC) from bicuspid aortic valve (BAV) patients (dotted line) were cultured in the presence of 10 ng/mL vascular endothelial growth factor (VEGF) before exposure to 0 to 4,800 μM t-butyl hydroperoxide (tBHP) for 3 hours and compared with SMCs from nonaneurysmal patients (solid line). Lines represent mean ± SEM, n = 3 and n = 4 for nonaneurysmal and BAV, respectively.
To begin to draw a connection between oxidative stress responses and ECM homeostasis, we quantified type I collagen gene expression in ascending aorta and primary aortic SMCs in vitro. Type I collagen gene expression was increased in BAV ascending aorta (Fig 3) relative to nonaneurysmal controls (p < 0.05). There was no change in type I collagen gene expression among primary aortic SMCs from nonaneurysmal (7.2 × 10−2 ± 2.50 × 10−2), TAV (2.8 × 10−2 ± 1.06 × 10−2), and BAV (5.1 × 10−2 ± 1.80 × 10−2; p = 0.31).
Fig 3.
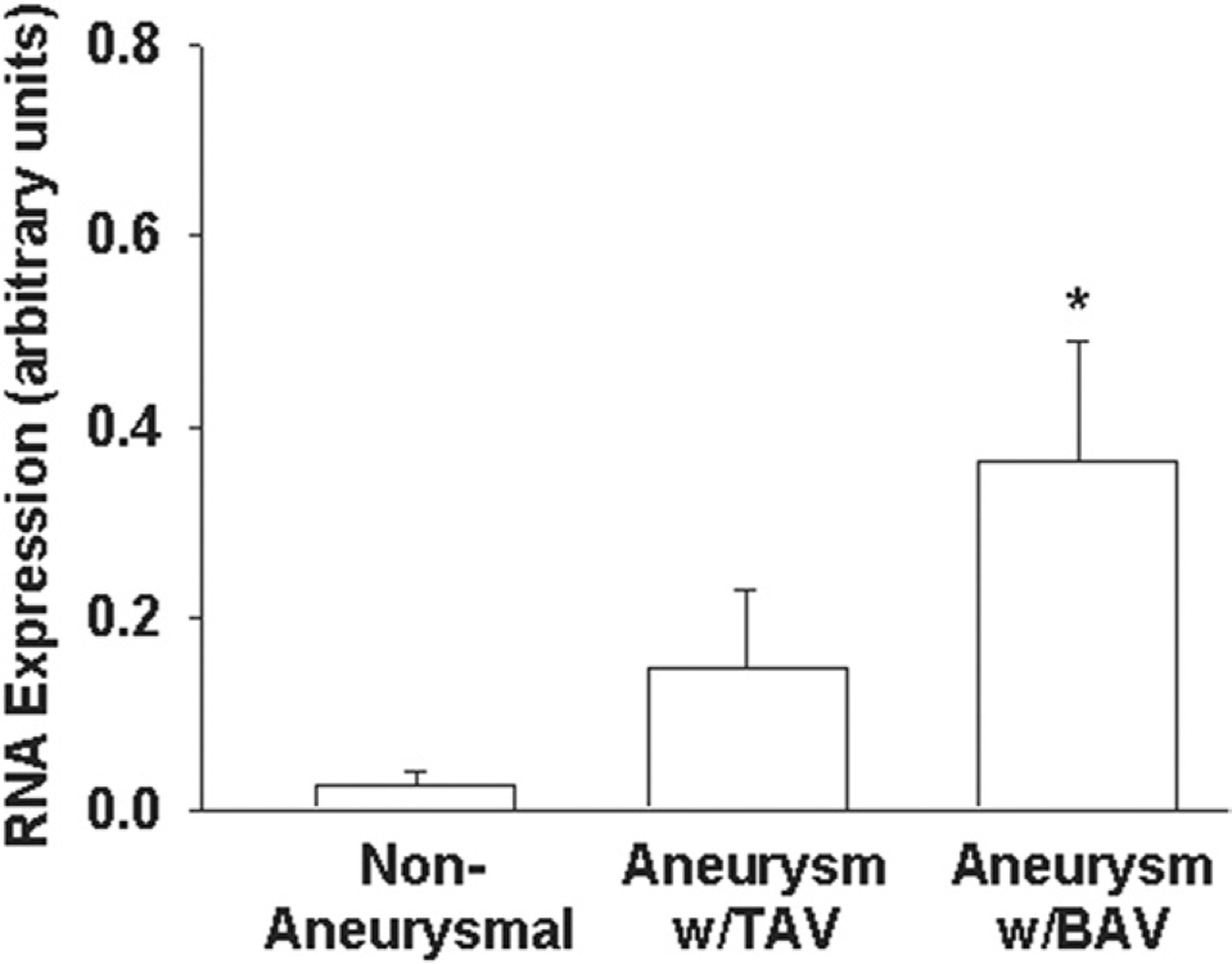
Type I collagen gene expression. (A) Human ascending aorta from nonaneurysmal patients (n = 23), aneurysmal with tricuspid aortic valve (TAV) patients (n = 21), and aneurysmal with bicuspid aortic valve (BAV) patients (n = 40). Bars represent mean ± SEM. *Significant from nonaneurysmal for p less than 0.05.
To further delineate the specific role of MT in aortic SMC oxidative stress response and ECM homeostasis, we employed the use of MT−/− mice. Primary ascending aortic SMCs from MT−/− mice exhibit reduced cell viability under conditions of ROS in vitro relative to wild-type (p < 0.05; Fig 4). Basal VEGF was elevated in MT−/− SMCs relative to wild-type (p < 0.05) and was not further induced with tBHP (p = 0.420; Fig 5), whereas wild-type cells induced VEGF in the presence of tBHP (p < 0.05). Expression of VEGF in mouse ascending aortic tissue was equivalent in wild-type and MT−/− (2.10 × 10−4 ± 3.36 × 10−5 versus 2.33 × 10−4 ± 3.86 × 10−5, respectively; p = 0.356). Type I collagen gene expression was similar in wild-type and MT−/− mice ascending aortic tissue (1.4 × 10−4 ± 7.64 × 10−5 versus 2.1 × 10−4 ± 1.23 × 10−4; p > 0.7).
Fig 4.
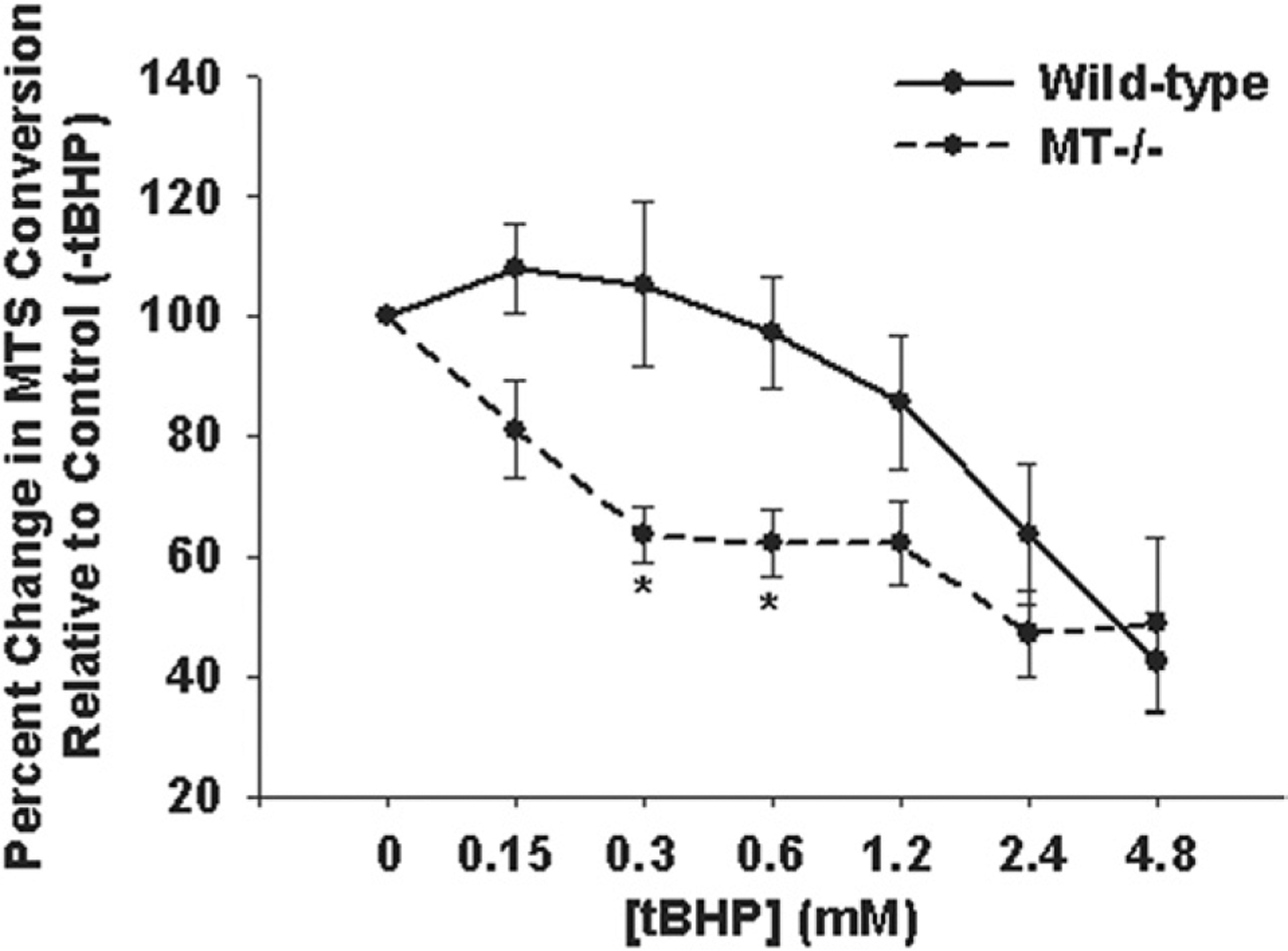
Cell viability of primary ascending aortic smooth muscle cells (SMC) from wild-type mice (solid line) and MT−/− mice (dashed line). *Significant from wild-type, p less than 0.02. (MT = metallothionein; tBHP = t-butyl hydroperoxide.)
Fig 5.
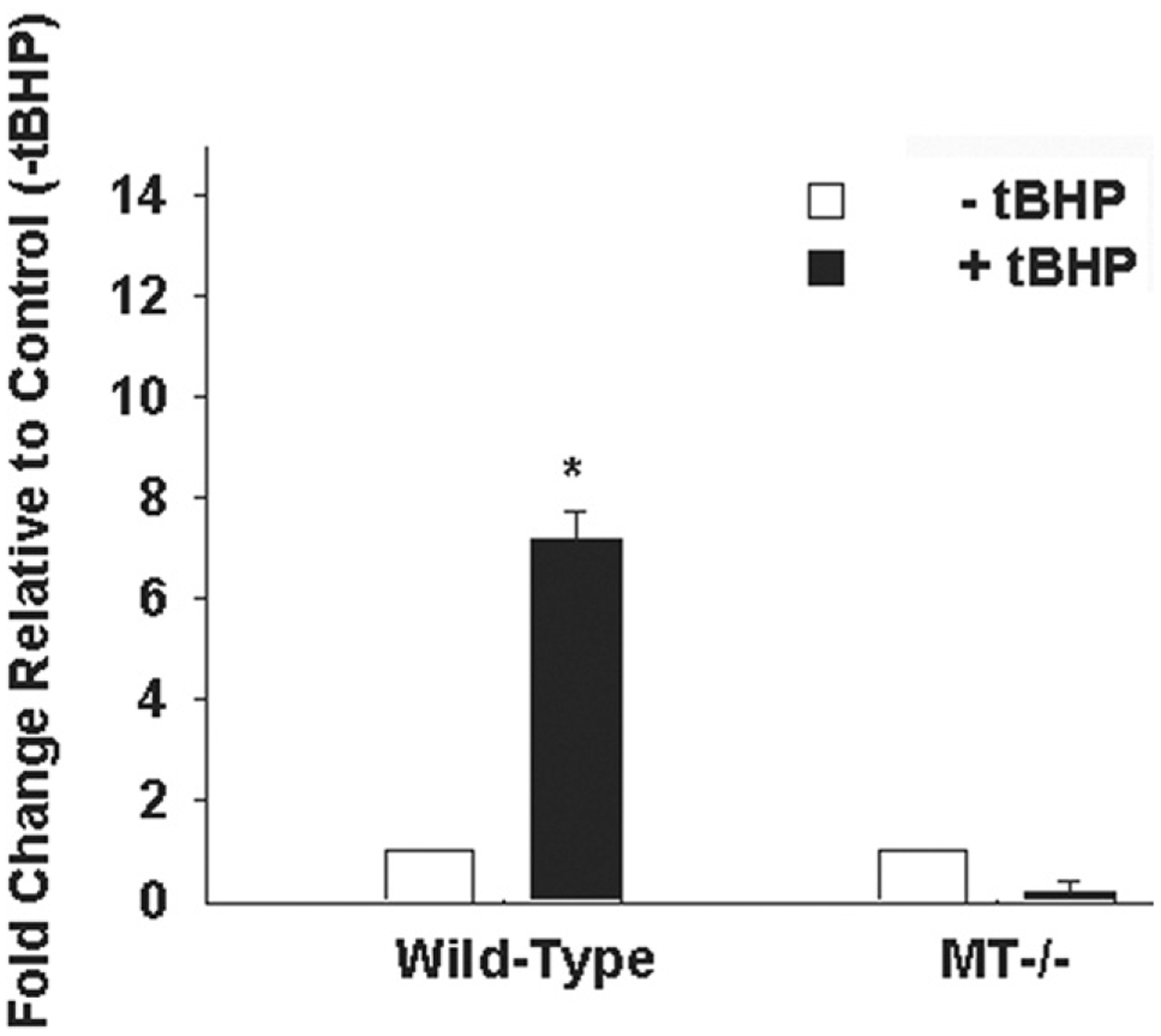
Expression of vascular endothelial growth factor (VEGF) gene expression in primary ascending aortic smooth muscle cells (SMC) from wild-type mice and MT−/− mice. The SMCs were cultured in the presence (solid bars) and absence (open bars) of 600 μM t-butyl hydroperoxide (tBHP) for 6 hours. *Significant from wild-type, p less than 0.05. (MT = metallothionein.)
Comment
Building on our evidence of low MT expression and induction [4], we now report impairment of the oxidative stress response in BAV patients involving reduced induction of VEGF gene expression, an important molecule in the cascade of cell survival under oxidative stress. Since addition of rVEGF restored cell viability of BAV SMCs to levels equivalent to nonaneurysmal SMCs, the compromised cellular response to ROS in BAV can be rescued by supplementing VEGF. These combined data reinforce the notion that MT is extremely important in the overall cellular defense of ascending aortic SMCs against oxidative stress and are consistent with other reports supporting the involvement of ROS in TAAs [8–12], and absence of MT reduces the expression of several growth factors including VEGF [13].
Vascular endothelial growth factor is antiapoptotic in several cell types [14] and has been extensively characterized in cancer models as a proponent of cell survival by signaling through phosphatidylinositol-3’-kinase and Akt [15]. Prechallenge with VEGF rescued endothelial cells from ROS generation [16], suggesting potential antiapoptotic mechanisms for VEGF. We reason that a lack of response to ROS could manifest as low VEGF induction and may play a role in apoptosis of BAV SMCs in the aortic media. Apoptosis has been proposed as a mechanism of noninflammatory SMC loss within the media of dilated aortas, aneurysm, and dissection and is associated with elastin fragmentation, disruption of the elastin lamellae, alterations in matrix metalloproteinase (MMP)/tissue inhibitor of MMP ratios, and fibrosis with an increase in collagen and a higher apoptotic index [17]. Although the pathways initiating MMP overexpression are not known, MMPs promote apoptosis [18] and are associated with increased presence of ROS within vascular tissues [11]. Sources of ROS in abdominal aortic aneurysms are thought to initiate from local macrophages that infiltrate the tissue as well as mechanical stress [19, 20]. These events trigger increases in MMPs, which contribute to tissue degradation [21, 22]. The source of ROS in BAV TAAs is the subject of our ongoing research, and we reason that increased ROS may contribute to alterations in MMP/tissue inhibitor of MMP levels deleterious to the aortic ECM.
Increased type I collagen gene expression in BAV TAAs implies a direct role for MT in regulating ECM homeostasis. Like BAV SMCs, MT−/− SMCs exhibit reduced cell viability under stress but there was no difference in type I collagen gene expression in wild-type versus MT−/− mice. Increased VEGF expression in MT−/− could indicate that other pathways or mechanisms may compensate for MT. Thus far, our unpublished observations of MT−/− ascending show no aortic dilation or dissection and a lack of histologic evidence for medial degeneration. The MT−/− mice are nonlethal, and MT is not required for proper growth or development; however, the absence of MT-1 or -2 increased Cd-induced lethality and hepatotoxicity, and overexpression of MTs provided protection from Cd-induced death [23]. The MT−/− mice display vascular defects including a lack of myogenic reflexes in resistance arteries [24] and hypoxic pulmonary vasoconstriction [25]. However, compensatory pathways may also be dysregulated in BAV patients and result in overproduction of collagen. Type I collagen production is altered in pathologic conditions, including hypoxia [26], owing to hypoxia-response elements within the proximal promoter [27]. Our results are consistent with previous reports that demonstrate increased collagen content [28] and an overall decrease in collagen concentration [29] amidst aortic dissections and TAAs [30,31]. However, others have demonstrated reduced collagen in the setting of aortic dissection and aneurysm [32]. Future studies will be needed to determine if MT plays a direct role in the regulation of type I collagen expression. Discrepancies in VEGF and type I collagen gene expression between aorta and primary SMCs may be related to variations of SMC phenotype (contractile versus synthetic) ex vivo.
We propose that a disruption in the homeostasis of the aortic ECM in BAV TAAs involves a reduced oxidative stress response including MT and VEGF or factors up-stream, or both. The combination of reduced availability of cell survival factors coupled with overproduction of type I collagen may contribute to ECM breakdown and increased SMC loss within the media of the BAV aorta. Ongoing studies are focused on elucidating a putative causal role for MT-related oxidative stress responses and identifying other related factors and the mechanisms that govern these pathways that may disrupt ECM homeostasis. Knowledge of these pathways could translate into improved diagnostics and surveillance methods, and lead to the development of novel therapeutic and preventative treatments of the aneurysms that form in patients with BAV.
Acknowledgments
The authors thank the University of Pittsburgh Medical Center for financial support of this project. The authors gratefully acknowledge Michelle Shirey and Erica Butler for assistance with patient informed consent and IRB procedures, and Drs Lawrence Wei, Forozan Navid, Jay Bahma, Yoshiya Toyoda, and Christian Bermudez for assistance collecting the aortic tissue samples. The authors also thank Dr Nancy Sussman for her kind assistance with the statistical analyses, and Dr Ayse Aydemir for critical review of the manuscript.
Abbreviations and Acronyms
- BAV
bicuspid aortic valve
- ECM
extracellular matrix
- MMP
matrix metalloproteinase
- MT
metallothionein
- ROS
reactive oxygen species
- rVEGF
recombinant vascular endothelial growth factor
- SMC
smooth muscle cell
- TAA
thoracic aortic aneurysm
- TAV
tricuspid aortic valve
- tBHP
t-butyl hydroperoxide
- VEGF
vascular endothelial growth factor
References
- 1.Ward C. Clinical significance of the bicuspid aortic valve. Heart 2000;83:81–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gleason TG. Heritable disorders predisposing to aortic dissection. Semin Thorac Cardiovasc Surg 2005;17:274–81. [DOI] [PubMed] [Google Scholar]
- 3.Coady MA, Rizzo JA, Goldstein LJ, Elefteriades JA. Natural history, pathogenesis, and etiology of thoracic aortic aneurysms and dissections. Cardiol Clin 1999;17:615–35,vii. [DOI] [PubMed] [Google Scholar]
- 4.Phillippi JA, Klyachko EA, Kenny JPT, Eskay MA, Gorman RC, Gleason TG. Basal and oxidative stress-induced expression of metallothionein is decreased in ascending aortic aneurysms of bicuspid aortic valve patients. Circulation 2009;119:2498–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fang CX, Doser TA, Yang X, Sreejayan N, Ren J. Metallothionein antagonizes aging-induced cardiac contractile dysfunction: role of PTP1B, insulin receptor tyrosine phosphorylation and Akt. Aging Cell 2006;5:177–85. [DOI] [PubMed] [Google Scholar]
- 6.Feng W, Wang Y, Cai L, Kang YJ. Metallothionein rescues hypoxia-inducible factor-1 transcriptional activity in cardiomyocytes under diabetic conditions. Biochem Biophys Res Commun 2007;360:286–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kondo Y, Rusnak JM, Hoyt DG, Settineri CE, Pitt BR, Lazo JS. Enhanced apoptosis in metallothionein null cells. Mol Pharmacol 1997;52:195–201. [DOI] [PubMed] [Google Scholar]
- 8.Ejiri J, Inoue N, Tsukube T, et al. Oxidative stress in the pathogenesis of thoracic aortic aneurysm: protective role of statin and angiotensin II type 1 receptor blocker. Cardiovasc Res 2003;59:988–96. [DOI] [PubMed] [Google Scholar]
- 9.Fiorillo C, Becatti M, Attanasio M, et al. Evidence for oxidative stress in plasma of patients with Marfan syndrome. Int J Cardiol 2010. May 25 Epub. [DOI] [PubMed] [Google Scholar]
- 10.Pereira L, Lee SY, Gayraud B, et al. Pathogenetic sequence for aneurysm revealed in mice underexpressing fibrillin-1. Proc Natl Acad Sci USA 1999;96:3819–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rajagopalan S, Meng XP, Ramasamy S, Harrison DG, Galis ZS. Reactive oxygen species produced by macrophage-derived foam cells regulate the activity of vascular matrix metalloproteinases in vitro. Implications for atherosclerotic plaque stability. J Clin Invest 1996;98:2572–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang HH, van Breemen C, Chung AW. Vasomotor dysfunction in the thoracic aorta of Marfan syndrome is associated with accumulation of oxidative stress. Vasc Pharmacol 2010; 52:37–45. [DOI] [PubMed] [Google Scholar]
- 13.Penkowa M, Carrasco J, Giralt M, et al. Altered central nervous system cytokine-growth factor expression profiles and angiogenesis in metallothionein-I+II deficient mice. J Cereb Blood Flow Metab 2000;20:1174–89. [DOI] [PubMed] [Google Scholar]
- 14.Epstein RJ. VEGF signaling inhibitors: more pro-apoptotic than anti-angiogenic. Cancer Metastasis Rev 2007;26:443–52. [DOI] [PubMed] [Google Scholar]
- 15.Wu Y, Hooper AT, Zhong Z, et al. The vascular endothelial growth factor receptor (VEGFR-1) supports growth and survival of human breast carcinoma. Int J Cancer 2006;119: 1519–29. [DOI] [PubMed] [Google Scholar]
- 16.Yang Z, Mo X, Gong Q, et al. Critical effect of VEGF in the process of endothelial cell apoptosis induced by high glucose. Apoptosis 2008;13:1331–43. [DOI] [PubMed] [Google Scholar]
- 17.Lesauskaite V, Tanganelli P, Sassi C, et al. Smooth muscle cells of the media in the dilatative pathology of ascending thoracic aorta: morphology, immunoreactivity for osteopontin, matrix metalloproteinases, and their inhibitors. Hum Pathol 2001;32:1003–11. [DOI] [PubMed] [Google Scholar]
- 18.Williams H, Johnson JL, Jackson CL, White SJ, George SJ. MMP-7 mediates cleavage of N-cadherin and promotes smooth muscle cell apoptosis. Cardiovasc Res 2010;87:137–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thompson RW, Holmes DR, Mertens RA, et al. Production and localization of 92-kilodalton gelatinase in abdominal aortic aneurysms. An elastolytic metalloproteinase expressed by aneurysm-infiltrating macrophages. J Clin Invest 1995;96:318–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCormick ML, Gavrila D, Weintraub NL. Role of oxidative stress in the pathogenesis of abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol 2007;27:461–9. [DOI] [PubMed] [Google Scholar]
- 21.Grote K, Flach I, Luchtefeld M, et al. Mechanical stretch enhances mRNA expression and proenzyme release of matrix metalloproteinase-2 (MMP-2) via NAD(P)H oxidase-derived reactive oxygen species. Circ Res 2003;92:e80–6. [DOI] [PubMed] [Google Scholar]
- 22.Hishikawa K, Oemar BS, Yang Z, Luscher TF. Pulsatile stretch stimulates superoxide production and activates nuclear factor-kappa B in human coronary smooth muscle. Circ Res 1997;81:797–803. [DOI] [PubMed] [Google Scholar]
- 23.Klaassen CD, Liu J. Metallothionein transgenic and knock-out mouse models in the study of cadmium toxicity. J Toxicol Sci 1998;23(Suppl 2):97–102. [DOI] [PubMed] [Google Scholar]
- 24.Pearce LL, Wasserloos K, St Croix CM, Gandley R, Levitan ES, Pitt BR. Metallothionein, nitric oxide and zinc homeostasis in vascular endothelial cells. J Nutr 2000;130(Suppl):1467–70. [DOI] [PubMed] [Google Scholar]
- 25.Bernal PJ, Leelavanichkul K, Bauer E, et al. Nitric-oxide-mediated zinc release contributes to hypoxic regulation of pulmonary vascular tone. Circ Res 2008;102:1575–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Corpechot C, Barbu V, Wendum D, et al. Hypoxia-induced VEGF and collagen I expressions are associated with angiogenesis and fibrogenesis in experimental cirrhosis. Hepatology 2002;35:1010–21. [DOI] [PubMed] [Google Scholar]
- 27.Rossert J, Terraz C, Dupont S. Regulation of type I collagen genes expression. Nephrol Dial Transplant 2000;15(Suppl 6):66–8. [DOI] [PubMed] [Google Scholar]
- 28.Wang X, LeMaire SA, Chen L, et al. Increased collagen deposition and elevated expression of connective tissue growth factor in human thoracic aortic dissection. Circulation 2006;114(Suppl):I200–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Whittle MA, Hasleton PS, Anderson JC, Gibbs AC. Collagen in dissecting aneurysms of the human thoracic aorta. Increased collagen content and decreased collagen concentration may be predisposing factors in dissecting aneurysms. Am J Cardiovasc Pathol 1990;3:311–9. [PubMed] [Google Scholar]
- 30.Jones JA, Zavadzkas JA, Chang EI, et al. Cellular phenotype transformation occurs during thoracic aortic aneurysm development. J Thorac Cardiovasc Surg 2010. March 8 Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maleszewski JJ, Miller DV, Lu J, Dietz HC, Halushka MK. Histopathologic findings in ascending aortas from individuals with Loeys-Dietz syndrome (LDS). Am J Surg Pathol 2009;33:194–201. [DOI] [PubMed] [Google Scholar]
- 32.de Figueiredo Borges L, Jaldin RG, Dias RR, Stolf NA, Michel JB, Gutierrez PS. Collagen is reduced and disrupted in human aneurysms and dissections of ascending aorta. Hum Pathol 2008;39:437–43. [DOI] [PubMed] [Google Scholar]


