Abstract
Red-light bacteriophytochromes regulate many physiological functions through photoisomerization of a linear tetrapyrrole chromophore. Here, we mapped out femtosecond-resolved fluorescence spectra of excited Pr state and observed unique active-site relaxations on picosecond timescales with unusual spectral tuning of rises at the blue side and decays on the red side of the emission. We also observed initial wavepacket dynamics in femtoseconds with two low-frequency modes of 38 and 181 cm−1 as well as the intermediate product formation after isomerization in hundreds of picoseconds. With critical mutations at the active site, we observed similar dynamic patterns with different times of both relaxation and isomerization, consistent with the structural and chemical changes induced by mutations. The observed multiphasic dynamics clearly represent the active-site relaxations, not different intermediate reactions or excitation of heterogeneous ground states. The active-site relaxation must be considered in understanding overall isomerization reactions in phytochromes and such molecular mechanism should be general.
Keywords: unique spectral tuning, active-state relaxation, Pr-state isomerization, fluorescence dynamics, femtosecond spectroscopy
Graphical Abstract
Phytochrome photoreceptors convert light energy into biological signals and control a variety of cellular activities in plants, microorganisms, and fungi.1–3 Upon light absorption bacteriophytochromes can interconvert between the red absorbing (Pr, 15Z) and far-red absorbing (Pfr, 15E) states. The first step in Pr-to-Pfr photoconversion is the photo-induced isomerization around a double bond C15=C16 of the chromophore biliverdin IXα. The ultrafast photoisomerization is then followed by a series of protein structural evolution on micro- to millisecond time scales to finally reach the Pfr state. Several high-resolution structures of bacteriophytochromes have been recently solved.4–7 The protein domain architecture and chromophore-protein interactions inside the binding pocket are found to be highly conserved among bacteriophytochromes. Due to the significant structural similarity, a universal photoconversion mechanism is thus expected.
The Pr-state photoisomerization dynamics has been extensively studied recently by various spectroscopic methods.8–17 The dynamics were observed ranging from sub-picosecond to hundreds of picoseconds. But the origins of the observed multiple dynamics are still under debate. Two main kinetic models have been proposed based on protein structure and spectroscopic studies: one is a heterogeneous model, suggesting that each ground-state subpopulation has distinct excited-state lifetimes due to their different reaction barriers, leading to multiple excited-state reactions.8–11 The other one is a homogenous model, concluding that the reaction begins with a single ground-state conformation and the multiphasic excited-state dynamics represent various reaction intermediates.12–17 Interestingly, the effects of protein relaxation due to sudden excitation of the chromophore on its ultrafast photoisomerization have not been well addressed.
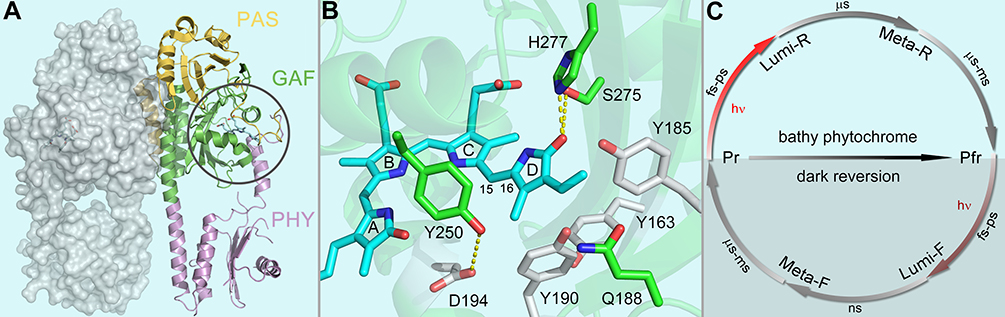
In this report, we study bacteriophytochrome from Pseudomonas aeruginosa (PaBphP), a phytochrome from the bathy phytochrome family. The dark-adapted ground state in bathy bacteriochromes is Pfr state while in canonical bacteriochromes is Pr state.18,19 Both bathy and canonical bacteriophytochrome photosensory modules share similar protein and chromophore structures, with the conserved photosensory module of PAS-GAF-PHY domain architecture and protein-chromophore interactions (Figure 1). Biliverdin IXα A-ring is covalently linked to a cysteine residue of the N-terminal extension of the PAS domain. The B- and C-ring propionic groups form extensive hydrogen-bond network with the GAF domain in both Pr and Pfr state. The chromophore D-ring is surrounded by a set of conserved aromatic and hydrogen-bonding sidechains. The chromophore is buried inside the GAF pocket and shielded from outside solvent.
Figure 1.
(A) X-ray crystallographic structure of Pr state PaBphP dimer (PDB ID: 3G6O) shown in both ribbon and surface representations. The chromophore is buried deeply inside the chromophore binding pocket (grey circle) formed by the GAF domain (green), the PAS domain N-terminal extension (yellow), and PHY domain tongue (purple). (B) Close-up view of the local configuration at the chromophore binding pocket with four critical residues (green), the chromophore (cyan) and other structurally important residues (grey). The mutants of each of the four key residues were examined for their contributions in the modulations of the photoisomerization dynamics. (C) Photocycle of bathy phytochrome with the initial photo-induced isomerization (red arrows) and the ground-state structural evolutions (grey arrows). Note that the dark reversion from Pr to Pfr state in bathy phytochrome.
Here, we report our characterization of Pr-state photoisomerization of PaBphP using both femtosecond (fs)-resolved fluorescence and absorption methods. We systematically studied the wild type (WT) and several critical mutants at the active site and examined the Pr-state photoreaction dynamics and the role of neighboring residues around the chromophore. By measuring the fs-resolved fluorescence signals of the chromophore, we were able to follow the entire evolution of excited state. We also examined excitation-wavelength dependence and deuterium isotope effect of Pr-state photoisomerization. We further studied the dynamics of various intermediates and photoproduct probed from 560 to 750 nm wavelengths. These results together finally revealed a unique dynamic pattern of excited-state evolution and thus elucidate the new molecular mechanism of bacteriophytochrome Pr-state photoisomerization.
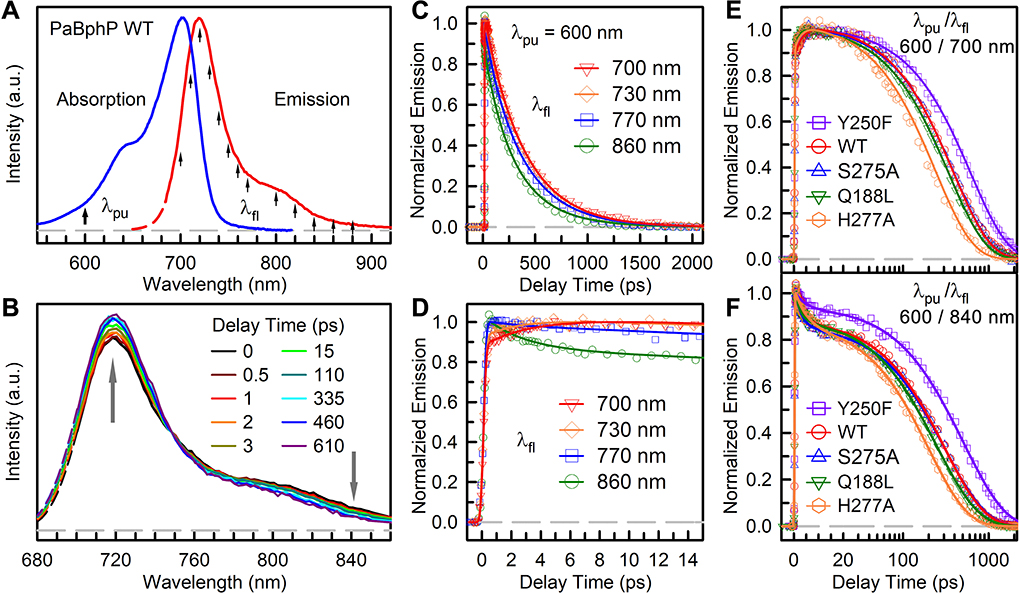
Figure 2A shows the Pr-state absorption and fluorescence spectra of the wild type. The WT absorption spectrum has its peak at 700 nm with a shoulder near 640 nm due to vibronic transitions and the fluorescence spectrum covers from 660 to 900 nm with a major peak at 718 nm and a red shoulder around 800 nm.16 The Pr-state absorption and fluorescence spectra exhibit a mirror symmetry. The Pr-state steady-state spectra of mutations of Q188L, S275A, Y250F and H277A are very similar to WT except the fluorescence spectrum of Y250F with a peak shifted to 722 nm and a more pronounced shoulder (Figures S1A and S1B). When incubated in the dark, WT reverted back to Pfr state from Pr state in ~50 min (Figures S1C and S2). All other four mutants showed significantly decreased dark reversion rate, especially H277A only with partial photoconversion and not reaching 100% Pfr state.
Figure 2.
(A) Steady-state spectra of PaBphP Pr state. Arrows mark the excitation wavelength and gated fluorescence wavelengths. (B) Measured TRES of WT PaBphP at selected delay times normalized at 746 nm. (C, D) Normalized femtosecond-resolved fluorescence transients of WT PaBphP gated at typical wavelengths with 600-nm excitation. (E, F) Normalized femtosecond-resolved fluorescence transients of WT PaBphP and mutants gated at 700 nm and 840 nm with 600-nm excitation wavelength. The gray dashed lines are the baseline at zero. Note that the delay time is shown on linear scale before 20 ps and logarithmic scale thereafter.
To evaluate the complete excited-state reaction dynamics, we measured the fs-resolved emission spectra (FRES, Figure 2B) and fluorescence transients at several typical wavelengths gated from the blue to red side of the emission within a 3.2-ns window (Figures 2C–2F, and Figures S3–S6). Figure 2B shows a unique FRES evolution normalized at 746 nm where the transient only shows its lifetime decay. Overall, the relative intensities around the major peak at the blue side increase while the red-side spectral intensities after 746 nm decrease. Figures 2C and 2D show the femtosecond-resolved fluorescence transients at four typical wavelengths. Besides the lifetime component of 375 ps, two more components ranging from 2.2 to 3.3 ps and 56 to 207 ps are needed to fit all the transients. Significantly, we observed that a component in a few picoseconds decays on the red side of the emission from 746 nm and rises at the blue side from 700 to 746 nm. The other decay component in 56–207 ps only exhibits after 746 nm with larger amplitudes at the redder wavelengths. This new observation of the blue-side rise and red-side decay is unusual and must reflect the dynamic interactions of local protein environment with the excited chromophore. The unique dynamic pattern in Pr state was first observed here, indicating the dynamic spectral tuning of bilin emission through perturbation of the potential energy surface at the active site,20–22 leading to distortion of the energy surface and causing the unique spectral shape evolution with the blue-side rise and the red-side shrink of FRES. This observation could be general to the Pr-state isomerization dynamics in phytochrome. Thus, the observed a few and 56–207 picoseconds are not the chromophore reaction dynamics, but they are the relaxation processes at the active site. With sudden excitation of the chromophore, the initial several picoseconds are from the local orientational relaxation and the long 50–207 ps is from the local sidechain and hydrogen-bond network rearrangements.
We further examined the anisotropy dynamics of the chromophore (Figure S4) and found the anisotropy is nearly constant in the time window of ~1 ns without any orientational relaxation on the picosecond timescale, indicating the local rigidity and the observed unique active-site relaxation from the surrounding proteins and hydrogen-bond network. Such observation also clearly shows the double-bond twisting happening in hundreds of picoseconds or longer unless the D-ring twisting does not change the excited-state transient dipole moment. We also examined the excited-state evolution in D2O buffer where all four pyrrole N-H groups can undergo H/D exchange and clearly observed slower dynamics.23 Both the active-site relaxation and photoisomerization reaction slow down, resulting from the heavy deuterium-bond network motions, as also observed by others.8,24 The observed kinetic isotope effect (KIE) of the reaction is 1.2 at D2O. Finally, we performed excitation wavelength dependence of the excited-state dynamics at 660 and 720 nm (Figures S5B and S5C) and the similar pattern of unique active-site relaxation was observed, indicating that the signal of vibrational cooling is negligible. But the different relaxation time and isomerization dynamics may indicate the different relaxation and reaction pathways on the potential energy surface.
We examined the effects of the critical active-site residues on the dynamics of relaxation and isomerization. We studied the dynamics of four critical mutants of Q188L, Y250F, S275A, and H277A at the active site. Figures 2E and 2F show the fs-resolved fluorescence transients of mutants with WT for comparison at two typical wavelengths of 700 nm (blue side) and 840 nm (red side) out of a series of detection wavelengths (Figures S7 and S8). A similar dynamic pattern, decay on the red side and rise at the blue side, was observed. The detailed relaxation processes and isomerization dynamics vary among mutants, indicating the significant roles of the critical residues in tuning the Pr-state dynamics of both the active-site relaxation and isomerization reaction (Table S1–S4). These excited-state dynamics of the mutants are closely correlated with local active-site properties, as discussed below.
In Pr state, the sidechains of S275 and H277 are within hydrogen-bonding distances to the chromophore D-ring. The highly conserved H277 residue functions as an anchor to the D-ring, which can effectively tune the excited-state dynamics. Upon isomerization, these hydrogen-bonding interactions will be disrupted; the D-ring must push away its surrounding bulky residues, including H277 and also several conserved Y residues, to reach the final isomerized state (Figure 1B).25 For the H277A mutant, we observed faster active-site relaxation from 54 to 166 ps and a shorter reaction time of 233 ps (Table S3). The removal of the hydrogen-bonding interaction and the bulky sidechain of H277 result in a much more flexible active site and thus both the active-site relaxation and isomerization reaction become faster. S275 is unique in bathy bacteriophytochrome PaBphP and is believed to provide extra stabilization to the Pr state.4,5 But, by mutation of S257A, even with the removal of hydrogen-bonding interactions to the D-ring, we did not observe noticeable changes of both dynamics, indicating the dominant interactions from H277, not S275. Thus, H277 is critical to having hydrogen bonding to the chromophore D-ring and the disruption of the interaction by mutation directly impacts the excited Pr-state dynamics, as also observed by others.8
Y250 is highly conserved and critical to the extensive hydrogen-bond network around the active site. The mutation of Y250F has significant effects on spectral tuning; both the absorption and fluorescence spectra are red-shifted. Although the mutation of Y250F disrupts the hydrogen bonding with the neighboring residues and could cause the local environment more flexible, we observed a longer isomerization in 600 ps, indicating that the electrostatics around the D-ring may not favor the twisting after mutation, assuming no noticeable structural changes from Y to F as observed by the X-ray structure.26 It would be interesting to use QM/MM to calculate the relative isomerization barriers and determine what factors affect the barriers. Q188 locates at the GAF-PHY interface and is only found in the bathy phytochromes.5 The Q188 residue is also in close proximity to the conserved aromatic residues, including Y163, Y185 and Y190, that impose steric constrains to the D-ring twisting (Figure 1B). In Q188L, we observed a faster isomerization time of 330 ps, primarily due to a more flexible local structure both at the PAS-GAF interface and around the chromophore D-ring as a result of replacing the polar Q with hydrophobic L. But the active-site relaxation remains similar, indicating that Q188 may not be directly involved in the local relaxation.
The multiphasic decays commonly observed in transient-absorption measurements in Pr-state phytochrome (also see below) are generally attributed to heterogeneity of protein population in the ground state. Here, our results of the fs-resolved fluorescence studies suggest that the multiple decay dynamics in a few to hundreds of ps result from the local protein-water relaxations and are not for isomerization reaction. These relaxations occur from the active-site sidechains, trapped water molecules, and hydrogen-bond network, as observed in photolyases and flavodoxins.27–29 Other studies by locking the D-ring twisting to eliminate the isomerization still show the relaxation dynamics in a few to hundreds of picoseconds from the local environment relaxation.30–31 Thus, with sudden change of the chromophore dipole upon excitation, the system in nonequilibrium relaxes into a new equilibrated state by the orientational relaxations and local rearrangements of polar and charged residues and trapped water molecules in a few to hundreds of picoseconds before reaching the isomerization process. These heterogeneous interactions by electrostatic and steric constrains during relaxation could lead to distortion of the potential energy surface, resulting in an unusual shape evolution of FRES with rises at the blue and decays on the red side. Only with fs-resolved fluorescence detection can we unambiguously disentangle the active-site relaxation dynamics from the excited-state isomerization reaction.
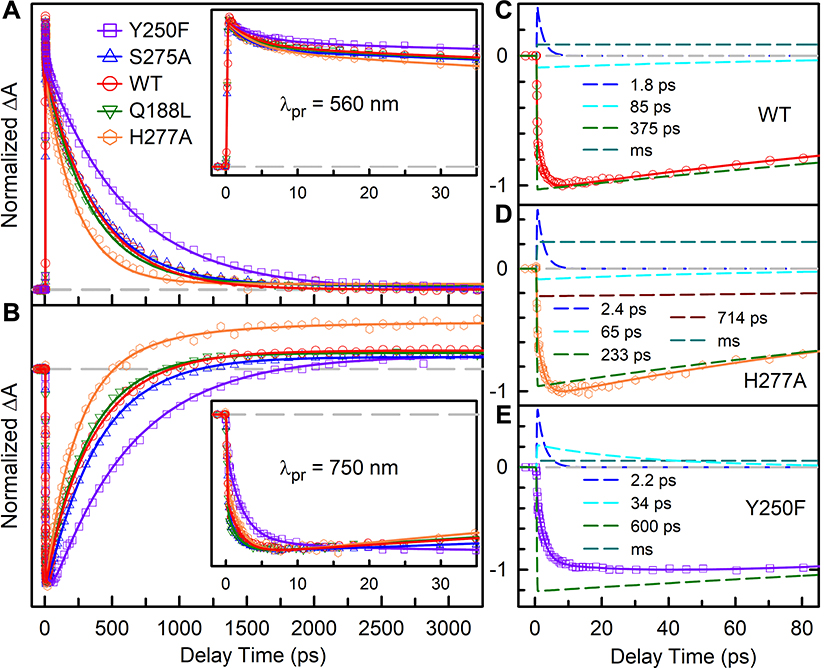
To further study the Pr excited-state dynamics and possible intermediates, we switched to the transient-absorption detection in the visible range (Figure 3 and 4, Figures S9–S13). Surprisingly, we observed coherent oscillation signals on the short time, especially when probed at around 725 nm. Figure 3 shows the clear oscillations in femtoseconds with ultrafast dephasing for WT and mutant transients. For WT, the residuals extracted from exponential fitting are Fourier transformed and two main low-frequency modes of ~38 cm−1 and ~181 cm−1 were found. The dephasing time for the 38 cm−1 mode oscillation is 0.54 ps, and for the 180 cm−1 mode oscillation is 0.25 ps. For all the mutants, the two low-frequency vibration modes were also observed with minor differences in the frequencies and dephasing times due to the alteration of the active-site environment (Table S5). Low-frequency coherent oscillation modes were also reported by our earlier study of the PaBphP Pfr state and in other phytochromes.32–34 In cyanobacteriochromes Slr1393g3, the coherent oscillation signals from three modes of ~20–40 cm−1, ~120–140 cm−1, and 280 cm−1 were reported, which are in good agreement with the observed frequencies here in PaBphP.33 Thus, upon excitation, the excited wavepacket starts to oscillate on the excited-state potential surface and such oscillations are rapidly damped within 1 ps.
Figure 3.
Absorption transients of WT PaBphP and mutants probed at 725 nm with clear oscillation signals. (Left panel) Comparison of exponential (blue) and oscillation (red) fit of experimental data. Note that the oscillation fit begins after 120 fs to avoid the influence of the instrument responses. The insets show the two major oscillation modes derived from the oscillation fit. (Right panel) Fourier transforms of the residuals from exponential fit. The gray dashed lines are the baseline at zero.
Figure 4.
Normalized femtosecond-resolved absorption transients of WT and mutants probed at 560 nm (A) and 750 nm (B). Insets show the absorption transients at early delay times. (C) The deconvolution of transients probed at 750 nm of WT, H277A, and Y250F into multi-exponential components. The gray dashed lines are the baseline at zero.
After the initial wavepacket motions within 1 ps, the transients probed by various wavelengths reflect the dynamics of both active-site relaxation and isomerization reaction. Specifically, for transient-absorption detections from 560 to 590 nm, transient signals are from the excited-state absorption and a minor ground-state bleaching (Figures S9–S13). Figure 4A shows the transients of WT and the mutants probed at 560 nm and the transients can be fitted with multiple exponential decays, comparable to the fluorescence results (Table S6–S10), indicating that the transient-absorption signals also represent both the relaxation and isomerization. From 656 to 670 nm, the signals are a mixture of the ground-state bleaching, excited-state absorption and ground-state product formation (Figures S9–S13). From 725 to 750 nm, we detected negative stimulated emission signal with a positive amplitude plateau due to the photoproduct Lumi-R formation. Figure 4B shows the transients of WT and all of the mutants probed at 750 nm. The relaxation dynamics can also be readily observed in Figures 4C to 4E with the deconvolution of WT, H277A, and Y250F into the relaxation dynamics of about 1.8–2.4 and 34–65 ps besides the isomerization and photoproduct formation components. The H277A exhibits a pronounced positive plateau from the photoproduct absorption when probed at 750 nm, indicating an increased Lumi-R quantum yield. Furthermore, a component in 714 ps was also observed from 725 to 750 nm and is assigned to the continuous structural relaxation of the isomerized photoproduct on the ground state. After the photoisomerization is completed, the active-site residues continue to relax to accommodate the twisted photoproduct on microsecond or even longer timescale. In H277A, such conformational dynamics are much faster and can readily be resolved in our detection window. Similarly, in the Pfr state, the photoproduct conformational relaxation becomes faster by abolishing the hydrogen-bonding constrain to the D-ring in the Q188L mutant.32
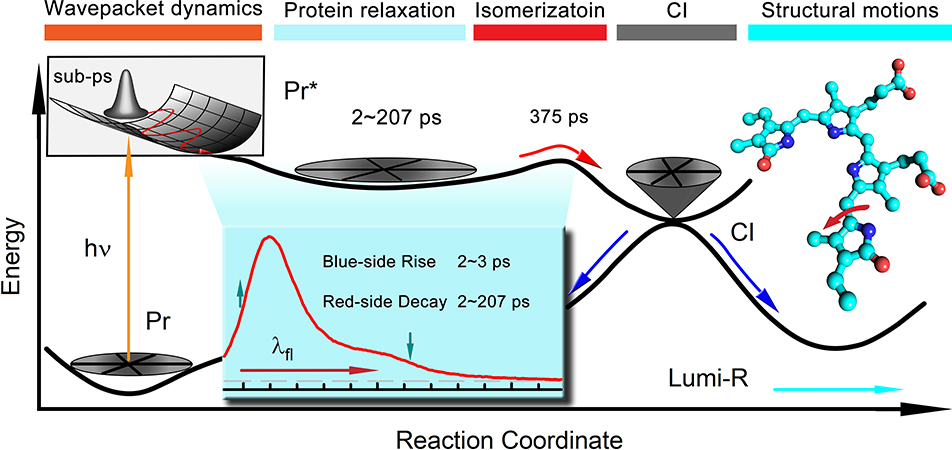
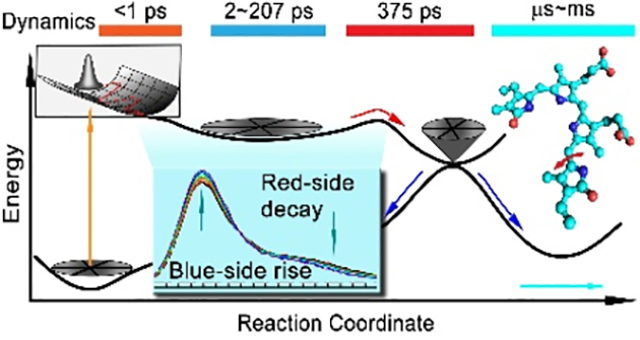
Combining the results of fs-resolved fluorescence and transient detections, the molecular mechanism of Pr-state photoisomerization is clear (Figure 5). Upon excitation, the wavepacket begins to oscillate within 1 ps on the excited-state potential surface, and then a series of structural relaxations of the active site including sidechains, trapped water and hydrogen-bond networks follows, resulting in the unique FRES shape evolution in a few to hundreds of picoseconds. Such local conformational relaxations finally lead the chromophore to overcome the energy barrier on the excited-state potential surface to enter conical intersection and by the D-ring twisting convert to intermediate product, ground-state Lumi-R conformations, which continues to relax on micro- to millisecond time scales to eventually form Pfr state (Figure 1C). The excited-state isomerization dynamics and the final quantum yield of product are dictated mainly by the active-site relaxation and conical intersection process.
Figure 5.
Schematic potential energy surface for PaBphP Pr state photoisomerization along the reaction coordinate. The sub-ps coherent nuclear wave packet movements of excited-state Pr* is followed by structural relaxation dynamics in a few picoseconds and hundreds of picoseconds. The excited-state population decay to the ground-state through the conical intersection (CI). The inset shows the steady-state emission spectrum of WT PaBphP and the arrows indicate the changes of the spectrum shape evolutions.
We reported here our systematic characterization of the Pr-state photoisomerization dynamics of bathy bacteriophytochrome PaBphP. With the fs-resolved fluorescence method, we unambiguously characterized a unique active-site relaxation process occurring from a few to hundreds of picoseconds and revealed a continuous structural evolution at the active site, leading to special tuning on the chromophore emission spectra with decays at the blue and rises on the red side. This observation is critical and resolves the long debate about the origin of the excited-state multiple exponential decays. In fact, the multiple dynamic behaviors are from the local relaxations, not isomerization reactions. Thus, the ground-state heterogeneity is unnecessarily invoked to explain the excited-state multiple dynamics.
Our transient absorption results are also consistent with the above dynamic picture. Furthermore, we observed the wavepacket dynamics with two low-frequency modes around 38 cm−1 and 181 cm−1 in femtoseconds as we also observed early in Pfr state. With several critical mutations at the active site, we observed the similar dynamic patterns but with different times of relaxations and isomerization reactions, consistent with the structural modification of mutations, i.e., the more flexible active site, the faster relaxation and isomerization. Thus, the molecular mechanism revealed here can be general to phytochrome and cyanobacteriochrome photoreceptors. The active-site relaxation must be taken into consideration of overall isomerization dynamics or other chemical reactions in proteins.35
Supplementary Material
ACKNOWLEDGMENT
We thank Prof. Bern Kohler for our use of the steady-state infrared fluorescence spectrometer. We also thank Dr. Zheyun Liu for the initial help in experiment. This work was supported in part by the National Institute of Health grants GM118332 to DZ and EY024363 to XY.
Footnotes
ASSOCIATED CONTENT
Supporting Information.
The following files are available free of charge.
Materials and methods, supporting figures and tables, and additional references. (PDF)
The authors declare no competing financial interests.
Contributor Information
Dihao Wang, Program of Biochemistry, The Ohio State University, Columbus, Ohio 43210, United States.
Yangzhong Qin, Department of Physics, The Ohio State University, Columbus, Ohio 43210, United States.
Meng Zhang, Program of Biophysics, The Ohio State University, Columbus, Ohio 43210, United States.
Xiankun Li, Department of Chemistry and Biochemistry, The Ohio State University, Columbus, Ohio 43210, United States.
Lijuan Wang, Department of Physics, The Ohio State University, Columbus, Ohio 43210, United States.
Xiaojing Yang, Department of Chemistry, University of Illinois at Chicago, Chicago, Illinois 60607, United States.
Dongping Zhong, Department of Physics, Department of Chemistry and Biochemistry, Program of Biophysics, Program of Chemical Physics, and Program of Biochemistry, The Ohio State University, Columbus, Ohio 43210, United States.
REFERENCES
- 1.Rockwell NC; Su Y; Lagarias JC Phytochrome Structure and Signaling Mechanisms. Annu. Rev. Plant Biol 2006, 57, 837–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Quail P Phytochrome Photosensory Signalling Networks. Nat. Rev. Mol. Cell Biol 2002, 3, 85–93. [DOI] [PubMed] [Google Scholar]
- 3.Nagatani A Phytochrome: Structural Basis for Its Functions. Curr. Opin. Plant Biol 2010, 13, 565–570. [DOI] [PubMed] [Google Scholar]
- 4.Yang X; Kuk J; Moffat K Crystal Structure of Pseudomonas aeruginosa Bacteriophytochrome: Photoconversion and Signal Transduction. Proc. Natl. Acad. Sci. U.S.A 2008, 105, 14715–14720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang X; Kuk J; Moffat K Conformational Differences between the Pfr and Pr States in Pseudomonas aeruginosa Bacteriophytochrome. Proc. Natl. Acad. Sci. U.S.A 2009, 106, 15639–15644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burgie ES; Zhang J; Vierstra RD Crystal Structure of Deinococcus Phytochrome in the Photoactivated State Reveals a Cascade of Structural Rearrangements during Photoconversion. Structure 2016, 24, 448–457. [DOI] [PubMed] [Google Scholar]
- 7.Schmidt A; Sauthof L; Szczepek M; Lopez MF; Escobar FV; Qureshi BM; Michael N; Buhrke D; Stevens T; Kwiatkowski D; Von Stetten D; Mroginski MA; Krauss N; Lamparter T; Hildebrandt P; Scheerer P Structural Snapshot of a Bacterial Phytochrome in its Functional Intermediate State. Nat. Commun 2018, 9, 4912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mathes T; Ravensbergen J; Kloz M; Gleichmann T; Gallagher KD; Woitowich NC; St Peter R; Kovaleva SE; Stojkovic EA; Kennis JTM Femto- to Microsecond Photodynamics of an Unusual Bacteriophytochrome. J. Phys. Chem. Lett 2015, 6, 239–243. [DOI] [PubMed] [Google Scholar]
- 9.Yang Y; Linke M; von Haimberger T; Matute R; Gonzalez L; Schmieder P; Heyne K Active and Silent Chromophore Isoforms for Phytochrome Pr Photoisomerization: An Alternative Evolutionary Strategy to Optimize Photoreaction Quantum Yields. Struct. Dyn 2014, 1, 14701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim PW; Rockwell NC; Freer LH; Chang C; Martin SS; Lagarias JC; Larsen DS Unraveling the Primary Isomerization Dynamics in Cyanobacterial Phytochrome Cph1 with Multipulse Manipulations. J. Phys. Chem. Lett 2013, 4, 2605–2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim PW; Rockwell NC; Martin SS; Lagarias JC; Larsen DS Dynamic Inhomogeneity in the Photodynamics of Cyanobacterial Phytochrome Cph1. Biochemistry 2014, 53, 2818–2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dasgupta J; Frontiera RR; Taylor KC; Lagarias JC; Mathies RA Ultrafast Excited-State Isomerization in Phytochrome Revealed by Femtosecond Stimulated Raman Spectroscopy. Proc. Natl. Acad. Sci. U.S.A 2009, 106, 1784–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spillane KM; Dasgupta J; Mathies RA Conformational Homogeneity and Excited-State Isomerization Dynamics of the Bilin Chromophore in Phytochrome Cph1 from Resonance Raman Intensities. Biophys. J 2012, 102, 709–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang C; Flanagan ML; McGillicuddy RD; Zheng H; Ginzburg AR; Yang X; Moffat K; Engel GS Bacteriophytochrome Photoisomerization Proceeds Homogeneously Despite Heterogeneity in Ground State. Biophys. J 2016, 111, 2125–2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bizimana LA; Epstein J; Brazard J; Turner DB Conformational Homogeneity in the Pr Isomer of Phytochrome Cph1. J. Phys. Chem. B 2017, 121, 2622–2630. [DOI] [PubMed] [Google Scholar]
- 16.Spillane KM; Dasgupta J; Lagarias JC; Mathies RA Homogeneity of Phytochrome Cph1 Vibronic Absorption Revealed by Resonance Raman Intensity Analysis. J. Am. Chem. Soc 2009, 131, 13946–13948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andel F; Hasson KC; Gai F; Anfinrud PA; Mathies RA Femtosecond Time-Resolved Spectroscopy of the Primary Photochemistry of Phytochrome . Biospectroscopy 1997, 3, 421–433 [Google Scholar]
- 18.Tasler R; Moises T; Frankenberg-Dinkel N Biochemical and Spectroscopic Characterization of the Bacterial Phytochrome of Pseudomonas aeruginosa. FEBS J. 2005, 272, 1927–1936. [DOI] [PubMed] [Google Scholar]
- 19.Rottwinkel G; Oberpichler I; Lamparter T Bathy Phytochromes in Rhizobial Soil Bacteria. J. Bacteriol 2010, 192, 5124–5133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kochendoerfer GG; Lin SW; Sakmar TP; Mathies RA How Color Visual Pigments are Tuned. Trends Biochem. Sci 1999, 24, 300–305 [DOI] [PubMed] [Google Scholar]
- 21.Philip AF; Nome RA; Papadantonakis GA; Scherer NF; Hoff WD Spectral Tuning in Photoactive Yellow Protein by Modulation of the Shape of the Excited State Energy Surface. Proc. Natl. Acad. Sci. U.S.A 2010, 107, 5821–5826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feliks M; Lafaye C; Shu X; Royant A; Field M Structural Determinants of Improved Fluorescence in a Family of Bacteriophytochrome-based Infrared Fluorescent Proteins: Insights from Continuum Electrostatic Calculations and Molecular Dynamics Simulations. Biochemistry 2016, 55, 4263–4274. [DOI] [PubMed] [Google Scholar]
- 23.Velazquez Escobar F; Piwowarski P; Salewski J; Michael N; Fernandez Lopez M; Rupp A; Muhammad Qureshi B; Scheerer P; Bartl F; Frankenberg-Dinkel N; Siebert F; Andrea Mroginski M; Hildebrandt P A Protonation-Coupled Feedback Mechanism Controls the Signalling Process in Bathy Phytochromes. Nat. Chem 2015, 7, 423–430. [DOI] [PubMed] [Google Scholar]
- 24.Singer P; Wörner S; Lamparter T; Diller R Spectroscopic Investigation on the Primary Photoreaction of Bathy Phytochrome Agp2-Pr of Agrobacterium fabrum: Isomerization in a pH-dependent H-bond Network. ChemPhysChem 2016, 17, 1288–1297. [DOI] [PubMed] [Google Scholar]
- 25.Yang Y; Linke M; von Haimberger T; Hahn J; Matute R; Gonzalez L; Schmieder P; Heyne K Real-Time Tracking of Phytochrome’s Orientational Changes during Pr Photoisomerization. J. Am. Chem. Soc 2012, 134, 1408–1411. [DOI] [PubMed] [Google Scholar]
- 26.Mailliet J; Psakis G; Feilke K; Sineshchekov V; Essen L; Hughes J Spectroscopy and a High-Resolution Crystal Structure of Tyr263 Mutants of Cyanobacterial Phytochrome Cph1. J. Mol. Biol 2011, 413, 115–127. [DOI] [PubMed] [Google Scholar]
- 27.Chang C; Guo L; Kao Y; Li J; Tan C; Li T; Saxena C; Liu Z; Wang L; Sancar A; Zhong D Ultrafast Solvation Dynamics at Binding and Active Sites of Photolyases. Proc. Natl. Acad. Sci. U.S.A 2010, 107, 2914–2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chang C; He T; Guo L; Stevens JA; Li T; Wang L; Zhong D Mapping Solvation Dynamics at the Function Site of Flavodoxin in Three Redox States. J. Am. Chem. Soc 2010, 132, 12741–12747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kundu M; He TF; Lu Y; Wang L; Zhong D Short-Range Electron Transfer in Reduced Flavodoxin: Ultrafast Nonequilibrium Dynamics Coupled with Protein Fluctuations. J. Phys. Chem. Lett 2018, 9, 2782–2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Linke M; Yang Y; Zienicke B; Hammam MAS; von Haimberger T; Zacarias A; Inomata K; Lamparter T; Heyne K Electronic Transitions and Heterogeneity of the Bacteriophytochrome Pr Absorption Band: An Angle Balanced Polarization Resolved Femtosecond VIS Pump-IR Probe Study. Biophys. J 2013, 105, 1756–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhu J; Shcherbakova DM; Hontani Y; Verkhusha VV; Kennis JTM Ultrafast Excited-state Dynamics and Fluorescence Deactivation of Near-infrared Fluorescent Proteins Engineered from Bacteriophytochromes. Sci. Rep 2015, 5, 12840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang D; Qin Y; Zhang S; Wang L; Yang X; Zhong D Elucidating the Molecular Mechanism of Ultrafast Pfr-State Photoisomerization in Bathy Bacteriophytochrome PaBphP. J. Phy. Chem. Lett 2019, 10, 6197–6201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Slavov C; Xu X; Zhao K; Gärtner W; Wachtveitl J Detailed Insight into the Ultrafast Photoconversion of the Cyanobacteriochrome Slr1393 from Synechocystis sp. Biochim. Biophys. Acta - Bioenerg 2015, 1847, 1335–1344. [DOI] [PubMed] [Google Scholar]
- 34.Müller MG; Lindner I; Martin I; Gärtner W; Holzwarth AR Femtosecond Kinetics of Photoconversion of the Higher Plant Photoreceptor Phytochrome Carrying Native and Modified Chromophores. Biophys. J 2008, 94, 4370–4382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gai F; Hasson KC; McDonald JC; Anfinrud PA Chemical Dynamics in Proteins: The Photoisomerization of Retinal in Bacteriorhodopsin. Science 1998, 279, 1886–1891. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.