We show how to foresee and account for risks in applying the SIBER framework to coral trophic ecology.
Abstract
In an era of major environmental changes, understanding corals’ resistance to bleaching is as crucial as it is challenging. A promising framework for inferring corals’ trophic strategies from Stable Isotope Bayesian Ellipses has been recently proposed to this end. As a contribution to this framework, we quantify a risk of bias inherent in its application and propose three alternative adjustments.
It is generally accepted that the symbiotic association between corals and their endosymbiotic algae (Symbiodiniaceae) is fundamental to the development of coral reefs as they transfer the major part of their photosynthates to the coral host (autotrophic nutrition) (1). However, corals are considered as mixotrophs, also acquiring energy through host consumption of exogenous organic resources (heterotrophy). Over the past decades, rising seawater temperatures have destabilized the symbiosis between corals and their algal endosymbionts, inducing massive bleaching events. Understanding the mechanisms underlying coral species’ resistance to environmental changes or resilience to bleaching is therefore a major research and conservation challenge (2). Previous works have suggested that heterotrophy was one determinant of corals’ mortality levels during and following bleaching events [e.g., (3)]. Conti-Jerpe et al. (4) recently provided a groundbreaking contribution to this field, showing that resistance to temperature rise is correlated with trophic strategy in symbiotic corals. Their mesocosm experiment demonstrated that heterotrophic corals were more tolerant to increasing temperatures. To evaluate the trophic strategy in a panel of coral genera, they proposed an innovative method relying on Stable Isotope Bayesian Ellipses [SIBER; (5)]. From both a theoretical and technical point of view, we think that some adjustments would increase the confidence associated with further applications of this promising method to the original mixotrophic nature of corals.
Inferring metabolic processes from statistics unavoidably smooths the path to approximations, whether purposeful or unintended. One risk in comparing trophic status from nitrogen and carbon isotopic niches is the missing of a potential nonhomogeneous distribution of the resource base in lower trophic levels (6, 7). Spatiotemporal variability in the stable isotope values of primary producers, i.e., nitrogen isoscapes, propagates up the food web and is reflected in the isotope values of consumers (8). In other words, different isotopic niches among consumers sampled at different places or times may not reflect differences in diet if their food sources had different isotope values (9). Similarly, divergent symbiont communities between closely located colonies of the same species could result in variable isotope values (10). The study of Conti-Jerpe et al. (4) relies on two distinct datasets: the isotope niche of corals sampled at 23 sites up to 45 km apart and a “temperature-resistance” mesocosm experiment conducted at one of these locations. SIBER Corrected Standard Ellipses (SEAcs), as applied to the first dataset, were designed specifically for isotopic niche comparisons and to deal with small sample sizes by including 40% of observations around the mean (5). This application of the maximum likelihood hypothesis to multivariate normal distributions is helpful for removing extreme values and to bring broad ecological patterns to light. However, extreme values may also represent ecologically relevant processes (5), particularly in datasets covering large spatiotemporal extents.
In corals, the consumer (host) and the producer (symbiont) belong to the same holobiont. This infra-individual scale implies a maximum difference in mean δ15N values of the two fractions around 3.4 ± 1.1‰, i.e., an average trophic step (11, 12). Technically, this limit reinforces the risk of missing ecologically relevant patterns when using SIBER’s SEAc (40%) for coral metapopulations. This risk can be illustrated using the summary statistics of stable isotope analyses for the genus Platygyra presented by Conti-Jerpe et al. (4). For the 40 individuals of this genus, sampled at 12 stations, located along a steep environmental gradient (13), δ13C values of host and symbionts were nearly identical, but δ15N means [∆Mean δ15N(Host-Symb)] differed by +2.5‰ with cumulated SDs of 2‰. In such a situation, the overlap between SEAc would be lower than 10% in 95% of cases (Fig. 1), and the Hotelling test would be significant in 100% of cases (2000 simulations). As a consequence, Conti-Jerpe et al. (4) defined this genus as heterotrophic. However, simulations suggest an 85% chance that one or more subgroups do not present distinct niches in the isotope biplot according to the Hotelling T2 test and may thus be considered autotrophic (Fig. 2). This risk of masking groups that do not have distinct niches is obviously reduced with higher ∆mean and ΣSD (Fig. 2). Our example highlights a risk from spatial variations in one dimension of the isotopic biplot, the δ15N values. Spatiotemporal variations in δ13C values of corals are also plausible (14–16) and would likely affect the bidimensional segregation of trophic niches in the same way. Note that this risk may be considered while applying SIBER to the isotope niche differentiation in other symbiotic organisms such as sponges (17).
Fig. 1. Probability of the three cutoff values [suggested by (1)] resulting from the use of SIBER’s SEAc as a function of the difference between the mean δ15N isotopic values of the two considered groups (Host and Symbionts) [N = 40; ΣSD(Host-Symb) = 2‰].
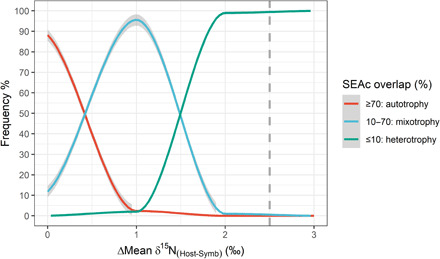
Dotted line represents the values obtained for Platygyra sp. [∆Mean δ15N(Host-Symb) = 2.5‰; ΣSD(Host-Symb) = 2‰]. Results were obtained from 3500 simulations.
Fig. 2. Chances that a pooled dataset contains at least one subgroup with overlapping isotopic niches as a function of the difference between the mean isotopic values of the two considered groups (Host and Symbionts).
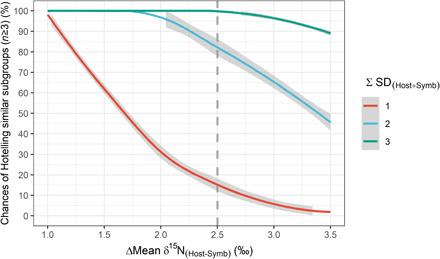
The curves correspond to different levels of SD associated with the means of the pooled fractions. Niche segregation is deduced from the P value of Hotelling tests (P > 0.05) for 182,000 simulated data per SD. Dotted line represents the values obtained for Platygyra sp. [ΔMean δ15N(Host-Symb) = 2.5‰ and ΣSD(Host-Symb) = 2].
From a theoretical perspective, the distribution of corals across a wide range of environmental conditions is certainly the result of adaptive trophic strategies (18). Many studies showed that shifts from autotrophy to heterotrophy can occur within species at varying depth (19) or turbidity (20) or depending on resource availability (21). We are thus convinced that accounting for the adaptive potential of species/genera toward a range of environmental conditions will be determinant in predicting the fate of symbiotic corals. To better reflect this potential at a fine spatiotemporal scale, we propose three adjustments to the analytical tools proposed by Conti-Jerpe et al. (4).
1) Hotelling tests should be conducted at the highest spatiotemporal resolution when possible. This would allow for applying SEAc to remove the least frequent behaviors while controlling for a potential plasticity in the study taxon. This approach may also be used to identify contrasting subgroups and help to consider the best scale for pooling data and designing SEAc. Note that Hotelling tests may be used to compare two groups of data from n = 2 but would be more reliable as much as the sample size increases, hence our choice of n ≥ 3 in the presented simulations (Fig. 2).
2) Subgrouping repeated measures demonstrated that SEAcs substantially help to deal with small sample sizes, while both standard ellipses (40% and 95% of observations) produce fair estimates of isotopic niche width for sample sizes >30 (5, 22). Thereby, for spatiotemporally heterogeneous datasets with n > 30, drawing ellipses including 95% of observations may be a reasonable compromise to lower the risk of mischaracterization due to spatiotemporal variability in isotope values.
3) Conti-Jerpe et al. (4) calculated the overlap between SEAc as a proportion of the host SEAc. Considering this overlap as the proportion of the nonoverlapping area may produce a metric more independent from variations of the host isotope niche width (5). While this third adjustment alone would tend to lower the overlap metric between host and symbiont fractions, combination of adjustment two along with this adjustment three will likely produce larger overlap metrics. The cutoff (10% and 70%) values proposed by Conti-Jerpe et al. (4) to this overlap metric would thereby make mixotrophic profiles more common. This sounds like a fair adjustment considering the established mixotrophic nature of most symbiotic coral genera, with species/colonies more autotrophic than others.
It is worth mentioning that these SIBER-derived metrics are insensitive to the sign of the ∆Mean δ15N(Host-Symb). This difference is found to be positive in most studies, including that of Conti-Jerpe et al. (4). However, some reported that host tissues can be 15N-depleted relative to the symbionts, with varying interpretations depending on the context (23, 24). Investigations beyond SIBER-derived inferences may thus be needed in such particular cases.
These “refinements” of the method would undoubtedly account for the ability of symbiotic corals to complement or temporarily replace autotrophic nutrition with heterotrophy, depending on fluctuations of their environment. Conti-Jerpe et al.’s (4) approach likely prioritized the characterization of broad trends that matched their observations at the mesocosm study site. We are confident that these comments could contribute to further developments of their promising analytical framework toward comprehensive predictions regarding the fate of coral reefs and a facilitated response to management stakes.
Acknowledgments
Author contributions: F.H. supervised the study. M.T. analyzed the data. M.T. and F.H. wrote the manuscript with the support from A.L. Competing interests: The authors declare that they have no competing interests. Data and materials availability: Data are available in the original paper (4).
REFERENCE AND NOTES
- 1.Muscatine L., Porter J. W., Reef corals: Mutualistic symbioses adapted to nutrient-poor environments. Bioscience 27, 454–460 (1977). [Google Scholar]
- 2.Grottoli A. G., Rodrigues L. J., Palardy J. E., Heterotrophic plasticity and resilience in bleached corals. Nature 440, 1186–1189 (2006). [DOI] [PubMed] [Google Scholar]
- 3.Anthony K. R. N., Hoogenboom M. O., Maynard J. A., Grottoli A. G., Middlebrook R., Energetics approach to predicting mortality risk from environmental stress: A case study of coral bleaching. Funct. Ecol. 23, 539–550 (2009). [Google Scholar]
- 4.Conti-Jerpe I. E., Thompson P. D., Wong C. W. M., Oliveira N. L., Duprey N. N., Moynihan M. A., Baker D. M., Trophic strategy and bleaching resistance in reef-building corals. Sci. Adv. 6, eaaz5443 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jackson L., Inger R., Parnell A. C., Bearhop S., Comparing isotopic niche widths among and within communities: SIBER—Stable Isotope Bayesian Ellipses in R. J. Anim. Ecol. 80, 595–602 (2011). [DOI] [PubMed] [Google Scholar]
- 6.Layman C. A., Post D. M., Can stable isotope ratios provide for community-wide measures of trophic structure? Reply. Ecology 89, 2358–2359 (2008). [DOI] [PubMed] [Google Scholar]
- 7.Hoeinghaus D. J., Zeug S. C., Can stable isotope ratios provide for community-wide measures of trophic structure? Comment. Ecology 89, 2353–2357 (2008). [DOI] [PubMed] [Google Scholar]
- 8.McMahon K. W., Hamady L. L., Thorrold S. R., Ocean ecogeochemistry: A review. Oceanogr. Mar. Biol. 51, 327–374 (2013). [Google Scholar]
- 9.Heikoop J. M., Dunn J. J., Risk M. J., Tomascik T., Schwarcz H. P., Sandeman I. M., Sammarco P. W., δ15 N and δ13 C of coral tissue show significant inter-reef variation. Coral Reefs 19, 189–193 (2000). [Google Scholar]
- 10.Wall C. B., Kaluhiokalani M., Popp B. N., Donahue M. J., Gates R. D., Divergent symbiont communities determine the physiology and nutrition of a reef coral across a light-availability gradient. ISME J. 14, 945–958 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Minagawa M., Wada E., Stepwise enrichment of 15N along food chains: Further evidence and the relation between δ15N and animal age. Geochim. Cosmochim. Acta 48, 1135–1140 (1984). [Google Scholar]
- 12.Hoegh-Guldberg O., Muscatine L., Goiran C., Siggaard D., Marion G., Nutrient-induced perturbations to δ13C and δ15N in symbiotic dinoflagellates and their coral hosts. Mar. Ecol. Prog. Ser. 280, 105–114 (2004). [Google Scholar]
- 13.Duprey N. N., Yasuhara M., Baker D. M., Reefs of tomorrow: Eutrophication reduces coral biodiversity in an urbanized seascape. Glob. Change Biol. 22, 3550–3565 (2016). [DOI] [PubMed] [Google Scholar]
- 14.Orejas C., Gori A., Rad-Menéndez C., Last K. S., Davies A. J., Beveridge C. M., Sadd D., Kiriakoulakis K., Witte U., Roberts J. M., The effect of flow speed and food size on the capture efficiency and feeding behaviour of the cold-water coral Lophelia pertusa. J. Exp. Mar. Biol. Ecol. 481, 34–40 (2016). [Google Scholar]
- 15.Treignier C., Tolosa I., Grover R., Reynaud S., Ferrier-Pagès C., Carbon isotope composition of fatty acids and sterols in the scleractinian coral Turbinaria reniformis: Effect of light and feeding. Limnol. Oceanogr. 54, 1933–1940 (2009). [Google Scholar]
- 16.Tanaka Y., Suzuki A., Sakai K., The stoichiometry of coral-dinoflagellate symbiosis: Carbon and nitrogen cycles are balanced in the recycling and double translocation system. ISME J. 12, 860–868 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Freeman C. J., Baker D. M., Easson C. G., Thacker R. W., Shifts in sponge-microbe mutualisms across an experimental irradiance gradient. Mar. Ecol. Prog. Ser. 526, 41–53 (2015). [Google Scholar]
- 18.Goreau T. F., Goreau N. I., Yonge C. M., Reef corals: Autotrophs or heterotrophs? Biol. Bull. 141, 247–260 (1971). [Google Scholar]
- 19.Palardy J. E., Grottoli A. G., Matthews K. A., Effects of upwelling, depth, morphology and polyp size on feeding in three species of Panamanian corals. Mar. Ecol. Prog. Ser. 300, 79–89 (2005). [Google Scholar]
- 20.Anthony K. R., Fabricius K. E., Shifting roles of heterotrophy and autotrophy in coral energetics under varying turbidity. J. Exp. Mar. Biol. Ecol. 252, 221–253 (2000). [DOI] [PubMed] [Google Scholar]
- 21.Fox M. D., Elliott Smith E. A., Smith J. E., Newsome S. D., Trophic plasticity in a common reef-building coral: Insights from δ13C analysis of essential amino acids. Funct. Ecol. 33, 2203–2214 (2019). [Google Scholar]
- 22.Syväranta J., Lensu A., Marjomäki T. J., Oksanen S., Jones R. I., An empirical evaluation of the utility of convex hull and standard ellipse areas for assessing population niche widths from stable isotope data. PLOS ONE 8, e56094 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rodrigues L. J., Grottoli A. G., Calcification rate and the stable carbon, oxygen, and nitrogen isotopes in the skeleton, host tissue, and zooxanthellae of bleached and recovering Hawaiian corals. Geochim. Cosmochim. Acta 70, 2781–2789 (2006). [Google Scholar]
- 24.Ferrier-Pagès C., Peirano A., Abbate M., Cocito S., Negri A., Rottier C., Riera P., Rodolfo-Metalpa R., Reynaud S., Summer autotrophy and winter heterotrophy in the temperate symbiotic coral Cladocora caespitosa. Limnol. Oceanogr. 56, 1429–1438 (2011). [Google Scholar]


