Abstract
Coronary in-stent restenosis and late stent thrombosis are the two major inadequacies of vascular stents that limit its long-term efficacy. Although restenosis has been successfully inhibited through the use of the current clinical drug-eluting stent which releases antiproliferative drugs, problems of late-stent thrombosis remain a concern due to polymer hypersensitivity and delayed re-endothelialization. Thus, the field of coronary stenting demands devices having enhanced compatibility and effectiveness to endothelial cells. Nanotechnology allows for efficient modulation of surface roughness, chemistry, feature size, and drug/biologics loading, to attain the desired biological response. Hence, surface topographical modification at the nanoscale is a plausible strategy to improve stent performance by utilizing novel design schemes that incorporate nanofeatures via the use of nanostructures, particles, or fibers, with or without the use of drugs/biologics. The main intent of this review is to deliberate on the impact of nanotechnology approaches for stent design and development and the recent advancements in this field on vascular stent performance.
INTRODUCTION
Atherosclerosis, a chronic inflammatory condition, occurs due to the build-up of fatty deposits within coronary arteries, which causes arterial narrowing, thereby hampering blood flow.1,2 The advent of percutaneous coronary intervention procedures that commenced with balloon angioplasty and presently intravascular stenting has relieved millions suffering from this coronary artery disease.3–5 Bare-metal stents (BMS) and drug-eluting stents (DES) are the two successful intravascular stent candidates that have revolutionized the field of interventional cardiology. Although BMS could restore occluded arteries and promote re-endothelialization of the denuded artery, it suffers from in-stent restenosis in nearly 30% of the cases.6,7 Early restenosis occurred due to the migration and hyperproliferation of vascular smooth muscle cells (VSMCs) into the intimal space in response to vascular injury, which is caused by stent deployment.8–11 DES, the current clinical gold standard, evolved from BMS, which elutes an antiproliferative drug that reduced SMC hyperplasia within the stent, thereby reducing restenosis rates.12 DES could significantly reduce the complications of in-stent restenosis, but long-term trial revealed another challenge, which is late and very late stent thrombosis.13–15 The antiproliferative effect of DES slows the process of re-endothelialization, thus triggering platelet activation and late stent thrombosis.16,17 Even though the incidence of stent thrombosis is low, it occurs suddenly with acute life-threatening symptoms and high mortality.15,18 Additionally, the polymeric coatings utilized for drug incorporation induce inflammatory reactions and other instability issues, augmenting its complications.19,20 The development of DES progressed through various generations. First-generation DES had a stainless steel stent coated with drugs, either sirolimus or paclitaxel. Paclitaxel (PTX) inhibits microtubule disassembly and interferes with the cell cycle, leading to cell cycle arrest in G0–G1 and G2-M phases.21 Sirolimus binds to FKBP12 and subsequently inhibits the mTOR and PI3 pathway, arresting cell cycle in the G1 phase.22,23 First-generation DES used synthetic polymers such as poly(ethylene-co-vinyl acetate), poly(n-butyl methacrylate) or tri-block copolymer poly(styrene-b-isobutylene-b-styrene). Observations of late stent thrombosis and inflammatory cells surrounding the stent struts pointed to polymer hypersensitivity as the major issue.24 Second-generation DES utilized cobalt–chromium stents with more biocompatible polymer coatings such as phosphorylcholine and co-polymer poly(vinylidene fluoride-co-hexafluoropropylene) that reduced inflammation. These stents possess decreased strut thickness, improved flexibility, deliverability, enhanced biocompatibility, and superior re-endothelialization and are now the predominant clinical stents.25–27 To further reduce inflammatory response, third-generation DES utilize completely bioabsorbable polymers such as poly-lactic acid (PLA), poly(lactide-co-glycolide) (PLGA), and polycaprolactone (PCL).28
Researchers are now keen to advance the stent technology and devise a novel stent material/surface that can promote re-endothelialization and concurrently inhibit restenosis, without altering the hemocompatibility or stent characteristics.29,30 In this direction, various novel schemes have been proposed and tested at lab scale or preclinically in small/large animal models, with a few taken forward to clinical trials. Among these innovations, stent surface engineering strategies that provide novel alterations in topography, chemistry, roughness, and wettability and also offer a platform for drug/biologics loading are thrust areas in coronary stent development.31,32 Additionally, the clinical needs of long-term safety and bio/hemocompatibility demand high standards for the choice of the stent material, design, or surface.33,34 Stent surface engineering is the process of modifying the surface of a stent material to enhance its overall performance characteristics. Engineering a surface for a desired outcome can be done either by chemically modifying the existing surface or by depositing a thin film with the desired properties onto the existing surface.35–37 This would obviate corrosion and ion leaching, improve biocompatibility and durability, and also enhance cell–material interactions. Nanosurface engineering encompasses engineering materials and/or technologies at the nanoscale, wherein one or more features are less than 100 nm in at least one dimension. This may refer to the size of individual crystals, grains, pores, particles, fiber diameter, etc.37 Specifically, by exploiting the high surface area to volume ratio, surface energy, roughness, reactivity, and wettability offered by nanostructures, nanosurface engineering of stents can be a way forward to design stents with improved biological performance.38,39 It is also necessary that the mechanical characteristics (stent deliverability, crimping and expansion profile, and coating durability) of the stents are retained without significant variations, after nanotexturing. Progress in nanotechnology now makes it possible to precisely design and modulate the surface properties of materials at the nanoscale via alterations in the method of processing, choice of the stent material, incorporation of drugs/biologics, etc.40–42 From the materials engineering perspective, physico-chemical properties of a biomaterial surface (namely, topographical features and roughness, surface chemistry, and hydrophilicity) can profoundly affect cellular mechanisms. Likewise, shape and size of the structures can regulate cellular functions by modifying the cytoskeleton organization. Nanoscale topographies can also critically control the signaling pathways at the molecular and subcellular levels.43,44 Thus, nanosurfaces can uniquely direct the overall biological response and hemocompatibility of the implanted stent material, mainly, its protein adsorption, vascular cell [smooth muscle cell and endothelial (EC) cell] adhesion and proliferation, etc.
Recent advances encompass a paradigm shift toward nanoengineered stent coatings to improve stent efficacy, which include polymer-less techniques of stent modification, coatings for controlled drug delivery, drug-free nanotopographical approaches, and nanoparticle (NP)-eluting/nanofiber-coated stents. Nanofiber coatings on stents developed by electrospinning are classified as nanotechnology, as the electrospun fiber diameters are at the nanoscale,45 although it does not represent “surface engineering.” A wealth of literature exists that delves on various nanotechnology-based techniques that help to generate nanoscale surface features and coatings on existing stent materials. Such nanoengineered stents alleviate the problems of restenosis, lack of re-endothelialization, local inflammatory response, and thrombus formation,41,46 which are common to BMS or DES. The future generation of cardiovascular stents will be nanotechnology-centric, due to the multifarious benefits it promises. Despite these advantages, a relevant clinical translation of stents utilizing this technology in the biomedical device industry is still awaited.47,48 This review throws light into the diverse nanosurface modification strategies (Fig. 1) that are widely adopted for developing novel stents and their associated cellular response in vitro and in vivo. To translate the research findings from bench to bedside entails the development of a viable stent prototype and its further preclinical evaluation in animal models, followed by regulatory approval and clinical testing. The journey of those few stents that surpassed these standards to the clinical trial stage is also presented.
FIG. 1.

Various nanoscale surface engineering strategies (nanostructured surface and thin films, nanoparticulate, and nanofibrous) adopted as coatings on coronary bare-metal stents to prevent in-stent restenosis and promote re-endothelialization.
Nanostructured surfaces and nano-thin-film coatings
Nanoscale architectures on stent
Texturing the stent surface at nanoscale may be beneficial, given the fact that nanosurface topography mimics the natural extracellular matrix and can regulate vascular cell adherence and proliferation.49 Cells when in contact with nanostructured surfaces not only respond to the type of material, but also to the surface topology.50 Surface properties such as topography and chemistry, roughness and wettability, are known to influence protein and cell adhesion.41,51 Moreover, creation of reservoirs and pores at nanoscale provides a platform to load drugs efficiently.52 Development of polymer-free stents can eliminate the problems such as polymer delamination and the long-term risk of inflammatory response, and thereby help to better endothelial regeneration.53 This section elaborates the diverse nanostructured surfaces on vascular stents and their biological response. Among the medically relevant metals such as titanium (Ti), magnesium (Mg), iron (Fe), and the alloys, viz., stainless steel (SS), cobalt–chromium (CC), nitinol (NiTi), etc., metallic stents are mainly based on the alloys of SS, CC, NiTi, Mg, and Fe.54 Significant efforts are under way to obtain nanostructured surfaces on these alloyed metals, which include the widely studied electrochemical anodization process, physical/chemical vapor deposition (CVD), thermochemical processing, lithography, etc.55
Nanotubular structures
Titanium dioxide nanotubes (titania nanotubes or TNT) can be fabricated using diverse methods including sol-gel,56,57 hydrothermal processes,58–60 template-assisted synthesis,61,62 seeded growth,63 and electrochemical anodization.64–67 Among all these methods, electrochemical anodization is widely used, because it provides a relatively simple and effective way of generating nanotubular structures. Moreover, it is a cost-effective process which offers the feasibility to tune the size and shape of nanotubular arrays to the desired dimensions. Furthermore, the tubes prepared via this method are highly ordered, well-defined with high aspect ratios, and are vertically oriented to the substrate, with good adherent strength.68–70 Most importantly, the dimensions of nanotubes such as diameter, length, wall thickness, etc., can be controlled precisely and modified easily by modulating the anodization parameters.71,72 However, such nanotubes have mostly been developed on metallic Ti or NiTi surfaces by anodization in acidic or alkaline conditions at different applied voltages for varied time intervals.73,74 By changing the anodization parameters (applied voltage and anodization time), TiO2 nanotubes having different diameters from 15 to 300 nm, and different lengths (nm to mm) can be obtained.75 The excellent potential of titanium nanotubes is mainly due to its high effective surface and the possibility to vary their geometry (diameter and length), which could be specifically designed for a desired biological response.74
Titanium, due to its low tensile strength and ductility, failed to make an impact as a sole stent material, because of its higher probability of tensile failure upon expansion when developed as stents.76,77 Despite these limitations, immense information has been garnered on the impact of nanoscale dimensions in modulating vascular cell behavior in vitro by utilizing anodized Ti surfaces. An optimal surface engineered stent should inhibit vascular smooth muscle cell proliferation to prevent in-stent restenosis, but simultaneously enhance endothelial cell adhesion and proliferation for restoration of a healthy endothelium.78 Several studies report TNTs as a promising platform for cardiovascular stent applications owing to their selective regulation of vascular cell response, specifically endothelial (EC) and smooth muscle cells (SMC), but the reason for this selective cell proliferation is still unknown and not clearly elucidated by researchers. The cell viability and activity on a nanostructured surface depends on various parameters such as the surface topography and roughness, surface wettability, and surface chemistry, which collectively dictate the cell response.39 Recently, a study was conducted to investigate the combined influence of nanotopography and surface chemistry on the in vitro biological response of TiO2 nanotubes. It was observed that nanotopography, surface chemistry, and wettability as well as morphology, cooperatively contributed to the reduced platelet adhesion and preferential vascular cell response.79 Several other studies report that TiO2 nanotubes of varied nanotube diameters (30–90 nm) promoted EC growth and proliferation, with concurrent inhibition of smooth muscle cells.80,81 The highlights of such in vitro studies include faster migration of ECs on nanotubular surface82 and lower inflammatory response, resulting in reduced TNFα-induced SMC proliferation,83 good hemo- and cytocompatibility with lessened platelet adhesion, and enhanced endothelial cell adhesion and proliferation for smaller diameter (30 nm) nanotubes.84 Rapid re-endothelialization, a key to the success of a cardiovascular implant device, has been achieved through several synergistic approaches on TiO2 nanotubular surfaces. Fibronectin (Fn), an extracellular matrix protein, when immobilized onto TNTs via an intermediate polydopamine (PDA) layer, has offered increased nitric oxide and prostaglandin (PGI2) secretion, indicating an increased functionality of ECs on these surfaces.85 Likewise, TNTs functionalized with polydopamine (PDA/NTs) showed a remarkable enhancement in the mobility of ECs with longer migration distances than that of bare Ti and TNTs, respectively.86 PDA/NTs incorporating a thrombin inhibitor, bivalirudin (BVLD), demonstrated high BVLD elution beyond 70 days. This synergism brought about a significant inhibitory effect on thrombin bioactivity, with concomitant less adhesion, activation, and aggregation of platelets, and selectivity for EC over SMC in a competitive growth environment.87 Recently, utilizing copper as a catalyst for effecting the release of nitric oxide from endogenous nitric oxide donors, Cu-loaded PDA nanoparticles were stacked onto TNTs. This surface yielded a controlled and steady release of Cu, sufficient to enable the release of NO within the physiological range. The in vivo effect induced by this synergy aided in preventing intimal hyperplasia and coagulation, with simultaneous rapid re-endothelialization after implantation in the abdominal aorta of rats.88
Another study utilized a nanotubular oxide layer as a drug reservoir on anodized Ti-8Mn alloy as a nickel and polymer-free matrix for drug-eluting stents. The highly ordered Ti-8Mn oxide NTs promoted cell (neonatal mice skin cells) proliferation in comparison with flat substrates. It was noted that alloying titanium with 8% manganese hindered charge transfer from fibrinogen to the material, thus preventing blood clots and thrombus formation. They demonstrated that the drug loading efficiency was higher on this alloyed nanotube surface, thus establishing Ti-8Mn oxide NTs to be a superior platform for drug loading than TNTs.89 Self-grown nanotubes of two nanotube morphologies, viz., homo, and hetero-NT, which are highly ordered and vertically aligned, with variations in tube diameter (80–190 nm), were developed on Ti–17Nb–6Ta substrate. Both NT morphologies showed significantly better results for endothelial cell proliferation, with homo-NTs displaying superior biological activity and drug loading capacity than hetero-NTs.90
Despite the abundant literature on TNT-based systems for cardiovascular stenting, no studies have yet proven the utility of this material for clinical translation. This could be due to the limitations of Ti as the base material for stent manufacturing and the complexity involved in translating TiO2 nanostructures onto clinically available coronary stent materials like SS and CC. This requires that titanium, which is deposited on SS or CC stents, be anodized to generate TNTs. Here, the restraints posed by the process of anodization (strong acidic/alkaline environment) can hamper the durability of the extremely thin stent struts (typically <100 μm) upon expansion and crimping. In a sole in vivo study reported thus far on a titanium stent prototype (Ti6Al4V) bearing nanotopographical cues of diameter 90 ± 5 nm and height 1800 ± 300 nm, significantly lower restenosis rates with minimal intimal hyperplasia and good stent strut coverage were observed after implantation in rabbit iliofemoral arteries as depicted in Fig. 2. This ascertained the importance of nanotopography in offering reduced in-stent restenosis, with concurrently enhanced endothelialization.91
FIG. 2.
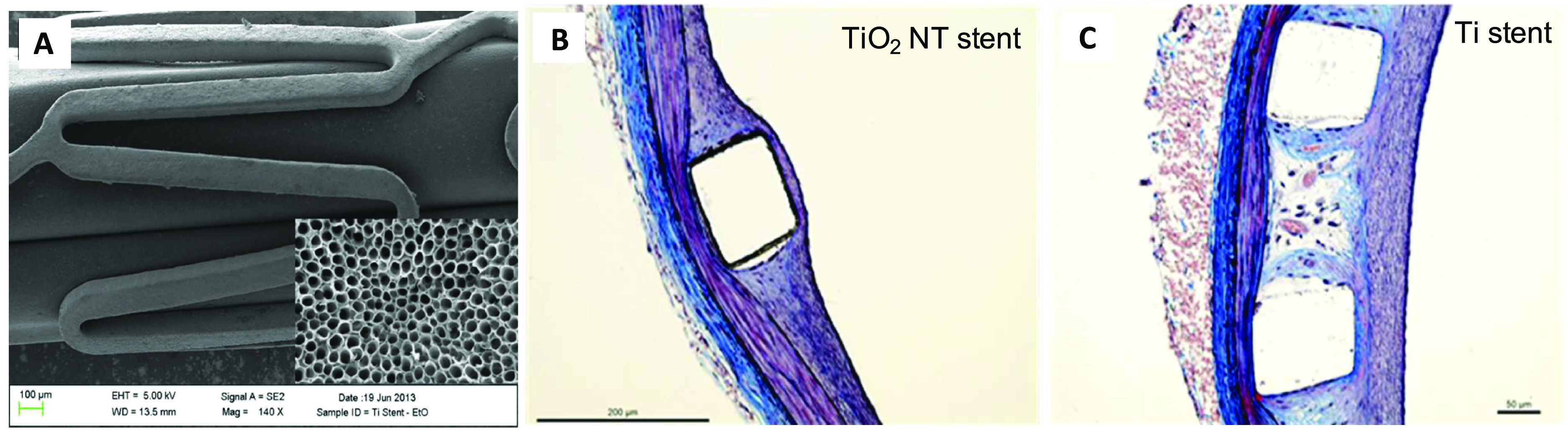
(a) Electron micrograph of titania nanotube coated stent. Inset: nanotubes with an average nanotube diameter of 90 nm (magnification ×250 000). Moffat trichrome-stained images of a stented artery. (b) Titania nanoengineered and (c) Ti stents, showing a 15.6% and 5.6% thinner neointima over the struts for TiO2 NT stents than Ti stent. Reprinted with permission from Nuhn et al., ACS Appl. Mater. Interfaces 9(23), 19677–19686 (2017). Copyright 2017 American Chemical Society.
In contrast to titanium-based stents, which find minimal use in coronary stenting, nitinol (NiTi), a widely explored alloy of titanium with nickel, finds applicability as coronary stents. The process of anodization helps to generate Ni–Ti–O nanotubular structures on the NiTi surface. The impact of nanotopography on vascular response to nitinol substrates was akin to titanium nanotubular structures when investigated in vitro using ECs and SMCs. These NTs showed reduced proliferation of SMCs, along with a decreased expression of collagen I and MMP-2, and parallelly enhanced EC spreading and migration.92 Moreover, nanotube diameter was found to influence EC and SMC response. While SMCs proliferated less, ECs showed increased proliferation and migration, with augmented production of elastin and collagen, on larger diameter (110 nm) NTs.93 Regardless of the limited investigations done on NiTi surfaces, nanotopography, especially NTs, showed promise for stenting applications and surprisingly these nanostructures always exhibited a preferential vascular response, the reason for which remains to be explored. Researchers have also developed a nanotubular α-Fe2O3 coating on biodegradable iron stents. PLGA coated on the NT surface incorporating the drug (Rapamycin) could efficiently reduce the initial burst with a sustained drug release of 30 days. These surfaces showed better EC viability than SMC along with good hemocompatibility.94
Other nanotopographies (nanoleaves, nanograss, nanoflakes, nanopillars, and nanowires) on stent surface
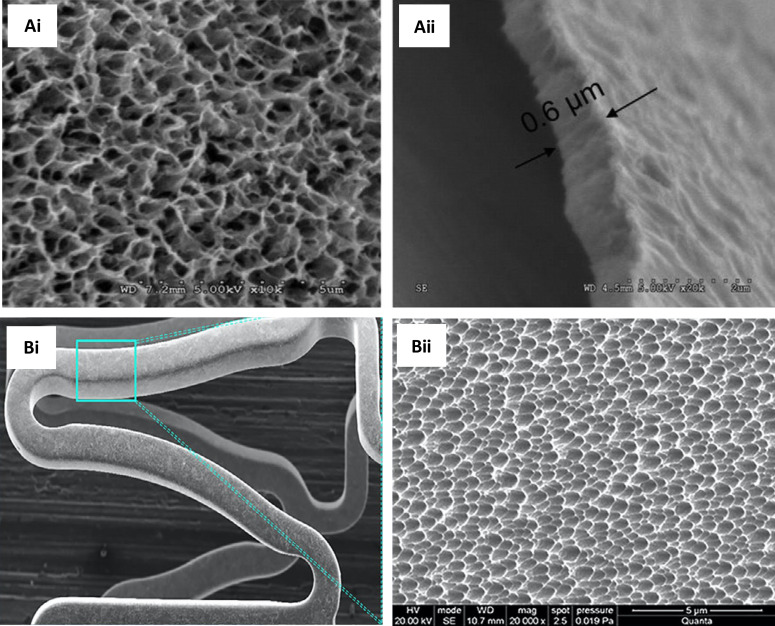
As an alternative to anodization, researchers have delved into chemical/thermochemical processing or lithography95,96 as a means to develop uniform and homogeneous nanostructures on metallic surfaces.31 Hydrothermal/thermochemical synthesis proposes various advantages such as low cost, simple experimental set up, and high yield.97 In a normal hydrothermal reaction, acidic or alkaline media are subjected to elevated temperature and pressure for a specific time, thus providing a one-step process for the generation of highly crystalline materials.98 The reaction parameters such as concentration and type of solvent, reaction temperature and time, offer significant effects on the formed nanostructures.99,100 Diverse titania nanotopographies were generated on Ti substrates using this facile thermochemical technique in NaOH at 200 °C, which exhibited a preferential vascular cell response, like for titania NTs.101 Static and dynamic blood contact studies done on Ti stent prototypes revealed these hydrothermally generated nanostructures to be antihemolytic, with minimal activation of coagulation cascade and platelets.102 Among the different topographies, a specific titania nanoleafy structure [Fig. 3(a)] yielded superior cyto- and hemocompatibility response in vitro, with high endothelialization and low SMC proliferation [Figs. 3(b) and 3(c)]. The uniqueness of this simple polymer-free and drug-free nanotexturing approach is that it could be readily translated onto any metallic stent substrate or on clinical stent materials of SS and CC. The nanoleafy structures were found to be extremely stable and adherent upon stent crimping and expansion with good corrosion resistance.103 This titania nanotexturing developed on SS bare-metal coronary stents presented minimal in-stent restenosis, effective endothelialization, and no thrombus formation after 8 weeks of implantation in a rabbit iliac artery model,104 as evident from Figs. 3(d)–3(f).
FIG. 3.
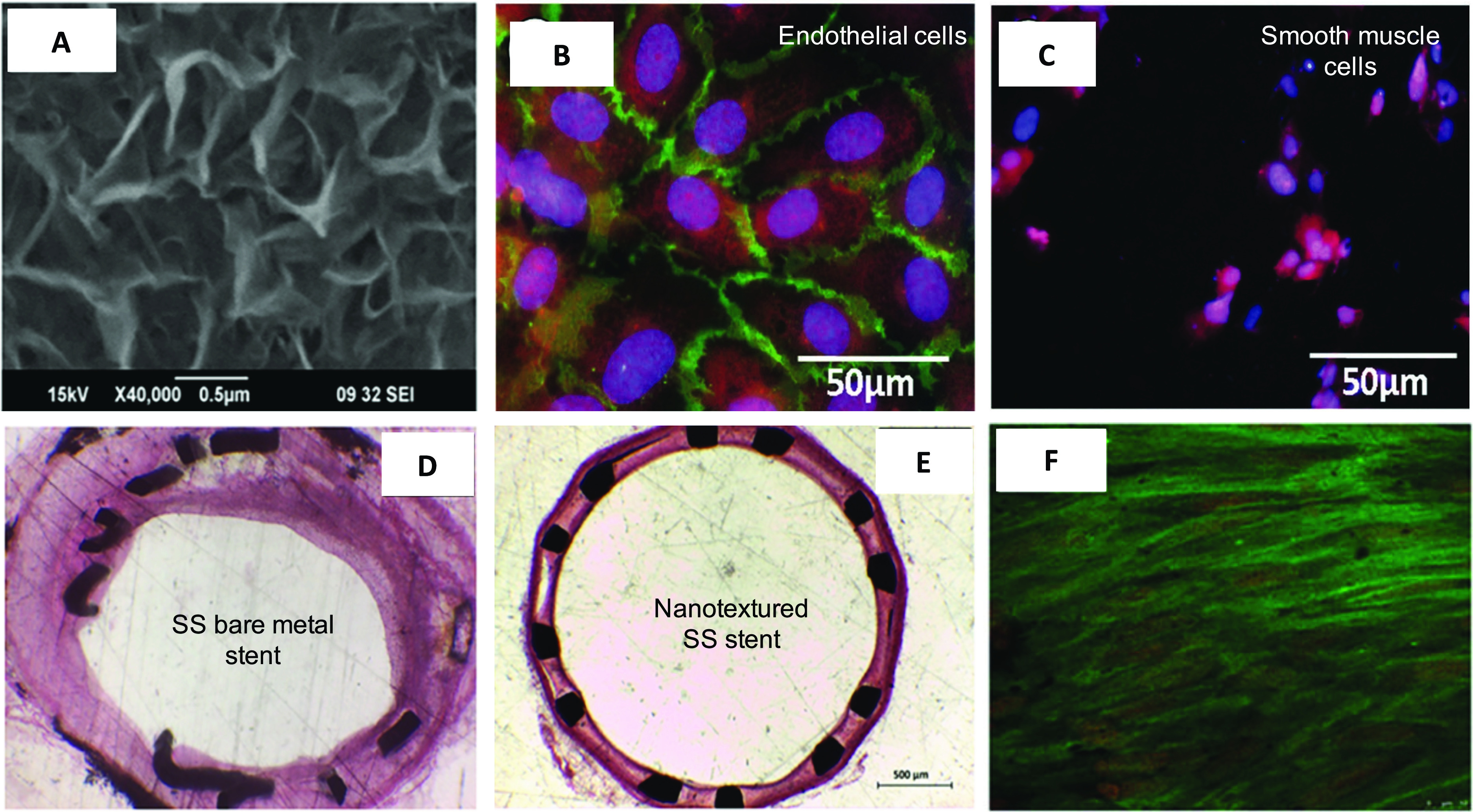
(a) Electron micrograph of titania nanoleafy textured stent surface (magnification ×40 000). Fluorescence imaging of (b) endothelial cells stained for F-actin (red) and PECAM1 (green) and (c) smooth muscle cells stained for F-actin (red) and nucleus (blue) on nanotextured SS surfaces, showing preferential adsorption and proliferation of ECs over SMCs on nanoleafy SS surface. Reprinted with permission from Mohan et al., Adv. Healthcare Mater. 6, 1601353 (2017). Copyright 2017 John Wiley and Sons. H&E images of rabbit iliac artery after 2 months implantation of (d) SS bare-metal stent and (e) nanotextured SS stent, showing nearly 50% decrease in neointimal stenosis for the nanotextured stent. (f) Immunofluorescent en-face stained images of wheat germ agglutinin on ECs in nanotextured stent implanted artery, showing complete endothelialization (scale bar: 10 μm). (a) and (d)–(f) Reprinted with permission from Cherian et al., ACS Omega 5, 17582–17591 (2020). Copyright 2020 American Chemical Society.
Likewise, direct nanotexturing of metallic substrates have also yielded nanotopographical features. For example, nanosized pyramidal structures were developed on SS substrates by hydrothermal treatment under alkaline conditions, which showed improved corrosion resistance, hemocompatibility, and EC growth, while inhibiting the proliferation of SMCs.105 A superhydrophilic nanoscale morphology with nanograss-like structures was likewise generated on Ni-free Ti–29Nb alloy after subjecting it to hydrothermal processing in alkaline sodium hydroxide solution at 250 °C for 10 h. This nanostructured material showed reduced hemolysis, minimal platelet adhesion, and activation upon contact with blood. The initially adsorbed intermediate water layer on this superhydrophilic surface might have caused resistance to platelet attachment, which can be attributed to the existence of a large number of hydrogen bonds.106 In the same manner, radially emanating metallic nanopillar structures were created on the surface of CC stent wires (MP35N) via controlled RF plasma processing technique.107 These uniformly coated nanopillar arrays of diameter 100 to 300 nm were developed directly on the stent wires. This surface displayed greater endothelial cell growth and functionality, continuous and complete endothelial monolayer formation, and minimal oxidative stress level in ECs.108 Increased EC and SMC adhesion has also been reported on nanostructured Ti and CC surfaces generated by compacting commercially pure metal particulates. Well-spread morphologies of both vascular cell types, with an increased ratio of viable ECs to SMCs, were noted on these nanostructured surfaces. A large number of particle boundaries at the surface of nanostructured metals were speculated to be responsible for improved adhesion of vascular cells on these surfaces.109 Such nanostructured Ti also showed greater competitive adhesion of ECs than SMCs.110,111 Utilizing a simple chemical conversion treatment of Mg−Nd−Zn−Zr alloys in 0.1 M potassium fluoride solution, they were surface textured to deposit MgF2 film with nanoscale flake-like features (∼200–300 nm-sized, having a thickness of 800 nm). These nanotextured films showed a significant reduction in corrosion rates and presented a favorable surface for enhanced viability, growth, and proliferation of ECs. Furthermore, implantation in rabbit abdominal aorta confirmed a complete and uninterrupted endothelial lining on the nano-MgF2 modified stent, along with minimal inflammatory reaction, thrombogenicity, and restenosis.112 Similar to the above studies, ultra-thin (300 nm) and chaotic one-dimensional (1D) aluminum oxide (Al2O3) nanostructures having a nanowire (NW) morphology were synthesized by chemical vapor deposition on a glass substrate. Al2O3 NWs presented a preference for EC adhesion and proliferation in comparison with SMC.113 ECs seem to favor growth on low-density NWs, while SMCs disliked this topography in contrast to commercially available microstructured Al2O3 plates.114 Recombinant filamentous bacteriophages (re-phage) with a cell adhesive peptide (RGD) were immobilized onto Al2O3 NWs by a simple dip-coating process for improvement of cell binding. This re-phage-coated material allowed a strong EC–nanostructure interaction, with increased cell population and viability, in comparison with Al2O3 NWs.115 A novel superhydrophobic hybrid coating that couples the effect of the topography of Al2O3 NWs and the low surface energy of poly (bis (2,2,2-trifluoroethoxy) phosphazene) (PTFEP) was developed by chemical vapor deposition (CVD) method and ultrasonic infiltration technique, for improved hemocompatibility of cardiovascular implants. The dual-scale surface roughness (micro/nano) and the superhydrophobic nature of the nanowired substrate reduced the contact area between the surface and blood, yielding a non-wetting surface that prevented platelet adhesion and activation. This reduced contact area and non-wetting nature imparted significant blood repellence to the surface.116
The impact of surface roughness in modulating EC response has also been investigated by various groups, especially on Ti surfaces. It is generally noted that ECs interact more efficiently on nanometer rough surfaces than on flat surfaces, with enhanced adhesion, proliferation, and migration. Nanoscale surface roughness on Ti enabled better and well-adherent endothelium under flow conditions as well.117 Such nanotexturing approaches have also been translated to metallic alloys and polymers. Commercially pure titanium, titanium alloy (Ti6Al4V), and polymers used to incorporate drugs in DES (e.g., polyethylene terephthalate, polytetrafluoroethylene, polyvinyl chloride, polyurethane, and nylon) were modified using an ionic plasma deposition and nitrogen ion implantation plasma deposition process to generate nanorough surface features. It was demonstrated that changes in surface chemistry and roughness at the nanoscale resulted in improved adhesion of ECs.118
Thus, results from literature point to the fact that nanostructured surfaces, irrespective of the method used or its surface chemistry, are able to modulate vascular cell response preferentially, with specific nanotopographies favoring endothelial cell adhesion and proliferation over smooth muscle cells. Various studies cited in this review,103–105 especially the titania nanotubes,80–83 have shown a selective response to the vascular cells, specifically ECs and SMCs. The exact reason for this preferential response still remains unclear and requires further investigation.
Patterned nanostructures
Femtosecond laser irradiation can produce periodic nanostructures on metals and semiconductors.119–121 Hierarchical micro/nanostructures possessing properties of dual-scale roughness were fabricated on Ni–Ti using a femtosecond laser for the surface modification of stents. Hydrophilic periodic nano- and hydrophobic micro/nanostructures formed could regulate the spreading of ECs, with more effect observed at the nanometer scale. Moreover, platelets failed to adhere to the micro/nanostructures.122 Utilizing femtosecond laser, micro-/nanobiomimetic surface patterns mimicking the morphology of VSMCs were generated on 316L SS stents. In vitro studies showed that this VSMC-biomimetic surface pattern of width ∼700 nm promoted adhesion, proliferation, and migration of ECs, with rapid re-endothelialization in vivo after 30 days.123 Ti patterning formed by plasma-based dry etching technique with a width and spacing varying from 750 nm to several micrometers presented significantly improved function and orientation, higher density, viability, and proliferation of rat aortic ECs compared to substrates with micro and random nanofeatures.111 Patterned TiO2 nanogratings as small as 70 nm significantly inhibited the proliferation of SMCs and concurrently enhanced EC proliferation. ECs could sense these nanogratings, yielding elongated morphologies with a larger number of focal adhesions on the patterned surfaces.124 Large-scale nanopatterns were developed on NiTi stents using target-ion-induced plasma sputtering (TIPS) onto which the PTFE layer was coated, resulting in a nanoporous surface having diameters ranging from 100 to 200 nm and depths of 600 nm, with infiltration of PTFE into nanoscale pores.125 Tantalum coating was provided on the same nanoroughened NiTi stents to generate distinct and uniform nanoscale surface structures (50–100 nm), as a means to reduce Ni ion release from the base material. These Ta-coated NiTi stents significantly improved EC attachment, proliferation, density, and coverage, in comparison with bare stents that had a considerable decrease in EC proliferation due to rapid dissolution of Ni ions.126 A similar process was used to develop a Ta-implanted nanoridge surface with a ripple-like surface pattern having round hills and steep valleys (depth of ∼470 nm) on CC stents. ECs that adhered on this stent surface formed numerous inter-endothelial adherent junctions through cell membrane protrusions, with faster migration rates and proliferation, besides exhibiting minimal platelet activation and fibrin formation. Synergistic effects of Ta and nanoscale surface features of this stent in rabbit iliac artery model resulted in very minimal intimal hyperplasia and lumen loss, with rapid re-endothelialization.127 Similarly, zirconium (Zr) ion implantation using metal vapor vacuum arc plasma source with pure Zr as the target material resulted in a nanopatterned Zr–NiTi alloy. Further, a thick Ni-depleted composite ZrO2/TiO2 nanofilm was developed on the surface of zirconium–NiTi (Zr–NiTi). Corrosion resistance was increased, depletion of Ni in the superficial surface layer resulted in reduced ion release rate of Zr–NiTi, and EC proliferation was favored after five and seven days of culture.128
Nanoporous architecture on stents for drug/biologics loading
In addition to the impact of nanotexturing on cellular response in vitro and in vivo, researchers have investigated the combined effects of nanotexturing with biologics/drug incorporation. Mostly, the nanotextures present on metallic surfaces are porous and these can be efficient sites for high drug loading.41 This polymer-free approach can be a viable strategy to circumvent the risks of using polymers as drug-eluting stent coatings.129,130 In one such study, an anti-CD146 antibody anchored onto a porous architecture bearing nanosized silicone filaments on CC stent surface helped to develop an endothelial progenitor cell (EPC) capturing stent. This stent enabled enhanced selective capture and adherence of circulating EPCs from blood and thereby induced rapid healing of endothelium at 1-week implantation in porcine, resulting in reduced neointimal thickening. Thus, the co-existence of the silicone nanofilaments and CD146 antibody provided synergistic effects for suppression of in-stent restenosis by promoting re-endothelialization.131 A nanoporous Al2O3 nanocoating (∼200 nm thick) on NiTi alloy substrate deposited via simple sputtering, followed by functionalization with VEGF, helped to significantly enhance EC adhesion, spreading, and proliferation. Additionally, higher levels of NO and prostaglandin (PGI2) secretion on the nanocoating indicated its advantage on EC functionality.132 Similarly, a ceramic stent coating of nanoporous alumina on SS stents served as a suitable carrier for the drug tacrolimus. These drug-coated nanoporous stents showed inhibition of neointimal proliferation in rabbits.133 However, after implantation in a porcine model, particle debris resulting from the cracking of ceramic coating during stent expansion resulted in increased neointimal growth and stenosis in these stents.134 A polymer-free sirolimus-eluting stent (PFSES) with a unique nanoporous surface was developed by adopting a simple electrochemical method to generate nanosized pores (∼400 nm) on the surface of SS stents. These stents when implanted in pigs showed low levels of neointima and inflammation than BMS, 3 months post-implantation.135 The same polymer-free nanoporous stent was loaded with the drug paclitaxel, revealing a significant reduction in neointimal hyperplasia and better endothelialization than polymer-based SES.136 This nanoporous stent, in another study, was spray-coated with sirolimus drug on the abluminal surface and immobilized with anti-CD34 antibodies on the blood-contacting luminal surface. This polymer-free stent completely re-endothelialized in 2 weeks with minimal restenosis in vivo.137 These nanoporous stents with anti-CD34 antibody immobilization alone facilitated effective capture of CD34+ ECs, with significantly high endothelialization.138 The same clinically tested platform as above, but containing CREG (a markedly upregulated gene during SMC differentiation) showed a similar degree of inhibition of SMCs as that of the drug sirolimus. However, EC proliferation was improved by CREG, in contrast to sirolimus which inhibits ECs. This CREG eluting stent attenuated neointimal formation with accelerated re-endothelialization after 4 weeks in porcine.139 BICARE is a novel version of the above nanoporous PFSES which elutes dual drugs (rapamycin and probucol). To assess the safety and efficacy of this PFSES-based dual drug delivery system (DDES), nanoporous SS stents loaded with probucol and rapamycin in combination were implanted in a porcine coronary artery. This DDES was found to be as safe as the commercial BMS and SES, but did not show any enhancement of re-endothelialization in porcine arteries.140
Nano-thin-films and their combination with drugs/biologics
Apart from the nanostructured topography generated on metallic surfaces, deposition of thin films on stents/substrates has also been widely examined. The concept of utilizing stent coatings was initially introduced as a means to mask the underlying stent surface, to prevent ion leaching from bare-metal stent surface into the bloodstream.41 Coating a stent with a thin film of biocompatible surface can improve blood compatibility, vascular cell response as well as the corrosion potential of the implant.4,141 These coatings can act as an inert barrier between the blood/tissue and metal with good biocompatibility.142,143 Deposition of thin films is a common and effective technique in surface engineering. Methods for thin-film deposition can be either physical or chemical, based on the nature of the deposition process. Chemical methods such as chemical vapor deposition (CVD), atomic layer deposition (ALD) and sol-gel involves gas- or liquid-phase chemical reactions, whereas physical methods involves sputter deposition, evaporation, and spraying.144,145 Such coatings can be based on polymers, inorganics, or other biocompatible materials.146
Titanium-oxide and titanium-oxy-nitride nano-thin-film coatings on stents
Titanium oxide-based coatings are the most promising coatings for cardiovascular stent applications among all inorganic materials, offering good blood compatibility, which is attributable to its surface energy and semiconducting behavior.147,148 The addition of nitrogen to TiO2 films has shown remarkable improvements in its blood compatibility due to the presence of nitride oxide on the surface.149 Adhesion of platelets and fibrinogen deposition were minimal for titanium–nitrideoxide (TiNOx) coatings in comparison with titanium oxide.150,151 TiNOx coating of thickness 500 nm was generated on metallic stents by reactive physical vapor deposition (PVD). The number of ECs on titanium oxide and titanium nitride was higher in comparison with control SS and NiTi substrates.152 Preclinical testing of these TiNOx-coated stents in a porcine model showed significantly less neointimal hyperplasia than SS stents at 6-weeks.149 The promising results from this study facilitated its transition to the clinical trial stage. Likewise, oxides of titanium, viz., Ti–O and TiO2, have been extensively investigated as stent coatings. A 25-nm-thick Ti–O film modified CC vascular stent developed via magnetron sputtering exhibited a faster rate of endothelialization without any thrombus, in-stent stenosis, or inflammatory reaction in vivo.153 Similarly, TiO2 thin-film layers consisting of embedded nanoscale TiO2 particles deposited on electro-polished SS using sol-gel dip coating showed enhanced blood compatibility in vitro, with longer blood clotting times and lesser platelet adhesion.154 Two types of titanium-based coatings having different thicknesses of 300 and 500 nm, respectively, deposited on 316L SS by a sol-gel route, displayed increased proliferation rates of ECs and also induced prolonged plasma recalcification time. In contrast, response to SMCs was not significantly altered by this chemistry155 and proved no superiority to BMS in vivo.156 In another study, a 100-nm-thick TiO2 film having a surface roughness of 35 nm deposited on magnesium–zinc (Mg–Zn) bioresorbable vascular scaffold helped to improve endothelial cell spreading with a good cytoskeletal arrangement. Moreover, the protective TiO2 layer had the potential to reduce the degradation rate of bare Mg–Zn alloy and retain its functionality.157 Likewise, Cu-doped TiO2 nanofilms containing Cu ion crystals sized 10 nm were deposited on wires through sol-gel method, wherein Cu behaved as a redox co-catalyst and promoted NO release. In vitro studies revealed a significant reduction of fibrinogen adsorption and platelet coverage, along with superior antithrombotic properties and anti-inflammatory ability. Reduction in neointimal thickening and suppression of inflammation, along with re-endothelialization, were noted in vivo within 4 weeks.158
Metallic coronary stents based on CC coated with TiO2 by a plasma-enhanced chemical vapor deposition (PECVD) process and loaded with drugs/biologics have also been tested for their ability to improve in vivo response after implantation in animal models. TiO2 films having a surface roughness of ∼10 nm and chemically grafted with heparin were found to reduce neointima formation with decreased inflammation and fibrin deposition.159 A similar study by the same group also investigated the effect of polymer-free TiO2 film-coated stent with abciximab or alpha-lipoic acid (ALA) in vivo. Both the thin-film-coated stents showed an effective reduction of in-stent restenosis and accelerated re-endothelialization.160 A similar TiO2 coating on stents, but having a dual-delivery system of abciximab (drug) and Kruppel-like factor 4 (KLF4)-plasmid (gene), showed analogous results.161 Yet another platform developed by this group for coating drugs like tacrolimus or everolimus on stents was nitrogen-doped TiO2 (N–TiO2) thin films. Drug coating was provided on the abluminal surface by electrospraying/dip coating, while N–TiO2 films were deposited by PECVD. The drug coating proved to be effective in reducing inflammation and in-stent restenosis, while the titania layer helped in increased re-endothelialization and reduced thrombosis in vivo (Fig. 4). The study yielded results that proved the non-inferiority of their stent to commercial controls.162,163 A 200-nm-thick nano-TiO2 ceramic film was deposited by radio frequency magnetron sputtering along with EC selective adhesion peptide Arg-Glu-Asp-Val (REDV) coating (polydopamine-coating technology) on 316L SS stents, which efficiently reduced nickel ion release from SS, and also promoted EC adhesion and proliferation with increased NO release. REDV/TiO2-coated stents when implanted in rabbit iliac arteries effectively reduced in-stent restenosis and promoted re-endothelialization as compared to TiO2-coated rapamycin-eluting stent and BMS.164
FIG. 4.
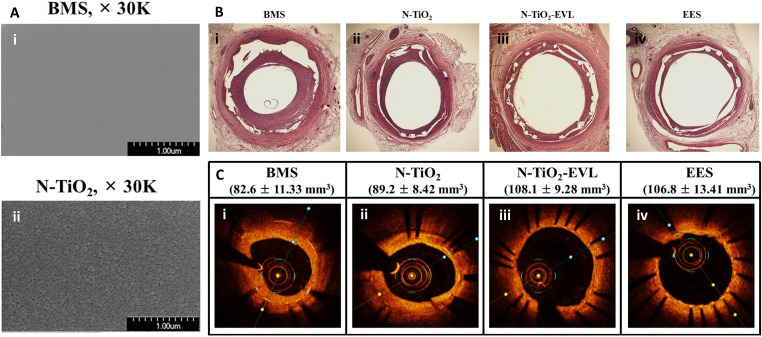
(a) SEM images of (i) BMS and (ii) N–TiO2 film deposited on a bare stent. (b) Histopathological H&E staining and (c) optical coherence tomography of porcine coronary arteries implanted with (i) BMS, (ii) N–TiO2, (iii) N–TiO2 with everolimus, and (iv) EES for 4 weeks. The restenosis area was significantly decreased in the N–TiO2-everolimus group compared to that in the BMS group and was at par with the commercial EES. Reprinted with permission from Park et al., Mater. Sci. Eng.: C 91, 615–623 (2018). Copyright 2018 Elsevier. (BMS: bare-metal stent; N–TiO2: nitrogen-doped TiO2 film; EES: everolimus-eluting stent.)
Carbon-based nano–thin-film stent coatings
Diamond-like carbon (DLC) thin films have also been investigated in earlier days as a stent coating because of their outstanding mechanical characteristics, and specifically the ability to reduce platelet activation and thrombus formation. DLC films of thickness 50 nm were deposited on CC stents using the PECVD method with excellent stability and crack resistance. Plasma irradiation of these films resulted in increased functional groups on its surface, making it possible to graft polymer with drugs to develop a DES that can continuously and slowly elute drugs.165,166 Biocompatible Si-doped DLC films deposited on Ti6Al7Nb alloy using magnetron sputtering exhibited a positive effect on the proliferation and viability of ECs.167 Nanothin DLC films deposited by physical vapor deposition (PVD) on CC stents had a nanostructured surface with well acceptable hemocompatibility and anti-inflammatory properties. These stents showed effective inhibition of fibrin deposition and thrombus activation, along with an early and complete endothelial healing (30 days) and decreased neointimal proliferation at 180 days in pigs.168 Neointimal hyperplasia was significantly lower for DLC-coated nitinol stents after implantation in a canine iliac artery model.169 Another class of diamond-like nanocomposite (DLN) stent coating showed reduced thrombogenicity and minimal neointimal hyperplasia in pigs. This coating when covered with another DLC showed enhanced inflammatory reaction without any added advantage, compared to single-layer DLN coating.170 Another fundamental study presented strong evidence for the influence of various material and processing parameters such as surface chemistry, nanotopography, and hydrophilicity, in mediating platelet adhesion as well as cell compatibility. Three different surface chemistries provided by amorphous hydrogenated carbon (C:H), Carbon Nanotube (CNT)/DLC, and titanium boron nitride (TiBxNy) thin films of ∼100 nm thickness, deposited by magnetron sputtering technique were compared. While the hydrophilicity and nanoscale roughness of C:H and TiBxNy films favorably influenced platelet behavior, the high roughness of CNT bundles and its hydrophobicity made the surface less thromboprotective.171 In another study, topography, stoichiometry, and surface properties were studied as the key parameters that regulate the thrombogenicity of a-C:H and titanium nitride (TiNx) nanocoatings developed by magnetron sputter deposition. By appropriate tuning of the deposition parameters (ion bombardment and content of hydrogen/nitrogen in plasma), the thrombogenic behavior of these nanocoatings could be tailored.172
Other inorganic coatings
As a biocompatible inert ceramic coating material for stents, iridium oxide has also been studied. The effect of the oxidation state of IrxTi1−x-oxide coatings formed on Ti substrate by thermal method showed a significant reduction in platelet adhesion and activation, rendering the surface blood compatible. Moreover, a substantial improvement in the ratio of EC/SMC count was also observed.173 Similarly, the antithrombogenic properties of amorphous silicon carbide (SiC) stent coating developed by chemical vapor deposition process could reduce the adhesion of platelets, leukocytes, and monocytes on the stent surface.174
Plasma coating using various materials has also been explored on coronary stents. Trimethylsilane (TMS) plasma coating of thickness 20–25 nm was formed on 316L SS coronary stents by direct current and radio frequency glow discharges, followed by an additional NH3/O2 plasma treatment. TMS plasma coatings imparted superior corrosion resistance to SS stents, thus hindering metallic ion release into the bloodstream.175 These coatings possessed good long-term chemical stability and displayed improved proliferation of ECs.176 SiCOH plasma nanocoatings of thickness 30–40 nm were also used to modify the surface of stents by low‐temperature plasma formed by a gas mixture of TMS and oxygen. This nanocoating showed excellent hemo‐ and cytocompatibility in vitro.177
Alumina coatings on stents have also been probed for their ability to impart antithrombogenicity and also as an inert layer that can inhibit metal ion leaching. Stents coated with sub-30 nm thick Al2O3 using plasma-enhanced atomic layer deposition (ALD) displayed improved antithrombogenicity, without altering its mechanical properties.178 Likewise, alumina coatings of thickness 10–20 nm deposited on NiTi by ALD technique could effectively better the corrosion resistance of NiTi, and inhibit the release of Ni atoms, thus reducing the serious problems of nickel allergic reactions.179
Another coating that has been investigated on clinical stents is the bioceramic hydroxyapatite, which has also been utilized as a substrate for drug loading. Porous nanothin hydroxyapatite coatings on stents were found to be stable for more than 4 months, with an in vivo anticipated lifetime between 9 months and 1 year for the coating, during which time the loaded drug would get released completely. A similar coating on CC stents aided the elution of low doses of sirolimus, which inhibited platelet adhesion and activation in vitro.180
Nanothin polyphosphazene polymer-based coating
Similar to the inorganic coatings on stents, polymeric coatings of nanothickness have also been extensively investigated. Specifically, polyzene-F (PzF) surface coating has gained immense attention because of the multifarious characteristics of this polymer, which include its biocompatibility, anti-inflammatory, and inherent thromboresistance.181,182 These properties can potentially help to overcome the deficits of the current clinically used DES, in patients having bleeding risk. Extensive preclinical studies including vascular cell response and platelet adhesion, followed by in vivo testing in various animal models were carried out on CC stents with a nanoscale PzF coating of ∼50 nm thickness. This nanocoating unfailingly exhibited minimal platelet adhesion and clotting, reduced inflammation, and accelerated endothelial healing. PzF stents have also shown significantly lower neointimal thickening, reduced thrombogenicity,183 and rapid healing, with complete re-endothelialization in rabbits in 1 week itself.184 Similar were the results when implanted in a pig coronary artery as well.185,186 This coating showed superior endothelial coverage than commercially available DES. This polymer coating, as a result of its excellent preclinical response, has found its place in clinical trials.
Thus, the vast literature on nanostent coatings cited above clearly underlines the impact of nanotechnology in offering the primary requisite of an ideal coronary stent, viz., its in vivo biological response. Table I lists the diverse nanoarchitectures and coatings developed on clinical stents and their in vivo outcomes.
TABLE I.
Diverse nanostructures and thin-film coatings developed on stents which have been tested in various animal models.
| Type of surface on stents | Description | Development technique | Drug/biologics | Animal model | Results | References |
|---|---|---|---|---|---|---|
| Nanotubular structures | Titanium dioxide nanotubes (Ti stent) | Anodization | ⋯ | In vivo rabbit iliac artery | Enhanced endothelialization and minimal in-stent restenosis | 89 |
| Nanostructures | Titanium dioxide nanoleaves (SS stent) | TiO2 sputter deposition followed by hydrothermal | ⋯ | In vivo rabbit iliac artery | Reduction of neointima and complete endothelialization | 102 |
| Nanoflaky MgF2 film (Mg–Nd–Zn–Zr stent) | Chemical conversion treatment | ⋯ | In vivo rabbit abdominal aorta | Complete endothelial lining with minimal thrombogenicity and restenosis | 110 | |
| VSMC biomimetic patterns (SS stent) | Femtosecond laser processing | ⋯ | In vivo rabbit iliac artery | Rapid re-endothelialization in thirty days | 121 | |
| Ta implanted nanoridges (CC stent) | Target-ion-induced plasma sputtering | ⋯ | In vivo rabbit iliac artery | Minimal neointimal hyperplasia and rapid re-endothelialization | 125 | |
| Nanosized silicone filament (CC stent) | Anti-CD164 antibody | In vivo porcine coronary artery | Improved selective EPC capture resulting in rapid endothelial healing in 1 week | 129 | ||
| Nanoporous alumina (SS stent) | Physical vapor deposition of aluminum followed by electrochemical conversion | Tacrolimus | In vivo rabbit iliac artery | Inhibited neointimal proliferation | 131 | |
| In vivo porcine coronary artery | Particle debris resulting from the cracking of ceramic coating during stent expansion resulted in increased neointimal growth and stenosis | 132 | ||||
| Nanoporous structures (SS stent) (Lepu Medical Technologies, China) | Electrochemical method to generate pores | Sirolimus and anti-CD34 antibody/anti-CD34 alone | In vivo porcine coronary artery | Endothelialization in 2 weeks with minimal restenosis | 135, 136 | |
| CREG gene | Accelerated endothelium in 4 weeks | 137 | ||||
| Rapamycin and probucol | As safe as BMS and SES without any significant enhancement in re-endothelialization | 138 | ||||
| Nano-thin-film coatings | Titanium nitride coating (SS stent) | Reactive physical vapor deposition | ⋯ | In vivo porcine coronary artery | Reduced neointimal hyperplasia | 147 |
| Ti–O film (CC stent) | Magnetron sputter deposition | ⋯ | In vivo rabbit abdominal aorta | Faster rate of endothelialization | 151 | |
| Titanium nano-thin-film coating (SS stent) | Sol-gel processing | ⋯ | In vivo porcine coronary artery | Non-inferior to BMS | 154 | |
| Copper-doped TiO2 nanofilms (Ti wire) | Sol-gel spin-coating | ⋯ | In vivo Rat abdominal aorta | Reduced neointimal hyperplasia and re-endothelialization in 4 weeks | 156 | |
| TiO2 thin films (CC stent) | Plasma-enhanced chemical vapor deposition | Heparin | In vivo porcine coronary artery | Reduced neointima, inflammation and fibrin deposition | 157 | |
| Abciximab/alpha lipoic acid | Effective reduction of in-stent restenosis and accelerated re-endothelialization | 158 | ||||
| Abciximab and Kruppel-like factor 4 gene | Reduced neointimal thickening and faster endothelialization | 159 | ||||
| Nitrogen-doped TiO2 thin films | Plasma-enhanced chemical vapor deposition | Tacrolimus | In vivo porcine coronary artery | Reduced in-stent restenosis and increased endothelial formation | 160 | |
| Everolimus | Decreased neointimal thickening and thrombosis with faster healing | 161 | ||||
| Nanothin TiO2 film (SS stent) | Radio frequency magnetron sputtering | REDV peptide | In vivo rabbit iliac artery | Reduced in-stent restenosis and promoted re-endothelialization | 162 | |
| Nanothin DLC (CC stent) | Physical vapor deposition | ⋯ | In vivo porcine coronary artery | Early and complete endothelial healing in 30 days and decreased neointimal proliferation at 180 days | 166 | |
| Nanothin DLC (NiTi stent) | Physical vapor deposition | ⋯ | In vivo canine iliac artery model | Significantly lower neointimal hyperplasia | 167 | |
| Nanothin polyzene F coating (CC stent) | Deposited from a solution and subsequently dried | ⋯ | In vivo rabbit iliac artery | Rapid healing in 1 week | 182 | |
| In vivo porcine coronary artery | Complete endothelial coverage and reduced neointimal hyperplasia and inflammation | 183, 184 |
Nanofibrous systems
Nanofibers (NF) have unique advantages as coating materials for cardiovascular stents on account of their nanoscale diameter, tunable surface morphology, flexibility, porosity, and higher length/diameter ratio. The large surface area to volume ratio of NFs permits them to be good platforms for incorporation of drugs/biologics with high drug loading.187 Nanofibrous matrix covered biomedical implants thus facilitate localized drug-eluting platforms, which provide sustained release of different kinds of drugs (anti-inflammatory, antithrombotic, antirestenotic) for prolonged durations at required doses188 and protect the vessel wall from direct metal contact.189 This can in turn help to reduce late stent thrombosis and restenosis risks, akin to the commercial DES. More predictable laminar flow also results from nanofiber-coated stents, thus reducing the probability of restenosis,190,191 but there is experimental and clinical evidence demonstrating that the covering of BMS with drug eluting polymers results in increased stent stiffness and thus modifies the mechanical properties of the stent platform,192 which needs to be addressed.
Fibrous polymeric nanoplatforms as coatings on BMS are commonly fabricated through a simple and cost-effective technique of electrospinning.193,194 This is a voltage-driven fiber production process, which uses electric force to draw charged threads of polymer solutions up to fiber diameters in the order of hundreds of nanometers.45,195,196 Diverse biodegradable polymeric materials [e.g., poly-L-lactic acid (PLA), poly(caprolactone) (PCL), poly-lactide-co-glycolide (PLGA), chitosan (CS)], and drugs have been studied as candidates for developing nanofiber-coated stents. For example, chitosan/β-cyclodextrin nanofibers loaded with simvastatin, a drug commonly used for restenosis prevention, was developed by electrospinning to cover a self-expandable NiTi stent. Drug release time was extended by altering the duration of electrospinning and blending with cyclodextrin in the NF matrix. A dose-dependent effect on vascular cells was observed using these NF-coated stents, with EC viability affected less than SMC in the presence of the drug.197 The same technique was extended to polymeric stents of PLA, wherein the stents were coated with NFs of PLA/chitosan, eluting the drug paclitaxel at different concentrations. This NF-coated stent offered controlled drug release in vitro and displayed effective cytotoxicity to normal fibroblast cells in culture.198 Chitosan/PLGA-PLA nanofibers incorporating β-estradiol in Eudragit nanoparticles were developed as electrospun coatings on metallic stents, which provided high endothelial proliferation rate and enhanced NO production. Moreover, these NFs also alleviated reactive oxygen species induced toxicity to ECs in vitro.190 Another stent coating studied is the nanofibrous matrix made from a blend of PCL, human serum albumin, and paclitaxel having a coating thickness of 150–180 μm and fiber diameter of ∼500 nm. After implantation in the rabbit iliac artery, these stents were less traumatic and induced weaker neointimal growth over 6 months, with increased blood flow as against that of BMS.199 Nanoscale cellulose acetate fibers containing rosuvastatin and heparin were developed and loaded on three different commercially available stents (SS, CC, and NiTi). This hybrid DES provided local and sustained delivery of high concentrations of the two drugs for 4 weeks, presenting a novel therapeutic method for patients who have a high risk of stent thrombosis, with minimal systemic side effects.200 Propylthiouracil (PTU), an antithyroid drug proven to suppress neointimal formation, was incorporated within biodegradable PLGA nanofibers with fiber diameter ranging from 112 to 622 nm and coated on BMS. These stents showed improved EC proliferation and migration, with increased NO production and eNOS activation, along with reduced platelet adhesion. A sustained release of PTU for 3 weeks, with a marked reduction in neointima formation and enhanced re-endothelialization, was observed in the injured aorta in rabbits.201 A blood-compatible nanofiber scaffold was developed using mesoporous silica nanoparticle (MSN) embedded PCL/gelatin electrospun nanofibers, for controlled dual delivery of 2-O-d-Glucopyranosyl-l-Ascorbic Acid (AA-2G) and heparin. Controlled release of AA-2G prevented initial oxidation and inflammation of blood cells, and the simultaneous release of heparin rendered long-term antithrombotic potential in vitro. Subcutaneous implantation in rats proved its biocompatibility and resistance to inflammation and thrombosis.202 Controlled and localized delivery of dipyridamole (DIP), a platelet aggregation inhibitor, using electrospun PCL scaffold has also been proposed as a stent coating. The released DIP accumulated in ECs without causing cytotoxicity, but inhibited EC proliferation in vitro, while concurrently increasing the gap junction coupling of ECs, which is a primary requirement in maintaining normal vascular physiology.203 In another study, PLA nanofibrous scaffolds consisting of DIP were developed by electrospinning as a cytocompatible coating for Co/Ni stents. Pharmacokinetics of the PLA/DIP nanofibers showed an initial burst, followed by a slow and controlled release for 7 months.204 Sustained and localized delivery of ticagrelor for about 4 weeks was achieved in vivo via the use of electrospun PLGA nanofiber coating on Gazelle stents. These stents reduced neointimal formation and favored endothelial recovery with lesser vasoconstrictor response and improved NO-mediated vasorelaxation after implantation in rabbit abdominal aorta as shown in Fig. 5.205 Likewise, PLGA nanofiber membrane-coated bare-metal SS stents were developed as a local and sustained delivery depot for acetylsalicylic acid. The electrospun PLGA/acetylsalicylic acid nanofibers had a diameter ranging from 50 nm to 8 μm. This hybrid stent was highly effective as inhibitors of platelet and monocytes and promoted re-endothelialization in rabbit denuded artery. NFs induced very minimal inflammatory reaction and were completely absorbed in 4 weeks.206 Nanofibrous coatings of antihyperglycemic drug vildagliptin have been developed on Gazelle stents as a strategy to treat diabetic vascular disease. Stents with vildagliptin loaded PLGA nanofibers showed effective drug delivery for more than 28 days in the abdominal aorta of diabetic rabbits. This stent accelerated the revival of diabetic endothelia and also decreased neointimal hyperplasia and type I collagen content in the vascular intima than that of the non-vildagliptin-eluting group.207 Poly-L-lactide films cut and rolled into a cable-tie type stent were coated with rapamycin-eluting biodegradable nanofibers, which exhibited excellent mechanical properties and delivered high drug concentrations for over 4 weeks. Moreover, these stents showed a substantial reduction in intimal hyperplasia in denuded rabbit arteries during 6 months follow-up.208
FIG. 5.
(a) Bare-metal stent with a nanofibrous membrane coating. (b) SEM micrographs of the electrospun nanofibrous membrane with ticagrelor. Magnification of ×3000. (c) SEM images of platelets on electrospun ticagrelor eluting membrane. Red arrow indicates activated platelets (scale bars = 10 μm). Magnification 3000×. (d) Hematoxylin–eosin stained section of arterial lesions in ticagrelor group exhibiting a complete lining of endothelial cells (red arrows). (e) Pathological arterial lesions in the ticagrelor group stained using HES5 markers at 4 weeks following stent implantation. The amount of formed neointima suggests less proliferation of SMCs in the media. (f) SEM images of the stented vessel showing complete endothelial coverage in the ticagrelor group. Reprinted with permission from Lee et al., Int. J. Nanomed. 13, 6039–6048 (2018). Copyright 2018 Dove Medical Press.
Drug-loaded coaxial nanofibers have also been tried as stent coatings. A drug-eluting stent coated with PCL/PU blend coaxial nanofibers containing the drug paclitaxel (PTX) in PCL, with PCL as the core and PU blended PCL as the shell, has been studied for controlled drug release. PCL/PU nanofibers containing PTX inhibited the proliferation of SMCs in vitro.209 Likewise, paclitaxel/chitosan (PTX/CS) core-shell NFs have been developed on Ni-Ti stents through the co-assembly of paclitaxel and chitosan, with very high drug loading. This nanofibrous coating displayed better EC viability in vitro than the drug alone, due to the presence of CS outside the NFs, which prevented direct cell contact with the drug.210 Using a similar coaxial spinning, polyvinyl alcohol (PVA)/gelatin nanofibers (with gelatin in the shell and PVA in the core of each nanofiber) were prepared as possible stent coating. This nanofibrous scaffold possessed the requisite biocompatibility (due to gelatin), mechanical strength, and stiffness (from PVA). It promoted EC migration and proliferation, with concurrent SMC inhibition.211 The same group also looked into the correlation between mechanical properties and hemocompatibility of this scaffold. It was observed that the increased stiffness of coaxial nanofibers resulted in higher rates of platelet activation and thrombin formation, in comparison with individual gelatin and PVA fibers, implying that mechanical stiffness and surface roughness are dominant factors that control platelet activity.212
Similar to drug-loaded stents, biological molecules have also been utilized for preparing nanofibrous coatings on stent materials. In one such study, a native endothelium extracellular matrix (ECM) mimicking self-assembled peptide amphiphile (PA) nanofibrous coating functionalized with fibronectin-derived EC-specific adhesion molecule, REDV, and mussel-adhesive protein inspired Dopa residue was formed on SS surfaces. REDV–PA was designed to enhance endothelial cell-specific activity and Dopa for immobilizing REDV-conjugated nanofibers on the stent surface. In vitro studies proved increased adhesion, spreading, and long-term viability of ECs, with significantly lower viability of SMCs.213 A similar endothelial ECM mimicking nanofibrous matrix was fabricated by self-assembly of PAs containing NO donating residues, EC adhesive YIGSR peptide ligands, and enzyme-mediated degradable sites (MMP-2). A rapid release, followed by a sustained release of NO from the nanofibrous matrix for over 30 days, promoted increased EC adhesion, proliferation and concurrently limited SMC proliferation, with a 150-fold reduction in platelet attachment.214 Similarly, functional peptide sequences containing enzyme-mediated degradable sites combined with either endothelial cell-adhesive ligands (YIGSR) or polylysine (KKKKK) nitric oxide (NO) donors were attached to the self-assembled PAs. Linkages of two different PAs (PA–YIGSR and PA–KKKKK) to pure NO helped to develop PA–YK–NO, which was self-assembled onto electrospun PCL nanofibers to fabricate a hybrid nanomatrix. NO release could trigger significant EC activity and suppress SMC and platelet adhesion, similar to the previous study.215 The same group could demonstrate mitigation of inflammation due to this NO release from PA-YK-NO stent coatings under static and dynamic physiological flow conditions in vitro.216 Table II summarizes those studies that have progressed to the in vivo (small animal) stage.
TABLE II.
Bare-metal stents coated with electrospun nanofibers tested in vivo.
| Type of coating | Description | Active agent | Animal model | Results | References |
|---|---|---|---|---|---|
| Electrospun nanofibrous coatings | PCL and human serum albumin | Paclitaxel | In vivo rabbit iliac artery | Induced weaker neointimal growth over 6 months | 197 |
| PLGA nanofibers on BMS | Propylthiouracil | In vivo rabbit injured aorta | Reduced neointimal hyperplasia and enhanced re-endothelialization | 199 | |
| PLGA nanofibers on BMS | Ticagrelor | In vivo rabbit abdominal aorta | Minimal neointimal formation and favored endothelial recovery | 203 | |
| PLGA nanofibers on SS stents | Acetylsalicylic acid | In vivo rabbit denuded artery | Promoted re-endothelialization | 204 | |
| PLGA nanofibers on BMS | Vildagliptin | In vivo rabbit abdominal aorta | Accelerated endothelial recovery and decreased SMC hyperplasia | 205 | |
| poly-L-lactic acid (PLLA) cable tie type stents | Rapamycin | In vivo rabbit denuded artery | Reduced neointimal hyperplasia | 206 |
Despite the significant efforts undertaken on nanofiber-coated stents demonstrating controlled drug/biologics release and synchronized cell response in vitro, research in this direction has not progressed further to the preclinical (large animal) or clinical stages. The stability and durability of these nanofibrous coatings upon stent expansion and crimping are important aspects that need to be assessed before proposing it for clinical translation.
Nanoparticulate systems
Nanoparticulate drug delivery systems are bestowed with significant benefits that can be readily capitalized in the cardiovascular field.217 This includes the possibility of target-specific drug delivery, enhanced intracellular uptake and high bioavailability, reduced drug dosage with less toxicity in tandem, tunable, and sustained drug release, etc.218,219 Nanoparticles (NP) loaded with drugs, when incorporated onto stent platforms, facilitate improved release kinetics and also promote a spatiotemporal delivery at the site of intervention.220 Nanoparticle encapsulation may also allow higher arterial wall concentrations and residence times than traditional drugs, two important factors for the prevention of restenosis.49,219 Additionally, drug stability is improved when loaded within a NP and an effective drug release from stents into the abluminal wall can be attained. This in turn enhances the intracellular uptake and local bioavailability of the drug at the stented site.49,221 After localized delivery from the stent surface, nanocarriers can infiltrate the vessel wall and form a depot, which offers a locally limited and sustained drug release into the arterial wall,222 thereby preventing in-stent restenosis. Local delivery of drug loaded nanoparticles, combined with antibody targeting strategies, can also permit high concentration, sustained drug therapy, which is required to prevent restenosis.46,223
Numerous studies have focused on enhancing localized drug delivery within arteries using nanoparticle formulations on stent platforms. Most commonly used polymers as stent coating include the bioabsorbable polymeric matrices of poly-D, L-lactic Acid (PDLLA), PLGA, PLA, PCL, etc., due to their excellent in vivo-biocompatibility.224 In one such study, sirolimus-loaded PDLLA nanoparticles exhibited good cellular and interstitial uptake as well as sufficient drug loading and revealed biphasic release kinetics with a short burst followed by a longer, slower release phase in vitro. However, these drug-loaded nanoparticles inhibited viability and proliferation of both EC and SMCs, although the nanodrug was less toxic to ECs compared to the free drug.225 A similar study in which bioresorbable PLLA stent surface was grafted with polyethylene vinyl acetate (PEVA) and PVP by plasma polymerization followed by coating with PDLLA nanoparticles carrying sirolimus displayed pronounced inhibition effect on SMCs than on ECs.226 Antirestenosis drugs, dexamethasone/rapamycin-loaded nanoparticles based on poly(ethylene oxide) and PLGA block copolymers, demonstrated a rapid burst release. This fast release kinetics was tuned by conjugating these nanoparticles with gelatin or albumin, which yielded a sustained release of dexamethasone and rapamycin for 17 and 50 days, respectively, after gelatin treatment.227 To address the problem of late stent thrombosis, an antiplatelet drug dipyridamole was loaded within PLA NPs, which showed a sustained drug release over 1 month in vitro.228 Stents coated with PLGA/chitosan nanoparticles containing a fluorescence marker (FITC) after 4 weeks of implantation in porcine coronary artery led to the specific uptake of these NPs by SMCs, yielding FITC fluorescence in the neointimal and medial layers of the stented artery in comparison with bare. However, the extent of neointima formation and re-endothelialization was comparable for the bare-metal and NP-eluting stents.229 This nanocarrier was utilized by the same group as a matrix for delivering various biomolecules/drugs. Imatinib mesylate [a platelet-derived growth factor (PDGF) receptor inhibitor] eluting PLGA/chitosan NPs attenuated the proliferation of SMCs associated with inhibition of the target molecule (phosphorylation of PDGF receptor-β), but showed no effect on EC proliferation in vitro. This observation was well reflected in vivo wherein a marked reduction (by 50%) of in-stent neointima formation and stenosis was observed, without any effect on re-endothelialization in pigs.230 Similarly, Pitavastatin loaded PLGA/chitosan NPs were as effective as the bare drug in inhibiting SMC proliferation and tissue factor expression even at very low doses. These NP eluting stents significantly reduced in-stent stenosis in a porcine model and also elicited endothelial healing effects for re-endothelialization of stented arteries.231 In vivo efficacy of a polymer-free stent that utilizes nanosized phospholipid particles to deliver sirolimus from a combined balloon-plus-stent platform was compared with a BMS and biolimus eluting stent in a porcine coronary model. Results revealed a larger lumen area with reduced neointimal thickness and stenosis, with completely covered stent struts after 28 days.232
Researchers have also probed into ways of selective targeting of drug/biologics to SMCs or ECs. Gene-eluting stents were developed to deliver Akt1 siRNA nanoparticles (ASNs) from a hyaluronic acid (HA)-coated stent surface to specifically suppress the pro-proliferative Akt1 protein in SMCs. This stent released Akt1 siRNA to the SMCs attached to the stent, thereby reducing cell proliferation in the implanted vasculature and also in-stent restenosis in a rabbit iliac artery model.233 A gene and drug co-delivering SS coronary stent coated with bi-layered PLGA NPs containing a VEGF plasmid in the outer layer and PTX in the inner core was developed. These stents could promote early endothelium healing and inhibit smooth muscle cell proliferation in the porcine coronary injury model.234 In another study, the possibility of using chitosan/PLGA NPs containing miR-126 dsRNA for efficient incorporation into ECs was investigated. These NPs enhanced EC proliferation and migration appreciably, while SMC proliferation was reduced in vitro. Implantation of stents coated with chitosan-modified PLGA NPs containing dsRNAs in a rabbit restenosis model significantly inhibited the progression of neointimal hyperplasia.235 Likewise, a substrate-mediated gene delivery system was prepared by using bioinspired PDA coating to which DNA complex nanoparticles, composed of protamine (PrS) and plasmid DNA encoding with hepatocyte growth factor (HGF-pDNA) gene, were immobilized. EC proliferation was specifically promoted due to HGF, with less influence on SMC growth.236 A nanobioactive stent platform was developed containing multiple angiogenic genes (VEGF and Ang1) carrying NPs entrapped in polyacrylic acid (PAA) functionalized CNTs and fibrin hydrogel. The developed stent coating could significantly reduce the loss of therapeutics while traversing through the vessel and during deployment, and showed significantly enhanced endothelial regeneration and inhibition of subsequent neointimal proliferation in vivo.237
Heparin, an antithrombotic agent, has been utilized for developing nanocarriers that encapsulate drugs/biologics, with supplemented targeting functionality, as stent coatings. VEGF-loaded heparin/poly-l-lysine nanoparticles immobilized on dopamine-coated SS surfaces indicated enhanced blood compatibility with reduced platelet adhesion and activation, SMC inhibition, and significantly improved EC response.238 The same group developed a biomimetic nanocoating of laminin loaded heparin/poly-L-lysine nanoparticles, which prevented platelet adhesion and thrombus formation and showed beneficial effects in promoting EC proliferation.239 These nanoparticles were also loaded with fibronectin in another study to enhance the anticoagulant properties of the surface and demonstrated effective improvement in EC adhesion and proliferation.240 A multifunctional endothelium mimicking coating was built by cystamine-modified heparin/polyethylenimine (PEI) nanoparticles immobilized on the polydopamine surface. Active heparin along with in situ NO generation by the cystamine moieties in NPs resulted in good anticoagulant activity, along with a significant inhibition of SMC proliferation, promotion of EC proliferation, and tissue safety after subcutaneous implantation in rats.241 Similarly, a stent coating was developed by grafting Hep/NONOates onto SS stent surface. Synergistic and complementary effects of the released heparin and NO resulted in superior blood compatibility and promoted re-endothelialization with a subsequent reduction in in-stent restenosis, after implantation in the atherosclerotic rabbit model as shown in Fig. 6.242 Heparin coating has also been explored for its potential to minimize ion leaching from stents. Chitosan-heparin nanoparticle-coated nitinol nanotube surface helped to reduce Ni ion release and offered improved EC response. The initial burst release of heparin followed by its slow and sustained release also yielded improved blood compatibility.243
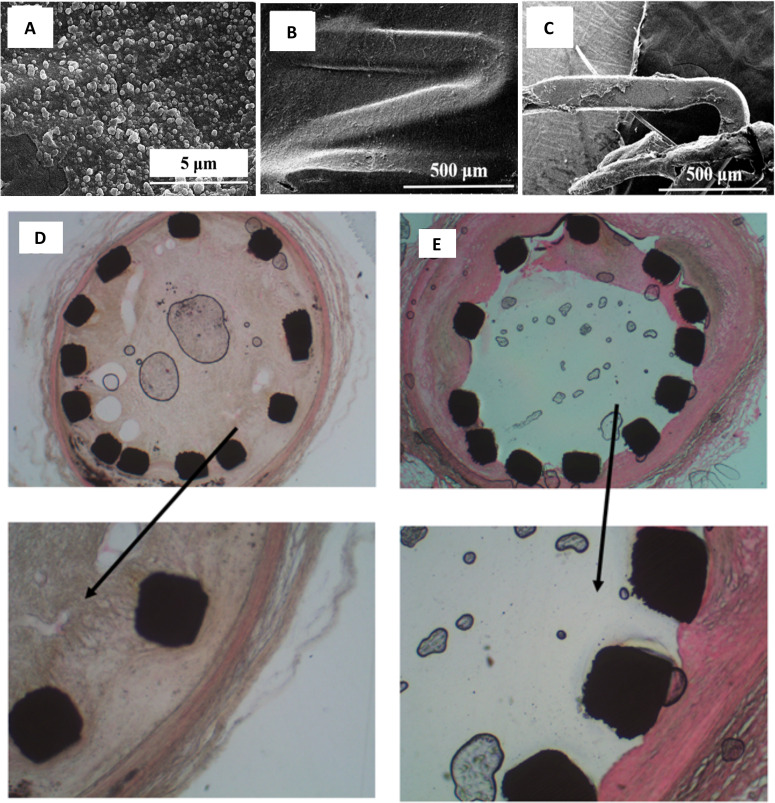
FIG. 6.
SEM images of (a) heparin/NONOate nanoparticles immobilized on polyglycidyl methacrylate (PGMA)-coated SS stents. Strut coverage on (b) SS-PGMA-Hep/NONOates and (c) control 316L SS stents harvested at 1 month. Histological hematoxylin−eosin stained images of (d) SS-PGMA-Hep/NONOates and (e) 316L SS stent after implantation for 1 month (arrows point to the higher magnification images). Reprinted with permission from Zhu et al., Langmuir 36, 2901–2910 (2020). Copyright 2020 American Chemical Society.
In addition to this, polymeric composites have also been explored for stent coatings and also as bioabsorbable stent platforms, mainly using amorphous calcium phosphate (ACP) as the ceramic component. ACP, an amorphous form of apatite with higher solubility and better bio absorbability, is capable of neutralizing the acidic by-products that are accumulated from the hydrolysis of polymers like PLGA, PLLA, etc., thereby reducing inflammation. The integration of small-dose ACP nanoparticles with PLLA resulted in the reduction of long-term chronic inflammatory response for up to 24 months after implantation in porcine arteries.244 SS stents coated with PLGA/ACP composites implanted into rat aortas displayed reduced restenosis with faster rates of re-endothelialization and lower inflammation. Likewise, PLLA/ACP studied as a stent platform showed significantly reduced inflammatory cell infiltration in the vessel walls of rabbit iliac arteries. No systemic toxicity was found in either PLGA/ACP or PLLA/ACP.245 A study on the use of PLLA/ACP scaffolds as bioresorbable stents in porcine showed larger lumen area and luminal patency rates with reduced late lumen loss and accelerated repair of the endothelium, after implantation in pigs.246 This fully bioabsorbable scaffold had less stent recoil and greater radial strength than PLLA scaffolds, suggesting its suitability for maintaining structural strength and functionality when implanted in porcine coronary arteries.247 PowerStent® Absorb Bioabsorbable drug-eluting stents (BDES), fabricated by co-formulating ACP nanoparticles with PLLA and paclitaxel were developed to overcome the current limitations of BDES, viz., its biocompatibility and radial strength. ACP facilitated accelerated hydrolytic degradation of PLLA and created nanometer pores that enlarged gradually to micrometer dimensions as degradation proceeds. The increased porosity also permitted endothelial ingrowth. This BDES showed a significant reduction of in-stent restenosis, inflammation, and stent recoil in pigs.248 The long-term safety and efficacy evaluation of these stents in porcine revealed minimal restenosis and complete re-endothelialization, with no stent thrombosis.249 A fully bioresorbable PLLA/ACP nanoparticle composite scaffold containing sirolimus that could release more than 70% of drug within 28 days, and completely degrade the polymer matrix within 2–3 years in vivo, was also studied. This stent showed a similar safety profile as sirolimus-eluting stents, with long-term inhibition of neointimal proliferation in pigs.250 Another material system investigated for rendering multifunctionality to stents is nanoscale copper-based metal-organic frameworks (MOFs). Nano-Cu–MOFs of size 10–100 nm immobilized on Ti/SS wires via polydopamine coating facilitated in situ delivery of Cu ions, which enabled a simultaneous catalytic generation of NO. Synergistic effects of NO and Cu ions released from nano-Cu–MOF surface resulted in reduced platelet activation, SMC and macrophage suppression, and EC proliferation. Wires coated with these nano-MOFs demonstrated excellent anticoagulation, re-endothelialization, and antihyperplasia properties after implantation in rat abdominal aorta.251 A non-biodegradable nanocomposite polymer based on polyhedral oligomeric silsesquioxanes (POSS) nanoparticles and poly(carbonate-urea)urethane (PCU) having antithrombogenic and in situ endothelialization properties were studied as coatings for stents. POSS–PCU has been used in in-man trials for various other applications owing to its superior biocompatibility and unique biophysical properties.252 NiTi stent was deposited with POSS–PCU by electrohydrodynamic atomization to eliminate the release of toxic ions from the underlying substrate. POSS–PCU coating on NiTi stents showed enhanced peel strength, surface resistance, and biocompatibility in vitro.253 This nanocomposite polymer with covalently attached anti-CD34 antibodies was also developed as a coating for BMS to enhance the capture of circulating EPCs and promote re-endothelialization. Antibody conjugation resulted in increased EPC capture, while maintaining in vitro biocompatibility and hemocompatibility.254 The same group developed small-caliber covered stents using POSS-PCU, wherein the metal struts were fully embedded within the membrane. Platelet studies supported the non-thrombogenicity of POSS-PCU in vitro.255
A polymer-free-composite was developed with an assembly of the first layer of thin carbon nanotube (CNT) film onto SS stents, followed by a second layer of mesoporous silica nanoparticles (MMSN)/CNTs coating. This nanostructured coating exhibited excellent mechanical flexibility, blood compatibility, drug loading, and continuous drug release for up to 2 weeks in vitro. In vivo implantation of this nanostructured DES in rabbit abdominal aorta showed an early-stage endothelialization.256 Likewise, silver nanoparticle (AgNPs) decorated TiO2–NT composites formed on Ti wire promoted protein-fouling resistance, anticoagulant and anti-inflammatory property, SMC inhibition, and low toxicity to ECs. Implantation of the functionalized Ti wire in rat abdominal aortic model revealed that photo-functionalized TiO2, AgNPs, and Ag+ released by AgNPs synergistically suppressed inflammation, excessive SMC proliferation, and tissue hyperplasia.257 Table III summarizes the different types of nanoparticulate coatings developed on stents tested in vivo for treating in-stent restenosis.
TABLE III.
Different types of nanoparticulate coatings developed on stents for treating in-stent restenosis, tested in vivo.
| Type of material | Description | Active agent | Animal model | Results | References |
|---|---|---|---|---|---|
| Nanoparticle coatings | PLGA/chitosan NPs | FITC | In vivo porcine coronary artery | Specific uptake of the NPs by SMCs. Extent of neointima and re-endothelialization were comparable for BMS and NP-eluting stents | 227 |
| Imatinib mesylate | Marked reduction (by 50%) of in-stent neointima formation and stenosis without any effect on re-endothelialization | 228 | |||
| Pitavastatin | Significantly reduced in-stent stenosis with elicited endothelial healing effects | 229 | |||
| Phospholipid NPs | Sirolimus | In vivo porcine coronary artery | Larger lumen area with reduced neointimal thickness and stenosis, with completely covered stent struts after 28 days | 230 | |
| Akt1 siRNA NPs | Akt1 siRNA | In vivo rabbit iliac artery | Reduced smooth muscle cell hyperplasia and thereby in-stent restenosis | 231 | |
| Bi-layered PLGA NPs | VEGF and paclitaxel | In vivo porcine injury model | Promoted early endothelium healing and inhibited smooth muscle cell proliferation | 232 | |
| Chitosan/PLGA NPs | miR-126 dsRNA | In vivo rabbit restenosis model | Inhibited the progression of neointimal hyperplasia | 233 | |
| NPs entrapped in polyacrylic acid functionalized CNTs and fibrin hydrogel | Vegf and Ang1 | In vivo injured canine femoral artery | Significantly enhanced endothelial regeneration and inhibited neointimal proliferation | 235 | |
| Hep/NONOate NP | Heparin and NONOate | Atherosclerotic rabbit model | Promoted re-endothelialization with subsequent reduction in in-stent restenosis | 240 | |
| Nanocomposite coatings | Amorphous calcium phosphate NPs with PLLA | ⋯ | In vivo porcine coronary artery | Reduced long-term chronic inflammatory response for up to 24 months | 242 |
| PLGA/ACP nanocomposites | ⋯ | In vivo rat abdominal aorta | Reduced restenosis with faster rates of re-endothelialization and lower inflammation | 243 | |
| PLLA/ACP nanoscaffolds | ⋯ | In vivo porcine coronary artery | Larger lumen area and luminal patency rates with reduced late lumen loss and accelerated repair of endothelium | 244, 245 | |
| PLLA/ACP nanocomposites | Paclitaxel | In vivo porcine coronary artery | Significant reduction of in-stent restenosis, inflammation and stent recoil at 1month and showed minimal restenosis and complete re-endothelialization at 6 months | 246, 247 | |
| Sirolimus | Long term inhibition of neointimal proliferation | 248 | |||
| Nano-Cu- metallic organic frameworks | ⋯ | In vivo rat abdominal aorta | Excellent anti-coagulation, re-endothelialization and anti-hyperplasia properties | 249 | |
| Mesoporous silica NPs/CNT | ⋯ | In vivo rat abdominal aorta | Early stage endothelialization | 254 | |
| Silver NPs decorated TiO2 NT | ⋯ | In vivo rat abdominal aorta | Suppressed inflammation, excessive SMC proliferation and tissue hyperplasia | 255 |
As evinced from the literature above, innumerable reports claim the benefits of using nanobased technologies and formulations for therapeutic and/or targeted delivery of drugs/biologics in cardiovascular applications. More extensive research is warranted for translating these findings to attain clinical benefits.
THE WAY FORWARD TO CLINICAL TRIALS
To translate a coronary stent from bench to bedside demands a multi-step process of (i) development of a viable stent prototype, (ii) functionality and toxicological evaluation, (iii) preclinical studies in large animal models, and finally (iv) clinical trials in humans.258 To surpass these phases and attain a clinically satisfactory product with all regulatory approvals entails a concerted effort from scientists and industry partners alike. Despite the enormous wealth of literature in this field of coronary stents utilizing nanotechnology, only a few have moved forward to the clinical phase. Majorly, the stents with inorganic coatings or polymeric thin films have emerged successful in clinical trials.
Among the inorganic coatings, the most effective products are the non-drug eluting, bioactive titania nitride oxide-coated SS stents (TiNOx), which have established significantly lower late lumen loss (LLL), angiographic restenosis, and reduced need for target lesion revascularization (TLR) than uncoated SS stents at 6 months follow-up259 and zotarolimus-eluting stents (ZES).260 Commercially available TitanTM (Hexacath, France) was associated with favorable clinical outcomes. A low incidence of stent thrombosis and repeat re-vascularization at 9 months follow-up was reported from the Titan Pori registry and Multi-center Titan registry from Israel.261,262 Long-term follow-up revealed a better clinical outcome of TiNOX stents vs paclitaxel-eluting stents (TITAX AMI)263 and everolimus-eluting stents (BASE ACS),264 proving that it can compete with DES and share a place in the stent market. The recent TIDES-ACS trial demonstrated the superiority of this bioactive stent vs the everolimus drug-eluting stent. This stent claims advantage of requiring only a short-term dual antiplatelet treatment post-stenting.265 Yet another successful candidate is the Diamond-like carbon (DLC) coated stents, which exhibited excellent clinical outcomes. MOMO® (Japan Stent Technology) is a drug-free CC BMS whose surface has a nanothin coating of DLC, which displayed non-inferiority over commercial BMS in a multi-center, non-randomized clinical trial. The DLC-coated stents showed low rates of target vessel failure (TVF) and angiographic restenosis.266,267 In contrast, the outcomes of human clinical trials on SiC-coated stents were not very satisfactory. SiC-coated stents did not exhibit any superiority over SS stents in terms of clinical and angiographic restenosis rates, despite the advantages observed in vitro. This is because, the direct decline in SMC growth by cytostatic drugs seems to be a crucial mechanism in the reduction of restenosis, rather than the drop in the number of platelets deposited on the stent surface.268,269 Likewise, inert SiO2 coatings on BMS after its first-in-man trial showed unsatisfactory suppression of neointimal hyperplasia.270
Polymeric coatings have also proven their effectiveness in clinical trials. The preclinical results of PzF nanothin coatings have translated into early convincing clinical data stating the ability of these nanocoated stents in promoting improved endothelial healing and reduced thrombosis.271 The COBRA Polyzene-F stent (Celenova Biosciences Inc.) satisfied all the performance goals for TVF and lumen loss at 9 months, with an excellent safety profile, rare occurrence of late myocardial infarction (low risk for MI), without any stent thrombosis.271,272 One-year follow-up with PzF-coated stents showed a TVF rate (Combination of all-cause mortality, myocardial infarction, or TVR) of only 12%, with no cases of stent thrombosis. This follow-up study demonstrated excellent clinical outcomes of COBRA PzF stent and compared favorably with current devices.273 Furthermore, various clinical trials aimed at shortening the duration of dual antiplatelet agents or administration of monoantiplatelet therapy after implantation of COBRA PzF stents to 1 month and a follow-up of 1-year have also produced excellent clinical outcomes, especially in patients with high bleeding risks.274,275
VESTAsync™ drug-eluting SS stent (MIV Therapeutics Inc.) utilizes a polymer-free nanothin hydroxyapatite surface coating having a microporous architecture impregnated with sirolimus drug [Fig. 7(a)]. The first-in-human investigation of this stent (VESTASYNC I trial) proved it to be a feasible and safe device that elicited minimal lumen loss and neointimal hyperplasia at 4-months, with a non-significant increase up to 9 months. There were no major adverse cardiac events (MACE) within 1 year of follow-up.276,277 In a randomized VESTASYNC II trial in a larger group of patients, the safety and efficacy of this stent were tested with the administration of antiplatelet medication for only 3 months.278 A nanoporous polymer-free SS stent eluting sirolimus (PFSES) (Nano+, Lepu Medical Technology) has entered clinical trials for safety and efficacy evaluation [Fig. 7(b)]. The nanopores of size ∼400 nm are capable of controlling drug release and maintaining the mechanical integrity of the stent platform. In a 3-month follow-up study, this drug-loaded nanoporous SS stent was effective in inhibiting neointimal proliferation and promoting early vascular healing, with high strut coverage.279
FIG. 7.
Representative SEM images of clinically tested porous stents. (a-i) Microporous hydroxyapatite-coated stent filled with sirolimus formulation (a-ii) cross section of the nanothin hydroxyapatite coating (∼600 nm). Reprinted with permission from Costa et al., JACC: Cardiovasc. Interventions 2(5), 422–427 (2008). Copyright 2008, Elsevier. (b-i) Nano+TM polymer-free stent showing strut microstructure after expansion. Strut thickness is approximately 91 μm having a large number of sirolimus-filled pores (∼400 nm) on the abluminal stent surface. (b-ii) Electron microscopy images of the nanopores (magnification ×20 000). Reprinted with permission from Liu et al., Catheterization Cardiovasc. Interventions 95(S1), 658–664 (2020). Copyright 2020 John Wiley and Sons.
This PFSES stent, on implantation in patients with inadequate vascular healing in 3 months, yielded complete healing at 6 months, as revealed by optical coherence tomography.280 In a prospective, single-blinded, multicenter, randomized clinical trial (Nanotrial) designed to investigate its safety and efficacy, nano-PFSES was non-inferior to SES at the primary end point (9 months).281 One-year clinical outcomes of this Nano+TM polymer-free SES implantation were excellent, with low rates of target lesion failure and stent thrombosis.282 By utilizing the same platform, but loaded with dual drugs, viz., sirolimus and probucol, the preliminary feasibility and safety of this DDES (BICARE, Lepu Medical Technology) in the first-in-human study showed absence of any early adverse events, favorable angiographic suppression of in-stent restenosis and excellent healing at 4-months.283 A large-scale clinical trial of this stent in 3002 patients showed its non-inferiority to the new generation polymer-based zotarolimus-eluting stent (ZES). No differences in angiographic stenosis and late lumen loss were observed for this stent in comparison with ZES for up to 12 months, but with a lower incidence of stent thrombosis.284 Figure 6 displays two representative stent structures of a nanoporous, polymer-free, drug-eluting stent (Nano+) and microporous, nanothin hydroxyapatite-coated stent (VESTAsync) that are currently in the clinical trial phase.
Table IV elaborates the various stents with surface modification at the nanoscale that have entered the clinical trial phase.
TABLE IV.
Stents with surface modification at the nanoscale in clinical trials.
| Stent | Clinical trial | Clinical endpoints | Result | References |
|---|---|---|---|---|
| Titan (Hexacath, France) titanium nitride oxide coating | TITAX AMI | MACE (16.4% vs 25.1%) and 5-year rates of cardiac death (1.9% vs 5.7%), recurrent MI (8.4% vs 18.0%) and rate of definite ST (0.9% vs 7.1%) were significantly lower in patients with TiNOx stent compared to Paclitaxel eluting stent (PES). | Better clinical outcome of TiNOx stent vs PES in patients with acute myocardial infarction | 261 |
| BASE ACS | At 5-year follow-up, TiNOx stent was non-inferior to everolimus eluting stent (EES) for primary endpoint of MACE (14.4% vs 17.8%). The rate of non-fatal MI was lower in TiNOx stent group (5.9% vs 9.7%) and the rates of cardiac death (2.8% vs 3.8%) and ischemia-driven TLR (8.3% vs 9.9%) were comparable for both groups. | Better clinical outcome and non-inferiority of TiNOx stent vs EES in patients with acute myocardial infarction | 262 | |
| TIDES-ACS | TiNOx stents were associated with lower rates of cardiac death (0.6% vs. 2.6%) and MI (2.2% vs. 5.0%) than everolimus eluting stent (EES) at 18 months of follow-up. | TiNOx-coated stents were non-inferior to platinum–chromium–based biodegradable polymer EES at 12 months | 263 | |
| MOMO (Japan Stent Technology) Diamond-like carbon coating | Multi-center, non-randomized clinical trial | No myocardial infarction, stent thrombosis, or cardiac death were observed in patients with MOMO stent. Binary restenosis was 12.5% (n = 5), and the LLL was 0.54 ± 0.3 mm. | Safety and feasibility of MOMO cobalt–chromium carbon-coated stent | 264 |
| No in-stent thrombosis or myocardial infarction was observed in patients with MOMO stent. The binary restenosis rate at the 6-month was 10.5 % for MOMO stent, which is lower than commercially available bare-metal stents (BMS). | Non-inferiority over commercial BMS | 265 | ||
| SiC-coated stent | Tenax-vs Nir-stent Study | MACE occurred in 12% of Tenax recipients and 14.3% of Nir recipients. Premature target lesion revascularization was performed in 6.9% patients in Tenax group and 5.1% patients in Nir group. | Both SiC-coated (Tenax) and non-coated (Nir) stents had low rate of MACE, with no definite superiority of any of the devices. | 266 |
| Target lesion revascularization was performed in 2% of Tenax group and 1.6% of Nir group and subacute thrombosis was observed in 0.8% of Tenax patients. | No advantage of the SiC-coated stent over stainless steel stent with regard to clinical and angiographic restenosis rates | 267 | ||
| SiO2 coated stent | First-in-man trial | Angiographic in-stent LLL was 0.77 ± 0.44 mm, and binary restenosis occurred in 33.3% of lesions. At 12 months, cardiac death, target vessel myocardial infarction, and target lesion revascularization rate was 33.3%. | In contrast with the preclinical study, the SiO2 coated stent did not efficiently suppress neointimal hyperplasia in humans in this trial. | 268 |
| COBRA Pz-F stent (Celenova Biosciences Inc.) Nanothin Polyzene-F coating | One-year follow-up | Target vessel failure (composite of all-cause mortality, myocardial infarction or target vessel revascularization) rate was 12%. There were no cases of definite stent thrombosis. | The COBRA PzF stent was safe and effective and was associated with excellent clinical outcomes. | 271 |
| VESTAsync drug eluting stent (MIV Therapeutics Inc.) Nanothin-microporous hydroxyapatite surface coating | VESTASYNC I trial | In-stent LL and percentage neointimal hyperplasia were 0.3 ± 0.25 mm and 2.6% ± 2.2%, respectively, with a nonsignificant increase at 9 months (0.36 ± 0.23 mm and 4.0% ± 2.2%, respectively). There were no MACE at 1 year follow-up. | VESTAsync-eluting stent was effective in reducing LL and neointimal hyperplasia at 4 and 9 months. | 274, 275 |
| Nano+ (Lepu Medical Technology) Nanoporous polymer-free SS stent eluting sirolimus | Nanotrial | Nano+ was non-inferior to durable-polymer DES (SES) at primary endpoint of 9 months. The incidence of MACE in the Nano+ group (7.6%) was comparable to SES group (5.9%) at 2 years follow-up. The frequency of cardiac death (0.8% vs. 0.7%) and stent thrombosis (0.8% vs. 1.5%,) was low for both Nano+ and SES. | Nano+ showed similar safety and efficacy compared with SES in the treatment of patients with de novo coronary artery lesions. | 279 |
| The 1-year Target Lesion Failure rate was 3.1% with clinically driven TLR rates (1.3%), cardiac death (1.8%) and MI (0.4%). ST occurred in 0.4% of patients. | The 1-year clinical outcomes for Nano+ polymer-free SES implantation were excellent | 280 | ||
| BICARE (Lepu Medical Technology) Nanoporous polymer-free SS stent eluting dual drugs sirolimus and probucol | First-in-human trial | At 4 months, angiographic in-stent late loss was 0.14 ± 0.19 mm, and the in-stent binary restenosis rate was 3.1% and complete strut coverage was 98.2%. At 18 months, TLF occurred in 9.4% patients with no adverse safety events. | The preliminary feasibility and safety of polymer-free, dual- drug eluting stent, without any adverse safety events and favorable suppression of neointimal hyperplasia. | 281 |
CONCLUSIONS AND FUTURE PERSPECTIVES
A way forward to advance the lacunae of the clinical drug-eluting stents is to judiciously utilize the principles of nanotechnology in the field of coronary stenting. Extensive research has been performed in this area utilizing diverse nanomaterials/surfaces that have shown effective re-endothelialization and simultaneous inhibition of in-stent restenosis. Nanothin coatings, nanotextured surfaces, and nanofibrous and nanoparticulate coatings on stents, with or without the use of active pharmaceutical ingredients, are widely explored. Specifically, those stents devoid of polymers or drugs can be a facile and cost-effective alternative to DES. In vitro and preclinical evaluations in small and large animal models have confirmed the utility of such nanotechnology-based stents in providing enhanced therapeutic benefits, with very few in clinical trials. The possible reasons include the risks with nanosized coatings, its flaking and thereby integrity, which needs to be confirmed before a clinical translation. Scaling up and regulatory approvals are also possible deterrents. To advance more of these stent candidates to the clinics demands surpassing the regulatory standards of functionality (especially the long-term stability and durability of nanocoatings) and toxicology as well. The promising benefits of nanomaterials science and technology would certainly help to evolve and translate these stents possessing surface modifications at the nanoscale into reality in the immediate future. In summary, nanotechnology can shape the foundation of next-generation coronary stent coatings by addressing the challenges of present-day stents.
ACKNOWLEDGMENTS
The authors acknowledge the financial assistance provided by the Department of Biotechnology, Government of India, through a project grant number BT/PR3516/MED/32/192/2011. A.M.C. acknowledges the Indian Council of Medical Research (ICMR) for her Senior Research Fellowship and Dr. Gopikrishna J for the illustration (No. 5/3/8/48/ITR-F/2018-ITR). The authors also thank Amrita Vishwa Vidyapeetham, Kochi, for all infrastructural support to carry out the research work.
DATA AVAILABILITY
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
References
- 1. Lusis A. J., “ Atherosclerosis,” Nature 407, 233–241 (2000). 10.1038/35025203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Insull W., “ The pathology of atherosclerosis: plaque development and plaque responses to medical treatment,” Am. J. Med. 122(1 SUPPL), S3 (2009). 10.1016/j.amjmed.2008.10.013 [DOI] [PubMed] [Google Scholar]
- 3. Lovely Chhabra A., Zain M. A., and Siddiqui W. J., Coronary Stents ( StatPearls Publishing, Treasure Island, FL, 2018). [PubMed] [Google Scholar]
- 4. Borhani S., Hassanajili S., Ahmadi Tafti S. H., and Rabbani S., “ Cardiovascular stents: Overview, evolution, and next generation,” Prog. Biomater. 7(3), 175–205 (2018). 10.1007/s40204-018-0097-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Simard T., Hibbert B., Ramirez F. D., Froeschl M., Chen Y. X., and O'Brien E. R., “ The evolution of coronary stents: A brief review,” Can. J. Cardiol. 30, 35–45 (2014). 10.1016/j.cjca.2013.09.012 [DOI] [PubMed] [Google Scholar]
- 6. Iqbal J., Gunn J., and Serruys P. W., “ Coronary stents: Historical development, current status and future directions,” Br. Med. Bull. 106, 193–211 (2013). 10.1093/bmb/ldt009 [DOI] [PubMed] [Google Scholar]
- 7. Malenka D. J., Kaplan A. V., Lucas F. L., Sharp S. M., and Skinner J. S., “ Outcomes following coronary stenting in the era of bare-metal vs the era of drug-eluting stents,” JAMA 299, 2868 (2008). 10.1001/jama.299.24.2868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Waksman R. and Steinvil A., In-stent restenosis? Circulation: Cardiovascular Interventions ( Lippincott Williams and Wilkins, 2016). [DOI] [PubMed] [Google Scholar]
- 9. Her A. Y. and Shin E. S., “ Current management of in-stent restenosis,” Korean Circ. J. 48, 337–349 (2018). 10.4070/kcj.2018.0103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Marx S. O., Totary-Jain H., and Marks A. R., “ Vascular smooth muscle cell proliferation in restenosis,” Circulation 4(1), 104–111 (2011). 10.1161/CIRCINTERVENTIONS.110.957332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Buccheri D., Piraino D., Andolina G., and Cortese B., “ Understanding and managing in-stent restenosis: A review of clinical data, from pathogenesis to treatment,” J. Thorac. Dis. 8, E1150–E1162 (2016). 10.21037/jtd.2016.10.93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tung R., Kaul S., Diamond G. A., and Shah P. K., “ Narrative review: Drug-eluting stents for the management of restenosis: A critical appraisal of the evidence,” Ann. Intern. Med. 144, 913 (2006). 10.7326/0003-4819-144-12-200606200-00009 [DOI] [PubMed] [Google Scholar]
- 13. Lüscher T. F., Steffel J., Eberli F. R., Joner M., Nakazawa G., Tanner F. C., and Virmani R., “ Drug-eluting stent and coronary thrombosis: biological mechanisms and clinical implications,” Circulation 115, 1051 (2007). 10.1161/CIRCULATIONAHA.106.675934 [DOI] [PubMed] [Google Scholar]
- 14. Abbott J. D., “ Revealing the silver and red lining in drug-eluting stents with angioscopy,” Circulation 1, 7 (2008). 10.1161/CIRCINTERVENTIONS.108.802926 [DOI] [PubMed] [Google Scholar]
- 15. Finn A. V., Nakazawa G., Joner M., Kolodgie F. D., Mont E. K., Gold H. K., and Virmani R., “ Vascular responses to drug eluting stents: Importance of delayed healing,” Arterioscler., Thromb., Vasc. Biol. 27, 1500–1510 (2007). 10.1161/ATVBAHA.107.144220 [DOI] [PubMed] [Google Scholar]
- 16. Lee C. W., Park D. W., Lee B. K., Kim Y. H., Hong M. K., Kim J. J., Park S. W., and Park S. J., “ Predictors of restenosis after placement of drug-eluting stents in one or more coronary arteries,” Am. J. Cardiol. 97(4), 506 (2006). 10.1016/j.amjcard.2005.09.084 [DOI] [PubMed] [Google Scholar]
- 17. Gersh B. J., “ Incidence, predictors, and outcome of thrombosis after successful implantation of drug-eluting stents,” Yearbook of Cardiology ( Elsevier, 2006), p. 260. [DOI] [PubMed] [Google Scholar]
- 18. Habib A. and Finn A. V., “ Endothelialization of drug eluting stents and its impact on dual anti-platelet therapy duration,” Pharmacol. Res. 93, 22–27 (2015). 10.1016/j.phrs.2014.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Commandeur S., van Beusekom H. M., and van der Giessen W. J., Polymers, Drug Release, and Drug-Eluting Stents ( Blackwell Publishing, Inc, 2006). [DOI] [PubMed] [Google Scholar]
- 20. Levy Y., Mandler D., Weinberger J., and Domb A. J., “ Evaluation of drug-eluting stents' coating durability: Clinical and regulatory implications,” J. Biomed. Mater. Res., Part B 91(1), 441–451 (2009). 10.1002/jbm.b.31420 [DOI] [PubMed] [Google Scholar]
- 21. Stone G. W., Ellis S. G., Cannon L., Mann J. T., Greenberg J. D., Spriggs D., O'Shaughnessy C. D., DeMaio S., Hall P., Popma J. J., Koglin J., and Russell M. E., “ Comparison of a polymer-based paclitaxel-eluting stent with a bare metal stent in patients with complex coronary artery disease: A randomized controlled trial,” J. Am. Med. Assoc. 294(10), 1215 (2005). 10.1001/jama.294.10.1215 [DOI] [PubMed] [Google Scholar]
- 22. Chatterjee S. and Pandey A., “ Drug eluting stents: Friend or foe? A review of cellular mechanisms behind the effects of paclitaxel and sirolimus eluting stents,” Curr. Drug Metab. 9(6), 554 (2008). 10.2174/138920008784892119 [DOI] [PubMed] [Google Scholar]
- 23. Marks A. R., “ Sirolimus for the prevention of in-stent restenosis in a coronary artery,” New Engl. J. Med. 349(14), 1307 (2003). 10.1056/NEJMp038141 [DOI] [PubMed] [Google Scholar]
- 24. Rizas K. D. and Mehilli J., “ Stent polymers: Do they make a difference?,” Circulation 9(6), e002943 (2016). 10.1161/CIRCINTERVENTIONS.115.002943 [DOI] [PubMed] [Google Scholar]
- 25. Kandzari D. E., Leon M. B., Meredith I., Fajadet J., Wijns W., and Mauri L., “ Final 5-year outcomes from the Endeavor zotarolimus-eluting stent clinical trial program: Comparison of safety and efficacy with first-generation drug-eluting and bare-metal stents,” JACC: Cardiovasc. Interventions 6(5), 504 (2013). 10.1016/j.jcin.2012.12.125 [DOI] [PubMed] [Google Scholar]
- 26. Leon M. B., Mauri L., Popma J. J., Cutlip D. E., Nikolsky E., O'Shaughnessy C., Overlie P. A., McLaurin B. T., Solomon S. L., Douglas J. S., Ball M. W., Caputo R. P., Jain A., Tolleson T. R., Reen B. M., Kirtane A. J., Fitzgerald P. J., Thompson K., and Kandzari D. E., “ A randomized comparison of the endeavor zotarolimus-eluting stent versus the TAXUS paclitaxel-eluting stent in de novo native coronary lesions. 12-Month outcomes from the ENDEAVOR IV trial,” J. Am. Coll. Cardiol. 55(6), 543 (2010). 10.1016/j.jacc.2009.08.067 [DOI] [PubMed] [Google Scholar]
- 27. Mauri L., Massaro J. M., Jiang S., Meredith I., Wijns W., Fajadet J., Kandzari D. E., Leon M. B., Cutlip D. E., and Thompson K. P., “ Long-term clinical outcomes with zotarolimus-eluting versus bare-metal coronary stents,” JACC: Cardiovasc. Interventions 3(12), 1240 (2010). 10.1016/j.jcin.2010.08.021 [DOI] [PubMed] [Google Scholar]
- 28. Busch R., Strohbach A., Rethfeldt S., Walz S., Busch M., Petersen S., Felix S., and Sternberg K., “ New stent surface materials: The impact of polymer-dependent interactions of human endothelial cells, smooth muscle cells, and platelets,” Acta Biomater. 10(2), 688 (2014). 10.1016/j.actbio.2013.10.015 [DOI] [PubMed] [Google Scholar]
- 29. Beshchasna N., Saqib M., Kraskiewicz H., Wasyluk Ł., Kuzmin O., Duta O. C., Ficai D., Ghizdavet Z., Marin A., Ficai A., Sun Z., Pichugin V. F., Opitz J., and Andronescu E., “ Recent advances in manufacturing innovative stents,” Pharmaceutics 12, 349 (2020). 10.3390/pharmaceutics12040349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kandaswamy E. and Zuo L., “ Recent advances in treatment of coronary artery disease: Role of science and technology,” Int. J. Mol. Sci. 19, 424 (2018). 10.3390/ijms19020424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nazneen F., Herzog G., Arrigan D. W. M., Caplice N., Benvenuto P., Galvin P., and Thompson M., “ Surface chemical and physical modification in stent technology for the treatment of coronary artery disease,” J. Biomed. Mater. Res., Part B 100(7), 1989 (2012). 10.1002/jbm.b.32772 [DOI] [PubMed] [Google Scholar]
- 32. Zhao J. and Feng Y., “ Surface engineering of cardiovascular devices for improved hemocompatibility and rapid endothelialization,” Advanced Healthcare Materials ( Wiley-VCH, Verlag, 2020). [DOI] [PubMed] [Google Scholar]
- 33. Helmus M. N., Gibbons D. F., and Cebon D., “ Biocompatibility: Meeting a key functional requirement of next-generation medical devices,” Toxicol. Pathol. 36(1), 70–80 (2008). 10.1177/0192623307310949 [DOI] [PubMed] [Google Scholar]
- 34. Yu Y., Wise S. G., Celermajer D. S., Bilek M. M. M., and Ng M. K. C., “ Bioengineering stents with proactive biocompatibility,” Interventional Cardiology ( Future Medicine Ltd., 2015), pp 571–584. [Google Scholar]
- 35. Chan C. M., Ko T. M., and Hiraoka H., “ Polymer surface modification by plasmas and photons,” Surf. Sci. Rep. 24(1-2), 1 (1996). 10.1016/0167-5729(96)80003-3 [DOI] [Google Scholar]
- 36. Oehr C., “ Plasma surface modification of polymers for biomedical use,” Nucl. Instrum. Methods Phys. Res., Sect. B 208, 40 (2003). 10.1016/S0168-583X(03)00650-5 [DOI] [Google Scholar]
- 37. Varíola F., Vetrone F., Richert L., Jedrzejowski P., Yi J. H., Zalzal S., Clair S., Sarkissian A., Perepichka D. F., Wuest J. D., Rosei F., and Nanci A., “ Improving biocompatibility of implantable metals by nanoscale modification of surfaces: An overview of strategies, fabrication methods, and challenges,” Small 5, 996 (2009). 10.1002/smll.200801186 [DOI] [PubMed] [Google Scholar]
- 38. Foerster A., Duda M., Kraśkiewicz H., Wawrzyńska M., Podbielska H., and Kopaczyńska M., “ Physico-chemical stent surface modifications,” Functionalised Cardiovascular Stents ( Elsevier, 2018), pp 137–148. [Google Scholar]
- 39. Variola F., Brunski J. B., Orsini G., Tambasco De Oliveira P., Wazen R., and Nanci A., “ Nanoscale surface modifications of medically relevant metals: State-of-the art and perspectives,” Nanoscale 3, 335–353 (2011). 10.1039/C0NR00485E [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tan C. H., Muhamad N., and Abdullah M. M. A. B., “ Surface topographical modification of coronary stent: A review,” IOP Conf. Ser. 209, 012031 (2017). 10.1088/1757-899X/209/1/012031 [DOI] [Google Scholar]
- 41. Bassous N., Cooke J. P., and Webster T. J., “ Enhancing stent effectiveness with nanofeatures,” Methodist DeBakey Cardiovasc. J. 12, 163 (2016). 10.14797/mdcj-12-3-163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jingan L., Some “Micro” and “Nano” Elements in the Surface Modification of Cardiovascular Biomaterials ( MedDocs Publishers LLC, 2020). [Google Scholar]
- 43. Nguyen A. T., Sathe S. R., and Yim E. K. F., “ From nano to micro: Topographical scale and its impact on cell adhesion, morphology and contact guidance,” J. Phys. 28, 183001 (2016). 10.1088/0953-8984/28/18/183001 [DOI] [PubMed] [Google Scholar]
- 44. Wang S., Li J., Zhou Z., Zhou S., and Hu Z., “ Micro-/nano-scales direct cell behavior on biomaterial surfaces,” Molecules 24, 75 (2018). 10.3390/molecules24010075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Xue J., Wu T., Dai Y., and Xia Y., “ Electrospinning and electrospun nanofibers: Methods, materials, and applications,” Chem. Rev. 119, 5298 (2019). 10.1021/acs.chemrev.8b00593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yin R. X., Yang D. Z., and Wu J. Z., “ Nanoparticle drug- and gene-eluting stents for the prevention and treatment of coronary restenosis,” Theranostics 4, 175 (2014). 10.7150/thno.7210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chandarana M., Curtis A., and Hoskins C., “ The use of nanotechnology in cardiovascular disease,” Appl. Nanosci. 8, 1607–1619 (2018). 10.1007/s13204-018-0856-z [DOI] [Google Scholar]
- 48. Jiang W., Rutherford D., Vuong T., and Liu H., “ Nanomaterials for treating cardiovascular diseases: A review,” Bioact. Mater. 2, 185–198 (2017). 10.1016/j.bioactmat.2017.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Caves J. M. and Chaikof E. L., “ The evolving impact of microfabrication and nanotechnology on stent design,” J. Vasc. Surg. 44, 1363 (2006). 10.1016/j.jvs.2006.08.046 [DOI] [PubMed] [Google Scholar]
- 50. Louizos L. A., Athanasopoulos P. G., and Varty K., “ Microelectromechanical systems and nanotechnology: A platform for the next stent technological era,” Vasc. Endovasc. Surg. 46, 605 (2012). 10.1177/1538574412462637 [DOI] [PubMed] [Google Scholar]
- 51. Martinez A. W. and Chaikof E. L., “ Microfabrication and nanotechnology in stent design,” Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol. 3, 256 (2011). 10.1002/wnan.123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bagheri M., Mohammadi M., Steele T. W. J., and Ramezani M., “ Nanomaterial coatings applied on stent surfaces,” Nanomedicine 11, 1309–1326 (2016). 10.2217/nnm-2015-0007 [DOI] [PubMed] [Google Scholar]
- 53. Rykowska I., Nowak I., and Nowak R., “ Drug‐eluting stents and balloons-materials, structure designs, and coating techniques: A review,” Molecules 25, 4624 (2020). 10.3390/molecules25204624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Mani G., Feldman M. D., Patel D., and Agrawal C. M., “ Coronary stents: A materials perspective,” Biomaterials 28, 1689–1710 (2007). 10.1016/j.biomaterials.2006.11.042 [DOI] [PubMed] [Google Scholar]
- 55. Soliva Beser A., “ Nanotechnology fabrication methods. Summary,” ET 1039-Nanotechnology Universitat Jaume, 2016. [Google Scholar]
- 56. Miao Z., Xu D., Ouyang J., Guo G., Zhao X., and Tang Y., “ Electrochemically induced sol-gel preparation of single-crystalline TiO2 nanowires,” Nano Lett. 2(7), 717 (2002). 10.1021/nl025541w [DOI] [Google Scholar]
- 57. López-Ayala S., Rincón M. E., and Pfeiffer H., “ Influence of copper on the microstructure of sol-gel titanium oxide nanotubes array,” J. Mater. Sci. 44(15), 4162 (2009). 10.1007/s10853-009-3617-2 [DOI] [Google Scholar]
- 58. Kim S. J., Lee N. H., Oh H. J., Jung S. C., Lee W. J., and Kim D. H., “ Photocatalytic properties of nanotubular-shaped TiO2 powders with anatase phase obtained from titanate nanotube powder through various thermal treatments,” Int. J. Photoenergy 2011, 1. 10.1155/2011/359161 [DOI] [Google Scholar]
- 59. Kasuga T., Hiramatsu M., Hoson A., Sekino T., and Niihara K., “ Formation of titanium oxide nanotube,” Langmuir 14(12), 3160 (1998). 10.1021/la9713816 [DOI] [Google Scholar]
- 60. Li G., Liu Z., Zhang Z., and Yan X., “ Preparation of titania nanotube arrays by the hydrothermal method,” Cuihua Xuebao/Chin. J. Catal. 30(1), 37 (2009). 10.1016/S1872-2067(08)60088-1 [DOI] [Google Scholar]
- 61. Sander M. S., Côté M. J., Gu W., Kile B. M., and Tripp C. P., “ Template-assisted fabrication of dense, aligned arrays of titania nanotubes with well-controlled dimensions on substrates,” Adv. Mater. 16(22), 2052 (2004). 10.1002/adma.200400446 [DOI] [Google Scholar]
- 62. Bae C., Yoo H., Kim S., Lee K., Kim J., Sung M. M., and Shin H., “ Template-directed synthesis of oxide nanotubes: Fabrication, characterization, and applications,” Chem. Mater. 20(3), 756 (2008). 10.1021/cm702138c [DOI] [Google Scholar]
- 63. Tian Z. R., Voigt J. A., Liu J., McKenzie B., and Xu H., “ Large oriented arrays and continuous films of TiO2-based nanotubes,” J. Am. Chem. Soc. 125(41), 12384 (2003). 10.1021/ja0369461 [DOI] [PubMed] [Google Scholar]
- 64. Macak J. M., Tsuchiya H., Ghicov A., Yasuda K., Hahn R., Bauer S., and Schmuki P., “ TiO2 nanotubes: Self-organized electrochemical formation, properties and applications,” Curr. Opin. Solid State Mater. Sci. 11, 3 (2007). 10.1016/j.cossms.2007.08.004 [DOI] [Google Scholar]
- 65. Zhao J., Wang X., Chen R., and Li L., “ Fabrication of titanium oxide nanotube arrays by anodic oxidation,” Solid State Commun. 134(10), 705 (2005). 10.1016/j.ssc.2005.02.028 [DOI] [Google Scholar]
- 66. Kaneco S., Chen Y., Westerhoff P., and Crittenden J. C., “ Fabrication of uniform size titanium oxide nanotubes: Impact of current density and solution conditions,” Scr. Mater. 56(5), 373 (2007). 10.1016/j.scriptamat.2006.11.001 [DOI] [Google Scholar]
- 67. Macák J. M., Tsuchiya H., and Schmuki P., “ High-aspect-ratio TiO2 nanotubes by anodization of titanium,” Angew. Chem.-Int. Ed. 44(14), 2100 (2005). 10.1002/anie.200462459 [DOI] [PubMed] [Google Scholar]
- 68. Indira K., Mudali U. K., Nishimura T., and Rajendran N., “ A review on TiO2 nanotubes: Influence of anodization parameters, formation mechanism, properties, corrosion behavior, and biomedical applications,” J. Bio-Tribo-Corros. 1, 28 (2015). 10.1007/s40735-015-0024-x [DOI] [Google Scholar]
- 69. Lim Y. C., Zainal Z., Tan W. T., and Hussein M. Z., “ Anodization parameters influencing the growth of titania nanotubes and their photoelectrochemical response,” Int. J. Photoenergy 2012, 1. 10.1155/2012/638017 [DOI] [Google Scholar]
- 70. Su Z., Zhou W., Jiang F., and Hong M., “ Anodic formation of nanoporous and nanotubular metal oxides,” J. Mater. Chem. 22(2), 535–544 (2012). 10.1039/C1JM13338A [DOI] [Google Scholar]
- 71. Yin H., Liu H., and Shen W. Z., “ The large diameter and fast growth of self-organized TiO2 nanotube arrays achieved via electrochemical anodization,” Nanotechnology 21(3), 035601 (2010). 10.1088/0957-4484/21/3/035601 [DOI] [PubMed] [Google Scholar]
- 72. Mura F., Masci A., Pasquali M., and Pozio A., “ Effect of a galvanostatic treatment on the preparation of highly ordered TiO2 nanotubes,” Electrochim. Acta 54(14), 3794 (2009). 10.1016/j.electacta.2009.01.073 [DOI] [Google Scholar]
- 73. Wang L. N., Jin M., Zheng Y., Guan Y., Lu X., and Luo J. L., “ Nanotubular surface modification of metallic implants via electrochemical anodization technique,” Int. J. Nanomed. 9, 4421–4435 (2014). 10.2147/IJN.S65866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Khudhair D., Bhatti A., Li Y., Hamedani H. A., Garmestani H., Hodgson P., and Nahavandi S., “ Anodization parameters influencing the morphology and electrical properties of TiO2 nanotubes for living cell interfacing and investigations,” Mater. Sci. Eng. C 59, 1125–1142 (2016). 10.1016/j.msec.2015.10.042 [DOI] [PubMed] [Google Scholar]
- 75. Hoseinzadeh T., Ghorannevis Z., Ghoranneviss M., Sari A. H., and Salem M. K., “ Effects of various applied voltages on physical properties of TiO2 nanotubes by anodization method,” J. Theor. Appl. Phys. 11(3), 243 (2017). 10.1007/s40094-017-0257-9 [DOI] [Google Scholar]
- 76. O'Brien B. and Carroll W., “ The evolution of cardiovascular stent materials and surfaces in response to clinical drivers: A review,” Acta Biomater. 5, 945–958 (2009). 10.1016/j.actbio.2008.11.012 [DOI] [PubMed] [Google Scholar]
- 77. Guildford A., Santin M., and Phillips G. J., “ Cardiovascular stents,” Biomaterials and Devices for the Circulatory System ( Elsevier, 2010), pp. 173–216. [Google Scholar]
- 78. Bedair T. M., ElNaggar M. A., Joung Y. K., and Han D. K., “ Recent advances to accelerate re-endothelialization for vascular stents,” J. Tissue Eng. 8, 204173141773154 (2017). 10.1177/2041731417731546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Junkar I., Kulkarni M., Benčina M., Kovač J., Mrak-Poljšak K., Lakota K., Sodin-Šemrl S., Mozetič M., and Iglič A., “ Titanium dioxide nanotube arrays for cardiovascular stent applications,” ACS Omega 5(13), 7280 (2020). 10.1021/acsomega.9b04118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Peng L., Eltgroth M. L., LaTempa T. J., Grimes C. A., and Desai T. A., “ The effect of TiO2 nanotubes on endothelial function and smooth muscle proliferation,” Biomaterials 30(7), 1268–1272 (2009). 10.1016/j.biomaterials.2008.11.012 [DOI] [PubMed] [Google Scholar]
- 81. Peng L., Barczak A. J., Barbeau R. A., Xiao Y., Latempa T. J., Grimes C. A., and Desai T. A., “ Whole genome expression analysis reveals differential effects of TiO2 nanotubes on vascular cells,” Nano Lett. 10(1), 143–148 (2010). 10.1021/nl903043z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Brammer K. S., Oh S., Gallagher J. O., and Jin S., “ Enhanced cellular mobility guided by TiO2 nanotube surfaces,” Nano Lett. 8(3), 786–793 (2008). 10.1021/nl072572o [DOI] [PubMed] [Google Scholar]
- 83. Cao Y. and Desai T. A., “ TiO2-based nanotopographical cues attenuate the restenotic phenotype in primary human vascular endothelial and smooth muscle cells,” ACS Biomater. Sci. Eng. 6(2), 923–932 (2020). 10.1021/acsbiomaterials.9b01475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Gong Z., Hu Y., Gao F., Quan L., Liu T., Gong T., and Pan C., “ Effects of diameters and crystals of titanium dioxide nanotube arrays on blood compatibility and endothelial cell behaviors,” Colloids Surf. B 184, 110521 (2019). 10.1016/j.colsurfb.2019.110521 [DOI] [PubMed] [Google Scholar]
- 85. Jin Z., Yan X., Liu G., and Lai M., “ Fibronectin modified TiO2 nanotubes modulate endothelial cell behavior,” J. Biomater. Appl. 33(1), 44–51 (2018). 10.1177/0885328218774512 [DOI] [PubMed] [Google Scholar]
- 86. Zhong S., Luo R., Wang X., Tang L. L., Wu J., Wang J., Huang R., Sun H., and Huang N., “ Effects of polydopamine functionalized titanium dioxide nanotubes on endothelial cell and smooth muscle cell,” Colloids Surf. B 116, 553–560 (2014). 10.1016/j.colsurfb.2014.01.030 [DOI] [PubMed] [Google Scholar]
- 87. Yang Z., Zhong S., Yang Y., Maitz M. F., Li X., Tu Q., Qi P., Zhang H., Qiu H., Wang J., and Huang N., “ Polydopamine-mediated long-term elution of the direct thrombin inhibitor bivalirudin from TiO2 nanotubes for improved vascular biocompatibility,” J. Mater. Chem. B 2(39), 6767–6778 (2014). 10.1039/C4TB01118J [DOI] [PubMed] [Google Scholar]
- 88. Jiang L., Yao H., Luo X., Zou D., Dai S., Liu L., Yang P., Zhao A., and Huang N., “ Polydopamine-modified copper-doped titanium dioxide nanotube arrays for copper-catalyzed controlled endogenous nitric oxide release and improved re-endothelialization,” ACS Appl. Bio Mater. 3(5), 3123–3136 (2020). 10.1021/acsabm.0c00157 [DOI] [PubMed] [Google Scholar]
- 89. Soliman A. M., Tolba S. A., Sharafeldin I. M., Gepreel M. A. H., and Allam N. K., “ Ni-free, built-in nanotubular drug eluting stents: Experimental and theoretical insights,” Mater. Sci. Eng. C 103, 109750 (2019). 10.1016/j.msec.2019.109750 [DOI] [PubMed] [Google Scholar]
- 90. Saleh Y. E., Gepreel M. A., and Allam N. K., “ Functional nanoarchitectures for enhanced drug eluting stents,” Sci. Rep. 7, 40291 (2017). 10.1038/srep40291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Nuhn H., Blanco C. E., and Desai T. A., “ Nanoengineered stent surface to reduce in-stent restenosis in vivo,” ACS Appl. Mater. Interfaces 9(23), 19677–19686 (2017). 10.1021/acsami.7b04626 [DOI] [PubMed] [Google Scholar]
- 92. Lee P. P., Cerchiari A., and Desai T. A., “ Nitinol-based nanotubular coatings for the modulation of human vascular cell function,” Nano Lett. 14(9), 5021–5028 (2014). 10.1021/nl501523v [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Lee P. P. and Desai T. A., “ Nitinol-based nanotubular arrays with controlled diameters upregulate human vascular cell ECM production,” ACS Biomater. Sci. Eng. 2(3), 409–414 (2016). 10.1021/acsbiomaterials.5b00553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Li M., Xu X., Jia Z., Shi Y., Cheng Y., and Zheng Y., “ Rapamycin-loaded nanoporous α-Fe2O3 as an endothelial favorable and thromboresistant coating for biodegradable drug-eluting Fe stent applications,” J. Mater. Chem. B 5(6), 1182–1194 (2017). 10.1039/C6TB02634F [DOI] [PubMed] [Google Scholar]
- 95. Tseng A. A., Chen K., Chen C. D., and Ma K. J., “ Electron beam lithography in nanoscale fabrication: Recent development,” IEEE Trans. Electron. Packag. Manuf. 26(2), 141 (2003). 10.1109/TEPM.2003.817714 [DOI] [Google Scholar]
- 96. Qin D., Xia Y., and Whitesides G. M., “ Soft lithography for micro- and nanoscale patterning,” Nat. Protoc. 5(3), 491 (2010). 10.1038/nprot.2009.234 [DOI] [PubMed] [Google Scholar]
- 97. Xi Y. Y., Hsu Y. F., and Chan W. K., “ Hydrothermal synthesis of nanostructures,” Recent Pat. Nanotechnol. 1(2), 121 (2008). 10.2174/187221007780859591 [DOI] [PubMed] [Google Scholar]
- 98. Rabenau A., “ The role of hydrothermal synthesis in preparative chemistry,” Angew. Chem., Int. Ed. 24, 1026 (1985). 10.1002/anie.198510261 [DOI] [Google Scholar]
- 99. Sikhwivhilu L. M., Sinha Ray S., and Coville N. J., “ Influence of bases on hydrothermal synthesis of titanate nanostructures,” Appl. Phys. A 94(4), 963 (2009). 10.1007/s00339-008-4877-4 [DOI] [Google Scholar]
- 100. Singaravelan R. and Alwar S. B. S., “ Effect of reaction parameters in synthesis, characterisation of electrodeposited zinc nanohexagons,” J. Nanostruct. Chem. 4(4), 109 (2014). 10.1007/s40097-014-0121-2 [DOI] [Google Scholar]
- 101. Mohan C. C., Sreerekha P. R., Divyarani V. V., Nair S., Chennazhi K., and Menon D., “ Influence of titania nanotopography on human vascular cell functionality and its proliferation in vitro,” J. Mater. Chem. 22(4), 1326–1340 (2012). 10.1039/C1JM13726C [DOI] [Google Scholar]
- 102. Mohan C. C., Chennazhi K. P., and Menon D., “ In vitro hemocompatibility and vascular endothelial cell functionality on titania nanostructures under static and dynamic conditions for improved coronary stenting applications,” Acta Biomater. 9(12), 9568–9577 (2013). 10.1016/j.actbio.2013.08.023 [DOI] [PubMed] [Google Scholar]
- 103. Mohan C. C., Cherian A. M., Kurup S., Joseph J., Nair M. B., Vijayakumar M., Nair S. V., and Menon D., “ Stable titania nanostructures on stainless steel coronary stent surface for enhanced corrosion resistance and endothelialization,” Adv. Healthcare Mater. 6(11), 1601353 (2017). 10.1002/adhm.201601353 [DOI] [PubMed] [Google Scholar]
- 104. Cherian A. M., Joseph J., Nair M. B., Nair S. v, Maniyal V., and Menon D., “ Successful reduction of neointimal hyperplasia on stainless steel coronary stents by titania nanotexturing,” ACS Omega 5(28), 17582–17591 (2020). 10.1021/acsomega.0c02045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Mohan C. C., Prabhath A., Cherian A. M., Vadukumpully S., Nair S. V., Chennazhi K., and Menon D., “ Nanotextured stainless steel for improved corrosion resistance and biological response in coronary stenting,” Nanoscale 7(2), 832–841 (2015). 10.1039/C4NR05015K [DOI] [PubMed] [Google Scholar]
- 106. Vishnu J., Calin M., Pilz S., Gebert A., Kaczmarek B., Michalska-Sionkowska M., Hoffmann V., and Manivasagam G., “ Superhydrophilic nanostructured surfaces of beta Ti29Nb alloy for cardiovascular stent applications,” Surf. Coat. Technol. 396, 125965 (2020). 10.1016/j.surfcoat.2020.125965 [DOI] [Google Scholar]
- 107. Loya M. C., Park E., Chen L. H., Brammer K. S., and Jin S., “ Radially arrayed nanopillar formation on metallic stent wire surface via radio-frequency plasma,” Acta Biomater. 6(4), 1671–1677 (2010). 10.1016/j.actbio.2009.11.015 [DOI] [PubMed] [Google Scholar]
- 108. Loya M. C., Brammer K. S., Choi C., Chen L. H., and Jin S., “ Plasma-induced nanopillars on bare metal coronary stent surface for enhanced endothelialization,” Acta Biomater. 6(12), 4589–4595 (2010). 10.1016/j.actbio.2010.07.007 [DOI] [PubMed] [Google Scholar]
- 109. Choudhary S., Berhe M., Haberstroh K. M., and Webster T. J., “ Increased endothelial and vascular smooth muscle cell adhesion on nanostructured titanium and CoCrMo,” Int. J. Nanomed. 1(1), 41 (2006). 10.2147/nano.2006.1.1.41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Choudhary S., Haberstroh K. M., and Webster T. J., “ Enhanced functions of vascular cells on nanostructured Ti for improved stent applications,” Tissue Eng. 13(7), 1421–1430 (2007). 10.1089/ten.2006.0376 [DOI] [PubMed] [Google Scholar]
- 111. Lu J., Rao M. P., MacDonald N. C., Khang D., and Webster T. J., “ Improved endothelial cell adhesion and proliferation on patterned titanium surfaces with rationally designed, micrometer to nanometer features,” Acta Biomater. 4(1), 192–201 (2008). 10.1016/j.actbio.2007.07.008 [DOI] [PubMed] [Google Scholar]
- 112. Mao L., Shen L., Chen J., Wu Y., Kwak M., Lu Y., Xue Q., Pei J., Zhang L., Yuan G., Fan R., Ge J., and Ding W., “ Enhanced bioactivity of Mg-Nd-Zn-Zr alloy achieved with nanoscale MgF2 surface for vascular stent application,” ACS Appl. Mater. Interfaces 7(9), 5320–5330 (2015). 10.1021/am5086885 [DOI] [PubMed] [Google Scholar]
- 113. Aktas C., Haidar A., Miró M. M., Dörrschuck E., Lee J., Veith M., and Abdul-Khaliq H., “ Improved endothelialisation on nanostructured surfaces,” Adv. Mater. Res. 324, 105–108 (2011). 10.4028/www.scientific.net/AMR.324.105 [DOI] [Google Scholar]
- 114. Aktas C., Dörrschuck E., Schuh C., Miró M. M., Lee J., Pütz N., Wennemuth G., Metzger W., Oberringer M., Veith M., and Abdul-Khaliq H., “ Micro- and nanostructured Al2O3 surfaces for controlled vascular endothelial and smooth muscle cell adhesion and proliferation,” Mater. Sci. Eng. C 32(5), 1017–1024 (2012). 10.1016/j.msec.2012.02.032 [DOI] [Google Scholar]
- 115. Lee J., Jeon H., Haidar A., Abdul-Khaliq H., Veith M., Aktas C., and Kim Y., “ Recombinant phage coated 1D Al2O3 nanostructures for controlling the adhesion and proliferation of endothelial cells,” BioMed Res. Int. 2015, 909807. 10.1155/2015/909807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Haidar A., Ali A. A., Veziroglu S., Fiutowski J., Eichler H., Müller I., Kiefer K., Faupel F., Bischoff M., Veith M., Aktas O. C., and Abdul-Khaliq H., “ PTFEP-Al2O3 hybrid nanowires reducing thrombosis and biofouling,” Nanoscale Adv. 1(12), 4659–4664 (2019). 10.1039/C9NA00436J [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Lu J., Yao C., Yang L., and Webster T. J., “ Decreased platelet adhesion and enhanced endothelial cell functions on nano and submicron-rough titanium stents,” Tissue Eng., Part A 18(13-14), 1389–1398 (2012). 10.1089/ten.tea.2011.0268 [DOI] [PubMed] [Google Scholar]
- 118. Pareta R. A., Reising A. B., Miller T., Storey D., and Webster T. J., “ Increased endothelial cell adhesion on plasma modified nanostructured polymeric and metallic surfaces for vascular stent applications,” Biotechnol. Bioeng. 103(3), 459–471 (2009). 10.1002/bit.22276 [DOI] [PubMed] [Google Scholar]
- 119. Das S. K., Dufft D., Rosenfeld A., Bonse J., Bock M., and Grunwald R., “ Femtosecond-laser-induced quasiperiodic nanostructures on TiO2 surfaces,” J. Appl. Phys. 105(8), 084912 (2009). 10.1063/1.3117509 [DOI] [Google Scholar]
- 120. Shinonaga T., Tsukamoto M., Kawa T., Chen P., Nagai A., and Hanawa T., “ Formation of periodic nanostructures using a femtosecond laser to control cell spreading on titanium,” Appl. Phys. B 119(3), 493 (2015). 10.1007/s00340-015-6082-4 [DOI] [Google Scholar]
- 121. Tsukamoto M., Asuka K., Nakano H., Hashida M., Katto M., Abe N., and Fujita M., “ Periodic microstructures produced by femtosecond laser irradiation on titanium plate,” Vacuum 80(11-12), 1346 (2006). 10.1016/j.vacuum.2006.01.016 [DOI] [Google Scholar]
- 122. Nozaki K., Shinonaga T., Ebe N., Horiuchi N., Nakamura M., Tsutsumi Y., Hanawa T., Tsukamoto M., Yamashita K., and Nagai A., “ Hierarchical periodic micro/nano-structures on nitinol and their influence on oriented endothelialization and anti-thrombosis,” Mater. Sci. Eng. C 57, 1–6 (2015). 10.1016/j.msec.2015.07.028 [DOI] [PubMed] [Google Scholar]
- 123. Liang C., Hu Y., Wang H., Xia D., Li Q., Zhang J., Yang J., Li B., Li H., Han D., and Dong M., “ Biomimetic cardiovascular stents for in vivo re-endothelialization,” Biomaterials 103, 170–182 (2016). 10.1016/j.biomaterials.2016.06.042 [DOI] [PubMed] [Google Scholar]
- 124. Muhammad R., Lim S. H., Goh S. H., Law J. B. K., Saifullah M. S. M., Ho G. W., and Yim E. K. F., “ Sub-100 nm patterning of TiO2 film for the regulation of endothelial and smooth muscle cell functions,” Biomater. Sci. 2(12), 1740–1749 (2014). 10.1039/C4BM00212A [DOI] [PubMed] [Google Scholar]
- 125. Lee M. K., Park C., Jang T. S., Kim H. E., and Jeong S. H., “ Enhanced mechanical stability of PTFE coating on nano-roughened NiTi for biomedical applications,” Mater. Lett. 216, 12–15 (2018). 10.1016/j.matlet.2017.12.139 [DOI] [Google Scholar]
- 126. Park C., Kim S., Kim H. E., and Jang T. S., “ Mechanically stable tantalum coating on a nano-roughened NiTi stent for enhanced radiopacity and biocompatibility,” Surf. Coat. Technol. 305, 139–145 (2016). 10.1016/j.surfcoat.2016.08.014 [DOI] [Google Scholar]
- 127. Jang T. S., Lee J. H., Kim S., Park C., Song J., Jae H. J., Kim H. E., Chung J. W., and Jung H. D., “ Ta ion implanted nanoridge-platform for enhanced vascular responses,” Biomaterials 223, 119461 (2019). 10.1016/j.biomaterials.2019.119461 [DOI] [PubMed] [Google Scholar]
- 128. Zhao T., Li Y., Xia Y., Venkatraman S. S., Xiang Y., and Zhao X., “ Formation of a nano-pattering NiTi surface with Ni-depleted superficial layer to promote corrosion resistance and endothelial cell-material interaction,” J. Mater. Sci. 24(1), 105–114 (2013). 10.1007/s10856-012-4777-1 [DOI] [PubMed] [Google Scholar]
- 129. McGinty S., Vo T. T. N., Meere M., McKee S., and McCormick C., “ Some design considerations for polymer-free drug-eluting stents: A mathematical approach,” Acta Biomater. 18, 213 (2015). 10.1016/j.actbio.2015.02.006 [DOI] [PubMed] [Google Scholar]
- 130. Kommineni N., Saka R., Khan W., and Domb A. J., “ Non-polymer drug-eluting coronary stents,” Drug Delivery Transl. Res. 8, 903 (2018). 10.1007/s13346-017-0414-3 [DOI] [PubMed] [Google Scholar]
- 131. Park K. S., Kang S. N., Kim D. H., Kim H. B., Im K. S., Park W., Hong Y. J., Han D. K., and Joung Y. K., “ Late endothelial progenitor cell-capture stents with CD146 antibody and nanostructure reduce in-stent restenosis and thrombosis,” Acta Biomater. 111, 91–101 (2020). 10.1016/j.actbio.2020.05.011 [DOI] [PubMed] [Google Scholar]
- 132. Shen W., Cai K., Yang Z., Yan Y., Yang W., and Liu P., “ Improved endothelialization of NiTi alloy by VEGF functionalized nanocoating,” Colloids Surf. B 94, 347–353 (2012). 10.1016/j.colsurfb.2012.02.009 [DOI] [PubMed] [Google Scholar]
- 133. Wieneke H., Dirsch O., Sawitowski T., Gu Y. L., Brauer H., Dahmen U., Fischer A., Wnendt S., and Erbel R., “ Synergistic effects of a novel nanoporous stent coating and tacrolimus on intima proliferation in rabbits,” Catheterization Cardiovasc. Interventions 60(3), 399–407 (2003). 10.1002/ccd.10664 [DOI] [PubMed] [Google Scholar]
- 134. Kollum M., Farb A., Schreiber R., Terfera K., Arab A., Geist A., Haberstroh J., Wnendt S., Virmani R., and Hehrlein C., “ Particle debris from a nanoporous stent coating obscures potential antiproliferative effects of tacrolimus-eluting stents in a porcine model of restenosis,” Catheterization Cardiovasc. Interventions 64, 85–90 (2005). 10.1002/ccd.20213 [DOI] [PubMed] [Google Scholar]
- 135. Chen M., Zheng B., Wu Z., Peng H. Y., Wang X. G., Zhang B., and Huo Y., “ Efficacy and safety of a novel nano-porous polymer-free sirolimuseluting stent in pigs,” Chin. Med. J. 126(24), 4731–4735 (2013). 10.3760/cma.j.issn.0366-6999.20123267 [DOI] [PubMed] [Google Scholar]
- 136. Jia H., Liu H., Kong J., Hou J., Wu J., Zhang M., Tian J., Liu H., Ma L., Hu S., Huang X., Zhang S., Zhang S., Yu B., and Jang I. K., “ A novel polymer-free paclitaxel-eluting stent with a nanoporous surface for rapid endothelialization and inhibition of intimal hyperplasia: Comparison with a polymer-based sirolimus-eluting stent and bare metal stent in a porcine model,” J. Biomed. Mater. Res., Part A 98(4), 629–637 (2011). 10.1002/jbm.a.33151 [DOI] [PubMed] [Google Scholar]
- 137. Yu Z., Zhu H., Lü S., and Yang X., “ Accelerated endothelialization with a polymer-free sirolimus-eluting antibody-coated stent,” J. Mater. Sci. 24(11), 2601–2609 (2013). 10.1007/s10856-013-5009-z [DOI] [PubMed] [Google Scholar]
- 138. Fu G., Yu Z., Chen Y., Chen Y., Tian F., and Yang X., “ Direct adsorption of anti-CD34 antibodies on the nano-porous stent surface to enhance endothelialization,” Acta Cardiol. Sin. 32(3), 273–280 (2016). 10.6515/ACS20150813A [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Deng J., Han Y., Sun M., Tao J., Yan C., Kang J., and Li S., “ Nanoporous CREG-eluting stent attenuates in-stent neointimal formation in porcine coronary arteries,” PLoS One 8(4), e60735 (2013). 10.1371/journal.pone.0060735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Zhang B., Zheng B., Wang X., Shi Q., Jia J., Huo Y., Pan C., Han J., and Chen M., “ Polymer-free dual drug-eluting stents evaluated in a porcine model,” BMC Cardiovasc. Disord. 17(1), 222 (2017). 10.1186/s12872-017-0654-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Kathuria Y. P., “ The potential of biocompatible metallic stents and preventing restenosis,” Mater. Sci. Eng. A 417(1-2), 40 (2006). 10.1016/j.msea.2005.11.007 [DOI] [Google Scholar]
- 142. Pendyala L., Jabara R., Robinson K., and Chronos N., “ Passive and active polymer coatings for intracoronary stents: Novel devices to promote arterial healing,” J. Interventional Cardiol. 22, 37 (2009). 10.1111/j.1540-8183.2009.00423.x [DOI] [PubMed] [Google Scholar]
- 143. Vladescu A., Surmeneva M. A., Cotrut C. M., Surmenev R. A., and Antoniac I. V., “ Bioceramic coatings for metallic implants,” Handbook of Bioceramics and Biocomposites ( Springer, Berlin, 2016). [Google Scholar]
- 144. Li P. H. and Chu P. K., “ Thin film deposition technologies and processing of biomaterials,” Thin Film Coatings for Biomaterials and Biomedical Applications ( Woodhead Publishing, 2016). [Google Scholar]
- 145. Seshan K., Handbook of Thin Film Deposition: Techniques, Processes, and Technologies, 3rd ed. ( William Andrew, 2012). [Google Scholar]
- 146.Thin Film Coatings for Biomaterials and Biomedical Applications, edited by Griesser H. J. ( Woodhead Publishing, 2016). [Google Scholar]
- 147. Huang N., Yang P., Leng Y. X., Chen J. Y., Sun H., Wang J., Wang G. J., Ding P. D., Xi T. F., and Leng Y., “ Hemocompatibility of titanium oxide films,” Biomaterials 24(13), 2177–2187 (2003). 10.1016/S0142-9612(03)00046-2 [DOI] [PubMed] [Google Scholar]
- 148. Chen J. Y., Leng Y. X., Tian X. B., Wang L. P., Huang N., Chu P. K., and Yang P., “ Antithrombogenic investigation of surface energy and optical bandgap and hemocompatibility mechanism of Ti(Ta + 5) O2 thin films,” Biomaterials 23, 2545 (2002). 10.1016/S0142-9612(01)00389-1 [DOI] [PubMed] [Google Scholar]
- 149. Windecker S., Mayer I., de Pasquale G., Maier W., Dirsch O., de Groot P., Wu Y. P., Noll G., Leskosek B., Meier B., and Hess O. M., “ Stent coating with titanium-nitride-oxide for reduction of neointimal hyperplasia,” Circulation 104(8), 928–933 (2001). 10.1161/hc3401.093146 [DOI] [PubMed] [Google Scholar]
- 150. Tsyganov I., Maitz M. F., Wieser E., Richter E., and Reuther H., “ Correlation between blood compatibility and physical surface properties of titanium-based coatings,” Surf. Coat. Technol. 200(1-4), 1041–1044 (2005). 10.1016/j.surfcoat.2005.02.093 [DOI] [Google Scholar]
- 151. Tsyganov I. A., Maitz M. F., Richter E., Reuther H., Mashina A. I., and Rustichelli F., “ Hemocompatibility of titanium-based coatings prepared by metal plasma immersion ion implantation and deposition,” Nucl. Instrum. Methods Phys. Res., Sect. B 257(1-2), 122–127 (2007). 10.1016/j.nimb.2006.12.169 [DOI] [Google Scholar]
- 152. Yeh H. I., Lu S. K., Tian T. Y., Hong R. C., Lee W. H., and Tsai C. H., “ Comparison of endothelial cells grown on different stent materials,” J. Biomed. Mater. Res., Part A 76(4), 835–841 (2006). 10.1002/jbm.a.30595 [DOI] [PubMed] [Google Scholar]
- 153. Liu H., Zhou S., Wu Q., Pan C., Huang N., and Liu Y., “ Investigation on endothelialization of Ti-O film modified vascular stent in vivo,” Integr. Ferroelectr. 189(1), 78–86 (2018). 10.1080/10584587.2018.1456081 [DOI] [Google Scholar]
- 154. Foruzanmehr M., Hosainalipour S. M., Tehrani S. M., and Aghaeipour M., “ Nano-structure TiO2 film coating on 316L stainless steel via sol-gel technique for blood compatibility improvement,” Nanomed. J. 1, 128 (2014). 10.7508/nmj.2014.03.002 [DOI] [Google Scholar]
- 155. Kopaczyńska M., Sobieszczańska B., Ulatowska-Jarza A., Hołowacz I., Buzalewicz I., Wasyluk Ł., Tofail S. A. M., Biały D., Wawrzyńska M., and Podbielska H., “ Photoactivated titania-based nanomaterials for potential application as cardiovascular stent coatings,” Biocybern. Biomed. Eng. 34(3), 189–197 (2014). 10.1016/j.bbe.2014.03.005 [DOI] [Google Scholar]
- 156. Wawrzyńska M., Kopaczyńska M., Sobieszczańska B., Ulatowska-Jarża A., Hołowacz I., Biały D., Pasławska U., Wasyluk Ł., Tofail S. A. M., Bauer J., and Podbielska H., Intracoronary Application of TiO2-Coated Cardiovascular Stents ( Imperial College Press, 2016). [Google Scholar]
- 157. Yang F., Chang R., and Webster T. J., “ Atomic layer deposition coating of TiO2 nano-thin films on magnesium-zinc alloys to enhance cytocompatibility for bioresorbable vascular stents,” Int. J. Nanomed. 14, 9955–9970 (2019). 10.2147/IJN.S199093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Jiang L., Yao H., Luo X., Zou D., Han C., Tang C., He Y., Yang P., Chen J., Zhao A., and Huang N., “ Copper-mediated synergistic catalytic titanium dioxide nanofilm with nitric oxide generation and anti-protein fouling for enhanced hemocompatibility and inflammatory modulation,” Appl. Mater. Today 20, 100663 (2020). 10.1016/j.apmt.2020.100663 [DOI] [Google Scholar]
- 159. Lim K. S., Jeong M. H., Bae I. H., Park J.-K., Park D. S., Shim J. W., Kim H. B., Jin M. R., Kim H. K., Kim S. S., Kim M. C., Sim D. S., Hong Y. J., Kim J. H., and Ahn Y., “ Effect of a non-polymer titanium dioxide thin film-coated stent with heparin in a porcine coronary restenosis model,” Clin. Exp. Thromb. Hemostasis 3(2), 17–23 (2017). 10.14345/ceth.17005 [DOI] [Google Scholar]
- 160. Lim K. S., Jeong M. H., Bae I. H., Park J. K., Park D. S., Kim J. M., Kim J. H., Kim H. S., Kim Y. S., Jeong H. Y., Song S. J., Yang E. J., Cho D. L., Sim D. S., Park K. H., Hong Y. J., and Ahn Y., “ Effect of polymer-free TiO2 stent coated with abciximab or alpha lipoic acid in porcine coronary restenosis model,” J. Cardiol. 64(5), 409–418 (2014). 10.1016/j.jjcc.2014.02.015 [DOI] [PubMed] [Google Scholar]
- 161. Kwon J. S., Song S. J., Yang E. J., Kim Y. S., Lim K. S., Kim D. G., Cho D. L., Jeong M. H., and Ahn Y., “ Novel abciximab-Kruppel-like factor 4-plasmid dual-delivery titanium dioxide-coated coronary stent,” Int. J. Cardiol. 168(5), 5104–5106 (2013). 10.1016/j.ijcard.2013.07.247 [DOI] [PubMed] [Google Scholar]
- 162. Park D. S., Bae I. H., Jeong M. H., Lim K. S., Hong Y. J., Shim J. W., Kim J. U., Kim M. K., Kim J. H., Hyun D. Y., Park J. K., Lim H. C., Kim H. B., Kim I. S., and Sim D. S., “ Anti-restenotic and anti-thrombotic effect of polymer-free N-TiO2 film-based tacrolimus-eluting stent in a porcine model,” Mater. Today Commun. 22, 100777 (2020). 10.1016/j.mtcomm.2019.100777 [DOI] [Google Scholar]
- 163. Park D. S., Bae I. H., Jeong M. H., Lim K. S., Sim D. S., Hong Y. J., Lee S. Y., Jang E. J., Shim J. W., Park J. K., Lim H. C., and Kim H. B., “ In vitro and in vivo evaluation of a novel polymer-free everolimus-eluting stent by nitrogen-doped titanium dioxide film deposition,” Mater. Sci. Eng. C 91, 615–623 (2018). 10.1016/j.msec.2018.05.064 [DOI] [PubMed] [Google Scholar]
- 164. Xu X., Wang L., Wang G., and Jin Y., “ The effect of REDV/TiO2 coating coronary stents on in-stent restenosis and re-endothelialization,” J. Biomater. Appl. 31(6), 911–922 (2017). 10.1177/0885328216675829 [DOI] [PubMed] [Google Scholar]
- 165. Okamoto K., Nakatani T., Yamashita S., Takabayashi S., and Takahagi T., “ Development of surface-functionalized drug-eluting stent with diamond-like carbon nanocoated by using PECVD method,” Surf. Coat. Technol. 202(22-23), 5750–5752 (2008). 10.1016/j.surfcoat.2008.06.153 [DOI] [Google Scholar]
- 166. Nakatani T., Okamoto K., Omura I., and Yamashita S., “ Application of diamond-like-carbon coating to a coronary artery drug-eluting stent,” J. Photopolym. Sci. Technol. 20, 221–228 (2007). 10.2494/photopolymer.20.221 [DOI] [Google Scholar]
- 167. Bociaga D., Sobczyk-Guzenda A., Komorowski P., Balcerzak J., Jastrzebski K., Przybyszewska K., and Kaczmarek A., “ Surface characteristics and biological evaluation of Si-DLC coatings fabricated using magnetron sputtering method on Ti6Al7Nb substrate,” Nanomaterials 9(6), 812 (2019). 10.3390/nano9060812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Castellino M., Stolojan V., Virga A., Rovere M., Cabiale K., Galloni M. R., and Tagliaferro A., “ Chemico-physical characterisation and in vivo biocompatibility assessment of DLC-coated coronary stents,” Anal. Bioanal. Chem. 405(1), 321–329 (2013). 10.1007/s00216-012-6449-x [DOI] [PubMed] [Google Scholar]
- 169. Kim J. H., Shin J. H., Shin D. H., Moon M. W., Park K., Kim T. H., Shin K. M., Won Y. H., Han D. K., and Lee K. R., “ Comparison of diamond-like carbon-coated nitinol stents with or without polyethylene glycol grafting and uncoated nitinol stents in a canine iliac artery model,” Br. J. Radiol. 84(999), 210–215 (2011). 10.1259/bjr/21667521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. de Scheerder I., Szilard M., Yanming H., Ping X. B., Verbeken E., Neerinck D., Demeyere E., Coppens W., and van de Werf F., “ Evaluation of the biocompatibility of two new diamond-like stent coatings (DylynTM) in a porcine coronary stent model,” J. Invasive Cardiol. 12(8), 389 (2000). [PubMed] [Google Scholar]
- 171. Karagkiozaki V., Karagiannidis P. G., Kalfagiannis N., Kavatzikidou P., Patsalas P., Georgiou D., and Logothetidis S., “ Novel nanostructured biomaterials: Implications for coronary stent thrombosis,” Int. J. Nanomed. 7, 6063–6076 (2012). 10.2147/IJN.S34320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Karagkiozaki V., “ Advances in stent coating technology via nanotechnology tools and process,” Eur. J. Nanomed. 1(1), 24–28 (2008). 10.3884/0001.6 [DOI] [Google Scholar]
- 173. Habibzadeh S., Li L., Omanovic S., Shum-Tim D., and Davis E. C., “ Biocompatibility of Ir/Ti-oxide coatings: Interaction with platelets, endothelial and smooth muscle cells,” Applied Surface Science ( Elsevier B.V., 2014), Vol. 301, pp. 530–538. [Google Scholar]
- 174. Schuler P., Assefa D., Ylanne J., Basler N., Olschewski M., Ahrens I., Nordt T., Bode C., and Peter K., “ Adhesion of monocytes to medical steel as used for vascular stents is mediated by the integrin receptor Mac-1 (CD11b/CD18; α M β 2) and can be inhibited by semiconductor coating,” Cell Commun. Adhes. 10, 17 (2003). 10.1080/15419060302065 [DOI] [PubMed] [Google Scholar]
- 175. Jones J. E., Chen M., and Yu Q., “ Corrosion resistance improvement for 316L stainless steel coronary artery stents by trimethylsilane plasma nanocoatings,” J. Biomed. Mater. Res., Part B 102(7), 1363–1374 (2014). 10.1002/jbm.b.33115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Jones J. E., Yu Q., and Chen M., “ A chemical stability study of trimethylsilane plasma nanocoatings for coronary stents,” J. Biomater. Sci., Polym. Ed. 28(1), 15–32 (2017). 10.1080/09205063.2016.1239947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Zhang Q., Shen Y., Tang C., Wu X., Yu Q., and Wang G., “ Surface modification of coronary stents with SiCOH plasma nanocoatings for improving endothelialization and anticoagulation,” J. Biomed. Mater. Res., Part B 103(2), 464–472 (2015). 10.1002/jbm.b.33229 [DOI] [PubMed] [Google Scholar]
- 178. Jugessur A. S., Textor A., and Grierson C., “ Nanometer scale coating using atomic layer deposition technique to enhance performance of bio-medical devices,” in Proceedings of the 12th IEEE International Conference on Nano/Molecular Medicine and Engineering (2018). [Google Scholar]
- 179. Lin H. C., Chang Y. L., Han Y. Y., Yang K. C., and Chen M. C., “ Atomic layer deposited Al2O3 films on NiTi shape memory alloys for biomedical applications,” Procedia Manuf. 37, 431–437 (2019). 10.1016/j.promfg.2019.12.070 [DOI] [Google Scholar]
- 180. Alviar C. L., Tellez A., Wang M., Potts P., Smith D., Tsui M., Budzynski W., Raizner A. E., Kleiman N. S., Lev E. I., Granada J. F., and Kaluza G. L., “ Low-dose sirolimus-eluting hydroxyapatite coating on stents does not increase platelet activation and adhesion ex vivo,” J. Thromb. Thrombolysis 34(1), 91–98 (2012). 10.1007/s11239-012-0696-8 [DOI] [PubMed] [Google Scholar]
- 181. Jinnouchi H., Mori H., Cheng Q., Kutyna M., Torii S., Sakamoto A., Guo L., Acampado E., Gupta A., Kolodgie F. D., Virmani R., and Finn A. v, “ Thromboresistance and functional healing in the COBRA PzF stent versus competitor DES: Implications for dual antiplatelet therapy,” EuroIntervention 15(4), e342–e353 (2019). 10.4244/EIJ-D-18-00740 [DOI] [PubMed] [Google Scholar]
- 182. Kumbar S. G., Bhattacharyya S., Nukavarapu S. P., Khan Y. M., Nair L. S., and Laurencin C. T., “ In vitro and in vivo characterization of biodegradable poly(organophosphazenes) for biomedical applications,” J. Inorg. Organomet. Polym. Mater. 16(4), 365 (2007). 10.1007/s10904-006-9071-6 [DOI] [Google Scholar]
- 183. Koppara T., Sakakura K., Pacheco E., Cheng Q., Zhao X. Q., Acampado E., Finn A. V., Barakat M., Maillard L., Ren J., Deshpande M., Kolodgie F. D., Joner M., and Virmani R., “ Preclinical evaluation of a novel polyphosphazene surface modified stent. Koppara preclinical testing of a PzF-coated stent,” Int. J. Cardiol. 222, 217–225 (2016). 10.1016/j.ijcard.2016.07.181 [DOI] [PubMed] [Google Scholar]
- 184. Maillard L., Corseaux D., Altié A., Ung A., Courageot J., Barakat M., Teiger E., and van Belle E., “ Time course of reendothelialization with polyzene-F nanocoated cobra PzFTM coronary stent on rabbit iliac arteries,” Cardiovasc. Revasc. Med. 21(2), 195–199 (2020). 10.1016/j.carrev.2019.11.005 [DOI] [PubMed] [Google Scholar]
- 185. Satzl S., Henn C., Christoph P., Kurz P., Stampfl U., Stampfl S., Thomas F., Radeleff B., Berger I., Grunze M., and Richter G. M., “ The efficacy of nanoscale polybis(trifluoroethoxy) phosphazene (PTFEP) coatings in reducing thrombogenicity and late in-stent stenosis in a porcine coronary artery model,” Invest. Radiol. 42, 303 (2007). 10.1097/01.rli.0000261439.90760.9d [DOI] [PubMed] [Google Scholar]
- 186. Stampfl U., Sommer C. M., Thierjung H., Stampfl S., Lopez-Benitez R., Radeleff B., Berger I., and Richter G. M., “ Reduction of late in-stent stenosis in a porcine coronary artery model by cobalt chromium stents with a nanocoat of polyphosphazene (polyzene-F),” Cardiovasc. Interventional Radiol. 31(6), 1184–1192 (2008). 10.1007/s00270-008-9392-7 [DOI] [PubMed] [Google Scholar]
- 187. Oh B. and Lee C. H., “ Nanofiber-coated drug eluting stent for the stabilization of mast cells,” Pharm. Res. 31(9), 2463 (2014). 10.1007/s11095-014-1341-3 [DOI] [PubMed] [Google Scholar]
- 188. Cleeton C., Keirouz A., Chen X., and Radacsi N., “ Electrospun nanofibers for drug delivery and biosensing,” ACS Biomater. Sci. Eng. 5(9), 4183–4205 (2019). 10.1021/acsbiomaterials.9b00853 [DOI] [PubMed] [Google Scholar]
- 189. Zhu Y. Q., Cui W. G., Cheng Y. S., Chang J., Chen N. W., and Yan L., “ Evaluation of biodegradable paclitaxel-eluting nanofibre-covered metal stents for the treatment of benign cardia stricture in an experimental model,” Br. J. Surg. 100(6), 784 (2013). 10.1002/bjs.9106 [DOI] [PubMed] [Google Scholar]
- 190. Oh B. and Lee C. H., “ Advanced cardiovascular stent coated with nanofiber,” Mol. Pharm. 10(12), 4432–4442 (2013). 10.1021/mp400231p [DOI] [PubMed] [Google Scholar]
- 191. Oh B. and Lee C. H., “ Nanofiber for cardiovascular tissue engineering,” Exp. Opin. Drug Delivery 10, 1565 (2013). 10.1517/17425247.2013.830608 [DOI] [PubMed] [Google Scholar]
- 192. Siminiak T., Link R., Wołoszyn M., Kałmucki P., and Baszko A., “ The effect of stent coating on stent deliverability: Direct randomised comparison of drug eluting and bare metal stents using the same stent platform,” Kardiol. Pol. 70(10), 998 (2012). [PubMed] [Google Scholar]
- 193. Barhoum A., Pal K., Rahier H., Uludag H., Kim I. S., and Bechelany M., “ Nanofibers as new-generation materials: From spinning and nano-spinning fabrication techniques to emerging applications,” Appl. Mater. Today 17, 1 (2019). 10.1016/j.apmt.2019.06.015 [DOI] [Google Scholar]
- 194. Toriello M., Afsari M., Shon H. K., and Tijing L. D., “ Progress on the fabrication and application of electrospun nanofiber composites,” Membranes 10, 204 (2020). 10.3390/membranes10090204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Jose Varghese R., Sakho E. H. M., Parani S., Thomas S., Oluwafemi O. S., and Wu J., “ Introduction to nanomaterials: Synthesis and applications,” Nanomaterials for Solar Cell Applications ( Elsevier, 2019). [Google Scholar]
- 196. Wang C., Wang J., Zeng L., Qiao Z., Liu X., Liu H., Zhang J., and Ding J., “ Fabrication of electrospun polymer nanofibers with diverse morphologies,” Molecules 24, 834 (2019). 10.3390/molecules24050834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Kersani D., Mougin J., Lopez M., Degoutin S., Tabary N., Cazaux F., Janus L., Maton M., Chai F., Sobocinski J., Blanchemain N., and Martel B., “ Stent coating by electrospinning with chitosan/poly-cyclodextrin based nanofibers loaded with simvastatin for restenosis prevention,” Eur. J. Pharm. Biopharm. 150, 156–167 (2020). 10.1016/j.ejpb.2019.12.017 [DOI] [PubMed] [Google Scholar]
- 198. Khashi M., Hassanajili S., and Golestaneh S. I., “ Electrospun poly-lactic acid/chitosan nanofibers loaded with paclitaxel for coating of a prototype polymeric stent,” Fibers Polym. 19(7), 1444–1453 (2018). 10.1007/s12221-018-8037-y [DOI] [Google Scholar]
- 199. Kuznetsov K. A., Murashov I. S., Chernonosova V. S., Chelobanov B. P., Stepanova A. O., Sergeevichev D. S., Karpenko A. A., and Laktionov P. P., “ Vascular stents coated with electrospun drug-eluting material: Functioning in rabbit iliac artery,” Polymers 12(8), 1741 (2020). 10.3390/polym12081741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Janjic M., Pappa F., Karagkiozaki V., Gitas C., Ktenidis K., and Logothetidis S., “ Surface modification of endovascular stents with rosuvastatin and heparin-loaded biodegradable nanofibers by electrospinning,” Int. J. Nanomed. 12, 6343–6355 (2017). 10.2147/IJN.S138261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Chang S. H., Lee C. H., Yeh Y. H., Liu S. J., Wang C. J., Hsu M. Y., and Chen W. J., “ Propylthiouracil-coated biodegradable polymer inhibited neointimal formation and enhanced re-endothelialization after vascular injury,” Int. J. Nanomed. 13, 1761–1771 (2018). 10.2147/IJN.S145528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Wang H., Xia Y., Liu J., Ma Z., Shi Q., and Yin J., “ Programmable release of 2-O-D-glucopyranosyl-L-ascorbic acid and heparin from PCL-based nanofiber scaffold for reduction of inflammation and thrombosis,” Mater. Today Chem. 17, 100303 (2020). 10.1016/j.mtchem.2020.100303 [DOI] [Google Scholar]
- 203. Repanas A., Bader A., Klett A., Ngezahayo A., and Glasmacher B., “ The effect of dipyridamole embedded in a drug delivery system made by electrospun nanofibers on aortic endothelial cells,” J. Drug Delivery Sci. Technol. 35, 343–352 (2016). 10.1016/j.jddst.2016.08.011 [DOI] [Google Scholar]
- 204. Bakola V., Karagkiozaki V., Tsiapla A. R., Pappa F., Moutsios I., Pavlidou E., and Logothetidis S., “ Dipyridamole-loaded biodegradable PLA nanoplatforms as coatings for cardiovascular stents,” Nanotechnology 29(27), 275101 (2018). 10.1088/1361-6528/aabc69 [DOI] [PubMed] [Google Scholar]
- 205. Lee C. H., Hsieh M. J., Liu K. S., Cheng C. W., Chang S. H., Liu S. J., Wang C. J., Hsu M. Y., Hung K. C., Yeh Y. H., Chen W. J., Hsieh I. C., Juang J. H., and Wen M. S., “ Promoting vascular healing using nanofibrous ticagrelor-eluting stents,” Int. J. Nanomed. 13, 6039–6048 (2018). 10.2147/IJN.S166785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Lee C. H., Lin Y. H., Chang S. H., Tai C. D., Liu S. J., Chu Y., Wang C. J., Hsu M. Y., Chang H., Chang G. J., Hung K. C., Hsieh M. J., Lin F. C., Hsieh I. C., Wen M. S., and Huang Y., “ Local sustained delivery of acetylsalicylic acid via hybrid stent with biodegradable nanofibers reduces adhesion of blood cells and promotes reendothelialization of the denuded artery,” Int. J. Nanomed. 9(1), 311–326 (2014). 10.2147/IJN.S51258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Lee C. H., Hsieh M. J., Chang S. H., Hung K. C., Wang C. J., Hsu M. Y., Juang J. H., Hsieh I. C., Wen M. S., and Liu S. J., “ Nanofibrous vildagliptin-eluting stents enhance re-endothelialization and reduce neointimal formation in diabetes: In vitro and in vivo,” Int. J. Nanomed. 14, 7503–7513 (2019). 10.2147/IJN.S211898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Lee C. H., Hsieh M. J., Chang S. H., Chiang C. L., Fan C. L., Liu S. J., Chen W. J., Wang C. J., Hsu M. Y., Hung K. C., Chou C. C., and Chang P. C., “ Biodegradable cable-tie rapamycin-eluting stents,” Sci. Rep. 7(1), 111 (2017). 10.1038/s41598-017-00131-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Son Y. J., Kim H. S., Choi D. H., and Yoo H. S., “ Multilayered electrospun fibrous meshes for restenosis-suppressing metallic stents,” J. Biomed. Mater. Res., Part B 105(3), 628–635 (2017). 10.1002/jbm.b.33583 [DOI] [PubMed] [Google Scholar]
- 210. Tang J., Liu Y., Zhu B., Su Y., and Zhu X., “ Preparation of paclitaxel/chitosan co-assembled core-shell nanofibers for drug-eluting stent,” Appl. Surf. Sci. 393, 299–308 (2017). 10.1016/j.apsusc.2016.10.015 [DOI] [Google Scholar]
- 211. Merkle V. M., Tran P. L., Hutchinson M., Ammann K. R., Decook K., Wu X., and Slepian M. J., “ Core-shell PVA/gelatin electrospun nanofibers promote human umbilical vein endothelial cell and smooth muscle cell proliferation and migration,” Acta Biomater. 27, 77–87 (2015). 10.1016/j.actbio.2015.08.044 [DOI] [PubMed] [Google Scholar]
- 212. Merkle V. M., Martin D., Hutchinson M., Tran P. L., Behrens A., Hossainy S., Sheriff J., Bluestein D., Wu X., and Slepian M. J., “ Hemocompatibility of poly(vinyl alcohol)-gelatin core-shell electrospun nanofibers: A scaffold for modulating platelet deposition and activation,” ACS Appl. Mater. Interfaces 7(15), 8302–8312 (2015). 10.1021/acsami.5b01671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Ceylan H., Tekinay A. B., and Guler M. O., “ Selective adhesion and growth of vascular endothelial cells on bioactive peptide nanofiber functionalized stainless steel surface,” Biomaterials 32(34), 8797–8805 (2011). 10.1016/j.biomaterials.2011.08.018 [DOI] [PubMed] [Google Scholar]
- 214. Kushwaha M., Anderson J. M., Bosworth C. A., Andukuri A., Minor W. P., Lancaster J. R., Anderson P. G., Brott B. C., and Jun H. W., “ A nitric oxide releasing, self assembled peptide amphiphile matrix that mimics native endothelium for coating implantable cardiovascular devices,” Biomaterials 31(7), 1502–1508 (2010). 10.1016/j.biomaterials.2009.10.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Andukuri A., Kushwaha M., Tambralli A., Anderson J. M., Dean D. R., Berry J. L., Sohn Y. D., Yoon Y. S., Brott B. C., and Jun H. W., “ A hybrid biomimetic nanomatrix composed of electrospun polycaprolactone and bioactive peptide amphiphiles for cardiovascular implants,” Acta Biomater. 7(1), 225–233 (2011). 10.1016/j.actbio.2010.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Alexander G. C., Vines J. B., Hwang P., Kim T., Kim J. A., Brott B. C., Yoon Y. S., and Jun H. W., “ Novel multifunctional nanomatrix reduces inflammation in dynamic conditions in vitro and dilates arteries ex vivo,” ACS Appl. Mater. Interfaces 8(8), 5178–5187 (2016). 10.1021/acsami.6b00565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Cicha I., Lyer S., Alexiou C., and Garlichs C. D., “ Nanomedicine in diagnostics and therapy of cardiovascular diseases: Beyond atherosclerotic plaque imaging,” Nanotechnol. Rev. 2, 449–472 (2013). 10.1515/ntrev-2013-0009 [DOI] [Google Scholar]
- 218. Brieger D. and Topol E., “ Local drug delivery systems and prevention of restenosis,” Cardiovasc. Res. 35, 405 (1997). 10.1016/S0008-6363(97)00155-7 [DOI] [PubMed] [Google Scholar]
- 219. Brito L. and Amiji M., “ Nanoparticulate carriers for the treatment of coronary restenosis,” Int. J. Nanomed. 2, 143 (2007). [PMC free article] [PubMed] [Google Scholar]
- 220. Rhee J. W. and Wu J. C., “ Advances in nanotechnology for the management of coronary artery disease,” Trends Cardiovasc. Med. 23, 39 (2013). 10.1016/j.tcm.2012.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Garg S., Bourantas C., and Serruys P. W., “ New concepts in the design of drug-eluting coronary stents,” Nat. Rev. Cardiol. 10, 248 (2013). 10.1038/nrcardio.2013.13 [DOI] [PubMed] [Google Scholar]
- 222. Cartiera M. S., Johnson K. M., Rajendran V., Caplan M. J., and Saltzman W. M., “ The uptake and intracellular fate of PLGA nanoparticles in epithelial cells,” Biomaterials 30(14), 2790–2798 (2009). 10.1016/j.biomaterials.2009.01.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Singh R. and Lillard J. W., “ Nanoparticle-based targeted drug delivery,” Exp. Mol. Pathol. 86, 215 (2009). 10.1016/j.yexmp.2008.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Strohbach A. and Busch R., “ Polymers for cardiovascular stent coatings,” Int. J. Polym. Sci. 2015, 1. 10.1155/2015/782653 [DOI] [Google Scholar]
- 225. Luderer F., Löbler M., Rohm H. W., Gocke C., Kunna K., Köck K., Kroemer H. K., Weitschies W., Schmitz K. P., and Sternberg K., “ Biodegradable sirolimus-loaded poly(lactide) nanoparticles as drug delivery system for the prevention of in-stent restenosis in coronary stent application,” J. Biomater. Appl. 25(8), 851–875 (2011). 10.1177/0885328209360696 [DOI] [PubMed] [Google Scholar]
- 226. Zhao J., Mo Z., Guo F., Shi D., Han Q. Q., and Liu Q., “ Drug loaded nanoparticle coating on totally bioresorbable PLLA stents to prevent in-stent restenosis,” J. Biomed. Mater. Res., Part B 106(1), 88–95 (2018). 10.1002/jbm.b.33794 [DOI] [PubMed] [Google Scholar]
- 227. Zweers M. L. T., Engbers G. H. M., Grijpma D. W., and Feijen J., “ Release of anti-restenosis drugs from poly(ethylene oxide)-poly(dl-lactic-co-glycolic acid) nanoparticles ,” J. Controlled Release 114(3), 317–324 (2006). 10.1016/j.jconrel.2006.05.021 [DOI] [PubMed] [Google Scholar]
- 228. Bakola V., Tsiapla A. R., Karagkiozaki V., Pappa F., Pavlidou E., Moutsios I., Gravalidis C., and Logothetidis S., “ Electrospray encapsulation of antithrombotic drug into poly (l-lactic acid) nanoparticles for cardiovascular applications,” Mater. Today 19, 102–109 (2019). 10.1016/j.matpr.2019.07.664 [DOI] [Google Scholar]
- 229. Nakano K., Egashira K., Masuda S., Funakoshi K., Zhao G., Kimura S., Matoba T., Sueishi K., Endo Y., Kawashima Y., Hara K., Tsujimoto H., Tominaga R., and Sunagawa K., “ Formulation of nanoparticle-eluting stents by a cationic electrodeposition coating technology. Efficient nano-drug delivery via bioabsorbable polymeric nanoparticle-eluting stents in porcine coronary arteries,” JACC: Cardiovasc. Interventions 2(4), 277–283 (2009). 10.1016/j.jcin.2008.08.023 [DOI] [PubMed] [Google Scholar]
- 230. Masuda S., Nakano K., Funakoshi K., Zhao G., Meng W., Kimura S., Matoba T., Miyagawa M., Iwata E., Sunagawa K., and Egashira K., “ Imatinib mesylate-incorporated nanoparticle-eluting stent attenuates in-stent neointimal formation in porcine coronary arteries,” J. Atheroscler. Thromb. 18, 1043 (2011). 10.5551/jat.8730 [DOI] [PubMed] [Google Scholar]
- 231. Tsukie N., Nakano K., Matoba T., Masuda S., Iwata E., Miyagawa M., Zhao G., Meng W., Kishimoto J., Sunagawa K., and Egashira K., “ Pitavastatin-incorporated nanoparticle-eluting stents attenuate in-stent stenosis without delayed endothelial healing effects in a porcine coronary artery model,” J. Atheroscler. Thromb. 20, 32 (2013). 10.5551/jat.13862 [DOI] [PubMed] [Google Scholar]
- 232. Takimura C. K., Galon M. Z., Gutierrez P. S., Sojitra P., Vyas A., Doshi M., and Lemos P. A., “ A new polymer-free drug-eluting stent with nanocarriers eluting sirolimus from stent-plus-balloon compared with bare-metal stent and with biolimus A9 eluting stent in porcine coronary arteries,” Cardiovasc. Diagn. Ther. 5(2), 113–121 (2015). 10.3978/j.issn.2223-3652.2015.03.08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Che H. L., Bae I. H., Lim K. S., Song I. T., Lee H., Lee D., Kim W. J., Jeong M. H., Park I. K., and Ahn Y., “ Therapeutic effect of Akt1 SiRNA nanoparticle eluting coronary stent on suppression of post-angioplasty restenosis,” J. Biomed. Nanotechnol. 12(6), 1211–1222 (2016). 10.1166/jbn.2016.2255 [DOI] [PubMed] [Google Scholar]
- 234. Yang J., Zeng Y., Zhang C., Chen Y. X., Yang Z., Li Y., Leng X., Kong D., Wei X. Q., Sun H. F., and Song C. X., “ The prevention of restenosis in vivo with a VEGF gene and paclitaxel co-eluting stent,” Biomaterials 34(6), 1635–1643 (2013). 10.1016/j.biomaterials.2012.11.006 [DOI] [PubMed] [Google Scholar]
- 235. Izuhara M., Kuwabara Y., Saito N., Yamamoto E., Hakuno D., Nakashima Y., Horie T., Baba O., Nishiga M., Nakao T., Nishino T., Nakazeki F., Ide Y., Kimura M., Kimura T., and Ono K., “ Prevention of neointimal formation using MiRNA-126-containing nanoparticle-conjugated stents in a rabbit model,” PLoS One 12(3), e0172798 (2017). 10.1371/journal.pone.0172798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Li B. C., Chang H., Ren K. F., and Ji J., “ Substrate-mediated delivery of gene complex nanoparticles via polydopamine coating for enhancing competitiveness of endothelial cells,” Colloids Surf. B 147, 172–179 (2016). 10.1016/j.colsurfb.2016.07.063 [DOI] [PubMed] [Google Scholar]
- 237. Paul A., Shao W., Shum-Tim D., and Prakash S., “ The attenuation of restenosis following arterial gene transfer using carbon nanotube coated stent incorporating TAT/DNAAng1+Vegf nanoparticles,” Biomaterials 33(30), 7655–7664 (2012). 10.1016/j.biomaterials.2012.06.096 [DOI] [PubMed] [Google Scholar]
- 238. Tan J., Cui Y., Zeng Z., Wei L., Li L., Wang H., Hu H., Liu T., Huang N., Chen J., and Weng Y., “ Heparin/poly-l-lysine nanoplatform with growth factor delivery for surface modification of cardiovascular stents: The influence of vascular endothelial growth factor loading,” J. Biomed. Mater. Res., Part A 108(6), 1295–1304 (2020). 10.1002/jbm.a.36902 [DOI] [PubMed] [Google Scholar]
- 239. Liu T., Hu Y., Tan J., Liu S., Chen J., Guo X., Pan C., and Li X., “ Surface biomimetic modification with laminin-loaded heparin/poly-l-lysine nanoparticles for improving the biocompatibility,” Mater. Sci. Eng. C 71, 929–936 (2017). 10.1016/j.msec.2016.11.010 [DOI] [PubMed] [Google Scholar]
- 240. Liu S., Hu Y., Tao R., Huo Q., Wang L., Tang C., Pan C., Gong T., Xu N., Liu T., and Li J., “ Immobilization of fibronectin-loaded polyelectrolyte nanoparticles on cardiovascular material surface to improve the biocompatibility,” BioMed Res. Int. 2019, 5478369. 10.1155/2019/5478369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Luo R., Zhang J., Zhuang W., Deng L., Li L., Yu H., Wang J., Huang N., and Wang Y., “ Multifunctional coatings that mimic the endothelium: surface bound active heparin nanoparticles with: In situ generation of nitric oxide from nitrosothiols,” J. Mater. Chem. B 6(35), 5582–5595 (2018). 10.1039/C8TB00596F [DOI] [PubMed] [Google Scholar]
- 242. Zhu T., Zhou M., Gao W., Fang D., Liu Z., Wu G., Wan M., Mao C., and Shen J., “ Coronary stents decorated by heparin/NONOate nanoparticles for anticoagulant and endothelialized effects,” Langmuir 36(11), 2901–2910 (2020). 10.1021/acs.langmuir.0c00112 [DOI] [PubMed] [Google Scholar]
- 243. Mohammadi F., Golafshan N., Kharaziha M., and Ashrafi A., “ Chitosan-heparin nanoparticle coating on anodized NiTi for improvement of blood compatibility and biocompatibility,” Int. J. Biol. Macromol. 127, 159–168 (2019). 10.1016/j.ijbiomac.2019.01.026 [DOI] [PubMed] [Google Scholar]
- 244. Feng G., Nguyen T. D., Yi X., Lyu Y., Lan Z., Xia J., Wu T., and Jiang X., “ Evaluation of long-term inflammatory responses after implantation of a novel fully bioabsorbable scaffold composed of poly-l-lactic acid and amorphous calcium phosphate nanoparticles,” J. Nanomater. 2018, 1519480. 10.1155/2018/1519480 [DOI] [Google Scholar]
- 245. Zheng X., Wang Y., Lan Z., Lyu Y., Feng G., Zhang Y., Tagusari S., Kislauskis E., Robich M. P., McCarthy S., Sellke F. W., Laham R., Jiang X., Gu W. W., and Wu T., “ Improved biocompatibility of poly(lactic-co-glycolic acid) and poly-l-lactic acid blended with nanoparticulate amorphous calcium phosphate in vascular stent applications,” J. Biomed. Nanotechnol. 10(6), 900–910 (2014). 10.1166/jbn.2014.1856 [DOI] [PubMed] [Google Scholar]
- 246. Gu D., Feng G., Kang G., Zheng X., Bi Y., Wang S., Fan J., Xia J., Wang Z., Huo Z., Wang Q., Wu T., Jiang X., Gu W., and Xiao J., “ Improved biocompatibility of novel biodegradable scaffold composed of poly-l-lactic acid and amorphous calcium phosphate nanoparticles in porcine coronary artery,” J. Nanomater. 2016, 1. 10.1155/2016/2710858 [DOI] [Google Scholar]
- 247. Dinh Nguyen T., Feng G., Yi X., Lyu Y., Lan Z., Xia J., Wu T., and Jiang X., “ Six-month evaluation of novel bioabsorbable scaffolds composed of poly-l-lactic acid and amorphous calcium phosphate nanoparticles in porcine coronary arteries,” J. Biomater. Appl. 33(2), 227–233 (2018). 10.1177/0885328218790332 [DOI] [PubMed] [Google Scholar]
- 248. Lan Z., Lyu Y., Xiao J., Zheng X., He S., Feng G., Zhang Y., Wang S., Kislauskis E., Chen J., McCarthy S., Laham R., Jiang X., and Wu T., “ Novel biodegradable drug-eluting stent composed of poly-l-lactic acid and amorphous calcium phosphate nanoparticles demonstrates improved structural and functional performance for coronary artery disease,” J. Biomed. Nanotechnol. 10(7), 1194–1204 (2014). 10.1166/jbn.2014.1868 [DOI] [PubMed] [Google Scholar]
- 249. Xiao J., Feng G., Kang G., Lan Z., Liao T., Kislauskis E., Chen J., Xia J., Wang Z., Huo Z., Wang Q., Xi T., McCarthy S., Jiang X., Wu T., and Laham R., “ 6-Month follow-up of a novel biodegradable drug-eluting stent composed of poly-l-lactic acid and amorphous calcium phosphate nanoparticles in porcine coronary artery,” J. Biomed. Nanotechnol. 11(10), 1819–1825 (2015). 10.1166/jbn.2015.2102 [DOI] [PubMed] [Google Scholar]
- 250. Wang X.-G., Hou C., Zheng B., Shi Q.-P., Zhang B., Qin Q., Chen D.-D., and Chen M., “ Evaluation of a novel fully bioresorbable poly-l-lactic acid/amorphous calcium phosphate scaffolds in porcine coronary arteries by quantitative coronary angiography and histopathology,” Int. J. Clin. Exp. Med. 10, 3131 (2017). [Google Scholar]
- 251. Fan Y., Zhang Y., Zhao Q., Xie Y., Luo R., Yang P., and Weng Y., “ Immobilization of nano Cu-MOFs with polydopamine coating for adaptable gasotransmitter generation and copper ion delivery on cardiovascular stents,” Biomaterials 204, 36–45 (2019). 10.1016/j.biomaterials.2019.03.007 [DOI] [PubMed] [Google Scholar]
- 252. Tan A., Farhatnia Y., and Seifalian A. M., “ Polyhedral oligomeric silsesquioxane poly(carbonate-urea) urethane (POSS-PCU): Applications in nanotechnology and regenerative medicine,” Crit. Rev. Biomed. Eng. 41, 495 (2013). [PubMed] [Google Scholar]
- 253. Bakhshi R., Darbyshire A., Evans J. E., You Z., Lu J., and Seifalian A. M., “ Polymeric coating of surface modified nitinol stent with POSS-nanocomposite polymer,” Colloids Surf. B 86(1), 93–105 (2011). 10.1016/j.colsurfb.2011.03.024 [DOI] [PubMed] [Google Scholar]
- 254. Tan A., Farhatnia Y., Goh D., de Mel A., Lim J., Teoh S.-H., Malkovskiy A. V., Chawla R., Rajadas J., Cousins B. G., Hamblin M. R., Alavijeh M. S., and Seifalian A. M., “ Surface modification of a polyhedral oligomeric silsesquioxane poly(carbonate-urea) urethane (POSS-PCU) nanocomposite polymer as a stent coating for enhanced capture of endothelial progenitor cells,” Biointerphases 8, 23 (2013). 10.1186/1559-4106-8-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Farhatnia Y., Pang J. H., Darbyshire A., Dee R., Tan A., and Seifalian A. M., “ Next generation covered stents made from nanocomposite materials: A complete assessment of uniformity, integrity and biomechanical properties,” Nanomedicine 12(1), 1–12 (2016). 10.1016/j.nano.2015.07.002 [DOI] [PubMed] [Google Scholar]
- 256. Wang Y., Zhang W., Zhang J., Sun W., Zhang R., and Gu H., “ Fabrication of a novel polymer-free nanostructured drug-eluting coating for cardiovascular stents,” ACS Appl. Mater. Interfaces 5(20), 10337–10345 (2013). 10.1021/am403365j [DOI] [PubMed] [Google Scholar]
- 257. Chen J., Dai S., Liu L., Maitz M. F., Liao Y., Cui J., Zhao A., Yang P., Huang N., and Wang Y., “ Photo-functionalized TiO2 nanotubes decorated with multifunctional Ag nanoparticles for enhanced vascular biocompatibility,” Bioact. Mater. 6(1), 45–54 (2021). 10.1016/j.bioactmat.2020.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258. Tan A., Farhatnia Y., de Mel A., Rajadas J., Alavijeh M. S., and Seifalian A. M., “ Inception to actualization: Next generation coronary stent coatings incorporating nanotechnology,” J. Biotechnol. 164, 151–170 (2013). 10.1016/j.jbiotec.2013.01.020 [DOI] [PubMed] [Google Scholar]
- 259. Windecker S., Simon R., Lins M., Klauss V., Eberli F. R., Roffi M., Pedrazzini G., Moccetti T., Wenaweser P., Togni M., Tüller D., Zbinden R., Seiler C., Mehilli J., Kastrati A., Meier B., and Hess O. M., “ Randomized comparison of a titanium-nitride-oxide-coated stent with a stainless steel stent for coronary revascularization: The TiNOX trial,” Circulation 111(20), 2617–2622 (2005). 10.1161/CIRCULATIONAHA.104.486647 [DOI] [PubMed] [Google Scholar]
- 260. Pilgrim T., Rber L., Limacher A., Löffel L., Wenaweser P., Cook S., Stauffer J. C., Togni M., Vogel R., Garachemani A., Moschovitis A., Khattab A. A., Seiler C., Meier B., Jüni P., and Windecker S., “ Comparison of titanium-nitride-oxidecoated stents with zotarolimus-eluting stents for coronary revascularization: A randomized controlled trial,” JACC: Cardiovasc. Interventions 4(6), 672–682 (2011). 10.1016/j.jcin.2011.02.017 [DOI] [PubMed] [Google Scholar]
- 261. Windecker S., Billinger M., and Hes O. M., “ Stent coating with titanium-nitride-oxide for prevention of restenosis,” EuroIntervention 2, 146 (2006). [PubMed] [Google Scholar]
- 262. Karjalainen P. P., Ylitalo A. S., Airaksinen K. E. J., and Juhani Airaksinen K. E., “ Reprint real world experience with the TITAN® stent: A 9-month follow-up report from the Titan PORI registry,” Eurointervention 2, 187 (2006). 10.4244/EIJV2I2A32 [DOI] [PubMed] [Google Scholar]
- 263. Tuomainen P. O., Ylitalo A., Niemelä M., Kervinen K., Pietilä M., Sia J., Nyman K., Nammas W., Airaksinen K. E. J., and Karjalainen P. P., “ Five-year clinical outcome of titanium-nitride-oxide-coated bioactive stents versus paclitaxel-eluting stents in patients with acute myocardial infarction: long-term follow-up from the TITAX AMI trial,” Int. J. Cardiol. 168(2), 1214–1219 (2013). 10.1016/j.ijcard.2012.11.060 [DOI] [PubMed] [Google Scholar]
- 264. Karjalainen P. P., Nammas W., Ylitalo A., de Bruyne B., Lalmand J., de Belder A., Rivero-Crespo F., Kervinen K., and Airaksinen J. K. E., “ Long-term clinical outcome of titanium-nitride-oxide-coated stents versus everolimus-eluting stents in acute coronary syndrome: Final report of the BASE ACS trial,” Int. J. Cardiol. 222, 275–280 (2016). 10.1016/j.ijcard.2016.07.267 [DOI] [PubMed] [Google Scholar]
- 265. Tonino P. A. L., Pijls N. H. J., Collet C., Nammas W., van der Heyden J., Romppanen H., Kervinen K., Airaksinen J. K. E., Sia J., Lalmand J., Frambach P., Penaranda A. S., de Bruyne B., and Karjalainen P. P., “ Titanium-nitride-oxide–coated versus everolimus-eluting stents in acute coronary syndrome: The randomized TIDES-ACS trial,” JACC: Cardiovasc. Interventions 13(14), 1697–1705 (2020). 10.1016/j.jcin.2020.04.021 [DOI] [PubMed] [Google Scholar]
- 266. Kesavan S., Strange J. W., Johnson T. W., Flohr-Roese S., and Baumbach A., “ First-in-man evaluation of the MOMO cobalt-chromium carbon-coated stent,” EuroIntervention 8(9), 1012–1018 (2013). 10.4244/EIJV8I9A156 [DOI] [PubMed] [Google Scholar]
- 267. Ando K., Ishii K., Tada E., Kataoka K., Hirohata A., Goto K., Kobayashi K., Tsutsui H., Nakahama M., Nakashima H., Uchikawa S., Kanda J., Yasuda S., Yajima J., Kitabayashi H., Sakurai S., Nakanishi K., Inoue N., Noike H., Hasebe T., Sato T., Yamasaki M., and Kimura T., “ Prospective multi-center registry to evaluate efficacy and safety of the newly developed diamond-like carbon-coated cobalt–chromium coronary stent system,” Cardiovasc. Intervention Ther. 32(3), 225–232 (2017). 10.1007/s12928-016-0407-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268. Unverdorben M., Sattler K., Degenhardt R., Fries R., Abt B., Wagner E., Koehler H., Scholz M., Ibrahim H., Tews K.-H., Hennen B., Daemgen G., Berthold H. K., and Vallbracht C., “ Comparison of a silicon carbide coated stent versus a noncoated stent in humans: The Tenax TM-versus Nir TM-Stent Study (TENISS),” J. Interventional Cardiol. 16(4), 325–333 (2003). 10.1034/j.1600-6143.2003.08058.x [DOI] [PubMed] [Google Scholar]
- 269. Unverdorben M., Sippel B., Degenhardt R., Sattler K., Fries R., Abt B., Wagner E., Koehler H., Daemgen G., Scholz M., Ibrahim H., Tews K. H., Hennen B., Berthold H. K., and Vallbracht C., “ Comparison of a silicon carbide-coated stent versus a noncoated stent in human beings: The Tenax versus Nir stent study's long-term outcome,” Am. Heart J. 145(4), E17 (2003). 10.1067/mhj.2003.90 [DOI] [PubMed] [Google Scholar]
- 270. Asano T., Suwannasom P., Katagiri Y., Miyazaki Y., Sotomi Y., Kraak R. P., Wykrzykowska J., Rensing B. J., Piek J. J., Gyöngyösi M., Serruys P. W., and Onuma Y., “ First-in-man trial of SiO2 inert-coated bare metal stent system in native coronary stenosis: The AXETIS FIM trial,” Circ. J. 82(2), 477–485 (2018). 10.1253/circj.CJ-17-0337 [DOI] [PubMed] [Google Scholar]
- 271. Bates M. C., Yousaf A., Sun L., Barakat M., and Kueller A., “ Translational research and early favorable clinical results of a novel polyphosphazene (polyzene-F) nanocoating,” Regener. Eng. Transl. Med. 5(4), 341–353 (2019). 10.1007/s40883-019-00097-3 [DOI] [Google Scholar]
- 272. Cutlip D. E., Garratt K. N., Novack V., Barakat M., Meraj P., Maillard L., Erglis A., Jauhar R., Popma J., Stoler R., Silber S., Cutlip D., Allaqaband S., Caputo R., Beohar N., Brown D., Garratt K., Jauhar R., George J., Varghese V., Huth M., Larrain G., Lee T., Malik A., Martin S., McGarry T., Phillips C., Shah A., Stoler R., Ball M., Price R. J., Rossi J., Taylor C., Tolleson T., Nicholson W., Kesanakurthy S., Shoukfeh M., Finn A., Devireddy C., Shoultz C., Robbins M., Kiesz R., Menon P., Weilenmann D., Sievert H., Erglis A., Stankovic G., Berland J., Delarche N., Lou Hirsch J., Maillard L., Shayne J., Serra A., Fernandez-Ortiz A., Monassier J. P., and Silber S., “ 9-Month clinical and angiographic outcomes of the COBRA polyzene-F nanocoated coronary stent system,” JACC: Cardiovasc. Interventions 10(2), 160–167 (2017). 10.1016/j.jcin.2016.10.037 [DOI] [PubMed] [Google Scholar]
- 273. Maillard L., Tavildari A., Barra N., Billé J., Joly P., Peycher P., Silvestri M., and Vochelet F., “ Immediate and 1-year follow-up with the novel nanosurface modified COBRA PzF stent,” Arch. Cardiovasc. Dis. 110(12), 682–688 (2017). 10.1016/j.acvd.2017.04.010 [DOI] [PubMed] [Google Scholar]
- 274. Maillard L., Vochelet F., Peycher P., Ayari A., Barra N., Billé J., Joly P., Silvestri M., Sévilla J., and Tavildari A., “ MAPT (mono antiplatelet therapy) as regular regimen after COBRA PzFTM nanocoated coronary stent (NCS) implantation,” Cardiovasc. Revasc. Med. 21(6), 785–789 (2020). 10.1016/j.carrev.2019.10.007 [DOI] [PubMed] [Google Scholar]
- 275. Maillard L., de Labriolle A., Brasselet C., Faurie B., Durel N., de Poli F., Bosle S., Madiot H., Berland J., and Belle L., “ Evaluation of the safety and efficacy of the cobra PzF NanoCoated coronary stent in routine, consecutive, prospective, and high‐risk patients: The E‐cobra study,” Catheterization Cardiovasc. Interventions (published online). 10.1002/ccd.29065 [DOI] [PubMed] [Google Scholar]
- 276. Costa J. R., Abizaid A., Costa R., Feres F., Tanajura L. F., Abizaid A., Maldonado G., Staico R., Siqueira D., Sousa A. G. M. R., Bonan R., and Sousa J. E., “ 1-Year results of the hydroxyapatite polymer-free sirolimus-eluting stent for the treatment of single de novo coronary lesions. The VESTASYNC I trial,” JACC: Cardiovasc. Interventions 2(5), 422–427 (2009). 10.1016/j.jcin.2009.02.009 [DOI] [PubMed] [Google Scholar]
- 277. Ribamar Costa J., Abizaid A., Costa R., Feres F., Fernando Tanajura L., Abizaid A., Alberto Mattos L., Staico R., Siqueira D., Sousa A. G. M. R., Bonan R., Eduardo Sousa J., and Paulo S., “ Preliminary results of the hydroxyapatite nonpolymer-based sirolimus-eluting stent for the treatment of single de novo coronary lesions a first-in-human analysis of a third-generation drug-eluting stent system,” JACC: Cardiovasc. Interventions 1, 545–551 (2008). 10.1016/j.jcin.2008.07.003 [DOI] [PubMed] [Google Scholar]
- 278. Costa J. R., Oliveira B. A., Abizaid A., Costa R., Perin M., Abizaid A., Chamié D., Tanajura L. F., Sousa A., and Sousa J. E. M. R., “ Clinical, angiographic, and intravascular ultrasound results of the VestSaync II trial,” Catheterization Cardiovasc. Interventions 84(7), 1073–1079 (2014). 10.1002/ccd.24909 [DOI] [PubMed] [Google Scholar]
- 279. Suwannasom P., Onuma Y., Benit E., Gach O., von Birgelen C., Hofma S. H., Sotomi Y., Bo X., Zhang Y. J., Gao R., García-García H. M., Wykrzykowska J. J., de Winter R. J., and Serruys P. W., “ Evaluation of vascular healing of polymer-free sirolimuseluting stents in native coronary artery stenosis: A serial follow-up at three and six months with optical coherence tomography imaging,” EuroIntervention 12(5), e574–e583 (2016). 10.4244/EIJV12I5A97 [DOI] [PubMed] [Google Scholar]
- 280. Suwannasom P., Benit E., Gach O., Von Birgelen C., Hofma S., Bo X., Zhang Y.-J., Nakatani S., Ishibashi Y., Onuma Y., García-García H. M., Gao R., and Serruys P. W., “ Short-term effects of nano+TM polymer-free sirolimus-eluting stents on native coronary vessels: An optical coherence tomography imaging study,” AsiaIntervention 1, 57–70 (2015). 10.4244/AsiaInterv_V1I1A11 [DOI] [Google Scholar]
- 281. Zhang Y. J., Chen F., Muramatsu T., Xu B., Li Z. Q., Ge J. B., He Q., Yang Z. J., Li S. M., Wang L. F., Wang H. C., He B., Li K., Qi G. X., Li T. C., Zeng H. S., Peng J. J., Jiang T. M., Zeng Q. T., Zhu J. H., Fu G. S., Bourantas C. V., Serruys P. W., and Huo Y., “ Nine-month angiographic and two-year clinical follow-up of polymer-free sirolimus-eluting stent versus durable-polymer sirolimus-eluting stent for coronary artery disease: The nano randomized trial,” Chin. Med. J. 127(11), 2153–2158 (2014). 10.3760/cma.j.issn.0366-6999.20133148 [DOI] [PubMed] [Google Scholar]
- 282. Liu Y., Zhang Y., Li Y., Qi T., Pan D., Wang H., Liu C., Ma D., Fang Z., Zhang R., Mou F., and Tao L., “ One-year clinical results of the NANO registry: A multicenter, prospective all-comers registry study in patients receiving implantation of a polymer-free sirolimus-eluting stent,” Catheterization Cardiovasc. Interventions 95(S1), 658–664 (2020). 10.1002/ccd.28734 [DOI] [PubMed] [Google Scholar]
- 283. Yu M., Xu B., Kandzari D. E., Wu Y., Yan H., Chen J., Qian J., Qiao S., Yang Y., and Gao R. L., “ First report of a novel polymer-free dual-drug eluting stent in de novo coronary artery disease: Results of the first in human BICARE trial,” Catheterization Cardiovasc. Interventions 83(3), 405–411 (2014). 10.1002/ccd.25129 [DOI] [PubMed] [Google Scholar]
- 284. Massberg S., Byrne R. A., Kastrati A., Schulz S., Pache J., Hausleiter J., Ibrahim T., Fusaro M., Ott I., Schömig A., Laugwitz K. L., and Mehilli J., “ Polymer-free sirolimus- and probucol-eluting versus new generation zotarolimus-eluting stents in coronary artery disease: The intracoronary stenting and angiographic results: Test efficacy of sirolimus- and probucol-eluting versus zotarolimus-eluting stents (ISAR-TEST 5) trial,” Circulation 124(5), 624–632 (2011). 10.1161/CIRCULATIONAHA.111.026732 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.