Abstract
Background
Coronavirus disease 2019 (COVID-19) represents a wide range of clinical manifestations, even if mild disease severity. It has been known that pulmonary function is affected by COVID-19 during infection and mid-to-long term. However, there is inadequate evidence about extrapulmonary features in post-COVID-19 patients.
Aims
This study aimed to investigate extrapulmonary features in post-COVID-19 patients who recovered from mild and moderate disease severity in the mid-term.
Methods
This cross-sectional study was carried out after at least 12 weeks from the COVID-19 diagnosis. Disease severity was defined using criteria for clinical severity of confirmed COVID-19 pneumonia. The peripheral muscle strength was measured using the dynamometer. Physical performance was assessed with five times sit-to-stand and 4-m gait speed. Physical activity level (PAL), mood, and sleep quality were assessed with the International Physical Activity Questionnaire, Hospital Anxiety, and Depression Scale, and Pittsburgh Sleep Quality Index, respectively.
Results
A total of 48 participants with post-COVID-19 (39.2 ± 7.9 years, 54.2% women) were included in the study. Handgrip and quadriceps weakness was observed in 39.6% and 35.4% of the participants, respectively. PAL was low in 39.6%, moderate in 33.3%, and high in 27.1% of the participants. Anxiety, depression, and poor sleep quality were observed in 33.3%, 29.2%, and 50% of the participants, respectively.
Conclusions
Extrapulmonary features are adversely affected in a substantial proportion of post-COVID-19 patients who recovered from mild and moderate disease severity in the mid-term. Comprehensive assessment and appropriate intervention strategies should also be considered for non-severe post-COVID-19 patients.
Keywords: COVID-19, Mood, Muscle strength, Physical activity, Sleep quality
Introduction
Coronavirus disease 2019 (COVID-19) arising from severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has been declared a global pandemic on March 11, 2020 [1]. As of March 28, 2021, more than 126.3 million people were infected from COVID-19, causing over 2.7 million deaths globally since the start of the pandemic [2]. Coronavirus-2 spikes protein binds to the angiotensin-converting enzyme (ACE2) which is distributed throughout the body and it induces various tissue damages [3]. Clinical manifestations may range from the most common symptoms such as fever, cough, dyspnea, myalgia, and fatigue to respiratory distress or respiratory failure [4]. Additionally, most of the patients recovered from COVID-19 present a wide variety of persistent symptoms, even if mild severity [5, 6].
It has been reported that most of the post-COVID-19 patients have impaired diffusion capacity and lower respiratory muscle strength in the early convalescence phase [7]. Also, the majority of the post-COVID-19 patients present persistent dyspnea with reduced exercise capacity and quality of life in the mid-term [8]. Moreover, a considerable proportion of post-COVID-19 patients have pulmonary diffusion abnormality at 6 months after hospitalization [9].
Contrary to existing knowledge regarding pulmonary functions, there is inadequate evidence about extrapulmonary features in post-COVID-19 patients who recovered from mild and moderate disease severity in the mid-term. Identifying affected extrapulmonary features in post-COVID-19 patients will be important to decide public health strategies and therapeutic management. Therefore, the objective of this study was to investigate peripheral muscle strength, physical performance, physical activity level (PAL), mood, and sleep quality in post-COVID-19 patients who recovered from mild and moderate disease severity in the mid-term. The secondary objective was to compare extrapulmonary outcomes between mild and moderate severity patients.
Methods
This cross-sectional study was performed at Dokuz Eylül University Hospital between January 7 and February 24, 2021. Patients with post-COVID-19 aged between 18 and 65 years were recruited to study after at least 12 weeks from the COVID-19 diagnosis. Inclusion criteria were recovery from mild to moderate disease severity, at least 12 weeks have passed since COVID-19 diagnosis, and volunteer to participate in the study. According to criteria for clinical severity of confirmed COVID-19 pneumonia, mild disease severity was defined as mild clinical symptoms and no imaging findings of pneumonia, whereas moderate disease severity was defined as fever, respiratory symptoms, and imaging findings of pneumonia [10]. Exclusion criteria were recovery from severe or critical disease severity, presence of cognitive, neurological, or orthopedic impairment.
This study was approved by the Ethics Committee of Dokuz Eylül University (No: 2020/28–07, Approval Date: 23.11.2020) and all participants signed written informed consent.
Sociodemographic and anthropometric characteristics of the participants including age, gender, weight, height, body mass index (BMI), marital, and smoking status were recorded. The time of COVID-19 diagnosis based on positive SARS-CoV-2 real-time reverse transcriptase-polymerase chain reaction (RT-PCR) on nasal swabs was collected from electronic medical records of participants. The presence of comorbidities was assessed with the modified Charlson Comorbidity Index [11]. Left ventricular ejection fraction evaluation was performed using a cardiac ultrasound device according to the recommendations of the American and European Society of Echocardiography [12] by a cardiologist who was blinded to the disease severity of participants.
Measurements
Body composition
Body composition was measured using a bioelectrical impedance analysis (BIA—Bodystat 1500, Bodystat Ltd., Douglas, UK) which is simple, quick, non-invasive, and reliable [13]. The gender, age, height, and weight of the participant were entered into the device. During supine position, two electrodes were attached on the right hand (the dorsal surface of the metacarpal heads and wrist) and two more electrodes on the right foot (the dorsal surface of the metatarsal heads and ankle) to measure by passing a safe signal through the body with a frequency of 50 kHz. Body fat mass (FM) and fat-free mass (FFM) were obtained from the device which is programmed to run on the manufacturer’s prediction equation and were expressed as kg and percentage [14].
Peripheral muscle strength
Handgrip strength was measured using a valid and reliable hand dynamometer (Jamar, Nottinghamshire, UK) [15]. The test was performed in accordance with the American Society of Hand Therapist recommendations. Three trials were carried out and the mean score was recorded for data calculation [16].
Quadriceps muscle strength was measured using a valid and reliable hand-held dynamometer (Lafayette, Indiana, USA). The participants were seated with hip and knee at 90° flexion and push the piston of the dynamometer which was placed on the anterior surface of proximal to ankle. The test was carried out three times [17].
The reference equations were used to calculate the percentages of the predicted normal values for handgrip and quadriceps muscle strength [18, 19]. A muscle strength below 80% of the predicted normal value was considered as muscle weakness [20].
Physical performance
Physical performance was measured using five times sit-to-stand (5STS) and 4 m (4-m) gait speed. To measure 5STS, the participants were instructed to stand up and sit 5 times as fast as possible and the time was recorded [21]. The test was done only after participants first demonstrated the ability to rise once without using their arms [22]. To measure 4-m gait speed, the participants were instructed to walk a distance of 4-m at the usual gait speed. The test was repeated twice and the faster of the two walks was used [22]. Usual gait speed was calculated by dividing the walking distance by the recorded time.
Physical activity level (PAL)
PAL was assessed with the Turkish version of the International Physical Activity Questionnaire Short-Form (IPAQ-SF) [23]. IPAQ-SF consists of 7 items that estimate four activity levels including vigorous-intensity activity, moderate-intensity activity, walking, and sitting for the last 1 week [24]. PAL was defined as low (< 600 MET minutes a week), moderate (600–1500 min a week), and high (> 1500 MET minutes a week) [25].
Mood
Mood was assessed with the Turkish version of the Hospital Anxiety and Depression Scale (HADS) [26]. HADS is a self-reported questionnaire containing 7-item Anxiety (HADS-A) subscale and 7-item Depression (HADS-D) subscale. Items are scored on a Likert scale ranging from 0 (not at all) to 3 (most of the time). A cut-off score of 8 for each subscale was used [27].
Sleep quality
Sleep quality was assessed with the Turkish version of the Pittsburgh Sleep Quality Index (PSQI) [28]. PSQI comprises 19 items that examine 7 components including sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbance, use of sleeping medications, and daytime dysfunction. Each component is scored range from 0 to 3. A cut-off point of 5 was used to determine the poor sleep quality [29].
Statistical analysis
Statistical analysis was performed using SPSS (Version 24.0, IBM Corp., Armonk, NY, USA) [30]. Continuous variables are presented as mean and standard deviation, while categorical variables are presented as numbers and percentages. The normality of distribution was determined by the Shapiro–Wilk test and histograms. To compare the two groups, the Student’s t-test was used for continuous variables with normal distribution, the Mann–Whitney U test was used for continuous variables with non-normal distribution, and the Chi-square test was used for categorical variables. According to the mood (HADS total score), the post hoc power of our study was 0.88. Statistical significance was set at p < 0.05 [31].
Results
A total of 48 participants with post-COVID-19 (39.2 ± 7.9 years, 54.2% women) were included in the study. Participants were divided into two groups as mild (n = 25) and moderate (n = 23) according to disease severity during COVID-19 infection. The mean time from COVID-19 diagnosis to the day of the participation in the study was 148.4 ± 39.1 days in the mild group and 140.3 ± 43.7 days in the moderate group (p = 0.353). During COVID-19 infection, 9 (39) participants were hospitalized without admission to the intensive care unit in the moderate group while none of the participants were hospitalized in the mild group (p < 0.001). The general features of participants are presented in Table 1. The majority of participants were married (72.9%) and had no previous smoking habits (93.7%). Mild and moderate groups were comparable in the terms of age, gender, BMI, FM, FFM, marital, and smoking status (p > 0.05). The modified Charlson Comorbidity Index was higher in the moderate group but not statistically different from the mild group (p > 0.05). The ejection fraction was in the normal range in all participants and similar between the groups (p > 0.05).
Table 1.
General features of the participants with post-COVID-19
| Total Mean ± SD (n = 48) |
Mild group Mean ± SD (n = 25) |
Moderate group Mean ± SD (n = 23) |
p | |
|---|---|---|---|---|
| Age (years) | 39.2 ± 7.9 | 38.5 ± 8.3 | 40.0 ± 7.5 | 0.523a |
| Women, n (%) | 26 (54.2) | 12 (48) | 14 (60) | 0.371c |
| BMI (kg/m2) | 27.0 ± 4.7 | 27.0 ± 3.9 | 27.0 ± 5.5 | 0.993a |
| FM (kg) | 22.4 ± 9.0 | 21.4 ± 8.5 | 23.4 ± 9.5 | 0.482a |
| FM (%) | 28.6 ± 8.5 | 27.1 ± 9.4 | 30.1 ± 7.4 | 0.274a |
| FFM (kg) | 55.3 ± 12.7 | 57.5 ± 13.1 | 52.9 ± 12.1 | 0.249a |
| FFM (%) | 71.3 ± 8.5 | 72.8 ± 9.4 | 69.8 ± 7.4 | 0.274a |
| Marital status | ||||
| Alone, n (%) | 13 (27.1) | 7 (28) | 6 (26.1) | 0.882c |
| Married, n (%) | 35 (72.9) | 18 (72) | 17 (73.9) | |
| Smoking status | ||||
| Current smoker, n (%) | 3 (6.3) | 2 (8.0) | 1 (4.3) | 0.532c |
| Never smoker, n (%) | 45 (93.7) | 23 (92.0) | 22 (95.7) | |
| Smoking exposure (packs-years) | 0.9 ± 4.0 | 0.9 ± 4.0 | 0.9 ± 4.2 | 0.623b |
| Modified Charlson Comorbidity Index | 0.7 ± 0.9 | 0.6 ± 0.8 | 0.8 ± 1.0 | 0.686b |
| Left ventricular ejection fraction (%) | 66.9 ± 4.4 | 67.1 ± 4.1 | 66.7 ± 4.8 | 0.810a |
BMI body mass index, FM fat mass, FFM fat-free mass
*p < 0.05
aStudent’s t-test
bMann–Whitney U test
cChi-square test
Handgrip and quadriceps weakness was observed in 19 (39.6%) and 17 (35.4%) of the participants, respectively. In the mild group, 9 (36%) of participants had handgrip muscle weakness, whereas in the moderate group, 10 (43.5%) of participants had handgrip muscle weakness (p = 0.597). A total of 6 (24%) of participants had quadriceps muscle weakness in the mild group while 11 (47.8%) of participants had quadriceps muscle weakness in the moderate group (p = 0.085) (see Fig. 1). Handgrip strength, quadriceps strength, and IPAQ were significantly lower in the moderate group than in the mild group (p < 0.05). PAL was low in 19 (39.6%), moderate in 16 (33.3%), and high in 13 (27.1%) of the participants. In the mild group, 7 (28%) of participants had low PAL, 8 (32%) of participants had moderate PAL, and 10 (40%) of participants had high PAL, whereas in the moderate group, 12 (52.2%) of participants had low PAL, 8 (34.8%) of participants had moderate PAL, and 3 (13%) of participants had high PAL (p = 0.03). The 5STS and IPAQ sitting were significantly higher in the moderate group than in the mild group (p < 0.05). Usual gait speed was similar between the groups (p = 0.415) (see Table 2). Anxiety, depression, and poor sleep quality were observed in 16 (33.3%), 14 (29.2%), and 24 (50%) of the participants, respectively. HADS-A was above the cut-off in 4 (16%) participants in the mild group and 12 (52.2%) participants in the moderate group (p = 0.008). HADS-D was above the cut-off in 3 (12%) participants in the mild group and 11 (47.8%) participants in the moderate group (p = 0.006). A total of 8 (32%) participants had poor sleep quality in the mild group, whereas 16 (69.6%) participants had poor sleep quality in the moderate group (p = 0.009) (see Fig. 2). HADS-A, HADS-D, HADS total, and PSQI total scores were significantly higher in the moderate group (p < 0.05) (see Table 2).
Fig. 1.
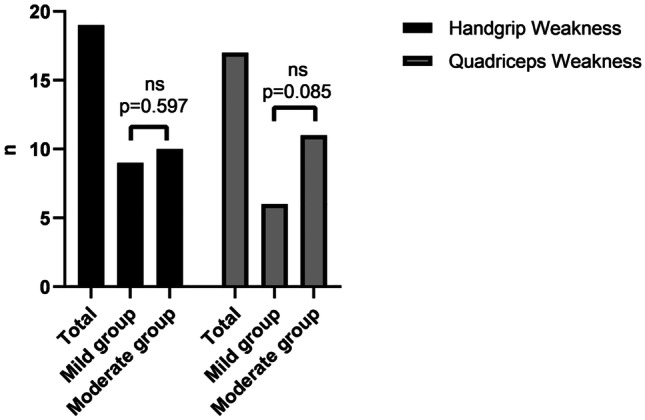
Comparison of muscle weakness in all post-COVID-19 patients and according to the disease severity. ns not significant
Table 2.
Extrapulmonary features of the participants with post-COVID-19
| Total Mean ± SD |
Mild group Mean ± SD |
Moderate group Mean ± SD |
p | |
|---|---|---|---|---|
| Dominant hand grip strength (kg) | 32.6 ± 13.8 | 36.7 ± 12.9 | 28.2 ± 13.7 | 0.032a,* |
| Non-dominant hand grip strength (kg) | 31.7 ± 13.8 | 35.8 ± 12.6 | 27.3 ± 14.0 | 0.033a,* |
| Dominant quadriceps muscle strength (kg) | 25.5 ± 7.3 | 27.9 ± 6.5 | 22.9 ± 7.3 | 0.018a,* |
| Non-dominant quadriceps muscle strength (kg) | 24.3 ± 7.4 | 26.5 ± 6.8 | 21.9 ± 7.3 | 0.033a,* |
| 5STS, s | 8.1 ± 2.2 | 7.3 ± 1.5 | 9.0 ± 2.6 | 0.002b,* |
| Usual gait speed, m/s | 1.2 ± 0.2 | 1.2 ± 0.2 | 1.2 ± 0.2 | 0.415b |
| IPAQ, MET/h/week | 1075.8 ± 869.3 | 1343.8 ± 910.6 | 784.6 ± 734.6 | 0.017b,* |
| Low PAL, n (%) | 19 (39.6) | 7 (28.0) | 12 (52.2) | |
| Moderate PAL, n (%) | 16 (33.3) | 8 (32.0) | 8 (34.8) | 0.030c,* |
| High PAL, n (%) | 13 (27.1) | 10 (40.0) | 3 (13.0) | |
| IPAQ-sitting, min/day | 387.9 ± 139.7 | 339.2 ± 134.9 | 440.8 ± 127.4 | 0.010a,* |
| HADS-A | 5.3 ± 3.9 | 3.5 ± 2.6 | 7.4 ± 4.2 | 0.001b,* |
| HADS-D | 5.8 ± 3.7 | 4.5 ± 3.1 | 7.3 ± 3.7 | 0.009a,* |
| HADS total score | 11.2 ± 6.9 | 8.0 ± 4.9 | 14.7 ± 7.3 | 0.001a,* |
| PSQI total score | 6.1 ± 3.7 | 4.8 ± 3.2 | 7.5 ± 3.8 | 0.017b,* |
5STS five times sit-to-stand, IPAQ International Physical Activity Questionnaire, PAL physical activity level, HADS-A Hospital Anxiety and Depression Scale—Anxiety, HADS-D Hospital Anxiety and Depression Scale—Depression, PSQI Pittsburgh Sleep Quality Index
*p < 0.05
aStudent’s t-test
bMann–Whitney U test
cChi-square test
Fig. 2.
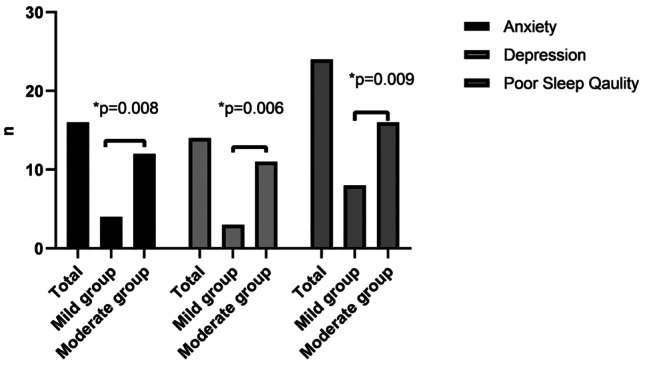
Comparison of anxiety, depression, and poor sleep quality in all post-COVID-19 patients and according to the disease severity. *p < 0.05
Discussion
To our best knowledge, this is the first study to investigate extrapulmonary features in post-COVID-19 patients who recovered from mild and moderate disease severity in the mid-term. The main findings of this study indicated that a considerable proportion of post-COVID-19 patients suffered from handgrip and quadriceps muscle weakness, low PAL, anxiety, depression, and poor sleep quality in the mid-term. Also, handgrip and quadriceps muscle strength, 5STS, PAL, anxiety, depression, and sleep quality were significantly different between the patients who recovered from mild and moderate disease severity.
Handgrip strength measurement is suggested to get an overall idea related to general health status in COVID-19 [32]. Also, muscle strength was found to be an independent risk factor for COVID-19 severity and per increase of 1 SD in handgrip strength is associated with a 16% decreased risk of hospitalization [33]. Tuzun et al. demonstrated the handgrip strength of all patients was below the age and gender specified normative values during hospitalization due to COVID-19 infection [34]. Additionally, Paneroni et al. reported that the vast majority of COVID-19 patients had quadriceps muscle weakness at the time of discharge [20]. We found that more than one third of the post-COVID-19 patients had handgrip and quadriceps muscle weakness in the mid-term and those recovered from moderate disease severity had lower handgrip and quadriceps muscle strength than those recovered from mild disease severity. Taken together, these findings point out muscle weakness at least 3 months after COVID-19 may persist despite non-severe disease severity.
Poor physical performance is associated with low muscle strength, but not low muscle mass [35]. COVID-19 patients exhibit a reduced physical performance at the hospital discharge [20]. In our study, physical performance was lower in the post-COVID patients who recovered from moderate disease severity in the mid-term. This observation is not surprising when considering those recovered from moderate disease severity had lower quadriceps muscle strength than those recovered from mild disease severity.
Lockdown and social-distancing in many countries to avoid the COVID-19 pandemic have led to restrictions in ordinary daily activities and social interaction, causing a decrease in physical activity that is associated with the prevention of cardiovascular disease and reduced risk of mortality [36, 37]. Another result of confinement regarding COVID-19 precautions is sleep disturbances which are related to psychological distress [38]. Moreover, COVID-19 patients suffer from anxiety and depression symptoms and poor sleep quality during hospitalization [39, 40]. In accordance with the literature, we found that a significant proportion of post-COVID-19 patients had low PALs, anxiety and depression symptoms, and poor sleep quality in the mid-term. Considering that physical activity leads to a decreased risk of anxiety and depression symptoms and improves sleep quality [37], strategies to increase physical activity may help to cope with these negative consequences in post-COVID-19 patients.
Limitations of the study
Firstly, our study did not include age- and sex-matched healthy controls. However, the reference equations and cut-off scores are available for the outcomes we used. Secondly, all post-COVID-19 patients consisted of healthcare professionals from a single center. Moreover, the measurements of the extrapulmonary features were conducted only at least 12 weeks after COVID-19 diagnosis, therefore no data available concerning during COVID-19 infection and early convalescence phase. Lastly, we did not recruit post-COVID-19 patients who recovered from the severe and critical disease severity; hence, this permitted us to focus on the effect of non-severe disease severity on extrapulmonary features, reducing confounding factors.
Conclusions
The present study provides informative data to understand the mid-term outcomes of COVID-19 on patients who recovered from mild and moderate disease severity. Our findings imply that peripheral muscle strength, PAL, mood, and sleep quality are adversely affected in a substantial proportion of post-COVID-19 patients. Also, patients who recovered from moderate disease severity have significant alterations in peripheral muscle strength, physical performance, PAL, mood, and sleep quality compared to patients who recovered from mild disease severity. Therefore, comprehensive assessment and appropriate intervention strategies should also be taken into consideration for non-severe post-COVID-19 patients, which may be ignored in the pandemic.
Author contribution
Conception and design: Aylin Tanriverdi, Sema Savci, Buse Ozcan Kahraman, Ebru Ozpelit; data collection: Aylin Tanriverdi, Ebru Ozpelit; statistical analyses: Aylin Tanriverdi, Buse Ozcan Kahraman; writing of manuscript: Aylin Tanriverdi, Sema Savci; revising the article for important intellectual content; and final approval: all authors.
Declarations
Ethics approval
This study conformed in accordance with the 2013 Declaration of Helsinki (study was not registered in a database). The study protocol was approved by the Ethics Committee of Dokuz Eylül University (reference number #2020/28–07).
Consent to participate
Written informed consent was obtained from all individual participants included in the study.
Consent for publication
Additional informed consent was obtained from all individual participants for whom identifying information is included in this article.
Conflict of interest
The authors declare no competing interests.
References
- 1.Cucinotta D, Vanelli M (2020) WHO declares COVID-19 a pandemic. Acta Biomed 91:157. 10.23750/abm.v91i1.9397 [DOI] [PMC free article] [PubMed]
- 2.WHO (2021) Weekly epidemiological update on COVID-19. https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---31-march-2021
- 3.Kai H, Kai M. Interactions of coronaviruses with ACE2, angiotensin II, and RAS inhibitors—lessons from available evidence and insights into COVID-19. Hypertens Res. 2020;43:648–654. doi: 10.1038/s41440-020-0455-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72,314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 5.Kamal M, Abo Omirah M, Hussein A, et al. Assessment and characterisation of post-COVID-19 manifestations. Int J Clin Pract. 2021;75:e13746. doi: 10.1111/ijcp.13746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kashif A, Chaudhry M, Fayyaz T et al (2021) Follow-up of COVID-19 recovered patients with mild disease. 10.21203/rs.3.rs-120819/v1 [DOI] [PMC free article] [PubMed]
- 7.Huang Y, Tan C, Wu J, et al. Impact of coronavirus disease 2019 on pulmonary function in early convalescence phase. Respir Res. 2020;21:1–10. doi: 10.1186/s12931-020-01429-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ybarra-Falcón C, García-Gómez M, Tobar J et al (2021) Post-COVID-19 syndrome: prospective evaluation of clinical and functional outcomes and systematic review. 10.2139/ssrn.3768533
- 9.Huang C, Huang L, Wang Y, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397:220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zu ZY, Jiang MD, Xu PP, et al. Coronavirus disease 2019 (COVID-19): a perspective from China. Radiology. 2020;296:E15–E25. doi: 10.1148/radiol.2020200490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beddhu S, Bruns FJ, Saul M, et al. A simple comorbidity scale predicts clinical outcomes and costs in dialysis patients. Am J Med. 2000;108:609–613. doi: 10.1016/s0002-9343(00)00371-5. [DOI] [PubMed] [Google Scholar]
- 12.Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015;16:233–271. doi: 10.1093/ehjci/jev014. [DOI] [PubMed] [Google Scholar]
- 13.Dehghan M, Merchant AT. Is bioelectrical impedance accurate for use in large epidemiological studies? Nutr J. 2008;7:1–7. doi: 10.1186/1475-2891-7-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghosh S, Meister D, Cowen S, et al. Body composition at the bedside. Eur J Gastroenterol Hepatol. 1997;9:783–788. doi: 10.1097/00042737-199708000-00009. [DOI] [PubMed] [Google Scholar]
- 15.Mathiowetz V, Weber K, Volland G, et al. Reliability and validity of grip and pinch strength evaluations. J Hand Surg Am. 1984;9:222–226. doi: 10.1016/s0363-5023(84)80146-x. [DOI] [PubMed] [Google Scholar]
- 16.Hamilton GF, McDonald C, Chenier TC. Measurement of grip strength: validity and reliability of the sphygmomanometer and jamar grip dynamometer. J Orthop Sports Phys Ther. 1992;16:215–219. doi: 10.2519/jospt.1992.16.5.215. [DOI] [PubMed] [Google Scholar]
- 17.Mentiplay BF, Perraton LG, Bower KJ, et al. Assessment of lower limb muscle strength and power using hand-held and fixed dynamometry: a reliability and validity study. PLoS One. 2015;10:e0140822. doi: 10.1371/journal.pone.0140822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Douma RK, Soer R, Krijnen WP, et al. Reference values for isometric muscle force among workers for the Netherlands: a comparison of reference values. BMC Sports Sci Med Rehabil. 2014;6:1–10. doi: 10.1186/2052-1847-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Y-C, Bohannon RW, Li X, et al. Hand-grip strength: normative reference values and equations for individuals 18 to 85 years of age residing in the United States. J Orthop Sports Phys Ther. 2018;48:685–693. doi: 10.2519/jospt.2018.7851. [DOI] [PubMed] [Google Scholar]
- 20.Paneroni M, Simonelli C, Saleri M et al (2021) Muscle strength and physical performance in patients without previous disabilities recovering from COVID-19 pneumonia. Am J Phys Med Rehabil 100:105–9. 10.1097/phm.0000000000001641 [DOI] [PubMed]
- 21.Paul SS, Canning CG. Five-repetition sit-to-stand. J Physiother. 2014;60:168. doi: 10.1016/j.jphys.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 22.Guralnik JM, Ferrucci L, Simonsick EM, et al. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332(9):556–561. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saglam M, Arikan H, Savci S, et al. International physical Activity Questionnaire: reliability and validity of the Turkish version. Percept Mot Skills. 2010;111:278–284. doi: 10.2466/06.08.pms.111.4.278-284. [DOI] [PubMed] [Google Scholar]
- 24.Craig CL, Marshall AL, Sjöström M, et al. International Physical Activity Questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35:1381–1395. doi: 10.1249/01.mss.0000078924.61453.fb. [DOI] [PubMed] [Google Scholar]
- 25.Forde C (2018) Scoring the international Physical Activity Questionnaire (IPAQ). University of Dublin
- 26.Aydemir O, Guvenir T, Kuey L, et al. Validity and reliability of Turkish version of hospital Anxiety and Depression Scale. Turk Psikiyatri Derg. 1997;8:280–287. [Google Scholar]
- 27.Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand. 1983;67(361):70. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 28.Agargün MY, Kara H, Anlar O. The validity and reliability of the Pittsburgh Sleep Quality Index. Turk Psikiyatri Derg. 1996;7:107–115. [Google Scholar]
- 29.Carpenter JS, Andrykowski MA. Psychometric evaluation of the Pittsburgh Sleep Quality Index. J Psychosom Res. 1998;45:5–13. doi: 10.1016/s0022-3999(97)00298-5. [DOI] [PubMed] [Google Scholar]
- 30.Green SB, Salkind NJ. Using SPSS for Windows and Macintosh: analyzing and understanding data. Prentice-Hall; 2012. [Google Scholar]
- 31.Munro BH (2005) Statistical methods for health care research. Lippincott williams & wilkins
- 32.Ekiz T, Kara M, Özçakar L. Measuring grip strength in COVID-19: a simple way to predict overall frailty/impairment. Heart Lung. 2020;49:853–854. doi: 10.1016/j.hrtlng.2020.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheval B, Sieber S, Maltagliati S, et al. Muscle strength is associated with COVID-19 hospitalization in adults 50 years of age and older. MedRxiv. 2021 doi: 10.1101/2021.02.02.21250909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tuzun S, Keles A, Yildiran T et al (2020) Assessment of musculoskeletal pain, fatigue and grip strength in hospitalized patients with COVID-19. 10.23736/S1973-9087.20.06563-6. [DOI] [PubMed]
- 35.Visser M, Deeg DJ, Lips P, et al. Skeletal muscle mass and muscle strength in relation to lower extremity performance in older men and women. J Am Geriatr Soc. 2000;48:381–386. doi: 10.1111/j.15325415.2000.tb04694.x. [DOI] [PubMed] [Google Scholar]
- 36.Wannamethee SG, Shaper AG. Physical activity in the prevention of cardiovascular disease. Sports Med. 2001;31:101–114. doi: 10.2165/00007256-200131020-00003. [DOI] [PubMed] [Google Scholar]
- 37.Violant-Holz V, Gallego-Jiménez MG, González-González CS, et al. Psychological health and physical activity levels during the COVID-19 pandemic: a systematic review. Int J Environ Res Public Health. 2020;17:9419. doi: 10.3390/ijerph17249419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Franceschini C, Musetti A, Zenesini C, et al. Poor sleep quality and its consequences on mental health during the COVID-19 lockdown in Italy. Front Psychol. 2020;11:3072. doi: 10.3389/fpsyg.2020.574475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Akıncı T, Başar HM. Relationship between sleep quality and the psychological status of patients hospitalised with COVID-19. Sleep Med. 2021;80:167–170. doi: 10.1016/j.sleep.2021.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mazza MG, De Lorenzo R, Conte C, et al. Anxiety and depression in COVID-19 survivors: role of inflammatory and clinical predictors. Brain Behav Immun. 2020;89:594–600. doi: 10.1016/j.bbi.2020.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]


