Summary
When the atomic nucleus of 125I decays by orbital electron capture followed by internal conversion, numerous very-low-energy electrons (Auger electrons) are emitted, so that the energy density in the immediate vicinity of the decay site is extremely high, 125I incorporated into DNA was as effective as densely ionising 5·3 MeV α-particles from 210Po in reducing the sperm-head population in mice. Hence the biological risks of Auger-electron emitting radionuclides widely used in biology and medicine ought to be reassessed.
INTRODUCTION
The increasing use of radionuclides in medical diagnosis and therapy, as well as their presence in the environment, necessitate investigation of the biological consequences of their radiations. Many of these radionuclides (eg, 55Fe, 99mTc 111In, 125I, 137Cs-137mBa, 201Tl) decay by electron capture (EC) or internal conversion (IC) or both, and the result is an inner atomic shell vacancy. The extremely rapid (approximately 10−15 s) atomic de-excitation that follows is dominated by Auger cascade transitions with the emission of numerous low-energy electrons.1 It is estimated1,2 that, on average, about 20 such electrons are emitted per 125I decay (table). Most of these electrons have subcellular ranges (table), with values of linear energy transfer (LET) ranging from about 10 to 25 keV/µm. In the immediate vicinity (< 20 nm) of the decay site, the density of absorbed energy is extremely high and similar to that found along the tracks of α-particles of high LET.1,3,4 Hence, radionuclides decaying by EC and IC may be expected to be highly radiotoxic when they are incorporated into the radiosensitive DNA of cells.
TABLE.
AVERAGE AUGER-ELECTRON SPECTRUM PER 125I DECAY
| Average energy1 (keV) |
LET32 (keV/µm) |
Yield per decay1 |
Range (µm) in unit density matter32 |
|---|---|---|---|
| 24·3 | 1·1 | 0·20 | 14·0 |
| 3·27 | 4·8 | 1·58 | 0·42 |
| 0·670 | 12·7 | 0·24 | 0·037 |
| 0·475 | 14·9 | 3·17 | 0·023 |
| 0·258 | 18·7 | 0·13 | 0·012 |
| 0·210 | 19·9 | 0·29 | 0·010 |
| 0·154 | 21·5 | 0·35 | 0·0075 |
| 0·110 | 23·0 | 0·82 | 0·0055 |
| 0·065 | 24·9 | 4·66 | 0·004 |
| 0·048 | 25·7 | 0·31 | 0·003 |
| 0·027 | 26·7 | 6·14 | 0·0015 |
| 0·016 | 27·3 | 1·36 | < 0·001 |
LET = linear energy transfer.
Because iododeoxyuridine (IUdR) is an analogue of thymidine (TdR), it is possible to incorporate 125I, in the form of 125IUdR, into the DNA of proliferating cells.5 In-vitro studies of the radiobiological effects of 125I thus incorporated on different mammalian cell lines5–7 have clearly shown that the Auger electron showers from 125I decays can cause cytocidal effects much like high LET radiations. Similar results8,9 are reported for other DNA-bound Auger emitters (123I,77Br). In contrast, studies with 3HTdR and 131IUdR show that the β-emitters 3H and 131I are much less radiotoxic even when they are similarly incorporated into the DNA.5,6
The high radiotoxicities of DNA-bound Auger-emitters observed in vitro prompted us to develop a very sensitive in-vivo model to determine the effects of tissue-incorporated radionuclides at low absorbed doses. By examining the effect on spermatogenesis of radionuclides distributed in the testis in mice, several Auger-emitters have been found to have relative biological effectiveness (RBE) considerably higher than 1 when compared with their β-emitting counterparts.10–12 The importance of these in-vivo results has been noted.13,14 Despite these findings, there are no experimental data directly comparing the toxicity of Auger-emitters with that of high-LET radiations such as α-particles. This paucity of data, and the increasing concern over the effects of tissue-incorporated Auger-emitting and α-emitting radionuclides, have prompted the present study, which compares directly the in-vivo toxicity of DNA-incorporated 125IUdR with the α-emitter 210Po. 210Po, a radon daughter, with a half-life of 138 days, emits 5·3 MeV α-particles which have a track-averaged LET of about 100 keV/µm and a range in tissue of approximately 50 µm.l5
The differentiated spermatogonial cells (types A1–A4, In, and B) in mouse testis are highly sensitive to ionising radiation. The complete process of spermatogenesis in mouse16–18 is similar to that in man but for the time scale—about 5 weeks for mouse and 10 weeks for man. Furthermore, human spermatogonial cells are at least as radiosensitive as those in mouse.16 Thus, spermatogenesis in mice is an experimental model relevant to man. Compared with the differentiated spermatogonia, their precursors and the postgonial cells (spermatocytes, spermatids, and Spermatozoa) are relatively radioresistant. Hence, any initial radiation insult to the testis will manifest itself as a reduced testicular sperm count after the time required for the differentiated spermatogonial cells to become spermatozoa.
METHODS
Our experimental procedures and protocols have been described in detail elsewhere.10 Briefly, 3 µl of solutions containing different amounts of either 125IUdR or 210Po-citrate were injected into the right testes of Swiss Webster mice (8–9 weeks of age, weight 30 g) anesthetised under ether. This mode of administration requires very small amounts of radioactivity and enables a clear delineation of the effects of low-energy electrons without the complications of whole-body irradiation inherent in intravenous or intraperitoneal modes of injection.10
125IUdR, dissolved in water, was obtained from ICN Radiochemicals (Irvine, California). Carrier-free 210Po in 3 mol/l nitric acid was obtained commercially (Amersham, Arlington Height, Illinois). The radiochemical 210Po-citrate was prepared by mixing the stock 210Po solution with 1 mol/1 sodium citrate (pH 4·7) in the ratio 1:9.
Testicular Clearance of the Radiochemicals
To determine the pattern of biological elimination of the radiochemicals from the testes, groups of 5 animals were killed under ether at various times after the initial injection, and the intratesticular activity determined—that of radioiodine with a NaI scintillation well detector and that of 210Po by digesting the testis in 10 ml ‘Fluorosol’ scintillation cocktail (National Diagnostics, Manville, New Jersey) before counting in an automatic liquid scintillation counter. The biological clearance did not depend on the amount of radioactivity injected.
Determination of Survival of Spermatogonial Cells
The survival of spermatogonial cells was determined by the sperm-head survival assay.10 The best time for this assay is when the testicular sperm-head population reaches a minimum after the initial radiation insult. This is determined experimentally. About 50 animals were injected with about 0·24 MBq of 125IUdR or about 0·16 kBq of 210Po-citrate, and killed at different post-injection times. The injected testes were removed, placed in 1 ml of deionised water, homogenised for 15 s, and sonicated for 30 s. The sperm-heads, which are resistant to sonication, were counted under a microscope in a haemocytometer; at least 200 were counted. The post-injection time required to achieve the minimum sperm count was 29 days for 125IUdR and 36 days for 210Po-citrate.
Thus on days 29 and 36 after injection of the radiochemicals animals were killed, and the injected testes removed and processed for sperm-head counting. Untouched mice and those injected with normal saline served as controls.
To verify that there were no chemotoxic effects the mice were injected with non-radioactive IUdR and with citrate solution alone (without polonium) in amounts corresponding to the highest doses. To determine macroscopic radionuclide distribution in the testes several injected testes were removed one day post-injection, frozen with ‘CRYOkwik’ (International Equipment Company, Needham Heights, Massachusetts), and sliced into ten sections. Each slice was weighed and the activity it contained assayed. The radioactivity per gram of tissue was essentially the same in all sections, thereby indicating fairly uniform distribution of the radioactive material.
Sperm-head survival fraction (S) was determined as a function of the average absorbed dose (D) to the testis. The absorbed dose to the testis was calculated, according to Medical Internal Radiation Dose (MIRD) procedures,12,19 from the biological and physical half-lives and the radiation data for the radionuclides (table and ref 20).
RESULTS
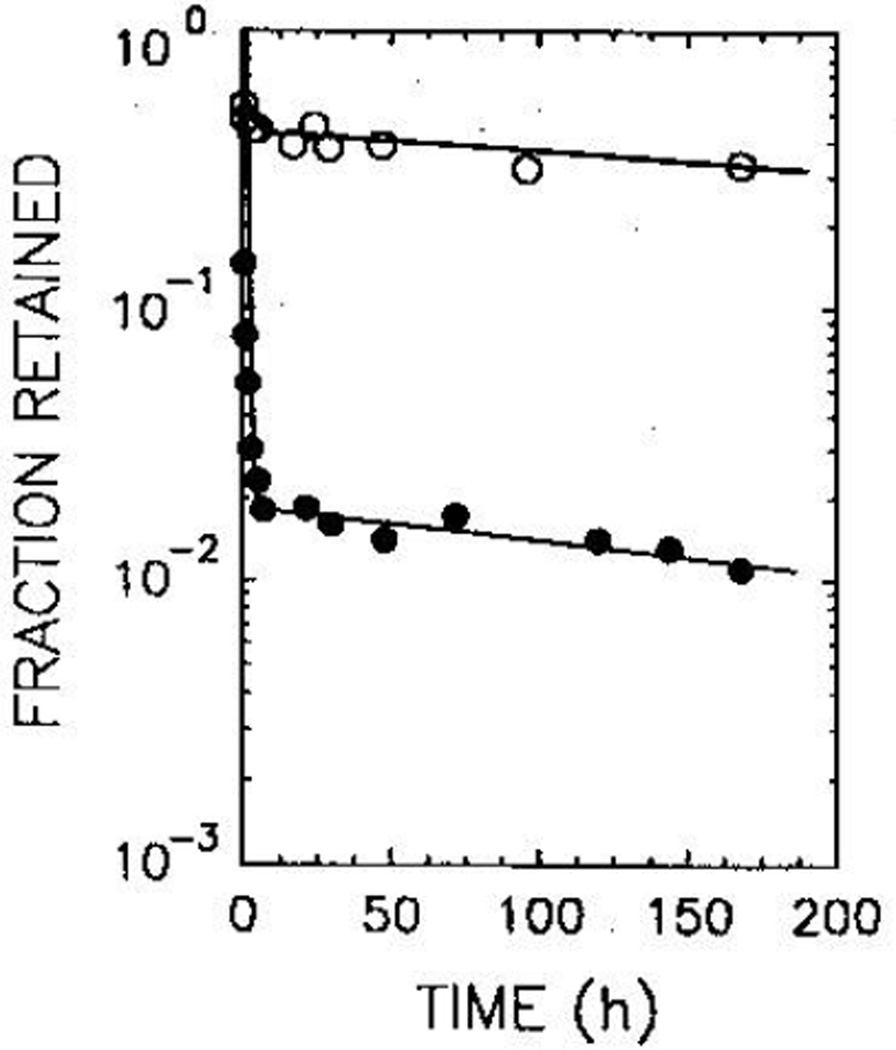
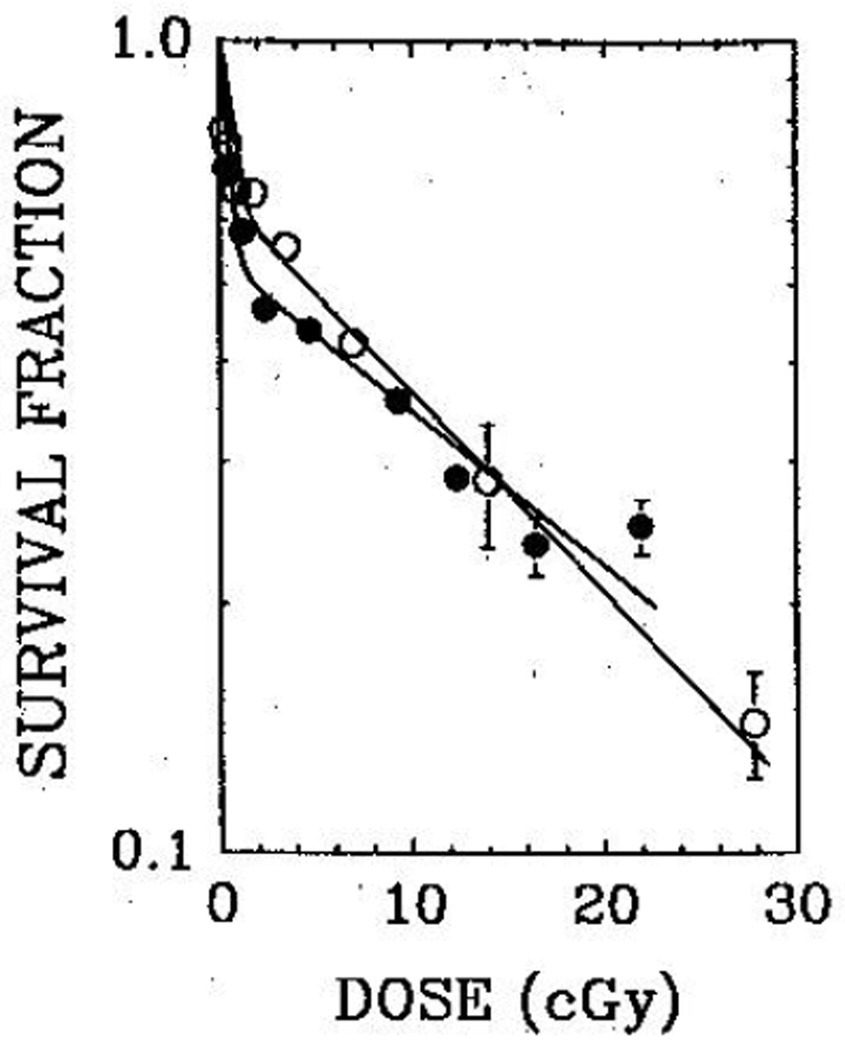
98% of the injected activity of 125IUdR was quickly eliminated from the testis, the biological half-life being 0·18 h. The remainder was cleared with a long half-life (308 h). For Po, however, a large fraction (about 40%) of the injected activity was retained, the biological half-life being 570 h (fig 1). Least-squares fitting of the survival data (fig 2) yielded mean lethal doses (D37) of 8·5 (SEM 2·1) cGy and 10 (1) cGy for 125IUdR and 210Po, respectively. When the mouse testes were selectively irradiated with 60 kVp and 120 kVp external X-rays, the 37% survival dose was 67 cGy.23 Therefore the RBE values for the DNA-incorporated Auger-emitter 125IUdR and the α-emitter 210Po are 7·9 (2·4) and 6·7 (1·4), respectively. Within the experimental uncertainties, both radiochemicals are equally effective in causing biological damage in the testis.
Fig 1.
Fraction of injected radiochemical retained by the testis as a function of post-injection time for 210Po-citrate (open circles) and 125IUdR (closed circles)
Data represent the average of 3 and 4 separate experiments for 125IUdR and 210Po-citrate, respectively.
Fig 2.
Fraction of surviving sperm-heads as a function of the testicular absorbed dose from 210Po-citrate (open circles) and 125IUdR (closed circles)
- S(125IUdR)=0·46 e−D/0·41+0·54 e−D/22·1,
- S(210Po)=0·30 e−D/0·20+0·70 e−D/15·6.
DISCUSSION
The two-component nature of the survival curves observed in these experiments (fig 2) is not an artifact of our protocols.10,11,21 Such survival curves were also observed when the radiochemicals were injected intraperitoneally, thereby introducing the activity into the testis by a different pathway. Furthermore, irradiation of the testis with external X-rays (60 kVp or 120 kVp) also yielded similar two-component curves.22,23 Perhaps, the differential radiosensitivities of the spermatogonial cell subpopulations A1–A4, In, and B may be responsible for the observed behaviour.11,24
The RBE values we obtained are in reasonable agreement with results of in-vitro tests for 125IUdR7 and the α-emitter 211At.25 Our study, which directly compares the effects of an Auger-emitter with an α-emitter, gives clear in-vivo evidence of the extreme toxicity and high-LET nature of DNA-incorporated Auger-emitters in an experimental model that is relevant to man.
The importance of our findings to medical dosimetry and radiation protection, in general, should be noted. The International Commission on Radiological Protection (ICRP) assigns a quality factor of 20 for α-particles.26 Hence, our RBE of 6·7 for spermatogonial cell killing with 5·3 MeV 210Po α-particles is not surprising and is in keeping with published results for cell inactivation with high-LET radiations.25,27,28 However, photons and electrons, irrespective of their localised patterns of energy deposition, are treated as low-LET radiations. Accordingly, a value of 1 is used for the RBE for 125I, an emitter of photons and seemingly harmless low-energy electrons. Thus, the risk associated with DNA-bound 125I is severely underestimated because of the assumptions used in the dosimetry procedures recommended by MIRD and IGRU Committees.19,29 They tacitly assume that the radionuclide and its deposited energy are uniformly distributed in the organ. This assumption is reasonably valid for radionuclides emitting penetrating photons of macroscopic mean free paths, and energetic electrons of low LET with ranges in tissue much larger than cell dimensions. These assumptions may not be tenable for Auger-emitters such as 125I. For example, a single 125I decay in the nucleus of a spermatogonial cell (9 µm cell diameter, 5 µm nucleus diameter) deposits 10·5 keV in the radiosensitive cell nucleus; this corresponds to a dose of 2·6 cGy;22 averaging this energy over the entire cell results in a six-fold reduction in the dose. The differences are of course even greater when the dose is calculated over molecular dimensions. Indeed, the conventional dosimetric approach trivialises the importance of deposition of small amounts of energy in extremely minute volumes3,11,14,30 by averaging the deposited energy over the entire organ. The highly localised nature of the deposition of energy is ignored. That this is particularly true when the Auger-electron emitter is bound to DNA in the cell nucleus is demonstrated by our in-vivo experiments.
In as much as the absorbed dose is used for risk assessment, the above considerations point to the need for some modifications in the current dosimetric approaches, particularly for tissue-incorporated Auger-emitters. The in-vitro and in-vivo evidence available presents a compelling case for biophysically meaningful dosimetry at subcellular instead of organ level.10–12,30 Knowledge of the cellular localisation and subcellular distribution of the Auger-emitter is essential. These, in turn, are governed by the chemical form of the radiolabelled agent. These conclusions are amply supported by radiobiological studies both in vitro8 and in our mouse testes model12,23 and have been endorsed by a recent forum on microdosimetry of radiopharmaceuticals.31 It is possible that as more experimental data are collected on the relation between the subcellular distribution and the biological effects, each radiochemical may be assigned a quality factor based on the radionuclide Auger-electron spectrum. Until such predictions can be made with reasonable accuracy, radiation protection guidelines must rely on experimental data obtained at low doses in radiosensitive models.
Acknowledgments
This work was supported in part by USPHS Grant No CA-32877 and New Jersey State Commission on Cancer Research Grant No 689-042.
REFERENCES
- 1.Sastry KSR, Rao DV. Dosimetry of low energy electrons. In: Rao DV, Chandra R, Graham M, editors. Physics of nuclear medicine: recent advances. Medical Physics Monograph no 10. American Institute of Physics; 1984. pp. 169–208. [Google Scholar]
- 2.Charlton DE, Booz J. A Monte Carlo treatment of the decay of 125I. Radiat Res. 1981;87:10–23. [PubMed] [Google Scholar]
- 3.Sastry KSR, Howell RW, Rao DV, et al. Dosimetry of Auger-emitters: physical and phenomenological approaches. In: Baverstock KF, Charlton DB, editors. DNA damage by Auger emitters. London: Taylor & Francis; 1988. pp. 27–38. [Google Scholar]
- 4.Charltnn DE. The range of high LET effects from 125I decays. Radiat Res. 1986;107:163–171. [PubMed] [Google Scholar]
- 5.Hofer KG, Hughes WL. Radiotoxicity of intranuclear tritium, iodine-125 and iodine-131. Radiat Res. 1971;47:94–109. [PubMed] [Google Scholar]
- 6.Hofer KG, Harris CR, Smith JM. Radiotmticity of intracellular Ga-67,I-125, H-3. Nuclear versus cytoplasmic radiation effects in murine L1210 leukaemia. Int J Radiat Biol. 1975;28:225–241. doi: 10.1080/09553007514550991. [DOI] [PubMed] [Google Scholar]
- 7.Kassis AI, Sastry KSR, Adelstein SJ. Kinetics of uptake, retention, and radiotoxicity of I-125 IUdR in mammalian cells: implications of localized energy deposition by Auger processes. Radiat Res. 1987;109:78–89. [PubMed] [Google Scholar]
- 8.Kassis AI, Howell RW, Sastry KSR, Adelstein SJ. Positional effects of Auger decays in mammalian cells in culture. In: Baverstock KF, Charlton DE, editors. DNA damage by Auger emitters. London: Taylor & Francis; 1988. pp. 1–14. [Google Scholar]
- 9.Kassis AI, Adelstein SJ, Haydock C, Sastry KSR, McElvany KD, Welch MJ. Lethality of Auger electrons from the decay of bromine-77 in the DNA of mammalian cells. Radiat Res. 1982;90:362–373. [PubMed] [Google Scholar]
- 10.Rao DV, Govelitz GF, Sastry KSR. Radiotoxicity of thallium-201 in mouse testes: inadequacy of conventional dosimetry. J Nucl Med. 1983;24:145–153. [PubMed] [Google Scholar]
- 11.Rao DV, Sastry KSR, Govelitz GF, Grimmond HE. In vivo effects of iron-55 and iron-59 on mouse testes: biophysical dosimetry of Auger electrons. J Nucl Med. 1985;26:1456–1465. [PubMed] [Google Scholar]
- 12.Rao DV, Sastry KSR, Grimmond HE, et al. Cytotoxicity of some indium radiopharmaceuticals in mouse testes. J Nucl Med. 1988;29:375–384. [PubMed] [Google Scholar]
- 13.Gaulclen ME. “Biological dosimetry” of radionuclides and radiation hazards: a teaching editorial. J Nucl Med. 1983;24:160–164. [PubMed] [Google Scholar]
- 14.Editorial. Auger cascades in nuclear medicine. Lancet. 1985;ii:533–534. [PubMed] [Google Scholar]
- 15.Cember H. Introduction to health physics. New York: Pergamon; 1976. pp. 119–121. [Google Scholar]
- 16.Meistrich ML, Samuels RC. Reduction in sperm levels after testicular irradiation of the mouse: a comparison with man. Radiat Res. 1985;102:138–147. [PubMed] [Google Scholar]
- 17.Oakberg EF, Diminno RL. X-ray sensitivity of primary spermatocytes of the mouse. Int J Radiat Biol. 1960;2:196–209. doi: 10.1080/09553006014550211. [DOI] [PubMed] [Google Scholar]
- 18.Oakberg EF. A discription of spermiogenesis in the mouse and its use in analysis of the cycle of the seminiferous epithelium and germ cell renewal. Am J Anat. 1956;99:391–409. doi: 10.1002/aja.1000990303. [DOI] [PubMed] [Google Scholar]
- 19.Loevinger R, Bertnan M. MIRD Pamphlet No 1, revised. New York: Society of Nuclear Medicine; 1976. A revised schema for calculating the absorbed dose from biologically distributed radionuclides. [Google Scholar]
- 20.Lederer CM, Hollander JM, Perlman I. Table of isotopes. 6th. New York: Wiley; 1967. [Google Scholar]
- 21.Sastry KSR, Rao DV. Biological damage caused by Auger cascades. Lancet. 1986;i:858–859. doi: 10.1016/s0140-6736(86)90970-0. [DOI] [PubMed] [Google Scholar]
- 22.Rao DV, Mylavarapu VB, Govelitz GF, Lanka VK, Sastry KSR, Howell RW. Biological and biophysical dosimetry of Auger-emitters in vivo: a review. In: Madhvanath U, Parthasarathy KS, Venkateswaran TV, editors. Selected topics in physics of radiotherapy and imaging. New Delhi: Tata McGraw-Hill; 1988. pp. 232–258. [Google Scholar]
- 23.Rao DV, Mylavarapu VB, Sastry KSR, Howell RW. Internal Auger emitters: effects on spermatogenesis and oogenesis in mice. In: Baverstock KF, Charlton DE, editors. DNA damage by Auger emitters. London: Taylor and Francis; 1988. pp. 15–26. [Google Scholar]
- 24.Monesi V. Relation between X-ray sensitivity and stages of the cell cycle in spermatogonia of the mouse. Radiat Res. 1962;17:809–838. [PubMed] [Google Scholar]
- 25.Kassis AI, Harris CR, Adelstein SJ, Ruth TJ, Lambrecht R, Wolf AP. The in vitro radiobiology of astatine-211 decay. Radiat Res. 1986;105:27–36. [PubMed] [Google Scholar]
- 26.Recommendations of the International Commission on Radiological Protection. ICRP Publication 26. Vol. 1. Oxford: Pergamon; 1977. p. 5. [Google Scholar]
- 27.Barendsen GW. Dose-survival curves of human cells in tissue culture irradiated with alpha-, beta-, 20-kV. X- and 200-kV. X-radiation. Nature. 1962;193:1153–1155. doi: 10.1038/1931153a0. [DOI] [PubMed] [Google Scholar]
- 28.Cox R, Thacker J, Goodhead DT, Munson RJ. Mutation and inactivation of mammalian cells by various ionising radiations. Nature. 1977;267:425–427. doi: 10.1038/267425a0. [DOI] [PubMed] [Google Scholar]
- 29.ICRU Report 32. Methods of assessment of absorbed dose in clinical use of radionuclides. Washington, DC: International Commission on Radiation Units and Measutements; 1979. [Google Scholar]
- 30.Adelstein SJ, Kassis AI, Sastry KSR. Cellular vs. organ approaches to dose estimates. In: Schlafke-Stelson AT, Watson EE, editors. Proceedings of the Fourth International Radiophamaceutical Dosimetry Symposium, Oak Ridge, Tennessee, November 1985. 1986. pp. 13–25. [Google Scholar]
- 31.Forum on the microdosimetry of radiopharmaceuticals: committee on effects of ionising radiation. Int J Radiat Biol. 1986;50:555–567. doi: 10.1080/09553008614550951. [DOI] [PubMed] [Google Scholar]
- 32.Cole A. Absorption of 20 eV to 50,000 eV electron beams in air and plastic. Radiat Res. 1969;38:7–33. [PubMed] [Google Scholar]