Abstract
Objectives
Studies on coronavirus disease 2019 (COVID-19) usually focus on middle-aged and older adults. However, younger patients may present with severe COVID-19 with potentially fatal outcomes. For optimized, more specialized therapeutic regimens in this particular patient group, a better understanding of the underlying pathomechanisms is of utmost importance.
Methods
Our study investigated relevant, pre-existing medical conditions, clinical histories, and autopsy findings, together with SARS-CoV-2-RNA, determined by qPCR, and laboratory data in six COVID-19 decedents aged 50 years or younger, who were autopsied at the Charité University Hospital.
Results
From a total of 76 COVID-19 patients who underwent an autopsy at our institution, six (7.9%) were 50 years old or younger. Most of these younger COVID-19 decedents presented with pre-existing medical conditions prior to SARS-CoV-2 infection. These included overweight and obesity, arterial hypertension, asthma, and obstructive sleep apnea, as well as graft-versus-host disease following cancer and bone marrow transplantation. Furthermore, clinical histories and autopsy results revealed a disproportionally high prevalence of thromboembolism and ischemic organ damage in this patient cohort. Histopathology and laboratory results indicated coagulopathies, signs of immune dysregulation, and liver damage.
Conclusions
In conclusion, pre-existing health conditions may increase the risk of severe and fatal COVID-19 in younger patients, who may be especially prone to developing thromboembolic complications, immune dysregulation, and liver damage.
Keywords: COVID-19, SARS-CoV-2, Cause of death, Autopsy, Younger
Introduction
The coronavirus disease 2019 (COVID-19) pandemic has caused more than two million deaths worldwide, mainly due to severe respiratory and vascular symptoms (Berlin et al., 2020; WHO report, 2020). Most studies have focused on middle-aged and older adults, who are particularly endangered by the virus and are more likely to suffer from serious, life-threatening courses of disease (Guan et al., 2020, Morley and Vellas, 2020). However, younger patients with no or very few pre-existing health conditions may be critically affected by severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2) infections, accompanied by serious health conditions and potentially fatal outcomes. For example, Gunasekaran et al. (2020) described a case of a 40-year-old female without any known pre-existing medical condition, who was tested positive for SARS-CoV-2, presented with flu-like symptoms, and, during the course of the disease, suffered from a large right-middle cerebral artery infarction. Another case report described the occurrence of fatal eosinophilic myocarditis in a 17-year-old male without pre-existing medical conditions (Craver et al., 2020). However, such cases appear to be rare, and little is known about COVID-19 pathomechanisms, courses of disease, and causes of death in younger adults.
Therefore, our study investigated relevant pre-existing medical conditions, clinical histories, and autopsy findings in six deceased COVID-19 patients aged 50 years or younger. Our study includes data on SARS-CoV-2 RNA in lung tissue as well as key laboratory values to analyze potential underlying pathomechanisms of the disease.
Methods
Study design
This study included all patients aged 50 years or younger who were hospitalized at the Charité University Hospital in Berlin (Germany) and died between March 1, 2020 and January 31, 2021 after confirmed SARS-CoV-2 infection with an autopsy request. Autopsies of these individuals were performed in the Institute of Pathology of the Charité University Hospital. Antemortem SARS-CoV-2 testing was performed by polymerase chain reaction (PCR) analysis of nasal and pharyngeal swab samples. For autopsies, informed consent was given by close relatives. Autopsies were undertaken in accordance with the §1 SRegG BE of the autopsy act of Berlin and §25(4) of the German Infection Protection Act. The study was approved by the Ethics Committee of the Charité (EA 1/144/13 and EA2/066/20) and the Charité-BIH COVID-19 research board, and adhered to the local principles of good clinical practice (‘Grundsätze der Charité zur Sicherung guter wissenschaftlicher Praxis’), as well as the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Autopsy procedure and tissue processing
External examination, autopsy, and tissue sampling were performed in all patients according to recent recommendations regarding safety precautions in suspected or confirmed COVID-19 cases, including the use of Filtering Face Piece 2 (FFP2) masks, protective suits, and cut-resistant gloves (Centers for Disease Control and Prevention, 2020). For histopathology, tissue samples were taken from each patient’s internal organs, from which the most representative areas were chosen upon macroscopic evaluation. The tissue samples were fixed in 4% buffered formalin. Paraffin-embedded, 4 μm-thick sections were stained with hematoxylin and eosin (HE), periodic acid–Schiff (PAS) stain, Van Gieson stain, and Prussian blue stain. Additionally, disease-specific immunohistochemical staining was performed if necessary. At least two pathologists examined all tissue slides by light microscopy.
Extraction of clinical data and pathological evaluation
Clinical data were extracted from available patient records and clinical death certificates. Data on clinical causes of death, as mentioned on the clinical death certificate, age, sex, onset of symptoms, documented pre-existing health conditions as well as laboratory parameters relevant for coagulation, inflammation and liver damage were selected.
SARS-CoV-2-specific PCR analysis
For PCR analysis of SARS-CoV-2 ribonucleic acid (RNA) in tissue samples, native tissue material, unfixed and, if possible, non-cryopreserved, was used. For RNA isolation and purification, the MagNA Pure 96 system was applied according to the manufacturer’s instructions, using approximately 50 mg of homogenized tissue obtained from organ samples. Quantitative real-time PCR targeting the SARS-CoV-2 E-gene was performed on RNA extracts, and quantification of the viral RNA was undertaken as described previously (Corman et al., 2020). Total DNA was quantified by applying the Qubit dsDNA HS assay kit (Thermo Fisher Scientific) according to the manufacturer’s instructions. The PCR analysis was replicated at least once per sample, if it tested positive. Detection of subgenomic RNA (sgRNA) was performed using oligonucleotides targeting the leader transcriptional regulatory sequence and region within the sgRNA coding for the E-gene, as described previously (Wölfel et al., 2020).
Statistical analysis
For data collection, statistical analysis, and graphical presentation, IBM SPSS Statistics, Version 23 (IBM, NY, USA) and GraphPad Prism 8.0 for Windows (GraphPad Software, SanDiego, CA, USA) were used.
Age and death were depicted as median with interquartile range (IQR) for consideration of deviations from a normal distribution. Categorical values were summarized and displayed as counts and percentages. Median survival was analyzed using the Kaplan–Meier method.
Results
Pre-existing medical conditions in most younger adults with severe COVID-19
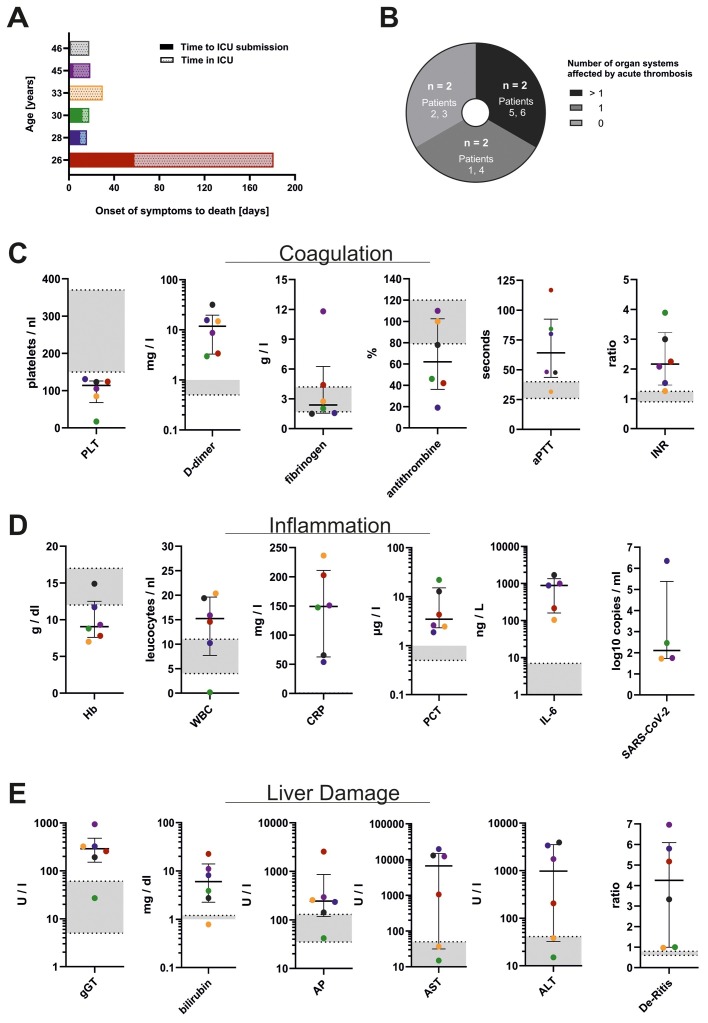
For this study, we performed autopsies on patients who died after COVID-19 disease with confirmed SARS-CoV-2 infection. Of all 76 SARS-CoV-2-positive autopsy cases between March 1, 2020 and January 31, 2021, six individuals met the age requirement, accounting for a total of 7.9%. Of these six decedents, three were male and three were female. The median age at death was 31.5 years (range 26–46 years). The median time from onset of COVID-19 symptoms to death was 18 days, ranging from 16 to 181 days (95% CI 15.6–20.4 days, Figure 1 A).
Figure 1.
Parameters for disease severity in six COVID-19 decedents aged <50 years. (A) Time from onset of symptoms to death, including time in intensive care unit (ICU). (B) Autoptic proof of thromboembolism. (C) Coagulation parameters in the peripheral blood drawn within the week prior to death. (D) Infection-associated parameters drawn from the peripheral blood prior to death and post-mortem pulmonary SARS-CoV-2 levels. (E) Liver parameters in the peripheral blood prior to death. The laboratory results in C–E are color-coded according to each patient’s age as depicted in A. Centre values represent the median of the entire cohort and error bars indicate the standard deviation. Standard values are displayed as grey zones.
Abbreviations: number of patients (n), platelets (PLT), activated partial thromboplastin time (aPTT), international normalized ratio (INR), hemoglobin (Hb), white blood cell count (WBC), C-reactive protein (CRP), procalcitonin (PCT), interleukin-6 (IL-6), severe acute respiratory syndrome virus 2 (SARS-CoV-2), gamma glutamyl transferase (gGT), alkaline phosphatase (AP), aspartate transaminase (AST), alanine transaminase (ALT), De Ritis ratio (ratio of AST:ALT).
Additional information on clinical presentation, symptoms, relevant pre-existing medical conditions, and the course of disease were available for all cases (Table 1, Table 2 ). Prior to death, all patients presented with COVID-19-related pulmonary symptoms and respiratory failure. Despite being of younger age, 83% (n = 5/6) of the included patients had pre-existing chronic medical conditions. Of these patients, 50% were overweight (n = 3/6), and one decedent fulfilled the criteria for obesity (n = 1/6). Chronic respiratory diseases were present in half of the cohort, since two patients suffered from bronchial asthma (n = 2/6) and one patient had a history of obstructive sleep apnea (n = 1/6). Furthermore, three patients presented cardiovascular conditions, such as arterial hypertension (n = 1/6), dilated cardiomyopathy with a pre-existing left ventricular thrombus (n = 1/6), and a history of pulmonary thromboembolism (n = 1/6). One patient presented with a history of Ewing sarcoma followed by chemotherapy and chemotherapy-related complications (n = 1/6). The cohort also included one patient with chronic anxiety disorder and consecutive use of benzodiazepines (n = 1/6). Notably, one patient presented without pre-existing chronic disease.
Table 1.
Clinical causes of death and documented comorbidities as reported in the death certificates of six young decedents with COVID-19.
| Case | Age | Gender | Symptoms to death | Immediate COD | Condition leading to COD | Underlying cause | Comorbidities/conditions |
|---|---|---|---|---|---|---|---|
| 1 | 26 years | Male | 181 days | Mesenteric ischemia | SARS-CoV-2 pneumonia | – | Coinfection with CMV/HHV-1 SSC History of pulmonary thromboembolism OSA Gastritis Nicotine abuse |
| 2 | 28 years | Female | 16 days | Acute liver failure | Celiac trunk ischemia | COVID-19 with ARDS | Coinfection with CandidaR ight heart insufficiency Asthma Obesity (WHO grade III) |
| 3 | 30 years | Female | 18 days | Septic MOF | Sepsis | Intestinal GvHD |
COVID-19 Coinfection with CMV Toxoplasmosis Dermal GvHD t-MDS (allo-HSCT, ruxolitinib) Ewing sarcoma (chemotherapy) Graves’ disease |
| 4 | 33 years | Male | 30 days | Hemorrhagic shock | Airway bleeding | COVID-19 | Acute pulmonary thromboembolism |
| 5 | 45 years | Female | 19 days | Septic MOF | SARS-CoV-2 pneumonia | Viral pneumonia |
Staph. aureus coinfection Mesenterial infarction Arterial hypertension Asthma COPD |
| 6 | 46 years | Male | 18 days | Cardiogenic shock | SARS-CoV-2 pneumonia | Dilated cardiomyopathy | Chronic left ventricular heart thrombus Anxiety disorder Benzodiazepine abuse |
Abbreviations: cause of death (COD), multiple organ failure (MOF), severe acute respiratory syndrome coronavirus type 2 (SARS CoV-2), coronavirus disease 2019 (COVID-19), cytomegalovirus (CMV), human herpes virus 1 (HHV-1), secondary sclerosing cholangitis (SCC), obstructive sleep apnea (OSA), World Health Organization (WHO), graft-versus-host disease (GvHD), therapy-induced myelodysplastic syndrome (t-MDS), allogeneic hematopoietic stem cell transplantation (allo-HSCT), Staphylococcus (Staph.), chronic obstructive pulmonary disease (COPD).
Table 2.
Causes of death and comorbidities as determined by autopsy in six young decedents with COVID-19.
| Case | Age | Gender | Symptoms to death | Immediate COD | Condition leading to COD | Underlying cause | Comorbidities/conditions |
|---|---|---|---|---|---|---|---|
| 1 | 26 years | Male | 181 days | Acute mesenteric ischemia | Cholangitis with liver failure | COVID-19 pneumonia | Thromboembolisms of brain vessels Hepatosplenomegaly Pulmonary emphysema Overweight (BMI 28.7 kg/m2)* |
| 2 | 28 years | Female | 16 days | MOF | Invasive pulmonary mycosis | COVID-19 pneumonia | Acute inflammatory cardiomyopathy Mild hepatic steatosis (5–7%)Obesity, WHO °III (BMI 41.2 kg/m 2)* |
| 3 | 30 years | Female | 18 days | Septic MOF | Invasive pulmonary mycosis Intestinal GvHD | Ewing sarcoma followed by t-MDS with therapy-related immunosuppression |
SARS-CoV-2 infection Lymphocytic myocarditis Cerebral bleeding Cerebral toxoplasmosis Overweight (BMI 28.1 kg/m2)* |
| 4 | 33 years | Male | 30 days | Hemorrhagic shock after intrathoracic bleeding | Pleural empyema (surgical treatment) | COVID-19 pneumonia | Acute pulmonary thromboembolism Hepatomegaly Heart failure |
| 5 | 45 years | Female | 19 days | Septic MOF | Disseminated intravascular coagulation (pulmonary, intestinal, cerebral) | COVID-19 pneumonia | Hepatic steatosis Coronary artery sclerosis Atherosclerosis |
| 6 | 46 years | Male | 18 days | Heart failure | COVID-19 pneumonia | Dilated cardiomyopathy | Acute pulmonary thromboembolism Chronic cardiac apical thrombus Mild atherosclerosis Overweight (BMI 29.1 kg/m2)* |
Abbreviations: cause of death (COD), multi-organ failure (MOF), severe acute respiratory syndrome coronavirus type 2 (SARS CoV-2), coronavirus disease 2019 (COVID-19), body mass index (BMI), *corrected for body cavity effusion), secondary sclerosing cholangitis (SCC), World Health Organization (WHO), graft-versus-host disease (GvHD), therapy-induced myelodysplastic syndrome (t-MDS), allogeneic hematopoietic stem cell transplantation (allo-HSCT).
Life-threatening events and death in younger, hospitalized adults with severe COVID-19
During hospitalization, acute pulmonary thromboembolism was detected in two patients, specifically the patient without previous symptoms and the patient with a history of pre-existing dilated cardiomyopathy with cardiac thrombus (n = 2/6). The decedent with previous asthma and obesity suffered from right ventricular heart failure and acute inflammatory cardiomyopathy (n = 1/6). Acute liver failure occurred in three patients (n = 3/6). More precisely, the patient presenting with pre-existing Ewing sarcoma suffered from acute hepatic graft-versus-host disease with restricted liver function. Another patient developed signs of secondary sclerosing cholangitis, while the third patient who had presented with asthma and obesity developed liver ischemia due to a stenosis of the celiac trunk. Acute renal failure co-occurred in four of the six patients.
All deaths occurred in the intensive care unit (ICU). Median time from admission to ICU to death was 15.5 days (range 6–123 days). In most individuals (n = 5/6), invasive ventilation as well as extracorporeal membrane oxygenation were documented as part of intensive patient care. Immediate causes of death as documented in the clinical death certificates were septic multi-organ failure (n = 2/6), mesenteric ischemia (n = 1/6), acute liver failure (n = 1/6), hemorrhagic shock (n = 1/6), and cardiogenic shock (n = 1/6). More detailed information on conditions leading to immediate causes of death and death-related underlying diseases can be found in Table 1.
Causes of death and comorbidities determined by autopsy results
In order to comprehend the clinically described comorbidities, courses of disease, and causes of death, full-body autopsies were performed on all six decedents. Immediate causes of death, conditions leading to cause of death, and underlying causes were defined based on pathological findings upon autopsy combined with the clinical data for each case (Table 2).
In three cases, (septic) multi-organ failure was identified as immediate cause of death (n = 3/6). In the other three cases, acute mesenteric ischemia (n = 1/6), heart failure (n = 1/6), or hemorrhagic shock (n = 1/6) were found to be the immediate cause of death. For conditions leading to cause of death, COVID-19 pneumonia (n = 1/6), invasive pulmonary mycosis (n = 2/6) with intestinal graft-versus-host disease (n = 1/6), disseminated arterial thrombosis (n = 1/6), cholangitis with liver failure (n = 1/6), and surgically treated pleural empyema (n = 1/6) were identified. As underlying causes, COVID-19 pneumonia was found to be most prevalent (n = 4/6), followed by dilated cardiomyopathy (n = 1/6) and history of chemoradiotherapy for Ewing sarcoma (n = 1/6). Other relevant macroscopically detected disease conditions included adipositas per magna (n = 1/6), cardiac apical thrombus (n = 1/6), mild atherosclerosis (n = 1/6), splenomegaly (n = 1/6), and hepatomegaly (n = 2/6). In combination, evidence of pre-existing chronic disease was present in five out of six younger COVID-19 decedents determined by autopsy.
Histopathology and laboratory results reveal coagulopathy, signs of immune dysregulation, and liver damage
Ante-mortem laboratory parameters revealed a constellation of (acute) liver damage, coagulopathy, anemia and general inflammation (Figure 2C and D).
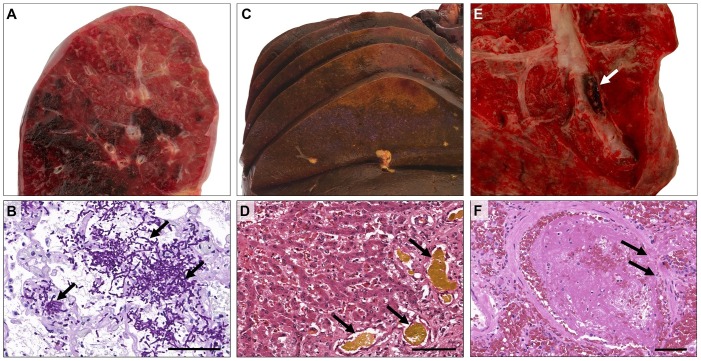
Figure 2.
Macroscopic and microscopic appearances in younger COVID-19 decedents. Macroscopically striking patchy hemorrhagic areas (A) and histological evidence of invasive pulmonary mycosis (B; PAS) in patient 3. The liver of patient 1 showed severe cholestasis (C + D; H&E) and focal ischemic damage (C). Macroscopic aspect (E, patient 6) and histological evidence (F, patient 5; H&E) of pulmonary thromboembolism. Scale bars correspond to 100 μm.
Post-mortem histopathology revealed thrombosis and/or ischemic organ damage in one or more organ systems in four, and severe bleeding in two out of six patients (Table 2, Figures 1B, 2 E and 2 F). Furthermore, fungal and/or parasitic infections were found in two out of the six patients, including pulmonary aspergillosis (n = 2/6) and cerebral toxoplasmosis (n = 1/6) (Figures 2A, 2 B, 3 E and 3 F), indicating immunodeficiency. Additionally, one case with lymphocytic myocarditis was observed (n = 1/6) (Figure 3B). Aside from acute symptoms, indicators for chronic metabolism-induced damage, such as hepatic steatosis (n = 2/6), mild to moderate atherosclerosis (n = 2/6), coronary artery sclerosis (n = 1/6), as well as chronic right-heart damage (n = 1/6) were found in four out of six patients.
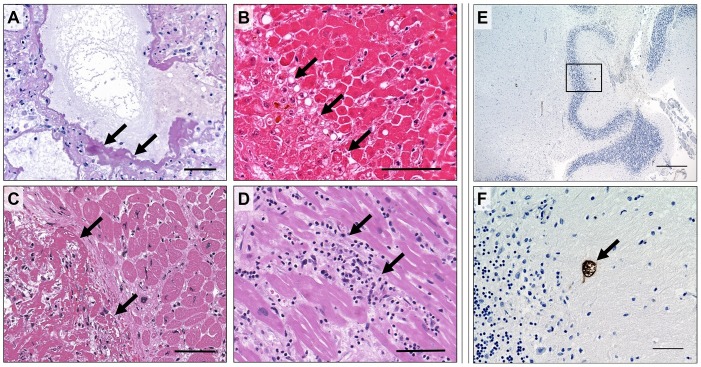
Figure 3.
Histopathological findings in younger COVID-19 decedents. The lung tissue of patient 2 showed hyaline membranes, a characteristic histological feature of COVID-19 indicating diffuse alveolar damage (A; PAS). Patient 2 also showed severe liver damage following celiac trunk ischemia (B; H&E). In patient 6 a large cardiac apical thrombus (C; H&E) was observed. Patient 3 showed a lymphocytic myocarditis, aside from the characteristics described in Figure 1 (D; H&E), and residual cerebral toxoplasmosis after treatment in the cerebellum (E + F, with F showing a close-up section of E; immunohistochemical staining). Scale bars correspond to 100 μm in A–D, 500 μm in E, and 50 μm in F.
Heterogeneous individual clinical histories
The youngest patient in this cohort, at 26 years of age, had a particularly long disease history. His pre-existing medical conditions were active nicotine abuse (15 pack years) and a history of perforated gastric ulcer in 2014. He presented initially with dyspnea. Due to increasing respiratory failure, the patient’s treatment required invasive ventilation and, later, extracorporeal membrane oxygenation. Amongst other complications, he developed an ischemia of the upper gastrointestinal tract refractory to therapy, hyperinflammation syndrome, acute liver failure with secondary sclerosing cholangitis (Figure 2C and D), reactivation of human herpes virus 1 (HHV-1), acute kidney injury, and a pulmonary thromboembolism during his hospitalization. Despite maximum therapeutic efforts, including the application of steroids, interleukin-1-receptor antagonists (Anakinra) and immunoglobulins, he died from an acute mesenteric ischemia.
A 28-year-old female patient with obesity, asthma, and right cardiac insufficiency, who developed a severe COVID-19 pneumonia with aspergillus superinfection of the lungs and trachea, died within 16 days from onset of symptoms after a suspected COVID-19 hyperinflammation syndrome, despite maximum therapeutic attempts, including newer therapeutic agents such as Anakinra and Ruxolitinib.
Another younger female patient in our cohort, who was aged 30 years, had suffered from a lengthy cancer history prior to SARS-CoV-2 infection and death. Her clinical records showed that she was diagnosed with Ewing sarcoma in 2013, treated with chemotherapy, which then resulted in therapy-associated myelodysplastic syndrome that was treated with an allogeneic hematopoietic stem cell transplantation in 2019. Consequently, she developed an acute intestinal graft-versus-host disease and toxoplasmosis with cerebral and pulmonal involvement (Figure 3E and F). During hospital treatment she was infected with SARS-CoV-2. Despite an optimized therapy regimen, her condition worsened due to aspiration, acute respiratory distress syndrome, acute renal failure, and, later, capillary leak, resulting in a lethal outcome of the disease. In addition to the clinical data, severe invasive mycosis of the lungs with hemorrhagic pneumonia (Figure 2A and B) was found by autopsy.
The fourth patient in our cohort was 33 years of age and did not have any known chronic disease prior to SARS-CoV-2 infection. He presented with a lung empyema and subsequently underwent surgery with pleural decortication. However, during the course of the disease, a pulmonary thromboembolism was diagnosed, which was treated with anticoagulation. Consequently, he developed a hematothorax, resulting in fulminant deterioration and death. Although this patient presented without pre-existing medical conditions, the fatal outcome in this case can be attributed to a treatment-associated major adverse event.
Next, our cohort included a 45-year-old patient who presented with flu-like symptoms, fever up to 39.5 °C, dry cough, headache, and myalgia. During the disease, she developed acute respiratory distress syndrome and required invasive ventilation. Despite maximum therapeutic efforts her condition worsened, and she developed acute liver failure and septic multi-organ failure. Histopathological analysis following autopsy found evidence of thrombotic vascular occlusion in different organ systems, including the lung, heart, kidneys, small intestine, and brain (Figure 2F).
Lastly, the cohort included one patient (46 years old) with chronic anxiety disorder and abuse of benzodiazepines. Despite the patient’s meticulous efforts of self-isolation, he was infected with SARS-CoV-2. Known pre-existing medical conditions were a dilated cardiomyopathy with highly reduced left ventricular ejection fraction (LVEF 15%), as well as a pre-existing chronic cardiac apical thrombus (Figure 3C) and a pulmonary thromboembolism, which he developed during the cause of the disease (Figure 2E). After initial signs of recovery, the patient’s respiratory and cardiac function declined, and he died from cardiogenic shock.
Discussion
This study provides a detailed insight into pre-existing chronic health conditions, clinical disease courses, and autopsy findings in younger adults with COVID-19-related death. To date, several autopsy studies of COVID-19 decedents have been published with observations on comorbidities and causes of death in unselected COVID-19 cohorts of all age groups. In these cohorts, the median age of investigated patients was approximately 70 years, and the proportion of younger adults was no more than 20% (Bryce et al., 2020, Chen et al., 2020, Elezkurtaj et al., 2021, Keresztesi et al., 2020, Onder et al., 2020, Wichmann et al., 2020). However, the analysis of younger decedents as a separate subgroup may be of particular interest, since younger patients tend to have fewer pre-existing medical conditions. Therefore, risk factors or predictors of outcome in severe COVID-19 may be narrowed down more specifically to certain conditions or circumstances in younger patients.
Our data show that the younger COVID-19 decedents in our cohort predominantly presented with relevant pre-existing medical conditions prior to SARS-CoV-2 infection. Common pre-existing conditions included overweight and obesity, asthma, and obstructive sleep apnea (OSA), as well as chronic cardiovascular disease.
Several studies have analyzed the influence of obesity on COVID-19 mortality and found that obese patients have a higher risk of a severe COVID-19 course of disease (Stefan et al., 2020), higher mortality from a SARS-CoV-2 infection (Hussain et al., 2020), and increased COVID-19 prevalence (Petrakis et al., 2020). Interestingly, for asthma, recent studies have observed higher rates of comorbid asthma in COVID-19 patients (Hughes-Visentin and Paul, 2020). However, additional studies have not shown an increased risk for hospitalization for patients classified as having asthma (Chhiba et al., 2020). Especially in patients with allergic asthma, no statistically significant correlation has been detectable between asthma and severe COVID-19 symptoms (Zhu et al., 2020). A possible explanatory role for this counterintuitive observation has been discussed in terms of type II inflammatory response cytokines and accumulation of eosinophils in asthma patients, which have been hypothesized to confer protective effects against COVID-19 (Hughes-Visentin and Paul, 2020). One patient in our cohort showed obstructive sleep apnea, which, especially with concurrent obesity, has been reported to contribute to worsening hypoxemia and cytokine release in COVID-19 patients (McSharry and Malhotra, 2020). Regarding arterial hypertension, studies have suggested that hypertension may be associated with an up to 2.5-fold higher risk of severe and fatal COVID-19 (Gao et al., 2020, Lippi et al., 2020; Tadic et al., 2020).
To summarize, studies investigating comorbidities have shown an increased risk of COVID-19 mortality, especially for obesity, OSA, and hypertension. These pre-existing conditions were prevalent in three of our six investigated cases, and may have contributed to each patient’s clinical history, despite their young age. However, these chronic health conditions are common among the general public, and many patients with these comorbidities still show moderate to mild courses of COVID-19.
From combined clinical data and autopsy results our study found that organ damage due to ischemic processes or development of thromboembolism occurred frequently (67%, n = 4/6). COVID-19-associated vasculitis and vasculopathy have been intensely discussed recently, as many research groups have observed macro- and microvascular thrombosis in different organs in COVID-19 patients (Becker, 2020). It has been reported that a hypercoagulable state may occur due to COVID-19, correlating strongly with mortality (Tang et al., 2020, Hanff et al., 2020). In another study published by our research group, showing autopsy results of all age groups, the occurrence of pulmonary thromboembolisms was diagnosed in approximately every fourth patient (Elezkurtaj et al., 2021). Higher rates of thromboembolic events have been reported by Schurink et al. (2020), with thromboembolic occurrences in 48% of decedents, and Wichmann et al. (2020), with venous thromboembolism in 58% and pulmonary thromboembolism as cause of death in 33.3% of decedents. Importantly, in earlier studies investigating COVID-19-related deaths, intensive anticoagulation had not yet been implemented into therapeutic regimens. Here, 66.7% of younger individuals had either ischemic organ damage, and/or thromboembolism, which appear to be more frequent in younger adults. A possible explanation for this observation may be that hyperinflammatory states, which have been described especially in children (Licciardi et al., 2020, Loomba et al., 2020, Riphagen et al., 2020), may lead to a hypercoagulable state, as described by Pourafkari et al. (2021). Furthermore, it is possible that in younger adults, who generally present in better health than older adults, more severe complications (such as thromboembolic events) are required to cause fatal outcomes. Conversely, older adults might succumb more easily to less severe complications.
A limitation of this study was the small sample size, which might have led to a selection bias towards younger patients with particularly severe COVID-19 and therefore a need for special intensive care admission. Additionally, autopsies might have been predominantly requested in patients with complicated or unforeseen clinical courses of disease. Larger cohort studies including control groups would be necessary for future validation of our results and for the determination of differences between age-related subgroups of COVID-19 decedents.
In conclusion, all the younger COVID-19 decedents in our cohort except one presented with pre-existing chronic medical conditions prior to SARS-CoV-2 infection, including obesity, OSA, and hypertension. These comorbidities most probably influenced the course of disease in these patients. Furthermore, our data suggest that younger SARS-CoV-2 infected adults may be particularly prone to thromboembolism and ischemic organ damage, as well as coagulopathies, immune dysregulation, and acute liver damage. The specific case histories emphasize that: (i) COVID-19 patients may develop serious and life-threatening health conditions, despite being young and healthy; and (ii) younger patients with severe pre-existing chronic health conditions are particularly vulnerable to a lethal COVID-19 outcome, especially as treatment options remain limited. In order to protect younger adults with relevant pre-existing health conditions, a prioritization of this patient group should be considered in vaccination strategies.
Conflicts of interest
All authors declare that they have no conflicts of interest with regard to this work.
Author contributions
SG and JI contributed equally to this study. SG, JI, MPD, SS, LH, DW, JM, TA, JS, IT, CAK, LM, HR, FLH, DH, and SE performed autopsies, histopathology, and clinical workup. JS and VMC performed viral RT-qPCR testing. DH and SE supervised the study. All authors analyzed the data, and wrote, revised, and approved the manuscript.
Funding
The authors acknowledge support from the German Research Foundation (DFG) and the Open Access Publication Fund of Charité — Universitätsmedizin Berlin for funding of open-access publication.
Acknowledgements
The authors are obliged to Anistan Sebastiampillai, Juliane Plaschke and Francisca Egelhofer for their excellent technical support during the autopsy procedure, as well as to Christoph Weber for his outstanding photography. The authors are thankful to Barbara Meyer-Bartell for her help with the laboratory workup. Furthermore, they would like to thank Sebastian Brünink and Tobias Bleicker for their help with SARS-CoV-2 RT-PCR testing.
References
- Becker R.C. COVID-19-associated vasculitis and vasculopathy. J Thromb Thrombolysis. 2020;50(3):499–511. doi: 10.1007/s11239-020-02230-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlin D.A., Gulick R.M., Martinez F.J. Severe Covid-19. NEJM. 2020;383(25):2451–2460. doi: 10.1056/NEJMcp2009575. [DOI] [PubMed] [Google Scholar]
- Bryce C., Grimes Z., Pujadas E., Ahuja S., Beasley M.B., Albrecht R. Pathophysiology of SARS-CoV-2: targeting of endothelial cells renders a complex disease with thrombotic microangiopathy and aberrant immune response. The Mount Sinai COVID-19 autopsy experience. medRxiv. 2020 doi: 10.1101/2020.05.18.20099960. 05.18.20099960. [DOI] [Google Scholar]
- Chen T., Wu D., Chen H., Yan W., Yang D., Chen G. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhiba K.D., Patel G.B., Vu T., Chen M.M., Guo A., Kudlaty E. Prevalence and characterization of asthma in hospitalized and nonhospitalized patients with COVID-19. J Allergy Clin Immunol. 2020;146(2):307–314.e4. doi: 10.1016/j.jaci.2020.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corman V.M., Landt O., Kaiser M., Molenkamp R., Meijer A., Chu D.K. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25(3):2000045. doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craver R., Huber S., Sandomirsky M., McKenna D., Schieffelin J., Finger L. Fatal eosinophilic myocarditis in a healthy 17-year-old male with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2c) Fetal Pedriatr Pathol. 2020;39(3):263–268. doi: 10.1080/15513815.2020.1761491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elezkurtaj S., Greuel S., Ihlow J., Michaelis E., Bischoff P., Kunze C.A. Causes of death and comorbidities in patients with COVID-19. Sci Rep. 2021;11:4263. doi: 10.1038/s41598-021-82862-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao C., Cai Y., Zhang K., Zhou L., Zhang Y., Zhang X. Association of hypertension and antihypertensive treatment with COVID-19 mortality: a retrospective observational study. Eur Heart J. 2020;41(22):2058–2066. doi: 10.1093/eurheartj/ehaa433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan S.P., Seet R., Kennedy B.K. Does eNOS derived nitric oxide protect the young from severe COVID-19 complications? Ageing Res Rev. 2020;64:101201. doi: 10.1016/j.arr.2020.101201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunasekaran K., Amoah K., Rajasurya V., Buscher M.G. Stroke in a young COVID-19 patient. QJM. 2020;113(8):573–574. doi: 10.1093/qjmed/hcaa177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanff T.C., Mohareb A.M., Giri J., Cohen J.B., Chirinos J.A. Thrombosis in COVID-19. Am J Hematol. 2020;95(12):1578–1589. doi: 10.1002/ajh.25982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes-Visentin A., Paul A. Asthma and COVID-19: what do we know now. Clinical medicine insights. Clin Med Circ Respir Pulm Med. 2020;14 doi: 10.1177/1179548420966242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain A., Mahawar K., Xia Z., Yang W., El-Hasani S. Obesity and mortality of COVID-19. Meta-analysis. ORCP. 2020;14(4):295–300. doi: 10.1016/j.orcp.2020.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Keresztesi A.A., Perde F., Ghita-Nanu A., Radu C.C., Negrea M., Keresztesi G. Post-mortem diagnosis and autopsy findings in SARS-CoV-2 infection: forensic case series. Diagnostics (Basel, Switzerland) 2020;10(12):1070. doi: 10.3390/diagnostics10121070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licciardi F., Pruccoli G., Denina M., Parodi E., Taglietto M., Rosati S. SARS-CoV-2-induced Kawasaki-like hyperinflammatory syndrome: a novel COVID Phenotype in children. Pediatrics. 2020;146(2) doi: 10.1542/peds.2020-1711. [DOI] [PubMed] [Google Scholar]
- Lippi G., Wong J., Henry B.M. Hypertension in patients with coronavirus disease 2019 (COVID-19): a pooled analysis. Pol Arch Intern Med. 2020;130(4):304–309. doi: 10.20452/pamw.15272. [DOI] [PubMed] [Google Scholar]
- Loomba R.S., Villarreal E.G., Flores S. COVID-19 and hyperinflammatory syndrome in children: Kawasaki disease with macrophage activation syndrome in disguise? Cureus. 2020;12(8):e9515. doi: 10.7759/cureus.9515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McSharry D., Malhotra A. Potential influences of obstructive sleep apnea and obesity on COVID-19 severity. J Clin Sleep Med. 2020;16(9):1645. doi: 10.5664/jcsm.8538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley J.E., Vellas B. Editorial: COVID-19 and older adults. J Nutr Health Aging. 2020;24(4):364–365. doi: 10.1007/s12603-020-1349-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onder G., Rezza G., Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020;323(18):1775–1776. doi: 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- Petrakis D., Margină D., Tsarouhas K., Tekos F., Stan M., Nikitovic D. Obesity — a risk factor for increased COVID-19 prevalence, severity and lethality (Review) Mol Med Rep. 2020;22(1):9–19. doi: 10.3892/mmr.2020.11127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourafkari L., Mirza-Aghzadeh-Attari M., Zarrintan A., Mousavi-Aghdas S.A. Clinical experience, pathophysiology, and considerations in the prophylaxis and treatment of hypercoagulopathy of COVID-19: a review study. Iran J Med Sci. 2021;46(1):1–14. doi: 10.30476/ijms.2020.87233.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention . 2020. Collection and submission of postmortem specimens from deceased persons with confirmed or suspected COVID-19 — interim guidance.https://www.cdc.gov/coronavirus/2019-ncov/hcp/guidance-postmortem-specimens.html [Google Scholar]
- Riphagen S., Gomez X., Gonzalez-Martinez C., Wilkinson N., Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 2020;395(10237):1607–1608. doi: 10.1016/S0140-6736(20)31094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schurink B., Roos E., Radonic T., Barbe E., Bouman C., de Boer H.H. Viral presence and immunopathology in patients with lethal COVID-19: a prospective autopsy cohort study. Lancet Microbe. 2020;1(7):e290–e299. doi: 10.1016/S2666-5247(20)30144-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefan N., Birkenfeld A.L., Schulze M.B., Ludwig D.S. Obesity and impaired metabolic health in patients with COVID-19. Nat Rev Endocrinol. 2020;16(7):341–342. doi: 10.1038/s41574-020-0364-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadic M., Cuspidi C., Grassi G., Mancia G. COVID-19 and arterial hypertension: hypothesis or evidence? J Clin Hypertens. 2020;22(7):1120–1126. doi: 10.1111/jch.13925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (WHO) 2020. Coronavirus disease (COVID-19) situation report of the World Health Organization — weekly epidemiological update.https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200816-covid-19-sitrep-209.pdf?sfvrsn=5dde1ca2_2 [Google Scholar]
- Wichmann D., Sperhake J.P., Lütgehetmann M., Steurer S., Edler C., Heinemann A. Autopsy findings and venous thromboembolism in patients with COVID-19: a prospective cohort study. Ann Intern Med. 2020;173(4):268–277. doi: 10.7326/M20-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wölfel R., Corman V.M., Guggemos W., Seilmaier M., Zange S., Müller M.A. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581(7809):465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- Zhu Z., Hasegawa K., Ma B., Fujiogi M., Camargo C.A., Jr., Liang L. Association of asthma and its genetic predisposition with the risk of severe COVID-19. J Allergy Clin Immunol. 2020;146(2):327–329.e4. doi: 10.1016/j.jaci.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]