Abstract
Pregnancy is hazardous with pulmonary arterial hypertension, but maternal mortality may have fallen in recent years. We sought to systematically evaluate pulmonary arterial hypertension and pregnancy-related outcomes in the last decade. We searched for articles describing outcomes in pregnancy cohorts published between 2008 and 2018. A total of 3658 titles were screened and 13 studies included for analysis. Pooled incidences and percentages of maternal and perinatal outcomes were calculated. Results showed that out of 272 pregnancies, 214 pregnancies advanced beyond 20 gestational weeks. The mean maternal age was 28 ± 2 years, mean pulmonary artery systolic pressure on echocardiogram was 76 ± 19 mmHg. Etiologies include idiopathic pulmonary arterial hypertension (22%), congenital heart disease (64%), and others (15%). Majority (74%) had good functional class I/II. Only 48% of women received pulmonary arterial hypertension-specific therapy. Premature deliveries occur in 58% of pregnancies at mean of 34 ± 1 weeks, most (76%) had Cesarean section. Maternal mortality rate was 12% overall (n = 26); even higher for idiopathic pulmonary arterial hypertension etiology alone (20%). Reported causes of death included right heart failure, cardiac arrest, pulmonary arterial hypertension crises, pre-eclampsia, and sepsis; 61% of maternal deaths occur at 0–4 days postpartum. Stillbirth rate was 3% and neonatal mortality rate was 1%. In conclusion, pulmonary arterial hypertension in pregnancy continues to be perilous with high maternal mortality rate. Continued prospective studies are needed.
Keywords: pulmonary hypertension, maternal risks, heart failure, survival
Introduction
Pulmonary arterial hypertension (PAH) is characterized by progressive obliterative pulmonary vascular disease that leads to eventual right ventricular (RV) failure and death. Pregnancy is contraindicated in women with PAH, as high rates of cardiac morbidity and mortality have been described in this precarious condition. A review of publications between 1978 and 1996 reported a 38% maternal mortality in pregnancies with PAH, 1 and a subsequent review between 1997 and 2007 reported a 28% maternal mortality. 2 However, previous reviews were limited to case reports or small series1–3 and used variable outcome definitions. Since earlier publications, there have been major advances in PAH therapies and specialized care; survival in the modern era may be better than historical reports. The aim of this systematic review was to evaluate maternal and perinatal outcomes in the last decade—the findings, although limited by the retrospective nature of the data and inevitable publication bias of smaller reports, would provide guidance for clinical teams.
Methods
The following databases were interrogated for publications in any language between 2008 and 2018; MEDLINE, Medline In-Process and other non-indexed citations, EMBASE, Cochrane Database of Systematic Reviews, and Cochrane Central Register of Controlled Trials. Both controlled vocabulary terms and text words (MesH and keywords) were used in the subject component blocks. The search strategy is detailed in online supplementary Table 1. Additional studies were identified by searching reference lists of articles, and the gray literature with the first 100 hits on Google Scholar after entering combinations of indexing terms. The maternal condition of interest was PAH (classified as World Health Organization Group I pulmonary hypertension 4 ), therefore pulmonary hypertension secondary to lung disease, left heart disease, and other unclear multifactorial conditions (classified as Groups II–V pulmonary hypertension) were not included. We also excluded case reports or series with less than five cases, studies that did not report on whether PAH therapy was used, and those which report only peripartum management without antepartum or postpartum follow-up details.
Two reviewers (T.T.L. and N.G.) independently performed title and abstract screening and data extraction for included studies. Conflicts were resolved by discussion and consensus. In cases of persistent disagreement, a third reviewer (C.K.S.) adjudicated. When appropriate, authors were contacted with data requests if there was a discrepancy in information or required information was not in the original publication. Patient baseline demographic and obstetric characteristics, PAH severity, PAH-targeted therapy, antepartum and obstetric management, and outcomes of pregnancies were collected.
The primary outcomes studied were: (a) maternal mortality and (b) perinatal outcomes in advanced pregnancies with PAH (>20 weeks gestation). Maternal mortality was defined as death during pregnancy, delivery, or up to six months postpartum. Data were collected and categorized into three etiology subgroups similar to previous reviews: idiopathic PAH (iPAH), PAH associated with congenital heart disease (PAH-CHD), or PAH associated with other conditions (oPAH). Perinatal outcomes included fetal or neonatal mortality, prematurity, and low birthweight. Prematurity was defined as birth of baby before 37 weeks and small for gestational age was defined as birthweight below the 10th percentile for gestational age.
The secondary adverse outcomes of interest were maternal and fetal mortality in early pregnancies (<20 weeks gestation). Fetal mortality in early pregnancies would include both spontaneous miscarriage and termination of pregnancy (TOP).
Numerical values reported in mean (± SD) and categorical variables within the group reported in percentages. Pooled incidences and percentages of maternal and perinatal outcomes were calculated. Comparison of maternal mortality to previously published reviews is shown graphically; however, statistical tests for differences between eras was not done because of difference in the methodologies used for the three systematic reviews.
Patients or the public were not involved in the design, or conduct, or reporting, or dissemination plans of our research.
Results
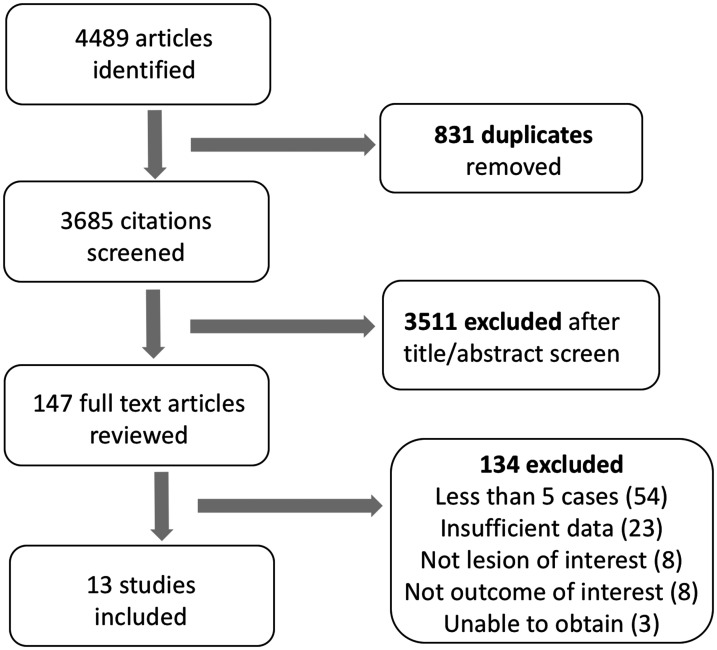
Our search results and study selection process are presented in Fig. 1. We screened 3658 citations, and selected 147 articles for full text reviews. Of these, 134 articles were excluded as they consist mostly of small series of less than five cases and/or had insufficient data.
Fig. 1.
Prisma flowchart for systematic review.
We included 13 studies, with a total of 272 pregnancies in 258 women with PAH.5–17 A total of 214 pregnancies were advanced beyond 20 weeks. The description of included studies is shown in Table 1. All studies were retrospective except one prospective multicenter study. 13 All pregnancies were managed in tertiary or quaternary referral centers. The largest cohort of advanced pregnancies (n = 34) was from the Registry of Pregnancy and Cardiac disease. 8 In the 10 studies (n = 221 pregnancies) that reported early pregnancy outcomes, 22% (n = 48) of pregnancies were terminated before 20 weeks.
Table 1.
Description of the 13 studies (n = 272 pregnancies) examining pregnancy outcomes in women with PAH included in the systematic review.
| Year Published | Author | Case collection | Country | Study type |
Women (n) |
Total number of pregnancies (n) | Pregnancies>20 WG (n) |
|---|---|---|---|---|---|---|---|
| 2017 | Meng | 2001–2015 | USA | Retrospective | 30 | 30 | 28 |
| 2016 | Ladouceur | 1997–2015 | French | Retrospective | 20 | 28 | 18 |
| 2016 | Duan | 2010–2014 | China | Retrospective | 11 | 11 | 11 |
| 2016 | Sliwa | 2008–2014 | ROPAC | Retrospective | 39 | 39 | 34 |
| 2014 | Zhang | 2007–2013 | China | Retrospective | 10 | 10 | 10 |
| 2013 | Subbiah | 2006–2012 | India | Retrospective | 30 | 30 | 30 |
| 2013 | Duarte | 1999–2009 | USA | Retrospective | 18 | 18 | 12 |
| 2012 | Smith | – | USA | Retrospective | 5 | 5 | 5 |
| 2012 | Katsuragi | 1982–2007 | Japan | Retrospective | 42 | 42 | 24 |
| 2012 | Curry | 1995–2010 | UK | Retrospective | 7 | 9 | 8 |
| 2012 | Jais | 2007–2010 | US, Eu, Aus | Prospective | 26 | 26 | 18 |
| 2012 | Rosengarten | – | Israel | Retrospective | 7 | 9 | 9 |
| 2009 | Kiely | 2002–2009 | UK | Retrospective | 13 | 15 | 10 |
USA: United States of America; ROPAC: Registry of Pregnancy and Cardiac Disease (European);UK: United Kingdom; Eu: Europe; Aus: Australia; WG: weeks gestation.
Baseline characteristics
The baseline characteristics are shown in Table 2. The mean maternal age was 28 ± 2 years, and most women were nulliparous (77%). More than half of these women were known to have PAH prior to pregnancy. There was only sporadic documentation as to whether these patients had pre-conception counseling. The baseline recorded mean pulmonary artery systolic pressure obtained by echocardiogram was 76 ± 19 mmHg (n = 155 pregnancies). Right heart catheterization in pregnancies was inconsistently reported. Many studies indicated that invasive hemodynamic assessments were performed only if the PAH diagnosis was new in pregnancy and supportive echocardiographic data were inadequate, or if there was hemodynamic decompensation. 6 ,11–13, 15 , 17
Table 2.
Baseline characteristics of women with pulmonary arterial hypertension and pregnancies carried beyond 20 weeks gestation.
| Combined baseline characteristics | Mean ± SDor % | Number of affected pregnancies | Total pregnancies in denominator | Number of studies with given data |
|---|---|---|---|---|
| Maternal age (years) | 28 ± 2 | 217 | 217 | 13 |
| Nulliparous (n) | 60.3% | 94 | 156 | 10 |
| Pulmonary artery systolic pressure on echocardiogram (mmHg) | 76 ± 19 | 154 | 154 | 10 |
| Functional status (n) | 73.7% | 126 | 171 | 10 |
| Functional Class I/II | 26.3% | 45 | 171 | 10 |
| Functional Class III/IV | ||||
| PAH etiologies (n) | ||||
| Idiopathic PAH | 21.5% | 46 | 214 | 13 |
| Congenital heart disease | 63.6% | 136 | 214 | 13 |
| Other etiologies | 15.0 % | 32 | 214 | 13 |
| PAH known before pregnancy (n)PAH-targeted therapy (n) | ||||
| PAH treatment before pregnancy | 58.0% | 79 | 136 | 9 |
| PAH treatment during | 37.9% | 25 | 66 | 6 |
| pregnancy | 47.7% | 92 | 193 | 13 |
| Anticoagulation treatment (n) | ||||
| Therapeutic anticoagulation | 49.0% | 51 | 104 | 8 |
| Prophylactic anticoagulation | 24.0% | 25 | 104 | 8 |
| Mode of anesthesia (n) | ||||
| General anesthesia | 30.7% | 54 | 176 | 11 |
| Regional anesthesia | 46.6% | 82 | 176 | 11 |
| Mode of delivery (n) | ||||
| Cesarean section | 75.7% | 159 | 210 | 13 |
| Vaginal delivery | 24.3% | 51 | 210 | 13 |
PAH: pulmonary arterial hypertension.
The most common diagnosis was PAH-CHD (n = 136, 64%), followed by iPAH (n = 46, 22%) and oPAH (n = 32, 15%). Eisenmenger syndrome constitutes 30% of patients who had PAH-CHD (n = 50). Underlying diagnoses for oPAH were connective tissue disease (n = 17), human immunodeficiency virus infection (n = 6), drug-related (n = 2), portal-pulmonary hypertension (n = 1), and the rest were undescribed (n = 6). Functional class (FC) pre-pregnancy was reported in 10 studies; most were in good FC I or II (73%).
PAH therapy and delivery characteristics in pregnancy
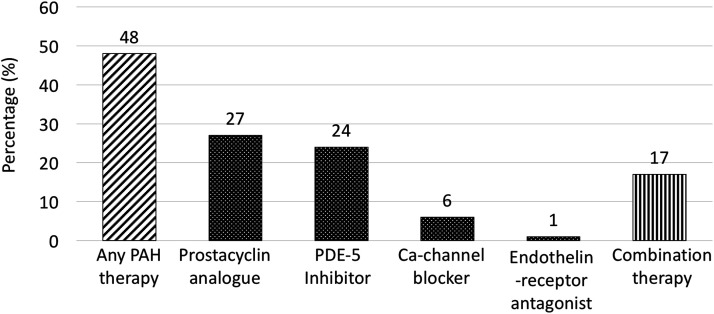
Approximately half of the cases (48%) were treated with PAH-targeted therapy in pregnancy (Fig. 2). In 214 pregnancies, prostacyclin analogues were most commonly used (27%), followed by phosphodiesterase-5 inhibitors (24%) and calcium channel blockers (6%).
Fig. 2.
PAH-targeted therapy in pregnancy.
Note: Use of specific pulmonary arterial hypertension medication is not mutually exclusive.
PAH: pulmonary arterial hypertension; PDE-5: phosphodiesterase-5; Ca-channel, calcium channel.
Only two patients were treated with endothelin receptor antagonists in pregnancy (1%). 8 Combination therapy was used in 17% of pregnancies (n = 32/193). The use of thromboprophylaxis was documented in 104 pregnancies; therapeutic anticoagulation was administered in almost half of the cases (49%), and prophylactic anticoagulation was given in about a quarter (24%) (Table 2).
The deliveries (n = 176) were often managed with regional anesthesia or general anesthesia (47% and 31% respectively). The mode of delivery was documented in 210 cases, and Cesarean deliveries were more common than vaginal deliveries (76 vs 24% respectively).
Maternal mortality outcomes
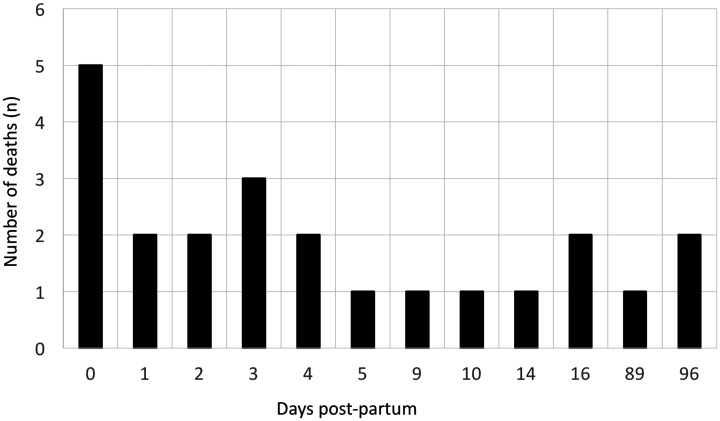
The overall maternal mortality rate in advanced pregnancies was 12% (n = 26); 9 maternal deaths occurred in 46 pregnancies with iPAH (20%) and 15 deaths in 136 pregnancies with PAH-CHD (11%). There were 2 deaths in 32 mothers with oPAH (6%). The causes of death included RV failure, cardiac arrest, pulmonary hypertension crises, pre-eclampsia, and sepsis (Table 3). Six patients required extra-corporal membrane oxygenation (ECMO) for life support. Out of these six patients on ECMO; four died (three Eisenmenger syndrome and one scleroderma), one survived with an emergent heart–lung transplant, and another one outcome was unspecified. All except one patient had ECMO initiated after delivery at the time of cardiac arrest. This patient with Eisenmenger syndrome was placed on venovenous ECMO two days before delivery, then converted to venoarterial ECMO on postpartum day 13 after a cardiopulmonary arrest, and did not survive. The timing of maternal demise was specified in 23 out of 28 recorded deaths and illustrated in Fig. 3. Most maternal deaths occurred within two weeks postpartum. The highest risk was in 0–4 days postpartum, which accounted for 61% (14 out of 23) of maternal demise.
Table 3.
Maternal, fetal, and neonatal outcomes in pregnancies carried beyond 20 weeks gestation (n = 214).
| Maternal mortality and morbidity outcomes | |
|---|---|
| Overall maternal mortality in PAH, n (%) | 26 (12) |
| Maternal mortality according to PAH etiologies | |
| ▪ Idiopathic PAH, n (%) | 9 (20) |
| ■ Congenital heart disease-associated PAH, n (%) | 15 (11) |
| ■ Other associated PAH, n (%) | 2 (6) |
| Reported causes of deatha | |
| ■ Heart failure or cardiogenic shock, n (%) | 12 (6) |
| ■ Cardiac arrest or sudden cardiac death, n (%) | 5 (2) |
| ■ Pulmonary hypertension crisis, n (%) | 2 (1) |
| ■ Pre-eclampsia, n (%) | 3 (1) |
| ■ Pulmonary infection/sepsis, n (%) | 5 (2) |
| Extra-corporal membrane oxygenation, n (%) | 6 (3)—4 died, 2 survived |
| Emergent heart lung transplant, n (%) | 1 (0.5)—survived |
| Perinatal mortality, fetal and neonatal outcomes | |
| Overall perinatal mortality, n (%) | 8 (4) |
| ■ Stillbirth rate, n (%) | 6 (3) |
| ■ Neonatal death rate, n (%) | 2 (1) |
| Prematurity, n (%) | 123 (58) |
| Small for gestation age, n (%) | 56 (36) |
| Mean birthweight, g | 1922 ± 292 |
| Mean gestational age at delivery, weeks | 34 ± 1 |
aReported causes of death here are not mutually exclusive.
PAH: pulmonary arterial hypertension.
Fig. 3.
Timing of death in pregnancies > 20 weeks.
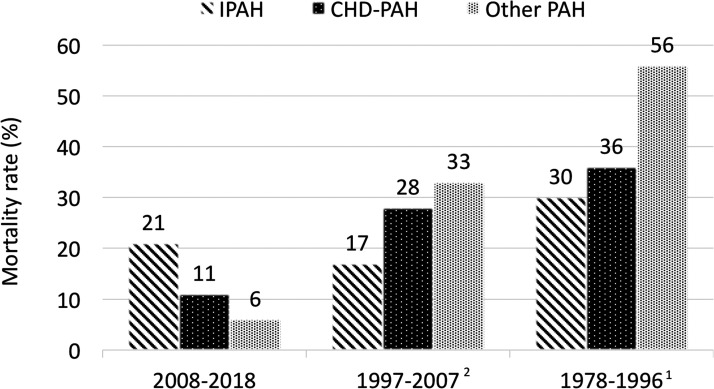
The comparison of maternal mortality across the last three decades was illustrated in Fig. 4. The overall maternal mortality rate appeared to have declined during this time period; however, the distribution of PAH etiology had also changed. In our study, the maternal mortality rate was highest amongst those with iPAH, when it was previously described to be the lowest amongst the three etiology subgroups.
Fig. 4.
Maternal mortality amongst parturients with pulmonary arterial hypertension across last three decades.
IPAH: idiopathic pulmonary arterial hypertension; CHD-PAH: congenital heart disease-associated pulmonary arterial hypertension; Other-PAH: other associated pulmonary arterial hypertension.
In addition, there were three maternal deaths reported in the late postpartum period. One woman with severe CHD-PAH died 89 days postpartum; she suffered a cardiac arrest and was placed on extra-corporal membranous oxygenation prior to her death. Another with severe CHD-PAH and baseline saturation below 85% had a sudden cardiac death 24 weeks after TOP. The third patient had iPAH and baseline FC I; she died at 24 weeks postpartum presenting with shock.
Perinatal mortality and adverse outcomes
The overall perinatal mortality was 4% (n = 8); 3% stillbirths, and 1% neonatal deaths (Table 3). The cause of the neonatal deaths were not described. There were two late TOP that occurred at 21 weeks, and these were excluded in calculating the perinatal mortality rate. Premature births occurred in more than half of the pregnancies (58%, n = 123), with a mean gestation age of 34 ± 1 weeks at delivery. In the eight studies that reported the cause of prematurity, most were iatrogenic; 84% (n = 63/75) secondary to a planned premature delivery usually for maternal indications. 5 , 6 , 9 , 11 , 12 , 14 , 16 , 17 In the eight studies that reported birthweights,5–7, 9 , 10 , 13 , 14 , 17 the mean birthweight was at 1922 ± 292 g and 36% of babies were reported to be small for gestational age.
Secondary maternal adverse outcomes
With regard to adverse events in early pregnancy, 55 women had pregnancies that ended before 20 weeks. Out of these women, 48 had TOP; 5 died after termination, and 1 died during the intervention. The overall maternal mortality in early pregnancy was 11% (n = 6/55). The rate of spontaneous miscarriage was 3.1% (n = 7/221) and the rate of early TOP was 21.7% (n = 48/221).
Discussion
In this comprehensive systematic review that examined pregnancy-related outcomes in women with PAH, we found that despite progress in the field of PAH with evolving treatment strategies across the years, maternal mortality remains significant. Maternal mortality was 12% in advanced pregnancies and 11% in early pregnancies. The highest risk period was in the immediate week postpartum, which accounted for two-thirds of maternal deaths (61%). Perinatal mortality was 4%. Amongst live births, more than half of the babies were delivered preterm (58%) with low birthweight overall. In the following paragraphs, we compare our findings in contrast to historical reviews, and discuss the bearing of our results on clinical care.
Consistent with previous reports, the immediate postpartum period poses a greater threat to maternal demise than the antepartum period. 1 , 2 Increased preload from relief of inferior vena cava obstruction, autotransfusion from the contracting uterus, fluid administration at the time of delivery, and volume shifts that occurs after delivery contribute to cardiac complications early postpartum, especially in those with decreased RV systolic function. After delivery, there is also abrupt increase in systemic and pulmonary vascular resistance to the non-pregnant state and reduced ventricular contractility. 18 , 19 In view of these hemodynamic stresses, it is imperative to monitor the women closely for the first week postpartum, especially in a high dependency or intensive care environment. Close outpatient follow-up is advisable upon discharge, as the effects of pregnancy on the cardiovascular system persist for several months after delivery. 19
The reported overall maternal mortality from a literature review in 1998 was 38%, 1 then 28% a decade later in 2009, 2 and 12% a decade later in our current review. However, it was not possible to conclude if maternal mortality had declined definitively across decades. The mortality rates cannot be directly compared as the study cohorts in these reviews were assembled differently1–3 and the numbers in all included series were relatively small. Nevertheless, it is conceivable that maternal outcomes may have improved from the remote past. Improved physician awareness, early diagnosis of PAH, advances in PAH-specific therapy, and greater availability of specialized multidisciplinary Heart Disease in Pregnancy teams are likely contributory to better survival.
The true current mortality rates may be even lower than described in our study. A recent Chinese study with a fairly large retrospective series of 57 patients with PAH, 20 described a 11% maternal mortality rate. Six deaths occurred within three months postpartum, all of whom had severe PAH to begin with (defined as systolic pulmonary arterial pressure above 50 mmHg, two of these patients had Eisenmenger syndrome), and were treated with combination therapy. This 11% mortality rate is without addition of two late deaths that occurred at 13 months and 21 months postpartum. This recent study was not included in our analysis as we limited the search to 2018, in comparing data across a decade of publications. We also found an abstract describing 170 women with PAH who had successful pregnancies at 100% survival. 21 A large study from the United States using National Inpatient Sample data described pregnancy outcomes in 906 cases of iPAH and 163 cases of PAH-CHD and reported extremely low maternal mortality of <1.1%. 22 However, this publication was excluded from our review as the data were restricted to ICD codes from delivery-related hospitalizations and the identification of diagnosis of pulmonary hypertension in this manner was not similar to the diagnostic approach used in other studies. There was also no information on disease severity, PAH-specific medication use, and perinatal complications.
Differences in maternal mortality were notable when analyzed within etiology subgroups; iPAH was previously associated with the best survival compared to CHD-PAH and oPAH, but in our study, we found iPAH carry the highest maternal mortality. It is important to note however, that the CHD-PAH group comprised entirely of Eisenmenger syndrome in the first 1989 review, 1 and there was an unknown proportion of Eisenmenger syndrome in the next 2009 review. 2 In comparing baseline characteristics between the cohorts, the most distinct difference was in functional status. In the last 2009 review, 2 61% of women were in FC III or IV compared to only 27% in our study (10 out of 13 publications reported FC, n = 171). Poor FC, in association with any cardiac disease in pregnancy, is a known predictor of major adverse cardiac events and poorer outcomes. 23 Although specific patient factors associated with good outcomes in pregnant PAH patients are not known, good FC pre-pregnancy is likely to confer a better prognosis.
Our study also revealed that even early pregnancy is risky for women with PAH, six had deaths occurred during and soon after TOP (out of 48 cases of TOP altogether).
Notwithstanding, these maternal deaths cannot be assumed to be the result of the intervention itself, as the circumstances of the demise and severity of PAH were not adequately described.
Termination could have been offered to women who were already clinically decompensating. Advances in PAH-specific therapy have revolutionized treatment strategies in the last decade. Surprisingly, a greater proportion of women were untreated in our study, 52% compared to 41% in a decade ago did not receive PAH-specific therapy. 2 Treatment trends, however, have changed overall. Previously, prostacyclin analogues were most frequently administered (41%, n = 30) and often commenced late when patients were unstable or developing heart failure, whereas Sildenafil was used only 7% (n = 5). 2 Comparatively, both prostacyclin analogues and phosphodiesterase-5 inhibitors were the most common treatment in our current study (27 and 24% respectively), and they were used in combination in 17% of women. In another review which included only pregnant patients who received PAH treatment, combination therapy was used in 39% (n = 30) of women. 3 Calcium channel blockers appeared to be effective treatment for patients who were responders in vasodilatory testing, and generally these patients carry a good prognosis for PAH anyway. 15 , 24 Endothelin receptor antagonists remained rarely used because of known teratogenic effects with animal data and labeled Food and Drug Administration pregnancy category X. 25 In our study, there was no birth anomaly for the two patients treated with endothelin receptor antagonists in pregnancy, 8 it was unclear when the drug was initiated. Initiation of targeted PAH therapy early well before delivery, perhaps as early as the first trimester, may have contributed to favorable outcomes. 1 , 17
Better patient selection is possibly a reason for better recent outcomes. In studies that stratify pregnant patients according to PAH severity, significantly more deaths occur in those defined to have severe disease, such as in patients with pulmonary artery systolic pressure (PASP) above 50 mmHg, FC III, or those with Eisenmenger syndrome. 9 , 10 , 13 , 20 Over the last few years, risk assessment tools have been developed from large multi-center PAH registries, 26 and a treat-to-goal strategy according to risk assessment criteria has been proposed by the guidelines. 18 It might be reasonable to use the European Society of Cardiology/European Respiratory Society risk assessment criteria to guide patient selection, 18 especially those who are asymptomatic and have just minor hemodynamic impairment. A stable patient who fulfills all parameters in the low-risk “green” category under treatment is likely to tolerate pregnancy better than if she were in the medium- or high-risk category. This cautious extrapolation might be more applicable for a non-pregnant woman with PAH who remains keen on trying for pregnancy after risk counseling, or for a pregnant woman with PAH deciding for TOP. Unfortunately, there is no evidence substantiating this approach to give the “green” light to pregnancy in patients with PAH. Clearly, it will be difficult to address this gap in evidence with a well-designed study because of ethical and practical concerns.
Other than PAH-specific therapy, the delivery strategy is paramount in managing this challenging patient cohort. The mode of anesthesia and delivery was relatively unchanged across the last decade. A third of the patients received general anesthesia as opposed to regional anesthesia for delivery, similar to the 2009 review. 2 A similar proportion of women had Cesarean section performed, 75% in our study and 72% in the 2009 review. 2 In the latest expert recommendations, 25 , 27 vaginal delivery appears to be equally safe in experienced hands under regional anesthesia, provided there is no obstetric indication. Given the paucity of data comparing epidural, spinal, and general anesthesia for pregnant patients with PAH, the current practice is likely based on the preference and experience of the unit. Intubation and mechanical ventilation should be used with great care in any patient with PAH as it can precipitate hemodynamic collapse, but may be necessary. With general anesthesia, intubation of these patients is often problematic owing to sedative effect on cardiac function and nonselective vasodilation leading to systemic hypotension and hemodynamic collapse. 28 Prior to delivery, the suitability for extracorporeal support should be discussed. Ancillary management with invasive monitoring, careful intravascular volume management, supplemental oxygen, and thromboprophylaxis are also crucial to successful outcomes.
The proportion of preterm births in our study was quite different from a decade ago, 58% in current study versus 85% in 2009 review. 4 Most preterm deliveries were planned. The optimal timing of delivery for pregnant women with PAH remains contentious. Experts have weigh in on planned Cesarean delivery around 32–36 weeks for best compromise between maternal health and sufficient fetal maturation, and to avoid the risk of the woman going into spontaneous labor during unsociable hours. 29 Current guidelines make no specific recommendations on optimal delivery timing with or without RV failure. 27 Compared to decades ago, improvements in neonatal care mean the overall risks of preterm delivery are lower. All but two live-born infants survived in our study; these two were born to mothers with Eisenmenger syndrome at 29 and 30 weeks and had severely low birthweight at 920 g and 970 g, respectively. It is encouraging that the 4% perinatal mortality rate in our study is substantially lower than 10% reported previously. 4
Limitations
As with any systematic review, the acquired data were limited to that provided in published literature, which was subjected to publication bias and selective reporting. Many larger publications had to be excluded, as the outcome data with PAH were reported as an aggregate with other causes of PH, including left heart disease, making it difficult to isolate outcomes in PAH group I for analysis. Finally, our review was not able to identify specific risk predictors for maternal or fetal outcomes such as degree of pulmonary artery pressure, RV size, and function or other co-morbidities, as the studies were mostly un-controlled and comorbidities were not routinely reported.
Conclusion
Maternal and perinatal mortality rates are high in this contemporary cohort of pregnant women with PAH. The global observations obtained from this systematic review provide a helpful reference when counseling women with PAH who are contemplating pregnancy. While there is no standardized approach for pregnant patients with PAH, favorable outcomes are more likely with organized multi-disciplinary care. Continued prospective studies are needed to identify subsets of women at lower risks, examine maternal morbidity, and understand which PAH therapy can improve pregnancy outcomes.
Acknowledgements
We would like to express our gratitude to Dr Ladouceur and Dr Meng who enthusiastically responded to our email queries on their publications, and generously shared more raw data with regards to their studies. Ethical approval, consent for participation and guarantor are not required for this study.
Author contributions: Dr T.T.L. performed the systematic review, data extraction, data analysis, and manuscript writing. Dr N. Guron performed the systematic review, data extraction, and manuscript writing. Dr R. Ducas assisted in the data analysis and editing of the manuscript. Dr K. Yamamura performed the data analysis and reviewed the manuscript. Dr P Charla assisted in screening and obtaining publications for systematic review and reviewed the manuscript. Dr J. Granton reviewed the manuscript. Dr C.K. Silversides contributed in performing the systematic review, data extraction, and writing the manuscript.
Conflict of interest: The author(s) declare that there is no conflict of interest.
Funding: The publication cost of this article is sponsored by the Women's Heart Health Clinic Fund, National University Heart Centre Singapore. This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD: Ting-Ting Low https://orcid.org/0000-0001-5359-9860
Supplemental Material: Supplemental material for this article is available online.
References
- 1.Weiss BM, Zemp L, Seifert B, et al. Outcome of pulmonary vascular disease in pregnancy: a systematic overview from 1978 through 1996. J Am Coll Cardiol 1998; 31: 1650–1657. [DOI] [PubMed] [Google Scholar]
- 2.Bédard E, Dimopoulos K, Gatzoulis MA. Has there been any progress made on pregnancy outcomes among women with pulmonary arterial hypertension? Eur Heart J 2009; 30: 256–265. [DOI] [PubMed] [Google Scholar]
- 3.Pieper PG, Lameijer H, Hoendermis ES. Pregnancy and pulmonary hypertension. Best Pract Res Clin Obstet Gynaecol 2014; 28: 579–591. [DOI] [PubMed] [Google Scholar]
- 4.Simonneau G, Gatzoulis MA, Adatia I, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 2013; 62: D34–D41. [DOI] [PubMed] [Google Scholar]
- 5.Meng ML, Landau R, Viktorsdottir O, et al. Pulmonary hypertension in pregnancy, a report of 49 cases at four tertiary north American sites. Obstet Gynecol 2017; 129: 511–520. [DOI] [PubMed] [Google Scholar]
- 6.Ladouceur M, Benoit L, Radojevic J, et al. Pregnancy outcomes in patients with pulmonary arterial hypertension associated with congenital heart disease. Heart 2017; 103: 287–292. [DOI] [PubMed] [Google Scholar]
- 7.Duan R, Xu X, Wang X, et al. Pregnancy outcome in women with Eisenmenger’s syndrome: a case series from west China. BMC Pregnancy Childbirth 2016. ; 16: 356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sliwa K, Van Hagen IM, Budts W, et al. Pulmonary hypertension and pregnancy outcomes: data from the Registry of Pregnancy and Cardiac Disease (ROPAC) of the European Society of Cardiology. Eur J Heart Fail 2016; 18: 1119–1128. [DOI] [PubMed] [Google Scholar]
- 9.Xu X, Zhang J, Wang H, et al. Severe idiopathic pulmonary arterial hypertension in pregnancy: a review of 10 cases. [Chinese]. Zhonghua fu chan ke za zhi 2014; 49: 419–423. [PubMed] [Google Scholar]
- 10.Subbaiah M, Kumar S, Roy KK, et al. Pregnancy outcome in women with pulmonary arterial hypertension: single-center experience from India. Arch Gynecol Obstet 2013; 288: 305–309. [DOI] [PubMed] [Google Scholar]
- 11.Duarte AG, Thomas S, Safdar Z, et al. Management of pulmonary arterial hypertension during pregnancy: a retrospective, multicenter experience. Chest 2013; 143: 1330–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith JS, Mueller J, Daniels CJ. Pulmonary arterial hypertension in the setting of pregnancy: a case series and standard treatment approach. Lung 2012; 190: 15560. [DOI] [PubMed] [Google Scholar]
- 13.Katsuragi S, Yamanaka K, Neki R, et al. Maternal outcome in pregnancy complicated with pulmonary arterial hypertension. Circ J 2012; 76: 2249–2254. [DOI] [PubMed] [Google Scholar]
- 14.Curry RA, Fletcher C, Gelson E, et al. Pulmonary hypertension and pregnancy – a review of 12 pregnancies in nine women. BJOG 2012; 119: 752–761. [DOI] [PubMed] [Google Scholar]
- 15.Jais X, Olsson KM, Barbera JA, et al. Pregnancy outcomes in pulmonary arterial hypertension in the modern management era. Eur Respir J 2012; 40: 881–885. [DOI] [PubMed] [Google Scholar]
- 16.Rosengarten D, Blieden LC, Kramer MR. Pregnancy outcomes in pulmonary arterial hypertension in the modern management era. Eur Respir J 2012; 40: 1304–1305. [DOI] [PubMed] [Google Scholar]
- 17.Kiely D, Condliffe R, Webster V, et al. Improved survival in pregnancy and pulmonary hypertension using a multiprofessional approach. BJOG 2010; 117: 565–574. [DOI] [PubMed] [Google Scholar]
- 18.Galiè N, Humbert M, Vachiery J, et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J 2016; 37: 67–119. [DOI] [PubMed] [Google Scholar]
- 19.Bassily-Marcus AM, Yuan C, Oropello J, et al. Pulmonary hypertension in pregnancy: critical care management. Pulmonary Med 2012; 2012: 709407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luo J, Shi H, Xu L, et al. Pregnancy outcomes in patients with pulmonary arterial hypertension: a retrospective study. Medicine (Baltimore) 2020; 99: e20285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zwicke D, Paulus S. Pregnancy and pulmonary arterial hypertension: 21st century approach . Chest 2018; 154: 1040A. [Google Scholar]
- 22.Thomas E, Yang J, Xu J, et al . Pulmonary hypertension and pregnancy outcomes: insights from the national inpatient sample. J Am Heart Assoc 2017; 6: e006144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Silversides CK, Grewal J, Mason J, et al. Pregnancy outcomes in women with heart disease. J Am Coll Cardiol 2018; 71: 2419–2430. [DOI] [PubMed] [Google Scholar]
- 24.McLaughlin VV, Shah SJ, Souza R, et al. Management of pulmonary arterial hypertension. J Am Coll Cardiol 2015; 65: 1976–1997. [DOI] [PubMed] [Google Scholar]
- 25.Elkayam U, Goland S, Pieper PG, et al. High-risk cardiac disease in pregnancy: part I. J Am Coll Cardiol 2016; 68: 502–516. [DOI] [PubMed] [Google Scholar]
- 26.Galiè N, McLaughlin VV, Rubin LJ, et al. An overview of the 6th world symposium on pulmonary hypertension. Eur Respir J 2019; 53: 1802148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Regitz-Zagrosek V, Roos-Hesselink JW, Bauersachs J, et al. 2018 ESC guidelines for the management of cardiovascular diseases during pregnancy. Eur Heart J 2018; 39: 3165–3241. [DOI] [PubMed] [Google Scholar]
- 28.Hoeper MM, Granton J. Intensive care unit management of patients with severe pulmonary hypertension and right heart failure. Am J Respir Crit Care Med 2011; 184: 1114–1124. [DOI] [PubMed] [Google Scholar]
- 29.Olsson KM, Channick R. Pregnancy in pulmonary arterial hypertension. Eur Respir Rev 2016; 25: 431–437. [DOI] [PMC free article] [PubMed] [Google Scholar]