Abstract
Objective:
The pathogenesis of sudden cardiac death may differ between younger and older adults in schizophrenia, but evidence remains scant. This study investigated the age effect on the incidence and risk of the physical and psychiatric comorbidity for sudden cardiac death.
Methods:
Using 2000 to 2016 data from the Taiwan National Health Insurance Research Database and Department of Health Death Certification System, we identified a national cohort of 170,322 patients with schizophrenia, 1,836 of whom had a sudden cardiac death. Standardized mortality ratios (SMRs) were estimated. Hazard ratios and population attributable fractions of distinctive comorbidities for sudden cardiac death were assessed.
Results:
The SMRs of sudden cardiac death were all >1.00 across each age group for both sexes, with the highest SMR in male patients aged <35 years (30.88, 95% CI: 26.18–36.18). The fractions of sudden cardiac death attributable to hypertension and congestive heart failure noticeably increased with age. By contrast, the fraction attributable to drug-induced mental disorder decreased with age. Additionally, chronic hepatic disease and sleep disorder increased the risk of sudden cardiac death in patients aged <35 years. Dementia and organic mental disorder elevated the risk in patients aged between 35–54 years. Ischemic heart disease raised the risk in patients aged ≥55 years.
Conclusions:
The risk is increased across the lifespan in schizophrenia, particularly for younger male patients. Furthermore, physical and psychiatric comorbidities have age-dependent risks. The findings suggest that prevention strategies targeted toward sudden cardiac death in patients with schizophrenia must consider the age effect.
Keywords: schizophrenia, sudden cardiac death, age effect, incidence, standardized mortality ratio, population attributable fraction
Abstract
Objectif:
La pathogenèse de la mort cardiaque subite peut différer entre adultes plus jeunes et plus âgés dans la schizophrénie, mais les données probantes demeurent faibles. La présente étude a recherché l’effet de l’âge sur l’incidence et le risque de morbidité physique et psychiatrique pour la mort cardiaque subite.
Méthodes:
À l’aide des données de 2000–2016 entre la Base de données nationale de recherche sur l’assurance maladie de Taïwan et le système de certification des décès du ministère de la santé, nous avons identifié une cohorte nationale de 170 322 patients souffrant de schizophrénie, dont 1 836 ont eu une mort cardiaque subite. Les taux de mortalité normalisés (TMN) ont été estimés. Les rapports de risques et les fractions attribuables à la population de comorbidités distinctes pour la mort cardiaque subite ont été évalués.
Résultats:
Les TMN de la mort cardiaque subite étaient tous > 1,00 dans chaque groupe d’âge pour les deux sexes, et le TMN le plus élevé chez les patients masculins âgés < 35 ans était (30,88; IC à 95% 26,18 à 36,18). Les fractions de mort cardiaque subite attribuables à l’hypertension et à l’insuffisance cardiaque congestive ont augmenté visiblement avec l’âge. Par contre, la fraction attribuable à un trouble mental induit par les drogues diminuait avec l’âge. En outre, la maladie hépatique chronique et les troubles du sommeil augmentaient le risque de mort cardiaque subite chez les patients âgés < 35 ans. La démence et un trouble mental organique élevaient le risque chez les patients âgés entre 35 et 54 ans. La cardiopathie ischémique haussait le risque chez les patients âgés > 55 ans.
Conclusions:
Le risque s’accroît au long de la durée de vie dans la schizophrénie, particulièrement chez les jeunes patients masculins. Par ailleurs, les comorbidités physiques et psychiatriques comportent des risques en fonction de l’âge. Les résultats suggèrent que des stratégies de prévention axées sur la mort cardiaque subite chez des patients souffrant de schizophrénie doivent tenir compte de l’effet de l’âge.
Introduction
Schizophrenia is a severe mental illness that typically first manifests in adolescence or young adulthood. 1 During the illness course, patients with schizophrenia are at an increased risk of numerous physical and psychiatric comorbidities. 2 –7 Furthermore, evidence has indicated that relative to the general population, patients with schizophrenia have an approximately 2 to 4 times higher mortality rate and an at least 10-year reduction in life expectancy. 4,8 –10 In particular, cardiovascular diseases are the major physical comorbidities contributing to the risk of death in patients with schizophrenia, whether they be from Asia or the West. 4,9 –11
Among the causes of cardiovascular mortality, sudden cardiac death has recently received increasing attention given its nature of sudden circulatory collapse and considerable impact on public health. 12,13 However, currently, risk stratification for sudden cardiac death still remains imperfect, 12 which are possibly attributable to the heterogeneous etiologies of sudden cardiac death. 12,13 For instance, although ischemic heart disease is the most common cardiac pathology underlying sudden cardiac death, arrhythmia and cardiomyopathy actually account for significant proportions of sudden cardiac deaths in those young adults aged <35 years. 13 Considering the evidence of a distinctive distribution of etiologies for sudden cardiac death across the lifespan, 13 studies on the presumed mechanisms underlying sudden cardiac death must include age in their analysis.
Studies have suggested that patients with schizophrenia are at an increased risk of sudden cardiac death. 14 –19 Notably, one postmortem study has found that myocardial infarction accounts for more than half of sudden deaths in older adult patients with schizophrenia. 16 By contrast, another postmortem study focusing on young adult patients revealed that >50% of sudden death cases remain unexplained, with arrhythmia as the suspected cause. 18 Considered together, these findings may imply an age effect on the pathogenesis and risk factors for sudden cardiac death in patients with schizophrenia. Thus far, only a few studies have directly examined the risk of physical and psychiatric comorbidities related to sudden cardiac death in patients with schizophrenia. 14,17 In these studies, dyslipidemia, diabetes mellitus, electrocardiographic abnormalities, and aggressive behaviors have been found to be associated with the risk of sudden cardiac death. However, the findings from these hospital-based studies, including ours, have potential selection bias as a shortcoming. Furthermore, these studies’ small sample sizes yield results of limited statistical power, making it difficult to determine the effect of age on the association between risk of comorbidity and sudden cardiac death.
To fill this gap in the literature, this prospective national cohort study investigated 23 million Taiwanese adults to examine the age effect on the incidence and risk of the physical and psychiatric comorbidity for sudden cardiac death, we stratified the patients into 3 age subgroups: <35 (younger aged), 35 to 54 (middle aged), and >55 years (older aged) by adopting definitions from previous studies. 13,16,18,20
Aims of the Study
To estimate the age effect on the incidence and standardized mortality ratio (SMR) of sudden cardiac death in patients with schizophrenia. In addition, we explored the age effect on the risk of the physical and psychiatric comorbidity for sudden cardiac death in schizophrenia. We hypothesized that distinctive comorbidities carry an age-dependent risk for sudden cardiac death in patients with schizophrenia.
Methods
Data Sources
The data source for this study was the entire Taiwan population (approximately 23,000,000 people) derived the National Health Insurance Research Database (NHIRD). The Institutional Review Board of Taipei City Hospital approved the protocol for this prospective nationwide cohort study, with a waiver of informed consent due to the de-identified and retrospective nature of the data.
The Taiwan NHIRD is maintained by the Health and Welfare Data Science Center and contains the registration files and medical claims data of beneficiaries who were covered by Taiwan’s National Health Insurance (NHI). Taiwan’s NHI program was implemented in 1995, and it provides comprehensive and accessible medical care to nearly 98% of the 23,000,000 Taiwanese population. The NHI covers visits to all NHI-contracted facilities in Taiwan, which makes the NHIRD database representative of the general population in Taiwan. Every year, the NHI authorities conduct a random review of the medical records to verify diagnosis codes. The Taiwan Joint Commission on Hospital Accreditation oversees the accreditation of most hospitals contracted with the NHI. To obtain accreditation for qualified psychiatric services, patients must receive a diagnosis from a board-certified psychiatrist. NHIRD claims’ data have been a useful source of data for numerous peer-reviewed epidemiological studies because the database contains detailed information on diagnoses and treatment. 21 –24
Identification of Schizophrenia Cohort
We selected a cohort of patients from the Taiwan NHIRD database who received a mental disorder diagnosis (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] codes: 290.x to 319.x; International Classification of Diseases, Tenth Revision [ICD-10] codes: F01–F99) between January 1, 2000, and December 31, 2016 (N = 10 ,422,350; Supplemental Figure 1). We then excluded patients who received a diagnosis of mood disorder (ICD-9-CM code: 296.x) between January 1, 2000, and December 31, 2016 (N = 188,902). After the exclusion, patients who received a consistent diagnosis of schizophrenia (ICD-9-CM code: 295.x; ICD-10 code: F20.x) between January 1, 2001, and December 31, 2016 (N = 170,322) were included in the study cohort.
Identification of Sudden Cardiac Death Events
The data for each cohort member were electronically synced with the Department of Health’s Death Certification System from January 1, 2001, through December 31, 2016, using the national identification number as an identifier (Supplemental Figure 1). Data on causes of death in the Department of Health’s Death Certification System for dates before and after December 31, 2014, were grouped according to ICD-9-CM and ICD-10 categories, respectively. The ICD-9-CM codes that were used to define sudden cardiac death were 390 to 398, 402, and 404 to 429, as per previous studies. 17,25 The ICD-10 codes that were used to define sudden cardiac death were, as per Ray et al., 26 I10, I11.9, I20, I21, I22, I23, I24, I25, I42.8, I42.9, I46, I47, I47.2, I49.0, I49.8, I49.9, I51.6, I51.9, I70.9, R96.1, and R98. In total, we identified 26,926 deaths, along with their causes of death. Among the 26,926 deaths, 1,836 were sudden cardiac deaths, defined to be so only if they were deaths reported as occurring out of hospital, or in the emergency room, or as “dead on arrival” with the cause of death reported to be cardiac disease. 17
Statistical Analysis
We calculated the survival (contributed) time of each cohort member from the date of first diagnosis of schizophrenia (baseline) to either the event of sudden cardiac death or the end of study (December 31, 2016). The crude mortality rates for sudden cardiac death were calculated as the incident cases divided by the contributed person-years. The SMR was estimated using the ratio of observed sudden cardiac deaths in the schizophrenia cohort to the expected deaths in the general population of Taiwan by utilizing our previously described methods. 27 The expected number of sudden cardiac deaths was computed by multiplying the number in the study cohort (standardized by sex and age) by the national incidence of sudden cardiac death between January 1, 2001, and December 31, 2016. To explore the age effect on the incidence of sudden cardiac death in the schizophrenia cohort, we stratified patients into 3 age subgroups: <35 years, 35 to 54 years, and >55 years. 13,16,18,20
Univariate Cox proportional hazards analyses were used to estimate the crude hazard ratios for each demographic and clinical variable. Using the backward stepwise selection method, we performed the multivariate Cox proportional hazards regression model to assess the hazard ratios for physical and psychiatric comorbidities. Variables with a significant association (P < 0.001) were retained in the final adjusted model. Furthermore, we calculated the population attributable fraction (PAF) by using the following equation: prevalence of individual physical or psychiatric comorbidity in cases with sudden cardiac death × [(hazard ratio − 1)/hazard ratio]. 28 The hazard ratio of individual physical or psychiatric comorbidity on sudden cardiac death was estimated based on the final regression model.
All statistical analyses were conducted using SAS statistical software (SAS System for Windows, version 9.4, SAS Institute, Cary, NC, USA). A P value of <0.05 indicated statistical significance.
Results
Incidence and SMR of the Schizophrenia Cohort
The study cohort included 170,322 patients with schizophrenia. During the cohort follow-up of 7.6 ± 4.5 years, 1,836 patients with schizophrenia had sudden cardiac death. The crude incidence of sudden cardiac death in the schizophrenia cohort was 133.8 cases per 100,000 person-years (Table 1) and 28.8, 132.9, and 531.1 among patients aged <35, 35 to 54, and >55 years, respectively (Table 1; Supplemental Figure 2). In addition, the increase in the cumulative incidence of sudden cardiac death was significantly higher among male patients (P < 0.001) than among female patients (Supplemental Figure 3).
Table 1.
Incidence and Standardized Mortality Ratio of Sudden Cardiac Death (N = 1,836) in Patients with Schizophrenia (N = 170,322) Stratified by Age, from January 1, 2001, through December 31, 2016.
| N | Follow-up Duration, years: Mean (SD) | Number of Sudden Cardiac Death Observed (N) | Total Person-Years | Crude Incidencea | Expected Number (N) | SMRb | 95% CI | P | |
|---|---|---|---|---|---|---|---|---|---|
| Agec (years) | |||||||||
| <35 | 76,325 | 9.0 (4.7) | 198 | 688,276.2 | 28.8 | 8.53 | 23.14 | 20.03 to 26.60 | <0.001 |
| 35 to 54 | 61,453 | 8.1 (4.8) | 665 | 500,483.2 | 132.9 | 98.90 | 6.73 | 6.22 to 7.26 | <0.001 |
| >55 | 32,544 | 5.6 (4.5) | 973 | 183,194.3 | 531.1 | 547.11 | 1.78 | 1.67 to 1.89 | <0.001 |
| Total | 170,322 | 7.6 (4.5) | 1,836 | 1,371,953.7 | 133.8 | 631.9 | 2.91 | 2.77 to 3.04 | <0.001 |
a Incidence rate: incident number/100,000 person-years.
b Standardized mortality ratio (SMR): the observed number of cases with sudden cardiac death/expected number of cases; the expected numbers of sudden cardiac death was obtained by multiplying the cumulative contributed person-years of patients in the specified stratum of the cohort with schizophrenia by the incidence of sudden cardiac death (2001 to 2016) in the general population.
c Indicates age at the baseline; we calculated SMR based on sex- and period (year) adjustment.
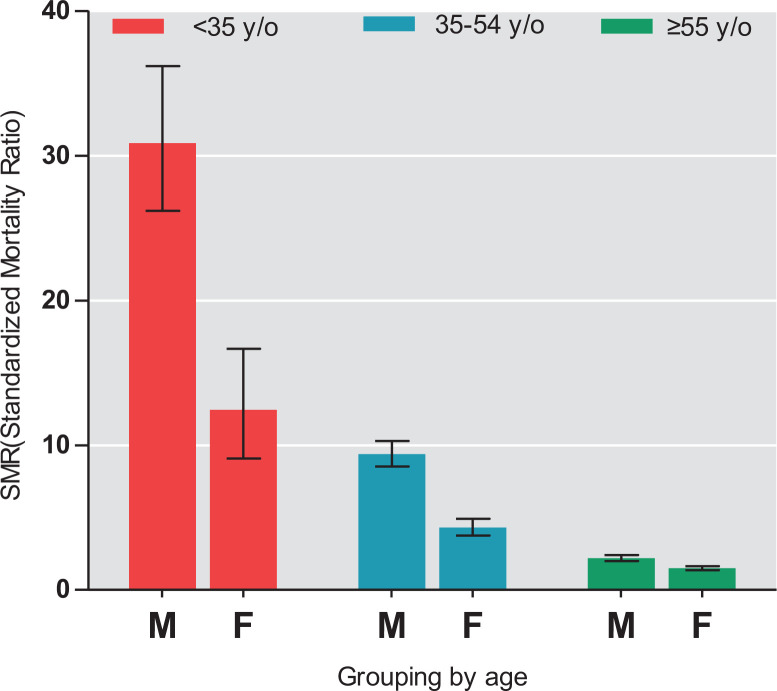
To estimate the risk of sudden cardiac death in patients with schizophrenia relative to the general population, SMRs were calculated. Significantly, the SMR of sudden cardiac death in the schizophrenia cohort was 2.91 (95% CI, 2.77 to 3.04; P < 0.001), which indicated a mortality gap between patients with schizophrenia and the general population. Furthermore, stratified analyses by age demonstrated that the SMRs of sudden cardiac death were all >1.00 across each age interval. The highest SMR was for patients aged <35 years (23.14; 95% CI, 20.03 to 26.60; P < 0.001), followed by those aged between 35 and 54 years (6.73; 95% CI, 6.22 to 7.26; P < 0.001) and those aged >55 years (1.78; 95% CI, 1.67 to 1.89; P < 0.001; Table 1). Additionally, stratified analyses by age and sex further revealed that male patients aged <35 years had the highest SMR (30.88; 95% CI, 26.18 to 36.18; Figure 1).
Figure 1.
Standardized mortality ratios of sudden cardiac death across the lifespan in a nationwide schizophrenia cohort. aError bars indicate 95% confidence intervals. bM: Male, F: Female.
Demographic Risk Factors for Sudden Cardiac Death in Patients with Schizophrenia
Table 2 presents the Cox proportional hazards model of the demographic risk factors for sudden cardiac death in patients with schizophrenia. Compared to male patients with schizophrenia, female patients had a significantly lower risk of sudden cardiac death (0.58; 95% CI, 0.53 to 0.63; P < 0.001). In addition, relative to patients who were unemployed, patients who were employed had a reduced risk of sudden cardiac death (0.76; 95% CI, 0.69 to 0.84; P < 0.001). Conversely, as compared with those living in highly urbanized areas, patients with schizophrenia who lived in moderately urbanized areas (1.28; 95% CI, 1.14 to 1.43; P < 0.001), subrural areas (1.24; 95% CI, 1.07 to 1.45; P = 0.005), and rural areas (1.21; 95% CI, 1.04 to 1.41; P = 0.014) had a higher risk of sudden cardiac death.
Table 2.
Cox Proportional Hazards Model of Demographic Variables on the Risk of Sudden Cardiac Death in Patients with Schizophrenia.
| Characteristics | Schizophrenia Cohort (N = 170,322) | Sudden Cardiac Death (N = 1,836) | Unadjusted Hazard Ratio | 95% CI | P | Adjusted Hazard Ratioa | 95% CI | P |
|---|---|---|---|---|---|---|---|---|
| N (%) | N (%) | |||||||
| Sex | ||||||||
| Male | 87,839 (51.6) | 1,072 (58.4) | Reference | Reference | ||||
| Female | 82,483 (48.4) | 764 (41.6) | 0.76 | 0.69 to 0.84 | <0.001 | 0.58 | 0.53 to 0.63 | <0.001 |
| Age, years | ||||||||
| <25 | 37,254 (21.9) | 40 (2.2) | Reference | Reference | ||||
| 25 to 34 | 39,071 (22.9) | 158 (8.6) | 4.00 | 2.83 to 5.66 | <0.001 | 4.07 | 2.87 to 5.76 | <0.001 |
| 35 to 44 | 34,919 (20.5) | 319 (17.4) | 9.42 | 6.78 to 13.09 | <0.001 | 9.96 | 7.16 to 13.86 | <0.001 |
| 45 to 54 | 26,534 (15.6) | 346 (18.9) | 14.76 | 10.64 to 20.47 | <0.001 | 15.88 | 11.42 to 22.10 | <0.001 |
| 55 to 64 | 15,402 (9.0) | 314 (17.1) | 27.32 | 19.66 to 37.97 | <0.001 | 27.39 | 19.62 to 38.24 | <0.001 |
| 65 to 74 | 8,225 (4.8) | 267 (14.5) | 49.88 | 35.77 to 69.56 | <0.001 | 43.02 | 30.63 to 60.43 | <0.001 |
| >75 | 8,917 (5.2) | 392 (21.4) | 113.41 | 81.79 to 157.26 | <0.001 | 85.80 | 61.22 to 120.25 | <0.001 |
| Urbanizationb | ||||||||
| Level 1 | 71,227 (41.8) | 672 (36.6) | Reference | Reference | ||||
| Level 2 | 45,721 (26.8) | 541 (29.5) | 1.36 | 1.21 to 1.52 | <0.001 | 1.28 | 1.14 to 1.43 | <0.001 |
| Level 3 | 18,543 (10.9) | 180 (9.8) | 1.03 | 0.87 to 1.21 | 0.727 | 0.98 | 0.83 to 1.16 | 0.836 |
| Level 4 | 17,078 (10.0) | 224 (12.2) | 1.41 | 1.21 to 1.64 | <0.001 | 1.24 | 1.07 to 1.45 | 0.005 |
| Level 5 | 16,966 (10.0) | 216 (11.8) | 1.37 | 1.18 to 1.60 | <0.001 | 1.21 | 1.04 to 1.41 | 0.014 |
| Employment | ||||||||
| No | 116,765 (68.6) | 1,277 (69.6) | Reference | |||||
| Yes | 53,557 (31.4) | 559 (30.5) | 0.96 | 0.87 to 1.06 | 0.420 | 0.76 | 0.69 to 0.84 | <0.001 |
a Based on multivariate regression model: the variables in Table 2 (demographical information) and the variables in Supplemental Table 1 (physical and psychiatric comorbidities) with P < 0.001 were remained in the final adjusted model.
b We applied urbanization stratification specifically used in Taiwan, and the level of urbanization was categorized as Level 1 (highly urbanized area), Level 2 (moderately urbanized area), Level 3 (township area), Level 4 (subrural area), and Level 5 (rural area).
Comorbidity Risk Factors for Sudden Cardiac Death in Patients with Schizophrenia
Table 3 presents the Cox proportional hazards model of the comorbidity risk factors for sudden cardiac death in patients with schizophrenia. Among physical comorbidities, hypertension (1.38; 95% CI, 1.23 to 1.55; P < 0.001), ischemic heart disease (1.30; 95% CI, 1.13 to 1.49; P < 0.001), and congestive heart failure (2.20; 95% CI, 1.87 to 2.58; P < 0.001) significantly increased the risk of sudden cardiac death in patients with schizophrenia. The proportions of sudden cardiac death that were attributable to hypertension, ischemic heart disease, and congestive heart failure were 9.91%, 3.98%, and 6.06%, respectively. As for psychiatric comorbidities, dementia and organic mental disorder (1.43; 95% CI, 1.24 to 1.64; P < 0.001) and drug-induced mental disorder (2.22; 95% CI, 1.78 to 2.77; P < 0.001) significantly increased the risk of sudden cardiac death in patients with schizophrenia; the fractions of sudden cardiac death attributable to the 2 aforementioned disorders were 3.73%, and 2.57%, respectively.
Table 3.
Cox Proportional Hazards Model of Physical and Psychiatric Comorbidity on the Risk of Sudden Cardiac Death in Patients with Schizophrenia.
| Characteristics | Schizophrenia Cohort | Sudden Cardiac Death | Adjusted Hazard Ratioa | 95% CI | P | Population Attributable Fraction (%) |
|---|---|---|---|---|---|---|
| N (%) | N (%) | |||||
| Total | 170,322 | 1,836 | ||||
| Physical illnesses | ||||||
| Hypertension | 23,101 (13.6) | 661 (36.0) | 1.38 | 1.23 to 1.55 | <0.001 | 9.91 |
| Ischemic heart disease | 8,604 (5.1) | 317 (17.3) | 1.30 | 1.13 to 1.49 | <0.001 | 3.98 |
| Congestive heart failure | 3,849 (2.3) | 204 (11.1) | 2.20 | 1.87 to 2.58 | <0.001 | 6.06 |
| Psychiatric illnesses | ||||||
| Dementia and organic mental disorder | 11,070 (6.5) | 228 (12.4) | 1.43 | 1.24 to 1.64 | <0.001 | 3.73 |
| Drug-induced mental disorder | 6,414 (3.8) | 86 (4.7) | 2.22 | 1.78 to 2.77 | <0.001 | 2.57 |
a Based on multivariate regression model in all of study subjects: the variables in Table 2 (demographics including sex, age, urbanization, employment) and the variables in Supplemental Table 1 (physical and psychiatric comorbidities) with P < 0.001 were remained in the final adjusted model.
Age Effect on Comorbidity Risk Factors for Sudden Cardiac Death in Patients with Schizophrenia
Considering that there was an age effect on the incidence and SMRs of sudden cardiac death in patients with schizophrenia (Table 1; Figure 1), stratified analyses were performed to assess the age effect on comorbidity risk factors for sudden cardiac death (Supplemental Tables 2, 3, and 4; organization of the tables were as per Table 4). Significantly, hypertension, congestive heart failure, and drug-induced mental disorder increased the risk of sudden cardiac death in patients with schizophrenia across the lifespan. In addition, chronic hepatic disease (2.38; 95% CI, 1.61 to 3.54; P < 0.001) and sleep disorder (1.77; 95% CI, 1.30 to 2.42; P < 0.001) raised the risk of sudden cardiac death among patients aged <35 years. Dementia and organic mental disorder (1.61; 95% CI, 1.26 to 2.05; P < 0.001) increased the risk of sudden cardiac death in patients aged between 35 and 54 years. Ischemic heart disease (1.53; 95% CI, 1.31 to 1.79; P < 0.001) elevated the risk of sudden cardiac death in patients aged >55 years.
Table 4.
Cox Proportional Hazards Model of Physical and Psychiatric Comorbidity on the Risk of Sudden Cardiac Death in Patients with Schizophrenia, Stratified by Age Subgroups.
| Characteristics | Schizophrenia Cohort (N = 170,322) | Sudden Cardiac Death (n = 1,836) | Adjusted Hazard Ratio | 95% CI | P | Population Attributable Fraction (%) |
|---|---|---|---|---|---|---|
| N (%) | N (%) | |||||
| Subgroup with age <35a | 76,325 | 198 | ||||
| Physical illnesses | ||||||
| Hypertension | 1,411 (1.9) | 18 (9.1) | 3.06 | 1.79 to 5.25 | <0.001 | 6.12 |
| Congestive heart failure | 201 (0.3) | 7 (3.5) | 6.91 | 3.05 to 15.68 | <0.001 | 3.03 |
| Chronic hepatic disease | 3,563 (4.7) | 32 (16.2) | 2.38 | 1.61 to 3.54 | <0.001 | 9.37 |
| Psychiatric illnesses | ||||||
| Sleep disorder | 15,638 (20.5) | 62 (31.3) | 1.77 | 1.30 to 2.42 | <0.001 | 13.62 |
| Drug-induced mental disorder | 3,339 (4.4) | 24 (12.1) | 2.53 | 1.64 to 3.92 | <0.001 | 7.33 |
| Subgroup with age 35 to 54b | 61,453 | 665 | ||||
| Physical illnesses | ||||||
| Hypertension | 7,421 (12.1) | 144 (21.7) | 2.08 | 1.71 to 2.53 | <0.001 | 11.24 |
| Congestive heart failure | 847 (1.4) | 33 (5.0) | 3.02 | 2.09 to 4.37 | <.001 | 3.32 |
| Psychiatric illnesses | ||||||
| Dementia and organic mental disorder | 4,018 (6.5) | 75 (11.3) | 1.61 | 1.26 to 2.05 | <0.001 | 4.27 |
| Drug-induced mental disorder | 2,658 (4.3) | 41 (6.2) | 1.79 | 1.30 to 2.46 | <0.001 | 2.72 |
| Subgroup with Age >55c | 32,544 | 973 | ||||
| Physical illnesses | ||||||
| Hypertension | 14,239 (43.8) | 499 (51.3) | 1.31 | 1.14 to 1.50 | <0.001 | 12.13 |
| Ischemic heart disease | 5,500 (16.9) | 267 (27.4) | 1.53 | 1.31 to 1.79 | <0.001 | 9.51 |
| Congestive heart failure | 2,801 (8.6) | 164 (16.9) | 2.22 | 1.85 to 2.67 | <0.001 | 9.27 |
| Psychiatric illnesses | ||||||
| Drug-induced mental disorder | 417 (1.3) | 21 (2.2) | 2.25 | 1.45 to 3.48 | <0.001 | 1.20 |
a Based on multivariate regression model in study subjects aged <35 years: sex, age, urbanization, employment, and the variables in Supplemental Table 2 (physical and psychiatric comorbidities) with P < 0.001 were remained in the final adjusted model.
b Based on multivariate regression model in study subjects aged between 35 and 54 years: sex, age, urbanization, employment, and the variables in Supplemental Table 3 (physical and psychiatric comorbidities) were with P < 0.001 remained in the final adjusted model.
c Based on multivariate regression model in study subjects aged >55 years: sex, age, urbanization, employment and the variables in Supplemental Table 4 (physical and psychiatric comorbidities) with P < 0.001 were remained in the final adjusted model.
As for the age effect on the PAF of the distinctive comorbidity for sudden cardiac death, sleep disorder had the highest PAF for sudden cardiac death (13.62%) in patients aged <35 years, followed by chronic hepatic disease (9.37%) and drug-induced mental disorder (7.33%). Among patients aged 35 to 54 years, hypertension had the highest PAF for sudden cardiac death (11.24%), followed by dementia and organic mental disorder (4.27%). In respect of patients aged >55 years, hypertension had the highest PAF for sudden cardiac death (12.13%), followed by ischemic heart disease (9.51%) and congestive heart failure (9.27%).
Discussion
To our knowledge, this is the first nationwide population-based cohort study to explore the age effect on the incidence and risk of the physical and psychiatric comorbidity for sudden cardiac death in patients with schizophrenia. We found that patients with schizophrenia had a 2.91-fold higher risk of sudden cardiac death relative to the general population. In addition, the SMRs in the schizophrenia cohort were >1.00 across all 3 age intervals and for both sexes, with the highest SMR in male patients aged <35 years (30.88; 95% CI, 26.18 to 36.18). Consistent with previous studies, 14,17 our findings from a Taiwanese national cohort suggest the urgency of formulating preventive strategies against sudden cardiac death in people with schizophrenia, particularly for younger male patients.
In this study, we determined a lower risk of sudden cardiac death among patients with schizophrenia who were employed or living in a highly urbanized area. Previous studies have shown that patients with schizophrenia are more likely to receive suboptimal medical care for their physical illnesses, which has resulted in excessive mortality. 29 –34 Such suboptimal medical care is due to complex reasons, possibly related to patients’ decreased ability for self-care and limited access to medical service. 35,36 Evidence has suggested that the association between risk of sudden cardiac death and residence in a rural area is related to the referral rate of specialist care. 37 In addition, higher neuropsychological abilities in patients with schizophrenia predict more favorable vocational outcomes. 38 Therefore, our finding of the decreased risk of sudden cardiac death in patients with schizophrenia who were employed or living in a highly urbanized area may reflect their greater ability for self-care and utilization of medical services.
Crucially, our study demonstrated that hypertension, congestive heart failure, and drug-induced mental disorder were the major comorbidities increasing the risk of sudden cardiac death across the lifespan in patients with schizophrenia. These findings are consistent with studies that have demonstrated that both physical and psychiatric comorbidities increase the risk of sudden cardiac death in patients with schizophrenia. 14,17 Furthermore, according to our stratified analyses, the fractions of sudden cardiac death attributable to hypertension and congestive heart failure increased with age. Conversely, the fraction of sudden cardiac death attributable to drug-induced mental disorder decreased with age. In Taiwan, methamphetamine is one of major classes of illicit drugs. 39 Our previous study revealed that methamphetamine increases the risk of arrhythmia particularly in younger patients, 40 which may partly explain the observed association between sudden cardiac death and drug-induced mental disorder in young patients with schizophrenia. Taken together, the findings from our prospective national cohort study suggest that, in contrast to psychiatric comorbidities, physical comorbidities, especially cardiovascular diseases, contribute more to sudden cardiac death among older adult patients with schizophrenia.
In addition to hypertension, congestive heart failure, and drug-induced mental disorder, we noted that chronic hepatic disease and sleep disorder increased the risk of sudden cardiac death in patients with schizophrenia aged <35 years. The reasons underlying these associations remain unknown but can be plausible.
To conjecture, systemic inflammation is a potential mechanism. Studies have demonstrated that patients with schizophrenia are characterized by inflammation-promoting risk genes. 41,42 Additionally, patients with chronic hepatic disease or sleep disorder have been observed to have increased levels of inflammatory markers such as interleukin-6 and highly sensitive C-reactive protein. 43,44 Interleukin-6 and highly sensitive C-reactive protein can impair calcium regulation in cardiomyocytes, causing QTc prolongation and arrhythmia 45,46 ; thus, patients with schizophrenia and comorbid chronic hepatic disease and sleep disorder may exhibit increased risks of sudden cardiac death. Moreover, studies have demonstrated that patients with schizophrenia have increased risks of body weight gain and obesity in their early adulthood. 47 In addition, obesity elevates the risk of numerous physical disorders, including cardiovascular diseases, nonalcoholic fatty liver disease, and obstructive sleep apnea. 48,49 Given that we did not specifically investigate obstructive sleep apnea in this study, further research must be conducted to determine whether obesity is a common factor linking nonalcoholic fatty liver disease, obstructive sleep apnea, and sudden cardiac death in young adult patients with schizophrenia.
Among patients with schizophrenia aged between 35 and 54 years, we identified dementia and organic mental disorder to collectively be an additional risk factor for sudden cardiac death. A recent nationwide population-based cohort study from Denmark determined a 2-fold higher risk of dementia among patients with schizophrenia. 6 Furthermore, a recent meta-analysis discovered that patients with schizophrenia who were younger than 65 years had a comparable relative risk of dementia to individuals aged 65 years and older. 50 Moreover, patients with schizophrenia and cognitive deficits may have decreased self-care ability, thus worsening physical health and causing, for example, hypertension and congestive heart failure, 35,36,51 our study’s findings suggest that clinicians must begin assessing the cognitive function and cardiovascular health of patients with schizophrenia before they enter into older age (at 55 years).
Limitations
Our study has several limitations. First, the diagnoses of schizophrenia and comorbidities in this study were based on only the ICD codes in NHIRD claims data. Nevertheless, the NHI Administration conducts yearly randomized reviews of the NHIRD by using medical records to verify the diagnoses therein. Therefore, the NHIRD is of acceptable accuracy with respect to the coding for epidemiological analysis. Second, laboratory test data could not be obtained from the NHIRD. These data can provide insights into the pathogenesis mechanisms of sudden cardiac death associated with schizophrenia. Third, due to the limitation inherent to the use of the NHIRD, data related to obesity-related measures (e.g., body mass index and waist circumference) and lifestyle variables (e.g., smoking and diet habits) were not available in the present analyses. Fourth, given that sudden cardiac death related to antipsychotic medication is usually attributed to arrhythmia and myocarditis, our present study design may not have been suitable for assessing the acute exposure effect of antipsychotic medications on the risk of sudden cardiac death. Future research with a case-crossover design is necessary to explore this crucial topic.
Conclusions
This prospective nationwide population-based cohort study noted a high risk of sudden cardiac death in patients with schizophrenia across the lifespan. Particularly, the SMR of sudden cardiac death is the highest among male patients aged <35 years. Furthermore, different comorbidities have distinctive age-dependent risks of sudden cardiac death. Given the facts that patients with schizophrenia are at an increased risk of excessive cardiac mortality, prevention strategies that account for the age effect are urgently required.
Supplemental Material
Supplementary_material for Age Effect on Incidence, Physical, and Psychiatric Comorbidity for Sudden Cardiac Death in Schizophrenia: Effet de l’âge sur l’incidence, la comorbidité physique et psychiatrique de la mort cardiaque subite dans la schizophrénie by Pao-Huan Chen, Shang-Ying Tsai, Chun-Hung Pan, Hu-Ming Chang, Yi-Lung Chen, Sheng-Siang Su, Chiao-Chicy Chen and Chian-Jue Kuo in The Canadian Journal of Psychiatry
Acknowledgments
The authors would like tom thank Wallace Academic Editing for editing the manuscript.
Authors’ Note: Drs. PH Chen, Chang, and Kuo led the conception and design of the study. Dr. Kuo acquired the data, and Mr. Su performed the statistical analysis. Drs. PH Chen and Kuo wrote and revised the manuscript. Drs. Pan and YL Chen made critical and substantive revisions to the manuscript. Drs. Tsai and CC Chen supervised the study. Please use the following website: http://nhird.nhri.org.tw/en/ to request information on the database this study used, such as the point of contact, data protection, and content of data files. The database is composed of medical claim files representative of the entire population in Taiwan.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by grants from Taiwan’s Ministry of Science and Technology (MOST 105-2314-B-532-006-MY3; MOST 108-2314-B-532-005) and Taipei City Hospital (10501-62-015; TPECH 106-77). The funding organizations had no involvement in the study design, data collection, analysis, interpretation of data, writing of the report, or decision to submit the paper for publication.
ORCID iD: Chian-Jue Kuo, MD, PhD  https://orcid.org/0000-0002-2773-1335
https://orcid.org/0000-0002-2773-1335
Supplemental Material: The supplemental material for this article is available online.
References
- 1. Marder SR, Cannon TD. Schizophrenia. N Engl J Med. 2019;381(18):1753–1761. [DOI] [PubMed] [Google Scholar]
- 2. Buckley PF, Miller BJ, Lehrer DS, Castle DJ. Psychiatric comorbidities and schizophrenia. Schizophr Bull. 2009;35(2):383–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wulff K, Dijk DJ, Middleton B, Foster RG, Joyce EM. Sleep and circadian rhythm disruption in schizophrenia. Br J Psychiatry. 2012;200(1):308–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Crump C, Winkleby MA, Sundquist K, Sundquist J. Comorbidities and mortality in persons with schizophrenia: a Swedish national cohort study. Am J Psychiatry. 2013;170(3):324–333. [DOI] [PubMed] [Google Scholar]
- 5. Schoepf D, Uppal H, Potluri R, Heun R. Physical comorbidity and its relevance on mortality in schizophrenia: a naturalistic 12-year follow-up in general hospital admissions. Eur Arch Psychiatry Clin Neurosci. 2014;264(2):3–28. [DOI] [PubMed] [Google Scholar]
- 6. Ribe AR, Laursen TM, Charles M, et al. Long-term risk of dementia in persons with schizophrenia: a Danish population-based cohort study. JAMA Psychiatry. 2015;72(11):1095–1101. [DOI] [PubMed] [Google Scholar]
- 7. Plana-Ripoll O, Pedersen CB, Holtz Y, et al. Exploring comorbidity within mental disorders among a Danish national population. JAMA Psychiatry. 2019;76(3):259–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Laursen TM, Munk-Olsen T, Vestergaard M. Life expectancy and cardiovascular mortality in persons with schizophrenia. Curr Opin Psychiatry. 2012;25(2):83–88. [DOI] [PubMed] [Google Scholar]
- 9. Olfson M, Gerhard T, Huang C, Crystal S, Stroup TS. Premature mortality among adults with schizophrenia in the United States. JAMA Psychiatry. 2015;72(12):1172–1181. [DOI] [PubMed] [Google Scholar]
- 10. Plana-Ripoll O, Pedersen CB, Agerbo E, et al. A comprehensive analysis of mortality-related health metrics associated with mental disorders: a nationwide, register-based cohort study. Lancet. 2019;394(10211):1827–1835. [DOI] [PubMed] [Google Scholar]
- 11. Correll CU, Solmi M, Veronese N, et al. Prevalence, incidence and mortality from cardiovascular disease in patients with pooled and specific severe mental illness: a large-scale meta-analysis of 3,211,768 patients and 113,383,368 controls. World Psychiatry. 2017;16(2):163–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Adabag AS, Luepker RV, Roger VL, Gersh BJ. Sudden cardiac death: epidemiology and risk factors. Nat Rev Cardiol. 2010;7(4):216–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hayashi M, Shimizu W, Albert CM. The spectrum of epidemiology underlying sudden cardiac death. Circ Res. 2015;116(12):1887–1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Manu P, Kane JM, Correll CU. Sudden deaths in psychiatric patients. J Clin Psychiatry. 2011;72(7):936–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sweeting J, Duflou J, Semsarian C. Postmortem analysis of cardiovascular deaths in schizophrenia: a 10-year review. Schizophr Res. 2013;150(2-3):398–403. [DOI] [PubMed] [Google Scholar]
- 16. Ifteni P, Correll CU, Burtea V, Kane JM, Manu P. Sudden unexpected death in schizophrenia: autopsy findings in psychiatric inpatients. Schizophr Res. 2014;155(1-3):72–76. [DOI] [PubMed] [Google Scholar]
- 17. Hou PY, Hung GC, Jhong JR, Tsai SY, Chen CC, Kuo CJ. Risk factors for sudden cardiac death among patients with schizophrenia. Schizophr Res. 2015;168(1-2):395–401. [DOI] [PubMed] [Google Scholar]
- 18. Risgaard B, Waagstein K, Winkel BG, et al. Sudden cardiac death in young adults with previous hospital-based psychiatric inpatient and outpatient treatment: a nationwide cohort study from Denmark. J Clin Psychiatry. 2015;76(9):e1122–e1129. [DOI] [PubMed] [Google Scholar]
- 19. Li KJ, Greenstein AP, Delisi LE. Sudden death in schizophrenia. Curr Opin Psychiatry. 2018;31(1):169–175. [DOI] [PubMed] [Google Scholar]
- 20. Cohen CI, Meesters PD, Zhao J. New perspectives on schizophrenia in later life: implications for treatment, policy, and research. Lancet Psychiatry. 2015;2(4):340–350. [DOI] [PubMed] [Google Scholar]
- 21. Wu CS, Wang SC, Cheng YC, Gau SS. Association of cerebrovascular events with antidepressant use: a case-crossover study. Am J Psychiatry. 2011;168(5):511–521. [DOI] [PubMed] [Google Scholar]
- 22. Kuo CJ, Yang SY, Liao YT, et al. Second-generation antipsychotic medications and risk of pneumonia in schizophrenia. Schizophr Bull. 2013;39(3):648–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wu CS, Wang SC, Gau SS, Tsai HJ, Cheng YC. Association of stroke with the receptor-binding profiles of antipsychotics-a case-crossover study. Biol Psychiatry. 2013;73(5):414–421. [DOI] [PubMed] [Google Scholar]
- 24. Chen PH, Tsai SY, Pan CH, et al. Mood stabilisers and risk of stroke in bipolar disorder. Br J Psychiatry. 2019;215(1):409–414. [DOI] [PubMed] [Google Scholar]
- 25. Zheng ZJ, Croft JB, Giles WH, Mensah GA. Sudden cardiac death in the United States, 1989 to 1998. Circulation. 2001;104(18):2158–2163. [DOI] [PubMed] [Google Scholar]
- 26. Ray WA, Chung CP, Murray KT, Hall K, Stein CM. Atypical antipsychotic drugs and the risk of sudden cardiac death. N Engl J Med. 2009;360(3):225–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kuo CJ, Chen WY, Tsai SY, Chen PH, Ko KT, Chen CC. Excessive mortality and causes of death among patients with personality disorder with comorbid psychiatric disorders. Soc Psychiatry Psychiatr Epidemiol. 2019;54(1):121–130. [DOI] [PubMed] [Google Scholar]
- 28. Greenland S. Applications of stratified analysis methods. In: Rothman KJ, Greenland S, Lash TL, editors. Modern epidemiology. 3rd ed. Philadelphia (PA): Lippincott Williams & Wilkins; 2008. p. 295–296. [Google Scholar]
- 29. Laursen TM, Munk-Olsen T, Agerbo E, Gasse C, Mortensen PB. Somatic hospital contacts, invasive cardiac procedures, and mortality from heart disease in patients with severe mental disorder. Arch Gen Psychiatry. 2009;66(7):713–720. [DOI] [PubMed] [Google Scholar]
- 30. Laursen TM, Mortensen PB, MacCabe JH, Cohen D, Gasse C. Cardiovascular drug use and mortality in patients with schizophrenia or bipolar disorder: a Danish population-based study. Psychol Med. 2014;44(8): 1625–1637. [DOI] [PubMed] [Google Scholar]
- 31. Kugathasan P, Horsdal HT, Aagaard J, Jensen SE, Laursen TM, Nielsen RE. Association of secondary preventive cardiovascular treatment after myocardial infarction with mortality among patients with schizophrenia. JAMA Psychiatry. 2018;75(1):1234–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Heiberg IH, Jacobsen BK, Balteskard L, et al. Undiagnosed cardiovascular disease prior to cardiovascular death in individuals with severe mental illness. Acta Psychiatr Scand. 2019;139(6):558–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hauck TS, Liu N, Wijeysundera HC, Kurdyak P. Mortality and revascularization among myocardial infarction patients with schizophrenia: a population-based cohort study. Can J Psychiatry. 2020;65(7):454–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kurdyak P, Vigod S, Duchen R, Jacob B, Stukel T, Kiran T. Diabetes quality of care and outcomes: comparison of individuals with and without schizophrenia. Gen Hosp Psychiatry. 2017;46:7–13. [DOI] [PubMed] [Google Scholar]
- 35. Liu NH, Daumit GL, Dua T, et al. Excess mortality in persons with severe mental disorders: a multilevel intervention framework and priorities for clinical practice, policy and research agendas. World Psychiatry. 2017;16(1):30–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Firth J, Siddiqi N, Koyanagi A, et al. The lancet psychiatry commission: a blueprint for protecting physical health in people with mental illness. Lancet Psychiatry. 2019;6(8):675–712. [DOI] [PubMed] [Google Scholar]
- 37. Parkash R, Wightman H, Miles G, et al. Primary prevention of sudden cardiac death with device therapy in urban and rural populations. Can J Cardiol. 2017;33(4):437–442. [DOI] [PubMed] [Google Scholar]
- 38. Mahmood Z, Keller AV, Burton CZ, et al. Modifiable predictors of supported employment outcomes among people with severe mental illness. Psychiatr Serv. 2019;70(9):782–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Feng LY, Yu WJ, Chang WT, Han E, Chung H, Li JH. Comparison of illegal drug use pattern in Taiwan and Korea from 2006 to 2014. Subst Abuse Treat Prev Policy. 2016;11:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Huang MC, Yang SY, Lin SK, et al. Risk of cardiovascular diseases and stroke events in methamphetamine users: a 10-year follow-up study. J Clin Psychiatry. 2016;77(10):1396–1403. [DOI] [PubMed] [Google Scholar]
- 41. Khandaker GM, Cousins L, Deakin J, Lennox BR, Yolken R, Jones PB. Inflammation and immunity in schizophrenia: implications for pathophysiology and treatment. Lancet Psychiatry. 2015;2(3):258–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Muller N. Inflammation in schizophrenia: pathogenetic aspects and therapeutic considerations. Schizophr Bull. 2018;44(5):973–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hung CS, Tseng PH, Tu CH, et al. Nonalcoholic fatty liver disease Is associated with QT prolongation in the general population. J Am Heart Assoc. 2015;4(7):e001820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Irwin MR, Olmstead R, Carroll JE. Sleep disturbance, sleep duration, and inflammation: a systematic review and meta-analysis of cohort studies and experimental sleep deprivation. Biol Psychiatry. 2016;80(1):40–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kazumi T, Kawaguchi A, Hirano T, Yoshino G. C-reactive protein in young, apparently healthy men: associations with serum leptin, QTc interval, and high-density lipoprotein-cholesterol. Metabolism. 2003;52(9):1113–1116. [DOI] [PubMed] [Google Scholar]
- 46. Lazzerini PE, Laghi-Pasini F, Bertolozzi I, et al. Systemic inflammation as a novel QT-prolonging risk factor in patients with torsades de pointes. Heart. 2017;103(22):1821–1829. [DOI] [PubMed] [Google Scholar]
- 47. Bioque M, Garcia-Portilla MAP, Garcia-Rizo C, et al. Evolution of metabolic risk factors over a two-year period in a cohort of first episodes of psychosis. Schizophr Res. 2018;193:188–196. [DOI] [PubMed] [Google Scholar]
- 48. Lavie CJ, Milani RV, Ventura HO. Obesity and cardiovascular disease: risk factor, paradox, and impact of weight loss. J Am Coll Cardiol. 2009;53(21):1925–1932. [DOI] [PubMed] [Google Scholar]
- 49. Polyzos SA, Kountouras J, Mantzoros CS. Obesity and nonalcoholic fatty liver disease: from pathophysiology to therapeutics. Metabolism. 2019;92(2): 82–97. [DOI] [PubMed] [Google Scholar]
- 50. Cai L, Huang J. Schizophrenia and risk of dementia: a meta-analysis study. Neuropsychiatr Dis Treat. 2018;14:2047–2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Storch Jakobsen A, Speyer H, Norgaard HCB, et al. Associations between clinical and psychosocial factors and metabolic and cardiovascular risk factors in overweight patients with schizophrenia spectrum disorders—baseline and two-years findings from the change trial. Schizophr Res. 2018;199:96–102. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary_material for Age Effect on Incidence, Physical, and Psychiatric Comorbidity for Sudden Cardiac Death in Schizophrenia: Effet de l’âge sur l’incidence, la comorbidité physique et psychiatrique de la mort cardiaque subite dans la schizophrénie by Pao-Huan Chen, Shang-Ying Tsai, Chun-Hung Pan, Hu-Ming Chang, Yi-Lung Chen, Sheng-Siang Su, Chiao-Chicy Chen and Chian-Jue Kuo in The Canadian Journal of Psychiatry