Abstract
Objectives
Cell entry of SARS-CoV-2 depends on angiotensin-converting enzyme II. Angiotensin-converting enzyme II is homologous with, but acts antagonistically to, angiotensin-converting enzyme and has the critical function of protecting the lungs. Angiotensin-converting enzyme inhibitors are major antihypertensive agents. Thus, we aimed to analyze the impact of the prevalence of preexisting hypertension on the local spread of COVID-19.
Methods
Data on SARS-CoV-2 infection and the estimated number of patients who received medical treatment on the basis of disease classification using the International Statistical Classification of Diseases and Related Health Problems (10th Revision) in each prefecture were obtained from the official Japanese notifications database. We analyzed the association between the proportion of patients with each disease and SARS-CoV-2-infection prevalence.
Results
The ratio of patients treated for diseases of the circulatory system, especially hypertensive disorders, per population demonstrated the most significant negative correlation with SARS-CoV-2-infection prevalence (Spearman’s rank correlation, P < 0.01). Age group analysis revealed a significant negative correlation in age groups 35–44, 45–54, 55–64, 75–84, and ≥85.
Conclusions
Our findings suggest that hypertension treatment may play a protective role against the local spread of SARS-CoV-2 infection.
Keywords: Angiotensin-converting enzyme II, Coronavirus disease 2019, COVID-19, Hypertension, Nitric oxide, SARS-CoV-2
Introduction
SARS-CoV-2 is the causative virus for COVID-19. Cell entry of SARS-CoV-2 depends on angiotensin-converting enzyme II (ACE2), which is a membrane-associated zinc peptidase, and transmembrane serine protease 2, which is a cellular serine protease (Zhou et al., 2020b, Hoffmann et al., 2020, Hirano and Murakami, 2020). ACE2 is homologous with, but acts antagonistically to, angiotensin-converting enzyme (ACE) and has the critical function of protecting the lungs from severe acute injury (Imai et al., 2005).
The renin-angiotensin-aldosterone system is an essential regulator of blood pressure. Thus, ACE inhibitors and angiotensin receptor blockers (ARBs) are commonly used as antihypertensive agents. Because both ACE inhibitors and ARBs increase ACE2 (Ferrario et al., 2005, Furuhashi et al., 2015), the possibility of these drugs having either the unfavorable effect of promoting SARS-CoV-2 cell entry or the beneficial effect of preventing acute lung distress in patients with hypertension remains controversial (Schiffrin et al., 2020, Shibata et al., 2020). Moreover, whether hypertension itself, as compared with other medical conditions, increases the risk of susceptibility to COVID-19 or disease severity, or impacts local COVID-19 spread, should be elucidated.
In a previous study, we demonstrated that routine infant Bacillus Calmette-Guérin (BCG) vaccination coverage in the younger generation had a significant impact on the prevention of local COVID-19 spread in Japan (Kinoshita and Tanaka, 2020). In this study, we analyzed the effect of the proportion of local patients with preexisting hypertension and other medical conditions per population on the local prevalence of patients with COVID-19 infection, SARS-CoV-2 polymerase chain reaction-positive (PCR+) individuals, and COVID-19 deaths in Japanese prefectures.
Materials and methods
Prevalence of patients with COVID-19, SARS-CoV-2 PCR+ individuals, and COVID-19 deaths in each prefecture
Anonymous data on COVID-19 infection in Japan were obtained, as described in our previous study (Kinoshita and Tanaka, 2020). In brief, the numbers of patients with COVID-19, SARS-CoV-2-PCR+ individuals, and COVID-19 deaths among Japanese residents in each prefecture were extracted from official notification records of the Ministry of Health, Labour and Welfare of Japan (Ministry of Health, Labour and Welfare of Japan, 2020). Data regarding patient age and sex were not available.
In Japan, COVID-19 diagnosis is on the basis of the Infectious Disease Surveillance System, in accord with the Act on the Prevention of Infectious Diseases and Medical Care for Patients with Infectious Diseases. Until March 29, 2020, the Act applied to individuals with symptoms suggestive of SARS-CoV-2 infection. Thereafter, the Act has also been applied to the asymptomatic population, and the official number of patients has also included PCR+ individuals presenting no clinical symptoms. In April 2020, the Japanese Prime Minister declared a state of emergency. Thus, in this study, data collected as of March 29, 2020, were used.
The crude prevalence of SARS-CoV-2 infection in each prefecture was calculated as the number of patients divided by the number of individuals in each prefecture on October 1, 2019, as estimated by the Statistics Bureau, Ministry of Internal Affairs and Communications of Japan (Statistics Bureau, Ministry of Internal Affairs and Communications of Japan, 2020).
Preexisting diseases
The estimated number of patients who received medical treatment in each prefecture was obtained from the Patient Survey of October 2017. The Patient Survey is run every 3 years by the Health Statistics Office, Ministry of Health, Labour and Welfare (Statistics Bureau, Ministry of Internal Affairs and Communications of Japan, 2020). The diseases were classified on the basis of the International Statistical Classification of Diseases and Related Health Problems (10th Revision) (Table 1 , Supplementary Tables 1A–1C). The ratio of the estimated number of patients treated for each disease per day to the estimated number of individuals on October 1, 2019, in each prefecture was used as a surrogate for the local prevalence of patients with the disease. Though the number of patients per day, as estimated by the Patient Survey, was much smaller than the crude number of patients, the data were suitable for correlation analyses among prefectures or comparison among diseases. Data on patient age and sex were not available.
Table 1.
The estimated number of patients nationwide (thousands, per day) according to the Patient Survey in 2017 and its correlation with COVID-19 prevalence (*P < 0.05, **P < 0.01).
| Classification of diseases | Number of patients | Spearman’s ρ |
|---|---|---|
| I. Certain infectious and parasitic diseases | 189.6 | −0.123 |
| Intestinal infectious diseases | 34.0 | −0.199 |
| Tuberculosis | 4.3 | 0.304* |
| Viral infections characterized by skin and mucous membrane lesions | 62.4 | −0.079 |
| Mycoses | 39.9 | 0.064 |
| II. Neoplasms | 391.7 | −0.398** |
| Malignant neoplasms | 309.7 | −0.417** |
| Carcinoma in situ of stomach | 32.4 | −0.342* |
| Carcinoma in situ of colon and rectum | 48.4 | −0.254 |
| Carcinoma in situ of trachea, bronchus, and lung | 34.9 | −0.342* |
| III. Diseases of the blood and blood-forming organs and disorders involving the immune system | 27.0 | −0.037 |
| IV. Endocrine, nutritional and metabolic diseases | 475.9 | −0.287 |
| Disorders of the thyroid gland | 33.1 | 0.000 |
| Diabetes mellitus | 242.9 | −0.299* |
| V. Mental and behavioural disorders | 512.9 | −0.360 |
| Schizophrenia, schizotypal and delusional disorders | 216.2 | −0.381** |
| Mood [affective] disorders | 119.5 | −0.140 |
| Neurotic, stress-related and somatoform disorders | 64.9 | −0.073 |
| VI. Diseases of the nervous system | 291.1 | −0.352* |
| VII. Diseases of the eye and adnexa | 370.2 | 0.020 |
| Cataract | 90.9 | −0.034 |
| VIII. Diseases of the ear and mastoid process | 101.8 | −0.236 |
| IX. Diseases of the circulatory system | 1117.5 | −0.469** |
| Hypertensive diseases | 652.5 | −0.456** |
| Other forms of heart disease | 198.2 | −0.296* |
| Ischaemic heart diseases | 70.6 | −0.257 |
| Cerebrovascular diseases | 231.9 | −0.387** |
| X. Diseases of the respiratory system | 725.8 | −0.098 |
| Acute upper respiratory infections | 249.7 | 0.058 |
| Pneumonia | 43.4 | −0.053 |
| Acute bronchitis/bronchiolitis | 93.5 | −0.432** |
| Bronchitis/emphysema | 30.8 | −0.036 |
| Asthma | 124.6 | −0.139 |
| XI. Diseases of the digestive system | 1359.3 | 0.097 |
| Dental caries | 277.1 | 0.061 |
| Gingivitis and periodontal diseases | 469.2 | 0.085 |
| Gastric/duodenal ulcer | 23.7 | 0.142 |
| Gastritis and duodenitis | 66.7 | −0.107 |
| Diseases of the liver | 34.3 | 0.001 |
| XII. Diseases of the skin and subcutaneous tissue | 315.2 | 0.081 |
| XIII. Diseases of the musculoskeletal system and connective tissue | 948.5 | −0.196 |
| Inflammatory polyarthropathies | 54.0 | −0.830 |
| Arthrosis | 219.4 | −0.087 |
| Dorsopathies | 443.2 | −0.220 |
| Disorders of bone density and structure | 61.7 | −0.211 |
| XIV. Diseases of the genitourinary system | 371.8 | −0.226 |
| Glomerular and renal tubulo-interstitial diseases, and renal failure | 188.7 | −0.103 |
| Hyperplasia of prostate | 33.3 | −0.304* |
| Disorders of breast and female pelvic organs | 94.2 | −0.106 |
| XV. Pregnancy, childbirth and the puerperium | 33.4 | −0.158 |
| Hypertensive disorders in pregnancy | 0.8 | 0.239 |
| XVI. Certain conditions originating in the perinatal period | 10.1 | −0.153 |
| XVII. Congenital malformations, deformations and chromosomal abnormalities | 19.8 | −0.053 |
| XVIII. Symptoms, signs and abnormal findings, not elsewhere classified | 93.2 | −0.172 |
| XIX. Injury, poisoning and certain other consequences of external causes | 436.7 | −0.057 |
| Fracture | 196 | 0.007 |
Statistical analyses
As per the Kolmogorov–Smirnov test, variables did not exhibit a normal distribution. Therefore, non-parametric Spearman’s rank correlation coefficient was employed to evaluate the correlation of SARS-CoV-2 prevalence with each preexisting disease using SPSS (version 16.0J; IBM Japan, Tokyo, Japan). The significance level was set at P = 0.01.
Results
Prevalence data of patients with COVID-19, SARS-CoV-2-PCR+ individuals, and COVID-19 deaths in each prefecture have previously been reported (Kinoshita and Tanaka, 2020). The 2017 Patient Survey demonstrated that circulatory system disease (Chapter IX, International Statistical Classification of Diseases and Related Health Problems [10th Revision]) was the second-largest classification of the estimated number of patients nationwide (Table 1).
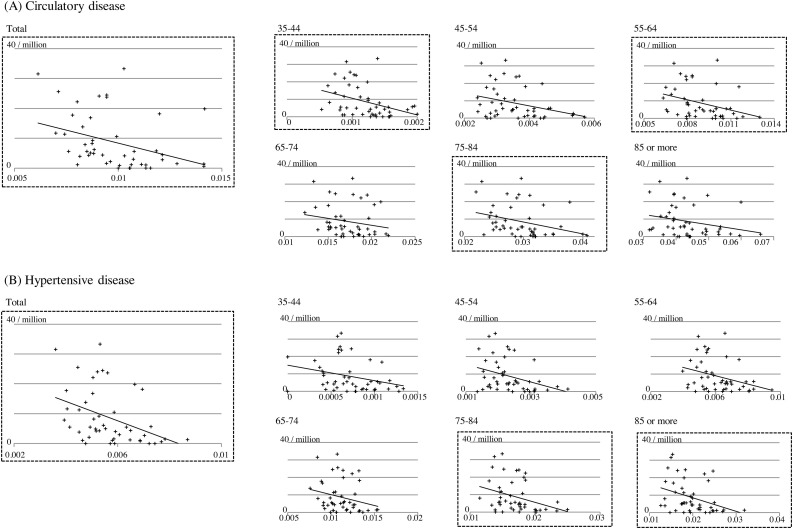
The prevalence of patients with COVID-19 on March 29, 2020, showed a significant negative correlation with the ratio of patients per population with diseases of the circulatory system, especially hypertensive disorders and cerebrovascular diseases that form its subcategory, and schizophrenia (P < 0.01) (Figure 1 , Table 1). Considering that our previous study demonstrated an inverse association between the elderly population and SARS-CoV-2-infection prevalence (Kinoshita and Tanaka, 2020) and that the proportion of patients with hypertensive disorders increases with age, we further analyzed the correlation between SARS-CoV-2 infection and hypertension by age group. A significant negative correlation was shown between SARS-CoV-2-infection prevalence and the ratio of hypertension in age groups 35–44, 45–54, 55–64, 75–84, and ≥85 (Figure 1 and Table 2 ).
Figure 1.
Correlation between the prevalence of patients with SARS-CoV-2 infection and (A) diseases of the circulatory system or (B) hypertensive diseases, by age group. Scatter plot of the prevalence of patients with SARS-CoV-2 infection (ordinate) against related factors (abscissa). Regression lines are shown for reference.
Table 2.
Correlation coefficient (Spearman’s ρ) between COVID-19 prevalence and diseases of the circulatory system, by age group.
| Classification of diseases | Total* | 35–44 | 45–54 | 55–64 | 65–74 | 75–84 | ≥85 |
|---|---|---|---|---|---|---|---|
| IX. Circulatory | −0.469** | −0.421** | −0.295* | −0.412** | −0.297* | −0.441** | −0.321* |
| Hypertensive | −0.456** | −0.306* | −0.370* | −0.335* | −0.266 | −0.367* | −0.383** |
P < 0.05.
P < 0.01.
Discussion
In this study, we analyzed the association of the ratio of local patients treated for preexisting hypertension and other medical conditions per population with SARS-CoV-2-infection prevalence in Japanese prefectures. The current study was the first to demonstrate that diseases of the circulatory system, especially treated hypertension, exhibited the strongest inverse correlation with the local spread of SARS-CoV-2 infection in Japan compared with a range of other diseases. Moreover, the correlation remained statistically significant after adjusting for the influence of age. Given prior reports indicating hypertension as a significant risk factor for COVID-19 incidence and severity (Zhou et al., 2020a, Wu et al., 2020), a plausible explanation for this unexpected inverse relationship is the potentially protective effect of antihypertensive medications.
The relationship between hypertension and COVID-19 has been controversial (Schiffrin et al., 2020). Initial studies in Wuhan, China, identified hypertension as the most common comorbidity in COVID-19 patients, especially those with lung injury (Zhou et al., 2020a, Wu et al., 2020). After the discovery of the cell-entry mechanism of SARS-CoV-2 (Zhou et al., 2020b, Hoffmann et al., 2020, Hirano and Murakami, 2020), the possibility of ACE inhibitors or ARBs having an unfavorable effect on COVID-19 secondary to the upregulation of ACE2 receptor sites theoretically facilitating viral cell entry has been a matter of great concern (Schiffrin et al., 2020, Shibata et al., 2020). However, in contrast to this line of reasoning, ACE2 has known protective pulmonary and vascular functions. Furthermore, decreased ACE2 expression is associated with worse outcomes in other respiratory diseases via down regulation of the ACE2/angiotensin-(1-7)/Mas axis. In concordance with this hypothesis, recent clinical studies in COVID-19 patients with hypertension have shown no increased risk of harm (Hasan et al., 2020) and further demonstrated a favorable role of renin-angiotensin system inhibitors (Meng et al., 2020, Adrish et al., 2020, Baral et al., 2020, Matsuzawa et al., 2020, Şenkal et al., 2020). A recent study revealed that ACE inhibitor use was associated with lower odds of COVID-19 among those aged ≥85 (An et al., 2021). In our study, the type of hypertensive medication could not be evaluated.
In Japan, calcium channel blockers are the most commonly used antihypertensives, and whereas ARBs are less often prescribed in hypertensive patients aged ≥75, they are almost equally used in patients <75. In patients <65, ARBs narrowly become the most common antihypertensive medication class (Ishida et al., 2018). With the prevalence of hypertension increasing with age and our previous study demonstrating an inverse association between ratios of the elderly population and SARS-CoV-2-infection prevalence (Kinoshita and Tanaka, 2020), an additional analysis was performed to adjust for the influence of age. Statistical significance was found in younger age groups, even those in their thirties or forties. The current study demonstrated a significant inverse correlation between cerebrovascular disease and COVID-19. Hypertension is one of the most important risk factors for cerebral infarction and cerebral hemorrhage, and absolute risk reduction can be achieved through optimal blood pressure control (Kannel et al., 1981, Tanaka et al., 1982, Thomopoulos et al., 2014). The use of ACE inhibitors or ARBs is recommended by the Japan Stroke Society considering the beneficial effects of improving insulin resistance, renal protection, and preventing atrial fibrillation (The Japan Stroke Society, 2019).
Severe COVID-19 outcomes are associated with endothelial dysfunction (Varga et al., 2020). ACE inhibitors and ARBs induce nitric oxide (NO) synthase, leading to the restoration of NO and improvement of endothelial dysfunction (Wagenaar et al., 2001, Palaniyappan et al., 2009). In addition, NO has direct antiviral activity in terms of preventing host-cell entry, virulence, or replication (Saura et al., 1999, Shulla et al., 2011, Green, 1995, Green, 2020). Host defense via NO can be activated by BCG inoculation in animal studies (Green et al., 1994). This mechanism is possibly reinforced in Japan by the universal BCG vaccination policy. The abundant dietary nitrate content of traditional Japanese foods is a source of NO (Yamasaki, 2020, Sobko et al., 2010).
In the present study, a significant inverse correlation was observed between schizophrenia and COVID-19. A recent study suggested the impact of a single nucleotide polymorphism (rs4702), located in the FURIN, on alveolar and neuron infection by SARS-CoV-2 in vitro (Dobrindt et al., 2020). The downregulation of FURIN expression, which is specific to the rs4702 G allele, has been associated with abnormal migration neurodevelopment leading to schizophrenia (Fromer et al., 2016, Hou et al., 2018, Schrode et al., 2019). The frequency of the rs4702 G allele in the Japanese population is relatively high (25.7%), whereas that in Yoruba Africans, the African ancestry in Southwest USA, or Luhya in Webuye, Kenya is negligible (SNPedia, 2020). Genetic preposition to SARS-CoV-2 infection warrants further studies.
In summary, the current study demonstrated that the proportion of patients treated for preexisting hypertension was inversely associated with local COVID-19 spread in Japan. Analysis by age group showed that the association remained statistically significant in patients in their thirties or forties. Antihypertensive medications, namely ACE inhibitors and ARBs, possibly play a protective role against COVID-19 via increases in ACE2 and NO.
Conflict of interest
None.
Funding source
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethical approval
Ethical approval for this study was waived because we used previously published data that did not contain any personal information.
Author contributions
MK, KS, and MT contributed to the conceptualization and investigation. MK, KS, BV, SJG, and MT contributed to the methodology. MK, KS, and MT performed data analysis. MK, KS, and TM contributed to the writing, original draft preparation, review, and editing. BV and SJG contributed to review and editing, and supervised the study. All authors contributed to the article and approved the submitted version.
Acknowledgement
We would like to thank Editage (www.editage.com) for English language editing.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.ijid.2021.05.071.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Adrish M., Chilimuri S., Sun H., Mantri N., Yugay A., Zahid M. The association of renin-angiotensin-aldosterone system inhibitors with outcomes among a predominantly ethnic minority patient population hospitalized with COVID-19: the Bronx experience. Cureus. 2020;12 doi: 10.7759/cureus.10217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An J., Wei R., Zhou H., Luong T.Q., Gould M.K., Mefford M.T. Angiotensin-converting enzyme inhibitors or angiotensin receptor blockers use and COVID-19 infection among 824 650 patients with hypertension from a US integrated healthcare system. J Am Heart Assoc. 2021;10 doi: 10.1161/JAHA.120.019669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baral R., White M., Vassiliou V.S. Effect of renin-angiotensin-aldosterone system inhibitors in patients with COVID-19: a systematic review and meta- analysis of 28,872 patients. Curr Atheroscler Rep. 2020;22:61. doi: 10.1007/s11883-020-00880-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrindt K., Hoagland D.A., Seah C., Kassim B., O’Shea C.P., Iskhakova M. Common genetic variation in humans impacts in vitro susceptibility to SARS-CoV2 infection. Stem Cell Rep. 2021;16:505–518. doi: 10.1016/j.stemcr.2021.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrario C.M., Jessup J., Chappell M.C., Averill D.B., Brosnihan K.B., Tallant E.A. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation. 2005;111:2605–2610. doi: 10.1161/CIRCULATIONAHA.104.510461. [DOI] [PubMed] [Google Scholar]
- Fromer M., Roussos P., Sieberts S.K., Johnson J.S., Kavanagh D.H., Perumal T.M. Gene expression elucidates functional impact of polygenic risk for schizophrenia. Nat Neurosci. 2016;19:1442–1453. doi: 10.1038/nn.4399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuhashi M., Moniwa N., Mita T., Fuseya T., Ishimura S., Ohno K. Urinary angiotensin-converting enzyme 2 in hypertensive patients may be increased by olmesartan, an angiotensin II receptor blocker. Am J Hypertens. 2015;28:15–21. doi: 10.1093/ajh/hpu086. [DOI] [PubMed] [Google Scholar]
- Green S.J., Scheller L.F., Marletta M.A., Seguin M.C., Klotz F.W., Slayter M. Nitric oxide: cytokine-regulation of nitric oxide in host resistance to intracellular pathogens. Immunol Lett. 1994;43:87–94. doi: 10.1016/0165-2478(94)00158-8. [DOI] [PubMed] [Google Scholar]
- Green S.J. Nitric oxide in mucosal immunity. Nat Med. 1995;1:515–517. doi: 10.1038/nm0695-515. [DOI] [PubMed] [Google Scholar]
- Green S.J. Covid-19 accelerates endothelial dysfunction and nitric oxide deficiency. Microbes Infect. 2020;22:149–150. doi: 10.1016/j.micinf.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan S.S., Kow C.S., Hadi M.A., Zaidi S.T.R., Merchant H.A. Mortality and disease severity among COVID-19 patients receiving renin-angiotensin system inhibitors: a systematic review and meta-analysis. Am J Cardiovasc Drugs. 2020;20:571–590. doi: 10.1007/s40256-020-00439-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano T., Murakami M. COVID-19: a new virus, but a familiar receptor and cytokine release syndrome. Immunity. 2020;52:731–733. doi: 10.1016/j.immuni.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181 doi: 10.1016/j.cell.2020.02.052. 271–80.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Y., Liang W., Zhang J., Li Q., Ou H., Wang Z. Schizophrenia-associated rs4702 G allele-specific downregulation of FURIN expression by miR-338-3p reduces BDNF production. Schizophr Res. 2018;199:176–180. doi: 10.1016/j.schres.2018.02.040. [DOI] [PubMed] [Google Scholar]
- Imai Y., Kuba K., Rao S., Huan Y., Guo F., Guan B. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436:112–116. doi: 10.1038/nature03712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida T., Oh A., Hiroi S., Shimasaki Y., Tsuchihashi T. Current use of antihypertensive drugs in Japanese patients with hypertension: analysis by age group. Geriatr Gerontol Int. 2018;18:899–906. doi: 10.1111/ggi.13276. [DOI] [PubMed] [Google Scholar]
- Kannel W.B., Wolf P.A., McGee D.L., Dawber T.R., McNamara P., Castelli W.P. Systolic blood pressure, arterial rigidity, and risk of stroke. The Framingham study. JAMA. 1981;245:1225–1229. [PubMed] [Google Scholar]
- Kinoshita M., Tanaka M. Impact of routine infant BCG vaccination on COVID-19. J Infect. 2020;81:625–633. doi: 10.1016/j.jinf.2020.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzawa Y., Ogawa H., Kimura K., Konishi M., Kirigaya J., Fukui K. Renin- angiotensin system inhibitors and the severity of coronavirus disease 2019 in Kanagawa, Japan: a retrospective cohort study. Hypertens Res. 2020;43:1257–1266. doi: 10.1038/s41440-020-00535-8. [DOI] [PubMed] [Google Scholar]
- Meng J., Xiao G., Zhang J., He X., Ou M., Bi J. Renin-angiotensin system inhibitors improve the clinical outcomes of COVID-19 patients with hypertension. Emerg Microbes Infect. 2020;9:757–760. doi: 10.1080/22221751.2020.1746200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ministry of Health, Labour and Welfare of Japan . 2020. About coronavirus disease 2019 (COVID-19)https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/0000164708_00001.html [Google Scholar]
- Palaniyappan A., Uwiera R.R.E., Idikio H., Jugdutt B.I. Comparison of vasopeptidase inhibitor omapatrilat and angiotensin receptor blocker candesartan on extracellular matrix, myeloperoxidase, cytokines, and ventricular remodeling during healing after reperfused myocardial infarction. Mol Cell Biochem. 2009;321:9–22. doi: 10.1007/s11010-008-9905-3. [DOI] [PubMed] [Google Scholar]
- Saura M., Zaragoza C., McMillan A., Quick R.A., Hohenadl C., Lowenstein J.M. An antiviral mechanism of nitric oxide: inhibition of a viral protease. Immunity. 1999;10:21–28. doi: 10.1016/S1074-7613(00)80003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Şenkal N., Meral R., Medetalibeyoğlu A., Konyaoğlu H., Kose M., Tukek T. Association between chronic ACE inhibitor exposure and decreased odds of severe disease in patients with COVID-19. Anatol J Cardiol. 2020;24:21–29. doi: 10.14744/AnatolJCardiol.2020.57431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffrin E.L., Flack J.M., Ito S., Muntner P., Webb R.C. Hypertension and COVID-19. Am J Hypertens. 2020;33:373–374. doi: 10.1093/ajh/hpaa057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrode N., Ho S.M., Yamamuro K., Dobbyn A., Huckins L., Matos M.R. Synergistic effects of common schizophrenia risk variants. Nat Genet. 2019;51:1475–1485. doi: 10.1038/s41588-019-0497-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata S., Arima H., Asayama K., Hoshide S., Ichihara A., Ishimitsu T. Hypertension and related diseases in the era of COVID-19: a report from the Japanese Society of Hypertension Task Force on COVID-19. Hypertens Res. 2020;43:1028–1046. doi: 10.1038/s41440-020-0515-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulla A., Heald-Sargent T., Subramanya G., Zhao J., Perlman S., Gallagher T. A transmembrane serine protease is linked to the severe acute respiratory syndrome coronavirus receptor and activates virus entry. J Virol. 2011;85:873–882. doi: 10.1128/JVI.02062-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SNPedia, rs4702. https://www.snpedia.com/index.php/Rs4702. [Accessed 26 September 2020].
- Sobko T., Marcus C., Govoni M., Kamiya S. Dietary nitrate in Japanese traditional foods lowers diastolic blood pressure in healthy volunteers. Nitric Oxide. 2010;22:136–140. doi: 10.1016/j.niox.2009.10.007. [DOI] [PubMed] [Google Scholar]
- Statistics Bureau, Ministry of Internal Affairs and Communications of Japan . 2020. e-Stat, Portal Site of Official Statistics of Japan.https://www.e-stat.go.jp [Google Scholar]
- Tanaka H., Ueda Y., Hayashi M., Date C., Baba T., Yamashita H. Risk factors for cerebral hemorrhage and cerebral infarction in a Japanese rural community. Stroke. 1982;13:62–73. doi: 10.1161/01.str.13.1.62. [DOI] [PubMed] [Google Scholar]
- The Japan Stroke Society . 2019. Japanese Guidelines for the Management of Stroke 2015, Revised.https://www.jsts.gr.jp/img/guideline2015_tuiho2019_10.pdf [Google Scholar]
- Thomopoulos C., Parati G., Zanchetti A. Effects of blood pressure lowering on outcome incidence in hypertension. 1. Overview, meta-analyses, and meta-regression analyses of randomized trials. J Hypertens. 2014;32:2285–2295. doi: 10.1097/HJH.0000000000000378. [DOI] [PubMed] [Google Scholar]
- Varga Z., Flammer A.J., Steiger P., Haberecker M., Andermatt R., Zinkernagel A.S. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagenaar L.J., Buikema H., Pinto Y.M., van Gilst W.H. Improvement of endothelial dysfunction in experimental heart failure by chronic RAAS-blockade: ACE-inhibition or AT1-receptor blockade? J Renin Angiotensin Aldosterone Syst. 2001;2(1_suppl):S64–9. doi: 10.1177/14703203010020011101. [DOI] [PubMed] [Google Scholar]
- Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180:934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki H. Blood nitrate and nitrite modulating nitric oxide bioavailability: potential therapeutic functions in COVID-19. Nitric Oxide. 2020;103:29–30. doi: 10.1016/j.niox.2020.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.