Intra-arterial cerebral fibrinolytic therapy for acute stroke was first described in 1983 by Zeumer et al (1). Subsequently, multiple anecdotal reports and small nonrandomized or controlled series have also supported that intra-arterial thrombolytic therapy may be useful for treatment of acute ischemic stroke (2–20). Results of the first randomized controlled trial of intra-arterial fibrinolytic therapy for acute stroke (PROACT II, Abbott Laboratories) were reported in February 1999 (21). One hundred eighty patients with proximal middle cerebral artery (MCA) occlusions were enrolled and treated within 6 hr of the onset of stroke. Additional inclusion criteria included a National Institutes of Health Stroke Scale score of 4 to 30 and lack of hemorrhage or early signs of infarction involving more than one-third of the MCA territory on CT scans. Exclusion criteria included rapidly improving neurologic deficit or sustained blood pressure of >180/100. Patients were randomized in a 2:1 ratio to receive either 9 mg of recombinant prourokinase infused into the MCA over 2 hr and IV administered heparin (2000 U bolus and 500 U/hr infusion for 4 hr) or heparin therapy alone. Arterial recanalization was achieved in 67% of the patients who received recombinant prourokinase and in 18% of the control patients. The primary efficacy outcome was the percentage of patients achieving a modified Rankin score of ≤2 at 90 days after therapy, which signifies slight or no disability. For this primary outcome measure, there was a 15% absolute benefit for the recombinant prourokinase group, which was significant at the 0.043 level. This represented a relative treatment benefit of 60%. Although the PROACT II Study was not powered to detect statistically significant differences in secondary outcome measures, the trends in secondary outcome measures also favored the recombinant prourokinase group. Despite an increased risk of early intracranial hemorrhage in the patients who received recombinant prourokinase (27.8% versus 5.5% within 24 hr), 90 day mortality rates were not significantly different between the two groups (24% recombinant prourokinase versus 27% control). This is the first randomized controlled trial of fibrinolytic therapy to show a statistically significant treatment benefit beyond 3 hr after stroke onset. At present, only IV administration of tissue plasminogen activator (TPA) in a narrowly defined patient population within 3 hr of symptom onset has been approved by the Food and Drug Administration (22). The PROACT Trial has, however, validated intra-arterial fibrinolytic therapy for treatment of selected acute nonhemorrhagic stroke within a 6-hr time to treatment (TTT) window.
Before PROACT II Trial results were obtained, it had been the consensus opinion of the ASITN that intra-arterial thrombolytic therapy for acute stroke was investigational. Although the results of the trial did not lead to Food and Drug Administration approval of a specific drug, the results of this trial are convincing evidence that intra-arterial thrombolytic therapy can now be considered an acceptable and appropriate therapy for acute stroke.
The ASITN maintains that use of the technique of intra-arterial thrombolysis in selected patients is appropriate and that ongoing research will better define the parameters of such intervention. Imaging findings, patient age, the magnitude of neurologic deficit, nature of the arterial occlusive lesion, and time to treatment are among the factors that should be considered when counseling patients and their families regarding the benefits and risks of intra-arterial thrombolytic therapy. The ASITN maintains that those who perform emergent cerebral thrombolysis should be well trained and experienced in cerebral angiography and should have appropriate credentials at their hospitals for doing so. These professionals should maintain records of their indications, successes, complications, and outcomes for cerebral angiography, according to the Quality Improvement Guidelines for Adult Diagnostic Neuroangiography put forth by the Cooperative Study of the American Society of Neuroradiology, the American Society of Interventional and Therapeutic Neuroradiology, and the Society of Cardiovascular and Interventional Radiology (23).
Although ideal experience and training in interventional stroke therapy are achieved in a recommended program, persons with formal training and experience might appropriately perform thrombolysis (24). The ASITN maintains that minimum standards for credentialing for cerebral angiography and thrombolytic intervention should reflect the principles espoused therein.
Comprehensive pre- and postoperative care of the patient who has suffered a stroke must be part of any interventional stroke therapy. Patient outcomes should be monitored using standardized measures, such as the National Institutes of Health Stroke Scale and Barthel and Rankin indices. Hemorrhagic complications are an unfortunate but inevitable part of fibrinolytic therapy for acute stroke. In both the PROACT II and National Institute of Neurological Disorders and Stroke IV TPA studies, the rate of intracerebral hemorrhage was significantly greater in the drug versus placebo group, yet clinical outcomes were significantly improved in those patients receiving fibrinolytic therapy. As a general guideline for acceptable complication rates, the clinical outcomes of patients receiving investigational intra-arterial therapy should not be worse than those of patients in the control groups of recent stroke trials.
Patient outcomes after cerebral infarction are variable, with some patients achieving almost full recovery and others progressing to severe neurologic deficit or death. The overall 1 month mortality rate after acute cerebral infarction was 12% in 16 series including 9738 patients (25–40). These studies included a broad spectrum of ischemic strokes, ranging from lacunae to large holo-hemispheric infarcts. Because standardized measures, such as the National Institutes of Health Stroke Scale and Barthel and Rankin indices, were not used, it is impossible to quantitate morbidity among these patients. However, the incidence of significant morbidity would probably be somewhat greater than the mortality rate. Mortality rates for patients receiving placebo in the National Institute of Neurological Disorders and Stroke and European Cooperative Stroke Study IV TPA Trials were 21% and 12.7%, respectively (1, 37). For patients with occlusion of the MCA, clinical outcomes are even worse. Saito et al (41) reported the clinical outcomes of 40 patients with angiographically proved MCA occlusions. At 3-month follow-up, 30% had died and 38% had severe deficits. Only seven (18%) patients were considered to have achieved good outcomes, and four of those patients had only small distal branch occlusions. The 27% 90 day mortality rate among the control group in the PROACT II Trial was almost identical to Saito's mortality data. Clinical outcomes of patients with basilar artery occlusion are even less favorable, with several authors reporting death among the majority of patients and severe deficits among most survivors (42–44).
Intra-arterial fibrinolytic therapy administered within the 3-hr time limit for approved use of IV TPA should not be considered to be unethical. The benefits of TPA were relatively modest in the National Institute of Neurological Disorders and Stroke Study (22). No angiography or other assessment of the vasculature was performed to assess thrombolytic efficacy. Based on analysis of a subset of the National Institute of Neurological Disorders and Stroke TPA Pilot Trial, evidence that IV TPA may be ineffective for proximal vessel occlusions was presented by Tomsick et al (45). Both baseline and 3-month follow-up National Institutes of Health Stroke Scale scores were significantly worse in cases with the hyperdense MCA sign, an indicator of proximal MCA thrombosis. An earlier dose escalation study of IV TPA, including pre- and post-treatment angiography, revealed a relatively low rate of recanalization of occluded arteries (46). The overall partial arterial recanalization rate was only 30%, and the complete recanalization rate was only 4.3%. Only partial recanalization of one (9%) occluded ICA was achieved. Partial recanalization of the proximal MCA was more frequent (24%), but total recanalization was uncommon (4.4%). Both of these reports suggest that IV TPA may be inadequate for lysis of large proximal vessel (ICA or MCA-M1) occlusions. Recanalization rates reported for intra-arterial administration of fibrinolytic agents are much higher (see table below).
Only one small randomized controlled study of intra-arterial recombinant TPA use has been reported. The Emergency Medical Services study randomized patients to treatment with IV recombinant TPA or placebo, then angiography, then intra-arterial thrombolysis (20 mg TPA up to 2 hr) if an arterial occlusive lesion was revealed (47). Fifteen patients had M1 or M2 occlusions, six in the placebo-IV group and nine in the IV recombinant TPA group. Ten of the 15 acieved Rankin 0–2 3-month outcomes (66%), five (55%) in the combined therapy group, and five (83%) in the intra-arterial-only group. Mean time to intraarterial treatment (TTT) was 4.2 hours. Although the concept of combined treatment is theoretically attractive, it may be the early TTT that promises to provide positive results suggested in this trial. Subsequent application of a similar treatment paradigm, in which IA TTT has been reduced to 3.3 hr, has led to similar results (48).
Intra-arterial fibrinolytic therapy may therefore be reasonable for patients with proximal arterial occlusions, even when these patients meet approved criteria for IV recombinant TPA therapy. Triage with MR imaging, CT angiography, transcranial Doppler ultrasonography, technetium-hexamethylpropyleneamineoxime isotope scanning, and even angiography may be useful for select patients who may be less likely to benefit from IV recombinant TPA therapy.
Except for data reported from the PROACT Study, it is very difficult to compile a database for efficacy and complications from other reported series because of the wide variability in the patient populations being treated and the technical factors of the procedures. Patient variables include patient age, vessels involved, volume of thrombus, presenting neurologic deficit (National Institutes of Health Stroke Scale score), concurrent medical problems, available collateral flow, and duration of time from symptom onset to lytic therapy. Technical variables are equally important, including lytic agent, method of administration (infusion, pulse spray location of the catheter tip [within clot, distal to clot, proximal to clot]), and whether mechanical disruption of the clot was performed. Reported outcomes of treatment usually include the technical ability to lyse the clot and perfuse the occluded territory and the patient's clinical outcome. Very few authors have used objective clinical evaluation criteria to measure outcome, such as the National Institutes of Health Stroke Scale and Barthel and Rankin indices.
Criteria for technical success and for failure and complication rates may be categorized into three vascular territories: 1) MCA, 2) vertebrobasilar system, and 3) ICA. This stratification is used to create relevant tables while providing greater specificity regarding efficacy and complication rates for the most commonly treated territories. The values presented in the three tables below were compiled from cited references (2–21, 49–55).
The following relative contraindications to cerebral fibrinolytic therapy have been described: intracerebral hemorrhage, recent surgery, extracerebral hemorrhage, recent arterial puncture or cervical venous puncture, excessive time between onset of symptoms and initiation and completion of lytic therapy, significant edema and swelling revealed by pretreatment CT, absence of visible collateral vascular supply to a large region on pretreatment angiograms, and absence of identifiable arterial thrombus or occlusion within an arterial territory consistent with the patient's clinical symptoms.
Standards for acceptable complication rates from the diagnostic angiographic portion of the procedure are listed elsewhere. As additional data from the PROACT II Trial and other trials are made available, standards of practice for thrombolytic therapy will be developed (56, 57). At present, it is impossible to define rigid standards for thresholds for appropriateness, success rate, and complications from intra-arterial fibrinolytic therapy. As a reasonable guideline for practice, intra-arterial fibrinolytic therapy performed with significant divergence from the protocol used in the PROACT II Trial might be considered investigational and subject to approval of the appropriate institutional review board.
Efficacy
 |
Safety
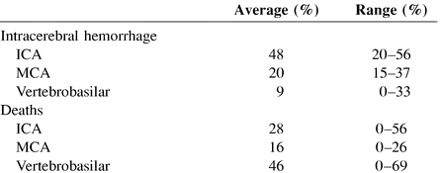 |
The average and range percentages are compiled from the available data in the articles listed as references.
References
- 1.Zeumer H, Hacke W, Ringelstein EB, Local intraarterial thrombolysis in vertebrobasilar thromboembolic disease. AJNR Am J Neuroradiol 1983;4:401-404 [PMC free article] [PubMed] [Google Scholar]
- 2.Mori E, Tabuchi M, Yoshida T, Yamadori A, Intracarotid urokinase with thromboembolic occlusion of the middle cerebral artery. Stroke 1988;19:802-812 [DOI] [PubMed] [Google Scholar]
- 3.del Zoppo G, Ferbert A, Otis S, Local intra-arterial fibrinolytic therapy in acute carotid territory stroke: a pilot study. Stroke 1988;19:307-313 [DOI] [PubMed] [Google Scholar]
- 4.Gonner F, Remonda L, Mattle H, Local intra-arterial thrombolysis in acute ischemic stroke. Stroke 1998;29:1894-1900 [DOI] [PubMed] [Google Scholar]
- 5.Zeumer H, Freitag HJ, Zanella F, Thie A, Arning C, Local intra-arterial fibrinolytic therapy in patients with stroke: urokinase versus recombinant tissue plasminogen activator (r-TPA). Neuroradiology 1993;35:159-162 [DOI] [PubMed] [Google Scholar]
- 6.Freitag HJ, Becker VU, Thie A, Lys-plasminogen as an adjunct to local intra-arterial fibrinolysis for carotid territory stroke: laboratory and clinical findings. Neuroradiology 1996;38:181-185 [DOI] [PubMed] [Google Scholar]
- 7.Barnwell SL, Clark WM, Nguyen TT, O'Neill OR, Wynn ML, Coull BM, Safety and efficacy of delayed intraarterial urokinase therapy with mechanical clot disruption for thromboembolic stroke. AJNR Am J Neuroradiol 1994;15:1817-1822 [PMC free article] [PubMed] [Google Scholar]
- 8.Ueda T, Hatakeyama T, Kumon Y, Sakaki S, Uraoka T, Evaluation of risk of hemorrhagic transformation in local intra-arterial thrombolysis in acute ischemic stroke by initial SPECT. Stroke 1994;25:298-303 [DOI] [PubMed] [Google Scholar]
- 9.Sasaki O, Takeuchi S, Koike T, Koizumi T, Tanaka R, Fibrinolytic therapy for acute embolic stroke: intravenous, intracarotid, and intra-arterial local approaches. Neurosurgery 1995;36:246-253 [DOI] [PubMed] [Google Scholar]
- 10.Jansen O, von Kummer R, Forsting M, Hacke W, Sartor K, Thrombolytic therapy in acute occlusion of the intracranial internal carotid artery bifurcation. AJNR Am J Neuroradiol 1995;16:1977-1986 [PMC free article] [PubMed] [Google Scholar]
- 11.Lanzieri CF, Tarr RW, Landis D, Cost-effectiveness of emergency intraarterial intracerebral thrombolysis: a pilot study. AJNR Am J Neuroradiol 1995;16:1987-1993 [PMC free article] [PubMed] [Google Scholar]
- 12.Brandt T, von Kummer R, Muller-Kuppers M, Hacke W, Thrombolytic therapy of acute basilar artery occlusion: variables affecting recanalization and outcome. Stroke 1996;27:875-881 [DOI] [PubMed] [Google Scholar]
- 13.Bendszus M, Urbach H, Ries F, Solymosi L, Outcome after local intra-arterial fibrinolysis compared with the natural course of patients with a dense middle cerebral artery on early CT. Neuroradiology 1998;40:54-58 [DOI] [PubMed] [Google Scholar]
- 14.Picard L, Bracard S, Maffei L, Selective intra-arterial thrombolysis for embolic complications of cerebral endovascular therapy. Intervent Neuroradiol 1996;2:263-269 [DOI] [PubMed] [Google Scholar]
- 15.Casto L, Caverni L, Camerlingo M, Intra-arterial thrombolysis in acute ischaemic stroke: experience with superselective catheter embedded in the clot. J Neurol Neurosurg Psychiatry 1996;60:667-670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nesbit GM, Clark WM, O'Neill OR, Barnwell SL, Intracranial intraarterial thrombolysis facilitated by microcatheter navigation through an occluded cervical internal carotid artery. J Neurosurg 1996;84:387-392 [DOI] [PubMed] [Google Scholar]
- 17.Theron J, Coskun O, Huet H, Oliveira G, Toulas P, Payella G, Local intraarterial thrombolysis in the carotid territory. Intervent Neuroradiol 1996;2:111-126 [DOI] [PubMed] [Google Scholar]
- 18.Yokogami K, Nakano S, Ohta H, Goya T, Wakisaka S, Prediction of hemorrhagic complications after thrombolytic therapy for middle cerebral artery occlusion: value of pre- and post-therapeutic computed tomographic findings and angiographic occlusive site. Neurosurgery 1996;39:1102-1107 [DOI] [PubMed] [Google Scholar]
- 19.Barr JD, Mathis JM, Wildehain SL, Wechsler L, Jungreis CA, Horton JA, Acute stroke intervention with intraarterial urokinase infusion. J Vasc Interv Radiol 1994;5:705-713 [DOI] [PubMed] [Google Scholar]
- 20.Suarez J, Sunshine J, Tarr R, Predictors of clinical improvement, angiographic recanalization, and intracranial hemorrhage after intra-arterial thrombolysis for acute ischemic stroke. Stroke 1999;30:2094-2100 [DOI] [PubMed] [Google Scholar]
- 21.Furlan A, Higashida R, Weschler L, Intra-arterial prourokinase for acute ischemic stroke: The PROACT II Study: a randomized controlled trial. JAMA 1999;282:2003-2011 [DOI] [PubMed] [Google Scholar]
- 22. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group Tissue plasminogen activator for acute ischemic stroke. N Engl J Med 1995;333:1581-1587 [DOI] [PubMed] [Google Scholar]
- 23. Cooperative Study between the ASNR, ASITN and SCVIR Quality improvement guidelines for adult diagnostic neuroangiography. AJNR Am J Neuroradiol 2000;21:146-150 [PMC free article] [PubMed] [Google Scholar]
- 24.Higashida RT, Hopkins LN, Berenstein A, Halbach VV, Kerber C, Program requirements for residency/fellowship education in neuroendovascular surgery/interventional neuroradiology: a special report on graduate medical education. AJNR Am J Neuroradiol 2000;21:1153-1159 [PMC free article] [PubMed] [Google Scholar]
- 25.Foulkes MA, Wolf PA, Price TR, Mohr JP, Hier DB, The Stroke Data Bank: design, methods, and baseline characteristics. Stroke 1988;19:547-554 [DOI] [PubMed] [Google Scholar]
- 26.Howard G, Toole JF, Becker C, Changes in survival following stroke in five North Carolina counties observed during two different periods. Stroke 1989;20:345-350 [DOI] [PubMed] [Google Scholar]
- 27.Ricci S, Celani MG, Guercini G, First-year results of a community-based study of stroke incidence in Umbria, Italy. Stroke 1989;20:853-857 [DOI] [PubMed] [Google Scholar]
- 28.Friday G, Lai SM, Alter M, Stroke in the Lehigh Valley: racial/ethnic differences. Neurology 1989;39:1165-1168 [DOI] [PubMed] [Google Scholar]
- 29.Kojima S, Omura T, Wakamatsu W, Prognosis and disability of stroke patients after 5 years in Akita, Japan. Stroke 1990;21:72-77 [DOI] [PubMed] [Google Scholar]
- 30.Martinez-Vila E, Guillen F, Villanueva JA, Placebo-controlled trial of nimodipine in the treatment of acute ischemic cerebral infarction. Stroke 1990;21:1023-1028 [DOI] [PubMed] [Google Scholar]
- 31.Sacco RL, Hauser WA, Mohr JP, Foulkes MA, One-year outcome after cerebral infarction in whites, blacks, and Hispanics. Stroke 1991;22:305-311 [DOI] [PubMed] [Google Scholar]
- 32.Candelise L, Pinardi G, Morabito A, and the Italian Acute Stroke Study Group Mortality in acute stroke with atrial fibrillation. Stroke 1991;22:169-174 [DOI] [PubMed] [Google Scholar]
- 33.Brainin M, Czvitkovits M, Seiser A, Pauley E, Stroke subtype is an age-independent predictor of early and late stroke mortality: the Klosterneuburg Stroke Data Bank. J Neurol 1991;vol:238–113
- 34.Monaco P, Pastore L, Cottone S, Conti S, Belinvia S, Effect of early treatment with GM1 on neurological impairment during ischemic stroke. J Neurol 1991;vol:238–120
- 35.Bamford J, Sandercock P, Dennis M, Burn J, Warlow C, Classification and natural history of clinically identifiable subtypes of cerebral infarction. Lancet 1991;337:1521-1526 [DOI] [PubMed] [Google Scholar]
- 36.D'Alessandro G, Di Giovanni M, Roveyaz L, Incidence and prognosis of stroke in the Valle d'Aosta, Italy: first-year results of a community-based study. Stroke 1992;23:1712-1715 [DOI] [PubMed] [Google Scholar]
- 37.Dennis MS, Burn JP, Sandercock PA, Bamford JM, Wade DT, Warlow CP, Long-term survival after first-ever stroke: the Oxfordshire Community Stroke Project. Stroke 1993;24:796-800 [DOI] [PubMed] [Google Scholar]
- 38.Censori B, Camerlingo M, Casto L, Prognostic factors in first-ever stroke in the carotid artery territory seen within 6 hours after onset. Stroke 1993;24:532-535 [DOI] [PubMed] [Google Scholar]
- 39.Sarti C, Tuomilehto J, Sivenius J, Stroke mortality and case-fatality rates in three geographic areas of Finland from 1983 to 1986. Stroke 1993;24:1140-1147 [DOI] [PubMed] [Google Scholar]
- 40.Hacke W, Kaste M, Fieschi C, Intravenous thrombolysis with recombinant tissue plasminogen activator for treatment of acute hemispheric stroke: The European Cooperative Acute Stroke Study. JAMA 1995;274:1017-1025 [PubMed] [Google Scholar]
- 41.Saito I, Segawa H, Shiokawa Y, Taniguchi M, Tsutsumi K, Middle cerebral artery occlusion: correlation of computed tomography and angiography with clinical outcome. Stroke 1987;18:863-868 [DOI] [PubMed] [Google Scholar]
- 42.Moscow NP, Newton TH, Angiographic implications in diagnosis and prognosis of basilar artery occlusion. Am J Roentgenol Radium Ther Nucl Med 1973;119:597-604 [DOI] [PubMed] [Google Scholar]
- 43.Thompson JR, Simmons CR, Hasso AN, Hinshaw DB Jr, Occlusion of the intradural vertebasilar artery. Neuroradiology 1978;14:219-229 [DOI] [PubMed] [Google Scholar]
- 44.Kubik CS, Adams RD, Occlusion of the basilar artery: clinical and pathological study. Brain 1946;69:73-121 [DOI] [PubMed] [Google Scholar]
- 45.Tomsick T, Barsan W, Brott T, Prognostic value of the hyperdense middle cerebral artery sign and stroke scale score before ultraearly thrombolytic therapy. AJNR Am J Neuroradiol 1996;17:79-85 [PMC free article] [PubMed] [Google Scholar]
- 46.Wolpert SM, Bruckmann H, Greenlee R, Neuroradiologic evaluation of patients with acute stroke treated with recombinant tissue plasminogen activator. AJNR Am J Neuroradiol 1993;14:3-13 [PMC free article] [PubMed] [Google Scholar]
- 47.Lewandowski C, Frankel M, Tomsick T, Combined intravenous and intra-arterial r-TPA versus intra-arterial therapy of acute Ischemic stroke: Emergency Management of Stroke (EMS) Bridging Trial. Stroke 1999;30:2598-2605 [DOI] [PubMed] [Google Scholar]
- 48.Ernst RT, Pancioli A, Tomsick TA, Combined intravenous and intra-arterial recombinant tissue plasminogen activator in acute ischemic stroke. Stroke 2000;31:2552-2557 [DOI] [PubMed] [Google Scholar]
- 49.Smalling R, Fuentes F, Matthews M, Sustained improvement in left ventricular function and mortality by intracoronary streptokinase administration during evolving myocardial infarction. Circulation 1983;68:131-138 [DOI] [PubMed] [Google Scholar]
- 50.Khaja F, Walton J, Brymer J, Intracoronary fibrinolytic therapy in acute myocardial infarction: report of a prospective randomized trial. N Engl J Med 1983;308:1305-1311 [DOI] [PubMed] [Google Scholar]
- 51.Rogers WJ, Mantle JA, Hood WP Jr, Prospective randomized trial of intravenous and intracoronary streptokinase in acute myocardial infarction. Circulation 1983;68:1051-1061 [DOI] [PubMed] [Google Scholar]
- 52.Tennant SN, Dixon J, Venable TC, Intracoronary thrombolysis in patients with acute myocardial infarction: comparison of the efficacy of urokinase with streptokinase. Circulation 1984;69:756-760 [DOI] [PubMed] [Google Scholar]
- 53.Kennedy J, Ritchie J, Davis K, Stadius M, Maynard C, Fritz J, The western Washington randomized trial of intracoronary streptokinase in acute myocardial infarction: a 12-month follow-up report. N Engl J Med 1985;312:1073-1078 [DOI] [PubMed] [Google Scholar]
- 54.Kambara H, Kawai C, Kajiwara N, Randomized, double-blinded multicenter study: comparison of intracoronary single-chain urokinase-type plasminogen activator, pro-urokinase (GE-0943), and intracoronary urokinase in patients with acute myocardial infarction. Circulation 1988;78:899-905 [DOI] [PubMed] [Google Scholar]
- 55.Natarajan D, Rai VN, Jain A, Roy T, Sharma PK, Nigam PD, Intracoronary versus intravenous streptokinase in acute myocardial infarction. Int J Cardiol 1985;19:181-189 [DOI] [PubMed] [Google Scholar]
- 56. Emergency interventional stroke therapy: a statement from the American Society of Neuroradiology and the Society of Cardiovascular and Interventional Radiology. Am J Neuroradiol AJNR 2001;22:54. [PMC free article] [PubMed] [Google Scholar]
- 57. Executive Committee of the ASITN Intraarterial thrombolysis: ready for prime time?. Am J Neuroradiol AJNR 2001;22:55-58 [PMC free article] [PubMed] [Google Scholar]


