Introduction
Subarachnoid hemorrhage-induced vasospasm is a well-recognized complication of aneurysm rupture and accounts for significant morbidity and mortality. The development and severity of vasospasm seems to be related to the amount of blood surrounding the vessel and the duration of exposure, indicating that some erythrocyte component, most likely hemoglobin, is ultimately responsible for its appearance. A variety of microscopic changes have been noted, including edema and thickening of the intima and media, necrosis and proliferation of the media, and adventitial inflammation resulting in luminal narrowing and rigidity of the vessel wall.
The development of vasospasm can be recognized by transcranial Doppler ultrasonographic evaluation, cerebral blood flow studies, and/or changes in the patient's neurologic examination results. Once detected, several therapeutic measures can be used to prevent or counteract vasospasm and its clinical sequelae, including medical treatment (eg, hypertensive, hypervolemic therapy) and pharmacologic regimens (eg, nimodipine). When these methods fail, timely application of endovascular procedures such as balloon angioplasty and intra-arterial infusion of papaverine may prove useful in reversing the effects of vasospasm.
The following guidelines are intended for use in quality improvement programs to evaluate the endovascular treatment of vasospasm. Assessment guidelines include indications for treatment, success rates for achieving treatment goals, and complication rates.
Discussion
The goal of endovascular therapy is the reestablishment of normal or nearly normal vascular caliber in an effort to enhance cerebral blood flow to ischemic tissue. Treatment of vasospasm by endovascular means falls into two categories: mechanical dilation of stenotic segments by compliant balloon angioplasty and chemical relaxation of the contracted vessel wall by the intra-arterial administration of drugs such as papaverine, which is a known smooth muscle relaxant. The procedure is performed in conjunction with diagnostic angiography, which confirms the clinical suspicion of vasospasm. Because vasospasm may involve more than one vascular territory, it is imperative that the angiographic findings correlate well with the clinical symptoms before treatment is undertaken. Patient selection may vary from institution to institution, but every effort should be made to evaluate and treat patients quickly, once a short course of medical or pharmacologic therapy has failed.
A variety of balloon angioplasty catheters and custom systems are available, but the most appropriate devices use compliant balloons, which conform to the shape of the vessel, making overdistension and rupture less likely. Some systems are introduced coaxially over a microguidewire, which can help navigate difficult turns but may carry a higher risk of vessel perforation or spasm.
Papaverine may be delivered close to the target vessel via an end-hole microcatheter, or more proximally into the cervical ICA via a diagnostic catheter. Papaverine has been generally efficacious in increasing lumen dimension, decreasing circulation time, and increasing cerebral blood flow. Papaverine often has a transient effect, and repeated treatments may be necessary.
Indications
Endovascular treatment of vasospasm is indicated for patients experiencing ischemia secondary to reduced cerebral blood flow, with angiographic evidence of vasospasm, for whom an appropriate trial of medical and/or pharmacologic therapy has failed.
Spastic vessels considered appropriate for balloon angioplasty are those that are ≥1.5 mm in diameter. Typical locations include the intracranial ICA, A1 and M1 segments, the distal vertebral arteries, basilar arteries, and P1 segments. Smaller vessels are prone to injury or rupture and should not be treated by this method. Balloon angioplasty is essentially permanent, and only in rare cases will subsequent treatment be necessary. Papaverine may also be used in these larger vessels, but it is particularly well suited for more distal territories.
Indicator thresholds may be used to assess the effectiveness and safety of therapy in a quality improvement program. Results that fall outside the threshold limits are used to prompt a procedural review to determine the cause of the problem and prompt recommendations for correction.
Threshold for endovascular therapy of vasospasm:
100%. When mechanical or chemical vasolytic therapy is administered to vessels that do not show vasospasm, a review should be conducted. Similarly, when treatment is undertaken of a spastic vessel in an asymptomatic patient, a review should be conducted.
Efficacy
The indicators of efficacy are technical and clinical success. Technical success is defined as angiographic evidence of improved vascular caliber approximating expected normal size and improved cerebral circulation time. Clinical success is defined as stabilization or improvement in the patient's neurologic status, usually measured as a ≥2-point increase in the Glasgow Coma Scale score.
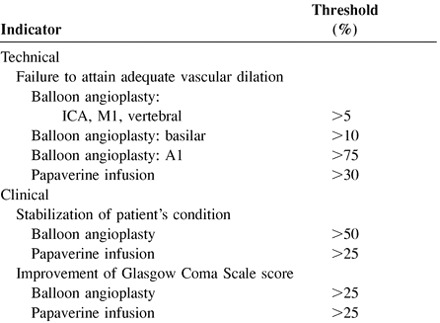 |
Safety
Undesirable outcomes and effects are known to occur in association with both mechanical and chemical vasolytic therapy. Technical complications may be classified as minor or severe and may result in transient or permanent neurologic sequelae. The potential complications and their rates vary between balloon angioplasty and papaverine infusion. Treatment of a vessel harboring an unsecured, ruptured aneurysm may lead to aneurysm rupture. Treatment of a large vascular territory that is obviously infarcted is avoided if possible.
Operators using the more proximal ICA infusion technique below the ophthalmic artery must be aware of pupillary dilation, which may occur and mimic neurologic decompensation. Papaverine infusion below the posterior-inferior communicating artery may result in respiratory depression and is not recommended unless ventilatory support is available. Similarly, reports of papaverine infusion causing increased intracranial pressure have been noted, and monitoring of intracranial pressure devices, when available, is recommended.
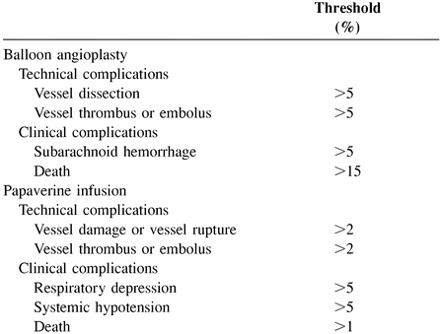 |
Bibliography
- Barr JD, Mathis JM, Horton JA, Severe transient brain stem depression during intraarterial papaverine infusion for cerebral vasospasm. AJNR Am J Neuroradiol 1994;15:719-723 [PMC free article] [PubMed] [Google Scholar]
- Clouston JE, Numaguchi Y, Zoarski GH, Aldrich EF, Simard JM, Zitnay KM, Intraarterial papaverine infusion for cerebral vasospasm after subarachnoid hemorrhage. AJNR Am J Neuroradiol 1995;16:27-38 [PMC free article] [PubMed] [Google Scholar]
- Coyne TJ, Montanera WJ, Macdonald RL, Wallace MC, Percutaneous transluminal angioplasty for cerebral vasospasm after subarachnoid hemorrhage. Can J Surg 1994;37:391-396 [PubMed] [Google Scholar]
- Eskridge JM, McAuliffe W, Song JK, Balloon angioplasty for the treatment of vasospasm: results of first 50 cases. Neurosurgery 1998;42:510-517 [DOI] [PubMed] [Google Scholar]
- Hendrix LE, Dion JE, Jensen ME, Phillips CD, Newman SA, Papaverine-induced mydriasis. AJNR Am J Neuroradiol 1994;15:716-718 [PMC free article] [PubMed] [Google Scholar]
- Higashida RT, Halbach VV, Cahan LD, Transluminal angioplasty for treatment of intracranial arterial vasospasm. J Neurosurg 1989;71:648-653 [DOI] [PubMed] [Google Scholar]
- Kaku Y, Yonekawa Y, Tsukahara T, Kazekawa K, Superselective intra-arterial infusion of papaverine for the treatment of cerebral vasospasm after subarachnoid hemorrhage. J Neurosurg 1992;77:842-847 [DOI] [PubMed] [Google Scholar]
- Kassell NF, Helm G, Simmons N, Phillips CD, Cail WS, Treatment of cerebral vasospasm with intra-arterial papaverine. J Neurosurg 1992;77:848-852 [DOI] [PubMed] [Google Scholar]
- Marks MP, Steinberg GK, Lane B, Intra-arterial papaverine for the treatment of vasospasm. AJNR Am J Neuroradiol 1993;14:822-826 [PMC free article] [PubMed] [Google Scholar]
- Mathis JM, DeNardo A, Jensen ME, Scott J, Dion JE, Transient neurologic events associated with intraarterial papaverine infusion for subarachnoid hemorrhage-induced vasospasm. AJNR Am J Neuroradiol 1994;15:1671-1674 [PMC free article] [PubMed] [Google Scholar]
- McAuliffe W, Townsend M, Eskridge JM, Newell DW, Grady MS, Winn HR, Intracranial pressure changes induced during papaverine infusion for treatment of vasospasm. J Neurosurg 1995;83:430-434 [DOI] [PubMed] [Google Scholar]
- Milburn JM, Moran CJ, Cross DT III, Diringer MN, Pilgram TK, Dacey RG Jr, Increase in diameters of vasospastic intracranial arteries by intraarterial papaverine administration. J Neurosurg 1998;88:38-42 [DOI] [PubMed] [Google Scholar]
- Newell DW, Eskridge JM, Mayberg MR, Grady MS, Winn HR, Angioplasty for the treatment of symptomatic vasospasm following subarachnoid hemorrhage. J Neurosurg 1989;71:654-660 [DOI] [PubMed] [Google Scholar]
- Vajkoczy P, Horn P, Bauhuf C, Effect of intra-arterial papaverine on regional cerebral blood flow in hemodynamically relevant cerebral vasospasm. Stroke 2001;32:498-505 [DOI] [PubMed] [Google Scholar]
- Zubkov YN, Nikiforov BM, Shustin VA, Balloon catheter technique for dilatation of constricted cerebral arteries after aneurysmal SAH. Acta Neurochir (Wien) 1984;70:65-79 [DOI] [PubMed] [Google Scholar]


