Abstract
Crustacean hyperglycemic hormones (CHHs) are a family of neuropeptides that were discovered in multiple tissues in crustaceans, but the function of most isoforms remains unclear. Functional discovery often requires comprehensive qualitative profiling and quantitative analysis. The conventional enzymatic digestion method has several limitations, such as missing posttranslational modification (PTM) information, homology interference and incomplete sequence coverage. Herein, by using a targeted top-down method, facilitated by higher sensitivity instruments and hybrid fragmentation modes, we achieved the characterization of two CHH isoforms from the sinus glands (SG-CHH) and the pericardial organs (PO-CHH) from the Atlantic blue crab, Callinectes sapidus, with improved sequence coverage compared to earlier studies. In this study, both label-free and isotopic labeling approaches were adopted to monitor the response of CHHs and CHH precursor-related peptide (CPRP) under low pH stress. The identical trends of CPRP and CHH expression indicated that CPRP could serve as an ideal probe in tracking the CHH expression level changes, which would greatly simplify the quantitative analysis of large peptides. Furthermore, the distinct patterns of changes in the expression of CHHs in the SG and the PO suggested their tissue-specific functions in the regulation of low pH stress. Ion mobility-mass spectrometry (IM-MS) was also employed in this study to provide conformation analysis of both CHHs and CPRPs from different tissues.
Keywords: Top-Down MS analysis, Neuropeptides, crustacean hyperglycemic hormone, CHH precursor-related peptide, peptide quantitation, ion mobility MS
Graphical Abstract
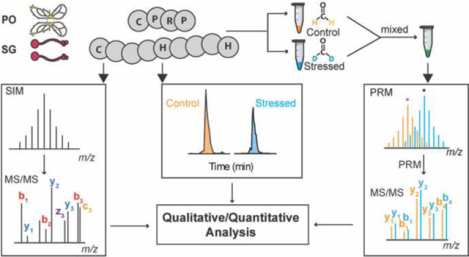
INTRODUCTION
Neuropeptides are endogenous signaling molecules that broadly participate in the regulation of numerous physiological process.1,2–5 The biosynthesis process usually begins with mRNA of neuropeptide gene being translated into preprohormones, which is followed by a series of enzymatic cleavages, producing signaling peptides, precursor-related peptides and mature, biologically active neuropeptides.6 Neuropeptides within the same family share similar structures and highly conserved sequence motifs,7,8 and some homologous neuropeptides are universal throughout various species.9 The decapod crustacean has been utilized extensively in neuropeptidomic studies in our group, focusing on the peptide profiling10–12 and stress-related function discovery13–16.
The crustacean hyperglycemic hormone (CHH) superfamily consists of a group of neuropeptides involved in metabolic control, energy homeostasis, molting, osmotic regulation and various biological activities.17–28 The superfamily can be divided into two distinct sub-groups, on the basis of their precursor and primary structures: type-1, CHH/ITP and type-2 MIH/MOIH/VIH/GIH.18,25 One of the major differences between the two subtypes is that preprohormones of type-1 peptides contain a CHH precursor related peptide (CPRP), which is absent in that of type-2 peptides.25,29 The hyperglycemic activity, which is only observed in type-1 peptides, is another aspect to differentiate the two categories.17,18,25
The CHH peptides (type-1 in CHH superfamily) are normally comprised of around 72 amino acids, containing three intramolecular disulfide bonds formed between six conserved cysteines22,30. Their hyperglycemic function was first reported in 1944, as a diabetogenic factor in the eyestalk of Atlantic blue crab, Callinectes sapidus and the sand fiddler crab, Uca pugilator.31 The first CHH structure was identified in the shore crab, Carcinus maenas, eyestalks in 1989.32 The characterization of the CHH peptides is always challenging due to the large molecular size, low concentration in vivo17,27 and PTM complexity.33,34,35 Edman degradation or the cDNA cloning strategies have been well-established to isolate and identify multiple CHH isoforms in various crustacean species.21,36–42 In recent decades, mass spectrometry (MS) has become an essential and powerful approach in neuropeptidome studies and has been widely adopted in previous CHH analyses43–45. With the help of high sensitivity and high-resolution instruments, accurate mass and sequence information of neuropeptides could be obtained from a small amount of sample. In addition, the development of electrospray ionization (ESI) broadened the detection mass range by generating multiply charged ions, which highly benefits the study of CHHs. Enzymatic digestion followed by LC-tandem MS bottom-up analysis generates detailed sequence information about pieces of such large peptides, which would further contribute to database matching32,37. However, the classical tryptic-digested method often causes problems such as incomplete coverage and missing PTM identification.46 Additionally, tryptic peptides from highly homologous peptides introduces interference when being assigned to the theoretical sequences. Top-down analysis would be an alternative solution where intact peptides/proteins are detected and fragmented.47–50Although this method can be informative for characterizing primary structure and modifications of intact molecules, it is also limited by difficulties in producing extensive gas-phase fragmentation ions, especially for those containing disulfide bonds. Usually, both bottom-up and top-down analysis are employed as complementary approaches to result in better coverage of the CHH peptides35,51.
Initial reports about the CHH peptides were focused on its occurrence in eyestalks, as well as its physiological function in glucose homeostasis.26,52,53 The studies demonstrated the discovery of CHH isoforms has been further extended to multiple extra-eyestalk regions, such as guts, pericardial organs, thoracic ganglia, etc.23,36,42,43,45,54,55 These CHH isoforms, although homologous, differ slightly from each other in their sequences, giving rise to diverse functional roles in physiological modulation and stress-induced regulation; for example, the abundance of CHH in blue crab eyestalk and thoracic ganglia showed different expression patterns throughout the molt cycle.42
CHH precursor-related peptide (CPRP) is C-terminally flanked by CHH in preprohormone.29,38,39,41 After being enzymatically cleaved, CHH and CPRP were co-localized in neural tissues23 and then co-released into hemolymph upon stimuli.43,56 Comparing to CHH, the CPRP exhibits a smaller molecular size, usually consisting of 33–38 amino acids, which is more facile to fragmentation. It is a circulating neurohormone in the crab; however, its function still remains unknown.56
Low pH stress is one of the most common stressors that crustaceans encounter due to their living habitat variation. The Atlantic blue crab, Callinectes sapidus, has been reported to be highly influenced when being exposed to an acidified environment57–59. However, responses of locomotive activities and body fluid pH in the blue crab were observed to exhibit a delay of about 2hrs from the initial exposure to the CO2 treatment58,59. The expression level changes of neuropeptides in the blue crab after 2hr incubation in acidified environment have been investigated to reveal potential neuropeptide players involved in modulation of the animal’s adaptation to acidification stimulus16. However, most of the reported neuropeptides were under 2 kDa. The function of CHHs in the low pH stress related regulation remains uncovered.
In this study, we applied a hybrid fragmentation method in targeted top-down MS analysis to enhance the profiling coverage and minimize experiment time. The expression levels of CHH and CPRP were quantified under low pH stress, contributing to the first report on the tissue-specific functional correlation between CHH and its precursor-related peptide.
METHODS
See Supporting Information for details about chemicals and materials, animal dissections, stress experiment setup, tissue extraction, reductive dimethylation and offline fractionation.
Briefly, pericardial organ and sinus glands were dissected out from the Atlantic blue crab, Callinectes sapidus.60 Neuropeptide extraction was performed using acidified methanol to precipitate large proteins. High pH RP-HPLC was applied to isolate the CHHs and CPRPs. In the stress-related study, CO2 gas was bubbled into the saltwater tank to acidify the water from normal (pH=8.3) to a moderate pH level (7.6–7.8) and air saturation 50% – 60%. The incubation period in low pH environment was 2 hours. Tissues collected from stressed and control animals were subjected to reductive dimethylation for further quantitation analysis.
Mass Spectrometry Analysis
For CHH sequence characterization, tissue extract was reduced by 5 mM dithiothreitol for 1 hour at room temperature and was immediately desalted using C18 Ziptip pipette tips, eluted into 10 μl 50/50/0.2 ACN/H2O/FA (v/v/v). The samples were always prepared fresh before analysis to avoid the re-formation of disulfide bonds. Top-down MS analysis was performed on an Orbitrap Fusion Lumos Tribrid mass spectrometer (Thermo Fisher Scientific) interfaced with a Dionex Ultimate 3000 UPLC system (Thermo Fisher Scientific). 2 μl purified sample was loaded onto a self-packed column (150 mm length of 1.7 μm C18 with a 3 μm C18 cap) for online separation. Mobile phase A was 0.1% formic acid in water and mobile phase B was 0.1% formic acid in ACN. A 120 min gradient was used at flow rate of 0.3 μl/min, starting from 97% A and 3% B, increasing to 10% B at 5 min, 55% B at 90 min, 95% B at 92 min (and remaining for 10 min) and then dropping back to 0% at 105 min. The MS/MS analysis was performed in selected ion mode with an inclusion list containing the mass of CHHs, which are selectively fragmented with the following settings selected ion mode (SIM), MS1 resolution, 120 000; Injection time, 250 ms; AGC target, 3e6; isolation window, m/z 6.0; dd-MS2, resolution, 60 000; Injection time, 250 ms; AGC target, 1e6. A set of collision energy parameters used in this experiment was listed in Figure S2.
For label free quantitation, tissue extract of control and stressed groups were desalted using C18 Ziptip pipette tips, dried down and resuspended in 10 μl 0.1% FA in water. The purified samples were subjected to online separation with a Waters nano-Acquity Ultra Performance LC system equipped with a self-packed column (same as described earlier) coupled to a Q-Exactive quadrupole Orbitrap mass spectrometer (Thermo Fisher Scientific, Bremen, Germany). LC gradient was the same as described above. The MS/MS analysis was performed in selected ion mode (SIM) with an inclusion list containing the mass of CHHs and CPRPs, which are selectively fragmented with the following settings: SIM, isolation window, m/z 6.0; resolution, resolution, 70 000; Injection time, 100 ms; AGC target, 3e6; dd-MS2, resolution, 35 000; Injection time, 100 ms; AGC target, 1e6; normalized collision energy, 30.
For the quantitation analysis using isotopic labeling strategy, labeled tissue extract mixture from low pH stress experiment was analyzed by top-down MS method on Q Exactive system in PRM mode. LC setup was identical as earlier section. The MS/MS analysis was performed in parallel reaction monitor (PRM) mode with an inclusion list containing the mass of CHHs, which are selectively fragmented with the following settings: resolution, 70 000; Injection time, 250 ms; AGC target, 1e6; isolation window, m/z 15.0; PRM, Injection time, 100 ms; AGC target, 5e5; normalized collision energy, 30. isolation window was increased here in order to select both heavy- and light-labeled peptides for fragmentation and further quantitation.
Offline HPLC fractions containing the CHHs and CPRPs were isolated and desalted before direct injection onto the Waters Synapt G2 Ion Mobility-Mass Spectrometer (IM-MS). Approximately 5 μL of each sample was loaded onto a home-made nanospray ion source, and a silver wire of 100 μm thickness was inserted into the borosilicate glass needle for high voltage application. Buffer of 10 mM NH4OAc was used. The MS instrument was run in positive ion mode. Nanospray voltages ranged between 1.0–2.0 kV and the sampling cone was used at 30 V. The MS cone temperature was 75 °C. In typical nanospray experiments, the size of the spray emitter was maintained at ~ 5 μm. The emitters were pulled from borosilicate glass capillaries using a P-2000 laser-based micropipette puller (Sutter Instruments, Novato, CA, USA). All IM-MS data were collected using Waters Synapt G2 instrument (Waters Corp., Manchester, UK) and analyzed in Waters MassLynx V4.1 (Waters Corp., Manchester, UK).
Data Processing
For sequence profiling, averaged tandem mass spectra were deconvoluted by Xtract CI-3.0 Software (Thermo Scientific Inc., Bremen, Germany), using S/N threshold at 1.5 and fit factor of 40%. The resultant spectra were assigned with b/y and c/z ions within 10 ppm error tolerance. Cas-SG-CHH contains N-terminus pyro-glutamate and C-terminus amidation. Cas-PO-CHH contains N-terminus pyro-glutamate.
For reductive dimethyl labeling, stress-to-control ratio were calculated out of the intensities of quantitative peak pairs in MS2 spectra acquired from PRM method. Three biological replicate experiments were carried out and each bio-replicate were repeated for three technical replicates. The quantitative results were evaluated by Student’s t-test. The p-value < 0.05 represents the statistical significance.
For label-free quantitation, XIC peak areas of most abundant charge states of CHHs and CPRPs, which were determined in “sequence profiling” panel, were added up, respectively. Four biological replicate experiments were carried out for Cas-PO-CHH and five for Cas-SG-CHH. Each bio-replicate was repeated for two technical replicates. The quantitative results were evaluated by Student’s t-test. The p-value < 0.05 represents the statistical significance.
RESULTS AND DISCUSSION
Sequence Profiling of SG-CHH and PO-CHH
The eyestalks were the first source where the existence of a factor that caused hyperglycemia in the crustacean was identified.31 The injection of eyestalk extracts back into the crabs resulted in elevation of blood sugar level. Furthermore, the sinus gland (SG) extract increased the in vivo sugar level almost 3-fold higher than the rise induced by SG-removed eyestalk extract, which indicated the primary location of the diabetogenic factor in the sinus gland. As the related studies expanded further, the factor was given the name of Crustacean Hyperglycemic Hormone (CHH). In the Atlantic blue crab, Callinectes sapidus, the cDNA sequences of CHH from the SG (Cas-SG-CHH) and that from the pericardial organ (Cas-PO-CHH) were identified using molecular cloning method.20,36 Cas-SG-CHH and its precursor-related peptide (Cas-SG-CPRP) are significant components in the sinus gland peptidome (Figure 1g), while the dominance is not observed in the pericardial organ (PO) (Figure 1h). The concentration of Cas-PO-CHH is about 10−11 M, which is 10-fold lower than the counterpart in the sinus gland at 10−10 M.20 In MS analysis, the precursor ions produced by ESI source are 6+, 7+ and 8+ for both SG and PO CHHs (Figure 1c, 1e) and 4+, 5+ and 6+ for their corresponding precursor-related peptides (Figure 1d, 1f).
Figure 1. Characterization of CHH using offline HPLC and MS.

Full MS spectra of Cas-SG-CHH (a) and Cas-PO-CHH (b) showing the 6 Da mass difference due to the DTT treatment. Most abundant charge states in MS1 spectra of the CHHs (c and e) and the CPRPs (d and f) using electrospray ionization. High pH HPLC separation of sinus gland extract (g) and pericardial organ tissue extract (h).
Mass spectrometry has been applied to sequence analysis of the CHH by our group previously.35,51,61 On top of the amino acid sequence study, post-translational modification (PTM) analysis is essential in functional discovery. Top-down approach outperforms the bottom-up method in analyzing PTMs, benefiting from eliminating artificial modifications or information loss during sample preparation. Intact CHH structures were restrained by three disulfide bonds. Both SG and PO extracts were reduced by DTT, however, no alkylation was performed. Due to the large size of CHHs, the alkylation usually tended to be incomplete35, which would cause peak split at MS1, leading to decreased precursor intensity. The reduction of three sets of disulfide bonds would cause an increment in mass by 6 Da (Figure 1a, 1b) and considerably promoted peptide fragmentation efficiency (Figure S1). Reduced Cas-PO-CHH and of Cas-SG-CHH were selectively fragmented on an Orbitrap Fusion Lumos Tribrid Mass Spectrometer under multiple collision energy levels (Figure S2). The protonated molecular mass and fragmented ions in representative MS2 spectra of both analytes (Figure 2 a–d) were measured at high accuracy, relative to the theoretical molecular mass (Figure 2 e–g). Overall, under the higher-energy collision dissociation (HCD), 54 and 56 peptide bonds were fragmented and consequently 46 out of 72 and 52 out of 71 amino acid residues were covered in the Cas-SG- and Cas-PO-CHH, respectively (Figure 3a, 3c). Both the fragmentation map and the tandem mass spectra exhibited that the cleavages preferred to occur on both termini, although the structure restriction imposed by the disulfide bonds has been released by DTT treatment and led to expanded conformation (Figure 6b). A hybrid fragmentation method -- electron-transfer/ higher-energy collision dissociation (EThcD) was applied to the characterization of two CHH peptides, generating both b/y ions and c/z ions. In the deconvoluted spectra, more fragment ions were aligned with residues in the middle region of the peptide chains (Figure 2b, 2d). In the earlier MS-based study on Cas-SG-CHH35, only 36 residues were identified with on-line top-down method and the combination of on-line and off-line top-down methods could reach the coverage of 54 residues. The bottom-up sequencing method enabled the identification of 58 residues. In our study, 53 residues were covered in a single on-line top-down method (Figure 3b), demonstrating that a much higher fragmentation efficiency (53/72 versus 36/72) was obtained with less time spent on sample preparation and instrument runs. For Cas-PO-CHH, almost every peptide bond was cleaved under EThcD, except for the one located next to the N-terminus pyro-glutamate and the one next to one of the six cysteines (Figure 3d). The identified ions were well spread out in the tandem mass spectra from the low mass range to the high mass range, producing a considerably enhanced fragmentation coverage. The top-down sequencing of Cas-PO-CHH strengthened the bottom-up result in the previous study and verified that the methionine oxidations found in that work35 were artificial modifications caused by sample preparation rather than endogenous PTMs.
Figure 2. Top-down tandem MS sequence profiling of CHH isoforms.

MS/MS spectra of Cas-SG-CHH (a and b) and Cas-PO-CHH (c and d) under HCD fragmentation (a and c) and EThcD fragmentation (b and d) acquired on the Fusion Lumos Orbitrap instrument. Zoom-in full MS spectra of protonated CHHs (e and g) and the representative fragment ion (f) with experimental and calculated masses annotated.
Figure 3. Fragmentation map and active labeling sites.

Fragmentation map of Cas-SG-CHH (a and b) and Cas-PO-CHH (c and d). Reductive dimethyl labeling active sites (lysine residue) in bold font. Identical residues between two homologous isoforms with grey background. Cas-SG-CHH contains N-terminus pyro-glutamate (pQ) and C-terminus amidation on Valine (Va). Cas-PO-CHH contains N-terminus pyro-glutamate (pQ).
Figure 6. Gas-phase conformational analysis using ion mobility mass spectrometry.
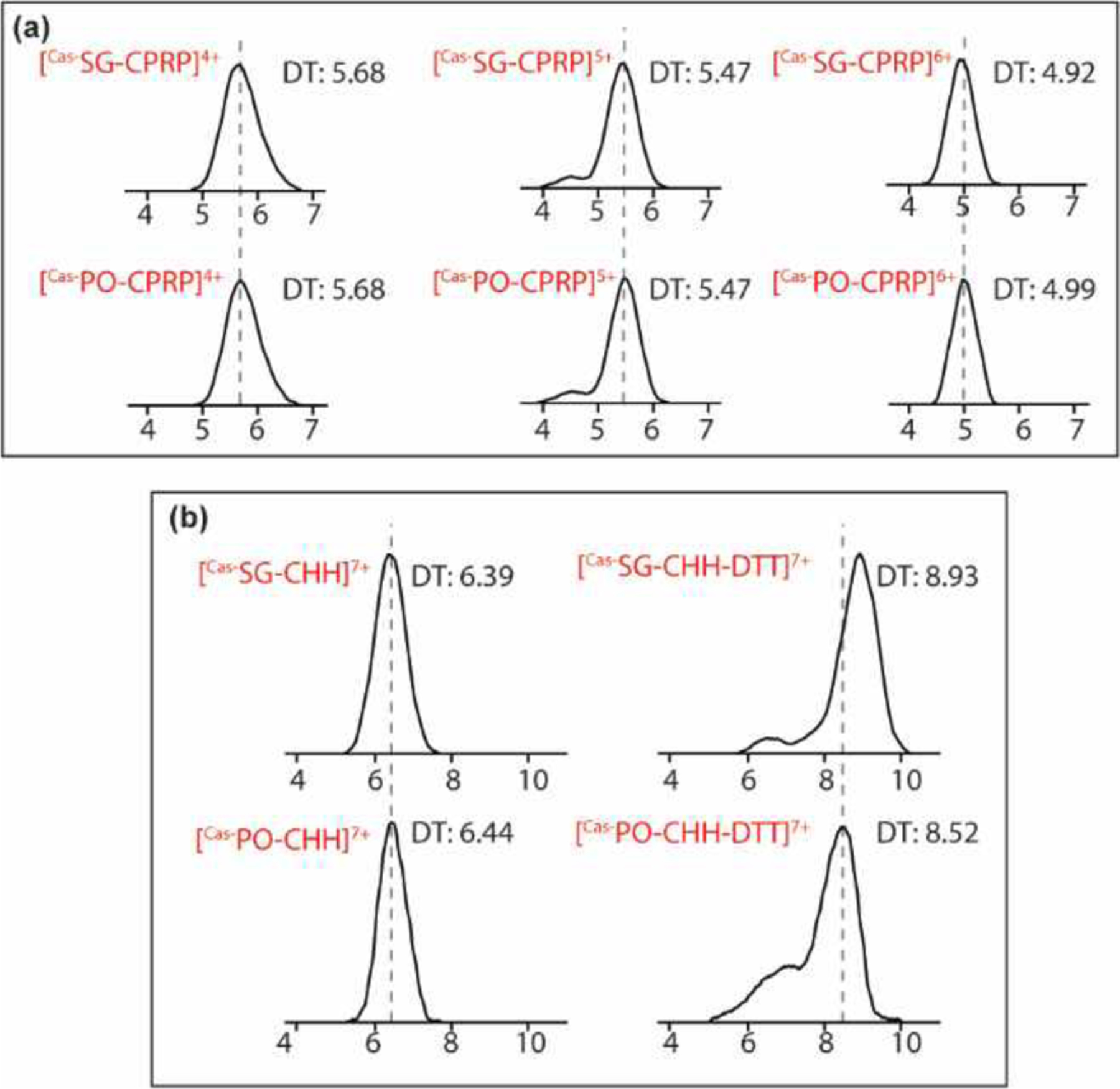
(a) Drift time alignment of Cas-SG-CPRP and Cas-PO-CPRP at their most abundant charge states. (b) Conformational change of Cas-SG-CHH and Cas-PO-CHH measured by shift in ion mobility drift time and CCS changes due to the DTT reduction treatment.
Isotopic Reductive Dimethyl Labeling-assisted Quantitation of SG-CHH in Response to Low pH Stress
The pH level drop is a common environmental fluctuation in blue crabs’ aqueous habitat. While encountering the stressful disturbance, crabs usually switch to an anaerobic energy metabolism, i.e., glycolysis, in which CHH plays essential regulatory roles.62 To investigate how Cas-SG-CHH is involved in the physiological modulation upon low pH stress, the control and stressed (2-hour incubation in acidified water) samples were labeled by formaldehyde-H2 (light) and formaldehyde-D2 (heavy), respectively, followed by LC-MS analysis using Q-Exactive Hybrid Quadrupole Orbitrap. With the free N-terminus being blocked, the active sites for reductive dimethylation labeling on the Cas-SG-CHH are the two lysine residues (bold font in Figure 3a, 3b), giving rise to 8 Da mass difference in molecular mass. Precursor ions of both channels exhibited great overlap (Figure 4a) due to the isotopic distribution and the small difference in mass-to-charge ratio (m/z), which is less desirable for quantification purpose. Therefore, we adopted the parallel reaction monitoring (PRM) method to simultaneously select light and heavy labeled precursors for HCD fragmentation. In the tandem mass spectra, y-ions containing the isotopic labeled lysine (the 5th residue on the C-terminus) were considered as quantitative peak pairs (Figure 4c). There were 4 Da mass difference between labeled y-ions and the dynamic range of the labeling method has been demonstrated from 1:0.1 to 1:10 (Figure S3). The stress-to-control ratios of two quantitative peak pairs, y82+ and y92+, were averaged to represent the change of Cas-SG-CHH expression upon the low pH stress.
Figure 4. Top-down MS quantitation upon low pH stress assisted by reductive dimethyl labeling.

(a) MS1 spectrum of isotopic formaldehyde labeled Cas-SG-CHH. Expression level change of Cas-SG-CHH in response to acidification stress (b) calculated from quantitative peak pairs acquired on Q-Exactive Orbitrap in PRM mode (c). n=3. s/c, stress-to-control ratio. Error bar, standard deviation.
As a result, a modest and insignificant drop was observed in Cas-SG-CHH as a response to the 2-hour low pH stress (Figure 4b). The well-known functional role of CHH is to cause hyperglycemia, which elevates the level of glucose and provides energy for physiological process against exterior stress.63 CHH is also involved in a negative feedback regulation with glucose.26,52,64 Accumulation of glucose in the body fluid, i.e., hemolymph, will inhibit the release of CHH.61 As the need for energy metabolism and consumption of glucose increases, as the condition discussed here, this will trigger CHH secretion65 and last for several hours,62 which would deplete the CHH concentration in the neuroendocrine tissue.
The expression level change of Cas-SG-CHH was in alignment with that of its precursor-related peptide (Cas-SG-CPRP) induced by the same stress condition, reported in an earlier study.16 CPRP, as a co-released peptide with CHH, has a shorter amino acid chain, thus a higher fragmentation efficiency in MS analysis. In vivo, the half-life time of CPRP is approximately 60 min, which is much longer than that of mature CHH at only 5–10 min.17,27,56,62 Therefore, if CPRP was found to secrete simultaneously with CHH and remain aligned in expression level fluctuations when encountering stress, it would be considered as an ideal probe or surrogate to monitor the change of CHH.
Discovery of Tissue-specific Function of CHH and the Relationship to Its Precursor
Different from its counterpart in the SG, Cas-PO-CHH contains the only labeling site (lysine residue) next to the cysteine (bold font in Figure 3c, 3d), which hinders the fragmentation and makes the quantitative peak pairs unavailable. One of the alternative options is to couple the dimethyl labeling process with reduction and alkylation. However, we would like to develop a quantitative method which could be ideally applied to analyze CHHs or other large peptides in the body fluid, demanding minimal sample preparation time to avoid enzymatic degradation. Here, targeted top-down detections of both CHH and CPRP were performed. MS1 accumulated peak areas were calculated for label-free quantitation and MS2 spectra were used for identity validation. In order to discover the tissue-specific function of CHH in the PO and SG, the quantitation of Cas-SG-CHH and Cas-SG-CPRP were repeated using a label-free strategy.
Both Cas-SG-CHH and Cas-PO-CHH have blocked N-terminus, which is the major isoform. Another minor species with free N-terminus was also observed during the experiment (Figure 5c 5d). The abundance distribution of isoforms with or without the N-terminus pyro-glutamate is a common pattern throughout many other species and the isoforms showed similar biological functions.37,66 Similar case was also observed for the CPRPs, the isoform with methionine oxidation being the major species (Figure 5a 5b). In this study, both isoforms were utilized for the quantification. As a result, Cas-SG-CHH and Cas-SG-CPRP responded to the low pH stress in an unnoticeable variation (Figure 5e), while expression levels for both Cas-PO-CHH and Cas-PO-CPRP dropped significantly (stress to control ratio (s/c) =0.45, p-value=0.018 and s/c=0.60, p-value=0.005, respectively) (Figure 5f). The label-free quantitation results of the Cas-SG-CHH, the Cas-SG-CPRP and the Cas-PO-CPRP were in agreement with the isotopic label-assisted measurements,16 indicating the minimum variance in run-to-run reproducibility and negligible sample loss in label-free and labeling strategies, respectively. In each tissue, the CPRPs exhibited the same changing trend as the CHHs. Therefore, the CPRPs can serve as an ideal probe in analyzing the in vivo expression level change of the CHHs upon low pH stress, which is often limited by the rapid degradation after secretion and their much bigger size for MS analysis. However, whether the characterization of CPRP as an indicator to CHH can be applied to a diverse set of stress studies, still needs further investigations.
Figure 5. Tissue-specific functional discovery using label-free strategy.

(a-d) Accumulation peak areas in extracted ion chromatogram (XIC) of CPRPs and CHHs. Stress-induced variation of CHHs and their precursors in the SG (e) and the PO (f) after 2-hour incubation at low pH environment. n=5 for Cas-SG-CHH and n=4 in Cas-PO-CHH. Student’s t-test was applied to evaluate the significance of expression level change. Error bar, standard deviation, asterisk, p-value < 0.05.
A tissue-specific functional role triggered by the acidification stress could be inferred from the different trends observed in the SG and PO. A possible mechanism is that the Cas-SG-CHH and Cas-SG-CPRP were irrelevant to the physiological modulation caused by low pH stress. However, Cas-PO-CHH and its precursor were released and circulated through the body fluid and exerted their regulatory effect on certain targets. It has been reported that the binding sites for Cas-PO-CHH are poorly competed for by Cas-SG-CHH, indicating their different functional roles and regions.67 Thus, it is possible that more Cas-PO-CHH were secreted from the tissue to the hemolymph to compensate the consumption during stress accommodation, leading to the significant drop of its abundance in the tissue.
The diverse response to the same environment stressor by the two CHH isoforms could be inferred from their diversity in sequences. Cas-SG-CHH and Cas-PO-CHH sequences are identical in the first 40 amino acids, while the latter region towards C-termini exhibits great difference (Figure 3).20 The sequence variance did not produce obvious disparity in gas-phase conformations of the intact peptides due to the three conserved disulfide bonds. Nonetheless, it is noted that the ion mobility drift times deviate from each other significantly after DTT reduction (Figure 6b). Similar sequence homology pattern is also observed in many other species44,45 and is always accompanied with discrepancy in their functions: the PO-CHHs do not initialize hyperglycemia as how the SG-CHHs perform.45 Early studies reported that in the blue crab, both PO-CHH and SG-CHH increased in relative abundance under hypoxia stress. After recovery in normal oxygen level, SG-CHH abundance dropped lower than the basal level, while the concentration of PO-CHH was reset to the initial point.20 However, Cas-SG-CPRP and Cas-PO-CPRP share the identical sequence (RSAEGLGRMGRLLASLKSDTVTPLRGFEGETGHPLE)42 and in our study, no gas-phase conformational difference was observed in the two CPRP isoforms at all abundant charge states using ion mobility MS (Figure 6a). It is speculated that the tissue-specific functional roles of neuropeptides occur not only among different members within the same family, but also among identical peptides in different regions.
CONCLUSION
In this study, we applied targeted top-down MS strategies to characterize the crustacean hyperglycemic hormone (CHH) and to discover the functional roles of the CHH and its precursor-related peptide (CPRP) in response to low pH stress. Benefiting from the advanced instrumentation and the hybrid fragmentation method, EThcD, we characterized the Cas-SG-CHH with an improved coverage using shorter experiment time, compared to previous studies. The Cas-PO-CHH was, for the first time, profiled using top-down method, which validated the results acquired using bottom-up de novo sequencing. The quantitative results of Cas-SG-CHH from labeling-assisted and label-free method were in agreement, confirming the reliability of both strategies. After 2-hour incubation in low pH stress environment, Cas-SG-CHHs were not obviously altered in expression level, while the abundance of Cas-PO-CHHs was significantly reduced, showing their different regulatory roles involved in the physiological process. In each tissue, the concentration changes in the CPRPs aligned with their corresponding CHHs, indicating that the CPRP can act as an ideal quantitative probe in tracking the CHH expression level alteration during the low pH stress. Collectively, this study highlights the qualitative and quantitative analysis of CHHs and CPRPs with enhanced efficiency and accuracy. This targeted top-down strategy can be further applied to investigate CHH isoforms in circulating body fluid and to uncover the comprehensive stress-related functions of the CHHs and CPRPs. The experimental design and strategy reported here can be applied to the study of large signaling peptides in other biological systems in the future.
Supplementary Material
ACKNOWLEDGEMENT
This work has been supported in part by the National Science Foundation grant (CHE-1710140) and National Institutes of Health (NIH) grants (1R01DK071801, 1R56DK071801, R01NS029436). The Q-Exactive Orbitrap instrument and the Fusion Lumos Orbitrap instrument were purchased through the support of an NIH shared instrument grant (NCRR S10RR029531) and Office of the Vice Chancellor for Research and Graduate Education at the University of Wisconsin - Madison. Y.L. would like to acknowledge Nhu Vu in the Li Research Group for her valuable comments on this manuscript and all Li Lab members for their support. G.L. thanks the funding support for a Postdoctoral Career Development Award provided by the American Society for Mass Spectrometry (2019). L.L. acknowledges a Vilas Distinguished Achievement Professorship and Charles Melbourne Johnson Distinguished Chair Professorship with funding provided by the Wisconsin Alumni Research Foundation and University of Wisconsin-Madison School of Pharmacy.
Footnotes
The authors declare no competing financial interest.
Supporting information
The supporting Information is available free of charge on the ACS website.
Details of animal dissection, low pH stress experiment setup, peptide extraction, reductive dimethylation labeling and offline fractionation; Top-down MS spectra comparison of Cas-SG-CHH with and without DTT treatment; Sequence coverage map obtained under different collision energy; Dynamic range of dimethylation assisted quantitation. (PDF)
REFERENCE:
- (1).Li L; Sweedler JV Peptides in the Brain: Mass Spectrometry–Based Measurement Approaches and Challenges. Annual Rev. Anal. Chem 2008, 1 (1), 451–483. 10.1146/annurev.anchem.1.031207.113053. [DOI] [PubMed] [Google Scholar]
- (2).Panula P; Aarnisalo AA; Wasowicz K Neuropeptide FF, a Mammalian Neuropeptide with Multiple Functions. Prog. Neurobiol 1996, 48 (4–5), 461–487. [DOI] [PubMed] [Google Scholar]
- (3).Jensen J Regulatory Peptides and Control of Food Intake in Non-Mammalian Vertebrates. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology 2001, 128 (3), 469–477. 10.1016/S1095-6433(00)00329-9. [DOI] [PubMed] [Google Scholar]
- (4).Hiremagalur B; Kvetnansky R; Nankova B; Fleischer J; Geertman R; Fukuhara K; Viskupic E; Sabban EL Stress Elicits Trans-Synaptic Activation of Adrenal Neuropeptide Y Gene Expression. Brain Res. Mol. Brain Res 1994, 27 (1), 138–144. [DOI] [PubMed] [Google Scholar]
- (5).Blitz DM; Christie AE; Marder E; Nusbaum MP Distribution and Effects of Tachykinin-like Peptides in the Stomatogastric Nervous System of the Crab, Cancer Borealis. J. Comp. Neurol 1995, 354 (2), 282–294. 10.1002/cne.903540209. [DOI] [PubMed] [Google Scholar]
- (6).Hook V; Funkelstein L; Lu D; Bark S; Wegrzyn J; Hwang S-R Proteases for Processing Proneuropeptides into Peptide Neurotransmitters and Hormones. Annu. Rev. Pharmacol. Toxicol 2008, 48, 393–423. 10.1146/annurev.pharmtox.48.113006.094812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Hoyle CHV Neuropeptide Families and Their Receptors: Evolutionary Perspectives. Brain Research 1999, 848 (1), 1–25. 10.1016/S0006-8993(99)01975-7. [DOI] [PubMed] [Google Scholar]
- (8).Christie AE; Stemmler EA; Dickinson PS Crustacean Neuropeptides. Cell. Mol. Life Sci 2010, 67 (24), 4135–4169. 10.1007/s00018-010-0482-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Stemmler EA; Bruns EA; Gardner NP; Dickinson PS; Christie AE Mass Spectrometric Identification of PEGFYSQRYamide: A Crustacean Peptide Hormone Possessing a Vertebrate Neuropeptide Y (NPY)-like Carboxy-Terminus. General and Comparative Endocrinology 2007, 152 (1), 1–7. 10.1016/j.ygcen.2007.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).DeLaney K; Li L Data Independent Acquisition Mass Spectrometry Method for Improved Neuropeptidomic Coverage in Crustacean Neural Tissue Extracts. Anal. Chem 2019, 91 (8), 5150–5158. 10.1021/acs.analchem.8b05734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Chen R; Ouyang C; Xiao M; Li L In Situ Identification and Mapping of Neuropeptides from the Stomatogastric Nervous System of Cancer Borealis. Rapid Communications in Mass Spectrometry 2014, 28 (22), 2437–2444. 10.1002/rcm.7037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Jia C; Lietz CB; Ye H; Hui L; Yu Q; Yoo S; Li L A Multi-Scale Strategy for Discovery of Novel Endogenous Neuropeptides in the Crustacean Nervous System. Journal of Proteomics 2013, 91, 1–12. 10.1016/j.jprot.2013.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Zhang Y; Buchberger A; Muthuvel G; Li L Expression and Distribution of Neuropeptides in the Nervous System of the Crab Carcinus Maenas and Their Roles in Environmental Stress. Proteomics 2015, 15 (23–24), 3969–3979. 10.1002/pmic.201500256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Chen R; Xiao M; Buchberger A; Li L Quantitative Neuropeptidomics Study of the Effects of Temperature Change in the Crab Cancer Borealis. J Proteome Res 2014, 13 (12), 5767–5776. 10.1021/pr500742q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Schmerberg CM; Liang Z; Li L Data-Independent MS/MS Quantification of Neuropeptides for Determination of Putative Feeding-Related Neurohormones in Microdialysate. ACS Chem. Neurosci 2015, 6 (1), 174–180. 10.1021/cn500253u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Liu Y; Buchberger AR; DeLaney K; Li Z; Li L Multifaceted Mass Spectrometric Investigation of Neuropeptide Changes in Atlantic Blue Crab, Callinectes Sapidus, in Response to Low PH Stress. J. Proteome Res 2019, 18 (7), 2759–2770. 10.1021/acs.jproteome.9b00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Chung JS; Webster SG Dynamics of in Vivo Release of Molt-Inhibiting Hormone and Crustacean Hyperglycemic Hormone in the Shore Crab, Carcinus Maenas. Endocrinology 2005, 146 (12), 5545–5551. 10.1210/en.2005-0859. [DOI] [PubMed] [Google Scholar]
- (18).Nakatsuji T; Lee CY; Watson RD Crustacean Molt-Inhibiting Hormone: Structure, Function, and Cellular Mode of Action. Comp. Biochem. Physiol., Part A Mol. Integr. Physiol 2009, 152 (2), 139–148. 10.1016/j.cbpa.2008.10.012. [DOI] [PubMed] [Google Scholar]
- (19).Chung JS; Webster SG Binding Sites of Crustacean Hyperglycemic Hormone and Its Second Messengers on Gills and Hindgut of the Green Shore Crab, Carcinus Maenas: A Possible Osmoregulatory Role. Gen. Comp. Endocrinol 2006, 147 (2), 206–213. 10.1016/j.ygcen.2006.01.002. [DOI] [PubMed] [Google Scholar]
- (20).Chung JS; Zmora N Functional Studies of Crustacean Hyperglycemic Hormones (CHHs) of the Blue Crab, Callinectes Sapidus - the Expression and Release of CHH in Eyestalk and Pericardial Organ in Response to Environmental Stress. FEBS J. 2008, 275 (4), 693–704. 10.1111/j.1742-4658.2007.06231.x. [DOI] [PubMed] [Google Scholar]
- (21).Duangprom S; Kornthong N; Suwansa-ard S; Srikawnawan W; Chotwiwatthanakun C; Sobhon P Distribution of Crustacean Hyperglycemic Hormones (CHH) in the Mud Crab (Scylla Olivacea) and Their Differential Expression Following Serotonin Stimulation. Aquaculture 2017, 468, 481–488. 10.1016/j.aquaculture.2016.11.008. [DOI] [Google Scholar]
- (22).Fanjul-Moles ML Biochemical and Functional Aspects of Crustacean Hyperglycemic Hormone in Decapod Crustaceans: Review and Update. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology 2006, 142 (3), 390–400. 10.1016/j.cbpc.2005.11.021. [DOI] [PubMed] [Google Scholar]
- (23).Hsu Y-WA; Messinger DI; Chung JS; Webster SG; de la Iglesia HO; Christie AE Members of the Crustacean Hyperglycemic Hormone (CHH) Peptide Family Are Differentially Distributed Both between and within the Neuroendocrine Organs of Cancer Crabs: Implications for Differential Release and Pleiotropic Function. J. Exp. Biol 2006, 209 (Pt 16), 3241–3256. 10.1242/jeb.02372. [DOI] [PubMed] [Google Scholar]
- (24).Keller R Crustacean Neuropeptides: Structures, Functions and Comparative Aspects. Experientia 1992, 48 (5), 439–448. 10.1007/BF01928162. [DOI] [PubMed] [Google Scholar]
- (25).Lacombe C; Grève P; Martin G Overview on the Sub-Grouping of the Crustacean Hyperglycemic Hormone Family. Neuropeptides 1999, 33 (1), 71–80. 10.1054/npep.1999.0016. [DOI] [PubMed] [Google Scholar]
- (26).Santos EA; Keller R Effect of Exposure to Atmospheric Air on Blood Glucose and Lactate Concentrations in Two Crustacean Species: A Role of the Crustacean Hyperglycemic Hormone (CHH). Comparative Biochemistry and Physiology Part A: Physiology 1993, 106 (2), 343–347. 10.1016/0300-9629(93)90523-7. [DOI] [Google Scholar]
- (27).Webster SG Measurement of Crustacean Hyperglycaemic Hormone Levels in the Edible Crab Cancer Pagurus during Emersion Stress. J. Exp. Biol 1996, 199 (Pt 7), 1579–1585. [DOI] [PubMed] [Google Scholar]
- (28).Yasuda A; Yasuda Y; Fujita T; Naya Y Characterization of Crustacean Hyperglycemic Hormone from the Crayfish (Procambarus Clarkii): Multiplicity of Molecular Forms by Stereoinversion and Diverse Functions. Gen. Comp. Endocrinol 1994, 95 (3), 387–398. 10.1006/gcen.1994.1138. [DOI] [PubMed] [Google Scholar]
- (29).De Kleijn DPV; Van Herp F Molecular Biology of Neurohormone Precursors in the Eyestalk of Crustacea. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology 1995, 112 (4), 573–579. 10.1016/0305-0491(95)00126-3. [DOI] [PubMed] [Google Scholar]
- (30).Webster SG; Keller R; Dircksen H The CHH-Superfamily of Multifunctional Peptide Hormones Controlling Crustacean Metabolism, Osmoregulation, Moulting, and Reproduction. General and Comparative Endocrinology 2012, 175 (2), 217–233. 10.1016/j.ygcen.2011.11.035. [DOI] [PubMed] [Google Scholar]
- (31).Abramowitz AA; Hisaw FL; Papandrea DN The Occurrence of a Diabetogenic Factor in the Eyestalks of Crustaceans. Biological Bulletin 1944, 86 (1), 1–5. 10.2307/1537946. [DOI] [Google Scholar]
- (32).Kegel G; Reichwein B; Weese S; Gaus G; Peter-Kataliníc J; Keller R Amino Acid Sequence of the Crustacean Hyperglycemic Hormone (CHH) from the Shore Crab, Carcinus Maenas. FEBS Letters 1989, 255 (1), 10–14. 10.1016/0014-5793(89)81051-8. [DOI] [PubMed] [Google Scholar]
- (33).Katayama H; Ohira T; Aida K; Nagasawa H Significance of a Carboxyl-Terminal Amide Moiety in the Folding and Biological Activity of Crustacean Hyperglycemic Hormone. Peptides 2002, 23 (9), 1537–1546. 10.1016/S0196-9781(02)00094-3. [DOI] [PubMed] [Google Scholar]
- (34).Mosco A; Edomi P; Guarnaccia C; Lorenzon S; Pongor S; Ferrero EA; Giulianini PG Functional Aspects of CHH C-Terminal Amidation in Crayfish Species. Regulatory Peptides 2008, 147 (1), 88–95. 10.1016/j.regpep.2008.01.005. [DOI] [PubMed] [Google Scholar]
- (35).Jia C; Hui L; Cao W; Lietz CB; Jiang X; Chen R; Catherman AD; Thomas PM; Ge Y; Kelleher NL; Li L High-Definition De Novo Sequencing of Crustacean Hyperglycemic Hormone (CHH)-Family Neuropeptides. Mol Cell Proteomics 2012, 11 (12), 1951–1964. 10.1074/mcp.M112.020537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Choi CY; Zheng J; Watson RD Molecular Cloning of a CDNA Encoding a Crustacean Hyperglycemic Hormone from Eyestalk Ganglia of the Blue Crab, Callinectes Sapidus. Gen. Comp. Endocrinol 2006, 148 (3), 383–387. 10.1016/j.ygcen.2006.03.003. [DOI] [PubMed] [Google Scholar]
- (37).Chung JS; Wilkinson MC; Webster SG Amino Acid Sequences of Both Isoforms of Crustacean Hyperglycemic Hormone (CHH) and Corresponding Precursor-Related Peptide in Cancer Pagurus. Regul. Pept 1998, 77 (1–3), 17–24. [DOI] [PubMed] [Google Scholar]
- (38).Kleijn DPVD; Janssen KPC; Martens GJM; Herp FV Cloning and Expression of Two Crustacean Hyperglycemic-Hormone MRNAs in the Eyestalk of the Crayfish Orconectes Limosus. European Journal of Biochemistry 1994, 224 (2), 623–629. 10.1111/j.1432-1033.1994.00623.x. [DOI] [PubMed] [Google Scholar]
- (39).de Kleijn DP; de Leeuw EP; van den Berg MC; Martens GJ; van Herp F Cloning and Expression of Two MRNAs Encoding Structurally Different Crustacean Hyperglycemic Hormone Precursors in the Lobster Homarus Americanus. Biochim. Biophys. Acta 1995, 1260 (1), 62–66. 10.1016/0167-4781(94)00173-z. [DOI] [PubMed] [Google Scholar]
- (40).Newcomb RW Amino Acid Sequences of Neuropeptides in the Sinus Gland of the Land Crab Cardisoma Carnifex: A Novel Neuropeptide Proteolysis Site. J. Neurochem 1987, 49 (2), 574–583. 10.1111/j.1471-4159.1987.tb02902.x. [DOI] [PubMed] [Google Scholar]
- (41).Tensen CP; De Kleijn DP; Van Herp F Cloning and Sequence Analysis of CDNA Encoding Two Crustacean Hyperglycemic Hormones from the Lobster Homarus Americanus. Eur. J. Biochem 1991, 200 (1), 103–106. 10.1111/j.1432-1033.1991.tb21054.x. [DOI] [PubMed] [Google Scholar]
- (42).Zheng J; Chen H-Y; Choi CY; Roer RD; Watson RD Molecular Cloning of a Putative Crustacean Hyperglycemic Hormone (CHH) Isoform from Extra-Eyestalk Tissue of the Blue Crab (Callinectes Sapidus), and Determination of Temporal and Spatial Patterns of CHH Gene Expression. Gen. Comp. Endocrinol 2010, 169 (2), 174–181. 10.1016/j.ygcen.2010.07.013. [DOI] [PubMed] [Google Scholar]
- (43).Chung JS; Dircksen H; Webster SG A Remarkable, Precisely Timed Release of Hyperglycemic Hormone from Endocrine Cells in the Gut Is Associated with Ecdysis in the Crab Carcinus Maenas. PNAS 1999, 96 (23), 13103–13107. 10.1073/pnas.96.23.13103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Toullec J-Y; Serrano L; Lopez P; Soyez D; Spanings-Pierrot C The Crustacean Hyperglycemic Hormones from an Euryhaline Crab Pachygrapsus Marmoratus and a Fresh Water Crab Potamon Ibericum: Eyestalk and Pericardial Isoforms. Peptides 2006, 27 (6), 1269–1280. 10.1016/j.peptides.2005.12.001. [DOI] [PubMed] [Google Scholar]
- (45).Dircksen H; Böcking D; Heyn U; Mandel C; Chung JS; Baggerman G; Verhaert P; Daufeldt S; Plösch T; Jaros PP; Waelkens E; Keller R; Webster SG Crustacean Hyperglycaemic Hormone (CHH)-like Peptides and CHH-Precursor-Related Peptides from Pericardial Organ Neurosecretory Cells in the Shore Crab, Carcinus Maenas, Are Putatively Spliced and Modified Products of Multiple Genes. Biochem. J 2001, 356 (Pt 1), 159–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Chait BT Chemistry. Mass Spectrometry: Bottom-up or Top-Down? Science 2006, 314 (5796), 65–66. 10.1126/science.1133987. [DOI] [PubMed] [Google Scholar]
- (47).Cui W; Rohrs HW; Gross ML Top-Down Mass Spectrometry: Recent Developments, Applications and Perspectives. Analyst 2011, 136 (19), 3854–3864. 10.1039/c1an15286f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Catherman AD; Skinner OS; Kelleher NL Top Down Proteomics: Facts and Perspectives. Biochem Biophys Res Commun 2014, 445 (4), 683–693. 10.1016/j.bbrc.2014.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Boone C; Adamec J 10 - Top-Down Proteomics. In Proteomic Profiling and Analytical Chemistry (Second Edition); Ciborowski P, Silberring J, Eds.; Elsevier: Boston, 2016; pp 175–191. 10.1016/B978-0-444-63688-1.00010-0. [DOI] [Google Scholar]
- (50).Han X; Jin M; Breuker K; McLafferty FW Extending Top-down Mass Spectrometry to Proteins with Masses Greater than 200 Kilodaltons. Science 2006, 314 (5796), 109–112. 10.1126/science.1128868. [DOI] [PubMed] [Google Scholar]
- (51).Ma M; Chen R; Ge Y; He H; Marshall AG; Li L Combining Bottom-up and Top-down Mass Spectrometric Strategies for de Novo Sequencing of the Crustacean Hyperglycemic Hormone from Cancer Borealis. Anal. Chem 2009, 81 (1), 240–247. 10.1021/ac801910g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Santos EA; Keller R Regulation of Circulating Levels of the Crustacean Hyperglycemic Hormone: Evidence for a Dual Feedback Control System. J Comp Physiol B 1993, 163 (5), 374–379. 10.1007/BF00265641. [DOI] [Google Scholar]
- (53).Vinagre AS; Chung JS Effects of Starvation on Energy Metabolism and Crustacean Hyperglycemic Hormone (CHH) of the Atlantic Ghost Crab Ocypode Quadrata (Fabricius, 1787). Mar Biol 2016, 163 (1), 3. 10.1007/s00227-015-2797-3. [DOI] [Google Scholar]
- (54).Ohira T; Tsutsui N; Nagasawa H; Wilder MN Preparation of Two Recombinant Crustacean Hyperglycemic Hormones from the Giant Freshwater Prawn, Macrobrachium Rosenbergii, and Their Hyperglycemic Activities. jzoo 2006, 23 (4), 383–391. 10.2108/zsj.23.383. [DOI] [PubMed] [Google Scholar]
- (55).Webster SG; Dircksen H; Chung JS Endocrine Cells in the Gut of the Shore Crab Carcinus Maenas Immunoreactive to Crustacean Hyperglycaemic Hormone and Its Precursor-Related Peptide. Cell Tissue Res 2000, 300 (1), 193–205. 10.1007/s004410000176. [DOI] [PubMed] [Google Scholar]
- (56).Wilcockson DC; Chung SJ; Webster SG Is Crustacean Hyperglycaemic Hormone Precursor-Related Peptide a Circulating Neurohormone in Crabs? Cell Tissue Res. 2002, 307 (1), 129–138. 10.1007/s00441-001-0469-8. [DOI] [PubMed] [Google Scholar]
- (57).Lehtonen MP; Burnett LE Effects of Hypoxia and Hypercapnic Hypoxia on Oxygen Transport and Acid-Base Status in the Atlantic Blue Crab, Callinectes Sapidus, During Exercise. J Exp Zool A Ecol Genet Physiol 2016, 325 (9), 598–609. 10.1002/jez.2054. [DOI] [PubMed] [Google Scholar]
- (58).Stover KK; Burnett KG; McElroy EJ; Burnett LE Locomotory Fatigue during Moderate and Severe Hypoxia and Hypercapnia in the Atlantic Blue Crab, Callinectes Sapidus. Biol. Bull 2013, 224 (2), 68–78. 10.1086/BBLv224n2p68. [DOI] [PubMed] [Google Scholar]
- (59).Cameron JN Effects of Hypercapnia on Blood Acid-Base Status, NaCl Fluxes, and Trans-Gill Potential in Freshwater Blue Crabs,Callinectes Sapidus. J Comp Physiol B 1978, 123 (2), 137–141. 10.1007/BF00687841. [DOI] [Google Scholar]
- (60).Kutz KK; Schmidt JJ; Li L In Situ Tissue Analysis of Neuropeptides by MALDI FTMS In-Cell Accumulation. Anal. Chem 2004, 76 (19), 5630–5640. 10.1021/ac049255b. [DOI] [PubMed] [Google Scholar]
- (61).Jia C; Yu Q; Wang J; Li L Qualitative and Quantitative Top-down Mass Spectral Analysis of Crustacean Hyperglycemic Hormones in Response to Feeding. Proteomics 2014, 14 (10), 1185–1194. 10.1002/pmic.201300331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (62).Chung JS; Zmora N; Katayama H; Tsutsui N Crustacean Hyperglycemic Hormone (CHH) Neuropeptidesfamily: Functions, Titer, and Binding to Target Tissues. Gen. Comp. Endocrinol 2010, 166 (3), 447–454. 10.1016/j.ygcen.2009.12.011. [DOI] [PubMed] [Google Scholar]
- (63).Prymaczok NC; Pasqualino VM; Viau VE; Rodríguez EM; Medesani DA Involvement of the Crustacean Hyperglycemic Hormone (CHH) in the Physiological Compensation of the Freshwater Crayfish Cherax Quadricarinatus to Low Temperature and High Salinity Stress. J. Comp. Physiol. B, Biochem. Syst. Environ. Physiol 2016, 186 (2), 181–191. 10.1007/s00360-015-0954-0. [DOI] [PubMed] [Google Scholar]
- (64).Glowik RM; Golowasch J; Keller R; Marder E D-Glucose-Sensitive Neurosecretory Cells of the Crab Cancer Borealis and Negative Feedback Regulation of Blood Glucose Level. Journal of Experimental Biology 1997, 200 (10), 1421–1431. [DOI] [PubMed] [Google Scholar]
- (65).Stentiford GD; Chang ES; Chang SA; Neil DM Carbohydrate Dynamics and the Crustacean Hyperglycemic Hormone (CHH): Effects of Parasitic Infection in Norway Lobsters (Nephrops Norvegicus). Gen. Comp. Endocrinol 2001, 121 (1), 13–22. 10.1006/gcen.2000.7575. [DOI] [PubMed] [Google Scholar]
- (66).Chung JS; Webster SG Does the N-Terminal Pyroglutamate Residue Have Any Physiological Significance for Crab Hyperglycemic Neuropeptides? Eur. J. Biochem 1996, 240 (2), 358–364. 10.1111/j.1432-1033.1996.0358h.x. [DOI] [PubMed] [Google Scholar]
- (67).Katayama H; Chung JS The Specific Binding Sites of Eyestalk- and Pericardial Organ-Crustacean Hyperglycaemic Hormones (CHHs) in Multiple Tissues of the Blue Crab, Callinectes Sapidus. Journal of Experimental Biology 2009, 212 (4), 542–549. 10.1242/jeb.022889. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


