Abstract
Given the role of effective communication in improving patient adherence and satisfaction, high quality patient-clinician communication is critical. Building on previous communication interventions in oncology and pediatrics, we developed a tailored communication coaching intervention to improve empathic communication quality and patient-centered care. In this randomized controlled trial, cardiologists record their patient encounters for review by a communication coach who provides tailored feedback. We are recruiting 40 cardiologists and 400 patients, or 4 patients per cardiologist in the Pre-intervention phase and 6 patients per cardiologists in the Post-intervention phase, from outpatient cardiology clinics within the Duke Health System. The primary goal of the trial is to determine the efficacy of the clinician communication coaching versus usual care in the post-intervention phase (240 patient encounters). In this paper, we describe the development of the communication coaching intervention. We also describe the details of the methods and outcomes of the ongoing trial. Finally, we discuss the challenges, solutions, and lessons learned during the start-up phase of the study.
Keywords: Patient-clinician communication, patient-centered care, cardiovascular disease, heart disease
Introduction
Over the past 20 years, the medical community has placed increased emphasis on the “patient experience” with patient-centered care being the gold standard. The Institute of Medicine includes patient-centered care, or “providing care that is respectful of and responsive to individual patient preferences, needs, and values and ensuring that patient values guide all clinical decisions” as one of the six domains of health care quality.1 Effective patient-centered communication is associated with improved patient adherence to recommendations, greater satisfaction,2 and fewer malpractice suits.3 Attention to patient emotion may also increase a patien’s willingness to reveal pertinent health information.4,5 Clinicians also benefit from effective communication as they experience higher satisfaction, which leads to their making fewer medical errors.6
Effective communication improves all encounters and may play an important role in encounters that involve more complexity, such as cardiology encounters. Conversations between cardiologists and patients can include highly technical, procedural and difficult prognostic information that is difficult to understand and can trigger a variety of reactions and emotions. Further, conversations can become increasingly difficult as treatment options decrease and disease duration increases.7 Despite its importance, few have studied cardiology communication or developed interventions aimed at improving communication among cardiologists.8,9 We developed a communication coaching intervention to provide tailored clinical communication skills training. The Communication Coaching in Cardiology (CCC) study is designed to determine the effect of a communication coaching intervention on an objective measure of communication quality and patients’ perceptions of the quality of patient-centered care. The objective of this paper is to present the study rationale, details of methods and outcomes. We also describe challenges with study implementation including enrollment pre and post-COVID-19 pandemic solutions, and lessons learned.
Methods/Design
Overview
The study is a two-arm prospective, cluster-randomized controlled trial, in which the unit of randomization is the cardiologist, and the unit of evaluation is the encounter. The study includes cardiologists and their new and returned patients seen at outpatient cardiology clinics at Duke Health. We collect audio recordings from ten patient encounters of each enrolled cardiologist, four patients in the Pre-Intervention Phase and six patients in the Post-Intervention Phase. After collecting the Pre-Intervention audio recordings, we randomly assign cardiologists in a 1:1 fashion to the intervention or control conditions. We deliver the coaching intervention either in-person or via video conference.
Study Timeline
We pilot tested the data collection process in the Fall of 2018. Cardiologist and patient enrollment data collection began in Spring 2019. We plan to continue patient enrollment and data collection through the end of 2021 (see Figure 1).
Figure 1.
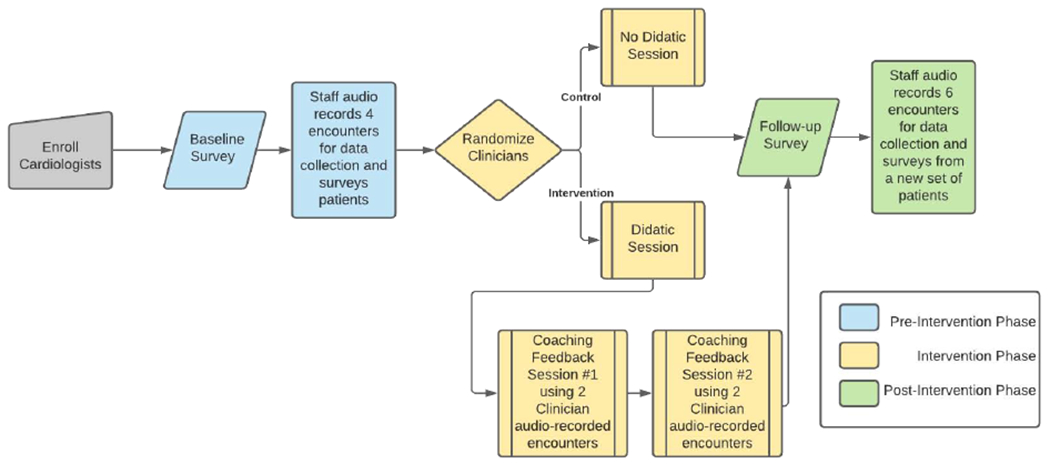
Study Flow
Cardiologists and Patients
We have recruited cardiologists and continue to recruit their patients from nine ambulatory cardiology outpatient clinics associated with Duke Health in Durham, North Carolina. The study and recruitment has occurred in pre-specified waves: one clinic every three to six months. The cardiology Division Chief first introduced the study to all clinic directors. Subsequently, clinic directors followed up with their staff and set up introductions for the study team. Cardiologists who provide direct patient care for at least 4 hours each month were asked to participate.
We include new and return patients of enrolled cardiologists who are 18 years or older, identify as Black/African-American or White, can read and speak English. Exclusion criteria include patients who are currently hospitalized and patients unable to provide informed consent. Caregivers accompanying patients to their appointment were also consented as their conversations may be captured in the recording. We do not survey and/or audio record the same individuals in the Pre- and Post-Intervention period. Therefore, patients who enroll in the study during the Pre-Intervention phase will not be eligible for Post-Intervention data collection.
Randomization and study arms
A computer algorithm allocates cardiologists in a 1:1 ratio via a method of minimization to ensure balance across strata.10 Unlike a standard stratified randomization, this dynamic allocation strategy examines the ongoing balance of stratification variables across arms and then assigns the next cardiologist to the arm which minimizes imbalance.10,11 The strata include clinician characteristics known to be correlated with communication skills including, cardiologist sex, prior communication training (yes/no), and type of cardiology specialty (procedural/non-procedural). We assign cardiologist after research staff collect complete data from four patients per enrolled clinician.
Data Collection Process: Pre-COVID
We identify patients in the electronic health record using the clinic schedule of enrolled cardiologists. On the day of the patien’s visit, front staff introduce the CCC study and give patients a tablet that has an app for the study. Our study staff are present in the waiting room and available to review study procedures and consent. We created an innovative mobile application that collects patient consent, patient baseline data, the audio recording, and the patient post-encounter survey and needs little study staff assistance. Patients use the app to audio record their visit with the cardiologist. Once the recording is complete, patients complete a post-encounter survey. The study application saves all data including the audio file to a REDCap (Research Electronic Data Capture) project, which protects the data and prevents personal health information from being saved locally on the device. Patients are unaware of their cardiologist’s study group assignment. Cardiologists know they are being audio recorded when patients bring the tablet in the room. We piloted this data collection process using approximately 20 patients of one cardiologist on the study team to identify study application or flow challenges. Once we identified and resolved app issues and developed a standard procedure for recruiting patients in the clinic, we began clinician and patient enrollment for the main trial.
Data Collection Process: Post-COVID
Due to the COVID-19 pandemic, we adjusted our recruitment techniques to limit study staff time in the clinic. However, our recruitment procedures now relies more heavily on research staff since we no longer ask clinic front desk staff to introduce the research study. As recommended by our Institutional Review Board, we now recruit and consent patients by phone. We have also attempted to recruit patients as they wait in their car upon arrival to the clinic.
Communication Coaching in Cardiology Intervention
The CCC intervention was developed based on evidence-based communication strategies in oncology and palliative care. Proven communication techniques, such as Motivational Interviewing (MI), promote discussion of patients’ values and priorities, emphasize listening to patients, and responding to patient emotion.12 The CCC intervention was developed by two experienced communication coaches (KP and NP).
After randomization, a cardiologist is assigned to one of the communication coaches. The coach meets with each cardiologist in the intervention arm three times to learn and review five evidence-based communication skills, using the acronym WISER (Figure 2).
Figure 2.

WISER Pocket Card
The coaching involves an initial 30-45 minute 1:1 session covering the WISER skills (Figure 2). Then each cardiologist audio records two encounters that they anticipate will be “challenging.” This was suggested to give the cardiologists more of an opportunity to apply WISER. We also ask cardiologists to collect recordings from a diverse set of patients. After recording is complete, the coaches code transcriptions of those encounters and meet with cardiologists for an individual 30-minute coaching session to provide feedback on these coded encounters. The coaches emphasize both the skills cardiologists did well and those they could improve. Then, the cardiologist audio records two more challenging encounters, followed by a second 30-minute coaching session. The time between the initial didactic session and each of the feedback sessions varies depending on when the cardiologists can audio record encounters. For some, it was within a month of each session. After each of the feedback sessions, the coach shares the coded transcripts and feedback.
Control condition
Cardiologists in the control arm rely on their existing communication skills or previous communication skills training to conduct patient visits. Because enrolled cardiologists may have varying levels of communication training experience, we stratify randomization based on previous communication training.
Outcomes
The primary outcome for the CCC study is the objective measures of the quality of communication. Communication quality is measured by coding post-intervention audio recordings for instances when the cardiologist asks open-ended questions, makes reflective statements, provides empathic responses, and revisits patient concerns. The secondary outcome is patient perceptions of the quality of patient-centered care, which is assessed at the post-encounter survey. We plan to examine treatment arm differences between White and Black patients.
Interpersonal Processes of Care.
We are using specific subscales of the IPC survey to measure interpersonal aspects of care.13 The scale has demonstrated internal-consistency reliability above 0.70. The IPC survey includes three key domains, communication, patient-centered decision making, interpersonal style, and discrimination. We included the following communication sub-domains: hurried communication (2 items), elicited concerns (3 items), and explained results (2 items). The two patient-centered decision-making items measure the extent to which the provider involved the patient in the treatment decisions. The two interpersonal style items measure the provider’s level of respect towards the patient. Patients were also asked to respond to two items regarding discrimination due to race/ethnicity. Patients are asked to respond to each item using a 5-point Likert scale from Never to Always.
Trust in Physician.
We are using the Trust in Physician scale, which is a brief scale to measure a patien’s perception of trust towards their physician.14 The 5-item scale had a Cronbach’s alpha of 0.87. Responses to each statement are scored 1 (Strongly disagree) to 5 (Strongly agree). A higher score is indicative of greater levels of trust.
The CARE Measure.
We are also including the consultation and relational empathy (CARE) measure to evaluate the encounter quality in terms of the patien’s overall perception of the doctor’s empathy.15 The measure was developed for patients across the socio-economic spectrum. The overall Cronbach’s alpha value is 0.93. Responses are on a 5-point Likert scale, ranging from Poor (1) to Excellent (5).
Self-rated health.
We include a single-item, self-report indicator of general health status on a scale from Poor (1) to Excellent (5).16
Data analysis
The primary aim is to determine the efficacy of the clinician communication coaching intervention versus usual care on an objective measure of the quality of communication. Additionally, we are interested the intervention effect within African American/Black and White patients separately. The quality of communication will be assessed at both baseline and post-training encounters by a summary of clinician-encounter counts derived from the audio recordings. We plan to use Poisson mixed-effects models fit via PROC GLIMMIX in SAS (SAS Inc., Cary, NC) as our primary analytic strategy because they will appropriately account for the intracluster correlation of multiple patient encounters for each clinician. The primary model for the overall intervention effect will include coefficients for intervention group, each cardiologist’s mean number of quality communication statements per conversation prior to the intervention, and the cardiologist stratification variables. This same model will be used to estimate the intervention efficient within Black and White patients separately. The differential intervention effect for Black compared to White patients will be assessed with a model which additionally includes a patient race by intervention interaction term. The secondary outcome, interpersonal processes of care, is a continuous measure assessed at Pre-intervention and Post-intervention. However, this is a self-reported outcome (i.e., the patient’s perception), and the same patients are not being followed longitudinally. Therefore, we will not incorporate the patients’ data collected in the Pre-intervention period in the analysis. We will estimate mean differences in the Post-intervention phase between intervention and control groups and test them via a linear mixed-effects model. We will conduct asensitivity analysis if we observe significant differences to adjust for potential confounders.
We transcribe audio recordings, and a team of 6 trained individuals code the transcripts for the primary, secondary, and exploratory outcomes. We developed a codebook with established definitions and examples for each code, including the WISER skills, patient participatory behaviors (asking questions and assertive responses), and global ratings of the cardiologists’ communication. The initial training involved the lead investigator reviewing and analyzing a patient recording with the team of coders. The codebook was then revised based on feedback from coders. The study coordinator meets regularly with the coding team to discuss disagreements and to review the codebook. After reaching a reliability of 80%, the coders began analyzing the recordings independently.
Statistical Power and Sample Size
The effect of interest for the study aim is the relative difference in the post-intervention phase between the intervention and usual care groups; the primary aim focuses on the overall difference and the difference within Black and White patients separately, while the secondary aim on the difference within Black patients compared to White patients.
For the primary outcome of the objective measure of communication, our sample size calculations are based upon the difference between two Poisson rates (incident rate ratio) in a cluster randomized design (i.e., patients clustered within cardiologist). Based on preliminary studies, the baseline mean number of quality communication statements is 1.0, and a conservative range of coefficients of variation is 0.2 to 0.5 to account for patients clustered within cardiologist. With a sample size of 240 patients in the Post-intervention period (6 per clinician) and a two-sided type-I error of 5%, we will have 80% power to detect incident rate ratios of 1.5 to 1.8 for the overall test, 1.6 to 1.9 for Black or White patients separately, and 1.8 to 2.5 for the interaction effect of the difference within Black patients compared to White patients.
For the Interpersonal Processes of Care outcome, our sample size calculations are based upon the difference between two means in a cluster randomized design. We present a conservative range of intraclass correlation coefficients (ICC) of 0.01 to 0.1 to account for patients clustered within clinician. With a sample size of 240 patients (6 per cardiologist) and a type-I error of 5%, we will have 80% power to detect mean differences of effect size 0.37 to 0.44 for the overall test, 0.52 to 0.56 for Black or White patients separately, and 0.65 to 0.91 for the interaction effect of the difference within Black patients compared to White patients. We used PASS 15 for all calculations.17
Discussion
We developed a tailored communication coaching intervention to improve cardiologists’ communication as well promote discussion of patients’ values and priorities. The strength of the study is the use of recordings of the enrolled cardiologists and their patients to tailor the intervention. In doing so, our study team has learned how future communication trials can reduce these challenges by 1) piloting their data collection process; 2) engaging clinic staff and maintaining close contact with intervention providers; 3) transitioning to remote data collection to reduce staff exposure. The progress of the CCC study has demonstrated the feasibility of empowering patients to be active participants by recording their encounters and using real encounters to provide tailed communication training. At the end of this trial, we will have the largest dataset of audio-recorded cardiology encounters to date.
Challenges
Challenge #1: Incomplete audio files
During this study, particularly during early enrollment, we encountered the unexpected challenge of missing or corrupt audio files. This is one of the first studies to ask patients to audio record their own encounters. During the first three months of study recruitment, 22% (10 out of 46) of enrolled patients had incomplete audio files. The three causes of missing or corrupt audio files were 1) ineffective recording instructions and reminders, 2) connectivity issues in the clinic and 3) length of the file size.
Solution #1: Patient training and app improvement
To reduce the number of missing audio files, study staff reminded patients to press the record button before the clinician entered the room. We also adjusted the design of the record button using the word “RECORD” instead of a red circle. We identified clinic locations with low Wi-Fi signal and asked patients to save the audio file in areas with a stronger signal to ensure the file was uploaded successfully. Finally,we identified a pattern where the data transfer process led to the corruption of audio recordings over ~45 minutes. We adjusted the data storage requirements to rectify this issue. Our current audio failure rate is now 10% or 27 out of 260 audio-recordings.
Challenge #2: Reduction of study staff oversight
One goal of the study is to reduce the involvement of study staff and automate the data collection process at multiple clinic locations by asking cardiologists to record their own audio recordings for coaching feedback and relying on clinic check-in staff to introduce the study to patients. We provide cardiologists with encrypted recording devices and instruct them to record two patient encounters after obtaining verbal consent. Many cardiologists, however, did not audio record on the days they expected to audio record. Reasons for this are 1) they forgot or 2) the device battery was too low. Because we are recording clinical encounters at multiple outpatient clinics, we encountered variations in clinic flow at each location. We initially met with clinic leadership to obtain permission to recruit in the clinics. Once we started recruiting, we realized we needed the support of the administrative and nursing staff to fully integrate the study into the clinic.
Solution #2: Facilitating cardiologists and front-desk staff
To improve audio recording timeliness, the communication coach helps cardiologists in the intervention arm identity times to record and sends calendar invitations. We also remind cardiologists to turn off the device when it is not in use. To gain support from clinic staff, we present the study and provide refreshments to the administrative and nursing staff in each clinic before patient recruitment. We also provide a script for front-desk staff to use to ensure the study was being introduced appropriately. This process helped us to develop a recruitment plan that did not interfere with clinic flow.
Challenge #3: In-person study activities during the COVID-19 pandemic
The outbreak of COVID-19 led to a suspension of clinical research activities from March 2020 to June 2020. During these months, enrolled cardiologists were unable to record their encounters for feedback until in-person clinical activities resumed.
Solution #3: Phone screening and verbal consent
We received permission to resume study activities in June 2020. To reduce staff time in clinic and limit participant and staff exposure, study staff began screening and consenting Pre- and Post-intervention patients by phone. This change has not caused any difference in patient refusal or other problems with recruitment. It has, however, been more time-consuming for study staff.
Conclusion
This is one of the only studies testing a communication coaching intervention for cardiologists in a large academic cardiovascular medicine practice. This study has the potential to support communication coaching in community-based clinics and other specialties. Consistent with the principles of community-engaged research, we also plan to present the findings of the study to the Division of Cardiology and acknowledge participants on the outcomes manuscript. Finally, we plan to publish the primary and secondary outcomes to add to the literature on clinician-patient communication interventions.
Acknowledgments
Funding:
This work was supported by the Duke Center for Research to Advance Health Equity (REACH Equity) sponsored by the National Institute of Minority Health and Health Disparities (1U54MD012530-01).
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to disclose.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Clinical Trial #: NCT03464110
References
- 1.Institute of Medicine Committee on Quality of Health Care in A. Crossing the Quality Chasm: A New Health System for the 21st Centu. ry. Washington (DC): National Academies Press (US) Copyright 2001 by the National Academy of Sciences. All rights reserved.; 2001. [Google Scholar]
- 2.Bertakis KD, Roter D, Putnam SM. The relationship of physician medical interview style to patient satisfaction. The Journal of family practice. 1991;32(2):175–81. Epub 1991/02/01.. [PubMed] [Google Scholar]
- 3.Levinson W, Roter DL, Mullooly JP, Dull VT, Frankel RM. Physician-patient communication. The relationship with malpractice claims among primary care physicians and surgeons. Jama. 1997;277(7):553–9. Epub 1997/02/19.. [DOI] [PubMed] [Google Scholar]
- 4.Ha JF, Longnecker N. Doctor-patient communication: a review. Ochsner J. 2010;10(1):38–43. [PMC free article] [PubMed] [Google Scholar]
- 5.(IOM) IoM. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academy Press, 2001. [PubMed] [Google Scholar]
- 6.West CP, Huschka MM, Novotny PJ, Sloan JA, Kolars JC, Habermann TM, Shanafelt TD. Association of perceived medical errors with resident distress and empathy: a prospective longitudinal study. Jama. 2006;296(9):1071–8. Epub 2006/09/07. doi: 10.1001/jama.296.9.1071.. [DOI] [PubMed] [Google Scholar]
- 7.Allen LA, Stevenson LW, Grady KL, Goldstein NE, Matlock DD, Arnold RM, Cook NR, Felker GM, Francis GS, Hauptman PJ, Havranek EP, Krumholz HM, Mancini D, Riegel B, Spertus JA. Decision Making in Advanced Heart Failure. Circulation. 2012;125(15):1928–52. doi: doi: 10.1161/CIR.0b013e31824f2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ha JF, Longnecker N. Doctor-patient communication: a review. The Ochsner journal. 2010;10(1):38–43. Epub 2011/05/24.. [PMC free article] [PubMed] [Google Scholar]
- 9.Brin D Back to Bedside Projects Energize Residents, Improve Patient Care [Online]. news.aamc.org: Association of American Medical Colleges; 2018. [updated May 08, 2018]. Available from: https://news.aamc.org/patient-care/article/back-to-bedside-improves-patient-care/. [Google Scholar]
- 10.Levy DE, Blood EA. Minimization: Testing A Dynamic Randomization Algorithm Dana-Farber Cancer Institute Available from: https://www.lexjansen.com/nesug/nesug04/ap/ap07.pdf. [Google Scholar]
- 11.McEntegart DJ. The Pursuit of Balance Using Stratified and Dynamic Randomization Techniques: An Overview. Drug Information Journal. 2003;37:293–308. [Google Scholar]
- 12.Pollak KI, Childers JW, Arnold RM. Applying motivational interviewing techniques to palliative care communication. J Palliat Med. 2011;14(5):587–92. doi: 10.1089/jpm.2010.0495.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stewart AL, Nápoles-Springer AM, Gregorich SE, Santoyo-Olsson J. Interpersonal processes of care survey: patient-reported measures for diverse groups. Health Serv Res. 2007;42(3 Pt 1):1235–56. doi: 10.1111/j.1475-6773.2006.00637.x.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dugan E, Trachtenberg F, Hall MA. Development of abbreviated measures to assess patient trust in a physician, a health insurer, and the medical profession. BMC Health Services Research. 2005;5(1):64. doi: 10.1186/1472-6963-5-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mercer SW, Maxwell M, Heaney D, Watt GC. The consultation and relational empathy (CARE) measure: development and preliminary validation and reliability of an empathy-based consultation process measure. Family Practice. 2004;21(6):699–705. doi: 10.1093/fampra/cmh621. [DOI] [PubMed] [Google Scholar]
- 16.Ware JE, Davies-Avery A, C D. Conceptualisation and measurement of health for adults in the health insurance study. Santa Monica, CA: Rand; 1978. [Google Scholar]
- 17.PASS 2015 Power Analysis and Sample Size Software. Kaysville, Utah, USA: NCSS, LLC; 2020. [Google Scholar]


