Abstract
The MAPK pathway is a major growth signal that has been implicated during the development of progenitors, neurons, and glia in the embryonic brain. Here, we show that the MAPK pathway plays an important role in the generation of distinct cell types from progenitors in the ventral telencephalon. Our data reveal that phospho-p44/42 (called p-ERK1/2) and the ETS transcription factor Etv5, both downstream effectors in the MAPK pathway, show a regional bias in expression during ventral telencephalic development, with enriched expression in the dorsal region of the LGE and ventral region of the MGE at E13.5 and E15.5. Interestingly, expression of both factors becomes more uniform in ventricular zone (VZ) progenitors by E18.5. To gain insight into the role of MAPK activity during progenitor cell development, we used a cre inducible constitutively active MEK1 allele (RosaMEK1DD/+) in combination with a ventral telencephalon enriched cre (Gsx2e-cre) or a dorsal telencephalon enriched cre (Emx1cre/+). Sustained MEK/MAPK activity in the ventral telencephalon (Gsx2e-cre; RosaMEK1DD/+) expanded dorsal lateral ganglionic eminence (dLGE) enriched genes (Gsx2 and Sp8) and oligodendrocyte progenitor cell (OPC) markers (Olig2, Pdgfrα, and Sox10), and also reduced markers in the ventral (v) LGE domain (Isl1 and Foxp1). Activation of MEK/MAPK activity in the dorsal telencephalon (Emx1cre/+; RosaMEK1DD/+) did not initially activate the expression of dLGE or OPC genes at E15.5 but ectopic expression of Gsx2 and OPC markers were observed at E18.5. These results support the idea that MAPK activity as readout by p-ERK1/2 and Etv5 expression is enriched in distinct subdomains of ventral telencephalic progenitors during development. In addition, sustained activation of the MEK/MAPK pathway in the ventral or dorsal telencephalon influences dLGE and OPC identity from progenitors.
Keywords: ETS factor, Etv5, Gsx2, Lateral ganglionic eminence (LGE), Oligodendrocyte progenitor cell (OPC)
Graphical Abstract
Introduction
The embryonic telencephalon can be generally divided into dorsal regions (i.e. pallium) that give rise to glutamatergic neurons and ventral regions (i.e. subpallium) that give rise to GABAergic neurons (reviewed in Marin and Rubenstein, 2001; Campbell, 2003; Hebert and Fishell, 2008). The ventral telencephalon is anatomically defined by two pronounced elevations called the medial and lateral ganglionic eminence (MGE and LGE). In addition, the caudal most LGE region, which is no longer adjacent to the MGE, is called the caudal ganglionic eminence (CGE) (Nery et al., 2002). The major brain structures generated from the ventral telencephalon are the striatum and globus pallidus which largely originate from LGE and MGE respectively (reviewed in Marin and Rubenstein, 2001; Campbell, 2003). The LGE is also divided into a dorsal LGE (dLGE) and ventral LGE (vLGE) which give rise to olfactory bulb interneurons and striatal projection neurons respectively (Yun et al., 2001; Stenman et al., 2003; Waclaw et al., 2006, Ehrman et al., 2013). In addition, multiple diverse cell types are also generated from the ventral telencephalon including interneurons from the MGE and CGE that migrate tangentially to occupy the striatum and cortex, and amygdala interneurons that migrate lateral from LGE/CGE (Anderson et al., 1997; Nery et al., 2002; Xu et al., 2004; Carney et al., 2006; Waclaw et al., 2010; Kuerbitz et al., 2018). Following neurogenesis, oligodendrocyte progenitor cells (OPCs) are temporally and spatially generated in the telencephalon in a ventral to dorsal wave with the earliest OPCs generated from MGE, then LGE, and finally from cortical progenitors (Kessaris et al., 2006). The influence of specific signaling pathways that are known to regulate neurogenesis and gliogenesis during development of the ventral telencephalon remains poorly understood. This study will address how the Mitogen Activated Protein Kinase (MAPK) pathway, a major growth signal, influences the generation of distinct cells types originating from progenitors in the ventral telencephalon.
The appropriate signaling of the MAPK pathway is key to normal development as mis-regulation through germline mutations in genes associated with this pathway result in a group of syndromes called RASopathies, which are identified by defects in multiple organ systems including but not limited to craniofacial, cardiovascular, neurodevelopmental, and growth abnormalities (reviewed in Tartaglia and Gelb, 2010). While previous research has revealed that RASopathy animal models of NF1, Noonan Syndrome, Costello Syndrome, and Cardiofaciocutaneous Syndrome (CFC) show neurodevelopmental defects including alterations in astrocytes, oligodendrocytes, and cortical neurons (Bennett et al., 2003; Dasgupta and Gutmann, 2005; Zhu et al., 2005; Mayes et al., 2013; Ehrman et al., 2014; Lopez-Juarez et al., 2017; Titus et al., 2017; Aiodi et al., 2018; Holter et al., 2019), the impact of RASopathy mutations and the specific influence of aberrant MAPK signaling in the developing ventral telencephalon remains largely under studied. Mutations in MAPK signaling or regulatory genes can also result in tumor formation as is the case with somatic BRAF-V600E mutation in low grade brain tumors and germline NF1 mutations in low- and high-grade glioma brain tumors (Dougherty et al., 2010, D’Angelo et al., 2019). Therefore, it is crucial to study the specific influence of the MAPK pathway during brain development to understand RASopathy disease mechanism and gain potential insight into early tumor formation.
The MAPK pathway has been extensively studied in the dorsal telencephalon. In fact, MEK1/2 and ERK1/2 signaling components have been implicated as regulators of early gliogenesis and aspects of neurogenesis during development of the cortex (Samuels et al., 2008; Li et al., 2012; Li et al., 2014). FGF signaling and Spry proteins, key regulators of the MAPK pathway, have also been implicated in cortical patterning during multiple neurogenesis phases (Borello et al., 2008; Paek et al., 2009; Kang et al., 2009; Storm et al., 2006; Cholfin and Rubenstein, 2008; Faedo et al., 2010). In the ventral telencephalon, activation of the MAPK pathway has been used as an indicator for defects in proliferation and growth in the MGE and CGE (Stanco et al., 2014). The signaling molecule Shp2 and fibroblast growth factor receptors (FGFRs), both upstream of the RAS/MAPK signaling, have also been shown to play crucial roles in early gliogenesis and OPC generation from the MGE and LGE (Ehrman et al., 2014 and Furusho et al., 2011). However, given that both Shp2 and FGFR are upstream of RAS, it seems likely that genetic disruption of these factors may impact other RAS related pathways, like AKT signaling, that would influence gliogenesis and OPC generation. Therefore, it remains unclear how MAPK signaling specifically influences regional progenitor domains and distinct cell type generation in the ventral telencephalon.
In this study, we directly address MAPK signaling through gene and protein expression and conditional transgenics manipulating MEK/MAPK activity. Our studies revealed that the MAPK effectors p-ERK1/2 and Etv5 are expressed in the dorsal region of the LGE and ventral region of the MGE during a developmental window (E13-E15). In addition, we show that manipulating MAPK activation through conditional expression of a constitutively active MEK allele in the LGE or the cortex results in increased dLGE identity cells and precocious OPC generation. Our results provide an understanding for the role of MAPK pathway activation in the developing telencephalon.
Methods
Animals
Animal protocols for experiments using mice were approved by the Institutional Animal Care and Use Committee at the Cincinnati Children’s Hospital Medical Center and carried out in accordance with National Institutes of Health guidelines. RosaMEKIDD/+ (Stock #012352) and Emx1cre/+ (Stock #005628) mice were obtained from Jax and genotyped from published protocols on Jax website. Gsx2e-cre mice were maintained and genotyped as described (Qin et al., 2016). For specific embryonic collections, vaginal plug indicates embryonic day 0.5 during timed matings. Embryo collections and processing for histology were completed as previously described (Waclaw et al., 2006, 2010; Ehrman et al., 2014).
Immunohistochemistry and Fluorescence.
Primary antibodies were used at the following concentrations: rbt-Etv5 (1:1500, from Abcam, catalog #AB102010), rbt-Foxp1 (1:2000, from Abcam, catalog #AB16645), rbt-Gsx2 (1:4000, Toresson et al., 2000), gt-Isl1 (1:500, from R&D Systems, catalog #AF1837), rbt-Nkx2.1 (1:2000, from Seven Hills Bioreagents, catalog # WRAB-1231), rbt-Olig2 (1:1000, from Millipore Sigma, catalog #AB9610), gt-Pdgfrα (1:500 from R&D Systems, catalog #AF1062), rbt-p-ERK1/2 (1:500 from Cell Signaling, catalog #9101 and #4370), rbt-Sox10 (1:500 from Cell Signaling, catalog #69661) and gt-Sp8 (1:5000 from Santa Cruz, catalog # sc-104661). The rbt-Etv5 antibody was preblocked with embryonic tissue to reduce background and tyramide amplification (ThermoFisher Scientific kit B40953 or B40956) was used to increase the signal. In situ hybridization and combination in situ hybridization and immunohistochemistry were completed as described (Kohli et al., 2017). Plasmids to generate Etv5 anti-sense probe were a gift from Dr. Xin Sun (UCSD) and Dr. Susan Mansour (The University of Utah) and published in Li et al., 2007 and Zhang et al., 2009. All brightfield pictures were captured on a Leica DM2500 microscope with either a Leica DFC500 or DMC6200 camera using Leica Acquisition Software (LAS-X). Fluorescence images were captured on a Nikon C2 confocal microscope using Nikon Elements software. Representative images were selected from at least n=3 embryos for each stain. In the case of RosaMEK1DD/+ experiments, “controls” refer to single positive transgenics in Figures 3-6 (Gsx2e-cre only or RosaMEK1DD/+ only) and Figures 7-8 (Emx1-cre only or RosaMEK1DD/+ only).
Quantification
Images were taken at either 10x (Foxp1, Sp8, and Gsx2 at E15) or 20x (Sox10, Gsx2, Sp8, and Pdgfra at E18) in 3 serial sections of each brain examined (n= 3 for control and GOF). For E15 embryos, images were taken in the striatum (Foxp1) or around the lateral ventricle (Sp8 and Gsx2). For E18 embryos, images were taken in the cortex, adjacent to the lateral ventricle. Raw images were processed through Cell Profiler to identify the number of cells in the image or identify the area of the image occupied by the stains, as indicated by the graph. Graphs were generated in GraphPad. Students t-test was used to determine significance.
Results
Regional expression of p-ERK1/2 and Etv5 in the LGE and MGE
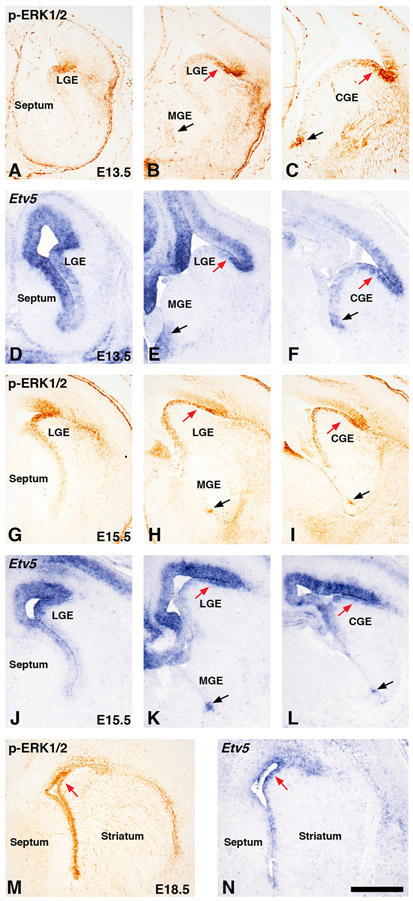
The MAPK pathway has generally been associated as a major growth signal in many organ systems (Dhillon et al., 2007). Indeed p-ERK1/2 expression has also been used as a growth pathway indicator or proliferation signal in LGE VZ progenitors (Ehrman et al., 2014; Stanco et al., 2014). Recent genetic evidence also implicates Mapk1/3 (Erk1/2) function in mature striatal projection neurons that originated from the LGE for striatal specific motor functions (Hutton et al., 2017). However, it remains largely unknown whether the MAPK pathway has distinct regional roles in the embryonic ventral telencephalon or if it is a general growth signal of neural progenitor cells. To address this, we describe here a detailed temporal characterization of p-ERK1/2 expression and the MAPK target gene Etv5 during development in progenitor cells of the ventral telencephalon. We analyzed 3 levels of the rostro-caudal axis to analyze (LGE only, LGE-MGE level, and CGE). Immunostaining for the p-ERK1/2 and in situ hybridization of Etv5 at E13.5 and E15.5 revealed a regional enrichment in expression in VZ cells of the ventral telencephalon with high expression in the dorsal region of the LGE or CGE (red arrows in Fig. 1B-C, E-F, H-I, K-L). We also detected regional expression in the ventral most MGE and CGE regions (black arrows in Fig. 1B-C, E-F, H-I, K-L). The unique ventral most location of this expression suggests a potential caudal extension from the MGE to the CGE regions. The expression of both p-ERK1/2 and Etv5 resolved to a more uniform pattern of expression in VZ progenitor cells (red arrows in Fig.1M-N) at later stages of development (E18.5). The expression of p-ERK1/2 and Etv5 was similar at all stages examined in the ventral telencephalon except for the rostral most (LGE only) levels at E13.5 where p-ERK1/2 labels the lateral telencephalon while Etv5 expression encompassed the lateral telencephalon and also medial telencephalon. The expression of p-ERK1/2 and Etv5 in the lateral telencephalon is similar to the expression of the orphan nuclear receptor Tlx/tailess which spans the pallio-subpallial boundary (Stenman et al., 2003). We also detected more robust Etv5 expression in cortex and septum compared to p-ERK1/2, which could be a result of a threshold of detection from p-ERK1/2 antibody or the transient nature of phosphorylation status of ERK1/2. Previous research and available gene expression databases on other MAPK effectors or readouts like Spry1/2 or Dusp6 appear to show a similar regional expression (Faedo et al., 2010; Diez-Roux et al., 2011) To directly compare Etv5 gene expression to protein expression, we identified an antibody that resembled Etv5 gene expression and double stained E13.5 and E15.5 tissue with Etv5 and p-ERK1/2 and found comparable pattern of expression in the lateral telencephalon (Fig. 2A-B). Similar to Etv5 gene expression in cortex and septum (Fig. 1), more Etv5+ cells were detected in cortical areas of lateral telencephalon further suggesting Etv5 robustly labels MAPK pathway activation.
Figure 1: MAPK activity during development in the ventral telencephalon.
Immunohistochemistry for phospho-ERK1/2 (called p-ERK1/2) (A-C, G-I, M) and in situ hybridization for Etv5 (D-F, J-L, N). Representative images were captured at rostral levels showing LGE only (A, D, G, J), Mid-levels showing LGE and MGE (B, E, H, K), and caudal levels showing CGE only (C, F, I, L) at E13.5 and E15.5 respectively. p-ERK1/2 and Etv5 showed high level of expression in dorsal LGE/CGE region (see red arrows in B-C, E-F, H-I, K-L) and in ventral most region of the MGE (black arrows in B, E, H, K) and CGE (C, F, I, L). Staining for both p-ERK1/2 and Etv5 appeared more general throughout the ventricular zone at E18.5 (see red arrows in M-N). Scale bar in N = 500μM. LGE=lateral ganglionic eminence, MGE=medial ganglionic eminence, CGE=caudal ganglionic eminence.
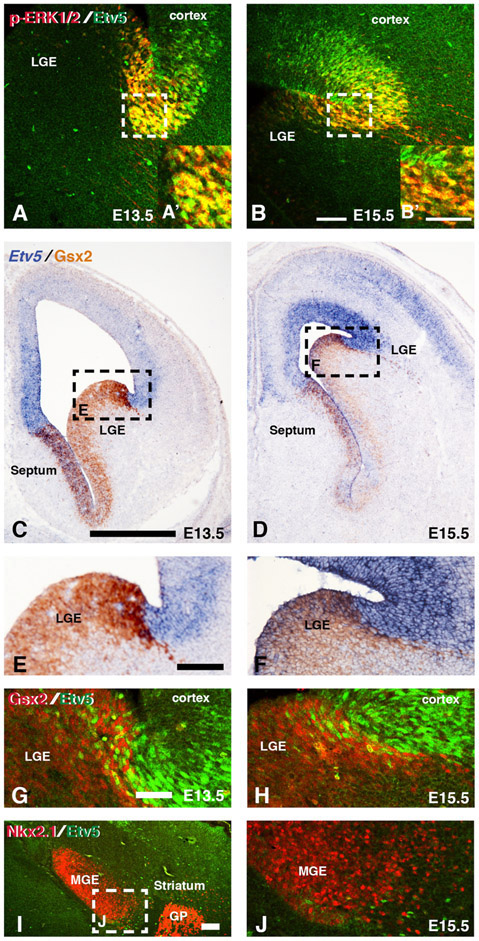
Figure 2: Etv5 expression in dorsal and ventral regions of the ventral telencephalon.
Representative confocal images from double immunofluorescence of p-ERK1/2 and Etv5 in dLGE region at E13.5 (A,A’) and E15.5 (B,B’). Dashed boxes in A and B refer to high magnification pictures in A’ and B’ respectively. Representative images from double In situ hybridization for Etv5 and immunohistochemistry for Gsx2 show overlap in the dorsal most LGE region (C-F). Dashed boxes in C and D refer to high magnification pictures in E and F respectively. Double immunofluorescence for Gsx2 and Etv5 show double positive cells at the pallio-subpallial boundary at both E13.5 (G) and E15.5 (H). Double immunofluorescence for the MGE marker Nkx2.1 and Etv5 reveals a small number of Etv5 positive cells in the ventral most MGE region at E15.5 (I-J). Dash box in I refers to high magnification image in J. Scale bars in B= 100μM for images A-B, B’= 50μM for images A’,B’, C= 500μM for images in C-D, E= 100μM for images in E-F, G= 50μM for images in G-H and J, I=100μM for image in I. LGE=lateral ganglionic eminence, MGE= medial ganglionic eminence, GP= globus pallidus
Etv5 expression in the dLGE and ventral MGE.
The expression of both p-ERK1/2 and Etv5 was largely enriched in VZ progenitors of the ventral telencephalon (Figure 1), which exhibit distinct molecular profiles that identify regional subdomains (Yun et al., 2001; Flames et al., 2007). One defining marker of the dLGE is the homeobox gene, Gsx2, which shows a high dorsal to low ventral gradient of expression in the LGE (Yun et al., 2001; Waclaw et al., 2009). To further characterize the regional expression of MAPK effectors in the ventral telencephalon, we performed in situ hybridization for Etv5 and immunohistochemistry for Gsx2 and double immunofluorescence for Etv5 and Gsx2. We detected scattered Etv5 cells in the dorsal Gsx2 domain of the LGE at both E13.5 and E15.5 (Fig. 2C-H). We also found an Etv5 only domain dorsal to the Gsx2 domain, which encompasses the ventral pallium regions (i.e. the ventral most region of the dorsal telencephalon). This data suggests that MAPK effectors are high in the lateral telencephalon that encompasses both the pallium (ventral pallium) and subpallium (dorsal LGE). We confirmed that p-ERK1/2 showed a similar pattern in the Gsx2 domain (data not shown). To further characterize the regional expression, we also double stained E15.5 sections with Etv5 and MGE marker Nkx2.1 to determine if the ventral most Etv5 expression is in the Nkx2.1 domain. We found isolated Etv5+ cells at the base and within Nkx2.1 domain (Fig. 2I-J). Our data support that Etv5 labels cells in the dLGE and ventral most MGE regions of the telencephalon.
Sustained activation of MEK/MAPK pathway throughout LGE and MGE in the ventral telencephalon
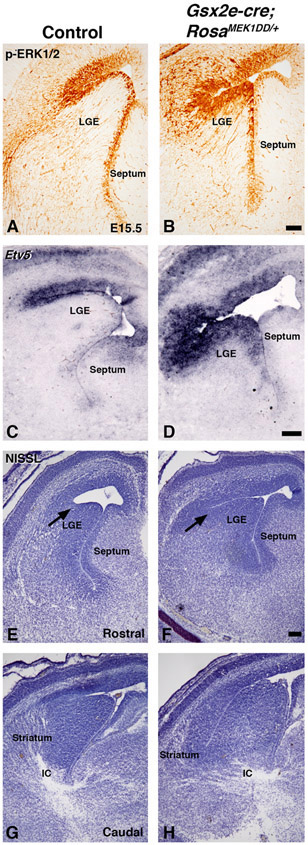
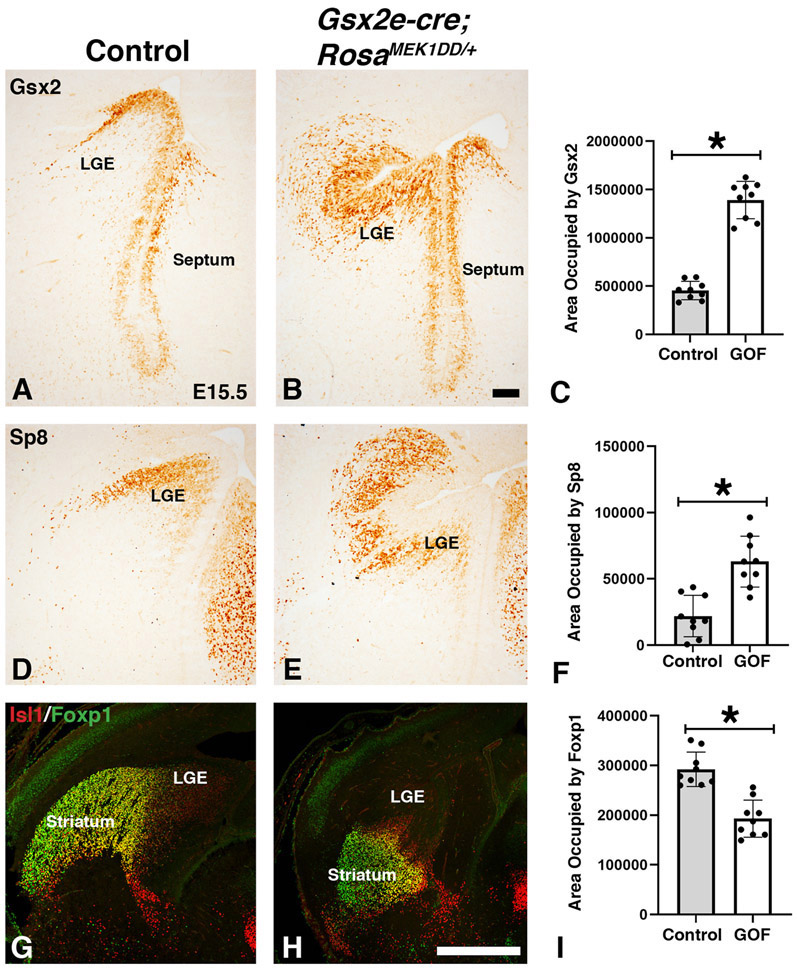
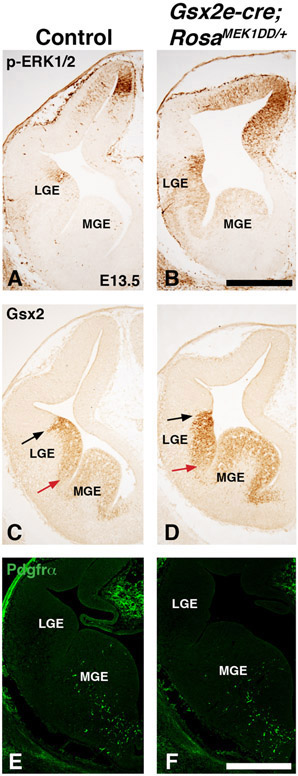
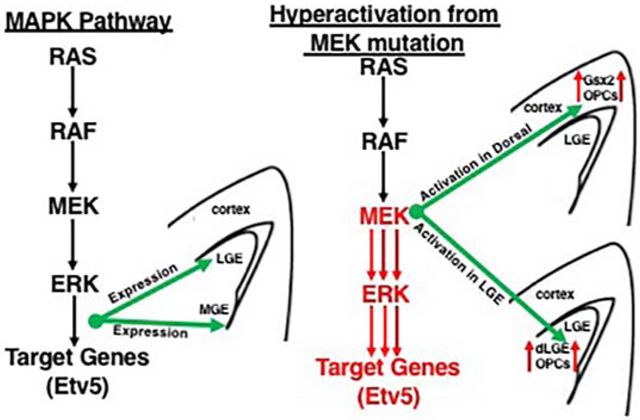
It has previously been shown that the MAPK pathway can be specifically activated in a transgenic mouse (RosaMEK1DD/+), that contains a cre inducible MEK1 allele containing 2 serine to aspartic acid substitutions (S218D/S222D) within the catalytic domain that promotes constitutive activity (Srinivasan et al., 2009). Since both p-ERK1/2 and Etv5 showed enriched expression in the dLGE of the Gsx2 domain, we developed a strategy to activate the MAPK pathway throughout the ventral telencephalon to test a role for pathway activation. We utilized the RosaMEK1DD/+ mice in combination with a Gsx2e-cre transgenic mouse that drives cre expression during early stages of LGE development (Qin et al., 2016). We collected double transgenic embryos at mid-gestation stages and confirmed robust activation of the pathway using p-ERK1/2 and Etv5 expression (compare Fig. 3B,D to A,C). The dorsolateral regions showed the highest levels of expression in Gsx2e-cre;RosaMEK1DD/+, which might reflect the transgene expression based on the high dorsal to low ventral gradient expression of Gsx2 (Waclaw et al., 2009; Qin et al., 2016). We noticed that Gsx2e-cre;RosaMEK1DD/+ embryos had abnormal morphology of the dorsolateral LGE region (arrows in Fig. 3E-F) and altered formation of the internal capsule at caudal levels (Fig. 3G-H). Based on the expression of the MAPK effectors in the dLGE region of the ventral telencephalon (Fig. 1-2), we analyzed dLGE markers Gsx2 in the VZ (Fig. 4A) and Sp8 in the SVZ (Fig. 4D). Gsx2e-cre;RosaMEK1DD/+ double transgenic animals showed abnormal expression of both Gsx2 and Sp8, with expanded expression in both ventral and dorsal regions (Fig. 4B and 4E). Quantification of area stained revealed a 2.77 fold increase for Gsx2 expression and 2.87 fold for Sp8 expression in double transgenic embryos. The ventral LGE (vLGE) domain in the SVZ is adjacent to the Sp8 dLGE domain and is labeled by Isl1 (Stenman et al., 2003, Waclaw et al., 2006; Ehrman et al., 2013). Immunostaining for Isl1 and the mature striatal marker Foxp1 shows that Gsx2e-cre;RosaMEK1DD/+ double transgenic embryos have an impaired vLGE domain and striatal complex (compare Fig. 4H to Fig. 4G). Gsx2e-cre;RosaMEK1DD/+ double transgenic embryos showed a 34% decrease in the striatal area labeled by Foxp1 expression These findings indicate that robust activation of the MAPK pathway using a constitutively active MEK1 allele results in expanded dLGE identity markers (Gsx2 and Sp8) and impairs the vLGE domain (Isl1) and striatum (Foxp1).
Figure 3: Sustained MEK/MAPK activity in the LGE of Gsx2e-Cre;RosaMEK1DD/+ embryos.
Representative images at E15.5 from controls (A, C, E, G) and double transgenic Gsx2e-Cre;RosaMEK1DD/+ (called MEK/MAPK GOF) embryos (B, D, F, and H). MEK/MAPK GOF embryos show increased p-ERK1/2 (B) and Etv5 (D) staining in the LGE ventricular zone compared to controls (A,C). NISSL staining shows abnormal morphology at the LGE sulcus (arrows in E-F), LGE, striatum, and forming axon tracts in the ventral telencephalon of MEK/MAPK GOF embryos (F,H) compared to controls (E,G). Scale bars in: B= 100μM for images in A-B, D= 200μM for images in C-D, F=200μM for images E-H. LGE= lateral ganglionic eminence, IC=internal capsule
Figure 4: Sustained MEK/MAPK activity in the LGE alters neuronal subtype specification.
Representative images at E15.5 from controls (A, D, G) and MEK/MAPK GOF double transgenic Gsx2e-Cre;RosaMEK1DD/+ (B, E, H) embryos. The Gsx2 expression domain is expanded in MEK/MAPK GOF embryos (B) compared to the high dorsal to low ventral gradient in the LGE ventricular zone of controls (A). The LGE SVZ is divided into a dorsal region labeled by Sp8 (D) and a ventral region that is labeled by the expression of Isl1 (G). The striatum is a vLGE derived structure and labeled by Foxp1 (G). MEK/MAPK GOF embryos show expanded expression of the Sp8 dorsal LGE domain (E) similar to Gsx2 (B). Isl1/Foxp1 double stain shows the vLGE and striatum are severely disrupted in MEK/MAPK GOF embryos (H). Area (pixels) occupied by stain quantified in C for Gsx2, F for Sp8, and I for Foxp1 (asterisk in C,F, and I= p<.001, significance determined by Students t-test). Scale bars in: B= 100μM for images in A-B,D-E, H= 500μM for images in G-H. LGE= lateral ganglionic eminence
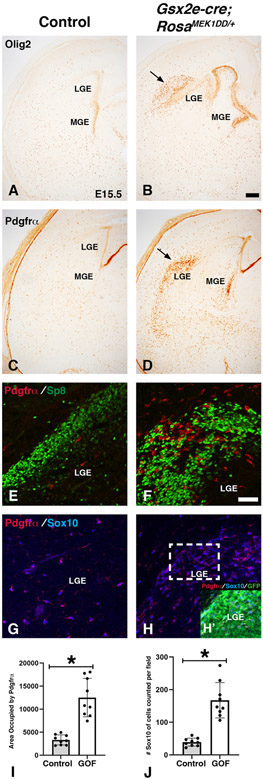
In addition to giving rise to dLGE and vLGE neuronal subdomains, the LGE also produces OPCs during development (Kessaris et al., 2006). Mice mutant for upstream MAPK regulators, Shp2 and FGFR1/2/3 show severe defects in OPC generation from the MGE and LGE at mid-gestation stages (Furusho et al., 2011; Ehrman et al., 2014). To address if sustained MAPK activity in Gsx2e-cre;RosaMEK1DD/+ double transgenic embryos impacts the generation of OPCs, we analyzed Olig2 and Pdgfrα at E15.5 (Fig. 5). Olig2 labels VZ progenitors in the MGE and LGE in addition to OPCs in the parenchyma where as Pdgfrα labels only OPCs in the parenchyma. We detected ectopic Olig2 and Pdgfrα adjacent to the LGE in Gsx2e-cre;RosaMEK1DD/+ embryos (see arrows in Fig. 5B and 5D) compared to controls (Fig. 5A and 5C). In addition, we detected abnormal ventral morphology as labeled by Olig2 (see MGE in Fig. 5B compared to 5A). The ectopic Olig2 and Pdgfrα cells were located in the same regions as the ectopic Gsx2 and Sp8 cells in double transgenic embryos (Fig. 4). To determine if these were unique populations, we double stained with Pdgfrα and Sp8 and found that these populations were largely distinct similar to controls (Fig. 5E-F). In addition, we confirmed that the Pdgfrα cells co-expressed another OPCs marker Sox10 (compare 5H to G). Quantification revealed a 3.75 fold increase in area stained with Pdgfrα and a 4.71 fold increase in Sox10 cells in double transgenic embryos (Fig. 5I-J). The RosaMEK1DD/+ allele contains a GFP to report recombination (Srinivasan et al., 2009). We confirmed the area with ectopic Pdgfrα and Sox10 cells was robustly labeled with GFP (Fig. 5H’), consistent with robust ventral recombination in the LGE/striatum of the Gsx2e-cre (Qin et al., 2016). Our results support previous studies showing that the timing of these two distinct populations are under the control of Gsx gene function as Gsx2 and Gsx1/2 mutants show precocious Pdgfrα expression and reduced Sp8 expression in the LGE (Chapman et al., 2013, 2018). These results suggest that sustained MAPK activity influences LGE progenitor cells to generate cells with dLGE or OPC identity during development.
Figure 5: Sustained MEK/MAPK activity in the LGE increases oligodendrocyte progenitor cell specification.
Representative images at E15.5 from controls (A, C, E, G) and MEK/MAPK GOF double transgenic Gsx2e-Cre;RosaMEK1DD/+ (B, D, F, H) embryos. At LGE/MGE levels, MEK/MAPK GOF embryos show altered morphology in ventral telencephalon and increased expression of Olig2 in both LGE/MGE VZ and near dorsal LGE (B) compared to controls (A). Unlike Olig2, Pdgfrα is not expressed in the VZ but does robustly label newly generated OPCs in the parenchyma (C). MEK/MAPK GOF embryos show precocious Pdgfrα staining in the MGE VZ and near the dLGE (arrows in D). The Pdgfrα staining pattern in MEK/MAPK GOF embryos resembled the expanded Sp8 expression in Figure 4. Double immunofluorescence revealed very little overlap between Pdgfrα and Sp8 (F) and nearly complete overlap with the OPC marker Sox10 (H). Inset in H reveals overlap with the MEK1DD allele (GFP) in Pdgfrα;Sox10 cells. Scale bars in: B= 200μM for images in A-D. F= 50μM for images in E-H. Pdgfrα was quantified as area (pixels) occupied by stain (I) and Sox10 was quantified as Sox10+ cells per field (asterisk in I and J= p<.001, significance determined by Students t-test).
To determine if LGE morphology was altered at early stages, we stained E13.5 Gsx2e-cre;RosaMEK1DD/+ double transgenic embryos with p-ERK1/2 and Gsx2. The LGE appears as a pronounced elevation by E13.5 and can be labeled by Gsx2 and p-ERK expression (Fig. 6A and C). Gsx2e-cre;RosaMEK1DD/+ double transgenic embryos already show abnormal LGE morphology and enhanced p-ERK1/2 immunoreactivity in the LGE at E13.5 (Fig. 6B) which is consistent with the timing of Gsx2e-cre expression (Qin et al., 2016). Gsx2 is expressed in double transgenic embryos but the normal pattern of high dorsal to low ventral expression detected in controls appears more uniform after MEK/MAPK activation (Compare arrows in Fig. 6D to C). Interestingly, despite the robust increase in Gsx2 and OPC markers at E15.5, early stage double transgenic embryos do not show expanded Gsx2+ cells outside of the LGE VZ or precocious Pdgfrα expression in the LGE. In addition, the Pdgfrα expression in the ventral MGE region is similar to controls (compare Fig. 6F to E). These results suggest that the robust changes in dLGE and OPC identity markers observed at E15.5 in GOF embryos are from MAPK activation either sustained over time or at these later stages of development.
Figure 6: Sustained MEK/MAPK activity alters LGE morphology at early stages.
Representative images at E13.5 from controls (A, C, and E) and MEK/MAPK GOF double transgenic Gsx2e-Cre;RosaMEK1DD/+ (B, D, and F) embryos. p-ERK1/2 expression is increased at E13.5 in MEK/MAPK GOF embryos (B) compared to controls (A). Gsx2 is expressed in presumptive LGE area of MEK/MAPK GOF embryos but the high dorsal to low ventral gradient observed in controls (arrows in C) is more uniform in GOF embryos (arrows in D). Unlike later stages, MEK/MAPK GOF embryos at E13.5 do not show precocious Pdgfrα staining in the LGE. The Pdgfrα staining pattern in the ventral MGE of MEK/MAPK GOF embryos (F) is similar to controls (E). Scale bars in: B= 500μM for images in A-D. F= 500μM for images in E-F.
Sustained activation of MEK/MAPK pathway throughout the dorsal telencephalon
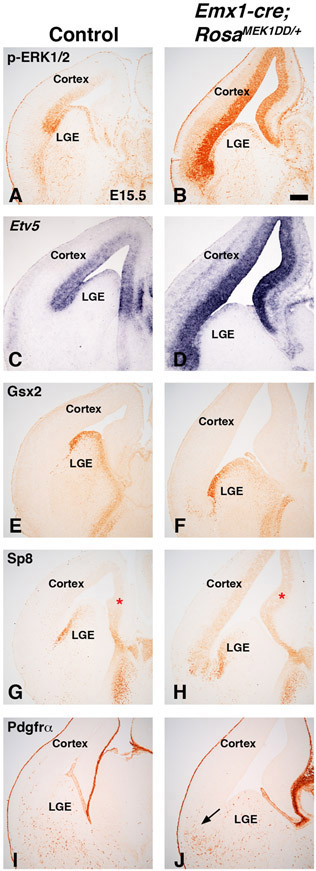
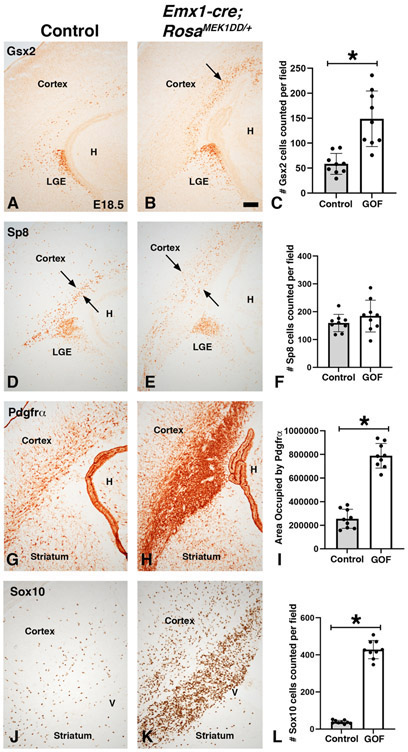
In addition to the scattered expression in the dLGE domain, p-ERK1/2 and Etv5 also robustly label cells dorsal to the Gsx2 domain in the dorsal telencephalon (Fig. 1-2). Previous work has shown that MEK1/2 and MAPK pathway activation play a key role in normal cortical radial glia development and OPC generation (Li et al., 2012; Li et al., 2014). To determine if sustained expression of MAPK activity in the dorsal telencephalon can induce the generation of dLGE identity cells in addition to OPCs, we generated double transgenic mice using Emx1cre/+ (Gorski et al., 2002) to drive expression in the developing cortex (Emx1cre/+;RosaMEK1DD/+). p-ERK1/2 and Etv5 were robustly expressed in the VZ of the developing cortex of Emx1cre/+;RosaMEK1DD/+ at E15.5 (compare Fig. 7B,D to 7A,C). Similar to the Gsx2e-cre driven experiments, we found abnormal brain morphology with an expanded ventricle region in double transgenic embryos. Unlike Gsx2e-cre;RosaMEK1DD/+ embryos, Gsx2 and Pdgfrα expression were not induced in the developing cortex of E15.5 double transgenic embryos (Fig. 7E-F and 7I-J). Interestingly, the Pdgfrα cells that are generated ventrally before E15.5 in MGE and LGE and migrate toward the cortex were disrupted in double transgenic embryos (arrows in Fig. 7J) compared to controls (Fig. 7I) suggesting that sustained MAPK activity in the cortex near the pallio-subpallial boundary might alter migration of cells generated from MGE and LGE. We also stained double transgenic embryos for Sp8, which is a dLGE identity marker but labels dorsomedial VZ progenitors as well (Borello et al., 2014). We found increased Sp8 immunoreactivity in the VZ of the developing cortex (compare Fig. 7H to 7G). We suspect this might be from an expansion of the dorsomedial Sp8 expression region (asterisk in 7G-H), which is known to be sensitive to changes in FGF activity (Sahara et al., 2007). It is also possible that Sp8 expression in both regions might be sensitive to MAPK activity. In contrast to the analysis of Emx1cre/+;RosaMEK1DD/+ double transgenic embryos at E15.5, later stage embryos (E18.5) revealed clear ectopic Gsx2 and disorganized Sp8 expressing cells in the cortex (see arrows in Fig. 8B and 8E). Quantification revealed a 2.55 fold increase in Gsx2 cells in the cortex of double transgenics (Fig. 8C). However, despite the disorganized appearance, there was no significant change in the number of Sp8+ cells (Fig. 8F). The OPC markers Pdgfrα and Sox10 were increased in the cortex of double transgenics at E18.5 (compare Fig. 8H,K to 8G,J). Quantification revealed a 3.10 fold increase in area occupied by Pdgfrα stain and a robust 11.31 fold increase in Sox10 cells in the cortex of double transgenic embryos (Fig. 8I,L). Our results suggest that sustained MAPK activity in the developing cortex strongly influences OPC specification. The increase in Gsx2 expression might be related to the OPC increase if the cortical Gsx2 cells are tripotent as recently suggested (Zhang et al., 2020). Another possibility is that ventral Gsx2 cells migrate from the pallio-subpallial boundary and “seed” the cortex since it has been shown that Emx1cre/+ mice recombine in the ventral Gsx2 dLGE domain (Waclaw et al., 2009). Alternatively, the cortical Gsx2 might reflect a new progenitor cell not related to the dLGE domain that contributes to postnatal olfactory bulb neurons (Li et al., 2021). Regardless of regional origin, sustained MAPK activity influences the expression of Gsx2 in addition to the robust effect on OPC identity.
Figure 7: Sustained MEK/MAPK activity in the cortex alters normal morphology but does not induce LGE or OPC genes at mid-gestation stages.
Representative images at E15.5 from controls (A, C, E, G, I) and MEK/MAPK GOF double transgenic Emx1-Cre;RosaMEK1DD/+ (B, D, F, H, J) embryos. p-ERK1/2 and Etv5 expression are increased in the VZ of cortical MEK/MAPK GOF embryos (compare B,D to A,C). Cortical MEK/MAPK GOF embryos showed an enlarged ventricle (compare B to A). Gsx2 expression was not detected in the cortex of MEK/MAPK GOF embryos (compare F to E). Sp8 expression was increased in the cortical VZ (compare H to G). Red asterisk refers to dorsal medial area of cortical Sp8 expression in controls (G) and expanded expression in MEK/MAPK GOF embryos (H). The OPC marker Pdgfrα was not detected in the cortex of MEK/MAPK GOF embryos (J). Arrow in J refers to clustering of migrating ventral Pdgfrα OPCs that are not observed in controls (I). Scale bars in: B= 200μM for images in A-J.
Figure 8: Sustained MEK/MAPK activity in the cortex results in the expression of dLGE and OPC genes at late embryonic stages.
Representative images at E18.5 from controls (A, D, G, and J) and MEK/MAPK GOF double transgenic Emx1-Cre;RosaMEK1DD/+ (B, E, H, and K) embryos. Increased numbers of Gsx2+ cells were observed in the cortex near the ventricular zone of cortical MEK/MAPK GOF embryos (arrows in B) compared to control embryos that only show sparse Gsx2+ cells in the cortex (A). Sp8 positive cells are observed near the cortical VZ of controls (D). Cortical MEK/MAPK GOF embryos show a disorganized pattern of Sp8 cells (compare arrows in E to D). The OPC markers Pdgfrα and Sox10 were robustly increased in MEK/MAPK GOF embryos in the cortical VZ and into the cortical mantle zone (compare H,K to G,J). Gsx2 (C), Sp8 (F), and Sox10 (L) were quantified cells per field. Pdgfrα (I) was quantified as area (pixels) occupied by stain. Asterisk in C, I, and L= p<.001, significance determined by Students t-test. Graph in F showed no significant change in Sp8 cell number (p=0.2659, determined by Students t-test). Scale bars in: B= 200μM for images in A-F.
Discussion
In this study, we evaluated the MAPK pathway by characterizing the expression of the downstream pathway readouts p-ERK1/2 and Etv5 in the ventral telencephalon. Our results indicate that p-ERK1/2 and Etv5 show similar expression patterns in the dorsal most LGE (dLGE) and ventral most MGE (vMGE) during midgestation stages at E12.5 and E15.5. However, as development proceeds, the expression of both factors are more uniform throughout LGE progenitors. Using a conditional approach to misexpress a constitutively active MEK1 allele (RosaMEK1DD/+) restricted to the ventral telencephalon (i.e. Gsx2e-cre), we identified that MEK/MAPK activation severely alters normal ventral brain morphology and promotes the expression of dLGE identity factors (Gsx2 and Sp8) and also OPC markers (Olig2, Pdgfrα, and Sox10). Moreover, we found that sustained misexpression of active MEK1 in the dorsal telencephalon using Emx1cre/+ promoted Gsx2 and OPC markers only in late stage embryos (E18.5). Our results suggest that robust MAPK pathway activation in dorsal or ventral progenitors influences dLGE and OPC identity markers in the telencephalon. These findings contribute significantly to understanding the role of MAPK pathway activation in the developing brain and more specifically identify a new role for pathway activation during development of the ventral telencephalon.
Even though MAPK signaling has long been associated as a growth signal in multiple cell types in the body (Dhillon et al., 2007), its enriched regional expression during development in the ventral telencephalon is noteworthy and suggests that this pathway may influence distinct cell types. Indeed, previous work has shown that MAPK pathway readouts show clear regional restrictions at early stages of cortical development in the dorsal telencephalon (Borello et al., 2008 and Faedo et al., 2010). We identified regional expression of both p-ERK1/2 and Etv5 in the ventral telencephalon at E13.5 and E15.5 in the dLGE and vMGE VZ progenitors. Specifically, we found Etv5+ cells located in the dorsal most Gsx2+ domain of the dLGE and the ventral most Nkx2.1+ domain in the vMGE. The subpallial ventricular zone is proposed to contain multiple progenitor domains based on expression of distinct transcription factors (Flames et al., 2007). Based upon this classification, the active MAPK cells (Etv5+p-ERK1/2+) are located in pLGE1/2 of the dLGE region (i.e. dorsal part of the Gsx2 domain) and in the ventral areas of pMGE5 in the vMGE region (i.e. part of the Nkx2.1 domain) at E13.5 and E15.5. Interestingly, the regional expression pattern changes to a more generalized expression pattern throughout the LGE progenitors at E18.5. Whether this expression change is linked to temporal differences in the generation of cellular diversity from progenitors remains unknown. The generation of a transgenic or knock-in reporter allele into Etv5 would be useful for future lineage studies or MAPK activation experiments in the telencephalon. Our data show that MAPK pathway activation readouts can help define regional progenitor domains during distinct stages of development in the ventral telencephalon.
Previous studies have linked MEK induced MAPK pathway activation to oligodendrocyte myelination in the postnatal telencephalon (Fyffe-Maricich et al., 2013; Ishii et al., 2013). In addition, it is known that MEK1/2 are crucial for normal radial glia development and OPC generation in the embryonic cortex in the dorsal telencephalon (Li et al., 2012). Also, transient electroporation of active RAS/RAF/MEK in the cortex increases p-ERK1/2, Etv5, and Ascl1 resulting in an ectopic expression of OPC markers (Li et al., 2014). However, little is known if MAPK pathway activation can influence regional development in the ventral telencephalon. Our study using a cre inducible constitutively active MEK1 allele combined with Gsx2e-cre shows that MAPK pathway activation throughout the LGE results in a robust anatomical defect with embryos showing abnormal ventral morphology and decreased striatal complex. Interestingly, we detected ectopic dLGE identity markers and OPC markers in double transgenic embryos. However, it appears these markers do not overlap, suggesting MEK/MAPK pathway activation promotes the generation of multiple cell types. Our results support previous work showing that FGF signaling upstream of MAPK activation is crucial for normal ventral development and also embryonic OPC generation (Gutin et al., 2006; Furusho et al., 2011). Our data show for the first time that MEK/MAPK activation influences regional identity by promoting dLGE- and OPC- like cells in the ventral telencephalon. Loss of function studies in the MAPK pathway in the brain are challenging given the redundancy of signaling components (Li et al., 2012; Xing et al., 2016). Given our GOF results, future loss of function studies on compound mutants of either MEK1/2, ERK1/2, or downstream factors Etv1,4,5 may reveal if the pathway is required for normal patterning and cell type generation in these ventral domains. In addition, it will be interesting to evaluate p-ERK1/2 or Etv5 expression in mutants for Gsx2 or Pax6 which are key dorso-ventral patterning regulators in the telencephalon (Corbin et al., 2000, Toresson et al., 2000; Yun et al., 2001). In fact, the expression of p-ERK1/2 and Etv5 seems to be enriched in the lateral telencephalon (i.e. both dLGE and ventral pallium), which is similar to the orphan nuclear receptor Tlx/tailess (Stenman et al., 2003). Tlx mutants show a dorsal shift in markers of the pallio-subpallial boundary including Gsx2 (Stenman et al., 2003). Given the robust effect of MEK/MAPK activation on Gsx2 expression, Tlx is an interesting candidate to consider with respect to the MAPK pathway in this region.
Multiple lines of evidence have implicated upstream or downstream factors of MAPK pathway as key regulators of cortical patterning in the dorsal telencephalon. In fact, FGF signaling molecules (namely Fgf8 and Fgf15) and Spry1/2 (MAPK feedback regulators) are key players at early patterning stages by establishing the rostral patterning centers in the most anterior region of the telencephalon (Fukuchi-Shimogori and Grove 2001; Borello et al., 2008, Faedo et al., 2010; Toyoda et al., 2010; Hoch et al., 2015). Loss of FGF signaling by compound FGF receptor mutants results in a nearly complete loss of all telencephalic structures (Paek et al., 2009). Also, previous work has provided direct evidence that MEK compound mutants or MEK GOF regulates cortical radial glia and cortical OPC generation (Li et al., 2012). To determine if ectopic MEK/MAPK activation in cortical progenitors influence dLGE or OPC identity, we utilized Emx1cre/+ to drive MEK1DD allele. Interestingly, despite robust p-ERK1/2 expression throughout cortical progenitors at E15.5, we did not observe ectopic dLGE or OPC markers. However, similar to the previous work (Li et al., 2012; Li et al., 2014), our GOF studies showed that sustained expression in cortical progenitors promoted OPC markers at late embryonic stages. In addition, our study reveals that Gsx2 is also increased at later stages. This is interesting given that at embryonic stages Gsx genes are thought to regulate the timing of LGE gliogenesis by repressing OPC markers (Chapman et al., 2013, 2018). Therefore, it may be that MAPK activity influences the generation of distinct markers that provide a balance in the appropriate numbers of cell types generated (OPC or dLGE identity). Alternatively, it is possible that MAPK activity has a selective effect on proliferation of distinct progenitors in the telencephalon resulting in expanded populations. It is also possible that the MAPK pathway is working together with other major developmental signaling pathways. In fact, a recent study found that late embryonic and early postnatal stage cortical neural stem cells generate tri-potential Gsx2+ cells that are regulated by SHH signaling (Zhang et al., 2020). Future experiments examining the intersection of SHH signaling, MAPK activation, and Gsx2+ cells will help define the complex balance between the major signaling pathways and progenitor cell diversity.
Dysregulation of the MAPK pathway is implicated in human disease. RASopathies are a group of syndromes caused by mutations in regulators or signaling components of the MAPK pathway. Therefore, studies on the MAPK pathway during normal brain development are crucial to understand the influence of appropriate signaling during brain development. Our results provide clues to cell types in the telencephalon that are sensitive to elevations in the pathway in a MEK activated mouse model. Indeed, evidence from RASopathy patients with Neurfibromatosis Type 1 (NF1) showing imaging abnormalities in subcortical brain regions and white matter tracts (Rosenbaum et al., 1999; Yokota et al., 2008; Payne et al., 2014) and several studies on RASopathy mouse models for NS, CFC, and NF1 showing defects during oligodendrocyte development (Bennett et al., 2003; Dasgupta and Gutmann, 2005; Zhu et al., 2005; Mayes et al., 2013; Ehrman et al., 2014; Lopez-Juarez et al., 2017; Titus et al., 2017; Aoidi et al., 2018; Holter et al., 2019) support our new data linking MAPK activation in ventral brain development to basal ganglia progenitor areas (LGE) and OPC cell types. Future studies focused on the postnatal consequence of developmental MAPK pathway activation in the telencephalon may provide additional clues to underlying defects in the cortex, striatum, and white matter caused by RASopathy mutations. It seems likely that each RASopathy mutation impacts the pathway at different levels and strength. In fact, a recent study identified mutations in the downstream effector MAPK1 (ERK2) that cause a severe neurodevelopmental disorder with some features of NS (Motta et al., 2020) further highlighting the importance of understanding p-ERK1/2 expression and function in the developing brain. It will be interesting to determine if activation of the MAPK pathway via RASopathy mutations reaches a critical threshold to impact the cell types highlighted in the paper, which would likely yield defects in basal ganglia or white matter development. Understanding the cells most sensitive to RASopathy mutations will provide clues for treatment of these conditions.
Highlights.
p-ERK1/2 and Etv5 show regional expression in the developing LGE and MGE.
Sustained MEK activity in the ventral telencephalon (Gsx2e-cre; RosaMEK1DD/+) expanded markers of the dorsal lateral ganglionic eminence region and oligodendrocyte progenitor cells
Activation of MEK activity in the dorsal telencephalon (Emx1cre/+; RosaMEK1DD/+) increased Gsx2 expression and oligodendrocyte progenitor cell markers at late embryonic stages.
Acknowledgments
This work was supported by NIH grant NS088529 to RRW. We would like to thank Dr. Kenneth Campbell (CCHMC) for the Gsx2 antibody and Gsx2e-cre transgenic mice in addition to critical reading of the manuscript. We would also like to thank Dr. Xin Sun (UCSD) and Dr. Susan Mansour (The University of Utah) for providing plasmids to generate Etv5 in situ probes.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson SA, Eisenstat DD, Shi L, Rubenstein JL, 1997. Interneuron migration from basal forebrain to neocortex: dependence on Dlx genes. Science 278, 474–476. [DOI] [PubMed] [Google Scholar]
- Aoidi R, Houde N, Landry-Truchon K, Holter M, Jacquet K, Charron L, Krishnaswami SR, Yu BD, Rauen KA, Bisson N, Newbern J, Charron J, 2018. Mek1(Y130C) mice recapitulate aspects of human cardio-facio-cutaneous syndrome. Dis Model Mech 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett MR, Rizvi TA, Karyala S, McKinnon RD, Ratner N, 2003. Aberrant growth and differentiation of oligodendrocyte progenitors in neurofibromatosis type 1 mutants. J Neurosci 23, 7207–7217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borello U, Cobos I, Long JE, McWhirter JR, Murre C, Rubenstein JL, 2008. FGF15 promotes neurogenesis and opposes FGF8 function during neocortical development. Neural Dev 3, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borello U, Madhavan M, Vilinsky I, Faedo A, Pierani A, Rubenstein J, Campbell K, 2014. Sp8 and COUP-TF1 reciprocally regulate patterning and Fgf signaling in cortical progenitors. Cereb Cortex 24, 1409–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell K, 2003. Dorsal-ventral patterning in the mammalian telencephalon. Curr Opin Neurobiol 13, 50–56. [DOI] [PubMed] [Google Scholar]
- Carney RS, Alfonso TB, Cohen D, Dai H, Nery S, Stoica B, Slotkin J, Bregman BS, Fishell G, Corbin JG, 2006. Cell migration along the lateral cortical stream to the developing basal telencephalic limbic system. J Neurosci 26, 11562–11574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman H, Riesenberg A, Ehrman LA, Kohli V, Nardini D, Nakafuku M, Campbell K, Waclaw RR, 2018. Gsx transcription factors control neuronal versus glial specification in ventricular zone progenitors of the mouse lateral ganglionic eminence. Dev Biol 442, 115–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman H, Waclaw RR, Pei Z, Nakafuku M, Campbell K, 2013. The homeobox gene Gsx2 controls the timing of oligodendroglial fate specification in mouse lateral ganglionic eminence progenitors. Development 140, 2289–2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cholfin JA, Rubenstein JL, 2008. Frontal cortex subdivision patterning is coordinately regulated by Fgf8, Fgf17, and Emx2. J Comp Neurol 509, 144–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbin JG, Gaiano N, Machold RP, Langston A, Fishell G, 2000. The Gsh2 homeodomain gene controls multiple aspects of telencephalic development. Development 127, 5007–5020. [DOI] [PubMed] [Google Scholar]
- D'Angelo F, Ceccarelli M, Tala, Garofano L, Zhang J, Frattini V, Caruso FP, Lewis G, Alfaro KD, Bauchet L, Berzero G, Cachia D, Cangiano M, Capelle L, de Groot J, DiMeco F, Ducray F, Farah W, Finocchiaro G, Goutagny S, Kamiya-Matsuoka C, Lavarino C, Loiseau H, Lorgis V, Marras CE, McCutcheon I, Nam DH, Ronchi S, Saletti V, Seizeur R, Slopis J, Sunol M, Vandenbos F, Varlet P, Vidaud D, Watts C, Tabar V, Reuss DE, Kim SK, Meyronet D, Mokhtari K, Salvador H, Bhat KP, Eoli M, Sanson M, Lasorella A, Iavarone A, 2019. The molecular landscape of glioma in patients with Neurofibromatosis 1. Nat Med 25, 176–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta B, Gutmann DH, 2005. Neurofibromin regulates neural stem cell proliferation, survival, and astroglial differentiation in vitro and in vivo. J Neurosci 25, 5584–5594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhillon AS, Hagan S, Rath O, Kolch W, 2007. MAP kinase signalling pathways in cancer. Oncogene 26, 3279–3290. [DOI] [PubMed] [Google Scholar]
- Diez-Roux G, Banfi S, Sultan M, Geffers L, Anand S, Rozado D, Magen A, Canidio E, Pagani M, Peluso I, Lin-Marq N, Koch M, Bilio M, Cantiello I, Verde R, De Masi C, Bianchi SA, Cicchini J, Perroud E, Mehmeti S, Dagand E, Schrinner S, Nurnberger A, Schmidt K, Metz K, Zwingmann C, Brieske N, Springer C, Hernandez AM, Herzog S, Grabbe F, Sieverding C, Fischer B, Schrader K, Brockmeyer M, Dettmer S, Helbig C, Alunni V, Battaini MA, Mura C, Henrichsen CN, Garcia-Lopez R, Echevarria D, Puelles E, Garcia-Calero E, Kruse S, Uhr M, Kauck C, Feng G, Milyaev N, Ong CK, Kumar L, Lam M, Semple CA, Gyenesei A, Mundlos S, Radelof U, Lehrach H, Sarmientos P, Reymond A, Davidson DR, Dolle P, Antonarakis SE, Yaspo ML, Martinez S, Baldock RA, Eichele G, Ballabio A, 2011. A high-resolution anatomical atlas of the transcriptome in the mouse embryo. PLoS Biol 9, e1000582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty MJ, Santi M, Brose MS, Ma C, Resnick AC, Sievert AJ, Storm PB, Biegel JA, 2010. Activating mutations in BRAF characterize a spectrum of pediatric low-grade gliomas. Neuro Oncol 12, 621–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrman LA, Mu X, Waclaw RR, Yoshida Y, Vorhees CV, Klein WH, Campbell K, 2013. The LIM homeobox gene Isl1 is required for the correct development of the striatonigral pathway in the mouse. Proc Natl Acad Sci U S A 110, E4026–4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrman LA, Nardini D, Ehrman S, Rizvi TA, Gulick J, Krenz M, Dasgupta B, Robbins J, Ratner N, Nakafuku M, Waclaw RR, 2014. The protein tyrosine phosphatase Shp2 is required for the generation of oligodendrocyte progenitor cells and myelination in the mouse telencephalon. J Neurosci 34, 3767–3778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faedo A, Borello U, Rubenstein JL, 2010. Repression of Fgf signaling by sprouty1–2 regulates cortical patterning in two distinct regions and times. J Neurosci 30, 4015–4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flames N, Pla R, Gelman DM, Rubenstein JL, Puelles L, Marin O, 2007. Delineation of multiple subpallial progenitor domains by the combinatorial expression of transcriptional codes. J Neurosci 27, 9682–9695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuchi-Shimogori T, Grove EA, 2001. Neocortex patterning by the secreted signaling molecule FGF8. Science 294, 1071–1074. [DOI] [PubMed] [Google Scholar]
- Furusho M, Kaga Y, Ishii A, Hebert JM, Bansal R, 2011. Fibroblast growth factor signaling is required for the generation of oligodendrocyte progenitors from the embryonic forebrain. J Neurosci 31, 5055–5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fyffe-Maricich SL, Schott A, Karl M, Krasno J, Miller RH, 2013. Signaling through ERK1/2 controls myelin thickness during myelin repair in the adult central nervous system. J Neurosci 33, 18402–18408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorski JA, Talley T, Qiu M, Puelles L, Rubenstein JL, Jones KR, 2002. Cortical excitatory neurons and glia, but not GABAergic neurons, are produced in the Emx1-expressing lineage. J Neurosci 22, 6309–6314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutin G, Fernandes M, Palazzolo L, Paek H, Yu K, Ornitz DM, McConnell SK, Hebert JM, 2006. FGF signalling generates ventral telencephalic cells independently of SHH. Development 133, 2937–2946. [DOI] [PubMed] [Google Scholar]
- Hebert JM, Fishell G, 2008. The genetics of early telencephalon patterning: some assembly required. Nat Rev Neurosci 9, 678–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoch RV, Clarke JA, Rubenstein JL, 2015. Fgf signaling controls the telencephalic distribution of Fgf-expressing progenitors generated in the rostral patterning center. Neural Dev 10, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holter MC, Hewitt LT, Koebele SV, Judd JM, Xing L, Bimonte-Nelson HA, Conrad CD, Araki T, Neel BG, Snider WD, Newbern JM, 2019. The Noonan Syndrome-linked Raf1L613V mutation drives increased glial number in the mouse cortex and enhanced learning. PLoS Genet 15, e1008108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton SR, Otis JM, Kim EM, Lamsal Y, Stuber GD, Snider WD, 2017. ERK/MAPK Signaling Is Required for Pathway-Specific Striatal Motor Functions. J Neurosci 37, 8102–8115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii A, Furusho M, Bansal R, 2013. Sustained activation of ERK1/2 MAPK in oligodendrocytes and schwann cells enhances myelin growth and stimulates oligodendrocyte progenitor expansion. J Neurosci 33, 175–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii A, Furusho M, Bansal R, 2013. Sustained activation of ERK1/2 MAPK in oligodendrocytes and schwann cells enhances myelin growth and stimulates oligodendrocyte progenitor expansion. J Neurosci 33, 175–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang W, Wong LC, Shi SH, Hebert JM, 2009. The transition from radial glial to intermediate progenitor cell is inhibited by FGF signaling during corticogenesis. J Neurosci 29, 14571–14580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessaris N, Fogarty M, Iannarelli P, Grist M, Wegner M, Richardson WD, 2006. Competing waves of oligodendrocytes in the forebrain and postnatal elimination of an embryonic lineage. Nat Neurosci 9, 173–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohli V, Nardini D, Ehrman LA, Waclaw RR, 2018. Characterization of Glcci1 expression in a subpopulation of lateral ganglionic eminence progenitors in the mouse telencephalon. Dev Dyn 247, 222–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuerbitz J, Arnett M, Ehrman S, Williams MT, Vorhees CV, Fisher SE, Garratt AN, Muglia LJ, Waclaw RR, Campbell K, 2018. Loss of Intercalated Cells (ITCs) in the Mouse Amygdala of Tshz1 Mutants Correlates with Fear, Depression, and Social Interaction Phenotypes. J Neurosci 38, 1160–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Scott DA, Hatch E, Tian X, Mansour SL, 2007. Dusp6 (Mkp3) is a negative feedback regulator of FGF-stimulated ERK signaling during mouse development. Development 134, 167–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Newbern JM, Wu Y, Morgan-Smith M, Zhong J, Charron J, Snider WD, 2012. MEK Is a Key Regulator of Gliogenesis in the Developing Brain. Neuron 75, 1035–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Mattar P, Dixit R, Lawn SO, Wilkinson G, Kinch C, Eisenstat D, Kurrasch DM, Chan JA, Schuurmans C, 2014. RAS/ERK signaling controls proneural genetic programs in cortical development and gliomagenesis. J Neurosci 34, 2169–2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Liu G, Yang L, Li Z, Zhang Z, Xu Z, Cai Y, Du H, Su Z, Wang Z, Duan Y, Chen H, Shang Z, You Y, Zhang Q, He M, Chen B, Yang Z, 2021. Decoding Cortical Glial Cell Development. Neurosci Bull. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Juarez A, Titus HE, Silbak SH, Pressler JW, Rizvi TA, Bogard M, Bennett MR, Ciraolo G, Williams MT, Vorhees CV, Ratner N, 2017. Oligodendrocyte Nf1 Controls Aberrant Notch Activation and Regulates Myelin Structure and Behavior. Cell Rep 19, 545–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin O, Rubenstein JL, 2001. A long, remarkable journey: tangential migration in the telencephalon. Nat Rev Neurosci 2, 780–790. [DOI] [PubMed] [Google Scholar]
- Mayes DA, Rizvi TA, Titus-Mitchell H, Oberst R, Ciraolo GM, Vorhees CV, Robinson AP, Miller SD, Cancelas JA, Stemmer-Rachamimov AO, Ratner N, 2013. Nf1 loss and Ras hyperactivation in oligodendrocytes induce NOS-driven defects in myelin and vasculature. Cell Rep 4, 1197–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motta M, Pannone L, Pantaleoni F, Bocchinfuso G, Radio FC, Cecchetti S, Ciolfi A, Di Rocco M, Elting MW, Brilstra EH, Boni S, Mazzanti L, Tamburrino F, Walsh L, Payne K, Fernandez-Jaen A, Ganapathi M, Chung WK, Grange DK, Dave-Wala A, Reshmi SC, Bartholomew DW, Mouhlas D, Carpentieri G, Bruselles A, Pizzi S, Bellacchio E, Piceci-Sparascio F, Lissewski C, Brinkmann J, Waclaw RR, Waisfisz Q, van Gassen K, Wentzensen IM, Morrow MM, Alvarez S, Martinez-Garcia M, De Luca A, Memo L, Zampino G, Rossi C, Seri M, Gelb BD, Zenker M, Dallapiccola B, Stella L, Prada CE, Martinelli S, Flex E, Tartaglia M, 2020. Enhanced MAPK1 Function Causes a Neurodevelopmental Disorder within the RASopathy Clinical Spectrum. Am J Hum Genet 107, 499–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nery S, Fishell G, Corbin JG, 2002. The caudal ganglionic eminence is a source of distinct cortical and subcortical cell populations. Nat Neurosci 5, 1279–1287. [DOI] [PubMed] [Google Scholar]
- Paek H, Gutin G, Hebert JM, 2009. FGF signaling is strictly required to maintain early telencephalic precursor cell survival. Development 136, 2457–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne JM, Pickering T, Porter M, Oates EC, Walia N, Prelog K, North KN, 2014. Longitudinal assessment of cognition and T2-hyperintensities in NF1: an 18-year study. Am J Med Genet A 164A, 661–665. [DOI] [PubMed] [Google Scholar]
- Qin S, Madhavan M, Waclaw RR, Nakafuku M, Campbell K, 2016. Characterization of a new Gsx2-cre line in the developing mouse telencephalon. Genesis 54, 542–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum T, Engelbrecht V, Krolls W, van Dorsten FA, Hoehn-Berlage M, Lenard HG, 1999. MRI abnormalities in neurofibromatosis type 1 (NF1): a study of men and mice. Brain Dev 21, 268–273. [DOI] [PubMed] [Google Scholar]
- Sahara S, Kawakami Y, Izpisua Belmonte JC, O'Leary DD, 2007. Sp8 exhibits reciprocal induction with Fgf8 but has an opposing effect on anterior-posterior cortical area patterning. Neural Dev 2, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels IS, Karlo JC, Faruzzi AN, Pickering K, Herrup K, Sweatt JD, Saitta SC, Landreth GE, 2008. Deletion of ERK2 mitogen-activated protein kinase identifies its key roles in cortical neurogenesis and cognitive function. J Neurosci 28, 6983–6995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan L, Sasaki Y, Calado DP, Zhang B, Paik JH, DePinho RA, Kutok JL, Kearney JF, Otipoby KL, Rajewsky K, 2009. PI3 kinase signals BCR-dependent mature B cell survival. Cell 139, 573–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanco A, Pla R, Vogt D, Chen Y, Mandal S, Walker J, Hunt RF, Lindtner S, Erdman CA, Pieper AA, Hamilton SP, Xu D, Baraban SC, Rubenstein JL, 2014. NPAS1 represses the generation of specific subtypes of cortical interneurons. Neuron 84, 940–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenman J, Toresson H, Campbell K, 2003. Identification of two distinct progenitor populations in the lateral ganglionic eminence: implications for striatal and olfactory bulb neurogenesis. J Neurosci 23, 167–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenman J, Yu RT, Evans RM, Campbell K, 2003. Tlx and Pax6 co-operate genetically to establish the pallio-subpallial boundary in the embryonic mouse telencephalon. Development 130, 1113–1122. [DOI] [PubMed] [Google Scholar]
- Storm EE, Garel S, Borello U, Hebert JM, Martinez S, McConnell SK, Martin GR, Rubenstein JL, 2006. Dose-dependent functions of Fgf8 in regulating telencephalic patterning centers. Development 133, 1831–1844. [DOI] [PubMed] [Google Scholar]
- Tartaglia M, Gelb BD, 2010. Disorders of dysregulated signal traffic through the RAS-MAPK pathway: phenotypic spectrum and molecular mechanisms. Ann N Y Acad Sci 1214, 99–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titus HE, Lopez-Juarez A, Silbak SH, Rizvi TA, Bogard M, Ratner N, 2017. Oligodendrocyte RasG12V expressed in its endogenous locus disrupts myelin structure through increased MAPK, nitric oxide, and notch signaling. Glia 65, 1990–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toresson H, Potter SS, Campbell K, 2000. Genetic control of dorsal-ventral identity in the telencephalon: opposing roles for Pax6 and Gsh2. Development 127, 4361–4371. [DOI] [PubMed] [Google Scholar]
- Toyoda R, Assimacopoulos S, Wilcoxon J, Taylor A, Feldman P, Suzuki-Hirano A, Shimogori T, Grove EA, 2010. FGF8 acts as a classic diffusible morphogen to pattern the neocortex. Development 137, 3439–3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waclaw RR, Allen ZJ 2nd, Bell SM, Erdelyi F, Szabo G, Potter SS, Campbell K, 2006. The zinc finger transcription factor Sp8 regulates the generation and diversity of olfactory bulb interneurons. Neuron 49, 503–516. [DOI] [PubMed] [Google Scholar]
- Waclaw RR, Ehrman LA, Pierani A, Campbell K, 2010. Developmental origin of the neuronal subtypes that comprise the amygdalar fear circuit in the mouse. J Neurosci 30, 6944–6953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waclaw RR, Wang B, Pei Z, Ehrman LA, Campbell K, 2009. Distinct temporal requirements for the homeobox gene Gsx2 in specifying striatal and olfactory bulb neuronal fates. Neuron 63, 451–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing L, Larsen RS, Bjorklund GR, Li X, Wu Y, Philpot BD, Snider WD, Newbern JM, 2016. Layer specific and general requirements for ERK/MAPK signaling in the developing neocortex. Elife 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Cobos I, De La Cruz E, Rubenstein JL, Anderson SA, 2004. Origins of cortical interneuron subtypes. J Neurosci 24, 2612–2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota O, Tsuchiya K, Hayashi M, Kakita A, Ohwada K, Ishizu H, Takahashi H, Akiyama H, 2008. Glial clusters and perineuronal glial satellitosis in the basal ganglia of neurofibromatosis type 1. Acta Neuropathol 116, 57–66. [DOI] [PubMed] [Google Scholar]
- Yun K, Potter S, Rubenstein JL, 2001. Gsh2 and Pax6 play complementary roles in dorsoventral patterning of the mammalian telencephalon. Development 128, 193–205. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Liu G, Guo T, Liang XG, Du H, Yang L, Bhaduri A, Li X, Xu Z, Zhang Z, Li Z, He M, Tsyporin J, Kriegstein AR, Rubenstein JL, Yang Z, Chen B, 2020. Cortical Neural Stem Cell Lineage Progression Is Regulated by Extrinsic Signaling Molecule Sonic Hedgehog. Cell Rep 30, 4490–4504 e4494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Verheyden JM, Hassell JA, Sun X, 2009. FGF-regulated Etv genes are essential for repressing Shh expression in mouse limb buds. Dev Cell 16, 607–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Harada T, Liu L, Lush ME, Guignard F, Harada C, Burns DK, Bajenaru ML, Gutmann DH, Parada LF, 2005. Inactivation of NF1 in CNS causes increased glial progenitor proliferation and optic glioma formation. Development 132, 5577–5588. [DOI] [PMC free article] [PubMed] [Google Scholar]