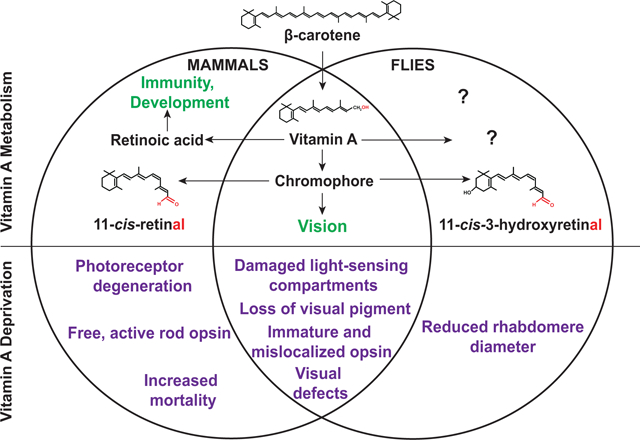
Abstract
Vitamin A deficiency can cause human pathologies that range from blindness to embryonic malformations. This diversity is due to the lack of two major vitamin A metabolites with very different functions: the chromophore 11-cis-retinal (vitamin A aldehyde) is a critical component of the visual pigment that mediates phototransduction, while the signaling molecule all-trans-retinoic acid regulates the development of various tissues and is required for the function of the immune system.
Since animals cannot synthesize vitamin A de novo, they must obtain it either as preformed vitamin A from animal products or as carotenoid precursors from plant sources. Due to its essential role in the visual system, acute vitamin A deprivation impairs photoreceptor function and causes night blindness (poor vision under dim light conditions), while chronic deprivation results in retinal dystrophies and photoreceptor cell death. Chronic vitamin A deficiency is the leading cause of preventable childhood blindness according to the World Health Organization. Due to the requirement of vitamin A for retinoic acid signaling in development and in the immune system, vitamin A deficiency also causes increased mortality in children and pregnant women in developing countries.
Drosophila melanogaster is an excellent model to study the effects of vitamin A deprivation on the eye because vitamin A is not essential for Drosophila development and chronic deficiency does not cause lethality. Moreover, genetic screens in Drosophila have identified evolutionarily conserved factors that mediate the production of vitamin A and its cellular uptake. Here, we review our current knowledge about the role of vitamin A in the visual system of mammals and Drosophila melanogaster. We compare the molecular mechanisms that mediate the uptake of dietary vitamin A precursors and the metabolism of vitamin A, as well as the consequences of vitamin A deficiency for the structure and function of the eye.
Keywords: vision, photoreceptor, vitamin A, rhodopsin, chromophore, carotene, retinoic acid, visual pigment, rhabdomere, outer segment, rod, cone, visual cycle
Graphical Abstract
1. Carotenoid-based chromophores, visual pigments, and other eye pigments
Carotenoids are organic pigments that are produced by plants, algae, and photosynthetic bacteria (von Lintig, 2010). They produce the colors of various fruits and vegetables that range from bright yellow to dark red. Carotenoids are a diverse group of lipophilic isoprenoids that can be divided into two main classes: the carotenes, which lack oxygen atoms, and the xanthophylls, which contain oxygen atoms. Some carotenoids have provitamin A activity, which means that they can be converted to vitamin A in our body. Since animals are unable to synthesize carotenoids, they need to obtain vitamin A either as preformed vitamin A from animal products or synthesize it from plant-derived precursors such as β-carotene (von Lintig, 2012). In mammals, β-carotene is converted in the gut to retinal (vitamin A aldehyde), which can be converted to retinol (vitamin A) or retinyl ester (Fig. 1).
Figure 1. Major carotenoid and retinoid molecules in mammalian vitamin A metabolism.
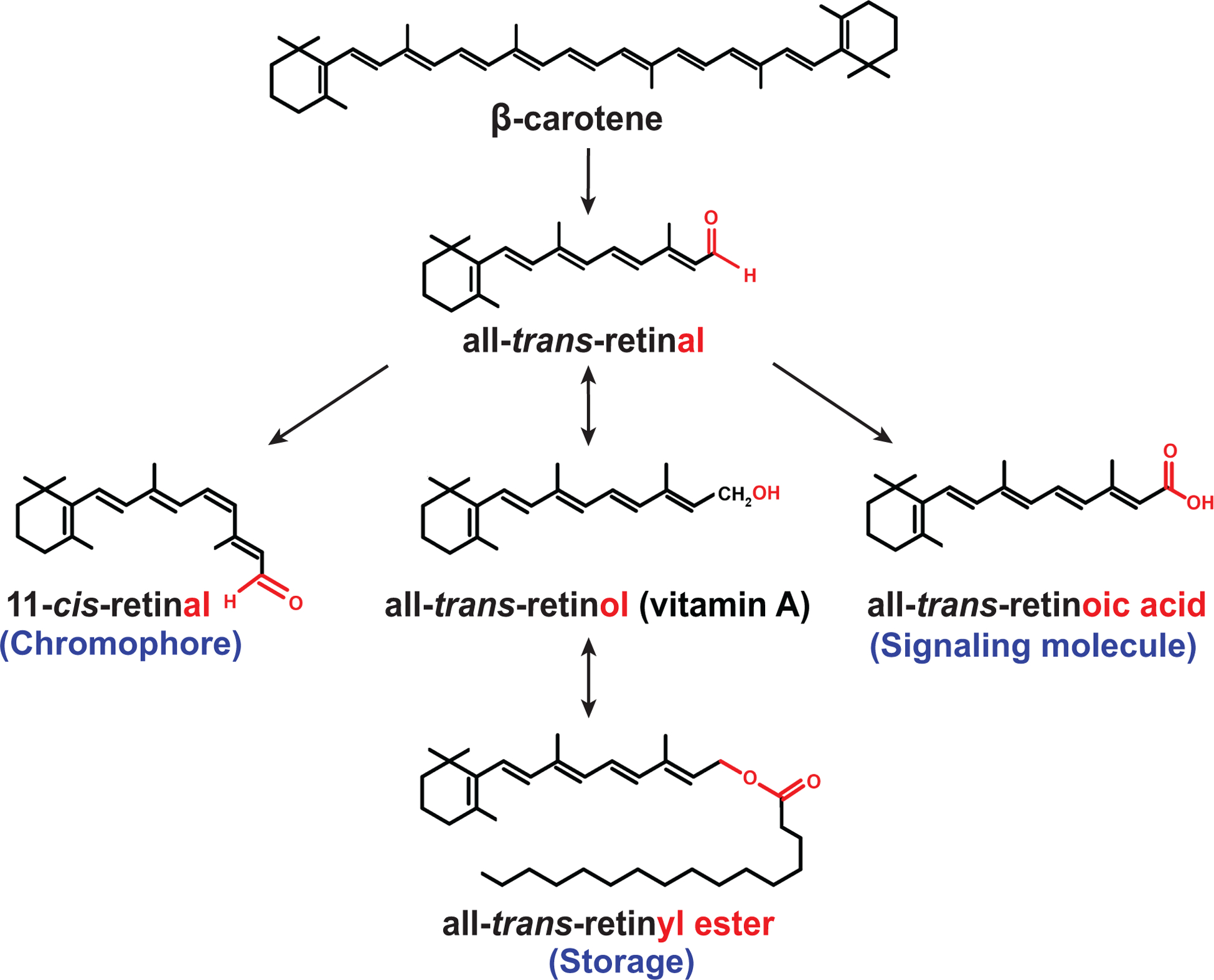
The oxidative cleavage of the dietary precursor β-carotene generates all-trans-retinal (vitamin A aldehyde), which can be isomerized to the visual chromophore 11-cis-retinal. Oxidation of all-trans-retinal results in the signaling molecule all-trans-retinoic acid, while reduction of all-trans-retinal generates all-trans-retinol (vitamin A) that can be esterified to all-trans-retinyl ester, an important transport and storage form of vitamin A in mammals.
The 11-cis-retinal chromophore (Fig. 1), or a closely related variant (e.g. 3,4-didehydroretinal in fish and amphibians or 11-cis-3-hydroxyretinal in some insects), is the basis of vision in the animal kingdom (Tsin and Santos, 1985; Vogt, 1984; Wald, 1968; Wald and Brown, 1956; Yau and Hardie, 2009). It is covalently attached via a Schiff base linkage to a specific opsin protein and thereby forms the visual pigment (Brown and Wald, 1964; Wald, 1968) (Fig. 2). Visual pigments are G protein-coupled seven transmembrane receptors that are embedded in the membranes of specialized light-sensing photoreceptor compartments, which are called outer segments in humans (Fig. 2) and rhabdomeres in flies (Kumar and Ready, 1995; Nickell et al., 2007). The wavelength sensitivity of a visual pigment is determined by interactions between the retinal chromophore and specific amino acids of its opsin partner (Kefalov et al., 2010; Salcedo et al., 2009; Zheng et al., 2015). Animals therefore commonly express different opsins that form different visual pigments in specific photoreceptor subtypes (Rister and Desplan, 2011). For instance, the human eye expresses a rod opsin (Rhodopsin) for vision at dim light conditions and three cone opsins that are sensitive to different wavelengths of light, while the Drosophila eye expresses six Rhodopsins with different wavelength sensitivities (Rister et al., 2013). The expression of visual pigments with different wavelength sensitivities in different photoreceptor subtypes is a prerequisite for color vision (Briscoe and Chittka, 2001).
Figure 2. Carotenoid uptake and vitamin A metabolism in mammals.
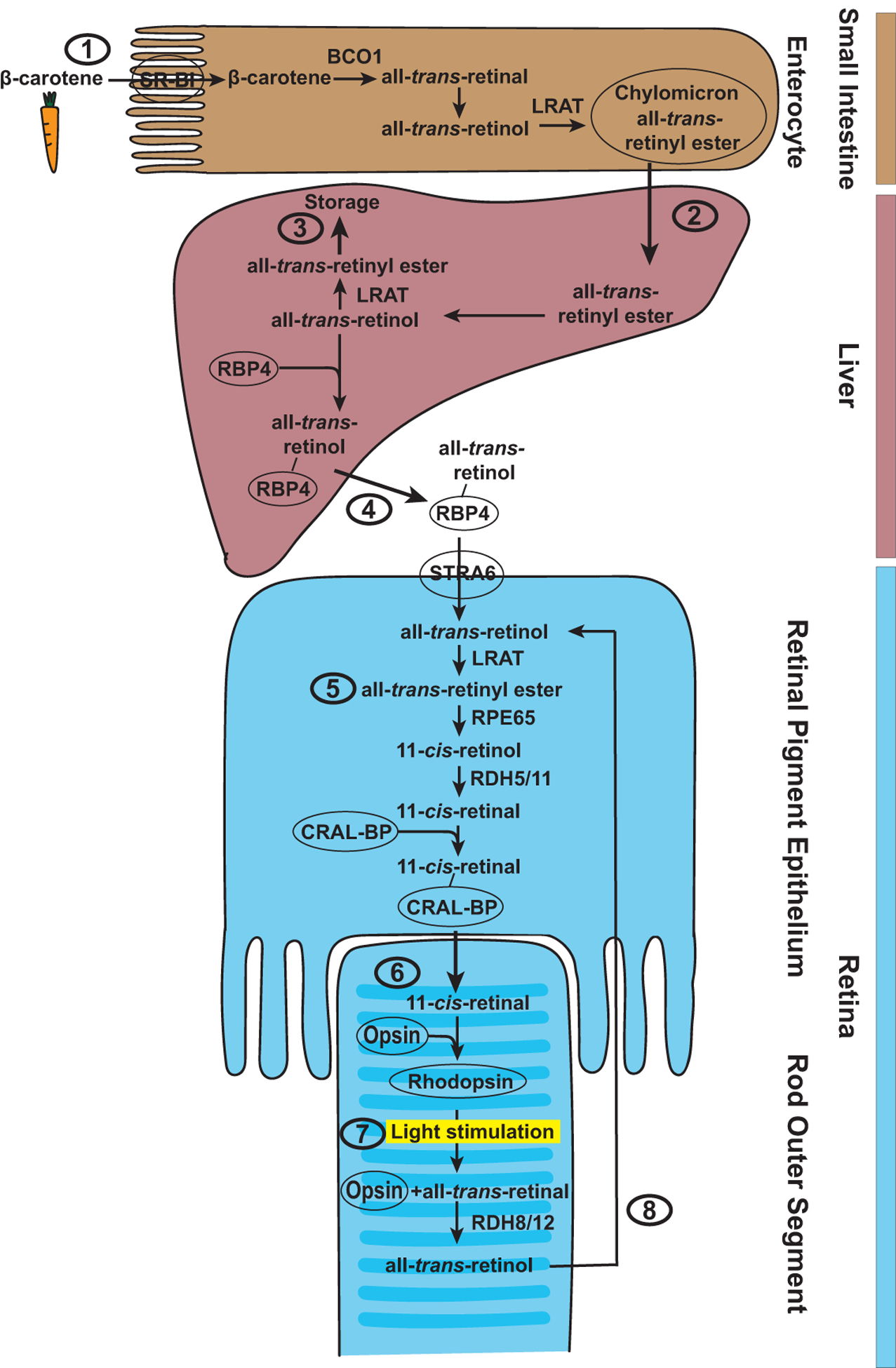
(1) β-carotene is absorbed in the enterocytes of the small intestine by SR-BI and broken down to two all-trans-retinal molecules by BCO1. All-trans-retinal is converted to all-trans-retinol and esterified by LRAT to all-trans-retinyl esters for transport in chylomicrons. (2) In the liver, all-trans-retinyl ester is hydrolyzed to all-trans-retinol, which can again be esterified into all-trans-retinyl ester by LRAT for storage (3) or bind to RBP4 for transport to the eye (4), where it is taken up in the retinal pigment epithelium by STRA6. (5) There, LRAT converts all-trans-retinol to all-trans-retinyl ester, which is converted in a series of enzymatic steps involving RPE65, RDH5, and RDH11 to the 11-cis-retinal chromophore. (6) CRAL-BP transports the chromophore to the outer segments of the photoreceptors, where it covalently binds to the opsin protein and forms the visual pigment Rhodopsin. (7) Light stimulation causes isomerization of the chromophore from cis to trans, a conformational change of the opsin, and dissociation of all-trans-retinal from the opsin. (8) Free all-trans-retinal is reduced to all-trans-retinol by RDH8 and RDH12 and transported to the retinal pigment epithelium.
While the visual pigment largely determines a photoreceptor’s wavelength sensitivity, the sensitivity can be shaped by the addition of carotenoid-based sensitizing or filtering pigments (Kirschfeld, 1979; Kirschfeld et al., 1977; Sharkey et al., 2020). In contrast to the visual pigment, these additional pigments are photostable, i.e. they are not chemically modified upon the absorption of light. For instance, birds use carotenoid-based filter pigments, which are stored in oil droplets in front of the visual pigment-containing outer segments, to fine-tune the spectral sensitivity of their short wavelength-sensitive cones and to enhance color vision (Toomey et al., 2016). In flies, the UV-sensitizing pigment 3-hydroxyretinol has been proposed to be attached via hydrogen bonds to the visual pigment Rhodopsin 1 (Rh1) (Kirschfeld and Vogt, 1986) to add UV sensitivity to Rh1’s maximal sensitivity in the blue-green range of the spectrum (Kirschfeld et al., 1977; Vogt and Kirschfeld, 1984). Another example for photostable carotenoid pigments in flies are the UV-sensitizing pigment and the blue-absorbing pigment that shape the sensitivity of the visual pigment Rh4 in ‘yellow R7’ photoreceptors (Hardie, 1986) to a maximal response in the UV range (Kirschfeld, 1979; Sharkey et al., 2020). The blue-absorbing pigment is a mixture of the xanthophylls lutein and zeaxanthin (Hardie, 1986), which function as a yellow filter (Kirschfeld, 1979). Lutein and zeaxanthin also produce the color of the ‘yellow’ spot (macula lutea) in the central human retina, which contains the fovea that mediates high resolution color vision. In the macula, the xanthophyll pigments serve as filters that absorb phototoxic UV as well as blue light and thereby improve visual performance (Renzi and Hammond, 2010; Stringham and Hammond, 2007). In addition, these pigments have been proposed to protect the macula due to their antioxidant properties (Ahmed et al., 2005).
2. Carotenoid uptake and vitamin A metabolism in mammals and flies
In this section, we review and compare the molecular mechanisms that mediate carotenoid uptake, vitamin A metabolism, chromophore formation, and visual pigment formation in mammals and in flies.
2.1. Carotenoid uptake and vitamin A metabolism in mammals
The generation of the visual chromophore and the visual pigment requires mechanisms for carotenoid uptake, transport, metabolism, delivery to target cells, and storage (Fig. 2). Vitamin A (retinol) and its derivatives (retinal and retinoic acid, Fig. 1) can be generated from plant-based precursors such as the provitamin A carotenoid β-carotene (von Lintig, 2010), which is highly lipophilic and therefore requires special mechanisms for uptake and transport. β-carotene is absorbed in the small intestine by the Scavenger Receptor class B type I (SR-BI) (van Bennekum et al., 2005) (Fig. 2). The first essential metabolic step is the breakdown of β-carotene by the β-carotene-15,15’-dioxygenase BCO1 to two molecules of all-trans-retinal (Hessel et al., 2007; Lindqvist and Andersson, 2002; von Lintig and Vogt, 2000; von Lintig and Wyss, 2001; Wyss et al., 2000). All-trans-retinal is then converted to all-trans-retinol and ultimately to all-trans-retinyl ester by the Lecithin Retinol Acyl-Transferase LRAT for transport (Batten et al., 2004; Wongsiriroj et al., 2008). The all-trans-retinyl esters are packed into chylomicrons for secretion into the lymph; once they reach the bloodstream, most of the all-trans-retinyl esters are taken up by the liver and stored there (Paik et al., 2004) (Fig. 2). In the liver, all-trans-retinyl ester is hydrolyzed back to all-trans-retinol (Goodman et al., 1965). All-trans-Retinol can then again be esterified to all-trans-retinyl ester by LRAT for storage (Batten et al., 2004) or bind to the Retinol Binding Protein RBP4 to be transported via the circulatory system to retinoid-metabolizing tissues such as the eye (Goodman, 1980).
Chromophore synthesis and response to light in mammals
In the eye (Fig. 2), RBP4-bound all-trans-retinol is taken up by the retinal pigment epithelium through the cell surface receptor and essential vitamin A transporter Stimulated by Retinoic Acid 6 (STRA6) (Kawaguchi et al., 2007). LRAT converts the received all-trans-retinol to all-trans-retinyl ester, which is used to produce the 11-cis-retinal chromophore of the visual pigment in a multistep process in the retinal pigment epithelium (von Lintig, 2012) (Fig. 2): All-trans-retinyl ester is converted and re-isomerized to 11-cis-retinol by the enzyme Retinal Pigment Epithelium 65 (RPE65) (Redmond et al., 1998). 11-cis-Retinol is subsequently oxidized to 11-cis-retinal by the Retinol Dehydrogenases RDH5 and RDH11 (Haeseleer et al., 2002). Since 11-cis-retinal is highly lipophilic, it requires the Cellular Retinaldehyde-binding Protein (CRAL-BP) that facilitates the transport to the outer segments of the photoreceptors (Saari et al., 2001) (Fig. 2). There, the 11-cis-retinal chromophore covalently binds to the opsin protein via a Schiff base linkage and thereby forms the visual pigment (Rhodopsin in rod photoreceptors) (Wald, 1968).
The visual pigment is a light-sensitive G protein-coupled receptor (Palczewski et al., 2000) that initiates phototransduction (Yau and Hardie, 2009), the amplification and conversion of a single photon response into an electrical signal that can be interpreted by the brain. Upon absorption of a photon, the 11-cis-retinal chromophore is isomerized from cis to trans, which causes a conformational change of the opsin to its photoactivated state, Metarhodopsin (Yau and Hardie, 2009). This activates the G protein and initiates the phototransduction cascade (Arshavsky et al., 2002), which ultimately results in a change in the membrane potential of the photoreceptor.
To prevent saturation of the photoreceptor and to maintain a high temporal resolution, Metarhodopsin is rapidly inactivated through GRK1-mediated phosphorylation and Arrestin binding (Yau and Hardie, 2009). As the mammalian visual pigment is photoisomerized (‘bleached’) (Wald and Brown, 1956) and Metarhodopsin is enzymatically hydrolyzed, the opsin dissociates from the all-trans-retinal (Fig. 2). The free opsin stays in a low-level activity state (Fain et al., 2001) and continuously triggers the phototransduction cascade in the absence of the chromophore (Fain, 2006). Especially after exposure to a bright light source, this ‘bleaching adaptation’ can significantly reduce the sensitivity of the rod photoreceptors to subsequent light stimulation (Fain et al., 2001; Lamb and Pugh, 2004; Pepperberg, 2003). The binding of the chromophore to the opsin therefore suppresses the prolonged, constitutive activity of the opsin and promotes the recovery of light sensitivity.
Chromophore regeneration in the visual cycle in mammals
To sustain vision after the dissociation of all-trans-retinal from the visual pigment, the chromophore needs to be regenerated (‘recycled’) in the mammalian eye in an energy-consuming process. Moreover, since the aldehyde group of the dissociated all-trans-retinal is highly reactive, the free chromophore needs to be rapidly cleared to avoid cellular damage (Maeda et al., 2008; Saari, 2016). Chromophore regeneration is achieved through a series of light-independent enzymatic steps in the so-called visual cycle (Kiser et al., 2014; Wald, 1935).
In the mammalian rod visual cycle (Fig. 3), the first step is the reduction of all-trans-retinal in the outer segments to all-trans-retinol by the Retinol Dehydrogenases RDH8 and RDH12 (Parker and Crouch, 2010). All-trans-retinol is then transported to the retinal pigment epithelium, where it gets esterified by LRAT (Batten et al., 2004). The retinyl esters can either be used to store vitamin A in lipid droplets called retinosomes (Imanishi et al., 2004) or be hydrolyzed and re-isomerized to 11-cis-retinol by RPE65. 11-cis-Retinol is subsequently oxidized in a final enzymatic step to 11-cis-retinal and re-transported to the outer segments, where it is again used for visual pigment synthesis. Taken together, the mammalian visual cycle ensures a sufficient supply of the chromophore to maintain vision and photoreceptor health; it also removes toxic products such as all-trans-retinal.
Figure 3. Mammalian visual cycle.
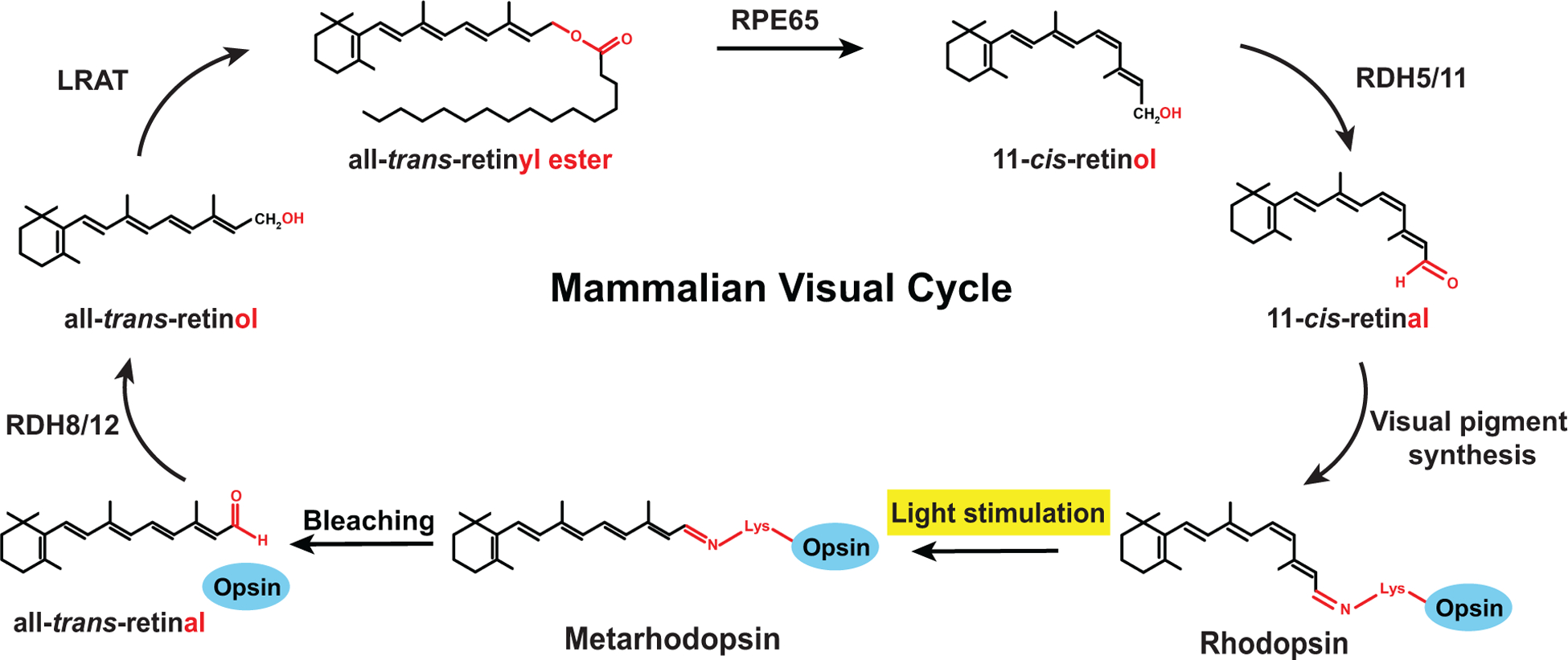
In the mammalian visual pigment, the 11-cis-retinal chromophore is covalently bound to the opsin (blue). Light stimulation causes a cis to trans isomerization (bleaching), hydrolysis of the Schiff base linkage, and release of all-trans-retinal. Chromophore regeneration is initiated by reduction of all-trans-retinal by RDH8/12 to all-trans-retinol and its transport to the retinal pigment epithelium. All-trans-retinol is esterified by LRAT to all-trans-retinyl ester, which is isomerized by RPE65 to 11-cis-retinol; the latter is oxidized by RDH5/11 to yield the 11-cis-retinal chromophore.
Another pathway for chromophore regeneration potentially involves a more recently identified RPE-retinal G protein-coupled Receptor (RGR)-dependent process (Chen et al., 2001; Morshedian et al., 2019; Zhang et al., 2019). RGR is a nonvisual opsin that has been suggested to act as a photoisomerase in a photic visual cycle that converts all-trans-retinal to 11-cis-retinal (Chen et al., 2001). However, the proposed role of RGR in a separate photic visual cycle is controversial because RGR has been shown to promote, in a light-independent manner, the conversion of retinyl esters to 11-cis-retinal by enhancing isomerohydrolase activity in the classical visual cycle (Wenzel et al., 2005).
As the chromophore regeneration by the classical visual cycle is insufficient to maintain light sensitivity of mammalian photoreceptors under bright daylight conditions, where cones are more important than rods (the latter are specialized in dim light vision), an attractive model is that RGR plays a role in an alternative cone visual cycle (Mata et al., 2002; Morshedian et al., 2019). This additional visual cycle has been proposed to involve Müller cells that take up all-trans-retinol that is released by rods and cones and subsequently oxidized to all-trans-retinal by RDH10. Upon absorption of a photon, all-trans-retinal is isomerized by RGR to 11-cis-retinal. RDH10 reduces 11-cis-retinal to 11-cis-retinol, which is then absorbed by the cones and oxidized to 11-cis-retinal by an unknown retinal dehydrogenase. 11-cis-Retinal binds to cone opsins via Schiff linkage to form the visual pigment. The absorption of a photon converts 11-cis-retinal to all-trans-retinal, which is released from the bleached cone opsin. RDH8 reduces all-trans-retinal to all-trans-retinol, which is then taken up by Müller cells; this completes this second visual cycle (Morshedian et al., 2019).
Mutations in key components of the visual cycle and vitamin A metabolism cause blinding eye diseases, either due to impaired chromophore synthesis or the accumulation of toxic products (Travis et al., 2007). For instance, mutations in RPE65 (Marlhens et al., 1997), LRAT (Thompson et al., 2001), or RDH12 (Perrault et al., 2004) cause Leber Congenital Amaurosis, an inherited eye disease that is characterized by early-onset retinal degeneration as well as severe visual impairment or blindness from infancy. Insights into the underlying mechanisms have been gained from knockout mouse models: For instance, Rpe65−/− mutant mice accumulate all-trans-retinyl esters (Fig. 3), lack 11-cis-retinoids, and display age-dependent photoreceptor degeneration (Redmond et al., 1998; Znoiko et al., 2005).
Retinoic acid signaling controls vitamin A production in mammals through negative feedback
Mammals use vitamin A not only for the generation of the chromophore, but also for the synthesis of the important signaling molecule retinoic acid that binds to nuclear receptors, which are ligand-regulated transcription factors that directly control gene expression upon activation (Cunningham and Duester, 2015). In the eye, retinoic acid is generated by the oxidation of retinal by Retinaldehyde Dehydrogenase (RALDH). Retinoic acid binds to the transporter Cellular Retinoic Acid Binding Protein (CRABP) that facilitates its transport into the nucleus (Kam et al., 2012). In the nucleus, retinoic acid binds to a heterodimer of the nuclear receptors Retinoic Acid Receptor (RAR) and Retinoic Acid X Receptor (RXR) (Huang et al., 2014; Zhang et al., 2015). The heterodimer binds to retinoic acid response elements in the promoters of target genes; the binding of retinoic acid to the RAR-RXR heterodimer causes corepressor release, coactivator recruitment, and target gene activation (Wei, 2003).
With respect to vitamin A metabolism, retinoic acid signaling plays a key role in controlling both the intestinal absorption of β-carotene as well as the subsequent conversion to vitamin A through negative feedback (von Lintig, 2012) (Fig. 4A). The uptake of dietary β-carotene leads to the synthesis of retinoic acid, which binds the RAR-RXR heterodimer and induces transcription of ISX (Lobo et al., 2010). ISX encodes the transcription factor Intestine Specific Homeobox (ISX) (Seino et al., 2008) that acts as a ‘gatekeeper’ (Lobo et al., 2010) of carotenoid metabolism in the small intestine by transcriptionally repressing SR-BI and BCO1. The consequence is the inhibition of β-carotene absorption and cleavage, respectively (Fig. 4A). The strength of ISX-mediated repression depends on the amount of vitamin A precursors in the diet: an abundance of β-carotene leads to an increase in retinoic acid that induces the repressor ISX and thus repression of vitamin A metabolism by negative feedback (Lobo et al., 2013).
Figure 4. Retinoic acid signaling controls vitamin A production in mammals.
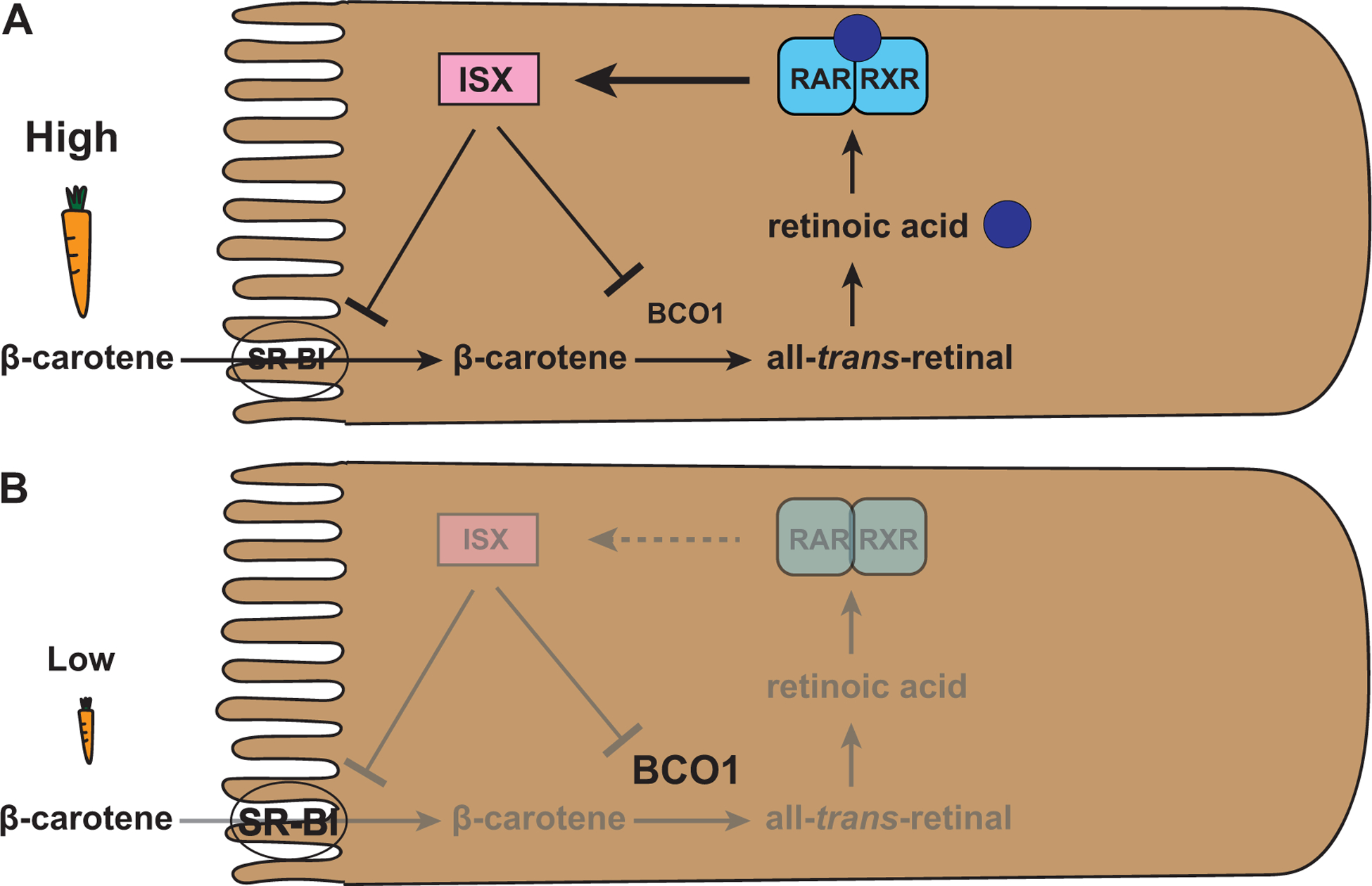
(A) Abundance of dietary β-carotene leads to the synthesis of retinoic acid that binds the RAR-RXR heterodimer and induces the expression of ISX. ISX is a repressor of SR-BI and BCO1 that are required for β-carotene uptake and processing, respectively. This retinoic acid based negative feedback mechanism limits vitamin A production.
(B) Vitamin A deprivation results in a lack of retinoic acid and thus a loss of activation of the repressor ISX. The lack of ISX leads to an increased expression of SR-BI and BCO1 and thus increased vitamin A production through increased β-carotene uptake and processing, respectively.
Conversely, under conditions of vitamin A deprivation (Fig. 4B), the lack of β-carotene and retinoic acid production causes a lack of expression of the repressor ISX and consequently an increased expression of SR-BI and BCO1, which both promote vitamin A production (Lobo et al., 2010). This negative feedback regulation by retinoic acid ensures that the absorption of β-carotene and the production of vitamin A match the requirements of the body (Lobo et al., 2013).
2.2. Carotenoid uptake and vitamin A metabolism in Drosophila melanogaster
Like mammals, Drosophila melanogaster cannot synthesize vitamin A de novo and employs evolutionarily conserved key factors for carotenoid uptake and vitamin A metabolism (Fig. 5). Dietary carotenoids are taken up in the Drosophila midgut by the homolog of SR-BI, Neither inactivation nor afterpotential D (NinaD) (Kiefer et al., 2002; Voolstra et al., 2006). Unlike mammals, however, flies do not immediately metabolize the carotenoids in the gut; instead, they reach the head via the hemolymph. In the head, the cellular uptake is mediated by another scavenger receptor, Santa-Maria (Scavenger receptor acting in neural tissue and majority of Rhodopsin is absent) (Wang et al., 2007) that is co-expressed in both neuronal and glial cells with NinaB (von Lintig and Vogt, 2000). NinaB is a β-carotene 15,15’-dioxygenase that combines the oxygenase and isomerase functions of its mammalian homologs BCO1 and RPE65, respectively. Hydroxylation yields the xanthophyll zeaxanthin, which is oxidatively cleaved by NinaB into one molecule of 11-cis-3-hydroxyretinal and one molecule of all-trans-3-hydroxyretinal (Oberhauser et al., 2008). It is unclear in which specific cell type(s) of the Drosophila head this step takes place, but it has been suggested that it involves extraretinal neurons of the central nervous system (Gu et al., 2004; Yang and O’Tousa, 2007). The enrichment of ninaB expression in the head and its lack of expression in the body (Yang and O’Tousa, 2007) contrasts the broad expression of its mammalian homologs in various tissues such as kidney, liver, testes, and muscle (Lindqvist and Andersson, 2002; Redmond et al., 2001). This difference in spatial expression is most likely due to the additional involvement of the mammalian homologs in retinoic acid signaling that regulates the development of various organs, which is not the case in flies (see below).
Figure 5. Carotenoid uptake and vitamin A metabolism in Drosophila.
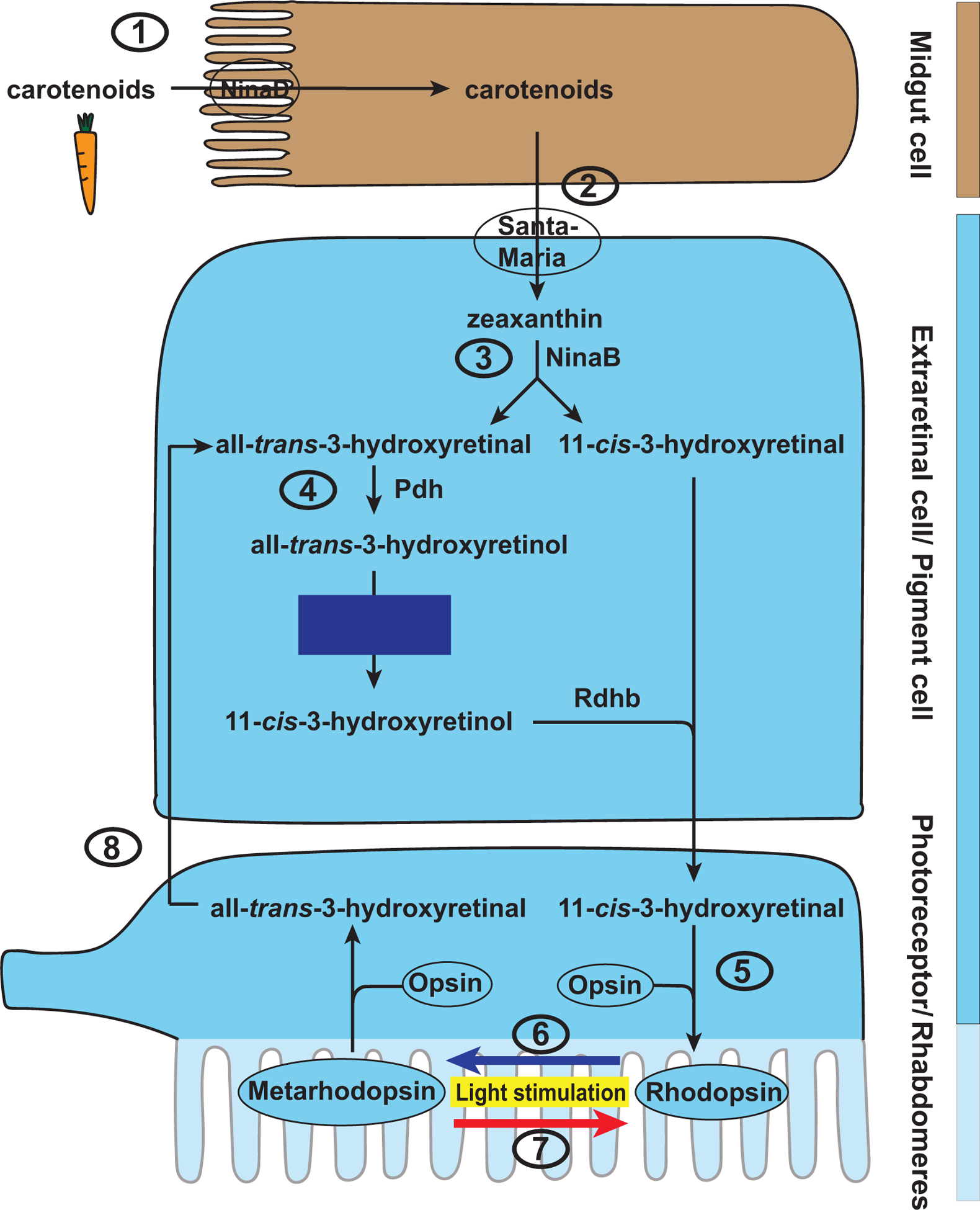
(1) Dietary carotenoids are taken up in the Drosophila midgut by NinaD, the homolog of SR-BI. (2) Carotenoids reach the head via the hemolymph and are taken up in unknown extraretinal cells by another scavenger receptor, Santa-Maria. (3) Carotenoids are converted to zeaxanthin that is cleaved by NinaB, a homolog of BCO1 and RPE65, to 11-cis-3-hydroxyretinal and all-trans-3-hydroxyretinal. (4) The latter is reduced and isomerized to 11-cis-3-hydroxyretinol in a Pdh- and blue light-dependent manner. Oxidation by Rdhb generates the 11-cis-3-hydroxyretinal chromophore. (5) The chromophore covalently binds to the opsin protein and thereby forms the visual pigment Rhodopsin. (6) The absorption of light causes a photoisomerization from cis to trans and converts Rhodopsin to its activated form, Metarhodopsin. Note that all-trans-3-hydroxyretinal remains bound to the opsin. Metarhodopsin can be reconverted to Rhodopsin by absorption of a photon of a specific wavelength (7). Some Metarhodopsin is degraded and the released all-trans-3-hydroxyretinal (8) can be recycled to 11-cis-3-hydroxyretinal in pigment cells.
Chromophore synthesis and response to light in Drosophila
11-cis-Retinal, which results from the NinaB-mediated cleavage of carotenoids such as zeaxanthin (Oberhauser et al., 2008), can be directly used to generate the chromophore 11-cis-3-hydroxyretinal. The chromophore then covalently binds to the opsin protein and forms the visual pigment called Rhodopsin (e.g. Rhodopsin 1, Rh1) (Fig. 5). Like in mammalian photoreceptors, the absorption of light causes the photoisomerization of the chromophore from the 11-cis to the all-trans configuration and converts Rhodopsin to Metarhodopsin (Hardie and Juusola, 2015). In contrast to mammalian Metarhodopsin, Drosophila Metarhodopsin does not bleach and all-trans-retinal remains bound to the opsin (Stavenga et al., 2017). Metarhodopsin can be rapidly re-isomerized to Rhodopsin by absorbing a photon of a specific wavelength (Hamdorf and Rosner, 1973; Ostroy et al., 1974; Stavenga et al., 2017). For instance, blue light converts Rh1 to Metarhodopsin and red light converts it back to Rh1 (Fig. 5).
Chromophore regeneration in Drosophila melanogaster
The Drosophila visual pigment does not bleach, but some Metarhodopsin is slowly degraded and releases all-trans-3-hydroxyretinal (Stavenga and Hardie, 2011) (Figs. 5 and 6). Another source of free all-trans-3-hydroxyretinal is the NinaB-mediated cleavage of zeaxanthin (Fig. 5 and see above). All-trans-3-hydroxyretinal from both sources can be converted to 11-cis-3-hydroxyretinal in a blue light-, Pdh-, and Rdhb-dependent manner (Schwemer, 1984; Wang et al., 2010; Wang et al., 2012) (Fig. 6). Since the fly visual pigment does not bleach, the fly visual cycle serves to regenerate the chromophore for maintaining Rhodopsin expression in adult flies. Consistent with this rationale, vitamin A-deprived flies that are supplemented with all-trans-retinal generate the chromophore and visual pigment when exposed to blue light; however, they fail to do so when kept in the dark (Satoh et al., 2005). The underlying mechanism remains to be elucidated.
Figure 6. Drosophila visual cycle.
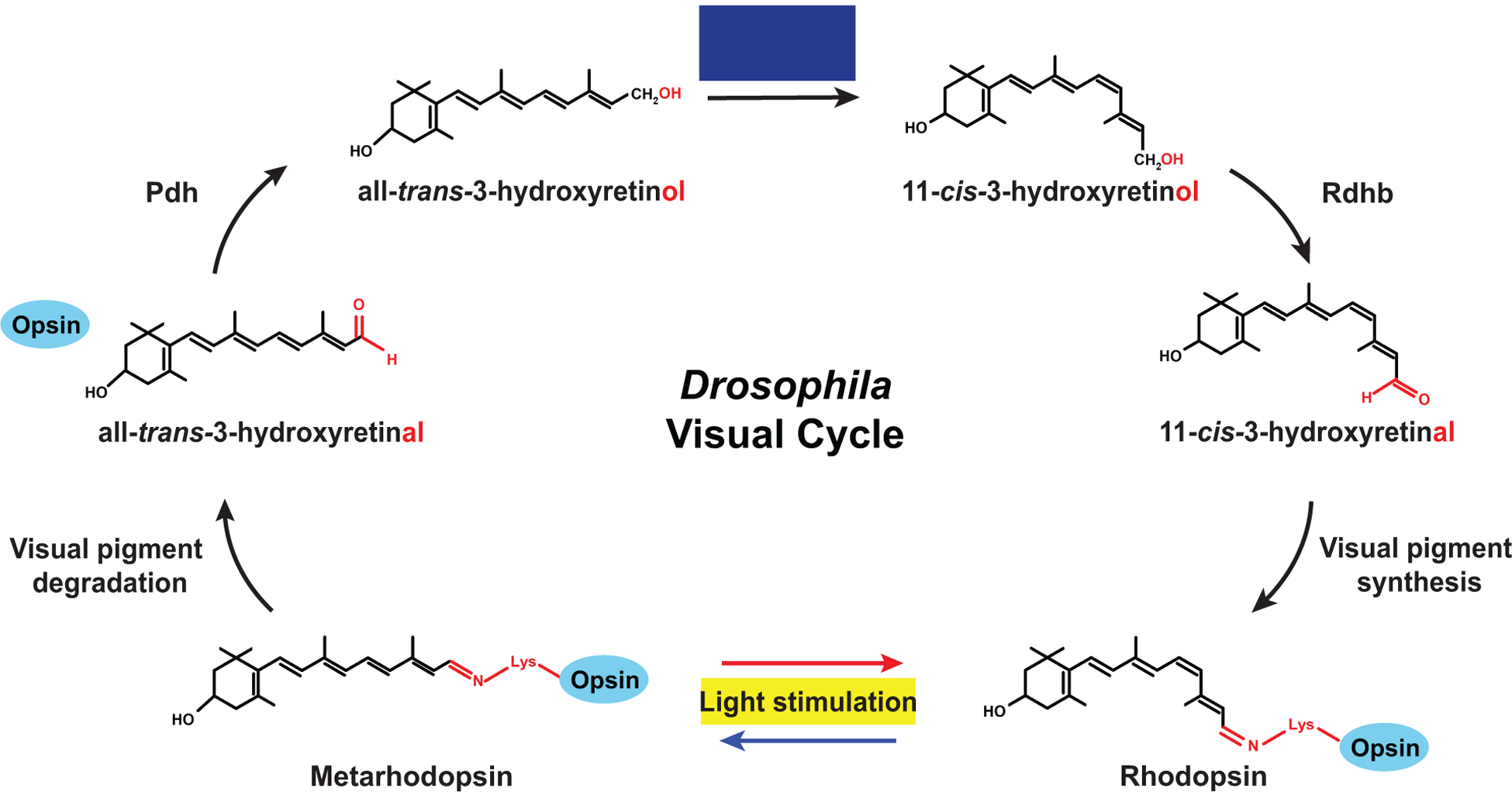
In the Drosophila visual pigment, the 11-cis-3-hydroxyretinal chromophore is covalently bound to the opsin (blue). After absorption of light and the resulting cis to trans isomerization, the chromophore remains bound to the opsin. Some of the activated visual pigment (Metarhodopsin) is slowly degraded and releases all-trans-3-hydroxyretinal, which is reduced to all-trans-3-hydroxyretinol by Pdh. All-trans-3-hydroxyretinol is isomerized in a blue light-dependent manner to 11-cis-3-hydroxyretinol, which is oxidized by Rdhb to the 11-cis-3-hydroxyretinal chromophore that is used for visual pigment synthesis.
The Drosophila pigment cells of the retina play a role in chromophore synthesis and regeneration that parallels the function of the retinal pigment epithelium in mammals (Wang et al., 2010) (Fig. 5). The pigment cells express the retinoid-binding protein PDA is not apparent (Pinta) that functions similarly to CRAL-BP in mammals (Saari et al., 2001) (Wang and Montell, 2005). Since Pinta preferentially binds all-trans-retinol, it has been proposed to either sequester all-trans-retinol to enrich vitamin A in pigment cells and/or to facilitate its oxidation to all-trans-retinal (Wang and Montell, 2005). The Photoreceptor dehydrogenase Pdh (Wang et al., 2010), which is functionally equivalent to mammalian RDH (Parker and Crouch, 2010), reduces all-trans-3-hydroxyretinal to all-trans-3-hydroxyretinol (Fig. 6). All-trans-3-hydroxyretinol is isomerized and oxidized by Rdhb to 11-cis-3-hydroxyretinal, which is transported to the photoreceptors and binds to the opsin to form the visual pigment (Wang et al., 2010).
The Drosophila visual cycle differs from the mammalian visual cycle in that retinal is converted to retinol in the pigment cells that are equivalent to the mammalian retinal pigment epithelium, rather than in the rhabdomeres that are equivalent to the outer segments. Moreover, retinyl esters (O’Byrne and Blaner, 2013) appear neither to be used for storage nor for transport of vitamin A in flies (Wang et al., 2010). The fly visual cycle also seems to have a rather low efficiency (Stavenga et al., 2017) and serves the de novo formation of chromophore when the adult eye is carotenoid deprived, and not as an indispensable chromophore recycling system like in mammals (Wang et al., 2010).
Retinoic acid signaling in Drosophila melanogaster
Mammals use vitamin A to synthesize retinoic acid, an important signaling molecule in various tissues (Kam et al., 2012). However, retinoic acid does not play a comparable role in Drosophila. While there is a Drosophila homolog of mammalian RXR called Ultraspiracle (Usp) (King-Jones and Thummel, 2005), Usp lacks the residues in the ligand binding domain that confer affinity for retinoic acid (Oro et al., 1990) and does not bind the RXR ligand 9-cis-retinoic acid (Bonneton et al., 2003). Usp’s natural ligand has not been unambiguously identified (Beck et al., 2009; Bonneton et al., 2003; Clayton et al., 2001), but it is well established that Usp has nuclear hormone receptor activity and forms a heterodimer with the Ecdysone Receptor (EcR) (King-Jones and Thummel, 2005). The hormone 20-hydroxyecdysone binds to the EcR-Usp heterodimer and controls major developmental transitions through ecdysone receptor response elements in target genes. While there is no known homolog of RAR in Drosophila, Usp can also heterodimerize with Dhr3, the Drosophila homolog of the RAR-related Orphan Receptor (ROR) (King-Jones and Thummel, 2005). While RORα binds to cholesterol derivatives (Bitsch et al., 2003; Kallen et al., 2002), RORβ binds to all-trans-retinoic acid (Stehlin-Gaon et al., 2003); however, Dhr3 has only a 35% similarity to human RORβ’s ligand binding domain (King-Jones and Thummel, 2005). In summary, these data suggest that flies lack canonical retinoic acid signaling.
Two studies suggested that vitamin A metabolites are required for apolipophorin (also known as retinoid and fatty acid binding glycoprotein) (Shim et al., 1997) and Rh1 expression (Picking et al., 1996). The latter is controversial because reduced Rh1 mRNA levels were only observed on Northern blots when Sang’s medium, but not other media that lack sources of vitamin A and cause dramatically reduced Rh1 protein levels, was used for vitamin A deprivation (Picking et al., 1996). It is also unclear whether the proposed effects are direct or indirect. Retinoic acid might serve other functions: For instance, one study showed the requirement for retinoid biosynthesis for Drosophila tissue regeneration (Halme et al., 2010) and another identified a retinoid-dependent carboxypeptidase that mediates the degradation of misfolded Rhodopsin (Huang et al., 2018).
3. Consequences of vitamin A deprivation for vision in mammals and Drosophila
In this section, we review and compare the structural and functional consequences of dietary or genetic vitamin A deprivation in mammals and flies.
Effects of vitamin A deprivation on mammalian photoreceptor morphology and function
In humans, vitamin A deficiency can be caused by suboptimal diets, eating disorders, or chronic impairment of tissues that mediate vitamin A absorption. Vitamin A deprivation causes multiple ocular and extraocular diseases such as xerophthalmia, Bitot’s spot, keratitis, and keratomalacia (Sommer, 2008). Chronic vitamin A deprivation is the leading cause of preventable childhood blindness according to the World Health Organization (https://www.who.int/data/nutrition/nlis/info/vitamin-a-deficiency).
Since vitamin A is critical for human vision as a precursor of the visual chromophore (Saari, 2016), insufficient vitamin A intake causes a lack of visual pigment. Consequently, a common initial symptom of acute vitamin A deficiency is impaired rod function and night blindness, i.e. compromised vision in dimly lit environments (Dowling and Wald, 1958, 1960). In contrast to the fly eye (see below), chromophore deficiency does not necessarily cause a concomitant loss of the rod opsin in the mammalian eye: while Rpe65 mutant mice are unable to generate 11-cis-retinal (Fig. 3) and their rods have disorganized outer segments that lack Rhodopsin, the opsin protein is still present and correctly localized in the severely functionally impaired rods (Redmond et al., 1998). Rpe65 mutant rods slowly degenerate, potentially due to the large amounts of free opsin that constitutively triggers the phototransduction cascade and impairs cellular calcium homeostasis (Cornwall and Fain, 1994; Fain, 2006; Melia et al., 1997). Injection of 11-cis-retinal in the Rpe65 mutant mice causes Rhodopsin regeneration and improves rod function (Ablonczy et al., 2002).
In contrast to the presence of free rod opsin, cone opsin synthesis and trafficking are defective in Rpe65 mutants (Rohrer et al., 2005). Moreover, cone degeneration begins much earlier and progresses faster than rod degeneration (Rohrer et al., 2005; Znoiko et al., 2005). Cone opsin localization, cone function, and cone survival are improved by early administration of 11-cis-retinal (Rohrer et al., 2005; Znoiko et al., 2005), suggesting that the lack of the chromophore causes the underlying defects. However, it is not known whether dietary vitamin A deficiency leads to comparable cone defects in the mammalian retina.
Since vitamin A is also the precursor of the major signaling molecule retinoic acid, which is indispensable for mammalian development and the functioning of the immune system (Sommer, 2008), chronic deprivation is difficult to study in mammalian model systems. To circumvent lethality, retinoic acid can be added to a vitamin A-depleted diet to rescue the developmental function and to limit vitamin A deprivation to the eye (Katz et al., 1993). This approach is possible because retinoic acid can neither be reduced to retinal nor to retinol in mammals (Dowling and Wald, 1960; Katz et al., 1993). Rats whose eyes are vitamin A deprived in this manner have dramatically reduced Rhodopsin levels (Dowling and Wald, 1960; Katz et al., 1993) and the size of the rod outer segments is reduced by 50% (Katz et al., 1993). These early defects, which eventually progress to photoreceptor degeneration, can be rescued by the injection of all-trans-retinol (Katz et al., 1993). Consistent with the deficiency in retinal and Rhodopsin, deprived rats require higher light intensities to reach electroretinogram (ERG) responses that are comparable to the non-deprived control rats (Katz et al., 1993).
In summary, the study of vitamin A deprivation in nocturnal mammals (mice and rats) yielded major insights into the consequences for rod structure and function. Because it is technically challenging to make mammalian eyes vitamin A deficient and, to our knowledge, there is a lack of an established model to study vitamin A deficiency in a diurnal mammal, the molecular consequences (particularly in cones) are still understudied.
Effects of vitamin A deprivation on Drosophila photoreceptor morphology and function
Since flies do not require vitamin A for their development or immune responses, chronic deprivation studies are conveniently feasible either through dietary deprivation (Figs. 7A–B) or genetic deprivation (Figs. 7D–F). The latter can be achieved by the mutation of genes that are essential for vitamin A metabolism but whose loss also does not cause lethality. When flies are chronically vitamin A deprived throughout their development, their light-sensing compartments, the rhabdomeres, are abnormally shaped and often improperly spaced (Fig. 7). Moreover, the surface area of the rhabdomeres is dramatically reduced (Lee et al., 1996) (Fig. 7). These defects resemble the structural damage in vitamin A-deprived mammalian outer segments. The size defect can be rescued by feeding the flies with carrot juice (Sapp et al., 1991). Moreover, like deprived rat photoreceptors, deprived Drosophila photoreceptors also show a dramatic decrease in Rhodopsin levels (Harris et al., 1977; Nichols and Pak, 1985). Without the chromophore, the opsin cannot complete the secretory pathway and instead remains in an immature, glycosylated state in the endoplasmic reticulum (Huber et al., 1994; Ozaki et al., 1993). This immature opsin is degraded faster in vitamin A deficient photoreceptors than in vitamin A replete photoreceptors (Huber et al., 1994). Taken together, chromophore deficiency in flies causes defective opsin maturation and trafficking, which resembles chromophore deficient mammalian cone opsins but contrasts the expression and proper localization of free mammalian rod opsin.
Figure 7. Dietary and genetic vitamin A deprivation in Drosophila.
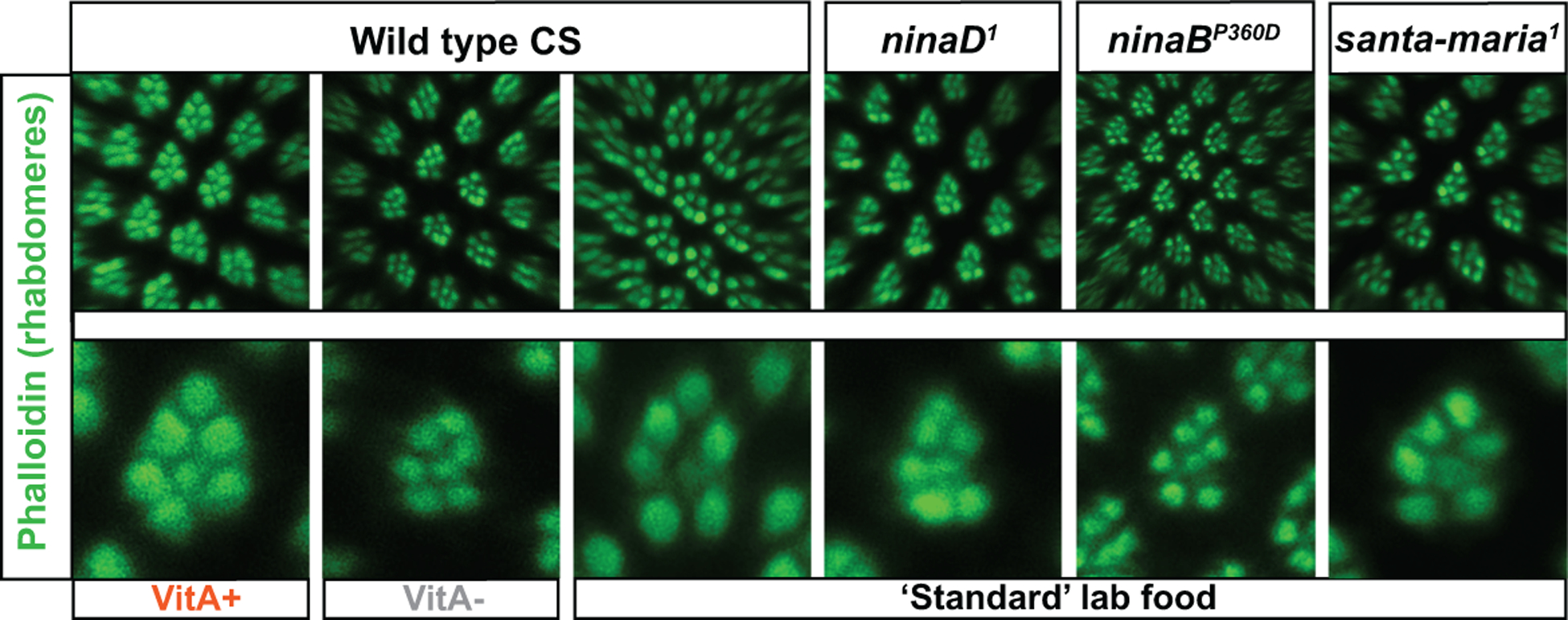
The light-sensing compartments (rhabdomeres) of adult Drosophila photoreceptors are labeled with Phalloidin (green). Scale bars, 10 μm.
(A-C) Dietary vitamin A deprivation causes structural rhabdomere damage.
(A) and (C) Wild type Canton S flies that were raised on minimal medium with β-carotene supplementation (VitA+) or ‘standard’ lab food have normally sized and shaped rhabdomeres (green).
(B) Wild type CS flies that were vitamin A deprived on minimal medium without β-carotene supplementation (VitA-) have dramatically reduced, abnormally shaped, and improperly spaced rhabdomeres.
(A’-C’) High magnification view of single unit eyes. Seven rhabdomeres are visible; note the reduction of the cross-sectional diameter, improper spacing, and abnormal shape caused by dietary vitamin A deprivation (B’).
(D-F) Genetic vitamin A deprivation through mutation of genes that are critical for vitamin A metabolism also causes rhabdomere damage (compare to wild type in C). All mutants were raised on ‘standard’ lab food.
(D’-F’) High magnification view of single mutant unit eyes. Seven rhabdomeres are visible; note the reduction of the cross-sectional diameter, improper spacing, and abnormal shape in all mutants (compare to wild type in C’). See text for details.
Like mammalian vitamin A deficient eyes, deprived Drosophila eyes show abnormal ERG responses due to their lack of visual pigment. For instance, the ERGs of both ninaD and ninaB mutants lack the prolonged depolarizing afterpotential (PDA), which is reflected in their names (nina stands for ‘neither inactivation nor afterpotential’) (Pak et al., 2012). The PDA is generated by a strongly depolarizing blue-light stimulus that converts a substantial amount (>20%) (Pak et al., 2012) of Rhodopsin to Metarhodopsin, saturates the response of the photoreceptors, and renders them unresponsive to another stimulus. In non-deprived flies, the PDA persists in the dark for several minutes after the stimulus is switched off, because the Metarhodopsin molecules outnumber the Arrestin molecules that are required for the termination of the response (Dolph et al., 1993). Due to the bistable nature of the fly visual pigment (see above), the PDA can be terminated by reconverting Metarhodopsin to Rhodopsin with an orange or red stimulus (Hamdorf and Rosner, 1973; Ostroy et al., 1974; Stavenga et al., 2017). The absence of the PDA in ninaD mutants is consistent with their lack of Rhodopsin - that would be required to overcome Arrestin levels - due to the lack of the chromophore.
In summary, Drosophila melanogaster is a powerful model to study the effects of vitamin A deficiency on the eye because vitamin A is not required for fly development or survival. However, little is known about the molecular consequences of vitamin A deprivation beyond opsin maturation. Moreover, like in mammalian models, most fly studies were focused on the rod-equivalent photoreceptor subtype (outer photoreceptors) and we know very little about how the cone-equivalent photoreceptor subtypes are affected.
Conclusions
The comparison of mammalian and fly models of vitamin A metabolism and deficiency reveals that similar mechanisms underlie the formation of the chromophore and the visual pigment, which includes evolutionarily conserved genes for absorption and processing of dietary β-carotene. Moreover, vitamin A deprivation causes a dramatic loss of the chromophore and the visual pigment, as well as similar structural and functional photoreceptor defects in rod or rod-equivalent photoreceptors in both model systems. The dependency of cone opsin maturation and fly Rhodopsin maturation on the retinal chromophore is also similar.
However, the mechanisms of vitamin A distribution and storage, the light response of the visual pigment (bleaching and chromophore release in mammals), and the role of the visual cycle are very different. Moreover, mammals show a much broader range of functions of vitamin A due to its role in retinoic acid-mediated gene regulation, while the role of the related nuclear receptors in flies is substantially divergent. It will be interesting to see whether more specialized roles of retinoic signaling in flies will be found in future studies. Moreover, it needs to be addressed in more detail in both model systems how different photoreceptor subtypes are affected by vitamin A deficiency.
In summary, mammalian and fly models have revealed deep insights into the molecular mechanisms of vitamin A metabolism and how the mutation of key players causes a variety of human pathologies. Given the remarkable recent progress, this field will continue to provide critical insights into the molecular basis of vision and inspire the development of therapies to prevent and treat degenerative eye diseases.
Highlights.
Comparison of mammalian and fly models to study the role of vitamin A in vision.
Vitamin A deprivation causes similar molecular and functional defects in the eye.
Shared mechanisms for chromophore and visual pigment formation.
Mechanisms for vitamin A distribution and storage are different.
Light response of the visual pigment and role of the visual cycle also differ.
Acknowledgements
This publication was supported by the National Eye Institute of the National Institutes of Health under Award Number R01EY029659. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ablonczy Z, Crouch RK, Goletz PW, Redmond TM, Knapp DR, Ma JX, Rohrer B, 2002. 11-cis-retinal reduces constitutive opsin phosphorylation and improves quantum catch in retinoid-deficient mouse rod photoreceptors. J Biol Chem 277, 40491–40498. [DOI] [PubMed] [Google Scholar]
- Ahmed SS, Lott MN, Marcus DM, 2005. The macular xanthophylls. Surv Ophthalmol 50, 183–193. [DOI] [PubMed] [Google Scholar]
- Arshavsky VY, Lamb TD, Pugh EN Jr., 2002. G proteins and phototransduction. Annu Rev Physiol 64, 153–187. [DOI] [PubMed] [Google Scholar]
- Batten ML, Imanishi Y, Maeda T, Tu DC, Moise AR, Bronson D, Possin D, Van Gelder RN, Baehr W, Palczewski K, 2004. Lecithin-retinol acyltransferase is essential for accumulation of all-trans-retinyl esters in the eye and in the liver. J Biol Chem 279, 10422–10432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck Y, Delaporte C, Moras D, Richards G, Billas IM, 2009. The ligand-binding domains of the three RXR-USP nuclear receptor types support distinct tissue and ligand specific hormonal responses in transgenic Drosophila. Dev Biol 330, 1–11. [DOI] [PubMed] [Google Scholar]
- Bitsch F, Aichholz R, Kallen J, Geisse S, Fournier B, Schlaeppi JM, 2003. Identification of natural ligands of retinoic acid receptor-related orphan receptor alpha ligand-binding domain expressed in Sf9 cells--a mass spectrometry approach. Anal Biochem 323, 139–149. [DOI] [PubMed] [Google Scholar]
- Bonneton F, Zelus D, Iwema T, Robinson-Rechavi M, Laudet V, 2003. Rapid divergence of the ecdysone receptor in Diptera and Lepidoptera suggests coevolution between ECR and USP-RXR. Mol Biol Evol 20, 541–553. [DOI] [PubMed] [Google Scholar]
- Briscoe AD, Chittka L, 2001. The evolution of color vision in insects. Annu Rev Entomol 46, 471–510. [DOI] [PubMed] [Google Scholar]
- Brown PK, Wald G, 1964. Visual Pigments in Single Rods and Cones of the Human Retina. Direct Measurements Reveal Mechanisms of Human Night and Color Vision. Science 144, 45–52. [DOI] [PubMed] [Google Scholar]
- Chen P, Hao W, Rife L, Wang XP, Shen D, Chen J, Ogden T, Van Boemel GB, Wu L, Yang M, Fong HK, 2001. A photic visual cycle of rhodopsin regeneration is dependent on Rgr. Nat Genet 28, 256–260. [DOI] [PubMed] [Google Scholar]
- Clayton GM, Peak-Chew SY, Evans RM, Schwabe JW, 2001. The structure of the ultraspiracle ligand-binding domain reveals a nuclear receptor locked in an inactive conformation. Proc Natl Acad Sci U S A 98, 1549–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornwall MC, Fain GL, 1994. Bleached pigment activates transduction in isolated rods of the salamander retina. J Physiol 480 (Pt 2), 261–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham TJ, Duester G, 2015. Mechanisms of retinoic acid signalling and its roles in organ and limb development. Nat Rev Mol Cell Biol 16, 110–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolph PJ, Ranganathan R, Colley NJ, Hardy RW, Socolich M, Zuker CS, 1993. Arrestin function in inactivation of G protein-coupled receptor rhodopsin in vivo. Science 260, 1910–1916. [DOI] [PubMed] [Google Scholar]
- Dowling JE, Wald G, 1958. Vitamin a Deficiency and Night Blindness. Proc Natl Acad Sci U S A 44, 648–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling JE, Wald G, 1960. The Biological Function of Vitamin a Acid. Proc Natl Acad Sci U S A 46, 587–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fain GL, 2006. Why photoreceptors die (and why they don’t). Bioessays 28, 344–354. [DOI] [PubMed] [Google Scholar]
- Fain GL, Matthews HR, Cornwall MC, Koutalos Y, 2001. Adaptation in vertebrate photoreceptors. Physiol Rev 81, 117–151. [DOI] [PubMed] [Google Scholar]
- Goodman DS, 1980. Plasma retinol-binding protein. Ann N Y Acad Sci 348, 378–390. [DOI] [PubMed] [Google Scholar]
- Goodman DW, Huang HS, Shiratori T, 1965. Tissue Distribution and Metabolism of Newly Absorbed Vitamin a in the Rat. J Lipid Res 6, 390–396. [PubMed] [Google Scholar]
- Gu G, Yang J, Mitchell KA, O’Tousa JE, 2004. Drosophila ninaB and ninaD act outside of retina to produce rhodopsin chromophore. J Biol Chem 279, 18608–18613. [DOI] [PubMed] [Google Scholar]
- Haeseleer F, Jang GF, Imanishi Y, Driessen C, Matsumura M, Nelson PS, Palczewski K, 2002. Dual-substrate specificity short chain retinol dehydrogenases from the vertebrate retina. J Biol Chem 277, 45537–45546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halme A, Cheng M, Hariharan IK, 2010. Retinoids regulate a developmental checkpoint for tissue regeneration in Drosophila. Curr Biol 20, 458–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamdorf K, Rosner G, 1973. Adaptation and photoregeneration in the eye of the blowfly. J Comp Physiol 86, 281–292. [Google Scholar]
- Hardie RC, 1986. The photoreceptor array of the dipteran retina. Trends Neurosci 9, 419–423. [Google Scholar]
- Hardie RC, Juusola M, 2015. Phototransduction in Drosophila. Curr Opin Neurobiol 34, 37–45. [DOI] [PubMed] [Google Scholar]
- Harris WA, Ready DF, Lipson ED, Hudspeth AJ, Stark WS, 1977. Vitamin A deprivation and Drosophila photopigments. Nature 266, 648–650. [DOI] [PubMed] [Google Scholar]
- Hessel S, Eichinger A, Isken A, Amengual J, Hunzelmann S, Hoeller U, Elste V, Hunziker W, Goralczyk R, Oberhauser V, von Lintig J, Wyss A, 2007. CMO1 deficiency abolishes vitamin A production from beta-carotene and alters lipid metabolism in mice. J Biol Chem 282, 33553–33561. [DOI] [PubMed] [Google Scholar]
- Huang HW, Brown B, Chung J, Domingos PM, Ryoo HD, 2018. highroad Is a Carboxypetidase Induced by Retinoids to Clear Mutant Rhodopsin-1 in Drosophila Retinitis Pigmentosa Models. Cell reports 22, 1384–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang P, Chandra V, Rastinejad F, 2014. Retinoic acid actions through mammalian nuclear receptors. Chemical reviews 114, 233–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber A, Wolfrum U, Paulsen R, 1994. Opsin maturation and targeting to rhabdomeral photoreceptor membranes requires the retinal chromophore. Eur J Cell Biol 63, 219–229. [PubMed] [Google Scholar]
- Imanishi Y, Gerke V, Palczewski K, 2004. Retinosomes: new insights into intracellular managing of hydrophobic substances in lipid bodies. J Cell Biol 166, 447–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallen JA, Schlaeppi JM, Bitsch F, Geisse S, Geiser M, Delhon I, Fournier B, 2002. X-ray structure of the hRORalpha LBD at 1.63 A: structural and functional data that cholesterol or a cholesterol derivative is the natural ligand of RORalpha. Structure 10, 1697–1707. [DOI] [PubMed] [Google Scholar]
- Kam RK, Deng Y, Chen Y, Zhao H, 2012. Retinoic acid synthesis and functions in early embryonic development. Cell Biosci 2, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz ML, Chen DM, Stientjes HJ, Stark WS, 1993. Photoreceptor recovery in retinoid-deprived rats after vitamin A replenishment. Exp Eye Res 56, 671–682. [DOI] [PubMed] [Google Scholar]
- Kawaguchi R, Yu J, Honda J, Hu J, Whitelegge J, Ping P, Wiita P, Bok D, Sun H, 2007. A membrane receptor for retinol binding protein mediates cellular uptake of vitamin A. Science 315, 820–825. [DOI] [PubMed] [Google Scholar]
- Kefalov VJ, Cornwall MC, Fain GL, 2010. Physiological studies of the interaction between opsin and chromophore in rod and cone visual pigments. Methods Mol Biol 652, 95–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiefer C, Sumser E, Wernet MF, Von Lintig J, 2002. A class B scavenger receptor mediates the cellular uptake of carotenoids in Drosophila. Proc Natl Acad Sci U S A 99, 10581–10586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King-Jones K, Thummel CS, 2005. Nuclear receptors--a perspective from Drosophila. Nat Rev Genet 6, 311–323. [DOI] [PubMed] [Google Scholar]
- Kirschfeld K, 1979. The function of photostable pigments in fly photoreceptors. Biophys Struct Mech 5, 117–128. [DOI] [PubMed] [Google Scholar]
- Kirschfeld K, Franceschini N, Minke B, 1977. Evidence for a sensitising pigment in fly photoreceptors. Nature 269, 386–390. [DOI] [PubMed] [Google Scholar]
- Kirschfeld K, Vogt K, 1986. Does retinol serve a sensitizing function in insect photoreceptors? Vision Res 26, 1771–1777. [DOI] [PubMed] [Google Scholar]
- Kiser PD, Golczak M, Palczewski K, 2014. Chemistry of the retinoid (visual) cycle. Chemical reviews 114, 194–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar JP, Ready DF, 1995. Rhodopsin plays an essential structural role in Drosophila photoreceptor development. Development 121, 4359–4370. [DOI] [PubMed] [Google Scholar]
- Lamb TD, Pugh EN Jr., 2004. Dark adaptation and the retinoid cycle of vision. Prog Retin Eye Res 23, 307–380. [DOI] [PubMed] [Google Scholar]
- Lee RD, Thomas CF, Marietta RG, Stark WS, 1996. Vitamin A, visual pigments, and visual receptors in Drosophila. Microsc Res Tech 35, 418–430. [DOI] [PubMed] [Google Scholar]
- Lindqvist A, Andersson S, 2002. Biochemical properties of purified recombinant human beta-carotene 15,15’-monooxygenase. J Biol Chem 277, 23942–23948. [DOI] [PubMed] [Google Scholar]
- Lobo GP, Amengual J, Baus D, Shivdasani RA, Taylor D, von Lintig J, 2013. Genetics and diet regulate vitamin A production via the homeobox transcription factor ISX. J Biol Chem 288, 9017–9027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo GP, Hessel S, Eichinger A, Noy N, Moise AR, Wyss A, Palczewski K, von Lintig J, 2010. ISX is a retinoic acid-sensitive gatekeeper that controls intestinal beta,beta-carotene absorption and vitamin A production. FASEB J 24, 1656–1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda A, Maeda T, Golczak M, Palczewski K, 2008. Retinopathy in mice induced by disrupted all-trans-retinal clearance. J Biol Chem 283, 26684–26693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlhens F, Bareil C, Griffoin JM, Zrenner E, Amalric P, Eliaou C, Liu SY, Harris E, Redmond TM, Arnaud B, Claustres M, Hamel CP, 1997. Mutations in RPE65 cause Leber’s congenital amaurosis. Nat Genet 17, 139–141. [DOI] [PubMed] [Google Scholar]
- Mata NL, Radu RA, Clemmons RC, Travis GH, 2002. Isomerization and oxidation of vitamin a in cone-dominant retinas: a novel pathway for visual-pigment regeneration in daylight. Neuron 36, 69–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melia TJ Jr., Cowan CW, Angleson JK, Wensel TG, 1997. A comparison of the efficiency of G protein activation by ligand-free and light-activated forms of rhodopsin. Biophys J 73, 3182–3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morshedian A, Kaylor JJ, Ng SY, Tsan A, Frederiksen R, Xu T, Yuan L, Sampath AP, Radu RA, Fain GL, Travis GH, 2019. Light-Driven Regeneration of Cone Visual Pigments through a Mechanism Involving RGR Opsin in Muller Glial Cells. Neuron 102, 1172–1183 e1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols R, Pak WL, 1985. Characterization of Drosophila melanogaster rhodopsin. J Biol Chem 260, 12670–12674. [PubMed] [Google Scholar]
- Nickell S, Park PS, Baumeister W, Palczewski K, 2007. Three-dimensional architecture of murine rod outer segments determined by cryoelectron tomography. J Cell Biol 177, 917–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Byrne SM, Blaner WS, 2013. Retinol and retinyl esters: biochemistry and physiology. J Lipid Res 54, 1731–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberhauser V, Voolstra O, Bangert A, von Lintig J, Vogt K, 2008. NinaB combines carotenoid oxygenase and retinoid isomerase activity in a single polypeptide. Proc Natl Acad Sci U S A 105, 19000–19005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oro AE, McKeown M, Evans RM, 1990. Relationship between the product of the Drosophila ultraspiracle locus and the vertebrate retinoid X receptor. Nature 347, 298–301. [DOI] [PubMed] [Google Scholar]
- Ostroy SE, Wilson M, Pak WL, 1974. Drosophila rhodopsin: photochemistry, extraction and differences in the norp AP12 phototransduction mutant. Biochem Biophys Res Commun 59, 960–966. [DOI] [PubMed] [Google Scholar]
- Ozaki K, Nagatani H, Ozaki M, Tokunaga F, 1993. Maturation of major Drosophila rhodopsin, ninaE, requires chromophore 3-hydroxyretinal. Neuron 10, 1113–1119. [DOI] [PubMed] [Google Scholar]
- Paik J, Vogel S, Quadro L, Piantedosi R, Gottesman M, Lai K, Hamberger L, Vieira Mde M, Blaner WS, 2004. Vitamin A: overlapping delivery pathways to tissues from the circulation. J Nutr 134, 276S–280S. [DOI] [PubMed] [Google Scholar]
- Pak WL, Shino S, Leung HT, 2012. PDA (prolonged depolarizing afterpotential)-defective mutants: the story of nina’s and ina’s--pinta and santa maria, too. J Neurogenet 26, 216–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palczewski K, Kumasaka T, Hori T, Behnke CA, Motoshima H, Fox BA, Le Trong I, Teller DC, Okada T, Stenkamp RE, Yamamoto M, Miyano M, 2000. Crystal structure of rhodopsin: A G protein-coupled receptor. Science 289, 739–745. [DOI] [PubMed] [Google Scholar]
- Parker RO, Crouch RK, 2010. Retinol dehydrogenases (RDHs) in the visual cycle. Exp Eye Res 91, 788–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepperberg DR, 2003. Bleaching desensitization: background and current challenges. Vision Res 43, 3011–3019. [DOI] [PubMed] [Google Scholar]
- Perrault I, Hanein S, Gerber S, Barbet F, Ducroq D, Dollfus H, Hamel C, Dufier JL, Munnich A, Kaplan J, Rozet JM, 2004. Retinal dehydrogenase 12 (RDH12) mutations in leber congenital amaurosis. Am J Hum Genet 75, 639–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picking WL, Chen DM, Lee RD, Vogt ME, Polizzi JL, Marietta RG, Stark WS, 1996. Control of Drosophila opsin gene expression by carotenoids and retinoic acid: northern and western analyses. Exp Eye Res 63, 493–500. [DOI] [PubMed] [Google Scholar]
- Redmond TM, Gentleman S, Duncan T, Yu S, Wiggert B, Gantt E, Cunningham FX Jr., 2001. Identification, expression, and substrate specificity of a mammalian beta-carotene 15,15’-dioxygenase. J Biol Chem 276, 6560–6565. [DOI] [PubMed] [Google Scholar]
- Redmond TM, Yu S, Lee E, Bok D, Hamasaki D, Chen N, Goletz P, Ma JX, Crouch RK, Pfeifer K, 1998. Rpe65 is necessary for production of 11-cis-vitamin A in the retinal visual cycle. Nat Genet 20, 344–351. [DOI] [PubMed] [Google Scholar]
- Renzi LM, Hammond BR, 2010. The effect of macular pigment on heterochromatic luminance contrast. Exp Eye Res 91, 896–900. [DOI] [PubMed] [Google Scholar]
- Rister J, Desplan C, 2011. The retinal mosaics of opsin expression in invertebrates and vertebrates. Dev Neurobiol 71, 1212–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rister J, Desplan C, Vasiliauskas D, 2013. Establishing and maintaining gene expression patterns: insights from sensory receptor patterning. Development 140, 493–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer B, Lohr HR, Humphries P, Redmond TM, Seeliger MW, Crouch RK, 2005. Cone opsin mislocalization in Rpe65−/− mice: a defect that can be corrected by 11-cis retinal. Invest Ophthalmol Vis Sci 46, 3876–3882. [DOI] [PubMed] [Google Scholar]
- Saari JC, 2016. Vitamin A and Vision. Sub-cellular biochemistry 81, 231–259. [DOI] [PubMed] [Google Scholar]
- Saari JC, Nawrot M, Kennedy BN, Garwin GG, Hurley JB, Huang J, Possin DE, Crabb JW, 2001. Visual cycle impairment in cellular retinaldehyde binding protein (CRALBP) knockout mice results in delayed dark adaptation. Neuron 29, 739–748. [DOI] [PubMed] [Google Scholar]
- Salcedo E, Farrell DM, Zheng L, Phistry M, Bagg EE, Britt SG, 2009. The green-absorbing Drosophila Rh6 visual pigment contains a blue-shifting amino acid substitution that is conserved in vertebrates. J Biol Chem 284, 5717–5722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapp RJ, Christianson JS, Maier L, Studer K, Stark WS, 1991. Carotenoid replacement therapy in Drosophila: recovery of membrane, opsin and visual pigment. Exp Eye Res 53, 73–79. [DOI] [PubMed] [Google Scholar]
- Satoh AK, O’Tousa JE, Ozaki K, Ready DF, 2005. Rab11 mediates post-Golgi trafficking of rhodopsin to the photosensitive apical membrane of Drosophila photoreceptors. Development 132, 1487–1497. [DOI] [PubMed] [Google Scholar]
- Schwemer J, 1984. Renewal of visual pigment in photoreceptors of the blowfly. J Comp Physiol A 154, 535–547. [Google Scholar]
- Seino Y, Miki T, Kiyonari H, Abe T, Fujimoto W, Kimura K, Takeuchi A, Takahashi Y, Oiso Y, Iwanaga T, Seino S, 2008. Isx participates in the maintenance of vitamin A metabolism by regulation of beta-carotene 15,15’-monooxygenase (Bcmo1) expression. J Biol Chem 283, 4905–4911. [DOI] [PubMed] [Google Scholar]
- Sharkey CR, Blanco J, Leibowitz MM, Pinto-Benito D, Wardill TJ, 2020. The spectral sensitivity of Drosophila photoreceptors. Scientific reports 10, 18242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim K, Picking WL, Kutty RK, Thomas CF, Wiggert BN, Stark WS, 1997. Control of Drosophila retinoid and fatty acid binding glycoprotein expression by retinoids and retinoic acid: northern, western and immunocytochemical analyses. Exp Eye Res 65, 717–727. [DOI] [PubMed] [Google Scholar]
- Sommer A, 2008. Vitamin a deficiency and clinical disease: an historical overview. J Nutr 138, 1835–1839. [DOI] [PubMed] [Google Scholar]
- Stavenga DG, Hardie RC, 2011. Metarhodopsin control by arrestin, light-filtering screening pigments, and visual pigment turnover in invertebrate microvillar photoreceptors. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 197, 227–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavenga DG, Wehling MF, Belusic G, 2017. Functional interplay of visual, sensitizing and screening pigments in the eyes of Drosophila and other red-eyed dipteran flies. J Physiol 595, 5481–5494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stehlin-Gaon C, Willmann D, Zeyer D, Sanglier S, Van Dorsselaer A, Renaud JP, Moras D, Schule R, 2003. All-trans retinoic acid is a ligand for the orphan nuclear receptor ROR beta. Nat Struct Biol 10, 820–825. [DOI] [PubMed] [Google Scholar]
- Stringham JM, Hammond BR Jr., 2007. The glare hypothesis of macular pigment function. Optometry and vision science : official publication of the American Academy of Optometry 84, 859–864. [DOI] [PubMed] [Google Scholar]
- Thompson DA, Li Y, McHenry CL, Carlson TJ, Ding X, Sieving PA, Apfelstedt-Sylla E, Gal A, 2001. Mutations in the gene encoding lecithin retinol acyltransferase are associated with early-onset severe retinal dystrophy. Nat Genet 28, 123–124. [DOI] [PubMed] [Google Scholar]
- Toomey MB, Lind O, Frederiksen R, Curley RW Jr., Riedl KM, Wilby D, Schwartz SJ, Witt CC, Harrison EH, Roberts NW, Vorobyev M, McGraw KJ, Cornwall MC, Kelber A, Corbo JC, 2016. Complementary shifts in photoreceptor spectral tuning unlock the full adaptive potential of ultraviolet vision in birds. eLife 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis GH, Golczak M, Moise AR, Palczewski K, 2007. Diseases caused by defects in the visual cycle: retinoids as potential therapeutic agents. Annual review of pharmacology and toxicology 47, 469–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsin AT, Santos FR, 1985. The 3, 4-didehydroretinal chromophore of goldfish porphyropsin. The Journal of experimental zoology 235, 181–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Bennekum A, Werder M, Thuahnai ST, Han CH, Duong P, Williams DL, Wettstein P, Schulthess G, Phillips MC, Hauser H, 2005. Class B scavenger receptor-mediated intestinal absorption of dietary beta-carotene and cholesterol. Biochemistry 44, 4517–4525. [DOI] [PubMed] [Google Scholar]
- Vogt K, 1984. The Chromophore of the Visual Pigment in Some Insect Orders. Z. Naturforsch 39, 196–197. [Google Scholar]
- Vogt K, Kirschfeld K, 1984. Chemical identity of the chromophores of fly visual pigment. Naturwissenschaften 71, 211–213. [Google Scholar]
- von Lintig J, 2010. Colors with functions: elucidating the biochemical and molecular basis of carotenoid metabolism. Annu Rev Nutr 30, 35–56. [DOI] [PubMed] [Google Scholar]
- von Lintig J, 2012. Metabolism of carotenoids and retinoids related to vision. J Biol Chem 287, 1627–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Lintig J, Vogt K, 2000. Filling the gap in vitamin A research. Molecular identification of an enzyme cleaving beta-carotene to retinal. J Biol Chem 275, 11915–11920. [DOI] [PubMed] [Google Scholar]
- von Lintig J, Wyss A, 2001. Molecular analysis of vitamin A formation: cloning and characterization of beta-carotene 15,15’-dioxygenases. Arch Biochem Biophys 385, 47–52. [DOI] [PubMed] [Google Scholar]
- Voolstra O, Kiefer C, Hoehne M, Welsch R, Vogt K, von Lintig J, 2006. The Drosophila class B scavenger receptor NinaD-I is a cell surface receptor mediating carotenoid transport for visual chromophore synthesis. Biochemistry 45, 13429–13437. [DOI] [PubMed] [Google Scholar]
- Wald G, 1935. Carotenoids and the Visual Cycle. J Gen Physiol 19, 351–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wald G, 1968. Molecular basis of visual excitation. Science 162, 230–239. [DOI] [PubMed] [Google Scholar]
- Wald G, Brown PK, 1956. Synthesis and bleaching of rhodopsin. Nature 177, 174–176. [DOI] [PubMed] [Google Scholar]
- Wang T, Jiao Y, Montell C, 2007. Dissection of the pathway required for generation of vitamin A and for Drosophila phototransduction. J Cell Biol 177, 305–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Montell C, 2005. Rhodopsin formation in Drosophila is dependent on the PINTA retinoid-binding protein. J Neurosci 25, 5187–5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Wang T, Jiao Y, von Lintig J, Montell C, 2010. Requirement for an enzymatic visual cycle in Drosophila. Curr Biol 20, 93–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Wang T, Ni JD, von Lintig J, Montell C, 2012. The Drosophila visual cycle and de novo chromophore synthesis depends on rdhB. J Neurosci 32, 3485–3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei LN, 2003. Retinoid receptors and their coregulators. Annual review of pharmacology and toxicology 43, 47–72. [DOI] [PubMed] [Google Scholar]
- Wenzel A, Oberhauser V, Pugh EN Jr., Lamb TD, Grimm C, Samardzija M, Fahl E, Seeliger MW, Reme CE, von Lintig J, 2005. The retinal G protein-coupled receptor (RGR) enhances isomerohydrolase activity independent of light. J Biol Chem 280, 29874–29884. [DOI] [PubMed] [Google Scholar]
- Wongsiriroj N, Piantedosi R, Palczewski K, Goldberg IJ, Johnston TP, Li E, Blaner WS, 2008. The molecular basis of retinoid absorption: a genetic dissection. J Biol Chem 283, 13510–13519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyss A, Wirtz G, Woggon W, Brugger R, Wyss M, Friedlein A, Bachmann H, Hunziker W, 2000. Cloning and expression of beta,beta-carotene 15,15’-dioxygenase. Biochem Biophys Res Commun 271, 334–336. [DOI] [PubMed] [Google Scholar]
- Yang J, O’Tousa JE, 2007. Cellular sites of Drosophila NinaB and NinaD activity in vitamin A metabolism. Mol Cell Neurosci 35, 49–56. [DOI] [PubMed] [Google Scholar]
- Yau KW, Hardie RC, 2009. Phototransduction motifs and variations. Cell 139, 246–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Choi EH, Tworak A, Salom D, Leinonen H, Sander CL, Hoang TV, Handa JT, Blackshaw S, Palczewska G, Kiser PD, Palczewski K, 2019. Photic generation of 11-cis-retinal in bovine retinal pigment epithelium. J Biol Chem 294, 19137–19154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Wang Y, Li R, Chen G, 2015. Transcriptional Factors Mediating Retinoic Acid Signals in the Control of Energy Metabolism. International journal of molecular sciences 16, 14210–14244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng L, Farrell DM, Fulton RM, Bagg EE, Salcedo E, Manino M, Britt SG, 2015. Analysis of Conserved Glutamate and Aspartate Residues in Drosophila Rhodopsin 1 and Their Influence on Spectral Tuning. J Biol Chem 290, 21951–21961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Znoiko SL, Rohrer B, Lu K, Lohr HR, Crouch RK, Ma JX, 2005. Downregulation of cone-specific gene expression and degeneration of cone photoreceptors in the Rpe65−/− mouse at early ages. Invest Ophthalmol Vis Sci 46, 1473–1479. [DOI] [PubMed] [Google Scholar]


