Abstract
Background:
The role of progestogens in colorectal cancer development are poorly characterized. To address this, our group developed a highly sensitive assay to measure concentrations of seven markers of endogenous progestogen metabolism among postmenopausal women.
Methods:
The markers were measured in baseline serum collected from postmenopausal women in a case-cohort study within the Breast and Bone Follow-up to the Fracture Intervention Trial (B~FIT). We followed women not using exogenous hormones at baseline (1992–1993) for up to twelve years: 187 women with incident colorectal cancer diagnosed during follow-up and a subcohort of 495 women selected on strata of age and clinical center. We used adjusted Cox regression models with robust variance to estimate risk for colorectal cancer (hazard ratios [HR], 95% confidence intervals [CI]).
Results:
High concentrations of pregnenolone and progesterone were not associated with colorectal cancer (quintile(Q)5 vs. Q1: pregnenolone HR 0.71, CI 0.40–1.25; progesterone HR 1.25, CI 0.71–2.22). A trend of increasing risk was suggested, but statistically imprecise across quintiles of 17-hydroxypregnenolone (Q2 to Q5 HRs 0.75 to 1.44, p-trend 0.06).
Conclusions:
We used sensitive and reliable assays to measure multiple circulating markers of progestogen metabolism. Progestogens were generally unassociated with colorectal cancer risk in postmenopausal women.
Impact:
Our findings are consistent with most prior research on circulating endogenous sex hormones, which taken together, suggest sex hormones may not be major drivers of colorectal carcinogenesis in postmenopausal women.
Keywords: Colorectal cancer, progestogens, hormones, prospective
Introduction
Colorectal cancer is both the third most commonly diagnosed cancer among women in the United States and also the third leading cause of cancer death.(1) There is a great need to identify potential biomarkers associated with both risk and prognosis. Colorectal cancer is more prevalent in men than in women;(2) differences in sex hormone production and metabolism could contribute to this observation. Although sex steroid hormone metabolism plays a role in the development of many cancers, especially gynecologic, its contribution to colorectal carcinogenesis is poorly understood.
Much of our knowledge about sex hormones and colorectal cancer stems from epidemiologic research on exogenous hormone use. In 2013, a meta-analysis from the Agency for Healthcare Research and Quality and the Centers for Disease Control and Prevention found evidence supporting a reduction in risk for colorectal cancer with prior oral contraceptive use.(3) However, this notion is complicated by inconsistent patterns of association across duration and recency of oral contraceptive use, coupled with null findings in recent large cohort analyses.(4),(5),(6),(7)
Combined estrogen and progestin menopausal hormone therapy (MHT) was linked to reductions in colorectal cancer risk after initial findings from the Women’s Health Initiative (WHI) Clinical Trial and other analyses conducted more than 20 years ago.(8) After updated analyses from the WHI Clinical Trial, a more complex story emerged. Combined MHT was associated with lower incidence of colorectal cancer, but this may be attributable to diagnostic delays; women in the intervention arm tended to be diagnosed with more advanced disease and there was no difference in colorectal cancer mortality by treatment arm.(9) Estrogen-only MHT was associated with increased colorectal cancer risk, but only among women with a history of polyp removal.(10) Analyses of WHI Observational Study data and other recent studies similarly do not support the idea that MHT use is a strong predictor of colorectal cancer risk.(4),(11)
Studying endogenous sex hormones may clarify their role in colorectal carcinogenesis. Progestogens specifically merit study because they serve as precursors to other steroid hormones (androgens, estrogens, and corticosteroids). Most colorectal cancers are diagnosed after menopause,(2) which has made research on this topic difficult to conduct. As others note, progestogens and their metabolites are found at low levels and can be difficult to measure among postmenopausal women.(12),(13) As a result, few epidemiologic studies explore circulating sex hormones and postmenopausal colorectal cancer risk; only three measured a progestogen (progesterone) and most do not identify associations with circulating hormones.(13),(14),(15),(16),(17),(18) In our research using data from the study population described below, estrogen metabolites were not linked to colorectal cancer risk.(14)
We wanted to understand if circulating markers of progestogen metabolism were associated with colorectal cancer among postmenopausal women. To do so, our team developed a highly sensitive liquid chromatography tandem mass spectrometry (LC-MS/MS) assay to measure the concentrations of seven markers of endogenous progestogen metabolism.(19)
Materials and Methods
We previously selected a case-cohort from the Breast and Bone Follow-up study (B~FIT) that was conducted within the Fracture Intervention Trial (FIT).(20) To enroll in FIT, women had to be at least 55 years of age and menopausal for at least 2 years, as determined during telephone screening interviews. A description of the study design for colorectal cancer outcomes was included in our analysis of estrogen biomarkers.(14)
To briefly summarize, from the 15 595 women in B~FIT, we selected all women who developed colorectal cancer over 12 years of follow-up (n=187; 1992–2004) and made comparisons to a subcohort of 495 women; 10 women who developed colorectal cancer were also sampled in the subcohort. Six women in the original subcohort were not included in the present analysis, as biospecimens were unavailable. All women were postmenopausal (mean age at baseline, cases: 67 years and subcohort: 70 years) and none had used MHT within the 4 months prior to blood draw; basic demographic information is summarized in Supplemental Table 1. Cancer diagnoses were confirmed by medical records and cancer registry information; vital status was confirmed through linkage to the National Death Index. Of the 187 women diagnosed with colorectal cancers, 20 had tumors located in the rectum, 80 had tumors in both the colon and rectum, and 87 had only colon tumors. All participants provided written informed consent and the study was approved by the Institutional Review Boards of all clinical sites and the National Cancer Institute.(14) Data can be made available upon request and with access agreements (https://dceg.cancer.gov/research/who-we-study/cohorts/bone-density-fit-study).
Participants provided pre-diagnostic serum at baseline; this was on average, six years before diagnosis for the women who developed colorectal cancer (standard deviation [SD] 3 years). The average time between baseline/serum collection and the end of follow-up among the subcohort was 10.2 years (SD 2.2, maximum 12.9 years). We measured seven circulating markers of progestogen metabolism: pregnenolone, progesterone, 17-hydroxypregnenolone, 17-hydroxyprogesterone, and three progesterone metabolites (5α-dihydroprogesterone, 3α-dihydroprogesterone, and 20α-dihydroprogesterone).
Among women in the subcohort, Spearman ranked correlations between these markers ranged from −0.07 (5α-dihydroprogesterone and 20α-dihydroprogesterone) to 0.73 (progesterone and 20α-dihydroprogesterone). In our population of postmenopausal women, total estradiol was not correlated with the measured progestogens (correlations ranging from −0.02 to 0.15); these data are described in detail elsewhere.(21) The LC-MS/MS assay that we used is highly sensitive and reliable.(19) All progestogens were assayed at the same time and there was little variation across batches; average laboratory coefficients of variation for blinded duplicate quality control samples within and across batches were less than 4%.(21) The lower limit of quantitation (LLOQ) for all measured progestogens was 0.5 ng/dL and the lower limit of detection was 0.1 ng/dL.(19, 21) No samples had undetectable levels for any of the hormone concentrations measured. Prior studies assessing circulating progesterone and colorectal cancer report a LC-MS/MS LLOQ of 100 pg/mL,(13) a radioimmunoassay “sensitivity” of 10 pg/ml,(15) and an electrochemiluminescence LLOQ of 1.57 ng/mL.(17)
For descriptive purposes, we estimated age-adjusted geometric mean concentrations of each progestogen among women in the subcohort and women who developed colorectal cancer. We then used Cox regression with robust variance to estimate risk for developing colorectal cancer during follow-up associated with circulating progestogens, categorized in quintiles according to the distribution of concentrations in the subcohort. We additionally estimated risk associated with ratios of these markers. Models were adjusted for baseline age and body mass index (both continuous), clinic enrollment site (10 sites), and enrollment arm in the original FIT trial. Age was used as the time metric; women in the subcohort entered the analysis upon age at baseline and contributed person-time until age at first diagnosis of colorectal cancer or censoring (death, end of follow-up). Women who developed colorectal cancer and were not in the subcohort contributed person time from 6 months before their age at diagnosis until that age at diagnosis (i.e., they contributed information only to their risk set).(21) Trends across quintiles were examined by treating the categorical quintile variables for each biomarker as continuous variables in models (reported as p for trend). As a sensitivity analysis, we also excluded persons with follow-up time of less than or equal to 2 years from baseline (20 cases, 1 of whom was also sampled in the subcohort); results were consistent with those presented in text. Though our previous work on circulating estrogens suggested no association with colorectal cancer risk (14), we explored model adjustment for circulating estradiol in the present analysis and did not find evidence that would change the conclusions presented here. To assess linearity of the exposure-risk relationships, we also plotted and visually examined risks associated with each progestogen modeled with 5-knot splines derived from the 10th, 25th, 50th, 75th, and 90th percentiles for each biomarker among the subcohort. All tests of statistical significance were two-sided and used an α of 0.05. SAS 9.4 was used for all analyses (SAS Institute Inc., Cary, North Carolina).
Results
Geometric mean concentrations of progestogens were comparable between women who developed colorectal cancer and those in the subcohort, though means for all markers except 3α-dihydroprogesterone and 20α-dihydroprogesterone were slightly higher among the cases (Table 1).
Table 1.
Age-adjusted geometric mean concentrations of circulating progestagens (pmol/L) among women who developed colorectal cancer and a comparison subcohort in the B~FIT study (1992–2004)
| Cases n=187 |
Subcohort n=495 |
|
|---|---|---|
| Geometric Mean (95% CI) | Geometric Mean (95% CI) | |
| Pregnenolone | 436.93 (408.93–466.84) | 461.07 (442.85–480.03) |
| Progesterone | 142.75 (136.89–148.85) | 138.85 (135.36–142.44) |
| 17-hydroxypregnenolone | 2,779.62 (2,620.11–2,948.83) | 2,636.50 (2,543.32–2,733.09) |
| 17-hydroxyprogesterone | 408.65 (382.25–436.86) | 402.57 (386.53–419.27) |
| 5α-dihydroprogesterone | 210.64 (196.52–225.77) | 221.86 (212.68–231.43) |
| 3α-dihydroprogesterone | 68.32 (63.00–74.10) | 64.90 (61.77–68.19) |
| 20α-dihydroprogesterone | 150.46 (144.01–157.19) | 145.89 (142.05–149.84) |
B~FIT=Breast and Bone Follow-up study to the Fracture Intervention Trial, pmol/L=picomole per liter, n=number, CI=confidence interval
There were 10 women who developed colorectal cancer who were also sampled into the subcohort.
Generally, high concentrations of progestogens relative to low concentrations, were not associated with developing colorectal cancer during follow-up (Table 2). We identified a trend of increasing risk across quintiles of 17-hydroxypregnenolone, but all confidence intervals were imprecise (quintile 5 vs. 1 hazard ratio [HR]: 1.44, 95% confidence interval (CI): 0.83–2.50, p for trend 0.06). Increased risks were suggested for all quintiles of 3α-dihydroprogesterone—but estimates were similarly imprecise and there was no trend of increasing risk across quintiles (quintile HRs ranging from 1.41 to 1.62, p for trend 0.21). Our data also did not support an association between colorectal cancer and increasing 3α-dihydroprogesterone relative to its precursor, progesterone (Table 2).
Table 2.
Associations between colorectal cancer and circulating progestagens, a case-cohort analysis from the B~FIT study (1992–2004)
| Progestagen | Q | Case1 n |
HR (95%CI)2 | P for trend3 |
|---|---|---|---|---|
| Pregnenolone | 1 | 46 | Reference | 0.308 |
| 2 | 34 | 0.72 (0.41–1.26) | ||
| 3 | 49 | 0.99 (0.58–1.67) | ||
| 4 | 31 | 0.77 (0.44–1.34) | ||
| 5 | 27 | 0.71 (0.40–1.25) | ||
| Progesterone | 1 | 35 | Reference | 0.461 |
| 2 | 41 | 1.15 (0.66–2.01) | ||
| 3 | 33 | 1.01 (0.56–1.81) | ||
| 4 | 38 | 1.13 (0.64–2.00) | ||
| 5 | 40 | 1.25 (0.71–2.22) | ||
| 17-hydroxypregnenolone | 1 | 40 | Reference | 0.064 |
| 2 | 26 | 0.75 (0.41–1.36) | ||
| 3 | 35 | 0.93 (0.53–1.64) | ||
| 4 | 40 | 1.09 (0.63–1.90) | ||
| 5 | 46 | 1.44 (0.83–2.50) | ||
| 17-hydroxyprogesterone | 1 | 39 | Reference | 0.360 |
| 2 | 33 | 0.83 (0.47–1.48) | ||
| 3 | 31 | 0.65 (0.36–1.16) | ||
| 4 | 44 | 0.95 (0.56–1.61) | ||
| 5 | 40 | 1.13 (0.65–1.97) | ||
| 5α-dihydroprogesterone (5αP) | 1 | 39 | Reference | 0.155 |
| 2 | 39 | 1.20 (0.68–2.12) | ||
| 3 | 44 | 1.14 (0.66–1.96) | ||
| 4 | 40 | 1.19 (0.67–2.12) | ||
| 5 | 25 | 0.67 (0.37–1.21) | ||
| 3α-dihydroprogesterone (3αHP) | 1 | 27 | Reference | 0.212 |
| 2 | 40 | 1.41 (0.79–2.51) | ||
| 3 | 39 | 1.49 (0.84–2.66) | ||
| 4 | 38 | 1.62 (0.90–2.92) | ||
| 5 | 43 | 1.50 (0.83–2.71) | ||
| 20α-dihydroprogesterone (20αHP) | 1 | 34 | Reference | 0.239 |
| 2 | 26 | 0.84 (0.45–1.56) | ||
| 3 | 47 | 1.46 (0.85–2.52) | ||
| 4 | 39 | 1.16 (0.67–2.01) | ||
| 5 | 41 | 1.33 (0.77–2.30) | ||
| Ratios | ||||
| 5αP : progesterone | 1 | 42 | Reference | 0.192 |
| 2 | 39 | 1.07 (0.60–1.90) | ||
| 3 | 42 | 0.86 (0.50–1.48) | ||
| 4 | 32 | 0.77 (0.44–1.35) | ||
| 5 | 32 | 0.77 (0.44–1.33) | ||
| 3αHP : progesterone | 1 | 34 | Reference | 0.945 |
| 2 | 26 | 0.71 (0.39–1.26) | ||
| 3 | 52 | 1.45 (0.84–2.50) | ||
| 4 | 43 | 1.12 (0.64–1.96) | ||
| 5 | 32 | 0.84 (0.46–1.55) | ||
| 20αHP : progesterone | 1 | 36 | Reference | 0.567 |
| 2 | 35 | 0.93 (0.53–1.65) | ||
| 3 | 38 | 1.10 (0.62–1.95) | ||
| 4 | 37 | 1.07 (0.61–1.87) | ||
| 5 | 41 | 1.13 (0.64–2.00) | ||
| 5αP : 3αHP | 1 | 44 | Reference | 0.407 |
| 2 | 37 | 0.97 (0.54–1.72) | ||
| 3 | 34 | 0.82 (0.47–1.45) | ||
| 4 | 39 | 0.95 (0.55–1.63) | ||
| 5 | 33 | 0.78 (0.44–1.37) | ||
| 5αP : 20αHP | 1 | 40 | Reference | 0.137 |
| 2 | 38 | 0.92 (0.54–1.57) | ||
| 3 | 45 | 0.89 (0.51–1.58) | ||
| 4 | 37 | 0.90 (0.52–1.58) | ||
| 5 | 27 | 0.64 (0.36–1.14) | ||
| Progesterone : estradiol4 | 1 | 31 | Reference | 0.970 |
| 2 | 40 | 1.15 (0.65–2.04) | ||
| 3 | 48 | 1.17 (0.66–2.08) | ||
| 4 | 35 | 1.11 (0.59–2.07) | ||
| 5 | 33 | 1.07 (0.57–2.02) |
B~FIT=Breast and Bone Follow-up study to the Fracture Intervention Trial, Q=quintile, n=number, HR=hazard ratio, CI=confidence interval
For all hormones/metabolites, there were 99 women from the subcohort in each quintile.
Models are adjusted for age, body mass index, clinic enrollment site, and enrollment arm in the original clinical trial.
P trend calculated by treating each quintile variable as a continuous exposure (using the median concentrations in each quintile).
Measured previously, please see Falk et al. (2015), manuscript reference #14.
Examination of spline results revealed non-linear relationships between progestogens and colorectal cancer risk, but these results similarly confirmed the imprecise and potentially null associations identified in the quintile-based analyses. Interestingly, the spline for 17-hydroxypregnenolone supported the increased cancer risks suggested for women with the highest circulating levels of this marker (Figure 1).
Figure 1.
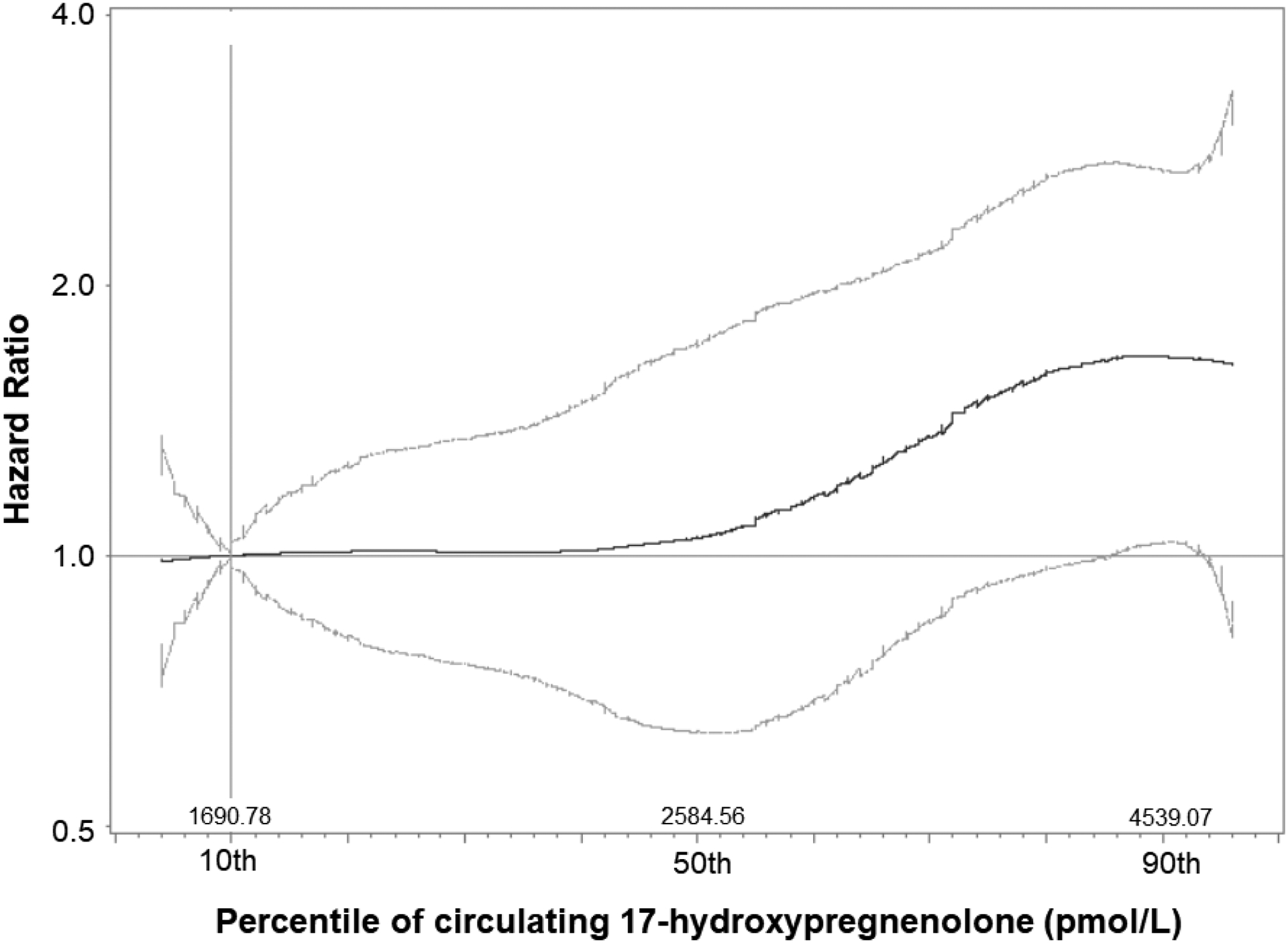
Hazard Ratios for colorectal cancer associated with increasing concentrations of circulating 17-hydroxypregnenolone, modeled with 5-knot splines, a case-cohort analysis from the B~FIT study (1992–2004)
Discussion
In our analysis, circulating progestogens were not associated with colorectal cancer in postmenopausal women. However, in measuring multiple progestogens, we were able to make important observations and comparisons with other research on hormone-associated carcinogenesis.
Progesterone-associated proliferation in breast cancers is thought to be largely mediated by nuclear factor kappa B pathways (e.g., RANKL/RANK), but progestogens are also metabolic precursors to androgens and estrogens, which along with their receptors, regulate DNA damage responses in breast and prostate cancers.(22, 23) These mechanisms, if at play in colorectal carcinogenesis, remain poorly understood. The progestogens included in our assay were initially selected because of hypothesized roles in breast cancer development. In particular, the metabolite 3α-dihydroprogesterone is linked to tumor suppression within in vitro breast cancer studies, whereas 5α-dihydroprogesterone may increase cancer cell proliferation and decrease rates of apoptosis.(24) Our analysis did not support an association between these metabolites and colorectal cancer.
The colorectal cancer association that we observed with 17-hydroxypregnenolone seemed relegated to those women with concentrations at the 90th percentile or higher—though at 4619.78 pmol/L, this appears to be within the range of normal values measured via radioimmunoassay among healthy women of postmenopausal age.(25) Interestingly, variants within the gene encoding 17 α -hydoxylase, the enzyme which converts progesterone and pregnenolone to their 17-hydroxy counterparts, were associated with colorectal cancer among women in the WHI Observational Study. However, functional experiments tying this variation to circulating or intratumoral hormone levels were not conducted.(26) In future studies, circulating 17-hydroxypregnenolone concentrations might contribute information in risk prediction models.
The strengths of our approach to understanding hormone-related colorectal carcinogenesis center on the use of highly sensitive assays to measure hormones and a case-cohort design, which facilitates estimating measures of association like the hazard ratio. To our knowledge, six studies have assessed postmenopausal colorectal cancer risk and circulating sex steroid hormones.(14),(15),(13),(16),(17),(18) Most do not identify statistically significant associations. Estradiol was the most commonly measured hormone, but the studies reach conflicting conclusions about its association with colorectal cancer. In our prior analysis among women in this B~FIT case-cohort, neither circulating estradiol or major estrogen metabolites were associated with colorectal cancer (also measured via a sensitive LC-MS/MS assay).(14) None of these studies measured 17-hydroxypregnenolone or its downstream metabolite dehydroepiandrosterone (DHEA), which is the immediate precursor to all androgens. A frequently cited nested case-control study of men, premenopausal women, and postmenopausal women, suggested that higher levels of radioimmunoassay-measured DHEA were associated with reduced colorectal cancer risk (n=117 cases), especially among men, but effect estimates were imprecise.(27)
Two analyses by other groups measured progesterone within nested case-control studies, but used immunoassays and electrochemiluminescence; neither found evidence supporting associations between progesterone levels and colorectal cancer.(15),(17) Clendenen and colleagues measured progesterone with an LC-MS/MS assay in their nested case-control study, but reported that “values were below the [LLOQ] for more than 93% of our samples.”(13) Another group used LC-MS/MS to measure circulating estradiol, estrone, and testosterone within a nested case-control study pooled from several prospective, U.S.-based cohorts, but the researchers similarly could not rule out null associations with colorectal cancer.(16) In all of these studies, as with ours, small sample sizes may limit power to detect very modest associations. It should also be noted that none of these studies conducted equivalence testing as a method to compare mean hormone levels between women who did and did not develop cancer.
When compared with other studies, our findings suggest that sex hormones may not be major drivers of colorectal cancer development in postmenopausal women.(14) To our knowledge, this is the most comprehensive exploration of progestogens and colorectal cancer risk, but metabolites that were not assayed may influence the development of this disease. Additionally, circulating levels of sex hormones may not fully reflect their local, intratumoral levels and actions; hormone activity would also vary with a person’s age and sex. Progesterone receptors appear to be expressed in the normal colon;(28) therefore, hormone receptor expression studies within colorectal tissues continue to be warranted. Of great value, will be studies that can contextualize sex steroid hormone action with epidemiologic information—such as potential colorectal cancer risk factors like diet, the microbiome, and family history. For example, comparing progesterone receptor expression in biopsy specimens from women with and without a history of exogenous hormone use (oral contraceptives and MHT), may provide insight on the long-term influences these exposures exert within the gastrointestinal tract.
Supplementary Material
Funding
This work was supported in part by the Intramural Research Program of the National Cancer Institute, National Institutes of Health. B~FIT was funded by the National Cancer Institute (contract #N02-CP-01019), National Institutes of Health, Department of Health and Human Services. The original FIT study was funded by Merck Research Laboratories.
The funding agency had no role in the design or conduct of the study, the analysis or interpretation of the data, the preparation of the manuscript, or the decision to submit the manuscript for publication.
Footnotes
Disclosures
The authors declare no conflicts of interest.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Fedewa SA, Ahnen DJ, Meester RGS, Barzi A, et al. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017;67(3):177–93. [DOI] [PubMed] [Google Scholar]
- 3.Gierisch JM, Coeytaux RR, Urrutia RP, Havrilesky LJ, Moorman PG, Lowery WJ, et al. Oral contraceptive use and risk of breast, cervical, colorectal, and endometrial cancers: a systematic review. Cancer Epidemiol Biomarkers Prev. 2013;22(11):1931–43. [DOI] [PubMed] [Google Scholar]
- 4.Kabat GC, Miller AB, Rohan TE. Oral contraceptive use, hormone replacement therapy, reproductive history and risk of colorectal cancer in women. Int J Cancer. 2008;122(3):643–6. [DOI] [PubMed] [Google Scholar]
- 5.Murphy N, Xu L, Zervoudakis A, Xue X, Kabat G, Rohan TE, et al. Reproductive and menstrual factors and colorectal cancer incidence in the Women’s Health Initiative Observational Study. Br J Cancer. 2017;116(1):117–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Charlton BM, Wu K, Zhang X, Giovannucci EL, Fuchs CS, Missmer SA, et al. Oral contraceptive use and colorectal cancer in the Nurses’ Health Study I and II. Cancer Epidemiol Biomarkers Prev. 2015;24(8):1214–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Michels KA, Pfeiffer RM, Brinton LA, Trabert B. Modification of the Associations Between Duration of Oral Contraceptive Use and Ovarian, Endometrial, Breast, and Colorectal Cancers. JAMA Oncol. 2018;4(4):516–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin JH, Giovannucci E. Sex hormones and colorectal cancer: what have we learned so far? J Natl Cancer Inst. 2010;102(23):1746–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simon MS, Chlebowski RT, Wactawski-Wende J, Johnson KC, Muskovitz A, Kato I, et al. Estrogen plus progestin and colorectal cancer incidence and mortality. J Clin Oncol. 2012;30(32):3983–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lavasani S, Chlebowski RT, Prentice RL, Kato I, Wactawski-Wende J, Johnson KC, et al. Estrogen and colorectal cancer incidence and mortality. Cancer. 2015;121(18):3261–71. [DOI] [PubMed] [Google Scholar]
- 11.Prentice RL, Pettinger M, Beresford SA, Wactawski-Wende J, Hubbell FA, Stefanick ML, et al. Colorectal cancer in relation to postmenopausal estrogen and estrogen plus progestin in the Women’s Health Initiative clinical trial and observational study. Cancer Epidemiol Biomarkers Prev. 2009;18(5):1531–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Missmer SA, Eliassen AH, Barbieri RL, Hankinson SE. Endogenous estrogen, androgen, and progesterone concentrations and breast cancer risk among postmenopausal women. J Natl Cancer Inst. 2004;96(24):1856–65. [DOI] [PubMed] [Google Scholar]
- 13.Clendenen TV, Koenig KL, Shore RE, Levitz M, Arslan AA, Zeleniuch-Jacquotte A. Postmenopausal levels of endogenous sex hormones and risk of colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2009;18(1):275–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Falk RT, Dallal CM, Lacey JV Jr., Bauer DC, Buist DS, Cauley JA, et al. Estrogen Metabolites Are Not Associated with Colorectal Cancer Risk in Postmenopausal Women. Cancer Epidemiol Biomarkers Prev. 2015;24(9):1419–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murphy N, Strickler HD, Stanczyk FZ, Xue X, Wassertheil-Smoller S, Rohan TE, et al. A Prospective Evaluation of Endogenous Sex Hormone Levels and Colorectal Cancer Risk in Postmenopausal Women. JNCI: Journal of the National Cancer Institute. 2015;107(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin JH, Zhang SM, Rexrode KM, Manson JE, Chan AT, Wu K, et al. Association between sex hormones and colorectal cancer risk in men and women. Clin Gastroenterol Hepatol. 2013;11(4):419–24.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mori N, Sawada N, Iwasaki M, Yamaji T, Goto A, Shimazu T, et al. Circulating sex hormone levels and colorectal cancer risk in Japanese postmenopausal women: The JPHC nested case-control study. Int J Cancer. 2019;145(5):1238–44. [DOI] [PubMed] [Google Scholar]
- 18.Gunter MJ, Hoover DR, Yu H, Wassertheil-Smoller S, Rohan TE, Manson JE, et al. Insulin, insulin-like growth factor-I, endogenous estradiol, and risk of colorectal cancer in postmenopausal women. Cancer Res. 2008;68(1):329–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trabert B, Falk RT, Stanczyk FZ, McGlynn KA, Brinton LA, Xu X. Reproducibility of an assay to measure serum progesterone metabolites that may be related to breast cancer risk using liquid chromatography-tandem mass spectrometry. Horm Mol Biol Clin Investig. 2015;23(3):79–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Black DM, Reiss TR, Nevitt MC, Cauley J, Karpf D, Cummings SR, et al. Design of the Fracture Intervention Trial. Osteoporos Int. 1993;3(3):29–39. [DOI] [PubMed] [Google Scholar]
- 21.Trabert B, Bauer DC, Buist DSM, Cauley JA, Falk RT, Geczik AM, et al. Association of Circulating Progesterone With Breast Cancer Risk Among Postmenopausal Women. JAMA Netw Open. 2020;3(4):e203645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ganguly S, Naik D, Muskara A, Mian OY. The Nexus of Endocrine Signaling and Cancer: How Steroid Hormones Influence Genomic Stability. Endocrinology. 2021;162(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trabert B, Sherman ME, Kannan N, Stanczyk FZ. Progesterone and Breast Cancer. Endocr Rev. 2020;41(2):320–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wiebe JP. Progesterone metabolites in breast cancer. 2006;13(3):717. [DOI] [PubMed] [Google Scholar]
- 25.Hill M, Lukác D, Lapcík O, Sulcová J, Hampl R, Pouzar V, et al. Age relationships and sex differences in serum levels of pregnenolone and 17-hydroxypregnenolone in healthy subjects. Clin Chem Lab Med. 1999;37(4):439–47. [DOI] [PubMed] [Google Scholar]
- 26.Lin JH, Manson JE, Kraft P, Cochrane BB, Gunter MJ, Chlebowski RT, et al. Estrogen and progesterone-related gene variants and colorectal cancer risk in women. BMC Med Genet. 2011;12(1):78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alberg AJ, Gordon GB, Hoffman SC, Comstock GW, Helzlsouer KJ. Serum dehydroepiandrosterone and dehydroepiandrosterone sulfate and the subsequent risk of developing colon cancer. Cancer Epidemiol Biomarkers Prev. 2000;9(5):517–21. [PubMed] [Google Scholar]
- 28.Asavasupreechar T, Saito R, Miki Y, Edwards DP, Boonyaratanakornkit V, Sasano H. Systemic distribution of progesterone receptor subtypes in human tissues. J Steroid Biochem Mol Biol. 2020;199:105599. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


