Abstract
Purpose:
The mTOR pathway has been identified as a key nutrient signaling hub that participates in metastatic progression of high-grade osteosarcoma. Inhibition of mTOR signaling is biologically-achievable with sirolimus, and might slow the outgrowth of distant metastases. In this study, pet dogs with appendicular osteosarcoma were leveraged as high-value biologic models for pediatric osteosarcoma, to assess mTOR inhibition as a therapeutic strategy for attenuating metastatic disease progression.
Experimental design:
324 pet dogs diagnosed with treatment-naïve appendicular osteosarcoma were randomized into a 2-arm, multicenter, parallel superiority trial whereby dogs received amputation of the affected limb followed by adjuvant carboplatin chemotherapy ± oral sirolimus therapy. The primary outcome measure was DFI, as assessed by serial physical and radiologic detection of emergent macroscopic metastases; secondary outcomes included overall 1- and 2-year survival rates, and sirolimus pharmacokinetic variables and their correlative relationship to adverse events and clinical outcomes.
Results:
There was no significant difference in the median DFI or overall survival between the two arms of this trial; the median DFI and survival for standard-of-care (SOC; defined as amputation and carboplatin therapy) dogs was 180 days (95% CI: 144-237), and 282 days (95% CI: 224-383); for SOC + sirolimus dogs, it was 204 days (95% CI: 157-217) and 280 days (95% CI: 252-332), respectively.
Conclusions:
In a population of pet dogs non-genomically segmented for predicted mTOR inhibition response, sequentially-administered adjuvant sirolimus, although well tolerated when added to a backbone of therapy, did not extend disease-free interval or survival in dogs with appendicular osteosarcoma.
Keywords: osteosarcoma, mTOR inhibition, canine, comparative oncology, clinical trials
Introduction
Osteosarcoma (OS) is a common and aggressive spontaneous malignancy arising from osteoblast lineage and affecting two primary species being human beings and canines. Comparatively, OS in both people and dogs share conserved clinical, molecular, genetic, and biological behaviors.1–6 Despite significant efforts to identify and implement treatment strategies that provide durable tumor control, metastatic OS progression continues to be a leading cause of death for both human and canine patients. For humans, significant improvements in outcome have not occurred in over 30 years since the implementation of multiagent chemotherapy alongside limb-salvaging surgical procedures, with approximately 30% of patients developing metastatic disease despite aggressive front-line treatment.7,8 Similarly, clinical data collected from pet dogs with naturally-occurring OS enrolled on both retrospective and prospective trials consistently demonstrates survival times which range from 242 to 306 days, with uniformly prescribed treatment being amputation of the affected limb followed by adjuvant cytotoxic chemotherapy.9 Collectively, there are resounding scientific and clinical justifications for exploring complementary and orthogonal modeling paradigms that might efficiently and rapidly validate molecularly-targeted agents for curbing metastatic OS progression.10
The Osteosarcoma Project is a joint initiative launched by the Morris Animal Foundation, the QuadW Foundation, and the National Cancer Institute’s Comparative Oncology Trial Consortium (NCI-COTC) to identify new therapeutic interventions that prevent or delay metastatic progression in OS via screening of novel agents in pet dogs with spontaneously-arising disease. The initiative is designed to compare investigational agents against a prospectively enrolled cohort of dogs receiving the current standard of care (SOC) for OS which is limb amputation followed by four doses of carboplatin chemotherapy. Through this collaborative partnership, the conductance of these rapid and scalable clinical trials in pet dogs is expected to provide unparalleled translational insights and discoveries related to OS metastatic progression, which might be leveraged by consortiums such as the Children’s Oncology Group (COG) or Sarcoma Alliance for Research through Collaboration (SARC) for clinical guidance and target prioritization in pediatric OS patients.
Using metrics for valuing preclinical data types for prioritizing and advancing agents to be assessed in pediatric OS trials,11 a group of clinician-scientists endorsed sirolimus as the first agent to be evaluated within the Osteosarcoma Project clinical trial framework. Robust scientific, preclinical, and translational justification for the selection of sirolimus as a favorable agent with presumed antimetastatic activities are multifactorial. First, the PI3K/mTOR pathway has been identified as a central signaling pathway responsible for mediating multiple aspects of OS progression and metastases.12,13 Second, inhibition of the mTOR pathway using sirolimus has been assessed in orthotopic mouse models of metastatic OS,14,15 and at clinically-achievable exposures, sirolimus exerts robust antimetastatic activities that is distinct from a modest effect on heterotopic primary tumor growth in mice.16 Third, clinical data collected within a series of canine comparative oncology trials carried out in normal and tumor-bearing dogs demonstrate that sirolimus administered parenterally is tolerable and provide pharmacokinetic exposures that are translatable to those achieved in human patients; and result in effective tumoral and surrogate peripheral blood mononuclear cell (PBMC) modulation of pS6RP, a proximate target of the mTOR pathway.17,18 Last, an intriguing study performed by the French Sarcoma Group reported the off-label use of sirolimus alone or in combination with cyclophosphamide for the management of refractory relapsed OS, and demonstrated that sirolimus could produce disease stabilization in a subset of patients with advanced metastatic OS.19
These existent data generated in preclinical models (mice/dogs) and clinical findings in human patients with advanced stage disease, in conjunction with provocative role of mTOR signaling in OS cellular biology related to invasion, proliferation, survival and metastasis,15,20–22 served as the impetus for conducting the clinical trial reported herein. The study hypothesis was that adjuvant sirolimus therapy administered sequentially following standard of care therapy will exert antimetastatic activities and extend the DFI for dogs receiving it compared to those receiving only SOC by at least 50%. The primary endpoints of the trial include disease-free interval (DFI) and overall survival assessed in a prospective, randomized clinical trial setting. A secondary objective of this study was to identify key factors related to tolerability and clinical efficacy of sirolimus when studied in the minimal residual disease setting, including pharmacokinetic parameters and patient/tumor related factors.
Materials and Methods
Trial design
The National Cancer Institute’s Comparative Oncology Trials Consortium (NCI-COTC)
The COTC infrastructure provides a facile means of conducting multi-center clinical trials in pet dogs to advance anti-cancer drug development and cancer biology questions that are not sufficiently asked or answered in other animal models.23,24 Eighteen COTC member institutions participated in this randomized 2-arm parallel superiority trial following CONSORT guidelines. The study period included the entirety of the dogs’ disease-free interval (DFI) after diagnosis, amputation of the affected limb, and administration of adjuvant carboplatin therapy with or without adjuvant sirolimus administration. Dogs were considered off-study at the time metastatic disease progression was detected and confirmed through standard clinical radiographic imaging methods and/or tissue analysis.
Participants
Patient enrollment procedures and eligibility criteria
Dogs met predetermined eligibility criteria (Supplemental Table 1) in order to participate in the trial. Each participating COTC member institution obtained and maintained approval from their respective Institutional Animal Care and Use Committee (IACUC) prior to enrolling patients in this trial. Dogs were actively recruited into the clinical trial over a span of 31 consecutive months, with patient-specific finalized outcomes reported up to 3 years post-enrollment.
Sample size calculation:
A sample size calculation was performed to estimate the number of dogs needed to detect a difference in DFI of six months (SOC: 282 days, SOC + S: 464 days) using a two-sided logrank test at 80% power and at a significance level of 0.05 (PASS 13 Power Analysis and Sample Size Software (2014). NCSS, LLC. Kaysville, Utah, USA, ncss.com/software/pass).
Randomization and allocation
Prior to limb amputation, dogs were randomized to either SOC or SOC + S arms in an allocation ratio of 1:1. Arms were stratified in a 2 x 2 matrix with regards to 2 consistent known prognostic factors25 being tumor location (proximal humerus vs. non-proximal humerus) and alkaline phosphatase (ALP) status (normal vs. elevated), by assigning dogs to one of the 4 blocks. Dogs were randomized using a pregenerated block randomization list (with 4 dogs in each block), through generation of random number sequences for each block (using RAND() function) in commercially available software (Microsoft Excel for Mac version 16.16, Microsoft, Redmond, WA). Randomization to SOC or SOC + S was instituted at the initiation of carboplatin chemotherapy instead of prior to surgery, and treatment allocation was not blinded to enrolling COTC investigators or dog owners.
Clinical procedures
Biologic sample collections and biobanking efforts
This study protocol included prospective collection of biologic samples (Figure 1a–b, Supplemental Figure 1) to enable post-hoc analyses of factors relating to metastatic behavior of the primary tumor. Each dog had whole blood, peripheral blood mononuclear cells (PBMC), serum, tumor and normal tissue collected at time of surgery, prior to initiation of any therapy. In addition, dogs enrolled on the SOC + S arm had whole blood, PBMC and serum collected during 5 pharmacokinetic (PK) sampling curves over 7 sampling timepoints across the 4 cycles of sirolimus exposure.
Figure 1.
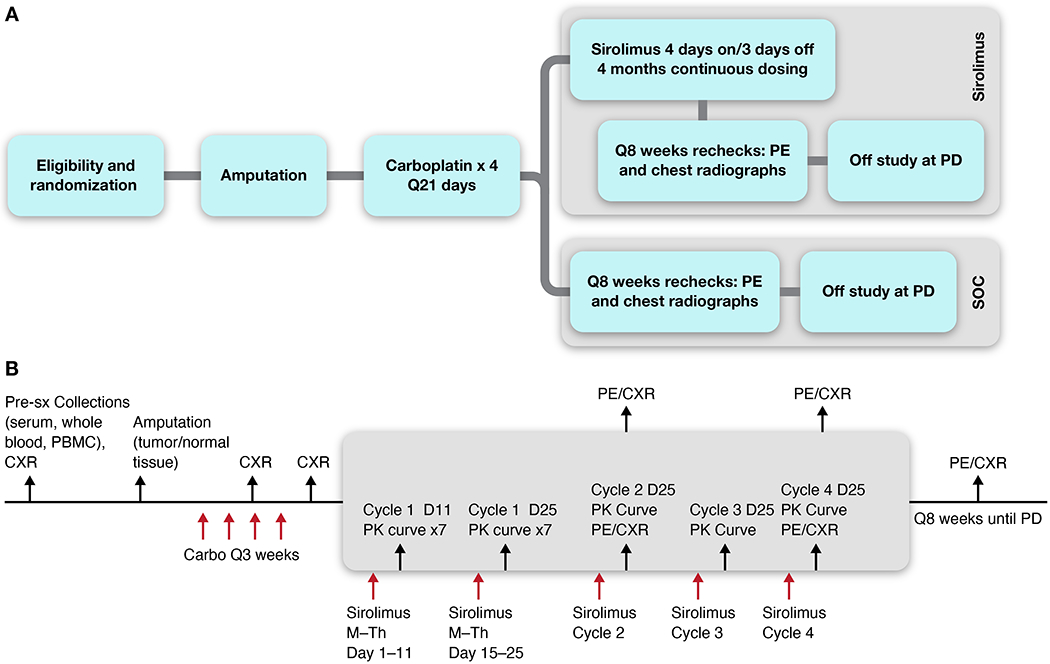
Overview of schema (A) and schedule of study procedures (B) for canine OS patients enrolled in the Standard of Care (SOC) vs. SOC + sirolimus (SOC + S) clinical trials. CXR = 3-view thoracic (chest) radiographic assessment. PBMC = Peripheral Blood Mononuclear Cells. PE = Physical Exam. PD = Progressive Disease. PK = pharmacokinetic assessment of drug levels.
Surgery
Dogs underwent either forelimb or hindlimb amputation surgery with regional lymphadenectomy to allow baseline biologic sample collection and tissue banking. At the time of surgery, tumor and normal tissue samples, serum, whole blood, and PBMCs were collected and stored for future analysis. Surgery occurred within 10 days of study enrollment.
Carboplatin chemotherapy
Between 10-21 days post-amputation, dogs began carboplatin chemotherapy at a dosage of 300 mg/m2 IV. If dogs had unacceptable clinical laboratory findings to allow for safe chemotherapy administration (e.g. Grade 1 or higher hematologic toxicity), the COTC clinician prescribed a dose delay of ≤ 7 days. In dogs with a history of a treatment delay due to Grade 2 or higher myelosuppression, a 10% reduction in carboplatin was prescribed for the ensuing cycle, but the q21 day treatment interval was preserved as often as possible.
Sirolimus administration
Sirolimus Walk-in trial
A dose-confirming study was conducted in tumor bearing dogs prior to initiation of the randomized trial of SOC ± sirolimus in order to determine the tolerability, pharmacokinetics, and optimized dosing regimen of oral sirolimus. A total of 22 tumor-bearing dogs received sirolimus orally at 0.1 mg/kg on either a Monday through Friday (M-F) schedule or Monday-Wednesday-Friday (M/W/F) schedule for 4 consecutive weeks. Whole blood samples were collected from a subset of dogs over a 48 hour period (0, 1, 2, 4, 6, 8, 24, 48 hours) following the first and last dose of sirolimus (administered on Day 1 and Day 26), as well as single timepoint measurements on Days 8 and 19, to monitor drug levels during treatment. Tolerability of both dosing schemes was assessed in all 22 dogs, while PK parameters were assessed in a subset of 4 dogs in the M-F dosing schedule, and 3 dogs in the M/W/F dosing schedule. Sirolimus tablets (0.5 mg and 2 mg) formulated for human use were used for treatment of pet dogs within both the walk-in and SOC + S trials (Sirolimus generic tablets for oral use, 0.5 mg and 2 mg, Greenstone® Brand, Greenstone LLC, Peapack, NJ, USA).
Adjuvant SOC + sirolimus arm
For dogs randomized to the SOC + S arm, treatment with sirolimus began within 7 days after completion of 4th cycle of carboplatin dosing at the Week 15 visit if dogs were confirmed free of macroscopic metastatic disease through the conductance of physical examination and thoracic radiography. Sirolimus was administered on a 4-days-on/3-days-off (treatment on Monday-Thursday, with no treatment on Friday-Sunday) at a dose of 0.1 mg/kg orally once a day for a 26-day cycle. The planned treatment interval was 4 consecutive cycles of sirolimus treatment. A seven-point whole blood pharmacokinetic (PK) sampling curve was collected on Days 11 and 25 of cycle 1 of sirolimus treatment; for cycles 2-4, only a Day 25 curve was conducted. Each dog had 3-view thoracic radiographs completed at the end of cycle 2 and 4, with evaluation by a board-certified veterinary radiologist.
Pharmacokinetic assessment of sirolimus-treated dogs
A validated method for extraction of a sirolimus analog was used as described to extract sirolimus from 100 μL of each blood sample by means of protein precipitation with 0.2M zinc sulfate followed by liquid-liquid extraction with 1 mL of ethyl acetate.26 The sirolimus concentration in each sample was determined by use of liquid chromatography-tandem mass spectrometry with tacrolimus as an internal standard as previously described.18
Initial PK analysis involved full time-course samples collected on Days 11 and 25 of cycle 1 for 20 random dogs. These full sample sets were used for the development of a limited sampling approach. This was done to determine the necessity of analyzing full time-course samples for the estimation of drug exposure via Area Under the Curve (AUC) and trough drug levels. Multiple stepwise linear regression modeling was done using 6 random subsets of the complete data set and a consensus model developed utilizing the 2- and 8-hour time points for estimation of AUC0-24h (MatLab vR2019a, MathWorks, Natick, MA, USA).
AUC (ng/ml x hr.) = 3.83 + (C2h x 3.15) + (C8h x 16.72)
The predictive capability of the limited sampling model was determined by calculation of the median absolute performance error (MAPE%), the median performance error (MPE%) and the root mean squared performance error (RMSPE%) as previously described.27 These analyses showed the MPE% = 1.89, MAPE% = 3.41 and RMSPE% = 6.37 and an accuracy ± precision (%CV) of the prediction as 95.3% ± 4.4%.
Clinical monitoring
Clinical monitoring was carried out through physical examination and thoracic radiography according to a standardized schedule (Figure 1a–b, Supplemental Figure 1). When clinically indicated, additional diagnostics to confirm or deny the presence of metastatic disease or other comorbidities were performed. Acute or chronic toxicities attributable to study procedures, surgery or drug treatments, or disease progression were prospectively assessed within this trial design. Adverse events (AEs) were given an attribution based upon a group consensus discussion between the COTC investigator, the study principal investigators (TMF, AKL) and the NCI COP study coordinator (CM). After completion of chemotherapy and/or sirolimus, dogs were reevaluated every 8 weeks with a physical examination and 3-view thoracic radiographs. If progressive pulmonary metastatic disease was suspected, repeat thoracic radiographs were performed after an additional 3-4 weeks to confirm the observation. All dogs were followed with this clinical monitoring schedule until confirmation of progressive disease, and/or until 3 years had passed from the date of surgery, whichever came first.
Statistical analysis
Categorical variables were described using frequencies and percentages. Continuous variables were assessed for normality using skewness, kurtosis and Shapiro-Wilk tests. Baseline characteristics were compared between SOC and SOC + S groups (age, weight, gender, tumor location, ALP status) using Chi-square or Fishers exact test for categorical variables and Kruskal Wallis tests for continuous variables.
Kaplan-Meier methods were used to generate survival curves for SOC and SOC + S groups and to calculate median DFI and median overall survival time with 95% confidence intervals (CI). The DFI was calculated as the number of days from the date of the limb amputation to the date of first detection of metastases. Dogs were censored in the DFI analysis if they did not have metastases documented at the time of last follow-up if alive and on study, or at death, or the date the dog went off study. The overall survival time was calculated as the number of days from limb amputation to death due to disease as gleaned from either clinicians’ observations or necropsy results (for disease-specific survival). Dogs were censored in the survival analysis if they were alive at last follow-up or were lost to follow-up. Logrank tests were used to evaluate for difference in DFI and overall survival time between SOC and SOC + S groups. Logrank tests were also used to assess for associations between baseline risk factors including serum ALP and anatomic tumor site within SOC and SOC + S groups. A subgroup analysis of dogs in the SOC + S group was performed using Cox proportional hazards analysis to assess for associations between sirolimus variables (Mean AUC baseline and grade 3 or above adverse events) variables on DFI, overall survival time and survival time after development of metastasis. Statistical significance was set at α = 0.05 and the statistical analysis was performed using commercially available software (SAS software, Version 9.4 of the SAS System for PC. Copyright © 2013 SAS Institute Inc. SAS and all other SAS Institute Inc. product or service names are registered trademarks or trademarks of SAS Institute Inc., Cary, NC, USA; GraphPad Prism for Windows, Version 7, San Diego, CA, USA)
An intent-to-treat and per-protocol analysis were performed and reported. The modified intent-to-treat analysis included all dogs randomized to each treatment group and prescribed the chemotherapy protocol. The per-protocol analysis included dogs that completed the prescribed chemotherapy protocol and were considered evaluable which was defined as reaching week 23 after limb amputation without disease progression. Given the susceptibility of this analysis to bias, the results of the per-protocol analysis are presented as supplementary data (Supplemental Table 2).
Results
Patient Demographics
Of the 324 dogs that were enrolled and randomized, 15 were removed due to a non-OS histologic diagnosis (n = 8); regional lymph node metastases (n = 4); or other factors (n = 3) (Figure 2). A total of 309 dogs formed the intent-to-treat population for further statistical analysis, with n = 157 in the SOC arm and n = 152 in the SOC + S arm. The median age was 8.1 years (range 1.4-15) and the median body weight was 38.8 kg (range 21.2-94.5), consistent with patient populations described in other studies of canine OS. No significant differences were seen in these patient characteristics between the 2 arms of the trial (Table 1).
Figure 2:
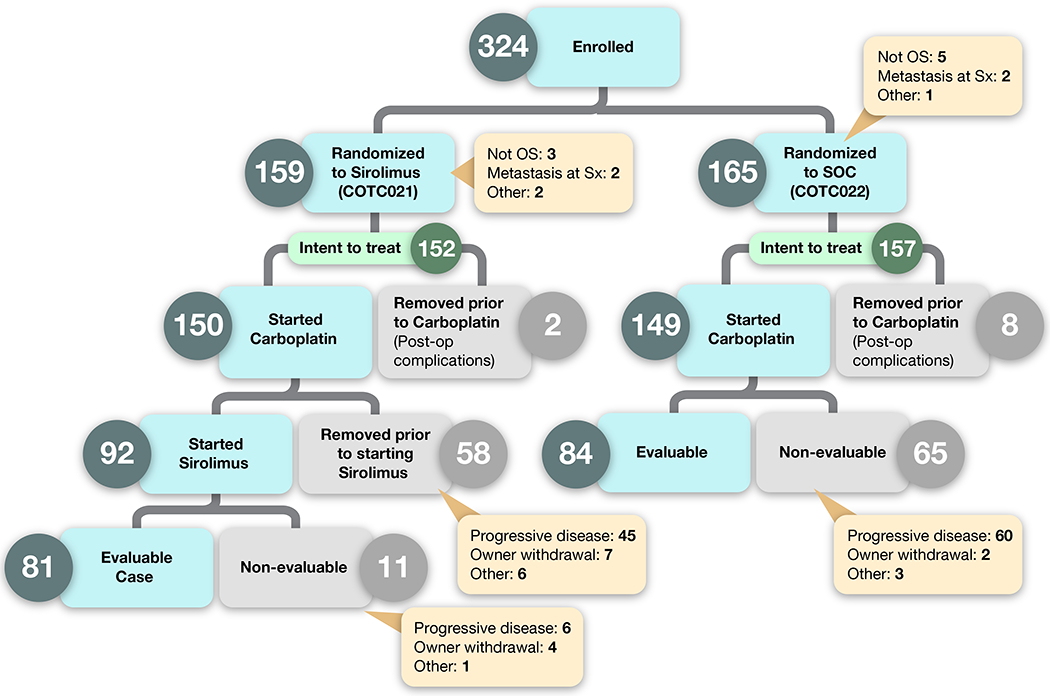
Events after enrollment are captured as dog were randomized to either Standard of Care (SOC) or SOC + sirolimus (SOC + S). Dogs that were removed from study prior to completing carboplatin chemotherapy were done so through their owners’ wishes. Sx = surgery.
Table 1:
Demographic features for pet dogs enrolled in the Standard of Care (SOC) or Standard of Care + adjuvant sirolimus (SOC + S) clinical trial arms. ALP refers to total serum alkaline phosphatase activity, indicated as normal or elevated based upon each COTC institution’s clinical pathology laboratory reference interval.
| SOC | SOC + S | |
|---|---|---|
| Intent to treat population | 157 | 152 |
| Age in years (Median, range) | 8.3 (1.4 – 15.6) | 7.9 (1.5 – 13) |
| Weight (kg) (Median, range) | 38.8 (25 - 94.5) | 39.0 (21.1 – 75.8) |
| Sex | ||
| Castrated Male | 83 (53%) | 90 (59%) |
| Spayed Female | 64 (41%) | 55 (36%) |
| Intact Male | 7 (4.5%) | 6 (4%) |
| Intact Female | 3 (1.5%) | 1 (1%) |
| ALP Status | ||
| Normal | 118 (75%) | 117 (77%) |
| Elevated | 39 (25%) | 35 (23%) |
| Tumor Location | ||
| Proximal humerus | 33 (21%) | 30 (20%) |
| Non-proximal humerus | 124 (79%) | 122 (80%) |
| Distal Radius | 57 | 53 |
| Distal Tibia | 27 | 22 |
| Distal Femur | 19 | 27 |
| Proximal Tibia | 9 | 9 |
| Ulnar | 4 | 5 |
| Other | 8 | 6 |
Carboplatin chemotherapy dose reductions and/or delays
Of the 309 dogs in the intent to treat population, 299 (97%) dogs, SOC (n=149) and SOC + S (n=150), started carboplatin therapy within 21 days of surgical amputation (Table 2). Ten dogs, SOC (n=8) and SOC + S (n=2), were removed from study prior to chemotherapy due to post-operative complications. Thirteen (4%) dogs, SOC (n=7) and SOC + S (n=6), had both carboplatin-attributable dose delays and dose reductions. Six (2%) dogs, SOC (n=1) and SOC + S (n=5), had a dose reduction. One hundred and forty-one (47%) dogs, SOC (n=75) and SOC + S (n=66), had a dose delay defined as an intertreatment carboplatin dosing interval of longer than 21 days. Carboplatin-attributable dose reductions and delays were due to drug-associated neutropenia and thrombocytopenia. The remaining 139 (46%) dogs, SOC (n=66) and SOC + S (n=73), received carboplatin per study protocol without deviation.
Table 2:
Clinical outcome measures for dogs within the Intent-to-treat population, enrolled in SOC and SOC + S trial arms.
| SOC | SOC + S | ||
|---|---|---|---|
| Intent to treat population | 157 | 152 | |
| Started SOC | 149 (95%) | 150 (99%) | |
| Completed SOC | 114 (73%) | 112 (74%) | |
| Started sirolimus treatment | N/A | 92 (61%) | |
| Completed sirolimus treatment | N/A | 64 (42%) | |
| Reason off-study | |||
| Disease Progression on Study | 52 (33%) | 83 (55%) | |
| Disease Progression during Follow-up Period | 69 (44%) | 34 (22%) | |
| Complicating Disease / Intercurrent Illness | 13 (8%) | 8 (5%) | |
| Follow-up period completed | 9 (6%) | 7 (5%) | |
| Refused further treatment | 3 (2%) | 12 (8%) | |
| Refused further follow up | 3 (2%) | 4 (3%) | |
| Death on Study | 2 (1%) | 3 (2%) | |
| Death during Follow-Up Period | 3 (2%) | 0 (0%) | |
| Adverse Events / Side Effects | 3 (2%) | 1 (<1%) | |
| Dogs dead during the study period | 146 (93%) | 143 (94%) | |
| Dead with evidence of metastatic disease* | 118 (75%) | 124 (82%) | |
| Dead without evidence of metastatic disease | 28 (18%) | 19 (13%) | |
| Dogs alive at the end of the follow-up period | 6 (4%) | 9 (6%) | |
| Alive with evidence of metastatic disease | 0 (0%) | 1 (<1%) | |
| Alive without evidence of metastatic disease | 6 (4%) | 8 (5%) | |
| Dogs lost to follow-up | 5 (3%) | 8 (5%) | |
N/A: not applicable
clinical, imaging, or necropsy evidence
Sirolimus therapy
Walk-in Tolerability
Oral sirolimus was tolerated in the majority of 22 tumor-bearing dogs receiving a dose of 0.1 mg/kg daily given either M-F or M/W/F schedules with weekends off for 4 consecutive weeks. However, a minority (20%) of patients demonstrated reduced appetite and gastrointestinal upset during the last 2 weeks of study, which was likely attributed to heavy disease burden and constitutional compromise. As drug tolerability was the endpoint, clinical response to sirolimus therapy was not assessed in the walk-in trial.
Walk-in Pharmacokinetics
Sirolimus administered at 0.1 mg/kg orally on a M-F schedule for 26 days provided an AUC/dose equivalent exposure of 4,367 [ng*hr/ml]/[mg/kg] (Supplemental Figure 2a), while the same dose administered on a M/W/F schedule produced lower AUC/dose equivalent exposures (Supplemental Figure 2b). Pharmacokinetic modelling based on these walk-in data were used to simulate a 4-day on/3-day off schedule (M-Th, Figure 3a, shaded curves). Simulation to include a 3-day weekend drug holiday was performed to address potential issues with tolerability of sirolimus when administered chronically (e.g., a planned treatment interval longer than 4 weeks). The AUC/dose equivalent from this simulation was 2403 ± 2246 [ng*hr/ml]/[mg/kg]. These actual and simulated values exhibit significant variability yet compare favorably to the AUC/dose equivalent of 3,555 [ng*hr/ml]/[mg/kg] predicted to exert an anti-metastatic effect in mouse models of OS.14 However, this simulation did not predict achievement of trough sirolimus concentrations approximating 10-15 ng/mL, which has been held as a PK target for solid tumor studies in pediatric patients receiving sirolimus treatment.28,29
Figure 3.
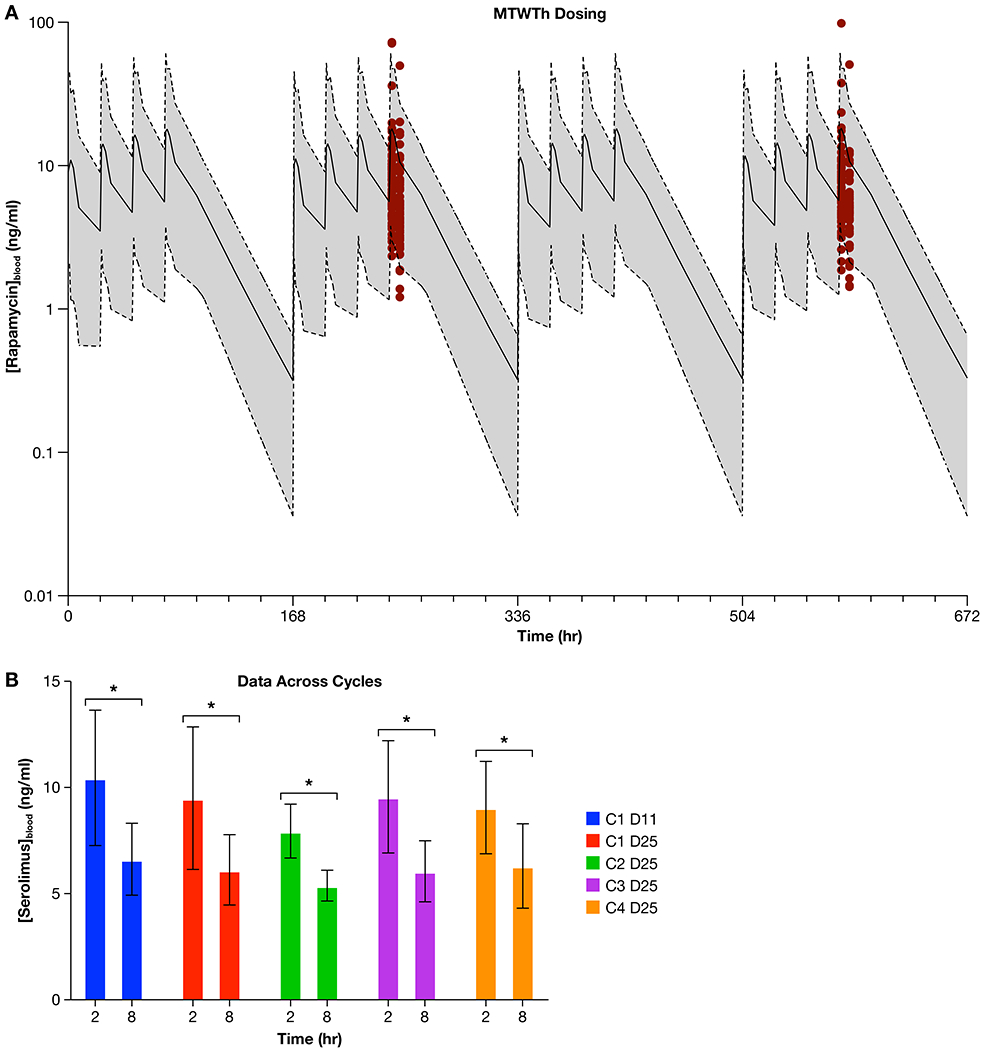
(a.) Red dots depict the sirolimus blood levels that were measured in dogs receiving 0.1 mg/kg orally on a Monday through Thursday basis within the SOC + S trial. These data were generated from whole blood of dogs (n = 61) on Cycle 1 Day 11 and Cycle 1 Day 25), and are overlaid with the simulated Area under the Curve (AUC) that was predicted from a simulation of Monday through Thursday dosing of 0.1 mg/kg (solid line = simulated mean sirolimus concentration; shaded area bound by dotted lines = simulated range of sirolimus concentration). The simulated sirolimus AUC data was generated from the walk-in study of sirolimus. Figure 3b. displays sirolimus concentrations in whole blood shown as the average value and 95% CI, obtained from dogs receiving 0.1 mg/kg of sirolimus orally within the SOC + S clinical trial, at the 2- and 8-hour time points across the 4 cycles of drug treatment (C1 D11 = Cycle 1, Day 11; C1 D25 = Cycle 1 Day 25; C2 D25 = Cycle 2 Day 25; C3 D25 = Cycle 3 Day 25; C4 D25 = Cycle 4 Day 25). ANOVA analysis shows that the 2 and 8 hour time points for each cycle are significantly different (* denotes significant difference, p < 0.0001) but none of the 2 or 8 hour average values were significantly different (p = 0.4825) when compared across dosing cycles.
Adjuvant SOC + S arm Tolerability
Ninety-two (64%) dogs started sirolimus treatment at week 15. Sixty-four dogs (70%) completed all 4 cycles, 5 (5.5%) completed 3 cycles, 15 (16.5%) completed 2 cycles, 4 (4%) completed 1 cycle, and 4 (4%) did not complete the first cycle. Of the 8 dogs that did not complete 2 cycles of sirolimus, 4 dogs were removed due to disease progression, 3 dogs due to owner removal, and 1 dog due to a concurrent complicating illness. One dog underwent a sirolimus dose reduction, 7-day drug holiday and a schedule modification to M/W/F dosing due to adverse events.
Adjuvant SOC + S arm Pharmacokinetics
Pharmacokinetic data from cycle 1-Day 11 and cycle 1-Day 25 from n=61 dogs treated M-Th in SOC + S arm (Figure 3a, red dots) was compared to the simulations of M-Th dosing generated within the Walk-in cohort of dogs (Figure 3a, shaded curves). These results show that the measured data (depicted by red dots at PK collection timepoints on cycle 1-Days 11 and -Day 25) are widely distributed across the simulated exposure curve. Further, the majority of dogs demonstrate measured sirolimus blood levels below 10 ng/mL. The AUC/dose equivalents for cycle 1-Day 11 and cycle 1-Day 25 timepoints were 1499 ± 1446 [ng*hr/ml]/[mg/kg] and 1357 ± 1475 [ng*hr/ml]/[mg/kg], respectively. To assess sirolimus pharmacokinetic variability across treatment duration (cycles 1-4), sirolimus levels were measured at 2- and 8- hours post dosing (Figure 3b). ANOVA showed that 2- and 8- hour timepoints were significantly different (p < 0.0001), but none of these average values were significantly different across cycles of treatment (p = 0.4825). Within this dataset, intra-patient variability was relatively small compared to inter-patient variability, suggesting that PK differences were largely patient-dependent. The magnitude of inter-patient PK variability was underscored by the wide range trough concentrations calculated from a subset of dogs (n=78) receiving sirolimus being 0.53 to 30.98 ng/mL, which span across predicted sub-therapeutic (< 10 ng/mL) and therapeutic (> 10 ng/mL) concentrations.
Adverse event reconciliation: severity and attribution
The most common recorded adverse events attributable to surgery were pain (n=33 dogs) and surgical site seromas (n=22 dogs). Carboplatin produced self-limiting adverse events including neutropenia (n=261), thrombocytopenia (n=178), anorexia (n=46), diarrhea (n=36) and vomiting (n=33). There were no Grade 5 events (fatal) attributable to carboplatin. Sirolimus exposure at 0.1 mg/kg was well tolerated, with only 17 (18%) of the 92 dogs experiencing events with the most common adverse clinical symptoms being lethargy/fatigue (n=7), diarrhea (n=6), anorexia (n=5), and nausea/ptyalism (n=4). There were only two Grade 3 events (fever), and one Grade 4 event (nausea). There were no Grade 5 events attributable to sirolimus. There were 5 deaths on study, none attributable to carboplatin or sirolimus treatment. One dog developed disseminated intravascular coagulation within 24 hours of surgery and died due to cardiopulmonary arrest 3 days after surgery. The additional 3 known causes of death were gastric dilatation with volvulus (GDV), endocarditis, and hemorrhage secondary to gastrointestinal ulcer perforation. An additional dog died at home due to unknown causes prior to starting carboplatin.
Clinical outcomes
Figure 2 depicts the progress of all 324 cases that enrolled, and of these, the 309 cases that comprise the intent-to-treat population from enrollment to evaluable/non-evaluable status. In order for a case to be deemed fully evaluable, dogs enrolled to the SOC arm had to complete carboplatin therapy and be deemed free of metastatic disease at the Week 23 visit. For the SOC + S arm, dogs had to complete carboplatin, be deemed free of metastatic disease at Week 15, and then complete 2 cycles of adjuvant sirolimus therapy (Week 23). Within the intent-to-treat population, the disease-free interval (180 vs 204 days, p = 0.87) and overall survival (282 vs 280 days, p = 0.98) were not significantly different between the SOC and SOC + S groups, (Figure 4).
Figure 4:
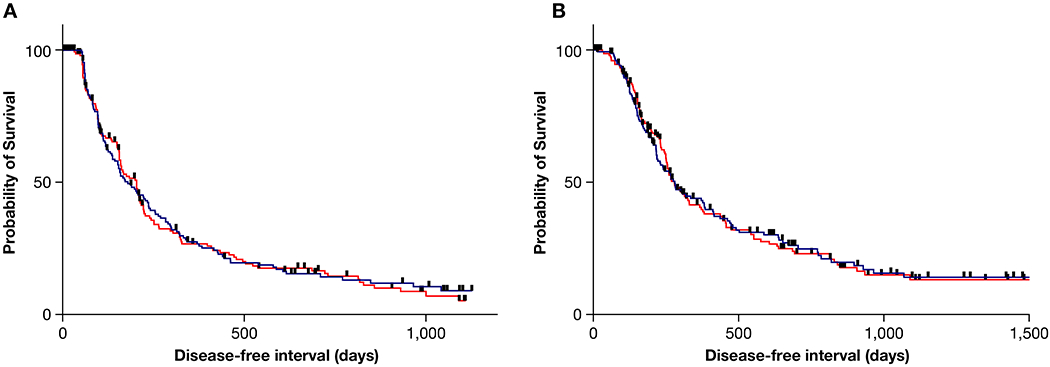
Kaplan-Meier event curves depicting the probability of survival with respect to disease-free interval (a) and overall survival (b) between the SOC (blue line) and SOC + S (red line) arms of the trial.
SOC
The median DFI for this group was 180 days (95% CI: 144-237), with 27.5% and 14.3% of dogs disease-free at 1- and 2- years post diagnosis, respectively (Figure 4, Supplemental Table 3). The median overall survival was 282 days (95% CI: 224-383), with 43.9% and 24.8% of dogs alive at 1- and 2- years post diagnosis, respectively. No differences were appreciated within the subgroups based on ALP status and tumor location within the SOC arm.
SOC + S
The median DFI for this group was 204 days (95% CI: 157-217), with 26.7% and 15.5% of dogs disease-free at 1- and 2- years post diagnosis, respectively (Figure 4, Supplemental Table 4). The median overall survival was 280 days (95% CI: 252-332), with 41.3% and 23.0% of dogs alive at 1- and 2- years post diagnosis, respectively. In contrast to the SOC arm, significant differences were identified between the subgroups represented by tumor location and ALP status. Within the assessment of DFI among subgroups, dogs with a non-proximal humeral tumor and normal ALP achieved the longest DFI (210 days), while dogs with a proximal humeral tumor and elevated ALP had the shortest DFI (131 days). This same pattern was not discernable in the overall survival analysis of the entire study arm inclusive of all 4 subgroupings, with the longest survival seen in dogs with a non-proximal humeral tumor and a normal ALP (320 days) and shortest in dogs with a non-proximal humeral tumor and an elevated ALP (203 days).
Disease progression and correlations with sirolimus PK
Metastatic pattern
Necropsy was performed in 42 and 41 dogs in SOC (27%) and SOC + S (27%) arms, respectively. Upon necropsy, fifty-six dogs (67%) were found to have disease in multiple sites, with the most common sites being lung, liver, kidney, heart, ribs, bone, and spine. Dogs that did not undergo a necropsy and had metastatic disease confirmed based on physical examination and radiographs, distant lesions were most often identified involving the lung (173 dogs) or bone (18 dogs).
Pharmacokinetic outcome association
No association was found between mean AUC on DFI (HR: 1.000; 95% CI: 0.998-1.003; p = 0.76) or overall survival time (HR: 1.000; 95% CI: 0.998-1.003). Additionally, no associations were found with development of grade 3 or worse adverse events and DFI (HR: 2.343; 95% CI: 0.927-5.920; p = 0.07) or overall survival time (HR: 1.496; 95% CI: 0.597-3.751; p = 0.39). However, this is likely due to the low number of sirolimus-attributable Grade 3 events (n=2; Grade 3 events (fever)), and one Grade 4 event (nausea).
Discussion
This clinical trial illustrates a convergence research approach, whereby a cooperative comparative oncology network (NCI-COTC) under the umbrella of the Osteosarcoma Project was able to rapidly generate biologically rich data within a naturally-occurring disease model such as the pet dog, which permits translational hypothesis testing to be explored in a mammalian species that more accurately recapitulates the natural history and progression of human OS. The results of this clinical trial showed that adjuvant sirolimus therapy administered sequentially following amputation and chemotherapy in dogs with appendicular OS was clinically tolerable, yet did not significantly improve DFI or survival over amputation and chemotherapy alone. While the absence of effect observed in this translational study was disappointing and discordant with existing preclinical metastatic OS mouse models,14,15 the clinical outcomes derived from this prospective clinical trial and associated study design should be viewed with compelling credibility. Given the abundance of scientific and clinical data supporting the intrinsic value of pet dogs with naturally-occurring OS to serve as sophisticated models for recapitulating the pathology of the human disease,2,11 the findings described in the current study should be leveraged to more fully understand the therapeutic framework and constraints associated with mTOR inhibition, as well as to promote the guidance for how PI3K/mTOR vulnerabilities can be more optimally targeted for curbing metastatic OS progression.
Signaling through PI3K/mTOR has been identified as a major oncogenic driver conserved across divergent tumor histologies,30 and substantive data supports this pathway to be frequently hyperactivated in both human and canine OS through various genomic changes including activating mutations in PI3K, amplifications of AKT, and PTEN downregulation.13,31–33 Collectively, these genomic perturbations exert pleiotropic pro-tumorigenic effects including OS invasion, cell cycle dysregulation, apoptosis evasion, angiogenesis, chemoresistance, and metastasis.34,35 Given its putative role in OS progression, inhibition of PI3K/mTOR signaling has been identified as a conserved therapeutic vulnerability for OS,12,13 and a large body of in vitro studies and preclinical metastatic OS mouse models have substantiated these genomically identified oncogenic susceptibilities when targeted by mTOR inhibitors, including sirolimus.14–16,22,36,37
Although existing genomic and biologic data strongly underscore the role of PI3K/mTOR signaling in OS progression, translation of these scientific and preclinical findings have largely remained unrealized for improving the clinical management of metastatic bone sarcomas. Despite multiple clinical reports describing the alluring potential of mTOR inhibition strategies for stabilizing or even regressing macroscopic recurrent or metastatic OS lesions using sirolimus19 or rapalogs,38–40 large randomized studies evaluating mTOR inhibition strategies to impede micrometastatic OS disease progression has not been systematically investigated in the context of Phase III human clinical trials. To date the most significant study which partially addresses this clinical gap in knowledge regarding the antitumor potential of mTOR inhibitors is the SUCCEED trial, an international randomized Phase III trial that evaluated the ability of ridaforolimus, a non-prodrug analog of sirolimus, to maintain benefits from prior cytotoxic chemotherapy for the prolongation of disease stability in patients with advanced sarcomas. In total, 711 patients with advanced sarcomas (soft tissue [n=642] and bone [n=69]) were enrolled, including a subgroup of 50 OS patients (1:1 ridaforolimus to placebo). Whereas ridaforolimus demonstrated statistically significant improvements in progression-free survival versus placebo (17.7 weeks versus 14.6 weeks, respectively) for the entire study population, subgroup analysis of OS patients was not adequately powered to detect any differences between treatment groups, but improvement in progression-free survival for the radaforolimus cohort was not detected in the limited OS population evaluated (n=50).41 Unfortunately, while ridaforolimus demonstrated statistically significant activity, the magnitude of clinical benefit was not sufficient to warrant new drug approval designation for the management of metastatic soft-tissue or bone sarcomas.
While the SUCCEED trial identified constraints of ridaforolimus in OS patients presenting with recurrent or relapsed disease, the translational potential of mTOR inhibition for thwarting micrometastatic OS progression has largely remained unanswered and predominantly limited to preclinical investigations reliant upon human xenograft mouse studies.14,15 Through the inclusion of pet dogs with naturally-occurring OS, this study was uniquely suited to provide high-value answers to this clinical gap in knowledge regarding the clinical impact of mTOR inhibition on OS micrometastatic progression. In the current study, the longitudinal outcomes in pet dogs failed to demonstrate any measurable effect on micrometastatic disease progression despite mTOR inhibition achieved by oral sirolimus when sequentially administered following the completion of systemic chemotherapies. Significant differences in progression-free and overall survival were not identified in dogs treated with or without sirolimus, and these findings do not support the inclusion of oral sirolimus sequentially following standard of care therapy (amputation + chemotherapy) for slowing micrometastatic progression in canine OS. Despite the negative clinical findings associated with sirolimus intervention, the conductance of this trial in pet dogs has allowed for the amassment of high value biologic samples which can be leveraged for ongoing and future studies to deeply study the mechanisms and vulnerabilities of OS metastatic progression.
Whereas the lack of improved progression-free and overall survival time in dogs treated with sirolimus was disappointing, any limitations of mTOR inhibition for delaying OS micrometastatic disease progression identified in the current study should be viewed contextually through the lens of disease biology, pharmacokinetics, and clinical trial design. First, while PI3K/mTOR has been identified as a therapeutic vulnerability for OS,12,13 given the genomic heterogeneity OS, it would be erroneous to assume that broad clinical benefit should be expected from a pan-mTOR inhibition strategy. Rather, recent scientific investigations strongly point towards a genome-informed targeted therapy approach for OS, whereby molecular subtypes of OS would be vulnerable to specific single or combination inhibition strategies.12,42 In the absence of genomic/molecular subtyping, pan-mTOR inhibition strategies might only benefit a minority of human or canine patients treated,13,33 and any positive treatment effects achieved in a small percentage of individuals could become indiscernible with aggregated data analysis. Second, in the current study, dogs were administered sirolimus at a biologically effective dose17,18 sufficient to inhibit mTOR signaling at 0.1 mg/kg M-Th schedule for up to 4 cycles (26 days/cycle). Despite historical studies demonstrating modulation of downstream mTOR targets (pS6RP/S6RP) with similar dosing strategies, trough concentrations of sirolimus in dogs predominantly ranged from 1-10 ng/mL, being slightly lower than target sirolimus concentrations in humans that exert immunosuppressive and anticancer activities (5-15 ng/mL).28,29,43 In contrast, in mouse models of OS whereby sirolimus either attenuates heterotopic primary tumor growth16 or delays orthotopic, spontaneous pulmonary metastases progression,14,15 biologically-active trough concentrations of sirolimus have been approximately 10-fold greater.14 Based upon the relatively low trough concentrations of sirolimus (< 10 ng/mL) achieved in the majority of dogs enrolled in this study, it remains a distinct possibility that insufficient drug exposure (concentration) or duration of exposure (maximum 4 cycles) might have contributed to the absence of antimetastatic activities in pet dogs receiving sirolimus. Last, prevailing evidence tightly links enhanced mRNA translational efficiency with successful OS metastasis biology,14 with activation of the mTOR signaling pathway and protein translational efficiency most critical during periods of cellular stress encountered along the metastatic cascade continuum, but likely accentuated during the most inefficient steps of metastasis, i.e. colonization.44 Given the importance of translational efficiency for successful metastasis, inhibition of the mTOR pathway would be expected to be most effective during periods when cancer cells are subjected to biological stressors (endogenous or exogenous). In this trial, dogs were treated sequentially with standard of care (amputation and chemotherapy) and than sirolimus; a purposeful clinical trial design to minimize undesirable hematologic toxicities associated with contemporaneous chemotherapy and mTOR inhibition strategies.45 However, the delayed introduction of sirolimus into the treatment protocol of dogs at Week 15 might have resulted in a “too little too late” effect, and it is reasonable to speculate that exposure to sirolimus, and consequent mTOR inhibition, would be more successful when administered concurrently with exogenous biologically stressors, such as chemotherapy. As such, concurrent and combinatorial strategies which temporally couple mTOR inhibitors with other therapies as employed in human clinical trials, would be predicted to have the greatest impact for improving the management of OS metastatic progression. Similarly, sirolimus exposure early in the natural disease course during active cancer cell colonization, as practiced in the generation of metastatic mouse models of OS,14,15 would be expected to increase the likelihood for observing beneficial antimetastatic activities.
Although not performed as a component of this trial, molecular profiling of canine OS is critical to understanding in what ways the canine disease recapitulates the genomic framework of human OS. Efforts are currently underway to characterize the samples obtained from dogs enrolled in this trial to identify correlative genomic subtypes within dog and human OS patients, explore tumor clonality and genomic evolution, and devise a model of platinum-based chemotherapy resistance in canine OS. These data will help to further support the basis for including the pet dog with OS as a naturally-occuring model for humans OS, building upon examples from the literature and supporting specific selection of canine OS subpopulations to participate in studies of molecularly-targeted agents.9, 48
Metastatic progression continues to be the life-limiting event for both human and canine OS patients, despite ongoing efforts to deconvolute the complex biology of OS and conduct clinical trials of novel agents in both species. The spontaneous development of OS in an immune-competent pet dog, coupled with the rapidity of disease progression, offers several advantages for OS drug development inclusive of immunotherapies.46–48 Given that the majority (∼90%) of dogs will succumb to OS progression and disease-related death within months to 2 years of diagnosis, the rapid conductance of Phase III-like clinical trial, as the one described herein, can generate mature results within 3-5 years. Indeed, the outcomes for dogs receiving standard of care therapy in this trial are consistent with other prior studies.9, 48 This deeply annotated clinical dataset provides an opportunity for data sharing efforts that enable comparison of novel adjuvant treatment strategies assessed in future trials. The experience gained through the conduct of this trial provides several opportunities to reflect on how to improve and optimize the design and implementation of future studies. Among these are the ability to pivot toward shorter observational periods occurring immediately after amputation, to emphasize improvement upon early failure rates, and a greater emphasis placed on combinatorial drug strategies in both the miminal residual disease and gross metastatic disease setting. As such, the inclusion of pet dogs as a unique model for pediatric OS can synergize and complement ongoing efforts which utilize rapid Phase II trial designs for the identification of the most promising agents to quickly advance towards upfront Phase III clinical trials.49,50
Supplementary Material
Statement of Translational Relevance.
Mammalian target of rapamycin (mTOR) is a serine/threonine kinase that serves as a key nutrient sensor that regulates diverse cellular functions including ribosome biosynthesis, protein translation, growth, and cytoskeletal rearrangement. Identified as a driver of metastasis, mTOR and its downstream signaling pathways mediated by mTORC1 and mTORC2, serve as attractive druggable targets for delaying metastatic progression in solid tumors including osteosarcoma. However, given osteosarcoma’s orphan disease status, the scalable and rapid assessment of mTOR inhibitors used in the upfront adjuvant setting would be protracted, necessitating the employment of higher throughput model systems that faithfully recapitulate the biologic complexities of the human disease. This study utilizes canine osteosarcoma patients as a means to efficiently evaluate the mTOR inhibitor, sirolimus, as a potential antimetastatic agent. Our results provide high-value biologic evidence for the translational value of canine osteosarcoma for prioritizing novel antimetastatic strategies that might be advanced for pediatric osteosarcoma clinical trials.
Acknowledgements
We extend our gratitude to the QuadW Foundation and to the Morris Animal Foundation Grants #D14CA-507, D16CA-518 and D16CA-519 for funding in part the work described herein. This work was supported (A. LeBlanc and C. Mazcko) by the Intramural Program of the National Cancer Institute, NIH (Z01-BC006161). This project has been funded (E. Berger) in whole or in part with Federal funds from the National Cancer Institute, National Institutes of Health, under Contract No. HHSN261201800001I. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. This study was supported in part by award number grant UL1TR002733 from the National Center for Advancing Translational Sciences and NCI P30 CA016058 (W. Kisseberth and M. Brown). Pharmacokinetic analysis was conducted by the Drug Development and Discovery Shared Resource of the University of Colorado Cancer Center with technical assistance from Nathaniel L. Gustafson, and was supported in part by NCI P30 CA046934 (D. Gustafson). We thank the University of California, Davis Veterinary Center for Clinical Trials. We thank The Virginia-Maryland College of Veterinary Medicine Clinical Trials Office coordinator, Mindy Quigley. Ohio State University College of Veterinary Medicine Integrated Oncology Service also includes Drs. Emma E. Warry, Joelle M. Fenger and Vincent A. Wavreille. We thank the Ohio State University Blue Buffalo Veterinary Clinical Trials Office, including Holly Borghese and Dana Nielsen.
Footnotes
The authors declare no conflicts of interest.
References
- 1.Fan TM, Khanna C. Comparative Aspects of Osteosarcoma Pathogenesis in Humans and Dogs. Vet Sci 2015;2(3):210–30 doi 10.3390/vetsci2030210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fenger JM, London CA, Kisseberth WC. Canine osteosarcoma: a naturally occurring disease to inform pediatric oncology. ILAR J 2014;55(1):69–85 doi 10.1093/ilar/ilu009. [DOI] [PubMed] [Google Scholar]
- 3.Gustafson DL, Duval DL, Regan DP, Thamm DH. Canine sarcomas as a surrogate for the human disease. Pharmacol Ther 2018;188:80–96 doi 10.1016/j.pharmthera.2018.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Angstadt AY, Thayanithy V, Subramanian S, Modiano JF, Breen M. A genome-wide approach to comparative oncology: high-resolution oligonucleotide aCGH of canine and human osteosarcoma pinpoints shared microaberrations. Cancer Genet 2012;205(11):572–87 doi 10.1016/j.cancergen.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 5.Paoloni M, Davis S, Lana S, Withrow S, Sangiorgi L, Picci P, et al. Canine tumor cross-species genomics uncovers targets linked to osteosarcoma progression. BMC Genomics 2009;10:625 doi 10.1186/1471-2164-10-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Angstadt AY, Motsinger-Reif A, Thomas R, Kisseberth WC, Guillermo Couto C, Duval DL, et al. Characterization of canine osteosarcoma by array comparative genomic hybridization and RT-qPCR: signatures of genomic imbalance in canine osteosarcoma parallel the human counterpart. Genes Chromosomes Cancer 2011;50(11):859–74 doi 10.1002/gcc.20908. [DOI] [PubMed] [Google Scholar]
- 7.Bishop MW, Janeway KA, Gorlick R. Future directions in the treatment of osteosarcoma. Curr Opin Pediatr 2016;28(1):26–33 doi 10.1097/MOP.0000000000000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grohar PJ, Janeway KA, Mase LD, Schiffman JD. Advances in the Treatment of Pediatric Bone Sarcomas. Am Soc Clin Oncol Educ Book 2017;37:725–35 doi 10.14694/EDBK_175378.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Selmic LE, Burton JH, Thamm DH, Withrow SJ, Lana SE. Comparison of carboplatin and doxorubicin-based chemotherapy protocols in 470 dogs after amputation for treatment of appendicular osteosarcoma. J Vet Intern Med 2014;28(2):554–63 doi 10.1111/jvim.12313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fan TM, Roberts RD, Lizardo MM. Understanding and Modeling Metastasis Biology to Improve Therapeutic Strategies for Combating Osteosarcoma Progression. Front Oncol 2020;10:13 doi 10.3389/fonc.2020.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khanna C, Fan TM, Gorlick R, Helman LJ, Kleinerman ES, Adamson PC, et al. Toward a drug development path that targets metastatic progression in osteosarcoma. Clin Cancer Res 2014;20(16):4200–9 doi 10.1158/1078-0432.CCR-13-2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gupte A, Baker EK, Wan SS, Stewart E, Loh A, Shelat AA, et al. Systematic Screening Identifies Dual PI3K and mTOR Inhibition as a Conserved Therapeutic Vulnerability in Osteosarcoma. Clin Cancer Res 2015;21(14):3216–29 doi 10.1158/1078-0432.CCR-14-3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perry JA, Kiezun A, Tonzi P, Van Allen EM, Carter SL, Baca SC, et al. Complementary genomic approaches highlight the PI3K/mTOR pathway as a common vulnerability in osteosarcoma. Proc Natl Acad Sci U S A 2014;111(51):E5564–73 doi 10.1073/pnas.1419260111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morrow JJ, Mendoza A, Koyen A, Lizardo MM, Ren L, Waybright TJ, et al. mTOR Inhibition Mitigates Enhanced mRNA Translation Associated with the Metastatic Phenotype of Osteosarcoma Cells In Vivo. Clin Cancer Res 2016;22(24):6129–41 doi 10.1158/1078-0432.CCR-16-0326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wan X, Mendoza A, Khanna C, Helman LJ. Rapamycin inhibits ezrin-mediated metastatic behavior in a murine model of osteosarcoma. Cancer Res 2005;65(6):2406–11 doi 10.1158/0008-5472.CAN-04-3135. [DOI] [PubMed] [Google Scholar]
- 16.Zhao S, Lu N, Chai Y, Yu X. Rapamycin inhibits tumor growth of human osteosarcomas. J BUON 2015;20(2):588–94. [PubMed] [Google Scholar]
- 17.Paoloni MC, Mazcko C, Fox E, Fan T, Lana S, Kisseberth W, et al. Rapamycin pharmacokinetic and pharmacodynamic relationships in osteosarcoma: a comparative oncology study in dogs. PLoS One 2010;5(6):e11013 doi 10.1371/journal.pone.0011013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Larson JC, Allstadt SD, Fan TM, Khanna C, Lunghofer PJ, Hansen RJ, et al. Pharmacokinetics of orally administered low-dose rapamycin in healthy dogs. Am J Vet Res 2016;77(1):65–71 doi 10.2460/ajvr.77.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Penel-Page M, Ray-Coquard I, Larcade J, Girodet M, Bouclier L, Rogasik M, et al. Off-label use of targeted therapies in osteosarcomas: data from the French registry OUTC’S (Observatoire de l’Utilisation des Therapies Ciblees dans les Sarcomes). BMC Cancer 2015;15:854 doi 10.1186/s12885-015-1894-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou Q, Deng Z, Zhu Y, Long H, Zhang S, Zhao J. mTOR/p70S6K signal transduction pathway contributes to osteosarcoma progression and patients’ prognosis. Med Oncol 2010;27(4):1239–45 doi 10.1007/s12032-009-9365-y. [DOI] [PubMed] [Google Scholar]
- 21.Wang X, Lai P, Zhang Z, Huang M, Wang L, Yin M, et al. Targeted inhibition of mTORC2 prevents osteosarcoma cell migration and promotes apoptosis. Oncol Rep 2014;32(1):382–8 doi 10.3892/or.2014.3182. [DOI] [PubMed] [Google Scholar]
- 22.Xie ZG, Xie Y, Dong QR. Inhibition of the mammalian target of rapamycin leads to autophagy activation and cell death of MG63 osteosarcoma cells. Oncol Lett 2013;6(5):1465–9 doi 10.3892/ol.2013.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gordon I, Paoloni M, Mazcko C, Khanna C. The Comparative Oncology Trials Consortium: using spontaneously occurring cancers in dogs to inform the cancer drug development pathway. PLoS Med 2009;6(10):e1000161 doi 10.1371/journal.pmed.1000161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.LeBlanc AK, Mazcko CN, Khanna C. Defining the Value of a Comparative Approach to Cancer Drug Development. Clin Cancer Res 2016;22(9):2133–8 doi 10.1158/1078-0432.CCR-15-2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boerman I, Selvarajah GT, Nielen M, Kirpensteijn J. Prognostic factors in canine appendicular osteosarcoma - a meta-analysis. BMC Vet Res 2012;8:56 doi 10.1186/1746-6148-8-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clavijo C, Strom T, Moll V, Betts R, Zhang YL, Christians U, et al. Development and validation of a semi-automated assay for the highly sensitive quantification of Biolimus A9 in human whole blood using high-performance liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 2009;877(29):3506–14 doi 10.1016/j.jchromb.2009.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sheiner LB, Beal SL. Some suggestions for measuring predictive performance. J Pharmacokinet Biopharm 1981;9(4):503–12 doi 10.1007/BF01060893. [DOI] [PubMed] [Google Scholar]
- 28.Scott JR, Courter JD, Saldana SN, Widemann BC, Fisher M, Weiss B, et al. Population pharmacokinetics of sirolimus in pediatric patients with neurofibromatosis type 1. Ther Drug Monit 2013;35(3):332–7 doi 10.1097/FTD.0b013e318286dd3f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Franz DN, Leonard J, Tudor C, Chuck G, Care M, Sethuraman G, et al. Rapamycin causes regression of astrocytomas in tuberous sclerosis complex. Ann Neurol 2006;59(3):490–8 doi 10.1002/ana.20784. [DOI] [PubMed] [Google Scholar]
- 30.Lawrence MS, Stojanov P, Mermel CH, Robinson JT, Garraway LA, Golub TR, et al. Discovery and saturation analysis of cancer genes across 21 tumour types. Nature 2014;505(7484):495–501 doi 10.1038/nature12912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moriarity BS, Otto GM, Rahrmann EP, Rathe SK, Wolf NK, Weg MT, et al. A Sleeping Beauty forward genetic screen identifies new genes and pathways driving osteosarcoma development and metastasis. Nat Genet 2015;47(6):615–24 doi 10.1038/ng.3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Freeman SS, Allen SW, Ganti R, Wu J, Ma J, Su X, et al. Copy number gains in EGFR and copy number losses in PTEN are common events in osteosarcoma tumors. Cancer 2008;113(6):1453–61 doi 10.1002/cncr.23782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gardner HL, Sivaprakasam K, Briones N, Zismann V, Perdigones N, Drenner K, et al. Canine osteosarcoma genome sequencing identifies recurrent mutations in DMD and the histone methyltransferase gene SETD2. Commun Biol 2019;2:266 doi 10.1038/s42003-019-0487-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang J, Yu XH, Yan YG, Wang C, Wang WJ. PI3K/Akt signaling in osteosarcoma. Clin Chim Acta 2015;444:182–92 doi 10.1016/j.cca.2014.12.041. [DOI] [PubMed] [Google Scholar]
- 35.Hu K, Dai HB, Qiu ZL. mTOR signaling in osteosarcoma: Oncogenesis and therapeutic aspects (Review). Oncol Rep 2016;36(3):1219–25 doi 10.3892/or.2016.4922. [DOI] [PubMed] [Google Scholar]
- 36.Gazitt Y, Kolaparthi V, Moncada K, Thomas C, Freeman J. Targeted therapy of human osteosarcoma with 17AAG or rapamycin: characterization of induced apoptosis and inhibition of mTOR and Akt/MAPK/Wnt pathways. Int J Oncol 2009;34(2):551–61. [PubMed] [Google Scholar]
- 37.Mu X, Isaac C, Schott T, Huard J, Weiss K. Rapamycin Inhibits ALDH Activity, Resistance to Oxidative Stress, and Metastatic Potential in Murine Osteosarcoma Cells. Sarcoma 2013;2013:480713 doi 10.1155/2013/480713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chawla SP, Staddon AP, Baker LH, Schuetze SM, Tolcher AW, D’Amato GZ, et al. Phase II study of the mammalian target of rapamycin inhibitor ridaforolimus in patients with advanced bone and soft tissue sarcomas. J Clin Oncol 2012;30(1):78–84 doi 10.1200/JCO.2011.35.6329. [DOI] [PubMed] [Google Scholar]
- 39.Fouladi M, Laningham F, Wu J, O’Shaughnessy MA, Molina K, Broniscer A, et al. Phase I study of everolimus in pediatric patients with refractory solid tumors. J Clin Oncol 2007;25(30):4806–12 doi 10.1200/JCO.2007.11.4017. [DOI] [PubMed] [Google Scholar]
- 40.Grignani G, Palmerini E, Ferraresi V, D’Ambrosio L, Bertulli R, Asaftei SD, et al. Sorafenib and everolimus for patients with unresectable high-grade osteosarcoma progressing after standard treatment: a non-randomised phase 2 clinical trial. Lancet Oncol 2015;16(1):98–107 doi 10.1016/S1470-2045(14)71136-2. [DOI] [PubMed] [Google Scholar]
- 41.Demetri GD, Chawla SP, Ray-Coquard I, Le Cesne A, Staddon AP, Milhem MM, et al. Results of an international randomized phase III trial of the mammalian target of rapamycin inhibitor ridaforolimus versus placebo to control metastatic sarcomas in patients after benefit from prior chemotherapy. J Clin Oncol 2013;31(19):2485–92 doi 10.1200/JCO.2012.45.5766. [DOI] [PubMed] [Google Scholar]
- 42.Sayles LC, Breese MR, Koehne AL, Leung SG, Lee AG, Liu HY, et al. Genome-Informed Targeted Therapy for Osteosarcoma. Cancer Discov 2019;9(1):46–63 doi 10.1158/2159-8290.CD-17-1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zimmerman JJ, Kahan BD. Pharmacokinetics of sirolimus in stable renal transplant patients after multiple oral dose administration. J Clin Pharmacol 1997;37(5):405–15 doi 10.1002/j.1552-4604.1997.tb04318.x. [DOI] [PubMed] [Google Scholar]
- 44.Luzzi KJ, MacDonald IC, Schmidt EE, Kerkvliet N, Morris VL, Chambers AF, et al. Multistep nature of metastatic inefficiency: dormancy of solitary cells after successful extravasation and limited survival of early micrometastases. Am J Pathol 1998;153(3):865–73 doi 10.1016/S0002-9440(10)65628-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu J, Tian D. Hematologic toxicities associated with mTOR inhibitors temsirolimus and everolimus in cancer patients: a systematic review and meta-analysis. Curr Med Res Opin 2014;30(1):67–74 doi 10.1185/03007995.2013.844116. [DOI] [PubMed] [Google Scholar]
- 46.Mason NJ. Comparative Immunology and Immunotherapy of Canine Osteosarcoma. Adv Exp Med Biol 2020;1258:199–221 doi 10.1007/978-3-030-43085-6_14. [DOI] [PubMed] [Google Scholar]
- 47.Wycislo KL, Fan TM. The immunotherapy of canine osteosarcoma: a historical and systematic review. J Vet Intern Med 2015;29(3):759–69 doi 10.1111/jvim.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mason NJ, Gnanandarajah JS, Engiles JB, Gray F, Laughlin D, Gaurnier-Hausser A, et al. Immunotherapy with a HER2-Targeting Listeria Induces HER2-Specific Immunity and Demonstrates Potential Therapeutic Effects in a Phase I Trial in Canine Osteosarcoma. Clin Cancer Res 2016;22(17):4380–90 doi 10.1158/1078-0432.CCR-16-0088. [DOI] [PubMed] [Google Scholar]
- 49.Lagmay JP, Krailo MD, Dang H, Kim A, Hawkins DS, Beaty O, 3rd, et al. Outcome of Patients With Recurrent Osteosarcoma Enrolled in Seven Phase II Trials Through Children’s Cancer Group, Pediatric Oncology Group, and Children’s Oncology Group: Learning From the Past to Move Forward. J Clin Oncol 2016;34(25):3031–8 doi 10.1200/JCO.2015.65.5381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Isakoff MS, Goldsby R, Villaluna D, Krailo MD, Gorlick R, Doski JJ, et al. Rapid Protocol Enrollment in Osteosarcoma: A Report From the Children’s Oncology Group. Pediatr Blood Cancer 2016;63(2):370–1 doi 10.1002/pbc.25754. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


