SUMMARY
Neurodegeneration in the central nervous system (CNS) is a defining feature of organismal aging that is influenced by peripheral tissues. Clinical observations indicate that skeletal muscle influences CNS aging but the underlying muscle-to-brain signaling remains unexplored. In Drosophila, we find that moderate perturbation of the proteasome in skeletal muscle induces compensatory preservation of CNS proteostasis during aging. Such long-range stress signaling depends on muscle-secreted Amyrel amylase. Mimicking stress-induced Amyrel upregulation in muscle reduces age-related accumulation of poly-ubiquitinated proteins in the brain and retina via chaperones. Preservation of proteostasis stems from the disaccharide maltose, which is produced via Amyrel amylase activity. Correspondingly, RNAi for SLC45 maltose transporters reduces expression of Amyrel-induced chaperones and worsens brain proteostasis during aging. Moreover, maltose preserves proteostasis and neuronal activity in human brain organoids challenged by thermal stress. Thus, proteasome stress in skeletal muscle hinders retinal and brain aging by mounting an adaptive response via amylase/maltose.
Keywords: muscle-to-brain signaling, aging, myokine, proteasome, stress response, proteostasis, amylase
eTOC blurb
Neurodegeneration in the central nervous system (CNS) occurs with aging and is influenced by crosstalk with peripheral tissues. Rai et al. identify muscle-to-CNS signaling mediated by a stress-induced amylase and maltose, which preserve proteostasis in the aging brain and retina via chaperones. Thus, muscle-derived signaling protects from neurodegeneration during aging.
Graphical Abstract
INTRODUCTION
The ubiquitin-proteasome system is a fundamental pathway for normal protein turnover and degradation of misfolded and pathogenic proteins (Collins and Goldberg, 2017). Proteasome dysfunction is causally associated with many age-related pathologies including neurodegeneration (Douglas and Dillin, 2010; Schmidt and Finley, 2014). Because of its fundamental roles, there are several cellular mechanisms that monitor proteasome function and dynamically adjust its abundance, composition, and activity in response to homeostatic challenges (Hanna et al., 2007; Lee et al., 2004; Lundgren et al., 2005; Park et al., 2011; Tsvetkov et al., 2015). For example, proteasome stress induces the expression of non-proteasomal proteases and peptidases, which partially compensate for proteasome dysfunction via their capacity to degrade polypeptides by exo- and endo-proteolytic cleavage (Geier et al., 1999; Glas et al., 1998; Lipinszki et al., 2013; Wang et al., 2000).
In addition to cell-autonomous (local) responses, there is increasing evidence that stress sensing in a tissue or group of cells induces cell non-autonomous (systemic) adaptations (Droujinine and Perrimon, 2016; Mitra and Ryoo, 2019; Taylor et al., 2014; van Oosten-Hawle et al., 2013; Wang et al., 2014). Such inter-tissue stress signaling may contribute to coordinated adaptations of distinct tissues to local and systemic challenges, so that the organism can better withstand and respond to homeostatic perturbations. However, it remains unknown whether proteasome stress in one tissue is sensed systemically.
The proteasome is the primary proteolytic system of skeletal muscle, which constitutes the bulk protein reserve of the organism (Bonaldo and Sandri, 2013). Skeletal muscle has emerged as an important tissue in the systemic regulation of aging (Demontis et al., 2014; Demontis et al., 2013; Gates et al., 2007; Hunt et al., 2019a; Keipert et al., 2013; Kim et al., 2013; Owusu-Ansah et al., 2013). In particular, clinical observations in humans and studies in model organisms indicate that skeletal muscle influences neurodegeneration and aging of the brain and retina (Boyle et al., 2009; Delezie and Handschin, 2018; Demontis and Perrimon, 2010; Lawson et al., 2014; Rai and Demontis, 2016; Voss et al., 2013). These systemic effects may arise from muscle-secreted factors known as myokines (Agudelo et al., 2014; Lourenco et al., 2019; Moon et al., 2016; Pedersen, 2019; Robles-Murguia et al., 2020). However, the myokines and mechanisms underlying such muscle-to-brain signaling remain unexplored.
On this basis, we have asked whether proteasome stress in skeletal muscle induces systemic adaptive stress responses in the aging brain and retina; whether such cell non-autonomous responses depend on the action of myokines; and whether myokines induced by proteasome stress influence protein quality control in the brain and retina during aging.
RESULTS
Muscle-Specific RNAi for Proteasome Subunits Reduces the Age-Related Accumulation of Proteasome Substrates in Distant Tissues
In Drosophila, a common strategy to induce proteasome stress consists in targeting proteasome subunits via RNAi (Lundgren et al., 2005; Tsakiri et al., 2019; Wojcik and DeMartino, 2002). To investigate whether moderate perturbation of the proteasome in skeletal muscle induces a compensatory stress response, RNAi for Prosβ1 (a component of the 20S proteasome catalytic core) was driven in thoracic muscles via the UAS/Gal4 system and the skeletal muscle-specific Mhc-Gal4 driver (Demontis and Perrimon, 2010; Schuster et al., 1996), and RNAi efficacy was confirmed by qRT-PCR (Supplemental Fig. S1A). RNA-seq revealed the induction of compensatory transcriptional changes in muscle with Prosβ1RNAi, compared to control whiteRNAi (Fig. 1A). Specifically, Prosβ1RNAi increased the expression of chaperones and proteases/peptidases (Fig. 1A), which promote the degradation of proteasome substrates and are induced by proteasome stress in other systems (Geier et al., 1999; Glas et al., 1998; Lipinszki et al., 2013; Wang et al., 2000). Similar transcriptional stress responses were also found with muscle-specific Prosβ5RNAi (Supplemental Fig. S1B) and with RNAi for Ter94 (Fig. 1A), the Drosophila homolog of p97/VCP (valosin-containing protein), which cooperates with the proteasome in the degradation of proteins from organellar membranes and multimolecular complexes (Piccirillo and Goldberg, 2012; van den Boom et al., 2016). Together, these findings indicate the induction of a local, transcriptional response to proteasome stress in skeletal muscle.
Fig. 1. Proteasome Stress in Skeletal Muscle Preserves Protein Quality Control in Distant Non-muscle Tissues.

(A) RNA-seq data indicates that RNAi for proteasome components and associated factors (Prosβ1 and Ter94/VCP) induces a local compensatory transcriptional response in muscle characterized by the increased expression of chaperones and proteases/peptidases. The most significant categories of upregulated genes are shown (logratio>1 and p<0.05; compared to whiteRNAi).
(B-C) Western blot analyses of thoraces (consisting primarily of skeletal muscle) and heads (consisting primarily of brains and retinas) from flies with muscle-specific Prosβ1RNAi and control whiteRNAi driven by Mhc-Gal4. Western blot analyses of detergent-insoluble fractions indicate that, although there are no substantial effects in muscle (B), muscle-specific Prosβ1RNAi improves protein quality control in heads, as indicated by the lower age-related accumulation of poly-ubiquitinated proteins in detergent-insoluble fractions (C).
(D-E) Similar improvement of proteostasis is also found in head tissues of flies with muscle-specific Ter94RNAi, compared to control whiteRNAi and vermillionRNAi. However, protein quality control is worsened in muscle (D), presumably due to insufficient compensation by transcriptional adaptive responses (A).
(F-G) Drug-induced Prosβ1RNAi expression in thoracic flight skeletal muscle reduces age-related accumulation of poly-ubiquitinated proteins in detergent-insoluble fractions, compared to uninduced controls and to control whiteRNAi (G).
The levels of ubiquitin, Ref(2)P/p62, α-tubulin, and/or or β-actin are shown in (B-G), together with the color-coded quantitation of ubiquitin levels normalized to α-tubulin or β-actin. The ages analyzed are 10, 30, and 60 days.
(H) Immunostaining of retinas from 30-day-old flies for ubiquitin (red), Ref(2)P/p62 (green), and F-actin (blue). Drug-induced (+RU486) expression of Prosβ1RNAi in thoracic flight skeletal muscle reduces the age-related accumulation of poly-ubiquitin protein aggregates in retinas during aging, compared to uninduced controls and to no transgene (+). The scale bar is 20μm.
(I) Proteasome stress induced in skeletal muscle via RNAi for proteasome subunits (indicated in the scheme) reduces the accumulation of proteasome substrates (poly-ubiquitinated proteins) in distant tissues (retina and brain) during aging.
Supplemental Fig. S1 and S2 report additional data related to Fig. 1.
To investigate whether muscle-specific RNAi for proteasome subunits induces adaptive stress responses in distant tissues, detergent-soluble and insoluble fractions from thoraces (consisting of skeletal muscle) and from heads (consisting mostly of retinas and brains) of Mhc>Prosβ1RNAi and control Mhc>whiteRNAi flies were analyzed by western blotting. Detergent-insoluble fractions contain insoluble ubiquitinated proteins that form protein aggregates, which are a hallmark of tissue aging (Demontis and Perrimon, 2010).
Muscle-specific Prosβ1RNAi led to limited changes in the age-related accumulation of poly-ubiquitinated proteins in detergent-insoluble fractions of muscle (Fig. 1B and Supplemental Fig. S1C), consistent with the induction of compensatory local (cell-autonomous) responses (Fig. 1A). However, muscle-specific Prosβ1RNAi reduced the age-related accumulation of proteasome substrates in head tissues, compared to controls (Fig. 1C and Supplemental Fig. S1D, I).
Different from Prosβ1RNAi (Fig. 1B), Ter94RNAi worsened proteostasis in muscle (Fig. 1D and Supplemental Fig. S1E), suggesting that local adaptive responses (Fig. 1A) do not completely compensate for the decline in protein quality control due to Ter94RNAi. However, as observed for Prosβ1RNAi, muscle-specific Ter94RNAi reduced the age-related accumulation of poly-ubiquitinated proteins in detergent-insoluble fractions from heads (Fig. 1E and Supplemental Fig. S1F, J).
Similar results were obtained via muscle-specific Prosβ1RNAi driven by the drug-inducible Act88F-GeneSwitch-Gal4, which is specific for thoracic indirect flight muscles (Robles-Murguia et al., 2019), (Fig. 1F–G), i.e. muscle-specific Prosβ1RNAi decreased the age-related increase in poly-ubiquitinated proteins that occurs in Drosophila heads (Fig. 1G), compared to uninduced controls and to whiteRNAi (Supplemental Fig. S1H). Similar results were also obtained with antibodies specific for K48-linked poly-ubiquitination (Supplemental Fig. S1K), which is considered the primary signal for targeting proteins to proteasomal degradation (Pickart and Fushman, 2004; Xu et al., 2009). There was also a good correlation between poly-ubiquitinated proteins and detergent-insoluble levels of Ref(2)P/p62, which binds to poly-ubiquitinated proteins tagged for degradation (Nezis et al., 2008; Seibenhener et al., 2004); (Fig. 1B–G).
Next, we examined the effect of RNAi for other proteasomal components. As observed for Prosβ1RNAi, muscle-specific RNAi for Prosα3, Prosα4, Prosα6, Prosβ2, Prosβ4, and Prosβ5 led to a reduction in the age-related accumulation of poly-ubiquitinated proteins in detergent-insoluble fractions from heads (Supplemental Fig. S2A–F), indicating that systemic improvements in protein quality can be induced by targeting several 20S proteasomal components in muscle. There were no substantial changes in total levels of Atg8/LC3 and in its conversion (i.e., Atg8-II versus Atg8-I levels), suggesting that preservation of proteostasis occurs independently from Atg8/LC3 lipidation in this context (Supplemental Fig. S1C–J and S2C–F).
We also tested whether preservation of protein quality control in head tissues by muscle-specific stress involves modulation of proteasome activity. To this purpose, caspase-like, chymotrypsin-like, and trypsin-like proteolytic activities were monitored in muscle and head homogenates from flies with muscle-specific proteasome stress (Mhc>Prosβ1RNAi and Mhc>Prosβ5RNAi) and controls (Mhc>whiteRNAi and Mhc>mCherryRNAi). In skeletal muscle, both Prosβ1RNAi and Prosβ5RNAi led to a decline in caspase-like proteolytic activity, compared to controls (Supplemental Fig. S2G). Prosβ1RNAi also led to a significant decline in chymotrypsin- and trypsin-like activities (Supplemental Fig. S2G). In head tissues, there was no significant change in any of the of the proteasomal proteolytic activities, suggesting that preservation of protein quality control in head tissues by muscle-specific proteasome stress does not arise from overall increased proteasome activity (Supplemental Fig. S2G).
Altogether, these findings indicate that local, moderate perturbation of proteasome function in thoracic skeletal muscle induces a systemic adaptive response that impedes the age-related accumulation of proteasome substrates in head tissues.
Next, we used immunostaining to test whether drug-induced, muscle-restricted Prosβ1RNAi regulates protein quality control in the retina during aging (Demontis and Perrimon, 2010). Muscle-specific Prosβ1RNAi led to a decrease in the age-related accumulation of poly-ubiquitinated protein aggregates in the retina compared to controls (Fig. 1H), indicating that proteasome stress in muscle improves protein quality control in the central nervous system (CNS) during aging (Fig. 1I).
Amyrel is a Myokine that is Induced by Muscle-Specific Proteasome Stress and that Regulates Systemic Protein Quality Control
The transcriptional response induced by proteasome stress in muscle included muscle-secreted proteins, i.e. myokines (Fig. 1A and Supplemental Fig. S1B), which may contribute to cell non-autonomous regulation of proteostasis.
By comparing RNA-seq data, we found several myokines that were regulated in a RNAi-specific manner whereas others were consistently induced by Prosβ1RNAi, Prosβ5RNAi and Ter94RNAi compared to control whiteRNAi (Fig. 2A). On this basis, we tested whether RNAi and/or overexpression of stress-regulated myokines modulates the accumulation of proteasome substrates in head tissues during aging.
Fig. 2. Amyrel is a key Myokine that Preserves Protein Quality Control in Distant Tissues in Response to Proteasome Stress in Muscle.
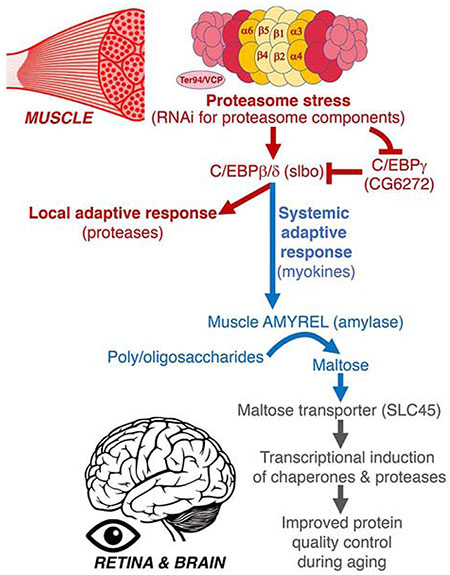
(A) RNA-seq data indicates muscle-secreted factors (myokines) that are commonly induced by RNAi for subunits of the 20S proteasome (Prosβ1 and Prosβ5) and Ter94/VCP. The logratio of Prosβ1RNAi, Prosβ5RNAi, and Ter94RNAi versus whiteRNAi is shown. Non-significantly regulated myokines are shaded in gray, whereas upregulated and downregulated myokines with p<0.05 are highlighted in red and blue, respectively. Some of the most-significantly regulated myokines (yellow) were selected for follow up biochemical analyses (B).
(B) Summary of the analysis of muscle-specific RNAi and overexpression for selected stress-induced myokines, based on the primary data shown in Supplemental Fig. S3 and S4. The levels of poly-ubiquitinated proteins found in the detergent-insoluble fractions of heads is indicated.
(C) Drug-induced Amyrel overexpression limited to the thoracic flight muscle reduces the age-related increase in poly-ubiquitinated proteins found in detergent-insoluble fractions of heads. No effect is seen with mock drug treatment (i.e. ethanol alone) and in the absence of the Amyrel transgene.
(D) GFP-tagged Amyrel expressed specifically in thoracic skeletal muscle is detected in muscle, in the circulation (hemolymph), and to a lower extent in the head, indicating that Amyrel is a muscle-secreted factor.
(E) Muscle-specific Prosβ1RNAi impedes the age-related accumulation of poly-ubiquitinated proteins in head tissues during aging. However, concomitant AmyrelRNAi blunts the effects of Prosβ1RNAi, compared to control yellowRNAi.
(F) Muscle-specific Amyrel overexpression improves protein quality control in heads of flies with pathogenic huntingtin (GMR-Htt-Q120) as indicated by the lower age-dependent increase in poly-ubiquitinated proteins found in detergent-insoluble fractions compared to controls. The ages analyzed are 10, 30, and 60 days.
(G) Drosophila retinas with transgenic expression of GFP-tagged pathogenic huntingtin (Htt-Q72-GFP) driven by GMR-Gal4. Amyrel overexpression decreases the overall amount of Htt-Q72-GFP protein aggregates in 30-day-old flies, compared to control mCherry and no transgene (+). SD, n≥18.
(H) Mutant tauV337M induces retinal degeneration during aging, as exemplified by the appearance of a rough eye phenotype due to the loss and derangement of photoreceptor neurons in 30-day-old females. Amyrel largely prevents such age-related neurodegeneration.
Supplemental Fig. S3 and S4 report additional data related to Fig. 2.
To this purpose, we analyzed detergent-soluble and -insoluble fractions obtained at 10, 30, and 60 days from heads of flies with muscle-specific overexpression and RNAi for some of the myokines that were most significantly regulated by proteasome stress: CG8093, Amyrel, and Ag5r2 (upregulated); and CG4666, takeout (to), and Akh (downregulated; Fig. 2A–B and Supplemental Fig. S3A–B and S4A–I).
Overall, Amyrel (a secreted amylase (Claisse et al., 2016)) consistently regulated protein quality control in a manner coincident with its transcriptional upregulation by proteasome stress (Fig. 2B–G and Supplemental Fig. S3C–E and S4E). Specifically, Amyrel overexpression in muscle reduced the age-related accumulation of proteasome substrates in head tissues (Fig. 2C) in a Atg8/LC3-independent manner (Supplemental Fig. S3C–D). Conversely, other myokines had minor or inconsistent effects (Fig. 2B and Supplemental Fig. S3A–B and S4A–I). Interestingly, although muscle-specific Amyrel overexpression promoted protein quality control in head tissues (Fig. 2C), it did not regulate proteostasis in skeletal muscle (Supplemental Fig. S4J), suggesting that muscle does not respond to Amyrel-mediated signaling. Consistent with its roles in the systemic regulation of protein quality control, GFP-tagged Amyrel overexpressed by skeletal muscle is also detected in the fly circulation (hemolymph) and in heads (Fig. 2D) whereas muscle-specific proteasome stress increases Amyrel mRNA levels in muscle (Fig. 2A) but not in head tissues (Supplemental Fig. S3F).
Next, we tested whether muscle-specific AmyrelRNAi impedes the systemic response induced by Prosβ1RNAi. In agreement with this hypothesis, the levels of poly-ubiquitinated proteins were higher in the detergent-insoluble fractions of heads from flies with muscle-specific Prosβ1RNAi+AmyrelRNAi compared to Prosβ1RNAi + controlRNAi (yellowRNAi; Fig. 2E and Supplemental Fig. S3D).
Age-related loss of protein quality control is accentuated by the concomitant expression of disease-associated aggregation-prone proteins (Balch et al., 2008; Douglas and Dillin, 2010). On this basis, we tested whether muscle-specific Amyrel overexpression improves proteostasis also in heads of flies with pathogenic Huntingtin (Htt-polyQ120) expression in the retina (Chan and Bonini, 2003) and found that this is the case (Fig. 2F and Supplemental Fig. S3E and S4E). Moreover, although GFP-tagged Huntingtin-polyQ72 aggregates (Zhang et al., 2010) increase with aging in the retina (Supplemental Fig. S3G), Amyrel decreased their age-related accumulation compared to controls (Fig. 2G).
We also examined the effect of Amyrel on the age-related neurodegeneration induced by mutant tauV337M, which causes frontotemporal dementia in humans (Nacharaju et al., 1999). In Drosophila, tauV337M induces retinal degeneration during aging, as exemplified by the appearance of a rough eye phenotype due to the loss and derangement of photoreceptor neurons. However, Amyrel largely prevents such age-related neurodegeneration (Fig. 2H and Supplemental Fig. S5I).
Together, these studies indicate that Amyrel is a key stress-induced myokine that improves proteostasis during aging and that prevents neurodegeneration induced by pathogenic proteins.
Proteasome Stress Induces Amyrel Expression via C/EBP Transcription Factors
We next examined the transcriptional mechanisms responsible for Amyrel induction by proteasome stress. Scanning of the proximal 1kb Amyrel promoter identified 2 binding sites for C/EBP transcription factors (Supplemental Fig. S3H), which are modulated by cell stress in other contexts (Chen et al., 2005; Hattori et al., 2003; Hungness et al., 2002; Shim and Smart, 2003).
To test whether C/EBPs sense proteasome stress in skeletal muscle, we examined the transcriptional changes induced by RNAi for CG6272/Irbp18, homologous to C/EBPγ, a dominant negative inhibitor of C/EBPβ and C/EBPδ. Therefore, CG6272RNAi promotes the transcriptional activity of C/EBPβ/δ (Lekstrom-Himes and Xanthopoulos, 1998).
There was substantial overlap (R2=0.447 for all genes, and R2=0.771 for p<0.05) in the gene expression changes induced by CG6272RNAi compared to Prosβ1RNAi and Prosβ5RNAi, but not compared to GFPRNAi (R2=0.053); (Fig. 3A–D; all RNAi are normalized by whiteRNAi). Some degree of similarity was also found between the transcriptional changes induced by Ter94RNAi and those induced by Prosβ5RNAi (Fig. 3E; R2=0.248) and CG6272RNAi (Fig. 3F; R2=0.202), but not by GFPRNAi (Fig. 3G; R2=0.068).
Fig. 3. C/EBP Transcription Factors Mimic the Response to Proteasome Stress and Induce Amyrel Expression.

(A) Similar gene expression changes (R2=0.808) are induced by muscle-specific Prosβ1RNAi and Prosβ5RNAi. (B) No correlation (R2=0.029) is found when Prosβ1RNAi and Prosβ5RNAi are each compared to GFPRNAi (all normalized to whiteRNAi; n = 3).
(C) Similar gene expression changes (R2=0.447; and R2=0.771 for p<0.05) are induced by muscle-specific Prosβ5RNAi and CG6272RNAi. (D) No correlation is found when compared control GFPRNAi (R2=0.053; and R2=0.104 for p<0.05).
(E-F) Comparison of muscle-specific Te94RNAi versus Prosβ5RNAi indicates some overlap in the gene expression changes induced (R2=0.248; and R2=0.305 for p<0.05), similar to the comparison of Te94RNAi versus CG6272RNAi (R2=0.202; and R2=0.257 for p<0.05). (G) No correlation (R2=0.068, and R2=0.097 for p<0.05) is found when Te94RNAi is compared with control GFPRNAi. In (A-G), the identity of x and y axes is specified in the figure panels. All RNA-seq datasets are normalized to whiteRNAi; GFPRNAi serves as additional control; n = 3.
(H) Gene categories upregulated in muscle by RNAi for CG6272 (C/EBPγ) include peptidases/proteases and many other gene categories (outlined) that are similarly modulated by Prosβ1RNAi and Prosβ5RNAi (Fig. 1 and Supplemental Fig. 1). Genes with p<0.05 and logratio>1 were used for these analyses.
(I) Myokines that are upregulated (red) and downregulated (blue) by proteasome stress (Fig. 2A) are similarly modulated by CG6272RNAi, including Amyrel.
(J-K) CG6272RNAi increases Amyrel expression in skeletal muscle, similar to overexpression of slbo, homologous to C/EBPβ and C/EBPδ compared to controls (L-M); SD, n = 3.
(N) RNAi for slbo reduces Amyrel expression in Prosβ1RNAi muscle, compared to control whiteRNAi. SD, n = 3.
(O) Proteasome stress in skeletal muscle is sensed via antagonizing functions of C/EBPβ/δ (slbo) and C/EBPγ (CG6272) transcription factors, which induce a local adaptive response based on the transcriptional induction of proteases, and a systemic adaptive response via modulation of muscle-secreted factors (myokines).
Supplemental Fig. S3 reports additional data related to Fig. 3.
Moreover, CG6272RNAi induced the expression of proteases/peptidases and secreted factors (Fig. 3H) similarly to Prosβ1RNAi and Prosβ5RNAi (Fig. 1A and Supplemental Figure S1B). Further analysis of 37 myokines regulated by proteasome stress (Fig. 2A) indicates that 28 of these (75%) are consistently and significantly regulated by CG6272RNAi (Fig. 3I), including Amyrel (Fig. 3J). Similar results were also obtained with drug-induced expression of CG6272RNAi by using Act88F-GS-Gal4 (Fig. 3K).
We next tested the function of Slbo, the Drosophila homolog of mammalian C/EBPβ/δ. As expected based on the analysis of CG6272 (C/EBPγ), slbo overexpression (slboOE) led to an increase in Amyrel expression (Fig. 3L–M) whereas slboRNAi reduced Amyrel expression in muscle with Prosβ1RNAi (Fig. 3N).
Altogether, these findings indicate a key role for C/EBPs in mediating the transcriptional response to proteasome stress in skeletal muscle, which in turn leads to both local and systemic adaptive responses (Fig. 3O).
Muscle-derived Amyrel Reduces the Age-Associated Accumulation of Proteasome Substrates in the Retina and Brain
We have found that muscle-derived Amyrel reduces the age-related increase in poly-ubiquitinated proteins in detergent-insoluble fractions of head tissues, which consist primarily of brains and retinas (Fig. 2C). On this basis, we next used immunostaining to further probe these findings.
Muscle-specific overexpression of Amyrel with Mhc-Gal4 and Act88F-GS-Gal4 significantly reduced the amount of poly-ubiquitin and Ref(2)P/p62-positive protein aggregates detected in old age in the retina and brain, compared to isogenic and uninduced controls (Fig. 4A–C), in agreement with biochemical analyses (Fig. 2C). Altogether, these findings indicate a key role for muscle-derived Amyrel in preserving protein quality control in the brain and retina during aging.
Fig. 4. Muscle-derived Amyrel Preserves Protein Quality Control in the Brain and Retina during Aging.

Immunostaining of retinas and brains from 60-day-old flies for poly-ubiquitinated proteins (red), Ref(2)P/p62 (green), and F-actin (blue).
(A) Skeletal muscle-specific overexpression of Amyrel (Mhc>Amyrel) reduces the age-related accumulation of poly-ubiquitin protein aggregates in the brain and retinas of flies, compared to isogenic controls (Mhc>+).
(B-C) Similar results are found in response to drug-induced (+RU486) expression of Amyrel in thoracic flight skeletal muscle with Act88F-GS-Gal4, compared to uninduced controls and no transgene (+), in both the retina (B) and brain (C).
(D) Muscle-specific Prosβ1RNAi systemically preserves protein quality control in the retina and brain during aging via Amyrel, as indicated by the higher levels of poly-ubiquitin protein aggregates found in the brains and retinas of flies with Prosβ1RNAi+AmyrelRNAi versus control Prosβ1RNAi+yellowRNAi. In (A-D), the scale bar is 20μm.
(A-D) Quantitation of the area of poly-ubiquitin protein aggregates in the brains and retinas of flies with muscle-specific modulation of Amyrel, Prosβ1RNAi+AmyrelRNAi, and controls. The n and SD is indicated.
In (A-D), higher (4x) magnification representative images are shown for each intervention.
(E) Proteasome stress in skeletal muscle improves protein quality control in the brain and retina during aging via the stress-induced myokine Amyrel.
Supplemental Fig. S3 reports additional data related to Fig. 4.
To further test the relevance of Amyrel downstream of Prosβ1RNAi, we examined the levels of poly-ubiquitinated proteins in the brains and retinas of flies with muscle-specific Prosβ1RNAi+AmyrelRNAi compared to Prosβ1RNAi+controlRNAi (yellowRNAi). Consistent with analyses in Fig. 2E, we found that Amyrel is necessary for the improvement of protein quality control in brains and retinas in response to muscle-specific Prosβ1RNAi (Fig. 4D). Altogether, these findings indicate that muscle-produced Amyrel preserves protein quality control in the CNS during aging in response to proteasome stress in muscle (Fig. 4E).
Muscle-derived Amyrel Induces Chaperone and Protease Expression in Head Tissues and Delays Age-Related Functional Decline
To dissect the mechanisms by which Amyrel regulates proteostasis, we examined head transcriptomes and found that several genes involved in proteostasis were induced in head tissues of flies with muscle-specific Amyrel overexpression compared to isogenic controls, including several chaperones and proteases (Fig. 5A–B). Htt-polyQ aggregates were increased by RNAi for the Amyrel-induced proteases CG9733 and CG7142 (Fig 5C), suggesting that they promote proteostasis in response to Amyrel. Amyrel also induced other gene categories relevant for aging and neurodegeneration, such as glutathione and lipid metabolism (Fig. 5B).
Fig. 5. Muscle-derived Amyrel Promotes the Expression of Chaperones and Proteases in Head Tissues and Preserves Neuronal Function during Aging.
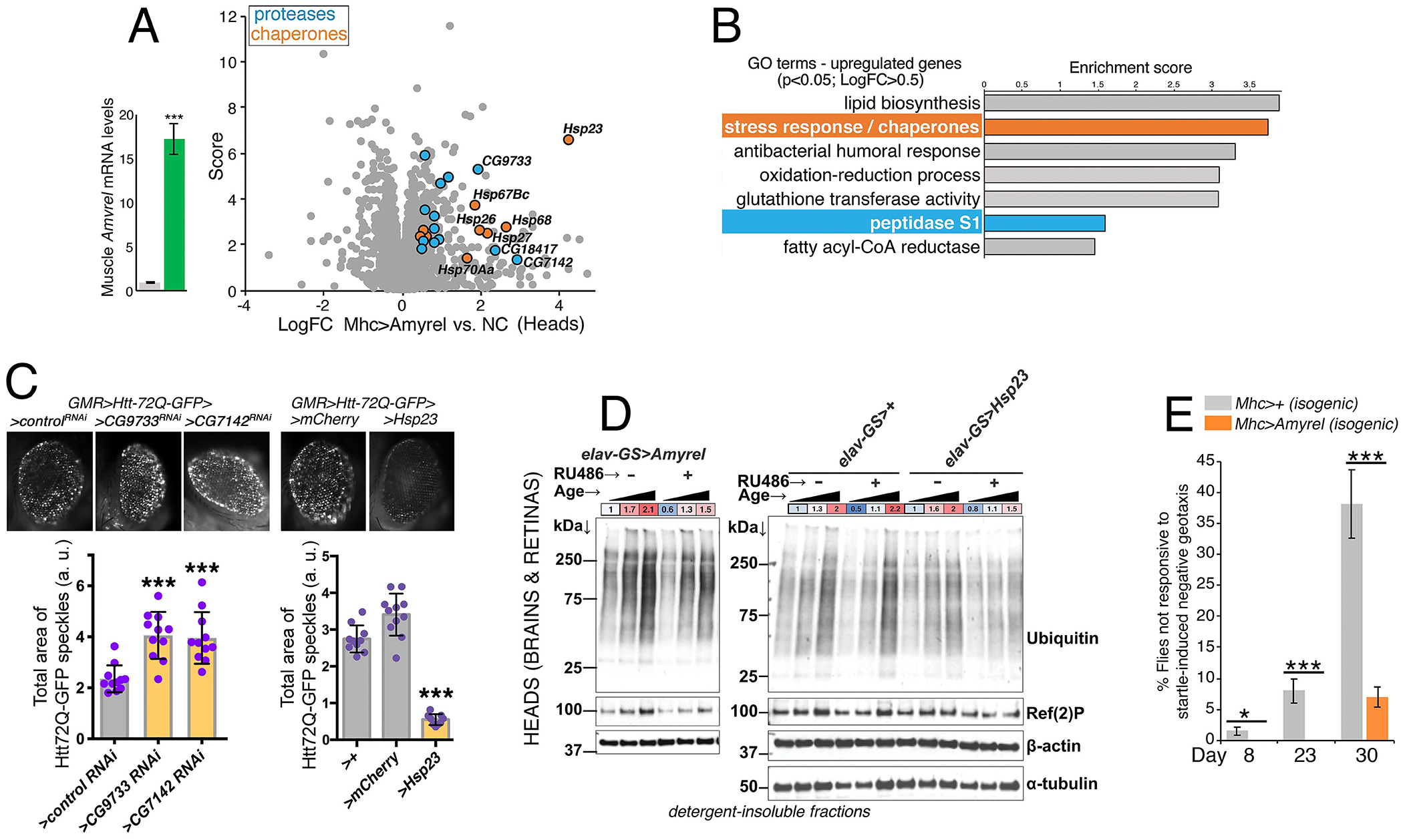
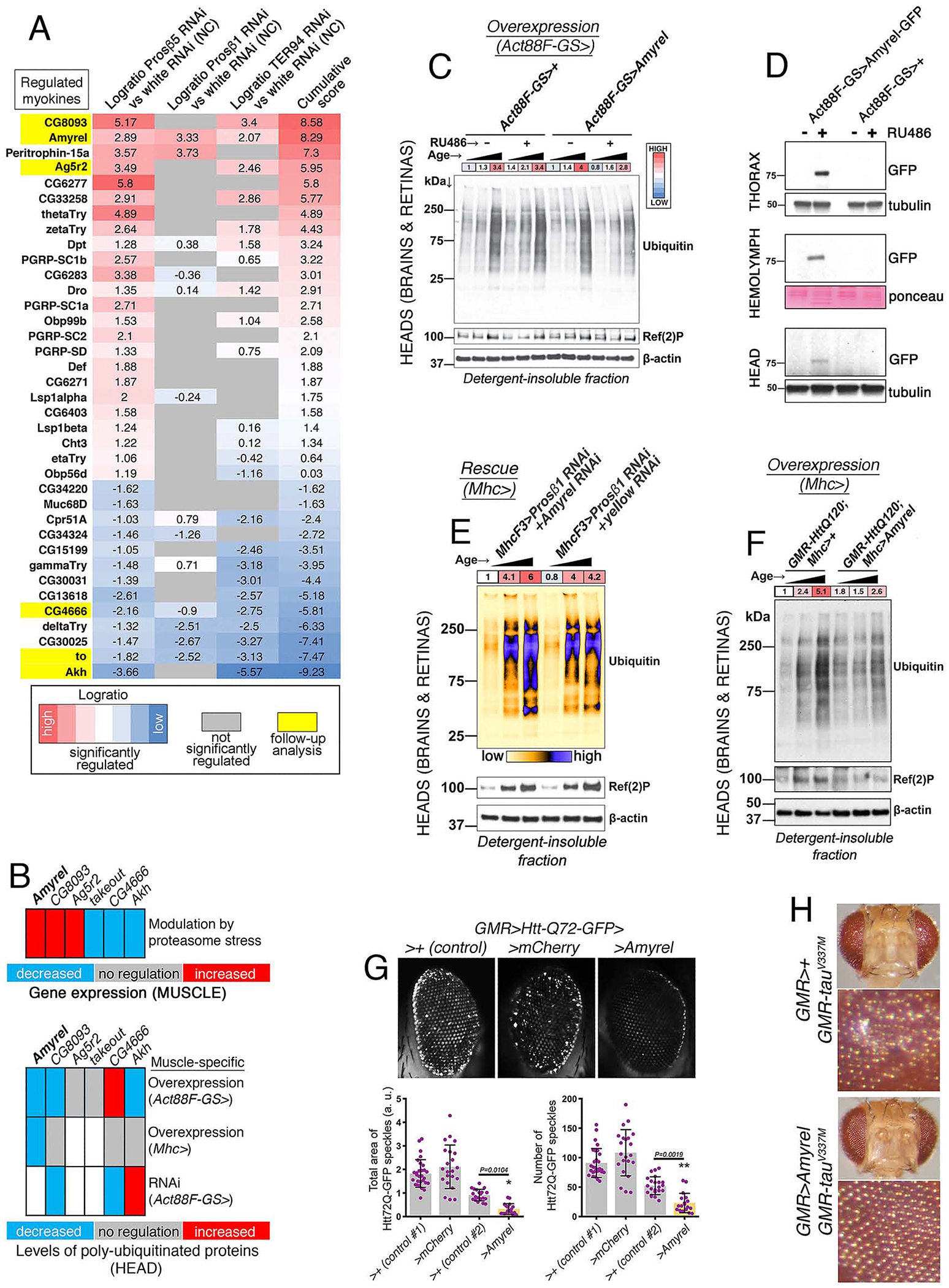
(A-B) Muscle-specific Amyrel overexpression induces transcriptional changes in head tissues (n = 3), including upregulation of chaperones (orange) and proteases (blue) (p<0.05 and LogFC>0.5). The score corresponds to −Log10(p-value).
(C) Hsp23 overexpression preserves protein quality control, as indicated by the lower amount of Htt-Q72-GFP protein aggregates compared to controls (SD, n≥9). Conversely, RNAi for CG9733 and CG7142 proteases (upregulated by Amyrel) increases Htt-Q72-GFP protein aggregates compared to control RNAi (SD, n=11).
(D) Drug-induced overexpression of Amyrel and Hsp23 in the CNS reduces the age-related increase in poly-ubiquitinated proteins (proteasome substrates) in detergent-insoluble fractions of heads (which consist primarily of brains and retinas). No effect is seen with mock drug treatments in the absence of transgenes. The ages analyzed are 10, 30, and 60 days.
(E) The capacity for startle-induced negative geotaxis declines during aging but is preserved by muscle-specific Amyrel overexpression, compared to isogenic controls (n[batches of 25 flies] = 11; SEM).
Supplemental Fig. S5 and S6 report additional data related to Fig. 5.
Chaperones can refold misfolded proteins, shield aggregation-prone proteins from interacting with endogenous proteins, and route such proteins to proteasomal degradation (Arndt et al., 2007; Labbadia and Morimoto, 2015; Tower, 2009). On this basis, Amyrel-induced heat shock proteins may promote protein quality control by chaperoning misfolded and aggregation-prone proteins and/or by facilitating their delivery to the proteasome (i.e., without impacting the proteolytic activity of the proteasome (Supplemental Fig. S2G)).
On this basis, we next examined the role of Hsp23, a small heat shock protein (sHsp) which is the gene most highly induced (>8-fold) by Amyrel (Fig. 5A). Hsp23 overexpression reduced Htt-polyQ72 aggregates (Fig. 5C), as observed with Amyrel (Fig. 2G). Conversely, RNAi for Hsp23 and other Amyrel-induced chaperones (Hsp68) led to an increase in Htt-polyQ72 aggregates (Supplemental Fig. S5A).
We next tested whether drug-induced Amyrel and Hsp23 overexpression in the brain (with elav-GS-Gal4) protects from age-related accumulation of poly-ubiquitinated proteins and found this to be the case (Fig. 5D and Supplemental Fig. S5B–C). This indicates that Hsp23 is a key Amyrel-induced gene that promotes brain proteostasis.
Preservation of protein quality control is necessary for ensuring neuronal activity and survival (Douglas and Dillin, 2010). Previously, neuron-restricted sHsp expression was found to preserve startle-induced locomotion with aging (Morrow et al., 2004). Because Amyrel induces sHsp expression (Fig. 5A–B), we tested whether Amyrel preserves startle-induced locomotion with aging and found this to be the case, compared to isogenic controls (Fig. 5E). Because Amyrel does not improve proteostasis in skeletal muscle (Supplemental Fig. S4J), preservation of startle-induced negative geotaxis likely reflects the action of Amyrel on the CNS. Together, these findings indicate that Amyrel preserves CNS function during aging.
Muscle Amyrel Increases Maltose Levels
Because muscle-produced Amyrel is detected both within skeletal muscle and in the circulation (Fig. 2D), its amylase activity (Claisse et al., 2016) in any of these compartments may produce a systemic factor that promotes proteostasis. Overexpression via GMR-Gal4 of other amylases (Amy-d and Amy-p) reduced the levels of Htt-polyQ72-GFP aggregates in the retina (Supplemental Fig. S5D), as found for Amyrel (Fig. 2G), suggesting a key role for the amylase activity of Amyrel in regulating proteostasis.
As observed for amylases in the digestive system, stress-induced muscle Amyrel may produce maltose and other disaccharides via the enzymatic degradation of polysaccharides and oligosaccharides. To test whether this occurs, we first examined the effect of muscle-specific Prosβ1RNAi and found that body (Fig. 6A) and head (Fig. 6B) maltose levels were higher, compared to control whiteRNAi, whereas glucose levels were not consistently regulated (Fig. 6A). There was also an increase in maltose levels upon Amyrel overexpression, in comparison with isogenic controls (Fig. 6A), in parallel with an increase in amylase activity (Supplemental Fig. S6A). Altogether, these findings indicate that Amyrel increases maltose levels, as expected based on its enzymatic activity.
Fig. 6. Amyrel-Produced Maltose and Maltose Transporters Promote Protein Quality Control in the Brain and Retina via the Transcriptional Induction of Chaperones and Proteases.
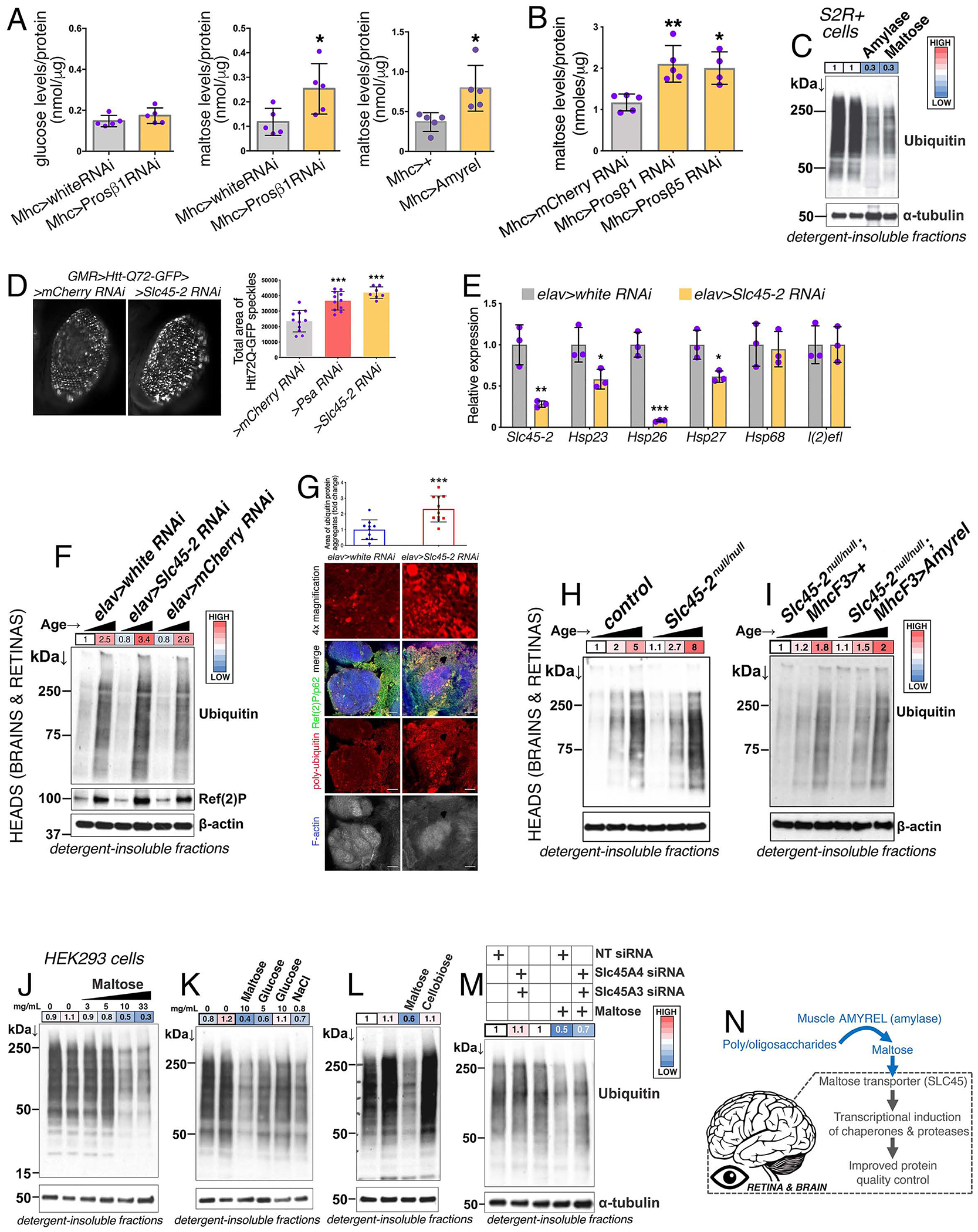
(A) Body levels of maltose increase in response to muscle-specific Prosβ1RNAi, whereas glucose is inconsistently regulated. Similarly, muscle-specific Amyrel overexpression increases body maltose levels. SD, n = 5.
(B) Head maltose levels increase in response to muscle-specific Prosβ1RNAi and Prosβ5RNAi compared to control mCherryRNAi; SD, n≥4.
(C) Western blot analysis of detergent-insoluble fractions from Drosophila S2R+ cells treated with porcine recombinant amylase or maltose and heat shocked for 6h at 37°C. Amylase and maltose decrease the heat-induced accumulation of poly-ubiquitinated proteins in detergent-insoluble fractions.
(D) RNAi for the maltose transporter Slc45-2 driven by GMR-Gal4 increases the overall amount of Htt-Q72-GFP protein aggregates in retinas, compared to control mCherryRNAi (n≥7, SD). PsaRNAi is a positive control.
(E) Slc45-2RNAi driven in the CNS by elav-Gal4 reduces the expression of chaperones that are upregulated by Amyrel. SD, n = 3.
(F) Slc45-2RNAi increases age-related accumulation of poly-ubiquitinated proteins in detergent-insoluble fractions of heads, compared to control RNAi. The ages analyzed are 10 and 30 days (elav>Slc45-2RNAi flies do not survive to 60 days).
(G) Immunostaining for poly-ubiquitin (red), Ref(2)P/p62 (green), and F-actin (blue) of brains from 30-day-old flies. Slc45-2RNAi leads to an increase in the age-related accumulation of poly-ubiquitinated protein aggregates in the brain compared to whiteRNAi.
(H) Head tissues from Slc45-2 null/null flies display age-dependent increase in poly-ubiquitinated proteins in detergent-insoluble fractions from heads, compared to controls.
(I) Muscle-specific Amyrel overexpression does not improve protein quality control of head tissues during aging in the absence of Slc45-2.
(J-L) Western blot analysis of detergent-insoluble fractions from human HEK293 cells treated with increasing maltose concentrations (0, 3, 5, 10, and 33 mg/mL) and iso-osmolar and iso-energetic controls (NaCl and glucose). Maltose preserves protein quality control in HEK293 cells that were heat shocked for 7h at 41.5°C, whereas treatment with the disaccharide cellobiose (10 mg/mL) does not.
(M) Western blot analysis of detergent-insoluble fractions from human HEK293 cells treated with 10 mg/mL maltose and either control NT siRNAs or combined SLC45A3+A4 siRNAs. SLC45 RNAi partially prevents the protective action of maltose.
(N) The stress-induced amylase Amyrel produces maltose, which improves proteostasis via SLC45 maltose transporters and transcriptional induction of chaperones.
Supplemental Fig. S5 and S6 report additional data related to Fig. 6.
SLC45 Maltose Transporters Ensure Protein Quality Control During Aging
Because Amyrel increases maltose levels, we next tested whether maltose is a key modulator of protein quality control by treating Drosophila S2R+ cells with recombinant porcine amylase and maltose. The effect of such treatments was tested by western blot after heat shock, which induces protein misfolding and increases the degradation burden for the proteasome (Balch et al., 2008; Douglas and Dillin, 2010). Similar to results obtained with transgenic Amyrel overexpression in vivo (Fig. 2 and 4), treatment with recombinant amylase and maltose reduced the accumulation of poly-ubiquitinated proteins in detergent-insoluble fractions upon heat shock (Fig. 6C and Supplemental Fig. S6B).
Because maltose improves protein quality control (Fig. 6C), we next examined whether Slc45-1 and Slc45-2 maltose transmembrane transporters (Meyer et al., 2011; Vitavska and Wieczorek, 2013) regulate proteostasis. We found that Slc45-1RNAi and Slc45-2RNAi (driven by GMR-Gal4) significantly increased Htt-polyQ72-GFP aggregates in Drosophila retinas (Fig. 6D and Supplemental Fig. S5G). Moreover, Slc45-1RNAi worsened age-related neurodegeneration induced by tauV3337M compared to control mCherryRNAi (Supplemental Fig. S5H–I). Overall, these findings indicate that Slc45 maltose transporters are necessary for protein quality control in the CNS during aging.
RNAi for SLC45 Maltose Transporters Reduces the Expression of Amyrel-Target Genes in the Brain
Disaccharides such as maltose are chemical chaperones that can stabilize membrane structure and protein folding (Kaplan and Guy, 2004; Levy-Sakin et al., 2014; Mensink et al., 2017). Therefore, intracellular transport of maltose through Slc45 may affect protein quality control via maltose chemical chaperone properties. Moreover, because Slc45-2 is highly expressed in the brain but has little or no expression in skeletal muscle (Supplemental Fig. S5E), its expression pattern may explain why protein quality control improves in the brain but not in muscle in response to Amyrel (Supplemental Fig. S4J). In addition, maltose may be responsible for Amyrel-induced gene expression changes. Consistent with this hypothesis, qRT-PCR determined that, in parallel with a decline in Slc45-2 mRNA levels, Slc45-2RNAi (driven by elav-Gal4) reduced the expression of key Amyrel-induced chaperones (Hsp23, Hsp26, Hsp27), indicating that Slc45-2 is necessary for Amyrel-induced target gene expression in the brain (Fig. 6E).
On this basis, we next assessed whether Slc45-2 regulates protein quality control during aging. In agreement with this model, Slc45-2RNAi (driven by elav-Gal4) led to an increase in poly-ubiquitinated proteins in head tissues with aging, compared to control whiteRNAi and mCherryRNAi (Fig. 6F and Supplemental Fig. S5F), as expected based on the decline in chaperone expression resulting from Slc45-2RNAi (Fig. 6E). Similar results were obtained via the immunostaining and analysis of poly-ubiquitin protein aggregates in the brain of elav>Slc45-2RNAi flies, compared to controls (Fig. 6G). Moreover, Slc45-2 null mutations (Xu and Wang, 2019) increased the age-related accumulation of detergent-insoluble poly-ubiquitinated proteins in head tissues, compared to wild-type controls (Fig. 5H and Supplemental Fig. S5J–K).
On this basis, we next tested whether Amyrel requires Slc45-2 to improve protein quality control in head tissues during aging. No substantial difference in the levels of detergent-insoluble poly-ubiquitinated proteins was found when comparing Slc45-2 null flies with concomitant muscle-specific Amyrel overexpression (Slc45-2null/null; MhcF3>Amyrel) with control Slc45-2 null flies with no Amyrel overexpression (Slc45-2null/null; MhcF3>+). These findings indicate that muscle-derived Amyrel does not improve protein quality control of head tissues during aging in the absence of Slc45-2 (Fig. 5I and Supplemental Fig. S5J, L).
Together, these studies indicate that maltose transporters are necessary for ensuring CNS proteostasis during aging and in response to Amyrel (Fig. 6C–I and Supplemental Fig. S5E–L and S6G–I), as expected based on the role of maltose (Fig. 6C) and of Amyrel in this process (Fig. 2, 4, 5).
In summary, these studies identify an unanticipated pathway for protein quality assurance that originates from maltose production by the stress-induced Amyrel and that requires Slc45 maltose transporters for chaperone induction and protein quality control in the brain and retina during aging (Fig. 6N).
Maltose Preserves Protein Quality in Human Cells in a SLC45-Dependent Manner
To further probe this model, we next tested the effect of maltose in human HEK293 cells (Lin et al., 2014; Shaw et al., 2002). As observed for S2R+ cells (Fig. 6C), maltose significantly prevented the heat shock-induced accumulation of poly-ubiquitinated proteins in detergent-insoluble fractions, compared to mock treatments (Fig. 6J and Supplemental Fig. S6C–D). These controls included iso-osmolar (NaCl, glucose, and the disaccharide cellobiose) and iso-energetic (glucose) treatments, which did not fully recapitulate the preservation of protein quality control seen with maltose (Fig. 6J–L and Supplemental Fig. S6E–F). Together, these findings suggest that the disaccharide maltose improves protein quality control downstream of Amyrel via mechanisms different from osmotic stress and metabolic utilization.
Next, we tested whether maltose transporters affect protein quality control in human HEK293 cells as observed in Drosophila. To this purpose, HEK293 cells were treated with either siRNAs for the maltose transporters SLC45A3 and SLC45A4 (the sole SLC45 family members expressed in HEK293 cells; Supplemental Fig. S6G), or with control NT siRNAs. Upon heat shock, there was accumulation of poly-ubiquitinated proteins in the detergent-insoluble fractions, which was largely prevented by maltose in HEK293 cells treated with control NT siRNAs, but less so in cells treated with SLC45A3+A4 siRNAs (Fig. 6M and Supplemental Fig. S6H–I). Altogether, these studies indicate that maltose preserves protein quality in human cells in a SLC45-dependent manner.
Maltose Preserves Protein Quality and Neuronal Activity in Human Cortical Brain Organoids Challenged by Heat Shock
Organoids (Supplemental Fig. S7A) are emerging as important models for aging and brain research (Hu et al., 2018; Qian et al., 2016). On this basis, we tested whether maltose regulates proteostasis in heat-shocked human brain organoids as observed in cultured cells (Fig. 6J–M). There was no modulation of poly-ubiquitinated proteins in detergent-soluble and insoluble fractions in maltose-treated versus control organoids in non-heat-shocked conditions (Supplemental Fig. S7B–C). Heat shock significantly increased the levels of poly-ubiquitinated proteins in detergent-insoluble fractions. However, this increase was mostly prevented by pre-treatment with maltose (Fig. 7A), as observed in HEK293 cells (Fig. 6G–J).
Fig. 7. Maltose Preserves Protein Quality Control in Human Cortical Brain Organoids Challenged by Thermal Stress.
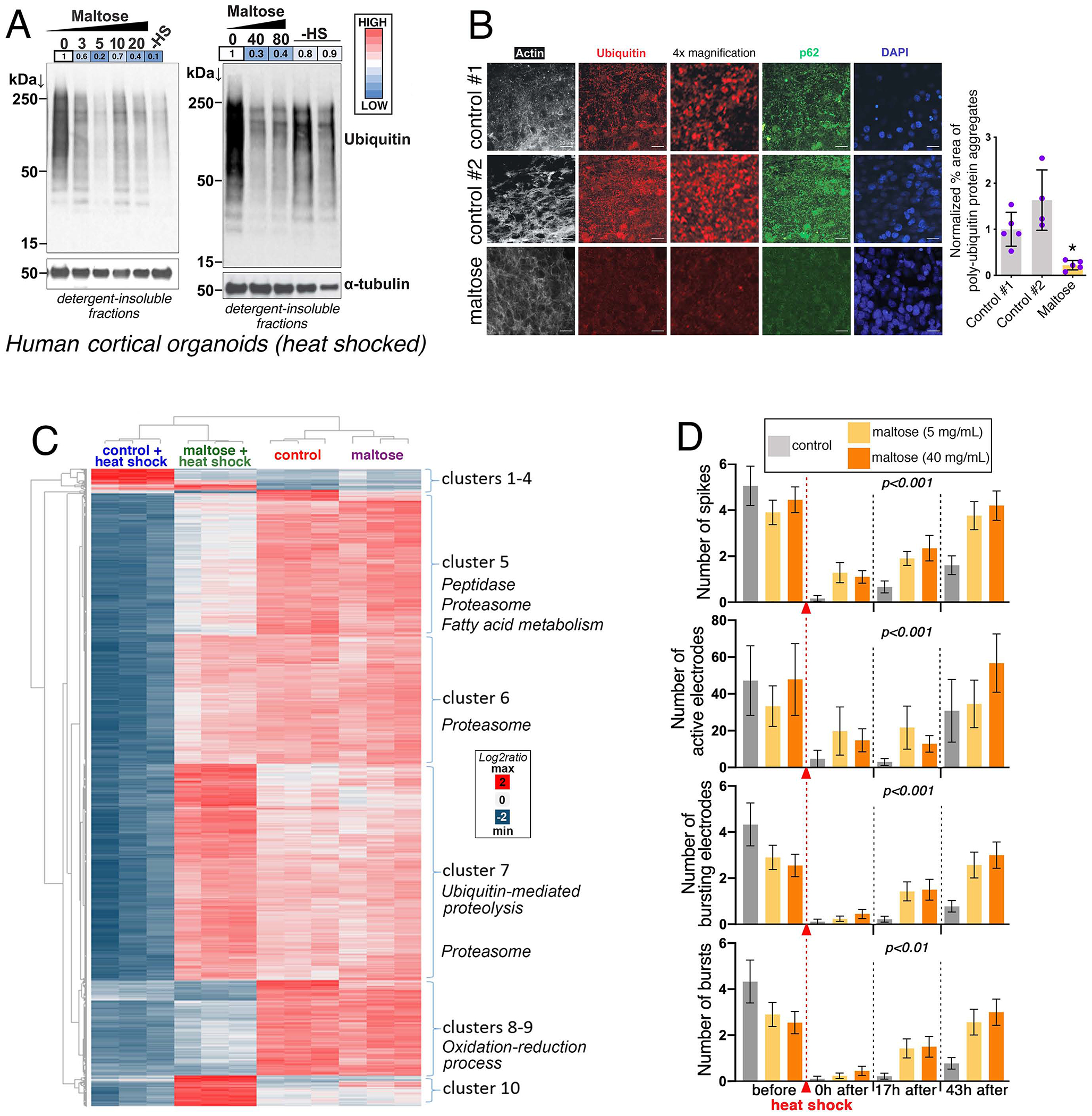
(A) Western blots of detergent-insoluble fractions from human cortical brain organoids treated with maltose (mg/mL) and heat shocked for 7h at 41.5°C. “-HS” denotes non heat-shocked control organoids.
(B) Immunostaining for ubiquitin and p62 indicates that maltose (40 mg/mL) prevents the accumulation of poly-ubiquitin protein aggregates after heat shock. The scale bar is 20 μm. The n and SD are indicated.
(C) Cluster analysis of RNA-seq data (n = 3; 2095 genes). Maltose partially prevents the gene expression changes induced by thermal stress and maintains the expression of genes involved in proteostasis.
(D) Multielectrode array recording of neuronal activity from cortical organoids treated with maltose and heat shocked (SEM with n=18 for each condition; each n represents a well with an organoid slice). Maltose treatment preserves neuronal activity, which is compromised by thermal stress. The p-value represents the row factor from two-way ANOVA, which indicates the effect of treatment at each time point (p<0.01).
Supplemental Fig. S7 reports additional data related to Fig. 7.
Similar results were obtained via immunostaining. Specifically, there were minimal levels of p62 and poly-ubiquitinated proteins in control non-heat-shocked conditions (Supplemental Figure S7D). However, maltose treatment significantly decreased p62 and ubiquitin immunoreactivity in heat-shocked organoids, compared to no-treatment controls (Fig. 7B).
We also examined gene expression changes and found that maltose prevents some of the gene expression changes induced by heat shock in human cortical brain organoids (Fig. 7C and Supplemental Fig. S7E). Treatment with maltose preserved the expression of several gene clusters involved in protein quality control, such as components of the proteasome and proteases/peptidases (Fig. 7C). Moreover, mRNA and protein levels of CRYA (α-crystallin, homologous to Drosophila Hsp23) were higher in human cortical organoids treated with maltose (Supplemental Fig. S7E–F).
Because preservation of proteostasis typically corresponds to improvement in neuronal function, we next tested whether the neural activity of organoids is improved by treatment with maltose. Measurements with microelectrode arrays (MEAs) indicated that neuronal activity of cortical organoids is not modulated by maltose at steady state but is significantly preserved by maltose immediately after and at 17h and 43h after heat shock (Fig. 7D). This activity was determined by measuring the average number of spikes, active electrodes, bursting electrodes, and network bursts displayed by multiple organoids in each condition. Interestingly, as low as 5 mg/mL of maltose is effective in preserving neuronal activity (Fig. 7D), coincident with the preservation of protein quality (Fig. 7A–B and Supplemental Fig. S7B–C). Altogether, these findings indicate that maltose has evolutionary conserved roles in preserving protein quality control in the brain.
DISCUSSION
Originally viewed as a disease that arises only from local changes in the brain, there is now increasing evidence that peripheral tissues contribute to age-related neurodegeneration (Chauhan et al., 2017; Pluvinage and Wyss-Coray, 2020; Sampson et al., 2016; Wang et al., 2017). Here, we have examined the effects of muscle-specific proteasome stress and found a key role for the amylase Amyrel, an atypical myokine, in muscle-to-brain stress signaling during aging (Fig. 2–5).
Amylases are normally confined to the digestive system, where they degrade poly/oligosaccharides into disaccharides such as maltose (Claisse et al., 2016). However, amylases are also expressed in non-exocrine tissues (Hokari et al., 2003). For example, the AMY1 amylase is expressed and secreted by skeletal muscle in mice (Deshmukh et al., 2015a; Deshmukh et al., 2015b). Moreover, AMY2A amylase expression increases in human HEK293 cells and in brain cortical organoids treated with the proteasomal inhibitor MG132 (Supplemental Fig. S7G–H), suggesting that amylases could be induced by proteasomal stress also in human tissues. Moreover, as observed for Drosophila Amyrel (Supplemental Fig. S7I), AMY1 mRNA and protein levels increase in mouse skeletal muscle during aging (Supplemental Fig. S7J), presumably in response to age-associated challenges to proteostasis (Jiao and Demontis, 2017). Similarly, the levels of AMY1 significantly increase in the human plasma during aging (Supplemental Fig. S7K; (Lehallier et al., 2019)), and in response to lifespan-extending interventions in mice (Supplemental Fig. S7L; (Tyshkovskiy et al., 2019)).
Altogether, this indicates that amylases can be produced and secreted by non-digestive tissues such as skeletal muscle upon challenges to proteostasis. Therefore, in addition to classical exocrine functions in carbohydrate digestion in the gut, amylases may function as stress-induced endocrine signaling factors released by non-digestive tissues.
We have found that mimicking stress-induced expression of Amyrel in skeletal muscle increases the levels of the disaccharide maltose in Drosophila (Fig. 6). Amyrel is induced locally in the stressed tissue (muscle) but is also secreted (presumably via a regulated process dependent on its signal peptide) and found in the circulation (hemolymph) and in head tissues (Fig. 2D). On this basis, the increase in body and head maltose levels (Fig. 6A–B) may depend on Amyrel amylase activity within the skeletal muscle, in the circulation, and/or in the brain.
Previously, an increase in maltose was found to occur in Drosophila in response to diverse challenges to protein homeostasis, such as cold and heat shock, ectopic amyloid expression, inbreeding, and starvation (Malmendal et al., 2006; Ott et al., 2016; Overgaard et al., 2007; Pedersen et al., 2008). Similarly, exposure to Aβ42 fibrils was found to increase AMY2A amylase activity in cultured human astrocytes (Byman et al., 2019). However, the physiological significance of such stress-induced surge in maltose and amylase was unknown.
Interestingly, stress-induced increases in amylase activity and maltose were found to promote protein folding in yeast (Zheng et al., 2019) and in plants (Kaplan and Guy, 2004, 2005; Tarkowski and Van den Ende, 2015). These effects were ascribed to the chemical chaperone properties of maltose (Kaplan and Guy, 2004; Levy-Sakin et al., 2014; Mensink et al., 2017). Consistently, in vitro studies have shown that maltose is the most effective chemical chaperone in preserving enzymatic activity under thermal stress, compared to other sugars (Levy-Sakin et al., 2014). However, it was unknown whether the chemical chaperone properties of maltose observed in vitro are relevant for protecting the proteome in vivo.
In this study, we have found that Amyrel-produced maltose induces an adaptive transcriptional response consisting of increased chaperone and protease expression, which promotes protein quality control (Fig. 6–7). Thus, in addition to its chemical chaperone properties, maltose promotes protein quality via the induction of a transcriptional stress response.
Although maltose is present in the human serum (Bao et al., 2009; Psychogios et al., 2011), little is known on the role that maltose/disaccharide intracellular transport and maltose-induced signaling plays in humans. Our finding that maltose protects human brain cortical organoids from heat shock-induced accumulation of poly-ubiquitinated proteins (Fig. 7) suggests that maltose and its intracellular transport may regulate protein quality control and contribute to the systemic propagation of proteasome stress from muscle to distant tissues also in humans.
Contrary to plants, where disaccharide transporters have been well characterized (Lalonde et al., 1999; Meyer et al., 2011), it was assumed that transport of sugars across plasma membranes is restricted to monosaccharides in animals (Meyer et al., 2011). However, this view has changed with the recent identification of maltose/disaccharide transporters of the solute carrier family 45 (SLC45) family, which are present in all vertebrates and Drosophila (Meyer et al., 2011; Vitavska and Wieczorek, 2013). Interestingly, disaccharide transporters are expressed not only in the intestine but also in non-digestive tissues such as the brain (Meyer et al., 2011; Vitavska and Wieczorek, 2013). Moreover, a SLC45 family member (SLC45A3) has been linked to human neurodegeneration (Vitavska and Wieczorek, 2013). Specifically, a SNP with significant association to Parkinson’s disease was found in proximity of SLC45A3 (Satake et al., 2009). Together, these findings suggest that disaccharide transport may impact proteostasis and neurodegeneration in humans. In summary, we have found unanticipated endocrine signaling functions for stress-induced muscle-derived Amyrel, an amylase which promotes protein quality control in the CNS during aging via maltose-dependent transcriptional induction of heat shock proteins.
Limitations of Study.
It is currently unknown whether Hsp induction stems from the chemical chaperone activity of maltose or, alternatively, from its capacity to modulate a maltose-sensing transcription factor.
STAR METHODS
RESOURCE AVAILABILITY
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Fabio Demontis (Fabio.Demontis@stjude.org).
Materials availability
There are no restrictions to the availability of tools generated in this study.
Data and code availability
The accession number for the RNA-seq data reported in this study (Drosophila muscle with proteasome stress; head tissues from Drosophila with muscle-specific Amyrel overexpression; and human cortical organoids treated with maltose/heat shock, and controls) is GEO: GSE149799. All other data supporting the findings of this study are available within the article and the supplementary information files and from the lead contact author upon request.
EXPERIMENTAL MODELS AND SUBJECT DETAILS
Drosophila
Drosophila husbandry
Flies were kept (~25 flies/tube) at 25°C, 60% humidity, and a 12h/12h light-dark cycle in tubes containing cornmeal/soy flour/yeast fly food, and aged to 10, 30, and 60 days at 25°C. The fly food was changed regularly every 2-3 days. For some experiments, as specified in the text and figure legends, flies were aged at 29°C. All experiments were done with male flies apart for the analysis of tauV337M-induced neurodegeneration, for which female flies were used as tau-induced degeneration is stronger in females. Fly stocks are listed in the Key Resource Table and were obtained from the Bloomington Drosophila Stock Center (BDSC), the Vienna Drosophila Resource Center (VDRC), the National Institute of Genetics (NIG, Japan), and the FlyORF. Transgene expression was confirmed by qRT-PCR.
Mhc-Gal4 and Act88F-GS-Gal4 were previously validated as skeletal muscle-specific Gal4 drivers (Demontis and Perrimon, 2010; Robles-Murguia et al., 2019; Robles-Murguia et al., 2020; Schuster et al., 1996) and further analyses have confirmed that they do not drive expression in the nervous system (Demontis and Perrimon, 2010; Robles-Murguia et al., 2020). Act88F-GS-Gal4 drives transgene expression only in the indirect flight muscles of the Drosophila thorax (Robles-Murguia et al., 2019; Robles-Murguia et al., 2020).
Muscle-specific MhcF3-Gal4 (Gajewski and Schulz, 2010) was used for epistasis analyses in Fig. 2E, 4D, and 6I.
Experimental Design of Aging Studies with Drosophila
Differences in the genetic and cytoplasmic backgrounds among various fly stocks are confounding variables for aging experiments. To avoid the contribution of these variables, we have followed the procedures described below, as done previously (Demontis et al., 2014).
To rule out any variation due to cytoplasmic background effects (e.g., changes in mitochondria across the stocks used), all crosses were set up with female virgins from the Gal4 line to ensure that all progenies receive the same cytoplasm (the zygote cytoplasm derives from the maternal oocyte).
To avoid any contribution of genetic background mutations to the observed phenotypes, UAS- transgenes were backcrossed through more than six generations against y1w1118 to obtain isogenic male siblings carrying either a UAS- or no transgene (distinguished by eye color: white+ and white−, respectively). Male siblings carrying either a UAS- or no transgene and having the same genetic background were then crossed to homozygous w1118;Mhc-Gal4 (rosy+ white−) females and the resulting male progenies were sorted (based on eye color) into isogenic transgene-expressing and transgene-nonexpressing cohorts. UAS- transgene expression was confirmed by qRT-PCR. To exclude muscle developmental effects, we used a Mhc-Gal4 line (Demontis and Perrimon, 2010; Schuster et al., 1996) that does not drive any meaningful transgene expression during muscle development at 25°C.
In addition to the procedures described above, the GeneSwitch expression system (Osterwalder et al., 2001) was also used to exclude the impact of genetic background mutations and developmental effects on the observed phenotypes. To this purpose, the muscle-specific Act88F-GS-Gal4 and the neuronal-specific elav-GS-Gal4 drivers (McGuire et al., 2004; Osterwalder et al., 2001; Robles-Murguia et al., 2020) were used. For these experiments, flies were raised for 4 days on normal food post-eclosion and then kept for 10, 30, or 60 days on food supplemented with 200 μM RU486 (mifepristone; Calbiochem #475838) dissolved in ethanol or with ethanol alone (control). Each RU486-treated genotype was compared to the corresponding ethanol-treated control. Effects deriving from the RU486 drug itself were excluded by examining the effect of drug-induced expression of control transgenes. In RNAi experiment, RNAi against white or mCherry was used as control to account for any non-specific effects deriving from the engagement of the RNAi machinery (Alic et al., 2012). In overexpression experiment, no transgene (+) and/or mCherry overexpression were used as controls.
For genetic epistasis experiments, the number of UAS transgenes to compare was kept constant in order to avoid Gal4 titration effects which may arise due to higher Gal4 activity if an uneven number of UAS transgenes is compared.
In all experiments, the fly food was changed regularly every 2-3 days.
Cell Culture
Drosophila S2R+ Cells
106 S2R+cells were seeded in 3 mL of Schneider’s cell culture medium (Gibco) containing heat-inactivated 10% FCS in each well of a 6 well-plate, with the addition of either 100 mg/mL of maltose, 100 mg/mL of glucose, 1.7 mg/mL of porcine amylase, or mock treatments. After culturing at 25°C for 3 days, the cells were kept for 6 hours at 37°C for heat shock. Subsequently, the cells and cell culture medium were scraped and centrifuged at maximum speed for 5 minutes, the supernatant removed, and the cell pellet frozen. The cell pellet was then processed as described above for the western blot analysis of detergent-soluble and -insoluble fractions.
HEK293T Cells
HEK293T cells were obtained from the ATCC and cultured as described before (Hunt et al., 2019b). Specifically, cells were maintained at 37°C with 5% CO2 in DMEM containing 10% fetal bovine serum, Glutamax and penicillin/streptomycin. HEK293T cells were screened regularly to ensure the absence of mycoplasma.
For experimental treatment, 200,000 HEK293 cells were plated in 2 mL in each well of a 6-well plate. After ~30 minutes, an additional 3 mL of culture medium was added to reach the final amount of 0, 15, 25, 50, and 100 mg of maltose per well (5 mL). After culturing at 37°C for 3 days, the cells were kept for 7 hours at 41.5°C for heat shock, or at 37°C (control). Subsequently, the cells were scraped and centrifuged at maximum speed for 5 minutes, the supernatant removed, and the cell pellet frozen. The cell pellet was then processed as described above for the western blot analysis of detergent-soluble and - insoluble fractions.
For the induction of proteasome stress in cultured cells, HEK293 cells were treated for 4 hours with 20, 40, and 80 μM MG132 dissolved in DMSO. The same volume of DMSO was used for mock treatment of control cells.
siRNA Treatment of HEK293 Cells
~200,000 HEK293 cells per well were seeded in a 6-well plate. 24 hours later, cells were transfected with siRNAs using Lipofectamine2000 to a final concentration of 200 nM siRNA per well. Specifically, a mixture of 16 μL of Lipofectamine2000, 200 μL Opti-MEM, and 8 μL of 50 μM siRNA was prepared and vortexed. After standing for 10 minutes, the mixture was added to each well (where 2 mL of fresh culture medium with serum had been added). For double siRNA transfections, 150 nM of each siRNA was used (i.e., a total of 300 nM siRNA per well). Specifically, a mixture of 24 μL of Lipofectamine2000, 200 μL Opti-MEM, 6 μL of 50 μM siRNA#1, and 6 μL of 50 μM siRNA#2 were added to each well. After 24 hours, the cell culture medium was replaced with medium supplemented with 10% FBS and 10 mg/mL maltose. After ~40 hours of maltose treatment, cells were heat shocked for 7 hours at 41.5°C and compared to control cells that were kept at 37°C.
Human ESC Culture
H9/WA09 cells (WiCell; NIH approval NIHhESC-10-0062) were cultured on hES-qualified Matrigel (5264004, Corning) in complete mTeSR1 (85850, STEMCELL Technologies) at 5% O2, 37°C and 5% CO2. The cultures were passaged with Versene (15040066, ThermoFisher).
Generation of Human Cortical Brain Organoids
Human cortical organoids were generated based on a novel method adapted from previously published protocols (Kadoshima et al., 2013; Lancaster et al., 2017; Lancaster et al., 2013). Briefly, H9 cultures were dissociated into single cells with Accutase (AT-104, Innovative Cell technologies), and plated into low-attachment 96-well V-bottom plates (MS-9096VZ, Sbio) at 9000 cells/well, in EB media (DMEM:F12, 20% Knockout Serum Replacement (10828, Life Technologies), 3% ES-FBS (ES-009-C, SIGMA), 100X Glutamax (Gibco), 1000X β-Mercaptoethanol (2020-07-30, Gibco), 100X antibiotic-antimycotic (Gibco)) supplemented with 5 μM SB-431542 (TGFβ inhibitor, 1614, Tocris), 2 μM Dorsomorphin (3093, Tocris), 3 μM IWR1e (Wnt inhibitor, 681669, EMD Millipore), and 20 μM ROCKi (Y-27632, 72304, Stemcell technologies). Half media was replaced on day 2. On days 4, 6, and 8, half media was replaced with GMEM KSR media (GMEM, 20% KSR, 100X NEAA (Gibco), 100X Pyruvate (Gibco), 500X β-Mercaptoethanol, 100X antibiotic-antimycotic) supplemented with 5 μM SB-431542, 3 μM IWR1e, 2.5 μM Cyclopamine (72074, Stemcell technologies) and 20 μM ROCKi. On days 10,12,14, half media was replaced with GMEM KSR media supplemented with 5 μM SB-431542, 3 μM IWR1e, and 20 μM ROCKi. Same on day 16, but without ROCKi. On days 18 and 20, half media was replaced with CBO N2 media (DMEM:F12, 100X chemically defined lipid concentrate (11905-031, Life technologies), 100X N2 supplement (17502-048, Gibco) and 100X antibiotic-antimycotic). On day 22, organoids were transferred to a mini-bioreactor (BWS-S03N0S-6, ABLE Corporation, Tokyo) in CBO N2 media supplemented with 50X B27 supplement without Vitamin A (12587-010, Gibco), 20 ng/mL bFGF (78003.1, Stemcell technologies) and 20 ng/mL EGF (AF-100-15-100UG, Peprotech), and spun at 55 rpm. Media was replaced on day 24, 26, 28. On day 30, media was changed to CBO FBS media (DMEM:F12), 100X chemically defined lipid concentrate (11905-031, Life technologies), 100X N2 supplement (17502-048, Gibco), 10% ES-FBS, 5 μg/mL Heparin and 100X antibiotic-antimycotic) supplemented with 1% v/v growth factor reduced-Matrigel (356230, Corning), 100X N2 supplement and 50X B27 supplement without Vitamin A, and replaced every 3 days until day 39. From day 42 to 48, media was changed to CBO FBS media supplemented with 50X B27 supplement without Vitamin A, 20 ng/mL BDNF (450-02, Peprotech) and 10 ng/mL GDNF (450-10, Peprotech), and replaced every 3 days. Day 51 onwards, media was changed to BrPhys media (05790, Stemcell technologies) supplemented with 100X N2 supplement, 50X B27 supplement without Vitamin A, 10 ng/mL BDNF and 10 ng/mL GDNF, and replaced every 3-4 days. After day 70, large cortical organoids were pinched off into 2-3 smaller pieces using a pair of sterile forceps, in order to avoid large necrotic centers.
Drug Treatment of Human Cortical Brain Organoids
An additional 0.5 mL of culture medium was added to reach the final amount of 1 mL with 0, 3, 5, 10, 20, 40, or 80 mg of maltose per mL, as indicated. After culturing at 37°C for 3 days, the organoids were kept for 7 hours at 41.5°C for heat shock, or at 37°C (control). Subsequently, the organoids were collected and snap frozen. The organoids were then processed as described below for the western blot analysis of detergent-soluble and insoluble fractions.
For the induction of proteasome stress, cortical organoids were treated for 4 hours with either 80 μM MG132 dissolved in DMSO or with the same volume of DMSO as control.
METHOD DETAILS
Whole-Mount Immunostaining of Drosophila Brains and Retinas
The immunostaining of brains and retinas was done as previously described (Demontis and Dahmann, 2009; Demontis and Perrimon, 2010; Hunt and Demontis, 2013). In brief, retinas and brains were dissected, fixed for 30 minutes in PBS with 4% paraformaldehyde and 0.2% Triton X-100 at room temperature, washed >3 times in PBS with 0.2% Triton X-100 at room temperature, and immunostained overnight at 4°C with rabbit anti-poly-ubiquitin (FK2; Enzo Life Sciences #BML-PW8810-0100) and anti-Ref(2)P/p62 antibodies (Abcam #178840). After washes with PBS with 0.2% Triton X-100, the samples were incubated with secondary antibodies and Alexa635-phalloidin for 2 hours at room temperature, washed and mounted in antifade medium. Subsequently, the samples were imaged on a Zeiss LSM 880 confocal microscope. Image analysis was done with NIH ImageJ 1,50i. An area of 7000 square microns was used for measuring aggregates across all the samples (both retina and brains).
Whole-Mount Immunostaining of Human Brain Organoids
Similar procedures as described above for Drosophila tissues (Demontis and Dahmann, 2009; Demontis and Perrimon, 2010; Hunt and Demontis, 2013) were followed for the whole-mount immunostaining of human cortical organoids with the following modifications: fixation and washes were done with 0.4% Triton X-100; the fixation was done for 50 minutes at room temperature; and the organoids were blocked for >1 hour with PBS with 0.4% Triton X-100 and 5% BSA before incubation with primary antibodies.
Whole-mount immunostaining was done with anti-poly-ubiquitin (FK2; Enzo Life Sciences #BML-PW8810-0100) and anti-p62/SQSTM1 antibodies (Cell Signaling Technologies, #5114). F-actin and nuclei were stained with Alexa635-conjugated phalloidin (1:100; Invitrogen) and DAPI (1 μg/mL), respectively. Organoids were mounted on slides with spacers and imaged with a Zeiss LSM 880 confocal microscope. In all cases, the peripheral regions of organoids were imaged. Image analysis was done with NIH ImageJ 1,50i.
Cloning and Fly Transgenesis
The coding sequences of Drosophila CG4666, CG8093, Amyrel, Amyrel-GFP-flag, Ag5r2, Amy-d, and Amy-p were generated as synthetic minigenes and cloned into the pUASTattB vector with the EcoRI-HF and KpnI-HF restriction enzymes. These plasmids were then injected into attP40 and/or attP2 by using the site-specific phiC31 integrase (Markstein et al., 2008) to generate transgenic flies.
Amylase Activity Assay
Colorimetric detection of amylase activity was performed by using the Amylase Activity Assay kit (Sigma-Aldrich, Cat #MAK009). In brief, 5 flies were decapitated and placed on ice into a 1.5 mL eppendorf tube. The decapitated bodies (to avoid interference from eye pigments) were homogenized in 150 μL of Amylase Assay Buffer (AAB). The homogenate was collected after high-speed centrifugation for 10 minutes. For this assay, 5 μL of the body homogenate or standard was added to a well in a 96-well plate and brought up to 50 μL with AAB or water, respectively. A master reaction mix was made to conduct a colorimetric assay, and 100 μL was added to each well. The plate was mixed well using a horizontal shaker. After 2-3 minutes the absorbance was measured at 405 nm with a Tecan Infinite 200 Pro. The plate was incubated at 25°C and the absorbance was measured at 405 nm every 5 minutes until the value of the most active sample exceeded the value of the highest standard.
Maltose Assays
To avoid interference from eye pigments (Al-Anzi and Zinn, 2010), flies with different eye colors (n=10/biological replicate) were decapitated and homogenized with a NextAdvance bullet blender and 0.5-mm zirconium beads in 100 μL of maltose assay buffer (MAB). Body homogenates were then collected after centrifugation at maximum speed for 10 minutes to remove cuticle debris. Maltose assays in Fig. 6B were done with heads (n=10/replicate) from flies with the same eye color.
Subsequently, the quantification of maltose concentration was done with the maltose assay kit (Sigma-Aldrich, #MAK019), according to manufacturer’s instructions. In brief, body homogenates were diluted 1:40 in MAB. 2.5 μL of the sample or of the standard were added per well in a 96-well plate. Then, 1 μL of α-D-Glucosidase was added to standards and samples to estimate maltose levels based on its degradation to glucose. In parallel, 1 μL of MAB was added to duplicate samples to estimate glucose background levels in the absence of glucosidase treatment. Subsequently, 46.5 μL of reaction mix (44.5 μL MAB, 1 μL maltose probe, 1 μL maltose enzyme mix) were added to each well. The plate was then incubated at 37°C shielded from light. After 1 hour, the absorbance was read at 570 nm with a Tecan Infinite 200 Pro. Blank (0 maltose standards) readings were subtracted from all readings. Maltose levels were calculated from the standard curve after subtracting the absorbance read in the absence of glucosidase treatment, which accounts for background glucose levels, from the absorbance read in duplicate samples treated with glucosidase.
Proteasome Assays
For each biological replicate, 10 thoraces or heads (as indicated) were collected and homogenized in 100 μL of PBS with a NextAdvance bullet blender and 0.5-mm zirconium beads. The homogenate was collected after centrifugation at high speed for 10 minutes. Chymotrypsin-like, trypsin-like, and caspase-like proteolytic activities of the proteasome were estimated with the Proteasome-Glo 3-substrate assay system (Promega, Cat. G8531). For this assay, 25 μL of tissue homogenate was added to a well of a 96-well white solid plate (Corning). 25 μL of Proteasome-Glo Reagent (consisting of Proteasome-Glo Buffer, Luciferin Detection Reagent, and the appropriate substrate) was added to each well. The plate was then mixed on a plate shaker at room temperature shielded from light. After 10 minutes, luminescence was recorded with a Tecan Infinite 200 Pro.
Hemolymph Preparation
Hemolymph was prepared from >30 flies, using standard procedures (Geminard et al., 2009; Robles-Murguia et al., 2020). Specifically, flies were decapitated, placed in a 0.5-mL eppendorf tube, centrifuged for 6 min at 1500 g, and the hemolymph collected in an underlying tube at 4°C. Equal volumes of hemolymph from all conditions were mixed with SDS-containing blue loading buffer with protease inhibitors, denatured by incubation at 95°C for 5 minutes, and used for SDS-PAGE and western blots.
Standard Western Blot Analyses
Standard western blot analyses were done as previously described according to routine protocols (Hunt et al., 2015). For the analysis of CRYAB protein levels, human organoids were homogenized in RIPA buffer, analyzed by SDS-PAGE, and the membranes probed with anti-CRYAB antibodies (Invitrogen #PA1-16950). For the detection of Amyrel-GFP-Flag, samples were homogenized in RIPA and examined with anti-GFP antibodies (Cell Signaling Technologies #2956).
Western Blots of Detergent-Soluble and Insoluble Protein Fractions from Drosophila Tissues
Western blots of detergent-soluble and insoluble fractions were obtained substantially as described before (Demontis and Perrimon, 2010). Specifically, thoraces or heads (as indicated) were dissected from 30 male flies and homogenized in 100 μL ice-cold Triton X-100 buffer (1% Triton X-100 in PBS containing protease inhibitors and phosphatase inhibitors) for 5 minutes at the highest speed. Homogenates were centrifuged at 14,000 rpm at 4°C for 10 minutes and the supernatant was collected (Triton X-100 soluble fraction). The remaining pellet was washed in 400 δL Triton X-100 buffer and centrifuged twice at 14,000 rpm for 5 minutes at 4°C. The pellet was then resuspended at room temperature in 100 μL RIPA buffer containing 8M urea and 5% SDS, centrifuged at 14,000 rpm at 4°C for 10 minutes, and the supernatant collected (Triton X-100 insoluble fraction). 6 μL of soluble/insoluble protein extracts was boiled with sample buffer containing DTT and used for SDS-PAGE. Detergent-soluble and -insoluble fractions were analyzed on 4-20% SDS-PAGE with anti-ubiquitin (Cell Signaling Technologies P4D1, #3936), anti-Ref(2)P/p62 (Abcam #ab178840), anti-Atg8/GABARAP (Abcam #ab109364), and/or anti-GFP (Cell Signaling Technologies, #2956), as indicated. Ponceau S staining, anti-α-tubulin antibodies (Cell Signaling Technologies, #2125), and/or anti-β-actin antibodies (Cell Signaling Technologies, #8457) were used as loading controls. Similar procedures were followed to obtain detergent-soluble and insoluble protein fractionations from Drosophila S2R+ cells.
Western Blots of Detergent-Soluble and Insoluble Protein Fractions from Human Cortical Organoids
Detergent-soluble and insoluble protein fractionations from human cortical organoids were prepared as described for Drosophila tissues with the following changes. Before homogenization, cortical organoids were washed with PBS to remove culture medium and pelleted by centrifugation at 2,000g for 5 minutes. 50 μL of buffer was then used for homogenization for 30 seconds, as described above. 5 μg of protein was boiled with sample buffer and DTT and used for SDS-PAGE. Western blotting was done as described above.
Western Blots of Detergent-Soluble and Insoluble Protein Fractions from HEK293 Cells
Western blots of detergent-soluble and insoluble fractions from HEK293 cells were obtained following the procedures described before for mammalian cells (Holden and Horton, 2009). Specifically, HEK293 cells were resuspended in ice-cold Triton X-100 buffer (1% TritonX-100 in PBS containing protease inhibitors and phosphatase inhibitors) by gentle pipetting. The cell suspension was centrifuged at 14,000 rpm to pellet the cells. Subsequently, the supernatant was resuspended (Triton X-100 soluble fraction), and the cell pellet was washed twice in ice cold Triton X-100 buffer and resuspended in urea buffer (RIPA buffer with 7M Urea and 100U/mL Benzonase) and allowed to sit at room temperature for 15-20 minutes to allow digestion of genomic DNA. After which, SDS solution (to a final concentration of 1%) was added. The samples were homogenized (without beads) at maximum speed for 30 seconds. The samples were then centrifuged at 14000 rpm for 10 minutes to pellet any remaining cell debris. The resulting supernatant is the Triton X-100 insoluble extract. 5 μg of protein was boiled with sample buffer and DTT and used for SDS-PAGE. Western blotting was done as described above.
Startle-Induced Negative Geotaxis
Startle-induced negative geotaxis was done as previously described (Demontis and Perrimon, 2010; Hunt et al., 2019a; Rhodenizer et al., 2008). Specifically, fly tubes were tapped and the number of flies that reached the top of the vial was scored after 20 seconds. For these experiments, male flies aged at 29°C were analyzed at different time points.
Analysis of Pathogenic Huntingtin Aggregates
Pathogenic Huntingtin-polyQ72-GFP protein aggregates (Zhang et al., 2010) were imaged with an epifluorescence ZEISS SteREO Discovery.V12 microscope at a specific exposure time and consistent settings. The acquired gray scale images were then analyzed in an automated manner to determine the number and/or total area of protein aggregates (Huntingtin-polyQ72-GFP speckles) by using Cell Profiler 3.0.0 (cellprofiler.org). This analysis was done with male flies after aging at 25°C for 30 days, and/or aging at 29°C for 20 days (the same conditions were applied to all the samples and the respective controls in any given experiment).
Analysis of Neurodegeneration Induced by TauV337M
Eyes of flies with expression of pathogenic tauV337M (human 2N4R MAPTV337M) in the retina were imaged with a ZEISS SteREO Discovery.V12 microscope. This analysis was done with female flies after aging at 29°C for 30 days. Expression of the tauV337M transgene was monitored by qRT-PCR from heads to ensure that the phenotypic changes observed in retinal pathology upon modulation of Amyrel and Slc45-1 are not due to changes in tauV337M expression.
qRT-PCR
qRT-PCR was performed as previously described (Demontis et al., 2014; Hunt et al., 2015). Total RNA was extracted with the TRIzol reagent (Life Technologies) from human cells, organoids, and Drosophila heads or thoraces (from >30 male flies/replicate), followed by reverse transcription with the iScript cDNA synthesis kit (Bio-Rad). qRT-PCR was performed with SYBR Green and a CFX96 apparatus (Bio-Rad). Three biological replicates were used for each genotype and time point. α-Tubuliin84B was used as a normalization reference for Drosophila qRT-PCR experiments whereas HPRT served as reference for qRT-PCRs with human samples. The comparative CT method was used for relative quantitation of mRNA levels.
For skeletal muscle-specific interventions, qRT-PCRs are done from thoraces to detect the mRNA levels of target genes in skeletal muscle, and (when specified) from heads to detect non-autonomous effects in head tissues stemming from muscle-specific interventions. For brain and retina-specific genetic interventions, qRT-PCRs are done from heads.
Table S1 (titled “qRT-PCR oligo sequences”) provides information on the primer sequences used for qRT-PCR.
RNA-Sequencing of Drosophila Tissues
As specified in the text, total RNA was extracted as described above from Drosophila heads, which consist primarily of brains and retinas, or Drosophila thoraces, which consist of skeletal muscle. Three or more biological replicates were prepared for RNA-seq with the TruSeq stranded mRNA library preparation kit (Illumina) and sequenced on the Illumina HiSeq 4000 platform, with 6 samples in each lane. Multiplexing was done on a per flowcell basis. Around 100 million reads were obtained for each sample. FASTQ sequences derived from mRNA paired-end 100-bp sequences were mapped to the Drosophila melanogaster genome (BDGP5 release 75) with the STAR aligner (version 2.5.3a; (Dobin et al., 2013)).
Transcripts were counted using HTSeq (version 0.6.1p1; (Anders et al., 2015)) based on the BDGP5 GTF release 75. Log2(FPKM) values were calculated and imported into Partek Genomic Suite 6.6 (www.partek.com/partek-genomics-suite/).
Read counts were imported into R 3.2.3, TMM normalized (Robinson et al., 2010), and fitted by voom to linear models (Law et al., 2014). Statistical tests and false discovery rates were derived from contrasts and refined with the eBayes function as implemented in the limma package in R 3.2.3 (Ritchie et al., 2015). The gene sets were analyzed by DAVID (Database for Annotation Visualization and Integrated Discovery; (Huang da et al., 2009)) to identify enriched functional classes of genes.
RNA-Sequencing of Human Cortical Brain Organoids
RNA sequencing libraries for each sample were prepared with 1 μg total RNA using the Illumina TruSeq RNA Sample Prep v2 Kit per the manufacturer’s instructions, and sequencing was completed on the Illumina HiSeq 2000. The 100 bp paired-end reads were trimmed, filtered against quality (Phred-like Q20 or greater) and length (50 bp or longer), and aligned to a human reference sequence GRCh38 (UCSC hg38), using CLC Genomics Workbench v12.0.1 (Qiagen). For gene expression comparisons, we obtained the TPM (transcript per million) counts from the RNA-Seq Analysis tool. A total of 19,749 genes with TPM>1 in at least one sample were used for the principal component analysis (PCA) and the gene expression analysis was performed using the non-parametric ANOVA using the Kruskal-Wallis and Dunn’s tests on log-transformed TPM counts between three replicates of each experimental group, implemented in Partek Genomics Suite v7.0 software (Partek Inc.). The expression of a gene was considered significantly different if the adjusted P-value was less than 0.05 and the expression change was more than two folds in at least one of the group comparisons. The z-scores of 2,095 significantly regulated genes were calculated and hierarchically clustered in a heat map, using correlation distance measure, implemented in Spotfire v7.5.0 software (TIBCO).
Amyrel Gene Promoter Analysis
Amyrel promoter analysis was initially done with Genomatix (www.genomatix.de), using the 1 kb proximal region (chr2R:16652699.. 16653699 from dm6). Subsequent analyses further defined the presence of C/EBP binding sites in the Amyrel promoter region by using FIMO (--thresh 1e-3 --motif-pseudo 0.0001 --no-q value) from MEME suite (version 4.11.3) to scan the collection of C/EBP-related motifs (from TRANSFAC) on the Amyrel gene promoter.
Multielectrode Array Recording of Human Cortical Brain Organoids
Spontaneous neuronal activity of ~60-day-old (D60) cortical organoids was recorded on the Axion Maestro Edge multielectrode array system. To this purpose, we optimized a method for plating and recording to ensure maximum contact with electrodes. Specifically, growth factor reduced matrigel (Corning) was diluted 1:3 in chilled media containing BrainPhys and 10% ES-FBS and kept on ice. D60 cortical organoids were first sliced in half using a pair of fine tweezers. Each half was then plated onto one well of a 24-well Cytoview plate (Axion Biosystems), where each well contained a grid of 16 electrodes. After placing the organoid slice onto a dry and empty well by using a sterile flat spatula, 15 μL of the chilled and diluted matrigel was added onto the slice. A P10 tip was used to position the slice to the center of the well over the grid of electrodes. The plates were left at 37°C for 3h for the matrigel to solidify over the organoid slices. After 3h, 500 μL of BrainPhys media supplemented with 100X N2 supplement, 50X B27 supplement without Vitamin A, 10 ng/ml BDNF and 10 ng/mL GDNF was added slowly to each well. Every three days, 300 mL media in each well was replaced with fresh media three days and six days after plating. Seven days after plating, the plates were treated with either control or maltose-containing media; all treatment conditions were included in each plate. Three days after treatment, spontaneous activity was recorded from each plate. The plates were then subjected to heat shock for 7h at 41.5°C. Spontaneous activity in each plate was recorded again after the heat shock. This recording was repeated after 17h and 43h from recovery. Data was analyzed using the Neural Metric tool (Axion Biosystems).
QUANTIFICATION AND STATISTICAL ANALYSIS
All experiments were performed with biological triplicates unless otherwise indicated. The unpaired two-tailed Student’s t-test was used to compare the means of two independent groups to each other. One-way ANOVA with Tukey’s post hoc test was used for multiple comparisons of more than two groups of normally distributed data. The “n” for each experiment can be found indicated in the figures and/or figure legends and represents independently generated samples for all experiments, including cell populations/wells for in vitro assays, and individual and/or batches of flies and fly tissues for in vivo experiments. Bar graphs present the mean ± SEM or ± SD, as indicated in the figure legend. Throughout the figures, asterisks indicate the significance of the p value: *p<0.05; **p<0.01; ***p<0.001. A significant result was defined as p<0.05. Statistical analyses were done with Excel and GraphPad Prism.
Supplementary Material
Table S1. qRT-PCR oligo sequences, Related to Star Methods.
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Rabbit anti-poly-ubiquitin (FK2) | Enzo Life Sciences | BML-PW8810-0100 |
| Rabbit anti-Ref(2)P/p62 | Abcam | 178840 |
| Rabbit anti-p62/SQSTM1 | Cell Signaling Technologies | 5114 |
| Mouse anti-ubiquitin (P4D1) | Santa Cruz | Sc-8017 |
| Rabbit anti-alpha-tubulin (11H10) | Cell Signaling Technologies | 2125S |
| Rabbit anti-Atg8/GABARAP | Abcam | Ab109364 |
| Rabbit anti-K48-poly-ubiquitin (D9D5), linkage specific | Cell Signaling Technologies | 8081 |
| Rabbit anti-GFP (D5.1) | Cell Signaling Technologies | 2956 |
| Rabbit anti-CRYAB | Invitrogen | PA1-16950 |
| Rabbit anti-beta-actin | Cell Signaling Technologies | 8457 |
| AlexaFluor555 anti-mouse | Life Technologies | A28180 |
| AlexaFluor488 anti-rabbit | Life Technologies | A11034 |
| AlexaFluor488 anti-mouse | Life Technologies | A11001 |
| AlexaFluor555 anti-rabbit | Life Technologies | A21428 |
| AlexaFluor488 anti-mouse IgG1 | Life Technologies | A21121 |
| AlexaFluor555 anti-mouse IgM | Life Technologies | A21426 |
| AlexaFluor647 anti-rat | Life Technologies | A21247 |
| AlexaFluor635 Phalloidin | Life Technologies | A22284 |
| Anti-mouse IgG, HRP-linked | Cell Signaling Technologies | 7076S |
| Anti-rabbit IgG, HRP-linked | Cell Signaling Technologies | 7074S |
| Chemicals, Peptides, and Recombinant Proteins | ||
| DAPI | Roche | 10236276001 |
| Trizol | Ambion | 15596018 |
| IQ Sybr Green supermix | Bio-Rad | 170-8885 |
| 6-well plates | Corning | REF3516 |
| 96-well PCR plates | Biorad | HSP9601 |
| Transparent 96-well plates | Corning | REF3599 |
| Lipofectamine 2000 | Invitrogen | 11668027 |
| Schneider’s cell culture medium | Thermo Scientific | 21720024 |
| Maltose | Sigma-Aldrich | M5895 |
| Glucose | Sigma-Aldrich | G8270 |
| Cellobiose | Sigma-Aldrich | C7252 |
| Porcine alpha-Amylase | MP Biomedicals | 191239 |
| High glucose DMEM, with glutamax | Gibco | 10566016 |
| Fetal bovine serum | Gibco | 10437-028 |
| OptiMEM | Gibco | 31985-070 |
| Penicillin streptomycin | Gibco | 15140122 |
| PBS | Gibco | 10010023 |
| Blue loading buffer pack | Cell Signaling | 7722 |
| Precision Plus protein standard | Bio-Rad | 1610374 |
| 4-20% Mini-PROTEAN TGX pre-cast gels | Bio-Rad | 4561096 |
| Immobilon-P PVDF membrane | Millipore | IPVH00010 |
| Ponceau S | Sigma-Aldrich | P7170-1L |
| 16% Paraformaldehyde | Electron Microscopy Sciences | 15710 |
| Benzonase nuclease | Sigma-Aldrich | E1014 |
| MG132 | Abcam | ab141003 |
| Critical Commercial Assays | ||
| Pierce BCA protein assay kit | Thermo Scientific | 23225 |
| Maltose assay kit | Sigma-Aldrich | MAK019 |
| iScript reverse transcriptase | Bio-Rad | 1708840 |
| Amylase activity kit | Sigma-Aldrich | MAK009-1KT |
| Proteasome-Glo Assays (3-substrate system) | Promega | G8531 |
| Deposited Data | ||
| RNA-seq data from Drosophila muscle with proteasome stress | This paper | Gene Expression Omnibus, GSE149799 |
| RNA-seq data from head tissues from Drosophila with muscle-specific Amyrel overexpression | This paper | Gene Expression Omnibus, GSE149799 |
| RNA-seq data from cortical organoids treated with maltose/heat shock, and controls | This paper | Gene Expression Omnibus, GSE149799 |
| Experimental Models: Cell Lines | ||
| Human: HEK293T cells | ATCC | CRL-3216 |
| Human: H9/WA09 cells | WiCell | NIHhESC-10-0062 |
| Drosophila: S2R+ cells | DRSC | N/A |
| Experimental Models: Drosophila Strains | ||
| w1118; Mhc-Gal4 | Demontis Lab collection | Demontis et al. 2010 |
| MhcF3-Gal4 | Demontis Lab collection | Gajewski et al. 2010 |
| Act88F-GS-Gal4 | Demontis Lab collection | Robles-Murguia et al. 2019 |
| w1118 (+) | Demontis Lab collection | N/A |
| Slc45-2null/null (lovit1/1) | Bloomington | #83374 |
| UAS-cherryRNAi | Bloomington | #35785 |
| UAS-whiteRNAi | Bloomington | #33623 |
| UAS-whiteRNAi | VDRC | #30033 |
| UAS-vermillionRNAi | NIG, Japan | #2155R-2 |
| UAS-vermillionRNAi | VDRC | #3349 |
| UAS-GFP RNAi | Bloomington | #41552 |
| UAS-Prosβ1RNAi | Bloomington | #34824 |
| UAS-Prosβ5RNAi | Bloomington | #34810 |
| UAS-Ter94RNAi | VDRC | #24354 |
| UAS-CG4666 | This paper | N/A |
| UAS-CG8093 | This paper | N/A |
| UAS-Amyrel | This paper | N/A |
| UAS-Amyrel-GFP-FLAG | This paper | N/A |
| UAS-Amy-d | This paper | N/A |
| UAS-Amy-p | This paper | N/A |
| UAS-Ag5r2 | This paper | N/A |
| UAS-Akh | Bloomington | #27343 |
| UAS-to | Bloomington | #27646 |
| UAS-mCherry | Bloomington | #35787 |
| UAS-yellowRNAi | NIG, Japan | 3757R-5 |
| UAS-AmyrelRNAi | NIG, Japan | 8221R-4; 8221R-1 |
| UAS-CG8093RNAi | VDRC | #19561 |
| UAS-AkhRNAi | Bloomington | #34960 |
| UAS-CG4666RNAi#1 | Bloomington | #55939 |
| UAS-CG4666RNAi#2 | VDRC | #109818 |
| UAS-slbo | FlyORF | F0038 (chrIII); F003286 (chrII) |
| UAS-slboRNAi | Bloomington | #53309, #27043 |
| UAS-CG6272RNAi | Bloomington | #29331, #33652 |
| GMR-Gal4, UAS-Htt-Q72-GFP | From Dr. Sheng Zhang, UTHealth | N/A |
| GMR-Htt-Q120 | Bloomington | #8533 |
| GMR-tauV337M (human 2N4R MAPTV337M) | Bloomington | #51367 |
| elav-Gal4 | Bloomington | #8765 |
| elav-GS-Gal4 | From Dr. Linda Partridge, UCL | N/A |
| UAS-Slc45-1RNAi#1 | VDRC | #5174 |
| UAS-Slc45-1RNAi#2 | VDRC | #5172 |
| UAS-Tret1-1 RNAi | VDRC | #8126 |
| UAS-Slc45-2/lovit RNAi | Bloomington | #40876 |
| UAS-PsaRNAi | Bloomington | #34674 |
| UAS-Prosβ2RNAi | Bloomington | #67363 |
| UAS-Prosβ4RNAi | Bloomington | #32390 |
| UAS-Prosα3RNAi | Bloomington | #77145 |
| UAS-Prosα4RNAi | Bloomington | #65161 |
| UAS-Prosβ5RNAi | Bloomington | #34786 |
| UAS-Prosα6RNAi | Bloomington | #53974 |
| UAS-Hsp23 | Bloomington | #30541 |
| UAS-Hsp23 RNAi | Bloomington | #80709, #82961 |
| UAS-CG9733 RNAi | Bloomington | #55149 |
| UAS-CG9733 RNAi | VDRC | #16546 |
| UAS-Hsp68 RNAi | VDRC | #35007 |
| UAS-CG7142 RNAi | VDRC | #5911 |
| Oligonucleotides | ||
| ON-TARGET Plus Non-targeting siRNA pool | Dharmacon | D-001810-10-05 |
| ON-TARGET Plus SLC45A3 siRNA pool | Dharmacon | L-004231-01-0005 |
| ON-TARGET Plus SLC45A4 siRNA pool | Dharmacon | L-025844-02-0005 |
| Primers for qRT-PCR, see Table S1 | This paper | N/A |
| Recombinant DNA | ||
| pUASTattB | from Dr. Konrad Basler | N/A |
| Software and Algorithms | ||
| GraphPad Prism | Graphpad | https://www.graphpad.com/ |
| Zeiss Zen | Zeiss | https://www.zeiss.com/microscopy/us/products/microscope-software/zen.html |
| Photoshop CSX | Adobe | https://www.adobe.com/products/photoshop.html |
| ImageJ | Schneider et al., 2012 | https://imagej.nih.gov/ij/ |
| CellProfiler 3.0.0 | CellProfiler | https://cellprofiler.org/ |
| CorelDRAW 2018 | CorelDRAW | https://www.coreldraw.com/en/ |
| Genomatix Matinspector | Genomatix | https://www.genomatix.de |
| SCope | SCope | https://scope.aertslab.org |
| Aging Plasma Proteome | Aging Plasma Proteome | https://twc-stanford.shinyapps.io/aging_plasma_proteome/ |
| GENtervention | GENtervention | http://gladyshevlab.org:3838/Gentervention/ |
Highlights.
Proteasome stress in skeletal muscle induces local and systemic adaptive responses
Muscle-to-brain stress signaling preserves proteostasis in the aging brain and retina
Long-range stress signaling depends on the muscle-secreted amylase Amyrel
Amyrel increases the levels of maltose, which preserves proteostasis via chaperones
ACKNOWLEDGMENTS
Fly stocks and cell lines were provided by the ATCC, DGRC, DRSC, VDRC, NIG-Fly, the FlyORF, and the Bloomington stock centers. This work was supported by research grants to F.D. from the Ellison Medical Foundation, Glenn Foundation for Medical Research, American Federation for Aging Research, Hartwell Foundation, American Parkinson Disease Association, and the National Institute on Aging of the NIH (R01AG055532 and R56AG063806).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DECLARATION OF INTERESTS
M.R. and F.D. are named co-inventors of a pending U.S. provisional patent application based in part on the research reported in this paper.
REFERENCES
- Agudelo LZ, Femenia T, Orhan F, Porsmyr-Palmertz M, Goiny M, Martinez-Redondo V, Correia JC, Izadi M, Bhat M, Schuppe-Koistinen I, et al. (2014). Skeletal muscle PGC-1alpha1 modulates kynurenine metabolism and mediates resilience to stress-induced depression. Cell 159, 33–45. [DOI] [PubMed] [Google Scholar]
- Al-Anzi B, and Zinn K (2010). Colorimetric measurement of triglycerides cannot provide an accurate measure of stored fat content in Drosophila. PLoS One 5, e12353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alic N, Hoddinott MP, Foley A, Slack C, Piper MD, and Partridge L (2012). Detrimental effects of RNAi: a cautionary note on its use in Drosophila ageing studies. PLoS One 7, e45367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anders S, Pyl PT, and Huber W (2015). HTSeq--a Python framework to work with high-throughput sequencing data. Bioinformatics 31, 166–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arndt V, Rogon C, and Hohfeld J (2007). To be, or not to be--molecular chaperones in protein degradation. Cell Mol Life Sci 64, 2525–2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balch WE, Morimoto RI, Dillin A, and Kelly JW (2008). Adapting proteostasis for disease intervention. Science 319, 916–919. [DOI] [PubMed] [Google Scholar]
- Bao Y, Zhao T, Wang X, Qiu Y, Su M, Jia W, and Jia W (2009). Metabonomic variations in the drug-treated type 2 diabetes mellitus patients and healthy volunteers. J Proteome Res 8, 1623–1630. [DOI] [PubMed] [Google Scholar]
- Bonaldo P, and Sandri M (2013). Cellular and molecular mechanisms of muscle atrophy. Disease models & mechanisms 6, 25–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle PA, Buchman AS, Wilson RS, Leurgans SE, and Bennett DA (2009). Association of muscle strength with the risk of Alzheimer disease and the rate of cognitive decline in community-dwelling older persons. Arch Neurol 66, 1339–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byman E, Schultz N, Netherlands Brain B, Blom AM, and Wennstrom M (2019). A Potential Role for alpha-Amylase in Amyloid-beta-Induced Astrocytic Glycogenolysis and Activation. J Alzheimers Dis 68, 205–217. [DOI] [PubMed] [Google Scholar]
- Chan HY, and Bonini NM (2003). Drosophila models of polyglutamine diseases. Methods Mol Biol 217, 241–251. [DOI] [PubMed] [Google Scholar]
- Chauhan R, Chen KF, Kent BA, and Crowther DC (2017). Central and peripheral circadian clocks and their role in Alzheimer’s disease. Dis Model Mech 10, 1187–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JJ, Huang WC, and Chen CC (2005). Transcriptional regulation of cyclooxygenase-2 in response to proteasome inhibitors involves reactive oxygen species-mediated signaling pathway and recruitment of CCAAT/enhancer-binding protein delta and CREB-binding protein. Molecular biology of the cell 16, 5579–5591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claisse G, Feller G, Bonneau M, and Da Lage JL (2016). A single amino-acid substitution toggles chloride dependence of the alpha-amylase paralog amyrel in Drosophila melanogaster and Drosophila virilis species. Insect biochemistry and molecular biology 75, 70–77. [DOI] [PubMed] [Google Scholar]
- Collins GA, and Goldberg AL (2017). The Logic of the 26S Proteasome. Cell 169, 792–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delezie J, and Handschin C (2018). Endocrine Crosstalk Between Skeletal Muscle and the Brain. Front Neurol 9, 698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demontis F, and Dahmann C (2009). Characterization of the Drosophila ortholog of the human Usher Syndrome type 1G protein sans. PLoS ONE 4, e4753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demontis F, Patel VK, Swindell WR, and Perrimon N (2014). Intertissue control of the nucleolus via a myokine-dependent longevity pathway. Cell reports 7 1481–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demontis F, and Perrimon N (2010). FOXO/4E-BP signaling in Drosophila muscles regulates organism-wide proteostasis during aging. Cell 143, 813–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demontis F, Piccirillo R, Goldberg AL, and Perrimon N (2013). The influence of skeletal muscle on systemic aging and lifespan. Aging Cell 12, 943–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshmukh AS, Cox J, Jensen LJ, Meissner F, and Mann M (2015a). Secretome Analysis of Lipid-Induced Insulin Resistance in Skeletal Muscle Cells by a Combined Experimental and Bioinformatics Workflow. J Proteome Res 14, 4885–4895. [DOI] [PubMed] [Google Scholar]
- Deshmukh AS, Murgia M, Nagaraj N, Treebak JT, Cox J, and Mann M (2015b). Deep proteomics of mouse skeletal muscle enables quantitation of protein isoforms, metabolic pathways, and transcription factors. Mol Cell Proteomics 14, 841–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, and Gingeras TR (2013). STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas PM, and Dillin A (2010). Protein homeostasis and aging in neurodegeneration. J Cell Biol 190, 719–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droujinine IA, and Perrimon N (2016). Interorgan Communication Pathways in Physiology: Focus on Drosophila. Annu Rev Genet 50, 539–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajewski KM, and Schulz RA (2010). CF2 represses Actin 88F gene expression and maintains filament balance during indirect flight muscle development in Drosophila. PLoS One 5, e10713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gates AC, Bernal-Mizrachi C, Chinault SL, Feng C, Schneider JG, Coleman T, Malone JP, Townsend RR, Chakravarthy MV, and Semenkovich CF (2007). Respiratory uncoupling in skeletal muscle delays death and diminishes age-related disease. Cell Metab 6, 497–505. [DOI] [PubMed] [Google Scholar]
- Geier E, Pfeifer G, Wilm M, Lucchiari-Hartz M, Baumeister W, Eichmann K, and Niedermann G (1999). A giant protease with potential to substitute for some functions of the proteasome. Science 283, 978–981. [DOI] [PubMed] [Google Scholar]
- Geminard C, Rulifson EJ, and Leopold P (2009). Remote control of insulin secretion by fat cells in Drosophila. Cell Metab 10, 199–207. [DOI] [PubMed] [Google Scholar]
- Glas R, Bogyo M, McMaster JS, Gaczynska M, and Ploegh HL (1998). A proteolytic system that compensates for loss of proteasome function. Nature 392, 618–622. [DOI] [PubMed] [Google Scholar]
- Hanna J, Meides A, Zhang DP, and Finley D (2007). A ubiquitin stress response induces altered proteasome composition. Cell 129, 747–759. [DOI] [PubMed] [Google Scholar]
- Hattori T, Ohoka N, Inoue Y, Hayashi H, and Onozaki K (2003). C/EBP family transcription factors are degraded by the proteasome but stabilized by forming dimer. Oncogene 22, 1273–1280. [DOI] [PubMed] [Google Scholar]
- Hokari S, Miura K, Koyama I, Kobayashi M, Matsunaga T, Iino N, and Komoda T (2003). Expression of alpha-amylase isozymes in rat tissues. Comparative biochemistry and physiology Part B, Biochemistry & molecular biology 135, 63–69. [DOI] [PubMed] [Google Scholar]
- Holden P, and Horton WA (2009). Crude subcellular fractionation of cultured mammalian cell lines. BMC Res Notes 2, 243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu JL, Todhunter ME, LaBarge MA, and Gartner ZJ (2018). Opportunities for organoids as new models of aging. The Journal of cell biology 217, 39–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang da W, Sherman BT, and Lempicki RA (2009). Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 4, 44–57. [DOI] [PubMed] [Google Scholar]
- Hungness ES, Robb BW, Luo GJ, Pritts TA, Hershko DD, and Hasselgren PO (2002). Proteasome inhibitors activate the transcription factors C/EBP-beta and delta in human intestinal epithelial cells. Biochemical and biophysical research communications 290, 469–474. [DOI] [PubMed] [Google Scholar]
- Hunt LC, and Demontis F (2013). Whole-mount immunostaining of Drosophila skeletal muscle. Nat Protoc 8, 2496–2501. [DOI] [PubMed] [Google Scholar]
- Hunt LC, Jiao J, Wang YD, Finkelstein D, Rao D, Curley M, Robles-Murguia M, Shirinifard A, Pagala VR, Peng J, et al. (2019a). Circadian gene variants and the skeletal muscle circadian clock contribute to the evolutionary divergence in longevity across Drosophila populations. Genome Res 29, 1262–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt LC, Stover J, Haugen B, Shaw TI, Li Y, Pagala VR, Finkelstein D, Barton ER, Fan Y, Labelle M, et al. (2019b). A Key Role for the Ubiquitin Ligase UBR4 in Myofiber Hypertrophy in Drosophila and Mice. Cell reports 28, 1268–1281 e1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt LC, Xu B, Finkelstein D, Fan Y, Carroll PA, Cheng PF, Eisenman RN, and Demontis F (2015). The glucose-sensing transcription factor MLX promotes myogenesis via myokine signaling. Genes Dev 29, 2475–2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao J, and Demontis F (2017). Skeletal muscle autophagy and its role in sarcopenia and organismal aging. Curr Opin Pharmacol 34, 1–6. [DOI] [PubMed] [Google Scholar]
- Kadoshima T, Sakaguchi H, Nakano T, Soen M, Ando S, Eiraku M, and Sasai Y (2013). Self-organization of axial polarity, inside-out layer pattern, and species-specific progenitor dynamics in human ES cell-derived neocortex. Proc Natl Acad Sci U S A 110, 20284–20289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan F, and Guy CL (2004). beta-Amylase induction and the protective role of maltose during temperature shock. Plant physiology 135, 1674–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan F, and Guy CL (2005). RNA interference of Arabidopsis beta-amylase8 prevents maltose accumulation upon cold shock and increases sensitivity of PSII photochemical efficiency to freezing stress. Plant J 44, 730–743. [DOI] [PubMed] [Google Scholar]
- Keipert S, Ost M, Chadt A, Voigt A, Ayala V, Portero-Otin M, Pamplona R, Al-Hasani H, and Klaus S (2013). Skeletal muscle uncoupling-induced longevity in mice is linked to increased substrate metabolism and induction of the endogenous antioxidant defense system. Am J Physiol Endocrinol Metab 304, E495–E506. [DOI] [PubMed] [Google Scholar]
- Kim KH, Jeong YT, Oh H, Kim SH, Cho JM, Kim YN, Kim SS, Kim do H, Hur KY, Kim HK, et al. (2013). Autophagy deficiency leads to protection from obesity and insulin resistance by inducing Fgf21 as a mitokine. Nat Med 19, 83–92. [DOI] [PubMed] [Google Scholar]
- Labbadia J, and Morimoto RI (2015). The biology of proteostasis in aging and disease. Annu Rev Biochem 84, 435–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalonde S, Boles E, Hellmann H, Barker L, Patrick JW, Frommer WB, and Ward JM (1999). The dual function of sugar carriers. Transport and sugar sensing. Plant Cell 11, 707–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster MA, Corsini NS, Wolfinger S, Gustafson EH, Phillips AW, Burkard TR, Otani T, Livesey FJ, and Knoblich JA (2017). Guided self-organization and cortical plate formation in human brain organoids. Nat Biotechnol 35, 659–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster MA, Renner M, Martin CA, Wenzel D, Bicknell LS, Hurles ME, Homfray T, Penninger JM, Jackson AP, and Knoblich JA (2013). Cerebral organoids model human brain development and microcephaly. Nature 501, 373–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law CW, Chen Y, Shi W, and Smyth GK (2014). voom: Precision weights unlock linear model analysis tools for RNA-seq read counts. Genome Biol 15, R29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson EC, Han MK, Sellers JT, Chrenek MA, Hanif A, Gogniat MA, Boatright JH, and Pardue MT (2014). Aerobic exercise protects retinal function and structure from light-induced retinal degeneration. J Neurosci 34, 2406–2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CS, Tee LY, Warmke T, Vinjamoori A, Cai A, Fagan AM, and Snider BJ (2004). A proteasomal stress response: pre-treatment with proteasome inhibitors increases proteasome activity and reduces neuronal vulnerability to oxidative injury. Journal of neurochemistry 91, 996–1006. [DOI] [PubMed] [Google Scholar]
- Lehallier B, Gate D, Schaum N, Nanasi T, Lee SE, Yousef H, Moran Losada P, Berdnik D, Keller A, Verghese J, et al. (2019). Undulating changes in human plasma proteome profiles across the lifespan. Nat Med 25, 1843–1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lekstrom-Himes J, and Xanthopoulos KG (1998). Biological role of the CCAAT/enhancer-binding protein family of transcription factors. The Journal of biological chemistry 273, 28545–28548. [DOI] [PubMed] [Google Scholar]
- Levy-Sakin M, Berger O, Feibish N, Sharon N, Schnaider L, Shmul G, Amir Y, Buzhansky L, and Gazit E (2014). The influence of chemical chaperones on enzymatic activity under thermal and chemical stresses: common features and variation among diverse chemical families. PloS one 9, e88541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YC, Boone M, Meuris L, Lemmens I, Van Roy N, Soete A, Reumers J, Moisse M, Plaisance S, Drmanac R, et al. (2014). Genome dynamics of the human embryonic kidney 293 lineage in response to cell biology manipulations. Nat Commun 5, 4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipinszki Z, Klement E, Hunyadi-Gulyas E, Medzihradszky KF, Markus R, Pal M, Deak P, and Udvardy A (2013). A novel interplay between the ubiquitin-proteasome system and serine proteases during Drosophila development. The Biochemical journal 454, 571–583. [DOI] [PubMed] [Google Scholar]
- Lourenco MV, Frozza RL, de Freitas GB, Zhang H, Kincheski GC, Ribeiro FC, Goncalves RA, Clarke JR, Beckman D, Staniszewski A, et al. (2019). Exercise-linked FNDC5/irisin rescues synaptic plasticity and memory defects in Alzheimer’s models. Nat Med 25, 165–1 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundgren J, Masson P, Mirzaei Z, and Young P (2005). Identification and characterization of a Drosophila proteasome regulatory network. Molecular and cellular biology 25, 4662–4675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmendal A, Overgaard J, Bundy JG, Sorensen JG, Nielsen NC, Loeschcke V, and Holmstrup M (2006). Metabolomic profiling of heat stress: hardening and recovery of homeostasis in Drosophila. Am J Physiol Regul Integr Comp Physiol 291, R205–212. [DOI] [PubMed] [Google Scholar]
- Markstein M, Pitsouli C, Villalta C, Celniker SE, and Perrimon N (2008). Exploiting position effects and the gypsy retrovirus insulator to engineer precisely expressed transgenes. Nat Genet 40, 476–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire SE, Roman G, and Davis RL (2004). Gene expression systems in Drosophila: a synthesis of time and space. Trends Genet 20, 384–391. [DOI] [PubMed] [Google Scholar]
- Mensink MA, Frijlink HW, van der Voort Maarschalk K, and Hinrichs WL (2017). How sugars protect proteins in the solid state and during drying (review): Mechanisms of stabilization in relation to stress conditions. European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik eV 114, 288–295. [DOI] [PubMed] [Google Scholar]
- Meyer H, Vitavska O, and Wieczorek H (2011). Identification of an animal sucrose transporter. J Cell Sci 124, 1984–1991. [DOI] [PubMed] [Google Scholar]
- Mitra S, and Ryoo HD (2019). The unfolded protein response in metazoan development. J Cell Sci 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon HY, Becke A, Berron D, Becker B, Sah N, Benoni G, Janke E, Lubejko ST, Greig NH, Mattison JA, et al. (2016). Running-Induced Systemic Cathepsin B Secretion Is Associated with Memory Function. Cell Metab 24, 332–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow G, Samson M, Michaud S, and Tanguay RM (2004). Overexpression of the small mitochondrial Hsp22 extends Drosophila life span and increases resistance to oxidative stress. FASEB J 18, 598–599. [DOI] [PubMed] [Google Scholar]
- Nacharaju P, Lewis J, Easson C, Yen S, Hackett J, Hutton M, and Yen SH (1999). Accelerated filament formation from tau protein with specific FTDP-17 missense mutations. FEBS Lett 447, 195–199. [DOI] [PubMed] [Google Scholar]
- Nezis IP, Simonsen A, Sagona AP, Finley K, Gaumer S, Contamine D, Rusten TE, Stenmark H, and Brech A (2008). Ref(2)P, the Drosophila melanogaster homologue of mammalian p62, is required for the formation of protein aggregates in adult brain. J Cell Biol 180, 1065–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterwalder T, Yoon KS, White BH, and Keshishian H (2001). A conditional tissue-specific transgene expression system using inducible GAL4. Proc Natl Acad Sci U S A 98, 12596–12601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott S, Vishnivetskaya A, Malmendal A, and Crowther DC (2016). Metabolic changes may precede proteostatic dysfunction in a Drosophila model of amyloid beta peptide toxicity. Neurobiology of aging 41, 39–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overgaard J, Malmendal A, Sorensen JG, Bundy JG, Loeschcke V, Nielsen NC, and Holmstrup M (2007). Metabolomic profiling of rapid cold hardening and cold shock in Drosophila melanogaster. J Insect Physiol 53, 1218–1232. [DOI] [PubMed] [Google Scholar]
- Owusu-Ansah E, Song W, and Perrimon N (2013). Muscle mitohormesis promotes longevity via systemic repression of insulin signaling. Cell 155, 699–71 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, Kim W, Tian G, Gygi SP, and Finley D (2011). Structural defects in the regulatory particle-core particle interface of the proteasome induce a novel proteasome stress response. The Journal of biological chemistry 286, 36652–36666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen BK (2019). Physical activity and muscle-brain crosstalk. Nat Rev Endocrinol 15, 383–392. [DOI] [PubMed] [Google Scholar]
- Pedersen KS, Kristensen TN, Loeschcke V, Petersen BO, Duus JO, Nielsen NC, and Malmendal A (2008). Metabolomic signatures of inbreeding at benign and stressful temperatures in Drosophila melanogaster. Genetics 180, 1233–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccirillo R, and Goldberg AL (2012). The p97/VCP ATPase is critical in muscle atrophy and the accelerated degradation of muscle proteins. The EMBO journal 31, 3334–3350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickart CM, and Fushman D (2004). Polyubiquitin chains: polymeric protein signals. Curr Opin Chem Biol 8, 610–616. [DOI] [PubMed] [Google Scholar]
- Pluvinage JV, and Wyss-Coray T (2020). Systemic factors as mediators of brain homeostasis, ageing and neurodegeneration. Nat Rev Neurosci 21, 93–102. [DOI] [PubMed] [Google Scholar]
- Psychogios N, Hau DD, Peng J, Guo AC, Mandal R, Bouatra S, Sinelnikov I, Krishnamurthy R, Eisner R, Gautam B, et al. (2011). The human serum metabolome. PloS one 6, e16957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian X, Nguyen HN, Song MM, Hadiono C, Ogden SC, Hammack C, Yao B, Hamersky GR, Jacob F, Zhong C, et al. (2016). Brain-Region-Specific Organoids Using Mini-bioreactors for Modeling ZIKV Exposure. Cell 165, 1238–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai M, and Demontis F (2016). Systemic Nutrient and Stress Signaling via Myokines and Myometabolites. Annu Rev Physiol 78, 85–107. [DOI] [PubMed] [Google Scholar]
- Rhodenizer D, Martin I, Bhandari P, Pletcher SD, and Grotewiel M (2008). Genetic and environmental factors impact age-related impairment of negative geotaxis in Drosophila by altering age-dependent climbing speed. Exp Gerontol 43, 739–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, and Smyth GK (2015). limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res 43, e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MD, McCarthy DJ, and Smyth GK (2010). edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robles-Murguia M, Hunt LC, Finkelstein D, Fan Y, and Demontis F (2019). Tissue-specific alteration of gene expression and function by RU486 and the GeneSwitch system. NPJ Aging Mech Dis 5, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robles-Murguia M, Rao D, Finkelstein D, Xu B, Fan Y, and Demontis F (2020). Muscle-derived Dpp regulates feeding initiation via endocrine modulation of brain dopamine biosynthesis. Genes Dev 34, 37–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson TR, Debelius JW, Thron T, Janssen S, Shastri GG, Ilhan ZE, Challis C, Schretter CE, Rocha S, Gradinaru V, et al. (2016). Gut Microbiota Regulate Motor Deficits and Neuroinflammation in a Model of Parkinson’s Disease. Cell 167, 1469–1480 e1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satake W, Nakabayashi Y, Mizuta I, Hirota Y, Ito C, Kubo M, Kawaguchi T, Tsunoda T, Watanabe M, Takeda A, et al. (2009). Genome-wide association study identifies common variants at four loci as genetic risk factors for Parkinson’s disease. Nat Genet 41, 1303–1307. [DOI] [PubMed] [Google Scholar]
- Schmidt M, and Finley D (2014). Regulation of proteasome activity in health and disease. Biochimica et biophysica acta 1843, 13–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster CM, Davis GW, Fetter RD, and Goodman CS (1996). Genetic dissection of structural and functional components of synaptic plasticity. II. Fasciclin II controls presynaptic structural plasticity. Neuron 17, 655–667. [DOI] [PubMed] [Google Scholar]
- Seibenhener ML, Babu JR, Geetha T, Wong HC, Krishna NR, and Wooten MW (2004). Sequestosome 1/p62 is a polyubiquitin chain binding protein involved in ubiquitin proteasome degradation. Mol Cell Biol 24, 8055–8068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw G, Morse S, Ararat M, and Graham FL (2002). Preferential transformation of human neuronal cells by human adenoviruses and the origin of HEK 293 cells. FASEB J 16, 869–871. [DOI] [PubMed] [Google Scholar]
- Shim M, and Smart RC (2003). Lithium stabilizes the CCAAT/enhancer-binding protein alpha (C/EBPalpha) through a glycogen synthase kinase 3 (GSK3)-independent pathway involving direct inhibition of proteasomal activity. J Biol Chem 278, 19674–19681. [DOI] [PubMed] [Google Scholar]
- Tarkowski LP, and Van den Ende W (2015). Cold tolerance triggered by soluble sugars: a multifaceted countermeasure. Front Plant Sci 6, 203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor RC, Berendzen KM, and Dillin A (2014). Systemic stress signalling: understanding the cell non-autonomous control of proteostasis. Nature reviews Molecular cell biology 15, 211–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tower J (2009). Hsps and aging. Trends Endocrinol Metab 20, 216–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsakiri EN, Gumeni S, Vougas K, Pendin D, Papassideri I, Daga A, Gorgoulis V, Juhasz G, Scorrano L, and Trougakos IP (2019). Proteasome dysfunction induces excessive proteome instability and loss of mitostasis that can be mitigated by enhancing mitochondrial fusion or autophagy. Autophagy, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsvetkov P, Mendillo ML, Zhao J, Carette JE, Merrill PH, Cikes D, Varadarajan M, van Diemen FR, Penninger JM, Goldberg AL, et al. (2015). Compromising the 19S proteasome complex protects cells from reduced flux through the proteasome. eLife 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyshkovskiy A, Bozaykut P, Borodinova AA, Gerashchenko MV, Ables GP, Garratt M, Khaitovich P, Clish CB, Miller RA, and Gladyshev VN (2019). Identification and Application of Gene Expression Signatures Associated with Lifespan Extension. Cell Metab 30, 573–593 e578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Boom J, Wolf M, Weimann L, Schulze N, Li F, Kaschani F, Riemer A, Zierhut C, Kaiser M, Iliakis G, et al. (2016). VCP/p97 Extracts Sterically Trapped Ku70/80 Rings from DNA in Double-Strand Break Repair. Molecular cell 64, 189–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Oosten-Hawle P, Porter RS, and Morimoto RI (2013). Regulation of organismal proteostasis by transcellular chaperone signaling. Cell 153, 1366–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitavska O, and Wieczorek H (2013). The SLC45 gene family of putative sugar transporters. Mol Aspects Med 34, 655–660. [DOI] [PubMed] [Google Scholar]
- Voss MW, Vivar C, Kramer AF, and van Praag H (2013). Bridging animal and human models of exercise-induced brain plasticity. Trends in cognitive sciences 17, 525–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang EW, Kessler BM, Borodovsky A, Cravatt BF, Bogyo M, Ploegh HL, and Glas R (2000). Integration of the ubiquitin-proteasome pathway with a cytosolic oligopeptidase activity. Proceedings of the National Academy of Sciences of the United States of America 97, 9990–9995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Gu BJ, Masters CL, and Wang YJ (2017). A systemic view of Alzheimer disease - insights from amyloid-beta metabolism beyond the brain. Nat Rev Neurol 13, 612–623. [DOI] [PubMed] [Google Scholar]
- Wang L, Karpac J, and Jasper H (2014). Promoting longevity by maintaining metabolic and proliferative homeostasis. J Exp Biol 217, 109–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojcik C, and DeMartino GN (2002). Analysis of Drosophila 26 S proteasome using RNA interference. The Journal of biological chemistry 277, 6188–6197. [DOI] [PubMed] [Google Scholar]
- Xu P, Duong DM, Seyfried NT, Cheng D, Xie Y, Robert J, Rush J, Hochstrasser M, Finley D, and Peng J (2009). Quantitative proteomics reveals the function of unconventional ubiquitin chains in proteasomal degradation. Cell 137, 133–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, and Wang T (2019). LOVIT Is a Putative Vesicular Histamine Transporter Required in Drosophila for Vision. Cell Rep 27, 1327–1333 e1323. [DOI] [PubMed] [Google Scholar]
- Zhang S, Binari R, Zhou R, and Perrimon N (2010). A genomewide RNA interference screen for modifiers of aggregates formation by mutant Huntingtin in Drosophila. Genetics 184, 1165–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng F, Zhang W, Sui Y, Ding R, Yi W, Hu Y, Liu H, and Zhu C (2019). Sugar Protectants Improve the Thermotolerance and Biocontrol Efficacy of the Biocontrol Yeast, Candida oleophila. Front Microbiol 10, 187. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. qRT-PCR oligo sequences, Related to Star Methods.
Data Availability Statement
The accession number for the RNA-seq data reported in this study (Drosophila muscle with proteasome stress; head tissues from Drosophila with muscle-specific Amyrel overexpression; and human cortical organoids treated with maltose/heat shock, and controls) is GEO: GSE149799. All other data supporting the findings of this study are available within the article and the supplementary information files and from the lead contact author upon request.


