SUMMARY
Through a synthetic lethal screen, ERK activation was found to mediate resistance to FAK inhibition in GNAQ-mutant uveal melanoma. With PLCB-PKC-ERK and Trio-FAK-Yap representing compensatory effectors of mutant Gαq signaling, combined inhibition of both pathways may be a promising therapeutic strategy in metastatic uveal melanoma.
MAIN TEXT
In this issue of Clinical Cancer Research, Paradis and colleagues used a kinome-wide CRISPR-Cas9 sgRNA library screen to identify ERK activation as mediator of resistance to FAK inhibition in GNAQ-mutant uveal melanoma (UM) (1). UM is the most common primary cancer of the eye and arises from cranial neural crest-derived melanocytes of the uveal tract, comprising the choroid, ciliary body, and iris. The local control rate following treatment of the primary tumor with radiotherapy or surgical resection is over 95%, with less than 2% of patients having detectable metastasis at initial presentation. Yet, up to half of patients develop metastatic disease with a strong liver tropism for which there is still no approved treatment. The genomic landscape of UM differs considerably from cutaneous, mucosal and acral melanomas, being characterized by two mutation hubs that arise early during evolution of the primary tumor (2). The first hub consists of mutually exclusive gain-of-function mutations in members of the Gαq/11 signaling pathway (GNAQ, GNA11, CYSLTR2, and PLCB4). These mutations represent initiating events that are present in over 95% of UM. The second hub comprises prognostically significant mutations in BAP1, SF3B1, or EIF1AX (“BSE” mutations), which are largely mutually exclusive and are associated with high, intermediate, and low metastatic risk, respectively. By the time the primary tumor is diagnosed, a BSE mutation is usually present in most or all tumor cells, suggesting that the Gαq/11 mutation may create a selective pressure to acquire a BSE mutation, after which a relative fitness maximum is achieved, with subsequent genomic aberrations accumulating through neutral or undirected evolution.
Whereas canonical Gαq/11 intracellular signaling is mediated through the PLCβ-PKC cascade, the authors previously presented data suggesting that mutant Gαq/11 can also signal through a non-canonical pathway that involves Hippo/YAP pathway activation mediated at least in part through FAK. In the present study, they sought to identify resistance mechanisms associated with FAK inhibition. After treating UM cells with a FAK inhibitor, synthetic lethal interactors were found to be enriched for members of both the PLC-PKC and MEK-ERK signaling modules. In cellular validation experiments, the MEK inhibitor trametinib was more effective in suppressing ERK activation than was a PKC inhibitor. As a result, MEK inhibition was chosen for further investigation in combination with FAK inhibition. Synergy between MEK and FAK inhibition was observed using 4 different MEK inhibitors and 2 different FAK inhibitors, consistent with a general drug class interaction. In a series of rigorous experiments, combined FAK and MEK inhibition exhibited potent synergy against UM cells, resulting in decreased proliferation and increased apoptosis in vitro and in vivo. Consistent with their expected mechanisms of action, these effects were associated with decreased phospho-ERK and nuclear YAP localization.
Many downstream effectors of Gαq/11 signaling have been implicated in UM development, including PLCβ-PKC, MEK-ERK, ARF6, PI3K-AKT-mTOR, Trio-Rho/Rac, FAK, and Hippo/YAP, which likely function through complex and interacting mechanisms (3). Elucidating the relative value of each effector as a therapeutic target remains a major challenge. The authors provide an important contribution in these ongoing efforts to identify mediators of therapeutic response and escape in UM. Their finding that ERK activation in response to FAK inhibition is noteworthy and consistent with recently published work by Faiao-Flores and colleagues, who found that MEK inhibition in UM cells resulted in increased FAK signaling (4). Together, these studies convincingly point to compensation between MEK-ERK and FAK signaling in Gαq/11-mutant UM.
While these results are promising, more research is needed to define the optimal strategy for blocking oncogenic Gαq/11 signaling. For example, Faiao-Flores and colleagues showed that, in addition to its effects on FAK signaling, MEK inhibition also caused an activation in PI3K-AKT signaling, and that HDAC inhibition blocked the increased output from both AKT and YAP signaling. These findings suggest that epigenetic modulators may allow simultaneous inhibition of multiple compensatory pathways, potentially allowing greater efficacy with less toxicity than dual kinase inhibition. Additionally, compounds are being developed that may directly inhibit mutant Gαq/11, which could eliminate the need for multiple drugs to target Gαq/11 oncogenic signaling.
It is also unclear whether pharmacologic targeting of Gαq/11 mutations alone will be sufficient to achieve effective results in metastatic UM. While Gαq/11 mutations are evidently important for tumor initiation and cellular proliferation, they do not cause malignant transformation when engineered into normal uveal melanocytes, and they are not sufficient for tumor progression and metastasis in human uveal melanocytic neoplasia. UM cell lines demonstrate “oncogene addiction” to mutant Gαq/11 in vitro, but this has not been proven to be the case in human metastatic tumors, where additional genomic and epigenetic aberrations appear to drive disease progression. Indeed, metastasizing UM usually harbor inactivating mutations in the tumor suppressor BAP1, which are present in about 40% of primary tumors and a larger percentage of metastatic tumors, whereas UM without BAP1 mutations do not tend to metastasize (5). BAP1 is a deubiquitinating enzyme involved in histone modifications, chromatin structure, development, and differentiation in multiple lineages, including neural crest from which UM is derived. Unfortunately, efforts to screen for pharmacologic inhibitors of BAP1-deficient UM cells have been limited, mostly because BAP1-wildtype cell lines proliferate more readily in culture than BAP1-deficient UM cells and are thus more amenable to synthetic lethal screening strategies. Studies to date have suggested a potential role for HDAC and PARP inhibitors in BAP1-mutant UM.
It has also become evident that alterations in the tumor immune microenvironment are critical determinants of metastasis and therapeutic response in UM. However, there are currently no UM animal models available that accurately recapitulate the genomic and cellular landscape of UM (Fig. 1). These deficiencies may help explain why there have been no drugs to date that have shown efficacy in pre-clinical UM models that were subsequently validated in human clinical trials. An important future direction will be to develop high throughput screening methods such as PDX-derived 3D cultures that allow for study of BAP1-deficient UM cells, as well as pre-clinical validation systems that more accurately depict the tumor microenvironment, such as co-cultures, organoids, small animal embryos, and humanized, syngeneic and genetically engineered mouse models. The findings of Paradis and colleagues add to our understanding of oncogenic Gαq/11 signaling, and they identify a promising strategy for combination therapy that warrants further investigation.
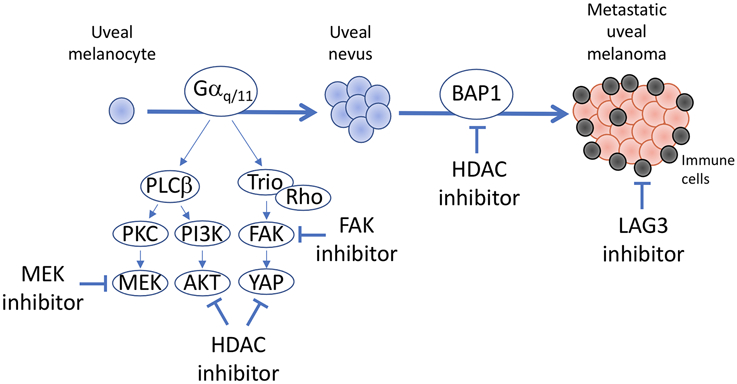
FIGURE 1.
Potential pharmacologic targets in metastatic uveal melanoma. Most uveal melanomas containing initiating mutation in a member of the Gαq/11 pathway (GNAQ, GNA11, CYSLTR2, and PLCB4), which trigger oncogenic signaling through multiple effectors, including PKC-MEK, PI3K-AKT, and FAK-YAP. The data presented by Paradis and colleagues (1) identifies dual inhibition of MEK and FAK as a synergistic combination in preclinical models of uveal melanoma. Other work has shown that HDAC inhibitors may also increase the efficacy of MEK inhibitors by blocking AKT and YAP signaling (4). Gαq/11 mutations result in benign melanocytic lesions unless additional genetic and genomic events occur. The mutations most strongly associated with metastasis in uveal melanoma occur in the metastasis suppressor BAP1, which are associated with extensive epigenetic and transcriptional rewiring that can be at least partly reversed by inhibition of HDAC activity. BAP1-mutant uveal melanomas are also enriched for exhausted cytotoxic T cells, alternatively activated macrophages, and other cells associated with immune dysfunction that likely contribute to metastatic competence and therapeutic resistance. LAG3 was recently shown to be a predominant checkpoint marker in uveal melanoma and its inhibition is currently the subject of a clinical trial in patients with metastatic uveal melanoma.
Acknowledgments
Funding: National Institute of Health grants R01 CA125970 (J.W.H.), P30CA240139 (Sylvester Comprehensive Cancer Center), and NEI P30EY014801 (Bascom Palmer Eye Institute), Research to Prevent Blindness Unrestricted Grant (Bascom Palmer Eye Institute), Department of Defense grant W81XWH-15-1-0578 (J.W.H.), Research to Prevent Blindness, Inc. Senior Scientific Investigator Award (J.W.H.), and a generous gift from Dr. Mark J. Daily (J.W.H).
Footnotes
Conflicts of Interest: Dr. Harbour is an inventor of intellectual property related to prognostic testing in uveal melanoma. He is a paid consultant for Castle Biosciences, licensee of this intellectual property, and he receives royalties from its commercialization.
REFERENCES
- 1.Paradis JS, Acosta M, Saddawi-Konefka R, Kishore A, Lubrano S, Gomes FG, et al. Synthetic Lethal Screens Reveal Co-Targeting FAK and MEK as a Multimodal Precision Therapy for GNAQ-Driven Uveal Melanoma. Clin Cancer Res 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Field MG, Durante MA, Anbunathan H, Cai LZ, Decatur CL, Bowcock AM, et al. Punctuated evolution of canonical genomic aberrations in uveal melanoma. Nat Commun 2018;9:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chua V, Lapadula D, Randolph C, Benovic JL, Wedegaertner PB, Aplin AE. Dysregulated GPCR Signaling and Therapeutic Options in Uveal Melanoma. Mol Cancer Res 2017;15:501–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Faiao-Flores F, Emmons MF, Durante MA, Kinose F, Saha B, Fang B, et al. HDAC Inhibition Enhances the In Vivo Efficacy of MEK Inhibitor Therapy in Uveal Melanoma. Clin Cancer Res 2019;25:5686–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harbour JW, Onken MD, Roberson ED, Duan S, Cao L, Worley LA, et al. Frequent mutation of BAP1 in metastasizing uveal melanomas. Science 2010;330:1410–3 [DOI] [PMC free article] [PubMed] [Google Scholar]