Abstract
Background:
Vitamin A is necessary for an adequate immune response to infections. Infection also alters vitamin A biomarkers, which interferes with assessment of vitamin A deficiency and thus impairs clinical management. Here we apply multiple strategies to adjust vitamin A biomarkers for inflammation during acute infection and evaluate associations between adjusted vitamin A status and immunologic response markers.
Methods:
We measured biomarkers in pediatric patients presenting with acute febrile illness in Guayaquil, Ecuador at paired acute and convalescent visits. Four adjustment strategies were applied to retinol-binding protein (RBP) concentrations: Thurnham correction factor (TCF), BRINDA regression correction (BRC), CRP-only adjustment factor (CRP), and proof-of-concept for a proposed interleukin 6 regression model (IL-6 RM). Adjusted RBP concentrations were compared between visits using the paired Wilcoxon signed-rank test. Multivariate regression analysis was used to assess associations between adjusted vitamin A status and immunologic response markers.
Results:
A sample of 57 participants completed the acute visit 1, and 18 of these individuals completed the convalescent visit 2. The IL-6 RM was the only strategy resulting in adjusted RBP concentrations that were not significantly different between paired visits (p = 0.20). Following RBP adjustment, 0.0% of participants were classified as vitamin A deficient (RBP ≤ 0.70 μmol/L) and 14.0% were classified as vitamin A insufficient (RBP ≤ 1.05 μmol/L). Adjusted vitamin A insufficiency was associated with an increase in macrophage inflammatory protein 1-alpha (MIP-1α, p = 0.03) and a pro-inflammatory immune response profile (p = 0.03) during the acute visit.
Conclusions:
We introduce a strategy for adjusting vitamin A in the context of clinical illness based on IL-6 concentrations that will need to be validated in larger studies. Assessment of vitamin A during infection allows for further understanding of how vitamin A status modulates immunopathology and enables targeting strategies for vitamin A supplementation in the context of infection among children in settings with high burdens of undernutrition and infectious diseases.
Keywords: vitamin A, acute febrile illness, cytokines, children
BACKGROUND
Vitamin A is an essential nutrient that supports several critical functions, including immune function [1]. In children, vitamin A deficiency is linked to infection-related mortality and morbidity [2–4]. Understanding the role of vitamin A in immune function is complicated by the fact that infection and inflammation interfere with the assessment of vitamin A status. Estimating underlying vitamin A status during an infection is critical for comprehensive clinical management. While universal vitamin A supplementation (VAS) programs were established based on demonstrated reductions in all-cause mortality [5], VAS has also led to adverse outcomes in the context of certain infections, such as HIV [2, 6]. Underlying vitamin A status may be a factor explaining such differential outcomes, therefore making vitamin A assessment at the individual level a priority, particularly for children in settings with high burden of infection and malnutrition.
Dietary vitamin A is stored in the liver and secreted into circulation at a constant rate in the form of retinol bound to retinol-binding protein (RBP) [7]. While measuring hepatic vitamin A concentration via liver biopsy is the gold standard for vitamin A assessment, circulating biomarkers of retinol or RBP are commonly used at the population level [8–11]. During acute infection or inflammation, hepatic secretion of retinol and RBP is temporarily reduced, which can invalidate vitamin A assessment [12].
Mechanisms of the acute phase response (APR), induced by acute infection, are responsible for reductions in circulating vitamin A. The APR is characterized by increases in pro-inflammatory cytokines, including interleukin 1-beta (IL-1β), interleukin 6 (IL-6), and tumor necrosis factor alpha (TNFα). Of these, IL-6 acts on the liver to alter the production and secretion of acute phase proteins [13, 14], resulting in increased C-reactive protein (CRP), increased alpha-1-acid glycoprotein (AGP), and decreased RBP concentrations [15–18]. As an episode of acute infection resolves, concentrations of CRP, AGP, and RBP return to previous baseline concentrations [19]. Longitudinal studies have demonstrated that reductions in circulating vitamin A concentrations in the days following acute infection onset were recovered after a period of 7–28 days [14, 20–23], while hepatic vitamin A stores remain unchanged [18, 24–27]. Using data from nationally representative cross-sectional surveys, several vitamin A adjustment strategies have been developed to estimate the burden of vitamin A deficiency at the population level. Such strategies calculate an adjusted vitamin A concentration using either correction factors or regression coefficients based on corresponding measures of circulating CRP and/or AGP [28–30]. Given the biological influence of IL-6 on hepatic acute phase proteins, we explore IL-6 as a candidate biomarker for vitamin A adjustment in the context of acute infection.
A nationally representative nutritional survey in Ecuador reported vitamin A deficiency proportions (serum retinol ≤ 0.70 μmol/L) of 14.2% and 9.3% for children ages 6–59 months and 5–9 years at the national level [31], and 20.6% and 11.7% for those same age groups residing in the city of Guayaquil. In the present study, we recruited pediatric patients from Guayaquil with acute febrile illness and measured circulating concentrations of RBP, acute phase proteins, and immunologic response markers during acute and convalescent visits. Our study objectives were two-fold: 1) To apply adjustment strategies to RBP, including a newly developed strategy based in IL-6, and compare adjusted concentrations across paired study visits; and 2) To investigate associations between adjusted vitamin A status and immunologic response markers during episodes of acute febrile illness.
MATERIALS AND METHODS
Population
All pediatric patients presenting with acute febrile illness to the Hospital Roberto Gilbert E. in Guayaquil, Ecuador from January-June 2018 were recruited and invited to return for a follow-up convalescent visit 2–4 weeks post enrollment. The inclusion criteria were reported fever with duration of at least three days and clinical blood work requested by an attending physician. Exclusion criteria included otitis media, sinusitis, urinary tract infection, allergic reaction, or physical injury. A parent or legal guardian provided written informed consent in Spanish. Protocol approval was obtained from the Hospital Luis Vernaza and Cornell University institutional review boards.
At enrollment, study physicians interviewed participants and collected demographic information and health history using electronic data capture, and de-identified data were synced to a secure third-party server daily [32]. Venous blood was collected at both acute and convalescent study visits, processed by staff phlebotomists, and serum was stored daily in a −80 °C freezer located at the Biomedicine Laboratory, ESPOL, Guayaquil, Ecuador.
Data Analysis
RBP (μmol/L), CRP (mg/L), and AGP (g/L) were measured by ELISA, (DRB400, DCRP00, DAGP00; R&D Systems, Inc., Minneapolis, MN). A panel of 29 immunologic response markers, listed in the Supplementary Information, was measured using a magnetic bead multiplex assay and reported in units of median fluorescent intensity (MFI), (HCYTMAG60PMX29BK, EMD Millipore Corporation, Billerica, MA).
RBP concentrations were adjusted using population-based strategies previously described, including the Thurnham correction factor (TCF), the BRINDA regression correction (BRC), and the CRP-only adjustment factor (CAF) [28–30]. RBP was also adjusted using an IL-6 regression model (IL-6 RM), developed with data from the present cohort. Adjustments were applied to RBP measures meeting adjustment criteria for each visit separately. Vitamin A deficiency was defined as RBP ≤ 0.70 μmol/L, and vitamin A insufficiency was defined as RBP ≤ 1.05 μmol/L[33]. Clusters of immunologic response markers were identified using a correlation matrix of one minus the squared correlation and a hierarchical cluster analysis of average distances with a threshold of < 0.64, which corresponds to a correlation of > 0.6. Immune response markers of each identified cluster were then weighted into a single continuous variable using principal component analysis, where the first principal component variable explained at least 0.8 proportion of the variance.
Thurnham Correction Factor
The Thurnham correction method is based on four stages of inflammation: 1) CRP < 5 mg/L and AGP < 1 g/L; 2) CRP ≥ 5 mg/L and AGP < 1 g/L; 3) CRP ≥ 5 mg/L and AGP > 1 g/L; and 4) CRP < 5 mg/L and AGP ≥ 1 g/L. For inflammation stages 1–4, the respective Thurnham correction factors for vitamin A adjustment are 1.0, 1.13, 1.24, and 1.11. Each measure of RBP is multiplied by the corresponding stage correction factor to yield the TCF-adjusted RBP concentration [30].
BRINDA Regression Correction
The BRINDA regression correction (BRC) model includes inflammation biomarkers of CRP and/or AGP and malaria infection status. Malaria was not included in this analysis as it was not assessed, and prevalence is low in Ecuador [34]. The BRC strategy establishes CRP and AGP reference values defined as the maximum value of the lowest decile concentration for the sample and adjusts RBP concentrations for individuals with CRP or AGP greater than each respective reference value. A regression equation modeling the natural logarithm of RBP equal to CRP and AGP generates CRP and AGP beta coefficients, which are then applied to the BRINDA equation (Equation 1) [29]. All values were natural log-transformed for application of the equation.
| (1) |
CRP-only Adjustment Factor
The CRP-only adjustment method partitions the sample into three groups based on CRP concentrations: 1) <5 mg/L; 2) ≥5 and <15 mg/L; 3) ≥15 mg/L and calculates the mean RBP for each group. The CRP-only adjustment factor (CAF) for each group is defined as the mean RBP for group 1 minus the respective group mean RBP. To generate the CAF-adjusted RBP value, the group-specific CAF value is added to the RBP concentration [28].
Interleukin-6 Regression Model
The IL-6 RM establishes an IL-6 reference value (IL-6Ref) defined as the maximum value of the lowest decile concentration for the sample, and the application of the IL-6 RM is limited to individuals with IL-6 concentrations greater than the IL-6Ref. A regression equation modeling the natural logarithm of RBP equal to IL-6 generates a beta coefficient (βIL-6), which is then applied to the IL-6 RM equation (Equation 2). All values are log-transformed for application of the equation.
| (2) |
Comparisons of participant characteristics within the sample at the acute visit were assessed using the non-parametric Wilcoxon rank sum test for continuous measures and Fisher’s Exact test for discrete categories. Comparisons between paired acute and convalescent visits were assessed using the non-parametric paired Wilcoxon signed-rank test for continuous measures and the McNemar test for discrete categories. Beta coefficients were generated using linear regression models. Multivariate linear regression was used to assess associations between either adjusted vitamin A status or sex with individual immune response markers at the acute visit and with correlated clusters of immune response markers at the acute visit. Fully adjusted regression models included variables of age, sex, body mass index (BMI), residential province, and either the duration of days between self-reported illness onset and the acute visit or the number of days between acute and convalescent visits. Measures of IL-6 were excluded from all regression analyses following adjustment of RBP using the IL-6 RM. Results are reported as median values with the interquartile range (IQR) unless otherwise stated. P values < 0.05 were considered significant. Statistical analyses were conducted using SAS® Studio 3.8 (SAS Institute Inc., Cary, NC).
RESULTS
Participant Characteristics and Biomarker Measures
A sample of 57 participants completed the acute visit 1 and provided sufficient serum volume (≥ 250 μL) for biomarker measures. Of the sample, a subset of 18 participants returned and completed the convalescent visit 2 (Figure 1). The median time between visits 1 and 2 was 15.5 days (14.0–20.0). Characteristics of the study population are presented in Table 1, including comparisons that demonstrate that there were no significant differences between the subset (n = 18) and participants who did not return for visit 2 (n = 39). Participant characteristics were also compared by sex (n = 57, Supplementary Table 1), and female age, height, and weight were significantly higher compared to males, yet BMI was not significantly different between sexes.
Figure 1.
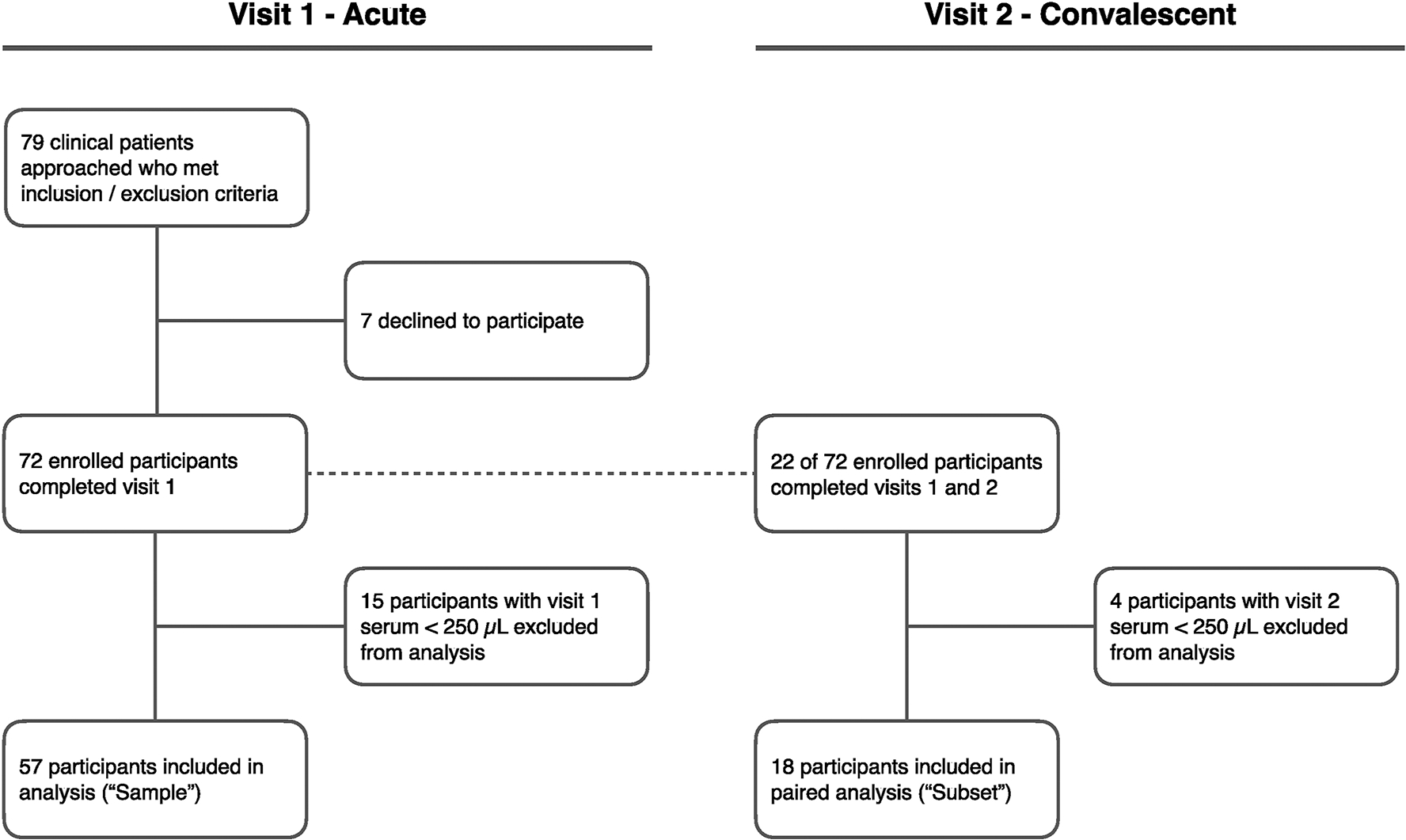
Flowchart
Table 1.
Participant characteristics, acute visit 1
| Median (Q1-Q3) or n (%) | p valuea | ||
|---|---|---|---|
| Sample (n = 57) | Paired subset (n = 18) | ||
| Sex | |||
| Male | 34 (59.6) | 10 (55.6) | |
| Female | 23 (40.4) | 8 (44.4) | 0.77 |
| Age (years) | 1.7 (0.9–3.1) | 1.3 (0.8–3.1) | 0.45 |
| Age group | |||
| 0–5 months | 2 (3.5) | 2 (11.1) | |
| 6–59 months | 45 (78.9) | 12 (66.7) | |
| 5–9 years | 7 (12.3) | 3 (16.7) | |
| 10–19 years | 3 (5.2) | 1 (5.5) | 0.15 |
| Anthropometry | |||
| Height (cm) | 83.0 (72.0–98.0) | 77.5 (70.0–98.0) | 0.48 |
| Weight (kg) | 11.0 (9.1–14.3) | 10.1 (8.6–14.8) | 0.25 |
| BMI (kg/m2) | 16.8 (15.6–18.0) | 16.6 (15.9–17.4) | 0.51 |
| MUAC (cm) | 15.2 (14.0–16.5) | 15.0 (14.0–16.5) | 0.66 |
| Province | |||
| Esmeraldas | 1 (1.7) | 0 (0) | |
| Guayas | 51 (89.5) | 17 (94.4) | |
| Los Ríos | 2 (3.5) | 0 (0) | |
| Manabí | 2 (3.5) | 1 (5.6) | |
| Santa Elena | 1 (1.7) | 0 (0) | 1.00 |
Differences between participants at the acute visit 1: subset (n = 18) compared to non-subset (n = 39), Wilcoxon rank sum test for continuous measures or Fisher’s Exact test for discrete categories.
BMI, body mass index; MUAC, mid-upper arm circumference.
Biomarker measures are reported in Table 2. For the sample at the acute visit 1 (n = 57), unadjusted median RBP (μmol/L) was 1.05 (0.89–1.20), and proportions of unadjusted vitamin A deficiency and insufficiency were 12.3% and 49.1%, respectively. For the subset completing paired acute and convalescent visits (n = 18), median RBP concentrations (μmol/L) were 1.02 (0.90–1.21) and 1.57 (1.26–1.75), respectively; median CRP concentrations (mg/L) were 10.9 (3.1–18.9) and 1.1 (0.1–3.5), respectively; and median AGP concentrations (g/L) were 1.3 (1.1–1.5) and 0.8 (0.5–1.1), respectively.
Table 2.
Biomarker measures.
| Median (Q1-Q3) or n (%) | |||
|---|---|---|---|
| Sample (n = 57) | Paired subset (n = 18) | ||
| Vitamin A (unadjusted) | Acute visit 1 | Acute visit 1 | Convalescent visit 2 |
| RBP (μmol/L) | 1.05 (0.89–1.20) | 1.02 (0.90–1.21) | 1.57 (1.26–1.75) |
| Deficiency (RBP ≤ 0.70 μmol/L) | 7 (12.3) | 2 (11.1) | 0 (0) |
| Insufficiency (RBP ≤ 1.05 μmol/L) | 28 (49.1) | 9 (50.0) | 1 (5.6) |
| Acute phase proteins | |||
| CRP (mg/L) | 14.4 (1.8–41.7) | 10.9 (3.1–18.9) | 1.1 (0.1–3.5) |
| CRP ≥ 5 mg/L | 38 (66.7) | 13 (72.2) | 4 (22.2) |
| AGP (g/L) | 1.4 (1.1–1.7) | 1.3 (1.1–1.5) | 0.8 (0.5–1.1) |
| AGP ≥ 1 g/L | 43 (75.4) | 14 (77.8) | 6 (33.3) |
| Immune response marker (MFI) | |||
| EGF | 269.0 (91.5–535.0) | 340.8 (91.5–745.0) | 608.5 (127.0–943.5) |
| Eotaxin | 328.0 (234.0–432.0) | 323.8 (228.0–400.0) | 386.8 (270.0–546.0) |
| G-CSF | 50.0 (37.0–82.0) | 51.0 (39.0–136.0) | 27.5 (21.5–37.0) |
| GM-CSF | 20.0 (17.0–25.0) | 22.0 (18.0–24.5) | 17.5 (15.0–24.0) |
| IFNα2 | 16.0 (13.0–21.0) | 19.8 (14.0–33.0) | 13.5 (12.0–16.0) |
| IFNγ | 60.0 (34.0–113.0) | 65.5 (43.0–107.5) | 30.3 (23.0–47.0) |
| IL-10 | 191.0 (107.0–535.0) | 337.0 (138.0–752.0) | 47.8 (35.0–82.5) |
| IL-12P40 | 36.0 (28.0–52.0) | 44.5 (33.0–63.0) | 31.3 (21.0–46.0) |
| IL-12P70 | 18.0 (15.0–27.0) | 17.0 (15.5–22.0) | 16.0 (13.0–28.0) |
| IL-13 | 30.0 (18.5–53.5) | 22.5 (16.0–31.0) | 22.3 (18.5–31.5) |
| IL-15 | 38.0 (32.0–55.0) | 41.0 (36.5–55.0) | 26.0 (23.0–29.0) |
| IL-17a | 34.0 (26.0–75.0) | 31.8 (25.0–39.0) | 27.5 (23.0–77.0) |
| IL-1RA | 65.0 (43.0–112.0) | 67.3 (42.0–112.0) | 24.5 (20.0–38.5) |
| IL-1α | 35.0 (26.0–45.5) | 35.0 (28.0–45.5) | 23.8 (20.0–37.0) |
| IL-1β | 22.5 (18.0–31.5) | 21.0 (18.0–28.5) | 18.5 (15.0–25.0) |
| IL-2 | 34.0 (30.0–41.5) | 32.3 (30.0–42.0) | 30.0 (28.0–34.0) |
| IL-3 | 14.0 (12.0–15.5) | 13.0 (12.0–16.0) | 12.5 (11.0–15.0) |
| IL-4 | 14.0 (12.0–20.0) | 13.3 (12.0–26.0) | 12.0 (10.5–15.0) |
| IL-5 | 21.5 (17.0–39.0) | 23.3 (17.0–40.0) | 19.3 (16.0–24.0) |
| IL-6 | 82.5 (43.0–154.5) | 56.0 (40.0–151.0) | 27.0 (22.0–88.0) |
| IL-7 | 19.0 (17.0–22.3) | 17.8 (16.0–20.0) | 18.5 (17.0–21.0) |
| IL-8 | 493.0 (278.0–781.0) | 590.0 (281.0–1221.0) | 202.5 (148.0–377.0) |
| IP-10 | 11,363.5 (7,494.0–16,257.0) | 13,336.0 (9,485.0–20,976.0) | 1,560.0 (998.0–2,203.0) |
| MCP-1 | 5,641.0 (3,954.0–8,515.5) | 5,838.5 (4,609.5–10,090.0) | 4,364.8 (2,518.0–7,104.0) |
| MIP-1α | 92.0 (62.0–124.5) | 85.0 (62.0–124.5) | 66.0 (47.0–104.0) |
| MIP-1β | 80.0 (52.0–123.5) | 92.3 (64.0–161.0) | 114.3 (78.5–160.0) |
| TNFα | 197.0 (154.3–265.0) | 238.0 (161.5–292.0) | 176.5 (142.0–258.0) |
| TNFβ | 26.0 (15.0–70.0) | 18.8 (14.0–32.0) | 19.5 (13.0–31.0) |
| VEGF | 54.5 (28.5–89.5) | 52.8 (26.0–81.0) | 43.3 (25.0–197.0) |
RBP, retinol-binding protein; CRP, C-reactive protein; AGP, alpha-1 acid glycoprotein; APR, acute phase response; MFI, median fluorescent intensity
RBP Adjustment Strategies: Development of the IL-6 Regression Model
Associations between the change in RBP concentration across visits and several acute phase protein and pro-inflammatory biomarker concentrations measured at visit 1 were assessed (Table 3). IL-6 was the only biomarker measured at visit 1 significantly associated with the change in RBP concentration across acute and convalescent visits (p = 0.03), a finding made possible given the longitudinal study design. Based on this association, IL-6 was further explored as a biomarker measure for vitamin A adjustment, leading to development of the IL-6 RM. In the sample at visit 1 (n = 57), MFI levels of IL-6 were compared to concentrations of hepatic acute phase proteins (Table 4), and IL-6 was significantly associated with CRP, AGP, and RBP concentrations. This finding influenced the application of the IL-6 RM independent of CRP or AGP concentrations.
Table 3.
Univariate linear regression of the change in RBP across visits and biomarkers measured at visit 1 (n = 18)
| Visit 1 biomarkersa | Regression coefficient (β) | R-squared | p value |
|---|---|---|---|
| CRP (mg/L) | 0.11478 | 0.03 | 0.54 |
| AGP (g/L) | 0.48651 | 0.02 | 0.60 |
| IL-1β (MFI) | 0.68066 | 0.13 | 0.17 |
| IL-6 (MFI) | 0.56949 | 0.30 | 0.03 |
| TNFα (MFI) | 0.73823 | 0.19 | 0.11 |
All values in the regression model were natural log-transformed.
RBP, retinol-binding protein; CRP, C-reactive protein; AGP, alpha-1 acid glycoprotein; IL-1β, interleukin 1-beta; IL-6, interleukin 6; TNFα, tumor necrosis factor alpha; MFI, median fluorescent intensity
Table 4.
Univariate linear regression of IL-6 and measures of acute phase proteins in the visit 1 sample (n = 57).
| Biomarkersa | Regression coefficient (β) | R-squared | p value |
|---|---|---|---|
| CRP (mg/L) | 0.80506 | 0.25 | <0.0001 |
| AGP (g/L) | 0.15971 | 0.35 | <0.0001 |
| RBP (μmol/L) | −0.17198 | 0.49 | <0.0001 |
All values in the regression model were natural log-transformed.
APR, acute phase response; CRP, C-reactive protein; AGP, alpha-1 acid glycoprotein; RBP, retinol-binding protein
RBP Adjustment Strategies: Application and Comparison Across Paired Study Visits
Vitamin A adjustment strategies were applied to measures of RBP for the sample at visit 1 (n = 57, Supplementary Table 2) and separately for the subset at each visit (n = 18, Figure 2 and Supplementary Table 3). RBP concentrations between visits were significantly higher at the convalescent visit 2 compared to the acute visit 1 for unadjusted RBP (p < 0.001) and after applying the TCF (p = 0.01), BRC (p = 0.03), and CAF (p = 0.001) strategies. Application of the IL-6 RM strategy resulted in RBP concentrations that were not significantly different between visits (p = 0.20).
Figure 2.
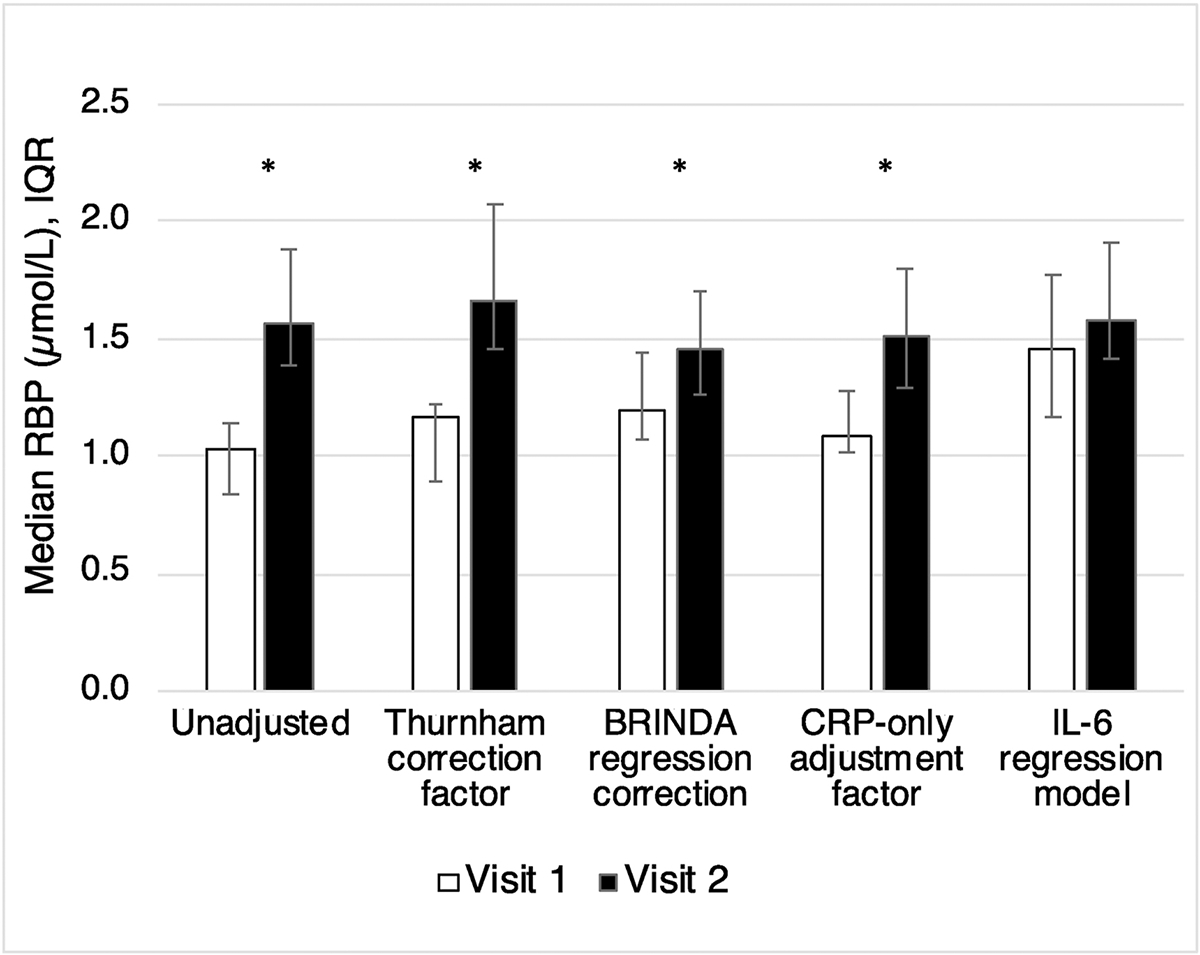
Adjustment strategies to applied to RBP concentrations at separate visits (n = 18). Asterisks represents significant differences between visits generated using non-parametric paired Wilcoxon signed-rank test for continuous measures. RBP, retinol-binding protein; IQR, interquartile range
Adjusted Vitamin A Status
The IL-6 RM adjustment strategy was applied to estimate vitamin A status for all subsequent analyses. Following IL-6 RM adjustment, none of the participants were classified as vitamin A deficient at either visit. After applying the IL-6 RM adjustment at visit 1, 14.0% of the sample (n = 57) and 16.7% of the subset (n = 18) were classified as vitamin A insufficient (Table 5).
Table 5.
Application of the IL-6 Regression Model adjustment strategy to RBP concentrations and corresponding proportions of vitamin A status
| n (%) | |||
|---|---|---|---|
| Sample (n = 57) | Paired subset (n = 18) | ||
| Vitamin A status | Acute visit 1 | Acute visit 1 | Convalescent visit 2 |
| Deficiency, unadjusted a | 7 (12.3) | 2 (11.1) | 0 (0) |
| Deficiency, IL-6 RM a | 0 (0) | 0 (0) | 0 (0) |
| Insufficiency, unadjustedb | 28 (49.1) | 9 (50.0) | 1 (5.6) |
| Insufficiency, IL-6 RMb | 8 (14.0) | 3 (16.7) | 2 (11.1) |
Vitamin A deficiency defined as RBP ≤ 0.70 μmol/L
Vitamin A insufficiency defined as RBP ≤ 1.05 μmol/L
RBP, retinol-binding protein; IL-6, interleukin 6; IL-6 RM, IL-6 regression model
Adjusted Vitamin A Status and Immunologic Response Markers
Associations between adjusted vitamin A insufficiency status and individual immunologic response markers were assessed in the sample at visit 1 (n = 57) using a multivariate linear regression model. Of the biomarkers measured, MIP-1α was the only individual marker associated with adjusted vitamin A insufficiency status (p = 0.03), with higher measures among those categorized as insufficient (Table 6). Immunologic response markers were also assessed by sex for the sample at visit 1. Several individual markers differed by sex with higher measures in females compared to males, including GM-CSF, IFNα2, IL-12p40, IL-1β, IL-2, and IL-8 (Supplementary Table 4).
Table 6.
Immune response markers by vitamin A status at visit 1 (n = 57).
| Median (Q1-Q3) | p valuea | ||
|---|---|---|---|
| Immune response marker (MFI) | Insufficient (n = 8) | Sufficient (n = 49) | |
| EGF | 769.5 (225.3–902.0) | 217.0 (91.5–449.0) | 0.18 |
| Eotaxin | 322.8 (286.0–536.0) | 335.0 (228.0–432.0) | 0.67 |
| G-CSF | 57.0 (42.0–125.5) | 50.0 (31.0–81.0) | 0.68 |
| GM-CSF | 19.5 (19.0–29.0) | 21.0 (17.0–25.0) | 0.62 |
| IFNα2 | 15.0 (14.5–27.0) | 16.0 (13.0–21.0) | 0.88 |
| IFNγ | 53.5 (46.3–243.3) | 70.0 (34.0–113.0) | 0.86 |
| IL-10 | 122.5 (93.5–435.3) | 206.0 (112.5–598.0) | 0.16 |
| IL-12P40 | 37.5 (31.5–80.8) | 36.0 (27.0–52.0) | 0.48 |
| IL-12P70 | 16.8 (16.0–26.5) | 18.0 (15.0–27.0) | 0.27 |
| IL-13 | 67.5 (21.3–118.5) | 29.5 (17.0–50.0) | 0.11 |
| IL-15 | 41.8 (37.5–56.5) | 38.0 (31.0–55.0) | 0.58 |
| IL-17a | 34.0 (26.8–72.8) | 33.0 (26.0–75.0) | 0.52 |
| IL-1RA | 134.5 (71.3–253.3) | 61.0 (42.0–91.0) | 0.25 |
| IL-1α | 37.5 (31.8–45.8) | 35.0 (25.5–45.5) | 0.34 |
| IL-1β | 25.8 (22.0–34.5) | 22.0 (18.0–31.0) | 0.21 |
| IL-2 | 38.8 (32.5–46.0) | 34.0 (30.0–41.0) | 0.06 |
| IL-3 | 14.8 (14.0–18.0) | 13.0 (12.0–15.0) | 0.55 |
| IL-4 | 15.0 (13.0–26.0) | 14.0 (12.0–19.0) | 0.41 |
| IL-5 | 33.0 (20.0–42.0) | 21.0 (17.0–28.0) | 0.77 |
| IL-7 | 16.5 (15.5–48.8) | 19.0 (17.0–22.3) | 0.44 |
| IL-8 | 556.0 (300.8–1771.8) | 493.0 (278.0–728.0) | 0.76 |
| IP-10 | 15,113.5 (11,658.817,277.8) | 10,241.0 (6,623.015,304.0) | 0.76 |
| MCP-1 | 4,863.3 (3,387.5–9,795.3) | 5,647.5 (3,954.0–8,143.0) | 0.18 |
| MIP-1α | 117.5 (92.0–484.0) | 80.0 (59.0–112.0) | 0.03 |
| MIP-1β | 121.0 (56.5–220.8) | 80.0 (52.0–108.0) | 0.26 |
| TNFα | 213.3 (166.8–547.8) | 191.5 (150.5–256.0) | 0.20 |
| TNFβ | 94.0 (15.0–194.0) | 26.0 (15.0–67.0) | 0.15 |
| VEGF | 72.3 (52.8–173.0) | 50.0 (27.0–81.0) | 0.14 |
Multivariate linear regression adjusted for variables of age, sex, body mass index, province, and number of days following the onset of symptoms. All values in the regression model were natural log transformed
In the sample at acute visit 1 (n = 57), hierarchical cluster analysis identified two separate clusters of correlated immune response markers: Cluster 1) IL-7, IL-17a, IL-12p70, IL-2, IL-1α, IL-1β; and Cluster 2) TNFα, IL-1RA, G-CSF, IL-10 (Figure 3). For each identified cluster, the corresponding immunologic response marker variables were weighted into a single continuous variable using principal component analysis, and the resulting principal component variables for Clusters 1 and 2 explained 0.89 and 0.87 proportions of the variance, respectively. Using multivariate linear regression, adjusted vitamin A insufficiency status was associated with the Cluster 1 variable (p = 0.03) (Table 7). Sex was not associated with either of the cluster variables (Supplementary Table 5).
Figure 3.
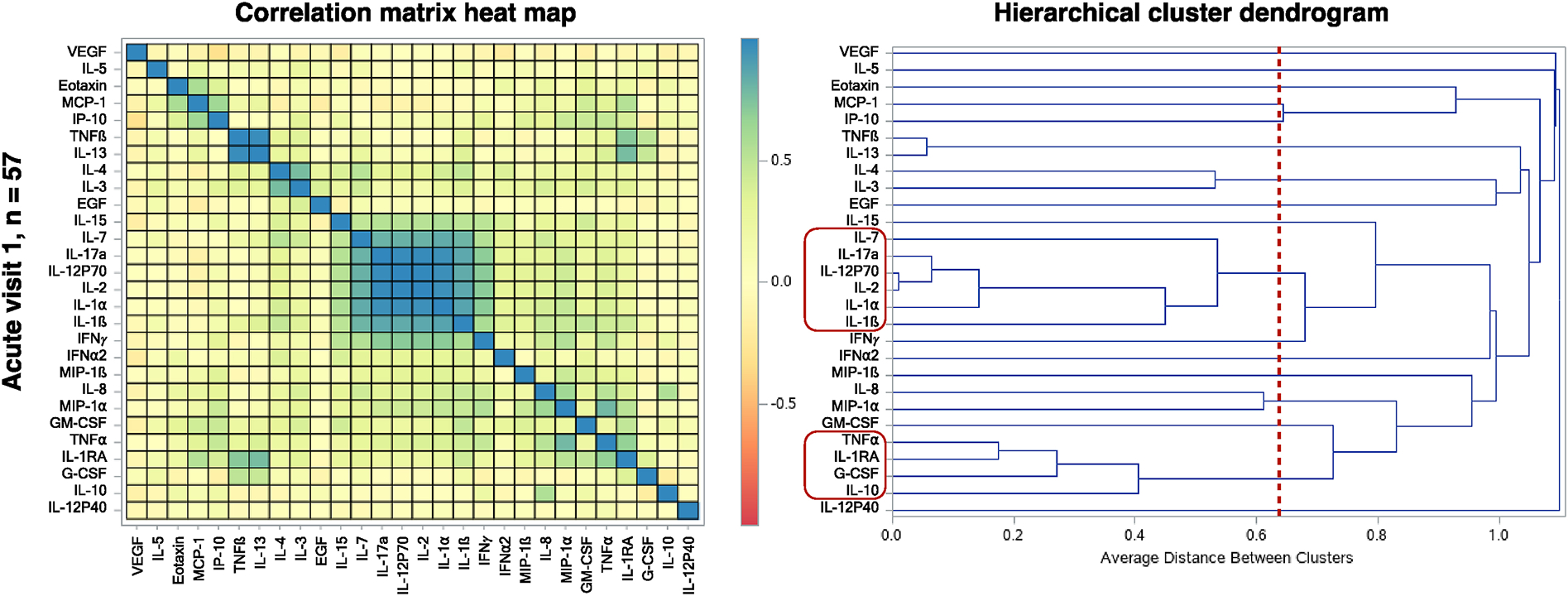
Correlated clusters of immune response markers at visit 1 (n = 57). The heat map panel was generated using a correlation matrix of one minus the squared correlation of immune response markers. In the hierarchical cluster dendrogram panel, the vertical red line indicates the threshold of 0.64 (equivalent to a correlation of 0.60). Immune response markers grouped in clusters greater than two and positioned left of the threshold line are indicated by the red outline boxes. These identified clusters can be visualized as shown on the correlation matrix heat map panel.
Table 7.
Immune response cluster variables by vitamin A status at visit 1 (n = 57)
| Clustered immune response markersb | Vitamin A statusa |
|
|---|---|---|
| Regression coefficient (β) | p valuec | |
| Cluster 1 variable: | ||
| IL-7 | −0.86199 | 0.03 |
| IL-17a | ||
| IL-12 P70 | ||
| IL-2 | ||
| IL-1α | ||
| IL-1β | ||
| Cluster 2 variable: | ||
| TNFα | 0.00091 | 1.00 |
| IL-1RA | ||
| IL-6 | ||
| G-CSF | ||
| IL-10 | ||
Vitamin A status categorized as sufficient (RBP > 1.05 μmol/L, N = 49) or insufficient (RBP ≤ 1.05 μmol/L, n = 8).
All values in the regression model were natural log-transformed.
Multivariate linear regression adjusted for variables of age, sex, body mass index, province, and number of days following the onset of symptoms.
DISCUSSION
Unadjusted RBP concentrations were significantly lower in individuals during an acute illness visit compared to a convalescent visit 2–4 weeks later, confirming that circulating RBP is temporarily reduced during acute febrile illness. Under the assumptions that the acute phase response does not reduce vitamin A liver stores and that stores remain relatively stable over a 4-week period, we expect adjusted circulating RBP measures to be similar across time points. After applying four adjustment strategies to paired visits separately, RBP concentrations remained significantly different between visits using the TCF, BRC, and CAF strategies, but not when using the IL-6 RM strategy. Wessells et al. (2019) also report that TCF and BRC adjustment strategies under-adjust for the effects of inflammation on RBP compared with longitudinal models, resulting in overestimates of vitamin A deficiency [35].
The IL-6 RM strategy was adapted from the BRINDA regression correction (BRC) strategy and was developed using data from the present analysis. The BRINDA strategy assumes that during infection or inflammation, increases in circulating CRP and/or AGP are proportional to decreases in circulating RBP and retinol. However, in our sample, IL-6 measured at visit 1 was the only biomarker associated with RBP change across visits. During the acute visit, our evidence demonstrates associations between IL-6 and hepatic acute phase proteins (CRP, AGP, and RBP; Table 4), which further supports the use of IL-6 as a candidate biomarker for vitamin A adjustment. The biological impact of IL-6 on hepatic acute phase proteins influenced the development of the IL-6 RM without integration of CRP or AGP measurements. Offering an adjustment model with just one additional biomarker may be more cost efficient for applying vitamin A adjustment in resource-limited settings.
After adjusting RBP concentrations using the IL-6 RM, none of the 57 participants presenting at the acute visit 1 was categorized as vitamin A deficient, while 14.0% were categorized as vitamin A insufficient. When assessing individual immune response markers, adjusted vitamin A insufficiency was associated with increased MIP-1α MFI levels during the acute visit 1. MIP-1α (CCL3) is a chemokine attractant of monocytes, macrophages, and neutrophils, elevated during the innate immune response. This finding is consistent with evidence describing a role between low vitamin A status and an enhanced macrophage-associated pro-inflammatory response during acute infection [24]. Independent immune response markers were also associated with sex during the acute visit 1. MFI levels of GM-CSF, IFNα2, IL-12p40, IL-1α, IL-1β, IL-2, and IL-8 were higher in females, which aligns with evidence that females exhibit stronger innate and adaptive immune responses compared to males [36–38].
Clusters of correlated immunologic response markers were identified in an effort to account for similar reactions of some cytokines and chemokines during episodes of acute febrile illness. The Cluster 1 variable, comprised of pro-inflammatory mediators of immune function, was associated with adjusted vitamin A insufficiency in the fully adjusted regression model. These results support a role for vitamin A status, adjusted for inflammation, as a mediator of immune function during episodes of acute febrile illness.
We acknowledge several limitations related to these findings. Previously published adjustment strategies for circulating vitamin A were designed for application in national surveys and may not be suitable for smaller studies with specific target demographics, particularly acute febrile illness. The paired sample size of 18 participants was small, and we only present data from two time points with a varying duration of days between visits. Given the existing evidence regarding vitamin A status and immune function during infections, we expected to observe evidence of impaired innate immune activation during acute visit 1 for those with vitamin A deficiency. However, none of the participants was categorized as vitamin A deficient following adjustment of RBP, and our analysis of vitamin A insufficiency status may not fully reflect the role of vitamin A in acute febrile illness immune responses in full. Additionally, we did not have pathogen-specific diagnoses for the cases of acute febrile illness. Viral, bacterial, and parasitic agents can dictate varying immune response profiles.
To our knowledge, this is the first analysis to use IL-6 as a correction coefficient for vitamin A adjustment. A recent cross-sectional analysis from MacDonell et al. (2018) used an IL-6 regression model to adjust concentrations of iron, zinc, and selenium biomarkers in adults older than 65 years in New Zealand [39]. Additional evaluation of the IL-6 RM should include a larger sample with multiple serial biomarker measures in an effort to evaluate and compare the kinetics of circulating IL-6 and acute phase proteins in the context of acute illness. The IL-6 RM should also be evaluated against vitamin A assessment by retinol isotope dilution, which estimates vitamin A total body stores. Further investigation into the changes in immunologic response markers across multiple visits during acute illness along with differential diagnosis would offer additional understanding of how adjusted vitamin A status may influence pathogenesis and illness outcomes.
Individual adjusted vitamin A assessment may also inform VAS strategies. VAS programs are associated with reduced mortality among children [2], and recommendations for implementing national VAS programs are based on population surveys of vitamin A deficiency [5, 40]. Subsequently, in countries meeting the threshold burden of vitamin A deficiency proportions among children, VAS is distributed universally, indiscriminate of individual vitamin A status. While VAS is important and effective for reducing mortality and morbidity due to diarrheal illness and measles [41, 42], evidence from clinical trials involving VAS among children with HIV, malaria, or pneumonia have yielded mixed results, including adverse outcomes [6, 43, 44]. Mechanisms for these worsened outcomes are not fully understood, but underlying vitamin A status may be a contributing factor. We do not suggest changes to national supplementation programs, however, the ability to estimate vitamin A status for individuals experiencing infection will offer targeted clinical management and opportunities for advancing research to determine mechanisms surrounding vitamin A treatment and immune response during infection.
CONCLUSION
Using data from a longitudinal cohort of children with acute febrile illness, we introduce a strategy for adjusting vitamin A biomarkers for individuals experiencing infection or inflammation based on IL-6. Following adjustment of RBP using the IL-6 RM strategy, RBP concentrations were not significantly different between acute and convalescent study visits. Adjusted vitamin A assessment allows for investigations to understand the impact of vitamin A status on immunopathogenesis during infection. Within this cohort, adjusted vitamin A insufficiency status during acute illness was associated with a pro-inflammatory immune response profile. Moving forward, the ability to estimate underlying vitamin A status in children experiencing acute infection or inflammation will expand research potential and allow for targeted supplementation therapy in settings with overlapping burdens of malnutrition and infection.
Supplementary Material
Acknowledgments:
This work was made possible by collaborations with the Complejo Hospitalario Alejandro Mann and the Laboratorio de Biomedicina at Escuela Superior Politécnica del Litoral (ESPOL) in Guayaquil, Ecuador. We would like to thank Vicky Simon of the Human Nutritional Chemistry Service Laboratory at Cornell University and Stephen Parry from the Cornell Statistical Consulting Unit. We would like to acknowledge Caleb Ruth for development and integration of the mobile electronic data capture platform.
Funding: This work was supported by awards from the National Institutes of Health, including the National Institute of Biomedical Imaging and Bioengineering [R01 EB021331] and the Office of Research on Women’s Health [R01 EB021331 S1].
Footnotes
Conflict of Interest: The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].World Health Organization. Global prevalence of vitamin A deficiency in populations at risk1995–2005. WHO global database on vitamin A deficiency. Geneva: World Health Organization; 2009. [Google Scholar]
- [2].Imdad A, Mayo-Wilson E, Herzer K, Bhutta ZA. Vitamin A supplementation for preventing morbidity and mortality in children from six months to five years of age. The Cochrane database of systematic reviews. 2017;3:Cd008524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Scrimshaw NS, Taylor CE, Gordon JE. Interactions of nutrition and infection. Monograph series World Health Organization. 1968;57:3–329. [PubMed] [Google Scholar]
- [4].Semba RD. Vitamin A, immunity, and infection. Clin Infect Dis. 1994;19:489–99. [DOI] [PubMed] [Google Scholar]
- [5].World Health Organization. Guideline: Vitamin A supplementation in infants and children 6–59 months of age. Geneva: World Health Organization; 2011. [Google Scholar]
- [6].Villamor E, Fawzi WW. Effects of vitamin a supplementation on immune responses and correlation with clinical outcomes. Clin Microbiol Rev. 2005;18:446–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].D’Ambrosio DN, Clugston RD, Blaner WS. Vitamin A metabolism: an update. Nutrients. 2011;3:63–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Olson JA. Serum levels of vitamin A and carotenoids as reflectors of nutritional status. Journal of the National Cancer Institute. 1984;73:1439–44. [PubMed] [Google Scholar]
- [9].Baeten JM, Richardson BA, Bankson DD, Wener MH, Kreiss JK, Lavreys L, Mandaliya K, Bwayo JJ, McClelland RS. Use of serum retinol-binding protein for prediction of vitamin A deficiency: effects of HIV-1 infection, protein malnutrition, and the acute phase response. American Journal of Clinical Nutrition. 2004;79:218–25. [DOI] [PubMed] [Google Scholar]
- [10].Gamble MV, Ramakrishnan R, Palafox NA, Briand K, Berglund L, Blaner WS. Retinol binding protein as a surrogate measure for serum retinol: studies in vitamin A-deficient children from the Republic of the Marshall Islands. American Journal of Clinical Nutrition. 2001;73:594–601. [DOI] [PubMed] [Google Scholar]
- [11].World Health Organization. Global prevalence of vitamin A deficiency in populations at risk 1995–2005. World Health Organization; 2009. [Google Scholar]
- [12].Stephensen CB, Gildengorin G. Serum retinol, the acute phase response, and the apparent misclassification of vitamin A status in the third National Health and Nutrition Examination Survey. Am J Clin Nutr. 2000;72:1170–8. [DOI] [PubMed] [Google Scholar]
- [13].Heinrich PC, Castell JV, Andus T. Interleukin-6 and the acute phase response. The Biochemical journal. 1990;265:621–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Tabone MD, Muanza K, Lyagoubi M, Jardel C, Pied S, Amedee-Manesme O, Grau GE, Mazier D. The role of interleukin-6 in vitamin A deficiency during Plasmodium falciparum malaria and possible consequences for vitamin A supplementation. Immunology. 1992;75:553–4. [PMC free article] [PubMed] [Google Scholar]
- [15].Baumann H, Gauldie J. The acute phase response. Immunology today. 1994;15:74–80. [DOI] [PubMed] [Google Scholar]
- [16].Gruys E, Toussaint MJ, Niewold TA, Koopmans SJ. Acute phase reaction and acute phase proteins. J Zhejiang Univ Sci B. 2005;6:1045–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Olson JA. Vitamin A metabolism during infection. Journal of Nutritional Immunology. 1995;4:17–34. [Google Scholar]
- [18].Rosales FJ, Ritter SJ, Zolfaghari R, Smith JE, Ross AC. Effects of acute inflammation on plasma retinol, retinol-binding protein, and its mRNA in the liver and kidneys of vitamin A-sufficient rats. J Lipid Res. 1996;37:962–71. [PubMed] [Google Scholar]
- [19].Beisel WR. Infection-induced depression of serum retinol--a component of the acute phase response or a consequence? Am J Clin Nutr. 1998;68:993–4. [DOI] [PubMed] [Google Scholar]
- [20].Barbosa KC, Cunha DF, Jordao AA Jr., Weffort VR, Cunha SF. Transient decreased retinol serum levels in children with pneumonia and acute phase response. Jornal de pediatria. 2011;87:457–60. [DOI] [PubMed] [Google Scholar]
- [21].Jacobs AL, Leitner ZA, Moore T, Sharman IM. Vitamin A in rheumatic fever. The Journal of clinical nutrition. 1954;2:155–61. [DOI] [PubMed] [Google Scholar]
- [22].Mitra AK, Alvarez JO, Guay-Woodford L, Fuchs GJ, Wahed MA, Stephensen CB. Urinary retinol excretion and kidney function in children with shigellosis. Am J Clin Nutr. 1998;68:1095–103. [DOI] [PubMed] [Google Scholar]
- [23].Velasquez-Melendez G, Okani ET, Kiertsman B, Roncada MJ. Vitamin A status in children with pneumonia. European journal of clinical nutrition. 1995;49:379–84. [PubMed] [Google Scholar]
- [24].Stephensen CB. Vitamin A, infection, and immune function. Annu Rev Nutr. 2001;21:167–92. [DOI] [PubMed] [Google Scholar]
- [25].Filteau SM. Vitamin A and the acute-phase response. Nutrition. 1999;15:326–8. [DOI] [PubMed] [Google Scholar]
- [26].Willumsen JF, Simmank K, Sotimehin SA, Naik R, Filteau SM. Vitamin A status following respiratory tract insult. International Vitamin A Consultative Group. Cairo: 1997. [Google Scholar]
- [27].Mitra AK, Alvarez JO, Wahed MA, Fuchs GJ, Stephensen CB. Predictors of serum retinol in children with shigellosis. Am J Clin Nutr. 1998;68:1088–94. [DOI] [PubMed] [Google Scholar]
- [28].Barffour MA, Schulze KJ, Coles CL, Chileshe J, Kalungwana N, Arguello M, Siamusantu W, Moss WJ, West KP Jr., Palmer AC. Comparability of inflammation-adjusted vitamin A deficiency estimates and variance in retinol explained by C-reactive protein and alpha1-acid glycoprotein during low and high malaria transmission seasons in rural Zambian children. Am J Trop Med Hyg. 2018;98:334–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Larson LM, Namaste SM, Williams AM, Engle-Stone R, Addo OY, Suchdev PS, Wirth JP, Temple V, Serdula M, Northrop-Clewes CA. Adjusting retinol-binding protein concentrations for inflammation: Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) project. Am J Clin Nutr. 2017;106:390s–401s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Thurnham DI, McCabe GP, Northrop-Clewes CA, Nestel P. Effects of subclinical infection on plasma retinol concentrations and assessment of prevalence of vitamin A deficiency: Meta-analysis. Lancet. 2003;362:2052–8. [DOI] [PubMed] [Google Scholar]
- [31].Freire WB, Ramírez-Luzuriaga MJ, Belmont P, Mendieta MJ, Silva-Jaramillo MK, Romero N, Sáenz K, Piñeiros P, Gómez LF, Monge R. The Ecuador National Health and Nutrition Survey of the Ecuadorian population from 0–59 years. ENSANUT-ECU 2012. Quito, Ecuador: Ministry of Public Health / National Institute of Statistics and Census; 2014. [Google Scholar]
- [32].Ruth CJ, Huey SL, Krisher JT, Fothergill A, Gannon BM, Jones CE, Centeno-Tablante E, Hackl LS, Colt S, Finkelstein JL, Mehta S. An Electronic Data Capture Framework (ConnEDCt) for Global and Public Health Research: Design and Implementation. J Med Internet Res. 2020;22:e18580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].World Health Organization. Serum retinol concentrations for determining the prevalence of vitamin A deficiency in populations. Vitamin and Mineral Nutrition Information System. Geneva: World Health Organization; 2011. [Google Scholar]
- [34].Pan American Health Organization. Report on the situation of Malaria in the Americas, 2014. Washington, D.C.: Pan American Health Organization; 2016. [Google Scholar]
- [35].Wessells KR, Peerson JM, Brown KH. Within-individual differences in plasma ferritin, retinol-binding protein, and zinc concentrations in relation to inflammation observed during a short-term longitudinal study are similar to between-individual differences observed cross-sectionally. Am J Clin Nutr. 2019;109:1484–92. [DOI] [PubMed] [Google Scholar]
- [36].Fish EN. The X-files in immunity: sex-based differences predispose immune responses. Nat Rev Immunol. 2008;8:737–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Garenne M Demographic evidence of sex differences in vulnerability to infectious diseases. J Infect Dis. 2015;211:331–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol. 2016;16:626–38. [DOI] [PubMed] [Google Scholar]
- [39].MacDonell SO, Miller JC, Harper MJ, Reid MR, Haszard JJ, Gibson RS, Houghton LA. A comparison of methods for adjusting biomarkers of iron, zinc, and selenium status for the effect of inflammation in an older population: a case for interleukin 6. Am J Clin Nutr. 2018;107:932–40. [DOI] [PubMed] [Google Scholar]
- [40].World Health Organization. Indicators for assessing vitamin A deficiency and their application in monitoring and evaluating intervention programmes: World Health Organization; Geneva; 1996. [Google Scholar]
- [41].Huiming Y, Chaomin W, Meng M. Vitamin A for treating measles in children. The Cochrane database of systematic reviews. 2005:CD001479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Imdad A, Herzer K, Mayo-Wilson E, Yakoob MY, Bhutta ZA. Vitamin A supplementation for preventing morbidity and mortality in children from 6 months to 5 years of age. The Cochrane database of systematic reviews. 2010:CD008524. [DOI] [PubMed] [Google Scholar]
- [43].Humphrey JH, Iliff PJ, Marinda ET, Mutasa K, Moulton LH, Chidawanyika H, Ward BJ, Nathoo KJ, Malaba LC, Zijenah LS, Zvandasara P, Ntozini R, Mzengeza F, Mahomva AI, Ruff AJ, Mbizvo MT, Zunguza CD, Group ZS. Effects of a single large dose of vitamin A, given during the postpartum period to HIV-positive women and their infants, on child HIV infection, HIV-free survival, and mortality. J Infect Dis. 2006;193:860–71. [DOI] [PubMed] [Google Scholar]
- [44].Mehta S, Fawzi W. Effects of vitamins, including vitamin A, on HIV/AIDS patients. Vitamins and hormones. 2007;75:355–83. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


