Abstract
Background:
We do not yet have validated biomarkers to predict response and outcome within hormone receptor-positive/HER2-positive (HR+/HER2+) breast cancer (BC). The PAM50-based chemo-endocrine score (CES) predicts chemo-endocrine sensitivity in HR+/HER2-negative BC. Here, we evaluate the relationship of CES with response and survival in HR+/HER2+ BC.
Methods:
Intrinsic subtype and clinicopathological data were obtained from 7 studies in which patients were treated with HER2-targeted therapy either with endocrine therapy (ET) or with chemotherapy (CTX). CES was evaluated as a continuous variable and categorically from low to high scores (CES-C [chemo-sensitive], CES-U [uncertain] and CES-E [endocrine-sensitive]. We first analyzed each dataset individually, and then all combined. Multivariable analyses were used to test CES association with pathologic complete response (pCR) and disease-free survival (DFS).
Results:
457 patients were included (112 with ET and 345 with CTX). In the combined cohort, CES-C, CES-U and CES-E were identified in 60%, 23% and 17% of the patients, respectively. High CES (i.e. CES-E) was associated with a lower probability of achieving pCR independently of clinical characteristics, therapy, intrinsic subtype, and study (adjusted odd ratio=0.42; p=0.016). 295 patients were analyzed for DFS with a median follow-up of 66 months. High CES was also associated with better DFS (adjusted hazard ratio=0.174, p=0.003) independently of pCR, clinical characteristics and intrinsic subtype. In patients with residual disease, the adjusted DFS hazard ratio of CES was 0.160 (p=0.012).
Conclusions:
In HER2+/HR+ BC, CES is useful for predicting chemo-endocrine sensitivity and provides additional prognostication beyond intrinsic subtype and clinicopathologic characteristics.
Keywords: intrinsic subtype, HER2-positive, Chemo-Endocrine Score, PAM50, breast cancer, gene expression
Introduction
Over the past 20 years, the prognosis of early-stage HER2-positive breast cancer has been improved by the implementation of HER2-targeted therapies in clinical practice(1) and neoadjuvant treatment has become the standard of care(2). In patients with hormone receptor (HR)-positive / HER2-positive (HR+/HER2+) breast cancer, neoadjuvant chemotherapy plus HER2-targeting results in pathologic complete response rates of up to 45%(3–6) and excellent survival outcomes. However, despite these standard therapies, 15-20% of HR+/HER2+ recur at distant sites(7–9) and standard regimens are complex and toxic, typified by polychemotherapy and 1–3 anti-HER2 drugs. Besides, while achievement of pCR after neoadjuvant treatment is a prognostic factor in HER2+ breast cancer, it has a stronger impact on disease-free survival (DFS) in the HR-negative/HER2+ compared to the HR+/HER2+ subgroup(10,11).
Molecular characterization studies have identified and extensively investigated the four main intrinsic subtypes (by PAM50 subtyping(12)) within HR+/HER2+ disease. Within HR+/HER2+, around 30% of tumors are HER2-Enriched (HER2-E), the subtype associated with high HER2/EGFR-pathway activation, increased proliferation rates, and an immune-activated stroma with elevated tumor-infiltrating lymphocyte levels(13–15). From a prognostic point of view, however, the HER2-E subtype is associated with worse prognosis(16,17), which appears in part to relate to drug sensitivity variability resulting in high pCR rates in about half, with attendant good outcomes, but much poorer outcome among those with residual disease(18). On the other hand, around 60-70% of HR+/HER2+, tumors are luminal A or B, which are estrogen receptor-dependent tumors, with lower HER2/EGFR pathway activation and a high rate of PIK3CA mutations(19), and which are associated with lower pCR rates to anti-HER2 treatment but better prognosis(16–18). Finally, less than the 10% are basal-like, which are characterized by the high expression of proliferation-related genes, intermediate expression of HER2-related genes, and low expression of luminal-related genes.
Thus, HR+/HER2+ disease is clinically and biologically heterogeneous and further subclassifications are needed to better tailor current and future treatments. We previously reported a Chemo-Endocrine Score (CES), which is based on the PAM50 gene expression-based assay plus expression of signatures related to response to chemotherapy or endocrine therapy (ET) in the neoadjuvant setting (20). In HR+ /HER2-negative disease, where decision-making centers on value of chemotherapy added to ET, high CES was associated with ET sensitivity and low CES was associated with high chemotherapy sensitivity beyond PAM50 Risk of Relapse (ROR) score, and beyond intrinsic subtype. In HR+/HER2+ disease, treatment options include either chemotherapy or ET added to HER2-targeting drugs, noting however, that we do not have an effective method to predict the likelihood of response or outcome to either approach. In this report, we evaluated the association of CES with pCR and DFS following anti-HER2-based therapy given with either chemotherapy or ET in HR+/HER2+ breast cancer across seven studies.
Materials and Methods
Study Designs and Participants
Clinicopathologic characteristics and PAM50 gene expression data from 457 patients with HR+/HER2+ early breast tumors were obtained from 7 independent neoadjuvant studies summarized in Table 1 and Table S1. The main inclusion criteria of the 7 cohorts have been previously reported(3,21-26), and all were either entirely or partly comprised of patients with HR+/HER2+ that were analyzed in this study. The trials differed by neoadjuvant therapy, which included HER2-targeting in all plus either chemotherapy or ET. Adjuvant therapy sometimes included chemotherapy but in all trials these patients were recommended to receive a total of 1 year of anti-HER2 adjuvant therapy with a trastuzumab-based regimen (none received trastuzumab emtansine) regardless of pCR status, and at least 5 years ET. These trials preceded the use of trastuzumab emtansine in residual disease.
Table 1:
Clinical-pathological characteristics and subtypes distribution of the overall study cohort.
| Parameter | Parameter Value | Pooled N (%) |
|---|---|---|
| Age, years | <50 | 211 (46.2) |
| ≥50 | 245 (53.8) | |
| Stage (%) | I | 46 (10.1) |
| II | 337 (73.7) | |
| III | 74(16.2) | |
| Tumor size | T1 | 81(17.7) |
| T2 | 289(63.2) | |
| T3 | 62(13.6) | |
| T4 | 13(2.8) | |
| Missing | 12(2.6) | |
| Nodal status | Negative | 232(50.8) |
| Positive | 216 (47.3) | |
| Missing | 9(1.9) | |
| HER2 treatment | Trastuzumab alone | 169 (37.0) |
| Lapatinib alone | 43 (9.4) | |
| Trastuzumab and lapatinib | 145 (31.7) | |
| Trastuzumab and pertuzumab | 100 (21.9) | |
| Chemotherapy | No | 112 (24.5) |
| Anthracyclines/Taxanes | 187 (40.9) | |
| Taxanes | 158 (34.6) | |
| pCR ypT0/is | Yes | 165 (36.1) |
| No | 292 (63.9) | |
| PAM50 | Luminal A | 110 (24.1) |
| Luminal B | 109 (23.9) | |
| HER2-E | 224 (49) | |
| Basal-like | 14 (3.1) | |
| CES | CES-E | 78 (17.0) |
| CES-U | 105 (23.0) | |
| CES-C | 274 (60.0) | |
PerELISA(21) (NCT02411344) was a single-arm phase II study of 64 patients with Stage I-III HR+/HER2+ disease. After diagnostic core biopsy including baseline Ki67 evaluation, the patients started letrozole for 2 weeks followed by a core biopsy for Ki67 central evaluation. Patients defined as molecular responders (Ki67 relative reduction >20% from baseline) started therapy with the combination of letrozole, trastuzumab and pertuzumab. Trastuzumab and pertuzumab were administered every 3 weeks for 5 cycles; letrozole, was continued until surgery was performed (within 3 weeks of the last dose of trastuzumab and pertuzumab). Patients defined as molecular non-responders discontinued letrozole and received weekly paclitaxel combined with pertuzumab and trastuzumab.
SOLTI-1114 PAMELA (NCT01973660)(22) was a single-arm phase II neoadjuvant trial within HER2+ breast cancer, where 151 patients were treated with lapatinib and trastuzumab for 18 weeks. Patients with HR+ breast cancer (N=75) also received neoadjuvant letrozole or tamoxifen according to menopausal status.
CALGB 40601 (NCT00770809)(3,18) was a phase III trial where 305 women (176 with HR+ tumors) with stage II-III HER2+ disease were randomized to receive paclitaxel weekly for 16 weeks with trastuzumab, lapatinib, or both. Patients were recommended to receive doxorubicin plus cyclophosphamide for four cycles and completion of one year trastuzumab adjuvantly.
CherLOB study (NCT00429299)(23) was a randomized phase II study of 121 patients with stage II-IIIA, HER2+ BC, 72 of which were HR+. These patients received preoperative chemotherapy with weekly paclitaxel followed by FEC plus trastuzumab, lapatinib, or both. Treatment after surgery was left to treating physician discretion.
SOLTI-1002 Opti-HER (NCT01669239)(24) was a phase II single-arm study of six 3-week cycles of non-pegylated liposomal doxorubicin, paclitaxel, trastuzumab, and pertuzumab as neoadjuvant therapy for 83 patients with stage II-IIIB HER2+ breast cancer, 57 of which were HR+.
The Hospital Clinic of Barcelona (HCB) cohort(25) is a consecutive series of 76 HR+/HER2+ tumor samples from 84 patients treated with neoadjuvant anti-HER2 chemotherapy according to routine clinical practice.
The Catalan Institute of Oncology (ICO) cohort(26) includes 44 HR+/HER2+ baseline tumors from a consecutive series of 150 patients with stage II-IIIC HER2+ breast cancer treated with trastuzumab added to neoadjuvant chemotherapy with weekly paclitaxel for 12 weeks followed by 4 cycles of FEC.
These studies were undertaken following the Good Clinical Practice guidelines and the World Medical Association Declaration of Helsinki. All patients provided written informed consent. Approvals for the studies were obtained from independent ethics committees. This study is reported according to REMARK recommendations(27).
Endpoints
This study’s primary aim was to investigate the association of CES as a continuous variable to pCR in primary HR+/HER2+ breast cancer treated with one or two HER2 targeting agents plus either ET or CTX. Secondary aims were to determine the association of CES groups to pCR using the previously reported cut-offs, association with letrozole monotherapy response and CES in PerELISA, and to test the relationship of CES to DFS.
pCR was defined as no invasive cells at a microscopic examination of the primary tumor at surgery (ypT0/Tis). DFS was defined as the interval from surgery to ipsilateral invasive breast tumor recurrence, regional recurrence, distant recurrence, or death of any cause, whichever occurred first. The studies with survival follow up were CALGB 40601, CherLOB, HCB and ICO.
PAM50 intrinsic subtyping
All tumors were assigned to an intrinsic molecular subtype of breast cancer (Luminal A, Luminal B, HER2-E, Basal-like) and the normal-like group using the research-based PAM50 subtype predictor. The PAM50 subtyping assay was performed using the nCounter as previously described (22,28,29), except in CALGB 40601 and CherLOB. In CALGB 40601, the RNAseq gene expression data from the PAM50 genes was first extracted and then normalized using a HER2 X ER subgroup-specific gene centering method (i.e. 4 subgroups) followed by the PAM50 predictor (18,30). In CherLOB, a research-based PAM50 microarray-based assay was used(31). Original subtype calls obtained from each study were used. Patients with Normal-like intrinsic subtype, which consists mostly of normal tissue, were eliminated from the analysis. The PAM 50 ROR was calculated using weighted coefficients to the four subtypes and a proliferation score using a previously reported and validated formula(12,32).
Chemo-endocrine sensitive score
The CES was calculated as previously reported(20). From the PAM50 classification algorithm, we calculated the correlation coefficients (CC) of each sample to the PAM50 Luminal A and Basal-like subtype centroids. We then subtracted the 2 values to determinate the CES (CES=CC to Luminal A – CC to Basal-like). Samples with a positive score were identified as being more Luminal A-like and as more endocrine-sensitive than chemotherapy-sensitive. In contrast, samples with a negative score were identified as more Basal-like and thus as more chemotherapy-sensitive than endocrine-sensitive. CES was evaluated as a continuous variable, and as group categories (CES-E [endocrine sensitive], CES-U [uncertain] and CES-C [chemo-sensitive]) using the previously reported cutoffs (CES-E vs. CES-U group, cutoff = 0.70; CES-U vs. CES-C group, cutoff=0.30)(20). These cutoffs were based on tertile groups determined in HR+/HER2-negative GEICAM 2006-03 samples.
Statistical analysis
To compare the distribution of variables between 2 groups, we used Fisher’s exact test. Proportions and 95% confidence interval (CI) were also provided. Univariate and multivariable logistic regression analyses were done to investigate the association of each variable with pCR. Odds ratios (ORs) and 95% CIs were calculated for each variable. Univariate and multivariable Cox proportional hazard regression analyses were performed to investigate each variable’s association with DFS. The significance level was set to a two-sided α of 0.05. Pearson correlation was assessed to analyze the relationship between continuous variables. To evaluate the accuracy of pCR predictors, the area under the ROC curve (AUC) was used. We used R version 3.3.1 for all the statistical analyses.
Results
Clinicopathologic characteristics of the combined cohort
A total of 457 patients with HR+/HER2+ breast cancer treated with anti-HER2-based neoadjuvant regimens were included in the analysis (Table 1 and Table S1). All datasets included all clinicopathological variables and pCR status. The mean age was 52.1 years and most patients had tumors no larger than 5 cm (80.9% T0-T2). 53.6% of patients received dual HER2-blockade with trastuzumab combined with pertuzumab or lapatinib, and 24.5% of patients received dual HER2-blockade treatment without chemotherapy (in the neoadjuvant setting).
Distribution of CES Within HR-Positive/HER2-Positive Breast Cancer
Of the 457 HR+/HER2+ tumors analyzed in the overall study cohort (Table 1 and Table S1), 17.0% were CES-E, 23.0% were CES-U, and 60.0% were CES-C. Figure 1A provides a comparison of CES across the different studies. Although CES distributions differed by the trial population (p=0.032), all CES groups were represented in each of the 7 cohorts.
Fig 1. PAM50 ROR, intrinsic subtype and CES in 457 primary breast cancers.
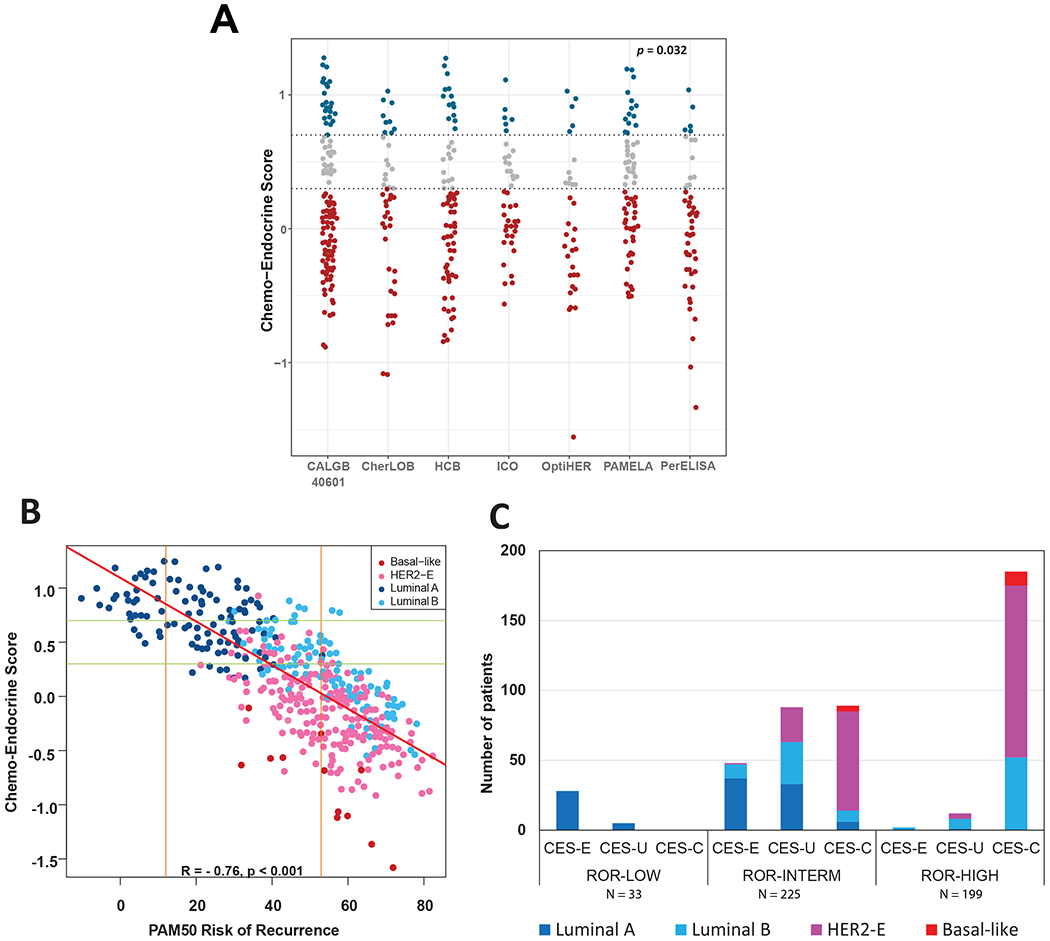
(A) CES stratified by Study. The two horizontal lines indicate the cutoffs of each CES group. P-value (p) was calculated by comparing mean values across all studies. (B) A scatter plot of CES score and ROR score, colored by subtype. The two horizontal lines indicate the cutoffs of each CES group. The two vertical lines indicate the cutoffs of each PAM50 ROR group. Red line represents the regression line. Pearson correlation coefficient (R) with significance (p value) is presented (C) Number of patients in each CES group based on ROR. Each bar is colored according to the subtype distribution.
As expected, a relationship between CES, intrinsic subtype and ROR was seen (Figure 1B, Fig S1). CES and ROR were found highly negatively correlated (correlation coefficient = −0.76). The results revealed that in the ROR-low group (N=33), 84.8% of cases were identified as CES-E and 100% were of the Luminal A subtype. In the ROR-high (N=199), 92% of the samples were identified as CES-C; non-luminal and Luminal B subtypes represented 73% and 27% of the ROR-high/CES-C cases, respectively. In the ROR-intermediate group (N=225), high heterogeneity was observed with all CES groups evenly represented. In terms of intrinsic subtype biology, Luminal A, Luminal B and non-Luminal subtypes represented 32%, 21% and 47%, respectively (Figure 1C) in the ROR-intermediate group.
Correlation of CES and pCR
In trials of chemotherapy plus HER2-targeting, pCR rates were significantly lower in the CES-E group (8%), compared with CES-U (31%) and CES-C groups (55%) (p<0.001). This relationship was also seen in trials of ET plus HER2-targeting (PAMELA, PerELISA molecular responders) (Fig 2). pCR was higher among patients with CES-E who were selected as molecular responders to letrozole alone in PerELISA, and approached the pCR rates of CES-C, although the numbers are small.
Fig 2.

Rates of pathological complete response (pCR) according to the chemo-endocrine score (CES) group in the 7 neoadjuvant clinical studies, in chemotherapy HER2 neoadjuvant trials and in the endocrine HER2 neoadjuvant trials. Bars denote 95% confidence intervals.
We next evaluated the association of baseline clinicopathological characteristics, intrinsic subtype, ROR and CES with pCR. In univariate analysis, neoadjuvant chemotherapy, HER2-E intrinsic subtype, high ROR, trial, and low CES (as a continuous variable or as group categories) were statistically significantly associated with pCR (Table 2). In a multivariable model including these 5 variables, HER2-E molecular subtype, neoadjuvant chemotherapy and CES remained significantly associated with pCR, and ROR was not; the adjusted OR of CES for achieving pCR was 0.39 (95% CI 0.19–0.81; p=0.011) (Table 2).
Table 2:
Logistic regression analyses of pathological complete response including CES, intrinsic subtypes and ROR in the entire cohort.
| Univariate Analysis | Multivariable Analysis | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variables | N | pCR rate | OR | Lower 95% | Upper 95% | P | OR | Lower 95% | Upper 95% | P |
| Chemotherapy | ||||||||||
| No | 112 | 20.54% | 1 | - | - | - | 1 | - | - | - |
| Yes | 373 | 43.43% | 2.707 | 1.631 | 4.490 | <0.001* | 5.086 | 1.097 | 23.576 | 0.031* |
| ROR-P (cont. variable per unit) | 457 | - | 1.029 | 1.018 | 1.041 | <0.001* | 0.999 | 0.977 | 1.014 | 0.523 |
| ROR-P (Group) | ||||||||||
| ROR-P low | 33 | 6.06% | 1 | - | - | - | ||||
| ROR-P intermedium | 225 | 33.77% | 7.906 | 1.843 | 33.920 | <0.005 | ||||
| ROR-P high | 199 | 43.71% | 12.040 | 2.804 | 51.694 | <0.001* | ||||
| Subtype | ||||||||||
| Non-Her2E | 233 | 20.17% | 1 | - | - | - | 1 | - | - | - |
| HER2E | 224 | 52.67% | 4.405 | 2.912 | 6.663 | <0.001* | 2.945 | 1.733 | 5.005 | <0.001* |
| CES (cont. variable per unit) | 457 | - | 0.216 | 0.138 | 0.338 | <0.001* | 0.391 | 0.188 | 0.808 | 0.016* |
| CES (Group) | ||||||||||
| CES-E | 78 | 7.69% | 1 | - | - | - | ||||
| CES-U | 105 | 24.76% | 3.949 | 1.537 | 10.144 | 0.004* | ||||
| CES-C | 274 | 48.54% | 11.319 | 4.761 | 26.907 | <0.001* | ||||
| Study | ||||||||||
| CALGB 40601 | 131 | 39.69% | 1 | - | - | - | 1 | - | - | - |
| CherLOB | 46 | 23.91% | 0.477 | 0.223 | 1.023 | 0.057 | 0.653 | 0.278 | 1.534 | 0.328 |
| HCB | 76 | 35.52% | 0.837 | 0.222 | 1.023 | 0.551 | 0.694 | 0.363 | 1.326 | 0.268 |
| ICO | 42 | 35.71% | 0.844 | 0.410 | 1.737 | 0.695 | 0.745 | 0.341 | 1.625 | 0.460 |
| OptiHER | 35 | 71.43% | 3.798 | 1.685 | 8.560 | 0.001* | 3.542 | 1.470 | 8.535 | 0.004* |
| PAMELA | 76 | 18.42% | 0.343 | 0.174 | 0.675 | 0.001* | 1.693 | 0.309 | 9.255 | 0.543 |
| PerELISA | 51 | 41.17% | 1.063 | 0.551 | 2.054 | 0.855 | 3.105 | 0.796 | 12.104 | 0.102 |
| Age (cont. variable) | 457 | - | 0.984 | 0.968 | 1.000 | 0.0519 | ||||
| Stage baseline | ||||||||||
| I | 46 | 36.95% | 1 | - | - | - | ||||
| II | 337 | 35.6% | 0.943 | 0.322 | 1.066 | 0.858 | ||||
| III | 81 | 37.84% | 1.038 | 0.485 | 2.222 | 0.923 | ||||
| Tumor size at baseline | ||||||||||
| cT1-2 | 370 | 37.02% | 1 | - | - | - | ||||
| cT3-4 | 75 | 29.33% | 0.706 | 0.411 | 1.211 | 0.206 | ||||
| Nodal status at baseline | ||||||||||
| 0 | 232 | 33.62% | 1 | - | - | - | ||||
| 1-2 | 216 | 38.42% | 1.232 | 0.837 | 1.813 | 0.290 | ||||
| Anti-HER2 | ||||||||||
| 1 | 227 | 37.00% | 1 | - | - | - | ||||
| 2 | 258 | 39.14% | 1.021 | 0.696 | 1.497 | 0.916 | ||||
When we did the same analysis limited to patients who received HER2 targeting plus neoadjuvant CTX and dual HER2 blockade, HER2-E intrinsic subtype, high ROR, trial, and low CES (as a continuous variable or as group categories) were statistically significantly associated with pCR in univariate analysis (Table S2). In a multivariable model including these 5 variables, HER2-E molecular subtype and CES remained significantly associated with pCR, and ROR was not; the adjusted OR of CES for achieving pCR was 0.41 (95% CI 0.19–0.89; p=0.024) (Table S2).
In the same analysis limited to those who received neoadjuvant ET (rather than CTX) and HER2 blockade, in univariate analysis, HER2-E intrinsic subtype, high ROR, study, and low CES (as a continuous variable or as group categories) were statistically significantly associated with pCR. In multivariable analysis, HER2-E molecular subtype remained significantly associated with pCR. CES. However, in the absence of chemotherapy, CES was not independently associated with pCR either as a continuous or categorical variable (Table S3). Finally, the AUC of CES to predict pCR was 0.71(95% CI 0.66-0.75) in the entire cohort, 0.70 in studies with neoadjuvant chemotherapy, and 0.69, and in studies without chemotherapy (Figure S2). The AUC for ROR to predict pCR were 0.63 (95% IC 0.58-0.67) in the entire cohort.
CES and endocrine sensitivity (PerELISA)
To further explore the CES’s ability to predict endocrine sensitivity and resistance in HR+ /HER2+, we evaluated the 51 (83.5%) samples from the PerELISA trial. Patients in this study received letrozole for 2 weeks followed by a core biopsy for Ki67 evaluation. Patients were defined as molecular responders if there was a Ki67 relative reduction >20% from baseline. As expected, CES was significantly associated with Ki67 decrease after 2 weeks in univariate analysis as a continuous variable (OR=27.45, 95% IC 3.50–215.51, p=0.001) (Fig 3). All patients with CES-E (n=5) or CES-U (n=10) tumors had a Ki67 relative reduction >20%. However, in the CES-C (n=36) only the 58.3% of patients had Ki67 relative reduction >20%.
Fig 3.

Probability of response (Ki67 relative reduction >20% from baseline) after 2 weeks of letrozole in monotherapy as a function of CES in PerELISA patients.
CES association with DFS
To better understand the relationship between prognosis and chemo-endocrine sensitivity in HR+/HER2+ breast cancer, we pooled survival data from CALGB 40601, ICO, HCB and CHERLOB for a total of 295 primary breast cancers treated with neoadjuvant chemotherapy plus HER2 blockade. The median follow-up was 72.7 months: 82.2 months for CALGB 40601, 66.6 months for CHERLOB, 38.6 months for HCB and 89.3 months for ICO. CES (as a continuous variable or as group categories) was found significantly associated with DFS (Fig 4A and Table S3). The hazard ratio between the CES-C group vs the CES-E group was 7.02 (95% CI 1.70-28.95, p<0.001). In multivariable analysis, pCR, baseline nodal status and CES provided independent predictive information for DFS, but intrinsic subtype and ROR did not; the adjusted hazard ratio of CES for DFS was 0.17 (95% CI 0.06–0.55; p=0.003) (Table S4).
Fig 4.

Survival curves in the combined HR-positive/HER2-positive breast cancer data set in 295 patients. A) Disease-free survival (DFS) according to CES group status. B) Disease-free survival according to pathological complete response and CES group status. Estimates of DFS were from Kaplan–Meier curves and tests of differences by two-sided log-rank test. Vertical ticks represent censoring events. DFS was defined as the interval from surgery to ipsilateral invasive breast tumor recurrence, regional recurrence, distant recurrence, or death of any cause, whichever occurred first.
Since pCR is a known prognostic factor in HR+/HER2+ breast cancer, combined survival analyses by pCR and CES status were carried out (Figure 4B). In the pCR and non-pCR group, patients with a CES-E and CES-U tumor have better survival than those with CES-C. Within patients that achieved a pCR, no variable was found to be significantly associated with DFS in univariate analyses. Within patients with residual disease, CES (as a continuous variable or as group categories) was found to be significantly associated with DFS in univariate and multivariable analyses after adjustment for ROR, PAM50 intrinsic subtypes and the other clinicopathological variables (adjusted hazard ratio 0.14; 95% CI 0.04–0.51.; p=0.003) (Table S5). Among them, nodal status before treatment was significantly associated with DFS (adjusted hazard ratio 2.21; 95% CI 1.13–4.34.; p=0.021). Finally, no statistically significant interaction (P = 0.783) was observed between CES (as a continuous variable) and pCR in DFS analysis.
Discussion
As previously described(33), the creation of drugs effective against HER2+ breast cancer has become more prevalent within the last 10 years. For example, since trastuzumab first arose, metastatic and/or early disease settings have borne witness to more compelling and tolerable anti-HER2 drugs and thereby, significant, and positive impact on survival outcomes(34–36). Nevertheless, HR+/HER2+ disease is clinically and biologically heterogeneous and current treatments do not confer the same degree of benefits onto all patients (37). For example, it is unclear how to choose between an ET or CTX backbone to add to the HER2-targeting. Optimized treatment tailoring using biomarkers will welcome the conception of prospective trials that aim to advance precision medicine in this subgroup of patients with breast cancer.
Herein, we evaluated the association of CES with response and survival outcomes in a large combined dataset of newly diagnosed patients with HR+/HER2+ disease treated with anti-HER2 neoadjuvant therapy and made the following observations. First, the CES predicts pCR in HR+/HER2+ breast cancer and its predictive value is independent of standard clinicopathological variables, and PAM50 ROR or intrinsic subtype. The maintained relationship of CES and pCR in patients treated on ET plus anti-HER2 drugs (rather than chemotherapy plus antiHER2) suggests that the CES predictive capability is driven more by HER2 than by HR. Second, CES provided independent prognostic information beyond standard clinicopathological variables, intrinsic subtype, and ROR. Third, within patients that do not achieve a pCR, the CES can identify a group of patients with excellent disease-free survival without trastuzumab emtansine (T-DM1). While additional validation of this prognostic tool is needed, this may be the CES’ greatest clinical utility given the absence of prognostic biomarkers to identify those truly benefiting from the current standard of escalating to T-DM1 for those with residual disease.
The prognostic abilities of the CES have been clinically validated in several studies in HR+/HER2-early breast cancer as providing value beyond PAM50 ROR and intrinsic subtype(20,38). In these previous studies, high CES values were associated with endocrine sensitivity and chemo-resistance and the low values associated with endocrine resistance and chemo-sensitivity. This study uniquely extended these findings to HER2+ disease treated with HER2-directed therapy, wich had not been previously examined. Overall, we found that in HR+/HER2+ early breast cancers, CES-E tumors show far lower sensitivity to antiHER2-based regimens in terms of pCR rates. In the PerELISA trial, HER2+/ER+ patients with a Ki67 drop after 2 weeks of letrozole (molecular responders) continued on letrozole, and trastuzumab/pertuzumab were added for another 12 weeks; non-responders were switched to paclitaxel with trastuzumab/pertuzumab(21). In this small but biologically intriguing molecular triaging study, CES was highly associated with likelihood of molecular response to ET, as expected. After molecular triaging, the pCR rate was 25% among molecular responders, with relatively similar pCR rates across CES groups selected on the basis of having excellent molecular response to ET. Among molecular non-responders, who by definition had inadequate response to ET alone and went on to chemotherapy plus HER2-targeting, virtually all had CES-C tumors and a remarkable pCR rate of 81%.
These findings support that CES may help us improve treatment for early stage HER2+ breast cancer, in whom new strategies are needed to optimize and de-intensify treatments. This is already a reality in HR+/HER-negative disease, where gene expression-based assays are routinely used to personalize treatment and, most importantly, to establish the benefits and needs of adjuvant chemotherapy(39). As noted, pCR is a well-validated clinically relevant endpoint that impacts on extent of surgery and the need for additional adjuvant therapy. A consistent finding over the past decade is the importance of intrinsic subtype in predicting pCR, and that HER2-E tumors have higher pCR rates (between 28-72%) when treated with anti-HER2 therapies (with/without chemotherapy), compared to other subtypes(40). We also found this to be true here. In our study CES provided predictive information independent of the HER2-E subtype, despite both being based in part on PAM50 subtyping.
There is currently an effort to de-escalate the treatment in HR+/HER2+ early disease, with different trials studying combinations with dual HER2 blockade, or antibody-drug conjugates and/or with hormone therapy. For example, the phase II clinical trial PHERGAIN (41) (NCT03161353) is evaluating the combination of pertuzumab and trastuzumab (and endocrine therapy if HR+) without chemotherapy for those patients who achieve a pCR or the Phase 2 TOUCH(42) (NCT03644186) trial is examined the role of neoadjuvant therapy with palbociclib, letrozole, pertuzumab and trastuzumab versus paclitaxel, trastuzumab and pertuzumab in postmenopausal women with HR+/HER2+ breast cancer. Presumably, patients with CES-E tumors will have a greater benefit from combinations with hormonal therapy compared to patients with CES-C tumors.
We were also able to examine CES and survival outcomes. Patients that achieve pCR appear to do well regardless of CES status. However, we found that CES was prognostic in patients with residual disease, which represents ~50% of HR+/HER2+ breast cancer. This might have implications for management of these patients. Our study suggests a low risk of cancer recurrence in the CES-E group with residual disease, which represented 25% of the residual disease population. Although the KATHERINE trial demonstrated that administration of adjuvant T-DM1 in patients with residual disease after neoadjuvant treatment was superior to trastuzumab regardless of HR status(35), it is possible that the absolute benefit of adjuvant T-DM1 might be low in CES-E HER2+ early breast cancer and may permit omission of this expensive drug with additional toxicity.
Our study has several limitations. First, the clinical cohorts in this study were powered for heterogeneous primary endpoints, which have been evaluated in primary publications. Second, although the data presented here validates CES from a clinical perspective, and the PAM50 assay on the nCounter platform allows the clinical implementation in a highly reproducible manner (43,44), further analytical validation of the CES methodology needed. Third, our data are mostly based on trastuzumab or the combination of trastuzumab plus lapatinib, and our findings will require confirmation in additional studies that test the combination of trastuzumab plus pertuzumab. Moreover, most of our trials included chemotherapy with HER2-targeting; pursuing findings in all-biologic regimens (i.e. no chemotherapeutics) will require larger sample sizes from cohorts of that type. Fourth,other promising molecular biomarkers, such as stromal tumor infiltrating-lymphocytes and immune gene signatures, were not uniformly available, and thus were not examined in this study.
Another important consideration of our study is that these cutoffs are based on tertiles in HR+/HER2-negative disease from the original publication and we did not attempt to identify an optimal cutoff(s) for CES in HR+/HER2+, but rather focused on the association of the continuous expression of CES with each endpoint. In any case, the fact that all seven testing sets gave very similar results and were found independently of the platform/protocol used argues in favor of a robust finding. In addition, considering that the CES is not scaled per dataset, it can be applied to any new dataset after calculating PAM50 subtype predictor.
To conclude, CES at diagnosis provides useful prognostic and predictive information for HR+/HER2+ patients. Further studies are needed to determine the role of CES in treatment decision-making at diagnosis in this population.
Supplementary Material
Statement of translational relevance.
Hormone receptor-positive/HER2-positive (HR+/HER2+) breast cancer (BC) is clinically and biologically heterogeneous, with increasingly complex treatment and efforts to tailor therapy based primarily on clinical features. To dateno predictive and/or prognostic biomarkers have been validated within this subgroup of patients, and with multiple treatment options, predictors of response and/or survival are urgently needed. Here, we present the PAM50-based Chemo-Endocrine Score (CES) clinical validation in 457 patients with early HR+/HER2+ BC treated with neoadjuvant anti-HER2-based therapy either combined with endocrine therapy or chemotherapy. Our study found CES to be strongly associated with pathologic complete response and disease-free survival beyond other clinicopathological and genomic biomarkers. In particular, high CES scores may be clinically useful in identifying patients with a low risk of recurrence despite not achieving a pCR after neoadjuvant therapy, and who may not need treatment escalation with additional systemic therapies such as T-DM1.
Acknowledgements
Fundación SEOM, Becas FSEOM para Formación en Investigación en Centros de Referencia en el Extranjero 2018 (to TP). Instituto de Salud Carlos III - PI16/00904 (to A.P.), Pas a Pas (to A.P.), Save the Mama (to A.P.), Breast Cancer Now - 2018NOVPCC1294 (to A.P.). Fundación SEOM, Becas FSEOM para Formación en Investigación en Centros de Referencia en el Extranjero 2016 (to A. F-M). BCRF (L.A.C, C.M.P), Susan G Komen (L.A.C, C.M.P), NCI SPORE (P50-CA58823), R01-CA229409 and Alliance (L.A.C., C.M.P).
Conflict of interest:
AP has declared an immediate family member being employed by Novartis, personal honoraria from Pfizer, Novartis, Roche, MSD Oncology, Lilly and Daiichi Sankyo, travel, accommodations and expenses paid by Daiichi Sankyo, research funding from Roche and Novartis, consulting/advisory role for NanoString Technologies, Amgen, Roche, Novartis, Pfizer and Bristol-Myers Squibb and patent PCT/EP2016/080056: HER2 AS A PREDICTOR OF RESPONSE TO DUAL HER2 BLOCKADE IN THE ABSENCE OF CYTOTOXIC THERAPY. PFC had declared consultant role for Novartis, Eli Lilly, Astra Zeneca and Tesaro, honoraria from BMS, Roche, Eli Lilly, Novartis and AstraZeneca, research funding from Novartis, Roche, BMS, Merck-KGa, Italian Ministry of Health, Veneto Secretary of Health and University of Padova. CMP is an equity stock holder and consultant of BioClassifier LLC and is also listed an inventor on patent applications on the Breast PAM50. LAC declares that Companies who have provided funds to her institution in the past 1-2 years either for her service on advisory/consultative programs or sponsored research were Genentech, Roche, Novartis, Seattle Genetics, G1 Therapeutics, Immunomedics and Innocrin. The other authors have nothing to declare.
Footnotes
Availability of data and materials:
The datasets generated and analyzed during this study are available from the corresponding authors on reasonable request.
CALGB 40601: FASTQ files from RNAseq data are available via the NCBI dbGAP repository under accession number phs001570.v2.p1. The star-salmon upper quartile normalized gene expression matrix is available in GEO under the accession number GSE116335.
CherLOB: Gene expression data are available in GEO under the accession number GSE66399.
Reference
- 1.Loibl S, Gianni L. HER2-positive breast cancer. The Lancet 2017;389:2415–29 [DOI] [PubMed] [Google Scholar]
- 2.Senkus E, Kyriakides S, Ohno S, Penault-Llorca F, Poortmans P, Rutgers E, et al. Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of oncology 2015;26:v8–v30 [DOI] [PubMed] [Google Scholar]
- 3.Carey LA, Berry DA, Cirrincione CT, Barry WT, Pitcher BN, Harris LN, et al. Molecular heterogeneity and response to neoadjuvant human epidermal growth factor receptor 2 targeting in CALGB 40601, a randomized phase III trial of paclitaxel plus trastuzumab with or without lapatinib. Journal of Clinical Oncology 2016;34:542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robidoux A, Tang G, Rastogi P, Geyer CE Jr, Azar CA, Atkins JN, et al. Lapatinib as a component of neoadjuvant therapy for HER2-positive operable breast cancer (NSABP protocol B-41): an open-label, randomised phase 3 trial. The lancet oncology 2013;14:1183–92 [DOI] [PubMed] [Google Scholar]
- 5.De Azambuja E, Holmes AP, Piccart-Gebhart M, Holmes E, Di Cosimo S, Swaby RF, et al. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): survival outcomes of a randomised, open-label, multicentre, phase 3 trial and their association with pathological complete response. The lancet oncology 2014;15:1137–46 [DOI] [PubMed] [Google Scholar]
- 6.Schneeweiss A, Chia S, Hickish T, Harvey V, Eniu A, Hegg R, et al. Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: a randomized phase II cardiac safety study (TRYPHAENA). Annals of oncology 2013;24:2278–84 [DOI] [PubMed] [Google Scholar]
- 7.Hwang K-T, Kim J, Jung J, Chang JH, Chai YJ, Oh SW, et al. Impact of breast cancer subtypes on prognosis of women with operable invasive breast cancer: a population-based study using SEER database. Clinical Cancer Research 2019;25:1970–9 [DOI] [PubMed] [Google Scholar]
- 8.Lambertini M, Campbell C, Gelber RD, Viale G, McCullough A, Hilbers F, et al. Dissecting the effect of hormone receptor status in patients with HER2-positive early breast cancer: exploratory analysis from the ALTTO (BIG 2-06) randomized clinical trial. Breast cancer research and treatment 2019;177:103–14 [DOI] [PubMed] [Google Scholar]
- 9.Cameron D, Piccart-Gebhart MJ, Gelber RD, Procter M, Goldhirsch A, de Azambuja E, et al. 11 years’ follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive early breast cancer: final analysis of the HERceptin Adjuvant (HERA) trial. The Lancet 2017;389:1195–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. The Lancet 2014;384:164–72 [DOI] [PubMed] [Google Scholar]
- 11.Huober J, Holmes E, Baselga J, de Azambuja E, Untch M, Fumagalli D, et al. Survival outcomes of the NeoALTTO study (BIG 1–06): updated results of a randomised multicenter phase III neoadjuvant clinical trial in patients with HER2-positive primary breast cancer. European journal of cancer 2019;118:169–77 [DOI] [PubMed] [Google Scholar]
- 12.Parker JS, Mullins M, Cheang MC, Leung S, Voduc D, Vickery T, et al. Supervised risk predictor of breast cancer based on intrinsic subtypes. Journal of clinical oncology 2009;27:1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prat A, Pascual T, De Angelis C, Gutierrez C, Llombart-Cussac A, Wang T, et al. HER2-enriched subtype and ERBB2 expression in HER2-positive breast cancer treated with dual HER2 blockade. JNCI: Journal of the National Cancer Institute 2020;112:46–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nuciforo P, Pascual T, Cortés J, Llombart-Cussac A, Fasani R, Paré L, et al. A predictive model of pathologic response based on tumor cellularity and tumor-infiltrating lymphocytes (CelTIL) in HER2-positive breast cancer treated with chemo-free dual HER2 blockade. Annals of Oncology 2018;29:170–7 [DOI] [PubMed] [Google Scholar]
- 15.Cejalvo JM, Pascual T, Fernández-Martínez A, Brasó-Maristany F, Gomis RR, Perou CM, et al. Clinical implications of the non-luminal intrinsic subtypes in hormone receptor-positive breast cancer. Cancer treatment reviews 2018;67:63–70 [DOI] [PubMed] [Google Scholar]
- 16.Conte PF, Griguolo G, Dieci MV, Bisagni G, Brandes AA, Frassoldati A, et al. PAM50 HER2-enriched subtype as an independent prognostic factor in early-stage HER2+ breast cancer following adjuvant chemotherapy plus trastuzumab in the ShortHER trial. American Society of Clinical Oncology; 2019. [Google Scholar]
- 17.Prat A, Carey LA, Adamo B, Vidal M, Tabernero J, Cortés J, et al. Molecular features and survival outcomes of the intrinsic subtypes within HER2-positive breast cancer. Journal of the National Cancer Institute 2014;106:dju152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fernandez-Martinez A, Krop IE, Hillman DW, Polley M-Y, Parker JS, Huebner L, et al. Survival, Pathologic Response, and Genomics in CALGB 40601 (Alliance), a Neoadjuvant Phase III Trial of Paclitaxel-Trastuzumab With or Without Lapatinib in HER2-Positive Breast Cancer. Journal of Clinical Oncology 2020:JCO. 20.01276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferrari A, Vincent-Salomon A, Pivot X, Sertier A-S, Thomas E, Tonon L, et al. A whole-genome sequence and transcriptome perspective on HER2-positive breast cancers. Nature communications 2016;7:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prat A, Lluch A, Turnbull AK, Dunbier AK, Calvo L, Albanell J, et al. A PAM50-Based Chemoendocrine Score for Hormone Receptor–Positive Breast Cancer with an Intermediate Risk of Relapse. Clinical Cancer Research 2017;23:3035–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guarneri V, Dieci M, Bisagni G, Frassoldati A, Bianchi G, De Salvo G, et al. De-escalated therapy for HR+/HER2+ breast cancer patients with Ki67 response after 2-week letrozole: results of the PerELISA neoadjuvant study. Annals of Oncology 2019;30:921–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Llombart-Cussac A, Cortés J, Paré L, Galván P, Bermejo B, Martínez N, et al. HER2-enriched subtype as a predictor of pathological complete response following trastuzumab and lapatinib without chemotherapy in early-stage HER2-positive breast cancer (PAMELA): an open-label, single-group, multicentre, phase 2 trial. The lancet oncology 2017;18:545–54 [DOI] [PubMed] [Google Scholar]
- 23.Guarneri V, Frassoldati A, Bottini A, Cagossi K, Bisagni G, Sarti S, et al. Preoperative chemotherapy plus trastuzumab, lapatinib, or both in human epidermal growth factor receptor 2–positive operable breast cancer: results of the randomized phase II CHER-LOB study. Journal of clinical oncology 2012;30:1989–95 [DOI] [PubMed] [Google Scholar]
- 24.Gavilá J, Oliveira M, Pascual T, Perez-Garcia J, Gonzàlez X, Canes J, et al. Safety, activity, and molecular heterogeneity following neoadjuvant non-pegylated liposomal doxorubicin, paclitaxel, trastuzumab, and pertuzumab in HER2-positive breast cancer (Opti-HER HEART): an open-label, single-group, multicenter, phase 2 trial. BMC medicine 2019;17:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Diaz-Redondo T, Lavado-Valenzuela R, Jimenez B, Pascual T, Gálvez F, Falcón A, et al. Different pathological complete response rates according PAM50 subtypes in HER2+ breast cancer patients treated with neoadjuvant Pertuzumab/Trastuzumab vs Trastuzumab plus standard chemotherapy: an analysis of Real World data. Frontiers in Oncology 2019;9:1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pernas S, Petit A, Climent F, Pare L, Perez-Martin J, Ventura L, et al. PAM50 subtypes in baseline and residual tumors following neoadjuvant trastuzumab-based chemotherapy in HER2-positive breast cancer: a consecutive-series from a single institution. Frontiers in oncology 2019;9:707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM. Reporting recommendations for tumor marker prognostic studies (REMARK). Journal of the National Cancer Institute 2005;97:1180–4 [DOI] [PubMed] [Google Scholar]
- 28.Vidal M, Peg V, Galvan P, Tres A, Cortes J, Ramon y Cajal S, et al. Gene expression-based classifications of fibroadenomas and phyllodes tumours of the breast. Mol Oncol 2015;9:1081–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prat A, Cheang MC, Galvan P, Nuciforo P, Pare L, Adamo B, et al. Prognostic Value of Intrinsic Subtypes in Hormone Receptor-Positive Metastatic Breast Cancer Treated With Letrozole With or Without Lapatinib. JAMA Oncol 2016;2:1287–94 [DOI] [PubMed] [Google Scholar]
- 30.Fernandez-Martinez A, Tanioka M, Fan C, Parker JS, Hoadley KA, Krop IE, et al. Genomic-based predictive biomarkers to anti-HER2 therapies: A combined analysis of CALGB 40601 (Alliance) and PAMELA clinical trials. American Society of Clinical Oncology; 2019. [Google Scholar]
- 31.Dieci M, Prat A, Tagliafico E, Paré L, Ficarra G, Bisagni G, et al. Integrated evaluation of PAM50 subtypes and immune modulation of pCR in HER2-positive breast cancer patients treated with chemotherapy and HER2-targeted agents in the CherLOB trial. Annals of Oncology 2016;27:1867–73 [DOI] [PubMed] [Google Scholar]
- 32.Nielsen TO, Parker JS, Leung S, Voduc D, Ebbert M, Vickery T, et al. A comparison of PAM50 intrinsic subtyping with immunohistochemistry and clinical prognostic factors in tamoxifen-treated estrogen receptor–positive breast cancer. Clinical cancer research 2010;16:5222–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schettini F, Pascual T, Conte B, Chic N, Brasó-Maristany F, Galván P, et al. HER2-enriched subtype and pathological complete response in HER2-positive breast cancer: A systematic review and meta-analysis. Cancer treatment reviews 2020;84:101965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Von Minckwitz G, Procter M, De Azambuja E, Zardavas D, Benyunes M, Viale G, et al. Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer. New England Journal of Medicine 2017;377:122–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Von Minckwitz G, Huang C-S, Mano MS, Loibl S, Mamounas EP, Untch M, et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. New England Journal of Medicine 2019;380:617–28 [DOI] [PubMed] [Google Scholar]
- 36.Martin M, Holmes FA, Ejlertsen B, Delaloge S, Moy B, Iwata H, et al. Neratinib after trastuzumab-based adjuvant therapy in HER2-positive breast cancer (ExteNET): 5-year analysis of a randomised, double-blind, placebo-controlled, phase 3 trial. The Lancet Oncology 2017;18:1688–700 [DOI] [PubMed] [Google Scholar]
- 37.Brandão M, Caparica R, Malorni L, Prat A, Carey LA, Piccart M. What is the real impact of estrogen receptor status on the prognosis and treatment of HER2-positive early breast cancer? Clinical Cancer Research 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Adamo B, Bellet M, Paré L, Pascual T, Vidal M, Fidalgo JAP, et al. Oral metronomic vinorelbine combined with endocrine therapy in hormone receptor-positive HER2-negative breast cancer: SOLTI-1501 VENTANA window of opportunity trial. Breast Cancer Research 2019;21:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krop I, Ismaila N, Andre F, Bast RC, Barlow W, Collyar DE, et al. Use of Biomarkers to Guide Decisions on Adjuvant Systemic Therapy for Women With Early-Stage Invasive Breast Cancer: American Society of Clinical Oncology Clinical Practice Guideline Focused Update. Journal of Clinical Oncology 2017;35:2838–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schettini F, Pascual T, Conte B, Chic N, Brasó-Maristany F, Galván P, et al. HER2-enriched subtype and pathological complete response in HER2-positive breast cancer: a systematic review and meta-analysis. Cancer Treatment Reviews 2020:101965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cortes J, Gebhart G, Ruiz Borrego M, Stradella A, Bermejo B, Escrivá S, et al. Chemotherapy (CT) de-escalation using an FDG-PET/CT (F-PET) and pathological response-adapted strategy in HER2 [+] early breast cancer (EBC): PHERGain Trial. American Society of Clinical Oncology; 2020. [Google Scholar]
- 42.Biganzoli L, Brain E, Malorni L, Risi E, Regan M. Phase II randomized trial of neoadjuvant trastuzumab and pertuzumab (TP) with either palbociclib+ letrozole (Pal+ L) or paclitaxel (Pac) for elderly patients with estrogen receptor & HER2 positive (ER+/HER2+) breast cancer (BC)(International Breast Cancer Study Group IBCSG 55–17, TOUCH). Annals of Oncology 2019;30:v96 [Google Scholar]
- 43.Nielsen T, Wallden B, Schaper C, Ferree S, Liu S, Gao D, et al. Analytical validation of the PAM50-based Prosigna Breast Cancer Prognostic Gene Signature Assay and nCounter Analysis System using formalin-fixed paraffin-embedded breast tumor specimens. BMC cancer 2014;14:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Prat A, Galván P, Jimenez B, Buckingham W, Jeiranian HA, Schaper C, et al. Prediction of response to neoadjuvant chemotherapy using core needle biopsy samples with the prosigna assay. Clinical Cancer Research 2016;22:560–6 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


