Abstract
Mental disorders diagnosis is based on specific clinical criteria. However, clinical studies found similarities and overlapping phenomenology across a variety of disorders, which suggests a common neurobiological substrate. Thus, there is a need to measure disease-related neuroanatomical similarities and differences across conditions. While structural alterations of the corpus callosum have been investigated in obsessive-compulsive disorder, schizophrenia, major depressive disorder and bipolar disorder, no study has addressed callosal aberrations in all diseases in a single study. Moreover, results from pairwise comparisons (patients vs. controls) show some inconsistencies, possibly related to the parcellation methods to divide the corpus callosum into subregions. The main aim of the present paper was to uncover highly localized callosal characteristics for each condition (i.e. obsessive-compulsive disorder, schizophrenia, major depressive disorder and bipolar disorder) as compared either to healthy control subjects or to each other. For this purpose, we did not rely on any sub-callosal parcellation method, but applied a well-validated approach measuring callosal thickness at 100 equidistant locations along the whole midline of the corpus callosum. One hundred and twenty patients (30 in each disorder) as well as 30 controls were recruited for the study. All groups were closely matched for age and gender, and the analyses were performed controlling for the impact of antipsychotic treatment and illness duration. There was a significant main effect of group along the whole callosal surface. Pairwise post hoc comparisons revealed that, compared to controls, patients with obsessive-compulsive disorder had the thinnest corpora callosa with significant effects almost on the entire callosal structure. Patients with schizophrenia also showed thinner corpora callosa than controls but effects were confined to the isthmus and the anterior part of the splenium. No significant differences were found in both major depressive disorder and bipolar disorder patients compared to controls. When comparing the disease groups to each other, the corpus callosum was thinner in obsessive-compulsive disorder patients than in any other group. The effect was evident across the entire corpus callosum, with the exception of the posterior body. Altogether, our study suggests that the corpus callosum is highly changed in obsessive-compulsive disorder, selectively changed in schizophrenia and not changed in bipolar disorder and major depressive disorder. These results shed light on callosal similarities and differences among mental disorders providing valuable insights regarding the involvement of the major brain commissural fibre tract in the pathophysiology of each specific mental illness.
Keywords: corpus callosum, obsessive-compulsive disorder, major depression, schizophrenia, bipolar disorder
Piras et al. compared highly localized callosal characteristics in four mental disorders (obsessive-compulsive disorder, schizophrenia, bipolar disorder and major depression). They showed that the corpus callosum is highly changed in obsessive-compulsive disorder, selectively changed in schizophrenia and not changed in bipolar disorder and major depression.
Graphical Abstract
Graphical Abstract.
Introduction
With more than 300 million fibres, the corpus callosum (CC) is the largest commissural white matter fibre tract in the human brain. Ensuing a topographical order, most of its fibres serve homotopic interconnections between the hemispheres1,2 playing a central role in cerebral information transferring and integration. Compartments of the anterior CC, including the rostrum, the genu and the rostral body, connect to prefrontal, premotor, and supplementary motor cortical areas, respectively. Fibres originating from the sensorimotor cortex are assumed to cross the CC through the middle and posterior body, whereas compartments of the posterior CC, including the isthmus and the splenium, connect to temporal, parietal, and occipital cortical regions. However, no macroscopic anatomical landmarks clearly delimit these callosal districts. Thus, several parcellation schemes have been used in neuroimaging studies to partition the CC into anatomically and functionally distinct subareas, in order to achieve more regionally definite measurements. Among them, the widest used method is the well-established Witelson3 segmentation criteria, by which the CC is arbitrarily divided into several regions according to maximal length (i.e. in thirds: the anterior third including the genu and the rostrum; the mid-third including the body; the posterior third including the isthmus and the splenium). Others have used a different approach by subjecting midsagittal callosal width measurements, made along the longitudinal axis, to factor analysis for generating a number of smaller clusters.4 However, parcellation schemes based on geometrical solutions (e.g. the Witelson scheme) could be biased by local variability in callosal shape,5 while measures based on statistically defined internal cohesiveness (using factor analysis) could produce factors that do not necessarily correspond to any functional boundaries. Such variability in anatomical sectioning affects structural MRI studies exploring CC morphology in mental disorders, contributing to inconsistent results. Indeed, despite the amount of interest that CC morphology has reached from the second half of the last century, a general consensus about its involvement in the pathophysiology of psychiatry phenomenology is still lacking.
Mental disorders are characterized, with different extent, by several and often overlapping clinical manifestations.6 These includes impairments in complex behaviours (e.g. social interactions and relationships), thought (e.g. reasoning and abstract thinking), high cognitive processes (e.g. attention, working memory, executive functions and inhibitory control), perception (e.g. illusions and hallucinations), mood regulation (e.g. mania or depression) and motor functions (e.g. psychomotor agitation/retardation, catatonia, compulsions). Cortical brain regions, from the most anterior prefrontal cortex to the most posterior occipital areas, are involved in and mediate these processes, requiring a finely coordinated activity for a proper functioning. Providing the physiological substrate for such homotopic inter-hemispheric cortical synchronizations,7 CC damage was therefore proposed to be differently involved in the pathophysiology of several mental disorders.
Several pieces of evidence revealed CC microstructural, volumetric and shape alterations in different disorders such as obsessive-compulsive disorder7–17 (OCD), schizophrenia18–28 (SZ), bipolar disorder30–39 (BD) and major depressive disorder40–47 (MDD). Specifically, evidence of CC involvement in SZ pathophysiology comes from the very first histological investigations48 up to recent studies involving advanced MRI methodologies.28 A recent review28 and a meta-analysis49 suggest that reduced callosal area characterize first episode drug naïve patients diagnosed with SZ. Increasing evidence implicates CC as a key component for the pathophysiology of mood disorders, indicating a disconnection between cortical regions as responsible for impairments in emotional processing.50 Specifically, structural MRI studies found reduced CC volume,33,51 signal intensity31 and microstructural integrity36,39 (see Bellani et al., 2009 for a Review) in patients suffering from BD, suggesting altered inter-hemispheric connectivity of bilateral homologous cortical areas. In MDD evidence suggests that CC abnormalities are present from the earlier46 up to the chronic stages of the illness.52 Finally, abnormalities of CC have recently received growing attention in OCD with evidence of either brain microstructural53 or morphological abnormalities.15
Brain structural54 and genetic55,56 studies indicate transdiagnostic overlaps among mental disorders, challenging the view that each brain disorder is exclusively characterized by specific patterns of brain alterations as well as by specific constellations of clinical symptoms.57–59 Since the diagnostic process is still based on a descriptive collection of such symptoms to categorize patients, the quantitative measurement of brain neuroanatomical similarities and differences between mental disorders represents a new frontier for psychiatric research. Among CC morphometric studies, the vast majority has been focussed on case–control analyses of one specific mental disorder at a time. As far as we know, to date no study has compared CC morphology among different conditions.
Here, we aimed at uncovering specifically localized CC morphological characteristics for different mental disorders, as compared to healthy control subjects (HC). In order to circumvent the risk of defining callosal sections with controversial fibre distribution, to avoid shape-induced biases, and to increase the spatial resolution of callosal measurements, we did not rely on any parcellation scheme. Indeed, we did perform analyses along the whole midline CC boundaries morphology applying an anatomical mesh-based geometrical modelling method to compute 100 pointwise indicators for callosal thickness.60,61 Furthermore, as no other study did, we compared CC abnormalities6–54 across patients diagnosed with OCD, SZ, MDD and BD, controlling for the potential confounding effect of stable antipsychotic drug dosages.62
Materials and methods
We used a priori power calculation in G*power to determine the minimum sample size needed for the study. Results indicated that a total sample size of at least 136 subjects (about 27 participants in each group) and a critical F(4; 126) = 1.387 has an actual power greater than 97% power to detect differences among groups, with a two tailed alpha value <0.05.
Subjects
We recruited 150 subjects (i.e. 120 patients and 30 HC) for the study. Patient groups included 30 participants diagnosed with SZ, 30 diagnosed with BD, 30 diagnosed with MDD and 30 diagnosed with OCD. In all diagnostic groups, individual patients were matched one to one for gender (100% concordance) and age (±1 year). Patients were preliminarily seen in outpatient clinics in central Italy and diagnosed according to the DSM-IV-TR.63 Psychiatrists who were treating the patients and knew their clinical history made a preliminary diagnosis of each patient. The clinicians were blind to the aims of the study. All diagnoses were confirmed at the Laboratory of Neuropsychiatry of the IRCCS Santa Lucia Foundation in Rome by a senior clinical psychiatrist (G.S.) using the structured clinical interview for DSM-IV-TR patient edition (SCID-P).64 In case of disagreement between the senior psychiatrist and the clinicians who had made the preliminary diagnosis, more data were requested to help resolve the discrepancies and the diagnostic process continued until a final consensus diagnosis was assigned (agreement among raters Cohen’s k > 0.80). If the diagnosticians could not reach an agreement, the patient was removed from the sample.
We also recruited 30 HC in the same geographical area, one to one paired-matched with the patients for gender (100% concordance) and age (±1 year). For all participants, inclusion criteria were (i) age between 18 and 65 years; (ii) at least five years of education; and (iii) suitability for MRI scanning. Exclusion criteria were (i) history of alcohol or drug abuse in the two years before the assessment; (ii) lifetime drug dependence; (iii) traumatic head injury with loss of consciousness; (iv) past or present major medical illness or neurological disorders; (v) any (for HC) or additional (for patients) psychiatric disorder or mental retardation; (vi) dementia or cognitive deterioration according to DSM-IV-TR criteria, and Mini-Mental State Examination (MMSE)65 score < 25, consistent with normative data in the Italian population66; (vii) low quality of T1-weighted images (i.e. presence of motion or scanner-generated artefacts); and (viii) any potential brain abnormality or microvascular lesion as apparent on conventional fluid attenuated inversion recovery (FLAIR) scans; in particular, the presence, severity and location of vascular lesions were computed according to the semi-automated method recently published by our group.67
The study was approved and undertaken in accordance with the guidance of our local Ethics Committee and written consent was obtained from all participants after a full explanation of the procedures of the study.
Clinical assessment
Information on clinical history was obtained from the patients, their relatives, psychiatrists and their clinical charts. All HC were screened for a current or lifetime history of DSM-IV-TR Axis I and II disorders using the Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-patient Edition (SCID-I-NP)68 and Structured Clinical Interview for DSM-IV Axis II Personality Disorders (SCID-II)69; they were also assessed to confirm that no first-degree relative had a history of OCD, mood- or SZ-related disorders.
For all 150 participants, global cognitive functioning was assessed by the MMSE.65 All patients were under stable pharmacologic treatment for at least six months, usually polypharmacy, including treatment with lithium or other mood-stabilizing agents, antidepressants and stable oral dosages of one or more antipsychotics. Illness duration was defined as the age at the MRI scanner time minus age at onset of first symptoms. Symptom severity, chlorpromazine equivalent dosage and illness duration are reported in Table 1.
Table 1.
Sociodemographic and clinical characteristics of 30 patients diagnosed with OCD, 30 with SZ, 30 with BD, 30 with MDD and 30 HC
| OCD (N = 30) | SZ (N = 30) | BD (N = 30) | MDD (N = 30) | HC (N = 30) | x 2, t or F | Df | P | |
|---|---|---|---|---|---|---|---|---|
| Male, N (%) | 15 (50%) | 15 (50%) | 15 (50%) | 15 (50%) | 15 (50%) | 0.00 | 4 | 1.000 |
| Age, mean (SD) | 39.87 (10.6) | 40.27 (9.7) | 40.27 (12) | 41.02 (10.8) | 40.77 (13.9) | 0.05 | 4; 145 | 0.995 |
| Educational level (years), mean (SD) | 13.03 (3.5) | 12.43 (3.4) | 14.7 (2.7) | 13.17 (3.9) | 15.97 (3.3) | 5.5 | 4; 145 | 0.0004 |
| Duration of illness (years), mean (SD) | 19.21 (12.3) | 24.1 (9) | 12 (10) | 9.57 (9.7) | – | 12.42 | 3; 114 | <0.0001 |
| Clorpromazine Equivalent (mg), mean (SD) | 61.1 (105.7) | 118.54 (209.4) | 80.9 (116.4) | 23.21 (53.5) | – | 2.38 | 3; 96 | 0.074 |
| HAM-D score, mean (SD) | – | – | 8.5 (6.2) | 16.5 (6.7) | – | −7.98 | 57 | <0.0001 |
| YMRS score, mean (SD) | – | – | 3 (2.7) | – | – | – | – | – |
| Y-BOCS, mean (SD) | 27.72 (7.9) | – | – | – | – | – | – | – |
| PANSS, mean (SD) | – | 77.5 (18.2) | – | – | – | – | – | – |
df, degrees of freedom; HAM-D, Hamilton Depression Rating Scale; PANSS, Positive and Negative Syndrome Scale; SD, standard deviation; YMRS, Young Mania Rating Scale; Y-BOCS, Yale-Brown Obsessive Compulsive Severity Scale.
MRI image acquisition and pre-processing
All 150 participants underwent the same imaging protocol, which included 3D T1-weighted, T2-weighted and FLAIR sequences using a 3T Achieva MR scanner (Philips Medical Systems, Best, The Netherlands) with a 32-channel receiving-only head coil. Whole-brain T1-weighted images were obtained using a fast-field echo sequence (echo time/repetition: time = 5.3/11 ms, flip angle = 9°, voxel size = 1 × 1 × 1 mm3). T2 and FLAIR sequences were acquired to screen for pathologies that would result in exclusion as described above. The T1-weighted images were preprocessed using SPM12 routines (www.fil.ion.ucl.ac.uk/spm/) in order to correct for magnetic field inhomogeneities and to ensure linear spatial alignment to MNI space using six-parameter rigid-body transformations.70,71 In addition, the total intracranial volumes (TIV) were estimated by skull-stripping the images and computing the volume using the FSLstats toolbox (https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/Fslutils#Tools) for later inclusion into the statistical model.
Subsequently, the point-wise callosal thickness was estimated in the preprocessed images as detailed elsewhere.60,72,73 Briefly, the upper and lower callosal boundaries were manually outlined by two experienced rater (DV and FK) in the midsagittal section of each brain. The intra-rater reliability for these manual traces was high (Cohen’s k = 0.90). The resulting upper and lower outlines were then resampled into 100 equidistant surface points each, and a midline was computed as the spatial average of these boundaries. Finally, the distances between 100 corresponding surface points from the upper and lower outline to this new midline were calculated as callosal distance values (CDVs, i.e. a measure of CC thickness) and entered as the dependent variable in the statistical analysis.
Statistical analysis
Comparisons among groups on sociodemographic variables and clinical characteristics were performed using ANOVA, student’s t or chi-square tests, using StatView statistical software (https://statview.software.informer.com), considering P < 0.05 as statistical threshold for significance.
Differences in point-wise callosal thickness among all groups were investigated in an ANCOVA using a mass-univariate general linear model. The 100 pointwise CDVs were considered the dependent variable(s) and the diagnosis was the fixed factor. Demographic (i.e. age, educational level), TIV and clinical variables (i.e. duration of illness and chlorpromazine equivalent dosage) were entered as covariates. In order to account for the inter-individual difference in clinical variables among patients (range of continuous values) and HC (only 0), the duration of illness and the chlorpromazine equivalent dosage were standardized by centering patients measures (distances from the mean) to their group mean (considered as 0). By rescaling these variables, we accounted for the inter-individual variability within the patient groups due to differences in illness duration and medication dose, while retaining the overall differences between patients groups and HC. We did not include sex as a covariate since it was 100% matched and highly correlated to TIV (r2 = 0.42, P < 0.0001), as already assessed also by previous studies.61,74
Post hoc comparisons were performed in case of significant ANCOVA results, holding the same set of covariates and comparing two groups at time for each CDVs, with univariate general linear models. Specifically, when post hoc comparisons included only patient samples (then without HC comparisons), duration of illness and chlorpromazine equivalent dosage were not standardized and considered as raw values.
As statistical tests were made at hundreds of CC surface points and as adjacent data points are highly correlated, statistical results were corrected for multiple comparisons using False Discovery Rate (FDR), with a 5% maximum allowable FDR.75,76
Data availability
De-identified data are available from the corresponding author upon reasonable request.
Results
Sociodemographic and clinical characteristics are reported in Table 1. As expected from the matching procedure, the groups did not differ for age and gender, while a significant difference was found for the educational level. Patients’ groups were also different in terms of illness duration and drug treatment (except for mood-stabilizers diverse from lithium), while the difference for chlorpromazine equivalent and benzodiazepine dosages approached statistical significance (Table 1).
Figure 1A shows the results of the ANCOVA. We found highly statistically significant morphological group differences along the vast majority of the CC (PFDRcorr < 0.005 for all comparisons), and slightly less significant differences in a little segment along the middle body surfaces (0.005 < PFDRcorr < 0.045). The very anterior and posterior ends of the CC did not differ among groups, however, these regions may be less sensitive as they also appear unaffected in severe neurodegenerative conditions.77Figure 1B shows mean CDVs for each diagnosis along the callosal axis from the tip of the rostrum to the bottom of the splenium. Overall, the lowest values were evident in OCD, whereas the highest were observed in HC and intermediate values in MDD and BD.
Figure 1.
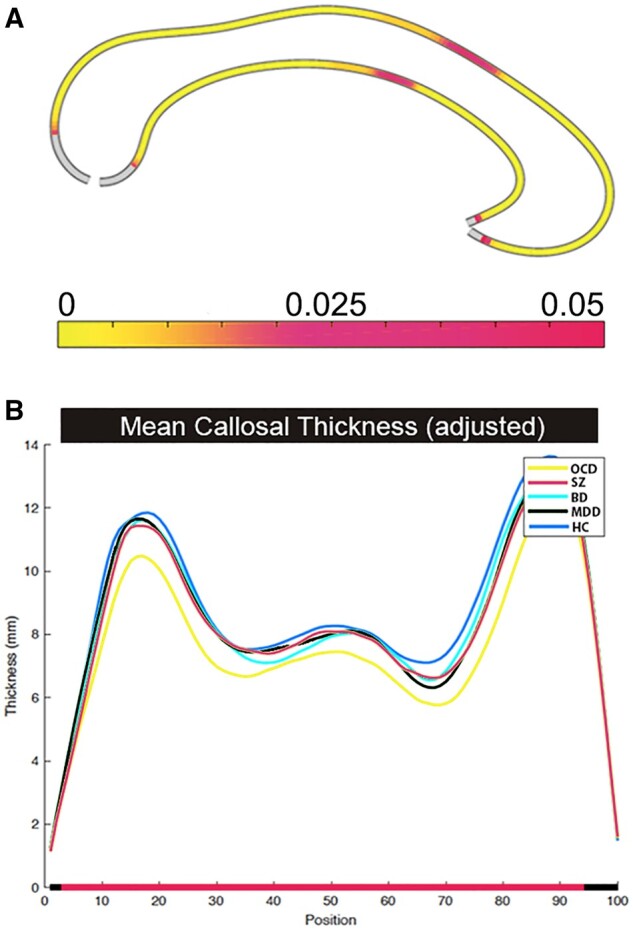
Differences in mean callosal thickness (adjusted) between HC, SZ, OCD, BD and MDD. (A) Significant group differences after FDR correction for multiple comparisons mapped back on the callosal outlines; The colour bar shows the corrected P-values, where magenta represents the weaker and yellow the strongest statistical significance. (B) Groups means of the callosal thickness at 100 points from the tip of the rostrum (Position 1) to the bottom of the splenium (Position 100) in healthy control (HC), obsessive-compulsive (OCD), schizophrenic (SZ), unipolar (MDD) and bipolar (BD) mood disorder groups.
Post hoc comparisons (Fig. 2) show that OCD and SZ were characterized by significantly thinned CC compared to HC. These effects were evident along the entire structure in OCD (PFDRcorr < 0.015 for all comparisons), but loco-regional in SZ [i.e. along the isthmus (0.005 < PFDRcorr < 0.035) and the rostrum (0.015 < PFDRcorr < 0.03)]. No significant differences were observed when comparing MDD, BD and HC. The comparisons among patient groups indicated that the CC was significantly thinned along the entire structure in OCD, with the exception of the middle body, compared to SZ (PFDRcorr < 0.015 for all comparisons), MDD (0.01 < PFDRcorr < 0.045) and BD (0.01 < PFDRcorr < 0.045) (Fig. 2). The extension of similar morphology of the middle body increased from OCD to SZ to BD to MDD.
Figure 2.
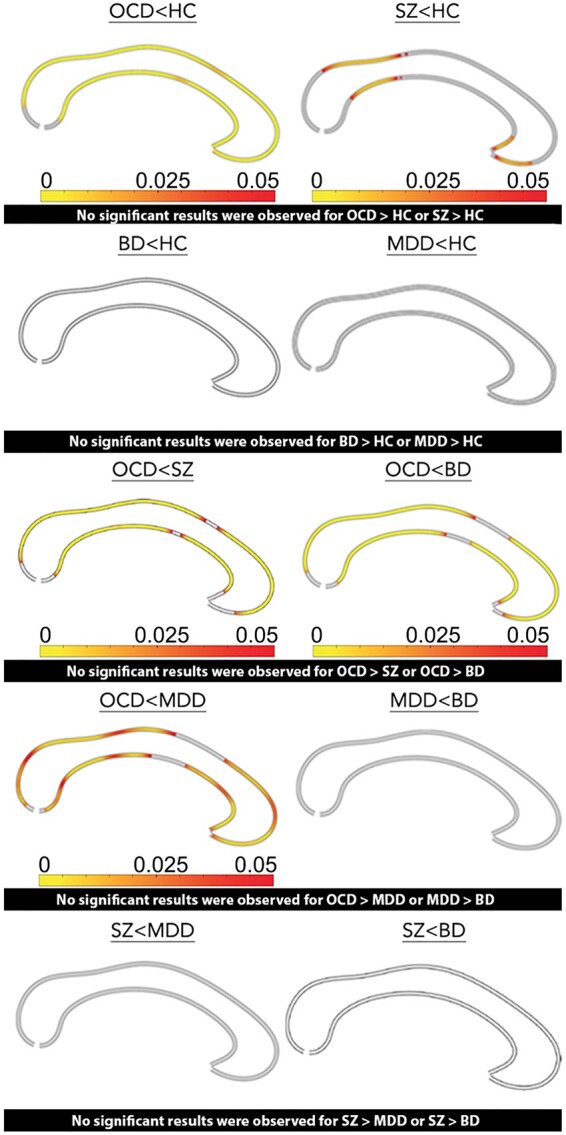
Pairwise comparisons of callosal thickness between HC, SZ, OCD, BD and MDD. Significant group differences are colour-coded and mapped onto the callosal outlines for the post hoc group comparisons, performed among of healthy control (HC), obsessive-compulsive (OCD), schizophrenic (SZ), unipolar (MDD) and bipolar (BD) mood disorder; The colour bar shows the FDR-corrected P-values, where red represents the weaker and yellow the strongest statistical significance.
Discussion
Here we explored highly localized CC morphometric features investigating, in a single study, patients diagnosed with OCD, SZ, BD, MDD and HC. We found three main results: first, CC thickness is reduced in both OCD and SZ, but not in BD or MDD, as compared to HC; second, these abnormalities are widespread in OCD and highly localized on the isthmus and rostrum surfaces in SZ; third, OCD have thinned CC along the entire surface except for the middle body, as compared to SZ, BD and MDD. Thus, we observed that CC morphology is highly abnormal in OCD, selectively abnormal in SZ and preserved in BD and MDD.
Although previous studies have investigated callosal size in OCD,15 SZ,21,22 BD30 and MDD,41 to the best of our knowledge, our study is the first to compare callosal thickness measures across all these major mental disorders.
Using different techniques, CC macrostructural15,78 alterations have been already described in OCD compared to HC, on the splenium, the isthmus and the anterior body and related to impaired inter-hemispheric connections among occipital, parieto-temporal and orbito-frontal associative cortices.1,2,79 Posterior CC changes in OCD have been related to impairments in visual-spatial performances15 while anterior CC abnormalities have been linked to executive prefrontal lobe disfunctions.80 Indeed, executive abilities such as response inhibition and set shifting, as well as working memory and planning, are often reported impaired in adults with OCD.81–84 Our results shed light on the potential involvement of the anterior CC in the pathophysiological processes mediating these cognitive disfunctions. Apart from the impairment of inter-hemispheric connections among cerebral associative cortices in OCD, here we describe an additional change of sensorimotor cortices connections, as mediated by CC fibres crossing the middle body. Sensorimotor cortices are part of the cortico-striato-thalamo-cortical loop, that has been widely described as a critical network in OCD pathophysiology.85,86 Indeed, reduction of sensorimotor cortical inhibition upon cortico-striato-thalamo-cortical impairs the ability to suppress internally triggered intrusive and repetitive movements (compulsion) and thoughts (obsessions).87 Thus, impairments in inter-hemispheric sensorimotor communication and integration may be also involved in the cortical inhibition impairment present in OCD.
In patients diagnosed with SZ21,22 in comparison with HC, CC structural changes have been previously described for the most anterior and for posterior portions of the CC. Here, we strengthen this evidence, confirming the presence of focal white matter changes in SZ.88 In particular, differently from OCD, callosal changes found in SZ were highly localized and restricted to the inter-hemispheric connections crossing the CC rostrum and the splenium. Fibres crossing through the rostrum mediate inter-hemispheric transfer of the ventromedial prefrontal and orbitofrontal cortices. Both these cortical regions89,90 are critical for a number of high cognitive processes, such as the representation of reward and value-based decision-making, emotion regulation and for multiple aspects of social cognition (i.e. facial emotion recognition, theory of mind ability and processing of self-relevant information). Such processes have been proved to be dysfunctional in SZ, both in neuroimaging91 and neuropsychological studies,92–95 and abnormal inter-hemispheric connections among the rostrum may be involved in such impaired functioning. Fibres crossing the isthmus mediate inter-hemispheric connections among posterior parietal cortices (PPC), that are multimodal associative areas also associated to high cognitive processes.96 Specifically, PPC functions mediate stimuli representations and the maintenance of attention over stimuli represented in sensory cortices.97 Moreover, there is strong evidence that PPC-based functions are central to working memory storage.98–101 Both attentional and working memory functions have been widely reported as impaired in SZ,102 and our results of thinned CC isthmus in SZ indicate that altered inter-hemispheric PPC connection might be one of the cerebral substrates of such cognitive impairment.
Here we did not find significant CC changes in BD as compared to HC. Previous studies, investigating morphometric features in BD, described alterations in CC thickness,33,103–105 microstructure106–108 or interhemispheric connectivity,109,110 though with some inconsistencies.111 Moreover, studies focussed on younger112 or fist-episode113 patients diagnosed with BD did not describe CC size changes but only curvature alterations. In order to explain inconsistences in the results, different sources of heterogeneity should be considered for MRI investigation in BD. Indeed, BD encompasses a broad of symptomatology and disease subtypes, leading to several issues in patient categorization. In particular, some of the citated studies were focussed only on patients diagnosed with BD type I,32,103,104 while the majority of them completely lacks to specify diagnostic subtype information.34,105,108,112,113 Furthermore, all but one104 among the cited studies lack to control statistical analysis for the potential impact of psychiatric/antipsychotic treatment, which effect on white matter structure is well known.114 Lastly, differences related to the technique and the measures employed for MRI investigations add a considerable amount of heterogeneity among CC studies, leading to problems in result comparisons. Indeed, the cited morphometric works often relayed on discrete CC parcellation schemes32 or used a smaller number of points on the CC surface in order to obtain thickness measurements (i.e. 39 points).103,113 Conversely, we obtained a continuous-like measure of the distances between upper and lower callosal surfaces employing 100 points of measurement, then performing a higher localized analysis along the whole CC surface.
Morphometric studies investigating callosal size in MDD also show a considerable amount of inconsistencies.40,41 Here, we are in line with results of unchanged CC thickness in this mood disorder. Specifically, abnormalities in MDD were found when the patient’s sample was stratified for illness onset41 (i.e. early versus late onset) or for familiar form of the diseases.38 It could be argued that callosal changes in MDD are present only in specific, or even in the more severe, forms of the disease, being not detectable for all types of MDD.
The third intriguing finding in our study was the significant thinned CC found in OCD patients compared to SZ, BD and MDD, on the majority of the investigated surfaces, indicating extensive CC implication in OCD mechanism in comparison with other major mental disorders. Indeed, only surfaces belonging to the middle body were not different among OCD and SZ, BD or MDD. The widespread changes in CC morphology of patients diagnosed with OCD indicates that interhemispheric integration of both associative and primary sensory visual cortices in OCD was the most impaired among the investigated disorders. This evidence could be useful also from a diagnostic point of view. Indeed, while mental diseases could overlap from a symptomatologic dimensional perspective, they result as categorially different referring to CC morphometric characteristics. Thus, a widespread thinned CC it could reasonably guide the diagnostic process towards a potential diagnosis of OCD.
Referring to callosal similarities among patient samples, fibres connecting pre- and post-central sensorimotor cortices by the middle body1,2,79 showed more homogeneous measures among patients. Focussed on OCD sample, it should be noted that the morphometric features of this CC area were the least affected as compared to HC. Being abnormally reduced in OCD patients only, it could be argued that inter-hemispheric sensorimotor integration is impaired in OCD but the extent of such impairment is not great enough to be significant in the comparisons with the other mental disorders. Future connectivity investigations should be performed in order to clarify this issue.
Limitations
Three main issues have to be considered before concluding. First, we didn’t control the analysis for the impact of antidepressant and mood stabiliser medicaments. Although this kind of treatment have been described as mostly related to changes in neuropils and grey matter structures,115–117 there is evidence that they may impact white matter as well (e.g. van Velzen et al.118). Future studies specifically focussed on the effect of such medications and with an adequate sample size are needed to address this complex issue in further detail. Second, the mental disorders here investigated may be better characterized by their clinical subtypes, in terms of symptom domains or severity, than by main diagnoses. However, stratifying patients in clinical sub-groups, characterized by more homogenous clinical manifestation, requires higher sample size to avoid false negative results. Further studies with larger sample sizes are needed to investigate this issue.
Third, our OCD sample was predominantly composed of patients with a late onset of the disease, according to the cut-off age of 19 proposed by Anholt and colleagues.119 Therefore, it could be argued that our results cannot not be generalized to OCD as whole, but limited to the late onset subtype. However, while it has been suggested that early and late onset adult OCD may differ in terms of neuropsychological features and grey matter volume (e.g. Kim et al.120), there is no clear evidence that they also differ in terms of white matter structure.
Conclusion
The comparison of CC morphometric features among OCD, SZ, BD, MDD and HC samples using an ultra-sensitive and highly localized morphometric technique allowed us to finely characterize CC morphometric features in four of the major mental disorders. Our approach revealed for the first-time similarities and differences among these patients, and points towards a thinned CC as a potential biomarker for OCD, as compared either to HC or to other major disorders. These results provide new insight into the involvement of the major brain commissural fibre tract into the pathophysiology of each of these specific mental disorders.
Funding
The study was partially funded by the Italian Ministry of Health, grants Ricerca Corrente, year 2021.
Competing interests
All authors declare no competing interests.
Glossary
- BD =
bipolar disorder
- CC =
corpus callosum
- CDV =
callosal distance values
- CSTC =
cortico-striato-thalamo-cortical
- FDR =
false discovery rate
- FLAIR =
fluid attenuated inversion recovery
- HC =
healthy controls
- MDD =
major depressive disorder
- MMSE =
mini-mental state examination
- OCD =
obsessive-compulsive disorder
- PPC =
posterior parietal cortices
- SZ =
schizophrenia
- TIV =
total intracranial volumes
References
- 1. Jarbo K, Verstynen T, Schneider W.. In vivo quantification of global connectivity in the human corpus callosum. Neuroimage. 2012;59(3):1988–1996. [DOI] [PubMed] [Google Scholar]
- 2. Hofer S, Frahm J.. Topography of the human corpus callosum revisited—Comprehensive fiber tractography using diffusion tensor magnetic resonance imaging. Neuroimage. 2006;32(3):989–994. [DOI] [PubMed] [Google Scholar]
- 3. Witelson SF. Hand and sex differences in the isthmus and genu of the human corpus callosum: A postmortem morphological study. Brain. 1989;112(3):799–835. [DOI] [PubMed] [Google Scholar]
- 4. Peters M, Oeltze S, Seminowicz D, Steinmetz H, Koeneke S, Jäncke L.. Division of the corpus callosum into subregions. Brain Cogn. 2002;50(1):62–72. [DOI] [PubMed] [Google Scholar]
- 5. Tomaiuolo F, Scapin M, Di Paola M, et al. Gross anatomy of the corpus callosum in Alzheimer’s disease: Regions of degeneration and their neuropsychological correlates. Dement Geriatr Cogn Disord. 2007;23(2):96–103. [DOI] [PubMed] [Google Scholar]
- 6. American Psychiatric Association. DSM-5 Task Force. Diagnostic and Statistical Manual of Mental Disorders: DSM-5. American Psychiatric Association;Arlington, VA, USA. 2013. [Google Scholar]
- 7. Filley CM. White matter: Organization and functional relevance. Neuropsychol Rev. 2010;20(2):158–173. [DOI] [PubMed] [Google Scholar]
- 8. Rosenberg DR, Keshavan MS, Dick EL, Bagwell WW, Mac Master FP, Birmaher B.. Corpus callosal morphology in treatment-naive pediatric obsessive compulsive disorder. Prog Neuro-Psychopharmacology Biol Psychiatry. 1997;21(8):1269–1283. [DOI] [PubMed] [Google Scholar]
- 9. Mac Master FP, Keshavan MS, Dick EL, Rosenberg DR.. Corpus callosal signal intensity in treatment-naive pediatric obsessive compulsive disorders. Prog Neuro-Psychopharmacology Biol Psychiatry. 1999;23(4):601–612. [DOI] [PubMed] [Google Scholar]
- 10. Saito Y, Nobuhara K, Okugawa G, et al. Corpus callosum in patients with obsessive-compulsive disorder: Diffusion-tensor imaging study. Radiology. 2008;246(2):536–542. [DOI] [PubMed] [Google Scholar]
- 11. Fontenelle LF, Harrison BJ, Yücel M, Pujol J, Fujiwara H, Pantelis C.. Is there evidence of brain white-matter abnormalities in obsessive-compulsive disorder? A narrative review. Top Magn Reson Imaging. 2009;20(5):291–298. [DOI] [PubMed] [Google Scholar]
- 12. Nakamae T, Narumoto J, Sakai Y, et al. Diffusion tensor imaging and tract-based spatial statistics in obsessive-compulsive disorder. J Psychiatr Res. 2011;45(5):687–690. [DOI] [PubMed] [Google Scholar]
- 13. Li F, Huang X, Yang Y, et al. Microstructural brain abnormalities in patients with obsessive-compulsive disorder: Diffusion-tensor MR imaging study at 3.0 T. Radiology. 2011;260(1):216–223. [DOI] [PubMed] [Google Scholar]
- 14. Oh JS, Jang JH, Jung WH, et al. Reduced fronto-callosal fiber integrity in unmedicated OCD patients: A diffusion tractography study. Hum Brain Mapp. 2012;33(10):2441–2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Di Paola M, Luders E, Rubino IA, et al. The structure of the corpus callosum in obsessive compulsive disorder. Eur Psychiatry. 2013;28(8):499–506. [DOI] [PubMed] [Google Scholar]
- 16. Spalletta G, Piras FF, Fagioli S, Caltagirone C, Piras FF.. Brain microstructural changes and cognitive correlates in patients with pure obsessive compulsive disorder. Brain Behav. 2014;4(2):261–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Anticevic A, Hu S, Zhang S, et al. Global resting-state functional magnetic resonance imaging analysis identifies frontal cortex, striatal, and cerebellar dysconnectivity in obsessive-compulsive disorder. Biol Psychiatry. 2014;75(8):595–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gonçalves Ó, Carvalho S, Leite J, Fernandes-Gonçalves A, Carracedo A, Sampaio A.. Morphometric and connectivity white matter abnormalities in obsessive compulsive disorder. Princ Pract Clin Res. 2016;3(1):1–11. [Google Scholar]
- 19. Nasrallah HA, McCalley-Whitters M, Bigelow LB, Rauscher FP.. A histological study of the corpus callosum in chronic schizophrenia. Psychiatry Res. 1983;8(4):251–260. [DOI] [PubMed] [Google Scholar]
- 20. Coger RW, Serafetinides EA.. Schizophrenia, corpus callosum, and interhemispheric communication: A review. Psychiatry Res. 1990;34(2):163–184. [DOI] [PubMed] [Google Scholar]
- 21. Woodruff PWR, McManus IC, David AS.. Meta-analysis of corpus callosum size in schizophrenia. J Neurol Neurosurg Psychiatry. 1995;58(4):457–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Downhill JE, Buchsbaum MS, Wei T, et al. Shape and size of the corpus callosum in schizophrenia and schizotypal personality disorder. Schizophr Res. 2000;42(3):193–208. [DOI] [PubMed] [Google Scholar]
- 23. Foong J, Maier M, Clark CA, Barker GJ, Miller DH, Ron MA.. Neuropathological abnormalities of the corpus callosum in schizophrenia: A diffusion tensor imaging study. J Neurol Neurosurg Psychiatry. 2000;68(2):242–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Keshavan MS, Diwadkar VA, Harenski K, Rosenberg DR, Sweeney JA, Pettegrew JW.. Abnormalities of the corpus callosum in first episode, treatment naive schizophrenia. J Neurol Neurosurg Psychiatry. 2002;72(6):757–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ardekani BA, Nierenberg J, Hoptman MJ, Javitt DC.. MRI study of white matter diffusion anisotropy in schizophrenia. Neuroreport. 2003;14(16):259–267. [DOI] [PubMed] [Google Scholar]
- 26. Whitford TJ, Kubicki M, Schneiderman JS, et al. Corpus callosum abnormalities and their association with psychotic symptoms in patients with schizophrenia. Biol Psychiatry. 2010;68(1):70–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rao NP, Venkatasubramanian G, Arasappa R, Gangadhar BN.. Relationship between corpus callosum abnormalities and schneiderian first-rank symptoms in antipsychotic-naïve schizophrenia patients. J Neuropsychiatry Clin Neurosci. 2011;23(2):155–162. [DOI] [PubMed] [Google Scholar]
- 28. Canu E, Agosta F, Filippi M.. A selective review of structural connectivity abnormalities of schizophrenic patients at different stages of the disease. Schizophr Res. 2015;161(1):19–28. [DOI] [PubMed] [Google Scholar]
- 29. Kelly S, Jahanshad N, Zalesky A, et al. Widespread white matter microstructural differences in schizophrenia across 4322 individuals: Results from the ENIGMA Schizophrenia DTI Working Group. Mol Psychiatry. 2018;23(5):1261–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Brambilla P, Nicoletti MA, Sassi RB, et al. Magnetic resonance imaging study of corpus callosum abnormalities in patients with bipolar disorder. Biol Psychiatry. 2003;54(11):1294–1297. [DOI] [PubMed] [Google Scholar]
- 31. Brambilla P, Nicoletti M, Sassi RB, et al. Corpus callosum signal intensity in patients with bipolar and unipolar disorder. J Neurol Neurosurg Psychiatry. 2004;75(2):221–225. [PMC free article] [PubMed] [Google Scholar]
- 32. Atmaca M, Ozdemir H, Cetinkaya S, et al. Cingulate gyrus volumetry in drug free bipolar patients and patients treated with valproate or valproate and quetiapine. J Psychiatr Res. 2007;41(10):821–827. [DOI] [PubMed] [Google Scholar]
- 33. Arnone D, McIntosh AM, Chandra P, Ebmeier KP.. Meta-analysis of magnetic resonance imaging studies of the corpus callosum in bipolar disorder. Acta Psychiatr Scand. 2008;118(5):357–362. [DOI] [PubMed] [Google Scholar]
- 34. Caetano SC, Silveira CM, Kaur S, et al. Abnormal corpus callosum myelination in pediatric bipolar patients. J Affect Disord. 2008;108(3):297–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Barnea-Goraly N, Chang KD, Karchemskiy A, Howe ME, Reiss AL.. Limbic and corpus callosum aberrations in adolescents with bipolar disorder: A tract-based spatial statistics analysis. Biol Psychiatry. 2009;66(3):238–244. [DOI] [PubMed] [Google Scholar]
- 36. Bellani M, Yeh PH, Tansella M, Balestrieri M, Soares JC, Brambilla P.. DTI studies of corpus callosum in bipolar disorder. Biochem Soc Trans. 2009;37(5):1096–1098. [DOI] [PubMed] [Google Scholar]
- 37. Sussmann JE, Lymer GKS, Mckirdy J, et al. White matter abnormalities in bipolar disorder and schizophrenia detected using diffusion tensor magnetic resonance imaging. Bipolar Disord. 2009;11(1):11–18. [DOI] [PubMed] [Google Scholar]
- 38. Matsuo K, Nielsen N, Nicoletti MA, et al. Anterior genu corpus callosum and impulsivity in suicidal patients with bipolar disorder. Neurosci Lett. 2010;469(1):75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sarrazin S, Poupon C, Linke J, et al. A Multicenter tractography study of deep white matter tracts in bipolar i disorder: Sychotic features and interhemispheric disconnectivity. JAMA Psychiatry. 2014;71(4):388–396. [DOI] [PubMed] [Google Scholar]
- 40. Lacerda ALT, Brambilla P, Sassi RB, et al. Anatomical MRI study of corpus callosum in unipolar depression. J Psychiatr Res. 2005;39(4):347–354. [DOI] [PubMed] [Google Scholar]
- 41. Ballmaier M, Kumar A, Elderkin-Thompson V, et al. Mapping callosal morphology in early- and late-onset elderly depression: An index of distinct changes in cortical connectivity. Neuropsychopharmacology. 2008;33(7):1528–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sun J, Maller JJ, Daskalakis ZJ, Furtado CC, Fitzgerald PB.. Morphology of the corpus callosum in treatment-resistant schizophrenia and major depression. Acta Psychiatr Scand. 2009;120(4):265–273. [DOI] [PubMed] [Google Scholar]
- 43. Kieseppä T, Eerola M, Mäntylä R, et al. Major depressive disorder and white matter abnormalities: A diffusion tensor imaging study with tract-based spatial statistics. J Affect Disord. 2010;120(1-3):240–244. [DOI] [PubMed] [Google Scholar]
- 44. Cyprien F, Courtet P, Malafosse A, et al. Suicidal behavior is associated with reduced corpus callosum area. Biol Psychiatry. 2011;70(4):320–326. [DOI] [PubMed] [Google Scholar]
- 45. Cole J, Chaddock CA, Farmer AE, et al. White matter abnormalities and illness severity in major depressive disorder. Br J Psychiatry. 2012;201(1):33–39. [DOI] [PubMed] [Google Scholar]
- 46. MacMaster FP, Carrey N, Marie Langevin L.. Corpus callosal morphology in early onset adolescent depression. J Affect Disord. 2013;145(2):256–259. [DOI] [PubMed] [Google Scholar]
- 47. Xu K, Jiang W, Ren L, et al. Impaired interhemispheric connectivity in medicationnaive patients with major depressive disorder. J Psychiatry Neurosci. 2013;38(1):43–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rosenthal R, Bigelow LB.. Quantitative brain measurements in chronic schizophrenia. Br J Psychiatry. 1972;121(562):259–264. [DOI] [PubMed] [Google Scholar]
- 49. Arnone D, McIntosh AM, Tan GMY, Ebmeier KP.. Meta-analysis of magnetic resonance imaging studies of the corpus callosum in schizophrenia. Schizophr Res. 2008;101(1-3):124–132. [DOI] [PubMed] [Google Scholar]
- 50. Sexton CE, Mackay CE, Ebmeier KP.. A systematic review of diffusion tensor imaging studies in affective disorders. Biol Psychiatry. 2009;66(9):814–823. [DOI] [PubMed] [Google Scholar]
- 51. Atmaca M, Ozdemir H, Yildirim H.. Corpus callosum areas in first-episode patients with bipolar disorder. Psychol Med. 2007;37(5):699–704. [DOI] [PubMed] [Google Scholar]
- 52. Wen M-C, Steffens DC, Chen M-K, Zainal NH.. Diffusion tensor imaging studies in late-life depression: Systematic review and meta-analysis. Int J Geriatr Psychiatry. 2014;29(12):1173–1184. [DOI] [PubMed] [Google Scholar]
- 53. Piras F, Piras F, Caltagirone C, Spalletta G.. Brain circuitries of obsessive compulsive disorder: A systematic review and meta-analysis of diffusion tensor imaging studies. Neurosci Biobehav Rev. 2013;37(10):2856–2877. [DOI] [PubMed] [Google Scholar]
- 54. Goodkind M, Eickhoff SB, Oathes DJ, et al. Identification of a common neurobiological substrate for mental illness. JAMA Psychiatry. 2015;72(4):305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Smoller JW, Kendler K, Craddock N, et al. Identification of risk loci with shared effects on five major psychiatric disorders: A genome-wide analysis. Lancet. 2013;381(9875):1371–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. O’dushlaine C, Rossin L, Lee PH, et al. Psychiatric genome-wide association study analyses implicate neuronal, immune and histone pathways. Nat Neurosci. 2015;18(2):199–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Buckley PF, Miller BJ, Lehrer DS, Castle DJ.. Psychiatric comorbidities and schizophrenia. Schizophr Bull. 2009;35(2):383–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Markon KE. Modeling psychopathology structure: A symptom-level analysis of Axis i and II disorders. Psychol Med. 2010;40:273–288. [DOI] [PubMed] [Google Scholar]
- 59. Vaidyanathan U, Patrick CJ, Iacono WG.. Examining the overlap between bipolar disorder, nonaffective psychosis, and common mental disorders using latent class analysis. Psychopathology. 2012;45(6):361–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Luders E, Narr KL, Zaidel E, Thompson PM, Jancke L, Toga AW.. Parasagittal asymmetries of the corpus callosum. Cereb Cortex. 2006;16(3):346–354. [DOI] [PubMed] [Google Scholar]
- 61. Luders E, Narr KL, Zaidel E, Thompson PM, Toga AW.. Gender effects on callosal thickness in scaled and unscaled space. Neuroreport. 2006;17(11):1103–1106. [DOI] [PubMed] [Google Scholar]
- 62. Kubicki M, Lyall AE.. Antipsychotics and their impact on cerebral white matter: Part of the problem or part of the solution? Am J Psychiatry. 2018;175(11):1056–1057. [DOI] [PubMed] [Google Scholar]
- 63. American Psychiatric Association. Diagnostic and statistical manual of mental disorders (4th ed., Text Revision). Washington DC, USA. 2000.
- 64. First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Clinical Trials Version (SCID-CT). New York: Biometrics Research, New York State Psychiatric Institute. 2002. [Google Scholar]
- 65. Folstein MF, Folstein SE, McHugh PR.. “Mini-mental state”. J Psychiatr Res. 1975;12(3):189–198. [DOI] [PubMed] [Google Scholar]
- 66. Measso G, Cavarzeran F, Zappalà G, et al. The mini‐mental state examination: Normative study of an Italian random sample. Dev Neuropsychol. 1993;9(2):77. [Google Scholar]
- 67. Iorio M, Spalletta G, Chiapponi C, et al. White matter hyperintensities segmentation: A new semi-automated method. Front Aging Neurosci. 2013;5(Dec):76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. First MB, Spitzer RL, Gibbon ML, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-patient Edition. (SCID-I/NP) New York: Biometrics Research. 2002. [Google Scholar]
- 69. First M, Gibbon M, Spitzer R.. Structured Clinical Interview for DSM-IV Axis II Personality Disorders (SCID-II). Am Psychiatr Press Inc, Washington DC, USA. 1997. [Google Scholar]
- 70. Ashburner J, Friston KJ.. Unified segmentation. Neuroimage. 2005;26(3):839–851. [DOI] [PubMed] [Google Scholar]
- 71. Luders E, Thompson PM, Kurth F.. Morphometry of the corpus callosum. In: Spalletta G, Gili T, Piras F, eds. Neuromethods, Vol 136. Humana Press Inc; 2018. 131–142. [Google Scholar]
- 72. Luders E, Narr KL, Bilder RM, et al. Positive correlations between corpus callosum thickness and intelligence. Neuroimage. 2007;37(4):1457–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Kurth F, Spencer D, Hines M, Luders E.. Sex differences in associations between spatial ability and corpus callosum morphology. J Neurosci Res. 2018;96(8):1380–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Luders E, Toga AW, Why PMT.. Size matters: Differences in brain volume account for apparent sex differences in callosal anatomy. Neuroimage. 2014;(84:):820–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Benjamini Y, Hochberg Y.. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J R Stat Soc Ser B. 1995;57(1):289–300. [Google Scholar]
- 76. Benjamini Y, Yekutieli D.. The control of the false discovery rate in multiple testing under dependency. Ann Stat. 2001;29(4):1165–1188. [Google Scholar]
- 77. Di Paola M, Luders E, Di Iulio F, et al. Callosal atrophy in mild cognitive impairment and Alzheimer’s disease: Different effects in different stages. Neuroimage. 2010;49(1):141–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Park HY, Park JS, Kim SH, et al. Midsagittal structural differences and sexual dimorphism of the corpus callosum in obsessive-compulsive disorder. Psychiatry Res Neuroimaging. 2011;192(3):147–153. [DOI] [PubMed] [Google Scholar]
- 79. Schmahmann JD, Pandya DN.. Fiber pathways of the brain. New York, Oxford: Oxford University Press; 2009. [Google Scholar]
- 80. Stuss DT. Functions of the frontal lobes: Relation to executive functions. J Int Neuropsychol Soc. 2011;17(5):759–765. [DOI] [PubMed] [Google Scholar]
- 81. Van Den Heuvel OA, Veltman DJ, Groenewegen HJ, et al. Frontal-striatal dysfunction during planning in obsessive-compulsive disorder. Arch Gen Psychiatry. 2005;62(3):301–310. [DOI] [PubMed] [Google Scholar]
- 82. Abramovitch A, Dar R, Schweiger A, Hermesh H.. Neuropsychological impairments and their association with obsessive-compulsive symptom severity in obsessive-compulsive disorder. Arch Clin Neuropsychol. 2011;26(4):364–376. [DOI] [PubMed] [Google Scholar]
- 83. Bannon S, Gonsalvez CJ, Croft RJ, Boyce PM.. Response inhibition deficits in obsessive-compulsive disorder. Psychiatry Res. 2002;110(2):165–174. [DOI] [PubMed] [Google Scholar]
- 84. Penadés R, Catalán R, Rubia K, Andrés S, Salamero M, Gastó C.. Impaired response inhibition in obsessive compulsive disorder. Eur Psychiatry. 2007;22(6):404–410. [DOI] [PubMed] [Google Scholar]
- 85. Tang W, Zhu Q, Gong X, Zhu C, Wang Y, Chen S.. Cortico-striato-thalamo-cortical circuit abnormalities in obsessive-compulsive disorder: A voxel-based morphometric and fMRI study of the whole brain. Behav Brain Res. 2016;313:17–22. [DOI] [PubMed] [Google Scholar]
- 86. Lapidus KAB, Stern ER, Berlin HA, Goodman WK.. Neuromodulation for obsessive-compulsive disorder. Neurotherapeutics. 2014;11(3):485–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Russo M, Naro A, Mastroeni C, et al. Obsessive-compulsive disorder: A “sensory-motor” problem? Int J Psychophysiol. 2014;92(2):74–78. [DOI] [PubMed] [Google Scholar]
- 88. Hulshoff Pol HE, Schnack HG, Mandl RCW, et al. Focal white matter density changes in schizophrenia: Reduced inter-hemispheric connectivity. Neuroimage. 2004;21(1):27–35. [DOI] [PubMed] [Google Scholar]
- 89. Hiser J, Koenigs M.. The multifaceted role of the ventromedial prefrontal cortex in emotion, decision making, social cognition, and psychopathology. Biol Psychiatry. 2018;83(8):638–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Rudebeck PH, Rich EL.. Orbitofrontal cortex. Curr Biol. 2018;28(18):R1083–R1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Lacerda ALT, Hardan AY, Yorbik O, Vemulapalli M, Prasad KM, Keshavan MS.. Morphology of the orbitofrontal cortex in first-episode schizophrenia: Relationship with negative symptomatology. Prog Neuro-Psychopharmacology Biol Psychiatry. 2007;31(2):510–516. [DOI] [PubMed] [Google Scholar]
- 92. Culbreth AJ, Westbrook A, Daw ND, Botvinick M, Barch DM.. Reduced model-based decision-making in schizophrenia. J Abnorm Psychol. 2016;125(6):777–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Sterzer P, Voss M, Schlagenhauf F, Heinz A.. Decision-making in schizophrenia: A predictive-coding perspective. Neuroimage. 2019;190:133–143. [DOI] [PubMed] [Google Scholar]
- 94. Bora E, Yucel M, Pantelis C.. Theory of mind impairment in schizophrenia: Meta-analysis. Schizophr Res. 2009;109(1-3):1–9. [DOI] [PubMed] [Google Scholar]
- 95. Bonfils KA, Ventura J, Subotnik KL, Nuechterlein KH.. Affective prosody and facial emotion recognition in first-episode schizophrenia: Associations with functioning & symptoms. Schizophr Res Cogn. 2019;18:100153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Ptak R. The frontoparietal attention network of the human brain: Action, saliency, and a priority map of the environment. Neuroscientist. 2012;18(5):502–515. [DOI] [PubMed] [Google Scholar]
- 97. Tu PC, Lee YC, Chen YS, Li CT, Su TP.. Schizophrenia and the brain’s control network: Aberrant within- and between-network connectivity of the frontoparietal network in schizophrenia. Schizophr Res. 2013;147(2-3):339–347. [DOI] [PubMed] [Google Scholar]
- 98. Postle BR. Working memory as an emergent property of the mind and brain. Neuroscience. 2006;139(1):23–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Xu Y, Chun MM.. Dissociable neural mechanisms supporting visual short-term memory for objects. Nature. 2006;440(7080):91–95. [DOI] [PubMed] [Google Scholar]
- 100. Mitchell DJ, Cusack R.. Flexible, capacity-limited activity of posterior parietal cortex in perceptual as well as visual short-term memory tasks. Cereb Cortex. 2008;18(8):1788–1798. [DOI] [PubMed] [Google Scholar]
- 101. Xu Y. Reevaluating the sensory account of visual working memory storage. Trends Cogn Sci. 2017;21(10):794–815. [DOI] [PubMed] [Google Scholar]
- 102. Dixit A. HB. Cognitive dysfunction in schizophrenia. Indian J Health Wellbeing. 2016;7(2):177–185. [Google Scholar]
- 103. Walterfang M, Wood AG, Barton S, et al. Corpus callosum size and shape alterations in individuals with bipolar disorder and their first-degree relatives. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:1050–1057. [DOI] [PubMed] [Google Scholar]
- 104. Bearden CE, Van Erp TGM, Dutton RA, et al. Mapping corpus callosum morphology in twin pairs discordant for bipolar disorder. Cereb Cortex. 2011;21:2415–2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Lloyd AJ, Ali HE, Nesbitt D, Moore PB, Young AH, Ferrier IN.. Corpus callosum changes in euthymic bipolar affective disorder. Br J Psychiatry. 2014;204(2):129–136. [DOI] [PubMed] [Google Scholar]
- 106. Yang C, Li L, Hu X, et al. Psychoradiologic abnormalities of white matter in patients with bipolar disorder: Diffusion tensor imaging studies using tract-based spatial statistics. J Psychiatry Neurosci. 2019;44(1):32–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Prunas C, Delvecchio G, Perlini C, et al. Diffusion imaging study of the corpus callosum in bipolar disorder. Psychiatry Res Neuroimaging. 2018;271:75–81. [DOI] [PubMed] [Google Scholar]
- 108. Wang F, Kalmar JH, Edmiston E, et al. Abnormal corpus callosum integrity in bipolar disorder: A diffusion tensor imaging study. Biol Psychiatry. 2008;64(8):730–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Yasuno F, Kudo T, Matsuoka K, et al. Interhemispheric functional disconnection because of abnormal corpus callosum integrity in bipolar disorder type II. BJPsych Open. 2016;2(6):335–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Leow A, Ajilore O, Zhan L, et al. Impaired inter-hemispheric integration in bipolar disorder revealed with brain network analyses. Biol Psychiatry. 2013;73(2):183–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Hauser P, Dauphinais ID, Berrettini W, Delisi LE, Gelernter J, Post RM.. Corpus callosum dimensions measured by magnetic resonance imaging in bipolar affective disorder and schizophrenia. Biol Psychiatry. 1989;26:659–668. [DOI] [PubMed] [Google Scholar]
- 112. Yasar AS, Monkul ES, Sassi RB, et al. MRI study of corpus callosum in children and adolescents with bipolar disorder. Psychiatry Res Neuroimaging. 2006;146(1):83–85. [DOI] [PubMed] [Google Scholar]
- 113. Walterfang M, Wood AG, Reutens DC, et al. Corpus callosum size and shape in first-episode affective and schizophrenia-spectrum psychosis. Psychiatry Res Neuroimaging. 2008;173:77–82. [DOI] [PubMed] [Google Scholar]
- 114. Szeszko PR, Robinson DG, Ikuta T, et al. White matter changes associated with antipsychotic treatment in first-episode psychosis. Neuropsychopharmacology. 2014;39(6):1324–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Vecchio D, Piras F, Piras F, et al. Lithium treatment impacts nucleus accumbens shape in bipolar disorder. NeuroImage Clin. 2020;25:102167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Sani G, Simonetti A, Janiri D, et al. Association between duration of lithium exposure and hippocampus/amygdala volumes in type I bipolar disorder. J Affect Disord. 2018;232:341–348. [DOI] [PubMed] [Google Scholar]
- 117. Manji HK, Moore GJ, Chen G.. Clinical and preclinical evidence for the neurotrophic effects of mood stabilizers: Implications for the pathophysiology and treatment of manic-depressive illness. Biol Psychiatry. 2000;48(8):740–754. [DOI] [PubMed] [Google Scholar]
- 118. van Velzen LS, Kelly S, Isaev D, et al. White matter disturbances in major depressive disorder: A coordinated analysis across 20 international cohorts in the ENIGMA MDD working group. Mol Psychiatry. 2020;25(7):1511–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Anholt GE, Aderka IM, Van Balkom AJLM, et al. Age of onset in obsessive-compulsive disorder: Admixture analysis with a large sample. Psychol Med. 2014;187:188–196. [DOI] [PubMed] [Google Scholar]
- 120. Kim T, Kwak S, Hur JW, et al. Neural bases of the clinical and neurocognitive differences between early-and late-onset obsessive–compulsive disorder. J Psychiatry Neurosci. 2020;45(4):234–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
De-identified data are available from the corresponding author upon reasonable request.


