Abstract
In the quest for the physical substrate of learning and memory, a consensus gradually emerges that memory traces are stored in specific neuronal populations and the synaptic circuits that connect them. In this review, we discuss recent progresses in understanding the reorganization of synaptic circuits and neuronal assemblies associated with learning and memory, with an emphasis on optical techniques for in vivo interrogations. We also highlight some open questions on the missing link between synaptic modifications and neuronal coding, and how stable memory persists despite synaptic and neuronal fluctuations.
Introduction
Learning and memory are vital brain functions, but their physical substrate long remains a mystery. In his Croonian Lecture delivered to the Royal Society of London in 1894 [1], Ramón y Cajal presciently remarked that with ‘ cerebral gymnastics’ or ‘mental exercise,’ ‘associations already established among certain groups of cells would be notably reinforced … in addition, completely new intercellular connections could be established thanks to the new formation of [axonal] collaterals and dendrites.’ Essentially, his view amounts to the idea that the plasticity of neural connections, both structural and functional, is fundamental to information processing and storage in the brain. This ‘connectionist’ tenet foreshadows Donald Hebb’s famous postulate [2], commonly summarized as ‘ fire together, wire together.’ As the discovery of long-term potentiation (LTP) [3] provides a plausible cellular substrate of sustained synaptic changes, this school of thought has received tremendous attention, culminating in the ‘synaptic plasticity and memory’ hypothesis [4] that ‘activity-dependent synaptic plasticity is induced at appropriate synapses during memory formation, and is both necessary and sufficient for the encoding and trace storage of [the] memory.’ Over the past several decades, a vast amount of knowledge [5,6] has accumulated on the molecular mechanisms and electrophysiology of various forms of synaptic plasticity, mostly with ex vivo preparations. As advancement in optical microscopy and molecular tools continue to promote in vivo interrogation of the synaptic circuit in learning and memory with single synapse-level resolution, our review will focus on findings arising from such endeavors.
Plasticity at individual synapses
Most early studies on synaptic plasticity used electrical stimulation of axonal inputs en bloc. This is a significant limitation, as synaptic inputs are hardly synchronous in vivo. The development of photo-uncaging of neurotransmitters addressed this problem [7]. In this technique, a photochemical protecting group is covalently linked to the bioactive molecule to render it inert; light irradiation severs the covalent bond and releases the caged substrate. The invention of two-photon (2P) microscopy [8], which confines excitation to a small volume around the focal point, further suggests that one may leverage 2P absorption for photo-uncaging with high spatial resolution. Matsuzaki et al. [9] showed that repeated photo-release of glutamate on single dendritic spines (postsynaptic sites of most excitatory neurons) induces a rapid, selective, and persistent enlargement of small spines, which is accompanied by electrophysiological changes consistent with LTP. This work establishes that LTP has a structural correlate and can indeed be induced in individual synapses. On the other hand, low-frequency glutamate uncaging results in the shrinkage of dendritic spines, a structural correlate of long-term depression (LTD) [10,11], and combining glutamate uncaging with optogenetic stimulation can induce spike timing-dependent plasticity at single spines [12]. To characterize the structure–function relationship of individual synapses in the living brain, Kasai et al. used in vivo patch clamp recording and 2P imaging to confirm a correlation between the maximum amplitude of electrical current induced by glutamate uncaging and the spine head volume [13]. Recently, the same group applied this technique to examine synaptic plasticity induced by 2P uncaging in vivo, and observed similar enlargement and shrinkage of spines in the neocortex as in hippocampal slices [14]. Furthermore, neurotransmitter uncaging can also induce spine formation [15] or elimination [16]. These studies demonstrate that the neuronal network can be reconfigured at the level of individual synapses.
Imaging learning-associated synaptic dynamics
As Bliss and Lømo prudently pointed out [3], the fact that the synapse can undergo LTP with repetitive stimuli ex vivo does not guarantee that this capacity is exploited in the intact animal under physiological conditions. To investigate synaptic plasticity in vivo during and after learning, researchers have followed structural changes of synapses with 2P microscopy.
Postsynaptic structural plasticity
To date, most works on the structural plasticity of synapses analyze the emergence and disappearance of spines, which are associated with synapse formation and elimination, respectively [17]. Such structural dynamics represents a topological reorganization of the neuronal network. Motor learning (e.g. single-pellet reaching, cued lever-press, running on an accelerating rotarod) promotes spine formation on pyramidal neurons in the mouse primary motor cortex (M1) [18–20] (Figure 1a–c). In addition, different sets of synapses may encode distinct motor memories (Figure 2a), as practicing novel, but not previously learned, motor tasks further promotes synaptogenesis [19]. Auditory fear conditioning increases synapse formation between lateral amygdala axons and L5 pyramidal neurons in the auditory cortex [21••]; fear extinction preferentially removes the new spines induced by fear conditioning [22]. Notably, learning-induced spine formation is spatially structured over the dendritic arbors (Figure 1c). Clustered spine formation has been observed both in M1 during motor learning [23] and in the retrosplenial cortex with contextual fear learning or training in the Morris water maze [24••]. Sibling dendritic branches of L5 pyramidal neurons also exhibit different degrees of spine formation enhancement after motor learning [25]. Cued lever-press promotes spine dynamics on the distal branches of the apical, but not the perisomatic, dendrites of L2/3 pyramidal neurons in M1 [20]. Such spatial structures of spine dynamics may reflect the combined effects of postsynaptic molecular signaling cross-talks [26] and presynaptic axons’ inhomogeneous distribution and differential activity patterns.
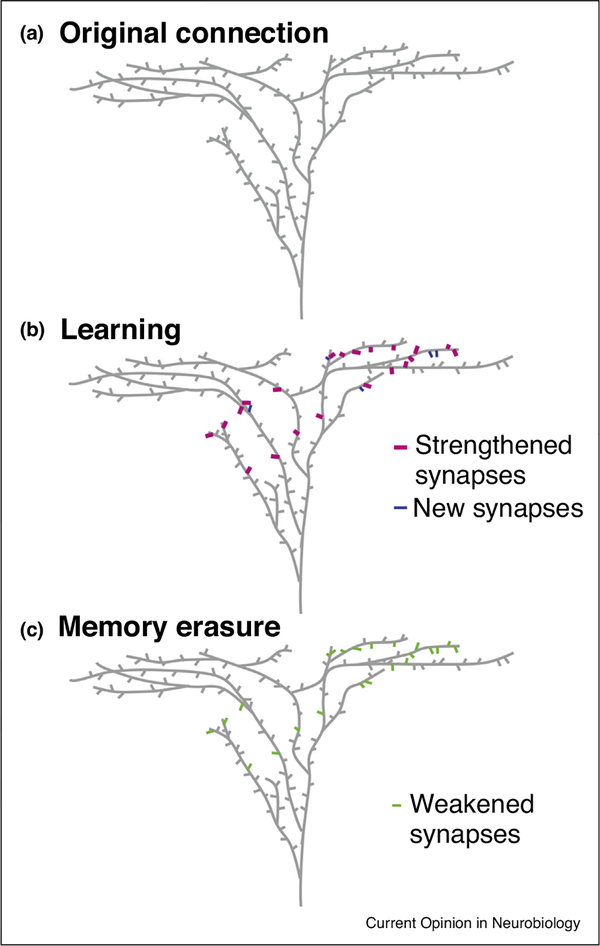
Figure 1.
Learning strengthens specific neuronal connections via synaptic modifications.
(a) A schematic drawing of spines on the apical dendritic arbor of a pyramidal neuron. (b) During learning some existing synapses (magenta) are strengthened and new synapses (blue) are formed, leading to stronger connections between the input axons (not shown) and the pyramidal neuron. (c) Selective weakening of the strengthened and newly formed synapses (green) erases the acquired memory.
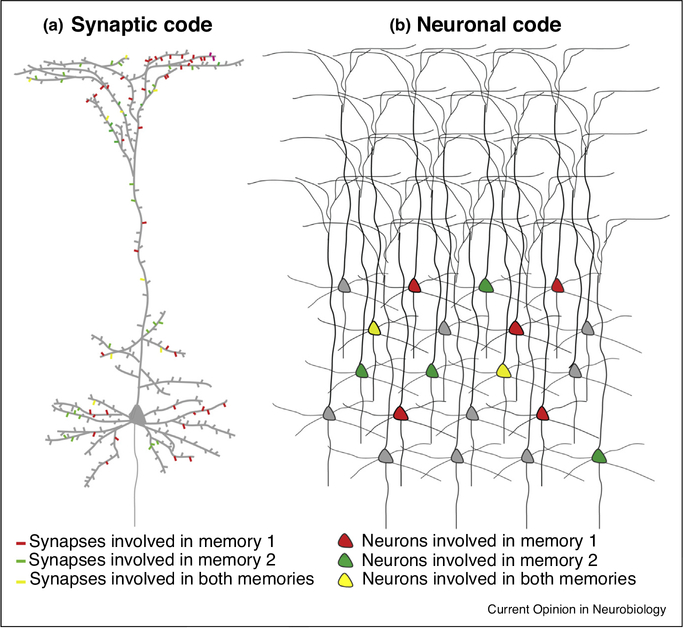
Figure 2.
Memory coding at synaptic and neuronal levels.
Two different memories may recruit two partially overlapping sets of synapses on the same neuron (a) and/or two partially overlapping sets of neurons (b).
Besides spine turnover, spine head size, a correlate of synaptic strength, also changes with learning. In the forebrain nucleus HVC of juvenile zebra finches, hearing a tutor song triggers the enlargement of stable spines [27]. In mouse M1, when spines form in a cluster, succedent new spine(s) are added as the first new spine is enlarged [23]. Recently, Roth et al. [28•] observed an increased a-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor level at a subset of spines in the mouse motor and visual cortices as the mouse learns a new motor skill. These results are consistent with mechanistic models of LTP and agree with the Hebbian theory of the strengthening of task-relevant neuronal circuits (Figure 1b,c).
Presynaptic structural plasticity
Learning also affects the structural dynamics on the presynaptic side. In the mouse M1, rotarod training differentially impact corticocortical and thalamocortical axonal boutons [29]. In the cerebellum, acrobatic motor learning (sequentially traversing elevated obstacles) reduces the formation of axonal varicosities on parallel fibers [30]. One plausible interpretation is that some inputs are strengthened by forming multi-synapse boutons (MSBs) [31], while the overall synaptic strength remains constant as the total number of presynaptic varicosities is reduced. This idea is consistent with electron microscopy reconstruction of Purkinje cell synapses after acrobatic learning, which reveals not only the selective induction of MSBs but also the comparatively small size of synapses near MSBs [32]. Such concerted synaptic strengthening and weakening are reminiscent of the findings in the hippocampus [33]. Interestingly, learning may also add new axonal boutons onto an existing dendritic spine, forming a multi-synapse spine [21••]. In principle, adding a new presynaptic or postsynaptic element to an existing synapse may strengthen it without changing the connectivity between the parent neurons, or create new fan-in or fan-out connections, or serve as a transitory state in swapping synaptic partners. The computational implication of such configurations calls for further studies.
Synaptic manipulation alters learning and memory
A demonstration of correlation does not establish a causal role of synaptic changes in learning and memory: one needs to employ manipulations. Nabavi et al. [34] made a significant progress in showing such a causal link. They generated associative memory in the rat by pairing a foot shock with optogenetic stimulation of auditory inputs to the amygdala, a region essential in fear conditioning. Optogenetically induced LTD inactivated the fear memory, which could be reinstated by optogenetic LTP. Similarly, optogenetic LTD in amygdalar neurons receiving thalamic afferents persistently attenuates learned fear [35]. Abdou et al. [36•] further showed that optogenetic LTP or LTD of synapses specific to one memory selectively affects the recall of that memory only. However, the plasticity induction protocols in these studies lack synapse-level precision and do not recapitulate the physiological conditions in natural learning.
To achieve spine-specific control, Hayashi-Takagi et al. [37] expressed AS-PaRac1 (a light-activatable version of the synaptic signaling protein Rac1), which can induce spine shrinkage with blue light, in the mouse motor cortex. They trained the mouse to run on a rotarod, and then selectively weakened the potentiated synapses to cause the mouse to forget the motor skill, showing the necessity of synaptic changes in memory formation (Figure 1d). It is conceivable that synapse-specific expression of optogenetic actuators [38], combined with novel microscopy that can access many targets in 3D with millisecond precision in parallel [39], will pave the way for the proof of sufficiency, that is, to synthesize a memory by artificially inducing synaptic changes without behavioral training.
From synaptic circuit to cell assembly
The selective strengthening of synaptic connections gives rise to the ‘cell assembly,’ subset of cells that can ‘[act] briefly as a closed system.’ [2]. Such cell assemblies provide a natural candidate for the physical substrate of memory traces or ‘engrams’ (Figure 2b), which have been demonstrated in a number of elegant works using activity-dependent cell labeling with optogenetics to activate, erase, and synthesize memory [40]. Recently the advent of genetically encoded calcium indicators has enabled researchers to characterize the establishment, maintenance, and evolution of neuronal assemblies by monitoring the activities of the same neurons longitudinally. Such studies have revealed interesting dynamism of neuronal assemblies. Generally, during motor learning, the movement-related activities of neuronal populations in the mouse motor cortex [41,42] and dorsolateral striatum [43] progressively converge and stabilize, corresponding to performance improvement. The functional reorganization of neuronal ensembles during learning is also cortical layer-specific [44].
On the other hand, a recent study [45] shows that when the mouse performs a hippocampus-dependent spatial memory task in a virtual environment, granule cells in the dentate gyrus exhibit a stable code, whereas neuronal ensembles in CA1 and CA2/3 represent the environment in a precise but dynamic way: although the number of place cells is similar for each context across days, individual cells shift their firing locations in the same context over days. This is consistent with the previous finding of a fluctuating membership of CA1 neurons in the place-coding ensemble when the free-moving mouse repeatedly explores an environment [46]. A spatial code with high day-to-day dynamism also emerges in the retrosplenial cortex [47]. Notably, such dynamism is not restricted to spatial memory. An earlier work on zebra finches [48] shows that the ensemble dynamics of projection neurons in the premotor nucleus HVC are globally stable, but individual neurons drift in and out of the ensemble. The dynamic representation of movement at single cell level is also observed in L2/3 neurons of M1 [44,49]. In mice repeatedly engaged in a virtual navigation task, neurons in the posterior parietal cortex exhibit a systemic representational drift. The drift makes the performance of fixed-weight linear decoders of kinematic information (position, velocity, and head direction) degrade significantly over time, which may be compensated by a biologically plausible synaptic plasticity rule [50••,51••]. Overall, these works suggest that the stability of a neuronal assembly, which accounts for the reproducibility of behavioral outputs, may be an emergent property at the ensemble level, while individual neurons only participate in the ensemble transiently. To use an analogy, when a vortex forms upon unplugging the bathtub, the structure of the vortex persists for some time, but the constituent water molecules are constantly in flux.
Outlooks and open questions
Many questions remain regarding how the cellular engram arises from synaptic interactions of its constituent neurons, and how neuronal network activities in turn modify the synaptic circuit. Here we highlight a few of them. To address these challenges, we need the synergy between conceptual innovations and technical advances.
Who are the presynaptic partners in structural dynamics of synapses?
Most in vivo studies on synaptic plasticity so far have focused on the postsynaptic spines only, due to the sparse neuronal labeling necessitated by the limited resolution of 2P microscopy. Yet the activity patterns experienced by synapses on the same dendritic branch may be dramatically disparate, dictated by their presynaptic partners [52,53]. Thus, identifying the parent neurons of the presynaptic axons becomes ineluctable for further dissection of the cellular engram. Molecular tools based on protein engineering [54] and viral vectors with low cytotoxicity [55] will enable trans-synaptic labeling of neuronal connections in vivo.
What are the functional roles of newly formed synapses in memory recall?
Only a fraction of newly formed synapses persist beyond the learning period [19]. What determines their fate? Are the persistent synapses active during memory recall in a consistent way? Novel volumetric imaging techniques [56,57] that can image synaptic activities will help address these questions.
How does the neuron integrate cell type-specific and pathway-specific synaptic inputs, and how does learning affect that?
In addition to the excitatory synapses discussed above, the placement and dynamics of inhibitory synapses exert a significant impact on the signal detection and integration of the neuron [58]. Even with the knowledge of the type and location of all synapses, the plethora of nonlinearities in dendritic integration and localized dendritic electrical events [59] highlight the need to treat a neuron, with its elaborate dendritic arbors and myriad of synaptic inputs, as a network rather than a point [60]. Further complicating this picture are the global changes in neuronal excitability, which may modulate the allocation of neurons to memory traces [61]. By combining anatomical and functional imaging data, one may build morphologically and biophysically realistic computational models with predictive power [62•] to address such questions.
How does the synaptic circuit maintain a stable representation of information despite spontaneous synaptic turnover and remodeling?
Numerous works have shown that synaptic connections in the living brain are volatile [63,64]. Properties such as spine and axonal bouton volume, which are correlated with synaptic strength, may fluctuate considerably under baseline conditions. Moreover, synapses may be formed or eliminated under baseline conditions without any explicit learning and memory process. These phenomena raise an acute question: how can a synaptic network maintain a stable memory with unstable synapses? A recent work [65•] proposes an interesting scheme to store memory as limit-cycle attractors. Such ideas await experimental tests and further theoretical elaborations.
How can a fluctuating neuronal assembly encode reproducible behavior? What are the underlying synaptic mechanisms?
If population dynamics are confined to a low-dimensional manifold, it may yield a stable representation of information in spite of single neuron variability [66,67]. However, certain synaptic plasticity may still be required to compensate for the neuronal drifting [51••]. It would be desirable to monitor synaptic and somatic activities of a neuronal population simultaneously to uncover such mechanisms.
Acknowledgements
We thank Dr. Weifeng Xu, Dr. Yang Yang, and Dr. Shaorong Ma for critical comments on the manuscript. This work is supported by the National Institutes of Health (grants R01MH109475 and R01NS104950) and a Max Planck Fellowship to Y.Z.
Footnotes
Conflict of interest statement
Nothing declared.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Ramón y Cajal S: The Croonian lecture — la fine structure des centres nerveux. Proc R Soc Lond 1894, 55:444–468 The section on the cerebral cortex is translated in De Felipe J, Jones EG: Cajal on the Cerebral Cortex: An Annotated Translation of the Complete Writings. New York: Oxford University Press; 1988. [Google Scholar]
- 2.Hebb DO: The Organization of Behavior: A Neuropsychological Theory. New York, NY: Wiley; 1949. [Google Scholar]
- 3.Bliss TV, Lømo T: Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol 1973, 232:331–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martin SJ, Grimwood PD, Morris RG: Synaptic plasticity and memory: an evaluation of the hypothesis. Annu Rev Neurosci 2000, 23:649–711. [DOI] [PubMed] [Google Scholar]
- 5.Herring BE, Nicoll RA: Long-term potentiation: from CaMKII to AMPA receptor trafficking. Annu Rev Physiol 2016, 78:351–365. [DOI] [PubMed] [Google Scholar]
- 6.Diering GH, Huganir RL: The AMPA receptor code of synaptic plasticity. Neuron 2018, 100:314–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ellis-Davies GCR: Two-photon uncaging of glutamate. Front Synaptic Neurosci 2018, 10:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Denk W, Strickler JH, Webb WW: Two-photon laser scanning fluorescence microscopy. Science 1990, 248:73–76. [DOI] [PubMed] [Google Scholar]
- 9.Matsuzaki M, Honkura N, Ellis-Davies GC, Kasai H: Structural basis of long-term potentiation in single dendritic spines. Nature 2004, 429:761–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nägerl UV, Eberhorn N, Cambridge SB, Bonhoeffer T: Bidirectional activity-dependent morphological plasticity in hippocampal neurons. Neuron 2004, 44:759–767. [DOI] [PubMed] [Google Scholar]
- 11.Zhou Q, Homma KJ, Poo MM: Shrinkage of dendritic spines associated with long-term depression of hippocampal synapses. Neuron 2004, 44:749–757. [DOI] [PubMed] [Google Scholar]
- 12.Zhang YP, Holbro N, Oertner TG: Optical induction of plasticity at single synapses reveals input-specific accumulation of alphaCaMKII. Proc Natl Acad Sci U S A 2008, 105:12039–12044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Noguchi J, Nagaoka A, Watanabe S, Ellis-Davies GC, Kitamura K, Kano M, Matsuzaki M, Kasai H: In vivo two-photon uncaging of glutamate revealing the structure-function relationships of dendritic spines in the neocortex of adult mice. J Physiol 2011, 589:2447–2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Noguchi J, Nagaoka A, Hayama T, Ucar H, Yagishita S, Takahashi N, Kasai H: Bidirectional in vivo structural dendritic spine plasticity revealed by two-photon glutamate uncaging in the mouse neocortex. Sci Rep 2019, 9:13922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kwon HB, Sabatini BL: Glutamate induces de novo growth of functional spines in developing cortex. Nature 2011, 474:100–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hayama T, Noguchi J, Watanabe S, Takahashi N, Hayashi-Takagi A, Ellis-Davies GC, Matsuzaki M, Kasai H: GABA promotes the competitive selection of dendritic spines by controlling local Ca2+ signaling. Nat Neurosci 2013, 16:1409–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knott GW, Holtmaat A, Wilbrecht L, Welker E, Svoboda K: Spine growth precedes synapse formation in the adult neocortex in vivo. Nat Neurosci 2006, 9:1117–1124. [DOI] [PubMed] [Google Scholar]
- 18.Yang G, Pan F, Gan WB: Stably maintained dendritic spines are associated with lifelong memories. Nature 2009, 462:920–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu T, Yu X, Perlik AJ, Tobin WF, Zweig JA, Tennant K, Jones T, Zuo Y: Rapid formation and selective stabilization of synapses for enduring motor memories. Nature 2009, 462:915–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen SX, Kim AN, Peters AJ, Komiyama T: Subtype-specific plasticity of inhibitory circuits in motor cortex during motor learning. Nat Neurosci 2015, 18:1109–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang Y, Liu DQ, Huang W, Deng J, Sun Y, Zuo Y, Poo MM: Selective synaptic remodeling of amygdalocortical connections associated with fear memory. Nat Neurosci 2016, 19:1348–1355.•• The authors used dual-color in vivo 2P imaging to monitor presynaptic and postsynaptic structures simultaneously. They found that synapse formation was predominantly additive rather than de novo, that is, by adding new partners to existing synapses. The implication of this phenomenon for microcircuit topology and function remains to be elucidated.
- 22.Lai CSW, Adler A, Gan WB: Fear extinction reverses dendritic spine formation induced by fear conditioning in the mouse auditory cortex. Proc Natl Acad Sci U S A 2018, 115:9306–9311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fu M, Yu X, Lu J, Zuo Y: Repetitive motor learning induces coordinated formation of clustered dendritic spines in vivo. Nature 2012, 483:92–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frank AC, Huang S, Zhou M, Gdalyahu A, Kastellakis G, Silva TK, Lu E, Wen X, Poirazi P, Trachtenberg JT et al. : Hotspots of dendritic spine turnover facilitate clustered spine addition and learning and memory. Nat Commun 2018, 9:422.•• The authors examined the relationship between spine dynamics in the mouse retrosplenial cortex and contextual or spatial learning. They found that pre-learning turnover rate is correlated with learning rate and performance, and that learning-related spine clustering tends to occur at hotspots of pre-learning turnover. These findings suggest that baseline spine turnover may serve the important role of sampling the synaptic configurational space, while learning selectively stabilizes the appropriate connections.
- 25.Yang G, Lai CS, Cichon J, Ma L, Li W, Gan WB: Sleep promotes branch-specific formation of dendritic spines after learning. Science 2014, 344:1173–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nishiyama J, Yasuda R: Biochemical computation for spine structural plasticity. Neuron 2015, 87:63–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roberts TF, Tschida KA, Klein ME, Mooney R: Rapid spine stabilization and synaptic enhancement at the onset of behavioural learning. Nature 2010, 463:948–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roth RH, Cudmore RH, Tan HL, Hong I, Zhang Y, Huganir RL: Cortical synaptic AMPA receptor plasticity during motor learning. Neuron 2020, 105:895–908 e895.• The authors imaged AMPA receptor dynamics in the mouse cortex during the acquisition of a motor task and found that motor learning increases AMPA receptor levels in a subset of clustered spines. It directly demonstrated learning-associated synaptic plasticity at the molecular level.
- 29.Hasegawa R, Ebina T, Tanaka YR, Kobayashi K, Matsuzaki M: Structural dynamics and stability of corticocortical and thalamocortical axon terminals during motor learning. PLoS One 2020, 15:e0234930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carrillo J, Cheng SY, Ko KW, Jones TA, Nishiyama H: The long-term structural plasticity of cerebellar parallel fiber axons and its modulation by motor learning. J Neurosci 2013, 33:8301–8307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Toni N, Buchs PA, Nikonenko I, Bron CR, Muller D: LTP promotes formation of multiple spine synapses between a single axon terminal and a dendrite. Nature 1999, 402:421–425. [DOI] [PubMed] [Google Scholar]
- 32.Lee KJ, Park IS, Kim H, Greenough WT, Pak DT, Rhyu IJ: Motor skill training induces coordinated strengthening and weakening between neighboring synapses. J Neurosci 2013, 33:9794–9799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bourne JN, Harris KM: Coordination of size and number of excitatory and inhibitory synapses results in a balanced structural plasticity along mature hippocampal CA1 dendrites during LTP. Hippocampus 2011, 21:354–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nabavi S, Fox R, Proulx CD, Lin JY, Tsien RY, Malinow R: Engineering a memory with LTD and LTP. Nature 2014, 511:348–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen M, Bi LL: Optogenetic long-term depression induction in the PVT-CeL circuitry mediates decreased fear memory. Mol Neurobiol 2019, 56:4855–4865. [DOI] [PubMed] [Google Scholar]
- 36.Abdou K, Shehata M, Choko K, Nishizono H, Matsuo M, Muramatsu SI, Inokuchi K: Synapse-specific representation of the identity of overlapping memory engrams. Science 2018, 360:1227–1231.• The authors used sophisticated mouse transgenic strategies and optogenetics to induce LTP or LTD at synapses specific to one auditory fear memory. They found that the recall of that memory, but not the other linked fear memory, is affected. This work shows that although the cellular engrams of distinct but related memories may overlap, the synapse-specific plasticity confers uniqueness to each memory trace.
- 37.Hayashi-Takagi A, Yagishita S, Nakamura M, Shirai F, Wu YI, Loshbaugh AL, Kuhlman B, Hahn KM, Kasai H: Labelling and optical erasure of synaptic memory traces in the motor cortex. Nature 2015, 525:333–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gobbo F, Marchetti L, Jacob A, Pinto B, Binini N, Pecoraro Bisogni F, Alia C, Luin S, Caleo M, Fellin T et al. : Activity-dependent expression of channelrhodopsin at neuronal synapses. Nat Commun 2017, 8:1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen IW, Papagiakoumou E, Emiliani V: Towards circuit optogenetics. Curr Opin Neurobiol 2018, 50:179–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tonegawa S, Morrissey MD, Kitamura T: The role of engram cells in the systems consolidation of memory. Nat Rev Neurosci 2018, 19:485–498. [DOI] [PubMed] [Google Scholar]
- 41.Peters AJ, Chen SX, Komiyama T: Emergence of reproducible spatiotemporal activity during motor learning. Nature 2014, 510:263–267. [DOI] [PubMed] [Google Scholar]
- 42.Adler A, Zhao R, Shin ME, Yasuda R, Gan WB: Somatostatin-expressing interneurons enable and maintain learning-dependent sequential activation of pyramidal neurons. Neuron 2019, 102:202–216 e207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sheng MJ, Lu D, Shen ZM, Poo MM: Emergence of stable striatal D1R and D2R neuronal ensembles with distinct firing sequence during motor learning. Proc Natl Acad Sci U S A 2019, 116:11038–11047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Masamizu Y, Tanaka YR, Tanaka YH, Hira R, Ohkubo F, Kitamura K, Isomura Y, Okada T, Matsuzaki M: Two distinct layer-specific dynamics of cortical ensembles during learning of a motor task. Nat Neurosci 2014, 17:987–994. [DOI] [PubMed] [Google Scholar]
- 45.Hainmueller T, Bartos M: Parallel emergence of stable and dynamic memory engrams in the hippocampus. Nature 2018, 558:292–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ziv Y, Burns LD, Cocker ED, Hamel EO, Ghosh KK, Kitch LJ, El Gamal A, Schnitzer MJ: Long-term dynamics of CA1 hippocampal place codes. Nat Neurosci 2013, 16:264–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mao D, Neumann AR, Sun J, Bonin V, Mohajerani MH, McNaughton BL: Hippocampus-dependent emergence of spatial sequence coding in retrosplenial cortex. Proc Natl Acad Sci U S A 2018, 115:8015–8018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liberti WA 3rd, Markowitz JE, Perkins LN, Liberti DC, Leman DP, Guitchounts G, Velho T, Kotton DN, Lois C, Gardner TJ: Unstable neurons underlie a stable learned behavior. Nat Neurosci 2016, 19:1665–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huber D, Gutnisky DA, Peron S, O’Connor DH, Wiegert JS, Tian L, Oertner TG, Looger LL, Svoboda K: Multiple dynamic representations in the motor cortex during sensorimotor learning. Nature 2012, 484:473–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Driscoll LN, Pettit NL, Minderer M, Chettih SN, Harvey CD: Dynamic reorganization of neuronal activity patterns in parietal cortex. Cell 2017, 170:986–999 e916.•• Driscoll et al. longitudinally tracked Ca activities of neurons in the parietal cortex while the mouse performed a virtual navigation task. They found that the relationship between individual cell’s activity and task features changed significantly across days, but statistical features of the population activity remained stable. The ensuing theoretical paper [51] further indicates that the observed drift would degrade a fixed readout but may be compensated by a synaptic plasticity rule. Together these two papers highlight the dynamic nature of the neuronal representations, and how synaptic plasticity, which is usually considered to confer the flexibility required by learning, can also serve to provide the stability needed by memory.
- 51.Rule ME, Loback AR, Raman DV, Driscoll LN, Harvey CD, O’Leary T: Stable task information from an unstable neural population. eLife 2020, 9.•• Driscoll et al. longitudinally tracked Ca activities of neurons in the parietal cortex while the mouse performed a virtual navigation task. They found that the relationship between individual cell’s activity and task features changed significantly across days, but statistical features of the population activity remained stable. The ensuing theoretical paper [51] further indicates that the observed drift would degrade a fixed readout but may be compensated by a synaptic plasticity rule. Together these two papers highlight the dynamic nature of the neuronal representations, and how synaptic plasticity, which is usually considered to confer the flexibility required by learning, can also serve to provide the stability needed by
- 52.Chen X, Leischner U, Rochefort NL, Nelken I, Konnerth A: Functional mapping of single spines in cortical neurons in vivo. Nature 2011, 475:501–505. [DOI] [PubMed] [Google Scholar]
- 53.Scholl B, Wilson DE, Fitzpatrick D: Local order within global disorder: synaptic architecture of visual space. Neuron 2017, 96:1127–1138 e1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim J, Zhao T, Petralia RS, Yu Y, Peng H, Myers E, Magee JC: mGRASP enables mapping mammalian synaptic connectivity with light microscopy. Nat Methods 2011, 9:96–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chatterjee S, Sullivan HA, MacLennan BJ, Xu R, Hou Y, Lavin TK, Lea NE, Michalski JE, Babcock KR, Dietrich S et al. : Nontoxic, double-deletion-mutant rabies viral vectors for retrograde targeting of projection neurons. Nat Neurosci 2018, 21:638–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lu R, Sun W, Liang Y, Kerlin A, Bierfeld J, Seelig JD, Wilson DE, Scholl B, Mohar B, Tanimoto M et al. : Video-rate volumetric functional imaging of the brain at synaptic resolution. Nat Neurosci 2017, 20:620–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kazemipour A, Novak O, Flickinger D, Marvin JS, Abdelfattah AS, King J, Borden PM, Kim JJ, Al-Abdullatif SH, Deal PE et al. : Kilohertz frame-rate two-photon tomography. Nat Methods 2019, 16:778–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Boivin JR, Nedivi E: Functional implications of inhibitory synapse placement on signal processing in pyramidal neuron dendrites. Curr Opin Neurobiol 2018, 51:16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stuart GJ, Spruston N: Dendritic integration: 60 years of progress. Nat Neurosci 2015, 18:1713–1721. [DOI] [PubMed] [Google Scholar]
- 60.Poirazi P, Papoutsi A: Illuminating dendritic function with computational models. Nat Rev Neurosci 2020, 21:303–321. [DOI] [PubMed] [Google Scholar]
- 61.Lisman J, Cooper K, Sehgal M, Silva AJ: Memory formation depends on both synapse-specific modifications of synaptic strength and cell-specific increases in excitability. Nat Neurosci 2018, 21:309–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Iascone DM, Li Y, Sumbul U, Doron M, Chen H, Andreu V, Goudy F, Blockus H, Abbott LF, Segev I et al. : Whole-neuron synaptic mapping reveals spatially precise excitatory/inhibitory balance limiting dendritic and somatic spiking. Neuron 2020, 106:566–578 e568.• The authors developed an imaging and analysis platform to map all excitatory and inhibitory synapses on the entire dendritic arbor of an individual neuron. They found a precise local balance of excitatory and inhibitory synapses across dendritic segments, which strongly impacts the neuronal input–output characteristics as suggested by computational modeling. This impressive work demonstrates the potential of combining imaging and modeling to understand how the integrative properties of neurons arise from their synaptic circuits.
- 63.Ziv NE, Brenner N: Synaptic tenacity or lack thereof: spontaneous remodeling of synapses. Trends Neurosci 2018, 41:89–99. [DOI] [PubMed] [Google Scholar]
- 64.Mongillo G, Rumpel S, Loewenstein Y: Intrinsic volatility of synaptic connections – a challenge to the synaptic trace theory of memory. Curr Opin Neurobiol 2017, 46:7–13. [DOI] [PubMed] [Google Scholar]
- 65.Susman L, Brenner N, Barak O: Stable memory with unstable synapses. Nat Commun 2019, 10:4441.• In this theoretical work, the authors proposed a scheme to store memory as time-varying attractors of neural dynamics, rendering the memory resilient to erosions caused by fluctuations in synaptic strength. It suggests an interesting route to solve the problem of maintaining stable memories in neural networks with ever-changing connections.
- 66.Montijn JS, Meijer GT, Lansink CS, Pennartz CM: Population-level neural codes are robust to single-neuron variability from a multidimensional coding perspective. Cell Rep 2016, 16:2486–2498. [DOI] [PubMed] [Google Scholar]
- 67.Gallego JA, Perich MG, Chowdhury RH, Solla SA, Miller LE: Long-term stability of cortical population dynamics underlying consistent behavior. Nat Neurosci 2020, 23:260–270. [DOI] [PMC free article] [PubMed] [Google Scholar]