Abstract
The aim of current study was to evaluate the acrylamide level in chicken, meat and shrimp nugget samples cooked in both traditional and industrial methods using “Quick, Easy, Cheap, Effective, Rugged, and Safe” QuEChERS extraction and gas chromatography-flame-ionization detection (GC-FID). Results revealed the traditional frying method has significant effect on the increase of acrylamide compared to industrial frying method and it was also found that the different cooking temperatures and time have significant effect on increase of acrylamide formation (p < 0.05), but type of edible oils had no significant effect. The highest acrylamide level found in shrimp nuggets (27 ± 1.5 ng/g) which fried by colza oil and traditional cooking method (6 min at 220 °C), while the lowest content of acrylamide found in chicken nuggets (7.3 ± 0.1 ng/g) which fried by corn oil and industrial method (3 min at 180 °C). Monte Carlo simulation (MCS) results indicated that the trend of potential non-carcinogenic risks on THQ for children was chicken nugget (3.51E-3) > meat nugget (1.36E-3) > shrimp nugget (1.43E-4) and for adults was chicken nugget (3.49E-4) > meat nugget (1.35E-4) > shrimp nugget (1.38E-5). The health risk of acrylamide for adults and children, was considerably lower than the safe risk limits (HQ >1 and CR > 1E-4) for Iranian population.
Keywords: Acrylamide, Nugget, Health risk assessment, Cooking methods
Introduction
Over the past few decades, rapid economic development and globalization of markets worldwide have led to fundamental changes in human lifestyles [1] Fast food cooking methods such as deep frying are one of the most common cooking methods in the industrial world, which are used to prepare delicious and spicy snacks. During the cooking process at high temperatures, various contaminants may occur in food that can have adverse effects on human health (acrylamide, polycyclic aromatic hydrocarbons, polyethylene terephthalate, etc.) [2–11]. The International Agency for Research on Cancer (IARC) and World Health Organization (WHO) reports that acrylamide is a potential human carcinogen (group 2A) considering its carcinogenicity in rodents [12]. In addition, it has recently been recognized that acrylamide has a toxic effect on human nervous system [13]. The presence of acrylamide in human daily diets causes concern among people in different countries of the world. In addition, estimates of acrylamide intake in diets have been established in many countries with different dietary records [13]. In human daily diets, the daily intake of acrylamide is estimated at 0.3 to 0.8 μg/kg body weight [14] . Acrylamide intake can vary from diet to diet in different countries around the world [15]. Acrylamide is formed in a wide variety of foods, especially foods rich in carbohydrate (starch), amino acids(asparagine) and oils(glycerol is degraded to acrolein), during the thermal frying process at temperatures above 120 °C [16, 17]. The main factors that affect the acrylamide formation process are: temperature and time of heating, blanching, humidity, pH, cooking method, etc. [18]. Several studies have been performed on the effect of various factors: cooking temperature, cooking method and cooking time, there was a strong correlation between temperature, method and cooking time and acrylamide formation [19–22]. However, the long cooking time combined with temperature (260 °C, 20 min) in various cooking methods reduced the acrylamide content in food [23]. Biedermann and Grob were found that acrylamide can also be formed at less than 100 °C [13]. Compared to conventional cooking conditions, classic cooking conditions with optimized temperature and relative humidity conditions reduce acrylamide formation up to 50% [13, 24]. Many studies indicated that relatively large amounts of acrylamide are also formed during frying or baking of carbohydrates and protein-containing foods such cereal products and nuggets (a small piece of chicken or shrimp covered with dry spices mix methods).Considerable quantities of nuggets consumed by the Iranian people are suspected of containing acrylamide. In Iran, no study has been carried out on the formation and quantitation of acrylamide in nuggets. Therefore, this study was conducted for the first time to evaluate the amount of acrylamide in cooked nuggets in two different methods using QuEChERS method and gas-flame ionization gas chromatography (GC-FID). Also, health risk assessment (non-carcinogenic and carcinogenic risk) of acrylamide for children and adults due to ingestion of chicken, meat and shrimp nuggets was estimated using MCS method.
Materials and methods
Chemical substances and reagents
The standard of acrylamide >99% (GC) and dimethyl phthalate as surrogate standard, zinc acetate Zn (CH3COO)2·2H2O, NaCl and anhydrous MgSO4 (analytical grades) were prepared from company of Merck (Darmstadt, Germany), and potassium hexacyanoferrate (II) 3-hydrate was obtained from company of PanReac (USA). All solvents used with analytical grade and purity >98% were purchased from company of Sigma-Aldrich (St. Louis, MO, USA). Primary and secondary amine (PSA) was obtained from company of Supelco (Bellefonte, Pennsylvania, USA).
Standard preparation procedure
In the first step, a stock solution (1000 ppm) was prepared using acrylamide dissolution in acetone. For recovery, calibration, quantification studies, a mix of standard solution prepared from stock solution using dissolving in acetone to reach the concentration of final (1, 5 and 10 ppm). The internal standard prepared by dissolving the 1.6 mg, dimethyl phthalate standard in 50 mL methanol and then 250 μL from this solution were diluted to 20 mL with GC grade distilled water. The solution of Carrez I was prepared by adding and mixing 10.6 g of trihydrate potassium hexacyanoproate in 100 mL of water and the solution carrez II prepared by adding and mixing 21.9 g of zinc acetate dehydrate and acetic acid)3 mL(in 100 mL of water. The stock standard solution stability was checked at least in repetitive measurements for each of the solutions.
Traditional cooking method
All commercial nugget samples were purchased and transported using cooler box to the laboratory and were kept at −18 °C. In this methods row nugget was placed in a pan containing edible oil. Afterward the content of the pan was fried in tow deferent temperatures and two deferent cooking time (to record the frying temperature and cooking time a digital thermal sensor and a digital timer was used). Then the cooked and dried (at 40 °C for 24 h) samples were milled and passed through a 20 μm mesh sieve. The passed particles were put in zip-lock bags (Polyethylene) and kept in a freezer (Ekofrigolab, Massa Martana, Italy) at −18 °C until analysis [25, 26] .
Industrial cooking method
Automatic industrial household deep fryer (ZOKOP Electric Fryer,12 L)was used for frying of nugget samples. In this method, the industrial household deep fryer containing the edible oil was pre-heated at 120 °C and 180 °C, before nugget samples being immersed. According to the manufacturer’s recommendation, 5 pieces of each frozen brand were fried during 3 and 6 min at 120 °C and 180 °C, respectively, using one of the edible oils. Then the cooked and dried (at 40 °C for 24 h) samples were milled and passed through a 20 μm mesh sieve. The passed particles were put in zip-lock bags (Polyethylene) and kept in a freezer (Ekofrigolab, Massa Martana, Italy) at −18 °C until analysis [25, 26].
Sample preparation
Two grams of homogenate nugget sample was placed in a centrifuge tube (50 mL). 100 μl of the appropriate 4 mg/L concentration of the dimethyl phthalate (internal standard) added and mixed. After about 1 h, 5 mL of n-hexane, 10 mL of acetonitrile, and 10 mL of deionized water were added into sample container. The container contain a sample was shaken for 2 min and then, 4 g of MgSO4 and 1 g NaCl added to it and then was mixed (vortex) for 4 min and centrifuged (R Universal 320, hettich, Germany) at 5000 g in −5 °C for 5 min. In the second step, the upper layer or hexane was discarded. Then, 100 μL carrez I and 100 μL carrezII were vortex and added to it and centrifuged at 8000 rpm for 5 min. Afterward, upperlayer was placed into a glass tube with 0.1 g PSA and 0.3 g MgSO4 and then vortexed and then centrifuged according to the previous description. Then, the extract (2 mL) was placed into a glass vial and dried under stable pure nitrogen gas flow condition. The residue was reconstructed by adding about 1 mL acetone and solution was vortexed for 3 min. Finally, the solution was passed through a 0.45 μm filter and 1 μL of it was injected into the GC -FID.
Gas chromatography- flame-ionization detection (GC-FID)
The extract (1 μL) was injected into a gas chromatograph equipped / flame ionization (Model CP-3800, Varian, Belrose, Australia) detector and a CP-Sil 8CB capillary column (30 m * 0.32 mm, 0.25 μm film thickness) (Agilent, Santa Clara, CA). Helium was applied as the carrier gas at an inlet pressure of 76 kPa at a split ratio of 1, 3, with a through-column flow rate of 2.9 mL min−1. The calibration curve for the internal standard technique was confirmed daily. The program of oven temperature was as follows: 35 °C isothermal for 2 min, 10 °C min−1 to 300 °C, isothermal for 5 min, and final temperature at 325 °C for 5 min. The temperatures of injection port and detector were 280 °C and 325 °C, respectively. Hydrogen, air, and makeup (nitrogen) flow rates for the flame ionization detector were 40, 450, and 30 mL min−1, respectively.
Method validation GC-FID
The analytical procedure validation for quantitative analysis of acrylamide in samples and its aqueous extracts was conducted by selective evaluation, working and linear ranges, limit of detection (LOD), limit of quantification (LOQ), repeatability and reproducibility (precision). Matrix effects were studied using standard addition method, by adding 100 μL of 3 level of standard solution and mixed to the original samples (08267Supelco, acrylamide, certified reference material, TraceCERT®). The recoveries were within 87% and 102% for all the studied acrylamide concentrations.
Health risk assessment
The Chronic Daily Intake (CDI) of detected acrylamide due to commercial nugget samples ingestion was calculated by Eq. 1
| 1 |
where, C is the acrylamide concentration (mg/kg); IRi as the daily intake of nuggets (chicken, meat and shrimp) were set as 1,4 g/day, 0,7 g/day and 0.2 g/day, respectively [27].Whereas no detailed information was available about the per capita consumption, Therefore, IR for nuggets samples is supposed ∼20% of chicken, meat and shrimp consumption per day. [27]; ED is the time-period of acrylamide (children and adults is 6 and 70 years, respectively); EFi, is the exposure frequency (365 days/year for both age groups) [28–30]; BWi, the average body weight (for children and adults is 15 and 70 kg, respectively), [31, 32] and AT is the mean time. The non-carcinogenic risk of detected acrylamide due to nuggets ingestion was estimated according to Eq. 2: [33, 34].
| 2 |
where, RfD represents the oral reference dose, which is 0.002 mg/kg.day for acrylamide.
The hazard index (HI) to evaluate the overall potential for effects of non-carcinogenic of cumulative acrylamide in nuggets (chicken, meat and shrimp) and the estimated according to the following Eq. 3:
| 3 |
If THQ < 1 and TTHQ ≤1 value, the health risk to human populations is acceptable [31, 35, 36].
The Carcinogenic Risk (CR) Potentials of acrylamide was also calculated Incremental Lifetime Cancer Risk (ILCR) and according to the following Eq. 4 [34, 37]:
| 4 |
where CDI is the chronic daily intake (mg/kg.day) over a life time; and SF is the cancer slope factor (SF) for acrylamide is 0.5 (mg/kg per day) [38].
Statistical analysis
The results were shown to be as mean ± SD and the comparisons of mean acrylamide levels were evaluated by Kruskal-Wallis non-parametric tests using the SPSS (version 22.0) program. Monte Carlo Simulation (MCS) is one of the arbitrary algorithms used to analytics of uncertainty and information regarding issues about health risks. The MCS analyses were carried out by Crystal Ball software (version 11.1.2.4.600, Oracle, Decisioneering, Denver, CO, USA) [39, 40].
Results and discussion
Method validation
Before determining the acrylamide concentration, the condition of necessary for preparation of sample and for GC-FID analysis were attentively chosen, in order to define the optimal settings for the analysis. After optimization, the selected methods indicated to be the most selective and sensitive, with limits of detection (LODs) and limit of quantification (LOQ) for acrylamide in nugget samples. The developed method exhibited good linearity over the range from 0.2 to 2.5 μg/mL with a correlation coefficient of 0.9992. The limit of detection (s/n = (3) and limit of quantification (s/n = 10) were 0.2 to 2 and 0.6–6.7 μg/ kg, respectively, and relative standard deviation (RSD) < 6%. Moreover, the recovery present of acrylamide ranged from 87% to104%. The acrylamide determination method in fried nuggets was convenient, fast, accurate and reliable, easy to promote the use.
Effect type of cooking methods in acrylamide formation
Due to the potential side effects of acrylamide, its formation in fried foods is one of the main concerns in food safety. As formation of acrylamide occurs mainly during the cooking process, the effects of two type of cooking methods were examined. Since industrial and traditional methods of dieting vary in moisture content, acrylamide formation is likely to vary widely. Also, depending on the type of cooking methods, the contamination formation in food can also be different [41–43]. In the traditional method, we have a decrease in the moisture content and color changes (yellow to brown) more than the industrial method, and in fact, water withdrawal is one of the ways to decarboxylation the Amadori compounds, and then the acrylamide formation [2, 19, 44, 45].
The results of acrylamide content of the analyzed commercial nugget samples subjected to traditional and industrial methods, changes of time and temperature and type of edible oils are presented Table 1. The results showed that traditional frying method was significantly effect on increase acrylamide than the industrial frying and indicated frying methods with colza oil were significantly effects on increase acrylamide than the other two oils (p < 0.05).
Table 1.
The mean ± SD concentration of acrylamide in commercial nuggets samples ng/g
| Oil type | Frying tem | Frying time | acrylamide (ng/g) | |||||
|---|---|---|---|---|---|---|---|---|
| Shrimp nuggets | Meat nuggets | Chicken nuggets | ||||||
| Traditional | Industrial | Traditional | Industrial | Traditional | Industrial | |||
| Corn | 180 | 3 | 17 ± 1.1 | 12 ± 0.1 | 14.6 ± 0.2 | 13 ± .3 | 7.9 ± 0.1 | 7.3 ± 0.1 |
| Corn | 180 | 6 | 18 ± 1.0 | 16.2 ± 0.1 | 15.3 ± 0.1 | 11 ± 0.1 | 13 ± 0.7 | 12.1 ± 0.8 |
| Corn | 220 | 3 | 19.8 ± 0.1 | 15.1 ± 0.4 | 14 ± 1.2 | 15 ± 0.5 | 14.5 ± 0.1 | 20 ± 1.1 |
| Corn | 220 | 6 | 25.8 ± 1.7 | 20.5 ± 0.1 | 17.8 ± 0.3 | 13 ± 0.8 | 21.7 ± 0.3 | 13.5 ± 0.2 |
| Sunflower | 180 | 3 | 17 ± 0.7 | 15 ± 0.1 | 15 ± 0.2 | 13 ± 0.7 | 14.1 ± 0.4 | 13.1 ± 0.2 |
| Sunflower | 180 | 6 | 18 ± 1.2 | 16.2 ± 0.1 | 16 ± 0.3 | 14 ± 0.5 | 15 ± 1.0 | 14 ± 0.1 |
| Sunflower | 220 | 3 | 18.4 ± 0.4 | 13 ± 0.1 | 13.9 ± 0.2 | 15 ± 0.6 | 14.8 ± 0.1 | 19 ± 0.3 |
| Sunflower | 220 | 6 | 25 ± 1.0 | 26 ± 1.6 | 20 ± 0.9 | 18 ± 0.8 | 19.2 ± 0.9 | 20 ± 1.2 |
| Colza | 180 | 3 | 18 ± 0.2 | 18 ± 1.1 | 14.9 ± 0.4 | 12 ± 0.1 | 17.4 ± 0.1 | 16 ± 0.2 |
| Colza | 180 | 6 | 17 ± .3 | 19.8 ± 1.0 | 16.7 ± 0.2 | 14 ± 0.3 | 17.5 ± 0.5 | 17 ± 0.8 |
| Colza | 220 | 3 | 25 ± .9 | 16 ± 1.5 | 16.8 ± 0.7 | 14.6 ± 0.5 | 18 ± 0.7 | 13 ± 0.5 |
| Colza | 220 | 6 | 27 ± 1.5 | 17 ± 0.3 | 19 ± 0.5 | 17 ± 0.3 | 22 ± 1.3 | 17 ± 0.1 |
The highest acrylamide level was in shrimp nuggets (27 ± 1.5 ng/g) which fried by traditional method (220 °C and 6 min), while the lowest content of acrylamide level was in chicken nuggets (7.3 ± 0.1 ng/g) which fried by industrial method (180 °C and 3 min).
In the traditional cooking method, due to the high temperature of cooking and direct heating, the amount of moisture or water in most samples decreases, therefore, reduction of water and moisture is one of the most important factors in decarboxylation of Amadori compound and then acrylamide formation. Assuming that acrylamide formation occurs mainly in the crust independently of the recipe or heat treatment. Previous studies have reported similar result that the amount of moisture, the formation of acrylamide, and the Maylard reaction rate were inversely related, and that the shell of shrimp is the main site where acrylamide is formed [13, 19, 46]. It can also be concluded that higher acrylamide content in shrimp than other nuggets can be due to the chemical composition of the shrimp acrylamide can be formed due to chemical interactions between carbohydrates, fats, proteins, etc. in some foods under the influence of high heat with the presence of other factors (moisture, PH, etc.) [47].
In the study of Soncu et al. [48] in chicken bread meat products showed that the average of acrylamide content in chicken drumsticks, wings of chicken, burgers of chicken, and nuggets of chicken were as 174.30, 20.75, 58.60, and 71.42 μg/kg respectively. Pacetti et al. [49] reported the highest mean content of acrylamide was in bakery products, such as biscuit (1104 μg/kg) and wafer (1449 μg/kg), and potato chips (916 μg/kg)respectively [49].
Effects of influence frying period and frying temperature in acrylamide formation
In both cooking methods, acrylamide content increases with the increase in frying temperature from 180 °C to 220 °C, and frying period 3, 6 min (p < 0.05) (Table 1). As formation of acrylamide occurs mainly during the process of cooking, the effects of frying period and temperature were examined. According to some studies, there is a positive relationship between food frying time and formation of acrylamide, so the longer the time, the higher the amount of acrylamide in food. Also, depending on the frying time and frying temperature, the formation of contamination in food can also be different [43, 45, 50].
Similar studies have been conducted by some researchers on the effect of food heating on the formation of acrylamide. They reported that increasing the frying time increased acrylamide formation [51–53]. Ottram D S, et al.(2002) revealed that the amount of acrylamide in potato strips initially increased exponentially with a change in time at a constant oven temperature (200 °C), but after a long heating period, the acrylamide content clearly decreased, because the ratio of the destruction of acrylic hope to its production increases [47]. Barutcu et al. revealed that increases in cooking time and temperature could also influence formation of acrylamide. This findings is in good agreement current study [54]. Gokmen et al. observed that acrylamide content french fries was associated not only with increasing temperature but also with frying time. However, frying temperature appeared to have a greater effect on acrylamide formation when compared to frying time [55]. Tareke et al. showed that heating potato slices for 12 min at 100 °C had no effect on acrylamide formation, while at 120 °C a slight increase of 30 μg/kg was observed. Also, between different heating periods (150 and 100 s), the highest concentration was related to 150 s [56]. Pedreschi et al., while measuring acrylamide on French fries, found that the main factors influencing acrylamide formation were the temperature and heating time of the food [57]. Shahrbabaki et al. found that increasing the cooking time and temperature in different Tah-digs is one of the main factors in the formation of acrylamide [7]. In fact, the findings of the present study are in good agreement with the results of the above research on food products.
Effects of type of edible oil in acrylamide formation
Due to the potential side effects of acrylamide, its formation in fried foods is one of the main concerns in food safety. As formation of acrylamide occurs mainly during the cooking process, the effects of three type of edible oil were examined by changing the temperature and time, corporates with the type of cooking methods. Since colza, corn, and sunflower oil are vary in triglyceride and fatty acid composition, their rates of degradation and acrylamide formation are likely to vary as well. The highest acrylamide contents were exhibited in shrimp nuggets which fried by colza oil in 220 °C and 6 min (27 ± 1.5 μg/kg), while the lowest content of acrylamide contents were exhibited in chicken nuggets which fried by corn oil in 180 °C and 3 min (7.3 ± 0.1 ng/g). Overall, the findings suggest that the type of frying oil has no significant impact upon acrylamide formation (p < 0.05) (Table 1). Daniali et al. [58] reported, rate of acrylamide formation can vary depending on the type of cooking oil, they showed that the formation of acrylamide was significantly lower in saturated animal fats (366 ng/g in lard and 211 ng/g in ghee) than in unsaturated vegetable oils(2447 ng/g in soy oil, 1442 ng/g in palm olein).
Behrouz Akbari-adergani B, et al. reported the highest level of acrylamide were observed by sunflower oil in potato tah-dig (194.091 mg /Kg) and the lowest level of acrylamide was obtained by solid oil in the bread tah-dig (48.54 mg /Kg).
SUN et al. [59] reported when the chickens are fried in fresh oil at 150 °C for 11 min, they have the highest moisture level and the lowest fat content. The appearance of golden color in the legs of fried chicken occurs when the ratio of fried oil to fresh oil was 1:1 and the degree of frying of chicken legs were 150 °C for 12 min. Also, the lowest amount of acrylamide were found in the skin of fried chicken, when the chicken was fried in fresh oil at 150 °C for 11 min. Also, when the chickens are fried in fresh oil and used oil mixed at 150 °C for 12 min, they have the lowest levels of acrylamide [2]. Mustadagh et al. (2005) reported that the type of frying oil is not effective in the acrylamide formation in fried potatoes, so the type of frying oils (cotton, olive, peanut, shortening, canola, meal, soybean, grain) no a significant effect [60] [61]. Animal fats and vegetable oils can produce acrolein (at high temperatures) as a result of thermal degradation of glycerol or lipids. Acrylic oxidation usually produces acrylic acid, which can react with nitrogen sources and produce acrylamide [60, 62, 63]. Acrolein intolerance to the Maillard reaction can be attributed to the fact that acrolein reacts immediately with various food components instead of being converted to acrylic acid by oxidation. This occurs if the normal frying temperature was higher than the boiling point of both acrolein (51 °C) and acrylic acid (140 °C) [64]. Therefore, compounds that result from the breakdown of lipids at high temperatures may quickly become gaseous, and therefore do not participate in the acrylamide formation in food such as nuggets. When fats or oils are heated, a high level of acrylamide can be formed in the food, especially if these oils contain high concentrations of unsaturated fatty acids. In this regard, they have low frying stability and show that the highest concentration of acrylamide is formed when using colza oil, followed by sunflower oil and corn oil respectively. In this way, the oxidation of acrolein to acrylic acid appears to be an important step in the acrylamide formation. Overall, although lipids and oils may be involved in the acrylamide formation and provide heat transfer to food, these findings suggest that they do not appear to play a main role in the formation of acrylamide compared to the main pathway is its formation (presence of starch and asparagine).
Health risk assessment
The health risk of exposure to acrylamide concentration in each nugget was estimated using MCS approach. The mean acrylamide exposure, difference percentiles for three individual nuggets products, is shown in Table 2.
Table 2.
The Chronic Daily Intake (mg/kg body-weight/day) of the analyzed nuggets
| CDI | Meat | Chicken | Shrimp | Meat | Chicken | Shrimp |
|---|---|---|---|---|---|---|
| Percentiles | Adults | Children | ||||
| 5% | 3.49E-7 | 7.07E-7 | 1.20E-8 | 3.54E-6 | 7.28E-6 | 1.22E-7 |
| 50% | 5.14E-7 | 1.05E-6 | 1.79E-8 | 5.23E-6 | 1.08E-5 | 1.84E-7 |
| 75% | 6.01E-7 | 1.24E-6 | 2.09E-8 | 6.12E-6 | 1.28E-5 | 2.15E-7 |
| 95% | 7.76E-7 | 1.58E-6 | 2.68E-8 | 7.98E-6 | 1.65E-5 | 2.73E-7 |
The 95th percentile from meat, chicken, shrimp nuggets based on CDI value in adults were 27.76E-7, 1.58E-6 and 2.68E-8 mg/kg bw/day and in children the 95th were 27.98E-6, 1.65E-5 and 2.73E-7 mg/kg bw/day, respectively (Table 2.)
Table 3.
Carcinogenic risk (CR) for acrylamide in nuggets simulated by montecarlo
| CR | Meat | Chicken | Shrimp | Meat | Chicken | Shrimp |
|---|---|---|---|---|---|---|
| Percentiles | Adults | Children | ||||
| 5% | 1.66E-7 | 3.41E-7 | 5.74E-9 | 1.66E-6 | 3.39E-6 | 5.87E-8 |
| 50% | 2.49E-7 | 5.28E-7 | 8.93E-9 | 2.58E-6 | 5.43E-6 | 9.13E-8 |
| 75% | 3.00E-7 | 6.33E-7 | 1.05E-8 | 3.08E-6 | 6.48E-6 | 1.11E-7 |
| 95% | 3.85E-7 | 8.10E-7 | 1.34E-8 | 3.92E-6 | 8.30E-6 | 1.42E-7 |
These results indicated that the highest and the lowest dietary exposure to acrylamide was observed in chicken nuggets and shrimp nuggets, respectively, which correlated with their consumption rat.
There are a series of studies which focused on the daily acrylamide intake, but a common result was not obtained due to the differences in acrylamide, based on consumption behaviors, consumption rate, and concentrations determined in some common food items. However, this intake is lower than the estimated total daily acrylamide intake based on consumption behaviors and concentrations determined in some common food items in some countries.
For example, in a Spanish total diet study, the mean dietary intake values to be 0.00053 mg/kg body-weight/day [65]; 0.00043 and 0.00069 mg/kg body-weight/day for French adults and children, respectively [66]; 0.00062, and 0.00033 mg/kg body-weight/day for Polish adults and children, respectively [67]; 0.00012 mg/kg body-weight /day in Croatia [68]; and, 0.00039 mg/kg body-weight/day in the USA [69]. As a common finding of these reports fast food, cereal-based products, coffee, potato crisps and French fries were measured as the major contributors to acrylamide intake.
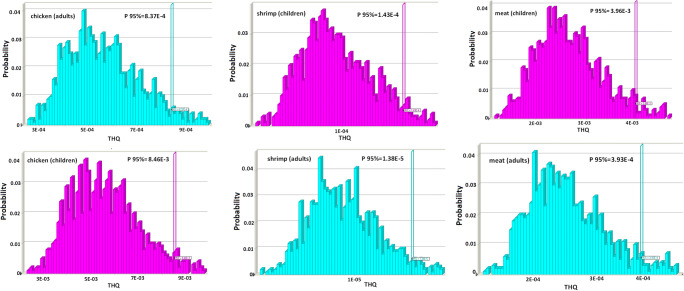
Estimated THQ values for the probability of a percentile of the exposure distribution for adults and children are presented in Fig. 1. The rank order of acrylamide level in children based on THQ was chicken nugget (8.46E-3) > meat nugget (3.96E-3) > shrimp nugget (1.43E-4) and for adults was chicken nugget (8.37E-4) > meat nugget (3.93E-4) > shrimp nugget (1.38E-5). In conclusion, the estimated THQ index indicates no health concern about dietary exposure for Iranian population. The HQs value of all samples in nugget products, for both adults and children, was lower than 1 value. Hence nugget products are not at considerable non-carcinogenic risk for consumers.
Fig. 1.
Uncertainly analysis for the THQ of acrylamide in nugget for children and adults
The result of cancer risk due to ingestion nugget products (acrylamide content) was presented in Table 3. The percentile 95% of the carcinogenic risk from meat, chicken, shrimp nugget due to acrylamide for adults was 3.85E-7, 8.10E-7 and 1.34E-08, respectively; and for children was 3.92E-6, 8.30E-6 and 1.42E-07, respectively. The hence carcinogenic risk for consumers is safe range, The percentile 95% ILCR acrylamide in nugget products was lower than 1E-4, the hence carcinogenic risk for consumers is safe range [70, 71].
Conclusion
From the perspective of acrylamide formation, it is possible that the different cooking methods and type of edible oil used can significantly influence the formation of acrylamide. The current study was performed to evaluate the acrylamide level in chicken, meat and shrimp nugget samples cooked in both traditional and industrial methods using QuEChERS extraction and gas chromatography-flame-ionization detection (GC-FID). The results revealed the traditional frying method has a significant effect on the increase of acrylamide compared to industrial frying method and it was also found that the different cooking temperatures and time have a significant effect on increase of acrylamide (p < 0.05), but type of edible oils had no significant effects. The highest acrylamide level was in shrimp nuggets (27 ± 1.5 ng/g) which fried by colza oil and traditional method (220 °C and 6 min), while the lowest content of acrylamide level was in chicken nuggets (7.3 ± 0.1 ng/g) which fried by corn oil and industrial method (180 °C and 3 min). MCS results indicated that the trend of potential non-carcinogenic risks on THQ for children were chicken nugget (3.51E-3) > meat nugget (1.36E-3) > shrimp nugget (1.43E-4) and for adults were chicken nugget (3.49E-4) > meat nugget (1.35E-4) > shrimp nugget (1.38E-5). The human health risk of acrylamide for adults and children, was considerably lower than the safe risk limits (HQ >1 and CR > 1E-4) for Iranians.
Acknowledgments
This work was conducted in laboratory of Department of Environmental Health of School of Health of Tehran University of Medical Sciences.
Declarations
Conflict of interest
The authors do not state any conflicts of interest in this research.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gadiraju TV, Patel Y, Gaziano JM, Djoussé L. Fried food consumption and cardiovascular health: a review of current evidence. Nutrients. 2015;7(10):8424–8430. doi: 10.3390/nu7105404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pedreschi F, Moyano P, Kaack K, Granby K. Color changes and acrylamide formation in fried potato slices. Food Res Int. 2005;38(1):1–9. [Google Scholar]
- 3.Shariatifar N, Dadgar M, Fakhri Y, Shahsavari S, Moazzen M, Ahmadloo M, Kiani A, Aeenehvand S, Nazmara S, Mousavi Khanegah A. Levels of polycyclic aromatic hydrocarbons in milk and milk powder samples and their likely risk assessment in Iranian population. J Food Compos Anal. 2020;85:103331. [Google Scholar]
- 4.Yousefi M, Shariatifar N, Tajabadi Ebrahimi M, Mortazavian AM, Mohammadi A, Khorshidian N, Arab M, Hosseini H. In vitro removal of polycyclic aromatic hydrocarbons by lactic acid bacteria. J Appl Microbiol. 2019;126(3):954–964. doi: 10.1111/jam.14163. [DOI] [PubMed] [Google Scholar]
- 5.Kiani A, Shariatifar N, Shahsavari S, Ahmadloo M, Moazzen M. Investigating the presence of polycyclic aromatic hydrocarbons in Doogh. J Mazandaran Univ Med Sci. 2019;29(178):10–23. [Google Scholar]
- 6.Moazzen M, Mahvi AH, Shariatifar N, Jahed Khaniki G, Nazmara S, Alimohammadi M, Ahmadkhaniha R, Rastkari N, Ahmadloo M, Akbarzadeh A, Dobaradaran S, Norouzian Baghani A. Determination of phthalate acid esters (PAEs) in carbonated soft drinks with MSPE/GC–MS method. Toxin Rev. 2018;37(4):319–326. [Google Scholar]
- 7.Shahrbabki PE, Hajimohammadi B, Shoeibi S, Elmi M, Yousefzadeh A, Conti GO, Ferrante M, Amirahmadi M, Fakhri Y, Mousavi Khaneghah A. Probabilistic non-carcinogenic and carcinogenic risk assessments (Monte Carlo simulation method) of the measured acrylamide content in Tah-dig using QuEChERS extraction and UHPLC-MS/MS. Food Chem Toxicol. 2018;118:361–370. doi: 10.1016/j.fct.2018.05.038. [DOI] [PubMed] [Google Scholar]
- 8.Kiani A, Ahmadloo M, Shariatifar N, Moazzen M, Baghani AN, Khaniki GJ, et al. Method development for determination of migrated phthalate acid esters from polyethylene terephthalate (PET) packaging into traditional Iranian drinking beverage (Doogh) samples: a novel approach of MSPE-GC/MS technique. Environ Sci Pollut Res. 2018;25(13):12728–12738. doi: 10.1007/s11356-018-1471-y. [DOI] [PubMed] [Google Scholar]
- 9.Moazzen M, Rastkari N, Alimohammadi M, Shariatifar N, Ahmadkhaniha R, Nazmara S, et al. Assessment of phthalate esters in a variety of carbonated beverages bottled in PET. J Environ Health Enginering. 2014;2(1):7–18. [Google Scholar]
- 10.Kouhpayeh A, Moazzen M, Jahed Khaniki GR, Dobaradaran S, Shariatifar N, Ahmadloo M, et al. Extraction and determination of phthalate esters (PAEs) in Doogh. J Mazandaran Univ Med Sci. 2017;26(145):257–267. [Google Scholar]
- 11.Roudbari A, Nazari RR, Shariatifar N, Moazzen M, Abdolshahi A, Mirzamohammadi S, et al. Concentration and health risk assessment of polycyclic aromatic hydrocarbons in commercial tea and coffee samples marketed in Iran. Environ Sci Pollut Res 2020;28:4827–4839. 10.1007/s11356-020-10794-0 [DOI] [PubMed]
- 12.FAO., food JFWCoHIoAi, staff WHO, organization WH, Programme WHOFS, WHO, et al. health implications of acrylamide in food: report of a joint FAO/WHO consultation, WHO headquarters, Geneva, Switzerland, 25-27 June 2002. World Health Organization; 2002.
- 13.Krishnakumar T, Visvanathan R. Acrylamide in food products: a review. J Food Process Technol. 2014;5(7):1. [Google Scholar]
- 14.Stroka J. Food additives & contaminants: Part A: Chemistry, analysis, control, exposure & risk assessment. Foreword. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2011;28(3):259. doi: 10.1080/19440049.2011.561599. [DOI] [PubMed] [Google Scholar]
- 15.Ono H, Chuda Y, Ohnishi-Kameyama M, Yada H, Ishizaka M, Kobayashi H, Yoshida M. Analysis of acrylamide by LC-MS/MS and GC-MS in processed Japanese foods. Food Addit Contam. 2003;20(3):215–220. doi: 10.1080/0265203021000060887. [DOI] [PubMed] [Google Scholar]
- 16.Boroushaki MT, Nikkhah E, Kazemi A, Oskooei M, Raters M. Determination of acrylamide level in popular Iranian brands of potato and corn products. Food Chem Toxicol. 2010;48(10):2581–2584. doi: 10.1016/j.fct.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 17.Lingnert H, Grivas S, Jägerstad M, Skog K, Törnqvist M, Åman P. Acrylamide in food: mechanisms of formation and influencing factors during heating of foods. Scand J Nutr. 2002;46(4):159–172. [Google Scholar]
- 18.Cengiz MF, Boyacı Gündüz CP. An eco-friendly, quick and cost-effective method for the quantification of acrylamide in cereal-based baby foods. J Sci Food Agric. 2014;94(12):2534–2540. doi: 10.1002/jsfa.6592. [DOI] [PubMed] [Google Scholar]
- 19.Shakeri F, Shakeri S, Ghasemi S, Troise AD, Fiore A. Effects of formulation and baking process on acrylamide formation in Kolompeh, a traditional cookie in Iran. J Chem. 2019;2019:1–6. [Google Scholar]
- 20.Foot R, Haase N, Grob K, Gonde P. Acrylamide in fried and roasted potato products: a review on progress in mitigation. Food Addit Contam. 2007;24(sup1):37–46. doi: 10.1080/02652030701439543. [DOI] [PubMed] [Google Scholar]
- 21.Kocadağlı T, Palazoğlu TK, Gökmen V. Mitigation of acrylamide formation in cookies by using Maillard reaction products as recipe modifier in a combined partial conventional baking and radio frequency post-baking process. Eur Food Res Technol. 2012;235(4):711–717. [Google Scholar]
- 22.Palazoğlu TK, Savran D, Gökmen V. Effect of cooking method (baking compared with frying) on acrylamide level of potato chips. J Food Sci. 2010;75(1):E25–EE9. doi: 10.1111/j.1750-3841.2009.01389.x. [DOI] [PubMed] [Google Scholar]
- 23.Capei R, Pettini L, Nostro AL, Pesavento G. Occurrence of acrylamide in breakfast cereals and biscuits available in Italy. J Prev Med Hyg. 2015;56(4):E190. [PMC free article] [PubMed] [Google Scholar]
- 24.Lindsay R, Jang S. Methods for suppressing acrylamide formation and restoring browned color and flavor: Google Patents; 2005.
- 25.Michalak J, Gujska E, Klepacka J. The effect of domestic preparation of some potato products on acrylamide content. Plant Foods Hum Nutr. 2011;66(4):307–312. doi: 10.1007/s11130-011-0252-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Albuquerque TG, Oliveira MBP, Sanches-Silva A, Bento AC, Costa HS. The impact of cooking methods on the nutritional quality and safety of chicken breaded nuggets. Food Funct. 2016;7(6):2736–2746. doi: 10.1039/c6fo00353b. [DOI] [PubMed] [Google Scholar]
- 27.Kalantari N, Ghafarpour M, Houshiarrad A, Kianfar H, Bondarianzadeh D, Abdollahi M, et al. National comprehensive study on household food consumption pattern and nutritional status, IR Iran, 2001–2003. Natl Rep. 2005;1(1).
- 28.Madani-Tonekaboni M, Rafiei Nazari R, Mirzamohammadi S, Abdolshahi A, Abbasi-bastami N, Arabameri M. Monitoring and risk assessment of Lead and cadmium in milks from east of Iran using Monte Carlo simulation method. Nutr Food Sci Res. 2019;6(2):29–36. [Google Scholar]
- 29.Taghizadeh SF, Goumenou M, Rezaee R, Alegakis T, Kokaraki V, Anesti O, Sarigiannis DA, Tsatsakis A, Karimi G. Cumulative risk assessment of pesticide residues in different Iranian pistachio cultivars: applying the source specific HQS and adversity specific HIA approaches in real life risk simulations (RLRS) Toxicol Lett. 2019;313:91–100. doi: 10.1016/j.toxlet.2019.05.019. [DOI] [PubMed] [Google Scholar]
- 30.Taghizadeh SF, Badibostan H, Hayes AW, Giesy JP, Karimi G. Residues levels of pesticides in walnuts of Iran and associated health risks. Hum Ecol Risk Assess: Int J 2019;1–14.
- 31.Jahanbakhsh M, Afshar A, Momeni Feeli S, Pabast M, Ebrahimi T, Mirzaei M, Akbari-Adergani B, Farid M, Arabameri M. Probabilistic health risk assessment (Monte Carlo simulation method) and prevalence of aflatoxin B1 in wheat flours of Iran. Int J Environ Anal Chem 2019;1–12. 10.1080/03067319.2019.1676421.
- 32.Shariatifar N, Rezaei M, Sani MA, Alimohammadi M, Arabameri M. Assessment of Rice marketed in Iran with emphasis on toxic and essential elements; effect of different cooking methods. Biol Trace Elem Res 2020;1–11. [DOI] [PubMed]
- 33.EPA U United States environmental protection agency. Quant Risk Assess Calculations. 2015;7–9:2015. [Google Scholar]
- 34.Samiee S, Fakhri Y, Sadighara P, Arabameri M, Rezaei M, Nabizadeh R, Shariatifar N, Mousavi Khaneghah A. The concentration of polycyclic aromatic hydrocarbons (PAHs) in the processed meat samples collected from Iran’s market: a probabilistic health risk assessment study. Environ Sci Pollut Res. 2020;27:21126–21139. doi: 10.1007/s11356-020-08413-z. [DOI] [PubMed] [Google Scholar]
- 35.Dadar M, Adel M, Nasrollahzadeh Saravi H, Fakhri Y. Trace element concentration and its risk assessment in common kilka (Clupeonella cultriventris caspia Bordin, 1904) from southern basin of Caspian Sea. Toxin Rev. 2017;36(3):222–227. [Google Scholar]
- 36.Ghasemidehkordi B, Malekirad AA, Nazem H, Fazilati M, Salavati H, Shariatifar N, Rezaei M, Fakhri Y, Mousavi Khaneghah A. Concentration of lead and mercury in collected vegetables and herbs from Markazi province, Iran: a non-carcinogenic risk assessment. Food Chem Toxicol. 2018;113:204–210. doi: 10.1016/j.fct.2018.01.048. [DOI] [PubMed] [Google Scholar]
- 37.Shariatifar N, Rezaei M, Alizadeh Sani M, Alimohammadi M, Arabameri M. Assessment of Rice marketed in Iran with emphasis on toxic and essential elements; effect of different cooking methods. Biol Trace Elem Res. 2020;198:721–731. doi: 10.1007/s12011-020-02110-1. [DOI] [PubMed] [Google Scholar]
- 38.Eslamizad S, Kobarfard F, Tsitsimpikou C, Tsatsakis A, Tabib K, Yazdanpanah H. Health risk assessment of acrylamide in bread in Iran using LC-MS/MS. Food Chem Toxicol. 2019;126:162–168. doi: 10.1016/j.fct.2019.02.019. [DOI] [PubMed] [Google Scholar]
- 39.Zhu Y, Duan X, Qin N, Lv J, Wu G, Wei F. Health risk from dietary exposure to polycyclic aromatic hydrocarbons (PAHs) in a typical high cancer incidence area in Southwest China. Sci Total Environ. 2019;649:731–738. doi: 10.1016/j.scitotenv.2018.08.157. [DOI] [PubMed] [Google Scholar]
- 40.Liao C-M, Chio C-P, Chen W-Y, Ju Y-R, Li W-H, Cheng Y-H, Liao VHC, Chen SC, Ling MP. Lung cancer risk in relation to traffic-related nano/ultrafine particle-bound PAHs exposure: a preliminary probabilistic assessment. J Hazard Mater. 2011;190(1–3):150–158. doi: 10.1016/j.jhazmat.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 41.Williams J. Influence of variety and processing conditions on acrylamide levels in fried potato crisps. Food Chem. 2005;90(4):875–881. [Google Scholar]
- 42.Ahrné L, Andersson C-G, Floberg P, Rosén J, Lingnert H. Effect of crust temperature and water content on acrylamide formation during baking of white bread: steam and falling temperature baking. LWT-Food Sci Technol. 2007;40(10):1708–1715. [Google Scholar]
- 43.Surdyk N, Rosén J, Andersson R, Åman P. Effects of asparagine, fructose, and baking conditions on acrylamide content in yeast-leavened wheat bread. J Agric Food Chem. 2004;52(7):2047–2051. doi: 10.1021/jf034999w. [DOI] [PubMed] [Google Scholar]
- 44.Michalak J, Gujska E, Czarnowska-Kujawska M, Nowak F. Effect of different home-cooking methods on acrylamide formation in pre-prepared croquettes. J Food Compos Anal. 2017;56:134–139. [Google Scholar]
- 45.Özkaynak E, Ova G. Effects of various cooking conditions on acrylamide formation in rolled patty. Food Addit Contam. 2009;26(6):793–799. doi: 10.1080/02652030902780257. [DOI] [PubMed] [Google Scholar]
- 46.Xia E-Q, Xu X-R, Chen Y-H, Wu S, Deng G-F, Zou Z-F, et al. Occurrence and analytical methods of acrylamide in food. Int J Food Nutr Saf. 2012;1(1):32–44. [Google Scholar]
- 47.Mottram DS, Wedzicha BL, Dodson AT. Acrylamide is formed in the Maillard reaction. Nature. 2002;419(6906):448–449. doi: 10.1038/419448a. [DOI] [PubMed] [Google Scholar]
- 48.Soncu ED, Haskaraca G, Kolsarıcı N. Presence of acrylamide and heterocyclic aromatic amines in breaded chicken meat products and dietary exposure of Turkish population from Ankara based on the food frequency questionnaire study. Eur Food Res Technol. 2018;244(3):501–511. [Google Scholar]
- 49.Pacetti D, Gil E, Frega NG, Álvarez L, Dueñas P, Garzón A, Lucci P. Acrylamide levels in selected Colombian foods. Food Addit Contam: Part B. 2015;8(2):99–105. doi: 10.1080/19393210.2014.995236. [DOI] [PubMed] [Google Scholar]
- 50.Kita A, Bråthen E, Knutsen SH, Wicklund T. Effective ways of decreasing acrylamide content in potato crisps during processing. J Agric Food Chem. 2004;52(23):7011–7016. doi: 10.1021/jf049269i. [DOI] [PubMed] [Google Scholar]
- 51.Rydberg P, Eriksson S, Tareke E, Karlsson P, Ehrenberg L, Törnqvist M. Investigations of factors that influence the acrylamide content of heated foodstuffs. J Agric Food Chem. 2003;51(24):7012–7018. doi: 10.1021/jf034649+. [DOI] [PubMed] [Google Scholar]
- 52.Elmore JS, Koutsidis G, Dodson AT, Mottram DS, Wedzicha BL. The effect of cooking on acrylamide and its precursors in potato, wheat and rye. Chemistry and safety of acrylamide in food: Springer; 2005. p. 255–69. [DOI] [PubMed]
- 53.Elmore JS, Koutsidis G, Dodson AT, Mottram DS, Wedzicha BL. Measurement of acrylamide and its precursors in potato, wheat, and rye model systems. J Agric Food Chem. 2005;53(4):1286–1293. doi: 10.1021/jf048557b. [DOI] [PubMed] [Google Scholar]
- 54.Barutcu I, Sahin S, Sumnu G. Acrylamide formation in different batter formulations during microwave frying. LWT-Food Sci Technol. 2009;42(1):17–22. [Google Scholar]
- 55.Gökmen V, Palazoğlu TK, Şenyuva HZ. Relation between the acrylamide formation and time–temperature history of surface and core regions of French fries. J Food Eng. 2006;77(4):972–976. [Google Scholar]
- 56.Tareke E, Rydberg P, Karlsson P, Eriksson S, Törnqvist M. Analysis of acrylamide, a carcinogen formed in heated foodstuffs. J Agric Food Chem. 2002;50(17):4998–5006. doi: 10.1021/jf020302f. [DOI] [PubMed] [Google Scholar]
- 57.Pedreschi F, Kaack K, Granby K. The effect of asparaginase on acrylamide formation in French fries. Food Chem. 2008;109(2):386–392. doi: 10.1016/j.foodchem.2007.12.057. [DOI] [PubMed] [Google Scholar]
- 58.Daniali G, Jinap S, Hajeb P, Sanny M, Tan C. Acrylamide formation in vegetable oils and animal fats during heat treatment. Food Chem. 2016;212:244–249. doi: 10.1016/j.foodchem.2016.05.174. [DOI] [PubMed] [Google Scholar]
- 59.Sun Z, Zhou G-h, Xu X-l, Wang P. Effect of frying conditions on the quality and security of fried chicken legs. Sci Technol Food Ind 2013;7.
- 60.Umano K, Shibamoto T. Analysis of acrolein from heated cooking oils and beef fat. J Agric Food Chem. 1987;35(6):909–912. [Google Scholar]
- 61.Mestdagh FJ, De Meulenaer B, Van Poucke C. Detavernier cl, Cromphout C, Van Peteghem C. influence of oil type on the amounts of acrylamide generated in a model system and in French fries. J Agric Food Chem. 2005;53(15):6170–6174. doi: 10.1021/jf0506683. [DOI] [PubMed] [Google Scholar]
- 62.Skog K, Alexander J. Acrylamide and other hazardous compounds in heat-treated foods: Woodhead Publishing; 2006.
- 63.Heshmati A, Sadati R, Ghavami M, Khaneghah AM. The concentration of potentially toxic elements (PTEs) in muscle tissue of farmed Iranian rainbow trout (Oncorhynchus mykiss), feed, and water samples collected from the west of Iran: a risk assessment study. Environ Sci Pollut Res 2019;1–10. [DOI] [PubMed]
- 64.Ehling S, Hengel M, Shibamoto T. Formation of acrylamide from lipids. Chemistry and safety of acrylamide in food. Springer; 2005. p. 223–33. [DOI] [PubMed]
- 65.Delgado-Andrade C, Mesías M, Morales FJ, Seiquer I, Navarro MP. Assessment of acrylamide intake of Spanish boys aged 11–14 years consuming a traditional and balanced diet. LWT-Food Sci Technol. 2012;46(1):16–22. [Google Scholar]
- 66.Commission E. Commission Recommendation of 10.1. 2011 on Investigations into the Levels of Acrylamide in Food. 2011.
- 67.Ghiasvand AR, Hajipour S. Direct determination of acrylamide in potato chips by using headspace solid-phase microextraction coupled with gas chromatography-flame ionization detection. Talanta. 2016;146:417–422. doi: 10.1016/j.talanta.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 68.Atwa MA, Emara M, Hamza AS, Elmeleigy K, Atwa M. Acrylamide levels in heat-treated Egyptian foods. J Food Dairy Sci. 2010;1(2):69–84. [Google Scholar]
- 69.Lineback DR, Coughlin JR, Stadler RH. Acrylamide in foods: a review of the science and future considerations. Annu Rev Food Sci Technol. 2012;3:15–35. doi: 10.1146/annurev-food-022811-101114. [DOI] [PubMed] [Google Scholar]
- 70.USEPA. U.S. Environmental Protection Agency, Supplemental guidance for assessing susceptibility from early-life exposure to carcinogens. 2016. http://www3.epa.gov/airtoxics/childrenssupplement final.pdf. Accessed 25 Jan 2016.
- 71.Huang C-L, Bao L-J, Luo P, Wang Z-Y, Li S-M, Zeng EY. Potential health risk for residents around a typical e-waste recycling zone via inhalation of size-fractionated particle-bound heavy metals. J Hazard Mater. 2016;317:449–456. doi: 10.1016/j.jhazmat.2016.05.081. [DOI] [PubMed] [Google Scholar]