Abstract
Purpose
Banana peel was used as a low-cost adsorbent for the removal of Cu and Pb ions from aqueous solution in a binary system.
Methods
The interactive effects of the operating parameters such as initial concentration, pH, adsorbent dosage and particle size were studied in a batch mode using central composite design. The characterizations of banana peels were done using point of zero charge (pHpzc), Fourier infrared transform (FTIR), scanning electron microscopy (SEM) and elemental composition (EDS).
Result
The point of zero charge of banana peels was determined to be 4.83. The FTIR, SEM and EDS showed the functional groups, surface morphology and elemental composition respectively before and after the adsorption process. The analysis of variance (ANOVA) showed a good fit of coefficient of determination (R2) for Cu and Pb being 0.998 and 0.988 respectively. The percentage removal of Cu and Pb increased with increasing adsorbent dosage, however, the bio-sorption capacity of Pb was greater than Cu. The optimized variable conditions for the bio-sorption of Cu and Pb using banana peel gave 99.79% and 88.94% removal for Pb and Cu respectively with initial concentration of 100 mg/L, pH 5, adsorbent dosage of 1 g and particle size of 75 μm. The above condition gave desirability of 0.959, which denotes that the optimum conditions are acceptable.
Conclusion
The regression model and the agreement between the experimental and predicted values confirmed the validity of second-order polynomial equation for the bio-sorption of Cu and Pb using banana peels.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40201-021-00632-x.
Keywords: Banana peels, Biosorption, Optimization, Wastewater, Response surface methodology
Introduction
Globally, pollution of the water body is a major challenge in both developed and the developing countries. It is a threat to humans, plants and animals wellbeing. Mining is one of the main activities in the industrial sector that generates high volumes of wastewater which greatly affect the environment. Wastewater produced from the mining activities is referred to as Acid mine drainage (AMD). AMD is highly toxic water with low pH and increased amounts of heavy metals and salts. AMD emanates from abandoned and ownerless mines or improper or failed treatment methods [1]. It contains heavy metals such as arsenic, cadmium, lead, manganese, mercury, zinc, chromium and copper which are hazardous to humans, plants and animals. When these pollutants enter surface water bodies, they have the potential to linger in natural ecosystems for a long period of time and accumulate in the biological chain thus generating severe and chronic diseases. The severe impact of AMD on the environment in some parts of the world has been explained and documented by various researchers [2–8]. The Environmental Protection Agency EPA has laws and regulations in place to minimise the formation and discharge of AMD into the environment. In South Africa, the limit for Cu(II) and Pb(II) discharge into water sources is 0.01 mg/L hence, it becomes imperative to treat the wastewater to an acceptable limit for portable use. The wastewater undergoes treatment in stages to conform to the permissible limit. At the tertiary stage of the wastewater treatment, the heavy metals concentrations are reduced, however, the presence of these heavy metals in wastewater is harmful at lower concentrations. Hence, it is highly important to treat the wastewater to a level whereby it causes no harm to humans, animals and plants life.
This work is focused on the treatment of wastewater to improve on the effluent quality of tertiary treatment process such that it can be healthy for plants, animals and human beings. There are many treatment methods that can be used to achieve better effluent quality such as ion exchange, osmosis, oxidation and reduction, flocculation and coagulation, complexation, solvent extraction, membrane separation, adsorption. All these methods except adsorption require high cost maintenance and level of expertise. Adsorption makes use of a phase known as the adsorbent that has the potential to adsorb different heavy metals. Recently, the use of adsorbents derived from natural or agricultural wastes known as bio-sorbents, have gained the interest of researchers for the treatment of wastewater as it is a way of reducing wastes in circulation. Many bio-sorbents have been used for removing various heavy metals from wastewater; moringa seeds, potato peels, cashew nut shell, watermelon rind, spinach, coffee, tea, corn cob [9–14]. These bio-sorbents have been tested and proven to have good adsorption capacity, readily available, application for industrial wastewater treatment, and can be reused in cycles and easily regenerated. Biosorption is cheap and eco-friendly, however, it is strongly affected by many environmental conditions such as, solution pH, initial concentration of the solution, adsorbent dosage, particle size of the adsorbent, time of mixing, temperature [15].
Banana is a fruit in high consumption worldwide. Unwanted peels of banana generate huge quantity of garbage/waste from household and marketplace. Banana peels consists of cellulose, hemicellulose, pectin, and lignin comprising of functional groups such as hydroxyl, carboxyl, carbonyl, and amine. These functional groups enhance the adsorption of metal ions to the active sites of the bio-sorbents [16–18]. Several studies have been reported on the bio-sorption of heavy metals using banana peels. The feasibility study of cadmium ion removal from aqueous solution using banana peel was carried out by investigating the effect of operating parameters such as; particle size, solution pH and initial metal ion concentration [19]. Adsorption of Cu2+ and Pb2+ was investigated using banana peel biochar in a batch experiment [20], natural banana peel was used as adsorbent for removal of fluoride from aqueous solution in batch experiment [21], adsorption of copper from water onto banana peel [22], banana peels nano-sorbent was used for the removal of radioactive mineral from mine water [23].
In the application of the bio-sorption process for industrial scale treatment of wastewater, it is crucial to study the effect of the co-existence of metal ions, as it is very rare for wastewater to contain a single metal ion. Therefore, to improve on the process efficiency, optimization of significant parameters is important, which can be achieved by means of response surface methodology approach (RSM).
This technique helps to explain the main effect of the most significant parameters, its interaction with other parameters and its quadratic effect that have impacts on the responses. RSM has been used extensively for optimization, prediction and interpretation of chemical processes using factorial designs [15, 24]. Some of the design methods applied for bio-sorption optimization with many variables include; Box-Behnken design (BBD), Central composite design (CCD), Doehlert design (DD) and a three level full factorial design [25]. Several studies have been carried out using RSM in the field of environmental pollution.
The present study therefore investigates the application of RSM to predict and optimize the bio-sorption capacity of banana peels for the binary removal of Cu(II) and Pb(II) ions from wastewater. Although, different reports exist on the bio-sorption of heavy metals using RSM, however, the bi-solute system of the heavy metals with the interaction of different operating parameters using RSM is an area of interest. The effects of operating parameters such as the solution pH, initial concentration of the solution, the adsorbent dosage and the particle size of the adsorbent was investigated and modelled. Mathematically designed RSM technique was used to optimize the above-mentioned parameters at specified ranges and explained their interactive effects on the responses (percentage removal of copper and lead).
Materials and methods
Preparation of bio-sorbent
Ripened banana peels were collected from a local market in Durban, South Africa. They were washed to remove dirt and thereafter rinsed with distilled water. Drying of the peels was then carried out in an oven (Prolab ovens and incubators) at 80 °C for 24 h. The dried peels were then crushed using attrition mill (Retsch GmbH 5657 HAAN, West-Germany). The bio-sorbent was re-washed to remove colour and dried again at 50 °C for 24 h. Thereafter, it was ground into smaller particles till it became powder using blender and then sieved through different meshes to get different particle sizes ranging from 75 to 455 μm.
Determination of point of zero charge of bio sorbent (pHzpc)
The point of zero charge of the banana peels was determined using 50 mL solution of 0.1 N KNO3 in eleven separate beakers. The solution pH was adjusted between 2 and 12 using 0.1 M HCl and 0.1 M NaOH. Then, 0.5 g of bio-sorbent was added to the solutions and the mixture was agitated at room temperature for 48 h. The final pH of the solution was measured and the difference between the initial pH and the final pH of the solution was plotted against the initial pH.
Preparation of synthetic solution
All the chemicals used were of analytical grade purchased from Laboratory Analytical Supplies Limited, South Africa. The stock solution of 1000 ppm was prepared by dissolving calculated amount of Pb(NO3)2 and Cu(NO3)2.3H2O in distilled water. Different initial metal concentrations were prepared by serial dilution of the stock solution. The pH of the solution was determined by a digital pH meter (HANNA edgepH HI 2002, USA) using digital pH electrode with integrated temperature sensor calibrated with a standard buffer solution. The pH of the solutions was adjusted by adding drops of 0.1 M H2SO4 or 0.1 M NaOH.
Analytical instruments used
Fourier transform infrared spectroscopy (FTIR) was used to determine the functional groups present in the bio-sorbent. The FTIR wavenumber and the percentage transmittance was generated to explain the range of each functional group (Perkin Elmer, Frontier, USA). Scanning electron microscopy (FEI Nova NanoSEM 230) was used to determine the surface morphology of bio-sorbent before and after adsorption. The elemental and bio-sorbent composition were identified using energy dispersive X-ray (Oxford X-Max detector and INCA software).
Batch bio-sorption experiment
The adsorption experiment was carried out by varying operating parameters which are initial concentration of the two-metal solution (mg/L), the adsorbent dosage (g), the pH of the solution and the adsorbent particle size (μm). Batch experiments were carried out using 250 mL conical flask containing 100 ml solution with varying initial concentrations (10–100 mg/L) of Cu(II) and Pb(II) and varying adsorbent dose (0.1–1 g). The particle size of the adsorbent was also varied (75–455 μm) and the pH of the solution ranged from 2 to 6 and was adjusted using 0.1 N H2SO4 or 0.1 N NaOH. The contact time was 120 min while the agitation speed was maintained at 180 rpm. After adsorption, the solutions were filtered using Whatman filter paper (150 mm) and the filtrate was filtered again using syringe filters (0.45 μm). Thereafter, the filtrate was analysed using micro-plasma atomic emission spectrophotometer (MP – AES, MY 18379001, Agilent, USA). The percentage removal of Pb(II) and Cu(II) was calculated using eqs. (1) and (2) respectively.
| 1 |
| 2 |
Where; Co,Pb and Co,Cu are the initial concentrations of Pb and Cu in the solution (ppm), Ce,Pb and Ce,Cu are the final concentrations of Pb and Cu in the solution after adsorption (ppm). The overall percentage removal of Pb and Cu was calculated using eq. (3).
| 3 |
Where; Co,Pb + Cu is the initial concentrations of Pb and Cu in the solution (ppm) and Ce,Pb + Cu is the final concentration of Pb and Cu in the solution after adsorption (ppm).
The amount of Pb and Cu adsorbed onto banana peels was calculated using the mass balance equation below.
| 4 |
The Langmuir and Freundlich isotherm models were used to determine the adsorption mechanism of Pb and Cu onto banana peels. The Langmuir isotherm assumes monolayer coverage of adsorbent, the model equation is stated below.
| 5 |
The Freundlich isotherm assumes the surface of the adsorbent is heterogenous and adsorption energy is exponentially distributed. The model equation is as expressed below.
| 6 |
Where qe and Ce are the quantity adsorbed (mg/g) and concentration at equilibrium (mg/L) respectively. Qmax is the maximum quantity adsorbed (mg/g) while b is the Langmuir constant, Kf and n are the Freundlich constant (L/g).
Central composite design (CCD)
The design expert software (11.1.0.1) was used to generate the statistical design of experiments and the data analysis. The CCD is one of the RSM design methods that makes use of a second-order model for improved optimization process. CCD offers high quality significant predictions of linear and quadratic interactive effects of the operating parameters influencing the process. It is a full factorial design consisting of two levels, a centre point that refers to the middle level of the factors and two axial points.
In this study, four operating parameters were selected for the bio-sorption of Cu(II) and Pb(II) ions using banana peels, namely; initial metal concentration (X1), pH (X2), adsorbent dosage (X3) and particle size (X4). The factor levels of the independent variables were coded as −1 (low), 0 (centre point) and + 1 (high) while the % removal of Cu (II) and Pb(II) ions were the dependent variables. A total of 30 experimental runs were generated from the design matrix. The experimental range and levels of the independent variables in coded form is depicted in Table 1. In the optimization process, the second order polynomial equation was used to explain the effects of the independent variables and their interactions. The quadratic model used to optimize the variables is as shown in Eq. 7,
| 7 |
Table 1.
Experimental range and level of independent variables
| Variables | Factor | Range and level | ||
|---|---|---|---|---|
| −1 | 0 | +1 | ||
| Initial metal concentration (mg/L) | A | 10 | 55 | 100 |
| pH | B | 2 | 4 | 6 |
| Adsorbent dosage (g) | C | 0.1 | 0.55 | 1 |
| Particle size (μm) | D | 75 | 265* | 455 |
*Actual value used in the experiment was 250 μm due to available sieve sizes
Where, Y is the response predicted, Xi and Xj are the independent variables, βo, βi, βii and βij are the regression coefficient and Ɛ is the residual error. The significant variables and the interpretation of the experimental results are explained using a set of mathematical functions called analysis of variance (ANOVA). The analysis of variance ANOVA is a statistical technique used to determine the significance of a factor in a multi-significant model. ANOVA helps to identify the most important factors in a model as well as the meaning of the experimental results. The F-value and p value are important coefficients for determining the fitness of the model and the significant of each factor represented in the model equation. The coefficient F-value is obtained as a quotient of the residual mean square and the mean square [15].
Results and discussion
Point of zero charge of banana peel
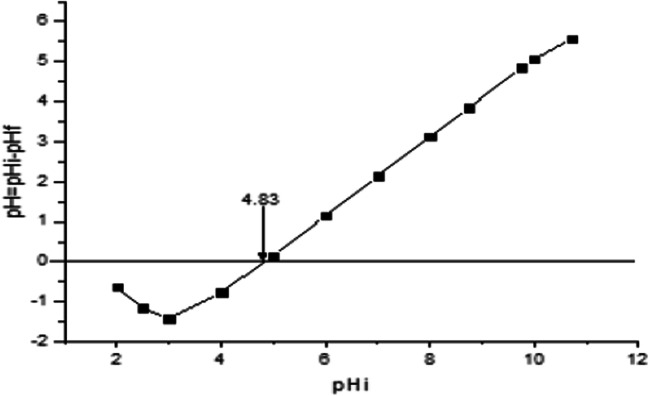
The point of zero charge pHpzc is the point at which the charge on an adsorbent is zero (neutral). It is obvious from Fig. 1 that the pHpzc of banana peel is 4.83. This suggests that the surface of banana peels contains acidic groups and therefore suitable for adsorption of cations. Adsorption of cations are favoured at pH higher than the pHpzc. However, it has been reported that when the pHpzc of banana peels is close to neutral value, it can be used for adsorption of cations and anions [26].
Fig. 1.
Point of zero charge of Banana peels
Characterization of bio-sorbent
Fourier transform infrared spectroscopy (FTIR)
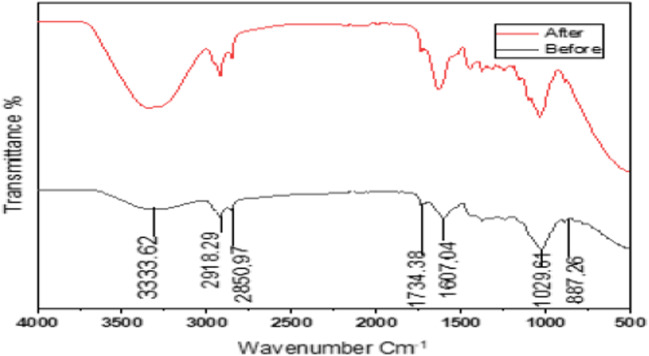
FTIR is an essential method of identifying the functional groups present on the surface of an adsorbent. There are several sharp peaks noticeable on the FT-IR graph of banana peels such as 3325 cm−1 that is attributed to -OH group, alcoholic stretching. The peaks at 2932 and 2855 cm−1 are the result of C-H stretching vibrations. The peak at 1728 cm−1 represents the carboxylic acids and ketones symmetrical stretching vibration of C=O. The peak showing at 1597 cm−1 indicates amine bending stretching vibration. The peaks at 1478, 1024 and 803 cm−1 represent -OH, C-OH and C-H stretching vibrations respectively. The FTIR spectra of copper and lead loaded banana peels (Fig. 2) showed the same characteristics of peaks as in natural banana peels with the percentage transmittance of the peaks either increases or decreases. The peaks on natural banana peels; 3325, 2932, 2855, 1728, 1597, 1478, 1024 and 803 cm−1 shifted to 3341, 2923, 2848, 1729, 1617, 1489, 1026 and 906 cm−1 respectively after adsorption of Cu(II) and Pb(II) ions. This shows that there were interactions of various functional groups present in banana peels with Cu (II) and Pb(II) ions. From the FTIR spectra (Fig. 2), the peak at 3325 cm−1 which agrees to -OH functional group became wider and shifted to 3341 cm−1 after adsorption. The percentage transmittance was also reduced after adsorption, suggesting the impact of -OH (hydroxyl group) on the adsorption of Cu(II) and Pb(II) ions. The peak corresponding to the alkyl group CH at 803 cm−1 was shifted to 906 cm−1 and became steep after adsorption, suggesting chemical interaction with Cu (II) and Pb(II) ions.
Fig. 2.
FTIR of banana peel before and after adsorption
Scanning electron microscope (SEM)
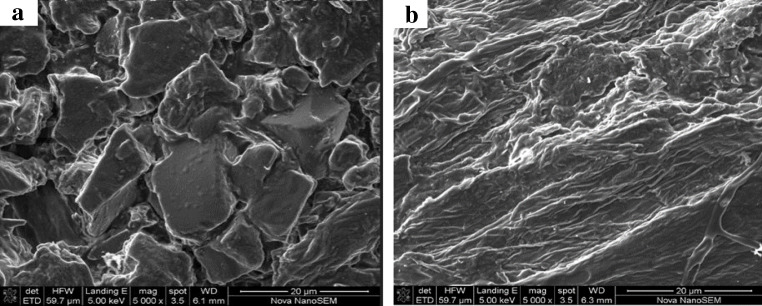
The SEM analysis of the banana peels was carried out before and after adsorption of Cu (II) and Pb (II) ions. This was used to explain the difference between the morphological structure of the banana peels before and after adsorption. Figure 3a shows the morphological structure of banana peels before adsorption, it reveals a rough, irregular and porous surface. The SEM of Cu(II) and Pb(II) ions loaded banana peels (Fig. 3b) shows a uniform, smooth and covered surface. This indicates the presence of Cu (II) and Pb (II) ions on the surface of the banana peels.
Fig. 3.
SEM of banana peels (a) before and (b) after adsorption
EDX analysis
The energy dispersive x-ray was done to determine the elemental composition of banana peel before and after adsorption as shown in Table 2. The presence of Cu(II) and Pb(II) ions after adsorption implies that banana peel has the potential to adsorb heavy metals while the absence of potassium after adsorption suggests ion exchange as the adsorption mechanism.
Table 2.
Elemental composition of Banana peels before and after adsorption
| Element Composition (%) |
C | O | Si | K | Pb | Cu |
|---|---|---|---|---|---|---|
| Before | 56.43 | 38.22 | 4.74 | 0.61 | – | – |
| After | 61.68 | 36.29 | 0.57 | – | 0.76 | 0.7 |
Experimental design
A total of 30 experimental runs were generated from design of experiment. The experimental design matrix and the responses for Cu(II) and Pb(II) ions biosorption are shown in Table 3. The centre of the design has six replicate runs that gave an approximate value of pure error variance. The second-order polynomial (Eq. 4) with multiple regression analysis was used to generate responses (Cu(II) and Pb(II) percentage removal) using the four design factors.
Table 3.
CCD experimental matrix in coded units and responses
| Run no | Variables | Responses | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Pb removal (%) | Cu removal (%) | Overall removal (%) | |||||||
| A | B | C | D | Y1exp | Y1pred | Y2exp | Y2pred | Yoverall | |
| 1 | 10 | 4 | 0.55 | 265 | 87.20 | 85.40 | 55.68 | 55.93 | 42.88 |
| 2 | 10 | 6 | 0.1 | 75 | 84.43 | 84.14 | 46.3 | 45.18 | 30.73 |
| 3 | 10 | 2 | 0.1 | 455 | 80.73 | 80.79 | 50.1 | 50.26 | 30.83 |
| 4 | 55 | 4 | 0.55 | 265 | 82.22 | 82.86 | 69.62 | 69.68 | 51.84 |
| 5 | 100 | 6 | 0.1 | 75 | 65.08 | 65.10 | 79.1 | 79.98 | 44.18 |
| 6 | 100 | 2 | 0.1 | 455 | 72.67 | 73.10 | 88.65 | 88.10 | 61.32 |
| 7 | 100 | 2 | 1 | 75 | 97.16 | 97.72 | 87.85 | 87.50 | 85.01 |
| 8 | 100 | 6 | 0.1 | 455 | 65.3 | 65.46 | 83.11 | 83.19 | 48.41 |
| 9 | 100 | 6 | 1 | 455 | 83.78 | 82.69 | 71.6 | 71.42 | 55.38 |
| 10 | 100 | 4 | 0.55 | 265 | 72.92 | 74.17 | 83.15 | 83.30 | 56.07 |
| 11 | 55 | 4 | 0.55 | 265 | 85.45 | 82.86 | 69.89 | 69.68 | 55.34 |
| 12 | 10 | 2 | 1 | 75 | 99.3 | 99.28 | 61.4 | 60.96 | 60.70 |
| 13 | 10 | 6 | 1 | 455 | 97.85 | 99.61 | 62.3 | 62.29 | 60.15 |
| 14 | 55 | 4 | 0.55 | 265 | 82.21 | 82.86 | 70.27 | 69.68 | 52.48 |
| 15 | 55 | 4 | 1 | 265 | 91.92 | 90.67 | 76.05 | 76.53 | 67.97 |
| 16 | 10 | 6 | 0.1 | 455 | 87.01 | 86.50 | 55.01 | 55.58 | 42.02 |
| 17 | 10 | 2 | 1 | 455 | 98.9 | 98.88 | 67.81 | 67.20 | 66.71 |
| 18 | 100 | 2 | 1 | 455 | 94.93 | 95.31 | 85.76 | 86.56 | 80.69 |
| 19 | 55 | 4 | 0.1 | 265 | 71.12 | 71.82 | 63.01 | 62.93 | 34.13 |
| 20 | 55 | 4 | 0.55 | 455 | 69.22 | 95.05 | 72.64 | 72.39 | 41.86 |
| 21 | 10 | 2 | 0.1 | 75 | 77.82 | 78.98 | 53.2 | 53.61 | 31.02 |
| 22 | 55 | 4 | 0.55 | 265 | 81.25 | 82.86 | 69.01 | 69.68 | 50.26 |
| 23 | 55 | 2 | 0.55 | 265 | 80.5 | 79.67 | 65.03 | 65.96 | 45.53 |
| 24 | 55 | 4 | 0.55 | 265 | 81.15 | 82.86 | 70.05 | 69.68 | 51.20 |
| 25 | 10 | 6 | 1 | 75 | 99.8 | 99.46 | 71.5 | 72.30 | 71.30 |
| 26 | 55 | 4 | 0.55 | 75 | 94.45 | 95.07 | 67.01 | 67.66 | 61.46 |
| 27 | 55 | 6 | 0.55 | 265 | 75.65 | 75.92 | 70.35 | 69.82 | 46.00 |
| 28 | 55 | 4 | 0.55 | 265 | 83.22 | 82.86 | 70.45 | 69.68 | 53.67 |
| 29 | 100 | 2 | 0.1 | 75 | 75.01 | 73.29 | 68.98 | 68.64 | 43.99 |
| 30 | 100 | 6 | 1 | 75 | 84.54 | 84.55 | 89.1 | 88.61 | 73.64 |
The quadratic regression model specifying the significant factors generated for the responses; percentage removal of Pb (Y1, %) and percentage removal of Cu (Y2, %) written with respect to coded factors and as a function of initial concentration (A), pH (B), adsorbent dosage (C) and the particle size (D) are stated in Eqs. (8)–(9) respectively.
| 8 |
| 9 |
The model equations were tested and analysed using t-test and the analysis of variance (ANOVA). The ANOVA showed 95% confidence level, which means the regression is statistically significant. Table 3 shows the experimental design matrix in coded factors and the responses for actual and predicted output of the respective metals (Y1exp, Y1pred., Y2exp and Y2pred.). It is obvious that the percentage removal of Pb is higher than Cu.
Analysis of variance and validation of model
The regression model was used to explain the interactions between the coded factors and the responses as presented in Tables 4 and 5. The regression model is well fitted when the model is significant, and the lack-of-fit is not significant. The responses for the bio-sorption of Pb and Cu were highly significant. The lack-of-fit was estimated using the pure error obtained from the experimental results and the residuals. The lack-of-fit p value was lower than 0.05 which implies that the quadratic model has good fit and significant for analysing the bio-sorption of Pb and Cu using banana peels. However, the p value of the model factor is expected to be lower than 0.05 then, the factor is regarded as significant.
Table 4.
ANOVA for the regression model
| Metal ion | Model factor | Coefficient estimate (coded factor) | Standard error | F-values | P values |
|---|---|---|---|---|---|
| Pb | Intercept | 82.78 | 0.457 | <0.0001 | |
| A | −5.65 | 0.346 | 267.17 | <0.0001 | |
| B | −1.86 | 0.346 | 29.07 | <0.0001 | |
| C | 9.38 | 0.346 | 736.68 | <0.0001 | |
| AB | −3.34 | 0.367 | 82.86 | <0.0001 | |
| AC | 1.03 | 0.367 | 7.90 | 0.0132 | |
| BC | −1.24 | 0.367 | 11.51 | 0.0040 | |
| A2 | −3.08 | 0.911 | 11.40 | 0.0042 | |
| B2 | −5.06 | 0.911 | 30.85 | <0.0001 | |
| D2 | 12.28 | 0.917 | 179.04 | <0.0001 | |
| Cu | Intercept | 69.87 | 0.2378 | <0.0001 | |
| A | 13.54 | 0.1798 | 5672.67 | <0.0001 | |
| B | 1.61 | 0.1798 | 80.03 | <0.0001 | |
| C | 6.39 | 0.1798 | 1265.33 | <0.0001 | |
| D | 2.36 | 0.1798 | 172.84 | <0.0001 | |
| AB | −2.56 | 0.1907 | 180.01 | <0.0001 | |
| AC | −4.62 | 0.1907 | 587.29 | <0.0001 | |
| AD | −1.80 | 0.1906 | 88.80 | <0.0001 | |
| BC | −2.56 | 0.1907 | 179.83 | <0.0001 | |
| BD | −4.06 | 0.1906 | 454.09 | <0.0001 | |
| CD | −5.10 | 0.1906 | 716.42 | <0.0001 | |
| B2 | −1.79 | 0.4738 | 14.32 | 0.0018 |
Table 5.
ANOVA validation
| Metal ions | Source | Sum of squares | Degree of freedom | Mean square | F-value | P value |
|---|---|---|---|---|---|---|
| Pb | Model | 2856.96 | 14 | 204.07 | 94.84 | <0.0001 |
| Residual | 32.28 | 15 | 2.15 | |||
| Cor. Total | 2889.23 | 29 | ||||
| Lack of fit | 19.55 | 10 | 1.95 | 0.7680 | 0.6631 | |
| Pure error | 12.73 | 5 | ||||
| R2 | 0.9888 | |||||
| Adjusted R2 | 0.9784 | |||||
| Predicted R2 | 0.9533 | |||||
| Cu | Model | 5504.47 | 14 | 393.18 | 675.95 | <0.0001 |
| Residual | 8.72 | 15 | 0.5817 | |||
| Cor. Total | 5513.20 | 29 | ||||
| Lack of fit | 7.39 | 10 | 0.7394 | 2.78 | 0.1354 | |
| Pure error | 1.33 | 5 | 0.2661 | |||
| R2 | 0.9984 | |||||
| Adj. R2 | 0.9969 | |||||
| Pred. R2 | 0.9903 |
For the percentage Pb bio-sorption (Y1), the significant factors in the model equation are A, B, C, AB, AC, BC, A2, B2 and D2 while D, AD, BD, CD and C2 are not significant which determines the influence of these variables on the bio-sorption process. The ANOVA results obtained from the regression model showed that the model is highly significant with F-value of 94.84 and p value less than 0.0001.
In addition, all terms represented in the quadratic model which include A, B, C, D, AB, AC, AD, BC, BD, CD and B2 are significant for the bio-sorption of Cu except A2, C2 and D2. The F-value for the regression model was 675.95 and the p value is lower than 0.0001.
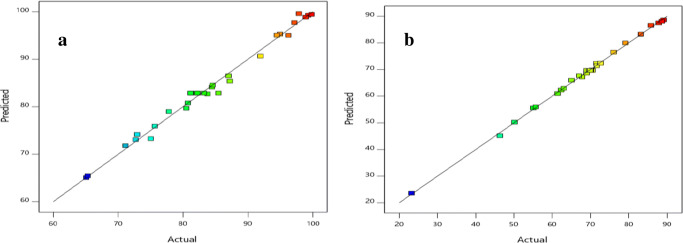
The validity for the mathematical model was done by comparing the actual values with the predicted values, this helps to verify the goodness of fit of the quadratic model. The coefficient of determination R2 (0.988; 0.998), adjusted R2 (0.978; 0.997) and predicted R2 (0.953; 0.990) for the bio-sorption of Pb and Cu respectively. A coefficient of determination close to 1 shows good correlation and relationship between the actual values and the predicted values as shown in Fig. 4. The difference between the adjusted R2 and the predicted R2 must also agree and not be more than 0.2 for a good fit.
Fig. 4.
Graph showing actual (%) vs predicted (%) removal data for (a) Pb and (b) Cu
Effect of initial concentration, pH, adsorbent dosage and particle size on Pb(II) and Cu(II) ions percentage removal
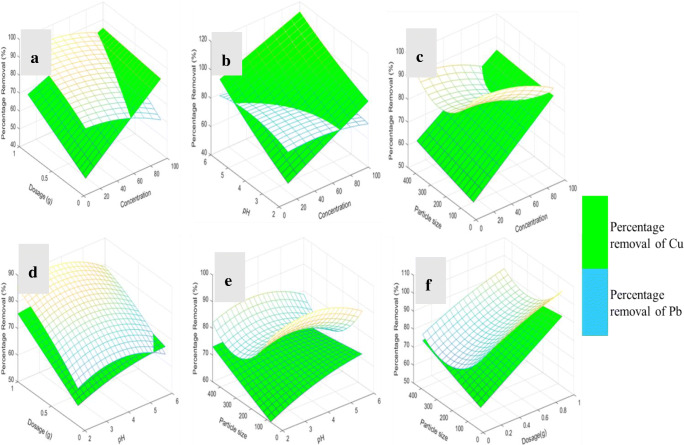
The effects of initial concentration, pH, adsorbent dosage and particle size on the percentage removal of Cu(II) and Pb(II) are explained using surface plot (Fig. 5). The surface plots were generated by writing code using MATLAB (MATLAB R2019a). Figure 5a, shows the interaction between the initial concentration and adsorbent dosage with the percentage removal of Cu(II) and Pb(II) ions. The bio-sorption capacity of Pb(II) increased with increasing adsorbent dosage while an increase in the initial concentration had no significant changes on the bio-sorption capacity of Pb(II) which implies that the surface of the bio-sorbent has reached saturation. Similarly, the percentage removal of Cu(II) increased as the adsorbent dosage and initial concentration increased. The effect of initial concentration and pH on percentage removal of Cu(II) and Pb(II) is shown in Fig. 5b. The percentage removal of Pb (II) increased with increasing pH from 2 to 6 while the initial concentration had no significant changes. Also, the percentage removal of Cu(II) increased with increasing pH and initial concentration. An intercept of the graphs occurred at the pH 5.5 and the initial concentration of 75 mg/L, this means that maximum adsorption of Cu(II) and Pb(II) ions occurred at pH 5.5. From Fig. 5c, it is obvious that the particle size has no influence on the percentage removal of Cu(II), however, it has significant effect on the percentage removal of Pb(II) ions.
Fig. 5.
3D surface plot of percentage removal of Cu and Pb with the interaction of the variables
From Fig. 5d showing the interactive effect of pH and adsorbent dosage on the percentage removal of Cu(II) and Pb(II) ions. It is shown clearly that the percentage removal of Cu(II) and Pb(II) ions increased with increasing adsorbent dosage and the pH. At pH 5 to 5.5 the bio-sorption of Cu(II) and Pb(II) was at the peak which agrees with the point of zero charge on the bio-sorbent as adsorption of cations are favoured at pH above the pHpzc [27]. The intersection of the two graphs gave pH 5.5 which implies maximum adsorption of these metal ions. In addition, the percentage removal of Cu(II) and Pb(II) ions increased with increasing dose of banana peels. This result is significant because a smaller mass of the bio-sorbent means a lower active site. Since, the surface of the bio-sorbent is acidic more active sites must be occupied for proton metal ion competition. In Fig. 5e, the particle size had little influence on the percentage removal of Pb(II) ion while no effect was noticed for the percentage removal of Cu(II) ion. The bio-sorption of Cu(II) ion showed a saddle point which means the highest bio-sorption capacity of the metal is between the maximum and the minimum points. Figure 5f showed the interactive effect of particle size and the adsorbent dosage on the percentage removal of Cu(II) and Pb(II) ions. The bio-sorption capacity of Pb(II) and Cu(II) ions increased with increasing dosage while the particle size has little effect on the percentage removal of Pb (II) ion only. However, the highest removal of Cu(II) ion was reached with particle size of bio-sorbent in the range 280 to 350 μm.
In conclusion, from Fig. 5(a – f) it is obvious that the adsorbent dose and the pH of the aqueous solution had greater influence on the adsorption process of Cu(II) and Pb(II) ions. The highest percentage removal of Cu(II) and Pb(II) ions occurred at a pH > pHpzc which is the pH that favours adsorption of positively charged metal ions. At pH > pHpzc, the surface of the bio-sorbent becomes negatively charged hence, the Cu(II) and Pb(II) ions in the solution get attracted to the surface.
Furthermore, bio-sorption of Pb(II) was greater than Cu(II) ions as can be seen in Table 2. Pb (II) ion is more reactive and has higher molecular weight than Cu (II) ion which explains why Pb(II) ion was more adsorbed than Cu(II) ion. Table 2 shows clearly that ion-exchange is the adsorption mechanism for the bio-sorption of Cu(II) and Pb(II) ions since potassium was not present on the surface of the bio-sorbent after adsorption. Before adsorption, potassium was present on the surface of banana peels in minute quantity. However, potassium was displaced by the presence of other metal ions.
Optimization of variables for the bio-sorption of cu (II) and Pb (II) ions
The aim of optimization is to determine the optimal points of the operating variables for the removal of Cu(II) and Pb(II) ions using banana peels. The main goal is to maximize the removal efficiency of the heavy metal ions to obtain the highest optimal percentage removal. The desirability is used to determine the most suitable conditions for optimal values. The real wastewater contains mixture of several metal ions that leads to selective adsorption among the heavy metals. Therefore, it is important to find the optimal condition for the collective removal of these heavy metals.
The optimized variable conditions for the bio-sorption of Cu(II) and Pb(II) ions using banana peels gave 88.94% and 99.79% removal for Cu(II) and Pb(II) ions respectively with initial concentration of 100 mg/L, pH 5, adsorbent dosage of 1 g and particle size of 75 μm. The above condition gave desirability of 0.959 which denotes that the optimum conditions are acceptable.
Mechanism of bio-sorption of Cu(II) and Pb(II) ions
The adsorption mechanism of Cu(II) and Pb(II) ions onto banana peels can be ascribed to the combination of the operating parameters to achieve maximum adsorption capacity. The characterization of banana peels played an important role in the adsorption process and established ion exchange as the sorption mechanism involved in the adsorption of Cu(II) and Pb(II). The presence of acid groups on the surface of banana peels as shown in Fig. 2, enhanced the removal of Cu(II) and Pb(II) ions. These acid groups become deprotonated at a solution pH above the pHpzc of the adsorbent hence, resulting in negatively charged adsorbent surface which favours uptake capacity of Cu(II) and Pb(II) ions by electrostatic repulsive interaction. Therefore, the condition for optimum uptake of Cu(II) and Pb(II) ions occurs at pH 5, which emphasized the characterization results of banana peels. Heavy metals adsorption onto agriculture-based wastes depends on the solution pH. [28, 29].
The EDX analysis in Table 2 for banana peels before and after adsorption of Cu(II) and Pb(II) showed the presence of K+ before adsorption, however, it disappeared after adsorption. This suggests exchange of ions among the cations in the solution.
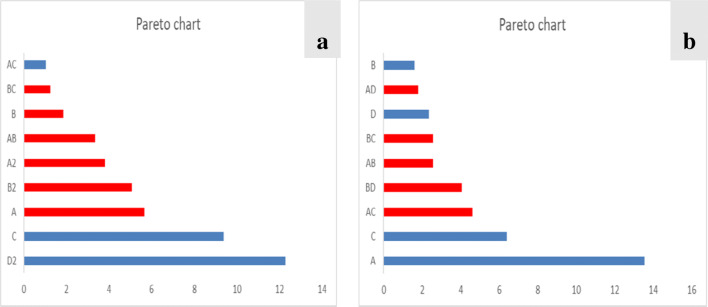
Pareto chart
The pareto chart (Fig. 6) shows the plot of coefficient of each parameter represented in the model equation. It helps to determine the influence of main effect, the interactions and the quadratic terms on the percentage removal of the metal ions. From Fig. 6a, D2 and C had greater effect on the bio-sorption of Pb(II) ions. Figure 6b, obviously shows A and C had greater influence on the bio-sorption of Cu(II) ions. Therefore, it can be concluded that the particle size has the greatest impact on the adsorption process for the bio-sorption of Pb(II) ion followed by the adsorbent dosage while initial concentration has a higher impact on the bio-sorption of Cu(II) ion followed by the adsorbent dosage. Hence, adsorbent dosage played a major role in the adsorption process.
Fig. 6.
Pareto chart of model variables for the removal of Pb (a) and Cu (b)
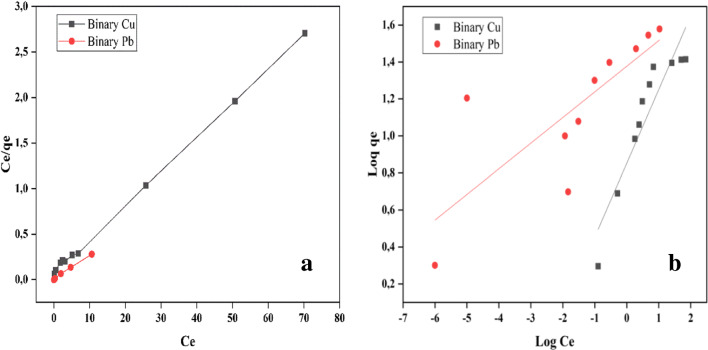
Equilibrium study
The binary solute isotherm experiments were done with mixture of Cu(II) and Pb(II) containing initial concentration in the ratio 1:1 and adsorbent dosage of 0.5 g while all other procedures for the batch experiments were followed. The isotherm studies were fitted into Langmuir and Freundlich isotherm models and the model parameters are presented in Table 6. The experimental data and the linear fit of the isotherm models are depicted in Fig. 7. The Langmuir isotherm constant KL is higher for Pb(II) than Cu(II) which suggests that the Pb(II) was more adsorbed than Cu(II) and Pb(II) could bind with varieties of functional groups present on the surface of banana peels [30]. Comparing the correlation value (R2) for Langmuir and Freundlich isotherm as presented in Table 6, it is obvious that Langmuir isotherm fitted the experimental data well for Cu(II) and Pb(II), signifying a monolayer adsorption mechanism.
Table 6.
Langmuir and Freundlich isotherm parameter
| Metal ion | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| qmax | KL | R2 | KF | n | R2 | |
| Cu | 26.89 | 0.45 | 0.999 | 7.12 | 2.51 | 0.930 |
| Pb | 37.69 | 7.89 | 0.999 | 23.82 | 7.22 | 0.791 |
Fig. 7.
Adsorption isotherms of Cu and Pb on banana peels in bi-solute system at pH 5. The symbols are the experimental results while the solid lines are the linear fittings of (a) Langmuir isotherm and (b) Freundlich isotherm
Comparison of Cu(II) and Pb(II) ions uptake using banana peels with other adsorbents
The maximum uptake of Cu(II) and Pb(II) ions are 29.26 mg/g and 39.32 mg/g respectively. The uptake of Pb(II) was higher than Cu(II) which suggests that banana peels have a higher affinity for Pb(II) than Cu(II) ions. The maximum adsorption of Cu(II) and Pb(II) ions using banana peels obtained in this study is compared with previous studies using banana peels and other bio-sorbents as presented in Table 7.
Table 7.
Comparison of adsorption capacity of Pb and Cu removal using banana peels with literature
| Bio-sorbent | Cu2+ uptake (mg/g) | Pb2+ uptake (mg/g) | Reference |
|---|---|---|---|
| Banana peels | 29.26 | 39.32 | This study |
| Banana peels | 28.57 | [22] | |
| Banana peels | 18.0 | [31] | |
| Banana peels | 2.18 | [32] | |
| Banana peels | 28 | 7.97 | [18] |
| Cabbage waste | 12.96 | 61.27 | [30] |
| Fumaria Indica | 6.62 | 9.15 | [33] |
| Mango plant | 22.51 | 24.4 | [34] |
| Orange peels | 33.99 | [35] |
Conclusion
Natural banana peels were used for the removal of Cu(II) and Pb(II) ions in a bi-solute system by varying operating conditions; initial concentration, pH, adsorbent dosage and the particle size. RSM based on four factors, three level central composite design was used to optimize the effect of the operation parameters on Cu(II) and Pb(II) ions bio-sorption process. The adsorbent dosage, initial concentration and the pH had the greatest impact on the bio-sorption process. The optimum conditions for the maximum percentage removal of Cu(II) and Pb(II) ions are initial concentration of 100 mg/L, pH 5, adsorbent dosage of 1 g and particle size of 75 μm with removal efficiency of 88.94% and 99.79% respectively. The percentage removal of Pb (II) ion was higher than Cu(II) ion suggesting banana peels have higher affinity for Pb(II) ion. The Langmuir isotherm model fitted the experimental data well for Cu(II) and Pb(II) bio-sorption onto banana peels. The FT-IR showed shifts in the peaks of the functional groups of banana peels after adsorption, most especially the carboxylic and hydroxylic groups. Also, the EDX showed increase in carbon composition after adsorption. Therefore, it can be concluded that ion exchange is the adsorption mechanism and natural banana peels is efficient for Cu (II) and Pb (II) ions removal from wastewater.
Supplementary Information
(DOCX 4009 kb)
Acknowledgements
The authors wish to appreciate National Research Foundation (NRF) of South Africa for their financial support, Durban University of Technology and Mangosuthu University of Technology for their support in using certain equipment for the analytical work conducted for this study.
Funding
The authors appreciated the financial support of National Research Foundation (Grant Nu: 105235).
Compliance with ethical standards
Conflict of interest
The authors declare that they have o conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Matsumoto S, Shimada H, Sasaoka TJNR. The key factor of acid mine drainage (AMD) in the history of the contribution of mining industry to the prosperity of the United States and South Africa: a review. Nat Resour. 2016;7:445–460. [Google Scholar]
- 2.Cherry DS, Currie RJ, Soucek DJ, Latimer HA, Trent GC. An integrative assessment of a watershed impacted by abandoned mined land discharges. Environ Pollut. 2001;111:377–388. doi: 10.1016/S0269-7491(00)00093-2. [DOI] [PubMed] [Google Scholar]
- 3.Neculita CM, Zagury GJ, Bussière B. Passive treatment of acid mine drainage in bioreactors using sulfate-reducing bacteria. J Environ Qual. 2007;36:1–16. doi: 10.2134/jeq2006.0066. [DOI] [PubMed] [Google Scholar]
- 4.Strosnider W, López FL, Nairn R. Acid mine drainage at Cerro Rico de Potosí I: unabated high-strength discharges reflect a five century legacy of mining. Environ Earth Sci. 2011;64:899–910. doi: 10.1007/s12665-011-0996-x. [DOI] [Google Scholar]
- 5.Strosnider WH, López FL, Nairn R. Acid mine drainage at Cerro Rico de Potosí II: severe degradation of the upper Rio Pilcomayo watershed. Environ Earth Sci. 2011;64:911–923. doi: 10.1007/s12665-010-0899-2. [DOI] [Google Scholar]
- 6.Wei TT, Yu Y, Hu ZQ, Cao YB, Gao Y, Yang YQ, Wang XJ, Wang PJ. Research progress of acid mine drainage treatment technology in China. Trans Tech Publ. 2013;409:214–220. [Google Scholar]
- 7.Antivachis DN, Chatzitheodoridis E, Skarpelis N, Komnitas K. Secondary sulphate minerals in a Cyprus-type ore deposit, Apliki, Cyprus: mineralogy and its implications regarding the chemistry of pit lake waters. Mine Water Environ. 2017;36:226–238. doi: 10.1007/s10230-016-0398-0. [DOI] [Google Scholar]
- 8.Balci N, Demirel C. Prediction of acid mine drainage (AMD) and metal release sources at the Küre copper mine site, Kastamonu. NW Turkey Mine Water Environ. 2018;37:56–74. doi: 10.1007/s10230-017-0470-4. [DOI] [Google Scholar]
- 9.Araujo CST, Carvalho DC, Rezende HC, Almeida ILS, Coelho LM, Coelho NMM, Marques TL, Alves VN. Bioremediation of waters contaminated with heavy metals using Moringa oleifera seeds as biosorbent 2013; 10.5772/56157.
- 10.Guechi EK, Hamdaoui O. Evaluation of potato peel as a novel adsorbent for the removal of cu (II) from aqueous solutions: equilibrium, kinetic, and thermodynamic studies. Desalin Water Treat. 2016;57:10677–10688. doi: 10.1080/19443994.2015.1038739. [DOI] [Google Scholar]
- 11.Kumar PS, Ramalingam S, Kirupha SD, Murugesan A, Vidhyadevi T, Sivanesan S. Adsorption behavior of nickel (II) onto cashew nut shell: equilibrium, thermodynamics, kinetics, mechanism and process design. J Chem Eng. 2011;167:122–131. doi: 10.1016/j.cej.2010.12.010. [DOI] [Google Scholar]
- 12.Lakshmipathy R, Sarada NC. A fixed bed column study for the removal of Pb2+ ions by watermelon rind. Environ Sci: Water Res Technol. 2015;1:244–250. [Google Scholar]
- 13.Lathan N, Edward S, Thomas C, Agwaramgbo L. Comparative study of Lead removal by extracts of spinach, coffee, and tea. J Environ Prot. 2013;04:250–257. doi: 10.4236/jep.2013.43029. [DOI] [Google Scholar]
- 14.Mahmoud MA. Kinetics studies of uranium sorption by powdered corn cob in batch and fixed bed system. J Adv Res. 2016;7:79–87. doi: 10.1016/j.jare.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Witek-Krowiak A, Chojnacka K, Podstawczyk D, Dawiec A, Pokomeda K. Application of response surface methodology and artificial neural network methods in modelling and optimization of biosorption process. Bioresour Technol. 2014;160:150–160. doi: 10.1016/j.biortech.2014.01.021. [DOI] [PubMed] [Google Scholar]
- 16.Kamsonlian S, Balomaju C, Chand S. Characterization of banana and orange peels: biosorption mechanism. Int J Sci Technol Manag. 2011;2:1–7. [Google Scholar]
- 17.Li XT, Yanru X, Zhexian L, Yinghui L, Fang L. Study on the preparation of orange peel cellulose adsorbents and biosorption of Cd2+ from aqueous solution. Sep Purif Technol. 2007;55:69–75. doi: 10.1016/j.seppur.2006.10.025. [DOI] [Google Scholar]
- 18.Arunakumara K, Walpola BC, Yoon MH. Banana Peel: a green solution for metal removal from contaminated waters. Korean J Environ Agric. 2013;32:108–116. doi: 10.5338/KJEA.2013.32.2.108. [DOI] [Google Scholar]
- 19.Kaewsarn P, Saikaew W, Wongcharee S. Dried biosorbent derived from banana peel: a potential biosorbent for removal of cadmium ions from aqueous solution. Thailand Chem Eng Appl Chem. 2008;2:552–558. [Google Scholar]
- 20.Amin MT, Alazba AA, Shafiq M. Removal of copper and Lead using Banana biochar in batch adsorption systems: isotherms and kinetic studies. Arab J Sci Eng. 2017;43:5711–5722. doi: 10.1007/s13369-017-2934-z. [DOI] [Google Scholar]
- 21.Mondal NK. Natural Banana (Musa acuminate) Peel: an unconventional adsorbent for removal of fluoride from aqueous solution through batch study. Water Conserv Sci Eng. 2016;1:223–232. doi: 10.1007/s41101-016-0015-x. [DOI] [Google Scholar]
- 22.Hossain MA, Ngo H, Hao Guo WS, Nguyen TV. Removal of copper from water by adsorption onto banana peel as bioadsorbent. Int J Geomate. 2012;2:227–234. [Google Scholar]
- 23.Oyewo OA, Onyango MS, Wolkersdorfer C. Application of banana peels nanosorbent for the removal of radioactive minerals from real mine water. J Environ Radioact. 2016;164:369–376. doi: 10.1016/j.jenvrad.2016.08.014. [DOI] [PubMed] [Google Scholar]
- 24.Montgomery DC. Design and analysis of experiments. 6th ed. Hoboken, N.J: John Wiley & Sons; 2005.
- 25.Nair AT, Makwana AR, Ahammed MM. The use of response surface methodology for modelling and analysis of water and wastewater treatment processes: a review. Water Sci Techn. 2014;69(3):464–478. doi: 10.2166/wst.2013.733. [DOI] [PubMed] [Google Scholar]
- 26.Pathak PD, Mandavgane SA, Kulkarni BD. Fruit peel waste: characterization and its potential uses. Curr Sci. 2017;113:444–454. doi: 10.18520/cs/v113/i03/444-454. [DOI] [Google Scholar]
- 27.Madala S, Mudumala VNR, Vudagandla S, Abburi K. Modified leaf biomass for Pb(II) removal from aqueous solution: application of response surface methodology. Ecol Eng. 2015;83:218–226. doi: 10.1016/j.ecoleng.2015.06.025. [DOI] [Google Scholar]
- 28.Amin M, Alazba A, Shafiq M. Batch and fixed-bed column studies for the biosorption of cu (II) and Pb (II) by raw and treated date palm leaves and orange peel. Global Nest J. 2017;19:464–478. doi: 10.30955/gnj.002294. [DOI] [Google Scholar]
- 29.Feng NC, Guo XY. Characterization of adsorptive capacity and mechanisms on adsorption of copper, lead and zinc by modified orange peel. Trans Nonferrous Met Soc China. 2012;22:1224–1231. doi: 10.1016/S1003-6326(11)61309-5. [DOI] [Google Scholar]
- 30.Hossain MA, Ngo H, Guo WS, Nghiem LD, Hai FI, Vigneswaran S, Nguyen TV. Competitive adsorption of metals on cabbage waste from multi-metal solutions. Bioresour Technol. 2014;160:79–88. doi: 10.1016/j.biortech.2013.12.107. [DOI] [PubMed] [Google Scholar]
- 31.da Silva Correia IK, Santos PF, Santana CS, Neris JB, Luzardo FHM, Velasco FG. Application of coconut shell, banana peel, spent coffee grounds, eucalyptus bark, piassava (Attalea funifera) and water hyacinth (Eichornia crassipes) in the adsorption of Pb2+ and Ni2+ ions in water. J Environ Chem Eng. 2018;6:2319–2334. doi: 10.1016/j.jece.2018.03.033. [DOI] [Google Scholar]
- 32.Anwar J, Shafique U, Zaman Waheed UZ, Saman M, Dar A, Anwar S. Removal of Pb(II) and cd(II) from water by adsorption on peels of banana. Bioresour Technol. 2010;101:1752–1755. doi: 10.1016/j.biortech.2009.10.021. [DOI] [PubMed] [Google Scholar]
- 33.Iqbal M, Khera RA. Adsorption of copper and lead in single and binary metal system onto Fumaria indica biomass. Chem Int. 2015;1:157b–163b. [Google Scholar]
- 34.Ashraf MA, Wajid A, Mahmood K, Maah MJ, Yusoff I. Removal of heavy metals from aqueous solution by using mango biomass. Afr J Biotechnol. 2011;10:2163–2177. doi: 10.5897/AJB11.1090. [DOI] [Google Scholar]
- 35.Kumar K, Patavardhan SS, Lobo S, Gonsalves R. Equilibrium study of dried orange peel for its efficiency in removal of cupric ions from water. Int J Phytoremediat. 2018;20:593–598. doi: 10.1080/15226514.2017.1405379. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 4009 kb)