Abstract
The pathogenesis of gestational diabetes mellitus (GDM) is multifactorial and it shares many features with type 2 diabetes mellitus. Growth differentiation factor 15 (GDF-15), a member of transforming growth factor-β superfamily, is expressed in a high amount in the placenta in addition to other organs. This cross-sectional study was performed to assess the difference of GDF-15 and pro-inflammatory cytokines between pregnant women with or without GDM, and to explore the possible association of GDF-15 with the parameters of dysglycemia (Serum insulin, HOMA-IR, fasting, 60 min, and 120 min post-75 gm oral glucose plasma glucose levels) and inflammation (IL-6 and TNF-α) in women with GDM at 24–28 weeks of gestation. Thirty-five women with GDM and 30 age-matched non-diabetic pregnant control (NDPC) subjects were recruited for the study. Mean serum GDF-15, IL-6, and TNF-α levels were significantly higher in GDM in comparison to the NDPC population. These differences persisted even after adjusting for the possible confounders like maternal age and BMI. GDF-15 level showed a positive correlation with parameters of dysglycemia (Serum insulin, HOMA-IR, fasting, 60 min, and 120 min post-75 gm oral glucose plasma glucose levels) but a variable correlation with the markers of inflammation. In conclusion, our study provides evidence that, in Indian women, serum GDF-15 level is higher in GDM in comparison to age-matched pregnant subjects without GDM in the early third trimester pregnancy. Moreover, in third trimester, GDF-15 level increases with increase in plasma glucose and insulin resistance.
Keywords: Diabetes, Gestational, Pregnancy, Growth differentiation factor 15, Pro-inflammatory cytokines
Introduction
Pregnancy is a physiological state of insulin resistance (IR). Gestational diabetes mellitus (GDM) is defined as diabetes diagnosed in the second or third trimester of pregnancy that was not clearly overt diabetes prior to gestation [1].
The prevalence of GDM in India varies between 3.8 and 22%. [2–5]. Uncontrolled gestational diabetes in pregnancy can lead to spontaneous abortion, preeclampsia, cesarean delivery, neonatal hypoglycemia, macrosomia, and fetal death. Not only the women with GDM, but the offspring may also have an increased risk of developing obesity and type 2 diabetes (T2DM) in later life [6, 7].The pathogenesis of GDM is possibly multifactorial and it shares many features with T2DM such as insulin resistance and inadequate beta-cell compensation. These two conditions also share many susceptibility genes [8–10].
Growth differentiation factor 15 (GDF-15), a member of transforming growth factor -β (TGF-β) superfamily, also known as macrophage inhibitory cytokine-1 (MIC-1), is expressed in a high amount in the placenta and also in heart, pancreas, liver, kidney, and colon [11–13]. GDF15 expression is increased in various acute and chronic inflammatory states, including tissue injury, cancer, cardiovascular disease, and diabetes [14–17]. Various animal and human studies have shown the association of GDF-15 and glyco-metabolic disorders. High plasma glucose and hyperinsulinemia significantly increase GDF-15 levels in humans [18]. Macia et al. found that the overexpression of GDF-15 reduces body weight and adiposity and leads to the improvement of glucose homeostasis in mice on a normal and obesogenic diet [19]. Patel et al. demonstrated that long term dietary stress induced by a high-fat diet induces GDF 15 secretion which promotes aversive response towards food [20]. These findings suggest that GDF-15 may act as a protective factor in hyperglycemia. A study has shown the role of GDF-15 in the improvement of insulin resistance in non-pregnant individuals [21]. GDF-15 level is also related to beta-cell function [22].
One previous study has shown that maternal serum GDF-15 level increases with the advancement of the gestational period [14]. Another recent study from China has shown that serum GDF-15 level has a positive correlation with the fasting, 60 min, and 120 min plasma blood glucose and Hemoglobin A1c (HbA1c) in the third trimester of the gestation [23]. Curry et al. demonstrated that TNF-α and IL-6 levels are higher in pregnancy in contrast to the non-pregnant state [24]. Also, the levels of these cytokines are higher in obese in comparison to non-obese pregnant women [24, 25]. However, to the best of our knowledge, the relationship between pro-inflammatory markers and GDF-15 has not yet been examined in women with GDM. Moreover, there are no data available on the relationship between GDF-15 and parameters of dysglycemia and pro-inflammatory cytokines in Indian pregnant women.
This study was done to assess the difference of GDF-15 and pro-inflammatory cytokines between pregnant women with and without GDM and to explore the possible association of GDF-15 with the parameters of dysglycemia and inflammation in women at 24–32 weeks of gestation.
Patients, materials and methods
Research subjects
This cross-sectional study was performed in the Institute of Post-Graduate Medical Education and Research and Seth Sukhlal Karnani Memorial Hospital (IPGME&R and SSKM Hospital), Kolkata, West Bengal, India. The women with GDM aged 18–35 years were included in the study. Non-diabetic pregnant women from the same age group were taken as controls. None of the participants were smokers, and the subjects with inflammatory bowel conditions, major systemic illness, hypothyroidism, and twin pregnancy were excluded. Based on the study of GDF-15 level in third trimester in women with and without GDM by Tang et.al. [23], the sample size was calculated by the following formula: n1 = [(σ12 + σ22/K)(z1 − α/2 + z1 − β)2]/Δ2, where Δ (absolute difference between two means) = 34; σ1, σ2 (variance of mean #1 and #2) ≃ 25; keeping α (probability of type I error) = 0.05 and β (probability of type II error) = 0.2; z = critical Z value for aforesaid α and β, the calculated sample size in each group was 8. However, to be on a safer side, consecutive 35 patients of GDM, who consented to participate in this study were recruited at 24–32 weeks of the gestation from the GDM clinic of the Department of Endocrinology and Metabolism, IPGME&R and SSKM Hospital. The GDM was diagnosed at the antenatal clinic of the same institution by using one-step OGTT with 75 gm anhydrous glucose at 24–28 weeks’ gestation before referring them to the GDM clinic. The diagnostic cutoffs of plasma glucose measured at fasting, 60 min, and 120 min were 92, 180, and 153 mg/dl, respectively, as per IADPSG criteria [1]. If a single value met or exceeded these cutoffs, the woman was diagnosed with GDM. Thirty consecutive gestational age-matched non-diabetic pregnant control (NDPC) subjects of 18–35 years of age were recruited from the antenatal clinic. Age, parity, gravidity, body mass index (BMI), and blood pressure were recorded.
Measurement methods
GDF-15 was quantified by ELISA kit (RayBiotech, USA) with a minimum detectable level of Human GDF-15 of 2 pg/ml with the intra- and inter-assay CV of 4.9% and 5.2%, respectively. IL-6 was quantified by ELISA kit (G-Biosciences, USA) with a minimum detectable level of 2.813 pg/ml with the intra and inter-assay CV of 3.3% and 4.2%, respectively. TNF-α was also quantified by ELISA kit (G-Biosciences, USA) with a minimum detectable level of < 1 pg/ml with the intra- and inter-assay CVs of 4.7% and 4.9%, respectively. Insulin was quantified by ELISA kit (RayBiotech, USA) with a minimum detectable level of 4 µIU/ml and the intra and inter-assay CV of 4.6% and 5.1%, respectively. Insulin resistance (IR) was estimated by the Homeostasis Model Assessment—Insulin Resistance (HOMA-IR) method using FPG and fasting serum insulin values.
Statistical analysis
The Shapiro–Wilk test was used to check the normality of the distribution of continuous variables. The averages of the variables were expressed as mean ± standard deviation (normally distributed data) or median ± IQR (non-normally distributed data). The unpaired t test analysis was used to compare normally distributed continuous variables among the two groups, whereas the Mann–Whitney test was used for the non-normally distributed counterpart. Comparison between proportions were done with the chi-square test. Adjustment for covariates was done with ANCOVA where applicable. The strength and direction of the relationships between continuous variables were analyzed by the Pearson’s or Spearmen’s correlation analysis as applicable. The statistical analyses were performed using JASP version 0.13 (the University of Amsterdam, The Netherlands). GraphPad Prism software (version 5.00) was used for the plotting.
Results
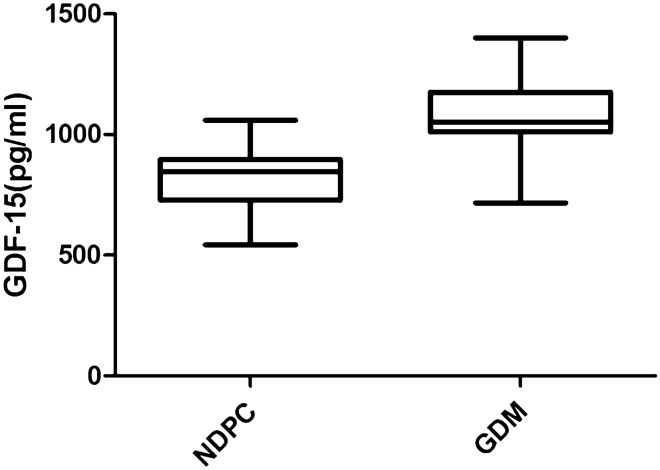
Clinical and biochemical parameters are summarized in Table 1. The mean maternal age was numerically higher in GDM Subjects in comparison to NDPC subjects, although the difference is not statistically significant. Significantly Higher proportion of subjects with GDM had family history of type 2 diabetes in at least one first-degree relatives (42.8% vs 30%, respectively), p = 0.0254. The BMI was significantly higher in GDM Subjects. There was no significant difference in systolic and diastolic blood pressure among the groups. As expected, fasting plasma glucose (FPG), 60 min, and 120 min OGTT plasma glucose were significantly higher in GDM Subjects. Fasting serum insulin, as well as HOMA-IR, were also significantly higher in GDM subjects. The mean serum level of GDF-15 was significantly higher in subjects with GDM in comparison to the NDPC population (Fig. 1). To adjust for the possible confounders such as maternal age and BMI, we did an ANCOVA analysis keeping GDF-15 as a dependent variable, presence or absence of GDM as an independent variable, and maternal age and BMI as covariates. After adjustment, the mean difference with a 95% confidence interval was 247.9 pg/ml (175.8, 320), p < 0.001. The effect size ω2 was 0.405 for the presence of GDM (p < 0.001), < 0.001 for BMI (p = 0.42), and 0.028 for maternal age (p = 0.045), respectively. Thus, the difference persisted even after the adjustment. We did not adjust for the gestational age as this parameter was very similar in both the groups.
Table 1.
Clinical and biochemical parameters: expressed as Mean ± SD except those marked as awhich are expressed as Median ± IQR
| Parameter | GDM (n = 35) | NDPC (n = 30) | P value |
|---|---|---|---|
| Age (year) | 27.3 ± 4 | 26 ± 4.3 | 0.112 |
| Gestational weeksa | 28 ± 1 | 28 ± 2 | 0.191 |
| Height (Cm) | 153.2 ± 5.8 | 151.8 ± 3.8 | 0.260 |
| Weight (kg) | 64.6 ± 12.3 | 58.5 ± 8.6 | 0.027 |
| BMI (kg/m2) | 27.5 ± 5.4 | 25.3 ± 3.4 | 0.029 |
| Systolic blood pressure (mm/Hg) | 113 ± 10 | 109 ± 19 | 0.148 |
| Diastolic blood pressure (mm/Hg) | 77 ± 9 | 78 ± 9 | 0.365 |
| FBS (mg/dl)a | 96 ± 29 | 84 ± 7 | < 0.001 |
| 60 min plasma glucose (mg/dl)a | 198 ± 88 | 160 ± 18 | < 0.001 |
| 120 min plasma glucose (mg/dl)a | 159 ± 60 | 117 ± 25 | < 0.001 |
| Fasting serum insulin (µU/L)a | 13.2 ± 2.3 | 8.8 ± 4.7 | < 0.001 |
| HOMA-IRa | 3.56 ± 1.51 | 1.87 ± 0.77 | < 0.001 |
| GDF-15(pg/ml) | 1061.1 ± 143.2 | 818.7 ± 140.9 | < 0.001 |
| TNF-α(pg/ml)a | 8.6 ± 6.1 | 4.9 ± 4.2 | 0.014 |
| IL-6(pg/ml)a | 5.5 ± 4.9 | 2.9 ± 1.6 | < 0.001 |
GDM Gestational diabetes mellitus, NDPC Non-diabetes pregnancy control
Fig. 1.
Average GDF-15 level in GDM population was significantly higher than NDPC subjects. p ≤ 0.001
The serum cytokines IL-6 and TNF-α levels were also higher in women with GDM. This difference in both the parameters remained significant even after adjusting for the aforementioned potential confounders.
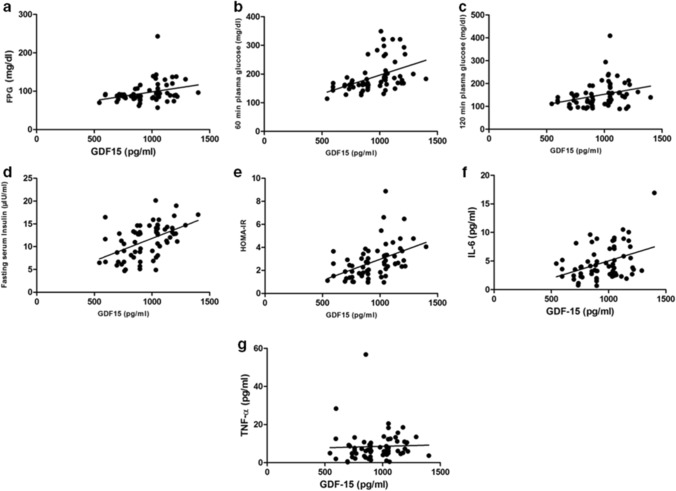
Correlation results were documented in Fig. 2. GDF-15 had a significant positive correlation with fasting serum insulin and HOMA-IR (r = 0.466, p ≤ 0.001; r = 0.464, p ≤ 0.001, respectively). Positive correlation of GDF-15 was found with FPG, 60 min and 120 min plasma glucose levels (r = 0.318, p = 0.009; r = 0.407, p = 0.001; r = 0.298, p = 0.019, respectively). However, GDF-15 had no correlation with BMI. (r = 0.188, p = 0.133). GDF-15 had a significant positive correlation with IL-6 (r = 0.298, p = 0.016) but not TNF-α (r = 0.233, p = 0.061).
Fig. 2.
Correlation of GDF-15 with different biochemical parameters. a Fasting plasma glucose (r = 0.318, p = 0.009), b post 60 min OGTT plasma glucose (r = 0.407, p = 0.001), c post 120 min OGTT plasma glucose (r = 0.298, p = 0.019), d fasting serum insulin (r = 0.466, p ≤ 0.001), e HOMA-IR (r = 0.464, p ≤ 0.001), f IL-6 (r = 0.298, p = 0.016), g TNF-α (r = 0.233, p = 0.061)
Discussion
In this cross-sectional study, serum GDF-15 level was found to be significantly higher in the case of GDM patients than the NDPC subjects in the early third trimester. There are very few studies that have explored the relationship between GDM and GDF-15. Sugulle M et al. from Norway have reported higher GDF-15 levels in pregnant women with diabetes (pre-existing type 1, type 2, and GDM) in comparison to non-diabetic pregnant women. However, such a difference was not observed when only the GDM subgroup was considered. [26]. Tang et al. from China has reported a higher GDF-15 level in women with GDM in the third trimester in comparison to matched non-diabetic pregnancy population. [23] This finding is concordant to ours. In our study, GDF-15 shows a significantly positive correlation with fasting serum insulin, HOMA-IR, and venous blood glucose levels. The one postulated mechanism for this finding may be the triggering of GDF-15 transcription and secretion by the hyperglycemia and hyperinsulinemia [18]. A study by Hong et al. documented a positive correlation between serum GDF-15 level and insulin resistance in subjects with prediabetes [27], although the relation is possibly indirect [28]. It has also been postulated that in addition to the placenta, excess GDF-15 is expressed in adipose tissue in pregnant women with diabetes [26]. Absolute GDF-15 levels of the NDPC subjects were lower in our study in comparison to the studies reported elsewhere. This is possibly due to the ethnic variation and the use of different detection methods.
Levels of pro-inflammatory cytokines were higher in GDM in comparison to control subjects in our study. Some of the previous studies have found a higher mean TNF-α level in GDM in comparison to women without GDM, whereas other studies did not find any significant differences. Similarly, the mean IL-6 level in women with GDM was found to be higher than controls in some studies, whereas the difference was not significant in others. However, many of these studies did not statistically adjust for the confounders [29]. In our study, the difference persisted even after statistical adjustment for the potential confounding factors.
As blood glucose levels in pregnancy are in a continuum, and plasma glucose cutoffs for GDM is somewhat arbitrary, we did the correlation studies with the GDM and NDPC population clubbed together. The GDF-15 level showed a weak positive correlation with IL-6 but no correlation was found with TNF-α. It has been found that increased blood glucose level in T2DM creates stress which ultimately increases GDF-15 expression [30]. Pro-inflammatory cytokines induce expression of GDF 15 in macrophages, which, in turn, limits further expression of macrophage and subsequent release of pro-inflammatory cytokines by them [31]. Complex interaction and feedback mechanism involving pro-inflammatory cytokines and GDF-15 possibly explain our finding of weak or no correlation between them. This study emphasizes the need for further research on the etio-pathological role of GDF-15 in the dysglycemia of pregnancy and its potential therapeutic implications in GDM.
Our study has some limitations. First, it is a cross-sectional study, therefore any change of GDF-15 level over time could not be documented. Second, the sample size was relatively small. Third, we do not have data on pre-pregnancy BMI. We calculated BMI based on weight during pregnancy which is not ideal for calculation of the same. However, it is useful for comparison purposes as it was measured in GDM and NDPC groups at similar gestational age. One of the strengths of our study was the exploration of the association between the inflammatory markers and GDF-15 during pregnancy. The other strength was that we compared GDF-15 and other relevant parameters between the groups after statistically adjusting for the potential confounders.
In conclusion, our study provides evidence that, in Indian women, mean GDF-15 is higher in GDM in comparison to age-matched pregnant subjects without GDM at 24–32 weeks of gestation. GDF-15 level showed a positive correlation with glycemic parameters and insulin resistance, which, if compared with the previous studies done in non-pregnant individuals with prediabetes and diabetes, possibly indicates that in early third trimester pregnancy, GDF-15 expression is increased with increasing hyperglycemia and insulin resistance. The GDF-15 level showed a positive correlation with IL-6 but no correlation was observed with TNF-α.
Funding
This study was funded by the Research Society for the Study of Diabetes in India, West Bengal, India. (Dated 15/03/17).
Compliance with ethical standards
Conflict of interest
The authors declare that there was no potential conflict of interest.
Ethical approval
The study protocol was approved by the Institutional Ethics Committee/ Research Oversight Committee of IPGME&R, Kolkata (Memo no. Inst/IEC/2016/504 dated 04/11/2016).
Informed consent
Written informed consent was taken from all participants. The study conformed to the provision of the Declaration of Helsinki.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Sudipta Banerjee, Email: sbcu2015@gmail.com.
Rana Bhattacharjee, Email: dr.r.bhatta@gmail.com.
Amitabh Sur, Email: consult.md2012@gmail.com.
Pieu Adhikary, Email: pieuadhikary1992@gmail.com.
Subhankar Chowdhury, Email: subhankar.chowdhury@gmail.com.
References
- 1.American Diabtic Association Diabetes Care. 2018;41(1):S13–S27. doi: 10.2337/dc18-S002. [DOI] [PubMed] [Google Scholar]
- 2.Raja M, Baba T, Hanga A, Bilquees S, Rasheed S, Haq I, et al. A study to estimate the prevalence of gestational diabetes mellites in an urban block of Kashmir valley (North India) Int J Med Sci Public Health. 2014;3(2):191–195. doi: 10.5455/ijmsph.2013.211120131. [DOI] [Google Scholar]
- 3.Bhatt AA, Dhore PB, Purandare VB, Sayyad MG, Mandal MK, Unnikrishnan AG. Gestational diabetes mellitus in rural population of Western India—results of a community survey. Indian J Endocrinol Metab. 2015;19(4):507–510. doi: 10.4103/2230-8210.159061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Swami SR, Mehetre R, Shivane V, Bandgar TR, Menon PS, Shah NS. Prevalence of carbohydrate intolerance of varying degrees in pregnant females in Western India (Maharashtra)—a hospital-based study. J Indian Med Assoc. 2008;106(11):712-4–735. [PubMed] [Google Scholar]
- 5.Mahalakshmi MM, Bhavadharini B, Maheswari K, Kalaiyarasi G, Anjana RM, Ranjit U, et al. Comparison of maternal and fetal outcomes among Asian Indian pregnant women with or without gestational diabetes mellitus: a situational analysis study (WINGS-3) Indian J Endocrinol Metab. 2016;20(4):491–496. doi: 10.4103/2230-8210.183469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holmes VA, Young IS, Patterson CC, Pearson DW, Walker JD, Maresh MJA, et al. Optimal glycemic control, pre-eclampsia, and gestational hypertension in women with type 1 diabetes in the diabetes and pre-eclampsia intervention trial. Diabetes Care. 2011;34(8):1683–1688. doi: 10.2337/dc11-0244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Waters TP, Dyer AR, Scholtens DM, Dooley SL, Herer E, Lowe LP, et al. Maternal and neonatal morbidity for women who would be added to the diagnosis of GDM using IADPSG criteria: a secondary analysis of the hyperglycemia and adverse pregnancy outcome study. Diabetes Care. 2016;39(12):2204–2210. doi: 10.2337/dc16-1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Catalano PM, Tyzbi ED, Wolfe RR, Roman NM, Amini SB, Sims EA. Longitudinal changes in basal hepatic glucose production and suppression during insulin infusion in normal pregnant women. Am J Obstet Gynecol. 1992;167(4 Pt 1):913–919. doi: 10.1016/s0002-9378(12)80011-1. [DOI] [PubMed] [Google Scholar]
- 9.Reece EA, Leguizamon G, Wiznitzer A. Gestational diabetes: the need for a common ground. Lancet. 2009;373(9677):1789–1797. doi: 10.1016/S0140-6736(09)60515-8. [DOI] [PubMed] [Google Scholar]
- 10.Lowe WL, Jr, Scholtens DM, Lowe LP, Kuang A, Nodzenski M, Talbot O, et al. Association of gestational diabetes with maternal disorders of glucose metabolism and childhood adiposity. JAMA. 2018;320(10):1005–1016. doi: 10.1001/jama.2018.11628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yokoyama-Kobayashi M, Saeki M, Sekine S, Kato S. Human cDNA encoding a novel TGF-βsuperfamily protein highly expressed in placenta. J Biochem. 1997;122(3):622–626. doi: 10.1093/oxfordjournals.jbchem.a021798. [DOI] [PubMed] [Google Scholar]
- 12.Koopmann J, Buckhaults P, Brown DA, Zahurak ML, Sato N, Fukushima N, et al. Serum macrophage inhibitory cytokine 1 as a marker of pancreatic and other periampullary cancers. Clin Cancer Res. 2004;10(7):2386–2392. doi: 10.1158/1078-0432.CCR-03-0165. [DOI] [PubMed] [Google Scholar]
- 13.Tan M, Wang Y, Guan K, Sun Y. PTGF-beta, a type beta transforming growth factor (TGF-beta) superfamily member, is a p53 target gene that inhibits tumor cell growth via TGF-beta signaling pathway. Proc Natl Acad Sci USA. 2000;97(1):109–114. doi: 10.1073/pnas.97.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marjono AB, Brown DA, Horton KE, Wallace EM, Breit SN, Manuelpillai U. Macrophage inhibitory cytokine-1 in gestational tissues and maternal serum in normal and preeclamptic pregnancy. Placenta. 2003;24(1):100–106. doi: 10.1053/plac.2002.0881. [DOI] [PubMed] [Google Scholar]
- 15.Xu J, Kimball TR, Lorenz JN, Brown DA, Bauskin AR, Klevitsky R, et al. GDF15/MIC-1 functions as a protective and antihypertrophic factor released from the myocardium in association with SMAD protein activation. Circ Res. 2006;98(3):342–350. doi: 10.1161/01.RES.0000202804.84885.d0. [DOI] [PubMed] [Google Scholar]
- 16.Ho JE, Mahajan A, Chen MH, Larson MG, McCabe E, Ghorbani A, Cheng S, et al. Clinical and genetic correlates of growth differentiation factor 15 in the community. Clin Chem. 2012;58(11):1582–1591. doi: 10.1373/clinchem.2012.190322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mehta RS, Song M, Bezawada N, Wu K, Garcia-Albeniz X, Morikawa T, et al. A prospective study of macrophage inhibitory cytokine-1 (MIC-1/GDF15) and risk of colorectal cancer. J Natl Cancer Inst. 2014;106(4):dju016. doi: 10.1093/jnci/dju016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schernthaner-Reiter MH, Kasses D, Tugendsam C, Riedl M, Peric S, Prager G, et al. Growth differentiation factor 15 increases following oral glucose ingestion: effect of meal composition and obesity. Eur J Endocrinol. 2016;175(6):623–631. doi: 10.1530/EJE-16-0550. [DOI] [PubMed] [Google Scholar]
- 19.Macia L, Tsai VW, Nguyen AD, Johnen H, Kuffner T, Shi YC, et al. Macrophage inhibitory cytokine 1 (MIC-1/GDF15) decreases food intake, body weight and improves glucose tolerance in mice on normal and obesogenic diets. PLoS ONE. 2012;7(4):e34868. doi: 10.1371/journal.pone.0034868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patel S, Alvarez-Guaita A, Melvin A, Rimmington D, Dattilo A, Miedzybrodzka EL, et al. GDF15 provides an endocrine signal of nutritional stress in mice and humans. Cell Metab. 2019;29(3):707–718.e8. doi: 10.1016/j.cmet.2018.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chrysovergis K, Wang X, Kosak J, Lee S-H, Kim JS, Foley JF, et al. NAG-1/GDF-15 prevents obesity by increasing thermogenesis, lipolysis and oxidative metabolism. Int J Obes (Lond) 2014;38(12):1555–1564. doi: 10.1038/ijo.2014.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schernthaner-Reiter MH, Itariu BK, Krebs M, Promintzer-Schifferl M, Stulnig TM, Tura A, et al. GDF15 reflects beta cell function in obese patients independently of the grade of impairment of glucose metabolism. Nutr Metab Cardiovasc Dis. 2019;29(4):334–342. doi: 10.1016/j.numecd.2018.12.008. [DOI] [PubMed] [Google Scholar]
- 23.Tang M, Luo M, Lu W, Wang S, Zhang R, Liang W, et al. Serum growth differentiation factor 15 is associated with glucose metabolism in the third trimester in Chinese pregnant women. Diabetes Res Clin Pract. 2019;156:107823. doi: 10.1016/j.diabres.2019.107823. [DOI] [PubMed] [Google Scholar]
- 24.Curry AE, Vogel I, Skogstrand K, Drews C, Schendel DE, Flanders WD, et al. Maternal plasma cytokines in early- and mid-gestation of normal human pregnancy and their association with maternal factors. J Reprod Immunol. 2008;77(2):152–160. doi: 10.1016/j.jri.2007.06.051. [DOI] [PubMed] [Google Scholar]
- 25.Christian LM, Porter K. Longitudinal changes in serum proinflammatory markers across pregnancy and postpartum: effects of maternal body mass index. Cytokine. 2014;70(2):134–140. doi: 10.1016/j.cyto.2014.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sugulle M, Dechend R, Herse F, Weedon-Fekjaer MS, Johnsen GM, Brosnihan KB, et al. Circulating and placental growth differentiation factor 15 in preeclampsia and in pregnancy complicated by diabetes mellitus. Hypertension. 2009;54(1):106–112. doi: 10.1161/HYPERTENSIONAHA.109.130583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hong JH, Chung HK, Park HY, Joung KH, Lee JH, Jung JG, et al. GDF15 is a novel biomarker for impaired fasting glucose. Diabetes Metab J. 2014;38(6):472–479. doi: 10.4093/dmj.2014.38.6.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vila G, Riedl M, Anderwald C, Resl M, Handisurya A, Clodi M, et al. The relationship between insulin resistance and the cardiovascular biomarker growth differentiation factor-15 in obese patients. Clin Chem. 2011;57(2):309–316. doi: 10.1373/clinchem.2010.153726. [DOI] [PubMed] [Google Scholar]
- 29.Gomes CP, Torloni MR, Gueuvoghlanian-Silva BY, Alexandre SM, Mattar R, Daher S. Cytokine levels in gestational diabetesmellitus: a systematic review of the literature. Am J Reprod Immunol. 2013;69(6):545–557. doi: 10.1111/aji.12088. [DOI] [PubMed] [Google Scholar]
- 30.Dostalova I, Roubicek T, Bartlova M, Mraz M, Lacinova Z, Haluzikova D, et al. Increased serum concentrations of macrophage inhibitory cytokine-1 in patients with obesity and type 2 diabetes mellitus: the influence of very low calorie diet. Eur J Endocrinol. 2009;161(3):397–404. doi: 10.1530/EJE-09-0417. [DOI] [PubMed] [Google Scholar]
- 31.Bootcov MR, Bauskin AR, Valenzuela SM, Moore AG, Bansal M, He XY, et al. MIC-1, a novel macrophage inhibitory cytokine, is a divergent member of the TGF-b superfamily. Proc Natl Acad Sci USA. 1997;94(21):11514–11519. doi: 10.1073/pnas.94.21.11514. [DOI] [PMC free article] [PubMed] [Google Scholar]