Abstract
Purpose
Per- and polyfluoroalkyl substances (PFAS) have been found to be widespread, extremely persistent and bioaccumulative with toxicity tendencies. Pre-synthesized nanocomposite-activated carbons, referred to, as physically activated maize tassel silver (PAMTAg) and chemically activated maize tassel silver (CAMTAg) were utilized in the present study. They were used for the removal of 10 PFAS from aqueous solutions.
Methods
The nanocomposite-activated carbons were characterized via scanning electron microscopy (SEM), Fourier transform infrared (FTIR) spectroscopy, Brunauer Emmett Teller (BET) and other techniques. Batch equilibrium experiments were conducted in order to investigate the effects of solution pH, adsorbent dosage, initial PFAS concentration and temperature on the removal of PFAS using PAMTAg and CAMTAg. Langmuir and Freundlich adsorption isotherm models were used to analyse the equilibrium data obtained.
Results
Maximum adsorption capacities of 454.1 mg/g (0.91 mmol/g) and 321.2 mg/g (0.78 mmol/g) were recorded for perfluorooctanesulfonate (PFOS) and perfluorooctanoate (PFOA), respectively using CAMTAg. The values recorded for the Gibbs’ free energy (ΔG°) for the adsorption of PFOS and PFOA onto PAMTAg and CAMTAg were negative; PFOS (−9.61, −9.99 and − 10.39), PFOA (−8.77, −9.76 and − 10.21) using PAMTAg; and PFOS (−13.70, −12.70 and − 12.37), PFOA (−12.86, −12.21 and − 11.17) using CAMTAg. Therefore, the adsorption processes were spontaneous and feasible. The values recorded for enthalpy (ΔH°) (kJ/mol) for the adsorption of PFOS (−26.15) and PFOA (−35.86) onto CAMTAg were negative, indicating that the adsorption mechanism is exothermic in nature. Positive values were recorded for ΔH° for the adsorption of PFOS (2.32) and PFOA (12.69) onto PAMTAg, indicative of an endothermic adsorption mechanism. Positive entropy (ΔS°) values (0.04 and 0.07) were recorded for PFOS and PFOA using PAMTAg; whereas negative values (−0.04 and − 0.08) were recorded for ΔS° using CAMTAg. A positive ΔS° indicates an increase in randomness of the adsorbate at the solid-solution interface and the reverse is the case for a negative ΔS°.
Conclusion
The interplay of electrostatic attraction and hydrophobic interactions enabled the removal of PFAS using PAMTAg and CAMTAg. Findings suggest that PAMTAg and CAMTAg are effective for the removal of PFAS from aqueous media and are good alternatives to commercially available activated carbons.
Graphical abstract
Supplementary Information
The online version contains supplementary material available at 10.1007/s40201-020-00597-3.
Keywords: Maize tassel, Activated carbon, Silver nanocomposite, Adsorption, PFOS, PFOA
Introduction
Background
The contamination of water by biological and chemical agents compromises the quality of water systems. The prevalence of organic contaminants in water have been an issue of great concern for the water industry [1]. Many organic contaminants have been found to have adverse environmental impacts. They have been reported as potentially carcinogenic, with the ability to impair the health of humans and animals, even at trace levels [2]. Per- and polyfluoroalkyl substances are among the compounds that have been found with adverse environmental impacts [3]. Per- and polyfluoroalkyl substances are organic compounds that have some or all of their hydrogen atoms that are linked to a carbon chain length, being replaced by fluorine atoms [3, 4]. Per- and polyfluoroalkyl substances are released into the environment through the use of products that contain PFAS. These products include aqueous film forming fire-fighting foams (AFFF), textile products, coating and surface-active agents (surfactants). Per- and polyfluoroalkyl substances are present in fire and chemical-resistant tubings, electrical wire casings, stain repellents, plumbing sealants and non-stick cooking wares [5]. The two most widely reported PFAS are PFOA and PFOS [6, 7].
Per- and polyfluoroalkyl substances have been detected in surface water and ground water globally [1, 6]. This development has caused a public concern over the potential risk of human exposure through consumption of contaminated drinking water, since surface water and ground water are key sources for drinking water production [8–10]. The consumption of PFAS-contaminated water has been related to potential adverse health impacts such as low birth weight of new-borns, kidney and liver diseases, impaired semen quality, degeneration of the thyroid hormone and reproductive systems in humans and wildlife [11, 12].
Several studies have reported that existing water treatment techniques such as oxidation using hydrogen peroxide and/or permanganate, aeration, coagulation, sedimentation and disinfection are unable to remove PFAS from water systems [1, 6, 13]. Ion exchange resins and the use of high-pressure membranes via reverse osmosis and nanofiltration can remove PFAS considerably but they are expensive to operate due to their high-energy consumption [7]. The use of commercially available granular activated carbon (GAC) is common but it needs frequent re-activation to maintain its performance [3]. Hence, there is a quest for sustainable and effective techniques for removing PFAS from aqueous media. The present study is focused on the application of adsorption as a technique for the removal of PFAS from aqueous media; via the use of pre-synthesized nanocomposite-activated carbons produced form maize tassel powder and silver nitrate.
Motivation for the present study
Maize tassel represents the male flower of the maize plant. The maize tassel occurs at the tip of the maize plant and is usually disposed of during the harvest of the maize crop [4]. It is a carbonaceous agro-waste biomass. Globally, maize (and by extension maize tassel) is one of the most abundant, readily available, widely grown and harvested lignocellulosic grain [14]. About 10–14 million tons of maize are harvested yearly in South Africa [14–16]. In addition to maize tassel being available, it can be preserved over extended periods of time and utilized all year-long. In essence, maize tassel is durable and remains viable for a long time. For instance, the authors have maize tassels that have been in dry storage since 2018 and are still usable. Literature has shown maize tassel to be efficacious in the removal of toxic metals from aqueous media; owing to the important functional groups (hydroxyl, carboxyl, ether and many others) that are found in maize tassel [17, 18]. Furthermore, maize tassel is non-hazardous, sustainable and renewable.
Silver belongs to the class of noble metals. Therefore, it exists in nature in its zero oxidation state due to its high standard reductive potential. Silver is resistant to corrosion and oxidation in moist air [19]. However, silver can still be oxidized during a chemical reaction; making it useful in the degradation of pollutants such as organohalides into less toxic compounds [20]. Of all the different types of metallic nanoparticles that abound, silver nanoparticles (AgNPs) is one of the most popular. This is due to the positive antecedents of silver. For centuries, even in medieval times, silver has been used for the disinfection of water, for preserving perishable items and for general domestic use [21]. Silver nanoparticles are excellent antimicrobial agents and are used as constituents of disinfectants. This is due to the biocidal properties of AgNPs [21–23]. This is an edge that AgNPs have, over other types of nanoparticles such as titanium dioxide, gold nanoparticles, carbonaceous nanoparticles, nanoiron composites and many others. Silver nanoparticles are unarguably one of the most commonly applied metallic nanoparticles; owing to their ability to increase the surface atoms and surface energy of their substrates, their catalytic properties, high reactivity and good electrical conductivity [24, 25].
Multiple advances have been made in the area of water purification via the deposition of AgNPs on substrates. Impregnating natural and synthetic materials with AgNPs is one practical approach of harnessing the anti-germ features of silver [26]. Several studies have reported the incorporation of AgNPs on natural and synthetic polymers as useful for antibacterial activity during water purification [24, 27, 28]. For instance, Pourmand et al. [28] reported a good antibacterial activity against Legionella bacteria at 4 °C using AgNPs. Legionnaires’ disease also known as Legionella pneumophila occurs when Legionella bacteria is allowed to build up in air-conditioning systems of large buildings, pipes, shower heads, connections and water tanks [21, 28]. Pourmand et al. [28] concluded in their studies that AgNPs can be used to kill L. pneumophila at cold conditions by adding AgNPs to the cooling water system. Olgun et al. [24] reported a 100% reduction in bacterial contamination, Escherichia coli (E.coli) using 0.08–0.12% of nanosilver. Motshekga et al. [27] prepared AgNPs loaded on bentonite clay, as well as AgNPs/zinc oxide loaded on bentonite clay. It was concluded in the study by Motshekga et al. [27] that both AgNPs-composites showed good antibacterial activity against gram-negative bacteria, E.coli.
Activated carbons possess large surface areas that are highly porous and afford suitable sites onto which contaminants can be adsorbed [29, 30]. Activated carbons are easy and safe to utilize. Several studies have reported activated carbons as highly effective in removing PFAS from aqueous media [29, 31, 32]. Nanocomposite formation involves the dispersion of a nanoparticle-material (≤ 100 nm) in either natural or synthetic polymers [33, 34]. Different metallic nanoparticles such as silver-, nickel oxide-, zinc oxide- nanoparticles and others have been synthesized and applied singly or used to functionalize materials such as clay, chitosan, powdered activated carbon (PAC), granular activated carbon (GAC) and carbon nanotubes (CNTs) [27, 33, 35]. Recently, many studies have been centered on the application of metallic nanoparticles and nanocomposites for water purification and for environmental remediation as a whole [2, 36, 37]. The rise in the use of nanoparticles and nanocomposites is due to their extremely high surface areas, increased chemical reactivity, increased functionality and mechanical strength, shortened distance for the occurrence of intraparticle diffusion and strong sorption capabilities [38–40]. For instance, it has been reported in literature that nanocomposites that were synthesized using CNTs as precursors exhibited greater adsorption efficiency in the removal of organic contaminants from aqueous solutions than plain activated carbon [38, 41]. Furthermore, Pal et al. [33] reported that 72.5% of methyl orange dye was removed using silver nanoparticle-activated carbon (AgNPs-AC) whereas 50% was achieved using plain activated carbon.
Over the last decade and more, AgNPs have been incorporated on activated carbon via ecofriendly techniques such as carbonization and thermal activation [33, 42, 43] and the present study. Excellent antibacterial properties were reported for the AgNPs-activated carbon prepared by Tuan et al. [42]. Ghaedi et al. [43] reported 98% as the maximum percentage removal for direct yellow 12 (DY12) dye from aqueous media using AgNPs-activated carbon. Silver nanoparticles-activated carbon was found to be effective for the removal of methyl orange dye from aqueous solutions in the studies by Pal et al. [33]. One of the goals of the present study was to produce an adsorbent that possesses both the excellent properties of activated carbons and the unique features of AgNPs. The authors wanted to explore the co-benefits of an adsorbent that can remove PFAS adequately and simultaneously possess some antibacterial activity against bacteria such as E.coli and others. The antibacterial activities of the synthesized nanocomposite-activated carbons will be investigated and evaluated in future studies.
Several materials have been applied for the adsorption and thereby removal of PFAS from aqueous systems. Zeolites [44], PAC [29, 31], GAC [44] and CNTs [45, 46] have been employed for the removal of PFAS from aqueous solutions. Agro-waste biomasses such as cocoa shell [47], sugar cane bagasse [48], coconut shell and husks [30], orange peel [49], rice straw [50], saw dust [51], peanut hull [52], corncobs [53] and many others reviewed in [54, 55] have been used to prepare activated carbons; for the removal of contaminants (besides PFAS) from aqueous solutions. To the best of the authors’ knowledge, a limited number of studies have applied agro-waste biomasses for the adsorption and removal of PFAS from aqueous solution. These studies include the removal of PFAS using; grape leaf-litter [56], rice husk [57], maize straw ash [45] and bamboo [58]. Omo-Okoro et al. [4], Liu et al. [59] and Kucharzyk et al. [32] showed that a knowledge gap exists. There is very scant published data on the application of agro-waste biomasses as adsorbents (activated carbons) for the removal of PFAS from aqueous media. In an attempt to close this knowledge gap, the authors have been fully involved in studies focused on the identification, production, characterization and application of novel agro-waste adsorbents for the removal of PFAS from water systems, including the present study.
Novelty and relevance of the present study
This study entails a first time assessment of PFAS removal from aqueous solutions using nanocomposite-activated carbons synthesized from maize tassel and silver nitrate. Therein, lies the novelty of the present study. Furthermore, the technique for preparing the adsorbents is safe and easy-to-replicate. Moreover, there is a growing need in the use of activated carbons and nanocomposites for water purification [37, 60, 61]. Therefore, the production of sustainable but effective activated carbons and nanocomposites is increasingly being encouraged. This growing need underpins the relevance of the present study.
Materials and methods
Standards and reagents
The analytical standards and reagents used include silver nitrate (AgNO3, ≥ 99.8%), phosphoric acid (H3PO4), perfluorooctanoic acid (PFOA) and perfluorooctanesulfonic acid (PFOS, potassium salt). They were purchased from Sigma Aldrich, Germany. Others include ammonium acetate (p.a. grade, ≥ 98.0%) (FlukaChemie GmbH, Buchs, Germany), water (HPLC Gradient Grade, Fisher Scientific, Loughborough, UK) and methanol (MeOH), LC/MS grade, Biosolveb.v., Valkenswaard, Netherlands) used for preparing the Liquid Chromatograph (LC) mobile phases. The ultrapure water was obtained using Merck Millipore Milli-Q® water system (MA, USA).
Preparation and characterization of nanocomposite-activated carbons
As reported in Omo-Okoro et al. [62], 0.8 g of maize tassel powder (particle size: ≤ 45 μm) was firstly dispersed in distilled water (55 mL) in different beakers. Then 25 mL of varying percentages of silver nitrate (1, 2.5, 5 and 10%) were added individually to the maize tassel powder solution in a ratio of 1:1 in the differently labelled beakers (in replicates). During optimization experiments, time (30 m, 45 m and 60 m), temperature (60 °C, 70o and 80 °C) and power (800 W and 1200 W) were also varied and the results were investigated. For each test during optimization, the maize tassel – silver nitrate suspensions in Teflon vessels were microwaved via an Ultraclave High Performance Microwave Reactor (Milestone, Italy), a process referred to hereafter, as microwave irradiation synthesis. After each microwave irradiation synthesis, the maize tassel – silver nitrate suspensions in the Teflon vessels were stirred thoroughly using a stirrer and then allowed to stand and equilibrate for 24 h. This was carried out to assess the stability of the formed maize tassel silver (MTAg) nanoparticles. They were then filtered using a filter paper (0.45 μm) and a filtration unit connected to a vacuum pump. The varying residues of MTAg (1, 2.5, 5 and 10%) were dried individually on filter papers placed over petri dishes [62].
To investigate the possibility of silver that may have leached, the different filtrates were centrifuged at 3000 rpm for 20 min. The supernatants were collected from each of the replicates and then analyzed for residual silver via inductively coupled plasma mass spectrometry (ICP-MS) analysis; using a Thermo Fisher Scientific iCAP Q7000 ICP-MS instrument. It is important to note that after each run during optimization studies, a leaching test and characterization tests such as transmission electron microscopy (TEM) and powder x-ray diffraction (XRD) were conducted to confirm the formation of silver nanoparticles. The final optimized conditions reached for the microwave irradiation synthesis were 60 °C, 1 h and 800 W [62]. Following the ICP-MS and Brunauer Emmett Teller (BET) results obtained [62], 5% silver nitrate (MTAg 1:1) was selected as the best and adopted for the rest of the studies.
The MTAg nanocomposite was then thoroughly characterized [62]. After the characterization protocol, the MTAg nanocomposite powder was packed into a quartz glass tube and the tube was placed inside a tubular electric furnace (Carbolite-Labotec, South Africa) for carbonization and then activation individually; physical activation (temperature of 400 °C using air) and chemical activation (using H3PO4 in a ratio of 1:1 at 500 °C for 1 h). The produced chemically activated maize tassel silver (CAMTAg) nanocomposite-activated carbon was washed several times using hot deionized water until a neutral pH (6.9–7.1) was achieved for the effluent [62].
Both the physically activated maize tassel silver (PAMTAg) and the CAMTAg samples were characterized in the present study via scanning electron microscopy (SEM), BET, Fourier transform infrared (FTIR) spectroscopy, XRD, energy dispersive x-ray spectroscopy (EDS) and TEM. A SEM instrument, JOEL JSM-7500F (Tokyo, Japan) was used to perform SEM analyses of the synthesized adsorbents. The TEM analyses of the synthesized silver nanocomposites were performed using a TEM detector present on the SEM instrument (JOEL JSM-7500F Tokyo, Japan). The XRD analyses was conducted using the XRD XPERT-PRO Panalytical, Netherlands at a variable slit of 45 kV and 40 mA; via Ni-filtered CuKα radiation (λ =1.5406 Ǎ). The XRD and TEM analyses were performed in order to confirm the crystallinity, shape and size of the AgNPs. The elemental composition of the synthesized MTAg nanocomposite in comparison with the raw MT was evaluated via EDS using a different SEM instrument (Zeiss Auriga SEM, Germany). The samples were deposited on aluminum stubs with the aid of a carbon tape. Carbon coating was performed to prevent the samples from charging during the SEM and EDS analyses.
Zeta potential analysis using a zeta sizer instrument, Malvern Zetasizer Nano ZS, was conducted to determine the pH at the point of zero charge (pHpzc) of the nanocomposite-activated carbons. The zeta potential indicates the electrical charge of particles when suspended in liquid. The pHpzc refers to the pH value at which a solid material exhibits a net electrical charge of zero (0 mV) on its surface when dispersed in an aqueous solution. Therefore, the pHpzc describes the surface charge of the material. Briefly, the samples of PAMTAg and CAMTAg were dispersed individually, in aqueous solutions at pre-selected ionic strengths. Thereafter, the suspensions were left to equilibrate for 12 h. Then they were re-dispersed using an ultrasonicator for 15 min. Subsequently, the zeta potential is measured using the Malvern Zetasizer. To identify the specific pHpzc, zeta potential (mV) (y-axis) against pre-selected pH values (x-axis) was plotted in a graph. The specific pH (on the x-axis) at which the electrical charge is 0 mV on the y-axis was then determined and noted as the pHpzc. Both PAMTAg and CAMTAg have been applied as adsorbents in the present study for the removal of PFAS from aqueous media.
Batch equilibrium experiments
Batch equilibrium experiments were performed using PAMTAg and CAMTAg to adsorb PFAS from PFAS-simulated water via the spiking of ultrapure water. These ranges; pH (2–9), adsorbent dosage (0.02 g – 0.1 g), initial PFAS concentration (0.025 mg/L – 0.1 mg/L) and temperature (25–45 °C) were tested during adsorption experiments evaluating the influence of pH, adsorbent mass, initial PFAS concentration and temperature. After batch equilibrium experiments, the mixture (containing adsorbent powder and spiked PFAS) was then filtered using a 0.45 μm filter (Whatman®) and then the filtrate was analysed for residual PFAS concentrations using the HPLC-MS/MS.
For the purpose of reproducibility, the adsorption of PFAS in the present research was studied via batch adsorption experiments as a function of pH (2–9), adsorbent dosage (0.02–0.1 g), contact time (1–120 min), initial PFAS concentration (0.025 mg/L – 0.1 mg/L), agitation speed (120–180 rpm) and temperature (25–45 °C). The percentage removal (% R) and the amount of adsorbate at equilibrium (qe) were obtained using Eqs. 1 and 2 below [56, 63].
| 1 |
| 2 |
where ‘Co’ is the initial PFAS concentration (mg/L) and ‘Ce’ is the equilibrium concentration (mg/L). The volume of the solution (L) and the mass (weight) of the adsorbent (g) are represented by ‘v’and ‘w’, respectively. The average value, standard deviation and coefficient of variation of the triplicates were then obtained. The Scheffe test was also used to compare the different mean values of the populations.
Adsorption isotherm experiments
Both PAMTAg and CAMTAg were also used for the adsorption isotherm and thermodynamic studies. A thermostatic water bath shaker was utilized for agitation and operated at 180 rpm for 24 h. The maximum adsorption capacities (mg/g) for the two major PFAS, PFOS and PFOA were determined by applying the Langmuir and Freundlich isotherm models.
Briefly, about 0.05 g of the prepared adsorbents (PAMTAg and CAMTAg) were added individually into 25 mL of PFAS simulated water in different labelled centrifuge tubes. The initial PFOS and PFOA concentrations were varied during the adsorption isotherm experiments to evaluate the effect of adsorbate concentration during the adsorption process. The ultrapure water was spiked using environmentally relevant concentrations for each of PFOS and PFOA. The initial PFAS concentrations tested ranged from 10, 20, 50, 70 and 100 ng/mL (5 levels). The range utilized was chosen based on the detection frequency and concentrations frequently reported in surface waters and wastewaters [9, 64, 65]. Experiments were performed initially at 25 °C, then at 35 °C and 45 °C.
PFAS determination
Polypropylene centrifuge tubes were utilized. Both negative (solution with spiked PFAS but no adsorbent) and positive (solution with specific concentration of PFAS plus adsorbent) blanks were used. The blanks were then used to quantify the PFAS loss to the centrifuge tubes. A correction factor was subsequently calculated and applied for the experiments. The losses were found to be negligible (≈ 4%). Samples were analysed as soon as possible, after experiments were completed; since there is a possibility that the longer samples are stored, the greater the chances of the PFAS being adsorbed onto the walls of the tubes.
The levels of the PFAS (before and after adsorption experiments) in aqueous solutions were quantified using a high performance liquid chromatograph (HPLC), Agilent 1290 instrument (Agilent Technologies, Palo, Alto, California, USA), linked to a QTRAP 5500 MS/MS (AB Sciex, Foster City, California, USA). The chromatographic separation was performed at 20 °C using a reversed phase 80ÄSynergi (4 μ) Fusion column (50 mm × 2 mm; Phenomenex, USA). A mobile phase A of methanol/5 mM ammonium acetate in HPLC water (55/45) (v/v) and a mobile phase B of methanol were utilized for gradient separation at a flow rate of 200 uL/min. The mass spectrometer was operated using the electrospray (negative) ionization mode (ESI) (450 °C and ion voltage 4500 V) using two multiple reaction monitoring (MRM) transitions for each PFAS. The method detection limits (MDLs) of the target PFAS ranged between 0.0001–0.1099 ng/L while the instrument quantification limits (IQLs) for the target PFAS ranged between 0.004–0.01 ng/mL.
Results and discussion
Characterization of adsorbents
SEM
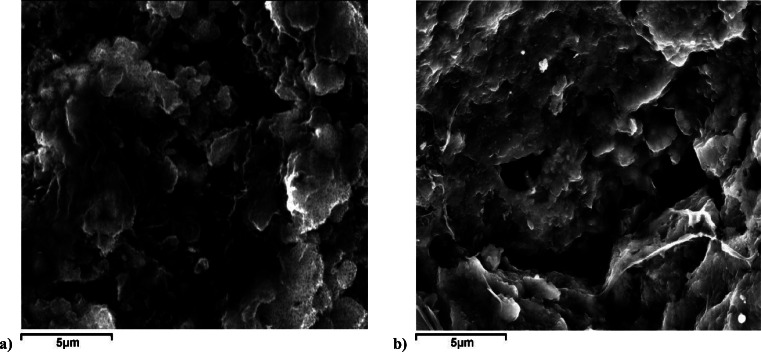
The SEM micrographs of PAMTAg and CAMTAg are presented in Fig. 1. Pores and cavities are observed for both PAMTAg and CAMTAg (Fig. 1). Though these pores and cavities are slightly wider and fewer in PAMTAg (Fig. 1a) than for CAMTAg (Fig. 1b). A rough morphology and a developed porous system are observed for both PAMTAg and CAMTAg (Fig. 1).
Fig. 1.
SEM micrographs of a PAMTAg and b CAMTAg
EDS
The elemental composition of the synthesized MTAg nanocomposite in comparison with the raw MT was evaluated via the EDS analyses using a SEM instrument (Zeiss Auriga SEM, Germany). The EDS spectra (Fig. 2) showed the absence of silver (Ag) in the spectrum of the raw MT (Fig. 2a) and the presence of Ag at a binding energy of 3 keV in the spectrum of synthesized MTAg nanocomposite (Fig. 2b). These findings are similar to related studies [66, 67]. The EDS analyses confirmed that the synthesized MTAg nanocomposite contained a mean value of (6.1 ± 0.1) % of Ag (out of 3 spectra). Typical constituents of plant cells such as sulphur, phosphorus, chlorine and potassium were also present in the EDS spectra (Fig. 2a and b).
Fig. 2.
EDS spectra of a Raw MT and b MTAg nanocomposite (Spectral aerial view 3; with different weight (Wt.) % for C, O, K, Cl, S, P and Mg for Raw MT and C, O, K, Cl, S, P and Ag for MTAg nanocomposite)
XRD and TEM analyses
The XRD analyses were conducted in order to assess the crystal growth on the synthesized MTAg nanocomposite in comparison to the raw MT (Fig. 3). The XRD image showed the presence of crystalline peaks of silver on the synthesized MTAg nanocomposite (Fig. 3; red spectrum) and the absence of these peaks on the XRD spectrum of raw MT (Fig. 3; blue spectrum). Figure 3 shows 5 crystalline peaks of silver at 2θ of about 37.8°, 43.6°, 64.6°, 76.8° and 81.3° on the XRD pattern for the synthesized MTAg nanocomposite (Fig. 3; red spectrum). These 5 peaks represent the diffraction planes (111), (200), (220), (311) and (222) of the crystalline face-centered cubic (fcc) silver compared with the powder diffraction file card. Ahmad et al. [68] and Zhang et al. [69] reported similar findings in their studies.
Fig. 3.

XRD spectra showing Raw MT (blue) and synthesized nanocomposite (MTAg) (red) (Adapted from the XRD image in ‘Omo-Okoro PN et al., 2020 - Microwave-assisted synthesis and characterization of an agriculturally derived silver nanocomposite and its derivatives’ in Waste and biomass valorization; 11: 2247–2259 (Source); License Number: 4921620894097. Alphabets (A-E) have been incorporated to highlight the 5 crystalline peaks of silver (Ag) at 2θ in the synthesized nanocomposite (MTAg) but absent in plain/raw MT)
The average crystalline size can be calculated from the XRD spectrum using the full width at half-maximum (FWHM) of the (111) peak on the XRD spectrum of the synthesized MTAg nanocomposite (Fig. 3; red spectrum); via the Scherrer’s equation but the XRD can only give the average size of a crystallite. A nanoparticle can contain many crystallites, that is, a nanoparticle can be polycrystalline. According to Londoño-Restrepo et al. [70] and Holder and Schaak [71], a more precise way of confirming the nano-size is via the TEM image (Fig. 4) of a synthesized silver nanocomposite. Therefore, in order to confirm the nano-size, the authors used ImageJ software (an image processing program) to determine the average size of the nanoparticles (nano-size) using a TEM image (Fig. 4b) (via a count of a 102 nanoparticles). A histogram was derived thereafter via ImageJ (Fig. 5) [62, 72]. The histogram is indicative of nanoparticles that are polydispersed and the average size of the nanoparticles was found to be 10.5 nm [62]. Buso et al. [72] and Ruggiero et al. [73] obtained the average size of synthesized nanoparticles (nano-size) in their studies via a histogram derived from ImageJ software.
Fig. 4.
TEM images of a Raw Mt, b Synthesized MTAg nanocomposite (Source: Omo-Okoro et al. [62];License number: 4930861311774), c Physically activated carbon (plain AC), d MTAg-activated carbon (PAMTAg), e Chemically activated carbon (plain AC) and f MTAg-activated carbon (CAMTAg)
Fig. 5.

Histogram derived via ImageJ showing the nano-size (average size = 10.5 nm; standard deviation = 4.99 nm) of the nanoparticles in the synthesized MTAg nanocomposite
The TEM analyses of the synthesized adsorbents were performed in order to confirm the shape and size of the AgNPs present on the silver nanocomposites (Fig. 4). The TEM images of the activated carbon without AgNPs and the activated carbon with AgNPs (PAMTAg and CAMTAg) have been provided (Fig. 4). The TEM images confirm the absence of AgNPs on the raw MT and the plain activated carbons; and the presence of AgNPs on the MTAg-nanocomposite and also on the nanocomposite-activated carbons (Fig. 4). Spherical, black and irregular-sized dots of AgNPs are evident on the TEM images of the synthesized MTAg nanocomposite, PAMTAg and CAMTAg (Fig. 4). The spherical dots observed in the micrographs (Fig. 4) are similar to those reported in the studies by Djerahov et al. [66] and Motshekga et al. [27]. Abreu et al. [74] and Li et al. [75] have reported a high antimicrobial activity against gram-positive and gram-negative microbes such as Staphylococcus aureus and E.coli, respectively, by silver nanocomposites with spherically shaped AgNPs. The antibacterial activities of PAMTAg and CAMTAg will be assessed in future studies.
FTIR
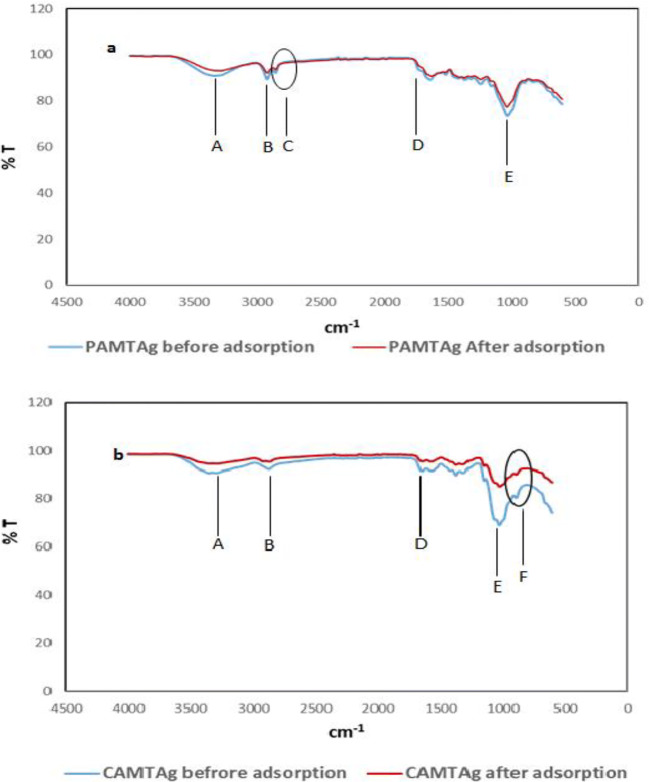
The FTIR spectra (before and after adsorption) of PAMTAg and CAMTAg are presented in Fig. 6. The main difference observed in the spectra is the shortening of vibrational peaks in the spectra (red line) of the adsorbents used (PAMTAg and CAMTAg) (Fig. 6). The shortened peaks (cm−1) include: A (3338; O-H stretching of hydroxyl group), B (2900; C-H stretching of methyl group), D (1730; carboxylic group) and E (1030; C-O-C stretching of ethers) [76, 77]. Also, the shoulder peak (C; 2800 cm−1) observed in the spectra of PAMTAg (before and after adsorption) (Fig. 6a) is completely lost in the spectra of CAMTAg (before and after adsorption) (Fig. 6b).
Fig. 6.
FTIR spectra of a PAMTAg and b CAMTAg
Furthermore, the peak synonymous with the phosphate group is observed at F; 950 cm−1, only in the spectra of CAMTAg (before and after adsorption) (Fig. 6b). This is possibly as a result of the activation process using H3PO4 [17, 77]. Carboxylics, phosphates, methyl groups, ethers, amines and other functional groups inherent in agricultural waste materials have been found to be associated with the efficacy of these agro-based adsorbents in the removal of contaminants from aqueous media [4, 18, 76].
The particle size distribution, pore size, pore system, BET surface area and points of zero charge (pHpzc) of PAMTAg and CAMTAg are presented in Table 1. The BET surface areas (18.56 and 331.09) m2/g were recorded for PAMTAg and CAMTAg, respectively (Table 1). Both PAMTAg and CAMTAg were found to be mesoporous; as their pore sizes fall within the 2–50 nm range denoted for mesoporous systems (Table 1) [78, 79]. The pHpzc recorded for PAMTAg and CAMTAg are 7.9 and 6.7, respectively (Table 1). Both PAMTAg and CAMTAg are positively charged when the pH of the solution fall below their pHpzc (pH < pHpzc) [18, 80, 81]. This is possibly associated with the high percentage removal of 10 PFAS (both short and long chain PFAS) recorded with the use of PAMTAg (79.2–81%) and CAMTAg (81.2–83%) (Fig. 7). This high percentage removal of PFAS could be attributed to the existence of electrostatic attraction occurring between the anionic PFAS (negatively charged) and the positively charged adsorbents (PAMTAg and CAMTAg) at pH 2, 4 and 6 (Fig. 7).
Table 1.
Pore and particle size characteristics of nanocomposite-activated carbons used in this study
| Adsorbent | Particle size distribution (nm) | Pore size (4 V/A by BET) (nm) | Pore system | BET surface area m2/g | pHpzc |
|---|---|---|---|---|---|
| PAMTAg | 400–446.3 | 3.87 | Mesoporous | 18.56 | 7.9 |
| CAMTAg | 150–175.2 | 2.39 | Mesoporous | 331.09 | 6.7 |
Fig. 7.
Effect of solution pH using a) PAMTAg and b) CAMTAg (Other constants include temperature of 25 °C, contact time of 24 h, initial concentration of 0.1 mg/L and adsorbent mass of 0.05 g) (the error bars represent the standard deviations of triplicates of each sample)
The authors hypothesized that;
The cellulose inherent in maize tassel powder can act as a reducing and stabilizing agent that will engender the attachment of AgNPs; thereby enabling the formation of maize tassel-silver nanocomposites;
Maize tassel powder that has been modified via nanostructuring, carbonization and activation (physical and chemical) will probably have a better developed structure with larger surface area than raw maize tassel powder which has a surface area of 2.52 m2/g;
The removal efficiency of PFAS is closely associated with the perfluorocarbon chain length and type (s) of functional group present.
Effect of pH
The effect of pH was tested using PAMTAg and CAMTAg as adsorbents for the removal of PFAS from aqueous media. It is evident from Fig. 7a that a high percentage removal was recorded for all the 10 PFAS at all the pH values but the optimum was found to be pH 2. Overall, PAMTAg can remove both short and long chain PFAS with a percentage removal range of 79.15–81% (Fig. 7a). Since the pHpzc of PAMTAg is 7.9, at pH values below 7.9, PAMTAg exists in its positively charged state. The availability of H+ on the adsorbent’s surface causes an electrostatic attraction between positively charged PAMTAg and the negatively charged PFAS [82]. At pH 9 (alkaline medium) when PAMTAg becomes negatively charged and PFAS is now neutral, a percentage removal of PFAS is still observed possibly as a result of hydrophobic interaction occurring between PFAS and PAMTAg. Yu et al. [31] reported the adsorption of PFAS onto activated carbon due to hydrophobic interaction between neutral PFOA and activated carbon.
The optimum pH for PFAS removal is also pH 2 when CAMTAg is utilized as an adsorbent; though the differences across the different pH levels was slight for the long chain PFAS (PFOS, PFNA and PFDA) (Fig. 7b). The pHpzc of CAMTAg is 6.7, so beyond 6.7, CAMTAg was negatively charged. The percentage removal observed at pH 9 (Fig. 7b) is also probably due to hydrophobic interaction and no longer electrostatic attraction.
The percentage removal of all 10 PFAS, both short and long chain was higher using CAMTAg (81.17–83%) than for PAMTAg (79.15–81%) (Fig. 7). It is obvious that both electrostatic and hydrophobic interactions were at play, with hydrophobic interaction as the dominant force at alkaline medium (pH 9) (Fig. 7); especially for the long chain PFAS. Guo et al. [83] reported that electrostatic attraction and hydrophobic interaction were responsible for the adsorption of PFOS using corn straw-biochar. It was concluded in that study that the difference in pH values affected the electrostatic interactions while hydrophobic interaction was the main mechanism between the two. The variation between sample triplicates is predominantly minimal and thereby represented by short error bars (Fig. 7).
Effect of adsorbent dosage
The masses of the powdered adsorbents 0.02, 0.05 and 0.1 g were applied as dosages during the batch equilibrium experiments investigating the effect of adsorbent dosage using PAMTAg and CAMTAg for the removal of PFAS from aqueous media. It can be observed from Fig. 8a, that as the adsorbent dosage increases from 0.02 to 0.05 g, the percentage removal of all 10 PFAS increases using PAMTAg. This is due to the increase in the binding sites on the surface of the adsorbent. This increase in PFAS percentage removal reaches a maximum and starts decreasing when the adsorbent dosage is increased beyond 0.05 g (Fig. 8a). Apparently, it seems that there is a threshold with respect to the amount of adsorbent (PAMTAg) needed for the effective removal of PFAS from aqueous solution. The optimum dosage of 0.05 g favoured the adsorption process (Fig. 8a). An increase beyond 0.05 g possibly causes an aggregation of the adsorbent material. Consequently, the aggregates start to block the active sites needed for adsorption to continue. When adsorption can no longer proceed due to the blockage of pores on the adsorbent’s surface, desorption sets in. Hence, a decrease in PFAS percentage removal is observed beyond 0.05 g (Fig. 8a).
Fig. 8.
Effect of adsorbent dosage using a PAMTAg and b CAMTAg (Other constants include: temperature of 25 °C, contact time of 24 h, initial concentration of 0.1 mg/L and solution pH of 2) (the error bars represent the standard deviations of triplicates of each sample)
The optimum dosage for the removal of PFAS using CAMTAg appears to be 0.05 g, because no significant increase in PFAS percentage removal is observed beyond 0.05 g. with the exception of PFBA (short chain PFAS) (Fig. 8b). The other PFAS (with longer perfluorocarbon chain and/different functional groups) were readily adsorbed using the medium dosage of 0.05 g. Kumar et al. [84] reported similar findings, where a maximum removal (98%) of rhodamine dye was observed using a medium dosage of the adsorbent (BioNC hydrogel). The increase in the removal of the dye stopped as the mass of BioNC hydrogel (adsorbent) was increased. No further change with respect to the percentage removal of the dye was observed beyond the use of the medium dosage of BioNC hydrogel. Therefore, Kumar et al. [84] concluded that an effective adsorptive material should be able to adsorb large quantities of adsorbate using moderate amounts of the adsorbent. This statement is in agreement with the findings in the present study where a moderate mass of the adsorbent (0.05 g) is able to remove PFAS from aqueous solutions.
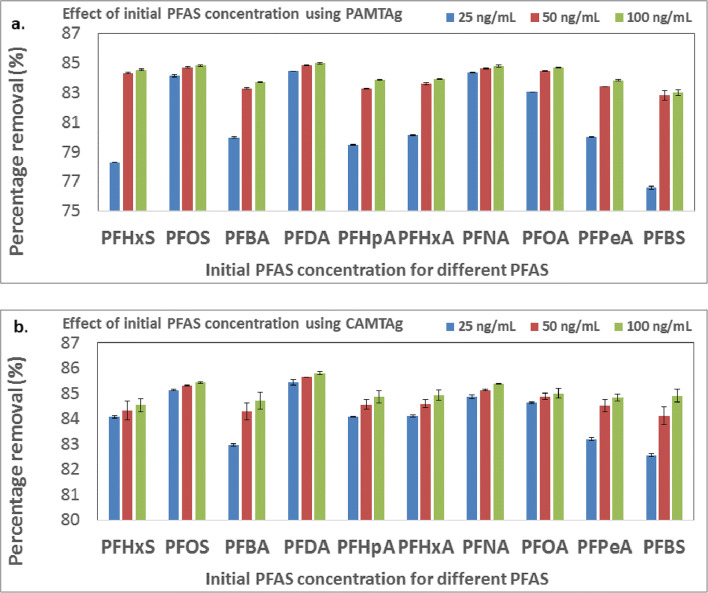
Effect of initial PFAS concentration
The PFAS percentage removal increased very slightly with increase in the initial PFAS concentration for the long chain PFAS (PFOS, PFNA and PFDA) using both PAMTAg and CAMTAg (Fig. 9a and b). On the other hand, the increase in PFAS percentage removal as the initial PFAS concentration increases was more conspicuous with the short chain PFAS using both PAMTAg and CAMTAg (Fig. 9a and b).
Fig. 9.
Effect of initial PFAS concentration using a PAMTAg and b CAMTAg (Other constants include: temperature of 25 °C, contact time of 24 h, adsorbent dosage of 0.05 g and solution pH of 2) (the error bars represent the standard deviations of triplicates of each sample)
The increase in PFAS removal as the initial PFAS concentration increases observed in Fig. 9 could be due to the increase in the rate of the adsorption mechanism driven by higher PFAS concentrations. Oyewo et al. [85] reported that an increase in the initial concentration of an adsorbate leads to a higher driving force which propels the adsorption mechanism and invariably causes a higher percentage removal of the adsorbate from aqueous solution. Furthermore, there is a direct relationship between PFAS concentration and the formation of PFAS micelles. An increase in PFAS concentration increases the formation of PFAS micelles on the surface of an adsorbent [13]. This consequently leads to an increase in the percentage removal of PFAS (Fig. 9).
Adsorption isotherm
Effect of temperature
Uptake of PFOS and PFOA onto PAMTAg increased from 25 °C to 35 °C and then decreased slightly with increasing temperature at 45 °C (Table 2). The maximum adsorption capacities for both PFOS and PFOA using PAMTAg occurred at 35 °C (Table 2). The increase in adsorption capacities of PFOS and PFOA as the temperature increases from 25 °C to 35 °C is representative of an endothermic process. The increase in adsorption capacity at 35 °C may have occurred because of the interaction between the adsorbate and the adsorbent, which may be due to electrostatic attraction or hydrophobic interaction or the combination of both. The decrease in PFOS and PFOA uptake and invariably, adsorption capacity using PAMTAg observed at temperature beyond 35 °C (Table 2), could be due to the occurrence of desorption caused by the increase in temperature (thermal energy). An increase in temperature beyond a threshold causes an increase in the mobility of molecules of an adsorbate which results in desorption and consequently reduces contaminant uptake levels [29, 86, 87]. The reverse was the case for the adsorption of PFOS and PFOA onto CAMTAg where low temperature (25 °C) favoured the adsorption process (Table 2). The highest adsorption capacities observed for both PFOS and PFOA using CAMTAg occurred at 25 °C (Table 2). This pattern is indicative of an exothermic adsorption process [88].
Table 2.
Isotherm parameters for the adsorption of PFOS and PFOA onto PAMTAg and CAMTAg
| Isotherm model | Temp (K) | Parameters | PAMTAg | CAMTAg | ||
|---|---|---|---|---|---|---|
| PFOS | PFOA | PFOS | PFOA | |||
| Langmuir | 298 | R2 | 0.90 | 0.95 | 0.89 | 0.86 |
| qm (mg/g) | 15.58 | 13.62 | 294.12 | 285.71 | ||
| KL (L/mg) | -0.99 × 10-3 | -1.14 × 10-3 | -0.23 × 10-3 | -0.6 × 10-3 | ||
| RL | 1.11 | 1.13 | 1.02 | 1.06 | ||
| 308 | R2 | 0.80 | 0.97 | 0.8 | 0.83 | |
| qm (mg/g) | 28.41 | 18.98 | 243.90 | 204.08 | ||
| KL (L/mg) | -1.25 × 10-3 | -1.5 × 10-3 | 0.35 × 10-3 | 0.38 × 10-3 | ||
| RL | 1.14 | 1.17 | 0.97 | 0.96 | ||
| 318 | R2 | 0.97 | 0.96 | 0.90 | 0.91 | |
| qm (mg/g) | 23.81 | 16.89 | 204.08 | 175.44 | ||
| KL (L/mg) | -1.20 × 10-3 | -1.29 × 10-3 | 0.23 × 10-3 | 0.25 × 10-3 | ||
| RL | 1.14 | 1.15 | 0.98 | 0.98 | ||
| Freundlich | 298 | R2 | 0.96 | 0.99 | 0.99 | 0.97 |
| KF | 18 | 18.82 | 454.09 | 321.24 | ||
| 1/n | 1.38 | 1.44 | 2.16 | 1.71 | ||
| n | 0.73 | 0.69 | 0.46 | 0.59 | ||
| 308 | R2 | 0.98 | 0.97 | 0.96 | 0.98 | |
| KF | 45.54 | 55.55 | 177.20 | 60.27 | ||
| 1/n | 1.68 | 1.77 | 1.26 | 1.59 | ||
| n | 0.60 | 0.56 | 0.79 | 0.63 | ||
| 318 | R2 | 0.96 | 0.98 | 0.97 | 0.96 | |
| KF | 35.40 | 46.95 | 69.17 | 21.45 | ||
| 1/n | 1.57 | 1.71 | 1.36 | 1.19 | ||
| n | 0.64 | 0.58 | 0.74 | 0.84 | ||
Other constants include pH 2, adsorbent dosage 0.05 g, contact time of 24 h and agitation speed of 180 rpm. Adsorption isotherm plots that corroborate the results in Table 2 are presented in Appendix A in the Supplementary Information
Adsorption isotherm modelling
Two common isotherm models, Langmuir and Freundlich were applied to the isotherm data obtained in the present study.
Langmuir isotherm model
The Langmuir isotherm model assumes that the particles of an adsorbate do not migrate into other layers of the adsorptive material and that the adsorption mechanism occurs at a specific homogenous site (monolayer) on the adsorbent. The Langmuir model is represented in Eq. (3) and linearized in Eq. (4) [56, 63].
| 3 |
| 4 |
where Co represents the initial concentration of the adsorbate, Ce represents the equilibrium concentration of the adsorbate (mg/L), qe denotes the quantity of adsorbate adsorbed per gram of the adsorbent at equilibrium (mg/g) and qm represents the maximum homogenous capacity (mg/g). The rate constant, KL is the Langmuir isotherm constant (L/mg). The values of qmcan be derived from the slope; (qm = 1/slope) and KL can be derived from the intercept; KL = 1/ (qmx intercept) [56, 87]. The slope and intercept are obtained from the equation from the plot between 1/qeversus1/Ce. A dimension-less separation factor, RL can be obtained from Eq. (5) [89].
| 5 |
The maximum adsorption capacities for the homogenous coverage of the Langmuir isotherm model, ‘qm’ for PFOS and PFOA were 28.41 mg/g and 18.98 mg/g, respectively, using PAMTAg at 35 °C (Table 2). The maximum ‘qm’ values were 294.12 mg/g and 285.71 mg/g for PFOS and PFOA, respectively, using CAMTAg at 25 °C (Table 2).
The values recorded for the coefficient of determination, R2 from the Langmuir modelling of the isotherm data for the adsorption of PFOS and PFOA using both PAMTAg and CAMTAg were in the range of 0.8–0.97 (Table 2). It was found from the generality of the results that the values recorded for the Langmuir constant, KL (L/mg) were negative. This is related to the RL values that were recorded. The RL values were greater than 1 in those instances (Table 2).
When the separation factor (RL) is greater than 1, the adsorption process is reported as unfavourable and therefore the model does not fit the data properly [89–91]. This validates the negative sign recorded for some of the KL values, therefore further reflecting the inadequacy of the Langmuir model to fully describe the data.
Conversely, when RL is less than 1, the adsorption process is favourable [89–91]. This statement is in agreement with four of the KL values that are positive (Table 2); where the RL values were less than 1 (0.96, 0.97 and 0.98) (Table 2).
Freundlich isotherm model
The Freundlich adsorption isotherm model describes a multilayer type of adsorption mechanism [56]. The empirical equation for the Freundlich isotherm model is expressed in Eq. (6) and linearized in Eq. (7) [87].
| 6 |
| 7 |
A plot of logqe against logCe produces a linear graph and a linear equation where KF and 1/n can then be obtained from the intercept and slope, respectively [92, 93]. The values of ‘1/n’ and ‘KF’ can be derived as follows; 1/n is equals to slope and the intercept is equals to ln KF [56, 87]. The rate constant, KF is the Freundlich isotherm constant on a heterogeneous surface and therefore the measured adsorption capacity; while ‘n’ represents the adsorption intensity. The value 1/n is used to represent the strength of the adsorption process of the adsorbent [91].
The maximum adsorption capacities in the present study were recorded with the Freundlich modelling of the adsorption of PFOS and PFOA onto CAMTAg (454.09 and 321.24 mg/g), respectively) (Table 2).
Higher R2 values in the range of 0.96–0.99 were recorded from the Freundlich modelling of the isotherm data for the adsorption of PFOS and PFOA using both PAMTAg and CAMTAg (Table 2). Furthermore, all the values recorded for ‘1/n’ using the Freundlich model were greater than 1 and all the values for ‘n’ were less than 1 (Table 2). When the value of ‘1/n’ is greater than 1 using the Freundlich model, this indicates that the adsorption process is favorable [94–96]. This therefore implies that when the value of ‘n’ is less than 1, the adsorption process is reported as favorable [91]. Therefore, the Freundlich model suits the data better than the Langmuir model in the present study. Also, an adsorption process that proceeds by physisorption is often better fitted with the Freundlich model [56].
It is also observed from the majority of the results in Table 2, that both PAMTAg and CAMTAg adsorbed PFOS more readily than PFOA. Sulphonates will be more readily adsorbed from an aqueous solution than carboxylates; and the heavier PFAS (PFOS; 500.13 g/mol) will be more readily adsorbed than PFOA (414.07 g/mol) [57, 58].
All the values recorded for the coefficient of determination, R2 in the present study were relatively good (Table 2). The generality of the R2 values were in the range of 0.9, with a few in the range of 0.8 (Table 2). Usually, R2 values that fall between 0.7 and 1 are acceptable [97, 98].
Thermodynamics
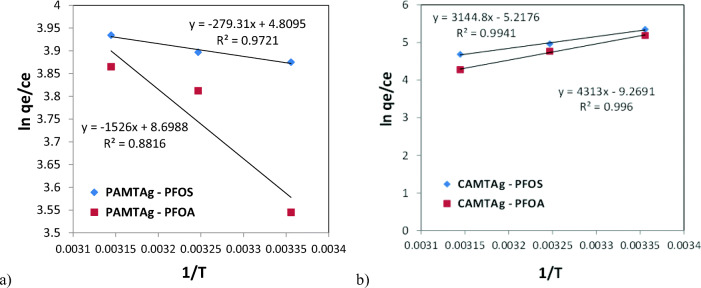
Thermodynamics studies for the adsorption of PFOS and PFOA onto PAMTAg and CAMTAg were also performed. The equations for deriving the thermodynamic parameters, ΔG°, ΔH° and ΔS° are expressed in Eqs. (8), (9) and (10); using the equilibrium coefficients derived at 298 K, 308 K and 318 K and obtained via Van’t Hoff’s plot of In KD versus 1/T (Fig. 10). The values of ΔG° at different temperatures can be derived from Eq. 9.
Fig. 10.
The plot of ln KD versus 1/T for the adsorption of PFOS and PFOA using a PAMTAg and b CAMTAg (Other constants were pH 2, 0.05 g and agitation speed of 180 rpm)
The value for ΔG° is obtained from the Van’t Hoff’s equations (Eqs. 8 and 9).
| 8 |
| 9 |
A linear equation is obtained and then expressed as Eq. (10) [87, 99].
| 10 |
The constant ‘ln KD’ is substituted as In qe/Ce, where ‘T’ is temperature in Kelvin (K) and ‘KD’ is the distribution coefficient. The values for ‘ln KD’ is then obtained from the values for ln qe/Ce at different temperatures (25, 35 and 45 °C) [87, 99]. The constant ‘R’ represents the universal gas constant, which is 8.314 J/mol.K (0.008314 kJ/mol.K). The value for ΔH° can be derived from the slope (−∆H0/R = slope) and the value for ΔS° can be derived from the intercept (∆S0/R = intercept) (Fig. 10) [99].
The values recorded for ΔG° (kJ/mol) for PFOS and PFOA were PFOS (−9.61, −9.99 and − 10.39), PFOA (−8.77, −9.76 and − 10.21) using PAMTAg; and PFOS (−13.70, −12.70 and − 12.37), PFOA (−12.86, −12.21 and − 11.17) using CAMTAg (Table 3). All the ΔG° values recorded for the adsorption of PFOS and PFOA onto PAMTAg and CAMTAg were negative; therefore, the adsorption processes were spontaneous and feasible. Furthermore, all the ΔG° values were less than -20 kJ/mol (Table 3). This strongly indicates a physical adsorption mechanism [100, 101].
Table 3.
Thermodynamic parameters for the adsorption of PFOS and PFOA onto PAMTAg and CAMTAg
| Adsorbent | Temp (K) | ln KD | ΔG° (kJ/mol) | PFOS | PFOA | ||||
|---|---|---|---|---|---|---|---|---|---|
| ΔH° (kJ/mol) | ΔS° (kJ/mol.K) | ln KD | ΔG° (kJ/mol) | ΔH° (kJ/mol) | ΔS° (kJ/mol.K) | ||||
| PAMTAg | 298 | 3.88 | −9.61 | 2.32 | 0.04 | 3.54 | −8.77 | 12.69 | 0.07 |
| 308 | 3.90 | −9.99 | 3.81 | −9.76 | |||||
| 318 | 3.93 | −10.39 | 3.86 | −10.21 | |||||
| CAMTAg | 298 | 5.35 | −13.70 | −26.15 | −0.04 | 5.19 | −12.86 | −35.86 | −0.08 |
| 308 | 4.96 | −12.70 | 4.77 | −12.21 | |||||
| 318 | 4.68 | −12.37 | 4.28 | −11.17 | |||||
Other constants include pH 2, adsorbent dosage 0.05 g, contact time of 24 h and agitation speed of 180 rpm
The values (0.97, 0.99 and 0.88) recorded for R2 in the plot of ln KD versus 1/T for the adsorption of PFOS and PFOA are relatively good (Fig. 10) [97, 98].
It was observed that the values for ΔH° (kJ/mol) were negative for the adsorption of PFOS (−26.15) and PFOA (−35.86) onto CAMTAg (Table 3). This indicates that the adsorption mechanism is exothermic in nature. Conversely, positive values were observed for ΔH° for the adsorption of PFOS (2.32) and PFOA (12.69) onto PAMTAg (Table 3). This denotes an endothermic adsorption mechanism. Generally, all the ΔH° values recorded for both PAMTAg and CAMTAg for the adsorption of PFOS and PFOA are quite low and were below ±80 kJ/mol (Table 3). This trend further indicates that the adsorption mechanism of PFOS and PFOA onto PAMTAg and CAMTAg proceeds by physisorption [56, 101]. Positive values were observed for ΔS° (kJ/mol.K) for the adsorption of PFOS and PFOA onto PAMTAg (0.04 and 0.07), respectively. Contrarily, negative values were recorded for ΔS° (kJ/mol.K) for the adsorption of PFOS and PFOA onto CAMTAg (−0.04 and − 0.08), respectively (Table 3). A positive ΔS° value signifies that there is an increase in the degree of randomness of the adsorbate at the solid-solution interface and the reverse is the case for a negative ΔS° value [90].
Further studies will be performed using more quantities of nanocomposite-activated carbons that will be synthesized via the previously used technique in [62]. The adsorption kinetics of the adsorption mechanism of PFOS and PFOA using PAMTAg and CAMTAG will then be studied via pseudo first order and pseudo second order kinetic models.
Reusability of the nanocomposite-activated carbons is important and would be evaluated. As a follow-up to the present studies, desorption studies will be conducted in order to examine the regeneration abilities and to evaluate the reusability of the spent activated carbons (PAMTAg and CAMTAg). Several adsorption-desorption cycles will be conducted using different desorbing solvents such as 90% methanol, 50% methanol, sodium hydroxide (NaOH), hydrochloric acid (HCl) and deionized water. Regeneration of the spent nanocomposite-activated carbons will be conducted using acidified hydrogen peroxide. Previous studies by Khan and Wahab [102] reported effective regeneration of chemically modified corncobs using acidified hydrogen peroxide. Reusability studies is of essence, as it will elucidate on how many times, one can utilize a produced adsorbent and still obtain relatively good results. The rate of reusability of an adsorbent will shed light on how cost-effective an adsorbent is. This will provide a motivation for the utilization of the same adsorbent by other researchers.
Both CAMTAg and PAMTAg will be applied as adsorbents for the removal of PFAS in surface water. The aim of this proposed real-life application is to investigate the adsorption mechanism of PFAS in surface water using the nanocomposite-activated carbons; in comparison to their application in spiked ultrapure water (present study).
It is important to note that a pH range of 2–9 was tested in the present study during the test on effect of pH and the best results were obtained at pH 2. Thereafter, in order to obtain maximum feasible results, the solution pH was maintained at pH 2 for the rest of the experiments using the spiked ultrapure water. But when using CAMTAg and PAMTAg to treat surface water, the solution pH will be maintained at the pH of surface water (≈ 7.65) throughout the experiments. This is because pH 7 is believed to be suitable for industrial applications. Moderate results are still expected at pH 7 since PFAS was readily removed in the present study at pH 6 and pH 9.
More research will also be performed in order to be able to deliver enough hydraulic head when the powdered adsorbents are packed. Therefore, as part of future studies, the possibility of producing PAMTAg and CAMTAg in the form of pellets will be explored. Pellets enable easier application and handling during column adsorption studies and for industrial operations.
The results recorded in the present study have been found to be comparable to other similar studies where other agro-based adsorbents have been utilized. Their adsorption capacities are listed in Table 4.
Table 4.
Adsorption capacities for PFOA and PFOS using other agro-based adsorbents
| Adsorbent | Adsorbate | Adsorption capacity | Initial PFAS concentration | References |
|---|---|---|---|---|
| Maize straw ash | PFOS | > 1.40 mmol/g | 100 mg/L | [45] |
| Aminated rice husk |
PFOS PFOA |
2.65 mmol/g 2.49 mmol/g |
< 400 mg/L < 400 mg/L |
[57] |
| Bamboo-GAC |
PFOS PFOA |
2.32 mmol/g 1.15 mmol/g |
100 mg/L 81 mg/L |
[58] |
| Grape leaf-litter-activated carbon |
PFOS PFOA |
0.15 mmol/g 0.19 mmol/g |
0.125–1.0 mg/L 0.125–1.0 mg/L |
[56] |
| Powdered activated carbon | PFOA | 0.06 mmol/g | 25 mg/L | [29] |
| Powdered activated carbon | PFOS | 1.04 mmol/g | 20–250 mg/L | [31] |
| CAMTAg |
PFOS PFOA |
0.91 mmol/g 0.78 mmol/g |
0.01–0.1 mg/L 0.01–0.1 mg/L |
This study This study |
| PAMTAg |
PFOS PFOA |
0.09 mmol/g 0.13 mmol/g |
0.01–0.1 mg/L 0.01–0.1 mg/L |
This study This study |
Conclusions
Commercially available activated carbons have been reported as effective for treating PFAS-contaminated water systems but they are expensive [29, 31]. The present study successfully applied pre-synthesized nanocomposite-activated carbons as adsorbents for the removal of PFAS from aqueous media.
The present study investigated the removal of PFAS from aqueous media using PAMTAg and CAMTAg. It was found that the optimum pH was pH 2, though relatively high percentage removal levels were achieved at other pH values including the alkaline pH value (pH 9). This indicates that electrostatic attraction between the adsorbents (PAMTAg and CAMTAg) and anionic PFAS; cannot be the only interaction driving the adsorption mechanism of PFAS onto PAMTAg and CAMTAg. It is possibly a combination of electrostatic attraction alongside hydrophobic interaction between the adsorbate and the adsorbent.
The activation energy results (kJ/mol) and all the values obtained for ΔH° and ΔG° strongly indicate the presence of a physisorption mechanism.
It was found from the results of the tests on effect of temperature and thermodynamics that the adsorption of PFOS and PFOA using PAMTAg is endothermic, whereas, the adsorption process by CAMTAg is exothermic.
The ΔG° values obtained in the present study show that the adsorption processes of PFOS and PFOA using both PAMTAg and CAMTAg are feasible and spontaneous.
It can be inferred from the generality of the results that CAMTAg and PAMTAg adsorbed PFOS more readily than PFOA; owing to the differences in the functional groups and molecular weights of the different PFAS.
Both PAMTAg and CAMTAg can efficiently remove PFAS from aqueous solutions and are therefore suitable alternatives to non-agro based adsorbents.
Supplementary Information
(PDF 162 kb)
Data availability
Data that are closely related to the present study showing the effects of pH, adsorption dosage and initial PFAS concentration on the adsorption mechanism of PFAS using inactivated maize tassel powder have been reviewed and stored in the Mendeley Data Repository. Data are available at; https://data.mendeley.com/datasets/ykb39rn322/1
Compliance with ethical clearance
Conflict of interest
There are no competing interests to be declared by the authors.
Footnotes
Highlights
• Electrostatic attraction is involved in the adsorption of PFAS in the present study
• Hydrophobic interaction also plays a significant role in the adsorption of PFAS
• Maximum adsorption capacities were recorded using CAMTAg
• The Freundlich model suits the isotherm data better than the Langmuir model
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Takagi S, Adachi F, Miyano K, Koizumi Y, Tanaka H, Mimura M, Watanabe I, Tanabe S, Kannan K. Perfluorooctanesulfonate and perfluorooctanoate in raw and treated tap water from Osaka. Jpn Chemosphere. 2008;72:1409–1412. doi: 10.1016/j.chemosphere.2008.05.034. [DOI] [PubMed] [Google Scholar]
- 2.Idris AO, Oseghe EO, Msagati TA, Kuvarega AT, Feleni U, Mamba B. Graphitic carbon nitride: a highly electroactive nanomaterial for environmental and clinical sensing. Sensors. 2020;20(20):5743. doi: 10.3390/s20205743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rahman MF, Peldszus S, Anderson WB. Behaviour and fate of perfluoroalkyl and polyfluoroalkyl substances (PFASs) in drinking water treatment: a review. Water Res. 2014;50:318–340. doi: 10.1016/j.watres.2013.10.045. [DOI] [PubMed] [Google Scholar]
- 4.Omo-Okoro PN, Daso AP, Okonkwo JO. A review of the application of agricultural wastes as precursor materials for the adsorption of per-and polyfluoroalkyl substances: a focus on current approaches and methodologies. Environ Technol Innov. 2018;9:100–114. doi: 10.1016/j.eti.2017.11.005. [DOI] [Google Scholar]
- 5.Mudumbi J, Ntwampe S, Muganza F, Okonkwo J. Perfluorooctanoate and perfluorooctane sulfonate in South African river water. Water Sci Technol. 2014;69(1):185–194. doi: 10.2166/wst.2013.566. [DOI] [PubMed] [Google Scholar]
- 6.Appleman TD, Higgins CP, Quiñones O, Vanderford BJ, Kolstad C, Zeigler-Holady JC, Dickenson ER. Treatment of poly-and perfluoroalkyl substances in US full-scale water treatment systems. Water Res. 2014;51:246–255. doi: 10.1016/j.watres.2013.10.067. [DOI] [PubMed] [Google Scholar]
- 7.Flores C, Ventura F, Martin-Alonso J, Caixach J. Occurrence of perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) in NE Spanish surface waters and their removal in a drinking water treatment plant that combines conventional and advanced treatments in parallel lines. Sci Total Environ. 2013;461:618–626. doi: 10.1016/j.scitotenv.2013.05.026. [DOI] [PubMed] [Google Scholar]
- 8.Banzhaf S, Filipovic M, Lewis J, Sparrenbom CJ, Barthel R. A review of contamination of surface-, ground-, and drinking water in Sweden by perfluoroalkyl and polyfluoroalkyl substances (PFASs) Ambio. 2017;46:335–346. doi: 10.1007/s13280-016-0848-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Post GB, Louis JB, Lippincott RL, Procopio NA. Occurrence of perfluorinated compounds in raw water from New Jersey public drinking water systems. Environ Sci Technol. 2013;47(23):13266–13275. doi: 10.1021/es402884x. [DOI] [PubMed] [Google Scholar]
- 10.Post GB, Louis JB, Cooper KR, Boros-Russo BJ, Lippincott RL. Occurrence and potential significance of perfluorooctanoic acid (PFOA) detected in New Jersey public drinking water systems. Environ Sci Technol. 2009;43(12):4547–4554. doi: 10.1021/es900301. [DOI] [PubMed] [Google Scholar]
- 11.Benford D, De Boer J, Carere A, Di Domenico A, Johansson N, Schrenk D, et al. Opinion of the scientific panel on contaminants in the food chain on perfluorooctane sulfonate (PFOS), perfluorooctanoic acid (PFOA) and their salts. EFSA J. 2008:1–131. https://hdl.handle.net/11245/1.293305. Accessed 6 Apr 2020. [DOI] [PMC free article] [PubMed]
- 12.Joensen UN, Bossi R, Leffers H, Jensen AA, Skakkebæk NE, Jørgensen N. Do perfluoroalkyl compounds impair human semen quality? Environ Health Perspect. 2009;117:923–927. doi: 10.1289/ehp.0800517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang F, Liu C, Shih K. Adsorption behavior of perfluorooctanesulfonate (PFOS) and perfluorooctanoate (PFOA) on boehmite. Chemosphere. 2012;89:1009–1014. doi: 10.1016/j.chemosphere.2012.06.071. [DOI] [PubMed] [Google Scholar]
- 14.Du Plessis, J. Maize production. Department of Agriculture Pretoria, South Africa. 2003
- 15.Adisa OM, Botai CM, Botai JO, Hassen A, Darkey D, Tesfamariam E, Adisa AF, Adeola AM, Ncongwane KP. Analysis of agro-climatic parameters and their influence on maize production in South Africa. Theor Appl Climatol. 2018;134(3–4):991–1004. doi: 10.1007/s00704-017-2327-y. [DOI] [Google Scholar]
- 16.CEC. 2020. South African Crop Estimates Committee (CEC). www.sagis.org.za/CEC. Accessed 10 Oct 2020.
- 17.Olorundare O, Krause R, Okonkwo J, Mamba B. Potential application of activated carbon from maize tassel for the removal of heavy metals in water. Phys Chem Earth Parts A/B/C. 2012;50:104–110. doi: 10.1016/j.pce.2012.06.001. [DOI] [Google Scholar]
- 18.Zvinowanda CM, Okonkwo JO, Sekhula MM, Agyei NM, Sadiku R. Application of maize tassel for the removal of Pb, se, Sr, U and V from borehole water contaminated with mine wastewater in the presence of alkaline metals. J Hazard Mater. 2009;164:884–891. doi: 10.1016/j.jhazmat.2008.08.110. [DOI] [PubMed] [Google Scholar]
- 19.Majdalawieh A, Kanan MC, El-Kadri O, Kanan SM. Recent advances in gold and silver nanoparticles: synthesis and applications. J Nanosci Nanotechnol. 2014;14(7):4757–4780. doi: 10.1166/jnn.2014.9526. [DOI] [PubMed] [Google Scholar]
- 20.Pradeep T. Noble metal nanoparticles for water purification: a critical review. Thin Solid Films. 2009;517(24):6441–6478. doi: 10.1016/j.tsf.2009.03.195. [DOI] [Google Scholar]
- 21.Esakkimuthu T, Sivakumar D, Akila S. Application of nanoparticles in wastewater treatment. Pollut Res. 2014;33(03):567–571. [Google Scholar]
- 22.Kim B, Park CS, Murayama M, Hochella MF., Jr Discovery and characterization of silver sulfide nanoparticles in final sewage sludge products. Environ Sci Technol. 2010;44(19):7509–7514. doi: 10.1021/es101565j. [DOI] [PubMed] [Google Scholar]
- 23.Nowack B, Krug HF, Height M. 120 years of nanosilver history: implications for policy makers. ACS Publ. 2011;45(4):1177–1183. doi: 10.1021/es103316q. [DOI] [PubMed] [Google Scholar]
- 24.Olgun U, Tunç K, Hoş A. Preparation and antibacterial properties of nano biocomposite poly (ε-caprolactone)-SiO 2 films with nanosilver. J Polym Res. 2019;26(2):24. doi: 10.1007/s10965-018-1686-0. [DOI] [Google Scholar]
- 25.Amin M, Alazba A, Manzoor U. A review of removal of pollutants from water/wastewater using different types of nanomaterials. Adv Mater Sci Eng. 2014; Article ID 825910: 24pp. 10.1155/2014/825910
- 26.Reidy B, Haase A, Luch A, Dawson KA, Lynch I. Mechanisms of silver nanoparticle release, transformation and toxicity: a critical review of current knowledge and recommendations for future studies and applications. Materials. 2013;6(6):2295–2350. doi: 10.3390/ma6062295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Motshekga SC, Ray SS, Onyango MS, Momba MN. Microwave-assisted synthesis, characterization and antibacterial activity of Ag/ZnO nanoparticles supported bentonite clay. J Hazard Mater. 2013;262:439–446. doi: 10.1016/j.jhazmat.2013.08.074. [DOI] [PubMed] [Google Scholar]
- 28.Pourmand M, Shahidi K, Nazari P, Moosavian SM, Samadi N, Pourmand G, Shahverdi A. The different antibacterial impact of silver nanoparticles against legionella pneumophila compared to other microorganisms. J Sci Islam Repub Iran. 2013;24(4):313–319. [Google Scholar]
- 29.Qu Y, Zhang C, Li F, Bo X, Liu G, Zhou Q. Equilibrium and kinetics study on the adsorption of perfluorooctanoic acid from aqueous solution onto powdered activated carbon. J Hazard Mater. 2009;169:146–152. doi: 10.1016/j.jhazmat.2009.03.063. [DOI] [PubMed] [Google Scholar]
- 30.BhatnagarA VVJ, Botelho CM, Boaventura RA. Coconut-based biosorbents for water treatment—a review of the recent literature. Adv. Colloid Interface Sci. 2010;160(1):1–15. doi: 10.1016/j.cis.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 31.Yu Q, Zhang R, Deng S, Huang J, Yu G. Sorption of perfluorooctane sulfonate and perfluorooctanoate on activated carbons and resin: kinetic and isotherm study. Water Res. 2009;43:1150–1158. doi: 10.1016/j.watres.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 32.Kucharzyk KH, Darlington R, Benotti M, Deeb R, Hawley E. Novel treatment technologies for PFAS compounds: a critical review. J Environ Manag. 2017;204:757–764. doi: 10.1016/j.jenvman.2017.08.016. [DOI] [PubMed] [Google Scholar]
- 33.Pal J, Deb MK, Deshmukh DK, Verma D. Removal of methyl orange by activated carbon modified by silver nanoparticles. Appl Water Sci. 2013;3(2):367–374. doi: 10.1007/s13201-013-0087-0. [DOI] [Google Scholar]
- 34.Goldstein N, Greenlee LF. Influence of synthesis parameters on iron nanoparticle size and zeta potential. J Nanopart Res. 2012;14(4):760. doi: 10.1007/s11051-012-0760-5. [DOI] [Google Scholar]
- 35.Igwegbe C, Rahdar S, Rahdar A, Mahvi A, Ahmadi S, Banach A. Removal of fluoride from aqueous solution by Nikel oxide nanoparticles: equilibrium and kinetic studies. Fluoride. 2019;52(4):569–579. [Google Scholar]
- 36.Ahmadi S, Igwegbe CA. Removal of methylene blue on zinc oxide nanoparticles: Nonlinear and linear adsorption isotherms and kinetics study. Sigma: J Eng Nat Sci/Mühendislik ve Fen Bilimleri Derg. 2020; 38(1).
- 37.Igwegbe CA, Onukwuli OD, Ighalo JO, Okoye PU. Adsorption of cationic dyes on Dacryodes edulis seeds activated carbon modified using phosphoric acid and sodium chloride. Environ Process. 2020;7:1–21. doi: 10.1007/s40710-020-00467-y. [DOI] [Google Scholar]
- 38.Qu X, Alvarez PJ, Li Q. Applications of nanotechnology in water and wastewater treatment. Water Res. 2013;47(12):3931–3946. doi: 10.1016/j.watres.2012.09.058. [DOI] [PubMed] [Google Scholar]
- 39.Zhang W, Zhang D, Liang Y. Nanotechnology in remediation of water contaminated by poly-and perfluoroalkyl substances: a review. Environ Pollut. 2019;247:266–276. doi: 10.1016/j.envpol.2019.01.045. [DOI] [PubMed] [Google Scholar]
- 40.Srivastava V, Zare EN, Makvandi P, Zheng XQ, Iftekhar S, Wu A, Padil VV, Mokhtari B, Varma RS, Tay FR. Cytotoxic aquatic pollutants and their removal by nanocomposite-based sorbents. Chemosphere. 2020; 127324. 10.1016/j.chemosphere.2020.127324 [DOI] [PubMed]
- 41.Pan B, Xing B. Adsorption mechanisms of organic chemicals on carbon nanotubes. Environ Sci Technol. 2008;42(24):9005–9013. doi: 10.1021/es801777n. [DOI] [PubMed] [Google Scholar]
- 42.Tuan TQ, Van Son N, Dung HT, Luong NH, Thuy BT, Van Anh N, Hoa ND, Hai NH. Preparation and properties of silver nanoparticles loaded in activated carbon for biological and environmental applications. J Hazard Mater. 2011;192(3):1321–1329. doi: 10.1016/j.jhazmat.2011.06.044. [DOI] [PubMed] [Google Scholar]
- 43.Ghaedi M, Sadeghian B, Pebdani AA, Sahraei R, Daneshfar A, Duran C. Kinetics, thermodynamics and equilibrium evaluation of direct yellow 12 removal by adsorption onto silver nanoparticles loaded activated carbon. Chem Eng J. 2012;187:133–141. doi: 10.1016/j.cej.2012.01.111. [DOI] [Google Scholar]
- 44.Ochoa-Herrera V, Sierra-Alvarez R. Removal of perfluorinated surfactants by sorption onto granular activated carbon, zeolite and sludge. Chemosphere. 2008;72(10):1588–1593. doi: 10.1016/j.chemosphere.2008.04.029. [DOI] [PubMed] [Google Scholar]
- 45.Chen X, Xia X, Wang X, Qiao J, Chen H. A comparative study on sorption of perfluorooctane sulfonate (PFOS) by chars, ash and carbon nanotubes. Chemosphere. 2011;83:1313–1319. doi: 10.1016/j.chemosphere.2011.04.018. [DOI] [PubMed] [Google Scholar]
- 46.Oyetade OA, Varadwaj GBB, Nyamori VO, Jonnalagadda SB, Martincigh BS. A critical review of the occurrence of perfluoroalkyl acids in aqueous environments and their removal by adsorption onto carbon nanotubes. Rev Environ Sci Biotechnol. 2018;17(4):603–635. doi: 10.1007/s11157-018-9479-9. [DOI] [Google Scholar]
- 47.Fisal A, Daud WMAW, Ahmad MA, Radzi R. Using cocoa (Theobroma cacao) shell-based activated carbon to remove 4-nitrophenol from aqueous solution: kinetics and equilibrium studies. Chem Eng J. 2011;178:461–467. doi: 10.1016/j.cej.2011.10.044. [DOI] [Google Scholar]
- 48.Akl MA, Dawy MB, Serage AA. Efficient removal of phenol from water samples using sugarcane bagasse based activated carbon. J Anal Bioanal Tech. 2014;5:2. doi: 10.4172/2155-9872.1000189. [DOI] [Google Scholar]
- 49.Fernandez ME, Nunell GV, Bonelli PR, Cukierman AL. Activated carbon developed from orange peels: batch and dynamic competitive adsorption of basic dyes. Ind Crop Prod. 2014;62:437–445. doi: 10.1016/j.indcrop.2014.09.015. [DOI] [Google Scholar]
- 50.Chang KL, Hsieh JF, Ou BM, Chang MH, Hseih WY, Lin JH. Adsorption studies on the removal of an endocrine-disrupting compound (Bisphenol a) using activated carbon from rice straw agricultural waste. Sep Sci Technol. 2012;47(10):1514–1521. doi: 10.1080/01496395.2011.647212. [DOI] [Google Scholar]
- 51.Malik PK. Use of activated carbons prepared from sawdust and rice-husk for adsorption of acid dyes: a case study of acid yellow 36. Dyes Pigments. 2003;56(3):239–249. doi: 10.1016/S0143-7208(02)00159-6. [DOI] [Google Scholar]
- 52.Zhong ZY, Yang Q, Li XM, Luo K, Liu Y, Zeng GM. Preparation of peanut hull-based activated carbon by microwave-induced phosphoric acid activation and its application in Remazol brilliant blue R adsorption. Ind Crop Prod. 2012;37(1):178–185. doi: 10.1016/j.indcrop.2011.12.015. [DOI] [Google Scholar]
- 53.Nwadiogbu J, Ajiwe V, Okoye P. Removal of crude oil from aqueous medium by sorption on hydrophobic corncobs: Equilibrium and kinetic studies. J Taibah Univ Sci. 2016;10(1):56–63. 10.1016/j.jtusci.2015.03.014.
- 54.Sud D, Mahajan G, Kaur M. Agricultural waste material as potential adsorbent for sequestering heavy metal ions from aqueous solutions–a review. Bioresour Technol. 2008;99(14):6017–27. 10.1016/j.biortech.2007.11.064. [DOI] [PubMed]
- 55.Wahi R, Chuah LA, Choong TS, Ngaini Z, Nourouzi MM. Oil removal from aqueous state by natural fibrous sorbent: an overview. Sep Purif Technol. 2013;113:51–63. 10.1016/j.seppur.2013.04.015.
- 56.Fagbayigbo BO, Opeolu BO, Fatoki OS, Akenga TA, Olatunji OS. Removal of PFOA and PFOS from aqueous solutions using activated carbon produced from Vitis vinifera leaf litter. Environ Sci Pollut Res. 2017;24:13107–13120. doi: 10.1007/s11356-017-8912-x. [DOI] [PubMed] [Google Scholar]
- 57.Deng S, Niu L, Bei Y, Wang B, Huang J, Yu G. Adsorption of perfluorinated compounds on aminated rice husk prepared by atom transfer radical polymerization. Chemosphere. 2013;91:124–130. doi: 10.1016/j.chemosphere.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 58.Deng S, Nie Y, Du Z, Huang Q, Meng P, Wang B, et al. Enhanced adsorption of perfluorooctane sulfonate and perfluorooctanoate by bamboo-derived granular activated carbon. J Hazard Mater. 2015;282:150–157. doi: 10.1016/j.jhazmat.2014.03.045. [DOI] [PubMed] [Google Scholar]
- 59.Liu L, Liu Y, Gao B, Ji R, Li C, Wang S. Removal of perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS) from water by carbonaceous nanomaterials: a review. Crit Rev Environ Sci Technol. 2020;50(22):2379–2414. doi: 10.1080/10643389.2019.1700751. [DOI] [Google Scholar]
- 60.Basta AH, Lotfy VF, Hasanin MS, Trens P, El-Saied H. Efficient treatment of rice byproducts for preparing high-performance activated carbons. J Clean Prod. 2019;207:284–295. doi: 10.1016/j.jclepro.2018.09.216. [DOI] [Google Scholar]
- 61.Mohan AN, Manoj B, Panicker S. Facile synthesis of graphene-tin oxide nanocomposite derived from agricultural waste for enhanced antibacterial activity against Pseudomonas aeruginosa. Sci Rep. 2019;9(1):1–12. doi: 10.1038/s41598-019-40916-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Omo-Okoro PN, Maepa CE, Daso AP, Okonkwo JO. Microwave-assisted synthesis and characterization of an agriculturally derived silver nanocomposite and its derivatives. Waste Biomass Valorization. 2020;11:2247–2259. doi: 10.1007/s12649-018-0523-3. [DOI] [Google Scholar]
- 63.Omo-Okoro PN, Curtis CJ, Karásková P, Melymuk L, Oyewo OA, Okonkwo JO. Kinetics, isotherm, and thermodynamic studies of the adsorption mechanism of PFOS and PFOA using inactivated and chemically activated maize tassel. Water Air Soil Pollut. 2020;231(9):1–21. doi: 10.1007/s11270-020-04852-z. [DOI] [Google Scholar]
- 64.Exner M, Färber H. Perfluorinated surfactants in surface and drinking waters. Environ Sci Pollut Res. 2006;13:299–307. doi: 10.1065/espr2006.07.326. [DOI] [PubMed] [Google Scholar]
- 65.Rumsby PC, Mclaughlin CL, Hall T. Perfluorooctane sulphonate and perfluorooctanoic acid in drinking and environmental waters. Philos Trans R Soc A Math Phys Eng Sci. 2009;367:4119–4136. doi: 10.1098/rsta.2009.0109. [DOI] [PubMed] [Google Scholar]
- 66.Djerahov L, Vasileva P, Karadjova I, Kurakalva RM, Aradhi KK. Chitosan film loaded with silver nanoparticles—sorbent for solid phase extraction of Al (III), cd (II), cu (II), co (II), Fe (III), Ni (II), Pb (II) and Zn (II) Carbohydr Polym. 2016;147:45–52. doi: 10.1016/j.carbpol.2016.03.080. [DOI] [PubMed] [Google Scholar]
- 67.Regiel A, Irusta S, Kyzioł A, Arruebo M, Santamaria J. Preparation and characterization of chitosan–silver nanocomposite films and their antibacterial activity against Staphylococcus aureus. Nanotechnology. 2012;24(1):015101. doi: 10.1088/0957-4484/24/1/015101. [DOI] [PubMed] [Google Scholar]
- 68.Ahmad MB, Tay MY, Shameli K, Hussein MZ, Lim JJ. Green synthesis and characterization of silver/chitosan/polyethylene glycol nanocomposites without any reducing agent. Int J Mol Sci. 2011;12(8):4872–4884. doi: 10.3390/ijms12084872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang Y, Gao X, Zhi L, Liu X, Jiang W, Sun Y, Yang J. The synergetic antibacterial activity of Ag islands on ZnO (Ag/ZnO) heterostructure nanoparticles and its mode of action. J Inorg Biochem. 2014;130:74–83. doi: 10.1016/j.jinorgbio.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 70.Londoño-Restrepo SM, Jeronimo-Cruz R, Millán-Malo BM, Rivera-Muñoz EM, Rodriguez-García ME. Effect of the nano crystal size on the X-ray diffraction patterns of biogenic hydroxyapatite from human, bovine, and porcine bones. Sci Rep. 2019;9(1):1–12. doi: 10.1038/s41598-019-42269-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Holder CF, Schaak RE. Tutorial on powder X-ray diffraction for characterizing nanoscale materials. ACS Publ. 2019;13:7359–7365. doi: 10.1021/acsnano.9b05157. [DOI] [PubMed] [Google Scholar]
- 72.Buso D, Pacifico J, Martucci A, Mulvaney P. Gold-nanoparticle-doped TiO2 semiconductor thin films: optical characterization. Adv Funct Mater. 2007;17(3):347–354. doi: 10.1002/adfm.200600349. [DOI] [Google Scholar]
- 73.Ruggiero I, Terracciano M, Martucci NM, De Stefano L, Migliaccio N, Tatè R, Rendina I, Arcari P, Lamberti A, Rea I. Diatomite silica nanoparticles for drug delivery. Nanoscale Res Lett. 2014;9(1):1–7. doi: 10.1186/1556-276X-9-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Abreu AS, Oliveira M, de Sá A, Rodrigues RM, Cerqueira MA, Vicente AA, Machado A. Antimicrobial nanostructured starch based films for packaging. Carbohydr Polym. 2015;129:127–134. doi: 10.1016/j.carbpol.2015.04.021. [DOI] [PubMed] [Google Scholar]
- 75.Li SM, Jia N, Ma MG, Zhang Z, Liu QH, Sun RC. Cellulose–silver nanocomposites: microwave-assisted synthesis, characterization, their thermal stability, and antimicrobial property. Carbohydr Polym. 2011;86(2):441–447. doi: 10.1016/j.carbpol.2011.04.060. [DOI] [Google Scholar]
- 76.Oyewo O, Onyango M, Wolkersdorfer C. Lanthanides removal from mine water using banana peels nanosorbent. Int J Environ Sci Technol. 2018;15:1265–1274. doi: 10.1007/s13762-017-1494-9. [DOI] [Google Scholar]
- 77.Zvinowanda C, Okonkwo J, Shabalala P, Agyei N. A novel adsorbent for heavy metal remediation in aqueous environments. Int J Environ Sci Technol. 2009;6:425–434. doi: 10.1007/BF03326081. [DOI] [Google Scholar]
- 78.Guo J, Lua AC. Characterization of adsorbent prepared from oil-palm shell by CO2 activation for removal of gaseous pollutants. Mater Lett. 2002;55:334–339. doi: 10.1016/S0167-577X(02)00388-9. [DOI] [Google Scholar]
- 79.Thommes M, Kaneko K, Neimark AV, Olivier JP, Rodriguez-Reinoso F, Rouquerol J, Sing KSW. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC technical report) Pure Appl Chem. 2015;87(9–10):1051–1069. doi: 10.1515/pac-2014-1117. [DOI] [Google Scholar]
- 80.Baccar R, Sarrà M, Bouzid J, Feki M, Blánquez P. Removal of pharmaceutical compounds by activated carbon prepared from agricultural by-product. Chem Eng J. 2012;211:310–317. doi: 10.1016/j.cej.2012.09.099. [DOI] [Google Scholar]
- 81.Omo-Okoro PN, Daso AP, Okonkwo JO. Per- and Polyfluoroalkyl substances: ubiquity, levels, toxicity and their removal from aqueous media using novel agro-based adsorbents. Organohalogen Compd. 2018b;80:309–12 http://dioxin20xx.org/organohalogen-compounds-database-search/. Accessed 10 Mar 2020.
- 82.Wang N, Liu J, Buck RC, Korzeniowski SH, Wolstenholme BW, Folsom PW, Sulecki LM. 6: 2 Fluorotelomer sulfonate aerobic biotransformation in activated sludge of waste water treatment plants. Chemosphere. 2011;82:853–858. doi: 10.1016/j.chemosphere.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 83.Guo W, Huo S, Feng J, Lu X. Adsorption of perfluorooctane sulfonate (PFOS) on corn straw-derived biochar prepared at different pyrolytic temperatures. J Taiwan Inst Chem Eng. 2017;78:265–271. doi: 10.1016/j.jtice.2017.06.013. [DOI] [Google Scholar]
- 84.Kumar N, Mittal H, Parashar V, Ray SS, Ngila JC. Efficient removal of rhodamine 6G dye from aqueous solution using nickel sulphide incorporated polyacrylamide grafted gum karaya bionanocomposite hydrogel. RSC Adv. 2016;6:21929–21939. doi: 10.1039/C5RA24299A. [DOI] [Google Scholar]
- 85.Oyewo OA, Onyango MS, Wolkersdorfer C. Application of banana peels nanosorbent for the removal of radioactive minerals from real mine water. J Environ Radioact. 2016;164:369–376. doi: 10.1016/j.jenvrad.2016.08.014. [DOI] [PubMed] [Google Scholar]
- 86.Mekonnen E, Yitbarek M, Soreta TR. Kinetic and thermodynamic studies of the adsorption of Cr (VI) onto some selected local adsorbents. S Afr J Chem. 2015;68:45–52. doi: 10.17159/0379-4350/2015/v68a7. [DOI] [Google Scholar]
- 87.Piccin J, Dotto G, Pinto L. Adsorption isotherms and thermochemical data of FD&C red n 40 binding by chitosan. Braz J Chem Eng. 2011;28:295–304. doi: 10.1590/S0104-66322011000200014. [DOI] [Google Scholar]
- 88.Al-Anber MA. Thermodynamics approach in the adsorption of heavy metals. Thermodynamics-Interaction Studies-Solids, Liquids and Gases. InTech; 2011. 30 pp.
- 89.Dada A, Olalekan A, Olatunya A, Dada O. Langmuir, Freundlich, Temkin and Dubinin–Radushkevich isotherms studies of equilibrium sorption of Zn2+ unto phosphoric acid modified rice husk. IOSR J Appl Chem. 2012;3(1):38–45. doi: 10.9790/5736-0313845. [DOI] [Google Scholar]
- 90.He J, Hong S, Zhang L, Gan F, Ho Y. Equilibrium and thermodynamic parameters of adsorption of methylene blue onto rectorite. Fresenius Environ Bull. 2010;19:2651–2656. [Google Scholar]
- 91.Sharma PK, Ayub S, Tripathi CN. Isotherms describing physical adsorption of Cr (VI) from aqueous solution using various agricultural wastes as adsorbents. Cogent Eng. 2016;3(1):1186857. doi: 10.1080/23311916.2016.1186857. [DOI] [Google Scholar]
- 92.Aziz HA, Yusoff MS, Adlan MN, Adnan NH, Alias S. Physico-chemical removal of iron from semi-aerobic landfill leachate by limestone filter. Waste Manag. 2004;24:353–358. doi: 10.1016/j.wasman.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 93.Ismail M, Weng CN, Rahman HA, Zakaria NA. Freundlich isotherm equilibrium equations in determining effectiveness of a low cost absorbent to heavy metal removal in wastewater (leachate) at Teluk Kitang landfill, Pengkalan Chepa, Kelantan, Malaysia. J Geogr Earth Sci. 2013;1:1–8. [Google Scholar]
- 94.Arsénio de Sá AS, Moura I, Machado AV. Polymeric materials for metal sorption from hydric resources, In Water Purification, Academic Press. 2017; 289–322. 10.1016/B978-0-12-804300-4.00008-3
- 95.Equilibria. Adsorption Equilbrium Principles [Online]. Marmara University, Turkey. 2017, 29 pp. available: http://mimoza.marmara.edu.tr/~zehra.can/ENVE401/3.%20Adsorption%20Equilibria.pdf. Accessed 6 Dec 2019.
- 96.Kumar U, Bandyopadhyay M. Sorption of cadmium from aqueous solution using pretreated rice husk. Bioresour Technol. 2006;97:104–109. doi: 10.1016/j.biortech.2005.02.027. [DOI] [PubMed] [Google Scholar]
- 97.Bansal G. What does coefficient of determination explain? (In terms of variation). University of Wisconsin. 2020. https://blog.uwgb.edu/bansalg/statistics-data-analytics/linear-regression/what-does-coefficient-of-determination-explain-in-terms-of-variation/ Accessed 5 Feb 2020.
- 98.Ratner B. The correlation coefficient: its values range between +1/−1, or do they? J Target Meas Anal Mark. 2009;17:139–142. doi: 10.1057/jt.2009.5. [DOI] [Google Scholar]
- 99.Ahmad MA, Puad NA, Bello OS. Kinetic, equilibrium and thermodynamic studies of synthetic dye removal using pomegranate peel activated carbon prepared by microwave-induced KOH activation. Water Resour Ind. 2014;6:18–35. doi: 10.1016/j.wri.2014.06.002. [DOI] [Google Scholar]
- 100.Adane B, Siraj K, Meka N. Kinetic, equilibrium and thermodynamic study of 2-chlorophenol adsorption onto Ricinus communis pericarp activated carbon from aqueous solutions. Green Chem Lett Rev. 2015;8:1–12. doi: 10.1080/17518253.2015.1065348. [DOI] [Google Scholar]
- 101.Hamad BK, Noor AM, Rahim AA. Removal of 4-chloro-2-methoxyphenol from aqueous solution by adsorption to oil palm shell activated carbon activated with K2CO3. J Phys Sci. 2011;22:39–55. doi: 10.1016/j.jaubas.2015.09.001. [DOI] [Google Scholar]
- 102.Khan MN, Wahab MF. Characterization of chemically modified corncobs and its application in the removal of metal ions from aqueous solution. J Hazard Mater. 2007;141(1):237–244. doi: 10.1016/j.jhazmat.2006.06.119. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 162 kb)
Data Availability Statement
Data that are closely related to the present study showing the effects of pH, adsorption dosage and initial PFAS concentration on the adsorption mechanism of PFAS using inactivated maize tassel powder have been reviewed and stored in the Mendeley Data Repository. Data are available at; https://data.mendeley.com/datasets/ykb39rn322/1