Abstract
Purpose
Dyes are highly toxic coloured compounds in nature that are largely applied in paper, food, textile and printing industries. Here, the adsorption technique was performed to remove methyl orange (MO) dye from water by polyethylene glycol (PEG) modified iron oxide nanoparticles (Fe3O4 NPs).
Methods
The method used for Fe3O4 NPs synthesis was chemical precipitation. The particles were analyzed by transmission electron microscope, magnetometer, BET analyzer, fourier-transform infrared spectroscopy, X-ray powder diffraction, zetasizer and particle size analyzer. The influence of pH (4.0 to 10.0), NaCl concentration (0.01 mM to 2 M), adsorbent dosage (1 to 10 mg), and the role of surface charge on adsorptive removal were investigated.
Results
The NPs size, zeta potential and surface area was found to be 26 ± 1.26 nm, 33.12 ± 1.01 mV and 119 m2/g respectively. The adsorption of MO on Fe3O4 NPs agreed best to Freundlich model (R2 = 0.965) when compared with Langmuir model (R2 = 0.249). By comparing pseudo-first-order kinetic model (R2 = 0.937), kinetic adsorption study was better followed by pseudo-second-order kinetic model (R2 = 1). The adsorption rate decreased with increasing NaCl concentration. At pH 4, maximum adsorption was noted. The particles were also exhibited excellent antibacterial and antibiofilm activities. The ROS formation, lipid peroxidation and oxidative stress were increased with increase in NPs concentration. The NPs precoated slides exhibited more than 50% growth inhibition.
Conclusion
The investigation denotes the versatile applications of the prepared particles for removing the dye stuffs from industrial effluents and as antibacterial and antibiofilm agent.
Keywords: Iron oxide particles, Methyl orange dye, Adsorption, Antimicrobial activity
Introduction
The huge amounts of liquid effluents are discharged from industries due to dying process that uses immeasurable amount of water [1]. The commercially used dyes in textile industries are azo dyes due to its availability and cost effectiveness [2]. Its complex chemical structure and higher stability, makes it biologically nondegradable [3]. The ideal compound of azo dye is methyl orange (MO). The textile industries consume azo dyes that account around 60–80% [4]. The methods used for the removal of MO are biological, physical and chemical [5, 6].
The dyes cannot be removed efficiently using chemical and biological methods. Some cases the product degraded may show higher toxic than initial product. Adsorption is a common physical method used to treat dye bearing effluents and currently various materials are used for the adsorption process [7, 8]. Adsorption technique has been recognized due to its easy operability, accessibility of adsorbents and low cost [9]. Solid materials are largely used for the adsorption process [10, 11]. There were several limitations associated with these materials including reusability and adsorbent cost has been overcome by the development of nanoparticles based adsorbent. The particle posses large surface area due to its smaller in size [12]. The biosorption, effluent water treatment and bio application of nano-sized Fe3O4 has great application in various types of adsorbents [13]. There were few reports on the application of polymer stabilized iron oxide NPs to remove dyes from the effluents [14, 15]. The aggregation of NPs occurs due to poor stabilizer and the increased production cost made them not suitable for effluent treatment. In order to overcome this drawback, the present study used polyethylene glycol (PEG) as stabilizer for magnetite NPs.
PEG is a biocompatible polymer that widely applied to increase NPs stability. PEG possesses unique characters like non-toxicity, water-solubility, non-antigenic nature, flexibility, biocompatibility, and hydrophilicity that make it a promising candidate for various environmental and biological applications. Comparing with bare counterpart, high chemical stability and dispersible nature was exhibited by PEG-coated NPs [16, 17]. The super paramagnetic property was exhibited by iron oxide NPs when attracted by magnetic field. After the magnetic field removal, the NPs magnetism were not retained. This magnetic property of Fe3O4 NPs makes it suitable for the purpose of pollutants removal when compared with nonmagnetic NPs [18].
This report deals about the adsorptive removal of MO from aqueous samples with the help of magnetite NPs. The conditions including pH, NaCl concentration and NPs dosage on adsorption were also optimized. The role of surface charge of PEG-modified magnetite NPs on enhancing dye removal was reported. The antibacterial property and anti-biofilm activity of this particle were also studied against Escherichia coli ATCC 25922, Micrococcus luteus (Clinical isolate) and Staphylococcus aureus ATCC 25923. The possible mechanism of action on antibacterial properties was elucidated by monitoring ROS generation, lipid peroxidation and oxidative stress. Also, toxic effect of magnetite NPs on bacterial biofilm was also evaluated. The study aims to project the versatile applications of magnetite NPs and a detailed study on related parameters were carried out. The present study concentrated on the application of magnetite NPs for environmental cleanup, antibacterial and antibiofilm agents.
Materials and method
Materials
The bacterial cultures used in the study were E. coli ATCC 25922, M. luteus (Clinical isolate) and S. aureus ATCC 25923. Ammonia, PEG (density – 1.125), MO, FeCl3.6H2O and FeCl2.4H2O were obtained from Merck (Germany). The procured chemicals are of analytical grade and used without further purification.
Iron oxide NPs synthesis
The Fe3O4 NPs were prepared based on chemical co-precipitation technique. FeCl3 and FeCl2 solutions were separately dissolved in distilled water (100 mL). The above solutions in molar ratio of 2:1 were taken and mixed and it is kept in stirrer for 1 h at 80 °C. Then the ammonia solution was added after adjusting the temperature to 90 °C followed by addition of PEG solution (0.3 M) and continued stirring for another 1 h. The precipitate was washed twice before lyophilization.
Characterization of nanoparticles
High resolution transmission electron microscopy (HRTEM) was used to study morphology of prepared NPs using JEOL JEM 2100 (Japan). Elemental analysis was performed with energy-dispersive X-ray spectroscopy (EDS) coupled with TEM. The X-ray powder diffraction (XRD) was analyzed using Bruker diffractometer (D8 Focus). Malvern zetasizer and particle size analyzer (Malvern, UK) were used to measure zeta potential and hydrodynamic size distribution of NPs respectively. Thermo Fisher Scientific Nicolet IS50 machine was used to perform fourier-transformed infrared spectroscopy (FTIR). BET surface area analyzer (Smart Instruments Co. Pvt. Ltd., Mumbai, India) was used to record surface area of NPs. The magnetic behavior of prepared particles was characterized via a vibrating sample magnetometer (VSM, Quantum Design, PAR 155, USA) at room temperature.
Adsorption studies
Different MO concentrations (0–250 mg/L) were prepared and exposed with NPs (5 mg) under shaking for 4 h at pH 7. There were two isothermal models used to demonstrate the equilibrium adsorption of the MO on magnetite NPs. The monolayer adsorption is indicated by Langmuir’s isotherm and multilayer of adsorption of dyes is considered by Freundlich’s model. Equilibrium adsorption was calculated with the help of below equation
| 1 |
where Ce (mg/L), C0 (mg/L) are the quantity of MO left out after adsorption and the concentration of initial dye, W (mg) is NPs weight used for adsorption, V(L) is the volume and qe is the quantity of MO adsorbed at equilibrium.
Influence of pH
Here, solution pH was adjusted from 4.0 to 10.0 to find the influence of pH on MO adsorption on magnetite NPs [19, 20]. For this purpose, 0.1 N of HCL and NaOH were used. Briefly, MO (100 mg/L) and 5 mg of magnetite NPs were kept for interaction (4 h) in orbital shaker (150 rpm). Then, the supernatant of the solution was collected by centrifugation and A465 was recorded to find the quantity of MO left in supernatant.
Influence of NaCl concentration
Here, 0.01 mM to 2 M NaCl concentrations were augmented to MO dye (100 mg/L) solution with NPs (5 mg). After the interaction (4 h), the supernatant was taken and A465 was recorded.
Influence of adsorbent dosage
Here, different amount of NPs from 1 to 10 mg were interacted with MO dye (100 mg/L) solution. Thereafter, the supernatant was collected by centrifugation and the quantity of MO was calculated by taking absorbance at 465 nm.
Adsorption kinetics
Here, Fe3O4 NPs (5 mg) and MO dye (250 mg/L) was interacted for 96 h. At different interval of time, an aliquot of the samples was collected, centrifuged and A465 was recorded. The following equation was used to calculate the adsorption amount at time t [21],
| 2 |
where Ct (mg/L) is MO quantity left at time t, C0 (mg/L) is the concentration of initial dye, W (mg) is NPs weight used for adsorption, V (L) is the volume of solution and at equilibrium qt is the MO quantity adsorbed at time t.
Antimicrobial study
The particles were interacted with E. coli, M. luteus and S. aureus to understand the antibacterial property of magnetite NPs. The overnight cultures were adjusted the cell number to 1 × 108 CFU/mL and the particles (0.1–100 μg/mL) were interacted with bacterial cultures separately for 4 h. Thereafter, plate counting technique was performed. The number of colony formed in the plates was equal to the no live cells remains in the solution.
Lipid peroxidation
Lipid peroxidation of bacterial cells exposed to magnetite NPs of different concentration 0.1, 1, 10 and 100 mg/L was estimated by thiobarbituric acid assay proposed by Bar-Or et al. [22] with minor variations. Here, CuCl2 (0.01 mM) in phosphate buffer (20 mM) solution of pH 7.4 was prepared mixed with the bacterial cells exposed to NPs and kept incubation (15 min). Afterward, to the sample mixture, 0.5% thiobarbituric acid (TBA) solution in 10% trichloroacetic acid (TCA) was augmented, heated (100 °C, 15 min) and cooled immediately by using ice bath. Then, the supernatant was collected after centrifuging the solution at 10,000×g (10 min). To quantify the presence of TBA-reactive species (TBARS) in the supernatant, A532 was recorded. The TBARS formed were compared with control. Control was maintained as bacterial cells without exposed to NPs.
ROS production
ROS formations in the cells are considered as the major reason for oxidative stress. The cellular ROS formation was estimated by using 2,7-dichlorodihydrofluorescein diacetate acetyl ester (H2DCF-DA). Here, the deacetylation of this membrane permeable probe to from 2,7-dichlorodihydrofluorescein (H2DCF), by cellular esterase activity. H2DCF was oxidized to 2,7-dichlorofluorescein (DCF), a fluorescent product, in ROS presence. The bacterial cells exposed to NPs were washed with phosphate buffer saline (PBS), and it was added with 80 μM of H2DCF-DA and the solution was incubated (37 °C) for 30 min. The samples fluorescence intensity was measured at 480 nm excitation and emission recorded at 530 nm (Elico, Inida). The bacterial cells without NPs interaction was taken as control.
Enzyme assay
Cellular oxidative status was monitored by measuring the activity of glutathione reductase, catalase and superoxide dismutase (SOD). The increase in reduction of nitro blue tetrazolium (NBT) by superoxide indicates the insufficient activity of superoxide dismutase enzyme to inhibit the superoxide. The catalase activity was performed by monitoring the disappearance of H2O2 using spectrophotometer. The glutathione reductase assay kit available commercially (Signal, USA) was used to detect the glutathione reductase enzyme activity. The assay buffer (350 μL) contains cell lysate (0.1 mL) and 2 mM GSSG (500 μL). Then, 50 μL of NADPH (2 mM) was added and the absorbance was taken for 2 min at 340 nm. Moron and Kepeierre [23] described the method to measure reduced glutathione (GSH) content. The formation of 5-thio (2-nitrobenzoic acid), a yellow by-product from GSH oxidation by 5,5-dithiobis (2-nitrobenzoic acid) (DTNB) which shows absorption peak at 412 nm.
Effect of NPs on preformed biofilm
The antibiofilm activity was performed against E. coli, M. luteus and S. aureus biofilm. In order to prepare bacterial biofilm, 2 mL of culture containing 1 × 108 CFU/mL was evenly spread on sterile and clean glass slide surface of 2.5 cm × 7.5 cm and incubated (37 °C, 24 h). Thereafter, the excess non-adherent cells and media were washed out twice with PBS. Then, the slides were interacted with various concentrations of magnetite NPs (0.1–100 μg/mL) and continue incubation (37 °C, 24 h). The toxicity was evaluated by crystal violet assay. Briefly, 0.5% crystal violet solution was poured on glass slides and allowed for 5 min. The dye was extracted using glacial acetic acid (30%) and absorbance was measured at 590 nm. The control biofilm was compared for calculating the toxicity.
Effect of pre-coated particles on biofilm formation
Here, the cultures were allowed to form biofilm on clean and neat glass slides which were previously coated with magnetite NPs. The coating of NPs on glass slides was performed by spin coating method using HOLMARC SPINCOATER (H0TH06). Then the toxicity was calculated as per the method discusses above.
Results and discussion
Characterization of NPs
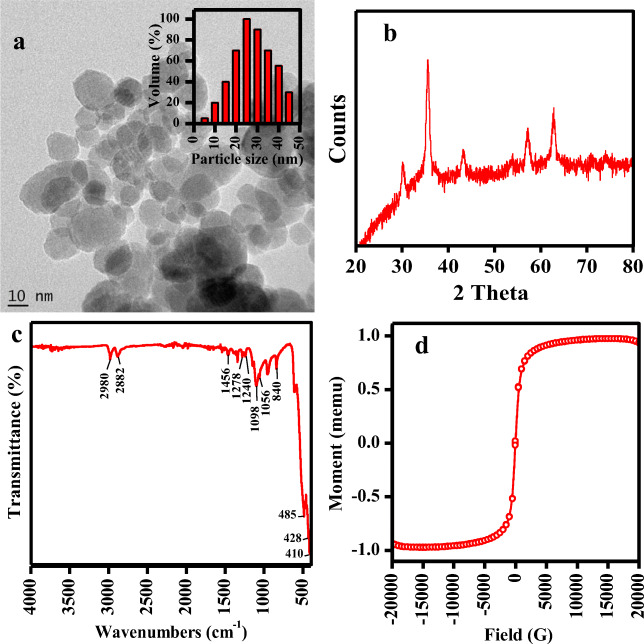
The morphology of magnetite NPs were analyzed by transmission electron microscope and the polydispersity of the particles can be seen in the image (Fig. 1a). The particle size distribution of PEG-Fe3O4 NPs is shown in Fig. 1a inset. The zeta potential and effective diameter were determined to be 33.12 ± 1.01 mV and 26 ± 1.26 nm respectively. EDS pattern confirmed the presence of Fe and O, represent the formation of Fe3O4. The Fe3O4 NPs showed the specific surface area of 119 m2/g which was determined by BET method. Jeong et al. [24] synthesized hydrogenated iron oxide NPs using hydrazine with underwater plasma discharge technique, which possessed the surface area of 85.07 m2/g. Bidast et al. [25] prepared different types of iron oxide NPs (goethite, hematite, and magnetite) by chemical method and the surface area of the samples was 54, 39 and 41 m2/g respectively. The reported NPs possessed high surface area compared to the above literature.
Fig. 1.
Characterization of Fe3O4 NPs: a TEM (inset – particle size distribution), b XRD, c FTIR and d VSM of NPs
The XRD pattern of synthesized Fe3O NPs is depicted in Fig. 1b. The peak positions at 2θ value of 30.14°, 35.59°, 43.25°, 56.23° and 62.83° were observed. The patterns observed agreed well with standard magnetite (Fe3O4) XRD patterns.
The capping of PEG is verified by FT-IR spectra (Fig. 1c). The IR bands at 410 to 485 cm−1 can be assigned to Fe-O-Fe bond [26]. Further peaks in region of 2882–2980 cm−1 assigned to C-H vibrations, 1340 and 1456 cm−1 denotes CH2 vibrations, 1146 and 1278 cm−1 indicates to C-O-C bending and stretching vibrations, 840 cm−1 assigned to C-H wagging, 1098 cm−1 indicate C-C vibration and 1056 cm−1 denoted C-O-H stretching vibration [27, 28].
Magnetite NPs are well known to exhibit magnetic property that can be estimated using VSM (Fig. 1d). The NPs magnetic saturation was attained in 97.44 emu g−1. Further super paramagnetic property was denoted by magnetic hysteresis loop, remanence and no coercivity observance [29]. This indicates the high magnetic nature that can be applied for separation of NPs from treated water.
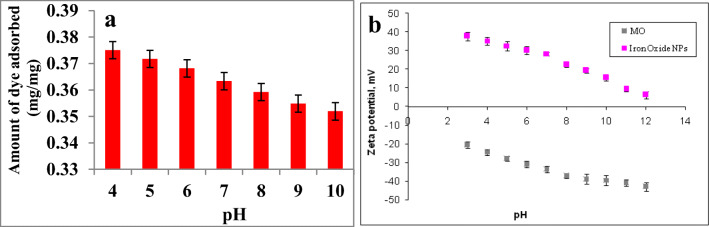
Effect of pH
The solution pH plays a crucial role in the adsorption process that controls the surface charge of adsorbent. The influence of pH on adsorption for MO on to the Fe3O4 NPs was investigated to find the optimum adsorption pH with fixed initial concentration (100 mg/L) in the range of pH (4.0–10.0). The data showed that the adsorption observed in all pH and maximum adsorption was observed at pH 4 (Fig. 2a). The mechanism of adsorption was determined by measuring zeta potential of NPs and dye at different pH and result is depicted in Fig. 2b. The results suggested that the surface charge of particles was positive and the dye was negative. Thus, the adsorption of negatively charged dye on the positively charged NPs was favored by electrostatic force of attraction. Up on increase of pH from 4 to 10 the surface charge of dye was decreased that leads to a slight decrease in adsorption. Paul Egwuonwn [30] reported the adsorption of MO and methyl red using various tree bark powder showed that the maximum adsorption of MO was noted at ph 6.5. The difference in adsorption may be due to difference in surface charge of the adsorbent.
Fig. 2.
a pH influence on MO dye adsorptive removal. b Zeta potential of NPs and dye at different pH. Values are the mean of n = 3 (mean ± standard error)
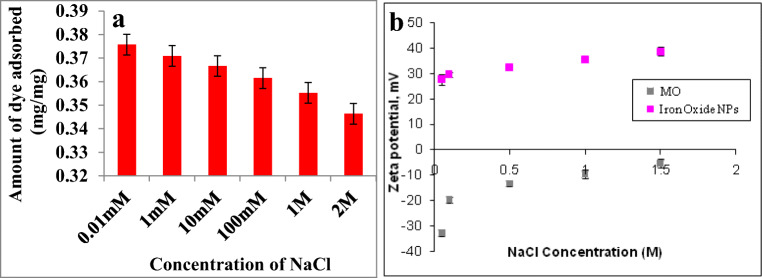
Effect of salinity
The influence of salinity in the effective adsorptive removal of dye is also important since the NaCl concentration in wastewater will be mostly very high. The results indicated that the decrease in adsorption observed upon increasing NaCl concentration (Fig. 3a). The mechanism of adsorption can be explained in terms of zeta potential. Figure 3b shows the zeta potential of MO and NPs at various concentrations of NaCl. The zeta potential of MO was found to be negative in all the NaCl concentrations. Same time magnetite nanoparticles showed an increase in positive zeta potential at increase in salinity level. At low NaCl concentration electrostatic force of attraction favors the adsorption of MO on magnetite NPs. At an increase in NaCl concentration the electrostatic force of attraction between MO and NPs decreased due to the low negative zeta potential of MO.
Fig. 3.
a NaCl concentration influence on MO dye adsorptive removal. b Zeta potential of NPs and dye at different salinity. Values are the mean of n = 3 (mean ± standard error)
Effect of adsorbent doses
The amount of MO adsorbed was significantly influenced by the various adsorbent doses. The amount of dye removed by Fe3O4 NPs was determined by interacting MO (250 mg/L) with various concentrations of NPs (1 mg - 10 mg/L). The increased dosage of NPs resulted in increased percentage of dye removal (Fig. 4). Ghaedi et al. [31] studied the capacity of adsorbent on removing dye and found that there was a significant increase in surface area and adsorptive capacity upon increased adsorbent dosage. It exhibited a rapid increase in percentage of dye removal till amount of adsorbent was raised to 0.02 g (silver and palladium atoms) [31].
Fig. 4.
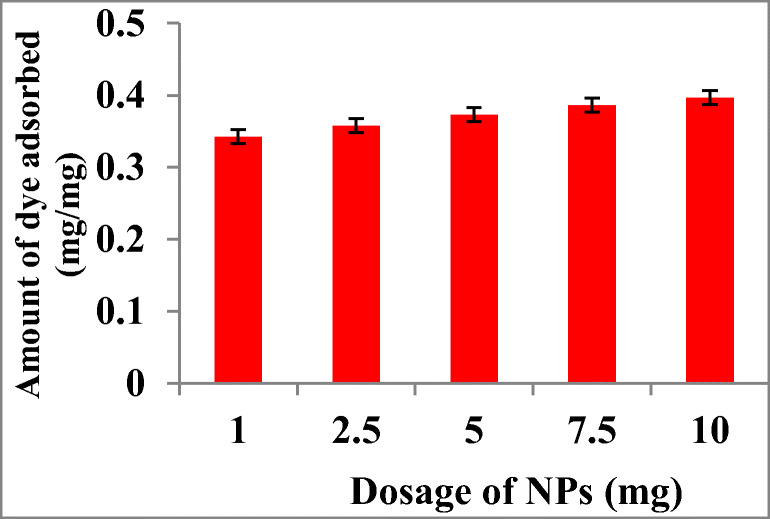
Effect of different absorbent doses of NPs on MO dye adsorptive removal. Values are the mean of n = 3 (mean ± standard error)
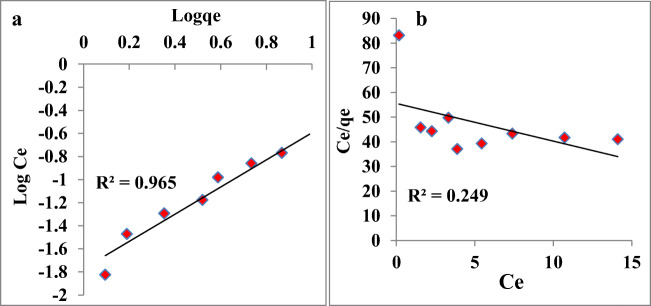
Adsorption isotherms
The investigation of adsorption isotherm is very important to obtain disposition of adsorbent and solute interaction information, and it is essential for adsorption system design. Freundlich and Langmuir models illustrate the adsorption process. The multilayer adsorption model is an assumption of the Freundlich model which demonstrates an increased concentration of dye in the interaction medium increases the amount of dye adsorption on adsorbent [32]. Our investigation on adsorption of MO dye was well fitted with Freundlich model, which suggest multilayer adsorption of MO on surfaces of Fe3O4 NPs (Fig. 5a). The Freundlich equation is represented as follows:
| 3 |
Fig. 5.
Langmuir isotherm model plotted as a function of log Ce vs log qe and b Freundlich isotherm model plotted as a function of Ce vs Ce/qe for MO dye adsorptive removal by Fe3O4 NPs
Where kF is Freundlich constant, Ce is the MO concentration after interaction with NPs in supernatant (mg/L), at equilibrium qe is the MO amount adsorbed. The linear form of Freundlich equation is represented as:
| 4 |
The best fitting of Freundlich experimental for MO adsorption is indicated by the high value of regression coefficient (R2) on the surface of Fe3O4 NPs.
The monolayer adsorption on outer surface of adsorbent is assumed by the Langmuir adsorption isotherm which suggests a rapid decline in intermolecular forces with distance [33]. The Langmuir equation can be represented as follows:
| 5 |
where qmax is maximum MO amount on NPs to form monolayer, Ce is the MO amount left in supernatant (mg/L), Ka is adsorption constant (L/mg) and at equilibrium qe is MO amount adsorbed. The linear form of Langmuir equation is:
| 6 |
The constants qmax and Ka were calculated from intercepts and linear plot slops of Ce/qe versus Ce. The adsorption process is better demonstrated by Freundlich isotherm than Langmuir model (Fig. 5b) due to its lower R2 value (regression coefficients). The parameters of Langmuir and Freundlich isotherm models for the adsorption of MO on Fe3O4 NPs are calculated and it is given in Table 1.
Table 1.
Langmuir and Freundlich model parameters for MO adsorption isotherms on iron oxide NPs
| Langmuir isotherm model | Freundlich isotherm model | ||||
|---|---|---|---|---|---|
| qmax (mg/mg) | ka (L/mg) | R2 | KF (mg/mg) (L/mg)1/n | n | R2 |
| 0.656 | 0.027 | 0.249 | 0.017 | 0.850 | 0.965 |
Adsorption kinetics
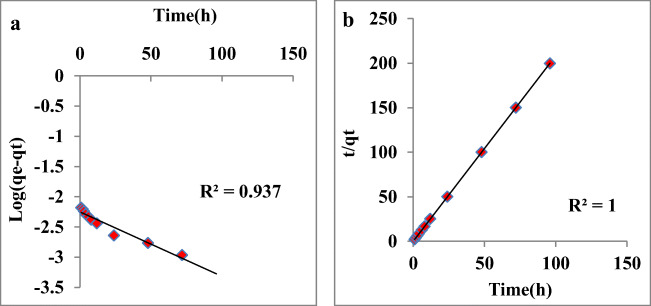
The contact time influence on MO adsorption on Fe3O4 NPs was studied for initial concentration of MO (250 mg/L) (Fig. 6). Two kinetic models, pseudo-1st-order [34] and pseudo-2nd-order [35] equations were used. Pseudo-first-order kinetic equation is represented as:
| 7 |
where qt is MO amount adsorbed at time t, at equilibrium qe is MO amount adsorbed and k1 is rate constant determined by straight line plot of log (qe-qt) versus time (h). Pseudo-second-order kinetic equation is expressed as:
| 8 |
Fig. 6.
Pseudo-first-order kinetics plotted as a function of log (qe-qt) versus time (h) and b pseudo-second-order kinetics plotted as a function of t/qt versus time (h) for the adsorption of MO on iron oxide NPs
The second order qe and k2 rate constant were calculated from intercepts and slopes. High R2 value in pseudo second order model (R2 = 1) denoted the linear relation between t/qt and time (h) than pseudo first order model (R2 = 0.937). The calculated k1 and k2 was 0.023 and 35.29 and qe was 0.006 and 0.480 for pseudo-first and second order reactions [36–38]. The calculated qe of pseudo second order reaction was near to the experimental qe (Table 2). Table 3 represents comparative data of various adsorbent used for dye removal and the adsorbent used in the present study [39–45].
Table 2.
Pseudo-first-order and second-order kinetic model of MO adsorption on iron oxide NPs
| qe (exp) (mg/mg) | Pseudo-first-order kinetic model | Pseudo-second-order kinetic model | ||||
|---|---|---|---|---|---|---|
| K1 (1/min) | qe (cal) (mg/mg) | R2 | K2 (1/min) | qe (cal) (mg/mg) | R2 | |
| 0.49 | 0.023 | 0.006 | 0.937 | 35.29 | 0.480 | 1 |
Table 3.
Comparison of the maximum adsorption capacity of MO with various adsorbents
| Adsorbent | Maximum adsorption capacity | pH | Reference |
|---|---|---|---|
| Graphene oxide | 16.83 mg/g | pH: 3 | [39] |
| SiO2–Al2O3 mixed-oxides | 23.09 mg/g | pH: 7 | [40] |
| Activated clay | 16.78 mg/g | pH: 7 | [41] |
| CO3O4 nanoparticles | 46.08 mg/g | pH: 6 | [42] |
| Maghemite/chitosan nanocomposite films | 29.41 mg/g | pH: 2.91 | [43] |
| Activated carbon /NiFe2O4 magnetic composite | 182 mg/g | pH: 3 | [44] |
| Tin oxide nanoparticles loaded on activated carbon | 250 mg/g | pH: 2 | [45] |
| Fe3O4-PEG | 0.49 mg/mg | pH: 4 | Present work |
Reusability
Here, the reusability of the adsorbent was also evaluated. It is a very important part since it reduces the cost of production. In addition to investigate the ability of the adsorbent materials to be reused, the XRD analysis was also performed for six cycles consecutively after adsorption studies. The particles showed almost similar XRD peak indicates the stability of adsorbent. The particles showed excellent adsorption ability similar to first which indicates in reusability.
Antibacterial studies
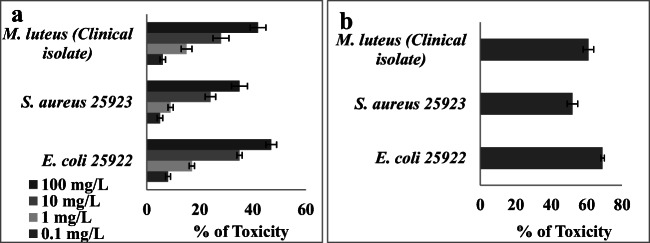
The antibacterial property of magnetite NPs was studied against E. coli, M. luteus and S. aureus and the results are shown in Fig. 7. At 100 mg/L NPs, all bacterial culture exhibited 100% toxicity. The toxicity decreased with decrease in NPs exposure. E. coli was highly sensitive to magnetite NPs, and low toxicity was observed with S. aureus. E. coli is a Gram negative bacterium and it posses thin cell wall, hence the NPs can easily damage and penetrate the bacterial cell that may be the reason for showing higher toxicity than M. luteus and S. aureus. Groiss et al. [46] reported an effective inhibition by Fe3O4 NPs against E. coli and S. epidermidis. Similar result was observed in the studies of Irshad et al. [47] that iron oxide NPs synthesized using Punicagranatum peel extract showed highest toxic effect against P. aeruginosa. Iron oxide NPs was reported to be toxic to Gram +ve and -ve bacteria, but it posses higher toxic effect to Gram -ve bacteria than Gram +ve one [48].
Fig. 7.
Antibacterial activity of magnetite NPs
Lipid peroxidation assessment
The membrane damage of bacterial cells was determined based on lipid peroxidation analysis. The estimation of amount of thiobarbituric acid reactive species (TBARS) indicates the peroxidation of bacterial membrane lipid due to nanoparticles exposure. The TBARS presence in the bacterial cells of E. coli, M. luteus and S. aureus were determined by recording the absorbance at 532 nm. E. coli showed higher lipid peroxidation rate with increase in NPs concentration from 0.1 mg/L to 100 mg/L than S. aureus and M. luteus (Fig. 8a). For 0.1 mg/L of NPs, 28% increase of lipid peroxidation observed compared to control and for 100 mg/L it was increased to 66%. In case of S. aureus it was increased from 22% to 60% for 0.1 to 100 mg/L NPs compared to control. M. luteus showed an increase lipid peroxidation from 28% to 66% for 0.1 to 100 mg/L compared to control. Jeng et al. [49] studied the toxicity of metal oxide NPs including Fe3O4, TiO2, Al2O3, ZnO and CrO3 in mammalian cells. They reported elevation in lipid peroxidation on cells in higher dosage of of NPs (>200 mg/L) which leads to cell damage and apoptosis. Singh et al. [50] reported the cellular toxicity caused due to DNA damage and oxidative stress by superparamagnetic iron oxide NPs. Radu et al. [51] reported the toxicity caused by hematite nanoparticles in MRC-5 cells and noticed an increased lipid peroxidation and depletion in glutathione up on interaction with NPs. The lipid peroxidation was increased to 81%, 189% and 110% after 24, 48 and 72 h respectively. Li et al. [52] studied the effect of water-brone nano-iron on Oryzia slatipes and reported the dose dependent oxidative damage caused by nano- iron.
Fig. 8.
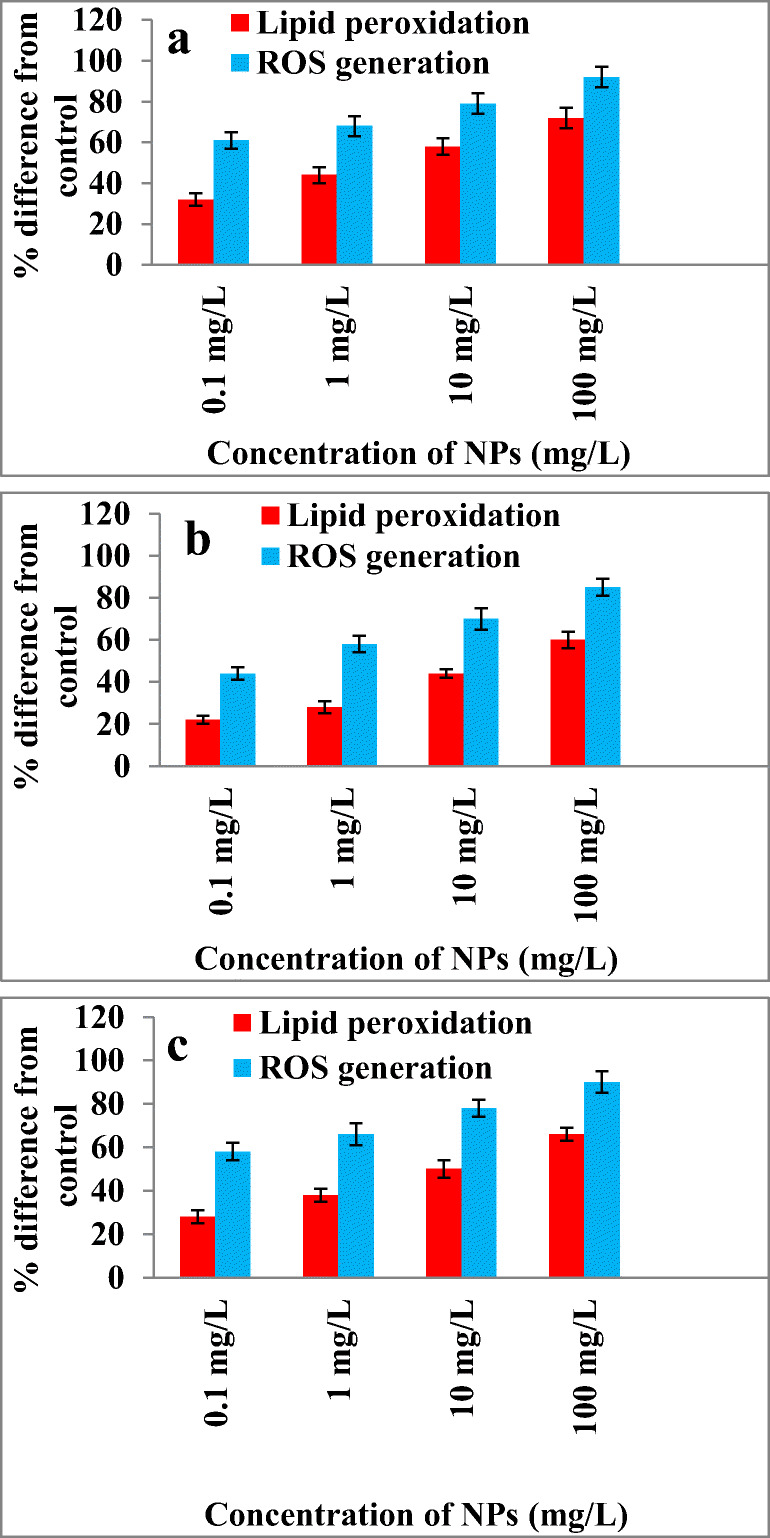
Effect of Fe3O4 NPs on ROS generation and lipid peroxidation in a E. coli 25,922, b S. aureus 25,923 and c M. luteus (Clinical isolate)
ROS estimation
The cellular ROS formation was estimated by measuring the increasing flurescence level of 2,7-dichlorofluorescein (DCF), a fluorescence product formed due to the deacetylation of 2,7-dichlorodihydrofluorescein diacetate acetyl ester (H2DCF-DA). Figure 8b shows effect of NPs on lipid peroxidation and ROS formation in E. coli, S. aureus and M. luteus. E. coli showed 61% increase in ROS for 0.1 mg/L and 92% for 100 mg/L with respect to control. S. aureus and M. luteus shows increase in ROS with increase in NPs exposure. Excessive generation of ROS makes the bacterial cell membrane damage and further cell lysis [53]. Several researchers reported on the ROS formation was one of the major reason for NPs toxicity [54–56]. Li et al. [57] studied the mechanism of toxicity induced by zinc and cerium NPs based on ROS formation in RAW 264.7 and BEAS-2B cell lines and they found oxidative stress and cytotoxicity increased up on NPs exposure.
Enzyme assay
The amount of antioxidants in the cell was gradually decreased with exposure of increase in NPs concentration. GSH content was determined by recording absorbance at 405 nm. Catalase activity was measured by recording absorbance of H2O2 at 240 nm for 5 min with 1 min time interval. SOD and glutathione reductase determination absorption was recorded at 350 and 405 nm respectively. Figure 9a, b & c shows effect on amount of superoxide dismutase (SOD), Catalase, Glutathione reductase, GSH and GSH+GSSG activity on E. coli, S. aureus and M. luteus respectively. The bacterial cells showed increased antioxidant enzyme activity at 0.1 mg/L and decrease in activity was noted with further increase in NPs concentration. Similarly, GSH and total GSH + GSSG content was also determined by comparing with control and it was found that the amount of GSH + GSSG was decreased with increase in NPs concentration. The key effect of nanomaterials on toxicity is due to the suppressing effect of antioxidant defense system results in oxidative stress [58]. Chen et al. [59] studied the different intracellular microenvironment in which iron oxide NPs (IONPs) were found to produce free radicals in human glioma U251 cells. Results showed that IONPs had a concentration-dependent enhanced cytotoxicity and H2O2-induced cell damage increased dramatically. Magnetite NPs induced the oxidative stress by depleting antioxidant enzymes in human cells [60]. Radu et al. [51] studied a time based exposure of hematite NPs on MRC-5 cells and they found the decreasing antioxidant enzymes content at increased NPs exposure time.
Fig. 9.

Effect of Fe3O4 NPs on bacterial antioxidant defense systems in a E. coli 25,922, b S. aureus 25,923 and c M. luteus (Clinical isolate)
Antibiofilm studies
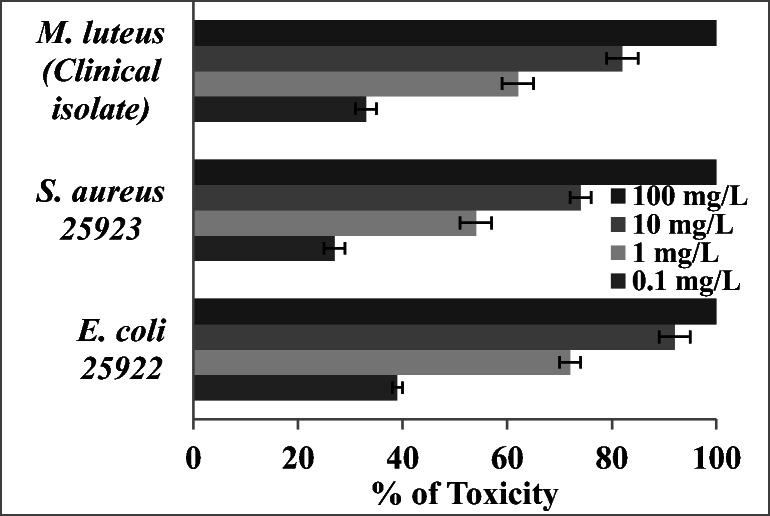
Antibiofilm activity of PEG capped magnetite NPs on preformed biofilm was evaluated and the results are shown in Fig. 10a. The toxic effect of magnetite NPs on biofilm was found to be 47, 42 and 35% for E. coli, M. luteus and S. aureus respectively when the biofilm was interacted with 100 mg/L NPs. No previous report on the anti-biofilm activity iron oxide NPs. Our previous study showed that ZnO stabilized with PVP shoed good antibiofilm activity [61]. There were several limitations associated ZnO NPs could be replaced with magnetite NPs due to its biocompatibility and low production cost. Figure 10b shows the anti-biofilm activity of magnetite NPs on pre-coated glass slides. When the glass slide was coated with iron oxide NPs the biofilm formation were greatly inhibited. E. coli, M. luteus and S. aureus showed the inhibition of 69, 61 and 52% respectively. One of the major mechanisms behind this toxic effect is reported to be due to the formation of ROS that leads to oxidative stress [47]. Researchers are suggested that magnetite NPs can be used as an effective tool for environmental cleanup and disinfection [48]. The present study report that PEG capped magnetite NPs is an efficient material for the adsorptive removal of dyes from the effluent and this materials can also be used to control the biofilm formation.
Fig. 10.
a Antibiofilm activity of Fe3O4 NPs on a preformed biofilm, b biofilm formation on precoated slides
Conclusion
The present study reports adsorptive removal of an azo dye, MO by using Fe3O4 NPs. Fe3O4 NPs have been characterized using TEM, BET, VSM, VSM, particle size analyzer and zeta sizer. The influence of NaCl, adsorbent doses and pH on MO removal were examined. The result indicated that, electrostatic force of attraction favors the adsorption of MO on magnetite NPs. The particles also exhibited good antibacterial and anti biofilm activity. Thus, the present study suggests that magnetite NPs can be an efficient material for the removal of dyes from the effluents. In addition the antibacterial and anti biofilm activity of magnetite NPs made them to use in self cleaning materials and surgical instruments.
Acknowledgements
Authors thank the management of Bannari Amman Institute of Technology for providing facility to carry out the work. The authors extend their appreciation to the Researchers supporting project number (RSP-2020/190) King Saud University, Riyadh, Saudi Arabia.
Compliance with ethical standards
Conflict of interest
None.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Fard RF, Sar MEK, Fahiminia M, Mirzaei N, Yousefi N, Mansoorian HJ. Efficiency of multi walled carbon nanotubes for removing direct blue 71 from aqueous solutions, Eurasian journal of analytical chemistry, 12. 2018. [Google Scholar]
- 2.Malek NNA, Jawad AH, Abdulhameed AS, Ismail K, Hameed BH. New magnetic Schiff's base-chitosan-glyoxal/fly ash/Fe3O4 biocomposite for the removal of anionic azo dye: an optimized process. Int J Biol Macromol. 2020;1461:530. doi: 10.1016/j.ijbiomac.2020.01.020. [DOI] [PubMed] [Google Scholar]
- 3.Ashrafi SD, Kamani H, Soheil Arezomand H, Yousefi N, Mahvi AH. Optimization and modeling of process variables for adsorption of basic blue 41 on NaOH-modified rice husk using response surface methodology. Desalin Water Treat. 2016;57:14051–14059. [Google Scholar]
- 4.Gupta VK, Mittal A, Krishnan L, Gajbe V. Adsorption kinetics and column operations for the removal and recovery of malachite green from wastewater using bottom ash. Sep Purif Technol. 2004;40:87–96. [Google Scholar]
- 5.Sandra JC, Lonnie RB, Donna FK, Daniel RD, Louis T, Frederick AB. Toxicity and metabolism of malachite green and leuco malachite green during short-term feeding to Fischer 344 rats and B6C3F1 mice. Chem Biol Interact. 1999;122:153–170. doi: 10.1016/s0009-2797(99)00119-2. [DOI] [PubMed] [Google Scholar]
- 6.Culp SJ, Beland FA. Malachite green: a toxicological review. Int J Toxicol. 1996;15:219–238. [Google Scholar]
- 7.Ghadiri SK, Alidadi H, Nezhad NT, Javid A, Roudbari A, Talebi SS, et al. Valorization of biomass into amine- functionalized bio graphene for efficient ciprofloxacin adsorption in water-modeling and optimization study. PLoS One. 2020;15:e0231045. doi: 10.1371/journal.pone.0231045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robinson T, Chandran B, Nigam P. Removal of dyes from a synthetic textile dye effluent by biosorption on apple pomace and wheat straw. Water Resources. 2002;36:2824–2830. doi: 10.1016/s0043-1354(01)00521-8. [DOI] [PubMed] [Google Scholar]
- 9.Javid A, Roodbari AA, Yousefi N, Fard MA, Barkdoll B, Talebi SS, et al. Modeling of chromium (VI) removal from aqueous solution using modified green-Graphene: RSM-CCD approach, optimization, isotherm, and kinetic studies. J Environ Health Sci Eng. 2020;18:1–15. doi: 10.1007/s40201-020-00479-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Srivastava S, Sinha R, Roy D. Toxicological effects of malachite green. Aquat Toxicol. 2004;66:319–329. doi: 10.1016/j.aquatox.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 11.Daneshvar N, Salari D, Khataee AR. Photocatalytic degradation of azo dye acid red 14 in water: investigation of the effect of operational parameters. J Photochemistry Photobiology A. 2003;157:111–116. [Google Scholar]
- 12.Bazgir A, Khorshidi A, Kamani H, Ashrafi SD, Naghipour D. Modeling of azo dyes adsorption on magnetic NiFe2O4/RGO nanocomposite using response surface methodology. J Environ Health Sci Eng. 2019;17:931–947. doi: 10.1007/s40201-019-00409-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kamari S, Shahbazi A. Biocompatible Fe3O4@SiO2-NH2 nanocomposite as a green nanofiller embedded in PES-nanofiltration membrane matrix for salts, heavy metal ion and dye removal: long-term operation and reusability tests. Chemosphere. 2020;243:125282. doi: 10.1016/j.chemosphere.2019.125282. [DOI] [PubMed] [Google Scholar]
- 14.Xu P, Zeng GM, Huang DL, Lai C, Zhao MH, Wei Z, et al. Adsorption of Pb(II) by iron oxide nanoparticles immobilized Phanerochaete chrysosporium: equilibrium, kinetic, thermodynamic and mechanisms analysis. Chem Eng J. 2012;203:423–431. [Google Scholar]
- 15.Mittal H, Mishra SB. Gum ghatti and Fe3O4 magnetic nanoparticles based nanocomposites for the effective adsorption of rhodamine B. Carbohydrate Polymer. 2014;101:1255–1264. doi: 10.1016/j.carbpol.2013.09.045. [DOI] [PubMed] [Google Scholar]
- 16.García-Jimeno S, Estelrich J. Ferro fluid based on polyethylene glycol-coated iron oxide nanoparticles: characterization and properties. Colloids Surf A Physicochem Eng Asp. 2013;420:74–81. [Google Scholar]
- 17.Kharazmi S, Taheri-Kafrani A, Soozanipour A. Efficient immobilization of pectinase on trichlorotriazine-functionalized polyethylene glycol-grafted magnetic nanoparticles: a stable and robust nanobiocatalyst for fruit juice clarification. Food Chem. 2020;325:126890. doi: 10.1016/j.foodchem.2020.126890. [DOI] [PubMed] [Google Scholar]
- 18.Faraji M, Yamini Y, Tahmasebi E, Saleh A, Nourmohammadian F. Cetyltrimethylammonium bromide-coated magnetite NPs as highly efficient adsorbent for rapid removal of reactive dyes from the textile companies’ wastewaters. J Iran Chem Soc. 2010;7:130–144. [Google Scholar]
- 19.Kamani H, Safari GH, Asgari G, Ashrafi SD. Data on modeling of enzymatic elimination of direct red 81 using response surface methodology. Data Brief. 2018;18:80–86. doi: 10.1016/j.dib.2018.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mehrabian F, Kamani H, Safari GH, Asgari G, Ashrafi SD. Direct blue 71 removal from aqueous solution by laccase-mediated system; a dataset. Data in Brief. 2018;19:437–443. doi: 10.1016/j.dib.2018.05.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim JH, Cha BJ, Kim YD, Seo HO. Kinetics and thermodynamics of methylene blue adsorption on the Fe-oxide nanoparticles embedded in the mesoporous SiO2. Adv Powder Technol. 2020;31:816–826. [Google Scholar]
- 22.Bar-Or D, Rael LT, Lau EP, Rao NK, Thomas GW, Winkler JV. An analog of the human albumin N-terminus (asp-Ala-his-Lys) prevents formation of copper-induced reactive oxygen species. Biochem Biophys Res Commun. 2001;284:856–862. doi: 10.1006/bbrc.2001.5042. [DOI] [PubMed] [Google Scholar]
- 23.Moron MS, Kepeierre JW. Levels of glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim Biophys Acta. 1979;582:67–68. doi: 10.1016/0304-4165(79)90289-7. [DOI] [PubMed] [Google Scholar]
- 24.Jeong Y, Park JI, Ha MG, Bae JS, Choi Y, Choi SA, et al. Effect of hydrogenated iron oxide nanoparticles with regular spherical shape by underwater plasma discharge treatment for high-efficiency water purification. Ceram Int. 2020;46:23582–23591. [Google Scholar]
- 25.Bidast S, Golchin A, Baybordi A, Zamani A, Naidu R. The effects of non-stabilised and Na-carboxymethylcellulose-stabilised iron oxide nanoparticles on remediation of co-contaminated soils. Chemosphere. 2020;261:128123. doi: 10.1016/j.chemosphere.2020.128123. [DOI] [PubMed] [Google Scholar]
- 26.Aghazadeh M, Karimzadeh I, Ganjali MR, Behzad A. Mn2+-doped Fe3O4 nanoparticles: a novel preparation method, structural, magnetic and electrochemical characterizations. J Mater Sci Mater Electron. 2017;28:18121–18129. [Google Scholar]
- 27.Aghazadeh M, Karimzadeh I, Ganjali MR. PVP capped Mn2+ doped Fe3O4 nanoparticles: a novel preparation method, surface engineering and characterization. Mater Lett. 2018;228:137–140. [Google Scholar]
- 28.Aghazadeh M. Electrochemical synthesis of dextran-and polyethyleneimine-coated superparamagnetic iron oxide nanoparticles and investigation of their physico-chemical characters. Analytical Bioanalytical Electrochem. 2019;11:362–372. [Google Scholar]
- 29.He HK, Gao C. Supraparamagnetic, conductive, and processable multifunctional graphene nanosheets coated with high-density Fe3O4 nanoparticles. ACS Appl Mater Interfaces. 2010;2:3201–3210. doi: 10.1021/am100673g. [DOI] [PubMed] [Google Scholar]
- 30.Dim PE. Adsorption of methyl red and methyl orange using different tree bark powder, academic research international, 4. 2013. [Google Scholar]
- 31.Ghaedi M, Heidarpour SH, Kokhdan SN, Sahraie R, Daneshfar A, Brazesh B. Comparison of silver and palladium nanoparticles loaded on activated carbon for efficient removal of methylene blue: kinetic and isotherm study of removal process. Powder Technol. 2012;228:18–25. [Google Scholar]
- 32.Jahangiri K, Yousefi N, Ghadiri SK, Fekri R, Bagheri A, Talebi SS. Enhancement adsorption of hexavalent chromium onto modified fly ash from aqueous solution; optimization; isotherm, kinetic and thermodynamic study. J Dispers Sci Technol. 2019;40:1147–1158. [Google Scholar]
- 33.Gholami Z, Ghadiri SK, Avazpour M, Fard MA, Yousefi N, Talebi SS, et al. Removal of phosphate from aqueous solutions using modified activated carbon prepared from agricultural waste (populous caspica): optimization, kinetic, isotherm, and thermodynamic studies. Desalin Water Treat. 2018;133:177–190. [Google Scholar]
- 34.Baocheng Q, Jiti Z, Xuemin X, Chunil Z, Hongxia Z, Xiaobai Z. Adsorption behavior of Azo dye C. I. Acid red 14 in aqueous solution on surface soils. J Environ Sci. 2008;20:704–709. doi: 10.1016/s1001-0742(08)62116-6. [DOI] [PubMed] [Google Scholar]
- 35.Ho YS, McKay G. Pseudo-second order model for sorption processes. Process Biochem. 1999;34:451–456. [Google Scholar]
- 36.Dalvand A, Nabizadeh R, Ganjali MR, Khoobi M, Nazmara S, Mahvi AH. Modeling of reactive blue 19 azo dye removal from colored textile wastewater using L-arginine-functionalized Fe3O4 nanoparticles: optimization, reusability, kinetic and equilibrium studies. J Magn Magn Mater. 2016;404:179–189. [Google Scholar]
- 37.Mahvi AH, Dalvand A. Kinetic and equilibrium studies on the adsorption of direct red 23 dye from aqueous solution using montmorillonite nanoclay. Water Quality Res J. 2020;55:132–144. [Google Scholar]
- 38.Pormazar SM, Ehrampoush MH, Ghaneian MT, Khoobi M, Talebi P, Dalvand A. Application of amine-functioned Fe3O4 nanoparticles with HPEI for effective humic acid removal from aqueous solution: modeling and optimization. Korean J Chem Eng. 2020;37:93–104. [Google Scholar]
- 39.Robati D, Mirza B, Rajabi M, Moradi O, Tyagi I, Agarwal S, et al. Removal of hazardous dyes-BR 12 and methyl orange using graphene oxide as an adsorbent from aqueous phase. Chem Eng J. 2016;284:687–697. [Google Scholar]
- 40.Arshadi M, Faraji AR, Mehravar M. Dye removal from aqueous solution by cobalt-nano particles decorated aluminum silicate: kinetic, thermodynamic and mechanism studies. J Colloid Interface Sci. 2015;440:91–101. doi: 10.1016/j.jcis.2014.10.040. [DOI] [PubMed] [Google Scholar]
- 41.Ma Q, Shen F, Lu X, Bao W, Ma H. Studies on the adsorption behavior of methyl orange from dye wastewater onto activated clay. Desalin Water Treatment. 2013;51:3700–3709. [Google Scholar]
- 42.Uddin MK, Baig U. Synthesis of Co3O4 nanoparticles and their performance towards methyl orange dye removal: characterisation, adsorption and response surface methodology. J Clean Prod. 2019;211:1141–1153. [Google Scholar]
- 43.Jiang R, Fu YQ, Zhu HY, Yao J, Xiao L. Removal of methyl orange from aqueous solutions by magnetic maghemite/chitosan nanocomposite films: adsorption kinetics and equilibrium. J Appl Polym Sci. 2012;125:540–549. [Google Scholar]
- 44.Jiang T, Liang YD, He YJ, Wang Q. Activated carbon/NiFe2O4 magnetic composite: a magnetic adsorbent for the adsorption of methyl orange. J Environ Chem Eng. 2015;3:1740–1751. [Google Scholar]
- 45.Ghaedi M, Rahimi MR, Ghaedi AM, Tyagi I, Agarwal S, Gupta VK. Application of least squares support vector regression and linear multiple regression for modeling removal of methyl orange onto tin oxide nanoparticles loaded on activated carbon and activated carbon prepared from Pistaciaatlantica wood. J Colloid Interface Sci. 2016;461:425–434. doi: 10.1016/j.jcis.2015.09.024. [DOI] [PubMed] [Google Scholar]
- 46.Groiss S, Selvaraj R, Varadavenkatesan T, Vinayagam R. Structural characterization, antibacterial and catalytic effect of iron oxide nanoparticles synthesised using the leaf extract of Cynometraramiflora. J Mol Struct. 2017;1128:572–578. [Google Scholar]
- 47.Irshad R, Tahir K, Li B, Ahmad A, Siddiqui AR, Nazir S. Antibacterial activity of biochemically capped iron oxide nanoparticles: a view towards green chemistry. J Photochem Photobiol B. 2017;170:241–246. doi: 10.1016/j.jphotobiol.2017.04.020. [DOI] [PubMed] [Google Scholar]
- 48.Muthukumar H, Chandrasekaran NI, Mohammed SN, Pichiah S, Manickam M. Iron oxide nano-material: physicochemical traits and in vitro antibacterial propensity against multidrug resistant bacteria. J Industrial Eng Chem. 2017;45:121–130. [Google Scholar]
- 49.Jeng HA, Swanson J. Toxicity of metal oxide nanoparticles in mammalian cells. J Environ Sci Health Part A. 2006;41:2699–2711. doi: 10.1080/10934520600966177. [DOI] [PubMed] [Google Scholar]
- 50.Singh N, Jenkins GJS, Asadi R, Doak SH. Potential toxicity of superparamagnetic iron oxide nanoparticles (SPION) Nano Rev. 2010;1:5358. doi: 10.3402/nano.v1i0.5358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Radu M, Munteanu MC, Petrache S, Serban AI, Dinu D, Hermenean A, et al. Depletion of intracellular glutathione and increased lipid peroxidation mediate cytotoxicity of hematite nanoparticles in MRC-5 cells. Acta Biochim Pol. 2010;57:355. [PubMed] [Google Scholar]
- 52.Li H, Zhou Q, Wu Y, Fu J, Wang T, Jiang G. Effects of waterborne nano-iron on medaka (Oryziaslatipes): antioxidant enzymatic activity, lipid peroxidation and histopathology. Ecotoxicol Environ Saf. 2009;72:684–692. doi: 10.1016/j.ecoenv.2008.09.027. [DOI] [PubMed] [Google Scholar]
- 53.Wahab R, Mishra A, Yun SI, Kim YS, Shin H-S. Antibacterial activity of ZnO nanoparticles prepared via non-hydrolytic solution route. Appl Microbiol Biotechnol. 2010;87:1917–1925. doi: 10.1007/s00253-010-2692-2. [DOI] [PubMed] [Google Scholar]
- 54.Xia T, Kovochich M, Liong M, Madler L, Gilbert B, Shi H, et al. Comparison of the mechanism of toxicity of zinc oxide and cerium oxide nanoparticles based on dissolution and oxidative stress properties. ACS Nano. 2008;2:2121–2134. doi: 10.1021/nn800511k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Klaine SJ, Alvarez PJJ, Batley GE, Fernandes TE, Handy RD, Lyon DY, et al. Nanomaterials in the environment: behavior, fate, bioavailability, and effects. Environ Toxicol Chem. 2008;27:1825–1851. doi: 10.1897/08-090.1. [DOI] [PubMed] [Google Scholar]
- 56.Kim JS, Kuk E, Yu KN, Kim JH, Park SJ, Lee HJ, et al. Antimicrobial effects of silver nanoparticles. Nanomedicine. 2007;3:95–101. doi: 10.1016/j.nano.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 57.Li N, Xia T, Nel AE. The role of oxidative stress in ambient particulate matter-induced lung diseases and its impli- cations in the toxicity of engineered nanoparticles. Free Radic Biol Med. 2008;44:1689–1699. doi: 10.1016/j.freeradbiomed.2008.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Carlson C, Hussain SM, Schrand AM, Braydich-Stolle LK, et al. Unique cellular interaction of silver nanoparticles: size- dependent generation of reactive oxygen species. J Phys Chem B. 2008;112:13608–13619. doi: 10.1021/jp712087m. [DOI] [PubMed] [Google Scholar]
- 59.Chen Z, Yin J-J, Zhou Y-T, Zhang Y, Song L, Song M, et al. Dual enzyme-like activities of iron oxide nanoparticles and their implication for diminishing cytotoxicity. ACS Nano. 2012;6:4001–4012. doi: 10.1021/nn300291r. [DOI] [PubMed] [Google Scholar]
- 60.Alarifi S, Ali D, Alkahtani S, Alhader MS. Iron oxide nanoparticles induce oxidative stress, DNA damage, and caspase activation in the human breast cancer cell line. Biol Trace Elem Res. 2014;159:416–424. doi: 10.1007/s12011-014-9972-0. [DOI] [PubMed] [Google Scholar]
- 61.Akhil K, Jayakumar J, Gayathri G, Sudheer Khan S. Effect of various capping agents on photocatalytic, antibacterial and antibiofilm activities of ZnO nanoparticles. J Photochem Photobiol B. 2016;160:32–42. doi: 10.1016/j.jphotobiol.2016.03.015. [DOI] [PubMed] [Google Scholar]