Abstract
This study reports temporal and spatial variations of 16 different species of particulate polycyclic aromatic hydrocarbons (particle-bonded PAHs) in the indoor and outdoor environments of three sampling sites in Bandar Mahshahr city, Iran. A low-volume air sampler was employed to collect size-segregated particulate matter during winter (October to December 2015), and summer (July to September 2016). The results showed that the annual concentrations of indoor and outdoor PM10 and PM2.5 were much higher than the related World Health Organization guidelines. The concentration of total particle-bonded PAHs (TPAHs) was higher in winter than in summer and a significant difference between the two sampling seasons was observed. The indoor and outdoor carcinogenic PAHs to TPAHs concentrations ratios in the sampling sites in summer and winter were as follow: for PM10 40.15–42.51%, PM2.5 41.30–42.97%, and PM1 43.07–44.36%, respectively; furthermore, the smaller the particle size, the higher the percentage of carcinogenic PAHs. 2 ring PAHs had a very small contribution to the total PAHs (about 1%), whereas PAHs with 3-to-4 rings had much larger contributions, ranging from 71.65% to 75.17%. The results demonstrated that as PM size decreased, the proportion of 5-to-6-ring PAHs to the total PAHs increased. Since 5-to-6- ring PAHs are considered to be more toxic, hence more attention should be paid to fine particles. The diagnostic ratios of indoor and outdoor of three sampling sites in both seasons suggested that petrogenic sources, as well as combustion of petroleum and other fossil fuels were the main PAHs sources.
Keywords: Atmospheric particulate matters, Polycyclic aromatic hydrocarbons, Spatial variation, Temporal variation, Bandar Mahshahr
Introduction
Atmospheric particulate matters (PM) especially fine and ultra-fine particulate matters have a significant role in air pollution and human health. Various related epidemiological, toxicological, and modeling studies have addressed that exposure to these particles can escalate and aggravate adverse health effects on human [1–6]. They can somewhat easily penetrate the respiratory system and deposit into deep regions of the lungs which result in acute or long-term cardiovascular and respiratory diseases, disorder the immune system functions, and also can proliferate the risk of adverse birth outcomes [7–9]. Fine particles, as the dominant culprits in visibility degradation [10], may be airborne for days or even weeks. Depending on meteorological conditions they may be transported over long distances since they deposit and remove gradually from the atmosphere [11]. Fine particles are also known as an active loading of toxic chemicals [12].
Two important characteristics of particulate matters, which can provide valuable information for understanding the sources, formation mechanisms and transportation patterns of particles, are their size distribution and chemical constituents [13]. The amount of particle deposition in various parts of the respiratory system is highly dependent on the PM size. In terms of chemical constituents, approximately around 20–90% in mass of atmospheric PM associated with assorted particulate organic compounds such as non-toxic alkanes [14, 15]. Nevertheless, the toxic compounds such as polycyclic aromatic hydrocarbons (PAHs), and their nitrated (nitroPAHs) and oxygenated (oxy-PAHs) derivatives form only 10% percent of atmospheric PM [16].
PAHs as a large group of organic compounds are comprised of two to seven aromatic rings in a linear, clustered, or angular arrangement. They are ubiquitous and persistent compounds formed during pyrolysis or incomplete combustion of organic matter, and from the release of petroleum products. Atmospheric PAHs can be emitted from natural sources such as forest fires and volcanic eruptions. Emissions from industries, motor-vehicle exhausts, commercial and residential heating using coal, wood or other biomass fuels, and indoors tobacco smoke are man-made sources of atmospheric PAHs [17]. PAHs can be moved over long distances in the atmosphere either in particle-bonded or gaseous form. Their distributions into the atmosphere depending on meteorological conditions and also the volatility of the individual compound [18, 19]. They are of noticeable concern principally due to their pervasive presence in the environment and well-documented mutagenicity, teratogenicity, and carcinogenicity [20]. They are carcinogenic to the lungs, skin and bladder [21]. For the same reasons, US Environmental Protection Agency (USEPA) mentioned 16 PAHs as priority pollutants [22].
International Agency for Research on Cancer (IARC) listed benzo[a]pyrene as Group 1 carcinogen. Dibenzo[a,l]pyrene and Dibenz[a,h]anthracene, which are categorized in Group 2A (probable carcinogens to humans), have been under fastidiousness because of their higher carcinogenic potency than benzo[a]pyrene. Moreover, some of PAHs are considered as Group 2B, possible carcinogen to humans, such as naphthalene, benz[a]anthracene, benzo[b]fluoranthene, benzo[j]fluoranthene, benzo[k]fluoranthene, chrysene, and indeno[1,2,3-cd] pyrene [23, 24].
PAHs are also categorized as endocrine-disrupting chemicals [25], as well as some of them demonstrating reproductive, developmental, neuro-, immuno-, hemato-, and cardio-toxicities in humans and animals [26]. It is crucial to figure out the impact of specific emission sources on the different compartments of the environment in order to assess the appropriate risk assessment of PAHs. PAHs diagnostic ratios may provide crucial data for the identification of main PAHs emission sources, which can be achieved by comparing ratios of pairs of frequently found PAH emissions [17].
To the best of our knowledge, the measurement of these compounds has not been done in Bandar Mahshahr city so far. Thus, the present study evaluates the particle-bonded PAHs concentrations in the indoor and outdoor air of this city. This city is located at southwest of Iran and has numerous industries especially petrochemical complexes and petroleum industries and also. The main aims of this study were: I) to investigate indoor and outdoor levels of 16 PAHs of size-fractionated particles considered by USEPA as priority pollutants II) to analyze PAHs temporal and spatial variability III) to apply the diagnostic ratios in order to identify possible emission sources of PAHs IV) to investigate risk assessment of the PAHs compounds.
Materials and methods
Sampling sites
As it has shown in Fig. 1 particulate matters (PM10, PM2.5 and PM1) mass concentrations and particle-bonded PAHs compounds were investigated at three sampling sites (S1, S2 and S3) on the basis of different anthropogenic activities and the local differences in the type, distribution and proximity of emission sources in Bandar Mahshahr city, Iran. S1 (30°33′25.9″N–49°11′20.8″E) is in a residential area with nearly low traffic fleets, S2 (30°33′27.3″N–49°11′58.1″E) is located in the center of the city which has high traffic flows with busy commercial activities and S3 (30°26′57.1″N–49°05′06.1″E) is in the industrial area and may be influenced by emissions from the industrial activities especially from adjacent petrochemical complexes.
Fig. 1.
Location of Bandar Mahshahr city in Khuzestan province and sampling stations
Air sample collection
Indoor and outdoor sampling were carried out twice a month in each sampling site during winter (October to December 2015), and summer (July to September 2016). Because of the main objectives of this study, ambient particulate samples were collected at the rooftop at a height of approximately 5 m above the ground level and also for indoor sampling the air sampling device in S1 and S2 sampling sites were installed in the living rooms and for S3 in the office where the staff frequently work at 1.5 m above the ground level. Size-segregated samples were collected on polytetrafluoroethylene (PTFE) filter papers (47 μm dia., 0.5 μm pore-size, from SKC) using low-volume air samplers (FRM OMNITM Air Sampler, multi-cut inlet; BGI, Inc., USA), operating at a flow rate of 5 l/min. The PTFE filter papers were used for collecting atmospheric PM samples because they are appropriate for both sampling and extraction of particle-bonded PAHs. Since the PTFE filters are thermally resistant, they can be heat-treated before sampling to remove any particle-bonded PAHs present in the blank filter. Each sampling time was 24 h. After collection, each filter was equilibrated in a desiccator at room temperature (25–35 °C), the filter was wrapped in cleaned aluminum foil, sealed in plastic bags, and then stored at −20 °C until being submitted for analysis. The meteorological data (e.g., wind speed and direction, humidity and ambient air temperature) was obtained from Bandar Mahshahr’s meteorological station.
Particulate matters (PM10, PM2.5 and PM1) mass concentrations
Mass concentrations of PM10, PM2.5, and PM1 were defined gravimetrically by subtracting the initial mean mass of the blank PTFE filter from the final mean mass of the exposed PTFE filter; the difference was then divided by the total volume of air that passed through the filter (at 25 °C and 101.3 kPa).
For the initial mean mass, the PTFE filters were equilibrated for 24 h in a conditioning environment (relative humidity held constant at a mean value of 40% ± 5% and the temperature held constant with a mean value between 25 and 35 °C) and then weighed by a microbalance (Mettler–Toledo, Columbus, OH, USA) with a sensitivity of 0.0001 mg.
The procedures for the final mean mass of the exposed PTFE filters were as follows: 24 h to equilibrate filters before weighing under controlled relative humidity (40 ± 5%) and temperature (25–35 °C) by using a microbalance (Mettler–Toledo, Columbus, OH, USA) with a sensitivity of 0.0001 mg followed by weighing during the following 24–48 h. If the measurements for one sample differed more than 10 mg, they were discarded and the filters were repeatedly weighed until three reproducible values were obtained. Then, the filters were packed in aluminum foils and stored at −20 °C until the subsequent extraction and chemical analysis [27, 28].
Analysis of PAHs with the gas chromatography mass spectrometry (GC/MS)
A quarter of each PTFE filter was cut into pieces and put in 10 mL centrifuge tubes, followed by the addition of 4 ml of methanol and dichloromethane (DCM) (1:1). We use an ultrasonic bath in order to extract the particle-bonded PAHs compounds from PTFE filters (30 min at 27 °C). To remove any solid material, the extract solution was filtered. A rotary evaporator (600 Torr) was used in order to concentrate the filtrates to approximately 0.5 mL and then, the residue was dissolved in 30 mL of n-hexane and evaporated to a rotary evaporator (180 Torr). Next, the residue was cleaned by silica gel cleanup technique (Sep-Pak Silica plus long cartridge 690 mg sorbent per cartridge 55–105 mm particle size 50/pk, Waters, Massachusetts, USA). Subsequently, the concentrated solution added to n-nonane and was evaporated under a gentle N2 stream in 60 °C water bath. Finally, for analysis of particle-bonded PAHs, the extract solution was concentrated to 0.1 mL and submitted to gas chromatography coupled with mass spectrometry (GC-MS).
Sixteen species of PAHs (EPA-PAHs) were detected including: Naphthalene (Naph), Acenaphthylene (Acy), Acenaphthene (Ace), Fluorene (Flu), Phenanthrene (Phen), Anthracene (Anth), Fluoranthene (Flt), Pyrene (Pyr), Benzo[a]anthracene (BaA), Chrysene (Chr), Benzo[b]fluoroanthene (BbF), Benzo[k]fluoroanthene (BkF), Benzo[a]pyrene (BaP), Dibenzo[a,h]anthracene (DahA), Benzo [ghi]perylene (BghiP), and Indeno[123-cd]pyrene (Ind). To obtain the standard curve (calibration curve) mixed standard solutions containing all of sixteen species of PAHs (labeled standards of PAHs) were used and achieved by dilution of the stock solutions with a proper amount of acetonitrile. In order to avoid photodegradation and volatilization, the solutions were stored at −20 °C in darkness.
In order to quantify particle-bonded PAHs, gas chromatography (Agilent Technologies 6890 N) coupled with mass spectrometry (Agilent Technologies 5973) in the selective ion-monitoring (SIM) mode was used. A fused silica DB5-ms (5% phenyl 95% dimethyl arylene siloxane) capillary column (30 m length, 0.25 mm i.d., 0.25 mm film thickness) was used for separation. The temperatures of the injector and ion source were 280 °C and 230 °C, respectively. The oven temperature was programmed from 50 °C held at this temperature for 2 min, after that temperature increased 15 °C min−1 to 180 °C, increased to 230 °C at 6 °C min−1, then increased at 8 °C min−1 to 270 °C and held at this temperature for 2 min, and finally ramped up to 320 °C at 20 °C min−1 and held for 15 min. MS detection was run in the selected target-ions monitoring (SIM) mode. The sample volume was 1 mL, and the injections were splitless. The carrier gas was High-purity helium. The flow rate of carrier gas was at the constant rate of 1.5 mL min−1. The identification of the individual PAHs was based on the retention times of target ion peaks (within ±0.05 min of the retention of the calibration standard) [27, 29–33].
Quality assurance and quality control
In this study, all analytic procedures were conducted according to rigid Quality Assurance and Quality Control (QA/QC) measures. Field and laboratory blanks, as well as spiked samples were analyzed along with the composite samples that were used for determining PAHs contents of the particulate matters. One blank sample for every four air samples was considered as laboratory blanks. The blank samples were treated similarly as the air samples were. Field blanks for the air samples were extracted and analyzed in the same manner as the samples were. The international standard reference materials (NIST Urban Dust SRM 1649) for PAHs were used for analytical control. The 16 species of PAHs standards were added to all of the samples to monitor the procedural performance and the matrix effects. The recoveries in the field samples of 16 species of PAHs were approximately 74% to 109%. For all compounds three times the standard deviation of the mean blank concentrations was set as the Method detection limits (MDLs). The detection limit of the process was stated as the concentration of the lowest calibration standard (0.12 ng/sample for the air samples).
Health risk assessment
Although some PAHs are typically carcinogenic, documents demonstrate that a combination of PAHs may be more carcinogenic to humans than each PAHs by itself. Moreover, PAHs may interact with other compounds and increase their risk of carcinogenicity. Therefore, we selected two different approaches to estimate the particulate PAH carcinogenic and mutagenicity potential. a) TEQ: toxic or carcinogenic equivalents b) MEQ: mutagenic equivalents.
TEQ = Concentrations of each PAH compound * Toxic Equivalency Factor (TEF) [34].
MEQ = Concentrations of each PAH compound * Mutagenic Equivalency Factor (MEF) [35, 36].
To estimate the carcinogenic and mutagenic effects of complex PAH mixtures relative to BaP, the TEF and MEF are used, respectively [37].
Results and discussion
PM mass concentrations
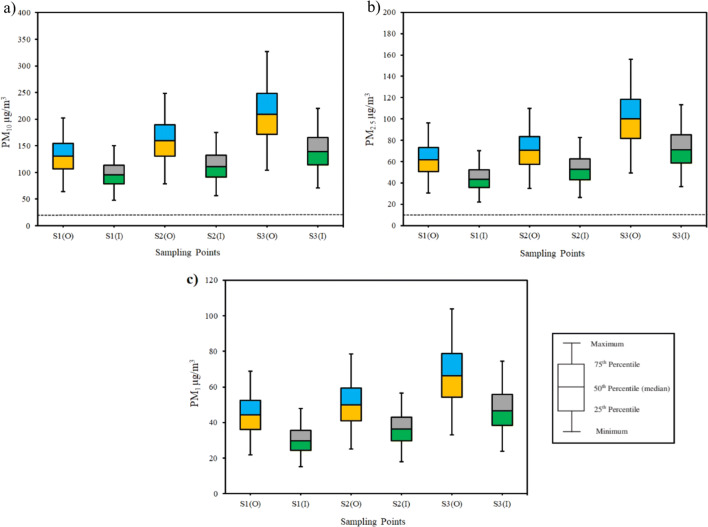
The indoor and outdoor, size-fractionated PM mass concentrations in the sampling sites are presented in Fig. 2. In the residential area (S1), outdoor PM10, PM2.5, and PM1 concentrations ranged from 64.29 to 204.46, 30.45 to 96.94, and 21.76 to 69.33 μg/m3, respectively. The corresponding indoor concentrations ranged from 47.74 to 151.09, 21.95 to 69.53, and 15.05 to 47.56 μg/m3. In the high traffic area (S2), the outdoor concentrations of PM10, PM2.5, and PM1 were in the range of 78.63 to 250.34, 34.81 to 110.68, and 24.98 to 79.07 μg/m3, respectively, while the corresponding indoor levels ranged from 56.03 to 176.84, 26.25 to 83.12, and 17.98 to 57.15 μg/m3. In the industrial area (S3), the outdoor concentrations of PM10, PM2.5, and PM1 were in the range of 103.81 to 329.73, 49.46 to 157.20, and 33.04 to 104.73 μg/m3, respectively, while the corresponding indoor levels ranged from 70.48 to 221.90, 36.32 to 114.15, and 23.86 to 74.92 μg/m3.
Fig. 2.
Box plot for indoor and outdoor a: PM10, b: PM2.5, and c: PM1 mass concentrations during the study period in the three sampling points. The dash lines show the Iranian national PM10 and PM2.5 standard levels
The Iranian national PM standard levels, which are the same as WHO guidelines, was used to compare the observed concentrations of PM masses with the standards in this field. The PM standard levels are 20 and 10 μg/m3 for annual average PM10 and PM2.5 concentrations, respectively. It is worth mentioning that no standard levels have been proposed for PM1 values in Iran since yet. As illustrated in Fig. 2, in the all three sampling sites, the annual lower quartile of indoor and outdoor PM10 and PM2.5 concentrations exceeded the WHO guidelines [38]. The comparison of PM2.5 mass concentrations measured in the present study with the results of other investigations around the world related to urban and rural areas such as Portugal [39], Taiwan [40], Greece [41], Spain [42], Japan [43], and Brazil [44] as well as industrial areas such as Italy [45], and Turkey [46] indicated that the concentrations of PM2.5 in the present study were quite higher. However, our results are in accordance with those obtained in China [47, 48].
Relatively high concentrations of PM at the sampling sites can be attributed to exposure to large amounts of particulate matters transported to this area as a result of the Middle East dust (MED) storms. As shown in Fig. 2 the concentrations of indoor and outdoor PM10, PM2.5, and PM1 were considerably higher in industrial area (S3) in comparison with other sampling sites. This factor indicated that emissions from the industrial activities especially from adjacent petrochemical complexes play an important role.
Two seasonal PAHs compositional patterns in PM10, PM2.5, and PM1 of the sampling sites
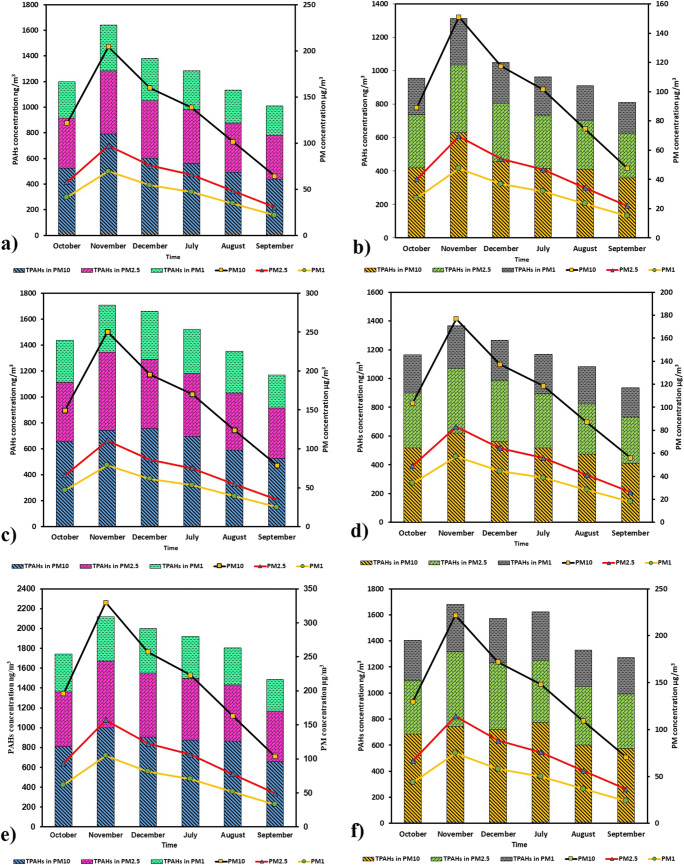
The average indoor and outdoor concentrations of particulate matters and also the concentrations of indoor and outdoor total PAHs (TPAHs, 16 species of particle-bonded PAHs) in sampling periods (the winter season (October to December 2015), and the summer season (July to September 2016)) at the three sampling sites with different pollution background in Bandar Mahshar City, Iran were presented in Fig. 3.
Fig. 3.
Temporal variation of TPAHs size distribution together with PM1, PM2.5 and PM10 in two seasons from a, b: outdoor and indoor of residential; c, d: outdoor and indoor of high traffic; and e, f: outdoor and indoor of industrial sampling sites, respectively
Based on Fig. 3, the average indoor and outdoor levels of particulate matters in the three sampling sites were higher in winter than summer. One of the main reasons for this trend is that the long-lasting sub adiabatic stability in the flat terrain of Khuzestan province, which is a frequent phenomenon, usually has been happening in winter.
The highest and lowest PM10, PM2.5 and PM1 concentrations of both indoor and outdoor in S1 were related to Nov 2015 and Sep 2016, respectively.
The average concentrations of outdoor TPAHs in PM10, PM2.5 and PM1 of S1 were 639.19, 444.50, and 324.05 ng/m3 in winter and 500.67, 347.34, and 263.49 ng/m3 in summer. On the other hand, the average concentrations of indoor TPAHs in PM10, PM2.5 and PM1 of mentioned station were 477.05, 357.82, and 252.49 ng/m3 in winter and 392.10, 292.50, and 205.64 ng/m3 in summer, respectively.
Comparing the outdoor and indoor TPAHs of S1 in winter and summer showed that there were significant differences between two sampling seasons so that their concentrations were higher in winter than in summer.
The average concentrations of outdoor TPAHs in PM10, PM2.5 and PM1 of S2 were 717.18, 531.86, and 353.58 ng/m3 in winter and 602.45, 440.44, and 305.53 ng/m3 in summer, correspondingly. Moreover, the average concentrations of indoor TPAHs in PM10, PM2.5 and PM1 of S2 were 565.09, 416.25, and 275.93 ng/m3 in winter and 471.89, 345.04, and 238.83 ng/m3 in summer, respectively.
Finally, for S3 the average concentrations of outdoor TPAHs in PM10, PM2.5 and PM1 were 903.79, 625.90, and 423.69 ng/m3 in winter and 799.33, 564.66, and 372.43 ng/m3 in summer, respectively. Moreover, the average concentrations of indoor TPAHs in PM10, PM2.5 and PM1 of S3 were 706.91, 489.85, and 330.69 ng/m3 in winter and 628.26, 442.33, and 291.13 ng/m3 in summer, separately.
The same trend was also observed for S2 and S3 as the indoor and outdoor concentrations of TPAHs were higher in winter than in summer. The results of this study are in compliance with the general trend observed for PAHs of airborne particles in other studies [40, 49]. Low PAHs levels in summer can be attributed to specific weather conditions in this period such as intense radiation, high temperature, and high humidity. These conditions promote thermo-, photo- and chemical oxidation reactions and as a result decreasing PAHs values [50]. In contrast, in winter PAHs levels were high due to the lack of suitable conditions for photochemical reactions, as well as presence of stable conditions such as frequent thermal inversion and weak vertical atmospheric dispersion potency due to the lower mixing layer in this season. Moreover, low ambient temperatures in winter can affect both the distribution balance between PAHs particle and gas phases and also PAH adsorption in particulate matters. Low temperatures can lead to relatively greater levels of PAHs partitioned to the particle phase. Furthermore, the lower the temperature the higher PAH adsorption in particulate matters [17, 51].
In general, the levels of PAHs in the gas phase increase in summer, while the concentrations of particle-bonded PAHs are predominant in winter [52, 53].
Tables 1, 2, 3 represent the statistical summary of PAHs concentrations in indoor and outdoor PM (PM10, PM2.5, and PM1) in the three sampling sites. As can be seen the concentrations of particulate PAHs in this study are much higher than those reported for other urban areas in Brazil [44]; China [54]; Delhi, India [31]; Greece [55]; and Los Angeles, CA, USA [56]. The existence of numerous industries in Bandar Mahshar City which produce PAHs compounds such as petrochemical complexes are the probable cause of this phenomenon.
Table 1.
PAHs concentration in indoor and outdoor environment of residential sampling site
| PAHs | Outdoor | Indoor | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PM10 | PM2.5 | PM1 | PM10 | PM2.5 | PM1 | |||||||
| Winter | Summer | Winter | Summer | Winter | Summer | Winter | Summer | Winter | Summer | Winter | Summer | |
| Nap | 7.27 ± 1.25 | 5.12 ± 0.69 | 4.67 ± 0.44 | 3.29 ± 0.34 | 3.44 ± 0.31 | 2.84 ± 0.33 | 4.49 ± 1.01 | 3.60 ± 0.30 | 3.76 ± 0.38 | 3.13 ± 0.22 | 2.42 ± 0.26 | 2.00 ± 0.20 |
| Acy | 6.37 ± 1.10 | 5.02 ± 0.66 | 3.82 ± 0.36 | 3.01 ± 0.28 | 3.07 ± 0.28 | 2.46 ± 0.28 | 4.43 ± 0.89 | 3.95 ± 0.28 | 3.08 ± 0.32 | 2.57 ± 0.18 | 2.42 ± .24 | 1.94 ± 0.17 |
| Ace | 3.40 ± 0.59 | 2.54 ± 0.36 | 2.31 ± 0.22 | 1.72 ± 0.17 | 1.84 ± 0.17 | 1.37 ± 0.16 | 2.27 ± 0.47 | 1.89 ± 0.15 | 1.86 ± 0.19 | 1.54 ± 0.11 | 1.37 ± 0.14 | 1.02 ± 0.10 |
| Flu | 83.45 ± 14.37 | 68.65 ± 7.88 | 51.72 ± 4.92 | 42.55 ± 3.79 | 37.16 ± 3.38 | 31.39 ± 3.63 | 59.61 ± 11.64 | 56.48 ± 3.47 | 41.64 ± 4.26 | 35.13 ± 2.49 | 30.57 ± 2.86 | 25.82 ± 2.21 |
| Phen | 100.58 ± 17.32 | 85.45 ± 10.83 | 77.02 ± 7.32 | 65.44 ± 5.59 | 48.64 ± 4.42 | 41.63 ± 4.81 | 69.85 ± 14.03 | 72.61 ± 4.50 | 62.00 ± 6.35 | 51.78 ± 3.67 | 41.33 ± 3.75 | 35.37 ± 2.92 |
| Anth | 40.06 ± 6.90 | 31.81 ± 3.45 | 29.82 ± 2.84 | 23.68 ± 2.14 | 21.19 ± 1.93 | 16.68 ± 1.93 | 30.81 ± 5.59 | 25.27 ± 1.57 | 24.00 ± 2.46 | 19.80 ± 1.40 | 16.83 ± 1.63 | 13.25 ± 1.17 |
| Flrt | 49.05 ± 8.75 | 37.26 ± 4.49 | 36.71 ± 3.49 | 27.89 ± 2.30 | 25.86 ± 2.35 | 18.93 ± 2.19 | 37.73 ± 6.84 | 28.31 ± 1.93 | 29.56 ± 3.03 | 21.31 ± 1.51 | 19.64 ± 1.99 | 14.38 ± 1.33 |
| Pyr | 51.41 ± 8.85 | 36.63 ± 5.36 | 38.21 ± 3.63 | 27.23 ± 2.59 | 27.02 ± 2.46 | 20.56 ± 2.38 | 38.08 ± 7.17 | 26.10 ± 2.22 | 30.76 ± 3.15 | 24.01 ± 1.70 | 19.25 ± 2.08 | 14.65 ± 1.44 |
| BaA | 56.78 ± 9.78 | 44.01 ± 4.77 | 36.35 ± 3.46 | 28.18 ± 2.49 | 27.78 ± 2.53 | 23.04 ± 2.66 | 44.01 ± 7.92 | 34.12 ± 2.17 | 29.26 ± 3.00 | 23.10 ± 1.64 | 21.54 ± 2.14 | 17.86 ± 1.62 |
| Chr | 73.15 ± 12.60 | 54.99 ± 6.39 | 55.61 ± 5.29 | 41.81 ± 4.05 | 45.15 ± 4.10 | 36.80 ± 4.26 | 55.00 ± 10.20 | 41.34 ± 2.91 | 44.77 ± 4.58 | 37.52 ± 2.66 | 33.94 ± 3.48 | 37.67 ± 2.59 |
| BbF | 55.75 ± 9.60 | 43.14 ± 5.92 | 36.86 ± 3.51 | 28.53 ± 2.68 | 28.66 ± 2.61 | 23.85 ± 2.76 | 43.28 ± 7.77 | 33.38 ± 2.45 | 29.67 ± 3.04 | 24.80 ± 1.76 | 22.18 ± 2.21 | 18.46 ± 1.68 |
| BkF | 31.59 ± 5.44 | 23.56 ± 3.48 | 21.21 ± 2.02 | 15.83 ± 1.45 | 15.64 ± 1.42 | 12.85 ± 1.49 | 24.62 ± 4.41 | 17.58 ± 1.44 | 17.08 ± 1.75 | 13.44 ± 0.95 | 11.67 ± 1.21 | 9.59 ± 0.90 |
| BaP | 10.51 ± 1.81 | 8.34 ± 1.13 | 7.39 ± 0.70 | 5.87 ± 0.55 | 4.73 ± 0.43 | 4.01 ± 0.46 | 8.11 ± 1.47 | 6.62 ± 0.47 | 5.95 ± 0.61 | 5.13 ± 0.36 | 3.76 ± 0.36 | 3.18 ± 0.28 |
| DahA | 33.15 ± 5.71 | 25.70 ± 3.71 | 21.94 ± 2.09 | 17.01 ± 1.62 | 17.46 ± 1.59 | 14.12 ± 1.63 | 23.94 ± 4.62 | 19.92 ± 1.54 | 17.66 ± 1.81 | 14.97 ± 1.06 | 13.53 ± 1.35 | 10.94 ± 0.99 |
| BghiP | 31.47 ± 5.42 | 22.78 ± 2.47 | 17.06 ± 1.62 | 12.35 ± 1.27 | 13.70 ± 1.25 | 10.76 ± 1.24 | 24.82 ± 4.39 | 16.49 ± 1.12 | 13.73 ± 1.41 | 11.74 ± 0.83 | 9.92 ± 1.06 | 7.79 ± 0.76 |
| Ind | 7.20 ± 1.24 | 5.67 ± 1.05 | 3.78 ± 0.36 | 2.98 ± 0.27 | 2.70 ± 0.25 | 2.21 ± 0.26 | 6.01 ± 1.00 | 4.46 ± 0.46 | 3.05 ± 0.31 | 2.54 ± 0.18 | 2.12 ± 0.21 | 1.74 ± 0.16 |
| PAHs | 639.19 ± 112.46 | 500.67 ± 62.47 | 444.50 ± 42.26 | 347.34 ± 31.57 | 324.05 ± 29.46 | 263.49 ± 30.46 | 477.05 ± 90.84 | 392.10 ± 26.45 | 357.82 ± 36.64 | 292.50 ± 20.74 | 252.49 ± 24.97 | 205.64 ± 18.51 |
| CANPAHs a | 268.12 ± 46.18 | 205.41 ± 26.35 | 183.16 ± 17.42 | 140.19 ± 13.11 | 142.13 ± 12.92 | 116.88 ± 13.51 | 204.97 ± 37.39 | 157.41 ± 11.13 | 147.44 ± 15.10 | 121.51 ± 8.62 | 108.74 ± 10.95 | 89.43 ± 8.21 |
| COMPAHs b | 401.30 ± 69.12 | 307.77 ± 38.33 | 268.21 ± 25.50 | 205.30 ± 19.15 | 209.20 ± 19.45 | 165.46 ± 19.13 | 303.54 ± 55.97 | 236.56 ± 16.31 | 215.90 ± 22.11 | 177.40 ± 12.58 | 154.95 ± 17.14 | 126.74 ± 11.63 |
aCarcinogenic PAHs includes: BaA, Chr, BbF, BkF, BaP, DahA, and Ind
bCombustion PAHs includes: Flu, Pyr, BaA, Chr, BbF, BkF, BaP, BghiP, and Ind
Table 2.
PAHs concentration in indoor and outdoor environment of high traffic sampling site
| PAHs | Outdoor | Indoor | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PM10 | PM2.5 | PM1 | PM10 | PM2.5 | PM1 | |||||||
| Winter | Summer | Winter | Summer | Winter | Summer | Winter | Summer | Winter | Summer | Winter | Summer | |
| Nap | 8.19 ± 0.49 | 7.22 ± 0.77 | 5.59 ± 0.62 | 4.71 ± 0.43 | 3.75 ± 0.23 | 3.29 ± 0.40 | 6.71 ± 0.46 | 5.91 ± 0.49 | 4.58 ± 0.26 | 3.86 ± 0.23 | 3.07 ± 0.13 | 2.70 ± 0.33 |
| Acy | 7.18 ± 0.43 | 6.08 ± 0.74 | 4.57 ± 0.51 | 3.87 ± 0.35 | 3.35 ± 0.21 | 2.85 ± 0.34 | 5.66 ± 0.40 | 4.79 ± 0.47 | 3.60 ± 0.21 | 3.05 ± 0.19 | 2.64 ± 0.11 | 2.25 ± 0.28 |
| Ace | 3.83 ± 0.23 | 3.07 ± 0.40 | 2.76 ± 0.31 | 2.32 ± 0.21 | 2.00 ± 0.12 | 1.59 ± 0.19 | 2.86 ± 0.22 | 2.29 ± 0.26 | 2.06 ± 0.13 | 1.73 ± 0.11 | 1.49 ± 0.07 | 1.19 ± 0.16 |
| Flu | 94.03 ± 5.62 | 83.2 ± 8.83 | 61.89 ± 6.89 | 52.9 ± 4.81 | 40.55 ± 2.51 | 36.4 ± 4.38 | 77.36 ± 5.29 | 68.44 ± 5.62 | 50.91 ± 2.85 | 43.52 ± 2.61 | 33.36 ± 1.36 | 29.94 ± 3.60 |
| Phen | 113.33 ± 6.77 | 95.48 ± 11.98 | 92.15 ± 10.26 | 77.97 ± 7.09 | 53.07 ± 3.28 | 48.27 ± 5.81 | 96.29 ± 6.38 | 81.12 ± 7.63 | 78.30 ± 4.25 | 66.24 ± 3.84 | 45.09 ± 1.78 | 41.01 ± 4.77 |
| Anth | 45.14 ± 2.70 | 38.55 ± 3.87 | 35.68 ± 3.97 | 29.82 ± 2.71 | 23.13 ± 1.43 | 19.34 ± 2.33 | 35.85 ± 2.54 | 30.62 ± 2.47 | 28.33 ± 1.64 | 23.68 ± 1.47 | 18.37 ± 0.78 | 15.36 ± 1.91 |
| Flrt | 55.27 ± 3.30 | 45.16 ± 5.01 | 43.93 ± 4.89 | 32.09 ± 2.92 | 28.21 ± 1.74 | 21.95 ± 2.64 | 41.99 ± 3.11 | 34.31 ± 3.18 | 33.37 ± 2.02 | 24.38 ± 1.58 | 21.43 ± 0.95 | 16.68 ± 2.17 |
| Pyr | 57.93 ± 3.46 | 44.41 ± 5.90 | 45.72 ± 5.09 | 36.15 ± 3.29 | 29.48 ± 1.82 | 23.84 ± 2.87 | 41.27 ± 3.26 | 31.64 ± 3.77 | 32.58 ± 2.11 | 25.75 ± 1.78 | 21.01 ± 0.99 | 16.98 ± 2.36 |
| BaA | 63.98 ± 3.82 | 53.33 ± 5.36 | 43.49 ± 4.84 | 34.79 ± 3.16 | 30.31 ± 1.87 | 26.72 ± 3.22 | 49.59 ± 3.6 | 41.34 ± 3.42 | 33.71 ± 2.00 | 26.96 ± 1.71 | 23.50 ± 1.02 | 20.71 ± 2.64 |
| Chr | 82.42 ± 4.92 | 71.47 ± 7.18 | 66.54 ± 7.41 | 56.5 ± 5.14 | 49.27 ± 3.04 | 42.68 ± 5.14 | 61.96 ± 4.64 | 53.73 ± 4.58 | 50.02 ± 3.07 | 42.48 ± 2.78 | 37.04 ± 1.65 | 32.08 ± 4.22 |
| BbF | 62.81 ± 3.75 | 50.73 ± 6.53 | 44.11 ± 4.91 | 37.34 ± 3.39 | 31.27 ± 1.93 | 27.65 ± 3.33 | 48.61 ± 3.53 | 39.26 ± 4.17 | 34.13 ± 2.03 | 28.90 ± 1.84 | 24.20 ± 1.05 | 21.40 ± 2.73 |
| BkF | 35.59 ± 2.13 | 28.57 ± 3.83 | 25.38 ± 2.83 | 20.24 ± 1.84 | 17.07 ± 1.05 | 14.90 ± 1.79 | 26.55 ± 2.00 | 21.31 ± 2.45 | 18.94 ± 1.17 | 15.10 ± 1.00 | 12.73 ± 0.57 | 11.11 ± 1.47 |
| BaP | 11.84 ± 0.71 | 10.11 ± 1.25 | 8.84 ± 0.98 | 7.72 ± 0.70 | 5.16 ± 0.32 | 4.64 ± 0.56 | 9.04 ± 0.67 | 8.03 ± 0.80 | 7.02 ± 0.41 | 6.13 ± 0.38 | 4.10 ± 0.17 | 3.69 ± 0.46 |
| DahA | 37.36 ± 2.23 | 31.15 ± 4.09 | 26.26 ± 2.92 | 22.54 ± 2.05 | 19.05 ± 1.18 | 16.37 ± 1.97 | 28.95 ± 2.10 | 24.15 ± 2.61 | 20.35 ± 1.21 | 17.47 ± 1.11 | 14.77 ± 0.64 | 12.69 ± 1.62 |
| BghiP | 35.46 ± 2.12 | 27.6 ± 2.77 | 20.41 ± 2.27 | 17.67 ± 1.61 | 14.95 ± 0.92 | 12.47 ± 1.50 | 25.67 ± 2.00 | 19.98 ± 1.77 | 14.77 ± 0.94 | 12.79 ± 0.87 | 10.82 ± 0.50 | 9.03 ± 1.23 |
| Ind | 8.12 ± 0.48 | 6.33 ± 1.13 | 4.53 ± 0.50 | 3.83 ± 0.35 | 2.94 ± 0.18 | 2.56 ± 0.31 | 6.39 ± 0.46 | 4.98 ± 0.74 | 3.56 ± 0.21 | 3.01 ± 0.19 | 2.31 ± 0.10 | 2.01 ± 0.25 |
| PAHs | 717.18 ± 44.97 | 602.45 ± 69.54 | 531.86 ± 59.21 | 440.44 ± 40.03 | 353.58 ± 21.85 | 305.53 ± 36.77 | 565.09 ± 43.18 | 471.89 ± 44.18 | 416.25 ± 24.5 | 345.04 ± 21.71 | 275.93 ± 11.88 | 238.83 ± 30.2 |
| CANPAHs a | 302.11 ± 18.04 | 251.68 ± 29.31 | 219.16 ± 24.40 | 182.96 ± 16.63 | 155.08 ± 9.58 | 135.52 ± 16.31 | 231.45 ± 17.00 | 192.78 ± 18.63 | 167.74 ± 10.1 | 140.05 ± 9.02 | 118.65 ± 5.21 | 103.70 ± 13.39 |
| COMPAHs b | 452.17 ± 27.01 | 375.74 ± 42.71 | 320.92 ± 35.73 | 267.13 ± 24.28 | 228.1 ± 11.47 | 191.86 ± 23.09 | 346.79 ± 25.45 | 288.70 ± 27.13 | 245.65 ± 14.78 | 204.64 ± 13.17 | 169.07 ± 4.05 | 146.96 ± 18.96 |
aCarcinogenic PAHs includes: BaA, Chr, BbF, BkF, BaP, DahA, and Ind
bCombustion PAHs includes: Flu, Pyr, BaA, Chr, BbF, BkF, BaP, BghiP, and Ind
Table 3.
PAHs concentration in indoor and outdoor environment of industrial sampling site
| PAHs | Outdoor | Indoor | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PM10 | PM2.5 | PM1 | PM10 | PM2.5 | PM1 | |||||||
| Winter | Summer | Winter | Summer | Winter | Summer | Winter | Summer | Winter | Summer | Winter | Summer | |
| Nap | 10.22 ± 0.87 | 9.65 ± 1.18 | 6.58 ± 0.53 | 6.03 ± 0.46 | 4.49 ± 0.32 | 4.02 ± 0.45 | 8.37 ± 0.29 | 7.91 ± 1.00 | 5.39 ± 0.70 | 4.94 ± 0.25 | 3.68 ± 0.22 | 3.29 ± 0.46 |
| Acy | 8.98 ± 0.77 | 8.08 ± 1.04 | 5.38 ± 0.44 | 5.00 ± 0.54 | 3.97 ± 0.33 | 3.48 ± 0.39 | 7.08 ± 0.23 | 6.37 ± 0.92 | 4.24 ± 0.57 | 3.94 ± 0.34 | 3.13 ± 0.26 | 2.74 ± 0.40 |
| Ace | 4.74 ± 0.41 | 4.07 ± 0.56 | 3.25 ± 0.26 | 3.00 ± 0.33 | 2.39 ± 0.16 | 1.94 ± 0.22 | 3.54 ± 0.17 | 3.04 ± 0.53 | 2.42 ± 0.35 | 2.24 ± 0.21 | 1.78 ± 0.11 | 1.45 ± 0.22 |
| Flu | 116.75 ± 10.09 | 109.54 ± 13.62 | 72.83 ± 5.89 | 68.39 ± 4.83 | 49.65 ± 3.54 | 44.37 ± 5.00 | 96.05 ± 3.90 | 90.11 ± 11.69 | 59.91 ± 7.76 | 56.26 ± 2.57 | 40.85 ± 2.46 | 36.50 ± 5.13 |
| Phen | 142.32 ± 12.16 | 128.17 ± 17.35 | 108.45 ± 8.78 | 99.43 ± 7.87 | 63.70 ± 4.63 | 58.84 ± 6.63 | 120.92 ± 3.12 | 108.90 ± 16.03 | 92.14 ± 11.56 | 84.48 ± 4.42 | 54.12 ± 3.31 | 49.99 ± 6.80 |
| Anth | 56.35 ± 4.82 | 50.77 ± 6.18 | 41.99 ± 3.4 | 38.09 ± 3.58 | 27.18 ± 2.36 | 23.58 ± 2.66 | 44.75 ± 1.57 | 40.32 ± 5.15 | 33.34 ± 4.48 | 30.25 ± 2.16 | 21.58 ± 1.91 | 18.73 ± 2.72 |
| Flrt | 69.41 ± 5.93 | 61.23 ± 8.14 | 51.7 ± 4.18 | 41.34 ± 3.01 | 33.71 ± 2.38 | 26.76 ± 3.02 | 52.73 ± 1.52 | 46.51 ± 7.43 | 39.27 ± 5.51 | 31.40 ± 1.63 | 25.61 ± 1.62 | 20.33 ± 3.09 |
| Pyr | 72.41 ± 6.19 | 58.74 ± 8.46 | 53.81 ± 4.35 | 46.10 ± 4.25 | 35.57 ± 2.70 | 29.06 ± 3.27 | 51.59 ± 1.92 | 41.85 ± 8.06 | 38.34 ± 5.74 | 32.58 ± 2.55 | 25.35 ± 2.01 | 20.70 ± 3.36 |
| BaA | 80.01 ± 6.83 | 70.23 ± 8.55 | 51.18 ± 4.14 | 44.15 ± 3.82 | 35.85 ± 3.01 | 32.57 ± 3.67 | 62.02 ± 2.08 | 54.44 ± 7.13 | 39.68 ± 5.46 | 34.22 ± 2.23 | 27.79 ± 2.4 | 25.25 ± 3.76 |
| Chr | 103.17 ± 8.81 | 95.66 ± 11.24 | 78.31 ± 6.34 | 72.25 ± 5.59 | 59.11 ± 4.29 | 52.02 ± 5.86 | 77.56 ± 2.59 | 71.91 ± 8.73 | 58.87 ± 8.35 | 54.31 ± 3.11 | 44.44 ± 3.05 | 39.11 ± 6.01 |
| BbF | 78.55 ± 6.71 | 67.29 ± 8.96 | 51.91 ± 4.2 | 47.62 ± 4.38 | 37.01 ± 3.10 | 33.71 ± 3.80 | 60.79 ± 2.05 | 52.07 ± 8.20 | 40.17 ± 5.53 | 36.85 ± 2.62 | 28.64 ± 2.46 | 26.09 ± 3.90 |
| BkF | 44.7 ± 3.82 | 37.73 ± 5.42 | 29.87 ± 2.42 | 26.33 ± 1.76 | 20.6 ± 1.56 | 18.16 ± 2.05 | 33.34 ± 0.98 | 28.14 ± 5.15 | 22.28 ± 3.18 | 19.64 ± 0.91 | 15.36 ± 1.16 | 13.55 ± 2.10 |
| BaP | 14.87 ± 1.27 | 13.3 ± 1.78 | 10.41 ± 0.84 | 9.78 ± 0.82 | 6.23 ± 0.47 | 5.66 ± 0.64 | 11.80 ± 0.33 | 10.56 ± 1.64 | 8.26 ± 1.11 | 7.76 ± 0.47 | 4.94 ± 0.35 | 4.49 ± 0.65 |
| DahA | 46.91 ± 4.01 | 42.53 ± 5.26 | 30.9 ± 2.50 | 29.95 ± 1.68 | 22.99 ± 1.74 | 19.96 ± 2.25 | 36.36 ± 1.03 | 32.96 ± 4.47 | 23.95 ± 3.29 | 23.22 ± 0.79 | 17.82 ± 1.3 | 15.57 ± 2.31 |
| BghiP | 44.2 ± 3.79 | 36.55 ± 4.57 | 24.02 ± 1.94 | 22.37 ± 1.87 | 17.70 ± 1.20 | 15.2 ± 1.71 | 31.99 ± 1.30 | 26.46 ± 3.94 | 17.39 ± 2.56 | 16.20 ± 1.08 | 12.81 ± 0.73 | 11.01 ± 1.76 |
| Ind | 10.19 ± 0.87 | 8.52 ± 1.33 | 5.33 ± 0.43 | 4.84 ± 0.41 | 3.55 ± 0.27 | 3.12 ± 0.35 | 8.02 ± 0.22 | 6.70 ± 1.30 | 4.19 ± 0.57 | 3.81 ± 0.23 | 2.79 ± 0.20 | 2.45 ± 0.36 |
| PAHs | 903.79 ± 77.19 | 799.33 ± 100.84 | 625.90 ± 50.66 | 564.66 ± 44.98 | 423.69 ± 31.78 | 372.43 ± 41.97 | 706.91 ± 23.26 | 628.26 ± 87.97 | 489.85 ± 66.72 | 442.36 ± 25.38 | 330.69 ± 23.44 | 291.13 ± 43.04 |
| CANPAHs a | 378.4 ± 32.31 | 335.26 ± 42.14 | 257.91 ± 20.87 | 234.92 ± 18.33 | 185.33 ± 14.36 | 165.2 ± 18.62 | 289.89 ± 9.27 | 256.80 ± 36.62 | 197.40 ± 27.49 | 179.82 ± 10.24 | 141.78 ± 10.9 | 126.40 ± 19.09 |
| COMPAHs b | 564.85 ± 48.29 | 497.57 ± 63.37 | 377.66 ± 30.56 | 341.83 ± 27.65 | 265.27 ± 19.95 | 233.87 ± 26.35 | 433.16 ± 15.35 | 382.26 ± 55.83 | 289.90 ± 40.26 | 261.90 ± 15.71 | 202.97 ± 14.76 | 179.14 ± 27.03 |
aCarcinogenic PAHs includes: BaA, Chr, BbF, BkF, BaP, DahA, and Ind
bCombustion PAHs includes: Flu, Pyr, BaA, Chr, BbF, BkF, BaP, BghiP, and Ind
To identify indoor and outdoor PAHs compositional patterns, the relative proportions of each PAH species were analyzed. The most plentiful indoor and outdoor PAHs in PM10, PM2.5, and PM1 and in the three sampling sites were as follow: Phen>Flu> Chr > BaA. These dominant PAHs are known as components of fuel combustion (e.g., vehicles, oil burning and coal combustion emissions) and were 3 rings and 4 rings PAHs.
PAHs components can be classified to a) combustion-derived PAHs (COMPAHs) b) carcinogenic PAHs (CANPAHs). Combustion-derived PAHs as their name implies, are known to be produced as a result of the combustion process including Flu, Pyr, BaA, Chr, BbF, BkF, BaP, BghiP, and Ind [44, 57]. Carcinogenic PAHs, which were used to evaluate the carcinogenic potential risk of the PAHs to the exposed respiratory of the human body, including BaA, Chr, BbF, BkF, BaP, DahA, and Ind [58].
The indoor and outdoor COMPAHs and CANPAHs values are presented in Tables 1-3. COMPAHs/TPAHs concentration ratios in the sampling sites in summer and winter, accounted for: PM10 59.02–60.65%, PM2.5 60.33–62.84%, and PM1 61.38–64.56%, respectively, proposing the use of different fuel combustion sources in the study area. The COMPAHs/TPAHs values obtained in winter are more than in summer, which can be attributed to the change in the types of fuel consumed in domestic and industrial sections of this area throughout the year.
The results showed that the indoor and outdoor CANPAHs/ TPAHs concentration ratios in the sampling sites in summer and winter were as follow: for PM10 40.15–42.51%, PM2.5 41.30–42.97%, and PM1 43.07–44.36%, respectively. Since the CANPAHs/ TPAHs values trends were similar to the registered temporal variation of PAHs concentrations (winter more than summer), it can be interpreted that both PAHs and CANPAHs were originated from the same sources. Furthermore, as seen the smaller the particle size, the higher the percentage of combustion-derived PAHs and carcinogenic PAHs.
Size distribution of PAHs components
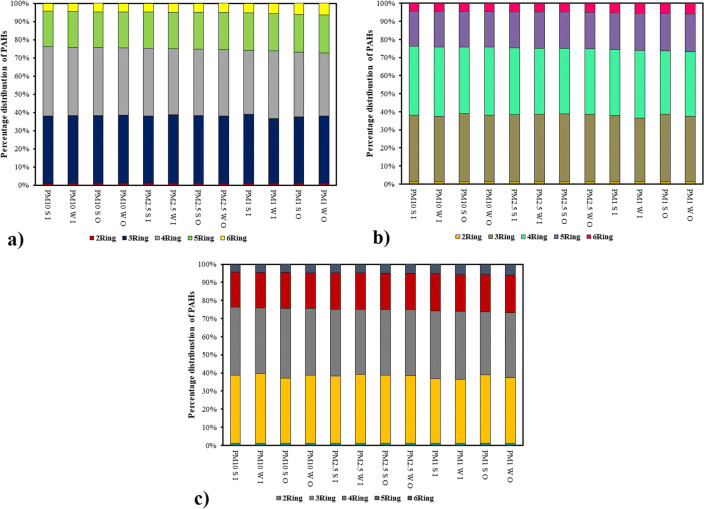
According to the number of aromatic rings that PAHs compounds have, they can be simply categorized into: 2-ring PAHs (include Nap); 3-ring PAHs (include Ace, Acy, Flu, Phen, and Anth); 4-ring PAHs (include Flt, Pyr, BaA, and Chr); 5-ring PAHs (include BbF, BkF, BaP, and DahA); and 6-ring PAHs (include BghiP and Ind) [59]. Figure 4 showed the percentage of PAHs with different number of rings to the total PAHs in indoor and outdoor PM of the sampling sites.
Fig. 4.
Seasonal percentage distribution of PAHs with different number of rings for size-segregated particles at a. residential, b. high traffic, and c. industrial
Two rings PAHs had a very small contribution to the total PAHs (about 1%), whereas PAHs with 3–4 rings had much larger contributions, ranging from 71.65% to 75.17%. PAHs with 2 or 3 rings usually exist in gaseous phase, however PAHs with 4 or more rings are less volatile [60]. The results demonstrated that as PM size decreased, the proportion of 5-to-6-ring PAHs to the total PAHs increased. The 5-to-6- ring PAHs are considered to be more toxic; hence more attention should be paid to smaller particles. As the Previous studies have found, the high tendency of PAHs components with 4-to-6-ring to be absorbed and condensed firmly by smaller particles, because of a large surface area availability, is one of the probable reasons [61–63]. In general, it can be suggested that a pollutant is presumably adsorbed or condensed on particles during emission since the pollutant geometric mean diameter is smaller than that of the PM mass [63].
Source identification of PAHs
Source apportionment is one of the most important steps in establishing the best control policies for environmental pollutants. PAHs diagnostic ratios may provide crucial data for the identification of main PAHs emission sources, which can be achieved by comparing ratios of pairs of frequently found PAH emissions [17].
Table 4 represents some PAHs isomer pair ratios which have often been used because of their relative stability to differentiate the possible types of PAH sources in the environment [64, 73] including: Phen/(Phen + Anth), Flrt/(Flrt + Pyr), Ind/(Ind + BghiP), BaA/(BaA + Chr), BaP/BghiP, Flu/(Flu + Pyr) and BaP/(BaP + Chy). To define the prevalence of Petrogenic, vehicles (gasoline or diesel), natural gas, oil, coal, grass, and wood combustion, as well as a number of other sources, these PAHs isomer pair ratios are often used [31].
Table 4.
Diagnostic ratios for different indoor and outdoor fraction sizes of particulate matter
| Ratios | Value | Sources | Reference |
|---|---|---|---|
| Ind/(Ind + BghiP) | <0.2 | Petrogenic | Yunker et al. [64] |
| 0.2–0.5 | Petroleum combustion | ||
| >0.5 | Grass, wood, and coal combustion | ||
| BaA/(BaA + Chr) | 0.38–0.64 | Diesel emissions | Manoli et al. [65] |
| 0.76 | Gasoline emissions | ||
| BaP/BghiP | <0.6 | Non-traffic emissions | Katsoyiannis et al. [66] |
| >0.6 | Traffic emissions | ||
| Flrt/(Flrt + Pyr) | <0.4 | Petrogenic | Yunker et al. [64] |
| 0.4–0.5 | Liquid fossil fuel | Yang et al. [67] | |
| >0.5 | Grass, wood, and coal combustion | Jiang et al. [68] | |
| Flu/(Flu + Pyr) | <0.5 | Gasoline emissions | Ravindra et al. [17, 51] |
| >0.5 | Diesel emissions | ||
| BaP/(BaP + Chy) | <0.5 | Diesel emissions | Khalili et al. [69] |
| >0.5 | Gasoline emissions | Guo et al. [70] | |
| Phen/(Phen + Anth) | >0.7 | Lubricant oils and fossil fuels | Mirante et al. [71] |
| BaA/ Chr | >0.35 | Fuel combustion | Krugly et al. [72] |
As shown in Table 5 and by comparing the values derived from indoor and outdoor diagnostic ratios of PAHs in the three sampling sites in both summer and winter seasons, it turns out that they have a little fluctuation. From this it can be concluded that indoor airborne PAHs are mainly influenced by outdoor sources. The results of this part are in compliance with [74].
Table 5.
Diagnostic ratios for different indoor and outdoor fraction sizes of particulate matter
| Ind/(Ind + BghiP) | BaA/(BaA + Chr) | BaP/BghiP | Flrt/(Flrt + Pyr) | Flu/(Flu + Pyr) | BaP/(BaP + Chy) | Phen/(Phen + Anth) | BaA/ Chr | |
|---|---|---|---|---|---|---|---|---|
| Out PM10a,w | 0.19 | 0.44 | 0.33 | 0.49 | 0.62 | 0.13 | 0.72 | 0.78 |
| Out PM10a,s | 0.20 | 0.44 | 0.37 | 0.50 | 0.65 | 0.13 | 0.73 | 0.80 |
| Out PM2.5 a,w | 0.18 | 0.40 | 0.43 | 0.49 | 0.58 | 0.12 | 0.72 | 0.65 |
| Out PM2.5 a,s | 0.19 | 0.40 | 0.48 | 0.51 | 0.61 | 0.12 | 0.73 | 0.67 |
| Out PM1 a,w | 0.16 | 0.38 | 0.35 | 0.49 | 0.58 | 0.09 | 0.70 | 0.62 |
| Out PM1 a,s | 0.17 | 0.39 | 0.37 | 0.48 | 0.60 | 0.10 | 0.71 | 0.63 |
| In PM10a,w | 0.19 | 0.44 | 0.33 | 0.50 | 0.61 | 0.13 | 0.69 | 0.80 |
| In PM10a.s | 0.21 | 0.45 | 0.40 | 0.52 | 0.68 | 0.14 | 0.74 | 0.83 |
| In PM2.5 a,w | 0.18 | 0.40 | 0.43 | 0.49 | 0.58 | 0.12 | 0.72 | 0.65 |
| In PM2.5 a,s | 0.18 | 0.38 | 0.44 | 0.47 | 0.59 | 0.12 | 0.72 | 0.62 |
| In PM1 a,w | 0.18 | 0.39 | 0.38 | 0.51 | 0.61 | 0.10 | 0.71 | 0.63 |
| In PM1 a.s | 0.18 | 0.39 | 0.41 | 0.50 | 0.64 | 0.10 | 0.73 | 0.65 |
| Out PM10b,w | 0.19 | 0.44 | 0.33 | 0.49 | 0.62 | 0.13 | 0.72 | 0.78 |
| Out PM10b,s | 0.19 | 0.43 | 0.37 | 0.50 | 0.65 | 0.12 | 0.71 | 0.75 |
| Out PM2.5 b,w | 0.18 | 0.40 | 0.43 | 0.49 | 0.58 | 0.12 | 0.72 | 0.65 |
| Out PM2.5 b,s | 0.18 | 0.38 | 0.44 | 0.47 | 0.59 | 0.12 | 0.72 | 0.62 |
| Out PM1 b,w | 0.16 | 0.38 | 0.35 | 0.49 | 0.58 | 0.09 | 0.70 | 0.62 |
| Out PM1 b,s | 0.17 | 0.39 | 0.37 | 0.48 | 0.60 | 0.10 | 0.71 | 0.63 |
| In PM10b,w | 0.20 | 0.44 | 0.37 | 0.50 | 0.65 | 0.13 | 0.73 | 0.80 |
| In PM10b.s | 0.20 | 0.43 | 0.40 | 0.52 | 0.68 | 0.13 | 0.73 | 0.77 |
| In PM2.5 b,w | 0.19 | 0.40 | 0.48 | 0.51 | 0.61 | 0.12 | 0.73 | 0.67 |
| In PM2.5 b,s | 0.19 | 0.39 | 0.48 | 0.49 | 0.63 | 0.13 | 0.74 | 0.63 |
| In PM1 b,w | 0.18 | 0.39 | 0.38 | 0.51 | 0.61 | 0.10 | 0.71 | 0.63 |
| In PM1 b.s | 0.18 | 0.39 | 0.41 | 0.50 | 0.64 | 0.10 | 0.73 | 0.65 |
| Out PM10c,w | 0.19 | 0.44 | 0.34 | 0.49 | 0.62 | 0.13 | 0.72 | 0.78 |
| Out PM10c,s | 0.19 | 0.42 | 0.36 | 0.51 | 0.65 | 0.12 | 0.72 | 0.73 |
| Out PM2.5 c,w | 0.18 | 0.40 | 0.43 | 0.49 | 0.58 | 0.12 | 0.72 | 0.65 |
| Out PM2.5 c,s | 0.18 | 0.38 | 0.44 | 0.47 | 0.60 | 0.12 | 0.72 | 0.61 |
| Out PM1 c,w | 0.17 | 0.38 | 0.35 | 0.49 | 0.58 | 0.10 | 0.70 | 0.61 |
| Out PM1 c,s | 0.17 | 0.39 | 0.37 | 0.48 | 0.60 | 0.10 | 0.71 | 0.63 |
| In PM10c,w | 0.20 | 0.44 | 0.37 | 0.51 | 0.65 | 0.13 | 0.73 | 0.80 |
| In PM10c.s | 0.20 | 0.43 | 0.40 | 0.53 | 0.68 | 0.13 | 0.73 | 0.76 |
| In PM2.5 c,w | 0.19 | 0.40 | 0.48 | 0.51 | 0.61 | 0.12 | 0.73 | 0.67 |
| In PM2.5 c,s | 0.19 | 0.39 | 0.48 | 0.49 | 0.63 | 0.13 | 0.74 | 0.63 |
| In PM1 c,w | 0.18 | 0.38 | 0.39 | 0.50 | 0.62 | 0.10 | 0.71 | 0.63 |
| In PM1 c.s | 0.18 | 0.39 | 0.41 | 0.50 | 0.64 | 0.10 | 0.73 | 0.65 |
Out outdoor, In indoor, a residential site, b high traffic site, c industrial site, w winter season, s summer season
The ratios of IcdP/ (IcdP + BghiP) varied between 0.16–0.21, revealing that petroleum sources (petrogenic) and petroleum combustion are the main sources of PAHs compounds emissions in the study area [64], and which can be corresponded to a large number of petrochemical industries in Bandar Mahshar City. Whereas BaA/ (BaA + Chy) varied between 0.38–0.45, indicating that diesel emissions are also was contributed [65].
The values of Flrt/ (Flrt + Pyr) ratios varied between 0.47 and 0.53. Thus, from this result, it was suggested that the source of combustion of liquid fossil fuel and coal [64] was contributed. The ratios of BaP/BghiP varied between 0.33 and 0.48, revealing that non-traffic emissions are a more important source [66] in Bandar Mahshar City (as the study area is affected by industrial complexes exhaust emissions).
The values of Flu/ (Flu + Pyr) ratios varied between 0.58 and 0.68, and BaP/ (BaP + Chy) ratios varied between 0.09 and 0.14. As our results, it was considered that the diesel exhaust emission was also a noticeable source [17, 51, 69, 70]. Table 5 also shows that the Phen/ (Phen + Anth) ratios varied between 0.69 and 0.74, and BaA/ Chr ratios varied between 0.61 and 0.83. Hence, according to this result, it was suggested that the sources of fuel combustion (especially oil and fossil fuels combustions) [71, 72] were contributed.
By comparing the data (Table 5), most of the diagnostic ratios of indoor and outdoor of three sampling sites in both seasons suggested that petrogenic sources, as well as combustion of petroleum and other fossil fuels were the main PAHs sources. Moreover, with relatively lower contributions diesel exhaust emissions.
In conclusion, it is suggested that local industrial emissions (from the combustion of liquid fossil fuels especially in winter because of shortage in gas supplies), and vehicle traffic may be the major sources of atmospheric PAHs in Bandar Mahshar. In winter, there was a possibility that the air mass polluted by the combustion of coal and mazut from industries in Bandar Mahshar city. Therefore, PAHs in atmospheric fine particles of the Bandar Mahshar city will pose a stronger toxicity and do greater harm to human health in winter.
It should also be noted that some studies have questioned the competence of these diagnostic ratios since each PAH compounds have different reactivities and atmospheric residence times. Moreover, atmospheric sources are to somewhat complex in real-world settings [20, 75, 76].
Health risk assessment
The estimated carcinogenic risk assessment indicators including BaP-TEQ and BaP-MEQ concentrations are presented in Table 6.
Table 6.
Seasonal profile of carcinogenic risk assessment
| Outdoor | Indoor | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PM10 | PM2.5 | PM1 | PM10 | PM2.5 | PM1 | |||||||
| Winter | Summer | Winter | Summer | Winter | Summer | Winter | Summer | Winter | Summer | Winter | Summer | |
| BaP-TEQa | 60.54 | 47.01 | 40.40 | 31.37 | 30.62 | 25.08 | 45.17 | 36.52 | 32.52 | 27.31 | 23.76 | 19.47 |
| BaP-MEQa | 51.64 | 39.80 | 33.64 | 25.96 | 25.16 | 20.72 | 39.70 | 30.70 | 27.08 | 22.70 | 19.39 | 15.97 |
| BaP-TEQb | 68.22 | 56.81 | 48.33 | 41.14 | 33.41 | 29.08 | 52.97 | 44.13 | 37.54 | 31.96 | 25.93 | 22.57 |
| BaP-MEQb | 58.19 | 47.76 | 40.22 | 34.18 | 27.46 | 24.02 | 44.84 | 36.83 | 31.06 | 26.38 | 21.16 | 18.52 |
| BaP-TEQc | 85.59 | 76.42 | 56.88 | 53.62 | 40.15 | 34.45 | 64.46 | 59.35 | 44.18 | 41.66 | 31.16 | 27.52 |
| BaP-MEQc | 72.90 | 63.58 | 47.37 | 43.87 | 32.82 | 29.28 | 56.18 | 49.03 | 36.55 | 33.86 | 25.29 | 25.57 |
As shown in Table 6, the annual concentrations of indoor and outdoor BaP-TEQ and BaP-MEQ were different in the three sampling sites, which could be possibly attributed to the different pollution background of these sampling sites.
The indoors and outdoors annual mean total concentrations of BaP-TEQ in three sampling points in summer ranged from 36.52 to 76.42, 27.31 to 53.62, and 19.47 to 34.45 ng/m3 for PM10, PM2.5 and PM1, respectively, and in winter ranged from 45.17 to 85.59, 35.52 to 56.88, and 23.76 to 40.15 ng/m3 for PM10, PM2.5 and PM1, respectively.
Moreover, the total concentrations of BaP-MEQ in summer were found to be ranging from 30.70 to 63.58, 22.70 to 46.87, and 15.97 to 29.28 ng/m3 for PM10, PM2.5, and PM1, respectively, and in winter ranged from 39.70 to 72.90, 27.08 to 47.37, and 19.39 to 32.82 ng/m3 for PM10, PM2.5 and PM1, respectively (Table 6).
The mean values of indoor and outdoor BaP-TEQ and BaP-MEQ in the residential and high traffic areas were lower than those obtained from the industrial area. This difference may be attributed to the presence of main PAHs sources such as petrochemical complexes in this area that have increased the BaPTEQ and BaP-MEQ concentrations. Based on Table 6, in all three sampling sites, the outdoor BaP-TEQ and BaPMEQ values were higher than those obtained from indoors. Using the TEF values proposed by Nisbet and LaGoy [34] for inhalation of particle-bonded PAHs, the average BaP-TEQ concentrations in indoor and outdoor PM measured in all three sampling sites significantly surpassed the maximum acceptable risk level of 1 ng/m3 for BaP. Based on the BaPTEQ values, outdoor environments have a higher potential cancer risk compared to indoor environments. The high concentrations of total BaP-TEQ and BaP-MEQ in Bandar Mahshar is a warning sign for human health risks associated with the high levels of PAHs. The WHO has suggested that exposure to BaP in the air at a value of 1 ng/m3 throughout lifetime can cause an excessive lung cancer in 1 out of 10,000 individuals [77].
Conclusions
PAHs compounds have noticeable influences on both human health and ecosystem stability. The concentrations of indoor and outdoor PM (PM10, PM2.5, and PM1) and bounded PAHs were measured in three sampling sites of Bandar Mahshar City, Iran from October to December 2015, and July to September 2016. Results indicated that the concentrations of indoor and outdoor PM10 and PM2.5 in the three sampling sites were much higher than the Iranian national PM standard levels. Distinct seasonal and spatial variability in atmospheric PAHs concentrations at sampling sites were observed. Seasonal values comparisons revealed that there were significant differences between PAHs concentrations in winter and summer. The highest concentrations of particle-bonded PAHs were recorded at the industrial sampling site, which were influenced by emissions from the industrial activities especially from adjacent petrochemical complexes. Based on the results presented in the study the most abundant PAHs were formed by three rings (Ace, Acy, Flu, Phen, and Anth) or four rings (Flt, Pyr, BaA, and Chr) PAHs, while the less abundant PAHs were formed by two rings (NaP). Diagnostic ratios suggested that industrial sources make the greatest contributions of PAHs to the environment; these primarily petrogenic sources included combustion of petroleum and other fossil fuels, with a relatively smaller contribution coming from diesel exhaust emissions. This proves the importance of the contribution of industrial sources to average daily PAHs concentrations.
Moreover, according to the results of the carcinogenic risk assessment, the average indoors and outdoors BaP-TEQ levels of the three sampling sites considerably exceeded the maximum permissible risk level of 1 ng/m3 for BaP. Furthermore, outdoor environments had a higher potential carcinogenic and mutagenic risk compared to indoor environments. It seems that establishing the best control policies is a crucial measure for mitigating the concentration of PAHs compounds especially from local petrochemical industries (as the main sources of PAHs compounds in Bandar Mahshahr city).
Acknowledgments
This paper is issued from APRD-9501 as a project number in Air Pollution and Respiratory Diseases research center and also M.Sc. thesis of Faezeh Jahedi. Financial support of this research was provided by Ahvaz Jundishapur University of Medical Sciences (AJUMS).
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Dehghan A, Khanjani N, Bahrampour A, Goudarzi G, Yunesian M. The relation between air pollution and respiratory deaths in Tehran, Iran-using generalized additive models. BMC pulmonary medicine. 2018; 18(1), 1-9. [DOI] [PMC free article] [PubMed]
- 2.Goudarzi G, Shirmardi M, Naimabadi A, Ghadiri A, Sajedifar J. Chemical and organic characteristics of PM2. 5 particles and their in-vitro cytotoxic effects on lung cells: The Middle East dust storms in Ahvaz, Iran. Science of The Total Environment. 2019; 655, 434-445. [DOI] [PubMed]
- 3.Li N, Han W, Wei X, Shen M, Sun S. Chemical characteristics and human health assessment of PM1 during the Chinese spring festival in Changchun, Northeast China. Atmospheric Pollut Res. 2019;10(6):1823–1831. [Google Scholar]
- 4.Farsani MH, Shirmardi M, Alavi N, Maleki H, Sorooshian A, Babaei A, et al. Evaluation of the relationship between PM10 concentrations and heavy metals during normal and dusty days in Ahvaz, Iran. Aeolian Research. 2018; 33, 12-22.
- 5.Marzouni MB, Alizadeh T, Banafsheh MR, Khorshiddoust AM, Ghozikali MG, Akbaripoor S, Sharifi R, Goudarzi G. A comparison of health impacts assessment for PM10 during two successive years in the ambient air of Kermanshah, Iran. Atmospheric Pollut Res. 2016;7(5):768–774. [Google Scholar]
- 6.Marzouni MB, Moradi M, Zarasvandi A, Akbaripoor S, Hassanvand MS, Neisi A, Goudarzi G, Mohammadi MJ, Sheikhi R, Kermani M, Shirmardi M. Health benefits of PM10 reduction in Iran. Int J Biometeorol. 2017;61:1389–1401. doi: 10.1007/s00484-017-1316-2. [DOI] [PubMed] [Google Scholar]
- 7.Chen C, Zhao B. Review of relationship between indoor and outdoor particles: I/O ratio, infiltration factor and penetration factor. Atmos Environ. 2011;45:275–288. [Google Scholar]
- 8.Dominici F, Peng RD, Bell ML, Pham L, McDermott A, Zeger SL, Samet JM. Fine particulate air pollution and hospital admission for cardiovascular and respiratory diseases. Jama. 2006;295:1127–1134. doi: 10.1001/jama.295.10.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pope CA, Dockery DW. Health effects of fine particulate air pollution: lines that connect. J Air Waste Manage Assoc. 2006;56:709–742. doi: 10.1080/10473289.2006.10464485. [DOI] [PubMed] [Google Scholar]
- 10.Watson JG. Visibility: science and regulation. J Air Waste Manage Assoc. 2002;52:628–713. doi: 10.1080/10473289.2002.10470813. [DOI] [PubMed] [Google Scholar]
- 11.Harrison RM, Smith D, Luhana L. Source apportionment of atmospheric polycyclic aromatic hydrocarbons collected from an urban location in Birmingham, UK. Environ Sci Technol. 1996;30:825–832. [Google Scholar]
- 12.Nation Research Council, (NRC), 1998. Nation research council. Research priorities for airborne particulate matter: I. Immediate Priorities and a Long-Range Research Portfolio. [PubMed]
- 13.Buczyńska AJ, Krata A, Van Grieken R, Brown A, Polezer G, De Wael K, Potgieter-Vermaak S. Composition of PM2.5 and PM1 on high and low pollution event days and its relation to indoor air quality in a home for the elderly. Sci Total Environ. 2014;490:134–143. doi: 10.1016/j.scitotenv.2014.04.102. [DOI] [PubMed] [Google Scholar]
- 14.Kanakidou M, Seinfeld J, Pandis S, Barnes I, Dentener F, Facchini M, Dingenen RV, Ervens B, Nenes A, Nielsen C. Organic aerosol and global climate modelling: a review. Atmos Chem Phys. 2005;5:1053–1123. [Google Scholar]
- 15.Turpin BJ, Saxena P, Andrews E. Measuring and simulating particulate organics in the atmosphere: problems and prospects. Atmos Environ. 2000;34:2983–3013. [Google Scholar]
- 16.Ré-Poppi N, Santiago-Silva M. Polycyclic aromatic hydrocarbons and other selected organic compounds in ambient air of Campo Grande City, Brazil. Atmos Environ. 2005;39:2839–2850. [Google Scholar]
- 17.Ravindra K, Sokhi R, Van Grieken R. Atmospheric polycyclic aromatic hydrocarbons: source attribution, emission factors and regulation. Atmos Environ. 2008;42:2895–2921. [Google Scholar]
- 18.Ma W-L, Sun D-Z, Shen W-G, Yang M, Qi H, Liu L-Y, Shen J-M, Li Y-F. Atmospheric concentrations, sources and gas-particle partitioning of PAHs in Beijing after the 29th Olympic games. Environ Pollut. 2011;159:1794–1801. doi: 10.1016/j.envpol.2011.03.025. [DOI] [PubMed] [Google Scholar]
- 19.Yan C, Zheng M, Yang Q, Zhang Q, Qiu X, Zhang Y, Fu H, Li X, Zhu T, Zhu Y. Commuter exposure to particulate matter and particle-bound PAHs in three transportation modes in Beijing, China. Environ Pollut. 2015;204:199–206. doi: 10.1016/j.envpol.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 20.Tobiszewski M, Namieśnik J. PAH diagnostic ratios for the identification of pollution emission sources. Environ Pollut. 2012;162:110–119. doi: 10.1016/j.envpol.2011.10.025. [DOI] [PubMed] [Google Scholar]
- 21.Mahler BJ, Metre PCV, Crane JL, Watts AW, Scoggins M, Williams ES. Coaltar-based pavement sealcoat and PAHs: implications for the environment, human health, and stormwater management. Environ Sci Technol. 2012;46(6):3039–3045. doi: 10.1021/es203699x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.US EPA, 1984, Guidelines for carcinogen risk assessment, EPA/630/P-03/001F, US Environmental Protection Agency, Washington, D.C., United States of America, 2005, http://www.epa.gov/raf/publications/pdfs/CANCER GUIDELINES FINAL 3-25-05.Pdf (accessed in may 2016).
- 23.IARC, 2002. Some traditional herbal medicines, some mycotoxins, naphthalene and styrene. World Health Organization. [PMC free article] [PubMed]
- 24.IARC Some non-heterocyclic polycyclic aromatic hydrocarbons and some related exposures. IARC Monogr Eval Carcinog Risks Hum. 2010;92:1. [PMC free article] [PubMed] [Google Scholar]
- 25.Bergman, Å., Heindel, J., Jobling, S., Kidd, K., Zoeller, R., 2013. State of the science of endocrine disrupting chemicals, 2012. United Nations environment Programme and the World Health Organization. Unep. Org/pdf/9789 241505031_eng. Pdf.
- 26.ATSDR, 1995. Toxicological profile for polycyclic aromatic hydrocarbons, Agency for Toxic Substances and Disease Registry, Atlanta, 1995, http://www.atsdr.cdc.gov/toxprofiles/tp69.html (accessed 16.02.16). [PubMed]
- 27.Hassanvand MS, Naddafi K, Faridi S, Nabizadeh R, Sowlat MH, Momeniha F, Gholampour A, Arhami M, Kashani H, Zare A, Niazi S. Characterization of PAHs and metals in indoor/outdoor PM10/PM2. 5/PM1 in a retirement home and a school dormitory. Sci Total Environ. 2015;527:100–110. doi: 10.1016/j.scitotenv.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 28.Pfeiffer R. Sampling for PM10 and PM2.5 particulates. Micrometeorology in Agricultural Systems. 2005;47:227–245. [Google Scholar]
- 29.NIOSH, 1994. Manual of analytical methods (NMAM): polynuclear aromatic hydrocarbons by GC. METHOD5515. National Institute for Occupational Safety and Health, Washington DC, pp. 2–7.
- 30.Pandey SK, Kim KH, Brown RJ. A review of techniques for the determination of polycyclic aromatic hydrocarbons in air. TrAC Trends Anal Chem. 2011;30(11):1716–1739. [Google Scholar]
- 31.Singh D, Gadi R, Mandal TK. Characterization of particulate-bound polycyclic aromatic hydrocarbons and trace metals composition of urban air in Delhi, India. Atmos Environ. 2011;45:7653–7663. [Google Scholar]
- 32.US EPA, 1999, Compendium method TO- 13A – determination of polycyclic aromatic hydrocarbons (PAH) in ambient air using gas chromatography/mass spectrometry (CG/MS). Center for environmental research information.
- 33.Zhou J, Wang T, Zhang Y, Zhong N, Medeiros PM, Simoneit BR. Composition and sources of organic matter in atmospheric PM10 over a two year period in Beijing. China Atmos Res. 2009;93:849–861. [Google Scholar]
- 34.Nisbet IC, LaGoy PK. Toxic equivalency factors (TEFs) for polycyclic aromatic hydrocarbons (PAHs) Regul Toxicol Pharmacol. 1992;16:290–300. doi: 10.1016/0273-2300(92)90009-x. [DOI] [PubMed] [Google Scholar]
- 35.Durant JL, Lafleur AL, Busby WF, Donhoffner LL, Penman BW, Crespi CL. Mutagenicity of C 24 H 14 PAH in human cells expressing CYP1A1. Mutation Research/Genetic Toxicology and Environmental Mutagenesis. 1999;446:1–14. doi: 10.1016/s1383-5718(99)00135-7. [DOI] [PubMed] [Google Scholar]
- 36.Durant JL, Busby WF, Jr, Lafleur AL, Penman BW, Crespi CL. Human cell mutagenicity of oxygenated, nitrated and unsubstituted polycyclic aromatic hydrocarbons associated with urban aerosols. Mutat Res Genet Toxicol. 1996;371(3–4):123–57. doi: 10.1016/s0165-1218(96)90103-2. [DOI] [PubMed] [Google Scholar]
- 37.Jung KH, Yan B, Chillrud SN, Perera FP, Whyatt R, Camann D, et al. Assessment of benzo (a) pyrene-equivalent carcinogenicity and mutagenicity of residential indoor versus outdoor polycyclic aromatic hydrocarbons exposing young children in New York City. Int. J. Environ. Res. Public Health. 2010;7:1889–1900. doi: 10.3390/ijerph7051889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.WHO, , 2005. Air quality guidelines: global update 2005: particulate matter, ozone, nitrogen dioxide, and sulfur dioxide. World Health Organization. [PubMed]
- 39.Oliveira M, Slezakova K, Delerue-Matos C, Pereira MC, Morais S. Assessment of polycyclic aromatic hydrocarbons in indoor and outdoor air of preschool environments (3–5 years old children) Environ Pollut. 2016;208:382–394. doi: 10.1016/j.envpol.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 40.Chen Y-C, Chiang H-C, Hsu C-Y, Yang T-T, Lin T-Y, Chen M-J, Chen N-T, Wu Y-S. Ambient PM 2.5-bound polycyclic aromatic hydrocarbons (PAHs) in Changhua County, Central Taiwan: seasonal variation, source apportionment and cancer risk assessment. Environ Pollut. 2016;218:372–382. doi: 10.1016/j.envpol.2016.07.016. [DOI] [PubMed] [Google Scholar]
- 41.Tolis EI, Saraga DE, Lytra MK, Papathanasiou AC, Bougaidis PN, Prekas-Patronakis OE, Ioannidis II, Bartzis JG. Concentration and chemical composition of PM2. 5 for a one-year period at Thessaloniki, Greece: a comparison between city and port area. Atmos Environ. 2015;113:197–207. [Google Scholar]
- 42.Callen MS, Iturmendi A, Lopez JM. Source apportionment of atmospheric PM2.5-bound polycyclic aromatic hydrocarbons by a PMF receptor model. Assessment of potential risk for human health. Environ. Pollut. 2014;195:167e177. doi: 10.1016/j.envpol.2014.08.025. [DOI] [PubMed] [Google Scholar]
- 43.Kume K, Ohura T, Noda T, Amagai T, Fusaya M. Seasonal and spatial trends of suspended-particle associated polycyclic aromatic hydrocarbons in urban Shizuoka. Jpn J Hazard Mater. 2007;144:513e521. doi: 10.1016/j.jhazmat.2006.10.079. [DOI] [PubMed] [Google Scholar]
- 44.Bourotte C, Forti M-C, Taniguchi S, Bícego MC, Lotufo PA. A wintertime study of PAHs in fine and coarse aerosols in São Paulo city, Brazil. Atmos Environ. 2005;39:3799–3811. [Google Scholar]
- 45.Masiol M, Hofer A, Squizzato S, Piazza R, Rampazzo G, Pavoni B. Carcinogenic and mutagenic risk associated to airborne particle-phase polycyclic aromatic hydrocarbons: a source apportionment. Atmos Environ. 2012;60:375e382. [Google Scholar]
- 46.Akyüz M, Çabuk H. Meteorological variations of PM2.5/PM10 concentrations and particle-associated polycyclic aromatic hydrocarbons in the atmospheric environment of Zonguldak. Turk. J. Hazard. Mater. 2009;170:13e21. doi: 10.1016/j.jhazmat.2009.05.029. [DOI] [PubMed] [Google Scholar]
- 47.Bandowe BAM, Meusel H, Huang RJ, Ho K, Cao J, Hoffmann T, Wilcke W. PM2.5-bound oxygenated PAHs, nitro-PAHs and parent-PAHs from the atmosphere of a Chinese megacity: seasonal variation, sources and cancer risk assessment. Sci. Total Environ. 2014;473e474:77e87. doi: 10.1016/j.scitotenv.2013.11.108. [DOI] [PubMed] [Google Scholar]
- 48.Liu J, Man R, Ma S, Li J, Wu Q, Peng J. Atmospheric levels and health risk of polycyclic aromatic hydrocarbons (PAHs) bound to PM2.5 in Guangzhou, China. Mar. Pollut. Bull. 2015;100:134e143. doi: 10.1016/j.marpolbul.2015.09.014. [DOI] [PubMed] [Google Scholar]
- 49.Teixeira E, Mattiuzi C, Agudelo-Castaneda D, de Oliveira Garcia K, Wiegand F. Polycyclic aromatic hydrocarbons study in atmospheric fine and coarse particles using diagnostic ratios and receptor model in urban/industrial region. Environ. Monit. Assess. 2013;185:9587e9602. doi: 10.1007/s10661-013-3276-2. [DOI] [PubMed] [Google Scholar]
- 50.Krumal K, Mikuska P, Vecera Z. Polycyclic aromatic hydrocarbons and hopanes in PM1 aerosols in urban areas. Atmos Environ. 2013;67:27e37. [Google Scholar]
- 51.Ravindra K, Wauters E, Van Grieken R. Variation in particulate PAHs levels and their relation with the transboundary movement of the air masses. Sci Total Environ. 2008;396:100–110. doi: 10.1016/j.scitotenv.2008.02.018. [DOI] [PubMed] [Google Scholar]
- 52.Lai IC, Lee CL, Zeng KY, Huang HC. Seasonal variation of atmospheric polycyclic aromatic hydrocarbons along the Kaohsiung coast 2011;92(8):2029–37. [DOI] [PubMed]
- 53.Mohanraj R, Dhanakumar S, Solaraj G 2012. Polycyclic aromatic hydrocarbons bound to PM 2.5 in urban Coimbatore, India with emphasis on source apportionment. The scientific world Journal, 2012. [DOI] [PMC free article] [PubMed]
- 54.Li J, Zhang G, Li X, Qi S, Liu G, Peng X. Source seasonality of polycyclic aromatic hydrocarbons (PAHs) in a subtropical city, Guangzhou, South China. Sci Total Environ. 2006;355:145–155. doi: 10.1016/j.scitotenv.2005.02.042. [DOI] [PubMed] [Google Scholar]
- 55.Tsapakis M, Stephanou EG. Occurrence of gaseous and particulate polycyclic aromatic hydrocarbons in the urban atmosphere: study of sources and ambient temperature effect on the gas/particle concentration and distribution. Environ Pollut. 2005;133:147–156. doi: 10.1016/j.envpol.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 56.Eiguren-Fernandez A, Miguel AH, Froines JR, Thurairatnam S, Avol EL. Seasonal and spatial variation of polycyclic aromatic hydrocarbons in vapor-phase and PM2. 5 in Southern California urban and rural communities. Aerosol Sci Technol. 2004;38:447–455. [Google Scholar]
- 57.del Rosario Sienra M, Rosazza NG, Préndez M. Polycyclic aromatic hydrocarbons and their molecular diagnostic ratios in urban atmospheric respirable particulate matter. Atmos Res. 2005;75:267–281. [Google Scholar]
- 58.Hong H, Yin H, Wang X, Ye C. Seasonal variation of PM 10-bound PAHs in the atmosphere of Xiamen, China. Atmos Res. 2007;85:429–441. [Google Scholar]
- 59.Kim K-H, Jahan SA, Kabir E, Brown RJ. A review of airborne polycyclic aromatic hydrocarbons (PAHs) and their human health effects. Environ Int. 2013;60:71–80. doi: 10.1016/j.envint.2013.07.019. [DOI] [PubMed] [Google Scholar]
- 60.Possanzini M, Di Palo V, Gigliucci P, Scianò MCT, Cecinato A. Determination of phase-distributed PAH in Rome ambient air by denuder/GC-MS method. Atmos Environ. 2004;38:1727–1734. [Google Scholar]
- 61.Kong S, Ding X, Bai Z, Han B, Chen L, Shi J, Li Z. A seasonal study of polycyclic aromatic hydrocarbons in PM 2.5 and PM 2.5–10 in five typical cities of Liaoning Province, China. J Hazard Mater. 2010;183:70–80. doi: 10.1016/j.jhazmat.2010.06.107. [DOI] [PubMed] [Google Scholar]
- 62.Sanderson EG, Farant J-P. Atmospheric size distribution of PAHs: evidence of a high-volume sampling artifact. Environ Sci Technol. 2005;39:7631–7637. doi: 10.1021/es0510111. [DOI] [PubMed] [Google Scholar]
- 63.Wang X, Cheng H, Xu X, Zhuang G, Zhao C. A wintertime study of polycyclic aromatic hydrocarbons in PM 2.5 and PM 2.5–10 in Beijing: assessment of energy structure conversion. J Hazard Mater. 2008;157:47–56. doi: 10.1016/j.jhazmat.2007.12.092. [DOI] [PubMed] [Google Scholar]
- 64.Yunker MB, Macdonald RW, Vingarzan R, Mitchell RH, Goyette D, Sylvestre S. PAHs in the Fraser River basin: a critical appraisal of PAH ratios as indicators of PAH source and composition. Org Geochem. 2002;33:489–515. [Google Scholar]
- 65.Manoli E, Kouras A, Samara C. Profile analysis of ambient and source emitted particle-bound polycyclic aromatic hydrocarbons from three sites in northern Greece. Chemosphere. 2004;56:867–878. doi: 10.1016/j.chemosphere.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 66.Katsoyiannis A, Terzi E, Cai Q-Y. On the use of PAH molecular diagnostic ratios in sewage sludge for the understanding of the PAH sources. Is this use appropriate? Chemosphere. 2007;69:1337–1339. doi: 10.1016/j.chemosphere.2007.05.084. [DOI] [PubMed] [Google Scholar]
- 67.Yang D, Qi S, Zhang Y, Xing X, Liu H, Qu C, Liu J, Li F. Levels, sources and potential risks of polycyclic aromatic hydrocarbons (PAHs) in multimedia environment along the Jinjiang River mainstream to Quanzhou Bay. China. Mar Pollut Bull. 2013;76(1–2):298–306. doi: 10.1016/j.marpolbul.2013.08.016. [DOI] [PubMed] [Google Scholar]
- 68.Jiang Y, Hu X, Yves UJ, Zhan H, Wu Y. Status, source and health risk assessment of polycyclic aromatic hydrocarbons in street dust of an industrial city. NW China. Ecotoxicol Environ Saf. 2014;106:11–8. doi: 10.1016/j.ecoenv.2014.04.031. [DOI] [PubMed] [Google Scholar]
- 69.Khalili NR, Scheff PA, Holsen TM. PAH source fingerprints for coke ovens, diesel and, gasoline engines, highway tunnels, and wood combustion emissions. Atmos Environ. 1995;29:533–542. [Google Scholar]
- 70.Guo H, Lee S, Ho K, Wang X, Zou S. Particle-associated polycyclic aromatic hydrocarbons in urban air of Hong Kong. Atmos Environ. 2003;37:5307–5317. [Google Scholar]
- 71.Mirante F, Alves C, Pio C, Pindado O, Perez R, Revuelta MA, Artiñano B. Organic composition of size segregated atmospheric particulate matter, during summer and winter sampling campaigns at representative sites in Madrid, Spain. Atmos Res. 2013;132:345–361. [Google Scholar]
- 72.Krugly E, Martuzevicius D, Sidaraviciute R, Ciuzas D, Prasauskas T, Kauneliene V, Stasiulaitiene I, Kliucininkas L. Characterization of particulate and vapor phase polycyclic aromatic hydrocarbons in indoor and outdoor air of primary schools. Atmos Environ. 2014;82:298–306. [Google Scholar]
- 73.Wu X, Lam JC, Xia C, Kang H, Xie Z, Lam PK. Atmospheric concentrations of DDTs and chlordanes measured from Shanghai, China to the Arctic Ocean during the third China Arctic research expedition in 2008. Atmos Environ. 2011;45:3750–3757. [Google Scholar]
- 74.Wang R, Liu G, Zhang J. Variations of emission characterization of PAHs emitted from different utility boilers of coal-fired power plants and risk assessment related to atmospheric PAHs. Sci Total Environ. 2015;538:180–190. doi: 10.1016/j.scitotenv.2015.08.043. [DOI] [PubMed] [Google Scholar]
- 75.Galarneau E. Source specificity and atmospheric processing of airborne PAHs: implications for source apportionment. Atmos Environ. 2008;42(35):8139–8149. [Google Scholar]
- 76.Perraudin E, Budzinski H, Villenave E. Kinetic study of the reactions of ozone with polycyclic aromatic hydrocarbons adsorbed on atmospheric model particles. J Atmos Chem. 2007;56(1):57–82. [Google Scholar]
- 77.WHO, 1987. Air quality guidelines for Europe. WHO Regional Publications, European Series No. 23Regional Office for Europe, Copenhagen. [PubMed]