Abstract
Aim
Increased crossing of finger nailfold capillaries could be a novel visual marker of early microvascular damage among type 2 diabetes mellitus patients. Although abdominal obesity is an important driver of early microvascular damage, its association with an increase in the percentage of crossing capillaries remains uncertain. We investigated the association between abdominal obesity and an increase in the percentage of crossing capillaries in the finger nailfold in patients with type 2 diabetes mellitus.
Methods
This cross-sectional study enrolled 123 type 2 diabetes mellitus patients (age 40–75 years) who visited the outpatient diabetic clinic at Osaka University Hospital between May and October 2019. Abdominal obesity was defined as a waist circumference ≥ 90 cm in women and ≥ 85 cm in men. Capillary morphology was assessed by nailfold capillaroscopy based on the simple capillaroscopic definitions of the European League Against Rheumatism Study Group. The association between abdominal obesity and a high percentage of crossing capillaries in the finger nailfold (defined as the highest tertile of crossing capillaries) was analyzed using multivariable logistic regression.
Results
After adjusting for age, sex, smoking status, regular exercise, duration of diabetes, glycated hemoglobin, hypertension, and dyslipidemia, abdominal obesity was significantly associated with a high percentage of crossing capillaries (multivariable-adjusted odds ratios [95% confidence interval] = 2.70 [1.05–6.90], p = 0.038).
Conclusions
Abdominal obesity may play an important role in the increase in the percentage of crossing capillaries in the finger nailfold in patients with type 2 diabetes mellitus.
Electronic supplementary material
The online version of this article (10.1007/s13340-020-00480-4) contains supplementary material, which is available to authorized users.
Keywords: Abdominal obesity, Microvascular dysfunction, Nailfold capillary, Nailfold capillaroscopy
Introduction
The current epidemic of type 2 diabetes mellitus (T2DM) [1] implies a resulting increase in micro and macro-vascular complications [2–5]. Recently, there has been increasing evidence that early microvascular changes precede and may predict these complications [6–9]. Thus, it has become increasingly important to detect microvascular damage at an early stage [9, 10].
Finger nailfold capillaries are the most easily and non-invasively accessible parts of the microcirculation [10]. Several previous studies have reported that patients with T2DM have a more abnormal capillary morphology than healthy control participants, especially in terms of the crossing capillaries [11–13]. A previous cross-sectional study demonstrated that among the abnormal features such as bushy capillary, neoformation, microhemorrhage, bizarre capillary, capillary ectasia, and aneurysm, the presence of crossing capillaries in the finger nailfold alone is significantly associated with an increased risk of diabetic retinopathy in patients with T2DM [12]. Furthermore, our previous study showed that an increase in the percentage of crossing capillaries in the finger nailfold is associated with diabetic retinopathy after adjusting for confounding factors [14]. Therefore, increased crossing of the finger nailfold capillaries could be an essential visual marker of early microvascular damage. However, the mechanism mediating the increase in the crossing of capillaries among those with T2DM remains uncertain.
The pathogenesis of microvascular damage resulting from diabetes is extremely complex, although, recent reviews have summarized that obesity, especially abdominal obesity, is an important driver of early abnormalities of the microcirculation [9, 15, 16]. This mechanism is related to the fact that abdominal obesity alters the secretion of adipokines leading to increased levels of free fatty acids and inflammatory mediators and decreased levels of adiponectin [16, 17]. Accordingly, abdominal obesity may contribute to increased crossing of capillaries. However, to the best of our knowledge, no study has investigated the relationship between abdominal obesity and an increase in the percentage of crossing capillaries in the finger nailfold in patients with T2DM.
Therefore, we examined the association between abdominal obesity and an increase in the percentage of crossing capillaries in the finger nailfold in patients with T2DM.
Materials and methods
Study population
In this cross-sectional study, outpatients with T2DM aged 40–75 years were recruited between May and October 2019 at the Department of Metabolic Medicine, Osaka University Hospital, Osaka, Japan. The exclusion criteria included a history of Raynaud’s phenomenon, collagen tissue disease, glaucoma, uveitis, dementia, cerebrovascular disease, coronary artery disease, dialysis, blindness, cancer treatment, current smoking status, removal of the fingernail cuticle, use of nail polish within 1 month before the test, and pregnancy. The study was approved by the Institutional Review Board of Osaka University on April 9, 2019 (approval no. 18546) and adhered to the principles of the Declaration of Helsinki and its later amendments. All participants provided written informed consent.
Assessment of anthropometric measures
For all measurements, patients were required to remove their shoes and put aside heavy objects such as smartphones, keys, and wallets. Waist circumference (WC) was measured using a non-elastic metric tape at the umbilical level at the end of the exhalation phase with the patient standing and exhaling. Height was measured using a stadiometer to the nearest 0.1 cm, and weight was measured using a digital scale to the nearest 0.1 kg. Abdominal obesity was defined as a WC ≥ 90 cm in women and ≥ 85 cm in men [18]. The waist-to-height ratio (WHtR) was calculated as the WC divided by height (in cm). Body mass index was calculated as the weight (kg) divided by height squared (m2). All measurements were conducted by the medical staff.
Assessment of the nailfold capillaries
A GOKO Bscan-Z microscope (GOKO Imaging Devices Co., Kanagawa, Japan), at approximately × 390 magnification, was used to examine finger nailfold capillaries (Fig. 1). The NTSC-USB 2.0 Converter was used to transmit the capillaroscopy images to a computer, and the images were stored using Power Director 8 (CyberLink Corp., New Taipei City, Taiwan).
Fig. 1.
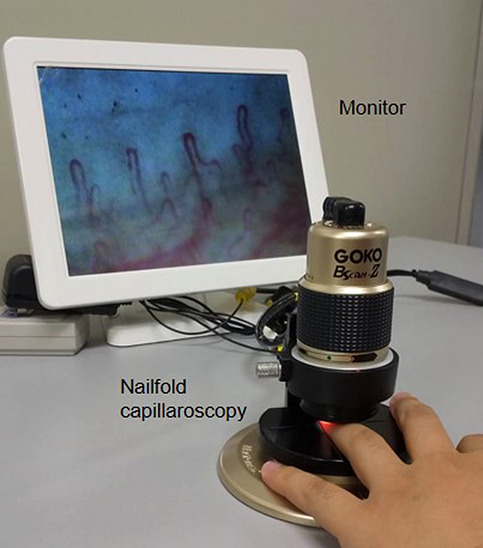
Representative image showing the process of finger nailfold capillaroscopy
To help participants acclimatize and relax before the test, they were seated in a temperature-controlled (23–26 °C) room for 15–20 min [19]. For each participant, three pictures per finger were taken in the middle of the nailfold, and eight fingers, excluding the thumbs, were examined [20]. A drop of vegetable oil was applied to the nailfold of each finger before observation to maximize the amount of light and improve the image quality [21]. This procedure was carried out by a trained operator with no knowledge of the patient’ s condition. The approximate size of the capillaroscopic image was 0.5 × 0.7 mm2, and, for each patient, fingers with physical injuries or without skin transparency were excluded from the analysis [21].
Capillaries in the distal row of the nailfold were analyzed by two masked raters. A regular capillary has a shape similar to that of a hairpin or an upside-down letter “U” but with a thinner arterial arm, an upper part, and a venous arm [21]. We used the simple capillaroscopic definitions of the European League Against Rheumatism Study Group [22, 23] to classify the nailfold capillary morphology into crossing (the limbs cross once or twice, and the capillary head is convex) or other types (Fig. 2). This particular definition, which is notably reliable, was developed to help standardize and simplify the definitions used to describe the morphology of a single capillary [23].
Fig. 2.
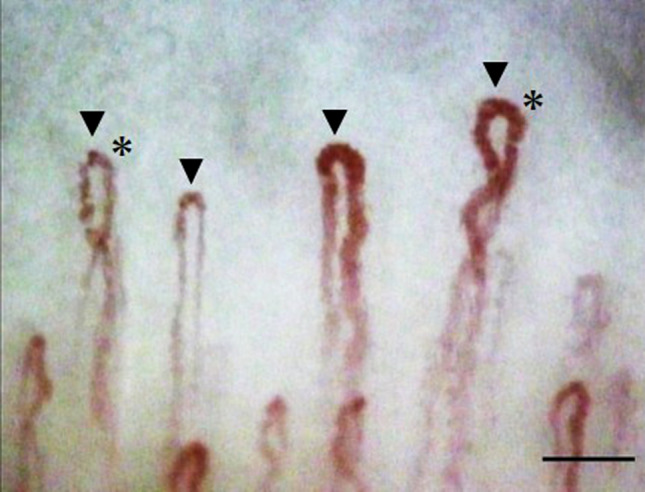
An example of finger nailfold capillaroscopy findings. Arrows indicate the nailfold capillaries assessed in the distal row. The asterisk (*) indicates a crossing capillary (the limbs cross once or twice and the capillary head is convex; scale bar, 0.1 mm)
In this study, the inter-rater reliability of simple capillaroscopic definitions was excellent (Cohen’s kappa: 0.84). To calculate the percentage of crossing capillaries, we divided the number of crossing capillaries by the number of assessed capillaries. Since the percentage of crossing capillaries has no defined cutoff point, we used cutoff points from our previous study as references, which showed that a high percentage of crossing capillaries (tertile ≥ 3) associated with the presence of retinopathy [14]; thus, those included in tertile ≥ 3 had a high percentage of crossing capillaries, and those included in tertiles 1 and 2 had low percentages of crossing capillaries.
Patients’ demographics and clinical characteristics
Blood pressure was measured twice by the medical staff in the same arm in a seated position using a calibrated electronic sphygmomanometer (HEM-8713; Omron Corporation, Kyoto, Japan), and the mean of the two measurements was calculated.
Information on age, sex, duration of diabetes, insulin treatment, presence or absence of diabetic retinopathy, medication use (antihypertensive medication, antilipidemic medication), the most recent glycated hemoglobin (HbA1c) value, estimated glomerular filtration rate (eGFR), high-density lipoprotein cholesterol (HDL-C) levels, low-density lipoprotein cholesterol (LDL-C) levels, triglyceride (TG) levels, and urine findings was collected from patients’ medical records. Blood sampling was usually carried out under fasting conditions. LDL-C levels were calculated using the equation developed by Friedewald et al. [24]. If the TG level exceeded 400 mg/dL or if data were missing for total cholesterol, HDL-C, or TG levels, we used the LDL-C value measured by the direct method. The eGFR was calculated using the formula specified by the Japanese Society of Nephrology [25]. Nephropathy (overt nephropathy or kidney failure) was defined based on the Classification of Diabetic Nephropathy 2014 in Japan; overt nephropathy was defined as urine albumin-to-creatinine ratio ≥ 300 mg/g creatinine or urinary protein-to-creatinine ratio ≥ 0.5 g/g creatinine or positive proteinuria by dipstick analysis examination; kidney failure was defined as eGFR < 30 ml/min/1.73 m2 [26].
Hypertension was defined as systolic blood pressure ≥ 140 mmHg, diastolic blood pressure ≥ 90 mmHg, and/or the use of antihypertensive drugs [27]. Dyslipidemia was defined as an LDL-C level ≥ 140 mg/dL, HDL-C level < 40 mg/dL, TG level ≥ 150 mg/dL, or the use of antilipidemic medications [28].
Regular exercise was defined as exercising two or more times per week for at least 30 min per session for at least 6 months. Regular alcohol consumption was defined as drinking any amount of alcohol one or more times per week.
Statistical analysis
To compare the patients’ characteristics according to the presence of abdominal obesity, we used Student’s or Welch’s t tests for continuous data and the Chi-square or Fisher’s exact tests for categorical data.
High and low percentages of crossing capillaries according to the presence of abdominal obesity were analyzed using the Chi-square test. The association between abdominal obesity and a high percentage of crossing capillaries was evaluated using multivariable logistic regression models (response variable: 1 = high percentage of crossing capillaries [≥ tertile 3], 0 = low percentage of crossing capillaries [< tertile 3]), using patients without abdominal obesity as the reference group. We adjusted for potential confounders such as age, sex, smoking status, regular exercise, duration of diabetes, HbA1c levels, hypertension, and dyslipidemia.
All p values were two-tailed, and p < 0.05 was considered significant. All analyses were performed using IBM SPSS Statistics, Version 24.0 (IBM SPSS, Chicago, IL).
Results
Of the 126 patients who participated in this study, those with missing data on metabolic profiles were excluded (n = 3); the data for the remaining patients (n = 123) were analyzed. The mean ± standard deviation (SD) age of the patients was 63.9 ± 8.6 years, and the majority of patients (n = 79, 64.2%) had abdominal obesity (Table 1). Table 1 summarizes the clinical and demographic characteristics of patients with or without abdominal obesity. Patients with abdominal obesity were likely to be young, and former smokers (all P < 0.05) compared with patients without abdominal obesity.
Table 1.
Clinical characteristics according to the presence of abdominal obesity
| Characteristic | All | Without abdominal obesity | With abdominal obesitya | p value |
|---|---|---|---|---|
| Number | 123 | 44 | 79 | |
| Age (years) | 63.9 ± 8.6 | 66.6 ± 6.7 | 62.4 ± 9.3 | 0.005* |
| Female, (%) | 45.5 | 52.3 | 41.8 | 0.262 |
| Smoking: former, (%) | 39.0 | 25.0 | 46.8 | 0.017* |
| Regular alcohol consumption, (%) | 26.0 | 31.8 | 22.8 | 0.274 |
| Regular exercise: absence, (%) | 50.4 | 40.9 | 55.7 | 0.116 |
| Duration of diabetes (years) | 14.6 ± 9.0 | 15.5 ± 9.5 | 14.2 ± 8.7 | 0.430 |
| HbA1c (%) | 7.2 ± 0.9 | 7.1 ± 0.9 | 7.3 ± 1.0 | 0.412 |
| Insulin, (%) | 18.7 | 15.9 | 20.3 | 0.554 |
| Diabetic nephropathy (overt nephropathy or kidney failure), (%)b,c | 10.9 | 7.5 | 12.9 | 0.298 |
| Diabetic retinopathy, (%)d | 28.7 | 35.0 | 25.0 | 0.267 |
| Hypertension, (%)e | 73.2 | 65.9 | 77.2 | 0.175 |
| Dyslipidemia, (%)f | 78.9 | 72.7 | 82.3 | 0.214 |
| Renin–angiotensin–aldosterone system inhibitor use, (%) | 47.2 | 38.6 | 51.9 | 0.158 |
| Statin use, (%) | 52.8 | 50.0 | 54.4 | 0.637 |
| Body mass index (kg/m2) | 25.9 ± 4.8 | 21.9 ± 2.4 | 28.1 ± 4.3 | < 0.001* |
| WC (cm) | ||||
| Female | 91.5 ± 11.8 | 80.2 ± 6.1 | 99.4 ± 7.6 | < 0.001* |
| Male | 93.3 ± 12.9 | 81.5 ± 2.9 | 98.7 ± 12.1 | < 0.001* |
Continuous data were analyzed using the Student’s t test or Welch’s t test, as appropriate, and are presented as means ± standard deviations
Categorical data were analyzed using the Chi-square test or Fisher’s exact test, as appropriate, and are presented as percentages (%)
WC waist circumference, HbA1c glycated hemoglobin
*Significant p values in comparing clinical characteristics according to the presence or absence of abdominal obesity
aAbdominal obesity was defined using the following cutoff points: female, WC ≥ 90 cm; male, WC ≥ 85 cm
bDiabetic nephropathy was assessed using the Classification of Diabetic Nephropathy 2014
cAvailable in 110 patients (excluded patients with other renal diseases or without the urine examination within 1 year)
dAvailable in 108 patients (excluded patients with no information about the presence or absence of diabetic retinopathy)
eHypertension was defined as a systolic blood pressure ≥ 140 mmHg, diastolic blood pressure ≥ 90 mmHg, and/or the use of antihypertensive drugs
fDyslipidemia was defined as a low-density lipoprotein cholesterol level ≥ 140 mg/dL, high-density lipoprotein cholesterol level < 40 mg/dL, triglyceride level ≥ 150 mg/dL, or the use of antilipidemic medications
Table 2 shows the characteristics of the nailfold capillaries. The median number of nailfold capillaries (25th percentile, 75th percentile) assessed per patient was 95.0 (73.0, 115.0). The median percentage of crossing capillaries (25th percentile, 75th percentile) was 60.3% (53.9, 69.5%). A high percentage of crossing capillaries was defined as ≥ 63.9% of crossing capillaries (tertile 3).
Table 2.
Characteristics of the nailfold capillaries according to the presence or absence of abdominal obesity
| Variables | All (n = 123) | Without abdominal obesity (n = 44) | With abdominal obesity (n = 79) |
|---|---|---|---|
| Number of assessed nailfold capillariesa | 95.0 (73.0, 115.0) | 96.5 (73.3, 115.5) | 94.0 (71.0, 115.0) |
| Number of assessed crossing capillariesb | 56.0 (43.0, 67.0) | 54.0 (40.3, 61.5) | 57.0 (43.0, 69.0) |
| Crossing capillary (%)c | 60.3 (53.9, 69.5) | 59.0 (46.6, 63.5) | 61.1 (55.6, 70.7) |
| Crossing capillaryd | |||
| < 57.1% (tertile 1) | 43 (35.0%) | 18 (40.9%) | 25 (31.6%) |
| 57.1–63.9% (tertile 2) | 39 (31.7%) | 16 (36.4%) | 23 (29.1%) |
| ≥ 63.9% (tertile 3) | 41 (33.3%) | 10 (22.7%) | 31 (39.2%) |
Continuous data are presented as medians (25th percentile, 75th percentile)
aNumber of assessed nailfold capillaries: the sum of the number of capillaries assessed in the distal row of the nailfold in eight fingers
bNumber of assessed crossing capillaries: the sum of the number of crossing capillaries assessed in the distal row of the nailfold in eight fingers
cCrossing capillary (%): the number of assessed crossing capillaries divided by the number of assessed nailfold capillaries
dThe percentage of crossing capillaries was categorized into tertiles and presented as the number of patients (%)
As shown in Table 3, the proportions of patients with a high percentage of crossing capillaries among those with and without abdominal obesity were 22.7% and 39.2%, respectively (p = 0.063). Compared to that in patients without abdominal obesity, the multivariable-adjusted odds ratio for a high percentage of crossing capillaries in patients with abdominal obesity was 2.70 (95% confidence interval [CI]: 1.05–6.90; Table 3). The results were not materially altered in an additional analysis wherein we defined abdominal obesity based on a WHtR of 0.5 instead of the WC cutoff points (Table S1). In contrast, no associations were observed between potential confounding factors such as age, sex, smoking status, regular exercise, duration of diabetes, HbA1c levels, hypertension, and dyslipidemia and a high percentage of crossing capillaries in the multivariable model.
Table 3.
Odds ratios for a high percentage of crossing capillaries according to the presence of abdominal obesity
| Variables | High percentage of crossing capillaries, | p value | Odds ratio (95% confidence interval) | p valueb | |
|---|---|---|---|---|---|
| % (case/n) | Model 1 | Model 2 | |||
| Abdominal obesitya | |||||
| Absence | 22.7 (10/44) | 0.063 | Reference | Reference | |
| Presence | 39.2 (31/79) | 2.32 (0.97–5.51) | 2.70 (1.05–6.90) | 0.038 | |
Abdominal obesity was analyzed using the Chi-square test
High percentage of crossing capillaries: crossing capillaries (%) ≥ 63.9% (tertile 3)
Response variable: 1 = high percentage of crossing capillaries (≥ 63.9%), 0 = low percentage of crossing capillaries (< 63.9%)
Model 1: Adjusted for age and sex
Model 2: Adjusted for age, sex, smoking status, regular exercise, duration of diabetes, glycated hemoglobin, hypertension, and dyslipidemia
WC waist circumference
aAbdominal obesity was defined using the following cutoff points–female: WC ≥ 90 cm, male: WC ≥ 85 cm
bp value from Model 2
Discussion
The main finding of this study was that abdominal obesity was positively associated with a high percentage of crossing capillaries in the finger nailfold in patients with T2DM. This association was independent of potential confounding factors, including age, sex, smoking status, regular exercise, duration of diabetes, HbA1c levels, hypertension, and dyslipidemia. Further, our study showed that not only the WC cutoff points but also a WHtR ≥ 0.5 (a different measurement of abdominal obesity) were significantly associated with an increased percentage of crossing capillaries in the finger nailfold. These results suggest that abdominal obesity might play an important role in the pathophysiology of increased crossing of capillaries of the finger nailfold, which could be a visual marker of microvascular damage in patients with T2DM.
Obesity, especially abdominal obesity, is thought to be a primary cause of early microvascular changes [9, 15, 16, 29]. Previous studies have demonstrated that abdominal obesity is associated with impaired functional microcirculation [17, 30]. For instance, the Maastricht study showed that WC was inversely associated with skin microvascular flow motion [30]. Another previous study showed that the amount of visceral adipose tissue was inversely associated with capillary recruitment in the nailfold [17]. Our study is the first to show that abdominal obesity is associated with abnormal morphological changes of the capillaries in patients with T2DM.
Early morphological changes of the capillaries in patients with T2DM are thought to be caused by the loss of pericytes in the vascular bed [31, 32]. Pericytes play critical roles in the preservation of the structural integrity of the microcirculation [32, 33]. Several animal studies have suggested that obesity induces pericyte loss [34, 35]. For example, obesity was shown to associate with the loss of pericyte coverage on cerebral microvessels in mice [34]. Furthermore, the number of pericytes in the retinas of diet-induced obese pigs with metabolic syndrome was significantly decreased compared with that in the retinas of lean pigs [35]. Pericytes are sensitive to inflammation and oxidative damage [32, 34, 35]. Therefore, it is possible that abdominal obesity-induced inflammation and oxidative stress might contribute to pericytes loss, resulting in increased crossing of capillaries.
In the present study, no associations between potential confounding factors and a high percentage of crossing capillaries were noted. Although, to the best of our knowledge, no previous studies have examined the factors associated with crossing capillaries using multivariate analyses [12, 36], the lack of a significant association of diabetes duration and HbA1c levels with a high percentage of crossing capillaries was particularly unexpected. The above-mentioned study examined the risk factors associated with skin microvascular flow motion in participants without cardiovascular diseases [30]; as in the present investigation, the study showed that WC was inversely associated with the skin microvascular flow motion, but unexpectedly, fasting plasma glucose and other metabolic or diagnostic measures of T2DM had no association with the skin microvascular flow motion. The explanation for these findings is not entirely clear. One possibility is that the participants in these studies had relatively well-controlled diabetes; thus, the effect of hyperglycemia on changes in microvascular function and morphology may have been small; moreover, our study may have lacked the statistical power to detect an association between other risk factors and a high percentage of crossing capillaries. Therefore, further studies to determine the association between other risk factors and a high percentage of crossing capillaries are warranted.
Early lifestyle changes resulting in a healthy diet, weight reduction, and increased physical activity are necessary to decrease the risk of coronary artery disease and all-cause death in patients with abdominal obesity and T2DM [37]. Nailfold capillaroscopy is an easy and noninvasive tool for the assessment of the microvascular structure and can be made available in a clinical setting [38]. Additionally, patients can visualize their crossing capillaries in real time through a monitor, motivating them to make the necessary lifestyle changes.
The strengths of this study included the assessment of crossing capillaries following a standardized quantitative assessment of the nailfold capillaries. Moreover, we controlled potential confounding factors, which could have biased the results. However, there were several limitations. First, because of the cross-sectional design of the current study, we could not determine the time course of the associations between abdominal obesity and crossing of finger nailfold capillaries. Second, our sample was limited to an Asian population, and thus, our results may not be generalizable to other regions or ethnic groups.
In conclusion, abdominal obesity was significantly associated with a high percentage of crossing capillaries in the finger nailfold in patients with T2DM after adjusting for confounding factors. Prospective and intervention studies are warranted to elucidate the temporal nature of this condition and to understand whether the percentage of crossing capillaries can be improved through weight loss.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This study was supported by grants from the Japan Health Foundation and Yamaji Fumiko Nursing Research Fund. The authors would like to thank the physicians and nurses at the Department of Metabolic Medicine, Osaka University Hospital, Osaka, Japan, for their assistance with patient recruitment and data collection. We would like to thank Editage (http://www.editage.com) for English language editing.
Author contributions
Study conception and design: MS, NS, AM, JK, IS, and YO; Data collection: MS, SS, JK, NM, MO, TAM, and IS; Analysis: MS, SS, and TT; Writing-original draft preparation: MS. All authors commented on the previous versions of the manuscript. All authors read and approved the final manuscript.
Compliance with ethical standards
Conflict of interest
Maiko Shikama, Nao Sonoda, Akiko Morimoto, Sayaka Suga, Tetsuya Tajima, Junji Kozawa, Norikazu Maeda, Michio Otsuki, Taka-Aki Matsuoka, Iichiro Shimomura, and Yuko Ohno declare that they have no conflict of interest.
Human rights
All procedures followed were in accordance with the ethical standards of the Institutional Review Board of Osaka University (April 9, 2019; approval no. 18546) and with the Helsinki Declaration of 1964 and later versions. Informed consent or substitute for it was obtained from all patients for being included in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Saeedi P, Petersohn I, Salpea P, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res Clin Pract. 2019;157:107843. doi: 10.1016/j.diabres.2019.107843. [DOI] [PubMed] [Google Scholar]
- 2.Koye DN, Magliano DJ, Nelson RG, et al. The global epidemiology of diabetes and kidney disease. Adv Chronic Kidney Dis. 2018;25:121–132. doi: 10.1053/j.ackd.2017.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Einarson TR, Acs A, Ludwig C, et al. Prevalence of cardiovascular disease in type 2 diabetes: a systematic literature review of scientific evidence from across the world in 2007–2017. Cardiovasc Diabetol. 2018;17:83. doi: 10.1186/s12933-018-0728-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feldman EL, Callaghan BC, Pop-Busui R, et al. Diabetic neuropathy. Nat Rev Dis Prim. 2019;5:41. doi: 10.1038/s41572-019-0092-1. [DOI] [PubMed] [Google Scholar]
- 5.Xavier RM, Banu R, Chee ML, et al. Incidence and progression of diabetic retinopathy: a systematic review. Lancet Diabetes Endocrinol. 2019;7:140–149. doi: 10.1016/S2213-8587(18)30128-1. [DOI] [PubMed] [Google Scholar]
- 6.Wong MS, Gu K, Heng D, et al. The Singapore impaired glucose tolerance follow-up study: does the ticking clock go backward as well as forward? Diabetes Care. 2003;26:3024–3030. doi: 10.2337/diacare.26.11.3024. [DOI] [PubMed] [Google Scholar]
- 7.Cheung CY, Ikram MK, Klein R, et al. The clinical implications of recent studies on the structure and function of the retinal microvasculature in diabetes. Diabetologia. 2015;58:871–885. doi: 10.1007/s00125-015-3511-1. [DOI] [PubMed] [Google Scholar]
- 8.Sörensen BM, Houben AJHM, Berendschot TTJM, et al. Prediabetes and type 2 diabetes are associated with generalized microvascular dysfunction: the Maastricht Study. Circulation. 2016;134:1339–1352. doi: 10.1161/CIRCULATIONAHA.116.023446. [DOI] [PubMed] [Google Scholar]
- 9.Stehouwer CDA. Microvascular dysfunction and hyperglycemia: a vicious cycle with widespread consequences. Diabetes. 2018;67:1729–1741. doi: 10.2337/dbi17-0044. [DOI] [PubMed] [Google Scholar]
- 10.Fuchs D, Dupon PP, Schaap LA, et al. The association between diabetes and dermal microvascular dysfunction non-invasively assessed by laser doppler with local thermal hyperemia: a systematic review with meta-analysis. Cardiovasc Diabetol. 2017;16:11. doi: 10.1186/s12933-016-0487-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barchetta I, Riccieri V, Vasile M, et al. High prevalence of capillary abnormalities in patients with diabetes and association with retinopathy. Diabet Med. 2011;28:1039–1044. doi: 10.1111/j.1464-5491.2011.03325.x. [DOI] [PubMed] [Google Scholar]
- 12.Uyar S, Balkarli A, Erol MK, et al. Assessment of the relationship between diabetic retinopathy and nailfold capillaries in type 2 diabetics with a noninvasive method: nailfold videocapillaroscopy. J Diabetes Res. 2016;2016:7592402. doi: 10.1155/2016/7592402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hsu PC, Liao PY, Chang HH, et al. Nailfold capillary abnormalities are associated with type 2 diabetes progression and correlated with peripheral neuropathy. Medicine (Baltimore). 2016;95:e5714. doi: 10.1097/MD.0000000000005714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shikama M, Sonoda N, Morimoto A, et al. Association of crossing capillaries in the finger nailfold with diabetic retinopathy in type 2 diabetes mellitus. J Diabetes Investig. 2020 doi: 10.1111/jdi.13444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jonk AM, Houben AJ, De Jongh RT, et al. Microvascular dysfunction in obesity: a potential mechanism in the pathogenesis of obesity-associated insulin resistance and hypertension. Physiology. 2007;22:252–260. doi: 10.1152/physiol.00012.2007. [DOI] [PubMed] [Google Scholar]
- 16.Karaca Ü, Schram MT, Houben AJHM, et al. Microvascular dysfunction as a link between obesity, insulin resistance and hypertension. Diabetes Res Clin Pract. 2014;103:382–387. doi: 10.1016/j.diabres.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 17.De Jongh RT, Ijzerman RG, Serné EH, et al. Visceral and truncal subcutaneous adipose tissue are associated with impaired capillary recruitment in healthy individuals. J Clin Endocrinol Metab. 2006;91:5100–5106. doi: 10.1210/jc.2006-1103. [DOI] [PubMed] [Google Scholar]
- 18.Examination Committee of Criteria for ‘Obesity Disease’ in Japan; Japan Society for the study of obesity new criteria for ‘obesity disease’ in Japan. Circ J. 2002;66:987–92. [DOI] [PubMed]
- 19.Etehad TM, Fatemi A, Karbalaie A, et al. Nailfold capillaroscopy in rheumatic diseases: which parameters should be evaluated? Biomed Res Int. 2015;2015:1–17. doi: 10.1155/2015/974530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cutolo M, Sulli A, Smith V. How to perform and interpret capillaroscopy. Best Pract Res Clin Rheumatol. 2013;27:237–248. doi: 10.1016/j.berh.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 21.Karbalaie A, Emrani Z, Fatemi A, et al. Practical issues in assessing nailfold capillaroscopic images: a summary. Clin Rheumatol. 2019;38:2343–2354. doi: 10.1007/s10067-019-04644-9. [DOI] [PubMed] [Google Scholar]
- 22.Smith V, Beeckman S, Herrick AL, et al. An EULAR study group pilot study on reliability of simple capillaroscopic definitions to describe capillary morphology in rheumatic diseases. Rheumatology (Oxford) 2016;55:883–890. doi: 10.1093/rheumatology/kev441. [DOI] [PubMed] [Google Scholar]
- 23.Cutolo M, Melsens K, Herrick AL, et al. Reliability of simple capillaroscopic definitions in describing capillary morphology in rheumatic diseases. Rheumatology (Oxford) 2018;57:757–759. doi: 10.1093/rheumatology/kex460. [DOI] [PubMed] [Google Scholar]
- 24.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. doi: 10.1093/clinchem/18.6.499. [DOI] [PubMed] [Google Scholar]
- 25.Matsuo S, Imai E, Horio M, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982–992. doi: 10.1053/j.ajkd.2008.12.034. [DOI] [PubMed] [Google Scholar]
- 26.Haneda M, Utsunomiya K, Koya D, et al. A new classification of diabetic nephropathy 2014: a report from Joint Committee on Diabetic Nephropathy. Clin Exp Nephrol. 2015;19:1–5. doi: 10.1007/s10157-014-1057-z. [DOI] [PubMed] [Google Scholar]
- 27.Shimamoto K, Ando K, Fujita T, et al. The Japanese Society of Hypertension guidelines for the management of hypertension (JSH 2014) Hypertens Res. 2014;37:253–390. doi: 10.1038/hr.2013.80. [DOI] [PubMed] [Google Scholar]
- 28.Teramoto T, Sasaki J, Ueshima H, et al. Diagnostic criteria for dyslipidemia. Executive summary of Japan Atherosclerosis Society (JAS) guideline for diagnosis and prevention of atherosclerotic cardiovascular diseases for Japanese. J Atheroscler Thromb. 2007;14:155–8. [DOI] [PubMed]
- 29.De Jongh RT, Serne EH, RG IJ, et al. Impaired microvascular function in obesity: implications for obesity-associated microangiopathy, hypertension, and insulin resistance. Circulation. 2004;109:2529–35. [DOI] [PubMed]
- 30.Muris DM, Houben AJ, Kroon AA, et al. Age, waist circumference, and blood pressure are associated with skin microvascular flow motion: the Maastricht Study. J Hypertens. 2014;32:2439–2449. doi: 10.1097/HJH.0000000000000348. [DOI] [PubMed] [Google Scholar]
- 31.Hammes HP, Lin J, Renner O, et al. Pericytes and the pathogenesis of diabetic retinopathy. Diabetes. 2002;51:3107–3112. doi: 10.2337/diabetes.51.10.3107. [DOI] [PubMed] [Google Scholar]
- 32.Warmke N, Griffin KJ, Cubbon RM. Pericytes in diabetes-associated vascular disease. J Diabetes Compl. 2016;30:1643–1650. doi: 10.1016/j.jdiacomp.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 33.Bodnar RJ, Satish L, Yates CC, et al. Pericytes: a newly recognized player in wound healing. Wound Repair Regen. 2016;24:204–214. doi: 10.1111/wrr.12415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tucsek Z, Toth P, Tarantini S, et al. Aging exacerbates obesity-induced cerebromicrovascular rarefaction, neurovascular uncoupling, and cognitive decline in mice. J Gerontol A Biol Sci Med Sci. 2014;69:1339–1352. doi: 10.1093/gerona/glu080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lim RR, Grant DG, Olver TD, et al. Young ossabaw pigs fed a western diet exhibit early signs of diabetic retinopathy. Invest Ophthalmol Vis Sci. 2018;59:2325–2338. doi: 10.1167/iovs.17-23616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ciaffi J, Ajasllari N, Mancarella L, et al. Nailfold capillaroscopy in common non-rheumatic conditions: a systematic review and applications for clinical practice. Microvasc Res. 2020;131:104036. doi: 10.1016/j.mvr.2020.104036. [DOI] [PubMed] [Google Scholar]
- 37.Lee M, Aronne LJ. Weight management for type 2 diabetes mellitus: global cardiovascular risk reduction. Am J Cardiol. 2007;99:68b–79b. doi: 10.1016/j.amjcard.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 38.Ingegnoli F, Ughi N, Dinsdale G, et al. An international survey on non-invasive techniques to assess the mIcrocirculation in patients with raynaud’s phenomenon (SUNSHINE survey) Rheumatol Int. 2017;37:1879–1890. doi: 10.1007/s00296-017-3808-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


