Abstract
The resistance of hospital bacterial isolates against traditional germicides, which used frequently, is one of the important factors contributing to emerging nosocomial infections. Moreover, due to having the side effects of chemical substances, the development of novel low-risk natural compounds seems necessary for control the spread of resistant pathogens in hospital environments. The aim of this study was to compare the effect of carvacrol and glutaraldehyde against two common hospital acquired pathogens, including Pseudomonas aeruginosa and Staphylococcus aureus. In this study 365 samples were collected from different wards of hospitals of Khorramabad, Iran. One hundred and sixty samples were identified as P. aeruginosa and S. aureus by using standard microbiological methods. Then the antibacterial effects of four combinations including carvacrol+ethanol, carvacrol+dimethyl sulfoxide (DMSO), glutaraldehyde 2%, and pure glutaraldehyde (50%) were evaluated and determined using dilution broth and disk diffusion methods. Our results showed that the carvacrol had more antibacterial effects against selected bacteria compared to glutaraldehyde. Moreover, the optimal time and concentration of carvacrol+ethanol against hospital isolates of P. aeruginosa and S. aureus was determined after 1 h at concentration of 64 μl/ml and 8 μl/ml, respectively. In conclusion by comparing the results of carvacrol and glutaraldehyde, seem that carvacrol, as an herbal and natural agent, may be a suitable alternative to glutaraldehyde in hospital equipment’s’ sterilization.
Keywords: Carvacrol, Nosocomial infection, Pseudomonas aeruginosa, Staphylococcus aureus
Introduction
The hospital-acquired infections, also known as nosocomial infections, include affect nearly 5–20% of hospitalized patients. Generally, the resistance of some bacterial strains against traditional germicides is considered as one of the important factors which can cause many social and economic losses [1]. Increased mortality due to the nosocomial infections caused by resistant pathogens become a serious health problem, especially in developing countries, where the controlling of these infections is a concern of medical care system [2]. The prevalence rate of hospital-acquired infections in high-income countries is 6.7%, and in low and middle income countries is 15.5% [3]. Various pathogens such as bacteria, viruses, fungi, and protozoa can cause nosocomial infections [4]. Commonly, Escherichia coli, Staphylococcus aureus, and Pseudomonas aeruginosa are the most frequent bacterial agents causing severe hospital infections [5, 6]. It has been demonstrated that bacteria such as P. aeruginosa and S. aureus are highly resistant to various disinfections [7, 8]. P. aeruginosa, as a Gram-negative opportunistic pathogen can cause several acute and chronic infections in human, especially in hospitalized patients due to its ability to form biofilms as well antimicrobial resistance [9]. In addition to the biofilm formation, a polycarboxy multicellular structure can lead to the accumulation of bacteria and results in the survival and resistance of bacteria to many agents such as antibiotics and disinfectants [10, 11]. It is estimated that P aeruginosa involves in 10% to 20% of hospital-acquired infections [10, 11]. Staphylococcus aureus is a Gram-positive and facultative anaerobic bacterium which generally found in the human upper respiratory tract as well as on the surface of normal skin [12, 13]. S. aureus is an important human pathogen that causes a wide variety of community-acquired infections such as skin infections, cutaneous or subcutaneous abscesses, pneumonia, osteomyelitis, septic arthritis, infective endocarditis and food poisoning [13, 14]. S. aureus shows resistance to various antibiotics and antimicrobial agents such as detergents and alcohol and also can produce several toxins that causes serious disease in humans by direct or indirect [12, 15]. This microorganism is considered as one of the most public health concerns for physicians and health care personnel in hospitals due to the cellular and tissue damage as well as high resistance to antimicrobial agents [16, 17]. Despite advances in the development of new antimicrobial agents for controlling nosocomial infections, but yet both S .aureus and P. aeruginosa infections are dramatically on the rise [18]. In the last decades, different strategies have been applied for prevention and control hospital infections includes, UV radiation, chemical substances, etc. [19]. Utilizing low-risk active substances is an alternatives approach to minimize adverse effects of chemical disinfectants, additionally herbal essential oils are an example of these substances, which, in comparison to chemical compounds have very low side effects on human health. Therefore because of environmental hazards in hospitals, using herbal ingredients has attracted a great interest in the recent years [20–22]. Carvacrol (2-methyl-5-(1-methylethyl)-phenol), is a monoterpenic phenol found in essential oils of numerous aromatic plants, and as safe active ingredient have various therapeutic effects [23]. Carvacrol has long been known to possess biological properties including anti-inflammatory, anticancer, antioxidant, antibacterial, antifungal, and insecticidal [24–32]. Conventionally glutaraldehyde 2%, which is known as a cold reference disinfectant, is used to sterilize hospital surfaces and heat-sensitive medical instruments such as surgical tools, suction bottles, dialysis equipment, endoscopes, bronchoscopes, ear, nose and throat equipment [33, 34]. Because of concern for great environmental toxicity of glutaraldehyde 2%, it is crucial to limit its use. Hence, safe antibacterial compounds as alternatives are potentially to develop [33]. Carvacrol can be interacts with two layers of lipids in the cytoplasmic membrane of bacteria due to the hydrophobic properties, which results in its break down and leakage of cellular materials such as ions, adenosine triphosphate (ATP) and nucleic acid. In other hand, the mechanism of action of carvacrol is similar to that of phenolic compounds, (i.e. increasing the permeability of bacterial membrane to hydrogen and potassium ions) resulting in ATP depletion and consequently cell death [25, 35, 36].
According to potential toxicity of the common chemical disinfectants such as glutaraldehyde to humans and animals, it seems necessary to search for novel safer antibacterial compounds without or with low toxicity. Therefore, the present study was conducted to assess the antibacterial activity of carvacrol against P. aeruginosa and S. aureus in comparison with the glutaraldehyde as a common chemical disinfectant.
Materials and methods
Bacterial strains and sample collection
Carvacrol essential oil with a purity of 92% was obtained from khorramman pharmaceutical company (Khorramabad- Iran) and was subjects to antibacterial activity. Chemicals, reagents and media cultures used in this study were supplied in laboratory grade from Merck (Germany) and Sigma Aldrich (USA) companies. P. aeruginosa ATCC 27853 and S. aureus ATCC 29213 were provided from Pasteur Institute of Iran and were used as standard strains. In addition, a total of 365 environmental samples were collected from different wards (surface and medical equipment) of the Shahid Rahimi, Shahid Madani, Shohadaie Ashaier and Shafa hospitals of the Khorramabad –city (Iran). All samples were obtained during twelve months from March 2016 to April 2017. The isolates from samples were identified and verified according to standard microbiological and biochemical characteristics. Of the 365 samples, a total of 160 clinical strains were isolated, of which 54 and 106 were confirmed as P. aeruginosa and S. aureus, respectively.
Antimicrobial susceptibility testing
The minimum inhibitory concentration (MIC) as well as the minimum bactericidal concentration (MBC) were used to determine susceptibilities of bacteria and also to evaluate the antibacterial efficacy. Ethanol and DMSO emulsifiers were applied as a solvent or co-solvent for better dissolution of the carvacrol phenolic solution in the culture media. The optimum solubility of carvacrol in the ethanol and DMSO were selected based on previous studies [25–27, 37, 38], in which 50 μL of carvacrol was added to 950 mL of emulsifiers. In parallel, 1 ml of 2% and 50% glutaraldehyde solutions in water (v/v) were used. Moreover, disc diffusion method was done for zone determination according to CLSI [39] for antibacterial susceptibility assay of the P .aeruginosa and S. aureus isolates.
MIC and MBC determination
To determine the MIC and MBC, a series of 10 sterile test tubes was labeled and numbered for each bacterial strains and antimicrobial agents with Nutrient Broth (NB) as the culture medium. Briefly, following standardized guidelines, the culture of the P. aeruginosa and S. aureus isolates done to obtain a cell concentration equal to 1.5 × 108 CFU/mL. Different concentrations of two-fold serial dilutions (V/V) of carvacrol and or 2% GA were well mixed with equivalent volumes of a sterile liquid growth medium (NB). Suspensions were then serially diluted in glass tubes contain NB. Subsequently 50 μL of suspended bacteria (1.5 × 108 CFU/mL) were added to each glass tubes and incubated at 37 °C for overnight. The MIC value were determined by measuring the turbidity within the glass tubes. The MBC value was determined by adding and spreading 100 μL of suspension from glass tubes on the Mueller-Hinton Agar (MHA) plates showing no visible growth until incubated at 37 °C for overnight. Each assay was done in triplicates at each concentration with positive and negative controls.
Disk diffusion method
The inhibition zone of both the carvacrol and 2% GA were determined using the Bauer-Kirby disk diffusion method (DDM) [40] against P .aeruginosa and S. aureus isolates. Briefly a culture of test bacteria was grown in a fresh nutrient broth (NB) medium at 37 °C to reach to the 0.5 Mc Farland turbidometry (108 CFU mL − 1). Then 100 μL of bacterial suspensions were loaded the plates containing the Mueller-Hinton agar and were directly spread with a swab onto the agar plate. Standard blank paper discs 6 mm in diameter (Padtan Teb Inc., Tehran, Iran) were saturated with 20 μL of various concentrations of carvacrol and 2% glutaraldehyde of MIC, 2MIC, and 4MIC per disc under sterile conditions. Subsequently, the paper discs were placed onto agar plates enriched with test bacterial inoculum and incubated at 37 °C overnight. Blank discs without adhesive and enriched with ethanol and or DMSO were applied as controls. The antibacterial activity was expressed by measuring the diameter of the growth inhibition zone (mm) around each paper disk using a ruler. An antibacterial activity with mean zones of inhibition were recorded using the following category; zone 0–10 mm (Resistance), 11–15 mm (Semi sensitive) and > 16 mm (Sensitive).
Time-kill kinetics assay analysis
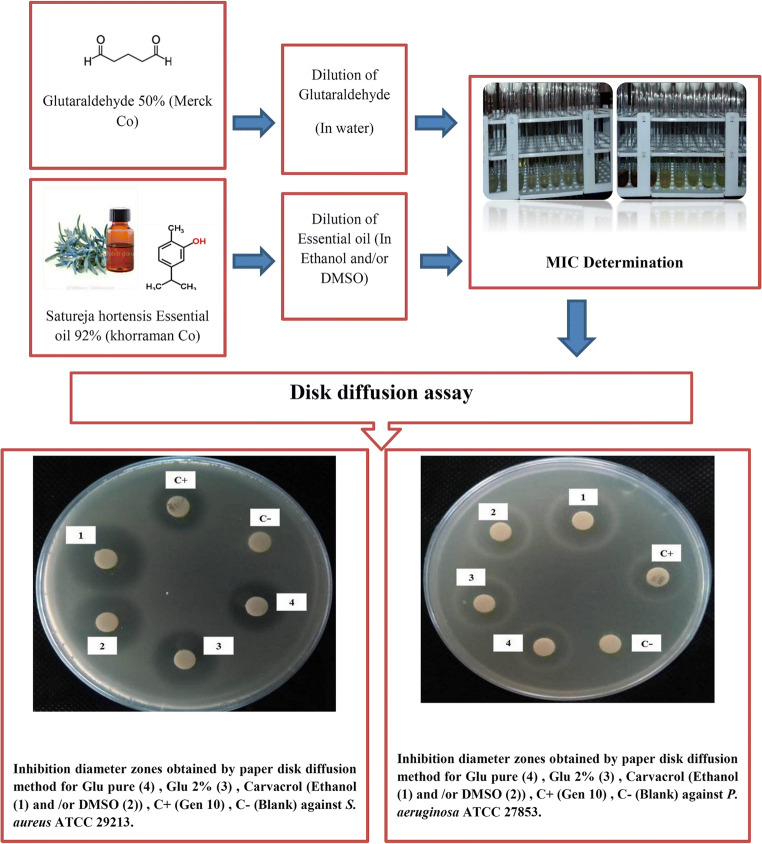
In order to determine the concentration and appropriate time for carvacrol disinfection, the kinetics of antibacterial of the carvacrol essential was performed based on a method described by May et al. [41] with slightly modification. The overnight broth culture of the tested microorganisms was adjusted to a 0.5 McFarland standard. Concentrations equal to MIC/2, MIC, 2MIC, 4MIC, 8MIC of the carvacrol were prepared and added to each tube contain bacterial strains, then all tubes incubated at 37 °C for 20 min, 1, 2, 4 and 6 h with shaken at 150 rpm. The assessment of CFU/ml counts procedure were performed by the inoculation 50 -μL of cultures onto pre-warmed plates of MHA and incubated for 24 h at 37 °C for each bacterial strain. The visible bacterial growth was checked. The least time and concentration, in which the bacteria were not grown, was considered as the optimal time and concentration of disinfection. Results were taken as positive and negative. The positive was considered that which showed visible growth after Incubation. The negative was considered that which showed no growth after incubation. Figure 1 summarizes schematically the experimental process in this study.
Fig. 1.
Schematic of the experimental process utilizing in this study, including: preparation of the used disinfectants, antimicrobial susceptibility testing, MIC and MBC determination
Statistical analysis
SPSS software (Version 20; SPSS Inc., Chicago, USA) was used for the statistical analysis. All tests were performed in triplicate and the results are expressedas mean ± standard deviation (SD) of these replicates. The results related to the zone of inhibition were then analyzed using one-way analysis of variance (ANOVA) followed by Tukey test with a p value of 0.05 or less as statistically significant.
Results
The MICs of carvacrol+ethanol ranged from 8 μg/mL against S. aureus (n = 106 strains) to 32 μg/mL against P. aeruginosa (n = 54 strains), while those of carvacrol + DMSO ranged from 8 μg/mL against S. aureus to 125 μg/mL against P. aeruginosa hospital-acquired strains (Table 1). Our results indicated that the MIC of the carvacrol (with both emulsion spheres) was significantly lower compared to the MIC of glutaraldehyde 2%. According to the disk diffusion results, carvacrol+ethanol and carvacrol + DMSO showed consistently stronger antimicrobial activity against tested bacteria than both glutaraldehyde 2% and pure glutaraldehyde at different diluted concentrations (MIC, 2MIC, and 4MIC per disk). The results of disk diffusion are shown in Table 2 and Fig. 1, in which represent the diameter of inhibition zone including diameter of paper disk (6 mm). The disk diffusion results also revealed that the antibacterial activity of carvacrol+ethanol and carvacrol + DMSO was non-significantly higher against S. aureus compared to P. aeruginosa strains. Table 3 indicates the power of antimicrobial activity of carvacrol compared to glutaraldehyde 2% and pure glutaraldehyde.
Table 1.
Results of Minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of carvacrol and glutaraldehydes against S, aureus and P. aeruginosa
| Antimicrobial agent | Pseudomonas aeruginosa | Staphylococcus aureus | ||||||
|---|---|---|---|---|---|---|---|---|
| Hospital strain | Standard strain | Hospital strain | Standard strain | |||||
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | |
| Carvacrol+ethanol | 32 | 64 | 32 | 64 | 8 | 8 | 16 | 16 |
| Carvacrol +DMSO | 125 | 250 | 64 | 125 | 8 | 8 | 16 | 16 |
| Glutaraldehyde 2% | 250 | 500 | 250 | 250 | 250 | 500 | 250 | 500 |
| Glutaraldehyde Pure | 8 | 16 | 8 | 8 | 8 | 8 | 8 | 8 |
| Ethanol | – | – | 500 | 500 | – | – | 500 | 1000 |
| DMSO | – | – | 1000 | 1000 | – | – | 500 | 1000 |
The serial two -fold dilutions from 1000 μl/ml to 2 μl/ml has been prepared with culture medium
Table 2.
The mean diameter of the growth inhibition zones (mm) of the bacterial strains based on the results of disc diffusion test for used antimicrobial agents
| Antimicrobial agents |
The diameter of inhibition zone (mm) (Mean ± SD) |
|||||
|---|---|---|---|---|---|---|
| Pseudomonas aeruginosa | Staphylococcus aureus | |||||
| MIC | 2MIC | 4MIC | MIC | 2MIC | 4MIC | |
| Carvacrol+ethanol | 17.5 ± 0.54a | 18 ± 0 | 20 ± 0 | 19 ± 0 | 19.5 ± 0.54 | 20.5 ± .54 |
| Carvacrol +DMSO | 15 ± 0 | 17.5 ± 0.54 | 19 ± 1.09 | 17 ± 0 | 19 ± 0 | 19 ± 0 |
| Glutaraldehyde 2% | 13.5 ± 0.54 | 15 ± 0 | 16 ± 0.09 | 13 ± 1.09 | 14.5 ± 0.54 | 17 ± 1.09 |
| Glutaraldehyde pure | 14 ± 0 | 14 ± 0 | 15 ± 0 | 12 ± 0 | 15 ± 0 | 17.5 ± 0.54 |
aThe mean diameter of the growth inhibition zones (mm) ± standard deviation (SD)
Table 3.
Results of antimicrobial agents’ performance against P. aeruginosa and S. aureus
| Antimicrobial agents | Antimicrobial activity function | |||||
|---|---|---|---|---|---|---|
| Pseudomonas aeruginosa | Staphylococcus aureus | |||||
| MIC | 2MIC | 4MIC | MIC | 2MIC | 4MIC | |
| Carvacrol+ethanol | Strength | Strength | Strength | Strength | Strength | Strength |
| Caravecrol +DMSO | Strength | Strength | Strength | Strength | Strength | Strength |
| Glutaraldehyde 2% | Medium | Strength | Strength | Medium | Medium | Strength |
| Glutaraldehyde Pure | Medium | Medium | Strength | Medium | Strength | Strength |
The optimal concentration and time of antibacterial activity of Carvacrol was also examined on S. aureus and P. aeruginosa strains using Time-Kill kinetics assay (Tables 4 and 5). Accordingly, our experiments showed that the optimal time of antibacterial activity of carvacrol+ethanol against S. aureus was 1 h at concentration of 64 μl/ml; and about carvacrol+DMSO was 2 h at concentration of 250 μl/ml. In addition, we found that the optimal time of antibacterial activity of carvacrol+ethanol against standard strain of P. aeruginosa was 1 h at concentration of 32 μl/ml (1MIC) and against hospital isolates of P. aeruginosa was 1 h at concentration of 64 μl/ml (2MIC). Moreover, the optimal time of antibacterial activity of carvacrol+DMSO was 4 h at concentration of 125 μl/ml (2MIC) and 250 μl/ml (2MIC) against standard strain and hospital isolates of P. aeruginosa, respectively. Based on the obtained results, the optimal time and concentration of carvacrol+ethanol against standard strain of S. aureus was determined after 2 h at concentration of 16 μl/ml (1MIC); and about hospital isolates was 1 h at concentration of 8 μl/ml (1MIC). Additionally, the optimal time and concentration of carvacrol+DMSO against standard strain of S. aureus was after 6 h at concentration of 16 μl/ml (1MIC); and about hospital isolates was 4 h at concentration of 16 μl/ml (2MIC).
Table 4.
Comparison of the antibacterial activity of carvacrol by the two organic solvents of ethanol and DMSO against P. aeruginosa using time-kill assay
| Emulsifier | Pseudomonas aeruginosa hospital sample | Standard strain of Pseudomonas aeruginosa | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Time | 20 min | 1 h | 2 h | 4 h | 6 h | 20 min | 1 h | 2 h | 4 h | 6 h | |
| The carvacrol concentrations | |||||||||||
| DMSO | MIC/2 | + | + | + | + | + | + | + | + | + | + |
| MIC | + | + | + | + | + | + | + | + | + | + | |
| 2MIC | + | + | + | – | – | + | + | + | – | – | |
| 4MIC | – | – | – | – | – | – | – | – | – | – | |
| 8MIC | – | – | – | – | – | – | – | – | – | – | |
| Ethanol | MIC/2 | + | + | + | + | + | + | + | + | + | + |
| MIC | + | + | + | + | + | + | – | – | – | – | |
| 2MIC | + | – | – | – | – | – | – | – | – | – | |
| 4MIC | – | – | – | – | – | – | – | – | – | – | |
| 8MIC | – | – | – | – | – | – | – | – | – | – | |
+ Represents: observable colony on an agar plate
–Represents: lack of colony growth on agar plate
Table 5.
Comparison of the antibacterial activity of carvacrol by the two organic solvents of Ethanol and DMSO against S. aureus using time-kill assay
| Emulsifier | Staphylococcus aureus hospital sample | Standard strain of Staphylococcus aureus | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Time | 20 min | 1 h | 2 h | 4 h | 6 h | 20 min | 1 h | 2 h | 4 h | 6 h | |
| The carvacrol concentrations | |||||||||||
| DMSO | MIC/2 | + | + | + | + | + | + | + | + | + | + |
| MIC | + | + | + | – | – | + | + | + | + | – | |
| 2MIC | – | – | – | – | – | + | + | – | – | – | |
| 4MIC | – | – | – | – | – | – | – | – | – | – | |
| 8MIC | – | – | – | – | – | – | – | – | – | – | |
| Ethanol | MIC/2 | + | + | + | + | + | + | + | + | + | + |
| MIC | + | – | – | – | – | + | + | – | – | – | |
| 2MIC | – | – | – | – | – | – | – | – | – | – | |
| 4MIC | – | – | – | – | – | – | – | – | – | – | |
| 8MIC | – | – | – | – | – | – | – | – | – | – | |
+ Represents: observable colony on an agar plate
–Represents: lack of colony growth on agar plate
Discussion
The development and production of novel natural antimicrobial products such as plant essential oils and their main active components is considered as an alternative strategy to fight antimicrobial-resistant bacteria. In addition, it is useful to combine existing antimicrobial agents with phytochemicals to enhance the efficacy of disinfectants [42]. Among natural antibacterial components, carvacrol, in which found in essential oils from Thymus vulgaris, Lepidium flavum, and Origanum vulgare, has been demonstrated to have strong antibacterial activity against pathogenic bacteria. The antimicrobial activity of carvacrol has been described more significant than that of other volatile compounds present in essential oils, which is due to the presence of the free hydroxyl group, hydrophobic substances, and the phenol moiety [43].
In this study, the antimicrobial activity of carvacrol was assessed against hospital environmental isolates of P. aeruginosa and S. aureus as well as the related-standard strains. Accordingly, we compared the antimicrobial activity of carvacrol with glutaraldehyde. Our experiments revealed a high antimicrobial activity of carvacrol against hospital environmental isolates of P. aeruginosa and S. aureus, with MICs lower than those found for glutaraldehyde 2%. Previously, Magi et al. suggested that bactericidal action of carvacrol is due to the interaction of its hydrophobic compounds with the membrane of bacterial cells and membrane damage [27].
In general, when comparing different used solvents, it was found that carvacrol with ethanol-aqueous solvent has stronger antibacterial activity power than DMSO. Indeed, our experimental findings revealed that the MIC of carvacrol in ethanol was less than DMSO emulsifier, which indicated a stronger antimicrobial activity of carvacrol when used with ethanol solvent. Regarding the antimicrobial activity of ethanol, it can be supposed that ethanol solvent increases the free hydroxyl group and the accessible level of carvacrol to the membrane of bacteria compared to DMSO. The impact of used solvents on antibacterial activity of essential oils was previously described by several authors. Quintas et al. investigated the effect of various solvents on antibacterial properties of natural components, and described that antimicrobial activities of these agents in the presence of the ethanol as solution were significantly higher than other used solvents [44]. Additionally, it should be noted that the concentration of carvacrol required to dissolve in ethanol is lesser than DMSO. Moreover, ethanol is more affordable and economical in terms of cost-effectiveness compared to DMSO. Our findings also indicated that carvacrol+ethanol showed a same MIC and MBC values against type strains and environmental isolates, while carvacrol + DMSO showed a higher MIC and MBS against hospital environmental isolates compared to type strains. Therefore, these data demonstrated that carvacrol+ethanol formulation performed better in antimicrobial activity than carvacrol + DMSO.
Our findings also indicated that S. aureus strains were more susceptible against carvacrol than P. aeruginosa strains. In agreement with our results, Ben Arfa et al. reported that carvacrol exhibited a weaker antimicrobial activity against Pseudomonas fluorescens compared to S. aureus [38]. This weakest antimicrobial activity could be attributed to the presence of outer membrane in addition to the cell membrane in gram negative bacteria, in which this double membrane structure plays a main role in preventing the accessibility of disinfectants to inside of bacterial cells [45].
According to the time-kill kinetics assay, the optimal time and concentration of carvacrol+ethanol disinfection on P. aeruginosa was determined 4 h at concentration of 64 μl/ml and on S. aureus was 1 h at concentration of 64 μl/ml. Xu et al. reported that 100 mg\L of carvacrol could not inhibit the growth of E. coli. However, the number of viable E. coli cells reduced significantly with the increased concentration of carvacrol after 6 h [25]. In another study, Khan et al. described that carvacrol completely prevent the growth of E. coli after 2 h of incubation at the concentration of 450 μg/mL, which was determined as 1MIC [46].
Conclusion
Overall, results of the current study suggest a potential use of carvacrol as a natural disinfectant against hospital environmental isolates of P. aeruginosa and S. aureus. Our findings revealed that the antibacterial activity of carvacrol is higher than that of glutaraldehyde2%. Moreover, the evidence presented in this study suggest a more-efficient antimicrobial activity provided by carvacrol+ethanol formulation compared to carvacrol + DMSO. In particular, carvacrol might be more active against hospital environmental isolates in combination with ethanol. Due to the fact that herbal medications are innocuous and have low toxicity, it can be a good option for cold disinfection using by carvacrol+ethanol formulation. To enhance the knowledge on this field, researchers are recommended to combine carvacrol with other herbal essential oils, various emulsifiers, and even nanoparticles, against opportunistic pathogens in various environmental conditions to achieve a proper and applicable outcome.
Acknowledgements
The authors would like to gratefully appreciate the Lorestan University of Medical Sciences and the khorramman pharmaceutical company, Khorramabad- Iran, for financially supporting this work under grant P/15/1190.
Declarations
Conflict of interest
The authors have no conflict of interest to declare.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Tayebeh Hasanvand, Email: tayebehhasanvand@gmail.com.
Mohsen Mohammadi, Email: mohamadi.m@lums.ac.ir, Email: mohamadi419@yahoo.com.
Foad Abdollahpour, Email: f.abdollahpour@modares.ac.ir.
Bahram Kamarehie, Email: b.kamarehie@gmail.com.
Ali Jafari, Email: jafari_a99@yahoo.com.
Afshin Ghaderpoori, Email: mghaderpoori@gmail.com.
Mohammad Amin Karami, Email: karami.mohammadamin@yahoo.com.
References
- 1.Sahlabadi F, Zandi H, Mokhtari M, Jamshidi S, Jasemizad T, Montazeri A, et al. The effectiveness evaluation of current disinfectants on pathogens isolated from surface of different parts of Shahid Sadughi accidents burns Hospital in City of Yazd. J Environ Health Eng. 2016;3(2):93–101. [Google Scholar]
- 2.Schabrun S, Chipchase L. Healthcare equipment as a source of nosocomial infection: a systematic review. J Hosp Infect. 2006;63(3):239–245. doi: 10.1016/j.jhin.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 3.Ducel G, Fabry J, Nicolle L. Prevention of hospital acquired infections: a practical guide. Prevention of hospital acquired infections: a practical guide. 2002. [Google Scholar]
- 4.Oliveira PS, Souza SG, Campos GB, da Silva DC, Sousa DS, Araújo SP, et al. Isolation, pathogenicity and disinfection of Staphylococcus aureus carried by insects in two public hospitals of Vitória da Conquista, Bahia, Brazil. Braz J Infect Dis. 2014;18(2):129–136. doi: 10.1016/j.bjid.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hadadi A, RASOULINEZHAD M, Afhami S, Mohraz M. Epidemiological, clinical, Para clinical aspects of brucellosis in imam Khomeini and Sina Hospital of Tehran (1998–2005) 2006. [Google Scholar]
- 6.Hadizadeh M, Norouzi A, Taghadosi R, Mohebi S, Mohammadi M, Hasanzade A, Moghadam MT. Prevalence of qnr, intI, and intII genes in extendedspectrum beta-lactamase (ESBL)-producing Escherichia coli isolated from clinical samples in Iran. Trop J Pharm Res. 2017;16(1):141–147. [Google Scholar]
- 7.Jarvis WR, Martone WJ. Predominant pathogens in hospital infections. J Antimicrob Chemother. 1992;29(suppl_A):19–24. doi: 10.1093/jac/29.suppl_a.19. [DOI] [PubMed] [Google Scholar]
- 8.Al-Jubory SA, Naher HS, Saleh RH. A study of efficacy of disinfectants and bacterial contamination in Al-Hilla teaching hospital. Med J Babylon. 2012;9(4):890–900. [Google Scholar]
- 9.Sharma G, Rao S, Bansal A, Dang S, Gupta S, Gabrani R. Pseudomonas aeruginosa biofilm: potential therapeutic targets. Biologicals. 2014;42(1):1–7. doi: 10.1016/j.biologicals.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 10.Manafi A, Kohanteb J, Mehrabani D, Japoni A, Amini M, Naghmachi M, et al. Active immunization using exotoxin a confers protection against Pseudomonas aeruginosa infection in a mouse burn model. BMC Microbiol. 2009;9(1):1–5. doi: 10.1186/1471-2180-9-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Finck-Barbançon V, Goranson J, Zhu L, Sawa T, Wiener-Kronish JP, Fleiszig SM, et al. ExoU expression by Pseudomonas aeruginosa correlates with acute cytotoxicity and epithelial injury. Mol Microbiol. 1997;25(3):547–557. doi: 10.1046/j.1365-2958.1997.4891851.x. [DOI] [PubMed] [Google Scholar]
- 12.Missiakas DM, Schneewind O. Growth and laboratory maintenance of Staphylococcus aureus. Curr Protoc Microbiol. 2013;28(1):9C. 1–9C. 1.9. doi: 10.1002/9780471729259.mc09c01s28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Control CD. Prevention. Outbreaks of community-associated methicillin-resistant Staphylococcus aureus skin infections--Los Angeles County, California, 2002–2003. MMWR Morb Mortal Wkly Rep. 2003;52(5):88. [PubMed] [Google Scholar]
- 14.Boucher HW, Corey GR. Epidemiology of methicillin-resistant Staphylococcus aureus. Clin Infect Dis. 2008;46(Supplement_5):S344–S3S9. doi: 10.1086/533590. [DOI] [PubMed] [Google Scholar]
- 15.Rasigade J-P, Vandenesch F. Staphylococcus aureus: a pathogen with still unresolved issues. Infect Genet Evol. 2014;21:510–514. doi: 10.1016/j.meegid.2013.08.018. [DOI] [PubMed] [Google Scholar]
- 16.Mihai MM, Holban AM, Giurcaneanu C, Popa LG, Buzea M, Filipov M, et al. Identification and phenotypic characterization of the most frequent bacterial etiologies in chronic skin ulcers. Rom J Morphol Embryol. 2014;55:1401–8. [PubMed]
- 17.Gebreyesus A, Gebre-Selassie S, Mihert A. Nasal and hand carriage rate of methicillin resistant Staphylococcus aureus (MRSA) among health care workers in Mekelle hospital, North Ethiopia. Ethiop Med J. 2013;51(1):41–47. [PubMed] [Google Scholar]
- 18.Khalafi T, Mohebbi SR, Moradi F, Khanipour F, Mahmoudian R, Montaseri M, et al. The trend of antibiotic resistance of Staphylococcus aureus isolated in clinical specimens in a referral hospital Shahid mohammadi hospital at Bandar abbas, south of Iran (2009-2014) Int Electron J Med. 2017;6(2):53–57. [Google Scholar]
- 19.Zschöck M, El-Sayed A, Eissa N, Lämmler C, Castañeda-Vazquez H. Resistencia a penicilina G y oxacilina, de cepas de Staphylococcus aureus aisladas de mastitis bovina subclínica. Vet Méx. 2011;42(3):207–217. [Google Scholar]
- 20.Velazquez-Meza ME, Hernández-Salgado M, Sánchez-Alemán MA. Evaluation of the antimicrobial activity of a super oxidized solution in clinical isolates. Microb Drug Resist. 2015;21(4):367–372. doi: 10.1089/mdr.2014.0266. [DOI] [PubMed] [Google Scholar]
- 21.Nostro A, Roccaro AS, Bisignano G, Marino A, Cannatelli MA, Pizzimenti FC, Cioni PL, Procopio F, Blanco AR. Effects of oregano, carvacrol and thymol on Staphylococcus aureus and Staphylococcus epidermidis biofilms. J Med Microbiol. 2007;56(4):519–523. doi: 10.1099/jmm.0.46804-0. [DOI] [PubMed] [Google Scholar]
- 22.Bagamboula C, Uyttendaele M, Debevere J. Inhibitory effect of thyme and basil essential oils, carvacrol, thymol, estragol, linalool and p-cymene towards Shigella sonnei and S. flexneri. Food Microbiol. 2004;21(1):33–42. [Google Scholar]
- 23.Liang WZ, Lu CH. Carvacrol-induced [Ca2+] i rise and apoptosis in human glioblastoma cells. Life Sci. 2012;90(17–18):703–711. doi: 10.1016/j.lfs.2012.03.027. [DOI] [PubMed] [Google Scholar]
- 24.Arunasree K. Anti-proliferative effects of carvacrol on a human metastatic breast cancer cell line, MDA-MB 231. Phytomedicine. 2010;17(8–9):581–588. doi: 10.1016/j.phymed.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 25.Xu J, Zhou F, Ji BP, Pei RS, Xu N. The antibacterial mechanism of carvacrol and thymol against Escherichia coli. Lett Appl Microbiol. 2008;47(3):174–179. doi: 10.1111/j.1472-765X.2008.02407.x. [DOI] [PubMed] [Google Scholar]
- 26.Marinelli L, Di Stefano A, Cacciatore I. Carvacrol and its derivatives as antibacterial agents. Phytochem Rev. 2018;17(4):903–921. [Google Scholar]
- 27.Magi G, Marini E, Facinelli B. Antimicrobial activity of essential oils and carvacrol, and synergy of carvacrol and erythromycin, against clinical, erythromycin-resistant group a streptococci. Front Microbiol. 2015;6:165. doi: 10.3389/fmicb.2015.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kachur K, Suntres Z. The antibacterial properties of phenolic isomers, carvacrol and thymol. Crit Rev Food Sci Nutr. 2020;60(18):3042–53. [DOI] [PubMed]
- 29.Memar MY, Raei P, Alizadeh N, Aghdam MA, Kafil HS. Carvacrol and thymol: strong antimicrobial agents against resistant isolates. Rev Med Microbiol. 2017;28(2):63–68. [Google Scholar]
- 30.Chavan PS, Tupe SG. Antifungal activity and mechanism of action of carvacrol and thymol against vineyard and wine spoilage yeasts. Food Control. 2014;46:115–120. [Google Scholar]
- 31.Nobrega RO, Teixeira APdC, Oliveira WAd, Lima EdO, Lima IO. Investigation of the antifungal activity of carvacrol against strains of Cryptococcus neoformans. Pharm Biol. 2016;54(11):2591–2596. doi: 10.3109/13880209.2016.1172319. [DOI] [PubMed] [Google Scholar]
- 32.Park J-H, Jeon Y-J, Lee C-H, Chung N, Lee H-S. Insecticidal toxicities of carvacrol and thymol derived from Thymus vulgaris Lin. against Pochazia shantungensis Chou & Lu., newly recorded pest. Sci Rep. 2017;7:40902. doi: 10.1038/srep40902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Herruzo-Cabrera R, Uriarte M, Rey-Calero J. Antimicrobial effectiveness of 2% glutaraldehyde versus other disinfectants for hospital equipment, in an in vitro test based on germ-carriers with a high microbial contamination. Rev Stomatol Chir Maxillofac. 1999;100(6):299. [PubMed] [Google Scholar]
- 34.Henn SA, Boiano JM, Steege AL. Precautionary practices of healthcare workers who disinfect medical and dental devices using high-level disinfectants. Infect Control Hosp Epidemiol. 2015;36(2):180–5. doi: 10.1017/ice.2014.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liolios C, Gortzi O, Lalas S, Tsaknis J, Chinou I. Liposomal incorporation of carvacrol and thymol isolated from the essential oil of Origanum dictamnus L. and in vitro antimicrobial activity. Food Chem. 2009;112(1):77–83. [Google Scholar]
- 36.McGucken PV, Woodside W. Studies on the mode of action of glutaraldehyde on Escherichia coli. J Appl Bacteriol. 1973;36(3):419–426. doi: 10.1111/j.1365-2672.1973.tb04123.x. [DOI] [PubMed] [Google Scholar]
- 37.Pol IE, Krommer J, Smid EJ. Bioenergetic consequences of nisin combined with carvacrol towards Bacillus cereus. Innovative Food Sci Emerg Technol. 2002;3(1):55–61. [Google Scholar]
- 38.Ben Arfa A, Combes S, Preziosi-Belloy L, Gontard N, Chalier P. Antimicrobial activity of carvacrol related to its chemical structure. Lett Appl Microbiol. 2006;43(2):149–154. doi: 10.1111/j.1472-765X.2006.01938.x. [DOI] [PubMed] [Google Scholar]
- 39.Clinical, Institute LS . Performance standards for antimicrobial susceptibility testing. Wayne, PA: Clinical and Laboratory Standards Institute; 2017. [Google Scholar]
- 40.Bayer A, Kirby W, Sherris J, Turck M. Antibiotic susceptibility testing by a standardized single disc method. Am J Clin Pathol. 1966;45(4):493–496. [PubMed] [Google Scholar]
- 41.May J, Chan C, King A, Williams L, French G. Time–kill studies of tea tree oils on clinical isolates. J Antimicrob Chemother. 2000;45(5):639–643. doi: 10.1093/jac/45.5.639. [DOI] [PubMed] [Google Scholar]
- 42.Saccucci M, Bruni E, Uccelletti D, Bregnocchi A, Sarto MS, Bossù M, et al. Surface disinfections: present and future. J Nanomater. 2018;2018:8950143. [Google Scholar]
- 43.Sharifi-Rad M, Varoni EM, Iriti M, Martorell M, Setzer WN, Del Mar CM, et al. Carvacrol and human health: a comprehensive review. Phytother Res. 2018;32(9):1675–1687. doi: 10.1002/ptr.6103. [DOI] [PubMed] [Google Scholar]
- 44.Quintas V, Prada-López I, Carreira MJ, Suárez-Quintanilla D, Balsa-Castro C, Tomás I. In Situ Antibacterial Activity of Essential Oils with and without Alcohol on Oral Biofilm: A Randomized Clinical Trial. Front Microbiol. 2017;8:2162. doi: 10.3389/fmicb.2017.02162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Heinzel M. Phenomena of biocide resistance in microorganisms. Int Biodeterior Biodegradation. 1998;41(3):225–234. [Google Scholar]
- 46.Khan I, Bahuguna A, Kumar P, Bajpai VK, Kang SC. Antimicrobial potential of carvacrol against uropathogenic Escherichia coli via membrane disruption, depolarization, and reactive oxygen species generation. Front Microbiol. 2017;8(2421):1–9. [DOI] [PMC free article] [PubMed]